Abstract
An abundant presynaptic protein, α-synuclein, is centrally involved in the pathogenesis of Parkinson's disease. However, conflicting data exist about the normal function of α-synuclein, possibly because α-synuclein is redundant with the very similar β-synuclein. To investigate the functions of synucleins systematically, we have now generated single- and double-knockout (KO) mice that lack α- and/or β-synuclein. We find that deletion of synucleins in mice does not impair basic brain functions or survival. We detected no significant changes in the ultrastructure of synuclein-deficient synapses, in short- or long-term synaptic plasticity, or in the pool size or replenishment of recycling synaptic vesicles. However, protein quantitations revealed that KO of synucleins caused selective changes in two small synaptic signaling proteins, complexins and 14-3-3 proteins. Moreover, we found that dopamine levels in the brains of double-KO but not single-KO mice were decreased by ≈20%. In contrast, serotonin levels were unchanged, and dopamine uptake and release from isolated nerve terminals were normal. These results show that synucleins are not essential components of the basic machinery for neurotransmitter release but may contribute to the long-term regulation and/or maintenance of presynaptic function.
The abundant soluble protein α-synuclein is highly enriched in nerve terminals (1-4) and has a central role in the pathogenesis of Parkinson's disease (5, 6). Pathologically, Parkinson's disease is characterized by Lewy bodies, which are eosinophilic inclusions in the neuronal cytoplasm that contain α-synuclein filaments (7). Genetically, a subset of familial Parkinson's disease patients harbors a mutant α-synuclein gene (6, 8-10). Thus, α-synuclein is linked to Parkinson's disease both genetically and neuropathologically.
Although the role of α-synuclein in Parkinson's disease is well established, its normal function remains unclear. Synuclein is absent from invertebrates, suggesting that it is not essential for synaptic transmission but may be involved in a vertebrate-specific specialization of synapses (e.g., synaptic plasticity). This conclusion is supported by the relatively late translocation of α-synuclein into presynaptic terminals during synaptogenesis, after functional synapses have been established (11). However, investigation of synapses lacking α-synuclein led to conflicting results. Whereas the original characterization of α-synuclein KO mice failed to detect major changes in the structure and function of synapses [only a minor use-dependent alteration of dopamine release was observed (12)], subsequent studies indicated that deletion of α-synuclein induces a massive loss of synaptic vesicles from nerve terminals, a decrease in synaptic responses during repetitive stimulation, and an impairment in the recovery of synaptic responses from use-dependent depression (13, 14). These studies have led to the notion that, consistent with its localization, α-synuclein may regulate synaptic plasticity (reviewed in ref. 15).
A confounding problem in studying α-synuclein is the presence of β- and γ-synucleins, two highly homologous isoforms (1-3, 16-20) (reviewed in ref. 21). The α- and β-synucleins are widely colocalized in presynaptic nerve terminals throughout the brain but absent from peripheral tissues. In contrast, γ-synuclein is largely absent from forebrain (13, 19) but is abundant in specialized neurons, such as dorsal root ganglia (17). It is also present in nonneuronal tissues (19, 20) and overexpressed in breast cancer cells (18). The high degree of coexpression of α- and β-synuclein indicates the potential for functional redundancy, which may have obscured an essential role of α-synuclein in α-synuclein KO mice. To address this possibility, we have now generated single- and double-KO mice of α- and β-synucleins. Our data demonstrate that the synucleins are not essential for basic synaptic functions (e.g., neurotransmitter release, synaptic vesicle numbers and pools, and synaptic plasticity) but are redundantly required for maintaining normal dopamine levels in the nigrostriatal system.
Materials and Methods
General. All data are given as mean ± SEM (except for ultrastructural data, which are given as mean ± SD), and all tests for statistical significance were performed with Student's t test.
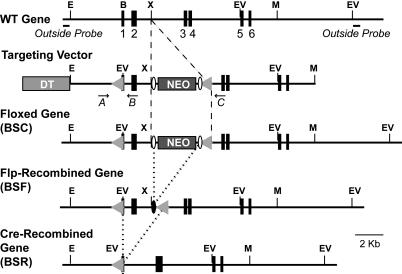
Generation of β-Synuclein KO Mice. By using genomic clones containing the entire β-synuclein gene, we constructed a targeting vector in which the first coding exon is flanked by loxP sites (Fig. 1). The vector was used in R1 embryonic stem cells (22) for standard homologous recombination experiments (23). The resulting mutant mice were crossed with protamine-cre transgenic mice (24) to remove the floxed exon and β-actin Flp transgenic mice (25) to remove the neomycin cassette. Genotyping was performed by PCR using the following oligonucleotide primers: SC02105, 5′-AGGACACCACTGGCCCCGAGTCC-3′; SC02106, 5′-GACGCACGTCCGCACGTCCACCC-3′; and SC01114, 5′-TGCCCCTGAAATGCTGCGCC-3′, which generated a 320-bp WT, a 360-bp floxed, and a 300-bp cre-excised product, respectively. We obtained α/β-synuclein double-KO mice by crossing the β-synuclein KO mice with previously generated (26) α-synuclein KO mice.
Fig. 1.
Targeting strategy for the generation of β-synuclein KO mice. Exons are represented by numbered black boxes, and positions of strategic restriction enzyme sites are indicated as follows: E, EcoRI; B, BamHI; X, XhoI; V, EcoRV; and M, MfeI. The locations of the outside probes used for Southern blotting are marked in the WT gene structure, and locations of PCR primers used for genotyping are marked in the targeting-vector structure. The diphtheria toxin (DT) and neomycin-resistance gene cassettes (NEO) that were introduced into the targeting vector for negative and positive selection, respectively, are shown as light and dark boxes, respectively. LoxP recombination sites are indicated by gray triangles, and frt recombination sites are indicated by open ovals. Dashed lines indicate insertion of DNA into the WT gene, and dotted lines indicate removal of DNA by flp or cre recombinase.
Protein Quantitations. Brain homogenates from four adult littermate α-/-β+/+ and α-/-β-/- mice (≈2 months old) were analyzed by quantitative immunoblotting using 125I-labeled secondary antibodies and PhosphorImager detection (Molecular Dynamics) (26) with GDP-dissociation inhibitor (GDI) and vasolin-containing protein (VCP) as internal standards.
Electrophysiology. Vibratome-cut transverse hippocampal slices (400 μm thick) from 3- to 5-week-old mice were used for field recordings by using standard procedures (see Supporting Materials and Methods, which is published as supporting information on the PNAS web site, for a detailed description).
Optical Imaging. Dissociated cortical neurons from 2- to 3-day-old mice were cultured at high density and examined by FM1-43 staining at 14 days in vitro (27) (see Supporting Materials and Methods).
Light and Electron Microscopy. Brain sections from 2-month-old mice were examined by light and electron microscopy (28). For quantitation of dopaminergic neurons, coronal brain sections (30 μm thick) were stained with an antibody to tyrosine hydroxylase (TH; 1:2,000 dilution; Protos Biotech, New York), and the unbiased stereological optical fractionator method was used to count substantia nigra TH-positive neurons [n = 177-398 neurons per animal, with n = 4 male mice per genotype (8 total) (29)]. For quantitation of synaptic parameters, randomized digital electron micrographs taken from cultured neurons at day 14 in vitro (30) were examined. Data shown were obtained from three independent cultures that contributed equally to the final numbers.
Quantification of Striatal Monoamines. The dorsal striatum was dissected and sonicated in ice-cold 0.1 M perchloric acid containing 3,4-dihydroxybenzylamine (Sigma) as an internal standard. After centrifugation, dopamine, serotonin, and metabolites were analyzed in the supernatant by HPLC and coulometry (ref. 31; see Supporting Materials and Methods for detailed experimental procedures).
Neurotransmitter-Release and 3H-Dopamine-Uptake Assays. Synaptosomes from dorsal striata of WT and mutant mice were incubated with 3H-dopamine [20-40 Ci/mmol (1 Ci = 37 GBq); PerkinElmer], and uptake was measured as described in ref. 32 (see Supporting Materials and Methods for detailed experimental procedures). Neurotransmitter release was measured with synaptosomes loaded with 270 nM 3H-dopamine (59 Ci/mmol) and 8 μM [14C]-γ-aminobutyric acid (GABA) (240 mCi/mmol). Stimulus-dependent release of dopamine and GABA was monitored in a superfusion chamber (33, 34). For each time point, the fractional-release rate of dopamine and GABA was calculated as the fraction of released radioactivity divided by the amount of radioactivity remaining on the filter.
Results
Generation of β-Synuclein Knockout (KO) Mice. By using genomic clones containing the entire murine β-synuclein gene, we constructed a β-synuclein targeting vector in which the first coding exon is flanked by loxP sites (Fig. 1). We used this targeting vector for homologous recombination experiments with R1 embryonic stem cells (26), and we generated mice that transmitted the mutant β-synuclein allele through the germ line. We bred the mutant mice with each other and with transgenic mice that express either flp or cre recombinase in the germ line (24, 25). In this manner, we generated homozygous mutant mice that contained either the initially targeted gene, the targeted gene without the neomycin cassette, or the targeted gene lacking the first coding exon (Figs. 1 and 7, which is published as supporting information on the PNAS web site).
Homozygous mice containing the initially targeted mutant β-synuclein gene (BSC) did not express β-synuclein, as determined by immunoblotting (Fig. 7B), probably because the neomycin-resistance cassette is present in the mutant gene. Removal of the neomycin-resistance gene cassette with flp recombinase restored β-synuclein expression, whereas subsequent deletion of the first coding exon with cre-recombinase abolished β-synuclein expression again. Thus, we produced with our targeting strategy both conditional β-synuclein KO mice (after flp recombination only) and constitutive β-synuclein KO mice (after cre recombination). The conditional KO mice may prove to be useful in analyses of β-synuclein function in specific brain regions. However, in the present study, we focused on the constitutive β-synuclein KO mice because our goal was to establish the baseline function of synucleins.
Characterization of the α/β-Synuclein Double-KO Animals. Systematic breeding experiments revealed that homozygous β-synuclein KO mice were viable and fertile, either as single β-synuclein or as α/β-synuclein double KOs. When we examined the frequency of genotypes in adult offspring of heterozygous matings, we detected no decrease in survival of single- or double-mutant mice. [Mendelian ratios of adult surviving mutant mice were as follows: 1.1:2.0:1.0 for β+/+α+/+/β+/-α+/+/β-/-α+/+ mice (n = 219), and 1.0:2.0:1.2 for β+/+α-/-/β+/-α-/-/β-/-α-/- mice (n = 277).] Because β-synuclein may act as an “anti-Parkinson” factor that prevents α-synuclein aggregation and, hence, neurodegeneration (35), we also tested whether aged β-synuclein KO animals exhibited signs of neurodegeneration or a shorter lifespan. However, β-synuclein KO mice developed no obvious age-dependent phenotype at >1.5 years, were indistinguishable from aged WT littermates (data not shown), and had a similar lifespan. These data demonstrate that simultaneous deletion of both synucleins does not impair survival.
Next, we used quantitative immunoblotting to measure the levels of 36 proteins (Table 1, which is published as supporting information on the PNAS web site). We compared α/β-synuclein double-KO mice with α-synuclein single-KO littermate control mice because studies have shown that the α-synuclein KO mice exhibit no significant changes in any studied protein (26). The levels of most proteins were not changed significantly, with three exceptions (Table 1): (i) γ-synuclein was increased by ≈50% in α/β-synuclein double-KO mice; (ii) 14-3-3ε protein was increased by ≈30%, whereas 14-3-3ζ protein was decreased by ≈30%; and (iii) complexin was increased by ≈30%. The γ-synuclein increase in the α/β-synuclein KO probably reflects a compensatory change, but because γ-synuclein levels in the CNS are very low (21), a 50% increase is very small. The 14-3-3 proteins are signaling molecules that recognize and dimerize a large number of target phosphoproteins (36), and they may interact with α-synuclein (37). Thus, the changes in 14-3-3 proteins in the synuclein KO mice could reflect a functional relationship. Complexins are small soluble presynaptic proteins like synucleins (38). Complexins bind to assembled SNARE complexes and regulate neurotransmitter release (39). The increase in complexin levels in the synuclein double-KO mice may reflect a functional relationship of synucleins with complexins or a compensatory mechanism to make up for the loss of an abundant soluble presynaptic protein in the synuclein KO mice.
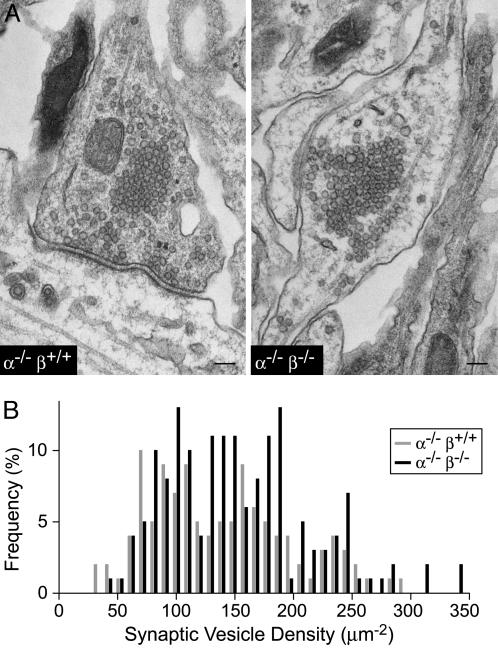
Synapse Structure in α/β-Synuclein Double-KO Mice. We found no structural abnormalities in the overall brain morphology of α/β-synuclein double-KO mice by Nissl staining (Fig. 8A, which is published as supporting information on the PNAS web site). Electron microscopy of brain sections also failed to reveal obvious abnormalities (data not shown). Similarly, in cultured cortical neurons from synuclein KO and control mice, we detected no deficits in the staining of synaptic markers, such as synaptophysin, rab3a, and synapsin (Fig. 8B, Supporting Materials and Methods, and data not shown). We then analyzed the structure of synapses in cultured neurons from α-synuclein single-KO and α/β-synuclein double-KO mice by quantitative electron microscopy (Fig. 2). We detected no significant changes in the presynaptic bouton area (α-/-β+/+ = 0.497 ± 0.37 μm2; α-/-β-/- = 0.398 ± 0.27 μm2; n = 114 and 157 synapses, respectively), density of synaptic vesicles (α-/-β+/+ = 135 ± 62 synaptic vesicles per μm2 bouton area; α-/-β-/- = 149 ± 63 synaptic vesicles per μm2 bouton area; n = 114 and 157 synapses, respectively), active zone length (α-/-β+/+ = 0.37 ± 0.14 μm; α-/-β-/- = 0.40 ± 0.16 μm; n = 65 and 95 synapses, respectively), or number of docked vesicles (α-/-β+/+ = 11.3 ± 4.9 synapses per μm active zone; α-/-β-/- = 11.4 ± 5.0 synapses per μm active zone; n = 65 and 95 synapses, respectively). To exclude the possibility that deletion of synucleins selectively depletes synaptic vesicles in a subgroup of synapses, we also measured the distribution of synaptic vesicle densities among different synapses, and again we found no significant difference between α-synuclein single-KO synapses and α/β-synuclein double-KO synapses (Fig. 2B).
Fig. 2.
Ultrastructural analysis of mutant synapses. (A) Representative electron micrographs of synapses in cultured cortical neurons from littermate α-synuclein KO (α-/-β+/+) and α/β-synuclein double-KO (α-/-β-/-) mice. (Calibration bar, 100 nm.) (B) Frequency distribution of the density of synaptic vesicles in synapses from α-synuclein KO (α-/-β+/+) and α/β-synuclein double-KO (α-/-β-/-) neurons. Synaptic vesicle densities were determined from randomized electron micrographs (see Supporting Materials and Methods).
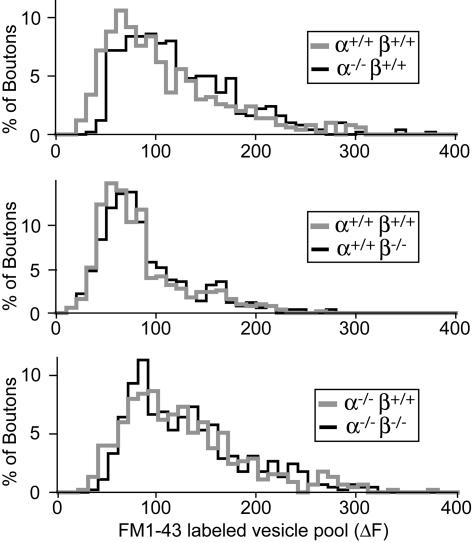
Synaptic Vesicle Pool Size. Recent reports (13, 14) have suggested that loss of α-synuclein severely decreases the number of reserve vesicles that are mobilized during repetitive stimulation, although an earlier study did not detect such a change (12). To determine whether synucleins are essential for reserve vesicles, we measured the total size of the synaptic vesicle recycling pool (which is composed of the readily releasable pool and the reserve pool) in neurons from α- and β-synuclein single-KO, double-KO, and littermate control mice. By using high-K+ stimulation (47 mM K+ for 90 s), we loaded recycling vesicles in cultured neurons with the styryl dye FM1-43. High-K+ stimulation induces synaptic vesicle exocytosis and endocytosis, which lead to the internalization of FM1-43 into synaptic vesicles (40). After washout of free dye, we monitored the release of FM1-43 from the vesicles when exocytosis is induced by high-frequency field stimulation (Fig. 9, which is published as supporting information on the PNAS web site), and we determined the total amount of FM1-43 fluorescence that is reversibly taken up into synaptic vesicles per bouton, a parameter that reflects the size of the total recycling vesicle pool (Fig. 3). We detected no significant change in the release rate of FM1-43 or in the size of the recycling vesicle pool in synuclein-deficient synapses. Thus, consistent with the normal vesicle numbers observed by electron microscopy and the normal levels of most synaptic proteins, synuclein single- and double-KO mice exhibited no decrease in the size of synaptic vesicle pools.
Fig. 3.
Functional synaptic vesicle pools in synuclein-deficient synapses. Synapses in cultured cortical neurons were loaded with FM1-43, and the size of the pool of actively recycling vesicles was determined as the amount of FM1-43 fluorescence that could be released upon repeated depolarization (3 × 90 mM K+ for 90 s) in the absence of exogenous FM1-43. Shown is the frequency distribution of FM1-43 labeling of synaptic boutons in cultures from α-synuclein (Top), β-synuclein (Middle), and α/β-synuclein double-KO (Bottom) neurons (in each case, compared with the corresponding distribution obtained with littermate control mice).
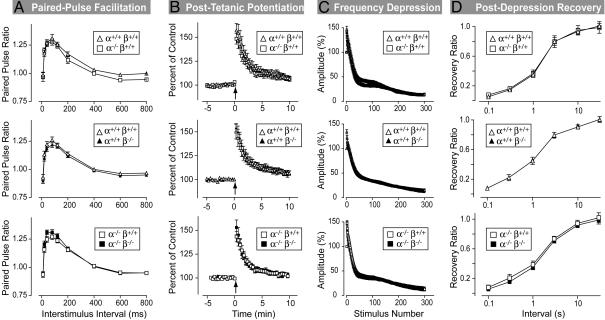
Synaptic Physiology. The vertebrate-specific expression of synucleins suggests a possible role for synucleins in synaptic plasticity (15). To investigate this possibility, we examined five principal forms of synaptic plasticity in hippocampal slices from littermate mice in three genotype comparisons (WT vs. α-synuclein KO mice, WT vs. β-synuclein KO mice, and α-synuclein KO vs. α/β-synuclein double-KO mice; Figs. 4 and 10, which is published as supporting information on the PNAS web site).
We first studied two forms of short-term plasticity, paired-pulse facilitation, and posttetanic potentiation by monitoring extracellular synaptic potentials. Paired-pulse facilitation is the enhancement of neurotransmitter release in the second of two closely spaced stimuli. Posttetanic potentiation is a longer-lasting enhancement of release induced by a short intense burst of action potentials. For all tested genotype combinations, both forms of plasticity were unchanged (Fig. 4 A and B).
Fig. 4.
Hippocampal synaptic plasticity in α- and β-synuclein single- and double-KO mice. All data are taken from recordings at excitatory Schaffer collateral/CA1 pyramidal cell synapses. (A) Paired-pulse facilitation. Data show the ratio of the second to the first synaptic response (paired-pulse ratio) to two closely spaced stimuli as a function of the interstimulus interval (data are given as number of mice/number of slices; n = 3:9 and 3:8 for α+/+β-/- and WT mice, respectively; n = 1:3 and 2:6 for α-/-β+/+ and WT mice, respectively; and n = 2:5 and 2:6 for α-/-β-/- and α-/-β+/+ control mice, respectively). (B) Posttetanic potentiation. Potentiation was induced by 30 stimuli at 100 Hz (arrow) in 40 μM AP5 (data are given as number of mice/number of slices; n = 3:9 and 3:8 for α+/+β-/- and WT mice, respectively; n = 1:3 and 2:6 for α-/-β+/+ and WT mice, respectively; and n = 2:5 and 2:7 for α-/-β-/- and α-/-β+/+ mice, respectively). (C) Synaptic depression. Synaptic responses during a 14-Hz stimulus train were normalized to the first response (data are given as number of mice/number of slices; n = 3:8 and 3:8 for α+/+β-/- and WT mice, respectively; n = 1:3 and 2:5 for α-/-β+/+ and WT mice, respectively; and n = 2:5 and 2:6 for α-/-β-/- and α-/-β+/+ mice, respectively). (D) Recovery after synaptic depression induced by a 100-Hz stimulus in the presence of AP5 and 5 mM Ca2+ (data are given as number of mice/number of slices; n = 3:12 and 3:12 for α+/+β-/- and WT mice, respectively; n = 1:4 and 2:8 for α-/-β+/+ and WT mice, respectively; and n = 2:8 and 2:8 for α-/-β-/- and α-/-β+/+ mice, respectively). In all graphs, open symbols are placed above filled symbols, and filled symbols are invisible if they precisely coincide with open symbols. All data are given as mean ± SEM.
We next probed use-dependent synaptic depression. We applied 14-Hz stimulus trains to WT and mutant hippocampal slices (Fig. 4C). In all genotypes, we observed a typical biphasic decrease in amplitudes during the 14-Hz stimulus train, without significant differences between WT and KO slices. This result is consistent with the FM labeling results that also failed to detect a change in the size of recycling and reserve vesicle pools in the synuclein KO mice. However, it is possible that, in synuclein KO mice, an increase in vesicle recycling compensates for a partial decrease in pool size. To rule out this possibility, we measured the time course with which synaptic responses recover after use-dependent depression was induced by high-frequency stimulation. Again, we detected no difference between the various genotypes (Fig. 4D), effectively ruling out a major problem in the vesicle numbers, pools, or dynamics in the α/β-synuclein double-KO mice. Last, we investigated whether deletion of synucleins had an effect on long-term potentiation, but as before, we detected no difference (Fig. 10).
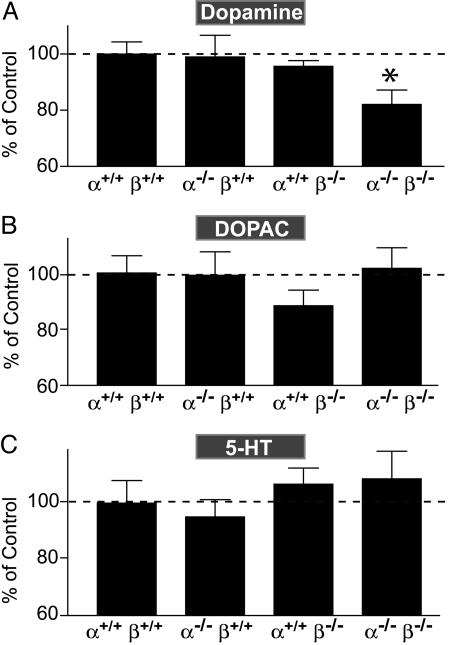
Dopaminergic System. In Parkinson's disease, mutations in the α-synuclein gene lead to a selective loss of dopaminergic neurons even though α-synuclein is widely expressed in the brain. Therefore, we examined whether deletion of α- and/or β-synuclein may have an effect on the dopaminergic system. We first quantified striatal levels of dopamine and its metabolites by HPLC. Compared with WT controls, dopamine levels in the striatum of synuclein double-KO mice, but not of individual α- or β-synuclein KO mice, were decreased by 18% (Fig. 5A). In contrast, the levels of the dopamine metabolites 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) were unchanged, suggesting that dopamine catabolism is not altered dramatically (Fig. 5B and data not shown). In addition, levels of the monoamine 5-hydroxytryptamine (5-HT) were also unaffected (Fig. 5C), indicating that the change in dopamine levels is selective.
Fig. 5.
Levels of dopamine (A), 3,4-dihydroxyphenylacetic acid (DOPAC) (B), and 5-hydroxytryptamine (5-HT) (C) in the dorsal striatum from synucleindeficient mice. Monoamine levels were quantified by HPLC (n = 13 α-/-β+/+, 13 α+/+β-/-, 14 α-/-β-/-, and 12 WT brains from 2- to 4-month-old mice). Data are normalized to WT levels (100%) and presented as mean ± SEM. *, P < 0.05, compared with WT, as determined by Student's t test.
A loss of dopaminergic neurons in the substantia nigra, as typically observed in Parkinson's disease, could cause the decrease in dopamine in the synuclein KO mice. To test this possibility, we stained dopaminergic neurons in the substantia nigra with an antibody to TH, and we counted dopaminergic neurons by using stereological methods (Fig. 11, which is published as supporting information on the PNAS web site). We found that the number of TH-positive cells was similar for WT and α/β-synuclein double-KO male mice (5,428 ± 881 vs. 5,418 ± 554 cells per substantia nigra; n = 4 per genotype), suggesting that synucleins are not essential for the development or survival of dopaminergic neurons.
We next investigated whether the protein levels of key monoamine biosynthetic enzymes, such as TH, were altered in synuclein KO mice, but we found no significant change (Table 1 and Fig. 7). In a final set of experiments, we evaluated the uptake and the release of dopamine from striatal synaptosomes. We found that total dopamine uptake into synaptosomes was only slightly decreased in α/β-synuclein double-KO samples compared with WT controls (94 ± 2.9% of controls). For release experiments, we loaded synaptosomes from littermate α-synuclein single-KO mice and α/β-synuclein double-KO mice with both 3H-dopamine and [14C]-GABA, and we used a superfusion system to stimulate neurotransmitter release by successive brief applications of a high-K+ and a hypertonic sucrose solution (33). The fractional-release rate for dopamine and GABA determined in this manner exhibited no difference between α-synuclein single-KO mice and α/β-synuclein double-KO mice (Fig. 6). Similar results were obtained when double KOs were compared with WT animals (data not shown). Collectively, these data suggest that the synthesis, release, and reuptake of dopamine are not impaired dramatically in the synuclein KO animals.
Fig. 6.
Analysis of dopamine release. Synaptosomes were loaded with 3H-dopamine (A) and 14C-GABA (B) and superfused with normal Krebs bicarbonate buffer. Release was triggered sequentially by membrane depolarization (30-s pulse of 25 mM K+) and hypertonic sucrose (30-s pulse of 0.5 M sucrose). Released transmitters were monitored continuously in the superfusate. Data are given as mean ± SEM from a representative experiment performed in duplicate and repeated independently three times.
Discussion
Much progress has been made in elucidating the role of α-synuclein in the pathogenesis of Parkinson's disease, but the normal function of this abundant protein remains a mystery. The high concentration of synucleins in presynaptic terminals suggested an essential role for synucleins in synapse formation, neurotransmitter release, or synaptic plasticity. Contrary to this expectation, the data presented here show that synucleins are not essential for any of these processes. We found that the deletion of α- and β-synucleins did not impair synaptic parameters, such as the structure of synapse, release of neurotransmitters, mobilization of synaptic vesicles, or forms of short- and long-term synaptic plasticity. Among other findings, these data demonstrate that the lack of a major phenotype in the observed α-synuclein KO mice (12, 26) is not due to redundancy between α- and β-synucleins. However, our results differ from those of Cabin et al. (14) who observed a dramatic loss of reserve vesicles and an increase in synaptic depression in α-synuclein KO mice. A difference in the mouse models that were used may explain this discrepancy. The α-synuclein KO mice that we (26) and Abeliovich et al. (12) generated were made by deleting exons 1 and 2, whereas Cabin et al. (14) targeted exons 4 and 5 of the α-synuclein gene and may have inadvertently disrupted an unknown downstream gene.
Based on in vitro studies, it was proposed that α- and β-synuclein have opposite effects on synuclein solubility and neuronal viability (35). However, our analysis of aged β-synuclein KO mice failed to reveal evidence of neurodegeneration or premature morbidity. Furthermore, we crossed β-synuclein KO mice with transgenic mice that overexpress mutant human α-synuclein, and we observed no change in the onset or severity of the age-dependent “Parkinson-like” neurodegenerative phenotype that is typically observed in these mice (S.C. and T.C.S., unpublished data). These results indicate that β-synuclein is not a major protective factor against α-synuclein-induced neurodegeneration. Moreover, the fact that single α- or β-synuclein KOs have no effect on dopamine levels but the double KO causes a decrease in dopamine levels argues for functional redundancy between the two synucleins and against opposite functions. Such redundancy is also consistent with their molecular similarity.
However, the lack of a major synaptic or neurodegenerative phenotype in the α/β-synuclein KO mice does not imply that synucleins have no function. Our experiments tested only basic synaptic parameters and not functions that might occur under special conditions (e.g., when neuronal repair is activated). An indication that such functions may be performed by α- and β-synuclein was provided by the small decrease in brain dopamine and moderate changes in two synaptic signaling molecules (complexins and 14-3-3 proteins) that we detected in the double-KO mice. These changes may reflect subtle homeostatic alterations in the regulation of synaptic transmission. These and other possible synuclein functions that may be activated only under distinct circumstances remain to be tested.
Supplementary Material
Acknowledgments
We thank Dr. A. Ho for invaluable help with striatal dissections; Dr. M. Khvotchev for guidance in neurotransmitter-release assays; I. Kornblum, E. Borowicz, and A. Roth for excellent technical assistance; and N. Hamlin and A. Mercado for help with mouse husbandry. This study was supported by National Institutes of Health Grant 1-R01-NS40057 (to T.C.S.) and a grant from the American Parkinson Disease Association (to S.C.).
Author contributions: S.C., F.F., X.L., R.E.H., D.C.G., P.E.C., and T.C.S. designed research; S.C., F.F., H.-B.K., U.Y., D.A., R.E.H., X.L., G.B., D.C.G., and P.E.C. performed research; D.C.G., P.E.C., and T.C.S. analyzed data; S.C. and T.C.S. wrote the paper; and T.C.S. organized the funding, arranged the collaborations, and organized the regulatory approvals.
Abbreviations: GABA, γ-aminobutyric acid; KO, knockout; TH, tyrosine hydroxylase.
References
- 1.Maroteaux, L., Campanelli, J. T. & Scheller, R. H. (1988) J. Neurosci. 8, 2804-2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tobe, T., Nakajo, S., Tanaka, A., Mitoya, A., Omata, K., Nakaya, K., Tomita, M. & Nakamura, Y. (1992) J. Neurochem. 59, 1624-1629. [DOI] [PubMed] [Google Scholar]
- 3.George, J. M., Jin, H., Woods, W. S. & Clayton, D. F. (1995) Neuron 15, 361-372. [DOI] [PubMed] [Google Scholar]
- 4.Jensen, P. H., Nielsen, M. S., Jakes, R., Dotti, C. G. & Goedert, M. (1998) J. Biol. Chem. 273, 26292-26294. [DOI] [PubMed] [Google Scholar]
- 5.Polymeropoulos, M. H., Lavedan, C., Leroy, E., Ide, S. E., Dehejia, A., Dutra, A., Pike, B., Root, H., Rubenstein, J., Boyer, R., et al. (1997) Science 276, 2045-2047. [DOI] [PubMed] [Google Scholar]
- 6.Spillantini, M. G., Schmidt, M. L., Lee, V. M., Trojanowski, J. Q., Jakes, R. & Goedert, M. (1997) Nature 388, 839-840. [DOI] [PubMed] [Google Scholar]
- 7.Galvin, J. E., Lee, V. M., Schmidt, M. L., Tu, P. H., Iwatsubo, T. & Trojanowski, J. Q. (1999) Adv. Neurol. 80, 313-324. [PubMed] [Google Scholar]
- 8.Kruger, R., Kuhn, W., Muller, T., Woitalla, D., Graeber, M., Kosel, S., Przuntek, H., Epplen, J. T., Schols, L. & Riess, O. (1998) Nat. Genet. 18, 106-108. [DOI] [PubMed] [Google Scholar]
- 9.Singleton, A. B., Farrer, M., Johnson, J., Singleton, A., Hague, S., Kachergus, J., Hulihan, M., Peuralinna, T., Dutra, A., Nussbaum, R., et al. (2003) Science 302, 841. [DOI] [PubMed] [Google Scholar]
- 10.Zarranz, J. J., Alegre, J., Gomez-Esteban, J. C., Lezcano, E., Ros, R., Ampuero, I., Vidal, L., Hoenicka, J., Rodriguez, O., Atares, B., et al. (2004) Ann. Neurol. 55, 164-173. [DOI] [PubMed] [Google Scholar]
- 11.Withers, G. S., George, J. M., Banker, G. A. & Clayton, D. F. (1997) Brain. Res. Dev. Brain Res. 99, 87-94. [DOI] [PubMed] [Google Scholar]
- 12.Abeliovich, A., Schmitz, Y., Farinas, I., Choi-Lundberg, D., Ho, W. H., Castillo, P. E., Shinsky, N., Verdugo, J. M., Armanini, M., Ryan, A., et al. (2000) Neuron 25, 239-252. [DOI] [PubMed] [Google Scholar]
- 13.Murphy, D. D., Rueter, S. M., Trojanowski, J. Q. & Lee, V. M. (2000) J. Neurosci. 20, 3214-3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cabin, D. E., Shimazu, K., Murphy, D., Cole, N. B., Gottschalk, W., McIlwain, K. L., Orrison, B., Chen, A., Ellis, C. E., Paylor, R., et al. (2002) J. Neurosci. 22, 8797-8807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Rosa, G., Puzzo, D., Sant'Angelo, A., Trinchese, F. & Arancio, O. (2003) Histol. Histopathol. 18, 1257-1266. [DOI] [PubMed] [Google Scholar]
- 16.Ueda, K., Fukushima, H., Masliah, E., Xia, Y., Iwai, A., Yoshimoto, M., Otero, D. A., Kondo, J., Ihara, Y. & Saitoh, T. (1993) Proc. Natl. Acad. Sci. USA 90, 11282-11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akopian, A. N. & Wood, J. N. (1995) J. Biol. Chem. 270, 21264-21270. [DOI] [PubMed] [Google Scholar]
- 18.Ji, H., Liu, Y. E., Jia, T., Wang, M., Liu, J., Xiao, G., Joseph, B. K., Rosen, C. & Shi, Y. E. (1997) Cancer Res. 57, 759-764. [PubMed] [Google Scholar]
- 19.Buchman, V. L., Hunter, H. J., Pinon, L. G., Thompson, J., Privalova, E. M., Ninkina, N. N. & Davies, A. M. (1998) J. Neurosci. 18, 9335-9341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Surguchov, A., Surgucheva, I., Solessio, E. & Baehr, W. (1999) Mol. Cell. Neurosci. 13, 95-103. [DOI] [PubMed] [Google Scholar]
- 21.Clayton, D. F. & George, J. M. (1998) Trends Neurosci. 21, 249-254. [DOI] [PubMed] [Google Scholar]
- 22.Nagy, A., Rossant, J., Nagy, R., Abramow-Newerly, W. & Roder, J. C. (1993) Proc. Natl. Acad. Sci. USA 90, 8424-8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schlüter, O. M., Schnell, E., Verhage, M., Tzonopoulos, T., Nicoll, R. A., Janz, R., Malenka, R. C., Geppert, M. & Südhof, T. C. (1999) J. Neurosci. 19, 5834-5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Gorman, S., Dagenais, N. A., Qian, M. & Marchuk, Y. (1997) Proc. Natl. Acad. Sci. USA 94, 14602-14607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dymecki, S. M. (1996) Proc. Natl. Acad. Sci. USA 93, 6191-6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schlüter, O. M., Fornai, F., Alessandri, M. G., Takamori, S., Geppert, M., Jahn, R. & Südhof, T. C. (2003) Neuroscience 118, 985-1002. [DOI] [PubMed] [Google Scholar]
- 27.Kavalali, E. T., Klingauf, J. & Tsien, R. W. (1999) Philos. Trans. R. Soc. London B 354, 337-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosahl, T. W., Spillane, D., Missler, M., Herz, J., Selig, D. K., Wolff, J. R., Hammer, R. E., Malenka, R. C. & Sudhof, T. C. (1995) Nature 375, 488-493. [DOI] [PubMed] [Google Scholar]
- 29.German, D. C., Nelson, E. L., Liang, C. L., Speciale, S. G., Sinton, C. M. & Sonsalla, P. K. (1996) Neurodegeneration 5, 299-312. [DOI] [PubMed] [Google Scholar]
- 30.Mozhayeva, M. G., Sara, Y., Liu, X. & Kavalali, E. T. (2002) J. Neurosci. 22, 654-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fornai, F., Lenzi, P., Gesi, M., Ferrucci, M., Lazzeri, G., Busceti, C. L., Ruffoli, R., Soldani, P., Ruggieri, S., Alessandri, M. G. & Paparelli, A. (2003) J. Neurosci. 23, 8955-8966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ho, A. & Blum, M. (1998) J. Neurosci. 18, 5614-5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khvotchev, M. & Südhof, T. C. (1998) J. Biol. Chem. 273, 21451-21454. [DOI] [PubMed] [Google Scholar]
- 34.Lonart, G., Janz, R., Johnson, K. M. & Sudhof, T. C. (1998) Neuron 21, 1141-1150. [DOI] [PubMed] [Google Scholar]
- 35.Hashimoto, M., Rockenstein, E., Mante, M., Mallory, M. & Masliah, E. (2001) Neuron 32, 213-223. [DOI] [PubMed] [Google Scholar]
- 36.Aitken, A., Baxter, H., Dubois, T., Clokie, S., Mackie, S., Mitchell, K., Peden, A. & Zemlickova, E. (2002) Biochem. Soc. Trans. 30, 351-360. [DOI] [PubMed] [Google Scholar]
- 37.Ostrerova, N., Petrucelli, L., Farrer, M., Mehta, N., Choi, P., Hardy, J. & Wolozin, B. (1999) J. Neurosci. 19, 5782-5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McMahon, H. T., Missler, M., Li, C. & Südhof, T. C. (1995) Cell 83, 111-119. [DOI] [PubMed] [Google Scholar]
- 39.Reim, K., Mansour, M., Varoqueaux, F., McMahon, H. T., Sudhof, T. C., Brose, N. & Rosenmund, C. (2001) Cell 104, 71-81. [DOI] [PubMed] [Google Scholar]
- 40.Betz, W. J. & Bewick, G. S. (1992) Science 255, 200-203. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.