Abstract
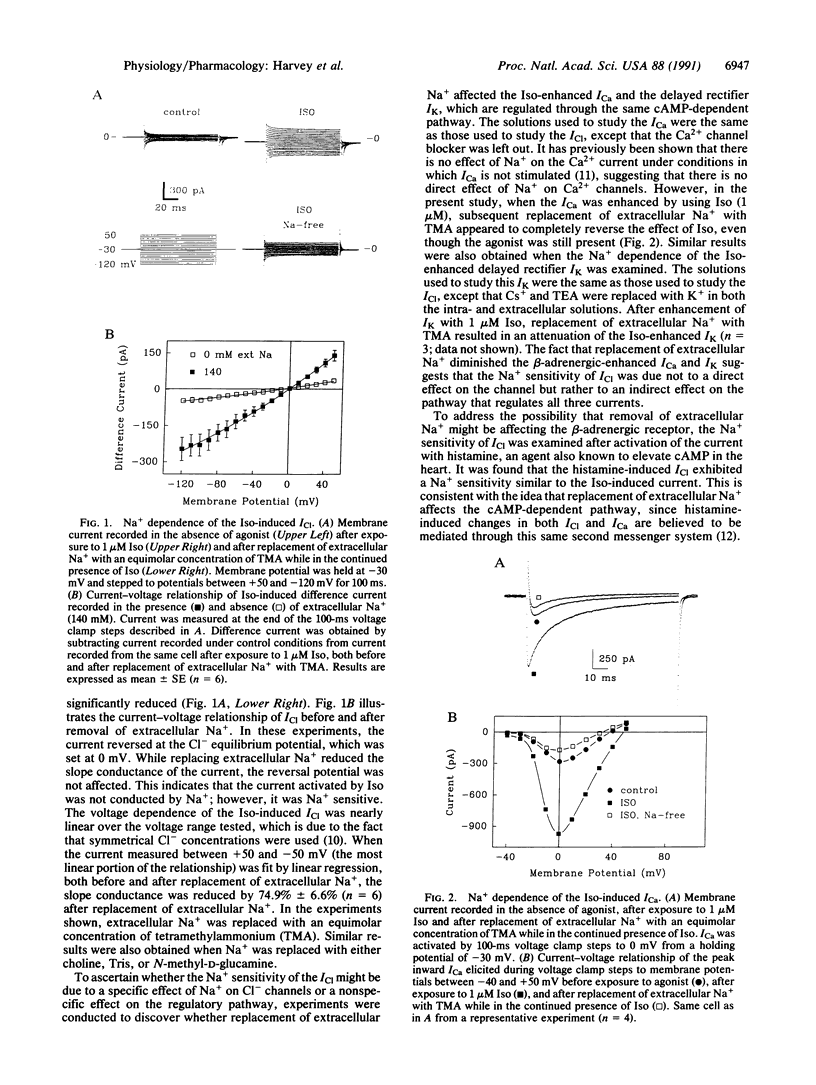
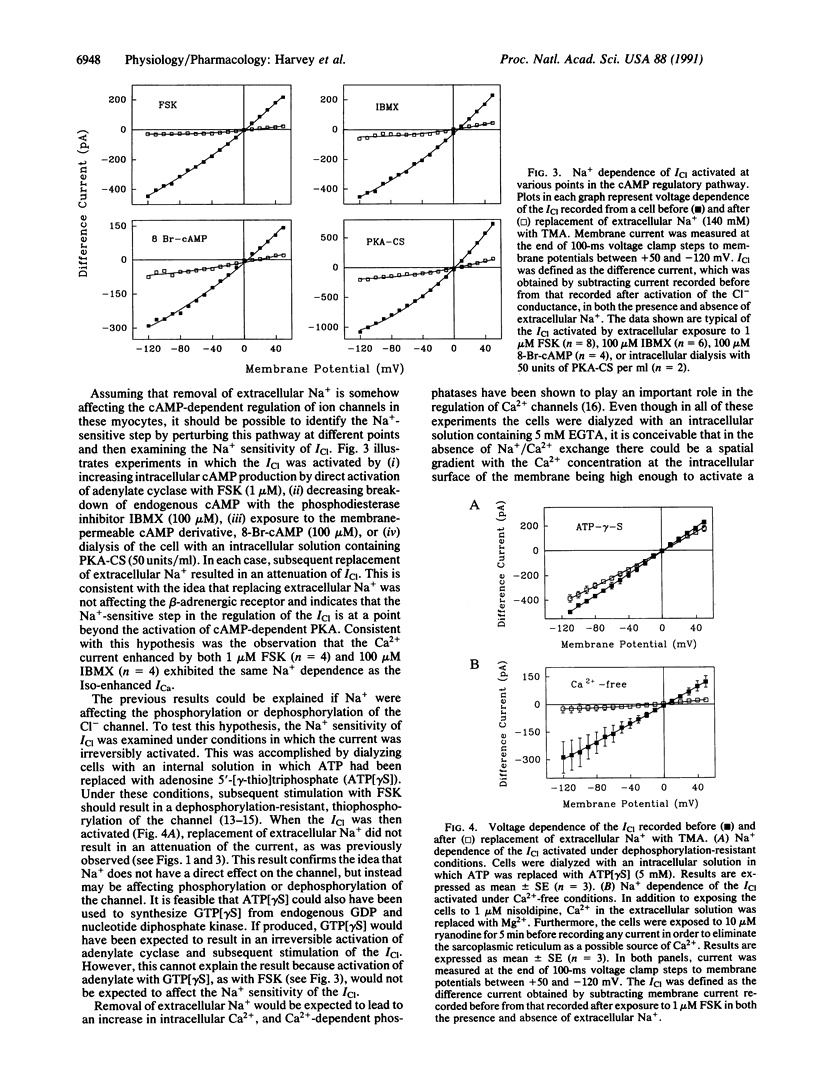
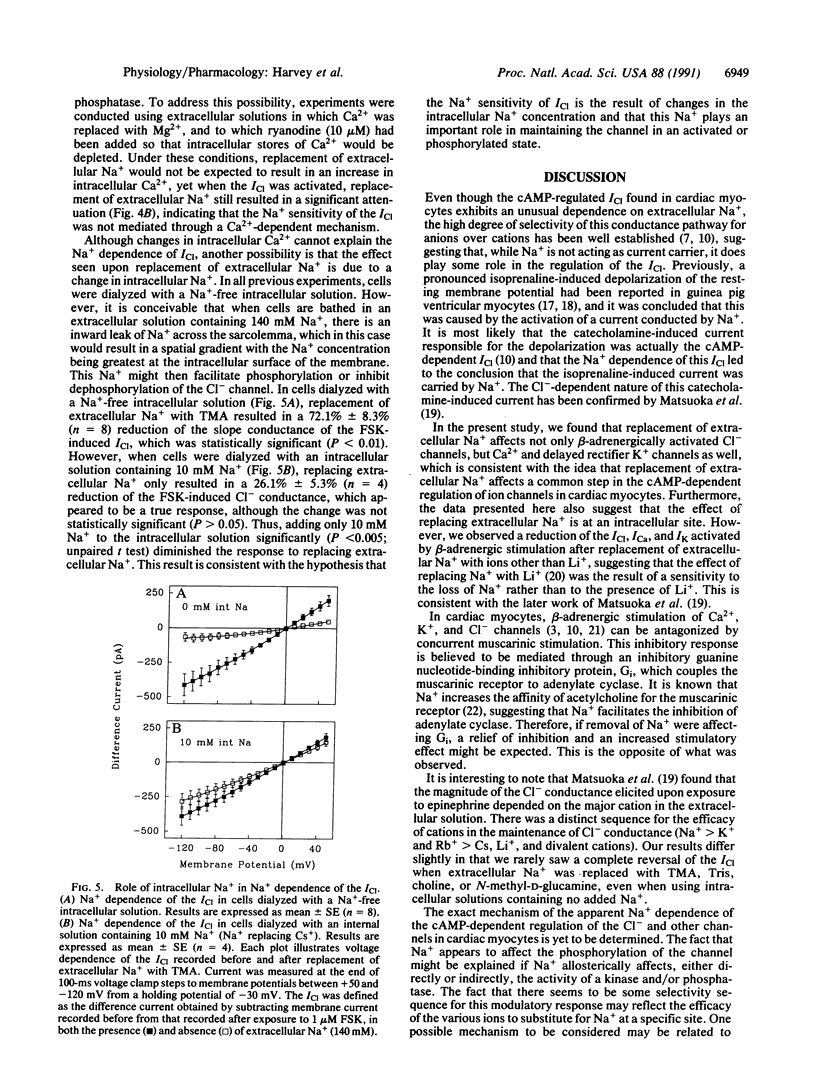
The cAMP-dependent regulation of ion channels was studied by using the whole-cell configuration of the patch clamp technique. In isolated cardiac ventricular myocytes, the beta-adrenergically regulated Cl- current (ICl) exhibited an unusual dependence on Na+, such that replacement of extracellular Na+ with compounds such as tetramethylammonium, choline, Tris, or N-methyl-D-glucamine resulted in a reduction in current amplitude without changing the reversal potential. Replacement of extracellular Na+ with tetramethylammonium also reduced the magnitude of the beta-adrenergically enhanced Ca2+ current and delayed rectifier K+ current, suggesting that removal of Na+ was affecting the cAMP pathway that regulates all three currents. Replacement of extracellular Na+ also reduced ICl that was stimulated by (i) direct activation of adenylate cyclase with forskolin, (ii) inhibition of phosphodiesterase with 3-isobutyl-1-methylxanthine, (iii) exposure to the membrane-permeable cAMP derivative 8-bromoadenosine 3',5'-cyclic monophosphate, or (iv) direct phosphorylation of the channel with protein kinase A catalytic subunit. This suggests that the Na+ dependence is at a point beyond the activation of protein kinase A. The Na+ dependence of ICl regulation could not be explained by changes in intracellular Ca2+. However, the sensitivity of the ICl to changes in extracellular Na+ depended significantly on the intracellular Na+ concentration, suggesting that intracellular Na+ plays an important role in the cAMP-dependent regulation of ion channels.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahinski A., Nairn A. C., Greengard P., Gadsby D. C. Chloride conductance regulated by cyclic AMP-dependent protein kinase in cardiac myocytes. Nature. 1989 Aug 31;340(6236):718–721. doi: 10.1038/340718a0. [DOI] [PubMed] [Google Scholar]
- Bean B. P., Nowycky M. C., Tsien R. W. Beta-adrenergic modulation of calcium channels in frog ventricular heart cells. 1984 Jan 26-Feb 1Nature. 307(5949):371–375. doi: 10.1038/307371a0. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D., Ferroni A., Mazzanti M., Tromba C. Properties of the hyperpolarizing-activated current (if) in cells isolated from the rabbit sino-atrial node. J Physiol. 1986 Aug;377:61–88. doi: 10.1113/jphysiol.1986.sp016177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchatelle-Gourdon I., Hartzell H. C., Lagrutta A. A. Modulation of the delayed rectifier potassium current in frog cardiomyocytes by beta-adrenergic agonists and magnesium. J Physiol. 1989 Aug;415:251–274. doi: 10.1113/jphysiol.1989.sp017721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan T. M., Noble D., Noble S. J., Powell T., Twist V. W. An isoprenaline activated sodium-dependent inward current in ventricular myocytes. Nature. 1987 Aug 13;328(6131):634–637. doi: 10.1038/328634a0. [DOI] [PubMed] [Google Scholar]
- Egan T. M., Noble D., Noble S. J., Powell T., Twist V. W., Yamaoka K. On the mechanism of isoprenaline- and forskolin-induced depolarization of single guinea-pig ventricular myocytes. J Physiol. 1988 Jun;400:299–320. doi: 10.1113/jphysiol.1988.sp017121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatman P. W. Magnesium transport across cell membranes. J Membr Biol. 1984;80(1):1–14. doi: 10.1007/BF01868686. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C. Regulation of cardiac ion channels by catecholamines, acetylcholine and second messenger systems. Prog Biophys Mol Biol. 1988;52(3):165–247. doi: 10.1016/0079-6107(88)90014-4. [DOI] [PubMed] [Google Scholar]
- Harvey R. D., Clark C. D., Hume J. R. Chloride current in mammalian cardiac myocytes. Novel mechanism for autonomic regulation of action potential duration and resting membrane potential. J Gen Physiol. 1990 Jun;95(6):1077–1102. doi: 10.1085/jgp.95.6.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey R. D., Hume J. R. Autonomic regulation of a chloride current in heart. Science. 1989 May 26;244(4907):983–985. doi: 10.1126/science.2543073. [DOI] [PubMed] [Google Scholar]
- Harvey R. D., Hume J. R. Autonomic regulation of delayed rectifier K+ current in mammalian heart involves G proteins. Am J Physiol. 1989 Sep;257(3 Pt 2):H818–H823. doi: 10.1152/ajpheart.1989.257.3.H818. [DOI] [PubMed] [Google Scholar]
- Hescheler J., Kameyama M., Trautwein W., Mieskes G., Söling H. D. Regulation of the cardiac calcium channel by protein phosphatases. Eur J Biochem. 1987 Jun 1;165(2):261–266. doi: 10.1111/j.1432-1033.1987.tb11436.x. [DOI] [PubMed] [Google Scholar]
- Hescheler J., Kameyama M., Trautwein W. On the mechanism of muscarinic inhibition of the cardiac Ca current. Pflugers Arch. 1986 Aug;407(2):182–189. doi: 10.1007/BF00580674. [DOI] [PubMed] [Google Scholar]
- Kameyama M., Hescheler J., Hofmann F., Trautwein W. Modulation of Ca current during the phosphorylation cycle in the guinea pig heart. Pflugers Arch. 1986 Aug;407(2):123–128. doi: 10.1007/BF00580662. [DOI] [PubMed] [Google Scholar]
- Kameyama M., Hofmann F., Trautwein W. On the mechanism of beta-adrenergic regulation of the Ca channel in the guinea-pig heart. Pflugers Arch. 1985 Oct;405(3):285–293. doi: 10.1007/BF00582573. [DOI] [PubMed] [Google Scholar]
- Matsuda H., Noma A. Isolation of calcium current and its sensitivity to monovalent cations in dialysed ventricular cells of guinea-pig. J Physiol. 1984 Dec;357:553–573. doi: 10.1113/jphysiol.1984.sp015517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka S., Ehara T., Noma A. Chloride-sensitive nature of the adrenaline-induced current in guinea-pig cardiac myocytes. J Physiol. 1990 Jun;425:579–598. doi: 10.1113/jphysiol.1990.sp018119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka S., Noma A., Powell T. Li+ inhibition of membrane current responses to epinephrine in guinea-pig ventricular cells. Pflugers Arch. 1989 Dec;415(3):384–386. doi: 10.1007/BF00370892. [DOI] [PubMed] [Google Scholar]
- Mullins L. J., Brinley F. J. Magnesium influx in dialyzed squid axons. J Membr Biol. 1978 Oct 19;43(2-3):243–250. doi: 10.1007/BF01933481. [DOI] [PubMed] [Google Scholar]
- Ono K., Kiyosue T., Arita M. Isoproterenol, DBcAMP, and forskolin inhibit cardiac sodium current. Am J Physiol. 1989 Jun;256(6 Pt 1):C1131–C1137. doi: 10.1152/ajpcell.1989.256.6.C1131. [DOI] [PubMed] [Google Scholar]
- Reuter H. Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature. 1983 Feb 17;301(5901):569–574. doi: 10.1038/301569a0. [DOI] [PubMed] [Google Scholar]
- Rodbell M. The role of hormone receptors and GTP-regulatory proteins in membrane transduction. Nature. 1980 Mar 6;284(5751):17–22. doi: 10.1038/284017a0. [DOI] [PubMed] [Google Scholar]
- Schubert B., VanDongen A. M., Kirsch G. E., Brown A. M. Beta-adrenergic inhibition of cardiac sodium channels by dual G-protein pathways. Science. 1989 Aug 4;245(4917):516–519. doi: 10.1126/science.2547248. [DOI] [PubMed] [Google Scholar]
- Walsh K. B., Begenisich T. B., Kass R. S. Beta-adrenergic modulation in the heart. Independent regulation of K and Ca channels. Pflugers Arch. 1988 Feb;411(2):232–234. doi: 10.1007/BF00582323. [DOI] [PubMed] [Google Scholar]
- White R. E., Hartzell H. C. Effects of intracellular free magnesium on calcium current in isolated cardiac myocytes. Science. 1988 Feb 12;239(4841 Pt 1):778–780. doi: 10.1126/science.2448878. [DOI] [PubMed] [Google Scholar]
- Yatani A., Brown A. M. Rapid beta-adrenergic modulation of cardiac calcium channel currents by a fast G protein pathway. Science. 1989 Jul 7;245(4913):71–74. doi: 10.1126/science.2544999. [DOI] [PubMed] [Google Scholar]



