Abstract
Background
Although Kawasaki disease (KD) is the most common cause of acquired heart disease in children and may result in coronary artery aneurysms (CAA) with an attendant risk of myocardial infarction, there is no recommended therapy to halt progression of arterial wall damage and prevent aneurysm formation in the acute phase of the vasculitis. While intravenous immunoglobulin (IVIG) reduces the risk of CAA, up to 20% of KD patients are IVIG resistant and have a higher risk for developing CAA. The IL-1 pro-inflammatory pathway is upregulated in children with acute KD and plays a critical role in the experimental animal model of KD. Thus, IL-1 is a logical therapeutic target.
Objectives
The goal of this study is to determine the safety, tolerability, pharmacokinetics, and immunomodulatory effects of anakinra, a recombinant human IL-1 receptor antagonist, in acute KD patients with coronary artery abnormalities on the baseline echocardiogram.
Design
This is a two-center dose-escalation Phase I/IIa trial in 30 acute KD patients ≥8 months old with a coronary artery Z score ≥3.0 in the right coronary artery and/or left anterior descending artery or an aneurysm. Subjects will receive a 2- to 6-week course of anakinra by daily subcutaneous injection and will be assessed for resolution of inflammation and dose limiting toxicities (leukopenia, anaphylactoid reaction, or severe infection).
Conclusion
The safety and tolerability of blocking both IL-1α and Il-1β by anakinra will be evaluated as a strategy to prevent or attenuate coronary artery damage in infants and children with acute KD.
Keywords: anakinra, interleukin 1, Kawasaki disease, coronary artery aneurysm
1. Introduction and rationale
Kawasaki disease (KD), the most common cause of acquired heart disease in children in Western developed countries and Asia, is a systemic vasculitis of unknown etiology. KD causes both a myocarditis and a vasculitis that damages the coronary arteries and other medium-sized muscular arteries leading to the formation of aneurysms (Tanaka 1979, Yonesaka, Nakada et al. 1989). The major sequelae of aneurysms include thrombosis, late coronary artery stenosis, myocardial ischemia, myocardial infarction, and death (Kato, Sugimura et al. 1996, Gordon, Kahn et al. 2009, McCrindle 2009). Treatment with intravenous immunoglobulin (IVIG) reduces the risk of aneurysm formation from 25% to 5% (Newburger, Takahashi et al. 1986). However, for patients with IVIG-resistance, the risk of aneurysms increases 3- fold (Tremoulet, Best et al. 2008). Currently, there is no recommended therapy beyond IVIG to halt the progression of arterial wall damage and prevent aneurysm formation. Since aneurysm prevention is a primary goal of treatment during the acute phase of KD, we have focused on intensification of therapy for patients with early coronary artery abnormalities (CAA) detected by transthoracic echocardiography.
In the KD mouse model, in which a coronary artery vasculitis, aortitis, and myocarditis are induced with an intraperitoneal injection of a cell wall extract from Lactobacillus casei (LCWE), mice exhibit systemic inflammation, increased body temperature, and elevated levels of IL-1β (Lehman, Walker et al. 1985, Yeung 2007, Schulte, Yilmaz et al. 2009). This LCWE-induced vasculitis occurs through the IL-1R signaling pathway via MyD88 in wild-type C57BL/6 but not IL-1R knockout mice (Rosenkranz, Schulte et al. 2005, Lee, Schulte et al. 2012). Studies to evaluate the effects of IL-1 blockade with anakinra in the LCWE KD mouse model have demonstrated clear benefit with reduction in inflammation. In addition, IL-1 related genes are upregulated in KD peripheral blood during acute phase of illness (Hoang, Shimizu et al. 2014).
Given the data supporting the role of IL-1 in the systemic inflammatory response in KD, we have chosen to pursue blocking of the IL-1 pathway in children with acute KD with anakinra, which competitively inhibits IL-1 binding to the IL-1 type 1 receptor. The rationale for choosing anakinra over other agents includes the rapid onset of IL-1 blockade, the ability to block both IL-1α and β, the excellent safety profile in young infants and children, and the short half-life in case of infectious disease complications (Table 1).
Table 1.
Justification for Selecting Anakinra to Block the IL-1 Pathway in KD
| PROS | CONS | |
|---|---|---|
| Anakinra |
|
|
| Rilonacept |
|
|
| Canakinumab |
|
|
sJIA, systemic juvenile idiopathic arthritis
2. Objectives
The goal of this study is to determine the safety, pharmacokinetics, and activity of anakinra in acute KD patients at least 8 months of age with a coronary artery Z score (internal dimension of the coronary artery normalized for body surface area and expressed as standard deviations from the mean) ≥3.0 in the right coronary artery (RCA) and/or left anterior descending (LAD) artery. This study is viewed as preparatory to a Phase III trial of anakinra to prevent or attenuate coronary artery damage in acute KD.
3. Study design
This two-center dose-escalation Phase I/IIa trial (See Dose Levels Table 2) will determine the safety, tolerability and immunomodulatory effects of anakinra in 30 acute KD patients at least 8 months old with CAA. The enrollment age limit is a condition of our IND and was imposed by the FDA until safety data for KD could be reviewed after completion of this trial. Enrollment will occur at two study sites: Rady Children’s Hospital San Diego and Children’s Hospital of Boston.
Table 2.
Dose levels
| Dose Cohort | Dose Level | Number of Subjects |
|---|---|---|
| 1 | 4 mg/kg/day | 3–6 |
| 2 | 6 mg/kg/day | 3–6 |
| 3 | 8 mg/kg/day | 3–6 |
| TOTAL | 9–18* | |
A total of 30 subjects will be enrolled in this study. Once the maximum tolerated dose (MTD) is determined, all remaining subjects will be enrolled at the MTD.
All subjects will be treated with IVIG, 2g/kg and aspirin (30–50 mg/kg/day divided every 6 hours; lowered to 3–5 mg/kg/day at the time of discharge or when afebrile for 48 h, whichever comes first), which is the standard of care. The first two doses of anakinra will be administered intravenously (i.e. 4 mg/kg/day will be 2 mg/kg IV every 12 hours for two doses) to ensure rapid drug effect and minimize discomfort related to medication administration in subjects. Anakinra will be administered intravenously by bolus over a 1 to 3 minute period (Opal, Fisher et al. 1997). All subsequent doses will be administered once daily subcutaneously starting 24 hours after the first dose of the medication. Treatment-resistance will be defined as persistent or recrudescent fever (T≥38.0 C orally or rectally) ≥36 hours and <7 days following end of IVIG infusion (Tremoulet, Best et al. 2008). Subjects who meet criteria for treatment-resistance will be treated at the Center PI’s discretion.
All subjects will receive at least 2 weeks of therapy. Only subjects with an echocardiogram at 2 weeks that shows either an LAD or RCA Z score ≥2.5 or an aneurysm (≥1.5 x the adjacent segment) of one of the coronary arteries will receive an additional 4-week course of anakinra. Although the inclusion criteria is a Z score ≥3.0, we consider a vessel with a Z score ≥2.5 two weeks after enrollment to be sufficiently abnormal to continue anakinra. All subjects will remain on study for the full 6 weeks whether or not they are receiving anakinra.
The dose-escalation protocol will enroll a minimum of three subjects per dose level (Table 2). A maximum of 100 mg/day will be administered. The 3+3 dose escalation design uses the number of dose limiting toxicities to determine the maximum tolerated dose (Table 3).
Table 3.
Dose Escalation Decision Rules
| Subjects with a DLT at a Given Dose | Escalation Decision Rule |
|---|---|
| 0 out of 3 | Enter 3 patients at the next dose level until maximum tolerated dose. |
| 1 out of 3 | Enter a total of 6 patients at this dose level.
|
| ≥ 2 | At least 3 additional patients will be entered at the next lowest dose level if only 3 patients were treated previously at that dose. This lower dose level will be declared the MTD. |
DLT = dose limiting toxicity; A DLT is defined as any of the following at the 2 or 6 week time point: (1) Serious infection qualifying as a serious adverse event and requiring intervention; (2) a decrease in the white blood cell count to <1500/mm3 (Grade 3 severity by NIH/NIAID) (NIH and DAIDS 2009); (3)an anaphylactoid reaction to an injection of anakinra. MTD = maximum tolerated dose; the MTD is defined as the highest dose of anakinra studied at which no more than on in six subjects experiences a DLT during the 6 weeks of treatment.
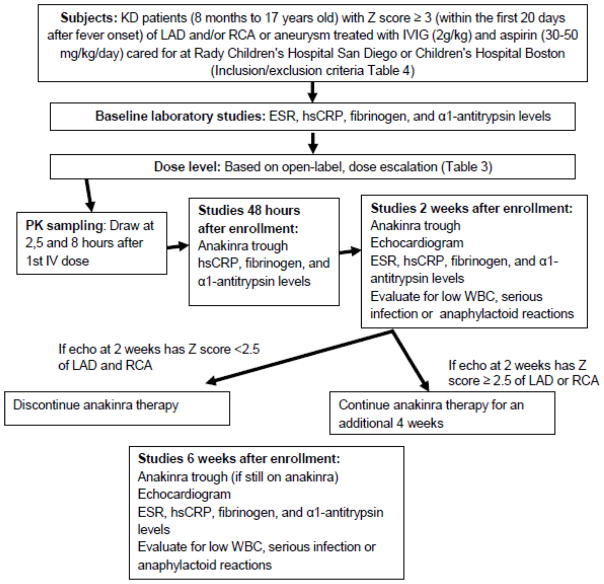
All patients will be evaluated at baseline as well as 48 hours and 2 and 6 weeks after enrollment (Figure). Relevant medical course, vital signs, and physical examination will be recorded. Pharmacokinetic samples will be taken at 2, 5 and 8 hours after the first IV dose of the medication and trough samples will be collected at 48 hours and 2 and 6 weeks (if still taking anakinra). Subjects will be monitored for adverse events and serious adverse events, and at 2 and 6 weeks will be assessed for a dose limiting toxicity, defined as a serious infection qualifying as a serious adverse event and requiring intervention, a decrease in the white blood cell count to <1500/mm3 (Grade 3 severity by NIH/NIAID) (NIH and DAIDS 2009) or an anaphylactoid reaction to an injection of anakinra. To assess for compliance with the study medication, the number of syringes dispensed as well as the remaining number of syringes at each study visit will be recorded. Echocardiograms will be performed at 2 and 6 weeks and read by a single cardiologist blinded to clinical and treatment status of the subjects. Additional echocardiograms for the clinical care of the subject will be at the discretion of the center investigator. The following testing will be performed on all subjects at baseline (pre-anakinra), 48 hours, 2 and 6 weeks after study entry: levels of hsCRP, α1-antitrypsin and fibrinogen, and white blood cell count. The erythrocyte sedimentation rate (ESR) will be measured at baseline, 2 and 6 weeks. Subjects will be assessed for compliance with the study medication at the 2 and 6 week visit.
Figure 1.
Study design and flow
LAD, left anterior descending coronary artery; RCA, right coronary artery; IVIG, intravenous immunoglobulin; WBC, white blood cell count; hsCRP, high sensitivity C-reactive protein; ESR, erythrocyte sedimentation rate; PK, pharmacokinetic
4. Inclusion and exclusion criteria
Infants and children at least 8 months of age (age limited by Food and Drug Administration) within the first 20 days after fever onset who meet American Heart Association criteria for KD and have a Z score of ≥3.0 of the LAD and/or RCA or an aneurysm are study eligible (Table 4). Subjects with an active, culture-positive bacterial infection will be excluded.
Table 4.
Inclusion and exclusion criteria
Inclusion criteria:
|
Exclusion Criteria:
|
5. Primary and secondary endpoints
The primary endpoint of the study is safety and tolerability of anakinra in the study population. The pre-specified secondary endpoints are the pharmacokinetics of anakinra, the changes in echocardiographic assessment of the internal diameters of the coronary arteries expressed as Z scores over the course of the study, and the change in biomarkers of inflammation including levels of hsCRP, α1-antitrypsin and fibrinogen, and the WBC and ESR. Innate immune cells and T cells will be studied prior to therapy and 2 and 6 weeks after therapy. Plasma and transcript levels of relevant cytokines and IL-1 pathway molecules, including IL-17, tumor necrosis factor (TNF)αR1 and R2, IL-8, IL-27, IFNg, sIL-1R1 and R2, IL-6, sIL-6R, and IL-10 will be assessed.
6. Ethics and informed consent
The study protocol was reviewed and approved by the Institutional Review Board at the University of California San Diego and Boston Children’s Hospital. Written informed consent will be obtained from the parents or legal guardians and assent, when appropriate, will be obtained from the patient. This trial is registered with clinicaltrials.gov (NCT02179853).
7. Statistical methods
Data analysis
All enrolled subjects at the maximum tolerated dose will be included in a case:control study and matched with historical controls from each center for age, sex, illness day, adjunctive therapies in addition to IVIG, and the worst coronary artery Z score for the RCA and LAD on the initial, pre-treatment echocardiogram. Measures of inflammation, including hsCRP, ESR and WBC, at two weeks will be compared to that of matched controls via paired statistical tests (e.g. McNemar’s test for categorical outcomes or the paired t-test for continuous outcomes). Non-parametric alternatives will be considered only if distributional assumptions are violated. Each marker will be initially summarized descriptively and analyzed individually. Separate multivariable mixed models repeated measures for clustered data (to account for the case-control paired nature of samples and repeated measures over time) will be used to characterize the markers of anakinra-treated subjects versus matched controls to adjust for any known confounders and additional time points (baseline, 48 hours, 2 weeks, and 6 weeks).
Sample size
This Phase I/IIa trial is not powered to show a difference in coronary artery dimensions or treatment response compared to controls. Instead, the sample size was determined based on detectable differences in inflammatory markers. A sample size of 30 KD patients treated with anakinra and 30 matched controls will have 80% power based on a two-sided, paired t-test with alpha set to 0.05 to compare the change from baseline to two weeks in several inflammatory markers, including α-1-antitrypsin and hsCRP.
8. Discussion
Despite timely treatment with high dose IVIG and aspirin, some infants and children with KD develop CAA. However, there is no recommended therapy to halt the progression of arterial wall damage and prevent aneurysm formation. Thus, aneurysm prevention is a primary goal of treatment during the acute phase of the disease and is the focus of this trial. Data from both animal studies and evaluation of the IL-1 pathway in patients with acute KD support IL-1 blockade as a therapeutic option.
Studies in the LCWE-induced mouse model of KD vasculitis have shown that the NLRP3 inflammasome, caspase 1 activation and IL-1β and IL-1α are important in KD vasculitis (Rosenkranz, Schulte et al. 2005, Lee, Schulte et al. 2012, Lee, Wakita et al. 2015). The IL-1 R antagonist, anakinra, which blocks both IL-1α and IL-1β, significantly blocked coronary arteritis, aortitis and myocarditis in this experimental model (Lee, Schulte et al. 2012).
In KD, the interleukin (IL)-1 pathway is upregulated, as demonstrated by increased transcript abundance by microarray and by increased levels of pathway proteins in the plasma (Maury, Salo et al. 1988, Leung, Cotran et al. 1989, Popper, Shimizu et al. 2007, Hoang, Shimizu et al. 2014). In a microarray study of acute and convalescent whole blood samples (PAXgene®) from 141 KD subjects, transcript abundance profiles from KD patients were compared to those of pediatric subjects with different acute infectious diseases and to pediatric healthy controls (Hoang, Shimizu et al. 2014). Differentially expressed transcripts were analyzed according to their participation in different biologic pathways. Common features of the top three pathways for KD were the abundance of transcripts related to the activation of the NLRP3 inflammasome, including IL-1α and β and caspase-1- related transcripts. The upregulation of key genes in the IL-1 pathway was validated using qRT-PCR in an independent cohort of 20 KD patients and 10 healthy controls (Hoang, Shimizu et al. 2014). IL-1α also plays a critical role in chronic inflammation, and recent studies suggest that IL-1α may regulate IL-1β secretion (Fettelschoss, Kistowska et al. 2011, Kamari, Shaish et al. 2011, Dinarello, Simon et al. 2012, Freigang, Ampenberger et al. 2013). It is intriguing that, IL-1 signaling drives proliferation of smooth muscle cells (SMC) and myofibroblast formation (Bonin, Fici et al. 1989, Sasu and Beasley 2000, Alexander, Moehle et al. 2012), a pathologic hallmark of subacute arteriopathy seen in KD.
Recent genome wide association studies have identified several risk loci and polymorphisms in patients with KD. Among, these, the SNPs in the inositol-trisphosphate 3-kinase C (ITPKC) gene were the most strongly associated polymorphisms with KD in both Japanese and US children (Onouchi, Gunji et al. 2008, Lou, Xu et al. 2012, Kuo, Hsu et al. 2014). Importantly, ITPKC regulates Ca2+ influx in the cell, and this mutation leads to sustained elevation of intracellular Ca2+, which is known to induce NLRP3 inflammasome activation (Lee, Subramanian et al. 2012, Murakami, Ockinger et al. 2012, Horng 2014, Rada, Park et al. 2014). The convergence of emerging genetic data with the findings that IL-1 signaling plays a crucial role in the experimental mouse model of KD, as well as the IL-1 pathway being upregulated in KD patients, provide a strong rationale for our hypothesis that IL-1 signaling plays a key role in KD vasculitis and coronary arteritis.
Anakinra is currently used in several inflammatory diseases in infants and children, including systemic juvenile idiopathic arthritis and many of the autoinflammatory syndromes including cryopyrin-associated periodic syndromes (CAPS) and tumor necrosis factor receptor-associated periodic syndrome (TRAPS) (Goldbach-Mansky, Dailey et al. 2006, Hoffman 2009, Ringold, Weiss et al. 2013). In a recent study of 26 patients with the autoinflammatory syndrome neonatal-onset multisystem inflammatory disease (NOMID), treatment with anakinra for 36 months had a low adverse event rate, with upper respiratory infection as the most commonly reported complication (Sibley, Plass et al. 2012). A similar study using anakinra in another autoinflammatory disorder, Muckle Wells syndrome, did not demonstrate any serious adverse events in the 12 patients treated with anakinra for a median of 11 months (range 5–14 months), of whom 5 were children (Kuemmerle-Deschner, Tyrrell et al. 2011).
At least 52 pregnant women, infants and children < 2 years of age have been treated with anakinra with doses ranging from 1 to 20 mg/kg without serious adverse events (Matsubara, Hasegawa et al. 2006, Aksentijevich, Masters et al. 2009, Hoffman 2009, Neven, Marvillet et al. 2010, Nigrovic, Mannion et al. 2011, Record, Beukelman et al. 2011, Stenerson, Dufendach et al. 2011, Cohen, Tacke et al. 2012, Minkis, Aksentijevich et al. 2012, Ruiz Gomez, Couce et al. 2012, Sibley, Plass et al. 2012, Rossi-Semerano, Piram et al. 2013, Chang, Spong et al. 2014, Paccaud, Berthet et al. 2014, Shafferman, Birmingham et al. 2014). In addition, through an informal survey of rheumatologists belonging to the Childhood Arthritis and Rheumatology Research Alliance (CARRA) Network, I was notified of an additional 10 infants (2 to 11 months old) who received anakinra (maximum dose ranging from 3 to 9 mg/kg) for inflammatory disorders without major adverse events. Thus, given the established safety of anakinra in young infants and children with inflammatory disorders, a Phase I/IIa study that evaluates safety, tolerability, and immunomodulatory effects in infants and children with acute KD and CAA is justified.
Anakinra has been used to treat three severe KD patients who were resistant to IVIG treatment (Cohen, Tacke et al. 2012, Shafferman, Birmingham et al. 2014, Tremoulet and Burns 2015). A 2 year old with giant aneurysms who failed therapy with IVIG and steroids was treated with a 6 week course of anakinra at 1 mg/kg (Cohen, Tacke et al. 2012). She suffered no adverse effects and at 6 months coronary angiography demonstrated resolution of the aneurysms, which was unexpected in such a severe case of KD. An 11 week old with KD and giant aneurysms was refractory to three doses of IVIG, steroids and a dose of infliximab (Shafferman, Birmingham et al. 2014). She was treated with anakinra initially at 6 mg/kg/day and then increased to 9 mg/kg/day. She too did not suffer any adverse effects and at an 8 month follow up visit her coronary artery status had also significantly improved. At our center, we treated a 2 month old White female infant who presented with a one week history of fever, classic signs of KD, and dilation of the left main coronary artery and left anterior descending coronary artery as well as an aneurysm of the right coronary artery (Z score max 8.2) by echocardiography. Given the severity of this infant’s illness, we added anakinra (2 mg/kg/day) on the eighth day of illness for five days. Unexpectedly, tissue remodeling, evidenced by decreasing Z score of the RCA, was documented within a month following anakinra treatment. In these three KD patients with CAA, treatment with anakinra did not result in any adverse events and coincided with a decrease in systemic inflammation and coronary artery dimensions.
The 3 + 3 study design allows a rapid escalation through three dosage levels while providing valuable data for PK analysis to inform the choice of dose for a subsequent Phase III efficacy trial. No assumptions are made about the dose/toxicity relationship and the design avoids exposing too many subjects to sub-optimal doses while preserving safety considerations for the toxicity endpoint. This adaptive study design is particularly well-suited to Phase I/II trials enrolling patients with rare or uncommon diseases for which the numbers of study-eligible subjects are limited but the trial requires rapid accrual. This dose finding scheme is a common approach in Phase I oncology trials given its simple design with defined stopping rules for escalation.
There are several strengths and limitations of this study. This is the first clinical trial to target the innate immune response to block progression of arterial wall damage in acute KD patients. Although enrollment can frequently be a major issue in KD clinical trials, our experience is that families in this setting are interested in participating in research that might help improve the future treatment and understanding of this uncommon condition. Obtaining permission from parents for phlebotomy is always a potential hurdle in any clinical trial. We have timed the research phlebotomy to coincide, as much as possible, with clinically-indicated phlebotomy to improve parent acceptance. The choice of a Z score of 3.0 as entrance criterion was based on our experience with 103 KD patients with CAA at Rady Children’s Hospital San Diego. Of these patients, 8 of 43 (or approximately 1 in 6 patients) with a Z score ≥3 went on to develop an aneurysm. This cut-point had a higher proportion of KD patients with an aneurysm than a Z score of 2.5 for which only 11 of 103 (or approximately 1 in 10 patients) developed an aneurysm. Thus, in order to capture a large proportion of the children who would develop an aneurysm yet limit the number of children treated with anakinra, we chose a cut-point Z score of 3. There is currently no robust biomarker to identify patients at risk for aneurysm development.
Conclusion
Thus, this dose-escalation Phase I/IIa trial will determine the safety, tolerability and immunomodulatory effects of anakinra in KD patients at least 8 months of age.
Acknowledgments
This work is supported by a University of California San Diego Department of Pediatrics Intramural Clinical Research grant (awarded to AHT), a Rady Children’s Hospital San Diego Academic Enrichment grant (awarded to AHT), and a grant from the Gordon and Marilyn Macklin Foundation grant (awarded to JCB). Ancillary study support was provided by the Clinical and Translational Study Unit at Boston Children’s Hospital. Anakinra was donated by the manufacturer, Sobi Pharmaceuticals.
Footnotes
Trial registration: Clinical Trials.gov # NCT02179853, registered June 28, 2014
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Adriana H Tremoulet, Associate Professor, Associate Director, Kawasaki Disease Research Center, Department of Pediatrics, University of California San Diego, La Jolla, California, USA, Rady Children’s Hospital San Diego, San Diego, California, USA.
Sonia Jain, Professor, Co-Director, Biostatistics Research Center, Department of Family Medicine and Public Health, University of California San Diego, La Jolla, California.
Susan Kim, Assistant Professor, Rheumatology Program, Division of Immunology, Boston Children's Hospital, Boston, MA, USA.
Jane Newburger, Professor, Department of Cardiology, Harvard Medical School, Boston, Massachusetts, USA, Department of Pediatrics, Children’s Hospital of Boston, Boston, Massachusetts, USA.
Moshe Arditi, Professor, Department of Pediatrics, Cedars Sinai Medical Center and UCLA School of Medicine.
Alessandra Franco, Associate Professor, Department of Pediatrics, University of California San Diego, La Jolla, California, USA.
Brookie Best, Professor, Department of Pediatrics and Skaggs School of Pharmacy, University of California San Diego, La Jolla, California, USA.
Jane C Burns, Professor, Director, Kawasaki Disease Research Center, Department of Pediatrics, University of California San Diego, La Jolla, California, USA, Rady Children’s Hospital San Diego, San Diego, California, USA.
References
- Aksentijevich I, Masters SL, Ferguson PJ, Dancey P, Frenkel J, van Royen-Kerkhoff A, Laxer R, Tedgard U, Cowen EW, Pham TH, Booty M, Estes JD, Sandler NG, Plass N, Stone DL, Turner ML, Hill S, Butman JA, Schneider R, Babyn P, El-Shanti HI, Pope E, Barron K, Bing X, Laurence A, Lee CC, Chapelle D, Clarke GI, Ohson K, Nicholson M, Gadina M, Yang B, Korman BD, Gregersen PK, van Hagen PM, Hak AE, Huizing M, Rahman P, Douek DC, Remmers EF, Kastner DL, Goldbach-Mansky R. An autoinflammatory disease with deficiency of the interleukin-1-receptor antagonist. N Engl J Med. 2009;360(23):2426–2437. doi: 10.1056/NEJMoa0807865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander MR, Moehle CW, Johnson JL, Yang Z, Lee JK, Jackson CL, Owens GK. Genetic inactivation of IL-1 signaling enhances atherosclerotic plaque instability and reduces outward vessel remodeling in advanced atherosclerosis in mice. The Journal of Clinical Investigation. 2012;122(1):70–79. doi: 10.1172/JCI43713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonin PD, Fici GJ, Singh JP. Interleukin-1 promotes proliferation of vascular smooth muscle cells in coordination with PDGF or a monocyte derived growth factor. Experimental cell research. 1989;181(2):475–482. doi: 10.1016/0014-4827(89)90104-3. [DOI] [PubMed] [Google Scholar]
- Chang Z, Spong CY, Jesus AA, Davis MA, Plass N, Stone DL, Chapelle D, Hoffmann P, Kastner DL, Barron K, Goldbach-Mansky RT, Stratton P. Anakinra use during pregnancy in patients with cryopyrin-associated periodic syndromes (CAPS) Arthritis Rheumatol. 2014;66(11):3227–3232. doi: 10.1002/art.38811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Tacke CE, Straver B, Meijer N, Kuipers IM, Kuijpers TW. A child with severe relapsing Kawasaki disease rescued by IL-1 receptor blockade and extracorporeal membrane oxygenation. Ann Rheum Dis. 2012;71(12):2059–2061. doi: 10.1136/annrheumdis-2012-201658. [DOI] [PubMed] [Google Scholar]
- Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11(8):633–652. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fettelschoss A, Kistowska M, LeibundGut-Landmann S, Beer HD, Johansen P, Senti G, Contassot E, Bachmann MF, French LE, Oxenius A, Kündig TM. Inflammasome activation and IL-1β target IL-1α for secretion as opposed to surface expression. Proc Natl Acad Sci U S A. 2011;108(44):18055–18060. doi: 10.1073/pnas.1109176108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freigang S, Ampenberger F, Weiss A, Kanneganti TD, Iwakura Y, Hersberger M, Kopf M. Fatty acid-induced mitochondrial uncoupling elicits inflammasome-independent IL-1α and sterile vascular inflammation in atherosclerosis. Nat Immunol. 2013;14(10):1045–1053. doi: 10.1038/ni.2704. [DOI] [PubMed] [Google Scholar]
- Goldbach-Mansky R, Dailey NJ, Canna SW, Gelabert A, Jones J, Rubin BI, Kim HJ, Brewer C, Zalewski C, Wiggs E, Hill S, Turner ML, Karp BI, Aksentijevich I, Pucino F, Penzak SR, Haverkamp MH, Stein L, Adams BS, Moore TL, Fuhlbrigge RC, Shaham B, Jarvis JN, O’Neil K, Vehe RK, Beitz LO, Gardner G, Hannan WP, Warren RW, Horn W, Cole JL, Paul SM, Hawkins PN, Pham TH, Snyder C, Wesley RA, Hoffmann SC, Holland SM, Butman JA, Kastner DL. Neonatal-onset multisystem inflammatory disease responsive to interleukin-1beta inhibition. N Engl J Med. 2006;355(6):581–592. doi: 10.1056/NEJMoa055137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JB, Kahn AM, Burns JC. When children with kawasaki disease grow up myocardial and vascular complications in adulthood. Journal of the American College of Cardiology. 2009;54(21):1911–1920. doi: 10.1016/j.jacc.2009.04.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang LT, Shimizu C, Ling L, Naim AN, Khor CC, Tremoulet AH, Wright V, Levin M, Hibberd ML, Burns JC. Global gene expression profiling identifies new therapeutic targets in acute Kawasaki disease. Genome Med. 2014;6(11):541. doi: 10.1186/s13073-014-0102-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman HM. Therapy of autoinflammatory syndromes. The Journal of allergy and clinical immunology. 2009;124(6):1129–1138. doi: 10.1016/j.jaci.2009.11.001. quiz 1139–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horng T. Calcium signaling and mitochondrial destabilization in the triggering of the NLRP3 inflammasome. Trends in Immunology. 2014 doi: 10.1016/j.it.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamari Y, Shaish A, Shemesh S, Vax E, Grosskopf I, Dotan S, White M, Voronov E, Dinarello CA, Apte RN, Harats D. Reduced atherosclerosis and inflammatory cytokines in apolipoprotein-E-deficient mice lacking bone marrow-derived interleukin-1α. Biochem Biophys Res Commun. 2011;405(2):197–203. doi: 10.1016/j.bbrc.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Kato H, Sugimura T, Akagi T, Sato N, Hashino K, Maeno Y, Kazue T, Eto G, Yamakawa R. Long-term consequences of Kawasaki disease. A 10- to 21-year follow-up study of 594 patients. Circulation. 1996;94(6):1379–1385. doi: 10.1161/01.cir.94.6.1379. [DOI] [PubMed] [Google Scholar]
- Kuemmerle-Deschner JB, Tyrrell PN, Koetter I, Wittkowski H, Bialkowski A, Tzaribachev N, Lohse P, Koitchev A, Deuter C, Foell D, Benseler SM. Efficacy and safety of anakinra therapy in pediatric and adult patients with the autoinflammatory Muckle-Wells syndrome. Arthritis Rheum. 2011;63(3):840–849. doi: 10.1002/art.30149. [DOI] [PubMed] [Google Scholar]
- Kuo HC, Hsu YW, Lo MH, Huang YH, Chien SC, Chang WC. Single-Nucleotide Polymorphism rs7251246 in ITPKC Is Associated with Susceptibility and Coronary Artery Lesions in Kawasaki Disease. PLoS ONE. 2014;9(3):e91118. doi: 10.1371/journal.pone.0091118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G-S, Subramanian N, Kim AI, Aksentijevich I, Goldbach-Mansky R, Sacks DB, Germain RN, Kastner DL, Chae JJ. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca(2+) and cAMP. Nature. 2012 doi: 10.1038/nature11588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Schulte DJ, Shimada K, Chen S, Crother TR, Chiba N, Fishbein MC, Lehman TJ, Arditi M. Interleukin-1beta is crucial for the induction of coronary artery inflammation in a mouse model of Kawasaki disease. Circulation. 2012;125(12):1542–1550. doi: 10.1161/CIRCULATIONAHA.111.072769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Schulte DJ, Shimada K, Chen S, Crother TR, Chiba N, Fishbein MC, Lehman TJ, Arditi M. Interleukin-1β is crucial for the induction of coronary artery inflammation in a mouse model of kawasaki disease. Circulation. 2012;125(12):1542–1550. doi: 10.1161/CIRCULATIONAHA.111.072769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Wakita D, Dagvadorj J, Shimada K, Chen S, Huang G, Lehman TJ, Fishbein MC, Hoffman HM, Crother TR, Arditi M. IL-1 Signaling Is Critically Required in Stromal Cells in Kawasaki Disease Vasculitis Mouse Model: Role of Both IL-1alpha and IL-1beta. Arterioscler Thromb Vasc Biol. 2015;35(12):2605–2616. doi: 10.1161/ATVBAHA.115.306475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman TJ, Walker SM, Mahnovski V, McCurdy D. Coronary arteritis in mice following the systemic injection of group B Lactobacillus casei cell walls in aqueous suspension. Arthritis & Rheumatism. 1985;28(6):652–659. doi: 10.1002/art.1780280609. [DOI] [PubMed] [Google Scholar]
- Leung DY, Cotran RS, Kurt-Jones E, Burns JC, Newburger JW, Pober JS. Endothelial cell activation and high interleukin-1 secretion in the pathogenesis of acute Kawasaki disease. Lancet. 1989;2(8675):1298–1302. doi: 10.1016/s0140-6736(89)91910-7. [DOI] [PubMed] [Google Scholar]
- Lou J, Xu S, Zou L, Zhong R, Zhang T, Sun Y, Lu X, Liu L, Li C, Wang L, Xiong G, Wang W, Gong F, Wu J. A functional polymorphism, rs28493229, in ITPKC and risk of Kawasaki disease: an integrated meta-analysis. Molecular biology reports. 2012 doi: 10.1007/s11033-012-2022-0. [DOI] [PubMed] [Google Scholar]
- Matsubara T, Hasegawa M, Shiraishi M, Hoffman HM, Ichiyama T, Tanaka T, Ueda H, Ishihara T, Furukawa S. A severe case of chronic infantile neurologic, cutaneous, articular syndrome treated with biologic agents. Arthritis and Rheumatism. 2006;54(7):2314–2320. doi: 10.1002/art.21965. [DOI] [PubMed] [Google Scholar]
- Maury CP, Salo E, Pelkonen P. Circulating interleukin-1 beta in patients with Kawasaki disease. N Engl J Med. 1988;319(25):1670–1671. doi: 10.1056/NEJM198812223192515. [DOI] [PubMed] [Google Scholar]
- McCrindle BW. Kawasaki disease: a childhood disease with important consequences into adulthood. Circulation. 2009;120(1):6–8. doi: 10.1161/CIRCULATIONAHA.109.874800. [DOI] [PubMed] [Google Scholar]
- Minkis K, Aksentijevich I, Goldbach-Mansky R, Magro C, Scott R, Davis JG, Sardana N, Herzog R. Interleukin 1 receptor antagonist deficiency presenting as infantile pustulosis mimicking infantile pustular psoriasis. Archives of Dermatology. 2012;148(6):747–752. doi: 10.1001/archdermatol.2011.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T, Ockinger J, Yu J, Byles V, McColl A, Hofer AM, Horng T. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proceedings of the National Academy of Sciences. 2012 doi: 10.1073/pnas.1117765109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neven B, Marvillet I, Terrada C, Ferster A, Boddaert N, Couloignier V, Pinto G, Pagnier A, Bodemer C, Bodaghi B, Tardieu M, Prieur AM, Quartier P. Long-term efficacy of the interleukin-1 receptor antagonist anakinra in ten patients with neonatal-onset multisystem inflammatory disease/chronic infantile neurologic, cutaneous, articular syndrome. Arthritis Rheum. 2010;62(1):258–267. doi: 10.1002/art.25057. [DOI] [PubMed] [Google Scholar]
- Newburger JW, Takahashi M, Burns JC, Beiser AS, Chung KJ, Duffy CE, Glode MP, Mason WH, Reddy V, Sanders SP, et al. The treatment of Kawasaki syndrome with intravenous gamma globulin. N Engl J Med. 1986;315(6):341–347. doi: 10.1056/NEJM198608073150601. [DOI] [PubMed] [Google Scholar]
- Nigrovic PA, Mannion M, Prince FH, Zeft A, Rabinovich CE, van Rossum MA, Cortis E, Pardeo M, Miettunen PM, Janow G, Birmingham J, Eggebeen A, Janssen E, Shulman AI, Son MB, Hong S, Jones K, Ilowite NT, Cron RQ, Higgins GC. Anakinra as first-line disease-modifying therapy in systemic juvenile idiopathic arthritis: report of forty-six patients from an international multicenter series. Arthritis and Rheumatism. 2011;63(2):545–555. doi: 10.1002/art.30128. [DOI] [PubMed] [Google Scholar]
- NIH and DAIDS. Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events. 2009 from http://www.niaid.nih.gov/LabsAndResources/resources/DAIDSClinRsrch/Documents/daidsaegradingtable.pdf.
- Onouchi Y, Gunji T, Burns JC, Shimizu C, Newburger JW, Yashiro M, Nakamura Y, Yanagawa H, Wakui K, Fukushima Y, Kishi F, Hamamoto K, Terai M, Sato Y, Ouchi K, Saji T, Nariai A, Kaburagi Y, Yoshikawa T, Suzuki K, Tanaka T, Nagai T, Cho H, Fujino A, Sekine A, Nakamichi R, Tsunoda T, Kawasaki T, Nakamura Y, Hata A. ITPKC functional polymorphism associated with Kawasaki disease susceptibility and formation of coronary artery aneurysms. Nature Genetics. 2008;40(1):35–42. doi: 10.1038/ng.2007.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opal SM, Fisher CJ, Jr, Dhainaut JF, Vincent JL, Brase R, Lowry SF, Sadoff JC, Slotman GJ, Levy H, Balk RA, Shelly MP, Pribble JP, LaBrecque JF, Lookabaugh J, Donovan H, Dubin H, Baughman R, Norman J, DeMaria E, Matzel K, Abraham E, Seneff M. Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: a phase III, randomized, double-blind, placebo-controlled, multicenter trial. The Interleukin-1 Receptor Antagonist Sepsis Investigator Group. Crit Care Med. 1997;25(7):1115–1124. doi: 10.1097/00003246-199707000-00010. [DOI] [PubMed] [Google Scholar]
- Paccaud Y, Berthet G, Von Scheven-Gete A, Vaudaux B, Mivelaz Y, Hofer M, Roth-Kleiner M. Neonatal treatment of CINCA syndrome. Pediatr Rheumatol Online J. 2014;12:52. doi: 10.1186/1546-0096-12-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popper SJ, Shimizu C, Shike H, Kanegaye JT, Newburger JW, Sundel RP, Brown PO, Burns JC, Relman DA. Gene-expression patterns reveal underlying biological processes in Kawasaki disease. Genome biology. 2007;8(12):R261. doi: 10.1186/gb-2007-8-12-r261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada B, Park JJ, Sil P, Geiszt M, Leto TL. NLRP3 inflammasome activation and interleukin-1β release in macrophages require calcium but are independent of calcium-activated NADPH oxidases. Inflammation Research. 2014 doi: 10.1007/s00011-014-0756-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Record JL, Beukelman T, Cron RQ. Combination therapy of abatacept and anakinra in children with refractory systemic juvenile idiopathic arthritis: a retrospective case series. Journal of Rheumatology. 2011;38(1):180–181. doi: 10.3899/jrheum.100726. [DOI] [PubMed] [Google Scholar]
- Ringold S, Weiss PF, Beukelman T, DeWitt EM, Ilowite NT, Kimura Y, Laxer RM, Lovell DJ, Nigrovic PA, Robinson AB, Vehe RK. 2013 update of the 2011 American College of Rheumatology recommendations for the treatment of juvenile idiopathic arthritis: recommendations for the medical therapy of children with systemic juvenile idiopathic arthritis and tuberculosis screening among children receiving biologic medications. Arthritis Rheum. 2013;65(10):2499–2512. doi: 10.1002/art.38092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz ME, Schulte DJ, Agle LM, Wong MH, Zhang W, Ivashkiv L, Doherty TM, Fishbein MC, Lehman TJ, Michelsen KS, Arditi M. TLR2 and MyD88 contribute to Lactobacillus casei extract-induced focal coronary arteritis in a mouse model of Kawasaki disease. Circulation. 2005;112(19):2966–2973. doi: 10.1161/CIRCULATIONAHA.105.537530. [DOI] [PubMed] [Google Scholar]
- Rossi-Semerano L, Piram M, Chiaverini C, De Ricaud D, Smahi A, Kone-Paut I. First clinical description of an infant with interleukin-36-receptor antagonist deficiency successfully treated with anakinra. Pediatrics. 2013;132(4):e1043–1047. doi: 10.1542/peds.2012-3935. [DOI] [PubMed] [Google Scholar]
- Ruiz Gomez A, Couce ML, Garcia-Villoria J, Torres A, Bana Souto A, Yague J, Vilaseca MA, Ribes A, Arostegui JI. Clinical, genetic, and therapeutic diversity in 2 patients with severe mevalonate kinase deficiency. Pediatrics. 2012;129(2):e535–539. doi: 10.1542/peds.2010-2192. [DOI] [PubMed] [Google Scholar]
- Sasu S, Beasley D. Essential roles of IkappaB kinases alpha and beta in serum- and IL-1-induced human VSMC proliferation. American journal of physiology. Heart and circulatory physiology. 2000;278(6):H1823–1831. doi: 10.1152/ajpheart.2000.278.6.H1823. [DOI] [PubMed] [Google Scholar]
- Schulte DJ, Yilmaz A, Shimada K, Fishbein MC, Lowe EL, Chen S, Wong M, Doherty TM, Lehman T, Crother TR, Sorrentino R, Arditi M. Involvement of innate and adaptive immunity in a murine model of coronary arteritis mimicking Kawasaki disease. J Immunol. 2009;183(8):5311–5318. doi: 10.4049/jimmunol.0901395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafferman A, Birmingham JD, Cron RQ. High dose anakinra for treatment of severe neonatal Kawasaki disease: a case report. Pediatr Rheumatol Online J. 2014;12:26. doi: 10.1186/1546-0096-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley CH, Plass N, Snow J, Wiggs EA, Brewer CC, King KA, Zalewski C, Kim HJ, Bishop R, Hill S, Paul SM, Kicker P, Phillips Z, Dolan JG, Widemann B, Jayaprakash N, Pucino F, Stone DL, Chapelle D, Snyder C, Butman JA, Wesley R, Goldbach-Mansky R. Sustained response and prevention of damage progression in patients with neonatal-onset multisystem inflammatory disease treated with anakinra: a cohort study to determine three- and five-year outcomes. Arthritis and Rheumatism. 2012;64(7):2375–2386. doi: 10.1002/art.34409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenerson M, Dufendach K, Aksentijevich I, Brady J, Austin J, Reed AM. The first reported case of compound heterozygous IL1RN mutations causing deficiency of the interleukin-1 receptor antagonist. Arthritis and Rheumatism. 2011;63(12):4018–4022. doi: 10.1002/art.30565. [DOI] [PubMed] [Google Scholar]
- Tanaka N. Pathological study of Kawasaki disease (MCLS): with special reference to sequelae. Jpn J Med Sci Biol. 1979;32(4):245–246. [PubMed] [Google Scholar]
- Tremoulet A, Burns J. Anakinra Treatment for IVIG-resistant Kawasaki Disease Patient with Coronary Artery Aneurysms: Clinical experience 2015 [Google Scholar]
- Tremoulet AH, Best BM, Song S, Wang S, Corinaldesi E, Eichenfield JR, Martin DD, Newburger JW, Burns JC. Resistance to intravenous immunoglobulin in children with Kawasaki disease. J Pediatr. 2008;153(1):117–121. doi: 10.1016/j.jpeds.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung RS. Lessons learned from an animal model of Kawasaki disease. Clin Exp Rheumatol. 2007;25(1 Suppl 44):S69–71. [PubMed] [Google Scholar]
- Yonesaka S, Nakada T, Sunagawa Y, Tomimoto K, Naka S, Takahashi T, Matubara T, Sekigami I. Endomyocardial biopsy in children with Kawasaki disease. Acta Paediatr Jpn. 1989;31(6):706–711. doi: 10.1111/j.1442-200x.1989.tb01384.x. [DOI] [PubMed] [Google Scholar]