Abstract
Glial cells are present in all organisms with a CNS and, with increasing brain complexity, glial cells have undergone substantive increases in cell number, diversity, and functions. Invertebrates, such as Drosophila, possess glial subtypes with similarity to mammalian astrocytes in their basic morphology and function, representing fertile ground for unraveling fundamental aspects of glial biology. Although glial subtypes in simple organisms may be relatively homogenous, emerging evidence suggests the possibility that mammalian astrocytes might be highly diversified to match the needs of local neuronal subtypes. In this Perspective, we review classic and new roles identified for astrocytes and oligodendrocytes by recent studies. We propose that delineating genetic and developmental programs across species will be essential to understand the core functions of glia that allow enhanced neuronal function and to achieve new insights into glial roles in higher-order brain function and neurological disease.
I. Introduction
Glial Functions since 1856
The term “glia” (from the ancient Greek for glue), coined by Rudolf Virchow in 1856, seems to carry both literal and figurative connotations. Virchow thought glia to be support cells, a putty holding things together. However, it is perhaps pertinent that in Virchow’s time glue was a rather ignoble substance made from the hooves of knackered horses. Whether intentional or not, his descriptor implied a passive and uninteresting function for glia, placing them low in the neural hierarchy. However, attitudes are shifting with new studies that show that glial cells are essential modulators of brain function and health.
In 2008, a previous Perspective on this topic in Neuron (Barres, 2008) highlighted many then newly identified and unexpected functions of glia and predicted many more. A mere 5 years later, the list of developmental mechanisms of and roles for macroglia—i.e., oligodendrocytes, astrocytes, and their precursors—has expanded significantly. Progress in the field has been comprehensively covered in many outstanding recent reviews (Aguzzi et al., 2013; Attwell et al., 2010; Emery, 2010; Eroglu and Barres, 2010; Freeman, 2010; Molofsky et al., 2012; Nave, 2010). This Perspective is not meant to be a comprehensive review of glial cell biology. Rather, we hope to highlight emerging ideas in the field, discuss how approaches are rapidly evolving, and suggest priorities for the future. We will focus primarily on macroglia (with apologies to microglia and Schwann cells) and adopt the speculative view-point that the long evolutionary time frame for codevelopment of neurons and glial cells, from simple organisms to higher organisms, indicates the fundamental importance of glia in invertebrates and predicts their increased diversity in vertebrates. We envisage that tools of developmental biology and cross-species analysis will yield exciting new insights into the precise functions of glial subtypes from the simplest invertebrates to man.
Brief Summary of Oligodendrocyte Generation and Functions
Oligodendrocytes are the myelinating cells of the CNS, which enable saltatory (or jumping) conduction of nerve impulses. The functional equivalent of the oligodendrocyte in the peripheral nervous system (PNS) is the Schwann cell. Oligodendrocytes and segmental/nodal myelination are a relatively recent evolutionary innovation appearing in jawed vertebrates (Zalc et al., 2008) (Figures 1 and 3), although analogous ensheathing cells and primitive myelinated membranes on axons are found in invertebrates (Hartline and Colman, 2007). Many aspects of myelination initiation remain poorly understood. On the one hand, oligodendrocytes can recognize even inert tubular structures of the appropriate axonal diameter to initiate myelin production; on the other, activity-driven and environmental cues also can regulate the timing and extent of myelination. In any case, myelination must be one of the most extraordinary examples of cellular hypertrophy in biology—an oligodendrocyte expands its surface area over 6,500-fold through the massive production of membrane in order to myelinate multiple (perhaps 50 or more) axon segments. Thus, oligodendrocytes must have a close association with the vasculature to support their extraordinary metabolic and substrate demands for myelination production and maintenance of myelin and axonal integrity (Lee et al., 2012).
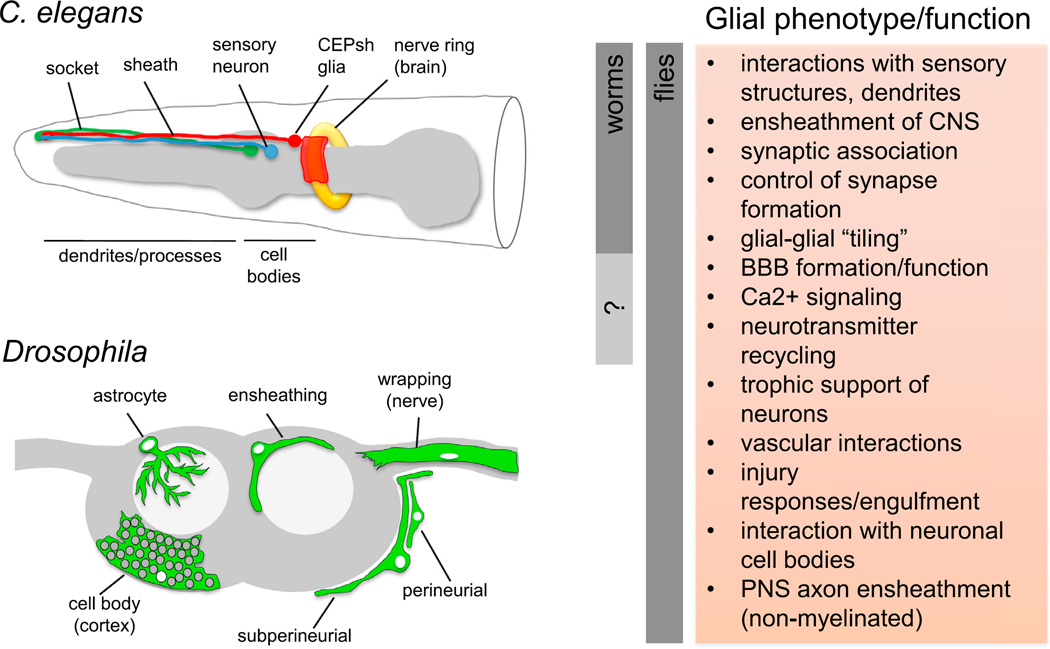
Figure 1. Complex Glia in Even Simple Organisms.
Glial cells in C. elegans and Drosophila. All C. elegans glia are associated with sensory structures, though the CEPsh glia also infiltrate the worm CNS. Drosophila have similar SOP-derived glial subtypes in the periphery (data not shown) and more elaborate and functionally distinct subclasses of glia in the CNS. A list of well-defined glial molecular or morphological phenotypes and functions that are conserved in worms and flies (indicated for each animal by gray bars to left) are listed. See text for details.
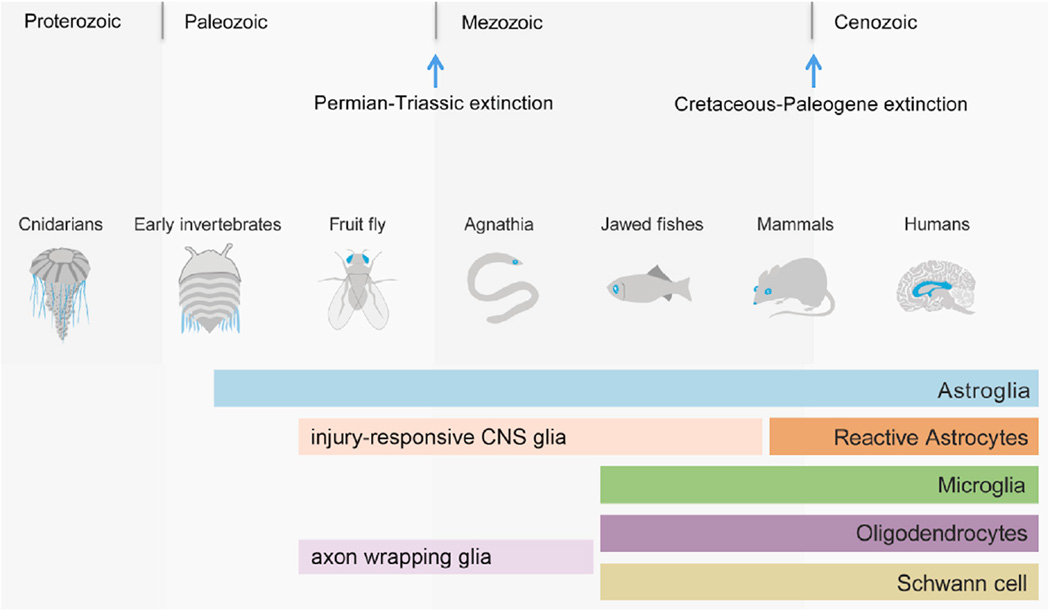
Figure 3. Evolutionary Glial Epochs: Increasing Complexity of the CNS Demanded New Glial Solutions and Diversity.
Cnidarians lack a nervous system and glia. The first vertebrates of the Paleozoic era (5–600 million years ago) most likely contained glial cells. Fruit flies contain 105 neurons and astroglia-like cells with many features in common with those of vertebrates, including responsiveness to injury. Invertebrates also contain nonmyelinating glia that ensheath long axons in the PNS, which may be related to oligodendrocytes and Schwann cells. In the Mesozoic era, jawless vertebrate fishes (agnatha) displayed neuronal diversity typical of vertebrates but lacked myelinated axon tracts. In contrast, jawed fishes possess myelinating oligodendrocytes of the CNS and Schwann cells of the peripheral nervous system. It is important to note that while zebrafish contain radial glial cells, definitive astrocytes have not yet been identified. Vertebrate astrocytes and reactive astrocytes are clearly present in mammals, such as the mouse, in which genetic tools allow dissection of potential astrocyte subtype heterogeneity. The human brain contains approximately 85 × 106 million neurons and as much as 90% of the human brain is glial cells. Human astrocytes possess more elaborate morphology than that of rodents.
Oligodendrocyte precursors (OPCs) recognized by expression of the chondroitin sulfate proteoglycan NG2 (hence the term “NG2 glia”) and other markers are the most proliferative cell type in the adult mammalian brain, outnumbering populations of persistent neural stem cells of the subventricular zone (SVZ) and hippocampus. Such OPCs are involved in turnover and routine maintenance of myelin; they receive synapses from neurons (Bergles et al., 2000; Lin et al., 2005) and respond to injury (Young et al., 2013). After demyelination, such as in multiple sclerosis (MS), caused by autoimmune attack of myelin, OPCs rapidly reinvest the lesion area and in some cases can perform myelination of denuded axons leading to functional recovery. Why some lesions of MS fail in remyelination, leading to chronic plaques, is unknown and might represent the environmental signals present in certain lesions and/or potentially variable capabilities of the OPCs in different lesions. OPCs are also among the first responders, even in injuries not requiring remyelination, and they are often present in glial scars, suggesting trophic or additional roles in CNS homeostasis.
While studies in the 1980s focused on the nature of glial precursors and their progeny lineages, the last decade has witnessed an explosion of developmental and genetic studies focused on glial subtypes, in particular oligodendrocytes. We now understand that all oligodendrocytes in the CNS are specified through a uniform process that requires function of Olig1/2 bHLH transcription factors. Many regulatory factors for subsequent lineage commitment, differentiation, proliferation, and maturation of oligodendrocytes have been identified. Progress in understanding the development of the oligodendrocyte lineage is a cogent example of how development can transform our understanding of diseases like MS, and other human disorders with prominent white matter injury including cerebral palsy, stroke, and spinal cord injury (Fancy et al., 2011). Speaking of “transformation,” recent studies suggest, unexpectedly, that oligodendrocytes serve as a cell of origin for glioma (Liu et al., 2011). Indeed, both OPCs and glioma can invade and migrate through tissues and proliferate in response to oncogenic signals of the RAS pathway. Exploiting such parallels in oligodendrocyte biology and gliomagenesis might provide insights into the genesis and biological properties of these deadly tumors as well as their therapy.
Brief Summary of Astrocyte Generation and Functions
Astrocytes are the most numerous cell type in the brain and a steady stream of work points to an increasingly wide spectrum of roles for these cells during development and in the mature CNS. Although the precise nature of astrocyte precursors remains poorly understood, radial glia comprise a substrate for generation and migration during development and may have additional roles in CNS organization (described below). During development, radial glial cells produce neuron, oligodendrocyte, and astrocyte precursors and then, finally, transform into astrocytes themselves (which explains why any cre recombinase fate map that includes even a transient stage of radial glia expression will mark a subset of astrocytes). It has become clear from recent work that a proliferating nonradial glial cell (“intermediate astrocyte precursor”) serves to expand local astrocyte populations in different CNS domains (Ge et al., 2012; Tien et al., 2012).
Classic astrocyte roles include structural and metabolic support, maintenance of the blood-brain barrier (BBB), regulation of cerebral blood flow, clearance of neurotransmitters at the synapses and maintaining ion balance, support of myelin structures in white matter tracts, and inflammatory reactivity after injury. Recent studies indicate that astrocytes are working hastily in the trenches during neural circuit formation and fine-tuning of synapses. We now appreciate that astrocytes can potently promote synaptogenesis through expression of thrombospondins, Sparc, and glypicans to allow for initial neuron-neuron contact and probably subsequent fine-tuning (Allen et al., 2012; Christopherson et al., 2005; Kucukdereli et al., 2011). Optimization of connectivity through synapse elimination can also be carried out directly by astrocyte engulfment of synapses through MerTK and MEGF10 (W.S. Chung and B. Barres, personal communication), a molecular event first described in Drosophila (Awasaki et al., 2006), or through modulating C1Q/complement cascade-mediated removal of synapses by microglia (Stevens et al., 2007). Emerging evidence also points to important roles for astrocytes in synaptic plasticity, which means astrocytes are required for the formation, fine-tuning, function, and plasticity of our brain circuitry. Finally, astrocytes are increasingly becoming implicated in a variety of human diseases from leukodystrophies, congenital epilepsy syndromes, to neurodevelopmental disorders, and beyond. Reactive astrocytes are a hallmark of nearly all major human CNS neurodegenerative conditions (Zamanian et al., 2012).
How do astrocytes do all this? Is there only one type of astrocyte? For many years, investigators have reported different morphologies of CNS astrocytes, but electrophysiological correlates have not been clearly demonstrated. Although astrocytes have traditionally been considered a homogeneous population of cells, steady reports of their increasingly diversified functional roles in mammals brings into question whether astrocyte subtypes may have been elaborated in complex brains to carry out enhanced regional functions. For example, expression profiling studies of astrocytes have generated databases suggesting heterogeneous functions that may be organized according to brain region. Astrocyte cocultures from brain and spinal cord can show differential effects in regulation of neural stem cells, and indeed, SVZ stem cells, which bear similarities to astrocytes, have been shown to be heterogeneous in terms of their progeny output (Merkle et al., 2007). A new type of radial glia stem cell, the outer radial glia (oRG), appears to function in the mammalian brain to contribute further rounds of progeny production increasing brain size and complexity (Rowitch and Kriegstein, 2010). These findings are augmented by the notion that evolutionary pressure might be a driving force for diversified astrocyte functions, discussed further below.
II. Glial Functions Starting in the Paleozoic Era
An Ancient Relationship with Neurons
The diversity of functional roles continuously carried out by glia make them indispensable for CNS function. For example, glia have to balance neuronal requests for energy, maintain the concentration of multiple extracellular ions, secrete growth factors, survey the nervous system for injury, all while reading neuronal activity and taking part in some aspects of signaling. The complexity of glial functions raises multiple experimental obstacles. First, because glia do so much, they are indispensable in higher organisms and their manipulation often leads to neuronal demise and death of the organism. Second, it is challenging to measure any of these functions in vivo, never mind measuring them all at once. Recording action potentials is relatively simple—how does one measure glial delivery of sugars, flux of ions across the membrane, and the activity of glia at individual synapses? Probably all of these are ultimately important for circuit function, but how do we integrate the impact of each on nervous system output? Third, because glia and neurons have developed a reciprocally interdependent relationship, removal of glia from an animal and placement in a dish breaks their physical associations with neurons (and other glia) and seems to transform their phenotypes morphologically and molecularly in dramatic ways. A prime example of this is astrocytes, which in culture appear more like reactive astrocytes. Therefore, while research in vivo is now widely accepted as essential, the field has been limited by a lack of genetic tools. Fortunately, a new enthusiasm for understanding glial biology is leading to the production of newer tools and experimental animal systems suitable for in vivo studies that can help propel our understanding of basic glial biology.
The Glial Menagerie: From Simple Beginnings to Staggering Complexity
About 600 million years ago, there was already great diversity of animal form, as displayed in the fossil record in deposits such as the Burgess Shale in the Canadian Rockies (Figure 2). The first moving multicellular animals, the Cnidarians, began floating about during the Protopaleozoic area. Jellyfish contain only rudimentary nerve nets and very simple light-sensing organelles; glial cells are not obviously present. It may be that nonneuronal support cells from mesodermal rather than ectodermal lineages perform very basic support roles for neurons, but this remains to be explored. The subsequent Paleozoic era is characterized by mass extinctions and intense selective pressure. In animals with slightly more sophisticated neural tissues that include peripheral sensory structures and simple centralized ganglia—such as in C. elegans—glial cells become much more obvious and even in this simple state neuron-glia interactions appear similar to those in higher organisms (Shaham, 2006; Stork et al., 2012). Such simple, ectodermally derived nonneuronal support cells may have originated once in a single common ancestor or multiple times in distinct lineages (e.g., through convergent evolution, atavism, etc.) (Hartline, 2011). If the former, then studying glial cells in simple model genetic organisms would be expected to bear great fruit in defining ancestral, and presumably the most essential, roles for these cells in neural tissues. If the latter, gaining a deeper understanding of both invertebrate and vertebrate glia would allow for the definition of key hurdles that must have been overcome with respect to neuronal function that allowed for the successful elaboration of more complex nervous systems. Much of the discussion below will rely on the morphology of neuron-glia interactions, because form can be indicative of function, but wherever possible molecular correlates will also be discussed. Upon close inspection, the morphological relationship between glia and neurons in flies and worms makes a strong argument that glia became highly dependent upon neurons very early on in evolution.
Figure 2. The Burgess Shale in the Canadian Rockies Provides a Rich Fossil Record of Paleozoic Invertebrates.
Glial Cells of C. elegans and Drosophila
Worms have a relatively simple nervous system composed of 302 neurons, 50 glial cells of ectodermal origin, and six glial cells of mesodermal origin (Shaham, 2006). All ectodermally derived glia in C. elegans are associated with sensory structures (Figure 1). The two major cell types are sheath cells, which associate closely with sensory dendrite endings, and socket cells, which form a pore to allow sensory neurons to interact with the environment. However, there are also four cephalic sheath (CEPsh) glia (a subset of sheath glia) that, in addition to associating with sensory dendrite endings in the periphery, also extend sheet-like processes to the nerve ring (essentially the worm brain). One can envision CEPsh being among the early primordial glia that reached their membrane processes from peripheral sensory organs toward the CNS (Heiman and Shaham, 2007). CEPsh glia are critical for nerve ring formation, and their processes enwrap the worm brain to potentially form what can loosely be called a type of “blood-brain” barrier, although this barrier function has not been investigated directly; CEPsh glia extend membrane processes deeply into the nerve ring, where they can associate closely with some synapses. Even in this apparently rudimentary state, CEPsh glia act like astrocytes, in that they control the placement of synapses. For example, displacement of CEPsh glial membranes results in a corresponding and predictable shift in the position of the AIY-RIA synaptic contacts (Colón-Ramos et al., 2007; Shao et al., 2013). Beyond simply wrapping the nerve ring and associating with synapses, CEPsh glia form sharply defined borders with one another, an arrangement reminiscent of the unique spatial domains occupied by mammalian astrocytes, called “tiling.” Based on the functional roles of worm glia, it appears that ancestral roles for glia include modulation of neurite outgrowth, organization of ganglia, regulation of synapse formation, and general support of neuronal function (Oikonomou and Shaham, 2011).
Drosophila larvae and adults have glial cells associated with both PNS and CNS neural tissues (Freeman, 2012; Stork et al., 2012). Much like C. elegans, Drosophila peripheral sensory organ precursor formation leads to the production of sheath and socket-type glial cells, as well as a glial cell that migrates along the sensory neuron axon toward the CNS. These peripheral sensory organs associated glia function in similar ways to worm sheath and socket glia, surrounding the sensory neuron and providing a suitable environment for sensory dendrites to receive information. The peripheral nerves connecting the CNS and PNS house sensory and motor axons, which are avidly ensheathed by glial cells, in a manner reminiscent of mammalian unmyelinated axon bundles in the PNS, termed Remak bundles. Although there is no myelin in Drosophila, these glial cells are probably the closest relatives to mammalian Schwann cells and oligodendrocytes due to their tight axonal association.
Based on morphology, molecular markers, and functional roles, two populations of glia in Drosophila appear analogous to mammalian astrocytes (Figure 1). First, cortical (“cell body”) glia associate with all neuronal cell bodies, isolating them from other neurons by apparently wrapping each neuronal soma individually. A second subtype of glia, referred to as Drosophila astrocytes, extends cellular processes deeply into the neuropil and associates closely with axons, dendrites, and synapses (Awasaki et al., 2008; Doherty et al., 2009). One might not think so, but fly CNS glia have evolved a complex association with the vasculature (containing hemolymph), likely to maintain neuronal health. Gas exchange in Drosophila occurs through a series of trachea, gas filled tubules that penetrate most tissues in the fly, including the CNS. Within the cell cortex, trachea are tightly surrounded by glial membranes, likely from cortex glia, and within the neuropil tracheal elements are in close proximity to astrocyte membranes (Pereanu et al., 2007). These glia-trachea contacts provide an obvious potential route of gas exchange between CNS neuronal cell bodies and the environment. And what about nutrient delivery? The Drosophila nervous system is surrounded by a multilayered BBB, which is composed of an outermost neural lamella (a carbohydrate-based extracellular matrix), a layer of glial cells termed perineurial glia, and then subperineurial glia (SPGs) (DeSalvo et al., 2011). SPGs are flattened, surround the entire CNS, and form pleated septate junctions with one another that act as a BBB. The entire BBB structure can be thought of as an inside-out blood vessel—hemolymph is on the outside and, to get in, the CNS molecules must pass through the neural lamella (a charge and size exclusion barrier). The PGs and SPGs (the latter sealed with tight junctions) are probably the site of exchange of ions, metabolites, growth factors, and other molecules that travel into and out of the CNS. The final subtype of CNS glia is ensheathing cells, which form a layer between the neuropil and cell cortex but also penetrate the neuropil to compartmentalize different regions of the brain. Rhombomeres of the vertebrate CNS are an example where compartmentalization of brain structures is important for segregating function; whether this is also the case for Drosophila glial brain segregation remains for the moment speculative. Certainly these cells are critical to maintain brain health—after injury, ensheathing glia become “reactive” and extend membranes to sites of injury, where they phagocytose degenerating neuronal material (MacDonald et al., 2006).
Certain functional roles for glial cells in the fly are analogous to those defined in mammals. Drosophila CNS glia express glutamate transporters, glutamine synthetase, and GABA transporters presumably to aid in neurotransmitter recycling (Rival et al., 2004; Soustelle et al., 2002). They guide axon outgrowth, dendrite morphogenesis, and provide trophic support required for neuronal survival (Edenfeld et al., 2005; Freeman and Doherty, 2006). Peripheral glial cells are important for maintaining nerve health, NMJ integrity, and growth. However, they can also actively destroy axons in situations in which the neuron is “dying back” through release of glial factors that activate a caspase-mediated axonal degradative pathway (Keller et al., 2011). Regional specialization of glial function is probably present in the fly. For example, cortex glia and astrocytes appear to have subdivided the roles of protoplasmic astrocytes in mammals by associating with the neuronal cell body and neural circuitry, respectively. However, functional heterogeneity within any of these subtypes has not yet been demonstrated.
Off the Scent of Evolutionary Conservation
Based on the remarkable successes of invertebrate model organisms in unraveling fundamental principles of neuronal development, physiology, and plasticity, one would think invertebrate model organisms like Drosophila and C. elegans would also be prime settings in which to explore basic aspects of glial cell biology. This is especially true based on the emerging widespread acceptance of the importance of studying glial cell function in the intact animal. However, the glial field has not fully embraced their potential and skepticism remains regarding their utility. A key factor shaping this point of view was almost certainly the identification of the glial cells missing (gcm) gene, which was met with great excitement when it was shown that Gcm was necessary and sufficient for specification of glial cell fate in the Drosophila embryo (Hosoya et al., 1995; Jones et al., 1995; Vincent et al., 1996). However, in contrast, the mammalian Gcm1 and Gcm2 orthologs have very little to do with glial cell fate despite the fact they functionally substitute for fly gcm (Kim et al., 1998). In response to this finding, a prominent Stanford glial biologist was overhead to say, “Maybe fly glia are from outer space”? Undoubtedly, outer space was on the mind of the investigator that named “astrocytes,” but the story is likely to be more complex. Follow-up studies in Drosophila have revealed that gcm is only part of the picture (Chotard et al., 2005) and that it was naive to expect to find a conserved “master regulator” for all astrocytes. Mounting evidence indicates that as we drill down into mechanisms, we can, in fact, learn quite a bit from the study of glial cells in model organisms like Drosophila and C. elegans. That said, the invertebrate brain is relatively small and simple compared with mammals, so adaptations specific to the more complex vertebrate brain may have been added over time. Each of these issues is discussed below.
III. Evolution of Brain Complexity: More and Diversified Glia Is, Evidently, Better
As described above, invertebrate glia carry out many functions that are analogous to their vertebrate counterparts. The Drosophila nervous system comprises about 105 neurons compared to 85 × 106 neurons in the human brain. Glia make up about 15% of the C. elegans and Drosophila nervous systems, but estimates range from 50%–90% of cells in the human brain, implying that greater glial numbers were essential for achieving increased brain complexity. The increased size of the brain required new mechanisms for proliferation and expansion of glial pool size and long-range conduction across white matter tracts. Beyond just increasing numbers, glia may also have acquired enhanced functions and diversity. Cell-intrinsic morphological and functional differences have been observed within mammals between mouse and human astrocytes (Han et al., 2013). Other examples of enhanced glial functions are described below.
Strategies to Enhance Nerve Conduction
Selective pressure for more rapid conduction of the nervous impulse, e.g., in escape or attack behaviors, increasing brain complexity, etc., resulted in two types of solutions: decreasing longitudinal resistance or increasing capacitance of axons. Invertebrates have ensheathing cells (Figure 1) but generally lack myelin. Exceptions are earthworms, copepods, and some crustacean nerves, but myelin and organized white matter tract, as such, are generally found only in vertebrates above the jawless fishes (Figure 3) (Hartline and Colman, 2007). In nonmyelinated axons, velocity of the action potential is directly proportional to the axon diameter. The major conduction speed augmentation strategy in invertebrates is reducing longitudinal resistance by increasing the diameter of axons. Prime examples of this are found in cephalopods that accommodate a very large diameter axon or the Drosophila giant fiber, which drives the escape response.
Vertebrates have other constraints that place limits on using this strategy, including limiting bony structures, greater size requiring longer axonal lengths in the CNS and PNS, and with increasing brain complexity there is the need to pack many more axons in a given space. The solution for accommodating many small-diameter axons is to reduce the effective capacitance and increase the effective membrane resistance, which is achieved by providing a layer of insulation, which is achieved with myelination. Myelin sheathes also organize sodium channels into clusters (nodes of Ranvier) for saltatory (jumping) conduction. For an axon of equivalent diameter, myelin can increase the velocity of nervous impulse conduction by 50- to 100-fold. It should also be noted that oligodendrocytes carry out other functions in support of axon integrity, likely an adaptation brought about to deal with energy and trophic demands of the extraordinarily long fast-firing axons found in many higher organisms. For example, a recent study showed that deficiency of a lactate transporter in oligodendrocytes led to axonopathy and degeneration (Lee et al., 2012).
Vascular Interactions and Regulation
The presence of blood vessels and the oxygen tension they carry evolved from invertebrates to air-breathing vertebrates. As glia comprise the majority of cells in the mammalian brain, one possibility is that they might interact with the stromal cells leading to vascular ingrowth at later stages of brain development. In any case, glial interactions with the mature vasculature are well established. Astrocyte end feet associate with vessels to form the blood-brain barrier, a feature found in almost all vertebrates; this process also requires pericytes. Astrocytes are known to regulate cerebral blood flow and are thought to release angiogenic factors. Given their high metabolic demands, oligodendrocytes and Schwann cells have a vested interest in regulating blood flow and having access to oxygen, glucose, and substrates for lipogenesis and proteolipid construction of myelin sheaths. Indeed, it is interesting to note that myelination is a largely postnatal process in the mammalian brain, a time frame distinguished by high oxygen tension compared with in utero conditions, and that postnatal hypoxia can delay developmental myelination in animal models. Might an oxygen-regulated trigger coordinate with activity-dependent inductive signals to time the onset of myelination? Oligodendrocyte cell-cell interactions with blood vessels and axons they invest represent fruitful areas for future research.
Microglia Are Immune Cells that Protect and Sculpt the Brain
It is not surprising that evolutionary progression is coupled to new glial subtypes with specialized functions in the CNS. Simple organisms like Drosophila have glial subtypes that act as nonprofessional phagocytes and respond to injury. The molecular mechanisms that drive these events, for example, the glial-expressed engulfment receptor Draper, are conserved in mammalian glia (Scheib et al., 2012; Wu et al., 2009). But the appearance of microglia added a new dimension to brain health. Responses to neuronal death or injury by these professional phagocytes are far more efficient than those of astrocytes, and microglia, as proper immune cells, also regulate inflammation, cytotoxicity, and antigen presentation. Perhaps unexpectedly, because they are thought of as mainly immune cells, microglia were recently shown to regulate the refinement of developing neural circuits through removal of exuberant synaptic connections.
IV. Evolutionary Case that Astroglial Heterogeneity Has Accompanied that of Neurons
At the moment, there is little evidence for functional heterogeneity in glial subclasses in simple organisms like Drosophila and C. elegans. In flies, perhaps all astrocytes are the same. However, the long evolutionary relationship between astroglia and neurons predicts a higher degree of astrocyte heterogeneity in vertebrates. Specialized vertebrate neuron subtypes generated through neural tube patterning and increased regionality and complexity of the CNS might have demanded diversified glial solutions that would have been coselected for over time. So, what is the evidence for astrocyte heterogeneity? Many studies have shown that astrocytes display morphological differences in white versus gray matter and in different brain regions. More recently, expression profiling has indicated that cells expressing the astrocyte marker Aldh1L1-GFP, or that encode TRAPP to label polysomal RNA, are regionally heterogeneous at the molecular level (Cahoy et al., 2008; Doyle et al., 2008; Heiman et al., 2008).
If we assume that astrocytes are regionally diversified to hundreds of subtypes tailored to their neuronal counterparts, how might regional heterogeneity be further demonstrated? The mouse CNS is a suitable system for dissecting regionality of astrocytes based on the notion that embryonic pattern formation is a key organizing process for glia. Indeed, this idea is supported by genetic studies of bHLH and homeodomain transcription factors in ventral neural tube that regulate glial subtype identity (Molofsky et al., 2012) and show segmental origins of astrocytes.
We fate mapped astrocyte origins throughout the brain and spinal cord using cre recombinase expressed in multiple region-restricted progenitor domains (Tsai et al., 2012). What we observed was surprisingly simple. Astrocytes in all domains migrated laterally along radial glial trajectories and never exhibited secondary migration from their domains of origin. Even adult astrocytes challenged by injury or depletion of astrocytes in particular domains by diphtheria toxin A (DTA) failed to provoke secondary emigration. Thus, the final location of astrocytes can predict their regional origins, raising the possibility that they become diversified for local functions in CNS. This “segmental model” for astrocyte allocation is illustrated in Figure 4.
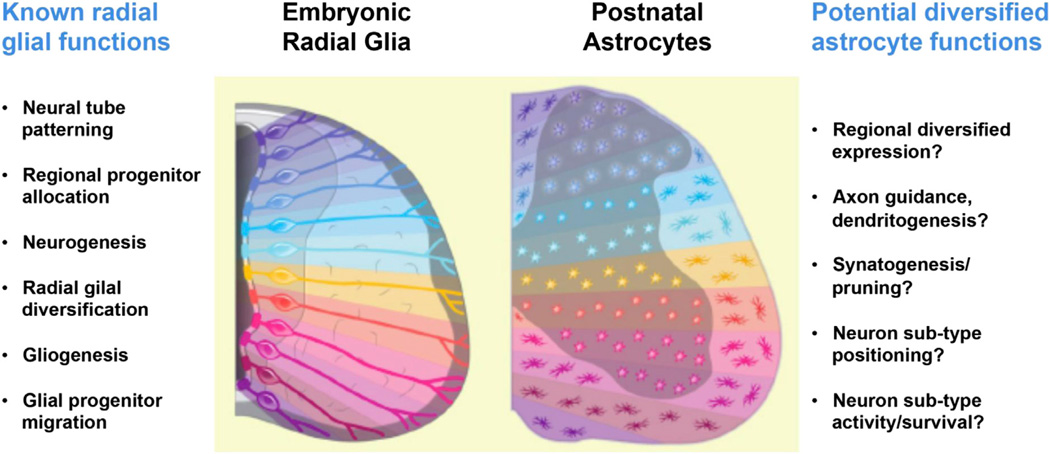
Figure 4. Speculative Concept of Astrocyte Scaffold and Functional “Astromeres”.
Embryonic patterning is known to generate diversified radial glial cells in response to complex positional cues in the dorsal-ventral and anterior-posterior axis. This in turn results in production of neuronal and glial subtypes (oligodendrocytes, astrocytes). The limited migration of astrocytes suggests the possibility that positional information might be retained in cells as they mature. If so, a variety of organizational roles and local functions to optimally support neuronal subtypes might be encoded in regionally distinct astrocyte domains called “astromeres.”
Regional Organization into Functional “Astromeres”?
In addition to allocation, recent work has shown that the “Segmental Model” holds true for understanding supracellular domain organization of astrocytes into functional units in cortex (Magavi et al., 2012), heterogeneity of type B stem cells of the SVZ (Merkle et al., 2007), and localized proliferation of intermediate astrocyte precursors (Tien et al., 2012). Future work might prove the existence of “astromeres” by showing specific astrocyte-encoded functions that play precise regional roles tailored to the particular locations that they occupy (Figure 4). The term astromere is meant to capture the immutable pattern of astrocyte segmental allocation, and the speculative notion that this could result in an astrocyte scaffold that retains positional information encoded during patterning. For instance, motor neurons of ventral spinal cord interact with multiple cell types as part of the sensory motor circuit responsible for most basic involuntary and voluntary movements. Their axons traverse long distances to reach targets in the periphery and they receive indirect inputs of long-range signaling from upper neurons in the brain. Astroglia in the locale of motor neurons might therefore have undergone intense selective pressure to optimally support their neuron neighbor. Indeed, a recent study showed that the initial trajectory of type 1a sensory axons was unaffected in FoxP1 mouse mutants with mislocalized MN targets (Sürmeli et al., 2011), suggesting the possibility that nonneuronal cells—perhaps astrocytes—encode the critical region-restricted guidance cues. We envisage that astromeres could function as local domains to direct axon guidance, as well as regional features involved in synapse formation/pruning, levels of neuronal activity, and even neuronal subtype survival.
Oligodendrocytes: Diversity or More of the Same?
It has been previously proposed that oligodendrocytes provided selective advantages based on enhanced motor neuron nerve conduction and that this relationship might underlie the common developmental-spatial origins of motor neurons and OPCs in the pMN domain of the ventral spinal cord (Richardson et al., 1997). If so, this would comprise a cogent example of glial “coselection.” It could be argued that there is strong selective pressure to keep their functions and cellular properties homogeneous, at least in terms of myelination. The issue of oligodendrocyte molecular and functional diversity remains an active area of debate in the field. Further elucidation of the precise developmental pathways involved might resolve these issues. For example, several studies have indicated that production of OPCs occurs in several temporal-spatial waves, with the general trend of early production of OPCs in the ventral regions of the brain and spinal cord being Sonic hedgehog regulated and later waves of production being from the more dorsal regions of spinal cord and brain (Rowitch and Kriegstein, 2010). It is possible that temporally distinct OPCs carry forward different properties that could be evaluated in terms of migration, myelination potential, and ability to function in repair after injury (Young et al., 2013). As discussed below, an enhanced understanding of precise functions of OPCs and oligodendrocytes during development and disease will equip us to look afresh at the issue of diversity.
V. Glial Biology’s Evolution: The Next 25 Years…
In the following sections, we look forward to new areas of research in glial cell biology. We propose that moving forward most efficiently will require defining the genetics of conserved mechanisms of glial function and developmental biology in the most experimentally accessible systems—worm, fly, and vertebrate systems including zebrafish and mouse. At the same time, we must explore how glial functions have diversified beyond basic functions in more sophisticated mammalian brains. Such an approach should lead to the production of new tools for investigating broad aspects of glial cell development and function and lead to a better understanding of the roles for glia in a variety of human neurological disorders.
New Tools, New Glial Functions: Exploit and Generate Genetically Accessible Systems
Future advances will rely heavily on the generation of new tools to study glial development. Invertebrate model organisms must be more heavily exploited to maximize progress in the field. Such preparations have been workhorses in pushing forward our understanding of the cell biology of the neuron, and their seminal contributions include defining the electrochemical basis of the axon potential, genetic characterization of mechanisms of neuronal cell fate specification, neural stem cell asymmetric cell division, specification of neuronal temporal identity, and axon guidance (this is by no means a complete list). Neuronal development and function is remarkably similar in worms, flies, mice, and humans. Based on the long evolutionary relationship between neurons and glia, coupled with our now more comprehensive understanding of glial cell biology in C. elegans and Drosophila, there is little reason to think that astroglia will be fundamentally different in invertebrates and mammals; in fact, molecular and morphological data argue the opposite. It seems that we must define what core developmental aspects and functional roles are conserved across these species and then explore their genetics in simple systems. The speed and precision with which one can explore basic aspects of cell biology—and then do rapid forward genetic screens for the underlying molecular machinery—is unmatched by more complex organisms, and such approaches are awaiting exploitation by the field.
A major limitation in the mammalian glial field is the lack of reliable cell-type- and stage-specific markers for vertebrate astrocytes. Once identified, further utility of relevant marker gene loci can be realized by utilizing their relevant cis-acting DNA regulatory sequences to drive transgenes for fate mapping and functional analysis, with conditional Brainbow or MADM reporters for precise glial lineage maps in vivo. The ever expanding collection of optogenetic tools also offers a noninvasive and reversible method for manipulating astrocytic function. We must better understand the language of astrocyte Ca2+ signals to manipulate and interpret potentially heterogeneous spatiotemporal dynamics of these signals in vivo.
New Roles for Oligodendrocytes and Their Precursors?
It is possible that oligodendrocytes have additional, as yet poorly appreciated, functions that can be investigated in mouse and human systems. What are the potential trophic roles of OPCs prior to myelination? What are the roles for such cells in the interaction and regulation of the synapse? Do OPCs have wider roles in the setting of vascularization during development and/or repair? How do properties of OPCs (highly migratory and invasive cells) translate into the production of glial neoplasms (Liu et al., 2011), which are lethal in large part because of their ability to invade brain tissue? For OPCs, zebrafish have proven themselves an excellent forward genetic system in which to explore myelinating glial cells in intact animals, and often with staggeringly beautiful live imaging studies. Studies in zebrafish have already defined key signaling pathways, such as GPR26, essential for myelination in both zebrafish and mouse. While not yet a topic of high focus, OPC biology seems a rich area for investigation in this highly accessible experimental system. Other functionally accessible animal systems to study CNS and PNS myelin include chick and Xenopus.
Glial Heterogeneity: Expression Profiling, Mapping, and the BRAIN Initiative
Further characterization of molecular heterogeneity could profoundly influence our understanding of glial functions. What are the factors required to determine astrocyte subtype identity? This problem is complex in execution since, in contrast to neurons, which develop during an early embryonic first wave, astrocytes might derive inductive signals from neurons. It remains unclear how much of glial identity is nature versus nurture. Simple misspecification of patterned domains by gain-of-function or loss-of-function approaches, as has been widely applied for the analysis of neuron subtype specification, is therefore complicated with respect to gliogenesis. To circumvent this problem, new tools would be required to alter the transcriptional code specifically at the onset of gliogenesis while leaving first wave neurogenesis intact. One example of this for spinal cord is Aldh1L1-cre, which shows onset of activity at embryonic day 13.5 in gliogenic radial glia (Tien et al., 2012).
Are all oligodendrocytes of one basic lineage (as held forth by “lumpers”) or do they comprise subtypes with fundamentally different developmental origins and potential (“splitters” view)? A more detailed understanding of genetic and epigenetic mechanisms that regulate the protean OPC will no doubt be required to address these issues. Further, fate mapping using the MADAM system in mice could help clarify the debate about OPC origins and potential. What is the nature of astrocyte precursors? Do all astrocytes derive from radial glia, or is there an additional stage of expansion that occurs through an “intermediate astrocyte precursor” (Ge et al., 2012; Tien et al., 2012), similar to those defined for neurons? Defining markers for astrocytes at early stages of development should clarify whether radial glia and/or intermediate astrocyte precursors represent valid astrocytic cells of origin.
It is also important to understand the mitogenic signaling pathways and cell-intrinsic factors that regulate the expansion of astrocyte and oligodendrocyte precursor populations. The density of astrocytes can be observed to differ between different domains of the spinal cord, and this may reflect the region-restricted expression of mitogenic cues or alternatively the cellular competence of certain populations of astrocytes to respond to such cues relative to others. For example, B-Raf-mediated RAS signaling has been shown to regulate the proliferation of astrocyte precursor cells in region-specific ways (Tien et al., 2012). The same principle could be applied to OPCs that derive from different regions to see whether this encodes a propensity for glioma formation. It is increasingly clear that developing astrocytes serve unique roles and are molecularly distinct from their adult counterparts.
How can this functional heterogeneity be further defined at the molecular level? First, it involves prospective identification of astrocyte cell-type-specific yet heterogeneous expression. This could be identified through interrogation of existing databases (e.g., the Allen Brain Atlas), enhancer trap studies, or discovery of developmental gene regulatory pathways specific for subsets of astrocytes. Whole-genome, proteome, and metabolomic approaches might distinguish functional subsets of astrocytes. An enhanced understanding of glial expression must then be followed by functional studies to prove astrocyte-specific roles in axon guidance, neuronal cell migration, trophic support, and survival, all of which might be regulated by astrocytes.
An important question is whether region-specific roles for astrocytes can be applied to better understand the nature of human neurological and neurodegenerative diseases. For example, given the evidence for an astroglial role in amyotrophic lateral sclerosis (ALS), which affects ventral horn motor neurons, it would be interesting to determine whether astrocytes in the region of motor neurons might be specifically affected by disease-causing mutations.
Finally, how can new imaging approaches be brought to mapping white matter tracts in the brain and myelinated nerves in the PNS with greater precision? MRI is the most common clinical technique to noninvasively assess white matter tracts in human, but because it is effectively measuring properties of proton (water) movement, it is not specific or particularly sensitive to detect myelin. Can we combine modalities as diverse as MRI and live animal confocal microscopy to image myelin in real time? This challenging goal might require new ways to label myelin quantitatively (e.g., chemical dyes, decorated myelin fusion proteins) but could help train new MRI techniques to gain sensitivity and specificity for myelin. Together, such high-risk projects might also have terrific yield and provide a way that glial biology could significantly impact the recent NIH BRAIN initiative and provide new insights into functions of greater than half the total cells in the brain.
Human Disease Insights
Understanding the role of astrocytes and oligodendrocytes in human neurological and psychiatric diseases requires a comprehensive picture of how they develop and what roles they play in the mature CNS. Conversely, human diseases could provide clues to subtle astrocyte and oligodendrocyte functions that may take years to manifest as abnormal behavior. The explosion of new disease-associated genes falling out from human genetics using next-generation sequencing methods might point to key glial genes and/or those expressed in neurons and glia with key glial contributions to pathology. We must be ready to recognize them as such, which requires the development of the database resources we discuss above, and we must have the tools in place to define their in vivo functions rapidly to understand their roles in disease. It will be important to understand how astrocytes modulate synaptic development and function in the circuits that mediate cognition, affect, and social function. An equally challenging question is whether gene-environment-developmental interactions might be regulated at the level of astrocyte or oligodendrocyte function.
Recent work has shown the feasibility of deriving functional astrocytes and oligodendrocytes from embryonic stem cells and from reprogrammed induced pluripotent stem cells (Han et al., 2013; Krencik et al., 2011; Krencik and Zhang, 2011) and self-organizing “minibrains” (Lancaster et al., 2013). Such technologies allow for an accessible human cellular system for studies of glial biology (with caveats noted above). Use of patient-derived astrocytes will be important to the study of many neurological and psychiatric disorders that involve astrocyte function, both those for which the genetic lesions are well understood (Rett’s, Fragile X, and the “RASopathies”) as well as those that are less well defined (schizophrenia, autism.) Another advantage of stem cell culture is that patterning molecules can be added during the neuroepithelial stage to specify progenitors to regionally distinct pools, mimicking the in vivo patterning described above in a controlled environment (Krencik et al., 2011). This might allow for the generation of various astrocyte subtypes to study intrinsic markers of human astrocyte diversity and might provide functionally specific astrocytes for studying region-specific diseases, e.g., midbrain astrocytes in the case of Parkinson’s disease or ventral-spinal astrocytes in the case of ALS.
Ultimately, new developments in understanding glial-based diseases must incorporate a more sophisticated understanding of glial development and incorporate new tools to study astrocyte and oligodendrocyte function in vivo. The formation of “glial chimeras,” i.e., mice with humanized oligodendrocytes and/or astrocytes (Han et al., 2013), provides an exciting approach to study the biology of human glia in a relatively complex milieu and might provide a preclinical model. Generation of future glial-based therapeutics will require a comprehensive understanding of cell-type-specific contributions to diseases of neurodevelopment and the mature brain. We envisage that the further evolution of glial biology in the next 25 years will yield new knowledge of fundamental neurobiology and therapies for human disease.
Acknowledgments
We would like to thank Ben Barres, Anna Molofsky, Carlos Lois, Bill Richardson, Dwight Bergles, and Bernhard Zalc for discussions and comments on the manuscript. The authors acknowledge funding from the NIH and HHMI.
REFERENCES
- Aguzzi A, Barres BA, Bennett ML. Microglia: scapegoat, saboteur, or something else? Science. 2013;339:156–161. doi: 10.1126/science.1227901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen NJ, Bennett ML, Foo LC, Wang GX, Chakraborty C, Smith SJ, Barres BA. Astrocyte glypicans 4 and 6 promote formation of excitatory synapses via GluA1 AMPA receptors. Nature. 2012;486:410–414. doi: 10.1038/nature11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasaki T, Tatsumi R, Takahashi K, Arai K, Nakanishi Y, Ueda R, Ito K. Essential role of the apoptotic cell engulfment genes draper and ced-6 in programmed axon pruning during Drosophila metamorphosis. Neuron. 2006;50:855–867. doi: 10.1016/j.neuron.2006.04.027. [DOI] [PubMed] [Google Scholar]
- Awasaki T, Lai SL, Ito K, Lee T. Organization and postembryonic development of glial cells in the adult central brain of Drosophila. J. Neurosci. 2008;28:13742–13753. doi: 10.1523/JNEUROSCI.4844-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres BA. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron. 2008;60:430–440. doi: 10.1016/j.neuron.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Bergles DE, Roberts JD, Somogyi P, Jahr CE. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature. 2000;405:187–191. doi: 10.1038/35012083. [DOI] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J. Neurosci. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chotard C, Leung W, Salecker I. Glial cells missing and gcm2 cell autonomously regulate both glial and neuronal development in the visual system of Drosophila. Neuron. 2005;48:237–251. doi: 10.1016/j.neuron.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P, Barres BA. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120:421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Colón-Ramos DA, Margeta MA, Shen K. Glia promote local synaptogenesis through UNC-6 (netrin) signaling in C. elegans. Science. 2007;318:103–106. doi: 10.1126/science.1143762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSalvo MK, Mayer N, Mayer F, Bainton RJ. Physiologic and anatomic characterization of the brain surface glia barrier of Drosophila. Glia. 2011;59:1322–1340. doi: 10.1002/glia.21147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty J, Logan MA, Taşdemir OE, Freeman MR. Ensheathing glia function as phagocytes in the adult Drosophila brain. J. Neurosci. 2009;29:4768–4781. doi: 10.1523/JNEUROSCI.5951-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle JP, Dougherty JD, Heiman M, Schmidt EF, Stevens TR, Ma G, Bupp S, Shrestha P, Shah RD, Doughty ML, et al. Application of a translational profiling approach for the comparative analysis of CNS cell types. Cell. 2008;135:749–762. doi: 10.1016/j.cell.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenfeld G, Stork T, Klämbt C. Neuron-glia interaction in the insect nervous system. Curr. Opin. Neurobiol. 2005;15:34–39. doi: 10.1016/j.conb.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Emery B. Regulation of oligodendrocyte differentiation and myelination. Science. 2010;330:779–782. doi: 10.1126/science.1190927. [DOI] [PubMed] [Google Scholar]
- Eroglu C, Barres BA. Regulation of synaptic connectivity by glia. Nature. 2010;468:223–231. doi: 10.1038/nature09612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fancy SP, Chan JR, Baranzini SE, Franklin RJ, Rowitch DH. Myelin regeneration: a recapitulation of development? Annu. Rev. Neurosci. 2011;34:21–43. doi: 10.1146/annurev-neuro-061010-113629. [DOI] [PubMed] [Google Scholar]
- Freeman MR. Specification and morphogenesis of astrocytes. Science. 2010;330:774–778. doi: 10.1126/science.1190928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman MR. Invertebrate glia. In: Ransom B, Kettenmann H, editors. Neuroglia. Third. Oxford: Oxford University Press; 2012. pp. 8–15. [Google Scholar]
- Freeman MR, Doherty J. Glial cell biology in Drosophila and vertebrates. Trends Neurosci. 2006;29:82–90. doi: 10.1016/j.tins.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Ge WP, Miyawaki A, Gage FH, Jan YN, Jan LY. Local generation of glia is a major astrocyte source in postnatal cortex. Nature. 2012;484:376–380. doi: 10.1038/nature10959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Chen M, Wang F, Windrem M, Wang S, Shanz S, Xu Q, Oberheim NA, Bekar L, Betstadt S, et al. Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell. 2013;12:342–353. doi: 10.1016/j.stem.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartline DK. The evolutionary origins of glia. Glia. 2011;59:1215–1236. doi: 10.1002/glia.21149. [DOI] [PubMed] [Google Scholar]
- Hartline DK, Colman DR. Rapid conduction and the evolution of giant axons and myelinated fibers. Curr. Biol. 2007;17:R29–R35. doi: 10.1016/j.cub.2006.11.042. [DOI] [PubMed] [Google Scholar]
- Heiman MG, Shaham S. Ancestral roles of glia suggested by the nervous system of Caenorhabditis elegans. Neuron Glia Biol. 2007;3:55–61. doi: 10.1017/S1740925X07000609. [DOI] [PubMed] [Google Scholar]
- Heiman M, Schaefer A, Gong S, Peterson JD, Day M, Ramsey KE, Suárez-Fariñas M, Schwarz C, Stephan DA, Surmeier DJ, et al. A translational profiling approach for the molecular characterization of CNS cell types. Cell. 2008;135:738–748. doi: 10.1016/j.cell.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoya T, Takizawa K, Nitta K, Hotta Y. Glial cells missing: a binary switch between neuronal and glial determination in Drosophila. Cell. 1995;82:1025–1036. doi: 10.1016/0092-8674(95)90281-3. [DOI] [PubMed] [Google Scholar]
- Jones BW, Fetter RD, Tear G, Goodman CS. glial cells missing: a genetic switch that controls glial versus neuronal fate. Cell. 1995;82:1013–1023. doi: 10.1016/0092-8674(95)90280-5. [DOI] [PubMed] [Google Scholar]
- Keller LC, Cheng L, Locke CJ, Müller M, Fetter RD, Davis GW. Glial-derived prodegenerative signaling in the Drosophila neuromuscular system. Neuron. 2011;72:760–775. doi: 10.1016/j.neuron.2011.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Jones BW, Zock C, Chen Z, Wang H, Goodman CS, Anderson DJ. Isolation and characterization of mammalian homologs of the Drosophila gene glial cells missing. Proc. Natl. Acad. Sci. USA. 1998;95:12364–12369. doi: 10.1073/pnas.95.21.12364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krencik R, Zhang SC. Directed differentiation of functional astroglial subtypes from human pluripotent stem cells. Nat. Protoc. 2011;6:1710–1717. doi: 10.1038/nprot.2011.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krencik R, Weick JP, Liu Y, Zhang ZJ, Zhang SC. Specification of transplantable astroglial subtypes from human pluripotent stem cells. Nat. Biotechnol. 2011;29:528–534. doi: 10.1038/nbt.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucukdereli H, Allen NJ, Lee AT, Feng A, Ozlu MI, Conatser LM, Chakraborty C, Workman G, Weaver M, Sage EH, et al. Control of excitatory CNS synaptogenesis by astrocyte-secreted proteins Hevin and SPARC. Proc. Natl. Acad. Sci. USA. 2011;108:E440–E449. doi: 10.1073/pnas.1104977108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Morrison BM, Li Y, Lengacher S, Farah MH, Hoffman PN, Liu Y, Tsingalia A, Jin L, Zhang PW, et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 2012;487:443–448. doi: 10.1038/nature11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SC, Huck JH, Roberts JD, Macklin WB, Somogyi P, Bergles DE. Climbing fiber innervation of NG2-expressing glia in the mammalian cerebellum. Neuron. 2005;46:773–785. doi: 10.1016/j.neuron.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Liu C, Sage JC, Miller MR, Verhaak RG, Hippenmeyer S, Vogel H, Foreman O, Bronson RT, Nishiyama A, Luo L, Zong H. Mosaic analysis with double markers reveals tumor cell of origin in glioma. Cell. 2011;146:209–221. doi: 10.1016/j.cell.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald JM, Beach MG, Porpiglia E, Sheehan AE, Watts RJ, Freeman MR. The Drosophila cell corpse engulfment receptor Draper mediates glial clearance of severed axons. Neuron. 2006;50:869–881. doi: 10.1016/j.neuron.2006.04.028. [DOI] [PubMed] [Google Scholar]
- Magavi S, Friedmann D, Banks G, Stolfi A, Lois C. Coincident generation of pyramidal neurons and protoplasmic astrocytes in neocortical columns. J. Neurosci. 2012;32:4762–4772. doi: 10.1523/JNEUROSCI.3560-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle FT, Mirzadeh Z, Alvarez-Buylla A. Mosaic organization of neural stem cells in the adult brain. Science. 2007;317:381–384. doi: 10.1126/science.1144914. [DOI] [PubMed] [Google Scholar]
- Molofsky AV, Krencik R, Ullian EM, Tsai HH, Deneen B, Richardson WD, Barres BA, Rowitch DH. Astrocytes and disease: a neurodevelopmental perspective. Genes Dev. 2012;26:891–907. doi: 10.1101/gad.188326.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nave KA. Myelination and support of axonal integrity by glia. Nature. 2010;468:244–252. doi: 10.1038/nature09614. [DOI] [PubMed] [Google Scholar]
- Oikonomou G, Shaham S. The glia of Caenorhabditis elegans. Glia. 2011;59:1253–1263. doi: 10.1002/glia.21084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereanu W, Spindler S, Cruz L, Hartenstein V. Tracheal development in the Drosophila brain is constrained by glial cells. Dev. Biol. 2007;302:169–180. doi: 10.1016/j.ydbio.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson WD, Pringle NP, Yu WP, Hall AC. Origins of spinal cord oligodendrocytes: possible developmental and evolutionary relationships with motor neurons. Dev. Neurosci. 1997;19:58–68. doi: 10.1159/000111186. [DOI] [PubMed] [Google Scholar]
- Rival T, Soustelle L, Strambi C, Besson MT, Iché M, Birman S. Decreasing glutamate buffering capacity triggers oxidative stress and neuropil degeneration in the Drosophila brain. Curr. Biol. 2004;14:599–605. doi: 10.1016/j.cub.2004.03.039. [DOI] [PubMed] [Google Scholar]
- Rowitch DH, Kriegstein AR. Developmental genetics of vertebrate glial-cell specification. Nature. 2010;468:214–222. doi: 10.1038/nature09611. [DOI] [PubMed] [Google Scholar]
- Scheib JL, Sullivan CS, Carter BD. Jedi-1 and MEGF10 signal engulfment of apoptotic neurons through the tyrosine kinase Syk. J. Neurosci. 2012;32:13022–13031. doi: 10.1523/JNEUROSCI.6350-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham S. Glia-neuron interactions in the nervous system of Caenorhabditis elegans. Curr. Opin. Neurobiol. 2006;16:522–528. doi: 10.1016/j.conb.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Shao Z, Watanabe S, Christensen R, Jorgensen EM, Colón-Ramos DA. Synapse Location during Growth Depends on Glia Location. Cell. 2013;154:337–350. doi: 10.1016/j.cell.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soustelle L, Besson MT, Rival T, Birman S. Terminal glial differentiation involves regulated expression of the excitatory amino acid transporters in the Drosophila embryonic CNS. Dev. Biol. 2002;248:294–306. doi: 10.1006/dbio.2002.0742. [DOI] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- Stork T, Bernardos R, Freeman MR. Analysis of glial cell development and function in Drosophila. Cold Spring Harb Protoc. 2012;2012:1–17. doi: 10.1101/pdb.top067587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sürmeli G, Akay T, Ippolito GC, Tucker PW, Jessell TM. Patterns of spinal sensory-motor connectivity prescribed by a dorsoventral positional template. Cell. 2011;147:653–665. doi: 10.1016/j.cell.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien AC, Tsai HH, Molofsky AV, McMahon M, Foo LC, Kaul A, Dougherty JD, Heintz N, Gutmann DH, Barres BA, Rowitch DH. Regulated temporal-spatial astrocyte precursor cell proliferation involves BRAF signalling in mammalian spinal cord. Development. 2012;139:2477–2487. doi: 10.1242/dev.077214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HH, Li H, Fuentealba LC, Molofsky AV, Taveira-Marques R, Zhuang H, Tenney A, Murnen AT, Fancy SP, Merkle F, et al. Regional astrocyte allocation regulates CNS synaptogenesis and repair. Science. 2012;337:358–362. doi: 10.1126/science.1222381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent S, Vonesch JL, Giangrande A. Glide directs glial fate commitment and cell fate switch between neurones and glia. Development. 1996;122:131–139. doi: 10.1242/dev.122.1.131. [DOI] [PubMed] [Google Scholar]
- Wu HH, Bellmunt E, Scheib JL, Venegas V, Burkert C, Reichardt LF, Zhou Z, Fariñas I, Carter BD. Glial precursors clear sensory neuron corpses during development via Jedi-1, an engulfment receptor. Nat. Neurosci. 2009;12:1534–1541. doi: 10.1038/nn.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KM, Psachoulia K, Tripathi RB, Dunn SJ, Cossell L, Attwell D, Tohyama K, Richardson WD. Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron. 2013;77:873–885. doi: 10.1016/j.neuron.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalc B, Goujet D, Colman D. The origin of the myelination program in vertebrates. Curr. Biol. 2008;18:R511–R512. doi: 10.1016/j.cub.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, Barres BA. Genomic analysis of reactive astrogliosis. J. Neurosci. 2012;32:6391–6410. doi: 10.1523/JNEUROSCI.6221-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]