Abstract
Phosphatidylinositol is a metabolic precursor of phosphoinositides and soluble inositol phosphates. Both sets of molecules represent versatile intracellular chemical signals in eukaryotes. While much effort has been invested in understanding the enzymes that produce and consume these molecules, central aspects for how phosphoinositide production is controlled and functionally partitioned remain unresolved and largely unappreciated. It is in these regards that phosphatidylinositol (PtdIns) transfer proteins (PITPs) are emerging as central regulators of the functional channeling of phosphoinositide pools produced on demand for specific signaling purposes. The physiological significance of these proteins is amply demonstrated by the consequences that accompany deficits in individual PITPs. Although the biological problem is fascinating, and of direct relevance to disease, PITPs remain largely uncharacterized. Herein, we discuss our perspectives regarding what is known about how PITPs work as molecules, and highlight progress in our understanding of how PITPs are integrated into cellular physiology.
Introduction
Inositol (Ins) and phosphoinositide signaling pathways are major intracellular regulatory systems of eukaryotic cells, and their derangements are underlying causes of many diseases. This topic is the theme around which the collection of reviews brought together in this volume is built. Yet, in spite of how much we have learned about phosphoinositide signaling and its regulation, central mechanisms for how phosphoinositide signaling is diversified are still poorly understood. This review addresses one such major gap by focusing on PITPs and their involvements in the mechanisms for specifying biological outcomes of phosphoinositide signaling. That PITP-deficiencies are relevant to neurological diseases, metabolic syndromes and cancer (e.g. as a proangiogenic factor in breast cancer) testifies to the biomedical impact of these proteins. At the cellular levels, evidence that PITPs regulate membrane trafficking, lipid droplet metabolism, developmental programs of polarized membrane biogenesis, and growth factor/morphogen receptor signaling identify exciting areas for inquiry. Yet, it is remarkable how little we know about what cellular processes are controlled by PITPs, or about the mechanistic details for how the conformational dynamics of lipid-exchange reactions catalyzed by these proteins govern the actions of PITPs as regulators of phosphoinositide signaling. This review discusses the mechanisms by which PITPs execute novel modes of signal control – i.e. by instructing biological outcomes of PtdIns kinase action. Studies of the Sec14-like PITPs provide an accurate picture of what is known, and what remains unknown, regarding mechanisms for how PITPs interface with phosphoinositide signaling in eukaryotic cells. We therefore focus in this review primarily on Sec14-like PITPs to illustrate progress that has been made, and what progress still needs to be made, regarding PITPs and how these proteins are integrated into the larger design of phosphoinositide signaling.
Inositol as a versatile signaling scaffold
Roles for phosphorylated D-myo-inositols (Ins-phosphates) and phosphoinositides (PIPs) in signal transduction are well documented (1-4), and phosphoinositides fulfill dual signaling roles in this capacity. First, phosphoinositides are metabolic precursors whose hydrolysis by phospholipases C (PLC) initiates production of the potent second messengers diacylglycerol and soluble Ins-phosphates. Historically, the most celebrated such case is PLC-mediated hydrolysis of phosphatidylinositol-4,5-bisphosphate (PtdIns(4,5)P2) to produce diacylglycerol and the soluble Ins-1,4,5-trisphosphate (InsP3) (5,6). The former lipid second messenger regulates activities of protein kinases C, whereas this particular isomer of IP3 both gates intracellular calcium channels and serves as the obligate precursor of the other soluble Ins-phosphates and Ins-pyrophosphates (7,8).
The chemical diversity of the Ins-phosphates, and the relationship of that diversity to the signaling capacities of these second messengers, lays the groundwork for arguments that Ins is evolution’s favorite molecule. As each position of the 6-member Ins ring can be phosphorylated (and, for some positions, pyro-phosphorylated), the potential Ins-phosphate cabal is a large one indeed. A simple binary code allows for 63 distinct molecules monophosphorylated at any position, and a trinary code balloons that potential to 728 distinct molecules if both mono- and pyro-phosphorylation is allowed at any position. Thus, the Ins headgroup is a versatile chemical scaffold upon which can be written a large set of unique signaling states, each distinguished from the others on the basis of how phosphates (or pyro-phosphates) are arranged around the Ins ring. It is therefore not surprising that the large numbers of chemically distinct Ins-phosphates produced by cells translate to diverse biological effects. These soluble compounds regulate a wide spectrum of cellular functions ranging from mRNA transcription, to mRNA transport across nuclear pores, to protein folding, and to regulation of enzyme activities (9-12).
Second, the phosphoinositide molecules themselves have intrinsic signaling capabilities independent of their roles as precursors for other second messenger molecules. This realization came from the discovery that phosphoinositides carrying phosphates on the 3-OH position of the Ins headgroup are not substrates for PLCs. As is the case for the Ins-phosphates, there are distinct phosphoinositide classes that differ in the position and number of phosphate groups that decorate the Ins ring. This chemical information is also decoded by proteins, and this recognition mechanism enables protein recruitment to membranes, and their subsequent activation, with spatial and temporal specificity. Phosphoinositides have roles in addition to recruitment of proteins to membranes. We now appreciate these lipids are important allosteric co-factors that modulate the activities of various enzymes and ion channels (3,13).
The chemical diversity of the soluble Ins-phosphates is not matched by that of the phosphoinositides. That is, the phosphoinositide cabal is far simpler. Yeast produce only five phosphoinositides (PtdIns3P, PtdIns4P, PtdIns5P, PtdIns(3,5)P2, and PtdIns(4,5)P2), of which only the two 4-OH phosphorylated species are essential for viability (2-4). Higher eukaryotes produce seven -- the five listed for yeast with the addition of PtdIns(3,4)P2 and PtdIns(3,4,5)P3. The chemical simplicity of phosphoinositides notwithstanding, the biological outcomes of phosphoinositide signaling remain exceedingly diverse. How this diversity in functional outcome is achieved from such a restricted set of molecules remains an important question in contemporary cell biology. It seems clear that solution of the diversification paradox requires a high degree of functional compartmentalization of phosphoinositide signaling pools.
Coincidence detection and phosphoinositide signaling
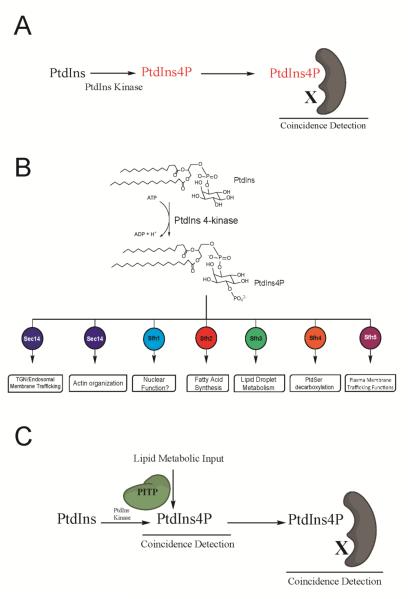
Functional diversification of phosphoinositide signaling is primarily attributed to the deciphering of phosphoinositide chemical codes by specific phosphoinositide-binding modules (e.g. PH-, ENTH-, FYVE-, BAR-domains, etc). Binding of a specific phosphoinositide species by such modules is subsequently translated to biological action by register of secondary effector binding cues – thereby generating a combinatorial coincidence detection code that confers signaling specificity Figure 1A . There is no question that cells employ this post-production, or ‘back-loaded’, strategy to diversify biological outcomes for phosphoinositide signaling.
Figure 1.
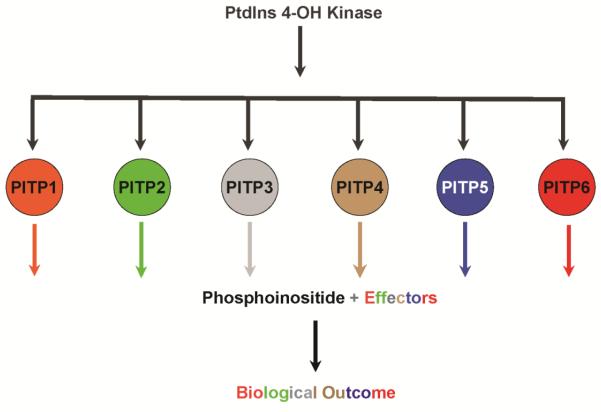
Diversity of PtdIns 4-OH kinase signaling in a unicellular cell. (A) Back-loaded models for diversifying phosphoinositide signaling interpret determination of biological outcome via a dual coincidence mechanism. In such models, distinction of phosphoinositide chemical identity is linked to recognition of some other effector after phosphoinositide synthesis has occurred (PtdIns 4-OH kinase signaling shown as example here). This combination specifies biological outcome. (B) Yeast have two essential PtdIns 4-OH kinases to which can be assigned a larger number of biological outcomes of which seven are shown. Each biological outcome tracks with a specific Sec14-like PITP. (C) PITP-mediated front-loaded model for diversifying phosphoinositide signaling. Front-loaded models interpret determination of biological outcome for phosphoinositide signaling via a primed channeling of PtdIns to an appropriate kinase (4-OH kinase signaling shown here). The channeling is determined at the synthesis event itself and is determined by the identity of the PITP interacting with the kinase. The differential lipid binding specificities of individual PITPs define the source of metabolic input to the PtdIns kinase, and the PITPs themselves could assemble distinct phosphoinositide binding effectors at the site where the signaling phosphoinositide is (or will be) produced. Such a strategy produces highly integrated signaling circuits with point resolution (dimensions of single protein complexes). This model also identifies PtdIns 4-OH kinases as fundamentally biologically insufficient enzymes, even as wild-type enzymes with all accessory subunits present. Combination of both front- and back-loaded strategies greatly amplifies the simple phosphoinositide chemical code into a large biological outcome space.
Yet, the ‘back-loaded’ concept does not satisfactorily account for the remarkable diversity in biological outcomes for phosphoinositide signaling. This shortcoming reflects the failure of this model to address compartmentation of phosphoinositide pools other than by rather low resolutions mechanisms involving differential intracellular localization of lipid kinases, etc. A striking example of the inadequacy of strictly ‘back-loaded’ mechanisms for diversifying phosphoinositide signaling is provided by the yeast Saccharomyces cerevisiae. In the example illustrated in Figure 1B, Stt4 PtdIns 4-OH kinase signaling in this organism has at least six distinct biological outcomes, and each outcome tracks with a specific PITP. This example, on stark display in a simple unicellular eukaryote, clearly reports that the biological outcomes of phosphoinositide signaling are neither exclusively determined by the chemical nature of the phosphoinositide, nor by identities of the PtdIns kinases that produce it (14-18). Rather, PITPs help determine the biological outcomes for phosphoinositide signaling via what we term a ‘front-loaded’ mechanism (Figure 1C). How might ‘front-loaded’ mechanisms work? Herein, we explore the concept that PITPs specify biological outcomes for PtdIns kinase activities via mechanisms that are coupled to the very act of phosphoinositide synthesis.
Phosphatidylinositol transfer proteins
PITPs are not enzymes, but proteins that mobilize energy-independent transfer of PtdIns, PtdCho and other lipids between membranes in vitro, and are therefore also inferred to transfer lipids in a manner not energized by ATP hydrolysis in vivo (19,20). PITPs are efficient in this regard as these proteins stimulate cell-free rates of PtdIns transfer between membranes by several orders of magnitude. It is by this operational ‘transfer-assay’ that PITPs are historically defined. But is this an unfortunate definition that miscasts PITPs as physiologically relevant inter-membrane lipid carriers, thereby sentencing interpretations of these activities to the prison of a superficial and essentially untestable model? We argue such is the case. It is our view that, after some four decades, the lipid transfer protein field still languishes under the conceptual straightjacket imposed upon it by the direct and rather uncritical linkage of in vitro transfer activities to in vivo functions as lipid carriers. We discuss alternative interpretations for the lipid-exchange activities, and these ideas suggest powerful new insights into how signaling can be highly compartmentalized – even in circumstances where similar enzymatic reactions are arranged in close quarters on the same membrane surface.
The biochemical and functional questions surrounding these proteins notwithstanding, PITPs are highly conserved across the eukaryotic kingdom. This property implies biological importance, and these proteins fall into 2 families. The Sec14-like PITPs define a superfamily that includes retinaldehyde binding proteins, Ras-GAPs, Rho-GEFs, tyrosine phosphatases, and other phosphoinositide-binding proteins (21-23). The founding member is the major yeast PITP Sec14 (14,24,25). Sec14 studies are driving the PITP and lipid transfer protein fields because structural, computational, biochemical and genetic approaches are being productively applied towards elucidation of functional mechanisms. The START-like PITPs belong to the StAR-related lipid transfer protein superfamily and are less well understood. These proteins are the primary subjects of recent reviews and readers are referred to those summaries for further details (26-28). Herein, we limit discussion of the START-like PITPs to the question of whether these proteins might also channel PtdIns kinase activities to dedicated biological outcomes (23,29).
PITPs and phosphoinositide signaling from a historical perspective
The experimental origins of lipid transfer proteins lie in studies that identified vesicle-trafficking-independent mechanisms of lipid trafficking in cells. These studies were of various designs, but all relied on measuring inter-compartmental trafficking of lipids in the face of some chemical or conditional inhibition of secretory pathway activity (30-33). Many of the experimental strategies also rested on necessarily rapid purifications of plasma membrane from endoplasmic reticulum using panning techniques. Rapid recoveries of highly purified plasma membrane fractions were absolutely required for interpretation of the data. With the benefit of hindsight informed by the advent of sensitive technologies with which to monitor membrane purity, and the newly rediscovered significance of such complicating structures as intermembrane contact sites (34), it is now apparent that rapid methods for membrane separation and purification were inadequate for the task at hand. The membrane fractions used for readout were not pure, and membrane contaminants preclude confident interpretation of the data. So, the experimental foundation for lipid trafficking via soluble protein carriers is less compelling than previously believed.
With regard to functional interpretations of PITPs, biochemical studies subsequently intersected with theoretical considerations stemming from the knowledge that the metabolic cycle for regenerating phosphoinositide molecules after their consumption by PLC during the course of agonist-stimulated signaling required navigation of a problem of biochemical compartmentation. This problem is grounded on the central presumption that the compartments where PtdIns, the metabolic precursor of all phosphoinositides, is synthesized is physically distant from the location where phosphoinositides are consumed. The specific case under discussion considers replenishment of PtdIns(4,5)P2 pools in the plasma membrane upon agonist stimulation of PLC activity. With respect to synthesis, PtdIns is produced de novo by the enzyme PtdIns synthase which converts Ins and cytidine-diphospho-diacylglycerol (CDP-DAG) to produce PtdIns and cytidine-monophosphate. CDP-DAG is produced from phosphatidic acid (PtdOH) and cytidine-trisphosphate. Both PtdIns- and CDP-DAG-synthases are integral membrane proteins of the endoplasmic reticulum. A solution to this topological problem was suggested where soluble lipid carrier proteins ferry PtdIns from the endoplasmic reticulum to the plasma membrane, and return DAG or PtdOH from the plasma membrane to the endoplasmic reticulum in a retrograde arm of the cycle (35; Figure 2). This conjecture set a framework for translating the biochemical properties of PITPs to physiological functions in support of phosphoinositide signaling (36). Results from permeabilized cells claiming an obligatory role for soluble PITPs in growth factor receptor signaling were subsequently taken as evidence in support of this basic concept (37; see below). However, the veracity of such claims in in vivo settings is now uncertain. Recent studies using sophisticated vital imaging studies reveal that the PtdIns synthase enzyme itself may be mobilized from the endoplasmic reticulum via a COPII vesicle-like mechanism, and positioned in the direct proximity of the plasma membrane (38). Moreover, available genetic studies with PITP mutant animals and cells also do not support the central predictions of the PtdIns supply model (39-41; see below).
Figure 2.
A lipid transfer cycle for coupling PtdIns synthesis to phosphoinositide signaling. Lipid-transfer proteins are proposed to drive a cycle of anterograde PtdIns transfer from the endoplasmic reticulum to the plasma membrane, and balanced retrograde transfer of either diacylglycerol (DAG) or phosphatidic acid (PtdOH), to fuel phosphoinositide synthesis and signaling. The model in its simplest and most integrated form predicts PITP deficits will result in reduced phosphoinositide signaling at the plasma membrane with diminished resynthesis of PtdIns at the endoplasmic reticulum.
Nonetheless, the interpretation of PITPs as lipid carriers remains the prevailing concept for how PITPs execute cellular functions (42,43). We view acceptance of this idea to be faith-based because there is no direct evidence that PITPs physically remove a PtdIns monomer from the endoplasmic reticulum and then transport it to the plasma membrane in cells where it is preferentially channeled into phosphoinositide synthesis. The experiment to appropriately test this model in cells is a technically difficult one of course, but there are other reasons to consider alternative ideas. For example, the first PITPs characterized were PtdIns and phosphatidylcholine (PtdCho) transfer proteins and it is for this reason that proteins with those particular properties are often referred to as classical (or canonical) PITPs. In the several cases where the integrity of robust PLC signaling pathways has been interrogated in cells or organisms lacking individual classical PITPs, the results are not consistent with the predictions of the PtdIns transfer cycle (see below).
The designation of PtdIns/PtdCho transfer proteins as classical PITPs will ultimately prove to be an awkward designation as non-classical PITPs (i.e. proteins that transfer PtdIns and some other ligand distinct from PtdCho) have been discovered, and these proteins outnumber the classical PITPs (22,29). Nonetheless, the non-classical PITPs are intriguing in part because the most parsimonious property of a PITP involved in the PtdIns supply cycle illustrated in Figure 2 is that of a non-classical PtdIns/DAG or PtdIns/PtdOH transfer protein. Support for PITPs being involved in a PtdIns carrier cycle would additionally involve two experimental demonstrations: (i) that acute loss of a PtdIns/PtdOH transfer protein inhibits sustained PLC signaling, and (ii) that loss of the PITP also reduces flux through the PtdIns biosynthetic pathway. While demonstrations that satisfy both criteria have not been reported to date, PITPs with the appropriate biochemical properties are now identified (see below).
The anatomy of the Sec14 phospholipid exchange cycle
To understand the functions of PITPs as molecules, no matter in what specific physiological settings these functions are discharged, it is essential to understand the complex dynamics associated with how PITPs execute lipid exchange. Structural and biophysical studies are contributing strongly in this regard. The superficial shape of the exchange cycle is simple. The PITP homes onto a membrane surface where it undergoes a conformational change to expose the hydrophobic pocket so that lipid exchange can ensue. Electron spin-labeling studies demonstrate the hydrophobicity gradient across the Sec14 lipid-binding pocket closely mimics that of a membrane leaflet (44). The exchange process, when viewed in basic terms, therefore rests on the concept that phospholipid (either bound or to be bound) is presented with a choice of partitioning into one of two chemically equivalent environments. That is, from the membrane into the hydrophobic pocket and vice-versa. A simple partitioning mechanism neatly accounts for the energy-independence of the lipid exchange cycle. However, PITP binding to the membrane must evoke a highly local destabilization of the membrane surface in order to ‘activate’ lipid molecules for chemical partitioning -- thereby identifying those activated molecules as lipids competent for exchange. The various questions surrounding the early steps in the exchange cycle remain to be investigated.
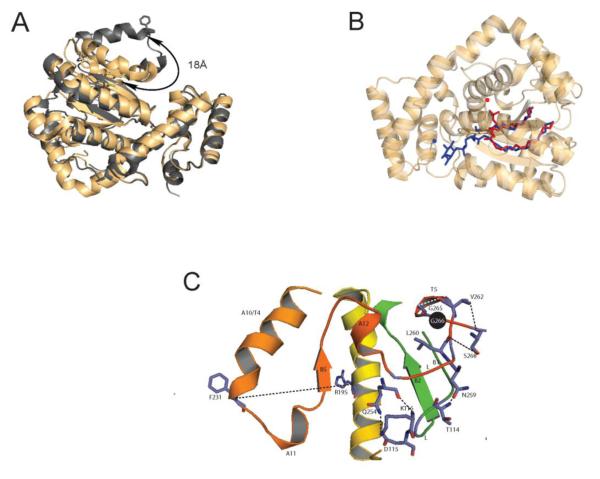
From the protein point of view, crystallographic studies define two major Sec14 conformers – i.e. the ‘open’ and ‘closed’ conformers (29,45,46). The ‘closed’ conformer is bound to a single phospholipid molecule, and this conformer is interpreted to represent the protein in its soluble (cytoplasmic) state. The ‘open’ conformer is interpreted as representing the membrane-associated PITP engaged in lipid exchange (45,46). In this form, the hydrophobic pocket is exposed and lipid-free due to an 18Å displacement of a helical gate element that governs access to the lipid binding pocket (Figure 3A). Reversible cross-linking experiments demonstrate that the large rigid body motions of the helical gate are essential for lipid exchange. Moreover, these conformational transitions are themselves controlled by a conformational switch element conserved across the Sec14-superfamily, the G-module. Survey of human disease alleles that involve Sec14-like proteins/domains highlight the importance of this element in the context of the Sec14-fold as a number of these alleles represent missense mutations that alter the G-module itself (47). Outstanding questions for future address include how activity of the G-module is regulated by membrane-binding. Indeed, the closer one examines the Sec14 conformational dynamics associated with lipid exchange, the more interesting these become. Directed evolution experiments indicate that G-module conformational transitions regulate the kinetics of gating of the hydrophobic pocket for lipid exchange, and that activation of the G-module responds to water organization and dynamics within the lipid binding pocket itself (48).
Figure 3.
Structural engineering of Sec14-like PITPs. (A) Access to lipid binding pocket is controlled by the dynamics of a helical gate. The α-carbon backbones of the open (silver) and closed (gold) structures of Sec14 and Sfh1 are rendered in ribbon diagram and superposed. The respective positions of the helical element that gates access to the lipid binding pocket, and the displacement of the gating element between the two conformers, are highlighted. (B) Classical Sec14-like PITPs bind PtdIns and PtdCho at distinct sites. The binding poses of PtdIns (blue) and PtdCho (red) within the closed Sec14 (Sfh1) conformer are shown. The respective headgroups are identified by the corresponding solid colored circles. (C) The Sec14 G-module is depicted in ‘open’ Sec14 conformer. This structural element regulates the opening and closing of the helical substructure that controls access to the Sec14 lipid binding pocket. The G-module is comprised of two sub-structures; the α-helix-loop-310-helix motif A12LT5 and the β-strand-loop-β-strand B1LB2 element. Residue G266 (highlighted as black sphere) is a critical component in organizing the extended H-bond network (dashed lines) that connects the G-module with A10/T4/A11 gate that controls access to the Sec14 hydrophobic pocket.
Although we are now beginning to understand the dynamics of the lipid exchange cycle from the Sec14 point of view, we know nothing about the cycle from the perspective of the phospholipid ligands. As will become clear below, evolving concepts for Sec14-like PITP activities not as lipid carriers, but as novel regulators of the biological outcomes of phosphoinositide signaling, demand an atomistic understanding of the mechanics of lipid exchange from both protein and lipid points of view. The challenges include solving how Sec14-like proteins dock onto membrane surfaces, what trajectories are drawn by PtdIns and secondary lipid ligands as these navigate entry and exit from the hydrophobic pocket, of the relative kinetics of lipid ligand entry and exit from the hydrophobic pocket, and how this relates to the interaction between the PITP and the PtdIns 4-OH kinase.
Lipid binding by Sec14-like proteins
Although the Sec14 conformational dynamics associated with lipid exchange provide a useful picture of the cycle from the protein point of view, an equally critical key to understanding mechanisms of PITP function demands understanding how the lipid ligands enter and exit the hydrophobic pocket. The fact that PtdIns and PtdCho are chemically similar molecules (with the exception of their respective headgroups) would seem to suggest that the trajectories with which these two phospholipids enter and/or exit the Sec14 hydrophobic pocket. But, the advent of high resolution crystal structures of Sec14 and other closely related Sec14-like proteins indicate this is not at all the case (29). Structural studies with Sec14 and its close homolog Sfh1 reveal the PtdCho and PtdIns headgroups are bound at physically distant sites. Whereas the PtdCho headgroup and glycerol backbone are buried deep within the hydrophobic pocket, the corresponding regions of bound PtdIns are positioned much closer to the protein surface (Figure 3B). Although Sec14 has a much higher affinity for PtdIns than for PtdCho, the selectivity for PtdIns vs PtdCho is estimated to be sufficiently small in energetic terms so that H2O rearrangements within the hydrophobic pocket are sufficient for negotiating the energy barriers that confront the heterotypic phospholipid exchange cycle. Of particular mechanistic relevance, a functional Sec14 molecule must house both PtdCho and PtdIns binding/exchange capability in order to stimulate production of PtdIns4P in cells (29,48). Those data demonstrate that both homotypic PtdCho- and homotypic PtdIns-exchange reactions are biologically futile activities, and that Sec14-mediated stimulation of PtdIns 4-OH kinase activity requires heterotypic exchange reactions (e.g. PtdIns for PtdCho or vice versa).
The concept of instructive regulation of PtdIns kinase activity by PITPs
Why is a heterotypic exchange cycle essential for Sec14-mediated support of optimal PtdIns 4-OH kinase activity and stimulated phosphoinositide synthesis in yeast cells? It is difficult to argue that PtdIns transfer mechanisms are involved because PtdIns naturally constitutes 20-25 mol% of bulk glycerophospholipid in yeast. Why would there be an obligate need for a PITP-driven PtdIns supply mechanism in membranes so rich in PtdIns? Alternative concepts need to be considered. In that regard, a kinetic trap model is proposed where slow PtdCho egress from the pocket results in abortive PtdIns entries – thereby exposing a frustrated PtdIns that is registered by the lipid kinase as an accessible substrate for phosphorylation (23,29).
This model has several key ideas, three of which are emphasized here. First, PITPs are required for biologically sufficient PtdIns4P production because PtdIns 4-OH kinases (at the least) are biologically inadequate interfacial enzymes when substrate PtdIns resides in a bilayer. This inadequacy is manifest even when PtdIns is a major bulk membrane phospholipid in the cell. Second, it describes stimulation of PtdIns 4-OH kinase activity in terms of a PITP-mediated PtdIns presentation mechanism where effective PtdIns presentation by Sec14 requires PtdCho binding. Third, the model identifies the optimal PtdIns substrate for PtdIns 4-OH kinase action as a lipid molecule that is frustrated in its entry into the Sec14 pocket. That is, a PtdIns molecule that never successfully enters the Sec14 pocket. In this model, Sec14 operates as a PtdCho sensor that couples PtdCho metabolic information to activation of PtdIns as a substrate for production of a dedicated signaling pool of PtdIns4P. The ability of Sec14 to prime PtdIns4P production in response to PtdCho metabolic cues outlines the basic principle of what we term instructive regulation of PtdIns 4-OH kinase activity. A detailed example of this idea is presented in the following section using Sec14-mediated regulation of membrane trafficking through the TGN/endosomal system as biological context.
The base concept that Sec14-like proteins specify privileged phosphoinositide signaling pools via instructive regulation of lipid kinases has interesting implications, and structural studies indicate this type of regulation might be very broad indeed. The structural signatures, or bar codes, that identify the elements directly involved in PtdIns headgroup coordination are conserved throughout the Sec14 superfamily, while the PtdCho-binding bar code is recognized only in a subset of these proteins (23,29,49). These features portend conservation of inositollipid binding capacity throughout the Sec14-superfamily with an accompanying diversification in the binding specificities for secondary lipid ligands. Using this idea to extend the principle of instructive lipid kinase regulation, Sec14-like proteins/domains can be interpreted as adaptors that interface the metabolism of diverse lipids/lipophiles with stimulated phosphoinositide synthesis for formation of functionally compartmentalized lipid signaling pools.
The concept of instructive regulation of inositol lipid kinases is gaining momentum from the recognition that naturally occurring human disease alleles of Sec14-superfamily proteins, including the most common inherited alleles associated with human retinal degeneration and acute vitamin E deficiencies, directly involve PtdIns (or phosphoinositide) binding bar-code residues (23,29,49-51). That remarkable correlation implies an inositol phospholipid is a genuine physiological ligand for the Sec14-like protein in question (cellular retinadehyde binding protein and α-tocopherol transfer protein, respectively). Indeed, recent structural and functional analyses of the α-tocopherol transfer protein strongly reinforce this concept (51). Other Sec14-like proteins/domains associated with human disease include caytaxin and neurofibromin, and loss-of-function mutations that compromise the presumptive PtdIns-binding bar-code motifs of these proteins have now been identified (23,29,49).
Instructive regulation from the perspective of yeast Sec14-like PITPs
The yeast system offers strong opportunities for exploring instructive regulation of PtdIns kinases by Sec14-like proteins. As discussed above, each of the six Saccharomyces Sec14-like PITPs specify unique biological outcomes for PtdIns 4-OH kinase signaling, and four of the six are non-classical on the basis of failing to exhibit measurable PtdCho-transfer activities and not displaying recognizable PtdCho-binding bar-codes (18,29,49,52). The Sfh3 PITP represents a particularly interesting case in this regard as, even though it is a PITP that activates PtdIns 4-OH kinase activity in a fashion analogous to the one discharged by Sec14 in the same cell, it nonetheless acts in a biologically antagonistic way to Sec14 (18). This functional antagonism reflects competing activities by these PITPs in channeling PtdIns 4-OH kinase activities towards disparate biological outcomes. Whereas Sfh3 directs PtdIns 4-OH kinase signaling towards developmental regulation of lipid droplet metabolism, Sec14 trains lipid kinase activity on membrane trafficking through the TGN/endosomal system. The idea of instructive stimulation of PtdIns 4-OH kinases by Sec14-like proteins posits that channeling of PtdIns 4-OH kinase activity to distinct biological outcomes is determined in part by the binding of these two PITPs to distinct secondary ligands. Indeed, the dimensions and chemical environment of the Sfh3 hydrophobic pocket differ radically from those of the classical PITPs Sec14 and Sfh1 (18,29) – i.e. a property consistent with diverged secondary lipid binding specificities. Identification of the secondary ligands hypothesized to prime PtdIns presentation to the lipid kinases is now an important endeavor, and there are data to suggest that such a ligand for Sfh3 may be a sterol (53).
How do Sec14-like PITPs interact with PtdIns 4-OH kinases? An enzyme::PITP complex offers the simplest solution for PtdIns presentation. In some instances this may well be the case. One such example involves the dual requirements for a Sec14-like PITP (Sfh4) and the Stt4 PtdIns 4-OH kinase in regulation of phosphatidylserine decarboxylation in yeast (15). The Sfh4 requirement shows high PITP-specificity as no other Sec14-like protein can operate as functional surrogate in this metabolic reaction (16), and the PITP specificity may reflect the need for a physical interaction between the Sfh4 and the decarboxylase enzyme (54); although the functional requirement for this interaction has not been shown. In other cases, a tight physical interaction does not seem essential. Stimulation of yeast Pik1 and Stt4 PtdIns 4-OH kinases by Sec14 are two cases in point. The possibility that PITPs might present PtdIns to the lipid kinase en passant, and thereby facilitate a ‘drive-by’ phosphorylation of the headgroup, is consistent with demonstrations that the structurally unrelated vertebrate START-like PITPs can perform as functional Sec14 surrogates in stimulating PtdIns 4-OH kinase activities when expressed at high levels in yeast (55,56).
Sec14 and the interface between lipid metabolism and membrane trafficking
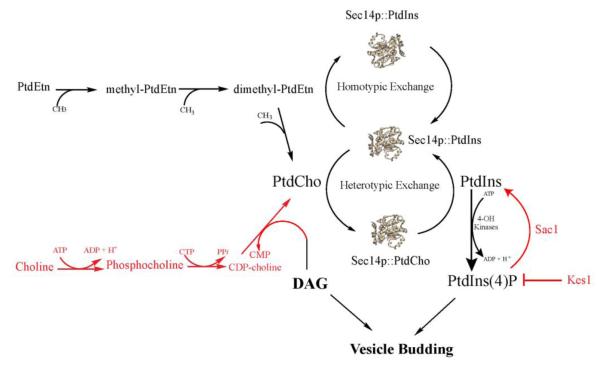
Why would cells employ specific Sec14-like PITPs to serve as PtdCho sensors when PtdCho is the most abundant cellular phospholipid? What possible signaling information could come from this curious design? This paradox can be examined by reviewing the results of genetic studies of Sec14 function in vivo, and interpreting those results through the conceptual lens of instructive regulation of the Pik1 PtdIns 4-OH kinase. The PITP activity of Sec14 is essential for yeast cell viability because this activity is required for efficient transport of secretory cargo from the trans-Golgi network (TGN) to the cell surface (14,24). Sec14 promotes TGN function primarily by regulating a retrograde membrane trafficking pathway that returns components of the anterograde trafficking machinery to the TGN from endosomes (57). Seminal insights into Sec14 function in the TGN/endosomal system came from study of ‘bypass Sec14’ mutations that circumvent the normally essential Sec14 requirement for membrane trafficking and cell viability (58-60). All of the ‘bypass Sec14’ alleles ultimately proved to be recessive – identifying these as loss-of-function mutations that inactivate functional antagonists of Sec14 activity. These mutations fall into two general categories; those that ablate enzymes of the CDP-choline pathway for PtdCho biosynthesis (25,59,61), and those that ablate proteins whose activities either degrade (e.g. the Sac1 PtdIns4P phosphatase) or sequester (e.g. the oxysterol binding protein homolog Kes1/Osh4) from PtdIns4P its pro-trafficking downstream effectors (58,62-66; Figure 4). Those genetic data revealed that Sec14 coordinates PtdCho metabolism with PtdIns4P signaling to promote membrane trafficking through TGN/endosomal compartments, and the PtdCho sensor function is proposed to constitute a mechanism for sensing metabolic flux through the CDP-choline pathway as a proxy for consumption of a pro-trafficking lipid diacylglycerol (23,67,68). Thus, the PtdCho sensor activity translates to activation of the Sec14 heterotypic exchange cycle for production of a second pro-trafficking lipid, PtdIns4P, by the Pik1 PtdIns 4-OH kinase (Figure 4). In this way, Sec14 facilitates the tuning of PtdIns4P production in response to PtdCho synthesis via the CDP-choline pathway and the consumption of DAG in the process. This model provides a coherent and physical picture for the general concept of crosstalk between PtdCho and PtdIns4P metabolic pathways.
Figure 4.
Instructive regulation of PtdIns 4-OH kinase by Sec14 and TGN/endosomal membrane trafficking. A model is drawn where Sec14 interfaces PtdCho metabolism (and consumption of the pro-secretory lipid DAG) with the amplitude of PtdIns4P pro-secretory signaling in the yeast TGN/endosomal membrane trafficking pathway. Sec14 serves as a sensor for flux through the CDP-choline pathway for PtdCho biosynthesis and, via PtdCho binding and activation of heterotypic exchange cycles, stimulates activity of the Pik1 PtdIns 4-OH kinase to produce PtdIns4P in response to CDP-choline pathway activity. Cellular activities whose loss of function result in ‘bypass Sec14’ are highlighted in red (see text).
Metazoan START-like PITPs
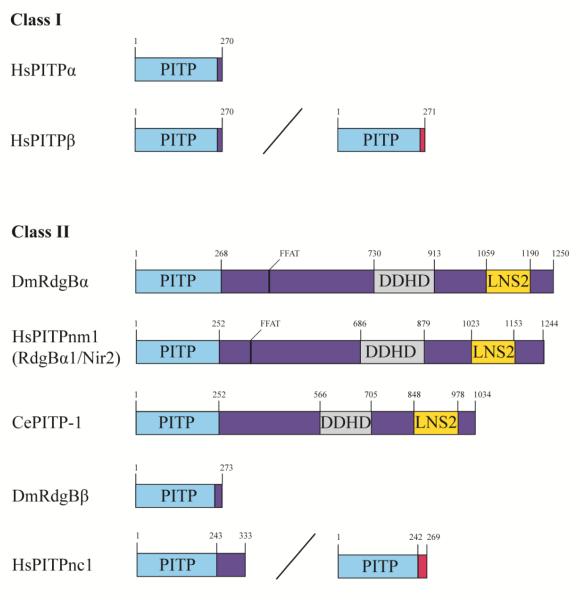
Metazoan START-like PITPs consist of either a free-standing PITP domain, or are multiple domain proteins (Figure 5), and numerous lines of evidence indicate that also regulate phosphoinositide signaling. Several of those annotated physiological contexts recapitulate the Sec14 case in that membrane trafficking events are involved. These include regulated and constitutive secretory processes in neuroendocrine and mast cells (69-71), and non-excitatory cells (72,73), retrograde membrane trafficking from the Golgi complex regulated by the START-domain protein PITPβ (74), speculation that endocytic trafficking of the angiotensin II receptor may be regulated by another START-domain protein PITPnc1 (75,76), and potentiation of anterograde trafficking from the mammalian TGN via regulation of diacylglycerol metabolism by a multi-domain START-like PITP termed PITPnm1. The TGN trafficking activity of PITPnm1 is of interest in that it, like Sec14, counters the antagonistic effects of the CDP-choline pathway for PtdCho biosynthesis on vesicular transport (77). Other signaling circuits established to show START-like PITP-dependence include the Drosophila visual and olfactory phototransduction pathways and chemosensation in the worm. These sensory systems depend on activity of the multi-domain START-like PITPs – e.g. fly RdgB and worm pitp-1, respectively, although a requirement for PITP activity in pitp-1 biological function has not been demonstrated (78-80). There are also reports of an obligate role for the START-domain PITPα and multi-domain PITPnm1 proteins in forward signaling of the epidermal growth factor receptor, and for PITPα in both epidermal growth factor receptor and netrin receptor signaling (37,81-83).
Figure 5.
Domain architecture of START-like PITPs. Class I PITPs include PITPα and two splice variants of PITPβ. Class II PITPs are orthologs of the Drosophila DmRdgBα and include the multi-domain human and C. elegans HsPITPnm1 and CePITP-1, respectively, the single-domain fly DmRdgBβ and two splice variants of HsPITPnc1. Alternative nomenclature is indicated in parentheses. Spliceoforms are designated by a diagonal line, and variant C-termini are indicated in pink. Domain location and length of proteins is marked in superscript: PITP (light blue), phosphatidylinositol transfer protein; DDHD (grey); Lipin/Ned1/Smp2; LNS2; FFAT, diphenylalanine in an acidic tract.
START-like PITPs and phosphoinositide signaling
The prevailing thought is these proteins are intracellular PtdIns carriers (20,26,42,43). In the case of the START-domain versions, these PITPs are considered to be soluble carriers while the membrane-associated multi-domain proteins are posited to act as shuttle factors that operate in focal areas of close membrane apposition – i.e. intermembrane contact sites (34,84,85). Unfortunately, the available data from model metazoan organisms – both vertebrate and invertebrate – are rather confusing from the perspective of offering some unified mechanism of function. While discussion of the various ramifications of the data is left for another venue, there is little solid evidence from animal systems to show that growth factor or morphogen receptors obligatory require START-domain PITPs (e.g. PITPα) for forward signaling from the plasma membrane. In cases where the question has been put to the acid experimental test, soluble canonical PITPs (e.g. PITPα) do not score as significant components of PtdIns supply pathways for sustained phosphoinositide signaling at the plasma membrane (39-41).
There is clear evidence of the physiological importance of the soluble START-like PITPs, however. Zebrafish PITPβ and PITPα hypomorphs show defects in establishment and/or maintenance of photoreceptor outer segments and embryogenesis, respectively (56). PITPα-deficient mice are born alive but suffer perinatal death brought on by multiple pathologies. These include a dramatic spinocerebellar neurodegenerative disease marked by a fulminating course of aponecrotic cell death and neuroinflammation, an inability of the animals to process and transport lipoproteins from the enterocyte, striking lipid homeostatic defects in the liver, and degeneration of pancreatic islets that leads to a catastrophic hypoglycemia (40). Interestingly, the high flux phosphoinositide-driven synaptic cycle remains fully intact in pitpα null neurons – even in performance assays where such cells are subjected to excitation trains of such intensities that the synaptic capacities of the test neurons are ultimately overwhelmed (41). A biologically functional PITPα must bind PtdIns as evidenced by the demonstration that mouse strains expressing a mutant protein specifically defective in PtdIns (but not PtdCho) binding, as sole source of PITPα activity in the animal, phenocopy the null mouse (41). Those data demonstrate an authentic role for PITPα in regulating inositol lipid signaling in the mouse, although there are no obvious derangements in bulk phosphoinositide pools in deficient cells or tissues (40).
Recently, the mammalian PITPnc1 and fly RdgBα were recognized to be PtdIns/PtdOH transfer proteins (76), and that the multi-domain PITPnm1 also binds PtdOH. In this case, PtdOh binding activity resides in a C-terminal LNS2 domain distinct from the PITP PtdIns/PtdCho-transfer domain (81). Non-canonical proteins such as PITPnc1 display the biochemical properties expected of a lipid shuttling activity involved in the phosphoinositide consumption-regeneration cycles depicted in Figure 2, and PITPs such as these will be particularly interesting to analyze with regard to the integrity of phosphoinositide signaling and capacity for PtdIns resynthesis in genuine in vivo contexts. In that regard, there is growing interest in PITPnc1 from the perspective of cancer biology. The microRNA mir-126 exhibits tumor suppressor activity (86-88), and one of its target genes is PITPNC1 (89). Downregulation of mir-126 and consequent elevation of PITPnc1 levels promotes recruitment of endothelial cells to tumors with subsequent increase in angiogenesis and increased metastatic potential (89).
START-like PITPs at inter-membrane contact sites
The multi-domain PITPs (e.g. PITPnm1) are envisioned to participate in such capacities via endoplasmic reticulum::plasma membrane contact sites that organize and integrate the calcium signaling machineries of those two membrane systems in response to cell stimulation (42,43). The primary physiological activities of these multi-domain PITPs are likely restricted to excitatory cells where the amplitudes of induced signaling are very high (e.g. neurons). Several observations are consistent with such a view. First, the Drosophila RdgBα is functionally important only in sensory organs involved in vision and olfaction. The null fly is otherwise viable. The pitp-1, also the sole multi-domain PITP in the worm, is similarly nonessential for base viability of this organism (80). Third, siRNA-mediated knockdown of PITPnm1 does not affect viability of the hypomorphic mammalian cells in an ex vivo context (77). This result is congruent with genetic data as the PITPnm1 null mice are viable – albeit with defects in cholesterol and calcium homeostasis and altered auditory functions (http://www.informatics.jax.org/marker/MGI:1197524; 90). An obligate role for the PITP-domain in discharge of these physiological activities remains to be demonstrated. The cellular and animal viability data are puzzling given the reported obligatory roles for this protein in promoting growth factor signaling and anterograde protein trafficking from the TGN. It remains formally possible that there is some functional redundancy between PITPnm1 and its close homolog PITPnm2 (91). However, the tissue expression profile for PITPnm2 is restricted to retina and dentate gyrus, and is therefore much more limited than that of the widely expressed PITPnm1. As such, PITPnm2 is likely the true mammalian ortholog of fly RdgBα. However, mice lacking PITPnm2 are healthy and show no defects in photoreceptor function (91).
START-like PITPs and instructive regulation of phosphoinositide signaling
Sec14-like and START-like PITPs exhibit completely unrelated structural folds (92-94), so it remains an open question as to whether or not these proteins execute mechanistically homologous activities. So, do START-like PITPs also instruct biological outcomes for PtdIns kinase signaling in metazoans? The question cannot be answered at this time because the appropriate experimental test has yet to be devised for the metazoan systems to which these proteins are native. The fact that the START-like PITP family is far more sparse than is the Sec14 superfamily in even the most complex vertebrates, suggests that instructive regulation is either not a physiologically important activity of these PITPs, or that the biological scope of any such activity is limited for these proteins. However, there are indications these PITPs can execute an instructive regulation when interrogated under quasi-physiological conditions. That evidence comes from yeast systems where both zebrafish and mammalian START-like PITPs efficiently stimulate PtdIns 4-OH kinase activities to produce PtdIns4P under conditions of PtdIns surfeit and high-level expression of the PITP (56). The fact that the RdgBα PITP domain can also perform Sec14-like functions when expressed at high levels in yeast, although it cannot fully substitute for Sec14, forecasts that it too has that biochemical capability (unpublished data).
That instructive regulation is a viable alternative for interpreting how the START-like PITPs function is also suggested by an old and puzzling observation. Although RdgBα is a multi-domain PITP of which the START-like PITP domain comprises only some 20% of the total protein sequence (78), expression of the RdgBα PITP domain alone is sufficient to fully complement the dramatic retinal degeneration phenotype of null flies (79). By contrast, expression of PITPα in RdgBα-deficient retinal is completely ineffective in this regard. The non-exchangeability of these two PITP modules is also on display in domain-swap experiments where PITPα replaced the RdgBα PITP domain in the context of the full-length protein (79). Perhaps the functional non-equivalence of these two PITPs reflects an ability of the RdgBα PITP to bind PtdOH whereas PITPα lacks this capacity. This biological specificity of PITP action, a result usually on display when PITP functions are interrogated in authentic biological contexts, and a central feature of instructive regulation models, is not recapitulated in permeabilized cell experiments where PITP functional promiscuity is the dominant theme (69-72,95).
That intermembrane contact sites are privileged sites of signaling and lipid transfer is an attractive model, and incorporation of multi-domain PITPs into such structures suggests how these proteins might facilitate intermembrane lipid transfer. Can instructive regulation work within the framework of a contact site? We suggest yes. Localization of the PITP to a contact site physically organizes where instructive regulation of a lipid kinase occurs in response to PtdOH production. In this scenario the PITP serves as a PtdOH sensor that monitors phosphoinositide signaling flux and couples PtdOH production to instruction of enhanced on-demand production of phosphoinositide at a precise site on the plasma membrane. There is much to be learned about the relationship between PITPs and contact sites, however. That expression of the PITP module alone is sufficient to satisfy the demands of the animal for multi-domain PITP function, in the two cases where the issue has been rigorously queried (i.e. fly RdgBα and worm pitp-1; 79,80), remains a remarkable and an altogether perplexing result.
PITPs as targets for chemical interference with phosphoinositide signaling
Finally, the concept of instructive regulation of PtdIns 4-OH kinases by PITPs identifies these proteins as highly discriminating portals through which privileged phosphoinositide signaling pools can be interrogated. Proof-of-concept is provided by recent discoveries of small molecule inhibitors with validated specificity for Sec14 (96,97). Treatment of yeast with biologically active inhibitors recapitulates the phenotypes associated with sec14 loss-of-function mutations, and there are no obvious off-target effects. Interestingly, Sec14 is subject to specific inhibition by several different chemical scaffolds (97), and the available data show that PITP-directed small molecule inhibitors offer superior strategies for interfering with phosphoinositide signaling pools that interface with specific biological pathways. Moreover, these PITP-targeted interventions can be applied with far greater specificities than those afforded by even the most specific inhibition of individual PtdIns-kinases (Figure 6), or even enzyme dimerization-based (e.g. rapalog) depletion of compartment-specific pools of individual phosphoinositides.
Figure 6.
PITPs as targets for chemical inhibition of phosphoinositide signaling. A diversity tree for biological outcomes of signaling via a PtdIns 4-OH kinase that produces only PtdIns4P but interfaces with multiple PITPs. Chemical inhibition of the kinase, even with absolute specificity, will compromise the entire outcome tree. Inhibition of any individual PITP offers a more surgical approach to dissecting phosphoinositide signaling.
Two objections against the idea of targeting PITPs for inhibition by small molecules can be imagined: (i) the concern that these proteins will bind lipophilic compounds nonspecifically due to the hydrophobic nature of the lipid binding pocket, and (ii) that candidate inhibitors will fail to discriminate between PITPs or even other unrelated proteins that bind lipophiles. What is particularly interesting is that even very closely-related Sec14-like PITPs are not inhibited by Sec14-active compounds. The available data indicate the biologically active small molecule inhibitors enter the Sec14 hydrophobic pocket in an exchange reaction and are trapped there by a set of specific Sec14::inhibitor interactions (96). The geometry of these interactions is both key to the binding mechanism and to the high specificity of these inhibitors for Sec14 as opposed to the other Sec14-like proteins. Inhibitor binding is not only sterically incompatible with Sec14::phospholipid interactions, but the interaction also effectively locks Sec14 into its closed conformer – thereby prohibiting lipid exchange.
The advent of strategies for discovery of PITP-directed inhibitors, and downstream exploitation of such inhibitors as experimental tool compounds, offers exciting new prospects for studying both PITP function and phosphoinositide signaling in a wide variety of experimental systems. First, the availability of appropriate and well-characterized inhibitors will enable functional analyses of PITPs and phosphoinositide signaling in organisms not amenable to application of standard genetic or gene-silencing approaches. Many eukaryotic parasites fall into this category. Second, PITP-directed inhibitors will facilitate genome-scale functional interaction screens in even the most complex cells by facilitating acute and specific inhibition of the target at the onset of the screen (98). This feature promises to effectively minimize the complications that arise when compensatory mechanisms come into play. Finally, PITP-directed inhibitors promise to facilitate resolution of biochemical systems where lipid signaling circuits have been biochemically reconstituted from wild-type components. Chemical intervention approaches obviate the need for biochemically reconstituting conditionally functional systems from mutant components – i.e. a strategy that, even when available, often proves problematic.
Conclusions
PITPs and other lipid transfer proteins, such as homologs of oxysterol binding proteins (Osh) and ceramide transfer proteins (CERT), have burst onto the main cell biology stage in the past few years. The growing interest in these enigmatic transfer proteins makes this an appropriate time to consider what it is that we actually know about how these proteins operate, what we do not know, and what has not yet been rigorously established. In this review, we used PITPs as a case study for discussing alternatives to the historical lipid carrier models that have dominated, and still dominate, the scene. Concepts such as instructive regulation of lipid metabolic enzymes, and roles for such mechanisms of metabolic control in diversifying biological outcomes for lipid signaling, offer new frameworks for interpreting the physiological actions of transfer proteins. Will there be a unifying resolution to how these various transfer proteins execute their biological functions? Perhaps other than general agreement for a central role for the lipid exchange cycle, we feel this unlikely. Whereas some proteins exhibit modes of action suggesting sensing roles, rather than transfer functions, others appear to be most readily interpreted in the context of transfer proteins. The various possibilities will need to be evaluated for each protein class on their own experimental merits. What is clear is that authentic physiological assays are needed for progress and, in the few cases where such assays have been exploited in detail, the results of such assays testify to the remarkable biology of these proteins. We anticipate that the emergence of as yet unappreciated linkages of PITPs to mammalian diseases will guide important future efforts.
Highlights.
** -- Historical views of PITPs as lipid transfer proteins are reassessed.
** -- PITPs channel lipid kinase activities to specific biological outcomes.
** -- Mechanisms of functional channeling rest on PITP ligand specificities.
** -- PITP lipid exchange cycle is an engine for potentiating lipid kinase catalytic efficiency.
Acknowledgements
Work in the authors’ laboratory is funded by a grant from the National Institutes of Health GM44530 and by the Robert A. Welch Foundation (BE0017) to VAB. The authors declare no financial conflicts. We are grateful to Raghu Padinjat (Bangalore) for helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Michell RH. Inositol derivatives: evolution and functions. Nat. Rev. Mol. Biol. 2008;9:151–161. doi: 10.1038/nrm2334. [DOI] [PubMed] [Google Scholar]
- 2.Fruman DA, Meyers RE, Cantley LC. Phosphoinositide kinases. Annu. Rev. Biochem. 1998;67:481–507. doi: 10.1146/annurev.biochem.67.1.481. [DOI] [PubMed] [Google Scholar]
- 3.Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–657. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- 4.Balla T. Phosphoinositides: Tiny lipids with giant impact on cell regulation. Physiol. Rev. 2013;93:1019–1137. doi: 10.1152/physrev.00028.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suh PG, Park JI, Manzoli L, Cocco L, Peak JC, Katan M, Fukami K, Kataoka T, Yun S, Ryu SH. Multiple roles of phosphoinositide-specific phospholipase C isozymes. BMB Rep. 1997;41:415–434. doi: 10.5483/bmbrep.2008.41.6.415. [DOI] [PubMed] [Google Scholar]
- 6.Rhee SG. Regulation of phosphoinositide-specific phospholipase C. Annu. Rev. Biochem. 2001;70:281–312. doi: 10.1146/annurev.biochem.70.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berridge MJ, Irvine RF. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984;312:315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- 8.Odom AR, Stahlberg A, Wente SR, York JD. A role for nuclear inositol 1,4,5-trisphosphate kinase in transcriptional control. Science. 2000;287:2026–2029. doi: 10.1126/science.287.5460.2026. [DOI] [PubMed] [Google Scholar]
- 9.Alcázar-Román AR, Elizabeth J, Tran EJ, Guo S, Wente SR. Inositol hexakisphosphate and Gle1 activate the DEAD-box protein Dbp5 for nuclear mRNA export. Nat. Cell Biol. 2006;8:711–716. doi: 10.1038/ncb1427. (2006) [DOI] [PubMed] [Google Scholar]
- 10.Macbeth MR, Schubert HL, Vandemark AP, Lingam AT, Hill CP, Bass BL. Inositol hexakisphosphate is bound in the ADAR2 core and required for RNA editing. Science. 2005;309:1534–1539. doi: 10.1126/science.1113150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan X, Calderon-Villalobos LIA, Sharon M, Zheng C, Robinson CV, Estelle M, Zheng N. Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature. 2007;446:640–645. doi: 10.1038/nature05731. [DOI] [PubMed] [Google Scholar]
- 12.Lee YS, Mulugu S, York JD, O’Shea EK. Regulation of a Cyclin/CDK/CDK inhibitor complex by inositol pyrophosphates. Science. 2007;316:109–112. doi: 10.1126/science.1139080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hilgemann DW, Feng S, Nasuhoglu C. The complex and intriguing lives of PIP2 with ion channels and transporters. Science, STKE. 2001 doi: 10.1126/stke.2001.111.re19. re19. [DOI] [PubMed] [Google Scholar]
- 14.Bankaitis VA, Aitken JR, Cleves AE, Dowhan W. An essential role for a phospholipid transfer protein in yeast Golgi function. Nature. 1990;347:561–562. doi: 10.1038/347561a0. [DOI] [PubMed] [Google Scholar]
- 15.Wu WI, Routt S, Bankaitis VA, Voelker D. A new gene involved in transport-dependent metabolism of phosphatidylserine, PSTB2/PDR17, shares sequence similarity with the gene encoding the PI-/PC-TP, Sec14p. J. Biol. Chem. 2000;275:14446–14456. doi: 10.1074/jbc.275.19.14446. [DOI] [PubMed] [Google Scholar]
- 16.Routt SM, Ryan MM, Tyeryar K, Rizzieri K, Roumanie O, Brennwald PJ, Bankaitis VA. Nonclassical PITPs activate phospholipase D via an Stt4p-dependent pathway and modulate function of late stages of the secretory pathway in vegetative yeast cells. Traffic. 2005;6:1157–1172. doi: 10.1111/j.1600-0854.2005.00350.x. [DOI] [PubMed] [Google Scholar]
- 17.Desfougeres T, Ferreira T, Bergés T, Régnacq M. SFH2 regulates fatty acid synthase activity in the yeast Saccharomyces cerevisiae and is critical to prevent saturated fatty acid accumulation in response to heme and oleic acid depletion. Biochem, J. 2005;409:299–309. doi: 10.1042/BJ20071028. [DOI] [PubMed] [Google Scholar]
- 18.Ren J, Lin CPC, Pathak M, Temple BRS, Nile AH, Mousley CJ, Duncan MC, Eckert D, Leiker TJ, Ivanova PT, Milne DS, Murphy RS, Brown HA, Verdaasdonk J, Bloom KS, Ortlund EA, Neiman AM, Bankaitis VA. A phosphatidylinositol transfer protein integrates phosphoinositide signaling with lipid droplet metabolism to regulate a developmental program of nutrient stress-induced membrane biogenesis. Molecular Biology of the Cell. 2014;25:712–727. doi: 10.1091/mbc.E13-11-0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karel WA, Wirtz KWA. Phospholipid transfer proteins in perspective. FEBS Lett. 2006;580:5436–5441. doi: 10.1016/j.febslet.2006.06.065. [DOI] [PubMed] [Google Scholar]
- 20.Cockcroft S, Carvou N. Biochemical and biological functions of class I phosphatidylinositol transfer proteins. Biochim Biophys Acta. 2007;1771:677–691. doi: 10.1016/j.bbalip.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 21.Phillips SE, Vincent P, Rizzieri K, Schaaf G, Gaucher EA, Bankaitis VA. The diverse biological functions of phosphatidylinositol transfer proteins in eukaryotes. Crit. Rev. in Bioch. & Mol. Biol. 2006;41:1–28. doi: 10.1080/10409230500519573. [DOI] [PubMed] [Google Scholar]
- 22.Saito K, Tautz L, Mustelin T. The lipid-binding SEC14 domain. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2007;1771:719–726. doi: 10.1016/j.bbalip.2007.02.010. (2007) [DOI] [PubMed] [Google Scholar]
- 23.Bankaitis VA, Mousley CJ, Schaaf G. Sec14-superfamily proteins and the crosstalk between lipid signaling and membrane trafficking. Trends in Biochemical Sciences. 2010;35:150–160. doi: 10.1016/j.tibs.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bankaitis VA, Malehorn DE, Emr SD, Greene R. R, The Saccharomyces cerevisiae SEC14 gene encodes a cytosolic factor that is required for transport of secretory proteins from the yeast Golgi complex. J, Cell Biol. 1989;108:1271–1281. doi: 10.1083/jcb.108.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cleves AE, Mcgee T, V.A., Bankaitis Phospholipid transfer proteins: a biological debut. Trends Cell Biol. 1991;1:30–34. doi: 10.1016/0962-8924(91)90067-j. [DOI] [PubMed] [Google Scholar]
- 26.Cockcroft S. Special issue: Lipid transporters in cell biology. BBA-Mol Cell Biol. 2007;1771:641–643. doi: 10.1016/j.bbalip.2022.159152. [DOI] [PubMed] [Google Scholar]
- 27.Cockcroft S, Garner K. Function of the phosphatidylinositol transfer protein gene family: is phosphatidylinositol transfer the mechanism of action? Crit Rev Biochem Mol Biol. 2011;46:89–117. doi: 10.3109/10409238.2010.538664. [DOI] [PubMed] [Google Scholar]
- 28.Shadan S, Holic R, Carvou N, Ee P, Li M, Murray-Rust J, Cockcroft S. Dynamics of lipid transfer by phosphatidylinositol transfer proteins in cells. Traffic. 2008;10:1743–s1756. doi: 10.1111/j.1600-0854.2008.00794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schaaf G, Ortlund E, Tyeryar K, Mousley C, Ile K, Woolls M, Garrett T, Raetz CRH, Redinbo M, Bankaitis VA. The functional anatomy of phospholipid binding and regulation of phosphoinositide homeostasis by proteins of the Sec14-superfamily. Molecular Cell. 2008;29:191–206. doi: 10.1016/j.molcel.2007.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sleight RG, Pagano RE. Rapid appearance of newly synthesized phosphatidylethanolamine at the plasma membrane. J. Biol. Chem. 1983;258:9050–9058. [PubMed] [Google Scholar]
- 31.Kaplan MR, Simoni RD. Intracellular transport of phosphatidylcholine to the plasma membrane. J. Cell Biol. 1985;101:441–445. doi: 10.1083/jcb.101.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vance JE, Aaslman EJ, Szarja R. Brefeldin A does not inhibit movement of phosphatidylethanolamine from its site of synthesis to the cell surface. J. Biol. Chem. 1991;266:8241–8247. [PubMed] [Google Scholar]
- 33.Wirtz KWA. Phospholipid transfer proteins. Annu. Rev. Biochem. 1991;60:73–99. doi: 10.1146/annurev.bi.60.070191.000445. [DOI] [PubMed] [Google Scholar]
- 34.Prinz WA. Bridging the gap: membrane contact sites in signaling, metabolism, and organelle dynamics. J Cell Biol. 2014;205:759–769. doi: 10.1083/jcb.201401126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michell RH. Inositol phospholipids and cell surface receptor function. Biochim. Biophys. Acta. 1975;415:81–147. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- 36.Van Paridon PA, Gadella TWJ, Somerharju PJ, Wirtz KWA. On the relationship between the dual specificity of the bovine brain phosphatidylinositol transfer protein and membrane phosphatidylinositol levels. Biochim. Biophys. Acta. 1987;903:68–77. doi: 10.1016/0005-2736(87)90156-8. [DOI] [PubMed] [Google Scholar]
- 37.Kauffmann-Zeh A, Thomas GM, Ball A, Prosser S, Cunningham E, Cockcroft S, Hsuan JJ. Requirement for phosphatidylinositol transfer protein in epidermal growth factor signaling. Science. 1995;268:1188–1190. doi: 10.1126/science.7761838. [DOI] [PubMed] [Google Scholar]
- 38.Kim YJ, Guzman-Hernandez ML, Balla T. A highly dynamic ER-derived phosphatidylinositol-synthesizing organelle supplies phosphoinositides to cellular membranes. Dev. Cell. 2011;21:813–824. doi: 10.1016/j.devcel.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alb JG, Jr., Phillips SE, Rostand K, Cotlin L, Pinxteren J, Manning T, Guo S, York JD, Sontheimer H, Collawn JF, Bankaitis VA. Ablation of phosphatidylinositol transfer protein function in murine cells. Mol. Biol Cell. 2002;13:739–754. doi: 10.1091/mbc.01-09-0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alb JG, Jr, Cortese JD, Phillips SE, Albin RL, Nagy TR, Hamilton BA, Bankaitis VA. Mice lacking phosphatidylinositol transfer protein alpha exhibit spinocerebellar degeneration, intestinal and hepatic steatosis, and hypoglycemia. J. Biol. Chem. 2003;278:33501–33518. doi: 10.1074/jbc.M303591200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alb JG, Jr., Phillips SE, Wilfley LR, Philpot BD, Bankaitis VA. The pathologies associated with functional titration of phosphatidylinositol transfer protein α activity in mice. J. Lipid Res. 2007;48:1857–1872. doi: 10.1194/jlr.M700145-JLR200. [DOI] [PubMed] [Google Scholar]
- 42.Prinz WA. Lipid trafficking sans vesicles: where, why, how? Cell. 2010;143:870–874. doi: 10.1016/j.cell.2010.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lev S. Non-vesicular lipid transport by lipid-transfer proteins and beyond. Nat. Rev. Mol. Cell. Bio. 2010;11:739–750. doi: 10.1038/nrm2971. [DOI] [PubMed] [Google Scholar]
- 44.Smirnova T, Chadwick TG, van Tol J, Ozarowski A, Poluektov O, Schaaf G, Ryan MM, Bankaitis VA. Local polarity and hydrogen bonding inside the Sec14p phospholipid-binding cavity: High-field multifrequency electron paramagnetic studies. Biophys. J. 2007;92:3686–3695. doi: 10.1529/biophysj.106.097899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sha B, Phillips SE, Bankaitis VA, Luo M. Crystal structure of the Saccharomyces cerevisiae phosphatidylinositol transfer protein Sec14p. Nature. 1998;391:506–510. doi: 10.1038/35179. [DOI] [PubMed] [Google Scholar]
- 46.Phillips S, Sha B, Topalof L, Xie Z, Alb J, Clenchin V, Swigart P, Cockcroft S, Luo M, Martin T, Bankaitis VA. Yeast Sec14p deficient in phosphatidylinositol transfer activity is functional in vivo. Molecular Cell. 1999;4:187–197. doi: 10.1016/s1097-2765(00)80366-4. [DOI] [PubMed] [Google Scholar]
- 47.Ryan MM, Temple BRS, Phillips SE, Bankaitis VA. Conformational dynamics of the major yeast phosphatidylinositol transfer protein Sec14p: Insights into the mechanisms of phospholipid exchange and diseases of Sec14p-like protein deficiencies. Mol. Biol. Cell. 2007;18:1928–1942. doi: 10.1091/mbc.E06-11-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schaaf G, Dynowski M, Mousley CJ, Shah SD, Yuan P, Winklbauer E, de Campos MKF, Trettin K, Quinones MC, Smirnova T, Yanagisawa LL, Ortlund E, Bankaitis VA. Resurrection of a functional phosphatidylinositol transfer protein from a pseudo-Sec14 scaffold by directed evolution. Mol. Biol. Cell. 2011;22:892–905. doi: 10.1091/mbc.E10-11-0903. (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nile AH, Bankaitis VA, Grabon A. Mammalian diseases of phosphatidylinositol transfer proteins and their homologs. Clinical Lipidology. 2010;5:867–897. doi: 10.2217/clp.10.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.He X, Lobsiger J, Stocker A. Bothnia dystrophy is caused by domino-like rearrangements in cellular retinaldehyde-binding protein mutant R234W. PNAS. 2009;106:18545–18550. doi: 10.1073/pnas.0907454106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kono N, Ohto U, Hiramatsu T, Urabe M, Uchida Y, Satow Y, Arai H. Impaired α-TTPPIPs interaction underlies familial vitamin E deficiency. Science. 2013;340:1106–1110. doi: 10.1126/science.1233508. [DOI] [PubMed] [Google Scholar]
- 52.Li X, Routt S, Xie Z, Cui X, Fang M, Kearns MA, Bard M, Kirsch D, Bankaitis VA. Identification of a novel family of nonclassical yeast PITPs whose function modulates activation of PLD and Sec14p-independent cell growth. Mol Biol. Cell. 2000;11:1989–2005. doi: 10.1091/mbc.11.6.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Holič R, Šimová Z, Ashlin T, Pevala V, Poloncová K, Tahotná D, Kutejová E, Cockcroft S, Griač P. Phosphatidylinositol binding of Saccharomyces Pdr16p represents an essential feature of this lipid transfer protein to provide protection against azole antifungals. Biochim. Biophys. Acta. 2014;1841:1483–1490. doi: 10.1016/j.bbalip.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Riekhof W, Wu WI, Jones JL, Nikrad M, Chan MM, Loewen CJR, Voelker DR. An assembly of proteins and lipid-binding domains regulates transport of phosphatidylserine to phosphatidyserine decarboxylase 2 in Saccharomyces cerevisiae. J. Biol. Chem. 2014;289:5809–5819. doi: 10.1074/jbc.M113.518217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Skinner HB, Alb JG, Whitters EA, Helmkamp GM, Jr., Bankaitis VA. Phospholipid transfer activity is relevant to but not sufficient for the essential function of the yeast SEC14 gene product. EMBO J. 1993;12:4775–4784. doi: 10.1002/j.1460-2075.1993.tb06166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ile KE, Kassen S, Cao C, Vihtehlic T, Shah SD, Huijbregts RPH, Alb JG, Jr., Stearns GW, Brockerhoff SE, Hyde DR, Bankaitis VA, V.A. The zebrafish class 1 phosphatidylinositol transfer protein family: PITPβ isoforms and double cone cell outer segment integrity in retina. Traffic. 2010;11:1151–1167. doi: 10.1111/j.1600-0854.2010.01085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mousley CJ, Tyeryar K, Ile KE, Schaaf G, Brost R, Boone C, Guan X, Wenk MR, Bankaitis VA. Trans-Golgi network and endosome dynamics connect ceramide homeostasis with regulation of the unfolded protein response and TOR signaling in yeast. Mol. Biol. Cell. 2008;19:4785–4803. doi: 10.1091/mbc.E08-04-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cleves AE, Novick PJ, Bankaitis VA. Mutations in the SAC1 gene suppress defects in yeast Golgi and yeast actin function. J. Cell Bio. 1989;109:2939–2950. doi: 10.1083/jcb.109.6.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cleves AE, McGee TP, Whitters EA, Champion KM, Aitken JR, Dowhan W, Goebl M, Bankaitis VA. Mutations in the CDP-choline pathway for phospholipid biosynthesis bypass the requirement for an essential phospholipid transfer protein. Cell. 1991;64:789–800. doi: 10.1016/0092-8674(91)90508-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fang M, Kearns BG, Gedvilaite A, Kagiwada S, Kearns M, Fung MKY, Bankaitis VA. Kes1p shares homology with human oxysterol binding protein and participates in a novel regulatory pathway for yeast Golgi-derived transport vesicle biogenesis. EMBO J. 1996;15:6447–6459. [PMC free article] [PubMed] [Google Scholar]
- 61.McGee TP, Skinner HB, Whitters EA, Henry SA, Bankaitis VA. A phosphatidylinositol transfer protein controls the phosphatidylcholine content of yeast Golgi membranes. J. Cell Biol. 1994;124:273–287. doi: 10.1083/jcb.124.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guo S, Stolz LE, Lemrow S, York JD. SAC1-like domains of yeast SAC1, INP52 and INP53, and human synaptojanin encode polyphosphoinositide phosphatases. J. Biol. Chem. 1999;274:12990–12995. doi: 10.1074/jbc.274.19.12990. [DOI] [PubMed] [Google Scholar]
- 63.Rivas MP, Kearns BG, Xie Z, Guo S, Sekar MC, Hosaka K, Kagiwada S, York JD, Bankaitis VA. Relationship between altered phospholipid metabolism, DAG, ‘bypass Sec14p’, and the inositol auxotrophy of yeast sac1 mutants. Mol. Biol. Cell. 1999;10:2235–2250. doi: 10.1091/mbc.10.7.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li X, Rivas MP, Fang M, Marchena J, Mehotra B, Chaudhary A, Feng L, Prestwich GD, Bankaitis VA. Analysis of oxysterol binding protein homologue Kes1p function in regulation of Sec14p-dependent protein transport from the yeast Golgi complex. J Cell Biol. 2002;157:63–77. doi: 10.1083/jcb.200201037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mousley C, Yuan P, Gaur NA, Trettin KD, Nile AH, Deminoff S, Dewar BJ, Wolpert M, Macdonald JM, Herman PK, Hinnebusch AG, Bankaitis VA. A sterol binding protein integrates endosomal lipid metabolism with TOR signaling and nitrogen sensing. Cell. 2012;148:702–715. doi: 10.1016/j.cell.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mesmin B, Bigay J, Moser von Filseck J, Lacas-Gervais S, Drin G, Antonny B. A four-step cycle driven by PI(4)P hydrolysis directs sterol/PI(4)P Exchange by the ER-Golgi tether OSBP. Cell. 2013;155:830–843. doi: 10.1016/j.cell.2013.09.056. [DOI] [PubMed] [Google Scholar]
- 67.Kearns BG, McGee TP, Mayinger P, Gedvilaite A, Phillips SE, Kagiwada S, Bankaitis VA. Essential role for diacylglycerol in protein transport from the yeast Golgi complex. Nature. 1997;387:101–105. doi: 10.1038/387101a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ile KE, Schaaf G, Bankaitis VA. Phosphatidylinositol transfer proteins and cellular nanoreactors for lipid signaling. Nature Chem. Biol. 2006;2:576–583. doi: 10.1038/nchembio835. [DOI] [PubMed] [Google Scholar]
- 69.Hay JC, Martin TFJ. Phosphatidylinositol transfer protein required for ATP-dependent priming of Ca2+-activated secretion. Nature. 1993;366:572–575. doi: 10.1038/366572a0. [DOI] [PubMed] [Google Scholar]
- 70.Ohashi M, de Vries KJ, Frank R, Snoek G, Bankaitis VA, Wirtz K, Huttner WB. A role for phosphatidylinositol transfer protein in secretory vesicle formation. Nature. 1995;377:544–547. doi: 10.1038/377544a0. [DOI] [PubMed] [Google Scholar]
- 71.Pinxteren JA, Gomperts BD, Phillips SE, Rogers D, Tatham PER, Thomas GMH. Phosphatidylinositol transfer proteins and protein kinase C make separate but non-interacting contributions to the phosphorylation state necessary for secretory competence in rat mast cells. Biochem.J. 2001;356:287–296. doi: 10.1042/0264-6021:3560287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jones SM, Alb JG, Jr., Bankaitis VA, Howell KE. A phosphatidylinositol-3-kinase and phosphatidylinositol transfer protein act synergistically in formation of constitutive transport vesicles from the trans-Golgi network. J. Biol. Chem. 1998;273:10349–10354. doi: 10.1074/jbc.273.17.10349. [DOI] [PubMed] [Google Scholar]
- 73.Simon JP, Morimoto T, Bankaitis VA, Gottlieb TA, Ivanov IE, Adesnik M, Sabatini DD. An essential role for phosphatidylinositol transfer protein in the scission of COPI-coated vesicles from the TGN. Proc. Natl. Acad. Sci. U.S.A. 1998;95:11181–11186. doi: 10.1073/pnas.95.19.11181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carvou N, Holic R, Li M, Futter C, Skippen A, Cockcroft S. Phosphatidylinositol- and phosphatidylcholine-transfer activity of PITPbeta is essential for COPI-mediated retrograde transport from the Golgi to the endoplasmic reticulum. J Cell Sci. 2010;123:1262–1273. doi: 10.1242/jcs.061986. S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Garner K, Li M, Ugwuanya N, Cockcroft S. The phosphatidylinositol transfer protein RdgBbeta binds 14-3-3 via its unstructured C-terminus, whereas its lipid-binding domain interacts with the integral membrane protein ATRAP (angiotensin II type I receptor-associated protein) Biochem J. 2011;439:97–111. doi: 10.1042/BJ20110649. [DOI] [PubMed] [Google Scholar]
- 76.Garner K, Hunt AN, Koster G, Somerharju P, Groves E, Li M, Raghu P, Holic R, Cockcroft S. Phosphatidylinositol transfer protein, cytoplasmic 1 (PITPNC1) binds and transfers phosphatidic acid. J Biol Chem. 2012;287:32263–32276. doi: 10.1074/jbc.M112.375840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Litvak V, Dahan N, Ramachandran S, Sabanay H, Lev S. Maintenance of the diacylglycerol level in the Golgi apparatus by the Nir2 protein is critical for Golgi secretory function. Nat Cell Biol. 2005;7(3):225–34. doi: 10.1038/ncb1221. [DOI] [PubMed] [Google Scholar]
- 78.Vihtelic TS, Hyde DR, O’Tousa JE. Isolation and characterization of the Drosophila retinal degeneration B (rdgB)gene. Genetics. 1991;127:761–768. doi: 10.1093/genetics/127.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Milligan SC, Alb JG, Jr., Elagina RB, Bankaitis VA, Hyde DR. The phosphatidylinositol transfer protein domain of Drosophila retinal degeneration B protein is essential for photoreceptor cell survival and recovery from light stimulation. J. Cell Biol. 1997;139:351–363. doi: 10.1083/jcb.139.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Iwata R, Oda S, Kunitomo H, Iino Y. Roles for class IIA phosphatidylinositol transfer protein in neurotransmission and behavioral plasticity at the sensory neuron synapses of Caenorhabditis elegans. Proc. Natl. Acad. Sci. U.S.A. 2011;108:7589–7594. doi: 10.1073/pnas.1016232108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim S, Kedan A, Marom M, Gavert N, Keinan O, Selitrennik M, Laufman O, Lev S. The phosphatidylinositol-transfer protein Nir2 binds phosphatidic acid and positively regulates phosphoinositide signaling. EMBO Reports. 2013;14:891–899. doi: 10.1038/embor.2013.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Larijani B, Allen-Baume V, Morgan CP, Li M, Cockcroft S. EGF regulation of PITP dynamics is blocked by inhibitors of phospholipase C and the Ras-MAP kinase pathway. Curr. Biol. 2003;13:78–84. doi: 10.1016/s0960-9822(02)01395-7. [DOI] [PubMed] [Google Scholar]
- 83.Xie Y, et al. Phosphatidylinositol transfer protein-alpha in netrin-1-induced PLC signalling and neurite outgrowth. Nat. Cell Biol. 2005;7:1124–1132. doi: 10.1038/ncb1321. [DOI] [PubMed] [Google Scholar]
- 84.Holthuis JC, Levine TP. Lipid traffic: Floppy drives and a superhighway. Nat Rev Mol Cell Biol. 2005;6:209–220. doi: 10.1038/nrm1591. [DOI] [PubMed] [Google Scholar]
- 85.Lev S. Nonvesicular lipid transfer from the endoplasmic reticulum. Cold Spring Harb Perspect Biol. 2012;4:a013300. doi: 10.1101/cshperspect.a013300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tavazoie SF, Alarcon C, Oskarsson T, Padua D, Wang QQ, Bos PD, Gerald WL, Massague J. Endogenous human microRNAs that suppress breast cancer metastasis. Nature. 2008;451:147–152. doi: 10.1038/nature06487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Guo C, Sah JF, Beard L, Willson JK, Markowitz SD, et al. The noncoding RNA, miR-126, suppresses the growth of neoplastic cells by targeting phosphatidylinositol 3-kinase signaling and is frequently lost in colon cancers. Genes Chromosomes Cancer. 2008;47:939–946. doi: 10.1002/gcc.20596. C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Feng R, Chen X, Yu Y, Su L, Yu B, Li J, Cai Q, Yan M, Liu B, Zhu Z. miR-126 functions as a tumour suppressor in human gastric cancer. Cancer Lett. 2010;298:50–63. doi: 10.1016/j.canlet.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 89.Png KJ, Halberg N, Yoshida M, Tavazoie SF. A microRNA regulon that mediates endothelial recruitment and metastasis by cancer cells. Nature. 2012;481:190–194. doi: 10.1038/nature10661. (2012) [DOI] [PubMed] [Google Scholar]
- 90.Carlisle FA, Pearson S, Steel KP, Louis MA. Pitpnm1 is expressed in hair cells during development but is not required for hearing. Neuroscience. 2013;248C:620–625. doi: 10.1016/j.neuroscience.2013.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lu C, Peng YW, Shang J, Pawlyk BS, Yu F, Li T. The mammalian retinal degeneration B2 gene is not required for photoreceptor function and survival. Neuroscience. 2001;107:35–41. doi: 10.1016/s0306-4522(01)00337-2. [DOI] [PubMed] [Google Scholar]
- 92.Yoder MD, Thomas LM, Tremblay JM, Oliver RL, Yarbrough LR, Helmkamp GM., Jr. Structure of a multifunctional protein: Mammalian phosphatidylinositol transfer protein complexed with phosphatidylcholine. J. Biol. Chem. 2001;276:9246–9252. doi: 10.1074/jbc.M010131200. [DOI] [PubMed] [Google Scholar]
- 93.Schouten A, Agianian B, Westerman J, Kroon J, Wirtz KW, Gros P. Structure of apo-phosphatidylinositol transfer protein alpha provides insight into membrane association. EMBO J. 2002;21:2117–2121. doi: 10.1093/emboj/21.9.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tilley SJ, Skippen A, Murray-Rust J, Swigart PM, Stewart A, Morgan CP, Cockcroft S, McDonald NQ. Structure-function analysis of phosphatidylinositol transfer protein alpha bound to human phosphatidylinositol. Structure. 2004;12:317–326. doi: 10.1016/j.str.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 95.Cunningham E, Ball A, Swigart P, Bankaitis VA, Cockroft S. The mammalian isoforms, PITPα and PITPβ, and yeast PITP all restore phospholipase C-mediated inositol lipid signalling in HL60 and RBL-2H3 cells. Proc. Natl. Acad. Sci. U.S.A. 1996;93:6589–6593. doi: 10.1073/pnas.93.13.6589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nile AH, Tripathi A, Yuan P, Mousley CJ, Suresh S, Wallace IM, Shah SD, Teiotico-Pohlhaus D, Temple B, Nislow C, Giaever G, Tropsha A, Davis RW, St. Onge RP, Bankaitis VA. PITPs as targets for selectively interfering with phosphoinositide signaling in cells. Nature Chemical Biology. 2014;10:76–84. doi: 10.1038/nchembio.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee AY, St.Onge RP, Proctor MJ, Wallace IM, Nile AH, Spagnuolo PA, Jitkova Y, Gronda M, Wu Y, Kim MK, Cheung-Ong K, Torres NP, Spear ED, Han MK, Schlecht U, Suresh S, Duby G, Heisler LE, Surendra A, Fung E, Urbanus ML, Gebbia M, Lissina E, Miranda M, Chiang JH, Aparicio AM, Zeghouf M, Davis RW, Cherfils J, Boutry M, Kaiser CA, Cummins CL, Trimble WS, Brown GW, Schimmer AD, Bankaitis VA, Nislow C, Bader GD, Giaever G. Mapping the cellular response to small molecules using chemogenomic fitness signatures. Science. 2014;344:208–211. doi: 10.1126/science.1250217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kampmann M, Bassik MC, Weissman JS. Integrated platform for genome-wide screening and construction of high-density genetic interaction maps in mammalian cells. Proc. Natl. Acad. Sci. U.S.A. 2013;110:E2317–26. doi: 10.1073/pnas.1307002110. [DOI] [PMC free article] [PubMed] [Google Scholar]