Abstract
Background
Intensity-modulated radiation therapy (IMRT) is a technologically advanced and resource-intensive method of delivering radiation therapy (RT) used to minimize toxicity for patients with head and neck cancers (HNC). Dependence on feeding tubes is a significant marker of toxicity of RT. The goal of this analysis was to compare the placement and duration of feeding tube use for patients with HNC from 1999-2011.
Methods
The cohort, demographics, and cancer-related variables were determined using the linked Surveillance, Epidemiology, and End Results (SEER)-Medicare database and analyzed regarding treatment details using claims data.
Results
A total of 2993 patients were identified. With a median follow-up of 47 months, 54.4% of patients had a feeding tube placed. The median duration from feeding tube placement to removal was 277 days. On zero-inflated negative binomial regression, patients treated with IMRT and 3DRT (non-IMRT) had similar rates of feeding tube placement (odds ratio (OR) 1.10; p=.35); however, patients treated with 3DRT had the feeding tube in place 1.18 times longer than those treated with IMRT (p=.03). The difference was only seen amongst patients treated with definitive radiation; patients treated with surgery and adjuvant radiation had no statistically significant difference in placement or duration.
Conclusions
Patients with HNC treated with definitive IMRT had significantly shorter duration of feeding tubes in place than those treated with 3DRT. These data suggest that there may be significant quality of life benefits to IMRT with respect to long-term swallowing function for patients.
Keywords: IMRT, Head and neck cancer, Feeding tube, Population-based data, Dysphagia
INTRODUCTION
Radiation therapy (RT) is a key component in the treatment of a majority of cancers of the head and neck. Although head and neck cancers (HNC) associated with smoking and alcohol are in the decline, the incidence of oropharyngeal carcinoma is increasing.1, 2 This trend is largely explained by the epidemic of human papillomavirus (HPV) infection, which is expected to cause even greater increases in the rates of HNC in the coming decades.3, 4 These patients are younger, with greater life expectancies in which to experience long-term toxicity. Patients treated for HNC with RT often have significant acute toxicity, including mucositis and dysphagia, which can lead to malnutrition and dehydration, as well as a risk for chronic toxicity, such as esophageal stricture, dysphagia, and aspiration.
During the course of RT, patients who develop significant side effects and are at risk for malnutrition are typically referred for placement of a feeding tube. The feeding tube provides a portal for nutrition and medication administration that bypasses the areas affected by the local symptoms, including dysphagia, odynophagia, dysgeusia, or severe xerostomia; it is anticipated that a feeding tube can prevent malnutrition, dehydration, and treatment breaks. There is significant controversy in the optimal timing of feeding tube placement during treatment, with some studies favoring prophylactic placement 5-12 and others only as needed.13-15 The argument against prophylactic placement arises in concerns about long-term feeding tube dependence in patients who are given upfront placement.14 Regardless of the philosophy of timing of placement, prior studies suggest that the majority of patients being treated with RT for locoregionally advanced-stage HNC (60% to 70%) will require a feeding tube at some point during their course of therapy.10, 16-18
Although the optimal strategy varies by center and practitioner, the impact of modern radiation techniques, namely intensity-modulated radiation therapy (IMRT), on the need for feeding tube placement has not been rigorously evaluated. IMRT was developed with the goal of sparing normal tissues, namely the parotid glands, while delivering curative dose to the tumor targets. IMRT has been widely adopted in the treatment of HNC.19, 20 Previous studies have demonstrated improved dose-volume parameters for key normal structures, improved quality of life, better xerostomia scores, and improved cause-specific survival for patients treated with IMRT.21-24 Although patients treated with IMRT have been included in prior studies examining feeding tube utilization, most reports have not addressed the relationship between IMRT and the placement and duration of feeding tube use. In theory, the use of IMRT could have benefit with regard to swallowing due to reducing high doses to the pharyngeal constrictors (superior, middle, and inferior), cricopharyngeus muscle, esophageal inlet, and glottic structures (supraglottis and glottis) while still providing adequate doses to the target structures. 25, 26
The goal of this study was to analyze the rates and duration of feeding tube use in patients treated with both definitive and adjuvant radiation for HNC using a large population-based registry and assess the impact of radiation technique (IMRT vs. 3DRT).
METHODS
Data Source
The SEER-Medicare linked database was queried to identify a cohort of HNC patients and measure the outcomes of interest. The linked SEER program, a National Cancer Institute-supported database, includes tumor registries in 17 areas: Greater California, San Francisco-Oakland, Los Angeles, San Jose, Connecticut, Detroit, Seattle-Puget Sound, Atlanta-Rural Georgia, Greater Georgia, Iowa, Louisiana, New Mexico, Utah, Hawaii, Kentucky, Louisiana, and New Jersey, comprising more than 1.5 million cancer cases in the United States.27 These data are linked to Medicare claims files by encrypted patient identifiers. Medicare provides payments for hospital, physician, and outpatient medical services for 97% of the US citizens who are ≥ 65 years of age.28, 29 Diagnoses and procedures for each patient were identified using the Patient Entitlement and Diagnosis Summary File (PEDSF), as well as all available Medicare claims. All data were de-identified and the University of Texas MD Anderson Cancer Center’s institutional review board exempted this study.
Cohort Identification
We queried the database for patients with HNC diagnosed between 1999 and 2011, as defined by SEER and International Classification of Diseases 9th revision (ICD-9) codes, treated with RT, including both definitive and adjuvant RT, with claims data through 2013. The primary site and ICD-O-3 histology codes SEER codes of interest were: cancers of the lip (20010), tongue (20020), floor of mouth (20040), gum and other mouth (20050), nasopharynx (20060), tonsil (20070), oropharynx (20080), hypopharynx (20090), other oral cavity and pharynx (20100), nose, nasal cavity, and middle ear (22010), and larynx (22020). For patients with subsites that could represent multiple categories, we used local treatment approach to determine anatomical location. For example, patients with tongue primaries who received surgery and post-operative RT as primary treatment were classified as oral cavity, while those treated with radiation therapy definitively were classified as oropharynx. For inclusion, the tumor needed to be pathologically confirmed, not diagnosed at death or autopsy, with a stage indicated and no evidence of distant disease, and treated with IMRT or 3D conventional radiation therapy; patients treated with 2D techniques were excluded. Patients with Stage I and II larynx cancer were excluded because IMRT was rarely used in this group; patients with salivary gland carcinomas were also excluded due to low rates of feeding tube use in this group. Patients were only included if they began definitive RT within 4 months or adjuvant RT within 6 months of diagnosis. Table 1 shows the algorithm for development of this cohort.
Table 1.
Algorithm for cohort identification from the SEER-Medicare database.
| Criteria | Number |
|---|---|
| Diagnosed with head and neck cancer as only or first cancer | 95,264 |
| Diagnosed 1999-2011 | 74,658 |
| Pathologically confirmed | 72,925 |
| Not diagnosed by autopsy or death certificate | 72,905 |
| In Medicare for age only | 56,617 |
| Age at diagnosis ≥66 and ≤80 years | 29,986 |
| Enrolled in Medicare Part A and B 1 year prior and post diagnosis with no HMO | 15,049 |
| No missing stage; exclude Stage I/II larynx | 9,949 |
| Received either definitive or adjuvant radiation | 5,418 |
| Feeding tube users have both placement and removal dates after diagnosis, excluding patients with both dates prior to RT | 4,699 |
| Received either IMRT or 3DRT | 4,570 |
| SEER tumor stage localized or regional disease only | 3,625 |
| Exclude salivary gland | 2,993 |
Outcome Identification
The primary outcomes analyzed in this study were the placement, use, and duration of a feeding tube. The date of placement of a feeding tube (CPT codes 43246, 43750, 44500, 44372, 74355, 74350, 49440, 49441 and ICD-9 code 43.11) or the start of claims for feeding tube supplies (HCPCS codes B4034-B4036, B4081-B4088, B4100, B4102-B4104, B4149-B4162) were used as the date of initiation of a feeding tube, and was subsequently correlated to the timing of the start of RT. In general, codes for removal of a feeding tube are not frequently used (ICD-9 97.51 and 97.52), since this is commonly performed during a routine office visit. Hence, the date of last claims for feeding tube supplies was used as a surrogate for removal and as the end date for feeding tube use. For patients who had a feeding tube, dates of both placement and removal were required for inclusion. The duration was calculated relative to the insertion date of the feeding tube. In addition, patients who had feeding tubes removed before the start of RT (n = 39) were excluded, as were those who had a second cancer diagnosis following their head and neck cancer (hence, excluding recurrent disease). Dobhoff tubes or nasogastric tubes, were not captured in this analysis.
Definition of Explanatory Variables
To ascertain the impact of potential demographic and clinic variables, we analyzed the impact of age at diagnosis, ethnicity, Charlson comorbidity index 30-33, tumor site, SEER tumor stage, type of RT, use of surgery and chemotherapy, and year of treatment on the placement and duration of feeding tubes.
To analyze the impact of RT technique, specific radiation treatment characteristics were identified from Medicare claims data through the ICD-9 procedure codes and CPT codes for RT planning and delivery. Patients were categorized as having received IMRT if there were any IMRT delivery or planning CPT codes (77418, 77301, GO174-IMRT, GO178-IMRT). For patients who did not have an IMRT planning or delivery code as part of their RT claims, they were categorized as having received 3-dimensional radiation therapy (3DRT) techniques if they had non-IMRT delivery codes as well as an accompanying planning code (77261, 77262, 77263). Those patients who did not have a planning code were categorized as having received 2-dimensional (2D) radiation therapy and excluded from the analysis (n = 129).
In addition to radiation therapy delivery, we identified treatment with surgery and chemotherapy using the claims codes for receipt of these modalities as part of primary management for a patient’s disease at the time of diagnosis.
Statistical Analysis
Statistical analyses were conducted using SAS statistical software (version 9.3) (SAS Institute; Cary, NC). The unadjusted associations of each potential explanatory variable with the outcomes of feeding tube use were assessed using the Chi-square test, and the duration of feeding tube use was assess using the Kruskal-Wallis test. We conducted multivariable modeling using the zero-inflated negative binomial regression analysis. This model estimates the likelihood of NOT having a feeding tube (zero-inflated binomial regression model) and the expected days of feeding tube (zero-inflated negative binomial regression model) use simultaneously.
RESULTS
Study cohort characteristics
We identified 2993 patients that met our criteria for inclusion. The median follow-up time for all patients was 47 months; the median follow-up time for all living patients was 67 months. Table 2 summarizes the demographic and treatment characteristics of the entire cohort, as well as the subsets of patients treated with surgery and adjuvant radiation vs. definitive radiation, respectively. Of the entire cohort, 1574 patients (52.6%) were treated with IMRT (median follow-up = 45 months), and 1419 (47.4%) were treated with 3DRT (median follow-up = 51 months). Of the entire cohort, 1110 patients (37.1%) were treated with surgery and adjuvant radiation, and 1883 patients (62.9%) were treated with definitive radiation.
Table 2.
Clinical factors and demographics for the entire cohort.
| Full N=2993 | Adjuvant N=1110 | Definitive N=1883 | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| N | % | N | % | N | % | |
|
|
|
|
||||
| Age at Diagnosis | ||||||
| 66-69 | 1,062 | 35.5 | 388 | 35.0 | 674 | 35.8 |
| 70-74 | 1,148 | 38.4 | 432 | 38.9 | 716 | 38.0 |
| 75-80 | 783 | 26.2 | 290 | 26.1 | 493 | 26.2 |
| Gender | ||||||
| Male | 2,003 | 66.9 | 687 | 61.9 | 1,316 | 69.9 |
| Female | 990 | 33.1 | 423 | 38.1 | 567 | 30.1 |
| Ethnicity | ||||||
| White non-Hispanic | 2,484 | 83.0 | 932 | 84.0 | 1,552 | 82.4 |
| Black non-Hispanic | 211 | 7.1 | 65 | 5.9 | 146 | 7.8 |
| Hispanic | 141 | 4.7 | 55 | 5.0 | 86 | 4.6 |
| Other | 157 | 5.3 | 58 | 5.2 | 99 | 5.3 |
| Charlson comorbidity Index | ||||||
| 0 | 1,670 | 55.8 | 657 | 59.2 | 1,013 | 53.8 |
| 1 | 707 | 23.6 | 257 | 23.2 | 450 | 23.9 |
| 2+ | 465 | 15.5 | 142 | 12.8 | 323 | 17.2 |
| Unknown | 151 | 5.1 | 54 | 4.9 | 97 | 5.2 |
| Tumor site | ||||||
| Oral cavity | 1,475 | 49.3 | 908 | 81.8 | 567 | 30.1 |
| Nasopharynx | 164 | 5.5 | 19 | 1.7 | 145 | 7.7 |
| Oropharynx | 733 | 24.5 | 13 | 1.2 | 720 | 38.2 |
| Hypopharynx | 317 | 10.6 | 69 | 6.2 | 248 | 13.2 |
| Nose, Nasal Cavity, and Middle Ear | 268 | 9.0 | 83 | 7.5 | 185 | 9.8 |
| Larynx | 36 | 1.2 | 18 | 1.6 | 18 | 1.0 |
| Type of radiation | ||||||
| 3D-RT | 1,419 | 47.4 | 520 | 46.9 | 899 | 47.7 |
| IMRT | 1,574 | 52.6 | 590 | 53.2 | 984 | 52.3 |
| Chemotherapy | ||||||
| No chemotherapy | 2,139 | 71.5 | 910 | 82.0 | 1,229 | 65.3 |
| Induction | 283 | 9.5 | 55 | 5.0 | 228 | 12.1 |
| Concurrent | 571 | 19.1 | 145 | 13.1 | 426 | 22.6 |
| Tumor stage | ||||||
| Localized | 759 | 25.4 | 309 | 27.8 | 450 | 23.9 |
| Regional | 2,234 | 74.6 | 801 | 72.2 | 1,433 | 76.1 |
Impact of clinical characteristics on feeding tube use for the entire cohort
Of the 2993 patients, a total of 1629 (54.4%) had a feeding tube placed after diagnosis of their HNC. There were several variables that correlated with higher rates of placement of a feeding tube on univariate analysis, including age (age 70-74 > age 66-69 > age 75-80; p=.0311), gender (male > female; p=.0032), ethnicity (Black non-Hispanic; p=.003), tumor site (oropharynx > hypopharynx > larynx > nasopharynx > oral cavity > nose/nasal cavity/middle ear; p<.0001), RT regimen (definitive > adjuvant; p<.0001), use of chemotherapy (concurrent > induction > none; p<.0001), SEER tumor stage (regional > localized; p<.0001), and use of IMRT (IMRT > 3DRT; p<.0001). The impact of clinical variables on feeding tube use is shown in Table 3. Very few feeding tubes were placed in patients with sinonasal cancers.
Table 3.
Impact of clinical factors on feeding tube use and duration
| Full N=2993 | Adjuvant N=1110 | Definitive N=1883 | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||||||||||
| Feeding Tube Use | Feeding Tube Duration | Feeding Tube Use | Feeding Tube Duration | Feeding Tube Use | Feeding Tube Duration | |||||||||||||||||||
|
| ||||||||||||||||||||||||
| No (N=1364) |
Yes (N=1629) |
Chi- square |
Tube Users only (N=1629) |
Kruskal- Wallis |
No (N=587) |
Yes (N=523) |
Chi- square |
Tube Users only (N=523) |
Kruskal- Wallis |
No (N=777) |
Yes (N=1106) |
Chi- square |
Tube Users only (N=1106) |
Kruskal- Wallis |
||||||||||
|
| ||||||||||||||||||||||||
| N | % | N | % | p-value | Mean | Median | p-value | N | % | N | % p-value | Mean | Median | p-value | N | % | N | % | p-value | Mean | Median | p-value | ||
| Age at Diagnosis | 0.031 | 0.079 | 0.573 | 0.931 | 0.034 | 0.023 | ||||||||||||||||||
| 66-69 | 498 | 46.89 | 564 | 53.11 | 549.2 | 227.5 | 209 | 53.87 | 179 | 46.13 | 552.7 | 238.0 | 289 | 42.88 | 385 | 57.12 | 547.6 | 223.0 | ||||||
| 70-74 | 489 | 42.60 | 659 | 57.40 | 576.6 | 283.0 | 220 | 50.93 | 212 | 49.07 | 569.6 | 255.0 | 269 | 37.57 | 447 | 62.43 | 579.9 | 300.0 | ||||||
| 75-80 | 377 | 48.15 | 406 | 51.85 | 610.8 | 337.5 | 158 | 54.48 | 132 | 45.52 | 532.0 | 261.5 | 219 | 44.42 | 274 | 55.58 | 648.7 | 361.0 | ||||||
| Gender | 0.003 | 0.198 | 0.682 | 0.528 | 0.004 | 0.162 | ||||||||||||||||||
| Male | 875 | 43.68 | 1,128 | 56.32 | 567.5 | 259.0 | 360 | 52.40 | 327 | 47.60 | 546.0 | 238.0 | 515 | 39.13 | 801 | 60.87 | 576.2 | 273.0 | ||||||
| Female | 489 | 49.39 | 501 | 50.61 | 594.0 | 303.0 | 227 | 53.66 | 196 | 46.34 | 568.1 | 268.5 | 262 | 46.21 | 305 | 53.79 | 610.6 | 331.0 | ||||||
| Ethnicity | 0.003 | 0.026 | 0.269 | 0.426 | 0.006 | 0.098 | ||||||||||||||||||
| White non-Hispanic | 1,150 | 46.30 | 1,334 | 53.70 | 572.8 | 250.5 | 504 | 54.08 | 428 | 45.92 | 548.1 | 240.0 | 646 | 41.62 | 906 | 58.38 | 584.6 | 257.5 | ||||||
| Black non-Hispanic | 74 | 35.07 | 137 | 64.93 | 578.7 | 364.0 | 28 | 43.08 | 37 | 56.92 | 607.0 | 398.0 | 46 | 31.51 | 100 | 68.49 | 568.2 | 364.0 | ||||||
| Hispanic | 58 | 41.13 | 83 | 58.87 | 654.2 | 309.0 | 26 | 47.27 | 29 | 52.73 | 686.6 | 328.0 | 32 | 37.21 | 54 | 62.79 | 636.9 | 308.0 | ||||||
| Other | 82 | 52.23 | 75 | 47.77 | 532.4 | 320.0 | 29 | 50.00 | 29 | 50.00 | 447.1 | 194.0 | 53 | 53.54 | 46 | 46.46 | 586.2 | 370.5 | ||||||
| Charlson comorbidity Index | 0.934 | 0.787 | 0.882 | 0.964 | 0.977 | 0.612 | ||||||||||||||||||
| 0 | 768 | 45.99 | 902 | 54.01 | 576.4 | 268.5 | 349 | 53.12 | 308 | 46.88 | 558.5 | 249.5 | 419 | 41.36 | 594 | 58.64 | 585.7 | 278.0 | ||||||
| 1 | 321 | 45.40 | 386 | 54.60 | 578.2 | 263.5 | 139 | 54.09 | 118 | 45.91 | 573.5 | 238.5 | 182 | 40.44 | 268 | 59.56 | 580.2 | 270.0 | ||||||
| 2+ | 206 | 44.30 | 259 | 55.70 | 545.8 | 317.0 | 71 | 50.00 | 71 | 50.00 | 597.6 | 240.0 | 135 | 41.80 | 188 | 58.20 | 526.2 | 333.5 | ||||||
| Unknown | 69 | 45.70 | 82 | 54.30 | 649.3 | 302.0 | 28 | 51.85 | 26 | 48.15 | 299.2 | 246.5 | 41 | 42.27 | 56 | 57.73 | 811.8 | 335.5 | ||||||
| Tumor site | <.0001 | 0.020 | <.0001 | 0.742 | <.0001 | 0.144 | ||||||||||||||||||
| Oral cavity | 724 | 49.08 | 751 | 50.92 | 543.3 | 230.0 | 467 | 51.43 | 441 | 48.57 | 549.8 | 241.0 | 257 | 45.33 | 310 | 54.67 | 534.0 | 226.5 | ||||||
| Nasopharynx | 75 | 45.73 | 89 | 54.27 | 597.7 | 303.0 | 8 | 42.11 | 11 | 57.89 | 559.5 | 366.0 | 67 | 46.21 | 78 | 53.79 | 603.0 | 296.0 | ||||||
| Oropharynx | 209 | 28.51 | 524 | 71.49 | 594.7 | 310.0 | 5 | 38.46 | 8 | 61.54 | 540.6 | 194.0 | 204 | 28.33 | 516 | 71.67 | 595.6 | 311.0 | ||||||
| Hypopharynx | 108 | 34.07 | 209 | 65.93 | 623.4 | 334.0 | 25 | 36.23 | 44 | 63.77 | 643.8 | 300.5 | 83 | 33.47 | 165 | 66.53 | 617.9 | 335.0 | ||||||
| Nose, Nasal Cavity, Middle Ear | 234 | 87.31 | 34 | 12.69 | 624.3 | 230.0 | 72 | 86.75 | 11 | 13.25 | 272.8 | 243.0 | 162 | 87.57 | 23 | 12.43 | 792.4 | 217.0 | ||||||
| Larynx | 14 | 38.89 | 22 | 61.11 | 606.7 | 370.5 | 10 | 55.56 | 8 | 44.44 | 706.5 | 321.5 | 4 | 22.22 | 14 | 77.78 | 549.7 | 370.5 | ||||||
| Chemotherapy | <.0001 | 0.679 | <.0001 | 0.477 | <.0001 | 0.705 | ||||||||||||||||||
| No chemotherapy | 1,115 | 52.13 | 1,024 | 47.87 | 592.0 | 271.0 | 512 | 56.26 | 398 | 43.74 | 567.4 | 240.0 | 603 | 49.06 | 626 | 50.94 | 607.6 | 300.0 | ||||||
| Induction | 96 | 33.92 | 187 | 66.08 | 532.7 | 285.0 | 26 | 47.27 | 29 | 52.73 | 486.7 | 247.0 | 70 | 30.70 | 158 | 69.30 | 541.2 | 300.0 | ||||||
| Concurrent | 153 | 26.80 | 418 | 73.20 | 554.8 | 282.0 | 49 | 33.79 | 96 | 66.21 | 520.4 | 305.5 | 104 | 24.41 | 322 | 75.59 | 565.0 | 272.5 | ||||||
| Type of radiation | <.0001 | <.0001 | 0.546 | 0.303 | <.0001 | <.0001 | ||||||||||||||||||
| IMRT | 663 | 42.12 | 911 | 57.88 | 473.1 | 234.0 | 307 | 52.03 | 283 | 47.97 | 481.0 | 222.0 | 356 | 36.18 | 628 | 63.82 | 469.6 | 236.0 | ||||||
| 3DRT | 701 | 49.40 | 718 | 50.60 | 705.6 | 334.5 | 280 | 53.85 | 240 | 46.15 | 640.7 | 281.5 | 421 | 46.83 | 478 | 53.17 | 738.2 | 357.5 | ||||||
| Regimen | <.0001 | 0.054 | - | - | - | - | ||||||||||||||||||
| Adjuvant | 587 | 52.88 | 523 | 47.12 | 554.3 | 245.0 | 587 | 52.88 | 523 | 47.12 | 554.3 | 245.0 | - | - | - | - | ||||||||
| Definitive | 777 | 41.26 | 1,106 | 58.74 | 585.7 | 292.5 | - | - | - | - | 777 | 41.26 | 1,106 | 58.74 | 585.7 | 292.5 | ||||||||
| Tumor stage | <.0001 | 0.160 | <.0001 | 0.066 | <.0001 | 0.759 | ||||||||||||||||||
| Localized | 461 | 60.74 | 298 | 39.26 | 546.7 | 246.0 | 206 | 66.67 | 103 | 33.33 | 456.7 | 207.0 | 255 | 56.67 | 195 | 43.33 | 594.2 | 284.0 | ||||||
| Regional | 903 | 40.42 | 1,331 | 59.58 | 582.1 | 281.0 | 381 | 47.57 | 420 | 52.43 | 578.2 | 259.0 | 522 | 36.43 | 911 | 63.57 | 583.9 | 293.0 | ||||||
On multivariable analysis with zero-inflated binomial regression analysis, which models the odds that a feeding tube was NOT placed and included the impact of year of diagnosis, patients treated with IMRT had similar rates of feeding tube placement as those patients treated with 3DRT (Table 4) (OR = 1.10; p=0.35). Patients of Black non-Hispanic ethnicity, those from 70-74 years of age, with cancers of certain sites and regional disease, and those treated with concurrent chemotherapy were more likely to have feeding tubes placed (Table 4).
Table 4.
Zero-inflated negative binomial (ZINB) regression analysis.
| Full N=2993 | Adjuvant N=1110 | Definitive N=1883 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||||||||
| Zero Model | NB Model | Zero Model | NB Model | Zero Model | Zero Model | |||||||||||||
| Est. | Exp(Est.) | P- Value |
Est. | Exp(Est.) | P- Value |
Est. | Exp(Est.) | P- Value |
Est. | Exp(Est.) | P- Value |
Est. | Exp(Est.) | P- Value |
Est. | Exp(Est.) | P-Value | |
|
|
|
|
||||||||||||||||
| Intercept Ethnicity | 0.83 | 2.29 | <.01 | 6.28 | 532.51 | <.01 | 0.75 | 2.13 | 0.01 | 6.46 | 640.72 | <.01 | 0.72 | 2.05 | <.01 | 6.12 | 456.96 | <.01 |
| White non-Hispanic | ||||||||||||||||||
| Black non-Hispanic | -0.48 | 0.62 | <.01 | 0.00 | 1.00 | 0.96 | -0.47 | 0.62 | 0.09 | 0.13 | 1.14 | 0.54 | -0.48 | 0.62 | 0.02 | -0.03 | 0.97 | 0.79 |
| Hispanic | -0.22 | 0.80 | 0.25 | 0.12 | 1.12 | 0.37 | -0.26 | 0.77 | 0.38 | 0.22 | 1.24 | 0.35 | -0.22 | 0.80 | 0.38 | 0.00 | 1.00 | 1.00 |
| Other | 0.15 | 1.16 | 0.41 | -0.10 | 0.91 | 0.47 | -0.27 | 0.77 | 0.37 | -0.19 | 0.83 | 0.43 | 0.38 | 1.46 | 0.10 | 0.03 | 1.03 | 0.85 |
| Age at Diagnosis | ||||||||||||||||||
| 66-69 | ||||||||||||||||||
| 70-74 | -0.19 | 0.82 | 0.04 | 0.05 | 1.06 | 0.40 | -0.19 | 0.83 | 0.21 | 0.04 | 1.04 | 0.72 | -0.19 | 0.83 | 0.12 | 0.08 | 1.08 | 0.32 |
| 75-80 | -0.10 | 0.91 | 0.34 | 0.08 | 1.08 | 0.28 | -0.15 | 0.86 | 0.38 | -0.06 | 0.94 | 0.66 | -0.06 | 0.94 | 0.65 | 0.17 | 1.18 | 0.05 |
| Tumor site | ||||||||||||||||||
| Oral cavity | ||||||||||||||||||
| Hypopharynx | -0.54 | 0.58 | <.01 | 0.06 | 1.06 | 0.52 | -0.55 | 0.58 | 0.04 | 0.09 | 1.09 | 0.66 | -0.53 | 0.59 | <.01 | 0.09 | 1.10 | 0.40 |
| Larynx | -0.18 | 0.83 | 0.61 | 0.18 | 1.20 | 0.46 | 0.36 | 1.43 | 0.47 | 0.16 | 1.17 | 0.70 | -0.83 | 0.44 | 0.16 | 0.26 | 1.30 | 0.38 |
| Nasopharynx | 0.01 | 1.01 | 0.95 | 0.13 | 1.14 | 0.34 | -0.26 | 0.77 | 0.61 | 0.22 | 1.25 | 0.55 | -0.01 | 0.99 | 0.95 | 0.15 | 1.16 | 0.31 |
| Nose, Nasal Cavity, and Middle Ear | 1.96 | 7.07 | <.01 | 0.06 | 1.06 | 0.76 | 1.93 | 6.88 | <.01 | -0.63 | 0.53 | 0.09 | 1.98 | 7.28 | <.01 | 0.35 | 1.41 | 0.15 |
| Oropharynx | -0.60 | 0.55 | <.01 | 0.09 | 1.10 | 0.24 | -0.48 | 0.62 | 0.43 | -0.22 | 0.80 | 0.61 | -0.60 | 0.55 | <.01 | 0.13 | 1.14 | 0.11 |
| Tumor stage | ||||||||||||||||||
| Localized Regional | -0.70 | 0.50 | <.01 | 0.10 | 1.10 | 0.17 | -0.80 | 0.45 | <.01 | 0.26 | 1.29 | 0.05 | -0.63 | 0.53 | <.01 | 0.02 | 1.02 | 0.84 |
| Type of radiation | ||||||||||||||||||
| IMRT | ||||||||||||||||||
| 3DRT | 0.10 | 1.10 | 0.35 | 0.17 | 1.18 | 0.03 | 0.08 | 1.09 | 0.63 | -0.11 | 0.90 | 0.47 | 0.10 | 1.11 | 0.47 | 0.30 | 1.35 | <.01 |
| Regimen | ||||||||||||||||||
| Adjuvant RT | ||||||||||||||||||
| Definitive RT | -0.16 | 0.85 | 0.10 | 0.00 | 1.00 | 1.00 | ||||||||||||
| Chemotherapy | ||||||||||||||||||
| No chemotherapy | ||||||||||||||||||
| Concurrent | -0.79 | 0.45 | <.01 | 0.05 | 1.05 | 0.51 | -0.88 | 0.41 | <.01 | 0.07 | 1.08 | 0.60 | -0.75 | 0.47 | <.01 | 0.04 | 1.04 | 0.59 |
| Induction | -0.36 | 0.69 | 0.01 | 0.02 | 1.02 | 0.83 | -0.35 | 0.70 | 0.25 | -0.01 | 0.99 | 0.97 | -0.34 | 0.71 | 0.04 | 0.02 | 1.02 | 0.84 |
| Year | ||||||||||||||||||
| 1999-2001 | ||||||||||||||||||
| 2002-2005 | -0.13 | 0.88 | 0.29 | -0.02 | 0.98 | 0.81 | 0.03 | 1.04 | 0.86 | -0.13 | 0.88 | 0.44 | -0.25 | 0.78 | 0.11 | 0.07 | 1.07 | 0.55 |
| 2006-2011 | -0.07 | 0.93 | 0.61 | -0.40 | 0.67 | <.01 | 0.20 | 1.22 | 0.37 | -0.65 | 0.52 | <.01 | -0.27 | 0.77 | 0.15 | -0.26 | 0.77 | 0.04 |
Zero model estimates the likelihood of no feeding tube use. Exponential value of the estimates (Exp(Est.)) is odds ratio of not using a feeding tube.
Negative binomial (NB) model estimates expected counted days with a feeding tube. Exponential value of the estimates (Exp(Est.)) is the ratio of expected days compared to the reference
Impact of clinical characteristics on feeding tube duration for the entire cohort
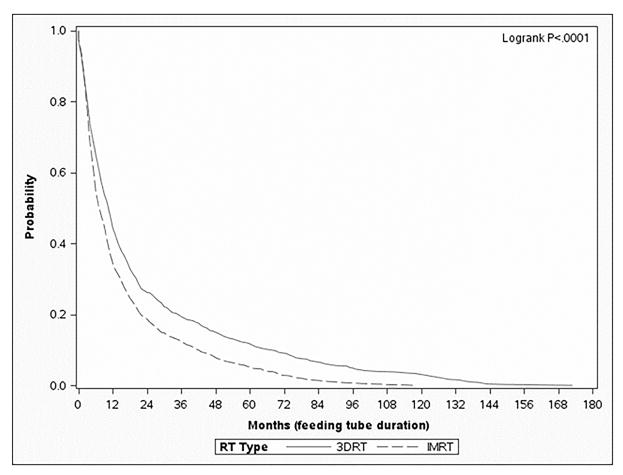
Of the 1629 patients that had a feeding tube in the entire cohort, the median duration with a feeding tube in place was 277 days, with an interquartile range of 123-640 days. Despite similar tendencies in placement of feeding tubes between IMRT and 3DRT on multivariable analysis, patients treated with IMRT had a statistically significant decrease in the median duration as compared to those treated with 3DRT (234 vs. 335 days, respectively, p<0.0001). On zero-inflated negative binomial analysis, a multivariable method of modeling the duration of feeding tube placement in days, patients treated with 3DRT had feeding tubes in place 1.18 times longer than those treated with IMRT (p=.03) (Table 4). Looking at the “survival” of the feeding tubes in the 1629 patients who received them, using a Kaplan-Meier analysis, patients treated with 3DRT consistently had increased rates of feeding tubes in place compared to those treated with IMRT (Figure 1A).
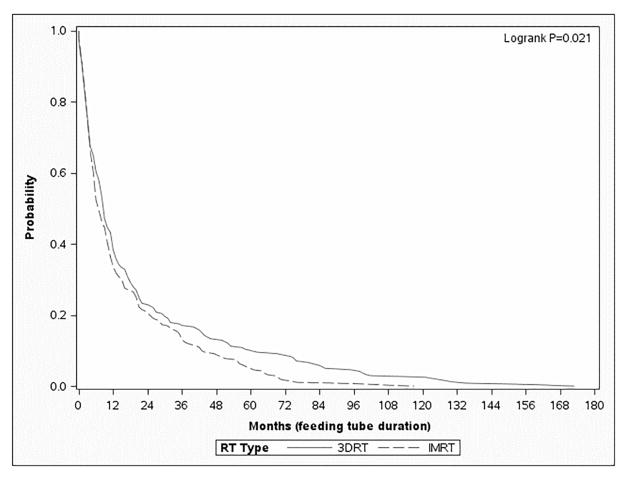
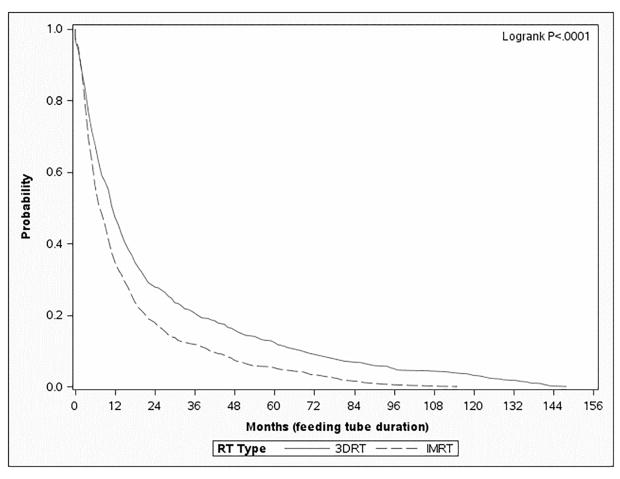
Figure 1.
Kaplan-Meier survival curve depicting “survival” of a feeding tube (in months) from the time of placement to the time of removal (IMRT – dashed, 3DRT – solid). Figure 1A depicts the entire cohort (n=2883), Figure 1B depicts the patients treated with surgery and adjuvant radiation (n=1110), and Figure 1C depicts the patients treated with definitive radiation (n=1883).
Impact of surgery on feeding tube placement and duration of use
Because patients treated with surgery and adjuvant radiation may have different feeding tube needs compared to those treated with definitive radiation therapy, we evaluated the role of surgery on both endpoints (placement of feeding tubes and the duration of use). To do this, the entire cohort was split into two groups: patients treated with primary surgery and adjuvant radiation (n=1110 patients) and patients treated with definitive radiation (n=1883 patients).
Patients treated with surgery and adjuvant radiation
Of the 1110 patients treated with surgery and adjuvant radiation, a total of 523 (47.1%) had a feeding tube placed after diagnosis of their HNC. The impact of clinical variables on feeding tube use is shown in Table 3.
On multivariable analysis with zero-inflated binomial regression analysis, which models the odds that a feeding tube was NOT placed and included the impact of year of diagnosis, patients treated with IMRT had similar rates of feeding tube placement as those patients treated with 3DRT (Table 4) (OR = 1.09; p=0.63) (Table 4). Furthermore, patients treated with IMRT had no statistically significant difference in the median duration of having a feeding tube in place compared to 3DRT (222 vs. 282 days, respectively). On zero-inflated negative binomial analysis, a multivariable method of modeling the duration of feeding tube placement in days, patients treated surgery and 3DRT had no statistically significant difference in feeding tube duration than those treated with surgery and IMRT (OR = 0.90; p=0.47) (Table 4). The presence of feeding tubes in place for patients treated with surgery and adjuvant radiation is shown in Figure 1B.
Patients treated with definitive radiation
Of the 1883 patients treated with definitive radiation, a total of 1106 (58.7%) had a feeding tube placed after diagnosis of their HNC. The impact of clinical variables on feeding tube use is shown in Table 3.
On multivariable analysis with zero-inflated binomial regression analysis, which models the odds that a feeding tube was NOT placed and included the impact of year of diagnosis, patients treated with IMRT had similar rates of feeding tube placement as those patients treated with 3DRT (Table 4) (OR = 1.11; p=0.47) (Table 4). On zero-inflated negative binomial analysis, a multivariable method of modeling the duration of feeding tube placement in days, patients treated with 3DRT had feeding tubes in place 1.35 times longer than those treated with IMRT (p<.01) (Table 4). The presence of feeding tubes in place for patients treated with definitive radiation is shown in Figure 1C.
DISCUSSION
In this population-based analysis, the data suggest that patients treated with definitive IMRT have similar rates of feeding tube placement, but a shorter duration of use, compared to those treated with 3DRT. In fact, even 12 and 24 months following RT, a greater proportion of those patients treated with non-IMRT have not recovered to the extent that a feeding tube can be removed (Figure 1); hence, the short-term need for a feeding tube translates into long-term debility. These data suggest that IMRT has a significant benefit to patients in return to functional swallowing for patients treated with definitive radiation therapy; this finding is not demonstrated in patients treated with surgery followed by adjuvant radiation, in which case there are no statistically significant differences between IMRT and 3DRT.
Despite multiple published reports documenting feeding tube rates in patients with HNC, there has been little analysis of the impact of IMRT up to now. IMRT has been used for HNC since 1999, and the rates of use have grown rapidly.19, 20 Three prior population-based analyses of feeding tube rates for patients treated for HNC have failed to explicitly discuss the impact of IMRT. Locher and colleagues published two analyses of the SEER-Medicare database, with patients treated from 2000 – 2005.34, 35 In the first analysis, the authors note a 35.1% rate of gastrostomy tube placement, with 16.9% placed prophylactically;35 the authors did not assess the impact of IMRT or the duration of feeding tube use. Overall, the rates published in this analysis are significantly less than those seen in our current analysis; this is likely due to higher rates of feeding tube placement in later years, which may reflect increasing use of prophylactic placement. The impact of prophylactic placement is beyond the scope of this discussion; however, there is a notable relationship between time and increasing use of both IMRT and prophylactic placement. In a second publication, Locher and colleagues analyze factors that contribute to feeding tube placement in patients treated from 2000 – 2005; they also did not assess the impact of IMRT.34 Of note, they found that patients with larynx and oropharynx cancer had increased rates of prophylactic tube placement; given the significant increase in the rates of oropharynx cancer over the last decade, this finding is also consistent with the increased rates observed in the current analysis. The third population-based analysis evaluated the outcomes of patients treated in the British Columbia Cancer Agency with prophylactic versus reactive feeding tube placement.36 The authors identified that 41% of patients treated at Center A (which generally used a reactive approach) and 47% of patients treated at Center B (which generally used a prophylactic approach) were treated with IMRT; there were no differences in overall survival or weight loss between the two centers. The impact of IMRT was not specifically discussed with regard to outcomes.
Several retrospective series have discussed the impact of radiation treatment technique on feeding tube placement and duration of use. Bhayani and colleagues reviewed 474 patients with oropharyngeal cancer; 93% were treated with IMRT.16 Overall, 62% of patients received feeding tubes. At 1 year, only 9% of patients remained dependent on enteral feeding. Strom and colleagues reported on 297 patients with oropharyngeal cancer treated with concurrent chemoradiation with IMRT; the majority of patients received prophylactic feeding tubes.37 Of the 128 patients (43.1%) that did not have a prophylactic feeding tube, 11.7% of patients required a reactive feeding tube with a median duration of 3.3 months (100.4 days). Naik and colleagues reviewed 147 patients with oropharynx cancer (130 HPV-positive) to understand the impact of HPV itself on swallowing outcomes.38 They noted that patients with HPV-positive cancers had a statistically significant decrease in feeding tube dependence (4% vs. 18%; p=.02). Hence, they hypothesize that improvements in swallowing outcomes may not just be due to improved techniques, but due to changing epidemiology as well.
Similar retrospective studies have investigated swallowing function after RT for hypopharynx cancers. Bhayani and colleagues reviewed the placement of feeding tubes in a series of 43 patients with hypopharyngeal cancer;17 69% were treated with IMRT. Overall, 69.8% of patients received feeding tubes. At 1 year, 25.6% of patients remained dependent on enteral feeding; this dropped to 3.4% at 2 years. The median duration was 23.8 weeks (167 days). Mok and colleagues evaluated 181 patients with hypopharynx cancer (90 3D and 91 IMRT).39 Prophylactic feeding tubes were recommended for all patients who were treated with chemoradiation and accelerated fractionation; more patients with IMRT had feeding tubes placed due to prophylactic placement. At 3 years, the IMRT group had higher locoregional control but similar rates of overall survival and feeding tube dependence (19% vs. 18%). This similar long-term feeding tube dependence may be due by the difference in placement practices.
Overall, this population-level analysis provides insight into the actual benefits of IMRT, one of our most widely used and resource-intensive technologies in radiation oncology, for patients treated with definitive radiation therapy. IMRT was largely adopted due to the ability to spare the parotid glands and improve xerostomia; this has been shown in randomized trials. However, the relative impact of IMRT on swallowing function has not truly been assessed. The ability of IMRT to selectively allocate dose, and spare normal tissues, may allow for improved swallowing through protection of the pharyngeal constrictors, glottic structures, and esophageal inlet or indirectly through improved salivary function that protects the upper aerodigestive mucosa. In this study, we demonstrate that patients treated with IMRT typically have similar rates of feeding tube placement, but those patients treated with definitive IMRT who did receive feeding tubes were more likely to recover swallowing function sufficient to stop using their tube more quickly than those treated with 3DRT. In fact, even 2 years following treatment, those patients treated with 3DRT have not yet returned to the same level of functionality as those treated with IMRT. This is perhaps the most compelling argument supporting IMRT use; those patients have improved swallowing rehabilitation and reduced need for long-term feeding tube rates than those treated with 3DRT. Given the relative good health and younger age of an ever increasing number of patients with HPV-associated HNC, the reduction of toxicity and optimization of function in survivorship is paramount. These data suggest that utilization of IMRT helps achieve these goals. While IMRT has become standard of care in the past decade, its unintended consequences to other normal tissues (due to increased low dose bath) have not been evaluated. These data further support that IMRT has benefits in terms of normal tissue toxicity, above and beyond xerostomia.
Interestingly, for patients treated with surgery and adjuvant radiation, the favorable impact of IMRT on feeding tube duration was not observed. Our analysis demonstrates no statistically significant difference in the rates of placement or the duration of use for these patients treated with upfront surgery followed by radiation therapy. This suggests that the potential benefits of IMRT may be abrogated in patients treated with surgery, at least with regard to feeding tube use. This may be due to the need to cover larger radiation portals in these cases, encompassing the entire operated region, with less opportunity to spare critical central structures.
Other notable findings of this analysis include the impact of chemotherapy and ethnicity on feeding tube rates and duration of use. Patients treated with concurrent chemotherapy have increased rates of feeding tube placement, but no statistically significant change in the duration of use, compared to those treated with no chemotherapy. In addition, patients of Black non-Hispanic ethnicity also have increased rates of placement, but no increased durations, compared to other ethnic groups. Both of these findings may reflect underlying clinical practice patterns and assessment of risk; further evaluation on the impact of these trends is warranted.
This study does share the limitations of observational studies using claims data. Claims data are not collected for scientific purposes; hence, there may be issues with completion and accuracy. The data does not reflect patient and physician choices and recommendations. In addition, the linked SEER-Medicare database only includes patients who are 65 years old or older; hence, patients who are younger and those with managed care are not included. The SEER-Medicare linked database also does not include radiation target, radiation dose, or HPV-status, all of which may significantly impact outcomes.
In addition to these general limitations, there are additional ones specific to this analysis. We have used claims data to understand placement and removal of the feeding tubes based on procedure codes; nasogastric tubes, which are placed in the office, have not been captured. Similarly, ongoing feeding tube use is indicated by claims for feeding tube supplies; hence patients who die with feeding tubes in place may be misallocated. In addition, rates of chronic feeding tube dependence underestimate the true burden of dysphagia after head and neck radiation as they do not account for those individuals who elect to eat, albeit risking pneumonia and secondary complications, despite chronic dysphagia, stricture, or aspiration. Finally, previous studies have shown improved cause-specific survival in patients treated with IMRT compared to 3DRT; 24 as a result, patients treated with 3DRT may have had early recurrences and never had the tube removed. If this is true, the lesser duration of feeding tubes observed with IMRT may reflect fewer recurrences (not improved functional recovery). In this analysis, we excluded patients with a subsequent diagnosis of head and neck cancer, hence attempting to eliminate this confounder; however, it is established that claims data are limited in the detection of recurrence. 40
In summary, this analysis suggests that patients treated with definitive IMRT had a statistically shorter duration of feeding tube use than those treated with 3DRT. IMRT has been approved and widely accepted as a technique that reduces xerostomia and improves quality of life for patients with head and neck cancer. Our findings suggest that it may also have an impact on long-term swallowing function after treatment.
Acknowledgments
We acknowledge that this study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the SEER Program tumor registries in the creation of the SEER-Medicare database.
Research Support: This research was funded in part by the Duncan Family Institute. Drs. Giordano and Guadagnolo are supported by CPRIT RP140020.
Footnotes
Author Contributions: Drs. Beadle and Guadagnolo are responsible for the overall content of the manuscript, all analyses and concepts, and drafted it in its entirety. Drs. Liao and Giordano contributed methodology, resources, data curation, data analysis, and manuscript review. Drs. Garden, Hutcheson, and Lai contributed specific concepts, investigation, and manuscript review. Each author fulfilled contributions as listed in the author contributions page, and the manuscript could not exist without each one.
Previous Presentations: This work was presented, in part, at the 2014 Annual Meeting of ASTRO, San Francisco, CA.
Disclaimers: There are no conflicts of interest for any author concerning this work.
References
- 1.Simard EP, Ward EM, Siegel R, Jemal A. Cancers with increasing incidence trends in the United States 1999 through 2008. CA Cancer J Clin. 2012 doi: 10.3322/caac.20141. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 3.Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26:612–619. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 4.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baschnagel AM, Yadav S, Marina O, et al. Toxicities and costs of placing prophylactic and reactive percutaneous gastrostomy tubes in patients with locally advanced head and neck cancers treated with chemoradiotherapy. Head Neck. 2014;36:1155–1161. doi: 10.1002/hed.23426. [DOI] [PubMed] [Google Scholar]
- 6.Chen AM, Li BQ, Lau DH, et al. Evaluating the role of prophylactic gastrostomy tube placement prior to definitive chemoradiotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys. 2010;78:1026–1032. doi: 10.1016/j.ijrobp.2009.09.036. [DOI] [PubMed] [Google Scholar]
- 7.Rutter CE, Yovino S, Taylor R, et al. Impact of early percutaneous endoscopic gastrostomy tube placement on nutritional status and hospitalization in patients with head and neck cancer receiving definitive chemoradiation therapy. Head Neck. 2011;33:1441–1447. doi: 10.1002/hed.21624. [DOI] [PubMed] [Google Scholar]
- 8.Ames JA, Karnell LH, Gupta AK, et al. Outcomes after the use of gastrostomy tubes in patients whose head and neck cancer was managed with radiation therapy. Head Neck. 2011;33:638–644. doi: 10.1002/hed.21506. [DOI] [PubMed] [Google Scholar]
- 9.Lee JH, Machtay M, Unger LD, et al. Prophylactic gastrostomy tubes in patients undergoing intensive irradiation for cancer of the head and neck. Arch Otolaryngol Head Neck Surg. 1998;124:871–875. doi: 10.1001/archotol.124.8.871. [DOI] [PubMed] [Google Scholar]
- 10.Morton RP, Crowder VL, Mawdsley R, Ong E, Izzard M. Elective gastrostomy, nutritional status and quality of life in advanced head and neck cancer patients receiving chemoradiotherapy. ANZ J Surg. 2009;79:713–718. doi: 10.1111/j.1445-2197.2009.05056.x. [DOI] [PubMed] [Google Scholar]
- 11.Silander E, Nyman J, Bove M, Johansson L, Larsson S, Hammerlid E. Impact of prophylactic percutaneous endoscopic gastrostomy on malnutrition and quality of life in patients with head and neck cancer: a randomized study. Head Neck. 2012;34:1–9. doi: 10.1002/hed.21700. [DOI] [PubMed] [Google Scholar]
- 12.Salas S, Baumstarck-Barrau K, Alfonsi M, et al. Impact of the prophylactic gastrostomy for unresectable squamous cell head and neck carcinomas treated with radio-chemotherapy on quality of life: Prospective randomized trial. Radiother Oncol. 2009;93:503–509. doi: 10.1016/j.radonc.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 13.Clavel S, Fortin B, Despres P, et al. Enteral feeding during chemoradiotherapy for advanced head-and-neck cancer: a single-institution experience using a reactive approach. Int J Radiat Oncol Biol Phys. 2011;79:763–769. doi: 10.1016/j.ijrobp.2009.12.032. [DOI] [PubMed] [Google Scholar]
- 14.Chen AM, Li BQ, Jennelle RL, et al. Late esophageal toxicity after radiation therapy for head and neck cancer. Head Neck. 2010;32:178–183. doi: 10.1002/hed.21164. [DOI] [PubMed] [Google Scholar]
- 15.Gillespie MB, Brodsky MB, Day TA, Lee FS, Martin-Harris B. Swallowing-related quality of life after head and neck cancer treatment. Laryngoscope. 2004;114:1362–1367. doi: 10.1097/00005537-200408000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Bhayani MK, Hutcheson KA, Barringer DA, et al. Gastrostomy tube placement in patients with oropharyngeal carcinoma treated with radiotherapy or chemoradiotherapy: factors affecting placement and dependence. Head Neck. 2013;35:1634–1640. doi: 10.1002/hed.23200. [DOI] [PubMed] [Google Scholar]
- 17.Bhayani MK, Hutcheson KA, Barringer DA, Roberts DB, Lewin JS, Lai SY. Gastrostomy tube placement in patients with hypopharyngeal cancer treated with radiotherapy or chemoradiotherapy: factors affecting placement and dependence. Head Neck. 2013;35:1641–1646. doi: 10.1002/hed.23199. [DOI] [PubMed] [Google Scholar]
- 18.Mekhail TM, Adelstein DJ, Rybicki LA, Larto MA, Saxton JP, Lavertu P. Enteral nutrition during the treatment of head and neck carcinoma: is a percutaneous endoscopic gastrostomy tube preferable to a nasogastric tube? Cancer. 2001;91:1785–1790. [PubMed] [Google Scholar]
- 19.Guadagnolo BA, Liu CC, Cormier JN, Du XL. Evaluation of trends in the use of intensity-modulated radiotherapy for head and neck cancer from 2000 through 2005: socioeconomic disparity and geographic variation in a large population-based cohort. Cancer. 2010;116:3505–3512. doi: 10.1002/cncr.25205. [DOI] [PubMed] [Google Scholar]
- 20.Sher DJ, Neville BA, Chen AB, Schrag D. Predictors of IMRT and conformal radiotherapy use in head and neck squamous cell carcinoma: a SEER-Medicare analysis. Int J Radiat Oncol Biol Phys. 2011;81:e197–206. doi: 10.1016/j.ijrobp.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 21.Graff P, Lapeyre M, Desandes E, et al. Impact of intensity-modulated radiotherapy on health-related quality of life for head and neck cancer patients: matched-pair comparison with conventional radiotherapy. Int J Radiat Oncol Biol Phys. 2007;67:1309–1317. doi: 10.1016/j.ijrobp.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 22.Tribius S, Bergelt C. Intensity-modulated radiotherapy versus conventional and 3D conformal radiotherapy in patients with head and neck cancer: is there a worthwhile quality of life gain? Cancer Treat Rev. 2011;37:511–519. doi: 10.1016/j.ctrv.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Nutting CM, Morden JP, Harrington KJ, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncology. 2011;12:127–136. doi: 10.1016/S1470-2045(10)70290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beadle BM, Liao KP, Elting LS, et al. Improved survival using intensity-modulated radiation therapy in head and neck cancers: a SEER-Medicare analysis. Cancer. 2014;120:702–710. doi: 10.1002/cncr.28372. [DOI] [PubMed] [Google Scholar]
- 25.Peponi E, Glanzmann C, Willi B, Huber G, Studer G. Dysphagia in head and neck cancer patients following intensity modulated radiotherapy (IMRT) Radiat Oncol. 2011;6:1. doi: 10.1186/1748-717X-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christianen ME, van der Schaaf A, van der Laan HP, et al. Swallowing sparing intensity modulated radiotherapy (SW-IMRT) in head and neck cancer: Clinical validation according to the model-based approach. Radiother Oncol. 2016;118:298–303. doi: 10.1016/j.radonc.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 27.Surveillance, Epidemiology, and End Results (SEER) Program Research Data (1973-2008) 2011 ( www.seer.cancer.gov)
- 28.Potosky AL, Riley GF, Lubitz JD, Mentnech RM, Kessler LG. Potential for cancer related health services research using a linked Medicare-tumor registry database. Med Care. 1993;31:732–748. [PubMed] [Google Scholar]
- 29.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40:IV-3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 30.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 31.Klabunde CN, Harlan LC, Warren JL. Data sources for measuring comorbidity: a comparison of hospital records and medicare claims for cancer patients. Med Care. 2006;44:921–928. doi: 10.1097/01.mlr.0000223480.52713.b9. [DOI] [PubMed] [Google Scholar]
- 32.Klabunde CN, Legler JM, Warren JL, Baldwin LM, Schrag D. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Ann Epidemiol. 2007;17:584–590. doi: 10.1016/j.annepidem.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 33.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 34.Locher JL, Bonner JA, Carroll WR, et al. Patterns of prophylactic gastrostomy tube placement in head and neck cancer patients: a consideration of the significance of social support and practice variation. Laryngoscope. 2013;123:1918–1925. doi: 10.1002/lary.24022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Locher JL, Bonner JA, Carroll WR, et al. Gastrostomy tube placement and use in patients with head and neck cancer. Head Neck. 2012;34:422–428. doi: 10.1002/hed.21753. [DOI] [PubMed] [Google Scholar]
- 36.Olson R, Karam I, Wilson G, Bowman A, Lee C, Wong F. Population-based comparison of two feeding tube approaches for head and neck cancer patients receiving concurrent systemic-radiation therapy: is a prophylactic feeding tube approach harmful or helpful? Support Care Cancer. 2013;21:3433–3439. doi: 10.1007/s00520-013-1936-y. [DOI] [PubMed] [Google Scholar]
- 37.Strom T, Trotti AM, Kish J, et al. Risk factors for percutaneous endoscopic gastrostomy tube placement during chemoradiotherapy for oropharyngeal cancer. JAMA Otolaryngol Head Neck Surg. 2013;139:1242–1246. doi: 10.1001/jamaoto.2013.5193. [DOI] [PubMed] [Google Scholar]
- 38.Naik M, Ward MC, Bledsoe TJ, et al. It is not just IMRT: Human papillomavirus related oropharynx squamous cell carcinoma is associated with better swallowing outcomes after definitive chemoradiotherapy. Oral Oncol. 2015;51:800–804. doi: 10.1016/j.oraloncology.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 39.Mok G, Gauthier I, Jiang H, et al. Outcomes of intensity-modulated radiotherapy versus conventional radiotherapy for hypopharyngeal cancer. Head Neck. 2015;37:655–661. doi: 10.1002/hed.23649. [DOI] [PubMed] [Google Scholar]
- 40.Warren JL, Mariotto A, Melbert D, et al. Sensitivity of Medicare Claims to Identify Cancer Recurrence in Elderly Colorectal and Breast Cancer Patients. Med Care. 2016;54:e47–54. doi: 10.1097/MLR.0000000000000058. [DOI] [PMC free article] [PubMed] [Google Scholar]