Abstract
Background
Oral immunotherapy (OIT) is an effective experimental food allergy treatment that is limited by treatment withdrawal and the frequent reversibility of desensitization if interrupted. Newly-diagnosed preschool children may have clinical and immunological characteristics more amenable to treatment.
Objective
To test the safety, effectiveness, and feasibility of early OIT (E-OIT) in the treatment of peanut allergy.
Methods
We enrolled 40 children aged 9–36 months with suspected or known peanut allergy. Qualifying subjects reacted to peanut during an entry food challenge and were block-randomized 1:1 to receive E-OIT at goal maintenance doses of 300 or 3000 mg/day in a double-blinded fashion. The primary endpoint, sustained unresponsiveness at four weeks after stopping E-OIT (4-SU), was assessed by DBPCFC either upon achieving four pre-specified criteria, or after three maintenance years. Peanut-specific immune responses were serially analyzed. Outcomes were compared to 154 matched standard-care controls.
Results
Of 40 consented subjects, three (7.5%) did not qualify. Overall, 29/37 (78%) in the intent-to-treat analysis achieved 4-SU (300 mg arm, 17/20 [85%]; 3000 mg, 12/17 [71%], p=0.43) over a median of 29 months. Per-protocol, the overall proportion achieving 4-SU was 29/32 (91%). Peanut-specific IgE levels significantly declined in E-OIT-treated children, who were 19 times more likely to successfully consume dietary peanut than matched standard-care controls, in whom peanut-specific IgEs significantly increased (RR 19.42 [95%CI 8.7 – 43.7], p<0.001). Allergic side effects during E-OIT were common but all were mild-moderate.
Conclusion
At both doses tested, E-OIT had an acceptable safety profile and was highly successful in rapidly suppressing allergic immune responses and achieving safe dietary reintroduction.
Keywords: Oral immunotherapy, Desensitization, Sustained unresponsiveness, Early intervention, Peanut allergy, Randomized clinical trial
CAPSULE SUMMARY
This randomized clinical trial generates critical new evidence supporting the safety and effectiveness of peanut OIT in newly-diagnosed young children, demonstrating superior outcomes after treatment as compared to matched standard-care controls.
INTRODUCTION
Over the last 20 years, peanut allergy has become a global public health problem affecting now 1.5–3% of children (1, 2). The lack of therapeutic options is a substantial unmet need. In previous randomized studies of children grade-school age and older, oral immunotherapy (OIT) has shown promise as an immunomodulatory treatment that can provide a margin of safety protecting against a potentially life-threatening accidental exposure (3–6). Yet because little evidence for cure exists, even OIT successes must continue vigilance with strict dietary restrictions and self-injectable epinephrine. Further, up to 20% cannot tolerate the treatment and there is substantial potential for relapse if treatment is interrupted (7). However, we previously showed that long-term treatment response was significantly associated with lower peanut-specific IgE (psIgE) levels at study entry. These subjects achieved “sustained unresponsiveness (SU)” to peanut after five years of treatment with goal maintenance doses of 4 gm/day, permitting them to stop OIT and safely introduce peanut-containing foods into the diet (8). This result suggests that the strength of allergic sensitization at baseline may largely influence durable OIT treatment success.
While it is now known that the production of food-specific IgE frequently begins in infancy (9–11), T cell receptor affinity is weak (12) and GATA-3 expression unstable (9). IgE production is further driven by progressive intensification of Th2 cytokine expression over the first two years of life, and is strongly correlated with the clinical expression of allergic disease (13–15). In the approximately 80% of affected patients for whom peanut allergy persists as a lifelong disease, psIgE production has been shown to increase over the first five years of life (15, 16). Taken together, these data suggest that the allergic program requires time to fully differentiate, and in the food allergy context, does so in the absence of oral exposure. We postulated that targeting newly diagnosed young peanut-allergic children would provide the best opportunity to enhance the clinical effectiveness of OIT as an immunomodulatory and disease-modifying treatment by interrupting allergic priming prior to its full maturation. We termed this approach early intervention OIT (E-OIT).
To test whether E-OIT would safely enhance favorable long-term outcomes and explore an effective dose range, we designed a randomized, double-blinded clinical trial of low- and high-dose peanut E-OIT among recently diagnosed peanut-allergic children aged 9–36 months and compared outcomes to a control group of untreated peanut-allergic patients. Our primary hypothesis was that ≥ 70% of participants receiving low-dose E-OIT would achieve SU to 5 grams of peanut protein during a double-blinded, placebo-controlled food challenge (DBPCFC) performed four weeks after discontinuing OIT.
METHODS
Study Design
This single-center clinical trial was appropriately registered (17) and carried out in accordance with the principles of the Declaration of Helsinki and the local ethics committee. Following written informed parental consent, eligible participants underwent a qualifying baseline open oral food challenge (OFC) to 4 grams of peanut protein (Supplemental Methods). Those who demonstrated clear objective evidence of an IgE-mediated allergic reaction were block-randomized 1:1 to receive low- (target maintenance dose, 300 mg/day peanut protein) or high-dose (3000 mg/day peanut protein) E-OIT. All randomized subjects represent the intent-to-treat (ITT) population. After an initial day escalation, all subjects in both groups up-dosed to a 3000 mg/day target maintenance dose in a double-blinded fashion before undergoing up to two exit DBPCFCs. Study product for the low-dose group consisted of 300 mg peanut flour plus 2700 mg of placebo filler. Further details about the investigational product and dosing schedule can be found in the Supplemental Methods section. All participants, site investigators, and study coordinators were blinded to treatment assignment. Efficacy, safety and immunological data were all analyzed in blinded fashion.
The primary endpoint was the proportion of ITT subjects achieving sustained unresponsiveness at four weeks (4-SU) after discontinuing E-OIT, defined as the ability to consume 5 grams of peanut protein without dose-limiting symptoms during an exit DBPCFC followed by one additional serving size feeding of peanut fed openly. As discussed further in Supplemental Methods, we pre-specified an analysis of a matched standard-care control group to compare the frequency of peanut consumption in the diet following OIT or standard care (i.e., allergen avoidance). Key secondary endpoints included the proportion of subjects achieving desensitization, the frequency of treatment-related AEs in each group, and longitudinal immunologic changes.
Study Population
We recruited children aged 9–36 months inclusive who were peanut-allergic or peanut-sensitized. Peanut-allergic children were enrolled within six months of a convincing first allergic reaction following oral exposure to a peanut-containing food, and had a psIgE of > 0.35 kUA/L and/or a peanut skin prick test (SPT) wheal diameter of ≥ 3 mm above the negative control. Children were also eligible with no known history of peanut ingestion and psIgE of ≥ 5 kUA/L. Exclusion criteria included: life-threatening peanut anaphylaxis (e.g., involving hypoxia, hypotension, or neurological compromise); wheat/oat allergy; severe atopic dermatitis according to the clinical judgment of the investigator (e.g., requiring systemic therapy); asthma requiring more than medium-dose inhaled corticosteroids as per the National Heart, Lung, and Blood Institute asthma guidelines; and participation in an interventional food allergy study within one year.
Standard care control group
A control cohort (N=154), matched on inclusion and exclusion criteria, was retrospectively collected from a pediatric allergy clinic database at Johns Hopkins (Supplemental Methods). These children were treated consistent with standard of care NIAID clinical guidelines (18) and the routine practice patterns of the attending physician(s). For example, not all diagnoses were routinely confirmed with OFC when the history was suggestive, and open oral food challenges were offered according to the judgment of the attending physician when he/she deemed natural tolerance likely to have occurred. Key clinical and immunologic variables were extracted from case histories by research assistants and were verified by the same pediatric allergist (C.K.), who was unaware of the trial results. IgEs at Johns Hopkins were measured by ImmunoCAP™ (Thermo Fisher).
Food challenge assessments
OFC techniques are described further in Supplemental Methods. Endpoints were assessed with two 5 gram exit DBPCFCs, the first at the end of treatment to confirm desensitization. If successful, then OIT was stopped and the DBPCFC repeated after four weeks of peanut abstinence to test for 4-SU. The protocol allowed for endpoint assessment upon achievement of pre-specified benchmarks (at least 12 months in the maintenance phase; psIgE ≤ 15 kUA/L; SPT ≤ 8 mm; and no severe peanut-related symptoms in the previous 6 months). All subjects not meeting these benchmarks were assessed for 4-SU once they completed a 36 month maintenance phase.
Mechanistic studies
SPT, PsIgE, total IgE, and psIgG4 were performed as previously described (19, 20).
Analysis plan
We computed averages, variances, frequencies, proportions, and graphical displays for all variables and examined them to ensure parametric distributional assumptions were met. Nonparametric test statistics were used as appropriate. Baseline demographics and categorical peanut consumption outcomes were compared between E-OIT and controls using Fisher’s exact test. Analyses were performed with GraphPad Prism 6 for Mac (La Jolla, CA) or Stata/SE 13.1 (College Station, TX). To achieve approximate normality and variance stabilization for longitudinal immune analyses, psIgE and psIgG4 were log-transformed, while for SPT raw data were employed. Models were fit separately for each group in R (www.r-project.org) for each outcome with functions of time using generalized estimating equations (21). Linear and quadratic, and cubic models in time were considered, with the best fitting model selected for each group for each outcome selected using QIC. All hypothesis tests were two-sided, with p<0.05 considered significant.
RESULTS
Subject enrollment and disposition
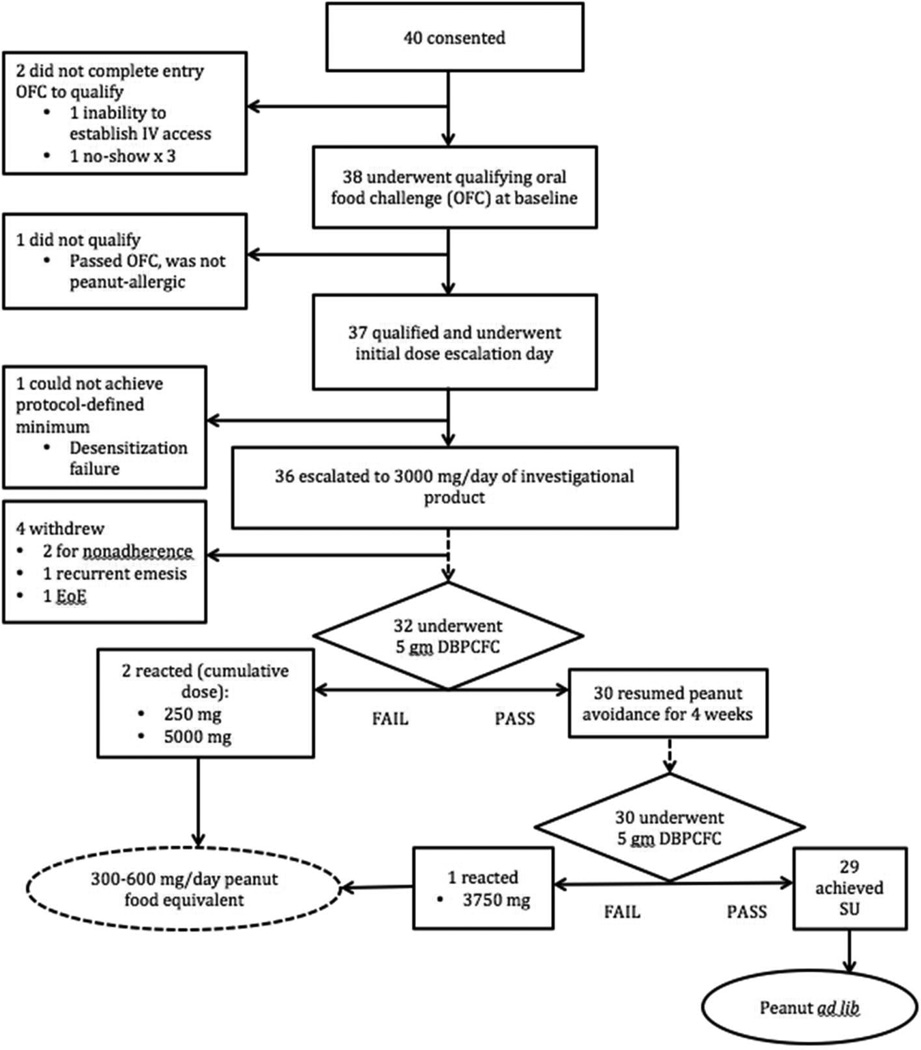
Based on our pre-study power calculations (Supplemental Methods) to compare E-OIT against standard-care controls, we consented and enrolled 40 participants (31 clinically allergic and 9 sensitized/never exposed). Study progression is shown in Figure 1. Baseline demographics, and those of the control group, are shown in Table 1. The study population was predominantly Caucasian and atopic, with median age at enrollment of 28.5 months (Interquartile range (IQR) 22–35). The ITT population consisted of 37 participants who reacted during the entry OFC at a median (IQR) of 21 (21–171) cumulative mg of peanut protein (Figure E1). Three (8%) of the 37 subjects were withdrawn from the study for treatment-related adverse events. Two additional subjects withdrew for nonadherence, leaving 32 participants with evaluable outcomes with respect to the primary endpoint. Four of the five withdrawals were from the high-dose arm.
Figure 1.
Progression of subjects through the study. DBPCFC, double-blinded, placebo-controlled food challenge; EoE, eosinophilic esophagitis; OFC, oral food challenge; SU, sustained unresponsiveness.
Table 1.
Baseline Demographics by Treatment Arm
| All subjects N (%) or median (IQR) |
High Dose N (%) or median (IQR) |
Low Dose N (%) or median (IQR) |
Controls N (%) or median (IQR) |
|
|---|---|---|---|---|
| Total | 37 randomized | 17 | 20 | 154 |
| Females | 12 (32%) | 4 (24%) | 8 (40%) | 47 (31%) |
|
Age (mo) at Starting OIT or observation (controls) |
||||
| 9–12 | 9 (24%)† | 4 (24%) | 5 (25%) | 15 (10%) |
| 13–24 | 17 (46%) | 9 (53%) | 8 (40%) | 67 (44%) |
| 25–36 | 11 (30%) | 4 (24%) | 7 (35%) | 72 (47%) |
| Race | ||||
| White | 33 (89%)†† | 16 (94%) | 17 (85%) | 92 (60%) |
| Black | 3 (8%) | 0 | 3 (15%) | 24 (16%) |
| Other | 1 (3%) | 1 (6%) | 0 | 38 (25%)* |
| History of: | ||||
| Asthma/recurrent wheeze | 10 (27%)** | 6 (35%) | 4 (20%) | 20 (13%) |
| Atopic Dermatitis | 26 (70%) | 14 (82%) | 12 (60%) | 130 (84%) |
| Allergic Rhinitis | 8 (22%) | 4 (24%) | 4 (20%) | 51 (33%) |
| Peanut SPT (mm) | 11.5 (8, 16.5) | 12.5 (8, 17.5) | 10.5 (8, 15.3) | n/a |
| Peanut IgE (kUA/L) | 14.4 (3.4, 48.6) | 12.3 (3.2, 61.5) | 22.4 (5.4, 43.4) | 21.9 (6.9, 73) |
|
Cumulative eliciting dose, entry OFC (mg) |
21 (21, 171) | 21 (21, 221) | 21 (9.8, 152) | n/a |
| Peanut IgG4 (mcg/mL) | 0.5 (0.2, 1.0) | 0.5 (0.2, 1.0) | 0.5 (0.1, 1.1) | n/a |
p=0.03 vs. controls
p=0.0005 vs. controls
p=0.001 vs. all E-OIT
p=0.04 vs. controls
Sustained unresponsiveness was achieved at high rates with both low- and high-dose OIT in young peanut-allergic children
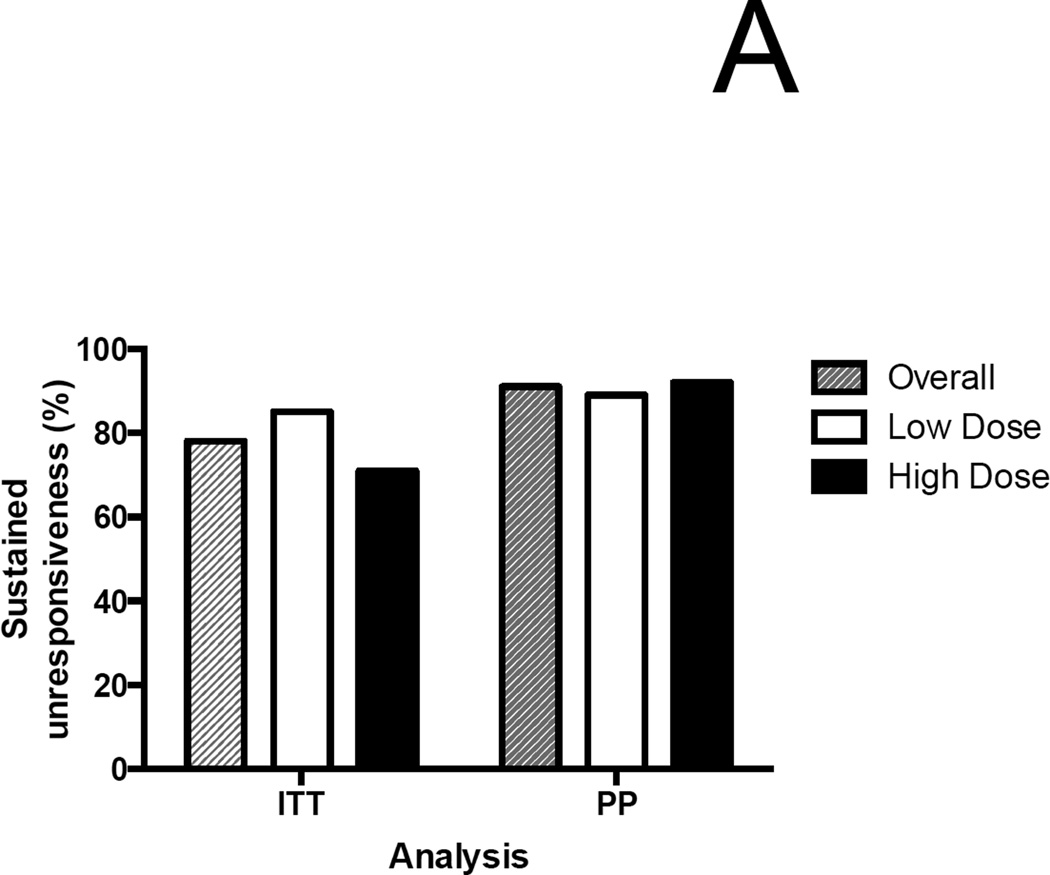
In the ITT analysis, 30/37 (81%) overall were desensitized at the end of treatment [low-dose, 17/20 (85%); high-dose, 13/17 (76%)]. 29/37 (78%) achieved 4-SU [low-dose, 17/20 (85%); high-dose, 12/17 (71%) (p=0.43 by Fisher’s exact test; difference in proportions 0.14 [95% CI: −0.12, 0.40])]. (Figure 2A). In the per-protocol (PP) analysis, the rate of desensitization was 30/32 (94%), with 29/32 (91%) achieving 4-SU. [low-dose, 17/19 (89%); high-dose 12/13 (92%)]
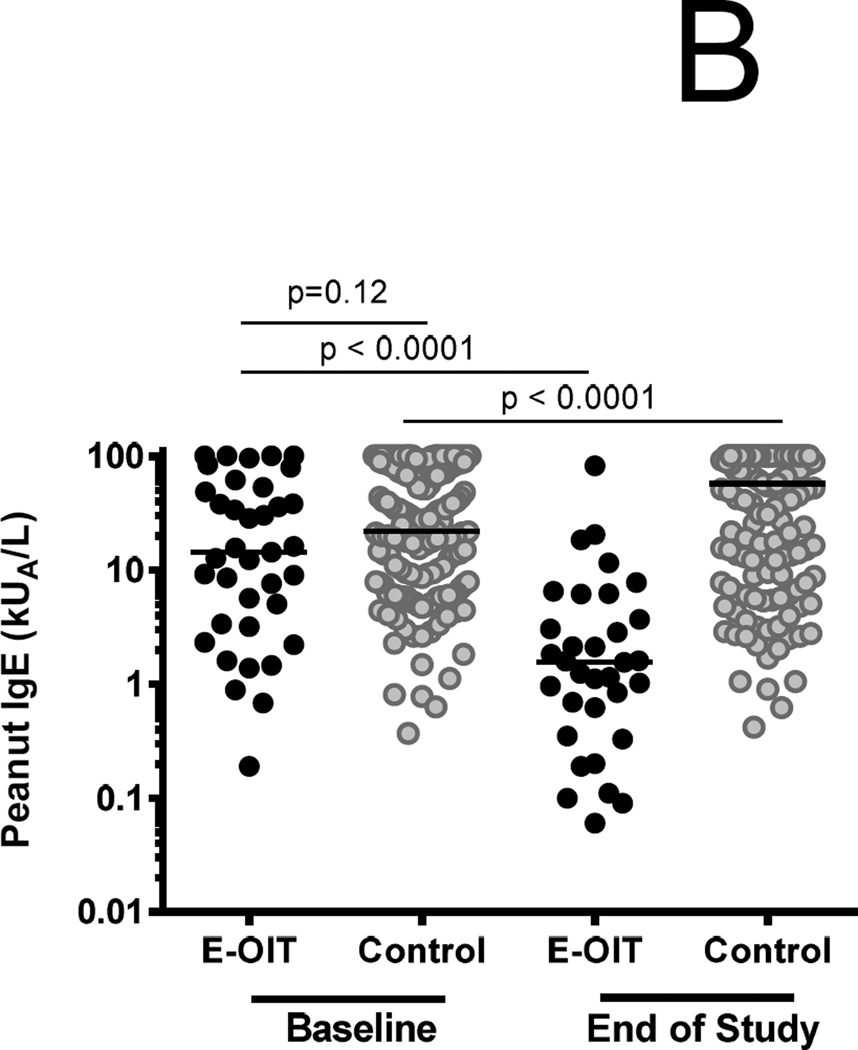
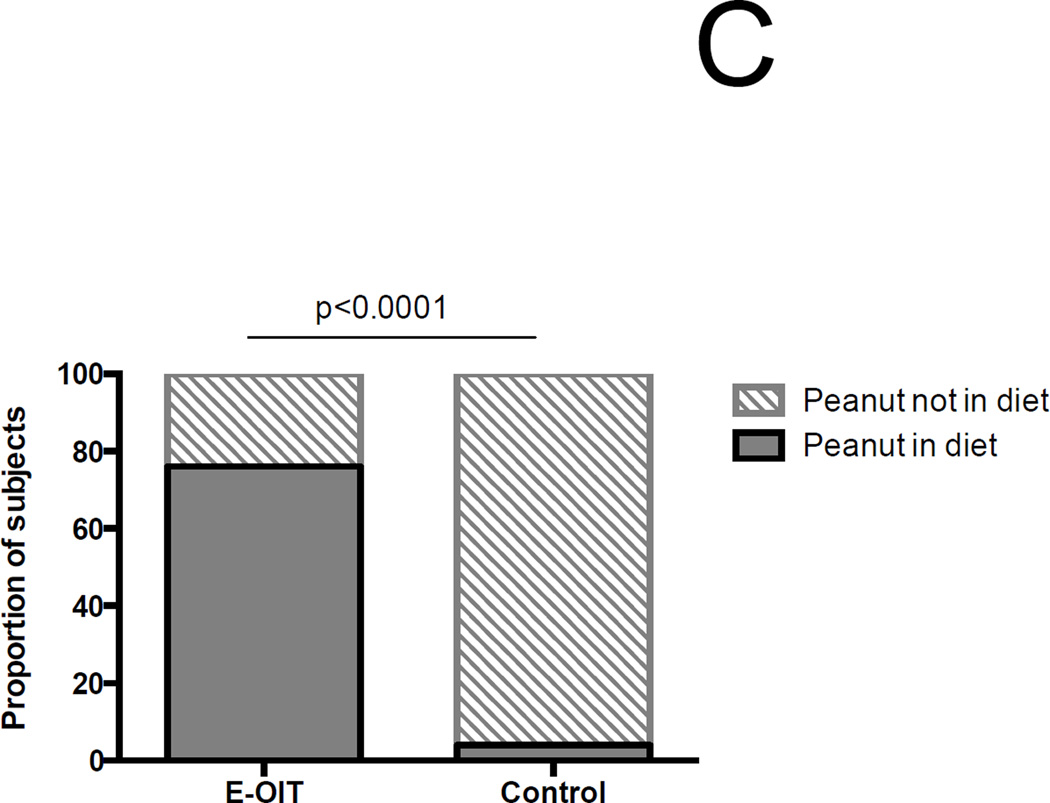
Figure 2.
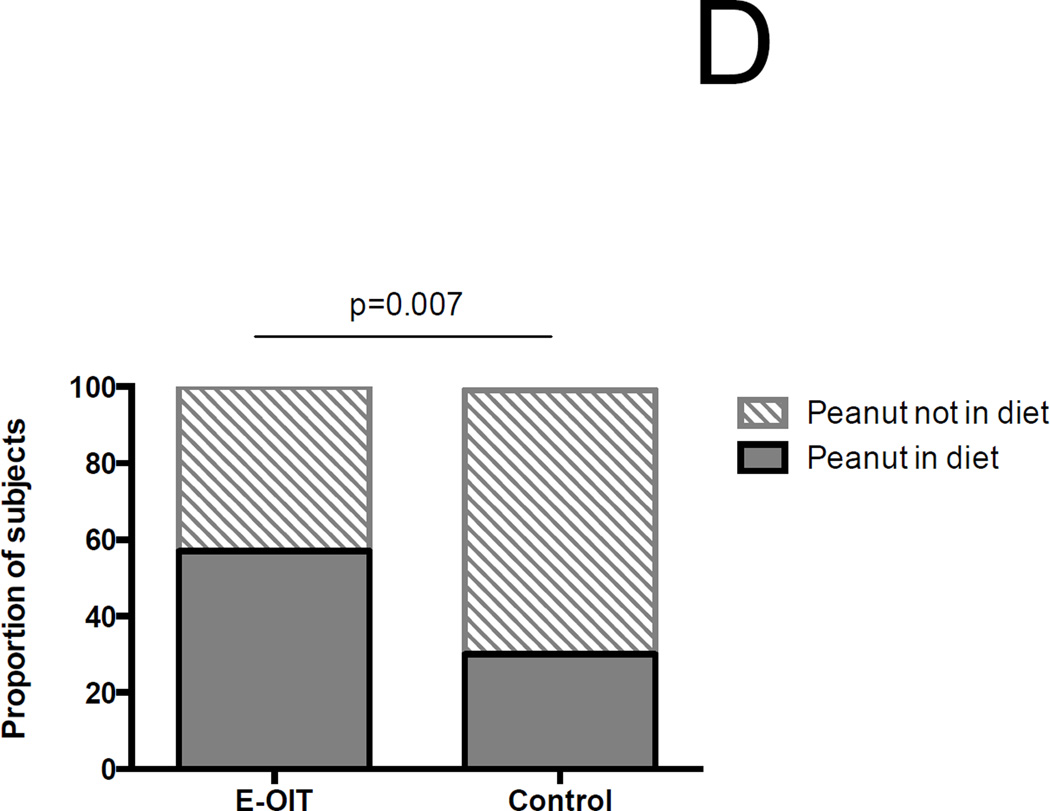
Outcomes of E-OIT and standard-care treatment. (A) Clinical outcomes of E-OIT. The proportion of overall subjects, and those in each treatment arm, achieving sustained unresponsiveness are shown for both intent-to-treat and per-protocol analyses.(B) The distributions of peanut-specific IgE among E-OIT participants and matched controls practicing allergen avoidance at baseline and end-of study periods (median 29 and 43 months, respectively). Note all peanut-specific IgE levels > 100 were transformed to 101 for these analyses because dilutional analysis was not available for all high-titer samples. (C) The proportions of E-OIT and control participants able to reintroduce peanut-containing foods in the diet at the end of study period. (D) The imputed proportions able to reintroduce peanut-containing foods in the diet with evidence-based worst-case assumptions.
Peanut exposure, not avoidance, suppresses psIgE and permits dietary consumption
Among 154 matched standard-care controls from the pediatric allergy clinic at Johns Hopkins, the median (IQR) baseline psIgE was 21.9 kUA/L (6.9–73), compared to 14.4 (3.3–51) in trial participants (p=0.12). However, over time, median (IQR) psIgE significantly declined in OIT subjects to 1.6 kUA/L (0.5–4.9), whereas in controls it significantly increased to 57.4 kUA/L (9–101) (Figure 2B). Based on standard-of-care treatment, 20 (13%) control patients were deemed OFC-eligible over an average follow up of 3.6 years (95%CI 3.3–3.8). Consistent with generally accepted clinical indications for OFC in this situation (22, 23), the median psIgE at OFC in the control cohort was 3.02 kUA/L [range 0.48–13.5]. 19/20 OFCs were completed, and 6/19 (32%) passed. The remaining 13 reacted at a cumulative eliciting dose of 1150 mg (range 250 – 5000), with three patients requiring epinephrine. No other control patients were noted to have developed spontaneous peanut tolerance. Therefore the known overall proportion of controls that successfully introduced peanut in the diet was 4%, compared to 78% in the OIT group (RR 19.42 [95%CI 8.7–43.7], Fisher’s exact p<0.001) (Figure 2C).
We performed a worst-case analysis of these outcomes based on well-established predictive values of peanut allergy. Nine of 37 (24%) of the randomized group began the study with peanut-specific IgE values < 5 kUA/L, one of who subsequently withdrew for nonadherence. In the control group, there were serial IgE values available for 147 subjects, of which 46 (31%) had peanut-specific IgE < 15 kUA/L at the end of the follow up period. For the purposes of this secondary analysis, we assumed that all nine of the subjects with peanut-specific IgE < 5 did not benefit from the intervention but would have outgrown it regardless (24), and were considered failures. We also assumed that all controls with psIgE ≥ 15 at the end of follow-up were allergic, while all those <15 were tolerant (25). In this scenario, the proportion of successes was still significantly higher (57%) in the OIT group than among controls (31%) (RR 1.8 [95%CI 1.3–2.6], p=0.007) (Figure 2D).
Treatment length and outcome are determined by baseline psIgE levels
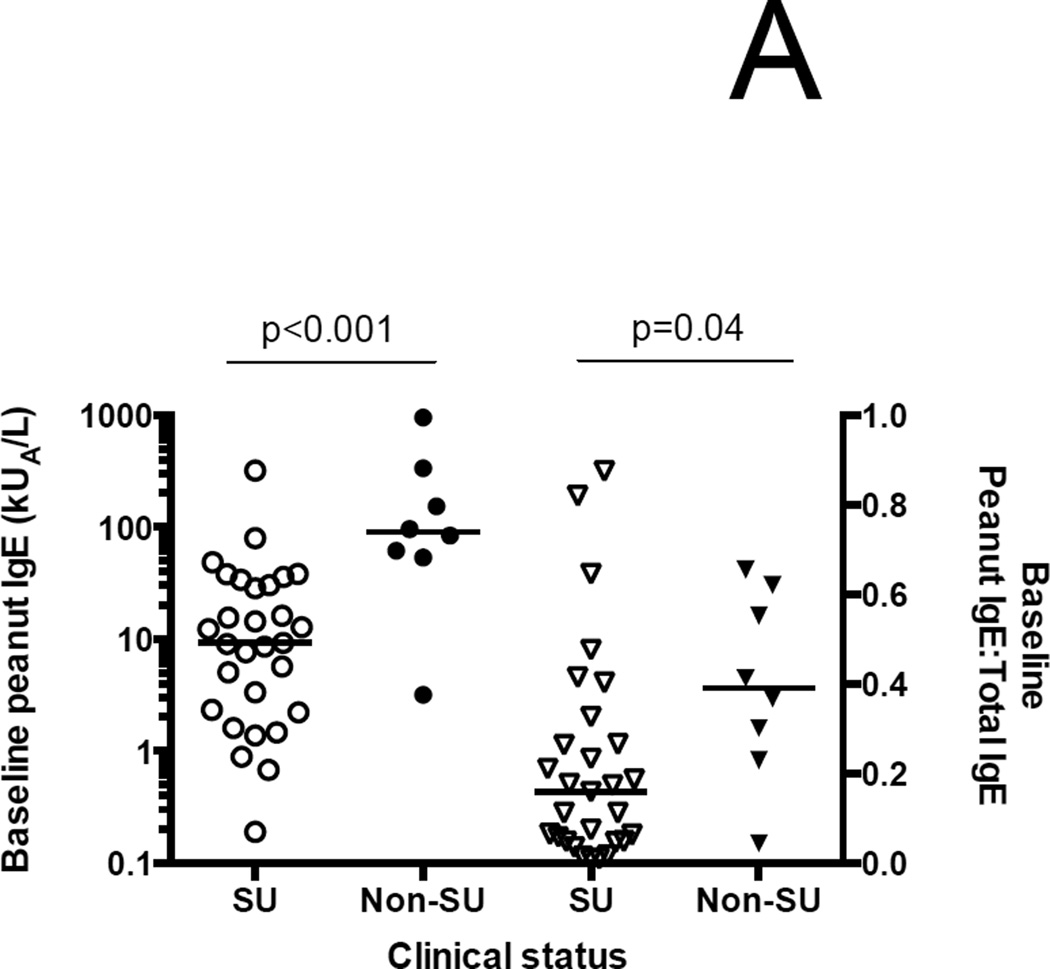
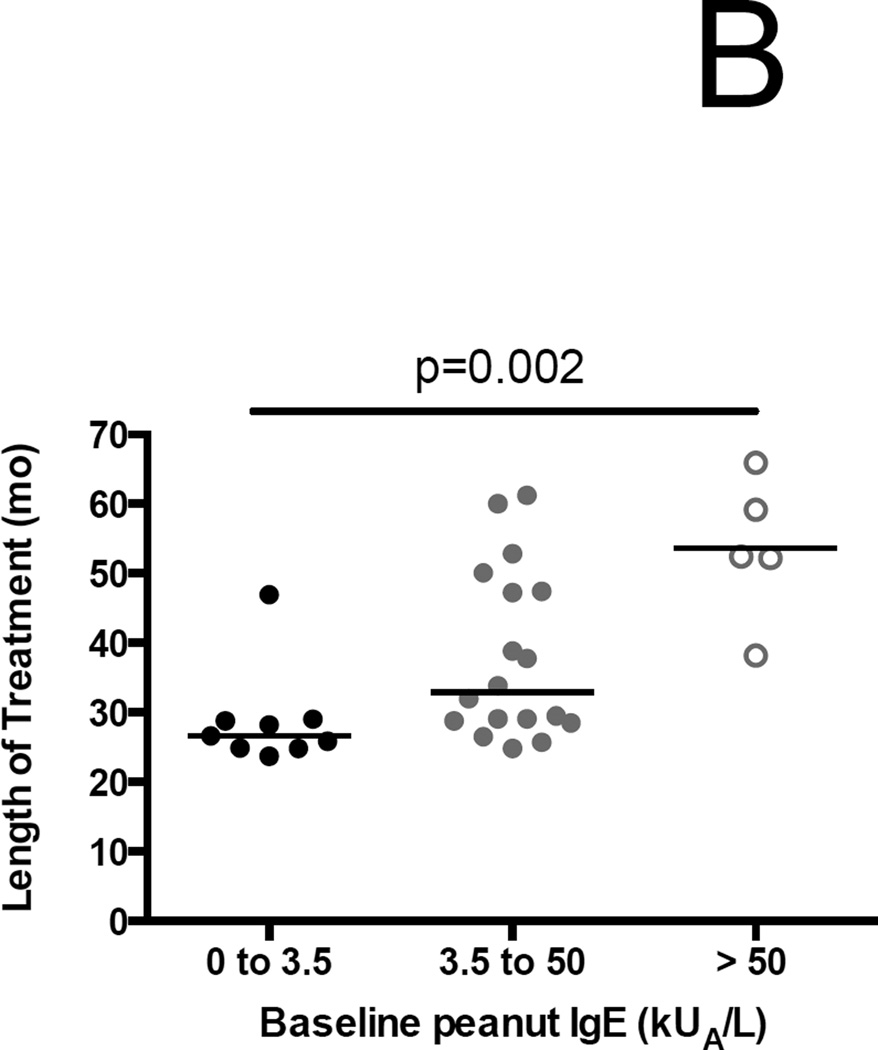
The 8 ITT failures had significantly higher median baseline psIgEs and psIgE:total IgE ratios than the 29 successes (90.1 kUA/L (IQR 55.4–288.8) versus 9.3 kUA/L (2.3–32), p<0.001; and 0.39 (0.25–0.6) versus 0.16 (0.05–0.3); p=0.04, respectively) (Figure 3A). As a result of the conditional endpoint assessment strategy, the median (IQR) duration of treatment was 29.1 months (25.3–47.3), and there was a significant stepwise increase in treatment duration from lowest to highest tertile of baseline psIgE (Figure 3B).
Figure 3.
Association of baseline peanut-specific IgE characteristics and outcomes. (A) Both the baseline peanut-specific IgE, and the ratio of peanut-specific to total IgE, are significantly lower among successes than failures in the ITT population. (B) Length of treatment broken down by tertiles of baseline peanut-specific IgE. Significance testing by one-way ANOVA.
Low- and high-dose peanut OIT both modulate allergic immune responses
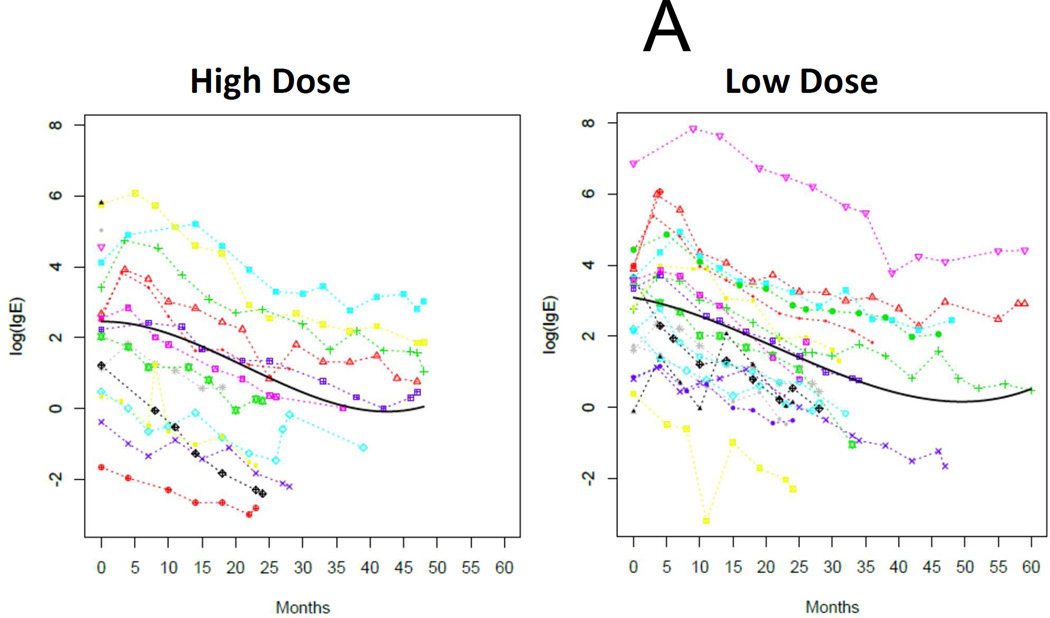
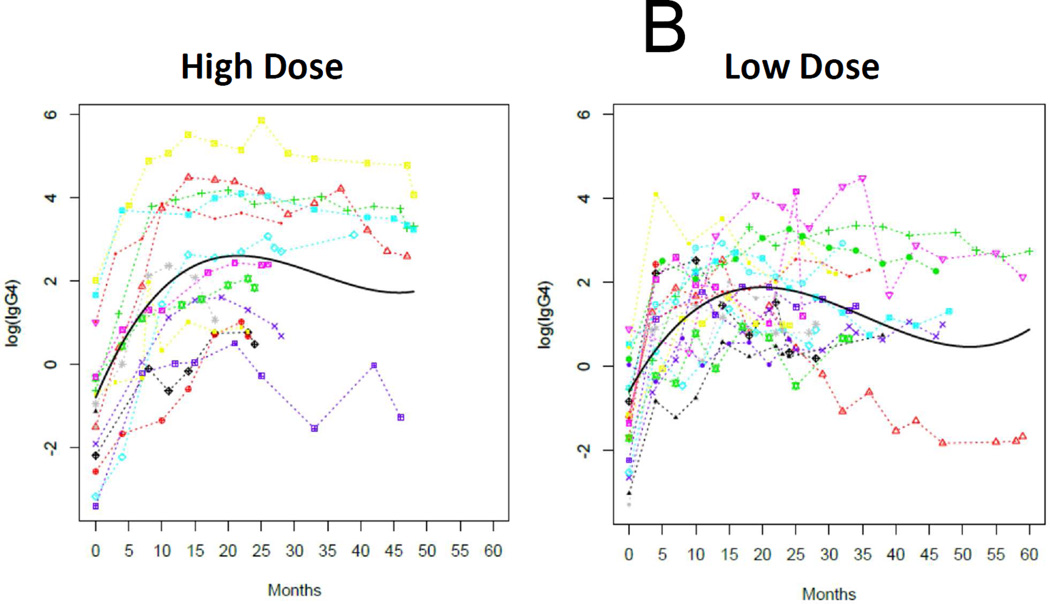
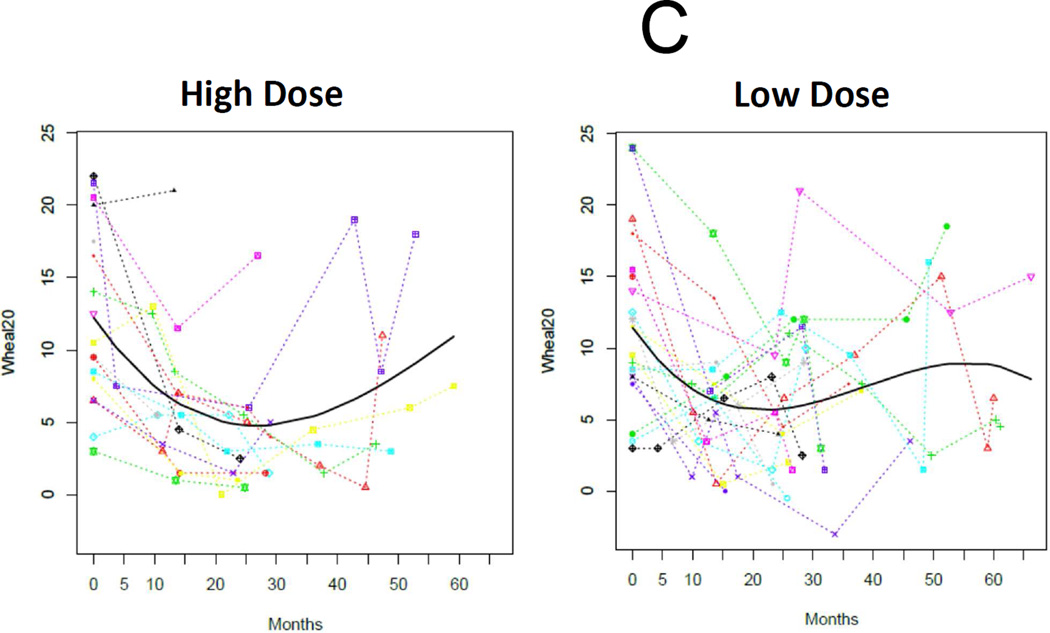
As expected, the baseline SPT, psIgE, and psIgG4 data were not normally distributed and were analyzed with nonparametric test statistics. Overall medians (IQR) for baseline psIgE and SPT were 14.4 kUA/L (3.4–48.6) and 11.5 mm (8–16.5), respectively, and not different between treatment arms (Table 1). We analyzed changes in these immunological outcomes with longitudinal mixed models for each group with functions of time. These models demonstrated strong temporal trends in the decline of psIgE and SPT, and the amplification of psIgG4 production, all of which are statistically significant in their change from baseline (Figures 4A, 4B, and 4C). Notably, there were no significant differences in the rate of change between treatment arms.
Figure 4.
Immunoregulation during E-OIT at both doses tested. Log-transformed peanut-specific IgE (A) and IgG4 (B), and raw data for mean wheal diameter of peanut skin tests (C) are plotted separately by group, with data from individuals connected by colored lines and the fitted time trajectory curve displayed with the raw data for these models. P-values comparing the parameter estimates for the two groups for each outcome are also provided.
E-OIT was overall safe and well tolerated
There were no treatment-related, protocol-defined severe adverse events, hospitalizations, or deaths. Overall, 95% of subjects were affected by AEs that were likely related to OIT, with an average per-dose rate of 0.8% (95%CI 0.3%-1.4%) overall (Supplementary Table E3). Reported treatment-related AEs occurred significantly more frequently during the build-up phase, compared to maintenance, and involved predominantly the GI tract and upper airway (Supplementary Figure E2). 85% of these AEs were mild, with 15% considered moderate and none severe. Two subjects withdrew with persistent GI-predominant adverse events. Withdrawing OIT resulted in prompt resolution of abdominal pain in the first. The other subject, who reported a history of “gastroesophageal reflux,” prior to starting OIT, underwent esophagogastroduodenoscopy while on OIT due to persistent regurgitation and vomiting refractory to high-dose ranitidine. Mucosal furrowing and ~30 eosinophils per high-powered field were noted, which persisted at repeat esophagogastroduodenoscopy three months later despite stopping OIT and resuming a peanut-free diet. Moderate-severity AEs were significantly more likely in the low-dose compared to high-dose group overall (p=0.04) and during the buildup phase (p=0.02) (Supplementary Table E3). Overall, 25% of events (47% of subjects) required treatment, with the vast majority being antihistamines only. No epinephrine was administered during a dose escalation visit, and once at home following a dose reaction (Supplementary Table E4).
DISCUSSION
This study is the first to target peanut-allergic children under the age of three for OIT treatment and also the first to prospectively study two peanut OIT doses in a randomized, blinded fashion. We show here that overall 78% of subjects receiving E-OIT demonstrated SU to peanut four weeks after stopping E-OIT and reintroduced peanut into the diet, the highest rate reported to date, after a median of only 29 months of treatment. Compared to a matched standard-care control group practicing avoidance, subjects receiving E-OIT had significantly lower psIgE levels and were estimated to be 19 times more likely to consume peanut in the diet over approximately a three-year period. Importantly, 300 mg/day was as effective as 3000 mg/day at regulating the allergic immune response, and produced 4-SU among 85% of the intent-to-treat and 89% of the per-protocol population. Confirming our previous results (8), we observed that SU was clearly associated with low baseline psIgE levels and psIgE:total IgE ratio. Allowing those with low psIgE to qualify early for exit challenges appeared to facilitate rapid introduction of peanut back into the diet. Taken together, these findings support our hypothesis that early intervention effectively disrupts peanut allergy and enhances outcomes, perhaps due to the lower average psIgE levels typically seen in young children and/or the plasticity of a relatively immature immune response, which we are exploring in greater detail in ongoing experiments.
Safety data from young children treated with OIT are sparse. We observed a favorable safety profile with E-OIT, with a side effect profile similar to other studies (7). Virtually all subjects experienced AEs likely related to OIT, but generally AEs were mild and required antihistamines, if any treatment was required. None were graded as severe. Like many other studies (26), GI allergic side effects were common and their persistence led to two withdrawals, one of which had EoE that importantly did not improve on a peanut-free diet, suggesting that peanut was not the specific trigger. Given his medical history of self-reported “gastroesophageal reflux,” and failure to respond to peanut elimination, it is most likely that his EoE was preexisting. Importantly, there were no AEs that met SAE criteria, and only one participant required epinephrine for one systemic reaction at home.
Almost 80% of the randomized study population was able to successfully introduce peanut-containing foods into the diet ad libitum four weeks after stopping treatment. This improvement has been termed sustained unresponsiveness (8, 27), and in part because we cannot be certain that these children have achieved permanent tolerance, we are continuing to follow them to better assess their long-term clinical outcomes. While we acknowledge that the lack of a placebo group in this first-in-preschoolers OIT study limits a precise estimate of the effect size, we believe that the high rates of success in dietary reintroduction are largely due to E-OIT. This conclusion is supported by the reduction in psIgE to very low levels that was sustained four weeks after stopping OIT, which has been previously associated with OIT success (8, 28). Though it is possible that spontaneous resolution may have occurred in the E-OIT group, several studies have shown that only a small minority of peanut-allergic children acquire natural tolerance (24, 29–31). Most recently, the HealthNuts study demonstrated, with high methodological rigor, a 22% rate of peanut allergy resolution by four years of age in an unselected population (24). The baseline characteristics of the active group, especially the low baseline OFC thresholds, as well as those of the controls in this study leave little reason to believe that we oversampled milder patients likely to spontaneously resolve (15, 24). We chose to use open challenges, rather than DBPCFCs, to confirm the diagnosis at baseline, and we required the presence of objective allergic signs in judging challenges to be positive. In other recent studies, including Learning Early About Peanut allergy (LEAP) and HealthNuts (2, 32), open OFCs were shown to be valid and sufficient for classifying food allergy in infants and young children (33). The prespecified primary efficacy outcome variable was based on the exit DBPCFC results only and did not include a comparison to the entry challenge.
Despite the similarities of the two participating food allergy centers and actively matching on inclusion and exclusion criteria, the control cohort was more ethnically diverse and was evaluated by standard-care criteria rather than required OFCs at the entry and exit of their observation period. Thus, during the period of observation 13% of the control patients were offered OFCs by their treating allergist based on clinical criteria suggesting the development of tolerance (21). Only one-third of those selected for challenge passed, suggesting that the treating physicians were aggressively evaluating for tolerance. While the selection of control patients for tolerance OFCs could introduce bias, our worst-case analysis showed a statistically significant effect favoring E-OIT even when we conservatively assumed that all patients with sIgE < 95% predictive values were tolerant. It should be noted that we understood in advance that we would be underpowered for a primary comparison of the two doses head-to-head. Given limited resources, we were unable to execute a study large enough to show what we assumed would be small between-group differences. Given how small the actually observed clinical and immunologic differences were in this study, this concern was justified. Our findings raise the possibility that the effective dose range in young children may be even lower than 300 mg.
Recently, the LEAP trial provided high-quality experimental evidence (32) supporting data obtained from other cohort studies (9, 11) that together construct a new paradigm concerning the early life origins of peanut allergy (34). In this paradigm, allergic sensitization to peanut begins in the first few months of life and progresses to production of psIgE and expression of clinical disease in a high proportion of high-risk infants. In the LEAP study, properly timed oral exposure appeared to interrupt this progression and prevent peanut allergy from developing in most of the selected population, which consisted of unexposed atopic infants with absent or minimal sensitization to peanut. However, children known to, or likely to, already have peanut allergy were deemed unlikely to benefit from primary or secondary prevention strategies, and were thus excluded from the LEAP study; and in a small proportion, the preventative intervention failed. The population of interest for treatment with E-OIT is this group of infants and toddlers whose sensitization to peanut has progressed to clinical disease. We show here that even after allergic hypersensitivity is apparent, immunological programming to peanut may still be effectively disrupted through oral exposure in the form of E-OIT.
In summary, E-OIT was immunoregulatory and resulted in a very high rate of 4-SU at 1/13th of the maintenance dose previously used in older peanut-allergic children,(8) and in as little as half the time. This suggests that allergic responses may be more easily and durably corrected in young children, and that in this context relatively low OIT doses are sufficiently potent in suppressing IgE responses and stimulating IgG4 production. We are further exploring these concepts in ongoing mechanistic studies. Within an average of two and a half years, children receiving E-OIT were able to stop treatment and begin eating peanut-containing foods at approximately 19-fold higher rates than similar peanut-allergic controls continuing to avoid peanut. If the promise of E-OIT is confirmed in other studies, one of which is ongoing with a randomized, placebo-controlled, multicenter design (35), we believe it has the potential to further transform the standard of care in the post-LEAP era. When initiated soon after the initial diagnosis, controlled oral peanut exposure through E-OIT may safely and effectively rescue many young children whose peanut allergy has already progressed to clinical disease expression.
Supplementary Material
CLINICAL IMPLICATIONS.
Treating peanut-allergic preschool children with OIT is feasible and may enhance long-term outcomes. In this study, relatively short, low-dose therapy achieved immunoregulation and a high rate of sustained unresponsiveness.
Acknowledgments
We thank the children and their families for their gracious participation in this trial. We are indebted to the Duke Clinical Research Institute and the North Carolina Translational and Clinical Sciences Institute for the use of clinical research facilities. J. Kamilaris, A. Edie, J. Hainline, D. Hamilton, and L. Herlihy provided nurse coordinator support. X. Yue, H. Zhang, N. Kamilaris, C. Opper, A. Beavers, and N. Szcezpanski provided invaluable laboratory and investigational drug manufacturing assistance. A. Kazatsky and B. Krueger assisted in extraction of clinical case histories at Johns Hopkins. The project described was supported by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through Grant Awards, Number UL1TR001111 and UL1TR001117; the National Institutes of Allergy and Infectious Disease, through Grant Numbers 5K23AI099083 (BPV), 1K23AI103187 and 1R21HD: 073557 (C.K.); the Thrasher Research Fund, award number NR-0101 (BPV); and departmental funds from the Departments of Pediatrics at Duke and UNC. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
ABBREVIATIONS
- 4-SU
Sustained unresponsiveness at four weeks after stopping OIT
- AE
Adverse event
- DBPCFC
Double-blinded, placebo-controlled food challenge
- EoE
Eosinophilic esophagitis
- IgE
Immunoglobulin E
- IQR
Interquartile range
- ITT
Intention-to-treat
- NIAID
National Institute of Allergy and Infectious Disease
- OFC
Oral food challenge
- OIT
Oral immunotherapy
- PP
Per protocol
- psIgE
peanut-specific IgE
- psIgG4
peanut-specific IgG4
- RR
Relative risk
- SAE
Serious adverse event
- SPT
Skin prick test
- SU
Sustained unresponsiveness
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Sicherer SH, Munoz-Furlong A, Godbold JH, Sampson HA. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. The Journal of allergy and clinical immunology. 2010;125(6):1322–1326. doi: 10.1016/j.jaci.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 2.Osborne NJ, Koplin JJ, Martin PE, Gurrin LC, Lowe AJ, Matheson MC, et al. Prevalence of challenge-proven IgE-mediated food allergy using population-based sampling and predetermined challenge criteria in infants. The Journal of allergy and clinical immunology. 2011;127(3):668–676. e1–e2. doi: 10.1016/j.jaci.2011.01.039. [DOI] [PubMed] [Google Scholar]
- 3.Varshney P, Jones SM, Scurlock AM, Perry TT, Kemper A, Steele P, et al. A randomized controlled study of peanut oral immunotherapy: clinical desensitization and modulation of the allergic response. The Journal of allergy and clinical immunology. 2011;127(3):654–660. doi: 10.1016/j.jaci.2010.12.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anagnostou K, Islam S, King Y, Foley L, Pasea L, Bond S, et al. Assessing the efficacy of oral immunotherapy for the desensitisation of peanut allergy in children (STOP II): a phase 2 randomised controlled trial. Lancet. 2014;383(9925):1297–1304. doi: 10.1016/S0140-6736(13)62301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Narisety SD, Frischmeyer-Guerrerio PA, Keet CA, Gorelik M, Schroeder J, Hamilton RG, et al. A randomized, double-blind, placebo-controlled pilot study of sublingual versus oral immunotherapy for the treatment of peanut allergy. The Journal of allergy and clinical immunology. 2014 doi: 10.1016/j.jaci.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bird J, Spergel J, Jones S, Rachid R, Assa’ad A, Wang J, et al. A novel characterized peanut allergen formulation (AR101) for oral immunotherapy (OIT) induces desensitization in peanut-allergic subjects: a Phase 2 clinical safety and efficacy study. Allergy. 2015;70(S101):110. [Google Scholar]
- 7.Keet CA, Wood RA. Emerging therapies for food allergy. The Journal of clinical investigation. 2014;124(5):1880–1886. doi: 10.1172/JCI72061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vickery BP, Scurlock AM, Kulis M, Steele PH, Kamilaris J, Berglund JP, et al. Sustained unresponsiveness to peanut in subjects who have completed peanut oral immunotherapy. The Journal of allergy and clinical immunology. 2014;133(2):468–475. doi: 10.1016/j.jaci.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sicherer SH, Wood RA, Stablein D, Burks AW, Liu AH, Jones SM, et al. Immunologic features of infants with milk or egg allergy enrolled in an observational study (Consortium of Food Allergy Research) of food allergy. The Journal of allergy and clinical immunology. 2010;125(5):1077–1083. doi: 10.1016/j.jaci.2010.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du Toit G, Roberts G, Sayre PH, Plaut M, Bahnson HT, Mitchell H, et al. Identifying infants at high risk of peanut allergy: the Learning Early About Peanut Allergy (LEAP) screening study. The Journal of allergy and clinical immunology. 2013;131(1):135–143. e1–e12. doi: 10.1016/j.jaci.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 11.Rowe J, Kusel M, Holt BJ, Suriyaarachchi D, Serralha M, Hollams E, et al. Prenatal versus postnatal sensitization to environmental allergens in a high-risk birth cohort. The Journal of allergy and clinical immunology. 2007;119(5):1164–1173. doi: 10.1016/j.jaci.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 12.Datta S, Milner JD. Altered T-cell receptor signaling in the pathogenesis of allergic disease. The Journal of allergy and clinical immunology. 2011;127(2):351–354. doi: 10.1016/j.jaci.2010.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holt PG, Rowe J, Kusel M, Parsons F, Hollams EM, Bosco A, et al. Toward improved prediction of risk for atopy and asthma among preschoolers: a prospective cohort study. The Journal of allergy and clinical immunology. 2010;125(3):653–659. doi: 10.1016/j.jaci.2009.12.018. 9 e1–9 e7. [DOI] [PubMed] [Google Scholar]
- 14.Rothers J, Halonen M, Stern DA, Lohman IC, Mobley S, Spangenberg A, et al. Adaptive cytokine production in early life differentially predicts total IgE levels and asthma through age 5 years. The Journal of allergy, clinical immunology. 2011;128(2):397–402. e2. doi: 10.1016/j.jaci.2011.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ho MH, Wong WH, Heine RG, Hosking CS, Hill DJ, Allen KJ. Early clinical predictors of remission of peanut allergy in children. The Journal of allergy and clinical immunology. 2008;121(3):731–736. doi: 10.1016/j.jaci.2007.11.024. [DOI] [PubMed] [Google Scholar]
- 16.Neuman-Sunshine DL, Eckman JA, Keet CA, Matsui EC, Peng RD, Lenehan PJ, et al. The natural history of persistent peanut allergy. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2012;108(5):326–331. e3. doi: 10.1016/j.anai.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 17.Mucosal Immunotherapy for Peanut Allergy in Young Children (DEVIL) Bethesda. [Available from: clinicaltrials.gov/ct2/show/NCT00932828.
- 18.Boyce JA, Assa’ad A, Burks AW, Jones SM, Sampson HA, Wood RA, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. The Journal of allergy and clinical immunology. 2010;126(6 Suppl):S1–S58. doi: 10.1016/j.jaci.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones SM, Pons L, Roberts JL, Scurlock AM, Perry TT, Kulis M, et al. Clinical efficacy and immune regulation with peanut oral immunotherapy. The Journal of allergy and clinical immunology. 2009;124(2):292–300. e1–e97. doi: 10.1016/j.jaci.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thyagarajan A, Jones SM, Calatroni A, Pons L, Kulis M, Woo CS, et al. Evidence of pathway-specific basophil anergy induced by peanut oral immunotherapy in peanut-allergic children. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2012;42(8):1197–1205. doi: 10.1111/j.1365-2222.2012.04028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan J, Fine J. Estimating equations for association structures. Statistics in medicine. 2004;23(6):859–874. doi: 10.1002/sim.1650. discussion 75–7, 79–80. [DOI] [PubMed] [Google Scholar]
- 22.Nowak-Wegrzyn A, Assa’ad AH, Bahna SL, Bock SA, Sicherer SH, Teuber SS, et al. Work Group report: oral food challenge testing. The Journal of allergy and clinical immunology. 2009;123(6 Suppl):S365–S383. doi: 10.1016/j.jaci.2009.03.042. [DOI] [PubMed] [Google Scholar]
- 23.Perry TT, Matsui EC, Kay Conover-Walker M, Wood RA. The relationship of allergen-specific IgE levels and oral food challenge outcome. The Journal of allergy and clinical immunology. 2004;114(1):144–149. doi: 10.1016/j.jaci.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 24.Peters RL, Allen KJ, Dharmage SC, Koplin JJ, Dang T, Tilbrook KP, et al. Natural history of peanut allergy and predictors of resolution in the first 4 years of life: A population-based assessment. The Journal of allergy and clinical immunology. 2015;135(5):1257–1266. e2. doi: 10.1016/j.jaci.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 25.Sampson HA. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. The Journal of allergy and clinical immunology. 2001;107(5):891–896. doi: 10.1067/mai.2001.114708. [DOI] [PubMed] [Google Scholar]
- 26.Lucendo AJ, Arias A, Tenias JM. Relation between eosinophilic esophagitis and oral immunotherapy for food allergy: a systematic review with meta-analysis. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2014 doi: 10.1016/j.anai.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 27.Burks AW, Jones SM, Wood RA, Fleischer DM, Sicherer SH, Lindblad RW, et al. Oral immunotherapy for treatment of egg allergy in children. The New England journal of medicine. 2012;367(3):233–243. doi: 10.1056/NEJMoa1200435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gorelik M, Narisety SD, Guerrerio AL, Chichester KL, Keet CA, Bieneman AP, et al. Suppression of the immunologic response to peanut during immunotherapy is often transient. The Journal of allergy and clinical immunology. 2014 doi: 10.1016/j.jaci.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fleischer DM, Conover-Walker MK, Christie L, Burks AW, Wood RA. The natural progression of peanut allergy: Resolution and the possibility of recurrence. Journal of Allergy and Clinical Immunology. 2003;112(1):183–189. doi: 10.1067/mai.2003.1517. [DOI] [PubMed] [Google Scholar]
- 30.Tang ML, Ponsonby AL, Orsini F, Tey D, Robinson M, Su EL, et al. Administration of a probiotic with peanut oral immunotherapy: A randomized trial. The Journal of allergy and clinical immunology. 2015;135(3):737–744. e8. doi: 10.1016/j.jaci.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 31.Skolnick HS, Conover-Walker MK, Koerner CB, Sampson HA, Burks W, Wood RA. The natural history of peanut allergy. J Allergy Clin Immunol. 2001;107(2):367–374. doi: 10.1067/mai.2001.112129. [DOI] [PubMed] [Google Scholar]
- 32.Du Toit G, Roberts G, Sayre PH, Bahnson HT, Radulovic S, Santos AF, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. The New England journal of medicine. 2015;372(9):803–813. doi: 10.1056/NEJMoa1414850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Venter C, Pereira B, Voigt K, Grundy J, Clayton CB, Gant C, et al. Comparison of open and double-blind placebo-controlled food challenges in diagnosis of food hypersensitivity amongst children. Journal of human nutrition and dietetics : the official journal of the British Dietetic Association. 2007;20(6):565–579. doi: 10.1111/j.1365-277X.2007.00828.x. [DOI] [PubMed] [Google Scholar]
- 34.Fleischer DM, Sicherer S, Greenhawt M, Campbell D, Chan E, Muraro A, et al. Consensus communication on early peanut introduction and the prevention of peanut allergy in high-risk infants. The Journal of allergy and clinical immunology. 2015;136(2):258–261. doi: 10.1016/j.jaci.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 35.Peanut Oral Immunotherapy in Children (IMPACT) Bethesda. [Available from: clinicaltrials.gov/ct2/show/NCT01867671.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.