Abstract
Background
BIOGF1K, a compound K-rich fraction prepared from the root of Panax ginseng, is widely used for cosmetic purposes in Korea. We investigated the functional mechanisms of the anti-inflammatory and antioxidative activities of BIOGF1K by discovering target enzymes through various molecular studies.
Methods
We explored the inhibitory mechanisms of BIOGF1K using lipopolysaccharide-mediated inflammatory responses, reporter gene assays involving overexpression of toll-like receptor adaptor molecules, and immunoblotting analysis. We used the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay to measure the antioxidative activity. We cotransfected adaptor molecules, including the myeloid differentiation primary response gene 88 (MyD88) and Toll/interleukin-receptor domain containing adaptor molecule-inducing interferon-β (TRIF), to measure the activation of nuclear factor (NF)-κB and interferon regulatory factor 3 (IRF3).
Results
BIOGF1K suppressed lipopolysaccharide-triggered NO release in macrophages as well as DPPH-induced electron-donating activity. It also blocked lipopolysaccharide-induced mRNA levels of interferon-β and inducible nitric oxide synthase. Moreover, BIOGF1K diminished the translocation and activation of IRF3 and NF-κB (p50 and p65). This extract inhibited the upregulation of NF-κB-linked luciferase activity provoked by phorbal-12-myristate-13 acetate as well as MyD88, TRIF, and inhibitor of κB (IκBα) kinase (IKKβ), and IRF3-mediated luciferase activity induced by TRIF and TANK-binding kinase 1 (TBK1). Finally, BIOGF1K downregulated the NF-κB pathway by blocking IKKβ and the IRF3 pathway by inhibiting TBK1, according to reporter gene assays, immunoblotting analysis, and an AKT/IKKβ/TBK1 overexpression strategy.
Conclusion
Overall, our data suggest that the suppression of IKKβ and TBK1, which mediate transcriptional regulation of NF-κB and IRF3, respectively, may contribute to the broad-spectrum inhibitory activity of BIOGF1K.
Keywords: anti-inflammatory activity, antioxidative activity, BIOGF1K, compound K, Panax ginseng
1. Introduction
Inflammation is defined as the complex biological response of the immune system against harmful pathogens [1]. To maintain the immune homeostasis system in the body, both acute and chronic inflammatory responses play an important role in the natural defense mechanisms of the body's innate immune system. Phagocytic uptake of infected materials via receptors, likely toll-like receptors (TLRs), that interact with molecular patterns of pathogen-derived materials including lipopolysaccharide (LPS) and peptidoglycan triggers upregulation of macrophage functions that mediate inflammatory responses [2], [3]. To activate inflammatory cells, intracellular signaling cascades linked to tyrosine kinases (e.g., Src), serine–threonine kinases, and inhibitor of κB (IκBα) kinase (IKK)-activating nuclear factor-κB (NF-κB) are required. Subsequent transcriptional activation of various inflammatory genes such as inducible nitric oxide (NO) synthase (iNOS) occurs [4], [5]. Consequently, several inflammatory mediators such as NO and proinflammatory cytokines such as interleukin (IL)-1, interferon (IFN)-β, and tumor necrosis factor-α are secreted to further stimulate other inflammatory cells [6]. These responses help protect the body against pathogenic infections. Nevertheless, prolonged inflammation causes severe diseases, including cancer, arthritis, diabetes, and atherosclerosis [7], [8], [9]. Thus, in recent decades, immunologists have focused on the development of safe and effective anti-inflammatory and antioxidative therapy to prevent chronic inflammatory diseases.
Numerous studies have expanded our perceptive on the TLR-signaling pathway [10]. After LPS stimulation, TLR4 is activated and delivers outside signals into the cytoplasm by recruiting two adaptor molecules, myeloid differentiation primary response protein-88 (MyD88) and Toll/IL-1 receptor-domain containing adaptor molecule-inducing interferon-β (TRIF), through an interaction between their Toll/interleukin-1 receptor (TIR) domains [11]. MyD88 and TRIF concurrently interact with various signaling proteins, such as IL-1 receptor-associated kinases (IRAK1 and IRAK4), protein kinase B (AKT), and TANK-binding kinase 1 (TBK1) [12]. When these proteins are activated, complex signaling cascades composed of Src, phosphoinositide 3-kinase, phosphoinositide-dependent kinase-1, and AKT trigger the NF-κB activation pathway linked to IKK, IκBα [13], [14], and the interferon regulatory factor 3 (IRF3) activation pathway [15]. These two major pathways participate in the release of proinflammatory cytokines and inflammatory mediators. Free radicals such as reactive oxygen species (ROS) are highly reactive molecules that are generated in micro- or macrolevel inflammatory responses [16], [17]. Oxidative stress can damage proteins, DNA, and small molecules other than lipids. Biologists and clinicians are interested in the capacity of antioxidants to defend the human body against damage by reactive free radicals found in immunological diseases such as cancer, atherosclerosis, and aging [18], [19].
The root of Korean ginseng (Panax ginseng Meyer) has been prescribed as a herbal medicine in Korea, China, and Japan, and its pharmacologically active compounds display antioxidant, anti-inflammatory, and anticancer effects [20], [21]. Recently, our group has developed an interesting fraction (BIOGF1K) with a higher amount of compound K, an active metabolite displaying anti-inflammatory, anticancer, and skin-protective activities [22], [23]. In the present study, we benchmark the molecular mechanisms of anti-inflammatory and antioxidative activities of BIOGF1K by exploring target proteins through molecular studies.
2. Materials and methods
2.1. Materials
LPS (Escherichia coli 0111:B4), ascorbic acid, phorbal-12-myristate-13 acetate (PMA), and (3-4-5-dimethylthiazol-2-yl)-2-5-diphenyltetrazolium bromide (MTT) were obtained from Sigma Chemical Co. (St. Louis, MO, USA). The luciferase construct with the NF-κB promoter binding site was used as reported earlier [3]. Fetal bovine serum and RPMI 1640 were purchased from Gibco (Grand Island, NY, USA). The cell lines (RAW264.7 and HEK293) used in the present experiments were obtained from ATCC (Rockville, MD, USA). All other chemicals were obtained from Sigma Chemical Co. Total or phospho-specific antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA). Plasmid constructs containing AKT, IKKβ, and TBK1 were used as reported previously [24], [25], [26].
2.2. Preparation of BioGF1K
BIOGF1K, a compound K-rich fraction, was gifted by Amorepacific R&D unit (Yongin, Korea). Briefly, P. ginseng root (2 kg) was pulverized into powder using a mechanical grinder. After weighing, P. ginseng roots were transferred to a flask, treated with 70% ethanol (4 L) for 2 kg of powdered P. ginseng root, refluxed to extract three times, and deposited for 6 d at 15°C. After that the extracts were suspended in water and subsequently fractionated using diethyl ether (1 L) and 1-butanol (500 mL). Dried 1-butanol extract (10 g) was continuously suspended into a citrate buffer solution (pH 4.0, 1 L) and added with pectinase (15 g, Multifect Pectinase FE, originating from Aspergillus niger) during agitation in a water bath for 48 h at 30°C. After the reaction, the incubation mixture was extracted using the same amount of ethylacetate three times and concentrated by speed vac. Finally, BIOGF1K (compound K, 3.2 g, and ginsenoside F1, 1.5 g) was obtained by separation using column chromatography (chloroform:methanol = 9:1); yield = 87.8%.
2.3. HPLC analysis
For determination of compound K in BIOGF1K, HPLC was performed as described previously [27], [28].
2.4. Cell culture
RAW264.7 and HEK293 cells were cultured in RPMI1640 and DMEM media, respectively, supplemented with 10% heat-inactivated fetal bovine serum and 1% antibiotics (penicillin and streptomycin) at 37°C under 5% CO2 [29].
2.5. Drug treatment
A stock solution of BIOGF1K dissolved in 100% dimethyl sulfoxide at a concentration of 100 mg/mL was further diluted with the culture medium, as reported previously [30], [31].
2.6. Production of NO
RAW264.7 macrophage cells (1 × 106 cells/ mL) were cultured for 18 h, pretreated with BIOGF1K (0–200 μg/ mL) for 1 h, and further incubated with LPS (1 μg/ mL) for 24 h. The inhibitory effect of BIOGF1K on NO was determined by Griess assay, as described previously [32].
2.7. Cell viability test
The cytotoxicity of BIOGF1K was determined with a conventional MTT assay, as described previously [33], [34].
2.8. Radical scavenging activity
A 2,2-diphenyl-1-picrylhydrazyl (DPPH) decoloration assay was carried out as reported previously [35]. Quenching of free radicals by each fraction and the standard compound was determined spectrophotometrically at 517 nm against the absorbance of the DPPH radical. A fresh batch of DPPH solution (20 μg/mL) was prepared for the experiment. The electron-donating activity described the difference in the absorbance between the mixture and the control solution, expressed as a percentage:
| electron-donating activity (%) = [(the absorbance of the control − the absorbance of the mixture)/the absorbance of the control] × 100. |
2.9. Analysis of mRNA by semiquantitative reverse transcriptase-polymerase chain reaction
To determine cytokine mRNA expression levels, RAW264.7 cells were pretreated with BIOGF1K (0–200 μg/mL) for 1 h before stimulation with LPS (1 μg/mL) for 6 h. Total RNA was isolated with TRIzol Reagent (Gibco) according to the manufacturer's instructions and stored at −70°C for further use. Semiquantitative reverse transcriptase-polymerase chain reactions were performed as described previously [36]. The primers listed in Table 1 were synthesized by Bioneer (Seoul, Korea).
Table 1.
PCR primers used in this study
| Name | Sequence (5′–3′) | |
|---|---|---|
| iNOS | F | CCCTTCCGAAGTTTCTGGCAGCAG |
| R | GGCTGTCAGAGCCTCGTGGCTTTGG | |
| IFN-β | F | CAAGTGGAGAGCAGTTGAGGACATC |
| R | TGAGGACATCTCCCACGTCAA | |
| GAPDH | F | CACTCACGGCAAATTCAACGGCA |
| R | GACTCCACGACATACTCAGCAC |
GAPDH, Glyceraldehyde 3-phosphate dehydrogenase; IFN-β, interferon beta; iNOS, inducible nitric oxide synthase; PCR, polymerase chain reaction
2.10. Plasmid transfection and luciferase reporter gene activity
For luciferase reporter assays, HEK293 cells (1 × 106 cells/mL in 24-well plates) were transfected with 1 μg of plasmid-containing β-galactosidase, NF-κB-Luc, or IRF3-Luc in the presence or absence of inducing molecules (IKKβ or TBK1), using the polyethyleneimine method. The cells were incubated with BIOGF1K for 24 h before harvest. Luciferase assays were carried out using the Luciferase Assay System (Promega, Madison, WI, USA), as described previously [37]. For immunoblotting analysis, HEK293 cells (1 × 107 cells/mL) were transfected with AKT for 48 h or with IKKβ and TBK1 for 24 h before harvesting. Lysates of AKT-overexpressing cells were subjected to immunoblotting analysis to check the amount of total or phospho forms of IKKα/β and β-actin.
2.11. Preparation of total lysates and nuclear extracts, and immunoblotting
Total lysates prepared from HEK293 or RAW264.7 cells (5 × 106 cells/ mL) were subjected to western blot analysis of various total or phospho-proteins. Nuclear lysates were extracted in a three-step procedure described previously [38]. Total or phosphorylated protein levels of transcription factors (p65 and p50), Akt, IκBα, IKKα/β, TBK1, IRF3, Src, HA, and β-actin (as a control) were visualized as described previously [39].
2.12. Statistical analysis
All data presented in this paper are the mean ± standard deviation of an experiment performed with six (Fig. 1) or three (Fig. 2, Fig. 3, Fig. 4) replicates. For statistical comparisons, these results were analyzed using analysis of variance/Scheffe's post hoc test and Kruskal–Wallis/Mann–Whitney U tests. A p value < 0.05 was considered statistically significant. All statistical tests were carried out using the computer program SPSS (version 22.0, 2013; IBM Corp., Armonk, NY, USA).
Fig. 1.
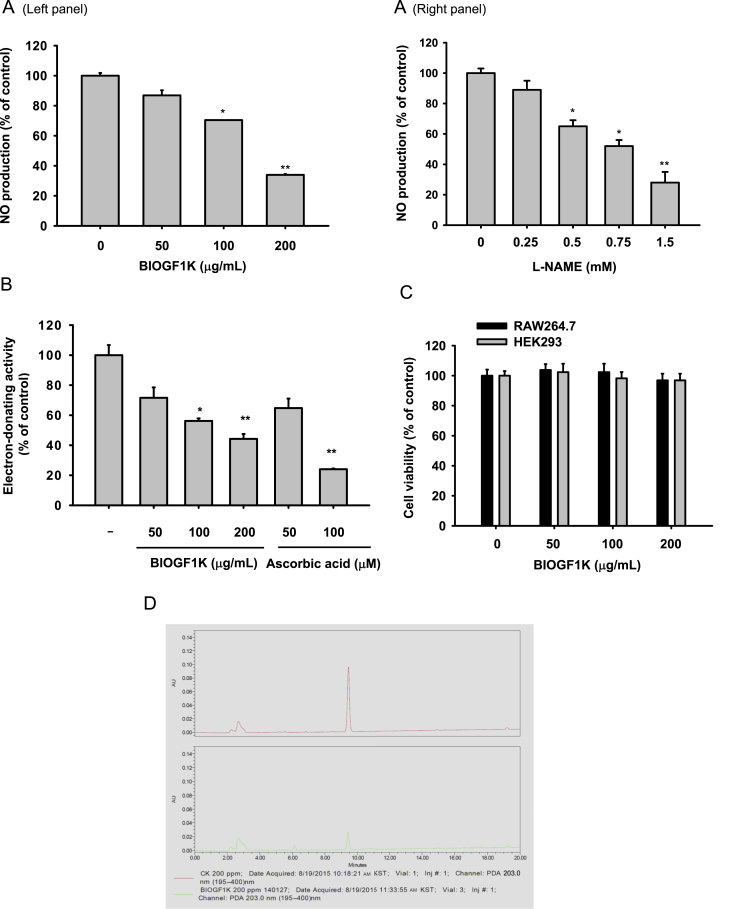
In vitro anti-inflammatory and antioxidative effects of BIOGF1K. (A) The NO level in the culture supernatant of RAW264.7 cells treated with LPS (1 μg/mL) in the presence or absence of BIOGF1K (left panel) or L-NAME (right panel) for 24 h was analyzed with a Griess assay. (B) The antioxidative activity (electron-donating activity) of BIOGF1K and ascorbic acid was measured by DPPH assay. After adding an ethanolic DPPH solution, the absorbance was monitored at 517 nm and enzyme activity was calculated as described in the “Materials and methods” section. (C) Cell viability of RAW264.7 and HEK293 cells under BIOGF1K treatment conditions was determined using the MTT assay. (D) The phytochemical analysis of the level of compound K in BIOGF1K was analyzed by HPLC. *p < 0.05, compared with the control. **p < 0.01, compared with the control. MTT, (3-4-5-dimethylthiazol-2-yl)-2-5-diphenyltetrazolium bromide; NO, nitric oxide; DPPH, 2,2-diphenyl-1-picrylhydrazyl.
Fig. 2.
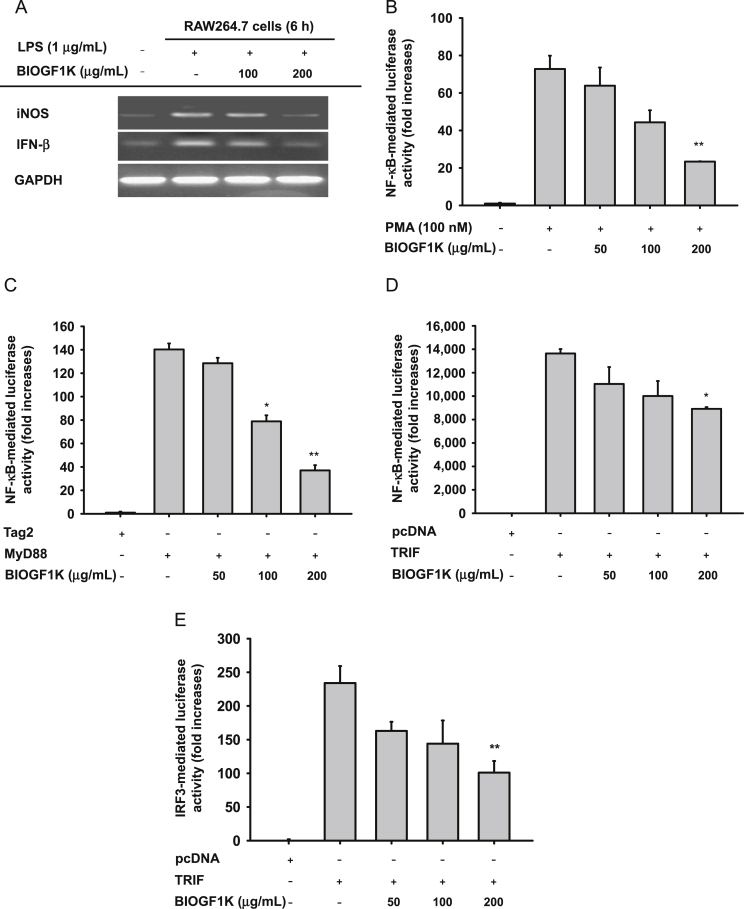
Effect of BIOGF1K on the expression of proinflammatory genes and their transcriptional activation. (A) The mRNA levels of iNOS and IFN-β were determined by semiquantitative RT-PCR. (B–E) The promoter binding activity of the transcription factor NF-κB was analyzed using a reporter gene assay in HEK293 cells transfected with plasmid constructs NF-κB-Luc (1 μg/mL), IRF3-Luc (1 μg/mL), β-gal (as a transfection control), PMA (100nM), MyD88 (1 μg/mL), or TRIF (1 μg/mL) in the presence or absence of BIOGF1K. Luciferase activity was measured using a luminometer. *p < 0.05, compared with the control. **p < 0.01, compared with the control. IFN-β, interferon-beta; iNOS, inducible nitric oxide synthase; IRF3, interferon regulatory factor 3; LPS, lipopolysaccharide; MyD88, myeloid differentiation primary response gene 88; NF-κB, nuclear factor-κB; PMA, phorbal-12-myristate-13 acetate; RT-PCR, reverse transcriptase-polymerase chain reaction; TRIF, Toll/interleukin-receptor domain containing adaptor molecule-inducing interferon-β; GAPDH, Glyceraldehyde 3-phosphate dehydrogenase.
Fig. 3.
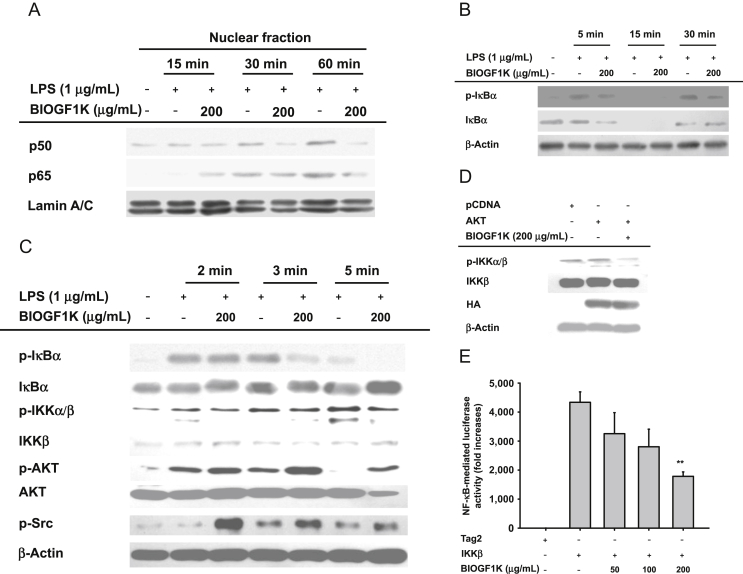
Effect of BIOGF1K on activation of NF-κB and its upstream signaling. (A) Levels of p65, p50, and lamin A/C in nuclear fractions were determined by nuclear fractionation and immunoblotting analysis. (B, C) Phospho-protein or total protein levels of IκBα, IKKα/β, AKT, Src, and β-actin in cell lysates were determined by immunoblotting analysis. (D) The effect of BIOGF1K on the activation of downstream signaling molecules (IKKα/β) induced by overexpression of AKT was analyzed with immunoblotting analysis. (E) Inhibition of IKKβ-induced NF-κB activation by BIOGF1K was analyzed using a reporter gene assay in HEK293 cells transfected with NF-κB-Luc (1 μg/mL) and IKKβ in the presence or absence of BIOGF1K. Luciferase activity was measured using a luminometer. **p < 0.01, compared with the control. IκBα, χ inhibitor of κB; IKK, inhibitor of κB kinase; LPS, lipopolysaccharide; NF-κB, nuclear factor-κB; HA, hemagglutinin.
Fig. 4.
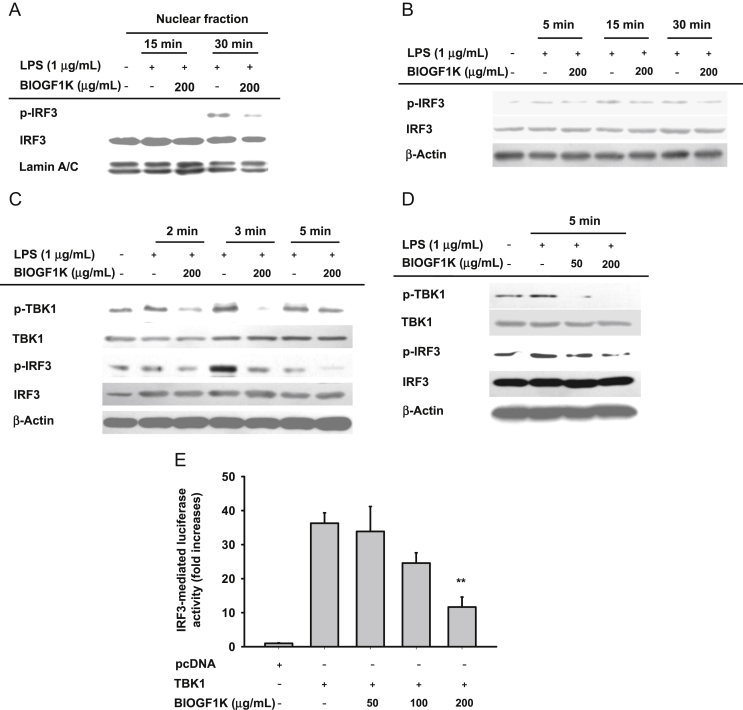
Effect of BIOGF1K on activation of IRF3 and its upstream signaling. (A) Levels of phospho-IRF3, IRF3, and lamin A/C in nuclear fractions were determined by nuclear fractionation and immunoblotting analysis. (B–D) Phospho-protein or total protein levels of IRF3, TBK1, and β-actin in whole cell lysates were determined by immunoblotting analysis. (E) Inhibition of TBK1-induced IRF3 activation by BIOGF1K was analyzed using a reporter gene assay in HEK293 cells transfected with IRF3-Luc (1 μg/mL) and TBK1 in the presence or absence of BIOGF1K. Luciferase activity was measured using a luminometer. **p < 0.01, compared with the control. IRF3, interferon regulatory factor 3; LPS, lipopolysaccharide; TBK1, TANK-binding kinase 1.
3. Results
3.1. Effects of BIOGF1K on NO production and radical scavenging activity
We first investigated BIOGF1K-induced suppression of NO production in LPS-treated RAW264.7 cells to determine the ability of this extract to modulate inflammatory responses. LPS upregulated NO levels in RAW264.7 cells (Fig. 1A), while 200 μg/mL BIOGF1K suppressed up to 67% of this increase (Fig. 1A, left panel). Meanwhile, the standard NO inhibitory compound L-NAME displayed a strong inhibitory activity (Fig. 1A, right panel), as expected [40]. To prove the radical-scavenging activity of BIOGF1K, we employed the DPPH assay (for electron-donating activities). BIOGF1K showed significant electron-donating activities (p < 0.01), which were comparable with that of the standard compound ascorbic acid (100μM; Fig. 1B). Cell viability was intact in RAW264.7 and HEK293 cells treated with BIOGF1K (Fig. 1C), implying that the NO inhibitory activity of this extract was not due to any distracted toxicity. Finally, the level of compound K in BIOGF1K was analyzed using HPLC. As Fig. 1D shows, a peak with a similar retention time to standard compound K was observed at 9.5 min in this extract.
3.2. Effect of BIOGF1K on the transcriptional activity of NF-κB
We examined mRNA levels of an NO-producing enzyme (iNOS) as well as IFN-β in LPS-treated RAW264.7 cells to determine whether BIOGF1K-mediated inhibition of NO release is modulated at the transcriptional or translational level. LPS exposure enhanced the mRNA levels of iNOS and IFN-β, whereas BIOGF1K remarkably inhibited the enhancement of these genes at 100 μg/mL and 200 μg/mL, implying that the transcription process is affected by BIOGF1K (Fig. 2A). As a confirmation, we employed a luciferase reporter gene assay with an NF-κB-Luc construct in HEK293 cells treated with PMA or adaptor molecules (MyD88 and TRIF) to measure the transcriptional activity of NF-κB [41]. MyD88, TRIF, and PMA alone strongly enhanced the luciferase activity up to 140-, 14,000-, or 76-fold, respectively, and addition of BIOGF1K clearly suppressed this activity by 72% at a dose of 200 μg/mL (Figs. 2B–2D), implying that BIOGF1K is able to block NF-κB transcriptional activity.
3.3. Effect of BIOGF1K on upstream signaling for NF-κB activation
Next, we examined whether the activation and translocation of NF-κB could be diminished by BIOGF1K using nuclear fractions, since the nuclear translocation of NF-κB is required for its activation [42]. As we expected, BIOGF1K suppressed the enhancement in the nuclear levels of the NF-κB p65 and p50 subunits at 30–60 min (Fig. 3A). To further investigate the effect of BIOGF1K on upstream signaling events for NF-κB translocation, we examined the phosphorylation patterns of relevant signaling molecules. As we anticipated, LPS treatment upregulated the phosphorylation of IκBα at 5 min, 15 min, and 30 min, whereas the increased patterns were time-dependently suppressed by BIOGF1K (Fig. 3B). Furthermore, phosphorylation of IKKα/β, an upstream kinase of IκBα, was appreciably blocked by BIOGF1K after 5 min of LPS treatment (Fig. 3C), signifying that these enzymes may be pharmacologically targeted by this extract. Furthermore, AKT appreciably enhanced the level of phospho-IKKα/β, and BIOGF1K suppressed such upregulated phosphorylation (Fig. 3D), implicating that IKKα/β might be a target of this extract in NF-κB signaling. To authenticate this molecular target, we performed NF-κB-mediated luciferase assay under IKKβ cotransfection. As we expected, BIOGF1K dose-dependently blocked NF-κB-mediated luciferase activity induced by IKKβ (Fig. 3E) in a dose-dependent manner.
3.4. Effect of BIOGF1K on activation of the IRF3 pathway
As Fig. 2E depicts, TRIF alone induced luciferase activity up to 234-fold, and 200 μg/mL of this extract dose-dependently and significantly (p < 0.01) downregulated luciferase activity up to 63%. BIOGF1K (200 μg/mL) also suppressed phosphorylated IRF3 in both nuclear fractions and whole lysates at 30 min (Figs. 4A, 4B). BIOGF1K also inhibited the phosphorylation of TBK1 (Fig. 4C), which is a critical event for IRF3 phosphorylation [15], indicating that TBK1 could be targeted in BIOGF1K-mediated inhibition of IRF3 activation. Moreover, we investigated the effect of BIOGF1K on upstream signaling events for IRF3 phosphorylation. LPS-induced phosphorylation of IRF3 was blocked by this extract at 5 min (Fig. 4C), while phosphorylation of TBK1 at 2 min and 3 min was strongly diminished. Fig. 4D shows that BIOGF1K was able to dose-dependently (50 μg/mL and 200 μg/mL) inhibit both events triggered by LPS at 5 min. Validation work with overexpressed TBK1 (Fig. 4E) showed upregulation of TBK1 phosphorylation and IRF3-mediated luciferase activity, confirming that TBK1 might be a target of BIOGF1K-mediated anti-inflammatory action.
4. Discussion
Here, we benchmarked in vitro anti-inflammatory activities of BIOGF1K with LPS-treated macrophages and investigated its electron-donating activity induced by DPPH assay, based on its use as a skin care product and moisturizer in the cosmetic industry. Additionally, to elucidate the prospect of developing this extract as a source of potential anti-inflammatory and antioxidative remedies, we identified the molecular and pharmacological mechanisms of this extract.
As shown in Fig. 1, Fig. 2, BIOGF1K inhibited production of the inflammatory mediator NO and the expression of the inflammatory genes iNOS and IFN-β in LPS-stimulated RAW264.7 cells. As iNOS expression is increased in various skin diseases such as atopic eczema and psoriasis [43], [44], the inhibitory effect of BIOGF1K on NO release can explain its known efficacy in cosmetic skin care formulations as well as in curing various skin inflammatory symptoms and diseases. In addition, the antioxidative activity of BIOGF1K (Fig. 1B), as assessed by a DPPH assay, can also protect the skin from stress or UV radiation-induced free radical generation [45]. In fact, strong antioxidants are popularly used in many skin care formulations and as a functional food source [46], [47]. Although BIOGF1K was prepared to increase the level of compound K from ginseng roots, as shown in Fig. 1D, these effects on NO production and antioxidative activity were similar to those reported from Korean Red Ginseng water extract and ginseng saponin fraction [20], [48]. Therefore, the anti-inflammatory and antioxidative components of Korean ginseng could also be extracted in BIOGF1K.
To elucidate the molecular mechanisms of BIOGF1K-mediated anti-inflammatory activity, we explored its effect on the activation of NF-κB, a key transcription factor modulating inflammation [49]. Overall, the data imply that the NF-κB pathway might be a target transcription factor in the BIOGF1K-mediated anti-inflammatory effect. BIOGF1K blocked nuclear translocation of NF-κB subunits (p50 and p65; Fig. 3A) and diminished PMA-induced and MyD88- or TRIF-mediated NF-κB activation (Figs. 2B–2D). BIOGF1K also suppressed IκBα phosphorylation, which is an essential step for NF-κB translocation [50] (Fig. 3B). Phosphorylation of IKKα/β was strongly diminished by this extract at 2–5 min under LPS-stimulated conditions (Fig. 3C). Overexpression of IKKβ increased NF-κB-mediated luciferase activities up to more than 4,000-fold, which was dose-dependently diminished by BIOGF1K (Fig. 3E). Moreover, molecular validation work with AKT and IKKβ overexpression in a reporter gene assay (Fig. 3E) and in immunoblotting analysis (Fig. 3D) clearly confirmed that IKKβ could directly be targeted for suppression of the NF-κB pathway by BIOGF1K. Other phytomedicinal plants such as Cordyceps pruinosa, Davallia bilabiata, Euonymus alatus, and Cynanchi atrati, and some flavonoids such as myricetin also display NF-κB-targeted anti-inflammatory activities through inhibition of IKK [4], [51], [52], [53]. Considering these findings, our data strongly indicate that the anti-inflammatory action of BIOGF1K is mediated by blockade of NF-κB after inhibition of IKK. In addition, compound K, as an active component of ginseng-derived ginsenosides, is able to suppress NF-κB activation in tumor necrosis factor-α-treated human astroglial cells [54] and under dextran sulfate sodium-induced colitic conditions [55]. Therefore, the IKKβ/NF-κB inhibitory action of BIOGF1K can be derived from its major component, compound K.
We previously reported that Korean Red Ginseng water extract and protopanaxadiol saponin fraction are capable of blocking IRF3 activation [20], [56]. Since the inhibitory activity of BIOGF1K on the IKKβ/NF-κB pathway is still marginal, it may have additional targets. BIOGF1K negatively regulated the activation of IRF3 (Fig. 2, Fig. 4), suggesting that the IRF3 pathway might be another target in BIOGF1K-mediated anti-inflammatory action. BIOGF1K diminished nuclear translocation of phospo-IRF3 (Fig. 4A), dose-dependently suppressed the TRIF-induced promoter binding activity of IRF3 (Fig. 2E), and suppressed IRF3 phosphorylation in LPS-stimulated RAW264.7 cells (Fig. 3B). In addition, BIOGF1K diminished TBK1 and IRF3 phosphorylation at 2–5 min under LPS-exposed conditions in a dose-dependent manner (Figs. 4C, 4D) and significantly inhibited TBK1/IRF3-mediated luciferase activity (Fig. 4E). Molecular validation work under TBK1-overexpression conditions also suggests that TBK1 could be another anti-inflammatory target protein of BIOGF1K (Fig. 4). Interestingly, other herbal plants such as Dryopteris crassirhizoma also exhibited IRF3-targeted anti-inflammatory activities through the suppression of TBK1 [20], [57]. Our data strongly imply that suppression of the TBK1/IRF3 pathway could, in part, contribute to the immunopharmacological activity of BIOGF1K.
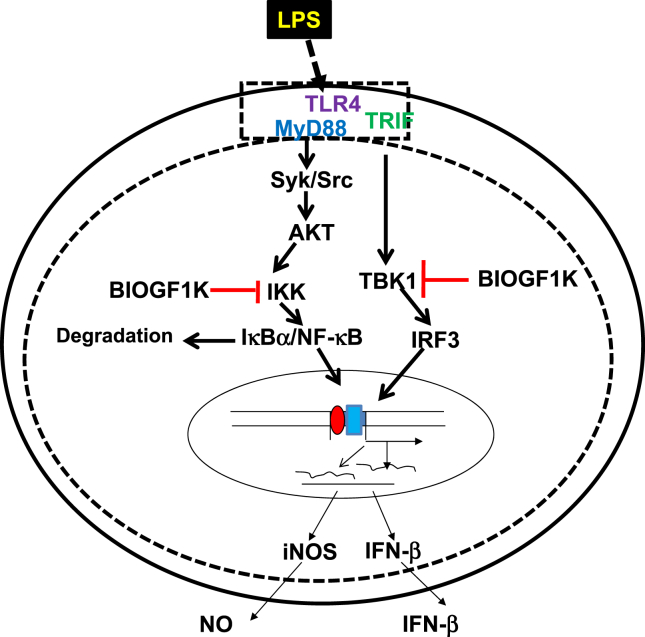
In summary, BIOGF1K possesses strong in vitro antioxidative and anti-inflammatory activities through blockade of both IKK and TBK1, and suppression of the downstream activation of NF-κB and IRF3, as described in Fig. 5. Further studies will focus on validating BIOGF1K as a new anti-inflammatory candidate in preclinical studies.
Fig. 5.
Putative mechanism of BIOGF1K-mediated anti-inflammatory responses. IFN-β, interferon-beta; IκBα. inhibitor of κB; IKK, inhibitor of κB kinase; iNOS, inducible nitric oxide synthase; IRF3, interferon regulatory factor 3; LPS, lipopolysaccharide; MyD88, myeloid differentiation primary response gene 88; NF-κB, nuclear factor-κB; NO, nitric oxide; TBK1, TANK-binding kinase 1; TLR, toll-like receptor; TRIF, Toll/interleukin-receptor domain containing adaptor molecule-inducing interferon-β.
Conflicts of interest
The authors report no conflicts of interest.
Contributor Information
Junseong Park, Email: superbody@amorepacific.com.
Jae Youl Cho, Email: jaecho@skku.edu.
References
- 1.Steel H.C., Cockeran R., Anderson R., Feldman C. Overview of community-acquired pneumonia and the role of inflammatory mechanisms in the immunopathogenesis of severe pneumococcal disease. Mediators Inflamm. 2013;2013:490346. doi: 10.1155/2013/490346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hiraiwa K., van Eeden S.F. Contribution of lung macrophages to the inflammatory responses induced by exposure to air pollutants. Mediators Inflamm. 2013;2013:619523. doi: 10.1155/2013/619523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hossen M.J., Baek K.S., Kim E., Yang W.S., Jeong D., Kim J.H., Kweon D.H., Yoon D.H., Kim T.W., Kim J.H. In vivo and in vitro anti-inflammatory activities of Persicaria chinensis methanolic extract targeting Src/Syk/NF-kappaB. J Ethnopharmacol. 2015;159:9–16. doi: 10.1016/j.jep.2014.10.064. [DOI] [PubMed] [Google Scholar]
- 4.Yang R.C., Chang C.C., Sheen J.M., Wu H.T., Pang J.H.S., Huang S.-T. Davallia bilabiata inhibits TNF-α-induced adhesion molecules and chemokines by suppressing IKK/NF-kappa B pathway in vascular endothelial cells. Am J Chin Med. 2014;42:1411–1429. doi: 10.1142/S0192415X1450089X. [DOI] [PubMed] [Google Scholar]
- 5.Weon J.B., Yun B.R., Lee J., Eom M.R., Ko H.J., Kim J.S., Lee H.Y., Park D.S., Chung H.C., Chung J.Y. Effect of Codonopsis lanceolata with steamed and fermented process on scopolamine-induced memory impairment in mice. Biomol Ther. 2013;21:405–410. doi: 10.4062/biomolther.2013.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Labow R.S., Meek E., Santerre J.P. Model systems to assess the destructive potential of human neutrophils and monocyte-derived macrophages during the acute and chronic phases of inflammation. J Biomed Mater Res. 2001;54:189–197. doi: 10.1002/1097-4636(200102)54:2<189::aid-jbm5>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 7.Ham M., Moon A. Inflammatory and microenvironmental factors involved in breast cancer progression. Arch Pharm Res. 2013;36:1419–1431. doi: 10.1007/s12272-013-0271-7. [DOI] [PubMed] [Google Scholar]
- 8.Lee M.S. Role of innate immunity in diabetes and metabolism: recent progress in the study of inflammasomes. Immune Netw. 2011;11:95–99. doi: 10.4110/in.2011.11.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woo Y., Jeong D., Chung D.H., Kim H.Y. The roles of innate lymphoid cells in the development of asthma. Immune Netw. 2014;14:171–181. doi: 10.4110/in.2014.14.4.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurt-Jones E.A., Popova L., Kwinn L., Haynes L.M., Jones L.P., Tripp R.A., Walsh E.E., Freeman M.W., Golenbock D.T., Anderson L.J. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol. 2000;1:398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- 11.Poltorak A., He X., Smirnova I., Liu M.Y., Van Huffel C., Du X., Birdwell D., Alejos E., Silva M., Galanos C. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi K., Hernandez L.D., Galán J.E., Janeway C.A., Medzhitov R., Flavell R.A. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell. 2002;110:191–202. doi: 10.1016/s0092-8674(02)00827-9. [DOI] [PubMed] [Google Scholar]
- 13.Sato S., Sanjo H., Takeda K., Ninomiya-Tsuji J., Yamamoto M., Kawai T., Matsumoto K., Takeuchi O., Akira S. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat Immunol. 2005;6:1087–1095. doi: 10.1038/ni1255. [DOI] [PubMed] [Google Scholar]
- 14.Hossen M.J., Kim S.C., Yang S., Kim H.G., Jeong D., Yi Y.S., Sung N.Y., Lee J.O., Kim J.H., Cho J.Y. PDK1 disruptors and modulators: a patent review. Expert Opin Ther Pat. 2015;25:513–537. doi: 10.1517/13543776.2015.1014801. [DOI] [PubMed] [Google Scholar]
- 15.Yu T., Yi Y.S., Yang Y., Oh J., Jeong D., Cho J.Y. The pivotal role of TBK1 in inflammatory responses mediated by macrophages. Mediators Inflamm. 2012;2012:979105. doi: 10.1155/2012/979105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He F., Zuo L. Redox roles of reactive oxygen species in cardiovascular diseases. Int J Mol Sci. 2015;16:27770–27780. doi: 10.3390/ijms161126059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim K.H., Kim T.S., Lee J.G., Park J.K., Yang M., Kim J.M., Jo E.K., Yuk J.M. Characterization of proinflammatory responses and innate signaling activation in macrophages infected with Mycobacterium scrofulaceum. Immune Netw. 2014;14:307–320. doi: 10.4110/in.2014.14.6.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halliwell B., Aeschbach R., Löliger J., Aruoma O. The characterization of antioxidants. Food Chem Toxicol. 1995;33:601–617. doi: 10.1016/0278-6915(95)00024-v. [DOI] [PubMed] [Google Scholar]
- 19.MatÉs J.M., Pérez-Gómez C., De Castro I.N. Antioxidant enzymes and human diseases. Clin Biochem. 1999;32:595–603. doi: 10.1016/s0009-9120(99)00075-2. [DOI] [PubMed] [Google Scholar]
- 20.Yang Y., Yang W.S., Yu T., Sung G.H., Park K.W., Yoon K., Son Y.J., Hwang H., Kwak Y.S., Lee C.M. ATF-2/CREB/IRF-3-targeted anti-inflammatory activity of Korean Red Ginseng water extract. J Ethnopharmacol. 2014;154:218–228. doi: 10.1016/j.jep.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Lee B., Sur B., Park J., Kim S.-H., Kwon S., Yeom M., Shim I., Lee H., Hahm D.H. Ginsenoside rg3 alleviates lipopolysaccharide-induced learning and memory impairments by anti-inflammatory activity in rats. Biomol Ther. 2013;21:381–390. doi: 10.4062/biomolther.2013.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim T.G., Lee C.C., Dong Z., Lee K.W. Ginsenosides and their metabolites: a review of their pharmacological activities in the skin. Arch Dermatol Res. 2015;307:397–403. doi: 10.1007/s00403-015-1569-8. [DOI] [PubMed] [Google Scholar]
- 23.Yang X.D., Yang Y.Y., Ouyang D.S., Yang G.P. A review of biotransformation and pharmacology of ginsenoside compound K. Fitoterapia. 2015;100:208–220. doi: 10.1016/j.fitote.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 24.Lee J.Y., Lee Y.G., Lee J., Yang K.J., Kim A.R., Kim J.Y., Won M.H., Park J., Yoo B.C., Kim S. Akt Cys-310-targeted inhibition by hydroxylated benzene derivatives is tightly linked to their immunosuppressive effects. J Biol Chem. 2010;285:9932–9948. doi: 10.1074/jbc.M109.074872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang Y., Yang W.S., Yu T., Yi Y.S., Park J.G., Jeong D., Kim J.H., Oh J.S., Yoon K., Kim J.H. Novel anti-inflammatory function of NSC95397 by the suppression of multiple kinases. Biochem Pharmacol. 2014;88:201–215. doi: 10.1016/j.bcp.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 26.Kim H.G., Yang W.S., Sung G.H., Kim J.H., Baek G.S., Kim E., Yang S., Park Y.C., Sung J.M., Yoon D.H. IKK beta-targeted anti-inflammatory activities of a butanol fraction of artificially cultivated Cordyceps pruinosa fruit bodies. Evid Based Complement Alternat Med. 2014;2014:562467. doi: 10.1155/2014/562467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Starkenmann C., Luca L., Niclass Y., Praz E., Roguet D. Comparison of volatile constituents of Persicaria odorata (Lour.) Sojak (Polygonum odoratum Lour.) and Persicaria hydropiper L. Spach (Polygonum hydropiper L.) J Agric Food Chem. 2006;54:3067–3071. doi: 10.1021/jf0531611. [DOI] [PubMed] [Google Scholar]
- 28.Almela L., Sanchez-Munoz B., Fernandez-Lopez J.A., Roca M.J., Rabe V. Liquid chromatographic–mass spectrometric analysis of phenolics and free radical scavenging activity of rosemary extract from different raw material. J Chromatogr A. 2006;1120:221–229. doi: 10.1016/j.chroma.2006.02.056. [DOI] [PubMed] [Google Scholar]
- 29.Hossen M.J., Jeon S.H., Kim S.C., Kim J.H., Jeong D., Sung N.Y., Yang S., Baek K.S., Kim J.H., Yoon D.H. In vitro and in vivo anti-inflammatory activity of Phyllanthus acidus methanolic extract. J Ethnopharmacol. 2015;168:217–228. doi: 10.1016/j.jep.2015.03.043. [DOI] [PubMed] [Google Scholar]
- 30.Yang Y., Yu T., Jang H.J., Byeon S.E., Song S.Y., Lee B.H., Rhee M.H., Kim T.W., Lee J., Hong S. In vitro and in vivo anti-inflammatory activities of Polygonum hydropiper methanol extract. J Ethnopharmacol. 2012;139:616–625. doi: 10.1016/j.jep.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 31.Hossen M.J., Kim S.C., Son Y.J., Baek K.S., Kim E., Yang W.S., Jeong D., Park J.G., Kim H.G., Chung W.J. AP-1-targeting anti-inflammatory activity of the methanolic extract of Persicaria chinensis. Evid Based Complement Alternat Med. 2015;2015:608126. doi: 10.1155/2015/608126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim M.Y., Cho J.Y. 20S-dihydroprotopanaxatriol modulates functional activation of monocytes and macrophages. J Ginseng Res. 2013;37:300–307. doi: 10.5142/jgr.2013.37.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim M.Y., Cho J.Y. 20S-dihydroprotopanaxadiol, a ginsenoside derivative, boosts innate immune responses of monocytes and macrophages. J Ginseng Res. 2013;37:293–299. doi: 10.5142/jgr.2013.37.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jho E.H., Kang K., Oidovsambuu S., Lee E.H., Jung S.H., Shin I.S., Nho C.W. Gymnaster koraiensis and its major components, 3,5-di-O-caffeoylquinic acid and gymnasterkoreayne B, reduce oxidative damage induced by tert-butyl hydroperoxide or acetaminophen in HepG2 cells. BMB Rep. 2013;46:513–518. doi: 10.5483/BMBRep.2013.46.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blois M.S. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–1200. [Google Scholar]
- 36.Yang Y., Yu T., Lee Y.G., Yang W.S., Oh J., Jeong D., Lee S., Kim T.W., Park Y.C., Sung G.H. Methanol extract of Hopea odorata suppresses inflammatory responses via the direct inhibition of multiple kinases. J Ethnopharmacol. 2013;145:598–607. doi: 10.1016/j.jep.2012.11.041. [DOI] [PubMed] [Google Scholar]
- 37.Back S.S., Kim J., Choi D., Lee E.S., Choi S.Y., Han K. Cooperative transcriptional activation of ATP-binding cassette sterol transporters ABCG5 and ABCG8 genes by nuclear receptors including Liver-X-Receptor. BMB Rep. 2013;46:322–327. doi: 10.5483/BMBRep.2013.46.6.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu T., Lee J., Lee Y.G., Byeon S.E., Kim M.H., Sohn E.H., Lee Y.J., Lee S.G., Cho J.Y. In vitro and in vivo anti-inflammatory effects of ethanol extract from Acer tegmentosum. J Ethnopharmacol. 2010;128:139–147. doi: 10.1016/j.jep.2009.12.042. [DOI] [PubMed] [Google Scholar]
- 39.Jeong Y.H., Hyun J.W., Kim Van Le T., Kim D.H., Kim H.S. Kalopanaxsaponin A exerts anti-inflammatory effects in lipopolysaccharide-stimulated microglia via inhibition of JNK and NF-kappaB/AP-1 pathways. Biomol Ther. 2013;21:332–337. doi: 10.4062/biomolther.2013.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang W.S., Ko J., Kim E., Kim J.H., Park J.G., Sung N.Y., Kim H.G., Yang S., Rho H.S., Hong Y.D. 21-O-angeloyltheasapogenol E3, a novel triterpenoid saponin from the seeds of tea plants, inhibits macrophage-mediated inflammatory responses in a NF-kappaB-dependent manner. Mediators Inflamm. 2014;2014:658351. doi: 10.1155/2014/658351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kang J.W., Park K.D., Choi Y., Baek D.H., Cho W.S., Choi M., Park J.H., Choi K.S., Kim H.S., Yoo T.M. Biodistribution and in vivo efficacy of genetically modified human mesenchymal stem cells systemically transplanted into a mouse bone fracture model. Arch Pharm Res. 2013;36:1013–1022. doi: 10.1007/s12272-013-0132-4. [DOI] [PubMed] [Google Scholar]
- 42.Aggarwal A., Agrawal D.K. Importins and exportins regulating allergic immune responses. Mediators Inflamm. 2014;2014:476357. doi: 10.1155/2014/476357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruzicka T., Ring J. Enhanced releasability of prostaglandin E2 and leukotrienes B4 and C4 from leukocytes of patients with atopic eczema. Acta Derm Venereol. 1987;67:469–475. [PubMed] [Google Scholar]
- 44.Bruch-Gerharz D., Fehsel K., Suschek C., Michel G., Ruzicka T., Kolb-Bachofen V. A proinflammatory activity of interleukin 8 in human skin: expression of the inducible nitric oxide synthase in psoriatic lesions and cultured keratinocytes. J Exp Med. 1996;184:2007–2012. doi: 10.1084/jem.184.5.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pouillot A., Polla A., Polla B.S. Iron and iron chelators: a review on potential effects on skin aging. Curr Aging Sci. 2013;6:225–231. doi: 10.2174/18746098112059990037. [DOI] [PubMed] [Google Scholar]
- 46.Oh J., Kim J.H., Park J.G., Yi Y.S., Park K.W., Rho H.S., Lee M.S., Yoo J.W., Kang S.H., Hong Y.D. Radical scavenging activity-based and AP-1-targeted anti-inflammatory effects of lutein in macrophage-like and skin keratinocytic cells. Mediators Inflamm. 2013;2013:787042. doi: 10.1155/2013/787042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim S.H., Park J.G., Sung G.H., Yang S., Yang W.S., Kim E., Kim J.H., Ha V.T., Kim H.G., Yi Y.S. Kaempferol, a dietary flavonoid, ameliorates acute inflammatory and nociceptive symptoms in gastritis, pancreatitis, and abdominal pain. Mol Nutr Food Res. 2015;59:1400–1405. doi: 10.1002/mnfr.201400820. [DOI] [PubMed] [Google Scholar]
- 48.Aravinthan A., Kim J.H., Antonisamy P., Kang C.W., Choi J., Kim N.S. Ginseng total saponin attenuates myocardial injury via anti-oxidative and anti-inflammatory properties. J Ginseng Res. 2015;39:206–212. doi: 10.1016/j.jgr.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Payne A.S., Freishtat R.J. Conserved steroid hormone homology converges on nuclear factor kappaB to modulate inflammation in asthma. J Investig Med. 2012;60:13–17. doi: 10.231/JIM.0b013e31823d7989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Magnani M., Crinelli R., Bianchi M., Antonelli A. The ubiquitin-dependent proteolytic system and other potential targets for the modulation of nuclear factor-kB (NF-kB) Curr Drug Targets. 2000;1:387–399. doi: 10.2174/1389450003349056. [DOI] [PubMed] [Google Scholar]
- 51.Fu R.H., Liu S.P., Chu C.L., Lin Y.H., Ho Y.C., Chiu S.C., Lin W.Y., Shyu W.C., Lin S.Z. Myricetin attenuates lipopolysaccharide-stimulated activation of mouse bone marrow-derived dendritic cells through suppression of IKK/NF-κB and MAPK signalling pathways. J Sci Food Agric. 2013;93:76–84. doi: 10.1002/jsfa.5733. [DOI] [PubMed] [Google Scholar]
- 52.Jeon J., Park K.A., Lee H., Shin S., Zhang T., Won M., Yoon H.K., Choi M.K., Kim H.G., Son C.G. Water extract of Cynanchi atrati Radix regulates inflammation and apoptotic cell death through suppression of IKK-mediated NF-κB signaling. J Ethnopharmacol. 2011;137:626–634. doi: 10.1016/j.jep.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 53.Oh B.K., Mun J., Seo H.W., Ryu S.Y., Kim Y.S., Lee B.H., Oh K.S. Euonymus alatus extract attenuates LPS-induced NF-κB activation via IKKβ inhibition in RAW 264.7 cells. J Ethnopharmacol. 2011;134:288–293. doi: 10.1016/j.jep.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 54.Choi K., Kim M., Ryu J., Choi C. Ginsenosides compound K and Rh(2) inhibit tumor necrosis factor-alpha-induced activation of the NF-kappaB and JNK pathways in human astroglial cells. Neurosci Lett. 2007;421:37–41. doi: 10.1016/j.neulet.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 55.Li J., Zhong W., Wang W., Hu S., Yuan J., Zhang B., Hu T., Song G. Ginsenoside metabolite compound K promotes recovery of dextran sulfate sodium-induced colitis and inhibits inflammatory responses by suppressing NF-kappaB activation. PLoS ONE. 2014;9:e87810. doi: 10.1371/journal.pone.0087810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang Y., Lee J., Rhee M.H., Yu T., Baek K.S., Sung N.Y., Kim Y., Yoon K., Kim J.H., Kwak Y.S. Molecular mechanism of protopanaxadiol saponin fraction-mediated anti-inflammatory actions. J Ginseng Res. 2015;39:61–68. doi: 10.1016/j.jgr.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang Y., Lee G.J., Yoon D.H., Yu T., Oh J., Jeong D., Lee J., Kim S.H., Kim T.W., Cho J.Y. ERK1- and TBK1-targeted anti-inflammatory activity of an ethanol extract of Dryopteris crassirhizoma. J Ethnopharmacol. 2013;145:499–508. doi: 10.1016/j.jep.2012.11.019. [DOI] [PubMed] [Google Scholar]