Abstract
Molting is an essential developmental process in nematodes during which the epidermal apical extracellular matrix, the cuticle, is remodeled to accommodate further growth. Using genetic approaches, we identified a requirement for three conserved ankyrin repeat-rich proteins, MLT-2/ANKS6, MLT-3/ANKS3, and MLT-4/INVS, in Caenorhabditis elegans molting. Loss of mlt function resulted in severe defects in the ability of larvae to shed old cuticle and led to developmental arrest. Genetic analyses demonstrated that MLT proteins functionally cooperate with the conserved NIMA kinase family members NEKL-2/NEK8 and NEKL-3/NEK6/NEK7 to promote cuticle shedding. MLT and NEKL proteins were specifically required within the hyp7 epidermal syncytium, and fluorescently tagged mlt and nekl alleles were expressed in puncta within this tissue. Expression studies further showed that NEKL-2–MLT-2–MLT-4 and NEKL-3–MLT-3 colocalize within largely distinct assemblies of apical foci. MLT-2 and MLT-4 were required for the normal accumulation of NEKL-2 at the hyp7–seam cell boundary, and loss of mlt-2 caused abnormal nuclear accumulation of NEKL-2. Correspondingly, MLT-3, which bound directly to NEKL-3, prevented NEKL-3 nuclear localization, supporting the model that MLT proteins may serve as molecular scaffolds for NEKL kinases. Our studies additionally showed that the NEKL–MLT network regulates early steps in clathrin-mediated endocytosis at the apical surface of hyp7, which may in part account for molting defects observed in nekl and mlt mutants. This study has thus identified a conserved NEKL–MLT protein network that regulates remodeling of the apical extracellular matrix and intracellular trafficking, functions that may be conserved across species.
Keywords: C. elegans, molting, NIMA kinase, endocytosis, ankyrin repeat proteins
MOLTING is a ubiquitous process in nematode species, including Caenorhabditis elegans, and is required for organismal growth and development. Although the observable biomechanics and major morphological features of C. elegans molting were first described nearly 40 years ago (Hirsh et al. 1976; Singh and Sulston 1978), the molecular and cellular mechanisms underlying this complex process are largely unknown. Notably, molting occurs in both pathogenic and nonpathogenic nematode species, and molting factors have been proposed as potential targets for antiparasitic drugs (Page et al. 2014; Gooyit et al. 2015).
Mechanistically, molting is the process by which nematodes remodel their epidermal apical extracellular matrix (ECM), termed the cuticle. In C. elegans larvae and adults, the cuticle serves as a mechanical barrier and provides physical and chemical protection from the environment (Page and Johnstone 2007). In addition, similar to the chitin-based exoskeleton of insects and other arthropods, the cuticle is required to facilitate organismal movement in conjunction with attached underlying muscles. Unlike arthropods, however, in nematodes the cuticle is comprised primarily of collagens (Page and Johnstone 2007) and thus may serve as a more proximate model for mammalian skin or for the ECM associated with mammalian tissues and organs. In mammals, ECM remodeling is essential for development, homoeostasis, and physiological wound healing (Cox and Erler 2011). Deregulation of ECM remodeling has also been linked to a number of disease states, most notably cancer cell growth and metastasis (Cox and Erler 2011; Yu et al. 2011). Furthermore, the inappropriate deposition of collagens can lead to severe fibrotic diseases such as pulmonary fibrosis (Raghu et al. 1989; Specks et al. 1995; Todd et al. 2012), liver cirrhosis (Murata et al. 1984; Nielsen et al. 2014), cardiovascular diseases (Zannad and Radauceanu 2005), and systemic sclerosis (Ponticos et al. 2015). C. elegans molting thus represents a powerful genetic system for studying conserved molecular mechanisms controlling ECM remodeling.
The C. elegans molting process has been traditionally separated into two stages termed lethargus (including apolysis and synthesis) and ecdysis (Singh and Sulston 1978; Page and Johnstone 2007; Chisholm and Xu 2012). Initially, the animal gradually decreases physical activity and feeding and becomes lethargic. During apolysis, the larva partially detaches its old cuticle, which then allows for the synthesis of an underlying new cuticle by the epidermis. During ecdysis, a series of stereotypical movements lead to the complete detachment of the old cuticle, followed by the resumption of normal feeding and activities (Singh and Sulston 1978; Page and Johnstone 2007; Chisholm and Xu 2012). The rapid and precise regulation of these steps, which includes the attachment of body wall muscles to the newly synthesized cuticle, is essential to minimize vulnerability and maximize growth potential.
Forward genetics and RNA interference (RNAi) screens have identified a variety of genes that promote molting in C. elegans (Frand et al. 2005). Positive regulators of molting include matrix metalloproteases (Davis et al. 2004; Hashmi et al. 2004; Suzuki et al. 2004; Altincicek et al. 2010; Kim et al. 2011; Stepek et al. 2011), which are important for digestion of the old cuticle or processing of the new cuticle precursors, as well as selenoproteins, which promote collagen cross-linking (Stenvall et al. 2011). Other functional classes of molecules critical for molting include sterol-binding nuclear hormone receptors (NHRs) (Kostrouchova et al. 1998, 2001; Gissendanner and Sluder 2000; Hayes et al. 2006; Monsalve and Frand 2012), enzymes controlling sterol and fatty acid synthesis (Jia et al. 2002; Kuervers et al. 2003; Entchev and Kurzchalia 2005; Li and Paik 2011), and hedgehog-related proteins (Zugasti et al. 2005; Hao et al. 2006), which are often modified by sterols (Wendler et al. 2006) and are dependent on NHRs for expression (Kouns et al. 2011). Accordingly, dietary cholesterol promotes normal molting (Yochem et al. 1999; Merris et al. 2003; Entchev and Kurzchalia 2005; Roudier et al. 2005), along with the LRP-1/megalin lipoprotein receptor, which is thought to support lipid uptake by the epidermis (Yochem et al. 1999; May et al. 2007). Although these and other identified molting factors have provided insights into the general processes involved in C. elegans molting, the specific molecular and cellular mechanisms that underlie molting remain poorly understood.
We recently described functions for two highly conserved members of the NIMA kinase family, NEKL-2 and NEKL-3, in C. elegans molting (Yochem et al. 2015). NEKL-2 is orthologous to mammalian NEK8, which has been implicated in ciliogenesis (Mahjoub et al. 2005; Quarmby and Mahjoub 2005; Otto et al. 2008; Shiba et al. 2010; Zalli et al. 2012). Correspondingly, mutations in NEK8 can lead to organogenesis defects resulting from ciliopathies (Mahjoub et al. 2005; Otto et al. 2008, 2011; Trapp et al. 2008; McCooke et al. 2012; Halbritter et al. 2013; Hoff et al. 2013; Manning et al. 2013; Delestre et al. 2015). NEKL-3 is the C. elegans ortholog of mammalian paralogs NEK6 and NEK7, which have been reported to control several processes associated with cell division and are linked to human cancer. Interestingly, our previous studies strongly implicate NEKL-2/NEK8 and NEKL-3/NEK6/NEK7 in cellular processes that are distinct from ciliogenesis and cell division and further implicate NEKL-2/NEK8 in the regulation of intracellular trafficking.
In this study, we have extended the NEKL protein network to include the conserved ankyrin repeat proteins MLT-2/ANKS6, MLT-3/ANKS3, and MLT-4/INVS. Our data suggest that this conserved NEKL–MLT network can be approximately divided into two functional units comprised of NEKL-2–MLT-2–MLT-4 and NEKL-3–MLT-3. Furthermore, we have shown that MLT proteins regulate the subcellular localization of NEKL kinases within the epidermis and may act as molecular scaffolds. Finally, we demonstrated that the NEKL–MLT protein network affects early steps in clathrin-mediated endocytosis in the epidermis. Our studies implicate conserved NIMA kinase family members and their ankyrin repeat partners in the regulation of fundamental cellular functions that have been previously overlooked.
Materials and Methods
Strains and maintenance
C. elegans strains were maintained according to standard protocols (Stiernagle 2006) and were propagated at 21°. Strains used in this study include N2/Bristol (wild type), LH374 [mlt-4(tm1484); mnEx173 (ZC15; pTG96)], RT1378 [pwIs528(gfp::chc-1)], SP2734 [mlt-4(sv9) V; mnEx173 (mlt-4(+); pTG96)], VC20044 [nekl-3(gk296269[E282K])], VC20136 [nekl-3(gk296270[P224L])], VC20396 [nekl-2(gk323150[S351N])], VC20590 [nekl-2(gk113450[A85T])], VC30086 [nekl-2(gk412380[G279E])], VC40715 [nekl-3(gk774978[G118S])], VC40946 [nekl-3(gk894345[D228N])], WY1098 [mlt-3(fd72); fdEx267 (WRM0610dD02; pTG96)], WY1117 [mlt-2(fd71); fdEx272 (WRM0610dG01; WRM0618cD05; WRM0620bC01; WRM0636cH02; WRM0633bB08; pTG96)], WY1114 [nekl-2(fd88[P283A,P284S,P285T])], WY1120 [nekl-2(fd79[Y84L,G87H,G88H]); fdEx273 (pDF166; pTG96)], WY1122 [nekl-2(fd81[Y84L,G88A])], WY1129 [nekl-2(fd83[Y84L,G88Q])], WY1130 [nekl-2(fd84[G87H,G88A,R92L]); fdEx278 (pDF166; pTG96)], WY1141 [nekl-3(gk894345) 3×-outcross], WY1145 [nekl-2(fd81); nekl-3(gk894345); fdEx286(pDF153[nekl-3(+)]; pTG96)]; fdEx286 (pDF153(nekl-3(+); pTG96)], WY1152 [nekl-2(fd89[Y84L,G87A,G88Q]); fdEx278 (pDF166; pTG96)], WY1155 [nekl-2(fd90[Y84L,G87A,G88A]); fdEx278], WY1165 [nekl-2(fd91[Y84L,G87A]; fdEx278)], WY1169 [mlt-2(fd95[mlt-2::mKate2::3xFlag])], WY1174 [nekl-2(fd100[nekl-2::NeonGreen::3×Flag])], WY1180 [nekl-3(fd106[nekl-3::mKate2::3×Flag])], WY1183 [mlt-3(fd109[mlt-3::mKate2::3×Flag])], WY1191 [mlt-4(fd114[mlt-4::gfp::3×Flag])], WY1193 [nekl-2(fd100[nekl-2::NeonGreen::3×Flag]); nekl-3(fd106[nekl-3::mKate2::3×Flag])], WY1194 [nekl-2(fd100[nekl-2::NeonGreen::3×Flag]); mlt-3(fd109[mlt-3::mKate2::3×Flag])], WY1195 [nekl-3(fd106[nekl-3::mKate2::3×Flag]); mlt-4(fd114[mlt-4::gfp::3×Flag])], WY1196 [mlt-2(fd95[mlt-2::mKate2::3×Flag]); mlt-4(fd114[mlt-4::gfp::3×Flag])], WY1230 [nekl-3(fd118[nekl-3::NeonGreen::3×Flag])], WY1237 [mlt-3(fd109[mlt-3::mKate2::3×Flag]); mlt-4(fd114[mlt-4::gfp::3×Flag])], WY1238 [mlt-3(fd109[mlt-3::mKate2::3×Flag]); nekl-3(fd118[nekl-3::NeonGreen::3×Flag])], WY1239 [nekl-2(fd100[nekl-2::NeonGreen::3×Flag]); mlt-4(sv9) V; mnEx173(zc15[mlt-4(+)]; pTG96)], WY1241 [nekl-3(fd118[nekl-3::NeonGreen::3×Flag]); mlt-4(sv9) V; mnEx173 (mlt-4(+); pTG96)], WY1242 [nekl-2(fd81); nekl-3(gk894345); pwIs528 (gfp::chc-1); fdEx256], WY1251 [nekl-3(gk894345); pwIs528], and WY1253 [nekl-2(fd81); pwIs528], WY1303 [nekl-2(gk839); fdEx256 (WRM0639aE11; WRM0636aD02; sur-5::rfp); nekl-3(gk506); mnEx174 (F19H6; pTG96)], WY1305 [mlt-2(fd71); fdEx309; mlt-3(fd72); fdEx267], WY1306 [mlt-2(fd71); fdEx309; mlt-4(tm1484); mnEx173] .
Isolation, mapping, and rescue of mlt-4(sv9)
mlt-4(sv9) was identified in a screen for recessive mutations that affect the completion of molting. N2 P0 worms were treated with 50 mM ethyl methanesulfonate (Brenner 1974), and F1 progeny were placed three per plate on OP50 plates. After growth at 25°, plates were examined for the presence of F2 progeny exhibiting defects in molting, including a failure to completely shed old cuticle. Because mutants exhibiting defective molting are often incapable of reaching adulthood, nonmutant siblings were individually isolated from candidate plates and strains were initially maintained as heterozygotes. Because none of the mutations proved to be temperature sensitive, a temperature of 20–22° was used for their maintenance and analysis.
sv9 was assigned to the right arm of LG V by means of bulk sequence analysis (Wicks et al. 2001) of single-nucleotide polymorphisms (SNPs) between the N2 strain and the Hawaiian strain CB4856. Mapping placed mlt-4 between unc-51 and rol-9; 21 cross-overs were observed between unc-51 and mlt-4, and one was observed between mlt-4 and rol-9. Viable Unc recombinants from a strain having the genotype unc-51mlt-4/CB4856 were examined for the segregation of SNPs that could be digested by a restriction enzyme. Likewise, viable Roll recombinants were examined from a strain with the genotype mlt-4rol-9/CB4856. Rescue of sv9 was initially obtained with the complete ZC15 cosmid. Rescue of sv9 not observed with individual injections of two overlapping PCR fragments, neither of which contained a complete ZC15.7 gene. In contrast, co-injection of these fragments resulted in robust rescue; homologous recombination within the overlapping region will create an intact ZC15.7 gene.
mRNA analysis
Total RNA was isolated from mixed-staged populations of N2 worms by means of TRIzol (Invitrogen, San Diego, CA). The 5′ end of mlt-4 was determined using FirstChoice RNA ligase-mediated rapid amplification of cDNA (RLM-RACE) kit (Ambion, Austin, TX). The 3′ ends were determined by means of reagents supplied with the kit. For both ends, gene-specific primers were used in combination with primers provided with the kit. Internal splice sites were verified by RT-PCR by means of a cMaster RTplusPCR System kit (Eppendorf, Westbury, NY) and gene-specific primers.
Bioinformatics tools
Protein motif and domain predictions were obtained using http://smart.embl-heidelberg.de (Letunic et al. 2015) and elm.eu.org (Dinkel et al. 2016) (Figure 1 and Figure 2 and data not shown). Sequence alignments were created using ClustalW in the Biology Workbench (http://workbench.sdsc.edu/; San Diego Supercomputer Center) (Supplemental Material, Figure S1), in the Jalview program using Muscle (Figure S3, A and B), and with Clustal sequence alignment services (Figure S3C) (Waterhouse et al. 2009). The phylogenetic tree was created by aligning amino acid sequences of selected proteins using http://www.ebi.ac.uk/Tools/msa/mafft/ (Katoh and Standley 2013; McWilliam et al. 2013; Li et al. 2015). Alignment was subsequently reformatted in http://www.ebi.ac.uk/Tools/sfc/emboss_seqret/ (Rice et al. 2000), and the tree was built using http://darwin.uvigo.es/software/prottest2_server.html (Abascal et al. 2005) and http://itol.embl.de/upload.cgi (Letunic and Bork 2016) (Figure S2). Frequency plot sequence logos were created using WebLogo (http://weblogo.berkeley.edu/logo.cgi) (Crooks et al. 2004) (Figure S3). Protein 3D structure predictions were created using I-TASSER (http://zhanglab.ccmb.med.umich.edu/I-TASSER) (Zhang 2008; Roy et al. 2010; Yang et al. 2015). For mutant versions of NEKL-2, the wild-type prediction was assigned as a template to guide I-TASSER modeling (Figure S4). Visualization, interatomic contacts, and surface analyses of predicted three-dimensional models were carried out using UCSF Chimera 1.11 (Pettersen et al. 2004) (Figure S4). Raytraced images were produced using POV-Ray (retrieved from http://www.povray.org/download/; Persistence of Vision Pty.) (Figure S4). Venn diagrams were created using Venny (http://bioinfogp.cnb.csic.es/tools/venny), created by J. C. Oliveros (Figure S4A).
Figure 1.
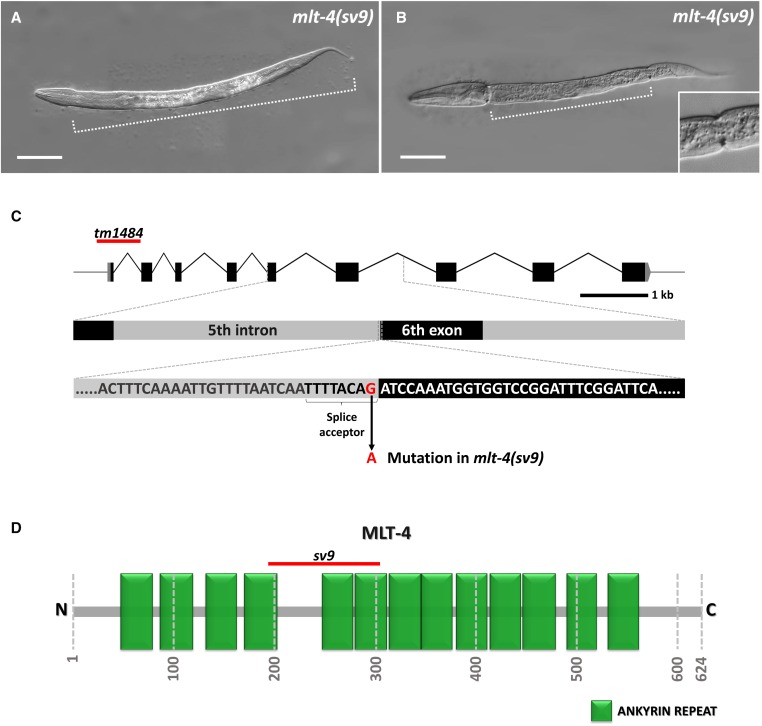
Molting defects in mlt-4(sv9) mutant animals. (A, B) DIC images of sv9 homozygous larvae entrapped within two layers of cuticle. Dotted line indicates the extent of the constricted region containing both the old and new cuticle layers. A larva in which only the head region is free of old cuticle is shown in (A), whereas the larva in (B) displays a classic corset phenotype in which both the head and tail regions have released the old cuticle. The inset in (B) shows an enlargement of the posterior constriction. (C) Schematic representation of the mlt-4 gene; the affected region with the splice acceptor site that is mutated in the sv9 allele is enlarged. The red line marks the location of the tm1484 deletion. (D) Schematic illustration of MLT-4 with annotated predicted ankyrin repeats (green boxes). The red line indicates the region that would be affected by mis-splicing in sv9 mutants. Numbers specify positions of amino acids. Bar, 50 µm (A, B).
Figure 2.
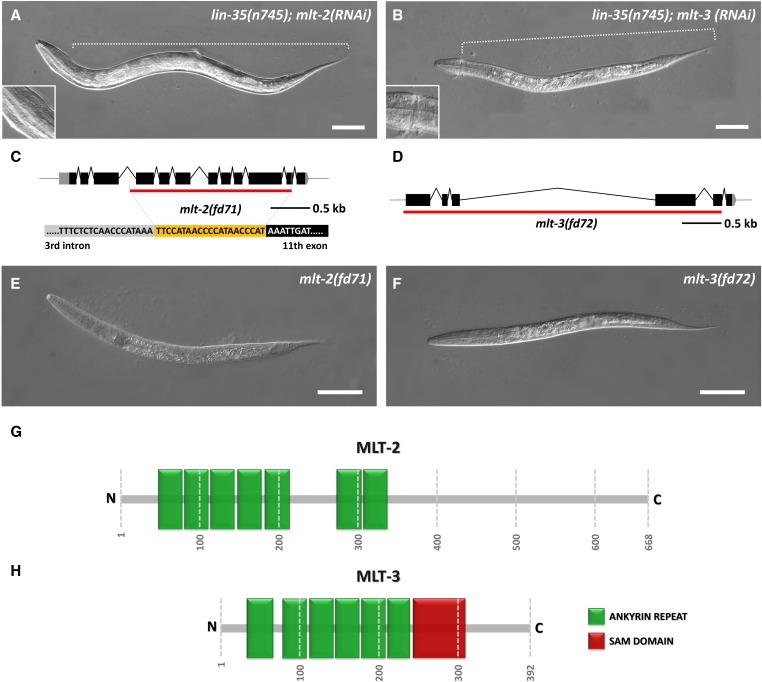
Molting defects in mlt-2 and mlt-3 mutants. (A, B) DIC images of lin-35(n745) animals, which display partial encasement within old cuticle following mlt-2(RNAi) (A) and mlt-3(RNAi) (B). Enlargements show constrictions within the head regions. (C, D) Schematic representations of mlt-2 (C) and mlt-3 (D); red lines indicate deleted regions in mlt-2(fd71) (C) and mlt-3(fd72) (D). The yellow region in (C) represents the inserted oligonucleotide that replaces the deleted region of mlt-2(fd71). (E, F) DIC images of homozygous mlt-2(fd71) (E) and mlt-3(fd72) (F) deletion mutants. (G, H) Schematic illustration of MLT-2 (G) and MLT-3 (H) proteins with annotated predicted ankyrin repeats (green boxes) and SAM domain (red box). Numbers specify positions of amino acids. Bar, 50 µm (A, B, E, and F).
Microscopy and measurements
Fluorescent images were acquired using an Olympus IX81 inverted microscope with a spinning-disc confocal head (CSU-X1 Yokogawa). Confocal illumination was provided by an ILE-4 laser launch (Spectral Applied Research). MetaMorph 7.7 software was used for image acquisition. During colocalization analyses, image registration of micrographs taken with different laser settings was performed with the Register Virtual Stack plugin in FIJI software. Registered images were merged, analyzed, and adjusted in ImageJ program. DIC images were acquired using a Nikon Eclipse epifluorescence microscope and Open Lab software. The same setup was used for measuring body length and width. Body width was measured in the central region of an animal, next to the vulva; 50 animals were scored for each category. Puncta movements were tracked and analyzed using MTrackJ plugin in ImageJ software. Animals were immobilized using a 0.1 M solution of levamisole. Nuclear staining was performed using Hoechst stain, which was added to levamisole to a final concentration of 0.2 µg/µl.
RNA interference
RNAi was performed using bacterial strains from Geneservice library. The standard feeding protocol was followed (Ahringer 2005). Because mlt-2(RNAi) does not cause molting defects in nonsensitized backgrounds, marker localization studies involving mlt-2(RNAi) were carried out using strains that were previously grown for one or two generations on lin-35(RNAi) plates, which increases RNAi susceptibility (Wang et al. 2005); this procedure was used for mlt-3(RNAi), although partially penetrant molting defects were observed for mlt-3(RNAi) in wild-type backgrounds. lin-35(RNAi) presensitization was not performed in other experiments. For the mlt-4(sv9) enhancement study, animals were fed with nekl-2(RNAi) or nekl-3(RNAi) for several generations prior to evaluation. Their progeny were then synchronized and analyzed after 2 days. gfp(RNAi) was used as an RNAi control except for experiments involving gfp::chc-1, in which case strains carrying pPD129.36 were used; pPD129.36 expresses an ∼200-bp dsRNA sequence that is not homologous to any C. elegans gene (Timmons et al. 2001).
Genetic mosaic analyses
Genetic mosaic analyses were performed using previously described methods (Yochem et al. 1998, 2000, 2006). Only healthy L4 and young adult mosaic animals were analyzed. Strains used in this study were SP2734, WY1098, and WY1117.
CRISPR-Cas9 generation of mlt-2 and mlt-3 deletion alleles
Using the CRISPR design tool (http://crispr.mit.edu), mlt-2 and mlt-3 sequences with high scores were chosen in regions close to the start and stop codons. Selected sequences were used for designing primers for site-directed mutagenesis to insert specific sequences into pDD162 (Peft-3::Cas9 + empty sgRNA) using the Q5 Site-Directed Mutagenesis Kit (New England Biolabs, Beverly, MA). Primers used in these experiments included mlt-2 5′ forward, 5′-taacacttgcatgtgcggggttttagagctagaaatagcaagt-3′; mlt-2 3′ forward, 5′-ataattccgtaaagcctgggttttagagctagaaatagcaagt-3′; mlt-3 5′ forward 1, 5′-tattgccagatctactcgagttttagagctagaaatagcaagt-3′; mlt-3 5′ forward 2, 5′-gatggaacatatgtgactcgttttagagctagaaatagcaagt-3′; mlt-3 3′ forward 1, 5′- ctctggagagcttaggatcgttttagagctagaaatagcaagt-3′; mlt-3 3′ forward 2, 5′-gagagcttaggatccggtcgttttagagctagaaatagcaagt-3′; and universal reverse, 5′-caagacatctcgcaatagg-3′.
Animals were injected with pDD162 derivatives and the pTG96 co-injection marker. Green F1 progeny were allowed to lay eggs before they were lysed and their genomic DNA was used for PCR screening for deletions. Because mlt-2 and mlt-3 are essential, viable heterozygotes were injected with appropriate fosmids and pTG96. For mlt-2 rescue, a mix of five fosmids was used: WRM0610dG01; WRM0618cD05; WRM0620bC01; WRM0636cH02; and WRM0633bB08. mlt-3 rescue was performed with fosmid WRM0610dD02. Candidate rescued homozygous strains were confirmed and characterized by DNA sequence analysis.
CRISPR-Cas9 generation of nucleotide substitutions
To generate nekl-2 hypomorphic alleles, genomic substitutions were made using the coconversion strategy for the induction of point mutations (Arribere et al. 2014; Paix et al. 2014). Briefly, pJA42 (Cas9 + rol-6 sgRNA construct) and rol-6(gof) donor ssDNA were co-injected with appropriate pDD162 derivatives and ssDNAs to create point mutations in desired genes. The following primers were used for site-directed mutagenesis of pDD162: nekl-2(fd88) forward, 5′-tcgaacctccaccgacggataaggtaatttttaattg-3′; nekl-2(fd79)/(fd81)/(fd83)/(fd84)/(fd89)/(fd90)/(fd91) forward, 5′-ttatgcagtacgcggaagggttttagagctagaaatagcaagt-3′; universal reverse, 5′-caagacatctcgcaatagg-3′. Repair oligos containing the desired mutations, together with novel restriction sites used for screening included nekl-2(fd88), ttgttcttccctatttgatatcaattcattgcgatttgggaagaatcgaagctagcacgacggataaggtaatttttaattgtattagtgttttgatttagagaaaaat; nekl-2(fd79), gtaaatttaattttaaaattgaattaaaattcgatatgttcagttatgcagctggcggaacatcacacattagagagattaataaatgatcagagagcgattaaagattcaaacatga; nekl-2(fd81), attttaaaattgaattaaaattcgatatgttcagttatgcagtacgcggaacatgccacattagagctcttaataaatgatcagagagcgattaaagattcaaacatgagagaatatttt; nekl-2(fd83), gtaaatttaattttaaaattgaattaaaattcgatatgttcagttatgcagctggcggaaggacaaacattagagagattaataaatgatcagagagcgattaaagattcaaacatga; nekl-2(fd84), attttaaaattgaattaaaattcgatatgttcagttatgcagtacgcggaacatgccacattagagctcttaataaatgatcagagagcgattaaagattcaaacatgagagaatatttt; nekl-2(fd89), gtaaatttaattttaaaattgaattaaaattcgatatgttcagttatgcagctggcggaagcacaaacattagagagattaataaatgatcagagagcgattaaagattcaaacatga; nekl-2(fd90), gtaaatttaattttaaaattgaattaaaattcgatatgttcagttatgcagctggcggaagcagcaacattagagagattaataaatgatcagagagcgattaaagattcaaacatga; and nekl-2(fd91), gtaaatttaattttaaaattgaattaaaattcgatatgttcagttatgcagctggcggaagcaggaacattagagagattaataaatgatcagagagcgattaaagattcaaacatga. The introduced restriction sites were as follows: NheI for nekl-2(fd88), PvuII for nekl-2(fd79)/(fd81)/(fd83)/(fd89)/(fd90)/(fd91), and SacI for nekl-2(fd84).
F1 progeny of injected animals were selected for the Rol phenotype, were allowed to lay eggs, and were then screened by single-worm PCR and restriction enzyme analysis to identify candidate heterozygotes containing the desired mutations. F2 progeny were screened as above, and, in the case of homozygous-lethal alleles, heterozygotes were injected with a nekl-2(+) construct (pDF166) and pTG96 to obtain homozygous rescued lines. All homozygous lines were confirmed by sequencing.
CRISPR-Cas9 generation of fluorescently tagged proteins
C-terminal fluorophore::3×Flag insertions were created using Cas9-mediated homologous recombination using the self-excising selection cassette method (Dickinson et al. 2015). pDD162-based constructs expressing Cas9 and sgRNAs were generated using Q5 Site-Directed Mutagenesis Kit and the following primers: nekl-2 forward, 5′-ttgcccacttccgaatgatgttttagagctagaaatagcaagt-3′; nekl-3 forward, 5′-ggagttgttgattggtcccgttttagagctagaaatagcaagt-3′; mlt-4 forward, 5′-ctcaaattcggtgctccgagttttagagctagaaatagcaagt-3′; universal reverse, 5′-caagacatctcgcaatagg-3′. For mlt-2 and mlt-3, the same 3′ Cas9-sgRNA constructs were used in the generation of mlt-2 and mlt-3 deletion alleles. Repair templates were created by inserting homology arms into AvrII- and SpeI-digested fluorophore-containing plasmids pDD268 (NeonGreen::3×Flag), pDD282 (gfp::3×Flag), and pDD285 (mKate2::3×Flag). Homology arms were either PCR amplified (∼700 bp) or ordered as gene blocks (440 bp) and inserted into constructs using NEBuilder HiFi DNA Assembly (New England Biolabs), which uses modified Gibson assembly methods. Homology arm PCR amplification primers were as follows: mlt-2 5′ arm forward, 5′-acgttgtaaaacgacggccagtcgccggcacaggaatgccaaagttttgctg-3′ and reverse, 5′-catcgatgctcctgaggctcccgatgctcccagtgatcggctaagaattgc-3′; mlt-3 3′ arm forward, 5′-cgtgattacaaggatgacgatgacaagagatgattttaattttttttttttgagaaag-3′ and reverse, 5′-ggaaacagctatgaccatgttatcgatttcgccccagaatgaatttctatg-3′; nekl-2 5′ arm forward, 5′-acgttgtaaaacgacggccagtcgccggcacttacttaaaacacacacgga-3′ and reverse, 5′-catcgatgctcctgaggctcccgatgctccatactttgaatgcacttgact-3′; nekl-3 3′ arm forward, 5′-cgtgattacaaggatgacgatgacaagagataaaaaaagctataacatttcaatttc-3′ and reverse, 5′-ggaaacagctatgaccatgttatcgatttcaatgtggcaaacacagtgtta-3′. PAM sites were mutated in repair templates, without amino acid changes. The presence of the homology arms was confirmed by sequencing. Co-injection markers were pTG96 and pCFJ90 (Pmyo-2::mCherry), and selection was based on the Rol phenotype and resistance to Hygromycin B. Positive homozygous animals were heat shocked at 32° for 4–6 h to activate the Cre recombinase, which removes the self-excising selection cassette flanked by LoxP sites. PCR amplification confirmed the presence of insertions within desired genes.
Yeast two-hybrid analysis
Identification of physical interactions between NEKL-3 and MLT-3 was performed using the ProQuest Two-Hybrid System (Invitrogen, Carlsbad, CA). The gateway cloning system was used to generate entry clones, bait, and prey plasmids. To generate entry clones, nekl-3 cDNA was amplified using 5′-ggggacaagtttgtacaaaaaagcaggcttcatggacaaaatttcgaacatcta-3′ and 5′-ggggaccactttgtacaagaaagctgggtcttagaattgcgttgaaggagttgtt-3′ primers, whereas mlt-3 amplification was accomplished with 5′-ggggacaagtttgtacaaaaaagcaggcttcatgtcgtttcgtgtccgtttttc-3′ and 5′- ggggaccactttgtacaagaaagctgggtctcaaacatgtgaggaaacttttaaa-3′ primers. The BP clonase recombination reaction between cDNA clones and pDONR221 was used to create entry vectors, which were used in the LR clonase recombination reaction with the bait destination vector pDEST32 and prey destination vector pDEST22. All constructs were sequenced to confirm the presence of the correct insert. Yeast strain MaV203 was cotransformed with all possible combinations of nekl-3/mlt-3/control empty vector bait and nekl-3/mlt-3/control empty vector prey constructs. Strains were tested for HIS3 self-activation by addition of 3-amino-1,2,4-triazole (3-AT) to SC-Leu-Trp-His medium. After the HIS3 self-activation threshold was determined, yeast strains were tested for their ability to grow on selective media SC-Leu-Trp-Ura, SC-Leu-Trp-His + 3-AT, and SC-Leu-Trp + 5-fluoroorotic acid (5-FOA). LacZ /β-gal activity was determined using chlorophenol red-β-d-galactopyranoside (CPRG) as a substrate. β-gal activity was determined as follows: β-gal units = (1000 × absorbance at 574 nm)/[(duration of reaction) × (sample volume) × (absorbance at 600 nm)]. Control constructs were provided by the manufacturer, including pEXP32/Krev1 bait (same for all controls), pEXP22/RalGDS-wt prey (for strong positive control), pEXP22/RalGDS-m1 prey (for weak positive control), and pEXP22/RalGDS-m2 prey (for negative control).
Data availability
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article. Reagents or specific data from this manuscript available upon request.
Results
C. elegans mlt-4 is required for normal molting and encodes a conserved ankyrin repeat protein
The sv9 allele was identified in a screen for recessive mutations that affect the completion of molting. Homozygous sv9 mutants typically arrest as L2- to L3-stage larvae, after failing to shed portions of old cuticle during molting cycles. In most sv9 homozygotes, the central body and tail regions are trapped within two layers of cuticle, whereas the head region contains only a single layer (Figure 1A). Other sv9 homozygotes display the “corset” phenotype in which both the head and tail regions are free of old cuticle (Figure 1B), similar to what is observed for nekl-3(sv3) mutants (Yochem et al. 2015).
sv9 was mapped to a region on LG V, and microinjection of cosmid ZC15, which contains a genomic insert corresponding to a portion of the implicated region, fully rescued the sv9 molting defect (see Materials and Methods). sv9 was subsequently rescued by a single open reading frame, ZC15.7 (hereafter referred to as mlt-4). Sequencing of the mlt-4 locus in sv9 mutants revealed a single G-to-A transition of the final nucleotide of the fifth intron (Figure 1C). This mutation affects the highly conserved G of the canonical 3′ splice acceptor site (TTTTCAG) and may reduce or eliminate incorporation of exon 6 into the mature mlt-4 mRNA. We note that mlt-4 activity was largely recalcitrant to RNAi and thus was not used in this study.
We also obtained a putative null deletion allele of mlt-4 (tm1484) (The C. elegans Deletion Mutant Consortium 2012), which contains a 637 bp in frame deletion, including 187 bp upstream from the start codon (Figure 1C). This deletion completely eliminates the 5′ UTR, the first exon, and most of the first intron of mlt-4. mlt-4(tm1484) homozygotes display 100% larval lethality, consistent with a strong loss-of-function or null mutation. We also examined the F1 progeny from eight germline-mosaic mlt-4(tm1484) mothers and observed complete encasement within old cuticle at the L1/L2 molt (n = 435).
The mlt-4 mRNA is derived from nine exons (Figure 1C) and encodes a predicted 624-aa protein. Using 5′ RACE, we determined that the mlt-4 mRNA begins 38 nucleotides upstream of the presumed AUG start codon and lacks a trans-spliced leader. Moreover, alternative forms of the mlt-4 mRNA are not abundant (also see WormBase). The predicted MLT-4 protein contains 13 tandem copies of an ankyrin repeat domain (Figure 1D). Splicing from exon 5 to exon 7 in sv9 mutants would be predicted to eliminate the fifth ankyrin repeat, as well as parts of the fourth and sixth repeats, but would not induce a frameshift. Ankyrin repeat domains are important for protein–protein interactions and can serve as molecular scaffolds (Mosavi et al. 2004; Voronin and Kiseleva 2008; Hollenbeck et al. 2012). Based on reciprocal BLAST and OrthoList (Shaye and Greenwald 2011), MLT-4 is orthologous to human inversin (INVS), and more similar in amino acid sequence than a previously reported INVS ortholog, NPHP-2 (Figure S1 and Figure S2) (Warburton-Pitt et al. 2012). Unlike MLT-4, however, INVS contains a C-terminal region that is largely intrinsically disordered. Although specific amino acid sequences within this region are not highly conserved in mammals, MLT-4 lacks the C-terminal region of mammalian INVS proteins altogether (Figure S1). Also unlike MLT-4, INVS contains two predicted IQ domains, which are implicated in Ca2+-independent calmodulin binding (Rhoads and Friedberg 1997).
The conserved ankyrin repeat proteins MLT-2 and MLT-3 are essential for normal molting
The C. elegans genome is predicted to encode ∼13 proteins with sequence similarity to MLT-4/INVS, most of which contain ankyrin repeats (Figure S2 and Table S1). To determine if any of these proteins play a role in the regulation of molting, we carried out RNAi against each gene in two RNAi-hyper-sensitive strains [lin-35(n745) and rrf-3(pk1426)] and scored for molting defects. Notably, downregulation of two genes, C01H6.2 and Y39C12A.1, led to partial entrapment within old cuticle, which can occur at any of the four molting stages (Figure 2, A and B, Table S1, and data not shown). We hereafter refer to C01H6.2 as mlt-2 and Y39C12A.1 as mlt-3.
mlt-2 encodes a predicted protein of 668 aa with seven N-terminal ankyrin repeat domains along with a C-terminal region that is structurally uncharacterized (Figure 2G). Several human proteins are similar to MLT-2, including the annotated ortholog ANKS6 (Shaye and Greenwald 2011) (Figure S1). Unlike MLT-2, however, ANKS6 contains a C-terminal sterile alpha motif (SAM) domain, which is implicated in both protein and RNA interactions (Kim and Bowie 2003). mlt-3 encodes a predicted 392-aa protein with six N-terminal ankyrin repeat domains and one C-terminal SAM domain (Figure 2H) and is orthologous to mammalian ANKS3 (Figure S1) (Shaye and Greenwald 2011).
Using CRISPR-Cas9 methods, we generated deletion alleles for both mlt-2 and mlt-3. fd71 contains an indel in which 2051 nucleotides spanning exons 4–11 of mlt-2 were deleted and replaced with a 21-bp insertion (Figure 2C). fd72 contains a 4028-nucleotide deletion, which removes approximately the first five exons of mlt-3 (Figure 2D). One hundred percent of homozygous mlt-2(fd71) and mlt-3(fd72) mutants arrested as L1–L2 larvae that were fully encased within two layers of cuticle (Figure 2, E and F), similar to what we observed for null deletion alleles of nekl-2 and nekl-3 (Yochem et al. 2015). Notably, the phenotypes of mlt-2(fd71); mlt-3(fd72) and mlt-2(fd71); mlt-4(tm1484) double mutants did not differ from the phenotypes observed in the single mutants (data not shown). Likewise, nekl-2(gk839); nekl-3(gk506) double null mutants showed identical phenotypes to single mutants (data not shown). Furthermore, the phenotypes of mlt-2(fd71), mlt-3(fd72), or mlt-4(tm1484) alleles were not enhanced by RNAi of mlt-2, mlt-3, nekl-2, or nekl-3 (data not shown). These results indicate that the MLTs and NEKLs do not have redundant functions earlier in development. Collectively, our findings demonstrate that conserved ankyrin repeat proteins are specifically required for the completion of molting in C. elegans.
mlt-2, mlt-3, and mlt-4 are required in the epidermal hyp7 syncytium
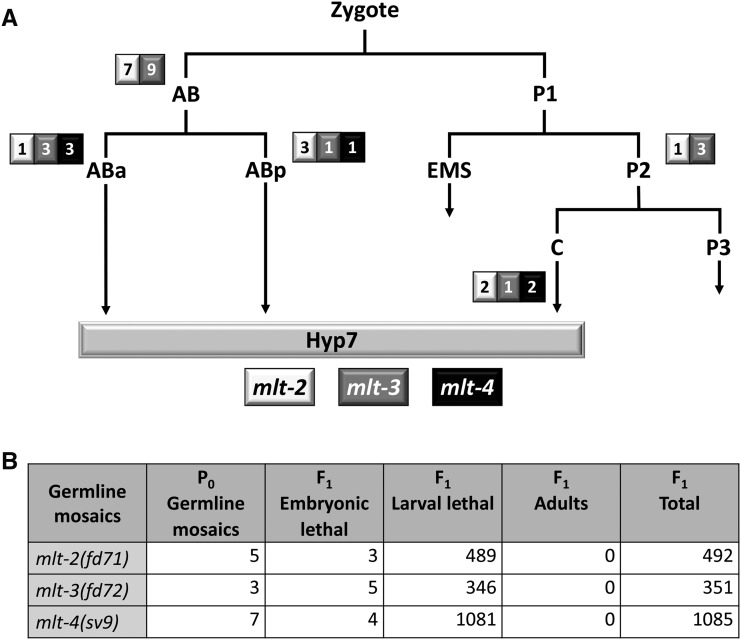
To elucidate the cell types in which mlt-2, mlt-3, and mlt-4 activities are essential, we carried out genetic mosaic analyses using standard methods (Yochem et al. 1998, 2000, 2006). For these studies, mutant strains containing GFP-marked mlt(+) rescuing extrachromosomal arrays were analyzed for array inheritance within the cell lineages of viable young adults or L4 larvae. The absence of the rescuing array within a specific cell lineage in aphenotypic animals implies that gene function is not required within that lineage or its derived tissues. Notably, this analysis implicated mlt-2, mlt-3, and mlt-4 as being specifically required in the hyp7 epidermal syncytium, a large multinucleate cell that covers most of the midbody of the worm. A series of cell fusion events during embryonic and postembryonic development generate hyp7, with contributions coming from the ABa, Abp, and C blastomeres (Sulston et al. 1983). Thus, segregation of the array within any one of these founder cells can be sufficient to rescue a gene requirement within hyp7.
In the case of mlt-2, mlt-3, and mlt-4 mutants, we observed that the presence of rescuing arrays within only ABa or ABp was able to fully suppress molting defects (Figure 3A). Moreover, in the case of mlt-4, viable adults were identified that contained positive clones in only ABarp (two mosaics), ABarpaa (one mosaic), or ABplapa (one mosaic). Correspondingly, the presence of a rescuing array within C only was able to rescue molting defects in all three mlt mutants (Figure 3A). Because the hyp7 syncytium is derived exclusively from descendants of the ABa, ABp, and C lineages, the most straightforward interpretation is that mlt-2, mlt-3, and mlt-4 are specifically required in hyp7, consistent with previous findings for nekl-2 and nekl-3 (Yochem et al. 2015). Furthermore, mosaic analyses indicated that there is no maternal requirement or functional contribution of mlt-2, mlt-3, or mlt-4. Namely, the progeny of animals in which rescuing arrays were absent from the maternal germline arrested with molting defects at the same developmental stage as (array-minus) mutants derived from germline-positive adults (Figure 3B). This finding is consistent with previous results for nekl-2 and nekl-3 and indicates that neither the NEKL nor MLT proteins play a critical role during embryogenesis. We note, however, that our results do not preclude a nonessential role for MLT and NEKL proteins in other epidermal syncytia.
Figure 3.
Mosaic analysis of mlt genes. (A) The early cell lineage of C. elegans is depicted showing contributions of ABa, ABp, and C to the epidermal syncytium, hyp7. The number of viable adult mosaic animals in which the rescuing array was present exclusively within AB, ABa, ABp, P2, or C in mlt-2(fd71), mlt-3(fd72), and mlt-4(sv9) mutants is shown in the adjacent boxes. For each mutant, the only descendant common to all the identified mosaic animals was hyp7. (B) Summary of phenotypes for the F1 progeny of mlt-2, mlt-3, and mlt-4 germline-mosaic animals. In all cases, larvae arrested with molting defects and no maternal contribution was observed.
Fluorescently tagged MLT-2, MLT-3, and MLT-4 proteins are expressed in the epidermis and show dynamic movement within puncta
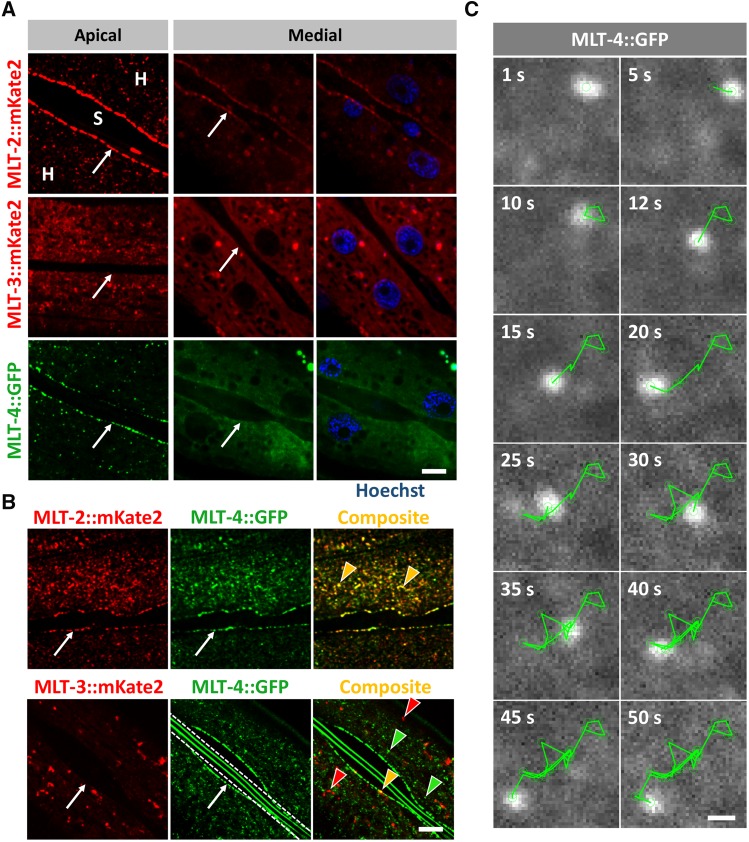
To determine the tissue and subcellular expression patterns of MLT-2, MLT-3, and MLT-4, we used CRISPR-Cas9 methods to add fluorescent tags to the endogenous loci. Expression of all three genes was detected exclusively within the epidermis, including hyp7, beginning in late embryogenesis (Figure 4A and data not shown). MLT-2::mKate2 and MLT-4::GFP displayed similar patterns of expression at the epidermal apical surface within well-separated puncta and also showed strong accumulation at the boundary of hyp7 and seam cells. In more medial planes, both MLT-2 and MLT-4 were more evenly dispersed throughout the cytosol with reduced accumulation at the seam cell boundary (Figure 4A). In contrast, accumulation of MLT-3::mKate2 was not highly enriched at the hyp7–seam cell boundary (Figure 4A and data not shown). Like MLT-2 and MLT-4, MLT-3 was expressed in apical puncta, although its expression was stronger and typically more dispersed than that observed for MLT-2 and MLT-4 (Figure 4A). In medial planes, expression of MLT-2, MLT-3, and MLT-4 was largely excluded from nuclei, but in some cases the tagged proteins exhibited partial enrichment or exclusion within other smaller compartments (Figure 4A). We note that animals homozygous for the modified mlt-3::mKate2 locus displayed a partially penetrant molting-defective phenotype, suggesting that fluorophore insertion may have slightly impaired the activity of the endogenous mlt-3 gene.
Figure 4.
Expression analysis of MLT proteins. (A) Confocal images of fluorescently labeled MLT-2::mKate2, MLT-3::mKate2, and MLT-4::GFP show expression in epidermis. Punctate accumulations were observed near the apical surface of the hyp7 syncytium (H), but not in the neighboring seam cells (S). MLT-2 and MLT-4 also show strong enrichment at the hyp7–seam cell boundary (white arrows). In medial planes, MLT-2, MLT-3, and MLT-4 are largely expressed in the cytosol and are largely absent from nuclei (blue) labeled with Hoechst. Bar, 5 µm. (B) Colocalization analyses revealed extensive overlap between apical puncta of MLT-2::mKate2 and MLT-4::GFP but not MLT-3::mKate2 and MLT-4::GFP. Images in A were modified to remove background fluorescence to highlight expression in puncta. Red and green arrowheads indicate representative puncta that express only one marker, whereas yellow arrowheads indicate colocalization. White arrows indicate hyp7–seam cell boundaries. White dashed lines demarcate autofluorescent alae. Bar, 5 µm. (C) Representative time lapse movie frames of MLT-4::GFP apical puncta. Green line traces the direction of particle movement. Bar, 0.5 µm.
To determine the extent of expression overlap between MLT-2, MLT-3, and MLT-4, we constructed doubly marked strains. Extensive colocalization was observed between MLT-2::mKate2 and MLT-4::GFP at apical puncta and at the hyp7–seam cell boundary, although some nonoverlapping puncta were also observed (Figure 4B). In addition, we noticed that MLT-2::mKate2 had some tendency to form larger accumulations, which were not observed for MLT-4::GFP (Figure 4, A and B and data not shown). In contrast to MLT-2 and MLT-4, MLT-3::mKate2 and MLT-4::GFP signals did not overlap significantly (Figure 4B). Taken together, our expression data are consistent with roles for MLT-2, MLT-3, and MLT-4 in the epidermis and suggest that MLT-2 and MLT-4 may have closely connected cellular functions.
Notably, all three MLT reporters were visible as highly dynamic structures in the epidermis (Figure 4C, File S1, File S2, File S3, File S4, File S5, and data not shown). In general, larger accumulations were less mobile and displayed short oscillating or linear movements, or no motility, within the time frame of the analysis (25–50 sec). In contrast, numerous smaller puncta throughout hyp7 displayed rapid linear and circular dynamics but were hard to trace given their movement in and out of the focal plane. We observed that smaller puncta in some cases branch out from larger accumulations or merge between each other and with bigger puncta. A high level of motility was observed at the hyp7–seam cell boundary region for all three MLTs including MLT-3::mKate2, which was not highly enriched in this region. Where possible, we quantified average velocities of puncta that were moving within a single horizontal plane, which included mostly oscillating larger puncta at the apical surface. For MLT-2::mKate2 the average velocity of puncta was 43.7 nm/sec, ranging from 13.3 to 117 nm/sec (51 puncta, eight animals). MLT-3::mKate2 had an average velocity of 59.2 nm/sec, ranging from 40.3 to 80.9 nm/sec (16 puncta, four animals). MLT-4::GFP puncta had an average velocity of 68.5 nm/sec, ranging from 29.3 to 139.6 nm/sec (71 puncta, seven animals). We note that particle movement was often erratic within the time frame of our observation and that MLT-puncta movement was not confined to a single stage but occurred throughout the life cycle of the animal.
Genetic analyses indicate functional cooperation of the NEKL and MLT proteins
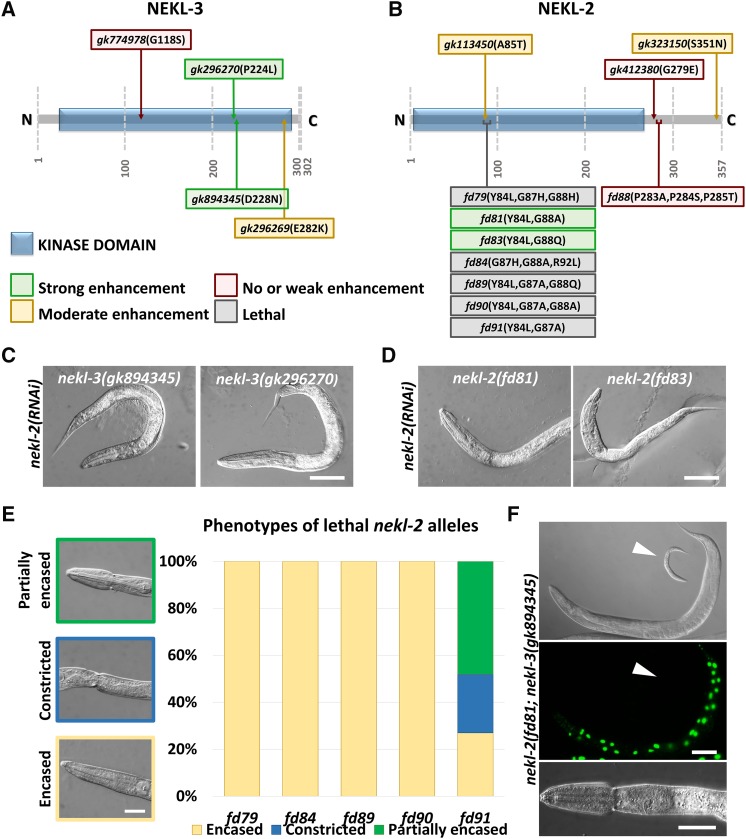
The mutant phenotypes of mlt-2, mlt-3, and mlt-4 are very similar to those previously described for nekl-2 and nekl-3 mutant animals (Yochem et al. 2015). To test for functional genetic interactions between the mlt and nekl genes, it was first necessary to obtain weak hypomorphic alleles of nekl-2 and nekl-3. Specifically, we postulated that viable hypomorphic alleles of nekl-2 or nekl-3 may be hyper-sensitive to further inhibition of pathway components, such as the mlt genes. We began by testing three aphenotypic alleles of nekl-2 and four aphenotypic alleles of nekl-3 generated by the Million Mutation project (Thompson et al. 2013). Two of the four tested nekl-3 alleles (gk296270 and gk894345) were strongly enhanced by nekl-2(RNAi), leading to a fully penetrant larval arrest (Figure 5, A and C). By contrast, nekl-2(RNAi) had no effect on wild-type worms (data not shown) and produced only weak-to-moderate effects on the two other nekl-3 strains tested (gk774978 and gk296269; Figure 5A). Similar results were also obtained for the four nekl-3 alleles following nekl-3(RNAi) feeding (data not shown). Notably, the most strongly enhanceable nekl-3 alleles contained amino acid substitutions within the predicted kinase domain (P224L and D228N). In addition, we observed that the level of enhancement roughly corresponded to the degree of conservation at the mutated site. In contrast to nekl-3, none of the Million Mutation nekl-2 alleles showed strong enhancement following RNAi of nekl-2 or nekl-3 (Figure 5B), although two strains showed moderate levels of molting defects.
Figure 5.
Isolation of nekl-2 and nekl-3 hypomorphic alleles. (A, B) Schematic representations of NEKL-3 (A) and NEKL-2 (B) proteins, with annotated predicted kinase domains (blue boxes). Mutant alleles indicated by text boxes, which are color coded according to the severity of the mutation: green, strong enhancement; yellow, moderate enhancement; red, no or weak enhancement; gray, homozygous lethal. (C, D) Representative DIC images of select nekl-3 (C) and nekl-2 (D) hypomorphic alleles treated with nekl-2(RNAi), which led to early larval lethality and molting defects. Bar, 50 µm. (E) Phenotypic analysis of homozygous-lethal nekl-2 alleles. Whereas fd79, fd84, fd89, and fd90 displayed early arrest with complete encasement, most fd91 homozygotes were partially encased and arrested later in development (n = 100 for each allele). Bar, 25 µm. (F) nekl-2(fd91); nekl-3(gk894345) double mutants (GFP–; white arrowhead) arrest as larvae with molting defects, including the corset phenotype (bottom panel). A GFP+ worm containing a nekl-3+ rescuing array (fdEx286), which is viable, is shown in the top two panels. Top bar, 100 µm, Bottom bar, 25 µm.
To obtain highly sensitized alleles of nekl-2 for enhancer analysis, we used CRISPR-Cas9 methods to generate changes in two regions of nekl-2. Whereas substitution of a PPP sequence (aa 283–285) outside the predicted kinase domain failed to provide sensitization, changes within a small region of the kinase domain (aa 84–92) led to a spectrum of loss-of-function phenotypes (Figure 5B). In particular, changes at glycines G87 and G88, together with changes at Y84, produced phenotypes ranging from uniform early larval lethality to worms that were largely aphenotypic but strongly enhanced by RNAi of nekl-2 or nekl-3 (Figure 5, B, D, and E and data not shown). Common to the homozygous-lethal alleles were changes at G87 including fd90 (Y84L, G87A, G88A) and fd84 (G87H, G88A, R92L), which led to arrest at the L1–L2 transition, and fd91 (Y84L, G87A), which led to arrest in late L2 or early L3 (Figure 5E). In contrast, changes affecting G88 [fd81 (Y84L, G88A) and fd83 (Y84L, G88Q)] did not produce lethality but led to strains that were highly sensitive to nekl-2 or nekl-3 RNAi feeding (Figure 5, B and D). Moreover, animals homozygous for hypomorphic alleles of both nekl-2(fd81) and nekl-3(gk894345) arrested at high frequency during larval development (98.5%, n = 1721) and displayed molting defects (Figure 5F), consistent with our previous studies indicating that nekl-2 and nekl-3 have functional overlap (Yochem et al. 2015).
Both G87 and G88 are completely conserved among NEKL-2/NEK8 orthologs and are largely conserved among members of the NIMA kinase family (Figure S3). To understand the basis for the differential effects of the G87 and G88 mutations, we carried out a computational structural analysis of wild-type NEKL-2 along with the fd91 (Y84L, G87A) and fd81 (Y84L, G88A) variants. Structural models predict that the more severe G87A substitution should cause much more extensive changes in intramolecular contacts as compared with the G88A substitution (Figure S4A). These changes are also reflected in the predicted surface structure of the protein, where residue 87 of the G87A variant is much less exposed than the equivalent residue in either wild type or the G88A variant (Figure S4B). Notably, the YAEG sequence is predicted to form an SH2 domain, and thus alterations in G87 may interfere with protein–protein interactions. In contrast, G88 is adjacent to the predicted ATP-binding pocket, and G88A may therefore exert a weak effect on the enzymatic activity of NEKL-2.
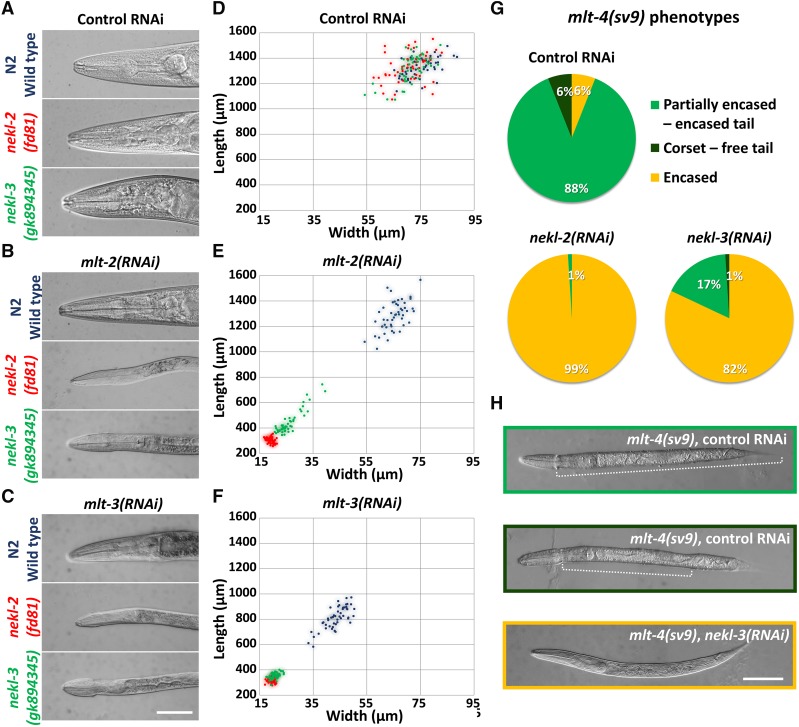
The hypomorphic alleles nekl-2(fd81) and nekl-3(gk894345), together with a wild-type control, were used to test for enhancement of molting defects by mlt-2(RNAi) and mlt-3(RNAi). Wild-type, nekl-2(fd81), and nekl-3(gk894345) animals developed normally on control gfp(RNAi) plates (Figure 6A) and displayed indistinguishable body size measurements (Figure 6D and Figure S5). As expected for components of a common network, mlt-2(RNAi) strongly enhanced both nekl-2(fd81) and nekl-3(gk894345) hypomorphic alleles, causing early larval arrest with accompanying molting defects, whereas wild-type worms were unaffected (Figure 6, B and E and Figure S5). Similar enhancer effects were also observed for RNAi of mlt-3, although mlt-3(RNAi) affected wild-type animals to a greater extent; treated N2 animals usually reached adulthood but were smaller and often displayed egg-laying defects, possibly because of some cuticle retention in the midbody region (Figure 6, C and F and Figure S5).
Figure 6.
Genetic enhancer analysis of mlt and nekl genes. (A–C) DIC images of wild-type, nekl-2(fd81), and nekl-3(gk894345) strains treated with control gfp(RNAi) (A), mlt-2(RNAi) (B), or mlt-3(RNAi) (C). Note that mlt-2(RNAi) and mlt-3(RNAi) induce molting defects and larval arrest in nekl-2(fd81) and nekl-3(gk894345) backgrounds but not in wild type. (D–F) Graphic representation of animal dimensions after RNAi treatment in different genetic backgrounds. Coordinates on the x axis indicate body width, whereas coordinates on the y axis represent body length. Each animal (n = 50 for each genotype and treatment) is represented by a colored dot corresponding to genotypes in A–C; blue, wild type; red, nekl-2(fd81); green, nekl-3(gk894345). Control gfp(RNAi) (D) had no effect on size, whereas RNAi for mlt-2 (E) or mlt-3 (F) caused nekl-2 and nekl-3 hypomorphs to have much smaller body dimensions as compared with stage-matched wild-type animals. (G) The severity of molting defects in mlt-4(sv9) homozygotes is enhanced by nekl-2(RNAi) and nekl-3(RNAi) treatment relative to the gfp(RNAi) control. (H) Representative DIC images of phenotypes described in G. Dotted lines mark the constricted region in partially encased animals. Bars, 50 µm.
Because RNAi of mlt-4 is largely ineffective (data not shown), we took a different approach to determine if mlt-4 genetically interacts with nekl-2 or nekl-3. As described above, mlt-4(sv9) homozygotes show a partially encased or corset phenotype and arrest in late stages of L2 or early L3. This property was used to determine if RNAi of nekl-2 or nekl-3 can enhance the severity of the sv9 phenotype and cause earlier developmental arrest. Indeed, complete encasement became the predominant phenotype in sv9 homozygotes treated with nekl-2(RNAi) or nekl-3(RNAi), a phenotype rarely seen with control gfp(RNAi) (Figure 6, G and H). Taken together, our genetic analyses implicate the MLT and NEKL proteins in a common pathway or network that controls molting.
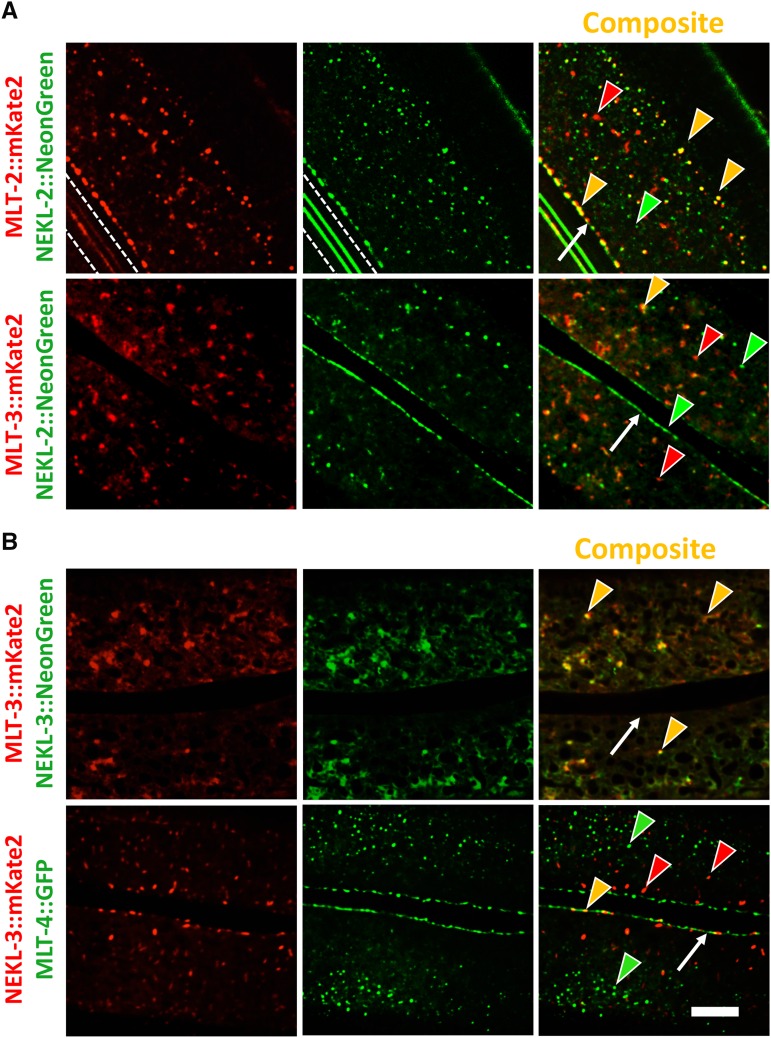
Colocalization of NEKL and MLT proteins
We next asked whether NEKL and MLT proteins colocalize in the epidermis. As for the mlt genes, we used CRISPR-Cas9 to generate fluorophore-tagged versions of nekl-2 and nekl-3. Consistent with our previous study (Yochem et al. 2015), these reporters were expressed strongly within the epidermis beginning in late embryonic development (Figure 7 and data not shown). NEKL-2::NeonGreen was present in cytosolic puncta that were enriched at the hyp7–seam cell boundary, similar to our observations for MLT-2 and MLT-4 (Figure 4A, Figure 7A, and data not shown). As anticipated, NEKL-2::NeonGreen showed extensive colocalization with MLT-2::mKate2 in the apical region of hyp7, although some puncta did not overlap (Figure 7A). In the case of NEKL-2 and MLT-3, coexpression was more variable. In apical regions, including the region encompassing the hyp7–seam cell boundary, most NEKL-2::NeonGreen and MLT-3::mKate2 puncta failed to overlap, although colocalization of some puncta was often observed (Figure 7A). Moreover, in some animals we observed moderate-to-strong overlap, particularly in larger apical accumulations and in more medial planes (data not shown).
Figure 7.
Colocalization of NEKL and MLT proteins. (A) Colocalization analyses of MLT-2::mKate2 and MLT-3::mKate2 with NEKL-2::NeonGreen showing extensive overlap of MLT-2 and NEKL-2 puncta throughout the hyp7 apical surface including the region adjacent to the seam cell. Though less frequent, some NEKL-2 puncta colocalized with MLT-3. White dashed lines in the first two panels demarcate autofluorescent alae. (B) NEKL-3::NeonGreen apical accumulations extensively overlapped with MLT-3::mKate2. In contrast, NEKL-3::mKate2 did not colocalize with MLT-4::GFP puncta. Red and green arrowheads indicate representative puncta that express only one marker, whereas yellow arrowheads indicate colocalization. White arrows indicate hyp7–seam cell boundaries. Bar, 5 µm.
NEKL-3 strains tagged with either mKate2 or NeonGreen displayed a pattern of expression similar to that of MLT-3, although both MLT-3 and NEKL-3 had somewhat variable patterns of expression between specimens. NEKL-3 was generally dispersed throughout the hyp7 cytosol and was present in variable-sized puncta (Figure 7B). NEKL-3::NeonGreen puncta strongly overlapped with MLT-3::mKate2 expression in many animals, although in some specimens there were more NEKL-3::NeonGreen puncta than MLT-3::mKate2 puncta (Figure 7B and data not shown). In contrast, NEKL-3 and MLT-4 did not show strong colocalization, consistent with the weak overlap between MLT-3 and MLT-4 (Figure 4B and Figure 7B). However, NEKL-3 puncta were observed in some animals near the hyp7–seam cell boundary and showed partial colocalization with MLT-4 in this region (Figure 7B). Likewise, NEKL-2 and NEKL-3 did not generally colocalize, consistent with our previous results using multicopy reporters (Yochem et al. 2015). Nevertheless, we did observe occasional overlap of NEKL-2 and NEKL-3 at the hyp7–seam cell boundary and, in some animals, we observed moderate-to-extensive colocalization of puncta in other regions of hyp7 (Figure S6 and data not shown).
As was observed for the MLTs, both NEKL-2 and NEKL-3 puncta were highly dynamic and included movement of both large and small particles (data not shown). The mobile nature of both MLT and NEKL puncta may in part account for the variability we observed in their degree of colocalization as well as in their overall expression patterns. Taken together, our expression data provide evidence for stable functional units or complexes comprised of NEKL-2–MLT-2–MLT-4 and NEKL-3–MLT-3, although additional interactions may also occur.
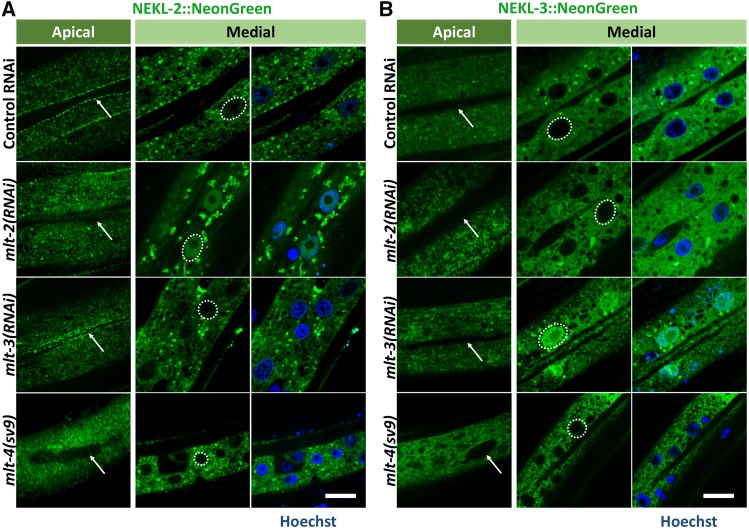
Regulation of NEKL localization by MLT proteins
Based on the above data and the known functions of ankyrin repeat proteins, we hypothesized that MLT proteins may act as molecular scaffolds for NEKL kinases and may control their subcellular localization. To test this we individually depleted mlt-2, mlt-3, and mlt-4 in NEKL-2::NeonGreen and NEKL-3::NeonGreen strains using RNAi or mutations and assayed for changes in localization. In the case of NEKL-2 expression, inhibition of mlt-2 by RNAi prevented normal accumulation at the hyp7–seam cell boundary in most animals (Figure 8A). Furthermore, mlt-2(RNAi) led to the accumulation of NEKL-2::NeonGreen in nuclei, which was, in some animals, stronger than the expression in the surrounding cytosol. This was not observed in wild-type animals, although NEKL-2::NeonGreen frequently was expressed at low-to-moderate levels in epidermal nuclei as animals got older (data not shown). Similar to the effect of mlt-2(RNAi), NEKL-2 accumulation was strongly reduced at the hyp7–seam cell boundary in mlt-4(sv9) mutants (Figure 8A). Unlike mlt-2, however, loss of mlt-4 activity did not lead to nuclear accumulation of NEKL-2 (Figure 8A). In contrast, we failed to observe corresponding changes in the localization patterns of MLT-2 or MLT-4 after inhibition of nekl-2 by RNAi (data not shown). Moreover, mlt-3(RNAi) did not perturb NEKL-2 localization at the seam cell boundary or affect its exclusion from nuclei, indicating distinct roles for the MLT proteins in their interactions with NEKL kinases (Figure 8A). A role for MLT-2 and MLT-4 in regulating NEKL-2 is consistent with the extensive colocalization of these proteins (Figure 4B and Figure 7A).
Figure 8.
Control of NEKL localization by MLTs. (A, B) Confocal images of NEKL-2::NeonGreen (A) and NEKL-3::NeonGreen (B) in strains treated with control gfp(RNAi), mlt-2(RNAi), or mlt-3(RNAi) and in the mlt-4(sv9) background. Shown are apical and medial planes of hyp7. DNA was stained with Hoechst (blue) and representative nuclei are outlined by a white dashed line. White arrows indicate the hyp7–seam cell boundary. Because mlt-4(sv9) homozygotes arrest at an earlier stage than animals treated with mlt-2(RNAi) or mlt-3(RNAi) (data not shown), they appear smaller in the panels. Bars, 5 µm.
In contrast to NEKL-2, NEKL-3 localization was strongly altered following mlt-3(RNAi) treatment but did not show dependence on either mlt-2 or mlt-4 activities (Figure 8B). Specifically, NEKL-3::NeonGreen became highly enriched in the nuclei of hyp7 after mlt-3 depletion, an effect that was also observed for NEKL-3::mKate2 (Figure 8B and data not shown). In reciprocal experiments, we failed to observe changes in MLT-3::mKate2 localization following nekl-3(RNAi), suggesting that cytosolic MLT-3 specifically prevents NEKL-3 from entering the nucleus. To further test this model, we carried out a yeast two-hybrid analysis. NEKL-3 bound MLT-3 in both bait and prey configurations (Figure S7), indicating that this functional interaction might be direct and is consistent with the colocalization data for NEKL-3 and MLT-3 (Figure 7B). Taken together, our findings indicate that ankyrin repeat proteins interact with specific NEKL kinase partners to control their subcellular localization.
Inhibition of the NEKL–MLT network affects intracellular trafficking
Inhibition of nekl-2 function leads to defects in intracellular trafficking in the epidermis (Yochem et al. 2015). Based on our results linking MLT proteins to NEKL-2 and NEKL-3 functions, we postulated that the MLTs may also affect intracellular trafficking during development. To test this we used a strain expressing an integrated fluorescently labeled clathrin heavy chain marker (GFP::CHC-1). This marker allows for the visualization of early events in endocytosis by labeling clathrin-coated pits and early endosomes. In control RNAi-treated animals, GFP::CHC-1 formed small, well-distributed puncta near the apical surface of hyp7 (Figure 9A). As an additional control, we examined GFP::CHC-1 expression in qua-1(RNAi) animals, which have penetrant molting defects but do not have perturbed intracellular trafficking (Yochem et al. 2015). As anticipated, GFP::CHC-1 appeared similar between control and qua-1(RNAi)-treated animals (Figure 9B).
Figure 9.
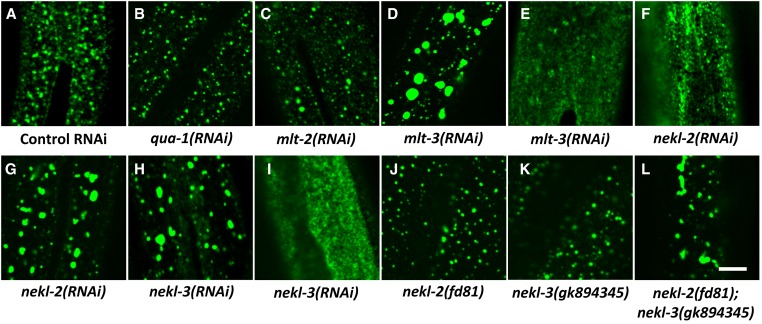
MLT and NEKL proteins control clathrin-mediated endocytosis. (A–L) Confocal images of GFP::CHC-1 apical epidermal expression. Normal punctate expression of GFP::CHC-1 (A) was not perturbed in qua-1(RNAi) animals (B), which showed penetrant molting defects. Whereas both mlt-2(RNAi) (C) and mlt-3(RNAi) (D, E) caused penetrant molting defects, mlt-3 depletion led to more severe alterations in GFP::CHC-1 expression, which varied from large accumulations (D) to diffuse expression at the apical surface (E). Abnormal accumulations and diffuse expression of GFP::CHC-1 were also observed in some nekl-2(RNAi) (F, G) and nekl-3(RNAi) (H, I) animals. Whereas hypomorphic alleles of nekl-2 (fd81) and nekl-3 (gk894345) did not perturb GFP::CHC-1 localization (J, K), nekl-2(fd81); nekl-3(gk894345) double mutants displayed extensive accumulation of the GFP signal (L). Bar, 5 µm
RNAi of mlt-2 led to a slight increase in the size of GFP::CHC-1 puncta but did not lead to gross changes in subcellular localization (Figure 9C). In contrast, mlt-3(RNAi) led to the formation of large and somewhat more basal accumulations of GFP::CHC-1 in a notable proportion of treated animals (Figure 9D). In some other animals, mlt-3(RNAi) led to diffuse expression of GFP::CHC-1 near the apical surface (Figure 9E). These variable defects in GFP::CHC-1 localization were also observed following nekl-2(RNAi), which led to both large accumulations and diffuse expression of the marker (Figure 9, F and G). We also observed partially penetrant mislocalization of GFP::CHC-1 following nekl-3(RNAi), as evidenced by either the enhanced-accumulation or diffuse-expression phenotypes (Figure 9, H and I). We further examined the roles of nekl-2 and nekl-3 in trafficking using the recently obtained weak hypomorphic alleles. Whereas nekl-2(fd81) and nekl-3(gk894345) single mutants showed normal GFP::CHC-1 localization, nekl-2(fd81); nekl-3(gk894345) double mutants displayed a highly penetrant accumulation phenotype (Figure 9, J–L), further supporting the conclusion that both NEKL-2 and NEKL-3 contribute to endocytic processes.
Discussion
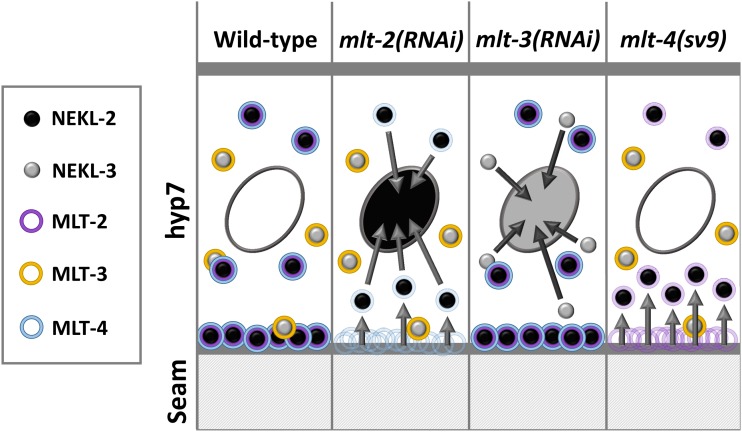
We have identified functions for the conserved ankyrin repeat proteins MLT-2/ANKS6, MLT-3/ANKS3, and MLT-4/INVS in the process of C. elegans molting. In addition, we have shown that the MLTs, together with NEKL-2 and NEKL-3, comprise a functional network that affects intracellular trafficking within the major epidermal syncytium, hyp7. Furthermore, NEKL–MLT expression and colocalization data, along with the observation that MLT proteins affect NEKL localization, point to the existence of two distinct functional units comprised of NEKL-2–MLT-2–MLT-4 and NEKL-3–MLT-3. Figure 10 summarizes the major findings from our expression studies. In wild-type animals, NEKL-2, MLT-2, and MLT-4 are coexpressed in puncta throughout the apical surface of hyp7 and show pronounced accumulation at the boundary with the epidermal seam cell. NEKL-3 and MLT-3 also colocalize to epidermal puncta, but these are largely distinct from NEKL-2–MLT-2–MLT-4 puncta and are not highly enriched at the hyp7–seam cell boundary. Nevertheless, occasional overlap of puncta can occur for all the examined members of the NEKL–MLT network and the extent of colocalization can be variable between animals. Furthermore, none of the MLTs and NEKLs is highly expressed in hyp7 nuclei.
Figure 10.
Summary model of MLT and NEKL proteins. Nuclei are indicated by ovals within hyp7. Arrows indicate changes in the subcellular localization of punctae. See Discussion for further details.
Consistent with colocalization data, loss of mlt-2 or mlt-4 activity leads to decreased levels of NEKL-2 at the hyp7–seam cell boundary (Figure 10). In addition, loss of mlt-2, but not mlt-4, results in variable levels of NEKL-2 accumulation within nuclei (Figure 10). Correspondingly, abnormal NEKL-3 nuclear accumulation occurs following the loss of mlt-3. In contrast, loss of mlt-2 or mlt-4 does not strongly affect NEKL-3 localization, and loss of mlt-3 does not affect NEKL-2 localization. We also note that we have observed some decreased localization of MLT-4 at the hyp7–seam cell boundary following depletion of mlt-2, suggesting that MLT-2 may help to stabilize a NEKL-2–MLT-2–MLT-4 complex (data not shown). Our collective findings are in accordance with our model that the NEKL–MLT protein network contains two largely distinct functional units comprised of NEKL-2–MLT-2–MLT-4 and NEKL-3–MLT-3 and demonstrate that MLT proteins control the subcellular localization of NEKL proteins. Notably, our genetic data do not indicate that members of these two functional units represent two parallel pathways. Rather, data from our enhancement studies suggest that MLTs and NEKLs are more likely part of a single extended network that controls molting (Figure 6).
Importantly, the NEKL–MLT network uncovered by our analysis is likely to be largely conserved in mammals. For example, the MLT-2 ortholog, ANKS6, physically associates both NEK8/NEKL-2 and INVS/MLT-4, although it is unknown if this interaction is direct or indirect (Hoff et al. 2013; Czarnecki et al. 2015). Our in vivo functional data support these findings and further show that NEKL-2 subcellular localization is dependent on both MLT-2 and MLT-4. However, whereas in mammalian cells ANKS6 localization to cilia is dependent on the presence of NEK8 (Czarnecki et al. 2015), we did not observe changes in the localization of MLT-2 following NEKL-2 depletion (data not shown). In addition, ANKS6/MLT-2 colocalizes with ANKS3/MLT-3 in kidney cells (Delestre et al. 2015), and these proteins physically interact through their SAM domains (Leettola et al. 2014). However, because MLT-2 does not contain a predicted SAM domain, this interaction may not be conserved in C. elegans, consistent with our observation that MLT-2 and MLT-3 do not extensively colocalize. It was also recently shown that ANKS3/MLT-3 and NEK7/NEKL-3 can be coprecipitated from cell lysates and that ANKS3 is important for the proper localization of NEK7 (Ramachandran et al. 2015). This finding is consistent with our expression studies in C. elegans and is further supported by our data from yeast two-hybrid studies demonstrating that these proteins likely directly bind to each other.
Our studies also provide new insights into the underlying molecular and cellular functions of the NEKL kinases and their ankyrin repeat partners, which are not well understood. In mammals, mutations affecting NEK8/NEKL-2, ANKS6/MLT-2, INVS/MLT-4, and ANKS3/MLT-3 have been directly linked to a class of diseases termed ciliopathies, which result from defects in the formation of primary cilia (Quarmby and Mahjoub 2005; Otto et al. 2008, 2011; Trapp et al. 2008; Halbritter et al. 2013; Hoff et al. 2013; Delestre et al. 2015). These include kidney disorders, such as nephronophthisis type 2 (Otto et al. 2003) and juvenile cystic kidney disease (Liu et al. 2002), as well as situs inversus, cardiovascular abnormalities, liver fibrosis, and defects in other organ systems (Frank et al. 2013; Hoff et al. 2013; Manning et al. 2013). These findings, as well as the expression of NEK8, INVS, ANKS6, and ANKS3 in the proximal ciliary compartment (Shiba et al. 2009, 2010; Hoff et al. 2013; Delestre et al. 2015), have led to the view that these proteins function primarily to control cilia formation. Our findings, however, suggest that ciliogenesis itself may not be the key central function of these proteins. Specifically, members of the C. elegans NEKL–MLT network play a critical role in an epidermal tissue that does not contain primary cilia, implying a more general cellular function. Consistent with this, mammalian NEK8 is detected in puncta throughout the cytoplasm, and only a minor fraction of NEK8 localizes to ciliary structures (Holland et al. 2002; Liu et al. 2002; Trapp et al. 2008; Shiba et al. 2010; Fukui et al. 2012; McCooke et al. 2012). In addition, INVS/MLT-4 regulates cortical actin during cell division (Werner et al. 2013), and NEK8 may affect gross actin levels as well as actin organization (Liu et al. 2002; Bowers and Boylan 2004).
In the case of the mammalian NEKL-3 orthologs, NEK6 and NEK7, the majority of studies have focused on the role of these kinases in the cell division process including centrosome separation (Sdelci et al. 2011), spindle formation, and cytokinesis (O’Regan et al. 2007; O’Regan and Fry 2009). These functions also link well to the reported involvement of NEK6 and NEK7 in tumorigenesis (Nassirpour et al. 2010; Wang et al. 2013; Zhou et al. 2016). Interestingly, we have not detected functions for NEKL-3, or any other component of the NEKL–MLT network, in cell division processes in C. elegans (Yochem et al. 2015). Although this could reflect divergent functions for these closely related orthologs between species, we have shown that nekl-3(sv3) mutants can be rescued by expression of mammalian NEK6 and NEK7 (data not shown). This finding, along with the high degree of sequence conservation among these proteins, suggests a more fundamental function for the NEKL-3/NEK6/NEK7 orthologs that may have been previously overlooked. Furthermore, the relative importance of mammalian NEK6 and NEK7 in mitosis is not fully resolved. Knockout of NEK6 in mice leads to no reported cell division defects (Bian et al. 2014), and NEK7–/– neonates are initially viable and display relatively minimal defects in cell division (Salem et al. 2010). Moreover, several observations suggest that NEK6 and NEK7 may also be associated with non-cell-cycle functions. For example, NEK6 and NEK7 are expressed at high levels in the cytoplasm during interphase (Kim et al. 2007; O’Regan and Fry 2009; de Souza et al. 2014), and NEK7 affects microtubule dynamics during interphase (Kang et al. 2013). In addition, most NEK6 and NEK7 interactors identified by proteomics are not linked specifically to cell cycle functions and include proteins associated with the cytoskeleton (e.g., TUBB, actin, CDC42, MAP7D1, MAP2, CLASP1, and CLASP2) and vesicular trafficking (e.g., CDC42, SNX26, RBLE1, ATP6V1G1, AP2A, AP2B, and RAB32) (Meirelles et al. 2011, 2014; de Souza et al. 2014).
Intriguingly, several studies have suggested possible functions for NIMA family kinases in intracellular trafficking. In one case, a high-throughput siRNA screen of 590 human kinases by Pelkmans et al. (2005) identified 208 candidate regulators of endocytosis including NEK6, NEK7, and NEK8. In addition, studies in nonmetazoan species have implicated members of the NIMA kinase family in a variety of functions unrelated to the cell cycle or ciliogenesis. For example, three Arabidopsis thaliana NEKs (related to NEKL-2) have been implicated in epidermal root tip growth through the possible regulation of microtubules (Sakai et al. 2008; Motose et al. 2011, 2012). Perhaps most notable was the recent report that Aspergillus nidulans nimA, which was originally identified for its role in mitosis, also controls polarized cell growth and localizes to microtubule plus ends in an EB1-dependent manner during interphase (Govindaraghavan et al. 2014). This study also showed that mutations in both the A. nidulans and Saccharomyces cerevisiae NIMA orthologs (nimA and kin3) are synthetically lethal with mutations in genes encoding ESCRT complex components, which promote membrane remodeling and endosome maturation (McCullough et al. 2013; Govindaraghavan et al. 2014). Although the functional connection between nimA and ESCRT proteins is unknown, this finding implicates NIMA kinases in processes linked to intracellular trafficking. Collectively, these studies, together with our own findings, support the model that at least some of the underlying conserved functions of NIMA family members are unrelated to cell cycle control or ciliogenesis and may encompass functions associated with vesicle trafficking and the interphase cytoskeleton.
Our observation that inhibition of MLT and NEKL proteins leads to defects in clathrin-dependent endocytosis in hyp7 raises the possibility that regulation of intracellular trafficking may be a key function of the NEKL–MLT network. In support of this model, we previously showed that NEKL-2 affects the expression of several endosomal markers including GFP::EEA-1, GFP::RAB-5, GFP::SNX-1, and GFP::CHC-1 (Yochem et al. 2015) and in the current study have further implicated NEKL-3, MLT-3, and, to a lesser extent, MLT-2 in controlling clathrin-dependent endocytosis. Interestingly, MLT-3 contains an FXDXF motif, which has been associated with accessory protein recruitment in clathrin-mediated endocytosis (Brett et al. 2002), although this motif is not conserved in human ANKS3. We also previously reported that NEKL-2 affects the localization of LRP-1/megalin, a member of the low-density lipoprotein receptor family (Yochem et al. 2015). LRP-1 is required for normal molting and is thought to function in the process of cholesterol uptake by the epidermis (Yochem et al. 1999; May et al. 2007). Consistent with this, cholesterol is essential for molting in C. elegans (Yochem et al. 1999; Merris et al. 2003; Entchev and Kurzchalia 2005; Roudier et al. 2005). In addition, studies on mammalian megalin suggest that C. elegans LRP-1 could promote molting in part by regulating the uptake of proteases and protease inhibitors (May et al. 2007; Marzolo and Farfan 2011; Etique et al. 2013). In addition to LRP-1, several other genes required for molting also affect intracellular trafficking and sterol–LRP-1 uptake including dab-1/Disabled, hgrs-1/VPS27, and sec-23/SEC23 (Kamikura and Cooper 2003; Roberts et al. 2003; Roudier et al. 2005; Holmes et al. 2007; Yochem et al. 2015).
The regulation of intracellular trafficking may not, however, be a primary or direct function of the NEKL–MLT network, and perturbation of trafficking may not be the main cause of molting defects in nekl and mlt mutants. Notably, the observed effects on endocytosis markers following inhibition of either the mlt or nekl genes are quite variable and incompletely penetrant, even in cases where the induced molting defects are fully penetrant. Moreover, we have not observed a clear correlation between the severity of molting defects (in individual animals or populations) and the extent of changes in the expression pattern of endosomal marker GFP::CHC-1. Thus, it seems likely that MLT and NEKL proteins control cellular processes that affect intracellular trafficking but are not absolutely required for this function.
Based on our current data, we suggest that defects in intracellular trafficking in nekl and mlt mutants may influence molting but that other cellular functions controlled by these genes are likely to further impact this process. For example, the NEKL–MLT network could affect the actin cytoskeleton, which is required for the formation of clathrin-coated vesicles as well as other fundamental functions and properties of cells (Qualmann et al. 2000; Smythe and Ayscough 2006; Mooren et al. 2012). Alternatively, the NEKL–MLT pathway could regulate interphase microtubules, which are also critical for vesicular trafficking, as well as cell polarity and organization (Soldati and Schliwa 2006; Siegrist and Doe 2007; Zhang et al. 2014). We note that the subcellular localization and dynamic nature of both NEKL and MLT puncta could be consistent with a variety of functions including intracellular trafficking and/or the regulation of cytoskeletal components. Future studies in C. elegans, including the further characterization of the NEKL–MLT subcellular compartments and the identification of NEKL target substrates, will greatly help to resolve questions regarding the conserved molecular functions of the NEKL–MLT network. In addition, these studies should shed light on our understanding of the widespread pathological conditions that arise from mutations in members of the NEKL–MLT network.
Acknowledgments
We foremost acknowledge the scientific input, generosity, and expertise of John Yochem, who started the molting project in the Fay Lab. We are also indebted to Bob Herman, Lihsia Chen, and Simon Tuck for their support of this project; to Amy Fluet for editing; to Jay Gatlin for microscopy support; to Barth Grant for providing strains and scientific input; and to Leslie Bell, Evguenia Karina, Daniel Dickinson, and Bob Goldstein for additional support. Some strains used in these studies were provided by the Caenorhabditis Genetics Center, which is funded by the United States National Institutes of Health (NIH) Office of Research Infrastructure Programs (P40 OD010440), and National BioResource Project, Tokyo (The C. elegans Deletion Mutant Consortium 2012). Cosmid clones were provided by the Wellcome Trust, UK. Support at the University of Wyoming was from the NIH grant GM-066868 to D.S.F.
Footnotes
Communicating editor: M. V. Sundaram
Supplemental material is available online at http://www.genetics.org/cgi/content/full/genetics.116.194464/DC1.
Literature Cited
- Abascal F., Zardoya R., Posada D., 2005. ProtTest: selection of best-fit models of protein evolution. Bioinformatics 21: 2104–2105. [DOI] [PubMed] [Google Scholar]
- Ahringer, J., 2005 Reverse genetics (April 6, 2006), WormBook, ed. The C. elegans Research Community WormBook, /10.1895/wormbook.1.47.1, http://www.wormbook.org.
- Altincicek B., Fischer M., Luersen K., Boll M., Wenzel U., et al. , 2010. Role of matrix metalloproteinase ZMP-2 in pathogen resistance and development in Caenorhabditis elegans. Dev. Comp. Immunol. 34: 1160–1169. [DOI] [PubMed] [Google Scholar]
- Arribere J. A., Bell R. T., Fu B. X., Artiles K. L., Hartman P. S., et al. , 2014. Efficient marker-free recovery of custom genetic modifications with CRISPR/Cas9 in Caenorhabditis elegans. Genetics 198: 837–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian Z., Liao H., Zhang Y., Wu Q., Zhou H., et al. , 2014. Never in mitosis gene A related kinase-6 attenuates pressure overload-induced activation of the protein kinase B pathway and cardiac hypertrophy. PLoS One 9: e96095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers A. J., Boylan J. F., 2004. Nek8, a NIMA family kinase member, is overexpressed in primary human breast tumors. Gene 328: 135–142. [DOI] [PubMed] [Google Scholar]
- Brenner S., 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett T. J., Traub L. M., Fremont D. H., 2002. Accessory protein recruitment motifs in clathrin-mediated endocytosis. Structure 10: 797–809. [DOI] [PubMed] [Google Scholar]
- Chisholm A. D., Xu S., 2012. The Caenorhabditis elegans epidermis as a model skin. II: differentiation and physiological roles. Wiley Interdiscip. Rev. Dev. Biol. 1: 879–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox T. R., Erler J. T., 2011. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis. Model. Mech. 4: 165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooks G. E., Hon G., Chandonia J. M., Brenner S. E., 2004. WebLogo: a sequence logo generator. Genome Res. 14: 1188–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarnecki P. G., Gabriel G. C., Manning D. K., Sergeev M., Lemke K., et al. , 2015. ANKS6 is the critical activator of NEK8 kinase in embryonic situs determination and organ patterning. Nat. Commun. 6: 6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. W., Birnie A. J., Chan A. C., Page A. P., Jorgensen E. M., 2004. A conserved metalloprotease mediates ecdysis in Caenorhabditis elegans. Development 131: 6001–6008. [DOI] [PubMed] [Google Scholar]
- Delestre L., Bakey Z., Prado C., Hoffmann S., Bihoreau M. T., et al. , 2015. ANKS3 co-localises with ANKS6 in mouse renal cilia and is associated with vasopressin signaling and apoptosis in vivo in mice. PLoS One 10: e0136781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza E. E., Meirelles G. V., Godoy B. B., Perez A. M., Smetana J. H., et al. , 2014. Characterization of the human NEK7 interactome suggests catalytic and regulatory properties distinct from those of NEK6. J. Proteome Res. 13: 4074–4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D. J., Pani A. M., Heppert J. K., Higgins C. D., Goldstein B., 2015. Streamlined genome engineering with a self-excising drug selection cassette. Genetics 200: 1035–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkel H., Van Roey K., Michael S., Kumar M., Uyar B., et al. , 2016. ELM 2016—data update and new functionality of the eukaryotic linear motif resource. Nucleic Acids Res. 44: D294–D300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entchev E. V., Kurzchalia T. V., 2005. Requirement of sterols in the life cycle of the nematode Caenorhabditis elegans. Semin. Cell Dev. Biol. 16: 175–182. [DOI] [PubMed] [Google Scholar]
- Etique N., Verzeaux L., Dedieu S., Emonard H., 2013. LRP-1: a checkpoint for the extracellular matrix proteolysis. BioMed Res. Int. 2013: 152163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frand A. R., Russel S., Ruvkun G., 2005. Functional genomic analysis of C. elegans molting. PLoS Biol. 3: e312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank V., Habbig S., Bartram M. P., Eisenberger T., Veenstra-Knol H. E., et al. , 2013. Mutations in NEK8 link multiple organ dysplasia with altered Hippo signalling and increased c-MYC expression. Hum. Mol. Genet. 22: 2177–2185. [DOI] [PubMed] [Google Scholar]
- Fukui H., Shiba D., Asakawa K., Kawakami K., Yokoyama T., 2012. The ciliary protein Nek8/Nphp9 acts downstream of Inv/Nphp2 during pronephros morphogenesis and left-right establishment in zebrafish. FEBS Lett. 586: 2273–2279. [DOI] [PubMed] [Google Scholar]
- Gissendanner C. R., Sluder A. E., 2000. nhr-25, the Caenorhabditis elegans ortholog of ftz-f1, is required for epidermal and somatic gonad development. Dev. Biol. 221: 259–272. [DOI] [PubMed] [Google Scholar]
- Gooyit M., Tricoche N., Javor S., Lustigman S., Janda K. D., 2015. Exploiting the polypharmacology of β-carbolines to disrupt O. volvulus molting. ACS Med. Chem. Lett. 6: 339–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindaraghavan M., McGuire Anglin S. L., Shen K. F., Shukla N., De Souza C. P., et al. , 2014. Identification of interphase functions for the NIMA kinase involving microtubules and the ESCRT pathway. PLoS Genet. 10: e1004248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbritter J., Porath J. D., Diaz K. A., Braun D. A., Kohl S., et al. , 2013. Identification of 99 novel mutations in a worldwide cohort of 1,056 patients with a nephronophthisis-related ciliopathy. Hum. Genet. 132: 865–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao L., Mukherjee K., Liegeois S., Baillie D., Labouesse M., et al. , 2006. The hedgehog-related gene qua-1 is required for molting in Caenorhabditis elegans. Dev. Dyn. 235: 1469–1481. [DOI] [PubMed] [Google Scholar]
- Hashmi S., Zhang J., Oksov Y., Lustigman S., 2004. The Caenorhabditis elegans cathepsin Z-like cysteine protease, Ce-CPZ-1, has a multifunctional role during the worms’ development. J. Biol. Chem. 279: 6035–6045. [DOI] [PubMed] [Google Scholar]
- Hayes G. D., Frand A. R., Ruvkun G., 2006. The mir-84 and let-7 paralogous microRNA genes of Caenorhabditis elegans direct the cessation of molting via the conserved nuclear hormone receptors NHR-23 and NHR-25. Development 133: 4631–4641. [DOI] [PubMed] [Google Scholar]
- Hirsh D., Oppenheim D., Klass M., 1976. Development of the reproductive system of Caenorhabditis elegans. Dev. Biol. 49: 200–219. [DOI] [PubMed] [Google Scholar]
- Hoff S., Halbritter J., Epting D., Frank V., Nguyen T. M., et al. , 2013. ANKS6 is a central component of a nephronophthisis module linking NEK8 to INVS and NPHP3. Nat. Genet. 45: 951–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland P. M., Milne A., Garka K., Johnson R. S., Willis C., et al. , 2002. Purification, cloning, and characterization of Nek8, a novel NIMA-related kinase, and its candidate substrate Bicd2. J. Biol. Chem. 277: 16229–16240. [DOI] [PubMed] [Google Scholar]
- Hollenbeck J. J., Danner D. J., Landgren R. M., Rainbolt T. K., Roberts D. S., 2012. Designed ankyrin repeat proteins as scaffolds for multivalent recognition. Biomacromolecules 13: 1996–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A., Flett A., Coudreuse D., Korswagen H. C., Pettitt J., 2007. C. elegans disabled is required for cell-type specific endocytosis and is essential in animals lacking the AP-3 adaptor complex. J. Cell Sci. 120: 2741–2751. [DOI] [PubMed] [Google Scholar]
- Jia K., Albert P. S., Riddle D. L., 2002. DAF-9, a cytochrome P450 regulating C. elegans larval development and adult longevity. Development 129: 221–231. [DOI] [PubMed] [Google Scholar]
- Kamikura D. M., Cooper J. A., 2003. Lipoprotein receptors and a disabled family cytoplasmic adaptor protein regulate EGL-17/FGF export in C. elegans. Genes Dev. 17: 2798–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y. L., Yochem J., Bell L., Sorensen E. B., Chen L., et al. , 2013. Caenorhabditis elegans reveals a FxNPxY-independent low-density lipoprotein receptor internalization mechanism mediated by epsin1. Mol. Biol. Cell 24: 308–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K., Standley D. M., 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30: 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C. A., Bowie J. U., 2003. SAM domains: uniform structure, diversity of function. Trends Biochem. Sci. 28: 625–628. [DOI] [PubMed] [Google Scholar]
- Kim T. H., Kim Y. J., Cho J. W., Shim J., 2011. A novel zinc-carboxypeptidase SURO-1 regulates cuticle formation and body morphogenesis in Caenorhabditis elegans. FEBS Lett. 585: 121–127. [DOI] [PubMed] [Google Scholar]
- Kim Y., Gentry M. S., Harris T. E., Wiley S. E., Lawrence J. C., Jr, et al. , 2007. A conserved phosphatase cascade that regulates nuclear membrane biogenesis. Proc. Natl. Acad. Sci. USA 104: 6596–6601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostrouchova M., Krause M., Kostrouch Z., Rall J. E., 1998. CHR3: a Caenorhabditis elegans orphan nuclear hormone receptor required for proper epidermal development and molting. Development 125: 1617–1626. [DOI] [PubMed] [Google Scholar]
- Kostrouchova M., Krause M., Kostrouch Z., Rall J. E., 2001. Nuclear hormone receptor CHR3 is a critical regulator of all four larval molts of the nematode Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 98: 7360–7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouns N. A., Nakielna J., Behensky F., Krause M. W., Kostrouch Z., et al. , 2011. NHR-23 dependent collagen and hedgehog-related genes required for molting. Biochem. Biophys. Res. Commun. 413: 515–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuervers L. M., Jones C. L., O’Neil N. J., Baillie D. L., 2003. The sterol modifying enzyme LET-767 is essential for growth, reproduction and development in Caenorhabditis elegans. Mol. Genet. Genomics 270: 121–131. [DOI] [PubMed] [Google Scholar]
- Leettola C. N., Knight M. J., Cascio D., Hoffman S., Bowie J. U., 2014. Characterization of the SAM domain of the PKD-related protein ANKS6 and its interaction with ANKS3. BMC Struct. Biol. 14: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letunic I., Bork P., 2016. Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 44: W242–W245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letunic I., Doerks T., Bork P., 2015. SMART: recent updates, new developments and status in 2015. Nucleic Acids Res. 43: D257–D260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Cowley A., Uludag M., Gur T., McWilliam H., et al. , 2015. The EMBL-EBI bioinformatics web and programmatic tools framework. Nucleic Acids Res. 43: W580–W584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Paik Y. K., 2011. A potential role for fatty acid biosynthesis genes during molting and cuticle formation in Caenorhabditis elegans. BMB Rep. 44: 285–290. [DOI] [PubMed] [Google Scholar]
- Liu S., Lu W., Obara T., Kuida S., Lehoczky J., et al. , 2002. A defect in a novel Nek-family kinase causes cystic kidney disease in the mouse and in zebrafish. Development 129: 5839–5846. [DOI] [PubMed] [Google Scholar]
- Mahjoub M. R., Trapp M. L., Quarmby L. M., 2005. NIMA-related kinases defective in murine models of polycystic kidney diseases localize to primary cilia and centrosomes. J. Am. Soc. Nephrol. 16: 3485–3489. [DOI] [PubMed] [Google Scholar]
- Manning D. K., Sergeev M., van Heesbeen R. G., Wong M. D., Oh J. H., et al. , 2013. Loss of the ciliary kinase Nek8 causes left-right asymmetry defects. J. Am. Soc. Nephrol. 24: 100–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzolo M. P., Farfan P., 2011. New insights into the roles of megalin/LRP2 and the regulation of its functional expression. Biol. Res. 44: 89–105. [DOI] [PubMed] [Google Scholar]
- May P., Woldt E., Matz R. L., Boucher P., 2007. The LDL receptor-related protein (LRP) family: an old family of proteins with new physiological functions. Ann. Med. 39: 219–228. [DOI] [PubMed] [Google Scholar]
- McCooke J. K., Appels R., Barrero R. A., Ding A., Ozimek-Kulik J. E., et al. , 2012. A novel mutation causing nephronophthisis in the Lewis polycystic kidney rat localises to a conserved RCC1 domain in Nek8. BMC Genomics 13: 393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough J., Colf L. A., Sundquist W. I., 2013. Membrane fission reactions of the mammalian ESCRT pathway. Annu. Rev. Biochem. 82: 663–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWilliam H., Li W., Uludag M., Squizzato S., Park Y. M., et al. , 2013. Analysis tool web services from the EMBL-EBI. Nucleic Acids Res. 41: W597–W600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meirelles G. V., Silva J. C., Mendonca Yde A., Ramos C. H., Torriani I. L., et al. , 2011. Human Nek6 is a monomeric mostly globular kinase with an unfolded short N-terminal domain. BMC Struct. Biol. 11: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meirelles G. V., Perez A. M., de Souza E. E., Basei F. L., Papa P. F., et al. , 2014. “Stop Ne(c)king around”: how interactomics contributes to functionally characterize Nek family kinases. World J. Biol. Chem. 5: 141–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merris M., Wadsworth W. G., Khamrai U., Bittman R., Chitwood D. J., et al. , 2003. Sterol effects and sites of sterol accumulation in Caenorhabditis elegans: developmental requirement for 4α-methyl sterols. J. Lipid Res. 44: 172–181. [DOI] [PubMed] [Google Scholar]
- Monsalve G. C., Frand A. R., 2012. Toward a unified model of developmental timing: a “molting” approach. Worm 1: 221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooren O. L., Galletta B. J., Cooper J. A., 2012. Roles for actin assembly in endocytosis. Annu. Rev. Biochem. 81: 661–686. [DOI] [PubMed] [Google Scholar]
- Mosavi L. K., Cammett T. J., Desrosiers D. C., Peng Z. Y., 2004. The ankyrin repeat as molecular architecture for protein recognition. Protein Sci. 13: 1435–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motose H., Hamada T., Yoshimoto K., Murata T., Hasebe M., et al. , 2011. NIMA-related kinases 6, 4, and 5 interact with each other to regulate microtubule organization during epidermal cell expansion in Arabidopsis thaliana. Plant J. 67: 993–1005. [DOI] [PubMed] [Google Scholar]
- Motose H., Takatani S., Ikeda T., Takahashi T., 2012. NIMA-related kinases regulate directional cell growth and organ development through microtubule function in Arabidopsis thaliana. Plant Signal. Behav. 7: 1552–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata K., Kudo M., Onuma F., Motoyama T., 1984. Changes of collagen types at various stages of human liver cirrhosis. Hepatogastroenterology 31: 158–161. [PubMed] [Google Scholar]
- Nassirpour R., Shao L., Flanagan P., Abrams T., Jallal B., et al. , 2010. Nek6 mediates human cancer cell transformation and is a potential cancer therapeutic target. Mol. Cancer Res. 8: 717–728. [DOI] [PubMed] [Google Scholar]
- Nielsen K., Clemmesen J. O., Vassiliadis E., Vainer B., 2014. Liver collagen in cirrhosis correlates with portal hypertension and liver dysfunction. APMIS 122: 1213–1222. [DOI] [PubMed] [Google Scholar]
- O’Regan L., Fry A. M., 2009. The Nek6 and Nek7 protein kinases are required for robust mitotic spindle formation and cytokinesis. Mol. Cell. Biol. 29: 3975–3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Regan L., Blot J., Fry A. M., 2007. Mitotic regulation by NIMA-related kinases. Cell Div. 2: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto E. A., Schermer B., Obara T., O’Toole J. F., Hiller K. S., et al. , 2003. Mutations in INVS encoding inversin cause nephronophthisis type 2, linking renal cystic disease to the function of primary cilia and left-right axis determination. Nat. Genet. 34: 413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto E. A., Trapp M. L., Schultheiss U. T., Helou J., Quarmby L. M., et al. , 2008. NEK8 mutations affect ciliary and centrosomal localization and may cause nephronophthisis. J. Am. Soc. Nephrol. 19: 587–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto E. A., Ramaswami G., Janssen S., Chaki M., Allen S. J., et al. , 2011. Mutation analysis of 18 nephronophthisis associated ciliopathy disease genes using a DNA pooling and next generation sequencing strategy. J. Med. Genet. 48: 105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page, A. P., and I. L. Johnstone, 2007 The cuticle (March 19, 2007), WormBook , ed. The C. elegans Research Community WormBook, /10.1895/wormbook.1.138.1, http://www.wormbook.org.
- Page A. P., Stepek G., Winter A. D., Pertab D., 2014. Enzymology of the nematode cuticle: a potential drug target? Int. J. Parasitol. Drugs Drug Resist. 4: 133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paix A., Wang Y., Smith H. E., Lee C. Y., Calidas D., et al. , 2014. Scalable and versatile genome editing using linear DNAs with microhomology to Cas9 sites in Caenorhabditis elegans. Genetics 198: 1347–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkmans L., Fava E., Grabner H., Hannus M., Habermann B., et al. , 2005. Genome-wide analysis of human kinases in clathrin- and caveolae/raft-mediated endocytosis. Nature 436: 78–86. [DOI] [PubMed] [Google Scholar]
- Pettersen E. F., Goddard T. D., Huang C. C., Couch G. S., Greenblatt D. M., et al. , 2004. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25: 1605–1612. [DOI] [PubMed] [Google Scholar]
- Ponticos M., Papaioannou I., Xu S., Holmes A. M., Khan K., et al. , 2015. Failed degradation of JunB contributes to overproduction of type I collagen and development of dermal fibrosis in patients with systemic sclerosis. Arthritis Rheumatol. 67: 243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qualmann B., Kessels M. M., Kelly R. B., 2000. Molecular links between endocytosis and the actin cytoskeleton. J. Cell Biol. 150: F111–F116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarmby L. M., Mahjoub M. R., 2005. Caught Nek-ing: cilia and centrioles. J. Cell Sci. 118: 5161–5169. [DOI] [PubMed] [Google Scholar]
- Raghu G., Masta S., Meyers D., Narayanan A. S., 1989. Collagen synthesis by normal and fibrotic human lung fibroblasts and the effect of transforming growth factor-β. Am. Rev. Respir. Dis. 140: 95–100. [DOI] [PubMed] [Google Scholar]
- Ramachandran H., Engel C., Muller B., Dengjel J., Walz G., et al. , 2015. Anks3 alters the sub-cellular localization of the Nek7 kinase. Biochem. Biophys. Res. Commun. 464: 901–907. [DOI] [PubMed] [Google Scholar]
- Rhoads A. R., Friedberg F., 1997. Sequence motifs for calmodulin recognition. FASEB J. 11: 331–340. [DOI] [PubMed] [Google Scholar]
- Rice P., Longden I., Bleasby A., 2000. EMBOSS: the European Molecular Biology Open Software Suite. Trends Genet. 16: 276–277. [DOI] [PubMed] [Google Scholar]
- Roberts B., Clucas C., Johnstone I. L., 2003. Loss of SEC-23 in Caenorhabditis elegans causes defects in oogenesis, morphogenesis, and extracellular matrix secretion. Mol. Biol. Cell 14: 4414–4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roudier N., Lefebvre C., Legouis R., 2005. CeVPS-27 is an endosomal protein required for the molting and the endocytic trafficking of the low-density lipoprotein receptor-related protein 1 in Caenorhabditis elegans. Traffic 6: 695–705. [DOI] [PubMed] [Google Scholar]
- Roy A., Kucukural A., Zhang Y., 2010. I-TASSER: a unified platform for automated protein structure and function prediction. Nat. Protoc. 5: 725–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T., Honing H., Nishioka M., Uehara Y., Takahashi M., et al. , 2008. Armadillo repeat-containing kinesins and a NIMA-related kinase are required for epidermal-cell morphogenesis in Arabidopsis. Plant J. 53: 157–171. [DOI] [PubMed] [Google Scholar]
- Salem H., Rachmin I., Yissachar N., Cohen S., Amiel A., et al. , 2010. Nek7 kinase targeting leads to early mortality, cytokinesis disturbance and polyploidy. Oncogene 29: 4046–4057. [DOI] [PubMed] [Google Scholar]
- Sdelci S., Bertran M. T., Roig J., 2011. Nek9, Nek6, Nek7 and the separation of centrosomes. Cell Cycle 10: 3816–3817. [DOI] [PubMed] [Google Scholar]
- Shaye D. D., Greenwald I., 2011. OrthoList: a compendium of C. elegans genes with human orthologs. PLoS One 6: e20085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba D., Yamaoka Y., Hagiwara H., Takamatsu T., Hamada H., et al. , 2009. Localization of Inv in a distinctive intraciliary compartment requires the C-terminal ninein-homolog-containing region. J. Cell Sci. 122: 44–54. [DOI] [PubMed] [Google Scholar]
- Shiba D., Manning D. K., Koga H., Beier D. R., Yokoyama T., 2010. Inv acts as a molecular anchor for Nphp3 and Nek8 in the proximal segment of primary cilia. Cytoskeleton (Hoboken) 67: 112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegrist S. E., Doe C. Q., 2007. Microtubule-induced cortical cell polarity. Genes Dev. 21: 483–496. [DOI] [PubMed] [Google Scholar]
- Singh R. N., Sulston J. E., 1978. Some observations on the moulting of Caenorhabditis elegans. Nematologica 24: 63–71. [Google Scholar]
- Smythe E., Ayscough K. R., 2006. Actin regulation in endocytosis. J. Cell Sci. 119: 4589–4598. [DOI] [PubMed] [Google Scholar]
- Soldati T., Schliwa M., 2006. Powering membrane traffic in endocytosis and recycling. Nat. Rev. Mol. Cell Biol. 7: 897–908. [DOI] [PubMed] [Google Scholar]
- Specks U., Nerlich A., Colby T. V., Wiest I., Timpl R., 1995. Increased expression of type VI collagen in lung fibrosis. Am. J. Respir. Crit. Care Med. 151: 1956–1964. [DOI] [PubMed] [Google Scholar]
- Stenvall J., Fierro-Gonzalez J. C., Swoboda P., Saamarthy K., Cheng Q., et al. , 2011. Selenoprotein TRXR-1 and GSR-1 are essential for removal of old cuticle during molting in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 108: 1064–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepek G., McCormack G., Birnie A. J., Page A. P., 2011. The astacin metalloprotease moulting enzyme NAS-36 is required for normal cuticle ecdysis in free-living and parasitic nematodes. Parasitology 138: 237–248. [DOI] [PubMed] [Google Scholar]
- Stiernagle, T., 2006 Maintenance of C. elegans (February 11, 2006), WormBook, ed. The C. elegans Research Community WormBook, /10.1895/wormbook.1.101.1, http://www.wormbook.org.
- Sulston J. E., Schierenberg E., White J. G., Thomson J. N., 1983. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev. Biol. 100: 64–119. [DOI] [PubMed] [Google Scholar]
- Suzuki M., Sagoh N., Iwasaki H., Inoue H., Takahashi K., 2004. Metalloproteases with EGF, CUB, and thrombospondin-1 domains function in molting of Caenorhabditis elegans. Biol. Chem. 385: 565–568. [DOI] [PubMed] [Google Scholar]
- The C. elegans Deletion Mutant Consortium , 2012. Large-scale screening for targeted knockouts in the Caenorhabditis elegans genome. G3 2: 1415–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson O., Edgley M., Strasbourger P., Flibotte S., Ewing B., et al. , 2013. The million mutation project: a new approach to genetics in Caenorhabditis elegans. Genome Res. 23: 1749–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmons L., Court D. L., Fire A., 2001. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene 263: 103–112. [DOI] [PubMed] [Google Scholar]
- Todd N. W., Luzina I. G., Atamas S. P., 2012. Molecular and cellular mechanisms of pulmonary fibrosis. Fibrogenesis Tissue Repair 5: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp M. L., Galtseva A., Manning D. K., Beier D. R., Rosenblum N. D., et al. , 2008. Defects in ciliary localization of Nek8 is associated with cystogenesis. Pediatr. Nephrol. 23: 377–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voronin D. A., Kiseleva E. V., 2008. Functional role of proteins containing ankyrin repeats. Cell Tiss. Biol. DOI: 10.1134/S1990519X0801001X. [PubMed] [Google Scholar]
- Wang D., Kennedy S., Conte D., Jr, Kim J. K., Gabel H. W., et al. , 2005. Somatic misexpression of germline P granules and enhanced RNA interference in retinoblastoma pathway mutants. Nature 436: 593–597. [DOI] [PubMed] [Google Scholar]
- Wang R., Song Y., Xu X., Wu Q., Liu C., 2013. The expression of Nek7, FoxM1, and Plk1 in gallbladder cancer and their relationships to clinicopathologic features and survival. Clin. Transl. Oncol. 15: 626–632. [DOI] [PubMed] [Google Scholar]
- Warburton-Pitt S. R., Jauregui A. R., Li C., Wang J., Leroux M. R., et al. , 2012. Ciliogenesis in Caenorhabditis elegans requires genetic interactions between ciliary middle segment localized NPHP-2 (inversin) and transition zone-associated proteins. J. Cell Sci. 125: 2592–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse A. M., Procter J. B., Martin D. M., Clamp M., Barton G. J., 2009. Jalview Version 2—a multiple sequence alignment editor and analysis workbench. Bioinformatics 25: 1189–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendler F., Franch-Marro X., Vincent J. P., 2006. How does cholesterol affect the way Hedgehog works? Development 133: 3055–3061. [DOI] [PubMed] [Google Scholar]
- Werner M. E., Ward H. H., Phillips C. L., Miller C., Gattone V. H., et al. , 2013. Inversin modulates the cortical actin network during mitosis. Am. J. Physiol. Cell Physiol. 305: C36–C47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicks S. R., Yeh R. T., Gish W. R., Waterston R. H., Plasterk R. H., 2001. Rapid gene mapping in Caenorhabditis elegans using a high density polymorphism map. Nat. Genet. 28: 160–164. [DOI] [PubMed] [Google Scholar]
- Yang J., Yan R., Roy A., Xu D., Poisson J., et al. , 2015. The I-TASSER Suite: protein structure and function prediction. Nat. Methods 12: 7–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yochem, J., 2006 Nomarski images for learning the anatomy, with tips for mosaic analysis (January 24, 2006), WormBook, ed. The C. elegans Research Community WormBook, /10.1895/wormbook.1.100.1, http://www.wormbook.org. [DOI] [PMC free article] [PubMed]
- Yochem J., Gu T., Han M., 1998. A new marker for mosaic analysis in Caenorhabditis elegans indicates a fusion between hyp6 and hyp7, two major components of the hypodermis. Genetics 149: 1323–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yochem J., Tuck S., Greenwald I., Han M., 1999. A gp330/megalin-related protein is required in the major epidermis of Caenorhabditis elegans for completion of molting. Development 126: 597–606. [DOI] [PubMed] [Google Scholar]
- Yochem J., Sundaram M., Bucher E. A., 2000. Mosaic analysis in Caenorhabditis elegans. Methods Mol. Biol. 135: 447–462. [DOI] [PubMed] [Google Scholar]
- Yochem J., Lazetic V., Bell L., Chen L., Fay D., 2015. C. elegans NIMA-related kinases NEKL-2 and NEKL-3 are required for the completion of molting. Dev. Biol. 398: 255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Mouw J. K., Weaver V. M., 2011. Forcing form and function: biomechanical regulation of tumor evolution. Trends Cell Biol. 21: 47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalli D., Bayliss R., Fry A. M., 2012. The Nek8 protein kinase, mutated in the human cystic kidney disease nephronophthisis, is both activated and degraded during ciliogenesis. Hum. Mol. Genet. 21: 1155–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannad F., Radauceanu A., 2005. Effect of MR blockade on collagen formation and cardiovascular disease with a specific emphasis on heart failure. Heart Fail. Rev. 10: 71–78. [DOI] [PubMed] [Google Scholar]
- Zhang J., Guo W. H., Wang Y. L., 2014. Microtubules stabilize cell polarity by localizing rear signals. Proc. Natl. Acad. Sci. USA 111: 16383–16388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., 2008. I-TASSER server for protein 3D structure prediction. BMC Bioinformatics 9: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Wang Z., Xu X., Wan Y., Qu K., et al. , 2016. Nek7 is overexpressed in hepatocellular carcinoma and promotes hepatocellular carcinoma cell proliferation in vitro and in vivo. Oncotarget 7: 18620–18630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zugasti O., Rajan J., Kuwabara P. E., 2005. The function and expansion of the Patched- and Hedgehog-related homologs in C. elegans. Genome Res. 15: 1402–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article. Reagents or specific data from this manuscript available upon request.