Version Changes
Revised. Amendments from Version 1
The section entitled Summary of outcome of GWAS studies in CD has been modified in accordance with the suggestions of Referee 2. Additional references have also been added.
Abstract
The cause of Crohn’s disease (CD) has posed a conundrum for at least a century. A large body of work coupled with recent technological advances in genome research have at last started to provide some of the answers. Initially this review seeks to explain and to differentiate between bowel inflammation in the primary immunodeficiencies that generally lead to very early onset diffuse bowel inflammation in humans and in animal models, and the real syndrome of CD. In the latter, a trigger, almost certainly enteric infection by one of a multitude of organisms, allows the faeces access to the tissues, at which stage the response of individuals predisposed to CD is abnormal. Direct investigation of patients’ inflammatory response together with genome-wide association studies (GWAS) and DNA sequencing indicate that in CD the failure of acute inflammation and the clearance of bacteria from the tissues, and from within cells, is defective. The retained faecal products result in the characteristic chronic granulomatous inflammation and adaptive immune response. In this review I will examine the contemporary evidence that has led to this understanding, and look for explanations for the recent dramatic increase in the incidence of this disease.
Keywords: Crohn’s, Inflammatory Bowel Disease, GWAS, Immunology, Infection, Bacteria, Gastroenteritis, Gene
General Introduction
The enigma that is the cause of Crohn’s disease (CD) has puzzled clinicians and scientists from time immemorial. It is generally accepted that CD results from an aberrant immune response to commensal microflora in genetically susceptible individuals 1, however, the nature of the immune defects, the responsible microflora and the genetic susceptibility remain incompletely defined and actively debated. With advances in genomic technologies our understanding of this puzzling condition is evolving, and answers forthcoming. The purpose of this article is to undertake a holistic review of the aetiopathogenesis of CD in which historical concepts are integrated with recent discoveries.
The bowel mucosa, an interface between faeces and the tissues
The distal ileum and colon contain >10 11 bacteria per gram of faecal material 2, which pose an immediate threat to life if they penetrate into the underlying tissues. The bowel microflora are isolated by a thin film of mucus and a single layer of columnar epithelial cells with a surface area of approximately 32m 2, 3. The requirement for the absorption of fluids and nutrients by the bowel mucosa means that the bowel lining cannot simply be a tough impermeable barrier, and as a consequence provision must be made to defend the vulnerable mucosal epithelial cell layer against its contents. Mucus secreted by goblet cells forms a continuous, weak, viscoelastic gel, lining, 5–500 μm thick 4. In addition to acting as a physical barrier and lubricant, the mucus is the site of action of a variety of antimicrobial mechanisms including secretory IgA, antimicrobial enzymes and peptides 5 and H 2O 2 generated by the DUOX electron transport chain 6. Despite these barriers, the separation of the tissues from the gut microbiome is not absolute, and even in health the mucosa is constantly penetrated by relatively small numbers of enteric organisms and soluble microbial products that gain access into the tissues 7– 10. Scattered amongst the epithelial cells overlying lymph follicles are Microfold (M) cells 11, 12, a unique intestinal epithelial cell (IEC) subset that are highly specialized for the phagocytosis and transcytosis of gut lumen macromolecules, particulate antigens and pathogenic or commensal microorganisms, which they transfer across the epithelium to mucosal macrophages and dendritic cells. This slow, constant, transit is important for the development, priming and maintenance of a potent immune system in the submucosa 13– 15. The protective role of the bowel immune system must be combined with tolerance to ingested antigens and commensal organisms to maintain homeostasis in a healthy bowel.
The immune system in the bowel
The bowel is the interface between a dense population of microbes and the immune system. Although an in depth review of the immune system in the bowel is well beyond the scope of this review, it is important to briefly cover this subject because defects in innate immunity are central to the development of CD whereas aberrant adaptive immunity causes bowel inflammation of a very different type, and a range of largely inaccurate animal models of CD.
Adaptive immunity. Most of what is known of classical adaptive immunology relates to the immune system of the bowel, but there are in addition some specialised features unique to the intestinal mucosa 16– 18.
The mucosae and exocrine glands harbour the largest activated B-cell system of the body, amounting to some 80–90% of all immunoglobulin (Ig)-producing cells in humans 19. The major product of these lymphocytes is polymeric (p)IgA (mainly dimers) with associated J chain. Both pIgA and pentameric IgM contain a binding site for the polymeric Ig receptor (pIgR), or secretory component (SC), which is a requirement for their active external transport through secretory epithelia into the overlying mucus 19.
M cells, and intestinal dendritic cells, that phagocytose bacteria interact with B and T cells in the Peyer’s patches, inducing B cells to produce IgA directed against intestinal bacteria 20. IgA+ B cells home to the intestinal lamina propria and secrete IgA that is transcytosed across the epithelium and deposited on the apical surface. The transcytosed IgAs bind to luminal bacteria, preventing microbial translocation across the epithelial barrier 21, 22.
After the initiation of the immune response by antigen processing and presentation to B and T cells in Peyer's patches, primed lymphocytes leave the mucosa via the thoracic duct. Finally they migrate back to the mucosa where they exert effector functions.
There has been considerable recent interest in IL-23 and IL-17 in relation to the aetiology of CD. IL-23 is secreted by macrophages and dendritic cells and transforms naïve T cells into Th (T-helper) 17 cells, and promotes their expansion and maintenance 23, and they then produce IL-17, IL-21 and IL-22 24. IL-17 induces numerous cell types including T-cells, mast cells, macrophages, neutrophils, keratinocyte, and natural killer cells to produce a raft of pro-inflammatory mediators including IL-1b, IL-6, IL-8, IL-11, Gro-α, G-CSF, GM-CSF, IL-4, IL-5, IL-13, IgE, and eotaxin 25. An important outcome of this cytokine cascade appears to be the recruitment of neutrophils to inflammatory sites 26. Despite the apparent importance of IL-17 for intestinal barrier function 27 and for diverse pro-inflammatory activities, there must be considerable redundancy in the pro-inflammatory repertoire as defects in the IL-17 pathway are associated with a very narrow predisposition to disease in the form of mucocutaneous candidiasis 28. None of the hundreds of patients with this condition had CD 29.
IL-17 has been considered to be detrimental in CD as a consequence of its apparent pro-inflammatory actions. It is therefore of interest that a trial of the treatment of CD with monoclonal antibodies against IL-17 had to be stopped because of the deterioration of the patients’ condition 30.
Adaptive immunity in the bowel protects against commensal organisms, or those previously encountered in infections that were successfully overcome. This is accomplished by the production of a barrier of secreted IgA that permeates the lining mucus layer and by the production of specific IgG and IgM that opsonise penetrating organisms for phagocytosis. Immunity to pathogenic bacteria like Salmonella, Shigella, Vibrio cholera and Escherichia coli is generally not very potent or long lasting, which, together with the propensity of bacteria to mutate, makes vaccines relatively ineffective 31, 32. A degree of immunity does develop as a result of repeated reinfection in endemic areas but because this is not permanent, it is gradually lost after emigration to cleaner environments, which might be an important factor in relation to the subsequent triggering of CD by infection in individuals moving from regions of low to high prevalence of this condition.
Innate immunity. The submucosa of the bowel is particularly vulnerable to microbial invasion if the mucosal barrier is breached as large numbers of organisms can achieve rapid access and the conditions are conducive to microbial proliferation. There is inadequate time for adaptive immunity to take effect and reliance must be placed on the innate system to contain and eliminate potentially harmful stimuli. At its heart this means the rapid and florid release of pro-inflammatory cytokines from lamina propria macrophages 33, recruited from blood monocytes 34, mast cells 35, 36, eosinophils and innate lymphoid cells 37– 39 when activated by bowel contents. Paneth cells are specialised intraepithelial secretory epithelium of the small intestine that reside in small clusters at the base of crypts of Lieberkühn in the small intestine. Large secretory granules in these cells contain a wide variety of proteins, the most abundant of which are antimicrobials such as the alpha defensins that are discharged into the crypt lumen. These effector molecules also diffuse from the crypt and disseminate into the mucous layer that overlies the mucosal epithelium, where they contribute to the mucosal antimicrobial barrier 40.
Pro-inflammatory cytokines induce changes in the microvasculature 41, 42 leading to the extravasation of plasma proteins and to the recruitment of neutrophils 43. A critical concentration of neutrophils is required to eliminate invading bacteria 44 and immediately after bacterial penetration of the mucosa there is direct competition between bacterial replication and neutrophil recruitment and bacterial phagocytosis and killing. In the absence of specific antibodies, uptake of the foreign material is enhanced by non-specific opsonins like pentraxins, collectins and complement 45. The neutrophils then undergo apoptosis or necrosis and the purulent collection is most probably discharged into the bowel lumen, with the residual debris being phagocytosed and cleared by macrophages 46.
Bowel inflammation in very early onset Inflammatory Bowel Disease (IBD)
Bowel homeostasis requires an intact mucosal barrier, itself requiring the integrated function of many different cell types and molecules, and the largest collection of immunological cells in the body to present an integrated defence against the intestinal microbiome. It is therefore not surprising that defects in genes coding for proteins required for the integrity of this barrier, or for normal immune surveillance, manifest as mucosal inflammation.
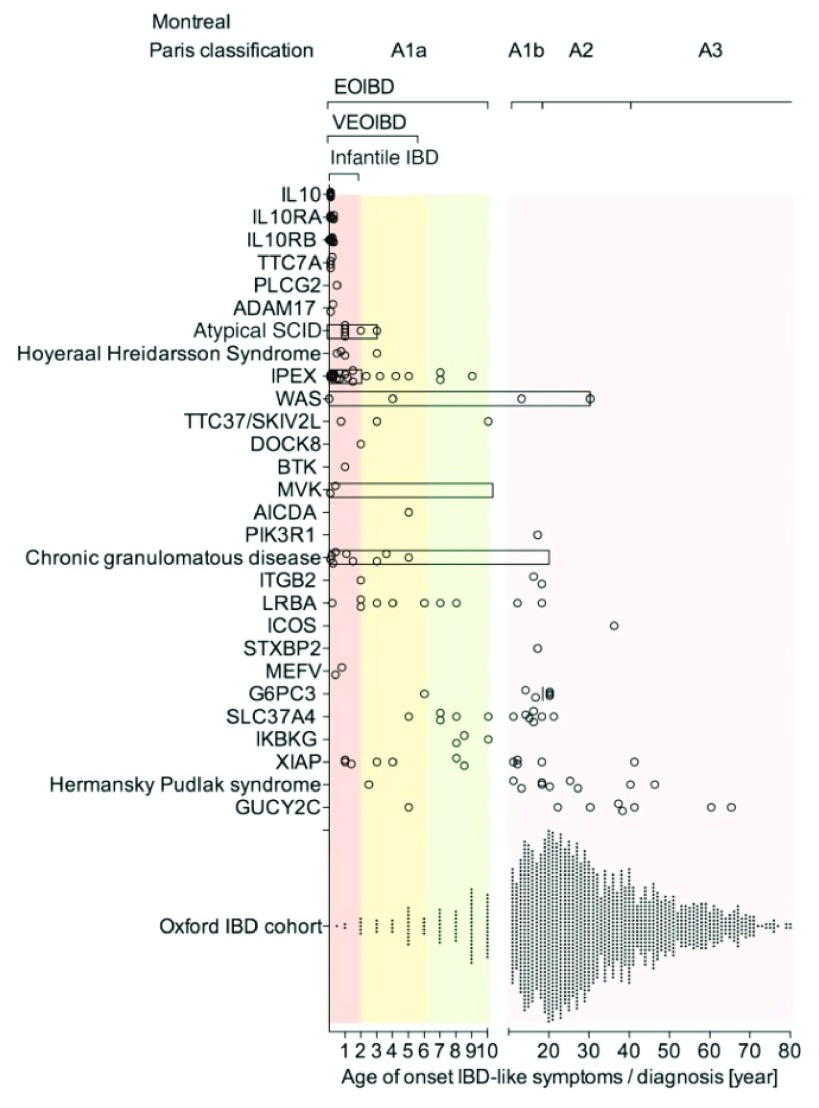
As might be expected, these conditions present very early in life, and because they affect the mucosa as a whole, they result in a diffuse, non-specific inflammation, predominantly in the large bowel where concentrations of bacteria are highest. Uhlig et al. ( Figure 1) 47 found that about 5% of their cases of IBD had infantile or very early onset disease. This will represent a much higher proportion of cases than that occurring in the general population, because most cases of IBD occur in adults and are handled in non-specialist facilities, whereas rare inherited diseases gravitate to specialist centres like those of Uhlig and his co-authors. The monogenic lesions identified provide important identifiers of the molecules required for bowel integrity and adaptive immunity.
Figure 1. Age of onset of IBD-like symptoms in patients with monogenic diseases.
Multiple genetic defects are summarized in the group of atypical Severe Combined Immunodeficiency (SCID), Hoyeraal–Hreidarsson syndrome, Chronic Granulomatous Disease (CGD), and Hermansky–Pudlak syndrome. By comparison, an unselected IBD population is presented (Oxford IBD cohort study; paediatric and adult referral-based IBD cohort, n = 1605 patients comprising CD, Ulcerative Colitis (UC), and IBD unclassified [IBDU]). Symbols represent individual patients. Bars represent the age range of case series if individual data were not available. Reproduced from 47 with permission from the publisher.
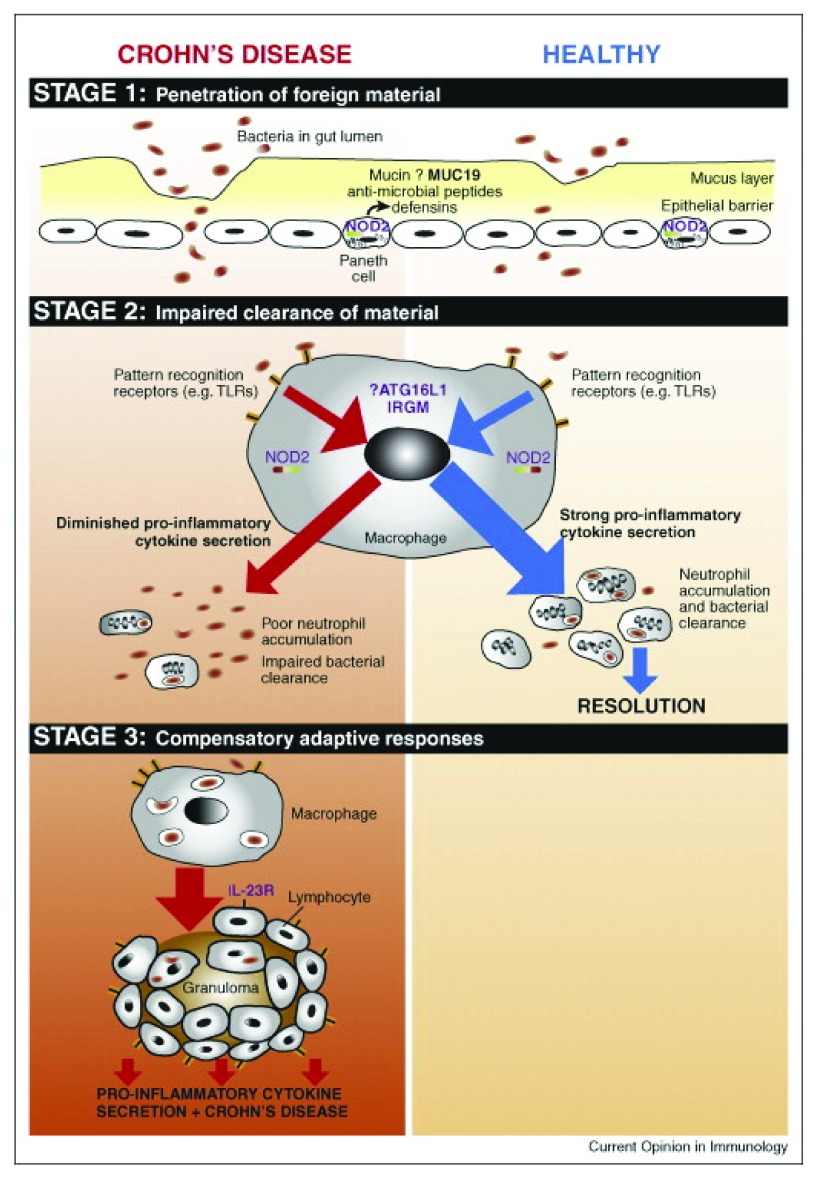
Figure 2. The immunopathogenesis of CD occurs in three temporally distinct stages.
Penetration of luminal contents into underlying tissues occurs in stage 1, which may be facilitated by environmental factors such as infection, or inherent defects in the mucosal barrier. In healthy individuals, resident macrophages secrete pro-inflammatory cytokines in response to this material, resulting in neutrophil accumulation, clearance of the material, and thereby resolution. In CD patients, defective secretion of pro-inflammatory cytokines by macrophages results in impaired neutrophil influx and clearance of foreign material (stage 2). Subsequently, chronic inflammatory responses (stage 3) will be triggered, giving rise to the characteristic features of the CD lesion. From 87 Figure 1 (reproduced with permission from the publisher).
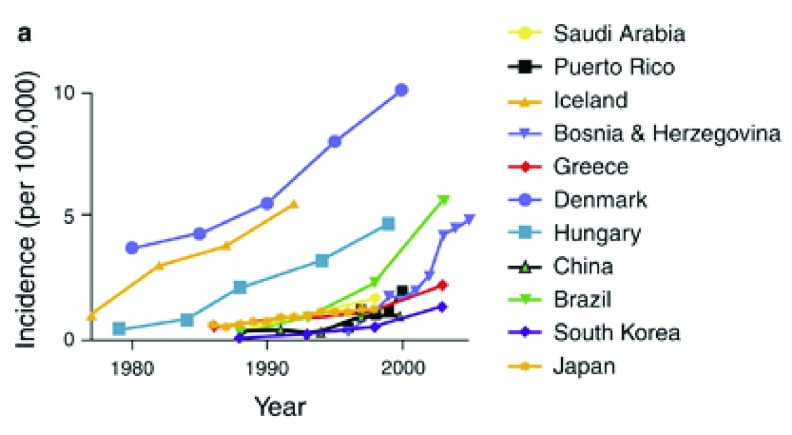
Figure 3. Increasing incidence of CD in several countries over time.
Reproduced from 94 (with permission).
Mutations in the barrier function genes such as COL7A1, FERMT1, TTC7A and ADAM17 generally result in infantile bowel dysfunction and inflammation.
The severe immunodeficiency syndromes such as atypical Severe Combined Immunodeficiency (SCID) and Immunodysregulation Polyendocrinopathy Enteropathy X-linked Syndrome (IPEX) also generally have a very early onset and also do not have intestinal changes characteristic of either CD or UC. In contradistinction, the neutrophil defects, exemplified by Chronic Granulomatous Disease (CGD), and including Hermansky-Pudlak, congenital neutropenia and leukocyte adhesion deficiency all have a CD phenotype clinically, endoscopically and histopathologically, with a lot of perianal disease and granulomata evident on biopsy 48. The neutrophil defects generally present later than the abnormalities of mucosal barrier function, or the severe immunological diseases.
Mutations in the genes coding for IL-10 and IL-10 receptor both present very early in life, as seen in mucosal abnormalities and immunodeficiencies, and exhibit the bowel phenotype characteristic of defective neutrophil function. These observations would appear to be at variance with the prevailing view that IL-10 down-regulates macrophage function, and that the bowel inflammation in its absence is a manifestation of uncontrolled macrophage activation 49. IL-10 does appear to be required for normal intestinal development. Multisystem abnormalities were observed in the original description of the IL-10 knock-out mouse 50 in which there was a general enterocolitis with greatest abnormalities in the duodenum and jejunum, not locations associated with a high burden of commensal bacteria. Under specific-pathogen free (SPF) conditions the bowel lesions persisted, but were limited to the proximal colon. In addition the mice exhibited a severe growth defect, and were severely anaemic with a paucity of erythroid precursors in the bone marrow that was filled with myeloid precursors. The anaemia was unrelated to the extent of bowel involvement. These phenotypic features indicate that IL-10 is required for the normal growth and development of the bowel mucosa and haemopoetic tissue, in addition to its suppressant effect on macrophages 51.
The observed phenotype of patients with IL-10 and IL-10 receptor deficiency is in keeping with that of defective neutrophil function 49, 52. Almost all the patients showed evidence of bacterial infections in the form of folliculitis, and ear and respiratory tract infections. Most revealing was the almost universal occurrence of perianal disease with abscesses, fissures and fistulae that are highly characteristic of the neutrophil deficiency diseases like CGD 53, 54, Hermansky-Pudluck 55 and glycogen storage disease 1b 56. If the IL-10 deficient phenotype results from the impairment of normal cellular development and an immunodeficiency rather than an excessive, unregulated, macrophage response as proposed, then it might be expected that treatment with immunosuppressive therapy would be ineffective, which was indeed found to be the case 52.
It is also noteworthy that bowel inflammation is not a feature of the classical autoinflammatory diseases in which deregulated macrophage activation is a feature. These diseases include cryopyrin-associated periodic syndrome (CAPS) 57 in which activating mutations in the NLRP3 gene result in increased excretion of IL-1β excretion and other pro-inflammatory cytokines, and the haemophagocytic syndrome 58, in which the uncontrolled activation of antigen-presenting cells (macrophages and histiocytes) and T cells produces an exaggerated inflammatory response and cytokine storm.
Of mice and men - mouse models of “IBD”
Mouse models of IBD have been extensively reviewed in the literature 59– 61. These models are very important because they are depended upon by clinicians and scientist trying to understand the causes of these diseases, and by the pharmaceutical industry attempting to produce drugs with which to treat them.
Induced inflammation. In general, impairment of the innate immune system as in mouse models of CGD or Wiscott-Aldrich does not result in the spontaneous development of bowel inflammation, although they do exhibit an exaggerated response to insult 62.
Predisposition to bowel inflammation may be exposed by reducing intestinal barrier function, thereby allowing access of the contained microbiome to the underlying tissues. Barrier function can be compromised through the genetic manipulation of proteins required for the production of mucus or the maintenance of epithelial integrity 63 or by the use of chemicals or infectious agents.
Chemical agents employed for this purpose fall into three main groups. Those that produce direct damage to the mucosa such as dextran sodium sulphate (DSS 64), acetic acid and carrageenan 65. The second group are those such as Haptens ( e.g. 2, 4-Dinitrochlorobenzene (DNCB 66) or Dinitrobenzene sulphonic acid (DNBS)) that induce an immune response. Finally infection with bacteria such as Salmonella, E.coli or Citrobacter, or parasites 60 may be utilised.
Genetic models. The advent of gene targeting technology has provided immunologists with powerful tools with which to explore the immune system. In the course of investigating its diverse components, hundreds of different genes have been knocked-out, some of which resulted in the spontaneous development of bowel inflammation. Because of this, these mice have been proposed as models of IBD.
Prominent examples of such mice include the IL-2 67, T cell receptor (TCR)α/β 68, and IL-10 50 knockout models. With the exception of IL-10-deficient mice, which possess some features of human CD, the majority of these models have diffuse colonic inflammation. A strain of mouse (TnfΔAREmice) was developed in which elements of the tumour necrosis factor (TNF) gene that are required to restrict the overproduction of this cytokine have been removed. In their absence the mice exhibit sustained over production of TNF which results in a diffuse arthritis and terminal ileal and caecal inflammation 69. IL-17 and IL-22 deficiency exacerbate induced colitis 70.
Spontaneous models. The C3H/HeJBir model of colitis was discovered by chance when mice in breeding colonies developed loose bowel actions 71. The pathology is characterised by spontaneous and chronic focal inflammation localised to the right colon and caecal region although not involving the small intestine. The colitis occurs in young mice and tends to resolve with age, without recurrence. The genetic mechanisms underlying these abnormalities remain to be identified.
SAMP1/Yit mice were developed from senescence-accelerated mice 72. They spontaneously developed ileitis and gastritis even under germ free conditions. The underlying aetiology is unknown but there is some evidence that the primary defect lies in the epithelial cell barrier and that B cells appear to play a role in the pathogenesis of inflammation at both sites 73.
Adoptive transfer. One of the most commonly cited models for the study of the role of T lymphocytes in bowel inflammation in mice (as a proposed model of CD) is the adoptive transfer model in which T cells are transfused into SCID mice 74, resulting in bowel inflammation. By observing the effects of varying the populations of cells infused, conclusions have been drawn as to the regulatory interaction of the various cell populations. It is important to understand that these host SCID mice have hardly any B or T lymphocytes and, as their name suggests, are hypogammaglobulinaemic and severely immunocompromised. In the CD45RBhigh transfer model, first described by Morrissey 75 a subset of lymph node CD4 + T-cells, expressing high levels of the marker CD45RB (CD45RB hi), were injected into SCID mice. The mice developed a wasting disease accompanied by massive hyperplasia of the intestinal mucosa with a dense infiltration of lymphocytes thought to be due to “an augmented, unregulated reaction towards higher levels of luminal-derived bacteria or bacterial products”. These changes were not seen when the animals were infused with unfractionated CD4 + or CD45RB lo cells, indicating that the extreme reaction to bacterial products 76 by the CD45RB Hi cells could be controlled by the CD45RB lo cells. Soon after, similar experiments were conducted by Powrie and colleagues 77, 78 who observed the same pathological changes in the bowel which they equated to those changes found in “inflammatory bowel disease” in humans. They showed that these changes could be prevented by antibodies to interferon-gamma and by recombinant IL-10 78, and identified the cells in the CD45RB lo population responsible for controlling CD45RB hi induced inflammation as the population of suppressor T-cells called T-reg cells 79.
These observations and their extrapolation to human IBD sparked a large body of work into the role of regulatory T cells in the pathogenesis of IBD. Over the past decade, multiple groups have failed to find abnormalities in these cells in the intestines or blood of patients with IBD 80, not altogether surprising given the extreme artificiality of the animal model from which their presumed role in human disease was derived.
These mouse inflammation models are undoubtedly of great value in dissecting out immunological mechanisms and attributing roles to specific cellular populations and their associated cytokines. However, equating genetic mutations leading to bowel inflammation in mice with causal mechanisms of diseases in humans can have serious consequences as it may misdirect clinicians and scientists as to the underlying pathophysiology, and mislead pharmaceutical companies as to the relevant biological pathways against which to attempt to develop drugs. On the other hand, mouse models can be of great value when the problem is turned the other way around and they, and other animals like zebrafish, are used to validate the causality of molecular lesions found in association with disease in humans, for example those involving IL-10 61 and ADAM17 81.
Classification of IBD
“Medicine is learned by the bedside and not in the classroom. Let not your conceptions of disease come from words heard in the lecture room or read from the book. See, and then reason and compare and control. But see first.”
Sir William Osler
IBD has referred to CD and UC because both can largely affect the colon and terminal ileum, however, although there may be overlap at the interface of these two conditions, their classical manifestations are quite different 82, 83. They are both syndromes, rather than specific diseases, where common clinical pictures are united by a common set of diagnostic criteria produced by similar pathophysiological mechanisms.
CD 84 usually involves the terminal ileum, and the caecum and colon to a variable extent, where the lesions are patchy, known as “skip lesions”, and associated with strictures, and fistulae between the bowel and other loops of bowel, the skin, and pelvic organs like the bladder and vagina. Outside the bowel, at the sites of transmural inflammation, the mesenteric adipocytes hypertrophy, covering the exterior of the bowel with a layer of protective fat, a process known as fat wrapping. Anal disease affects about 40% of these patients 85 exemplified by abscess, fistulation and skin tags. The inflammation is described as transmural, extending deep into the wall of the bowel, and contains diagnostic granulomata, collections of macrophages which represent a characteristic tissue response to retained foreign material. “The basic etiological factor in the case of all granulomas is probably the presence of a nidus of insoluble material which, if small enough is ingested by phagocytic cells, or, if too large, remains extracellular” 86. The central macrophages in these granulomata are surrounded by lymphocytes.
UC is very different in that it starts at the rectum and extends proximally, although occasionally, when it involves the whole large bowel there can also be involvement of the terminal ileum, a condition known as “backwash ileitis”. The inflammation in UC is superficial, being limited to the lamina propria, and the histological hallmarks are crypt abscesses and depletion of goblet cells that normally contain mucus.
Although a syndrome, the diagnostic features of classical CD are quite precise, and very different from the very rare cases of very early onset IBD, and the vast majority of genetically abnormal mice, both characterised by bowel inflammation rather than the clinical criteria used to diagnose CD or UC.
The three phases of Crohn’s disease
A unifying model of CD pathogenesis has been proposed in which this condition develops in three temporally distinct phases 87:
-
•
The trigger - gastrointestinal infection;
-
•
A defective response to the consequences of this infection;
-
•
A subsequent prolonged chronic inflammatory adaptive immune response.
The trigger - Breeching the mucosa – The infectious environmental factor
Epidemiology. There is strong evidence for the role of an infectious environmental factor in the pathogenesis of CD. This is most obviously seen when populations or families emigrate from one country to another. A high proportion of family members have been documented as developing the disease after moving from Morocco to Belgium 88, from Albania to Greece 89 and from India to Canada 90. After being imported into the household enteric infections can spread to family members 91. At a population level, an increased incidence of CD has been described in recent immigrants from Ethiopia to Israel 92, and from Eastern European and Iraq to Sweden 93.
The epidemiology of CD has been the subject of a large body of work and multiple reviews. Most pertinent to this paper are issues concerning environmental influences, several of which are clearly associated with CD as outlined below.
Temporal trends. There has been a steep rise in the incidence of CD over the last few decades in economically advanced countries across Europe, North America and Australasia 94– 96. This is not purely an effect of increasing economic affluence because the incidence of CD is much lower in other economically advanced countries such as Japan and South Korea, although the incidence is now also rising in these countries 97, 98.
CD is generally more common in urban females of higher socioeconomic status 96, with a male to female ratio of about 1.5–2:1.
The difference in prevalence of CD by country could be partly explained by genetic factors; however, evidence from migration studies emphasise the importance of the environment. A limited number of studies investigating the incidence of CD among recent immigrants have been undertaken. The most informative of these assessed the risk of IBD in first- and second-generation immigrants to Sweden from many different countries 93. They found that overall risk of CD was lower in many groups of first-generation immigrants than in the native-born Swedish reference group but that in most groups of second-generation immigrants these decreased risks disappeared, and in some cases even exceeded those in the native Swedish population. First generation Middle Eastern immigrants to Australia developed CD at a much later age (∼57 years) than the second-generation who developed it at about 28 years of age, roughly the standard age in Western society 99. CD is very rare in Ethiopia but emerged in Ethiopian Jews migrating to Israel after a median lag of about 12 years after arrival 92. CD is also more common in Bangladeshi immigrants to England 100. Combined, these studies imply that immigrants from underdeveloped countries initially have a resistance to CD that wanes over the subsequent decade or so.
Infection. Infection has long been considered to cause CD. Attempts were made to transmit a CD agent from gut or lymph node tissue of patients to wild-type or immunodeficient mice 101. More granulomata were found in the mice receiving CD tissue, but that could have been due to the fact that the inflamed tissue contained enteric organisms or inflammatory cytokines. In the first description by Danziel in 1913 of what was later to be called Crohn’s disease, the similarity between “chronic interstitial enteritis” and Johne's disease in cattle 102, which is caused by infection with Mycobacterium avium paratuberculosis, was commented upon 103. Evidence that this agent was also responsible for human CD has been extensively sought 104 but has not been forthcoming 105, 106.
Several prospective studies have followed the course of patients after infections with enteric organisms and all have found an increased incidence of IBD as compared with uninfected control subjects 107– 111. In one of these 110 the risk was similar whether or not an infecting agent was identified, suggesting that it was the damage to the bowel rather than a specific infection that was important.
Enteric infections are most commonly caused by viruses, particularly Norovirus 112 and by Campylobacter, Salmonellae, Shigella, Entamoeba histolytica, Cytomegalovirus and Yersinia 113, 114. Particular attention has been paid to an adherent-invasive subgroup of E. coli, that has been linked to the development of CD 115– 117. The natural lesions produced by these organisms might provide some insight into those most likely to trigger CD. Norovirus mainly affects the proximal small intestine 112, 118 and Amoebic and Salmonella infections generally produce a diffuse colitis whereas the other infections result in lesions located in the terminal ileum and colon, with a patchy distribution, similar to those of the lesions of CD 119– 126.
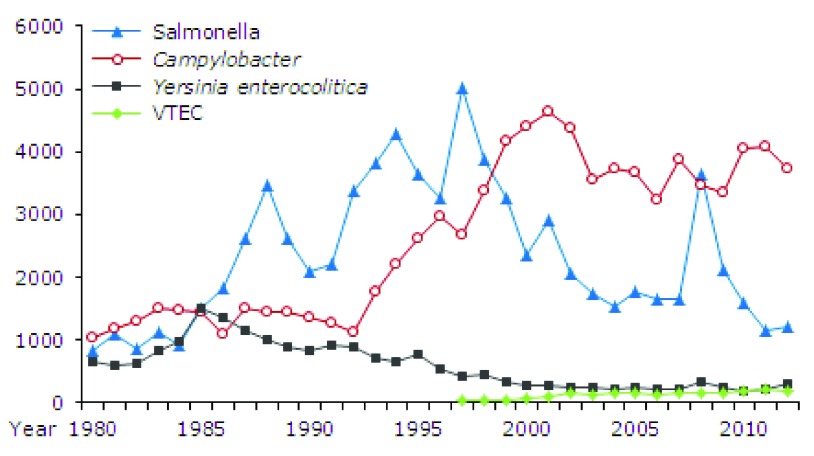
Whereas the incidence of most bacterial gastrointestinal infections is steady or falling, that induced by the commonest bacterial pathogen, Campylobacter is increasing in countries like North America, Europe, Scandinavia, Australia and New Zealand and Japan 127 ( Figure 4). This could be because this organism is a common contaminant of poultry, the consumption of which is increasing in these countries, however, broiler flocks are heavily contaminated with both Campylobacter and Salmonellae 128 and the incidence of infection by the latter is steady or falling ( Figure 4).
Figure 4. Number of recorded infections caused by Salmonella, Campylobacter, Yersinia enterocolitica and VTEC, 1980–2012.
EPI-NEWS 12, 2013 from Statens Serum Institut, Denmark (reproduced with permission).
“Relapses” in cases of IBD have been reported to be associated with infections with various organisms including Clostridium difficile, Shigella, Salmonella, Campylobacter, E. coli and Listeria 129. These subsequent infections might be inducing the development of a novel set of Crohn’s lesions in a predisposed bowel, rather than recrudescence of the original disease, as a result of infection by different organisms. These subsequent infections could be predisposed to by the immunosuppressive treatments commonly used in this condition including corticosteroids, cytotoxic and biological agents.
Most gastrointestinal infections do not generally produce homogeneous mucosal damage but lead to focal areas of ulceration 125, 126, often in the ileocaecal region of the bowel. Because infection with invasive gastrointestinal pathogens is a stochastic process 130, the age at which this occurs is highly variable, as is the outcome after the infection, which will depend upon the severity of the infection, extent of ulceration, quantity of bowel contents gaining access to the tissues and to the effectiveness of the innate immune response.
The microbiome, prebiotics, probiotics and faecal transplants. In the search for possible causal infectious agents, stool samples from CD patients have been extensively cultured and examined without a positive result (see for example 131). This is not entirely surprising because the average time from the onset of symptoms to diagnosis of CD is over six months 132 by which time an infectious organism will have been eliminated if it was a triggering agent rather than the cause of a chronic infection. With the advent of next generation 16S rRNA gene sequencing the phylogeny and taxonomy of samples from complex microbiomes can be determined without the need for them to be viable or culturable. Dysbiosis of the faecal microbiome is well recognised in CD 133, 134, with a decrease in the abundance and diversity of the Firmicutes phylum and an increased abundance of Proteobacteria, and alterations in the fungal composition 135. Differences were also found between the microbiotas of CD patients with ileal and with colonic disease 136. This could reflect an epiphenomenon secondary to the disease process. Major alterations in the microbiota are induced by diarrhoea 137, enteral nutrition 138, antibiotics 139, which most of these patients receive 140, and by iron therapy 141 which is often prescribed because these patients are generally anaemic. In general, gut and mouth microbiomes display universal dynamics, unlike microbial communities associated with certain skin sites that are probably shaped by differences in host environment 142.
Because CD predominantly occurs in those regions of the bowel with a high bacterial count, and given the differences in the microbiotas in CD described above, attempts have been made to alter the intestinal microbiota in the treatment of this condition. Prebiotics are typically non-digestible, fibre rich materials, which stimulate the growth or activity of advantageous bacteria that colonize the large bowel, whereas probiotics are live microorganisms that are directly administered by mouth. Neither prebiotics nor probiotics have been shown to be beneficial in CD 143– 145. An alternative means of directly altering the intestinal microbiota is by faecal microbiota transplantation, the transfer of faeces from a healthy donor, to restore the intestinal microbiota of a diseased individual. Whilst this is a logical treatment for Clostridium difficile infection, which generally develops in a colon depleted of its natural microbiome by antibiotics, it has not been found to be effective in the treatment of CD 146, 147.
The Hygiene Hypothesis. The considerable increase in the incidence of CD in developed countries in recent decades 148 has been attributed to immunological changes to alterations in the environment as outlined in the Hygiene Hypothesis 149. This hypothesis 150 was first described by Strachan in 1989 who stated that “over the past century declining family size, improvements in household amenities, and higher standards of personal cleanliness have reduced the opportunity for cross infection in young families. This may have resulted in more widespread clinical expression of atopic disease” 151. Subsequently modern living conditions have been held responsible for the increasing incidence of a variety of so called “auto-immune” diseases, including CD, which have been attributed to exposure a reduced load of microbes of decreased diversity. Certainly CD is less common in rural societies where there is exposure to animals, pets and soil, bedroom sharing is more common, and there is less access to hot water for ablutions 99.
According to this theory, standards of hygiene are lower in lower socioeconomic societies, leading to a greater abundance and variety of gastrointestinal pathogens. This would lead to a high incidence of gastrointestinal infections in infancy and childhood, resulting in death 152 or immunity 153. CD is very uncommon in underdeveloped societies in Asia 154, South America 155, China 156 and sub-Saharan Africa 157 and the increase in its incidence is closely associated with the improvement in income and living standards. Enteric infections are endemic in these developing societies in which diarrhoea is a major cause of death in children less than 5 years of age 158– 160. The population in underprivileged societies also host a large burden of gastrointestinal helminths 161 and the low incidence of CD recorded in developing countries has been attributed to the high rates of gastrointestinal infections with these organisms 162. Helminthic infection was found not to be protective against CD in Denmark 163 and the outcome of several trials of iatrogenic infection with helminths as therapy for CD are awaited, but current evidence does not suggest that they will be efficacious 162, 164.
Immunity to enteric organisms is transient 165– 167, and may be strain specific 168, and would be boosted by frequent reinfection in less advanced countries. This herd immunity would be lost over time after immigration to socially advanced, cleaner, societies, which would accord with the later age of onset of CD in first generation immigrants. One could envisage a situation in which the population of more socially advanced countries are living under increasingly hygienic conditions and are exposed to a less diverse repertoire of the enteric microorganisms capable of producing gastrointestinal infection. With less frequent gastrointestinal infection, the bowel is uninflamed, with less primed macrophages, mast cells and dendritic cells in the lamina propria and adaptive immunity is more restricted, and relatively feeble, through the lack of repeated boosts by infection, making the bowel vulnerable to attack by a novel or virulent organism.
If we postulate that the trigger for CD is enteric infection, how can the fact that the incidence of food-borne gastroenteritis is fairly steady in most developed countries 169 be reconciled with the rapidly increasing incidence of CD? Due to greater regulation and control of food production and distribution the incidence of foodborne outbreaks of disease have remained steady or have declined 170– 172.
It is important to consider the age distribution at which patients present with Crohn’s disease. It rises to a peak at between 20 and 30 years of age after which it demonstrates a steady decline, a pattern that is remarkably consistent, and very different from that of UC, across the geographical spectrum 98, 173– 175.
The peak incidence, generally at a later age than puberty, coincides with a stage in life accompanied by major lifestyle changes. These include the movement of individuals out of the family home, in which the ambient microbiome is likely to be relatively stable, into environments in which the risks of exposure to infection are much greater. The main two ways in which young adults are exposed to infectious enteric organisms is through the ingestion of contaminated food or fluids, or by person to person contact, the risk of both being increased by travel to places where exposure to novel organisms is more likely.
Sexual transmission
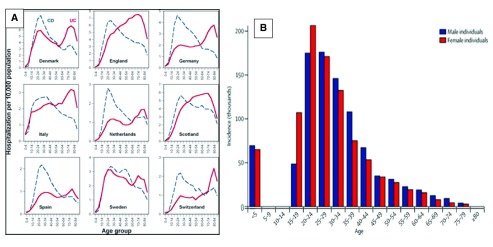
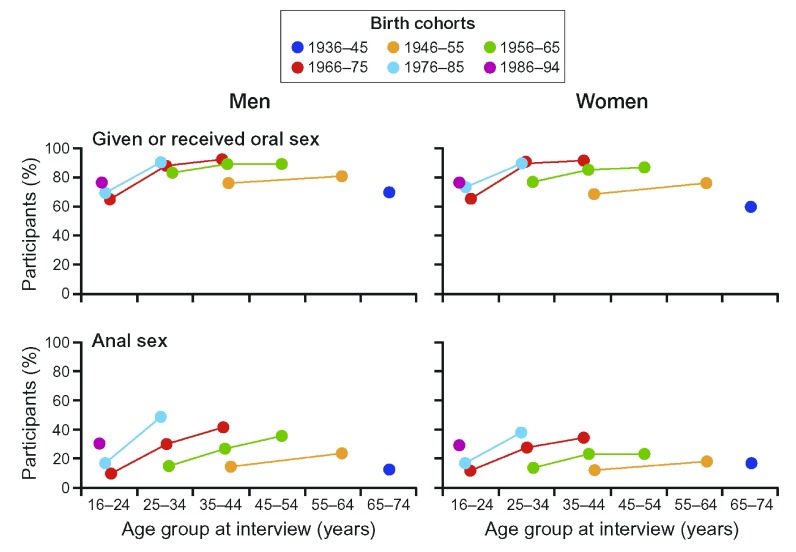
Although enteric infections are generally considered to be foodborne, only about one half are in fact transmitted in this way 177, most of the rest being transferred by person to person contact. Sexual transmission is worthy of consideration as a means of transmission of faecal organisms between individuals because, as might be expected, the peak age for the acquisition of sexually transmitted diseases is very similar to that of CD ( Figure 5).
Figure 5.
( A) The age distributions of Crohn's disease and ulcerative colitis in several European countries. (Reproduced from 173 with permission from the publisher). ( B) Global age-sex distribution of new HIV infections 176 (reproduced with permission).
Epidemiological studies from developed countries have reported an increasing prevalence of invasive infections by Entamoeba histolytica 178, Shigella 179, Cryptosporidia 180 and Campylobacter 181, among men who have sex with men (MSM), which is not surprising because of the increased risk of exposure to coliform organisms by oral, anal and oro-anal sexual practises 182. The ingestion of as few as 10 virulent Shigella organisms can confer full-blown dysentery 183. It is easier to establish the causality of infectious outbreaks in these groups of individuals as compared with the general population, because they fall into more readily identifiable groupings which facilitate the epidemiological studies. It would be important to establish the incidence of CD in MSM, but diagnosis in these individuals is complicated by the relatively small proportions of individuals attending gastroenterology facilities, the presence of compounding factors such as “gay bowel” 184 and of sexually transmitted diseases like lymphogranuloma venerium 185 that can masquerade as CD.
Given that oral and oro-anal sexual practises have been demonstrated to be responsible for the transmission of enteric infections in MSM, they must also pose a risk in other populations 186, 187. Although rectal bacterial flora are present on the perineum of both sexes 188 and in the vagina 189 it is unlikely that an increase in gastrointestinal infection would result from vaginal intercourse alone. Only about 5% of the sexually active individuals in countries like Britain 190 and the United States 191 are not heterosexual. In the heterosexual community the anal sex is practised by 30 – 40% of the population in England ( Figure 6) and North America, and fellatio and cunnilingus are almost universal 190, 192. In England the participation in anal sex has almost doubled over the last three decades, a similar increase to that of the incidence of CD. In terms of absolute numbers, approximately seven times more women than homosexual men engage in unprotected receptive anal intercourse 193. In addition, the ratio of homosexual to bisexual men is about 3:1, and the latter can act as “bridgers”, transmitting infections from men who have sex with men into the heterosexual community 194.
Figure 6. Heterosexual sexual practices in Britain.
Redrawn from from the three National Surveys of Sexual Attitudes and Lifestyles 190. Each line connects values for the same birth cohort at different ages.
Those countries with a high standard of living and low rate of CD like Japan 97, Taiwan, China 195 Korea 98, Saudi Arabia 196 and Malaysia 83, appear to have low rates of heterosexual anal sex http://www.data360.org/pdf/20070416064139.Global Sex Survey.pdf 197 and in these countries the sex ratio of the disease, which is commoner in females than in males 96 in Western countries, is reversed, implying that men are particularly vulnerable to infection in these places. This does not appear to be due to a reporting bias because the sex ratio of UC in these countries matches that of Europe and North America.
Monogamous heterosexual couples develop complementary microbiomes 198, 199 which would suggest that the highest risk to infection of either partner through sexual contact would be in the early stages of a relationship, and that the risk to an individual would be related to the numbers of sexual partners, some of whom might be asymptomatic carriers of pathogenic organisms 200– 202.
Clearly the above arguments are conjectural and their validity will require validation through sound sociological, epidemiological and microbiological investigations.
Four other factors, smoking, antibiotics, appendectomy and invasive pneumococcal disease have positive correlations with the incidence of CD.
Smoking
Smoking of tobacco is the strongest environmental influence on CD, roughly doubling the incidence 203 and relapse rate 204. Smoking and nicotine impair intestinal 205, 206 and gastric 207 mucosal blood flow. Adequate blood flow is central to the development of an effective acute inflammatory response. Smoking also reduces levels of acute inflammatory cytokines in the bowel wall 208 and lumen 209 in patients with CD.
Antibiotics and appendectomy
There is an increased frequency of antibiotic use in CD prior to diagnosis 96, 210. The increased frequency of antibiotic use may be explained by an increased number of childhood bacterial infections. Similarly, an increased frequency of tonsillectomies 210 has been reported in CD and this may be an indication of recurrent pharyngitis. A further indication of a predisposition to infection in CD comes from the demonstration that these patients are more susceptible to invasive pneumococcal infection 211.
A history of a greater frequency of appendectomy in CD is also in keeping with an increased susceptibility to childhood infection in this condition 96.
Phase two – a defective inflammatory response
“Any infectious agent associated with Crohn’s disease is likely to be a widely distributed organism to which some people react abnormally - that is, the disease is unlikely to show the characteristic features of an infectious disease 212 .”
As described above, there is very good reason to believe that the initiating lesion in CD is infection by one of a number of enteric pathogens. The key to comprehending how the pathological lesions of the disease then develop lies in understanding the response to that initial infection. The infection by the organisms described above is very unlikely to persist, or the causal connection would have been clearly established some time ago. This is also the reason that antibiotics are of only limited efficacy in the treatment of CD 213.
These patients are unlikely to be unduly susceptible to infection by these organisms, or else the onset would occur earlier, and systemic disease would be expected, as occurs with Salmonellae when the interferon-gamma/IL-12 axis is disrupted 214.
Immunoparesis of the acute inflammatory response is the underlying Crohn’s phenotype
The underlying pathology in Crohn’s disease is the ineffective manner in which the faecal material entering the tissues through the damaged mucosa is dealt with. Infective damage to the mucosa followed by the entry of faecal material with a bacterial count of greater than 10 11 bacteria per ml into the tissues poses an existential threat that must be dealt with vigorously. This is accomplished by the acute inflammatory response, a non-specific local reaction to tissue damage that recruits the innate immune system. It includes the secretion of inflammatory mediators from mast cells and macrophages, complement activation, markedly increased blood flow, capillary dilatation and permeability, the deposition of a fibrin network, and most importantly in the context of CD, a massive influx of neutrophil leukocytes, highly motile phagocytes that ingest and kill invading bacteria and fungi and digest foreign organic material.
The underlying, and unifying, predisposition to the development of CD is a systemic incompetence of this acute inflammatory response. I will deal with the evidence supporting this immunoparesis in some detail because these experiments have been performed on CD patients, and healthy control subjects, and in some cases patients with UC and represent a unique set of data that have not been repeated, possibly because of the invasive and uncomfortable investigations required to obtain them.
The delay in the recruitment of neutrophils to sites of trauma to the body by the innate immune response has been demonstrated in patients with CD in several different but complimentary ways. In 1976 I demonstrated that the accumulation of neutrophils in superficial abrasions on the arm called “skin windows”, was grossly deficient when compared with healthy subjects or patients with another chronic inflammatory condition, rheumatoid arthritis 215. It was observed that “This abnormality of neutrophil function in Crohn's disease appears to be secondary to a defective acute inflammatory response as the neutrophils themselves were found to behave normally on in-vitro testing. A weak acute inflammatory response to particulate or antigenic material in the bowel wall could result in the chronic inflammation observed in this condition.”
The next in these series of experiments was conducted on the ileal and rectal mucosa, and again on the skin 216. A small mucosal biopsy was taken from the ileum or rectum, and this was then followed 6 hours later by a further biopsy of the previous biopsy site, to determine the extent of the inflammatory response induced by the initial biopsy trauma. Once again there was a major delay in the recruitment of neutrophils in CD, and this was observed in both regions of the bowel. In addition to healthy subjects, control individuals with UC were studied and their neutrophil recruitment was normal. Trauma to the skin reproduced the impaired neutrophil recruitment into skin windows, as well as reduced secretion of IL-8 and IL-1β from them.
The direct injection of heat killed E.coli into the subcutaneous tissues of the forearm of normal subjects was followed by profound rise in local blood flow. This was considerably impaired in CD, but not in UC. Blood flow is important in recruiting innate immune cells to sites of inflammation and this already paltry vascular response in CD would be further compromised by smoking tobacco 217.
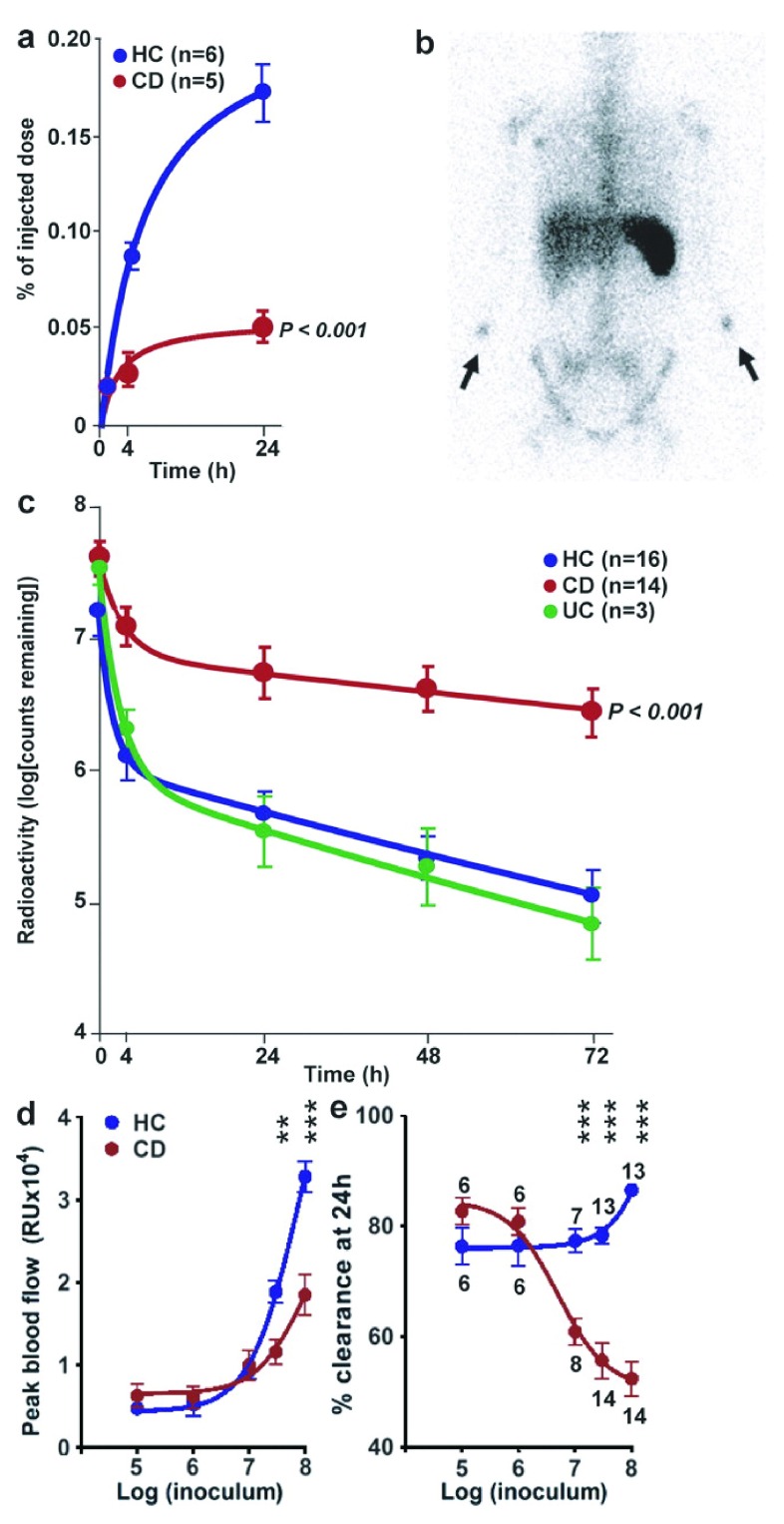
The third of these experiments directly measured the accumulation of neutrophils at the site at which E.coli had been injected subcutaneously, and the rate of clearance of these organisms. In this study peripheral blood neutrophils were purified from the individual under investigation, labelled with the gamma-ray emitting radioisotope Indium-111 218, and reinjected intravenously at the same time that unlabelled E.coli were injected subcutaneously into the forearms. The rate of accumulation of the radioactive neutrophils over the site of the injected bacteria was determined 219. A much smaller proportion of neutrophils were recruited to the injected bacteria in the CD subjects than in the HC or UC individuals ( Figure 7).
Figure 7. Neutrophil accumulation and subsequent clearance of E. coli from the tissues is markedly delayed in a dose-dependent manner in CD.
Reproduced from 219 with permission from the publisher. 111Indium-labeled autologous neutrophils were injected intravenously at the same time as killed E. coli were injected subcutaneously into each forearm. ( a) Radioactivity measured over the injection sites showed a much smaller proportion of labelled cells accumulating in CD subjects. ( b) γ-Camera image of a CD patient at 24 h after injection, demonstrating focal accumulations of radioactivity at bacterial injection sites (arrows) and confirming lack of bowel inflammation. ( c) 32P-labeled killed E. coli were injected into the subcutaneous tissues of the forearm and radioactivity was measured at the skin surface. Clearance of radioactivity was much slower in CD than in HC or UC. Extrapolating these curves indicated that almost complete removal (99%) would take 10.2 and 7.1 d in HC and UC subjects, respectively, compared with 44.3 d in CD. ( d and e) Effect of increasing bacterial dose from 10 5 to 10 8 on blood flow ( d) and bacterial clearance ( e). The numbers of subjects studied in the dose response experiment are depicted in e. All results are expressed as mean ± SEM (**, P < 0.01; ***, P < 0.001).
The next step was to radiolabel the E.coli with Phosphorus-32 and to then determine the rate of clearance of the bacteria from the tissues. This was a two phase process in HC and UC subjects with a very rapid initial clearance lasting about 4 hours followed by a slower phase, with total clearance being achieved by 7 to 10 days. In the CD subjects, initial clearance was much less efficient and total clearance was markedly delayed and was predicted to last from several weeks to infinity. This study showed unequivocally that coliform bacteria are cleared less efficiently from the tissues than normal in CD. It might be considered that this delayed recruitment of neutrophils to bacteria in the tissues should predispose these individuals to an increased incidence of clinically evident infections, which is not an obvious manifestation of CD. The reason for this apparent discrepancy is that the numbers of bacteria injected into the tissues were required to reach a certain critical load before the clearance defect was unmasked ( Figure 7e). In this study 10 6 organism were cleared normally whereas 10 7 were not, indicating that a significant bacterial load must enter the tissues before the clearance systems are overwhelmed. The bowel is the only location in the body where such a burden of microbes is readily available to enter the tissues.
Phase three - The consequences of the failure to clear intestinal contents from within the bowel wall.
In the absence of an adequate acute inflammatory response and the complete clearance of the inciting agent by neutrophils, the retained foreign material produces a granulomatous inflammation 220– 222. E.coli, Streptococci and Listeria have been demonstrated immunochemically in macrophages, giant cells and lymph nodes of CD patients 223, and E.coli DNA has been identified in Crohn’s granulomata isolated by laser capture microdissection 224. The retention of this faecal material within the bowel leads to an intense adaptive immune response and the tissues become infiltrated with large numbers of T-cells. It is not therefore surprising that when actively inflamed CD tissues are biopsied, any number and variety of adaptive immune cells can be identified and immune mechanisms evoked in the pathogenesis of the condition. The macrophages and adaptive immune cells, reacting to the foreign antigenic material, will produce cytokines such as IL-1β and TNFα 225, 226 that lead to local inflammation and systemic symptoms 227.
The clinical picture of an inflamed bowel containing large numbers of macrophages and T-cells 228, 229 has led to the erroneous belief that Crohn’s was an autoimmune disease 80. It is however clear that the cytokines produced by these inflammatory foci in their response to foreign faecal material contribute to the local and systemic inflammation, and failure of mucosal healing, as evidenced by the, often dramatic, responses to anti-TNF drugs. However, only about half the patients respond to this treatment, and in those that do the response is often partial and temporary 230.
Chronic inflammation leads to fibrosis, or scarring 231, which in a hollow muscular organ causes narrowing, or stricture formation. Under some circumstances the material in the bowel wall undergoes liquefaction, as may occur with tuberculosis 232. This material then tracks to adjoining organs, possibly driven by the osmotic pressure produced by the breakdown of the organic material within the abscess, and discharges into them. This can then produce fistulae 233 between the these organs for example between bowel and bowel, bowel and skin, bladder or vagina. The perianal fistulae between the rectum and perineum are characteristic of CD and of immunodeficiencies of the innate immune system, particularly those of neutrophil function 54.
This failure to clear organic material from the tissues offers an explanation for the false positive Kveim tests observed in CD 234. The Kveim test 235 was designed to diagnose sarcoidosis, another chronic granulomatous disease. The intradermal injection of a crude homogenate of an extract of sarcoid tissue, usually from lymph node, produced epithelioid cell granulomas in subjects with sarcoidosis, reproducing those diagnostic of this disorder. Initially it was thought that the injected material contained some sarcoid specific factor, such as an infectious agent or antigen 236 but it has been recognised more recently that it relates to an abnormal host response:
“The "immune paradox" (delayed type hypersensitivity anergy in a setting of exuberant systemic granulomatous response) resists explanation. Its relationship to the Kveim test is poorly understood. Immunological investigations generated the thesis that the characterizing systemic granuloma arise as a fall-back reaction to inefficient cellular immune processing, due most often to impaired myeloid dendritic cell function of unknown cause” 237.
This is precisely the nature of the pathogenic mechanism in CD and it is therefore not surprising that positive tests are found in both conditions 238 and that both diseases occasionally coexist in the same individual 239.
On the location of the CD lesions
Symptomatic lesions are largely confined to the terminal ileum, caecum and colon, probably due to the combination of mucosal damage by enteric infection coupled with the ready presence of massive numbers of bacteria to penetrate into the wall of the bowel when this happens. However, it is becoming apparent that the gastrointestinal tract is generally diffusely, sub-clinically, abnormal.
Oral manifestation of CD, particularly aphthous ulcers, are estimated to occur in 20–50% of patients 240. A prospective endoscopic study identified upper gastrointestinal (GI) manifestations of CD in 55% of 108 untreated, newly diagnosed adult patients with CD, irrespective of symptoms. All selected were free of H. pylori, infection with which, if anything, appears to protect against CD 241. About a quarter of the patients had lesions in both the stomach and duodenum and in about 20% they were in one or other of these organs. In roughly 2% of patients the gastric outlet is obstructed by a granulomatous inflammation requiring surgical intervention 242. Aphthous ulcers in the oesophagus were present in 7% of these subjects. Most of these lesions exhibited a granulomatous inflammation on histology.
In view of the systemic nature of the impairment of the innate immune system in CD, it is of great interest, although not altogether surprising that patients with CGD 54 exhibit very similar upper GI pathology. Aphthous ulceration and other oral lesions are common. Oesophageal, gastric and duodenal inflammation were detected in 21%, 74% and 37% of 78 patients 53. Large bowel lesions were present in the majority 53 and are indistinguishable from those of CD 48, 243. Between 4% 244 and 15% 245 of these patients also develop gastric outflow obstruction.
CGD is a condition in which there is a failure of microbial killing and digestion by neutrophils as a result of an absence of the respiratory burst produced by a NADPH oxidase, NOX2. Consequence, the pH of the phagocytic vacuole is too low for the efficient activity of the neutral protease digestive enzymes released into the vacuole from the cytoplasmic granules, and they fail to kill and digest the microbes 246, 247. The undigested material retained within the tissue is taken up by macrophages, producing the granulomata that give this condition its name.
Neutrophils play an important role in the debridement of wounds 248, 249, an important prelude and necessity for healing. It is possible that the upper GI inflammation that occurs in CD and CGD results from an impaired repair response to trauma and peptic digestion rather than infection in these locations.
Identifying the molecular cause/s of the CD phenotype
The abnormality of innate immunity in most cases of CD lies in the macrophages
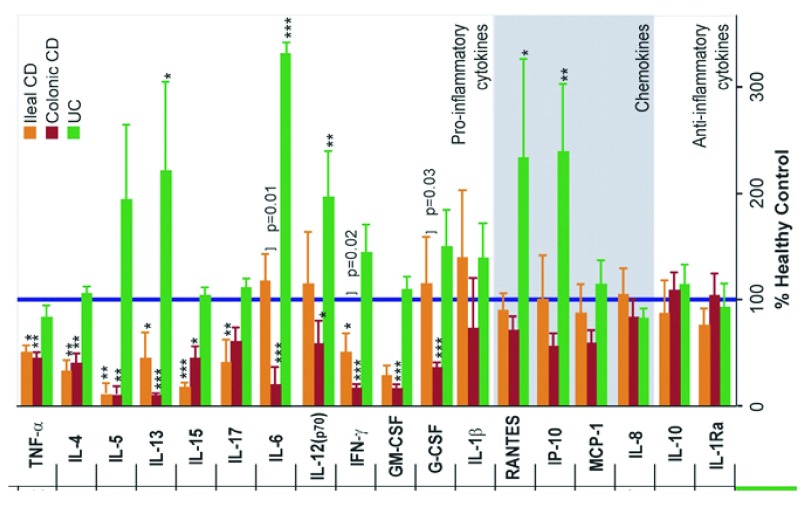
Defective secretion of pro-inflammatory cytokines in CD may be the explanation for the observed impairment in neutrophil recruitment 219, 250– 253. In CD, the neutrophils themselves are normal 254 and exhibit normal migration in vitro 215, 255, 256 and will migrate out of skin windows if chemoattractant substances are placed over them 216. In the absence of a primary abnormality of neutrophil function, CD macrophages showed defective secretion of pro-inflammatory cytokines, but normal release of chemokines, in response to stimulation with E.coli 219 ( Figure 8). The genes for these pro-inflammatory cytokines were transcribed and translated, but the proteins were misdirected to lysosomal degradation rather than secretion, suggestive of disordered vesicle trafficking.
Figure 8. Proinflammatory cytokine secretion by macrophages from CD patients is deficient in response to E. coli.
Cytokine and chemokine release expressed as a percentage of that secreted by HC cells (blue bar) from ileal and colonic CD patients (reproduced from 219 with permission from the publisher).
The question then arises as to how anti-TNF drugs can be effective against a condition in which the secretion of TNF and other pro-inflammatory cytokines is impaired? The answer is in the timing of the different components of the immune system. The call to arms of the innate immune system is a very early and explosive secretion of pro-inflammatory mediators, including TNF. If the clearance of faecal material from the tissues is incomplete, it becomes walled off by macrophages, endotoxin diffuses into the circulation, and cells of the adaptive immune system are recruited 257. They secrete a wide array of mediators over the next weeks including TNF which acts as an amplifier of the response 258. It is of interest that in a recent study of high-resolution gene expression profiling using RNA sequencing of inflamed biopsies from patients with CD, UC and controls, levels of pro-inflammatory cytokines like TNF, IL-1β, IL-6 and IL-23 were all elevated to a lesser extent in CD than in UC 259. The very early secretion of TNF and other mediators is required to prevent the development of the Crohn’s lesions whereas at a later stage it is the TNF and associated mediators that produce the symptoms, which in some cases respond to anti-TNF treatment. This explains why anti-TNF therapeutics can both cause 260 and alleviate symptoms of the disease 261.
What is the molecular cause of the impairment of acute inflammation?
There is a strong genetic component to the aetiology of CD. The sibling recurrence risk (risk of developing the disease in the context of an affected sibling) is approximately 13–36 262 and approximately 12% 263 of CD patients have at least one affected first degree relative. Furthermore, the study of over 300 twin pairs has demonstrated a higher concordance of disease phenotype in monozygotic (30%) compared with dizygotic twins (4%) 264. While the twin studies support the role of genetic susceptibility, they also indicate the requirement for additional environmental or other factors for the development of overt disease. By far the most likely such factor is an enteric infection of sufficient severity to overwhelm the ability of the innate immune system to adequately clear the faecal debris from the bowel wall. However, this risk may be further modulated by additional environmental factors, such as smoking. This phenomenon, whereby a genetic predisposition to disease manifests in the presence of environmental precipitants is exemplified by alpha-1-antitrypsin deficiency, in which the predisposition to emphysema is exposed by smoking 265.
Technological advances have provided the means of interrogating the genetic basis of CD.
1. Linkage and Genome Wide Association studies (GWAS)
Linkage. Linkage analysis (positional cloning) is a family based technique for identifying the possible location within the genome of causal mutations underlying genetic diseases 266. This is done by utilising markers of known location across the genome, such as microsatellites or single nucleotide polymorphisms (SNPs). The transmission of the markers through a family (or collection of families) is examined seeking those whose segregation closely follows the inheritance of the disease, thereby focussing attention on a small region (locus) in which the causal mutation might be found 266. Linkage analysis of affected sibling pairs with CD permitted the identification of a susceptibility locus on chromosome 16 (termed IBD1) in which mutations in the gene NOD2 were subsequently identified 267, 268. NOD2 mutations remain the most strongly associated common genetic variants associated with CD. Linkage is only a powerful tool when almost all cases of the disease in the families under study are caused by mutations in the same gene ( i.e. there is limited genetic heterogeneity) that are not seen in unaffected family members ( i.e. it is of high penetrance). Numerous factors can limit the effectiveness of linkage analysis such as: the presence of unaffected individuals that harbour the mutation (incomplete penetrance); individuals who develop the disease as a result of mutations in another gene or due to environmental factors (phenocopies); the requirement for the combined effect of two or more mutations (epistasis); or the requirement of the involvement of some environmental factor such as an infectious trigger (which will effectively reduce penetrance by not facilitating the manifestation of the underlying genetic predisposition in unexposed individuals). All of these factors are likely to have contributed to the limited success of linkage analysis in CD.
GWAS. Genes reside on chromosomes which undergo recombination at meioses. Population level haplotypes arise due to the non-random positioning of crossing-over events. Haplotypes are characterised by a particular set of SNP genotypes. Depending on the ancestral origin and frequency with which a mutation has arisen in the population, it may occur on a particular haplotype and thus the SNP genotypes defining that haplotype will be enriched in patients harbouring the disease-causing mutation. Therefore, when comparing a large population of diseased individuals with healthy controls, SNPs tagging the underlying mutation should be enriched in the affected compared with unaffected individuals. In GWAS, a set of SNPs are genotyped in an attempt to cover the whole-genome and the above comparison made 269. One of the major problems with analysing many hundreds of thousands (or millions) of markers across the genome is that the large number of comparisons undertaken risks producing false positives. This necessitates the utilisation of a stringent p-value threshold for significance of p<5×10 -8 270. As a result very large sample sizes are required 271, 272.
One of the technical strengths of GWAS as an investigative approach is that DNA is easily obtained and once purified it is stable, enabling it to be conveniently stored and transported. Technological advancements have permitted high throughput SNP genotyping of large numbers of samples on an industrial scale. Furthermore, the GWAS approach has the advantage of being comprehensive (compared with candidate gene studies) and objective (at least up until the stage of data interpretation). There are however a number of limitations, for example incomplete genomic coverage. In addition, a major drawback is that the SNPs employed as markers must be relatively common in the general population, in order to give the study adequate statistical power, so this approach is typically unable to identify low frequency mutations, however penetrant or important.
When a SNP is found to be statistically significantly associated with a disease by GWAS, it can be because the polymorphism is itself pathogenic or, more commonly that it is tagging a closely located genetic variant whose genotype correlates with that of the tagging polymorphism (the two variants are in linkage disequilibrium). The precise location of the causal variant(s) underlying the association signal within the identified locus may be interrogated further by fine mapping (in which higher resolution association studies are conducted) or by resequencing the locus looking for plausible pathogenic variants such as coding variants or those that affect gene-expression (eQTLs).
Increasingly large GWAS have been performed on CD and the results meta-analysed 273, 274. No single, or small number, of penetrant mutations have been found that independently cause the disease. The latest study of over 20,500 CD cases and 41,600 controls of European ancestry identified 145 loci associated with CD at p<5×10 -8. The mean OR of the top SNPs representing these 145 loci was 1.16 and the mean control allele frequency was 0.48. Four SNPs had an OR exceeding 1.5 of which three were within NOD2 and the fourth was in IL23R. The mean difference in allele frequency between cases and controls was only 0.02 275.
The very significant p-values obtained for the associated loci, led to the general perception that the molecular causes of CD have been identified. Individually the CD GWAS loci have very modest effect sizes ( i.e. a small difference in frequency in the control and CD populations), consistent with a polygenic model in which it is thought that that it is the combination of these minor influences that is causally important. However, in the latest meta-analysis, all 170 significantly associated loci combined account for only 10.9% of the disease “heritability” 273.
An important consideration is that about half of the healthy population also carry these variants, although (by definition) each at a slightly lower frequency than in the CD patients ( Figure 9). The healthy controls carrying these variants greatly outnumber the patients with CD in the population. With a prevalence of CD of about three patients in 1000 277, and taking the NOD2 frameshift mutation as an example because it has the greatest effect size at 3.32, for every 100,000 individuals in the population there will be 99,680 unaffected individuals of whom ∼2390 will carry this mutation. In this population there will be ∼320 CD cases of which ∼48 will have the mutation 274. This means that the penetrance of this mutation, with by far the greatest association with the disease, is only 2%. These effect sizes pale into insignificance when compared with the effect size of HLA-B27 in ankylosing spondylitis of approximately 94 278, 279, and HLA in type 1 diabetes and coeliac disease with effect sizes of approximately 25 and 50, respectively 280.
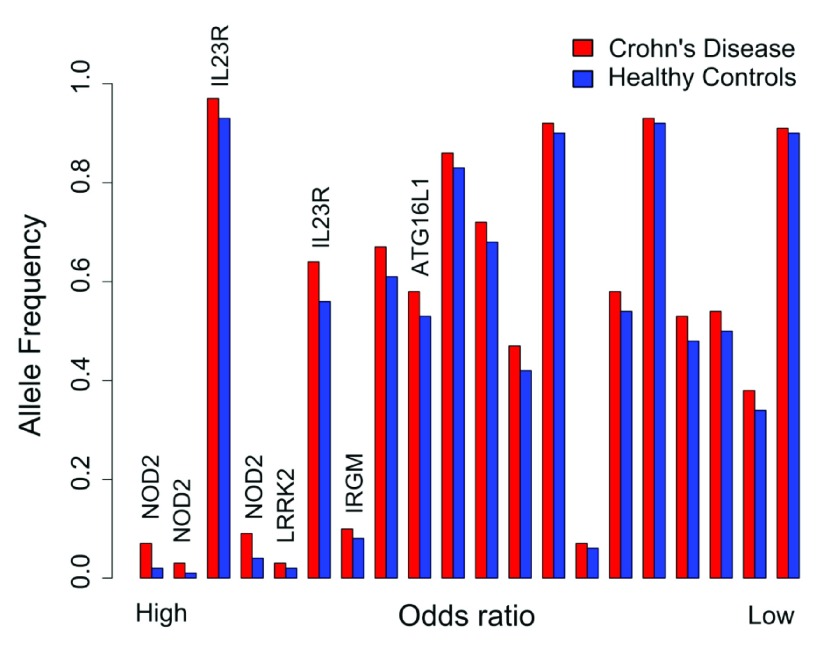
Figure 9. The allele frequency in Crohn’s disease patients and healthy controls for top 20 of 144 CD associated GWAS SNPs sorted by odds ratio.
The data were taken from the European cohort in 276. Loci harbouring genes of interest have been indicated.
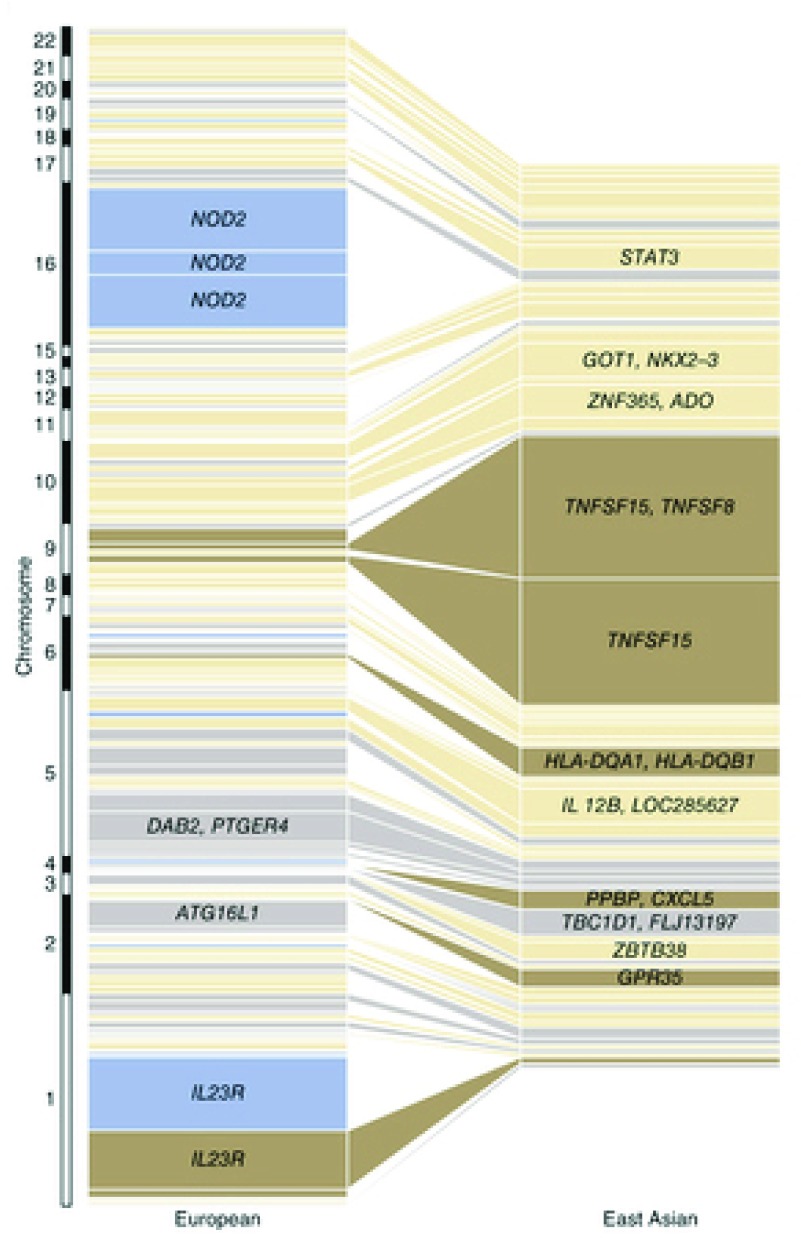
GWAS conducted on Europeans and East Asian populations have yielded noticeably different findings ( Figure 10) 276. In East Asian populations, the loci with largest effect sizes were those harbouring the genes TNFSF15/TNFSF8 (genes encoding cytokines that belong to the tumour necrosis factor (TNF) ligand family 281) and the major histocompatibility complex. Variants in NOD2 and ATG16L1 demonstrated no association with CD in these populations, and the effect size of the IL23R locus was minimal. The significant heterogeneity in common variant CD genetic architecture between different populations provides a further indication that the genes identified by GWAS are unlikely to play a primary causal role in the development of the disease, the manifestations of which are similar in patients regardless of their ethnicity.
Figure 10. Comparison of the variance explained per risk variant for Crohn's disease between East Asians and Europeans.
Each box represents an independently associated locus and the size of each box is proportional to the amount of variance in disease liability accounted for by that locus in the respective population. From 276 (reproduced with permission from the publisher).
GWAS have been performed for many different diseases and IBD associated loci have been shown to be shared with several other immunologically mediated diseases including rheumatoid arthritis, systemic lupus erythematosus (SLE), ankylosing spondylitis, coeliac disease and sarcoidosis 282. These associations are not surprising as comorbidities of some of these conditions are well recognised in the context of IBD 283– 285 and because almost all are associated with an increased incidence of similar pathologies such as arthritis 286, uveitis 287, 288 and bowel inflammation 289– 292.
Many of the associated genes common to these conditions have been implicated in pathways leading to activation or regulation of the immune response 282. It is possible that these genes were highlighted as being common to the chronic inflammatory conditions because they lead to more florid manifestations, causing signs and symptoms of the disease in those individuals with an underlying predisposition, thereby bringing them to the attention of the medical profession.
Despite their unimpressive effect sizes, the main GWAS CD associated molecules have generated considerable attention and are hence worth of a brief summary.
NOD2. NOD (Nucleotide-binding Oligomerisation Domain) 2 is a member of an extended family of inflammatory and immune proteins in plants (the resistance (R) genes 293), Drosophila (Toll-like receptors 294) and animals (NOD families). These proteins combine a central nucleotide-binding domain (NOD) with a C-terminal leucine-rich repeat (LRR) motif and an N-terminal caspase recruitment domain (CARD) or equivalent.
In general these proteins recognise a signal from an invading organism in their leucine-rich domain (LRR) domain that induces a polymerisation that triggers a signalling cascade which terminates in the production and release of pro-inflammatory molecules. NOD2 is activated by muramyl dipeptide (MDP) a component of the cell wall of both Gram negative and Gram positive bacteria. It seems to be taken into the cells within endocytic vacuoles; presumably the organisms are then digested within this compartment and the solubilised MDP is moved into the cytoplasm by peptide transporters like SLC15A3 295. Very recently it has been demonstrated that NOD1 and NOD2, do not only respond to bacterial stimuli, but are also important mediators of ER-stress-induced inflammation (described in more detail below) 296.
In the resting state NOD2 is doubled back on itself in an auto-inhibited conformation in the cytoplasm until activated by the attachment of MDP upon which opens it allowing self-oligomerisation and the binding of ATP 297, 298. A series of phosphorylation steps then end in the translocation of NF-κB to the nucleus and the production of pro-inflammatory cytokines and antimicrobial peptides 297. This theoretical model of NOD2 function as a pattern recognition receptor capable of inducing pro-inflammatory cytokine secretion has been validated by in vivo studies in humans in which the application of MDP to skin windows induced the production and release of pro-inflammatory cytokines in healthy and CD patients without NOD2 mutations, but not in those carrying the CD-associated mutations 216. The impaired secretion of these inflammatory mediators into skin windows in the absence of MDP, in CD patients without mutations in NOD2 in this study, demonstrates that other pro-inflammatory signals and pathways must be abnormal in these subjects, indicating that there are at least two parallel routes initiating the inflammatory response.
Expression of NOD2 is largely restricted to peripheral blood monocytes 299 and to Paneth cells at the base of intestinal crypts 300. Monocytes constitute approximately 5% of the circulating leukocytes and are generally regarded as functioning predominantly as circulating precursors of macrophages, without much in the way of a distinct set of functions of their own. It seems intuitively unlikely that a highly specialised cell with an active NADPH oxidase and granules containing myeloperoxidase, all of which are lost with the transformation to macrophages, would be produced to act predominantly as a stem cell.
It is generally assumed that pro-inflammatory cytokines are secreted by tissue macrophages, but these are quite widely dispersed amongst tissues and could not accumulate as rapidly as needed at inflammatory sites as monocytes that are carried there in capillaries perfusing the region 301, 302. Monocytes are rapidly recruited to sites of acute inflammation where they extravasate into the tissues 303 and make a large contribution to the production of pro-inflammatory cytokines 304– 306 before being transformed into inflammatory macrophages.
Autophagy and ATG16L1, CALCOCO2/NDP52, LRRK2 and Optineurin. First described in the new-born mouse kidney by Clark in 1957 307 and reviewed by De Duve and Wattiaux 308, autophagy was initially described as a process in the cytoplasm of cells directed to the remodelling of tissues and the removal or damaged or effete organelles and proteins, and to partial self-digestion under starvation conditions.
To undertake this process, cells must first identify the region of cytoplasm, effete organelles, or invading microbes, as objects for engulfment. This is achieved by labelling the surface of the target with chains of a small protein, ubiquitin 309, 310. The ubiquitinated material is then encircled by a double membranous structure, produced from elongated vesicles at the Golgi apparatus or (ER) 311, the ends of which then fuse to form the characteristic vacuole with a double membrane. This autophagocytic vacuole then fuses with lysosomes containing enzymes that digest the inner membrane and its contents 312.
Membrane vesicle extrusion, ubiquitination and fusion of granules with vesicles are general biological processes that are involved in many diverse cellular functions in addition to autophagy. Most of the cellular machinery required for these processes in autophagy was identified in mutant Saccharomyces cerevisiae 313 and homologues were then then found in Drosophila and man. The molecular basis of human autophagy has largely been investigated in promyelocytic HL60 and the Human Embryonic Kidney (HEK293) cell lines 314, 315. Thus the autophagy molecules have been identified in assays that measure autophagy in primitive cells. This does not mean that these proteins are necessarily exerting their effects exclusively through autophagy in more mature cells and tissues.
What is probably of particular relevance to CD is the specialised type of autophagy, known as xenophagy, that is designed to deal with bacteria that escape into the host cytosol (such as Shigella or Listeria) or those that reside in a modified intracellular vacuole (such as Salmonella and Mycobacteria), which is important for the entrapment and lysosome-mediated degradation of bacterial pathogens 316– 318. CD resulting from abnormal xenophagy is seen in Niemann-Pick disease type C1 and XIAP deficiency with NOD2 variants in Crohn's disease 319. Other proteins implicated in xenophagy are the autophagy receptor CALCOCO2/NDP52 320, Leucine-rich repeat kinase 2 (LRRK2) 321 and Optineurin 322, 323.
Despite the current interest in autophagy and in exploring the removal of intracytoplasmic organisms from intestinal cells and macrophages by xenophagy, it must not be forgotten that the vast majority of bacteria entering the body are phagocytosed, killed and digested by neutrophils, and that xenophagy only has to deal with a tiny minority escaping this process.
ATG (autophagy-related)16L1. Attention was focussed upon ATG16L1 when an association was demonstrated with CD in a GWAS study 324. The SNP was a common, non-synonymous variant resulting in an amino acid change from threonine to alanine at position 300 of the full-length protein (T300A) with an odds ratio of only 1.26 274. The ATG16L1 protein was shown to be mainly expressed in the thymus, prostate, liver, kidney and colon with little in the small bowel, and equal levels were found in the bowel from healthy and CD subjects with and without the polymorphism.
ATG16L1 is recruited to the ER at the initiation of the formation of the autophagosome at this site 315. It is involved in binding to ubiquitinated cytosolic Salmonella whilst these are enveloped in the phagophore to form the autophagosome 325 and could be important for the clearance of bacteria from within cells 315, 326– 328. This protein could be related to CD through the impaired clearance of intracellular organisms. It has been reported that the cellular architecture of Paneth cells is grossly distorted in knock-out mice severely depleted in this protein 329 and in CD patients homozygous for the T300A polymorphism. In the mice, there were many fewer granules in these cells, with their normal contents lying predominantly in the cytoplasm, together with multiple large vesicular structures that might represent the membranes that would normally surround granules. The rest of the bowel looked normal 329. It was also reported that CD patients with the T300A polymorphism demonstrated similar morphological changes with disorganized, or diminished, granules or exhibiting diffuse cytoplasmic lysozyme staining. This was claimed to be the first indication that Atg16L1 has a specific role in humans and mice in regulating the specialized properties of Paneth cells, and provides a novel and relevant mouse model that emulates one of the many diverse pathological hallmarks of human CD. Unfortunately the morphology of Paneth cells was not examined in healthy humans with the same polymorphism, which would not have been difficult given that 27% of this population is homozygous for the “risk” allele and 50% heterozygous, and which makes a pathological role for this SNP highly improbable 274.
It has been reported that the T300A significantly increases ATG16L1 sensitisation to caspase-3-mediated processing 330. Metabolic stress in human and murine macrophages increased degradation of the T300A (or mouse equivalent) variants of ATG16L1, respectively, resulting in diminished autophagy and knock-in mice harbouring the variant showed defective clearance of Yersinia enterocolitica and an elevated inflammatory cytokine response. Deletion of the caspase-3-encoding gene or elimination of the caspase cleavage site by site-directed mutagenesis rescued starvation-induced autophagy and pathogen clearance.
IRGM. Immunity related p47 guanosine triphosphatases (GTPases) or IRGs are a group of 47–48 kDa proteins that are implicated in the eradication of organisms like mycobacteria that are taken into cells within phagocytic vacuoles. IRGM has been characterised as an autophagy related molecule 331 on the basis that it was associated with vacuoles thought to contain mitochondria, a characteristic in common with autophagocytic vacuole. The evidence for the mitochondrial contents was the accumulation in the vacuoles of Mitotracker Red, a dye that partitions in mitochondria by virtue of the charge across their membranes. Mitochondria are marked for degradation by ubiquitination when they have lost their membrane potential 332 and this situation would not be improved by degradation within an autophagosome, so it is unlikely that such structures would attract Mitotracker Red. On the other hand, potentials are developed across the membrane of the phagocytic vacuole by the NADPH oxidase 333 and by electrogenic proton pumping of the vacuolar V-ATPases 334. The classification of IRGM as primarily involved in the autophagocytic processes on the evidence provided for mitophagy should be reconsidered.
The IRGs were discovered in mice as interferon-gamma inducible elements that were important for resistance to microbes engulfed into phagocytic vacuoles. There are 23 of these molecules in mice but only one or very few in humans 335. In human cells IRGM is largely expressed in immune cells whereas in mice it is almost exclusively in macrophages in which its expression is massively increased by LPS ( 336GeneAtlas MOE430 gcrma, Probeset: 1418825_at). Phagosomal maturation 337, 338 occurs through sequential fusion of the phagosome with early endosomes and lysosomes, resulting in acidification of the phagosome, release of hydrolytic proteases and death and digestion of the phagosome-bound pathogen. IRGs are located in the ER or Golgi compartments and after the host cell is infected, they are transported to pathogen-containing phagosomes/vacuoles, where they modulate the formation or processing of the phagosome, undermining pathogen survival either directly or by facilitating the action of intracellular effector molecules.
XBP1 (X-box binding protein 1) and ER stress, or unfolded protein response (UPR) 339, 340. The ER is the principal organelle involved in the synthesis, maturation, and post- or co-translational modification of secreted and membrane proteins, as well as in various metabolic processes including dynamic ion storage and biogenesis of membrane structures 341. Properly folded proteins are then directed to the Golgi apparatus, to other intracellular organelles, and to the extracellular surface by the secretory pathway 342. The ER stress or unfolded protein response develops when the protein synthetic process becomes disordered; leading to the accumulation of dysfunctional unfolded protein as a result of genetic or adverse environmental factors. To deal with this, the unfolded protein triggers the unfolded protein response (UPR) 343 which includes reducing protein synthesis by downregulating mRNA translation, synthesising more molecular chaperones to assist protein folding, and degrading misfolded proteins which are ubiquinated and directed to the proteasomes. XBP1 is a transcription factor that plays a central role in activating these ER stress responses 344, and unexpectedly, NOD1 and NOD2 have been shown to be important mediators of this process 296. Abnormalities in the ER stress response will produce unfolded proteins that will require removal by autophagy, so the linkage between these two processes is to be expected 345.
Major demands are made on the protein synthetic machinery in rapidly turning over cells like the intestinal mucosa, and this is particularly true of cells like Paneth and goblet cells that secrete proteins in addition to attending to their own homeostatic requirements. When additional requirements for protein synthesis are called upon, induced, for example, by infection or chemical toxins, defects in the ER stress response could weaken the mucosa leading to ulceration 346.
LRRK2. A considerable amount of work has been done on this protein because mutations in LRRK2 cause familial and sporadic Parkinson’s disease (PD). It is a large, 280kDa, protein with GTPase and kinase domains, the latter being constitutively active in PD. It is found in immune cells, in lamina propria macrophages, B-lymphocytes and dendritic cells, and levels are markedly increased in the bowel in CD 321 and in microglia in the nervous system 347. It interacts with the small GTPases Rab32 and Rab38 with which it co-locates to transport vesicles and recycling endosomes 348 and it is important for the elimination or intracellular Salmonellae 349 and Legionella 350. Rab32 and Rab38 play an important role in the biogenesis and traffic of melanosomes and lysosomes and this system is disordered in Hermansky-Pudlak syndrome 351, accounting for the characteristic partial albinism. If LRRK2 and its associated proteins are important for immunological resistance to the development of CD then it might be expected that CD would be more common in conditions in which the LRRK2 system is disordered, which is in fact the case. A clear association exists between CD and PD 352, 353 and CD and Hermansky-Pudlak 354. At least two recent studies have discovered a link between mutations in LRRK2 and CD in Ashkenazi Jews (submitted for publication).
Summary of outcome of GWAS studies in CD
The GWAS studies have provided a series of clear answers. No single gene, or a small number of genes, has been identified that is causal for CD. More than 170 GWAS hits combined contribute to about 10% of the “heritability” of CD. With the average individual contribution of only 0.1% it is unlikely that these variants will individually have major effects on cellular function, either in the CD patients or in experimental systems.
One of the founding premises of GWAS was that it was designed to identify genes that are involved in pathways relevant to disease pathogenesis. The value of this data, therefore, is that it points towards the specific pathways that are likely to be involved in disease pathogenesis, rather than identifying causal genes. Collectively, genes associated with CD by GWAS are enriched for Gene Ontology (GO) annotations (statements describing the functions of specific genes) related to host-microbe interactions, the regulation of cytokine production, lymphocyte activation and the response to molecules of bacterial origin 273. This is in keeping with the large body of data that is accumulating as to the role of the innate immune system and its interaction with intestinal microbes in the causal mechanisms in CD.
Other investigations to identify causal molecules
Macrophage expression profiling
In the knowledge that the release of pro-inflammatory cytokines by macrophages from CD subjects is depressed as a result of impaired vesicle trafficking 219, an attempt was made to identify genes contributing to this deficiency by looking for outlier levels of gene expression in these cells. The most commonly under-expressed gene identified was Optineurin, low expression levels of which were found in 10% of CD patients studied 355. ADAMDEC1 was under-expressed in about 7% of patients.
Optineurin (Optn) 322, 323 is a 67 kDa protein, ubiquitously expressed, and its expression can be induced by TNFα and interferons, probably as a result of NFκB activation, and it is localised in the cytosol and Golgi apparatus. In essence it is a linker, or adaptor, molecule and has several binding partners including Rab8, Huntingtin, the gene for which is mutated in Huntington disease, and Myosin VI, a multifunctional motor protein. Rab8 is a small GTPase involved in vesicular trafficking between the trans-Golgi network (TGN) and the plasma membrane. The function of Huntingtin itself is unknown but it is associated with several factors involved in vesicle trafficking. Myosin VI is attached by OPTN to the Golgi apparatus and then it participates in the transport of vesicles and their protein cargos from the from the Trans-Golgi network to be released at the cell surface. OPTN also contains a ubiquitin binding domain with the ability to bind polyubiquitinated cargoes and transport them to autophagosomes via its microtubule-associated protein 1 light chain 3-interacting domain 356.
Macrophages from patients with low expression of OPTN secreted abnormally low levels of pro-inflammatory cytokines as do macrophages from OPTN knock out mice 357. mRNA expression levels of these cytokines were normal, consistent with deranged secretion rather than synthesis. These mice were more susceptible to infection with Citrobacter, E. coli and Salmonella 358, and showed reduced levels of TNFα in their serum, diminished neutrophil recruitment to sites of acute inflammation and greater mortality, than wild-type mice. OPTN-knockdown zebrafish infected with Salmonella also had a higher mortality 357.
ADAMDEC1 (ADAM-like Decysin-1) is a member of the ADAM (A Disintegrin And Metalloproteinase) family, the expression of which is restricted to the macrophage/dendritic cell populations of the gastrointestinal tract. Its biological function is unknown but it has been hypothesised to play a role in immunity. Reduced ADAMDEC1 expression in macrophages from a subgroup of CD patients has provided evidence of a potential role in bowel inflammation 355. Adamdec1 -/- mice were more susceptible to the induction of bacterial and chemical induced colitis and they cleared Citrobacter rodentium less efficiently than wild-type mice after infection 359.
DNA sequencing
The development, availability and falling costs of high throughput DNA sequencing, has provided the means of directly identifying causal mutations in human disease 360, 361. Several studies employing such technology have been undertaken in CD 362, 363 and many more are likely to appear over the ensuing years. High-throughput DNA sequencing has also been absolutely crucial for the diagnosis of the rare primary immunodeficiencies 47 that produce bowel inflammation, rather than CD, as described earlier.
Initially the DNA of GWAS associated loci was sequenced, and a small number of rare variants were identified, most notably in the genes CARD9 and IL23R 364, 365.
The major problem in identifying causal genes by sequencing is the considerable individual variation in DNA sequence. Asymptomatic individuals carry, on average, approximately 100 genuine loss of function variants with ∼20 genes completely inactivated 366. This makes it very difficult to identify the disease causing mutation/s in any one individual.
Of note, an ongoing study in which whole-genome sequencing has been undertaken in 2,697 CD cases and 3,652 healthy controls failed to identify a single variant at genome-wide significance that had not already been identified by GWAS 367.
Alternative approaches have been taken to overcome the difficulty of the interpretation of individual variation. Several studies have focussed on the analysis of Ashkenazi Jews (AJ) because they have a roughly fourfold increased incidence of CD and demonstrate genetic homogeneity, having arisen from approximately 350 individuals about 30 generations ago 368.
Chuang et al. sequenced the exomes of 50 AJ CD patients and prioritised low frequency coding variants which were then genotyped in approximately 3,000 AJ CD cases and 3,000 controls. They identified a frameshift mutation in CSF2RB as a strong causal candidate which was associated with CD at p<3.5×10 -6 and an OR of 1.5 369. This variant is rare in the non-AJ population.
Levine et al. utilised an alternative family based approach 370. They characterised two very large AJ families with >800 and >200 members with 54 and 26 affected cases respectively, sequenced the exomes of all cases and imputed the genotypes of the unaffected family members. Low frequency coding variants that were predicted to be damaging and were enriched in affected compared with unaffected individuals were prioritised. In the large family they independently identified the identical frameshift mutation in CSF2RB as concurrently reported by Chuang et al. as a likely causal variant. Other strong candidate genes included NLRP2, a NOD-like receptor and a component of the inflammasome; ZC3H18 which is involved in IKK and NFκB activation 371, a pathway of established importance in CD; and MEGF10, a phagocytic receptor involved in apoptosis 372.
CSF2RB
Having been identified independently by two groups CSF2RB must be considered as a causal gene for CD in AJs 373. CSF2RB is the common or shared β subunit of the receptors for granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin (IL)-3, and IL-5 374. The distinct α chains of these receptors provide cytokine specificity whilst the β chain is responsible for high-affinity binding and is the major downstream signalling component of the receptor complexes.
GM-CSF is produced by myeloid cells, dendritic cells (DCs), T cells, B cells, and several non-immunological cells including epithelial cells 375 following exposure to inflammatory stimuli to promote the production and function of myeloid haemopoietic cells including haemopoietic progenitor cells and differentiated cells such as basophils, neutrophils, eosinophils, macrophages and certain dendritic cells 376 to deal with the cause of the inflammation.
IL-3 is predominantly produced by activated T cells, natural killer (NK) cells and mast cells. It acts on the early stages of haematopoiesis in synergy with other cytokines to induce progenitors of various lineages but it is a very important stimulus for the generation of mast cells and the regulation of mast cell function as well as basophil production and activation.
IL-5 stimulates mainly the production and function of eosinophils. The major source of IL-5 is T-cells with relatively lower amounts produced by mast cells and eosinophils 377.
DUOX2
Levine et al. also identified a damaging missense mutation in DUOX2 that impaired the function of the protein and showed a possible epistatic interaction with NOD2 370. DUOX2 is a member of the large NADPH oxidase (NOX) family of enzymes 378. Its expression in the bowel epithelium is induced by the microbiota 379. It generates H 2O 2 at the mucosal surface and this acts as substrate for lactoperoxidase catalysed oxidation of thiocyanate to microbicidal hypothiocyanite 6. It might also attract neutrophils to inflammatory sites 380. Knockdown of the DUOX2 homologue in invertebrates and mice resulted in an impaired tolerance to enteric bacteria 381. Of relevance, given the possible NOD2 epistatic interaction observed, a physical and functional interaction between these proteins has been demonstrated in epithelial and HEK293 cells 382.
Future treatment options
Treatment of CD poses a conundrum. The logical approach to correcting the underlying problem would be to develop means of enhancing innate immunity, although at present no such range of drugs is currently available. It would be dangerous to attempt to do this in the presence of ongoing bowel inflammation but it could be useful to maintain patients in remission after they had been cleared of disease by surgical resection, or through the use non-immunosuppressant therapies such as elemental diets 383. This approach was attempted with levamisole 384 as the immunostimulant, with varying results 385, 386. One problem with this form of treatment is that it is important to ensure that the patients are in remission before it is commenced; otherwise it is likely to exacerbate the inflammation. In two studies levamisole induced a severe reversible polyarthropathy 387, 388, indicating that the drug was in fact altering the immunological/inflammatory axis, and providing clues as to IBD associated arthritis, and to the immunopathology of the idiopathic arthritides in general.
Current drug and biological treatments are largely immunosuppressant. This, to varying degrees of efficiency, dampens down the secondary inflammation induced by the retained foreign material within the tissues. Anti-TNF treatments can be very helpful but do not provide a comprehensive answer. Only one third of patients will be in remission after one year on these treatments 389. Immunosuppressant treatment further compromises the underlying innate immune deficit to mucosal damage, thereby increasing the likelihood of further infection and the influx of bowel contents into the tissues, possibly converting CD from a sporadic to a chronic condition.
The primary pathology in most case of CD appears to affect macrophages recruited from the blood as monocytes 301. Advances in gene editing with the CRISPR-Cas 390, 391 technology make the corrective treatment of CD a real possibility in the relatively near future. Once a primary causal mutation has been identified, and validated in animal models, bone marrow could be extracted, edited and reinfused into a conditioned patient in much the same way as is being applied to gene therapy for primary immunodeficiencies 392.
Conclusion
After almost a century of concerted effort in clinical investigations combined with recent technological developments in genomic medicine, a consensus view is developing as to the causes of CD.
A genetic predisposition to the condition can exist, as evidenced by twin and family studies. Studies in patients have clearly demonstrated that there is a defect in the acute inflammatory response resulting in an impaired recruitment of neutrophils to inflammatory sites and a consequent delay in the clearance of bacteria from them. This results from an initial blunted response by monocytes and macrophages leading to deficient secretion of pro-inflammatory cytokines.
CD is a syndrome and the molecular basis of this deficiency will vary considerably. It might reflect the multifactorial extreme end of a normal distribution, or the critical loss of one or more important molecules. Molecules contributing to the former will be very difficult to identify whereas some of those playing a more singular role have been highlighted by linkage, GWAS and DNA sequencing studies. These extend across the spectrum of the interface between microbes and cells: from the derangement of cellular homeostasis, probably in intestinal and immune cells, through impairment of the ER stress response (XBP1 malfunction); defective signalling (aberrant NOD2); depressed killing and digestion of organisms within phagocytic vacuoles (IRGM) or xenophagic recovery of those that escape into the cytoplasm (ATG16L1); failure of vesicle trafficking resulting in disorganised lysosomal biology of reduced cytokine secretion (LRRK2, Optineurin) or ineffective signalling of cytokines on target cells because of aberrant receptors (CSF2RB). In addition, primary abnormalities of neutrophils, the final effector cells, constitute the ultimate predisposition.
However severe the covert underlying predisposition, it requires a triggering factor to be expressed, and all the evidence points to this being an enteric infection. The identity of the organism is of secondary importance to the fact that the mucosa is breached, allowing ingress of the faecal contents into the bowel wall. Increased frequency or potency of these infections, as a result of factors implicated in the “Hygiene Hypothesis” or through increased spread by travel, or changes in sexual practises, could account for the increasing incidence of CD in the Western world.
It is the abnormal response to the penetrating faeces that is the common denominator of the CD syndrome, and it is the failure to eliminate the foreign material in the tissues that leads to the classical chronic granulomatous inflammation, and subsequently to an adaptive immune response. The counter intuitive, but logical, conclusion is that a disease characterised by grossly exuberant inflammation can result from an initial failure of innate inflammation. A similar mechanism might be responsible for other chronic inflammatory conditions of unknown aetiology, like for example sarcoidosis, ankylosing spondylitis, psoriasis, rheumatoid arthritis and SLE.
Acknowledgements
I thank Holm Ulhig and Amnon Sonnenberg for permission to publish figures from their papers. I am indebted to Adam Levine for assistance with the preparation of this manuscript.
Funding Statement
I received financial support from the Medical Research Council, Wellcome Trust, Charles Wolfson Charitable Trust and the Irwin Joffe Memorial Trust.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 2; referees: 2 approved]
References
- 1. Malik TA: Inflammatory Bowel Disease: Historical Perspective, Epidemiology, and Risk Factors. Surg Clin North Am. 2015;95(6):1105–22, v. 10.1016/j.suc.2015.07.006 [DOI] [PubMed] [Google Scholar]
- 2. Franks AH, Harmsen HJ, Raangs GC, et al. : Variations of bacterial populations in human feces measured by fluorescent in situ hybridization with group-specific 16S rRNA-targeted oligonucleotide probes. Appl Environ Microbiol. 1998;64(9):3336–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Helander HF, Fändriks L: Surface area of the digestive tract - revisited. Scand J Gastroenterol. 2014;49(6):681–9. 10.3109/00365521.2014.898326 [DOI] [PubMed] [Google Scholar]
- 4. Allen A, Hutton DA, Pearson JP, et al. : Mucus glycoprotein structure, gel formation and gastrointestinal mucus function. Ciba Found Symp. 1984;109:137–56. 10.1002/9780470720905.ch10 [DOI] [PubMed] [Google Scholar]
- 5. Gill N, Wlodarska M, Finlay BB: Roadblocks in the gut: barriers to enteric infection. Cell Microbiol. 2011;13(5):660–9. 10.1111/j.1462-5822.2011.01578.x [DOI] [PubMed] [Google Scholar]
- 6. Rada B, Leto TL: Oxidative innate immune defenses by Nox/Duox family NADPH oxidases. Contrib Microbiol. 2008;15:164–87. 10.1159/000136357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Berg RD: Bacterial translocation from the gastrointestinal tract. Trends Microbiol. 1995;3(4):149–54. 10.1016/S0966-842X(00)88906-4 [DOI] [PubMed] [Google Scholar]
- 8. Smith SD, Cardona MA, Wishnev SA, et al. : Unique characteristics of the neonatal intestinal mucosal barrier. J Pediatr Surg. 1992;27(3):333–6; discussion 336–8. 10.1016/0022-3468(92)90857-4 [DOI] [PubMed] [Google Scholar]
- 9. Duerkop BA, Vaishnava S, Hooper LV: Immune responses to the microbiota at the intestinal mucosal surface. Immunity. 2009;31(3):368–76. 10.1016/j.immuni.2009.08.009 [DOI] [PubMed] [Google Scholar]
- 10. Kyd JM, Cripps AW: Functional differences between M cells and enterocytes in sampling luminal antigens. Vaccine. 2008;26(49):6221–4. 10.1016/j.vaccine.2008.09.061 [DOI] [PubMed] [Google Scholar]
- 11. Mabbott NA, Donaldson DS, Ohno H, et al. : Microfold (M) cells: important immunosurveillance posts in the intestinal epithelium. Mucosal Immunol. 2013;6(4):666–77. 10.1038/mi.2013.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ohno H: Intestinal M cells. J Biochem. 2016;159(2):151–60. 10.1093/jb/mvv121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kamada N, Seo SU, Chen GY, et al. : Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol. 2013;13(5):321–35. 10.1038/nri3430 [DOI] [PubMed] [Google Scholar]
- 14. Rhee KJ, Sethupathi P, Driks A, et al. : Role of commensal bacteria in development of gut-associated lymphoid tissues and preimmune antibody repertoire. J Immunol. 2004;172(2):1118–24. 10.4049/jimmunol.172.2.1118 [DOI] [PubMed] [Google Scholar]
- 15. Ohnmacht C, Park JH, Cording S, et al. : MUCOSAL IMMUNOLOGY. The microbiota regulates type 2 immunity through RORγt + T cells. Science. 2015;349(6251):989–93. 10.1126/science.aac4263 [DOI] [PubMed] [Google Scholar]
- 16. Maynard CL, Elson CO, Hatton RD, et al. : Reciprocal interactions of the intestinal microbiota and immune system. Nature. 2012;489(7415):231–41. 10.1038/nature11551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wijburg OL, Strugnell RA: Mucosal Immune Responses to Escherichia coli and Salmonella Infections. EcoSal Plus. 2006;2(1). 10.1128/ecosalplus.8.8.12 [DOI] [PubMed] [Google Scholar]
- 18. Perez-Lopez A, Behnsen J, Nuccio SP, et al. : Mucosal immunity to pathogenic intestinal bacteria. Nat Rev Immunol. 2016;16(3):135–48. 10.1038/nri.2015.17 [DOI] [PubMed] [Google Scholar]
- 19. Brandtzaeg P, Farstad IN, Johansen FE, et al. : The B-cell system of human mucosae and exocrine glands. Immunol Rev. 1999;171(1):45–87. 10.1111/j.1600-065X.1999.tb01342.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Macpherson AJ, Uhr T: Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004;303(5664):1662–5. 10.1126/science.1091334 [DOI] [PubMed] [Google Scholar]
- 21. MacPherson AJ, Gatto D, Sainsbury E, et al. : A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science. 2000;288(5474):2222–6. 10.1126/science.288.5474.2222 [DOI] [PubMed] [Google Scholar]
- 22. Doe WF: The intestinal immune system. Gut. 1989;30(12):1679–85. 10.1136/gut.30.12.1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Geremia A, Jewell DP: The IL-23/IL-17 pathway in inflammatory bowel disease. Expert Rev Gastroenterol Hepatol. 2012;6(2):223–37. 10.1586/egh.11.107 [DOI] [PubMed] [Google Scholar]
- 24. Burkett PR, Meyer zu Horste G, Kuchroo VK: Pouring fuel on the fire: Th17 cells, the environment, and autoimmunity. J Clin Invest. 2015;125(6):2211–9. 10.1172/JCI78085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Isailovic N, Daigo K, Mantovani A, et al. : Interleukin-17 and innate immunity in infections and chronic inflammation. J Autoimmun. 2015;60:1–11. 10.1016/j.jaut.2015.04.006 [DOI] [PubMed] [Google Scholar]
- 26. Song X, He X, Li X, et al. : The roles and functional mechanisms of interleukin-17 family cytokines in mucosal immunity. Cell Mol Immunol. 2016;13(4):418–31. 10.1038/cmi.2015.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee JS, Tato CM, Joyce-Shaikh B, et al. : Interleukin-23-Independent IL-17 Production Regulates Intestinal Epithelial Permeability. Immunity. 2015;43(4):727–38. 10.1016/j.immuni.2015.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cypowyj S, Picard C, Maródi L, et al. : Immunity to infection in IL-17-deficient mice and humans. Eur J Immunol. 2012;42(9):2246–54. 10.1002/eji.201242605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Toubiana J, Okada S, Hiller J, et al. : Heterozygous STAT1 gain-of-function mutations underlie an unexpectedly broad clinical phenotype. Blood. 2016;127(25):3154–64. 10.1182/blood-2015-11-679902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kaser A: Not all monoclonals are created equal - lessons from failed drug trials in Crohn’s disease. Best Pract Res Clin Gastroenterol. 2014;28(3):437–49. 10.1016/j.bpg.2014.04.005 [DOI] [PubMed] [Google Scholar]
- 31. Holmgren J: Mucosal immunity and vaccination. FEMS Microbiol Immunol. 1991;4(1):1–9. 10.1111/j.1574-6968.1991.tb04964.x [DOI] [PubMed] [Google Scholar]
- 32. Holmgren J, Svennerholm AM: Bacterial enteric infections and vaccine development. Gastroenterol Clin North Am. 1992;21(2):283–302. [PubMed] [Google Scholar]
- 33. Farache J, Zigmond E, Shakhar G, et al. : Contributions of dendritic cells and macrophages to intestinal homeostasis and immune defense. Immunol Cell Biol. 2013;91(3):232–9. 10.1038/icb.2012.79 [DOI] [PubMed] [Google Scholar]
- 34. Bain CC, Mowat AM: Macrophages in intestinal homeostasis and inflammation. Immunol Rev. 2014;260(1):102–17. 10.1111/imr.12192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bischoff SC: Role of mast cells in allergic and non-allergic immune responses: comparison of human and murine data. Nat Rev Immunol. 2007;7(2):93–104. 10.1038/nri2018 [DOI] [PubMed] [Google Scholar]
- 36. Mekori YA, Metcalfe DD: Mast cells in innate immunity. Immunol Rev. 2000;173(1):131–40. 10.1034/j.1600-065X.2000.917305.x [DOI] [PubMed] [Google Scholar]
- 37. Spits H, Cupedo T: Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annu Rev Immunol. 2012;30:647–75. 10.1146/annurev-immunol-020711-075053 [DOI] [PubMed] [Google Scholar]
- 38. Philip NH, Artis D: New friendships and old feuds: relationships between innate lymphoid cells and microbial communities. Immunol Cell Biol. 2013;91(3):225–31. 10.1038/icb.2013.2 [DOI] [PubMed] [Google Scholar]
- 39. Eberl G, Di Santo JP, Vivier E: The brave new world of innate lymphoid cells. Nat Immunol. 2015;16(1):1–5. 10.1038/ni.3059 [DOI] [PubMed] [Google Scholar]
- 40. Bevins CL, Salzman NH: Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol. 2011;9(5):356–68. 10.1038/nrmicro2546 [DOI] [PubMed] [Google Scholar]
- 41. Pober JS, Sessa WC: Inflammation and the blood microvascular system. Cold Spring Harb Perspect Biol. 2014;7(1):a016345. 10.1101/cshperspect.a016345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Goddard LM, Iruela-Arispe ML: Cellular and molecular regulation of vascular permeability. Thromb Haemost. 2013;109(3):407–15. 10.1160/TH12-09-0678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kumar V, Sharma A: Neutrophils: Cinderella of innate immune system. Int Immunopharmacol. 2010;10(11):1325–34. 10.1016/j.intimp.2010.08.012 [DOI] [PubMed] [Google Scholar]
- 44. Li Y, Karlin A, Loike JD, et al. : Determination of the critical concentration of neutrophils required to block bacterial growth in tissues. J Exp Med. 2004;200(5):613–22. 10.1084/jem.20040725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Foo SS, Reading PC, Jaillon S, et al. : Pentraxins and Collectins: Friend or Foe during Pathogen Invasion? Trends Microbiol. 2015;23(12):799–811. 10.1016/j.tim.2015.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kantari C, Pederzoli-Ribeil M, Witko-Sarsat V: The role of neutrophils and monocytes in innate immunity. Contrib Microbiol. 2008;15:118–46. 10.1159/000136335 [DOI] [PubMed] [Google Scholar]
- 47. Uhlig HH, Schwerd T, Koletzko S, et al. : The diagnostic approach to monogenic very early onset inflammatory bowel disease. Gastroenterology. 2014;147(5):990–1007.e3. 10.1053/j.gastro.2014.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Marks DJ, Miyagi K, Rahman FZ, et al. : Pathological features of inflammatory bowel disease in CGD are indistinguishable from those observed in Crohn’s disease. Gastroenterology. 2007;132(Suppl):156–7. [Google Scholar]
- 49. Glocker EO, Kotlarz D, Boztug K, et al. : Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med. 2009;361(21):2033–45. 10.1056/NEJMoa0907206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kühn R, Löhler J, Rennick D, et al. : Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75(2):263–74. 10.1016/0092-8674(93)80068-P [DOI] [PubMed] [Google Scholar]
- 51. Bogdan C, Vodovotz Y, Nathan C: Macrophage deactivation by interleukin 10. J Exp Med. 1991;174(6):1549–55. 10.1084/jem.174.6.1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Engelhardt KR, Shah N, Faizura-Yeop I, et al. : Clinical outcome in IL-10- and IL-10 receptor-deficient patients with or without hematopoietic stem cell transplantation. J Allergy Clin Immunol. 2013;131(3):825–30. 10.1016/j.jaci.2012.09.025 [DOI] [PubMed] [Google Scholar]
- 53. Khangura SK, Kamal N, Ho N, et al. : Gastrointestinal Features of Chronic Granulomatous Disease Found During Endoscopy. Clin Gastroenterol Hepatol. 2016;14(3):395–402.e5. 10.1016/j.cgh.2015.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Marks DJ, Miyagi K, Rahman FZ, et al. : Inflammatory bowel disease in CGD reproduces the clinicopathological features of Crohn’s disease. Am J Gastroenterol. 2009;104(1):117–24. 10.1038/ajg.2008.72 [DOI] [PubMed] [Google Scholar]
- 55. Hazzan D, Seward S, Stock H, et al. : Crohn’s-like colitis, enterocolitis and perianal disease in Hermansky-Pudlak syndrome. Colorectal Dis. 2006;8(7):539–43. 10.1111/j.1463-1318.2006.01046.x [DOI] [PubMed] [Google Scholar]
- 56. Dieckgraefe BK, Korzenik JR, Husain A, et al. : Association of glycogen storage disease 1b and Crohn disease: results of a North American survey. Eur J Pediatr. 2002;161(Suppl 1):S88–92. 10.1007/s00431-002-1011-z [DOI] [PubMed] [Google Scholar]
- 57. Kuemmerle-Deschner JB: CAPS--pathogenesis, presentation and treatment of an autoinflammatory disease. Semin Immunopathol. 2015;37(4):377–85. 10.1007/s00281-015-0491-7 [DOI] [PubMed] [Google Scholar]
- 58. Ramos-Casals M, Brito-Zerón P, López-Guillermo A, et al. : Adult haemophagocytic syndrome. Lancet. 2014;383(9927):1503–16. 10.1016/S0140-6736(13)61048-X [DOI] [PubMed] [Google Scholar]
- 59. Goyal N, Rana A, Ahlawat A, et al. : Animal models of inflammatory bowel disease: a review. Inflammopharmacology. 2014;22(4):219–33. 10.1007/s10787-014-0207-y [DOI] [PubMed] [Google Scholar]
- 60. Nell S, Suerbaum S, Josenhans C: The impact of the microbiota on the pathogenesis of IBD: lessons from mouse infection models. Nat Rev Microbiol. 2010;8(8):564–77. 10.1038/nrmicro2403 [DOI] [PubMed] [Google Scholar]
- 61. Ray A, Dittel BN: Interrelatedness between dysbiosis in the gut microbiota due to immunodeficiency and disease penetrance of colitis. Immunology. 2015;146(3):359–68. 10.1111/imm.12511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rodrigues-Sousa T, Ladeirinha AF, Santiago AR, et al. : Deficient production of reactive oxygen species leads to severe chronic DSS-induced colitis in Ncf1/p47 phox-mutant mice. PLoS One. 2014;9(5):e97532. 10.1371/journal.pone.0097532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pastorelli L, De Salvo C, Mercado JR, et al. : Central role of the gut epithelial barrier in the pathogenesis of chronic intestinal inflammation: lessons learned from animal models and human genetics. Front Immunol. 2013;4:280. 10.3389/fimmu.2013.00280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ni J, Chen SF, Hollander D: Effects of dextran sulphate sodium on intestinal epithelial cells and intestinal lymphocytes. Gut. 1996;39(2):234–41. 10.1136/gut.39.2.234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kim HS, Berstad A: Experimental colitis in animal models. Scand J Gastroenterol. 1992;27(7):529–37. 10.3109/00365529209000116 [DOI] [PubMed] [Google Scholar]
- 66. Zheng L, Gao ZQ, Wang SX: A chronic ulcerative colitis model in rats. World J Gastroenterol. 2000;6(1):150–2. 10.3748/WJG.v6.i1.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Waidmann M, Bechtold O, Frick JS, et al. : Bacteroides vulgatus protects against Escherichia coli-induced colitis in gnotobiotic interleukin-2-deficient mice. Gastroenterology. 2003;125(1):162–77. 10.1016/S0016-5085(03)00672-3 [DOI] [PubMed] [Google Scholar]
- 68. Mizoguchi A, Mizoguchi E, Chiba C, et al. : Cytokine imbalance and autoantibody production in T cell receptor-alpha mutant mice with inflammatory bowel disease. J Exp Med. 1996;183(3):847–56. 10.1084/jem.183.3.847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kontoyiannis D, Pasparakis M, Pizarro TT, et al. : Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity. 1999;10(3):387–98. 10.1016/S1074-7613(00)80038-2 [DOI] [PubMed] [Google Scholar]
- 70. Morrison PJ, Ballantyne SJ, Kullberg MC: Interleukin-23 and T helper 17-type responses in intestinal inflammation: from cytokines to T-cell plasticity. Immunology. 2011;133(4):397–408. 10.1111/j.1365-2567.2011.03454.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Elson CO, Cong Y, Sundberg J: The C3H/HeJBir mouse model: a high susceptibility phenotype for colitis. Int Rev Immunol. 2000;19(1):63–75. 10.3109/08830180009048390 [DOI] [PubMed] [Google Scholar]
- 72. Matsumoto S, Okabe Y, Setoyama H, et al. : Inflammatory bowel disease-like enteritis and caecitis in a senescence accelerated mouse P1/Yit strain. Gut. 1998;43(1):71–8. 10.1136/gut.43.1.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ernst PB, Wiznerowicz EB, Feldman SH, et al. : Pathogenesis of gastritis in ileitis-prone SAMP1/Yit mice. Keio J Med. 2011;60(2):65–8. 10.2302/kjm.60.65 [DOI] [PubMed] [Google Scholar]
- 74. Bosma GC, Custer RP, Bosma MJ: A severe combined immunodeficiency mutation in the mouse. Nature. 1983;301(5900):527–30. 10.1038/301527a0 [DOI] [PubMed] [Google Scholar]
- 75. Morrissey PJ, Charrier K, Braddy S, et al. : CD4+ T cells that express high levels of CD45RB induce wasting disease when transferred into congenic severe combined immunodeficient mice. Disease development is prevented by cotransfer of purified CD4+ T cells. J Exp Med. 1993;178(1):237–44. 10.1084/jem.178.1.237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Morrissey PJ, Charrier K: Induction of wasting disease in SCID mice by the transfer of normal CD4 +/CD45RB hi T cells and the regulation of this autoreactivity by CD4 +/CD45RB lo T cells. Res Immunol. 1994;145(5):357–62. 10.1016/S0923-2494(94)80200-9 [DOI] [PubMed] [Google Scholar]
- 77. Powrie F, Leach MW, Mauze S, et al. : Phenotypically distinct subsets of CD4 + T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int Immunol. 1993;5(11):1461–71. 10.1093/intimm/5.11.1461 [DOI] [PubMed] [Google Scholar]
- 78. Powrie F, Leach MW, Mauze S, et al. : Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RB hi CD4 + T cells. Immunity. 1994;1(7):553–62. 10.1016/1074-7613(94)90045-0 [DOI] [PubMed] [Google Scholar]
- 79. Toms C, Powrie F: Control of intestinal inflammation by regulatory T cells. Microbes Infect. 2001;3(11):929–35. 10.1016/S1286-4579(01)01454-X [DOI] [PubMed] [Google Scholar]
- 80. Lord JD: Promises and paradoxes of regulatory T cells in inflammatory bowel disease. World J Gastroenterol. 2015;21(40):11236–45. 10.3748/wjg.v21.i40.11236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Chalaris A, Adam N, Sina C, et al. : Critical role of the disintegrin metalloprotease ADAM17 for intestinal inflammation and regeneration in mice. J Exp Med. 2010;207(8):1617–24. 10.1084/jem.20092366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ooi CJ, Fock KM, Makharia GK, et al. : The Asia-Pacific consensus on ulcerative colitis. J Gastroenterol Hepatol. 2010;25(3):453–68. 10.1111/j.1440-1746.2010.06241.x [DOI] [PubMed] [Google Scholar]
- 83. Ooi CJ, Makharia GK, Hilmi I, et al. : Asia Pacific Consensus Statements on Crohn's disease. Part 1: Definition, diagnosis, and epidemiology: (Asia Pacific Crohn's Disease Consensus--Part 1). J Gastroenterol Hepatol. 2016;31(1):45–55. 10.1111/jgh.12956 [DOI] [PubMed] [Google Scholar]
- 84. Law DH: Regional enteritis. Gastroenterology. 1969;56(6):1086–110. [PubMed] [Google Scholar]
- 85. Safar B, Sands D: Perianal Crohn's disease. Clin Colon Rectal Surg. 2007;20(4):282–93. 10.1055/s-2007-991027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Warren KS: A functional classification of granulomatous inflammation. Ann N Y Acad Sci. 1976;278:7–18. 10.1111/j.1749-6632.1976.tb47011.x [DOI] [PubMed] [Google Scholar]
- 87. Sewell GW, Marks DJ, Segal AW: The immunopathogenesis of Crohn's disease: a three-stage model. Curr Opin Immunol. 2009;21(5):506–13. 10.1016/j.coi.2009.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Joossens M, Simoens M, Vermeire S, et al. : Contribution of genetic and environmental factors in the pathogenesis of Crohn's disease in a large family with multiple cases. Inflamm Bowel Dis. 2007;13(5):580–4. 10.1002/ibd.20086 [DOI] [PubMed] [Google Scholar]
- 89. Katsanos KH, Karetsos V, Tsianos EV: A family report of Crohn's disease in three children immigrating from Albania to Greece and review of the literature. J Crohns Colitis. 2010;4(5):582–5. 10.1016/j.crohns.2010.03.007 [DOI] [PubMed] [Google Scholar]
- 90. Freeman HJ, Hershfield NB: Anticipation in an Indo-Canadian family with Crohn’s disease. Can J Gastroenterol. 2001;15(10):695–8. 10.1155/2001/518043 [DOI] [PubMed] [Google Scholar]
- 91. Newman RD, Zu SX, Wuhib T, et al. : Household epidemiology of Cryptosporidium parvum infection in an urban community in northeast Brazil. Ann Intern Med. 1994;120(6):500–5. 10.7326/0003-4819-120-6-199403150-00009 [DOI] [PubMed] [Google Scholar]
- 92. Bar-Gil Shitrit A, Koslowsky B, Kori M, et al. : Inflammatory bowel disease: an emergent disease among Ethiopian Jews migrating to Israel. Inflamm Bowel Dis. 2015;21(3):631–5. 10.1097/MIB.0000000000000311 [DOI] [PubMed] [Google Scholar]
- 93. Li X, Sundquist J, Hemminki K, et al. : Risk of inflammatory bowel disease in first- and second-generation immigrants in Sweden: a nationwide follow-up study. Inflamm Bowel Dis. 2011;17(8):1784–91. 10.1002/ibd.21535 [DOI] [PubMed] [Google Scholar]
- 94. Talley NJ, Abreu MT, Achkar JP, et al. : An evidence-based systematic review on medical therapies for inflammatory bowel disease. Am J Gastroenterol. 2011;106(Suppl 1):S2–25; quiz S26. 10.1038/ajg.2011.58 [DOI] [PubMed] [Google Scholar]
- 95. Economou M, Zambeli E, Michopoulos S: Incidence and prevalence of Crohn’s disease and its etiological influences. Ann Gastroenterol. 2009;22(3):158–67. Reference Source [Google Scholar]
- 96. Timmer A: Environmental influences on inflammatory bowel disease manifestations. Lessons from epidemiology. Dig Dis. 2003;21(2):91–104. 10.1159/000073242 [DOI] [PubMed] [Google Scholar]
- 97. Asakura K, Nishiwaki Y, Inoue N, et al. : Prevalence of ulcerative colitis and Crohn’s disease in Japan. J Gastroenterol. 2009;44(7):659–65. 10.1007/s00535-009-0057-3 [DOI] [PubMed] [Google Scholar]
- 98. Lee KM, Lee JM: Crohn’s disease in Korea: past, present, and future. Korean J Intern Med. 2014;29(5):558–70. 10.3904/kjim.2014.29.5.558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ko Y, Kariyawasam V, Karnib M, et al. : Inflammatory Bowel Disease Environmental Risk Factors: A Population-Based Case-Control Study of Middle Eastern Migration to Australia. Clin Gastroenterol Hepatol. 2015;13(8):1453–63.e1. 10.1016/j.cgh.2015.02.045 [DOI] [PubMed] [Google Scholar]
- 100. Tsironi E, Feakins RM, Probert CS, et al. : Incidence of inflammatory bowel disease is rising and abdominal tuberculosis is falling in Bangladeshis in East London, United Kingdom. Am J Gastroenterol. 2004;99(9):1749–55. 10.1111/j.1572-0241.2004.30445.x [DOI] [PubMed] [Google Scholar]
- 101. Mitchell DN, Rees RJ: Agent transmissible from Crohn’s disease tissue. Lancet. 1970;2(7665):168–71. 10.1016/S0140-6736(70)92532-8 [DOI] [PubMed] [Google Scholar]
- 102. Wu CW, Livesey M, Schmoller SK, et al. : Invasion and persistence of Mycobacterium avium subsp. paratuberculosis during early stages of Johne’s disease in calves. Infect Immun. 2007;75(5):2110–9. 10.1128/IAI.01739-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Dalzeil TK: Chronic interstitial enteritis. Br Med J. 1913;2(2756):1068–70. Reference Source [Google Scholar]
- 104. Hermon-Taylor J, Bull TJ, Sheridan JM, et al. : Causation of Crohn’s disease by Mycobacterium avium subspecies paratuberculosis. Can J Gastroenterol. 2000;14(6):521–39. 10.1155/2000/798305 [DOI] [PubMed] [Google Scholar]
- 105. Chiodini RJ, Chamberlin WM, Sarosiek J, et al. : Crohn’s disease and the mycobacterioses: a quarter century later. Causation or simple association? Crit Rev Microbiol. 2012;38(1):52–93. 10.3109/1040841X.2011.638273 [DOI] [PubMed] [Google Scholar]
- 106. Gitlin L, Borody TJ, Chamberlin W, et al. : Mycobacterium avium ss paratuberculosis-associated diseases: piecing the Crohn’s puzzle together. J Clin Gastroenterol. 2012;46(8):649–55. 10.1097/MCG.0b013e31825f2bce [DOI] [PubMed] [Google Scholar]
- 107. Helms M, Simonsen J, Mølbak K: Foodborne bacterial infection and hospitalization: a registry-based study. Clin Infect Dis. 2006;42(4):498–506. 10.1086/499813 [DOI] [PubMed] [Google Scholar]
- 108. García Rodríguez LA, Ruigómez A, Panés J: Acute gastroenteritis is followed by an increased risk of inflammatory bowel disease. Gastroenterology. 2006;130(6):1588–94. 10.1053/j.gastro.2006.02.004 [DOI] [PubMed] [Google Scholar]
- 109. Ternhag A, Törner A, Svensson A, et al. : Short- and long-term effects of bacterial gastrointestinal infections. Emerg Infect Dis. 2008;14(1):143–8. 10.3201/eid1401.070524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Gradel KO, Nielsen HL, Schønheyder HC, et al. : Increased short- and long-term risk of inflammatory bowel disease after salmonella or campylobacter gastroenteritis. Gastroenterology. 2009;137(2):495–501. 10.1053/j.gastro.2009.04.001 [DOI] [PubMed] [Google Scholar]
- 111. Jess T, Simonsen J, Nielsen NM, et al. : Enteric Salmonella or Campylobacter infections and the risk of inflammatory bowel disease. Gut. 2011;60(3):318–24. 10.1136/gut.2010.223396 [DOI] [PubMed] [Google Scholar]
- 112. Glass RI, Parashar UD, Estes MK: Norovirus gastroenteritis. N Engl J Med. 2009;361(18):1776–85. 10.1056/NEJMra0804575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Rutgeerts P, Geboes K, Ponette E, et al. : Acute infective colitis caused by endemic pathogens in western Europe: endoscopic features. Endoscopy. 1982;14(6):212–9. 10.1055/s-2007-1021624 [DOI] [PubMed] [Google Scholar]
- 114. Ina K, Kusugami K, Ohta M: Bacterial hemorrhagic enterocolitis. J Gastroenterol. 2003;38(2):111–20. 10.1007/s005350300019 [DOI] [PubMed] [Google Scholar]
- 115. Agus A, Massier S, Darfeuille-Michaud A, et al. : Understanding host-adherent-invasive Escherichia coli interaction in Crohn’s disease: opening up new therapeutic strategies. Biomed Res Int. 2014;2014: 567929. 10.1155/2014/567929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Rhodes JM: The role of Escherichia coli in inflammatory bowel disease. Gut. 2007;56(5):610–2. 10.1136/gut.2006.111872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Rolhion N, Darfeuille-Michaud A: Adherent-invasive Escherichia coli in inflammatory bowel disease. Inflamm Bowel Dis. 2007;13(10):1277–83. 10.1002/ibd.20176 [DOI] [PubMed] [Google Scholar]
- 118. Ueda N: Gastroduodenal Perforation and Ulcer Associated With Rotavirus and Norovirus Infections in Japanese Children: A Case Report and Comprehensive Literature Review. Open Forum Infect Dis. 2016;3(1): ofw026. 10.1093/ofid/ofw026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Colgan T, Lambert JR, Newman A, et al. : Campylobacter jejuni enterocolitis. A clinicopathologic study. Arch Pathol Lab Med. 1980;104(11):571–4. [PubMed] [Google Scholar]
- 120. Khuroo MS, Mahajan R, Zargar SA, et al. : The colon in shigellosis: serial colonoscopic appearances in Shigella dysenteriae I. Endoscopy. 1990;22(1):35–8. 10.1055/s-2007-1012784 [DOI] [PubMed] [Google Scholar]
- 121. Parry CM, Hien TT, Dougan G, et al. : Typhoid fever. N Engl J Med. 2002;347(22):1770–82. 10.1056/NEJMra020201 [DOI] [PubMed] [Google Scholar]
- 122. Dilauro S, Crum-Cianflone NF: Ileitis: when it is not Crohn’s disease. Curr Gastroenterol Rep. 2010;12(4):249–58. 10.1007/s11894-010-0112-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Taniwaki S, Kataoka M, Tanaka H, et al. : Multiple ulcers of the ileum due to Cytomegalovirus infection in a patient who showed no evidence of an immunocompromised state. J Gastroenterol. 1997;32(4):548–52. 10.1007/BF02934098 [DOI] [PubMed] [Google Scholar]
- 124. Yamamoto T, Ishii T, Sanaka M, et al. : Ileal ulcers due to Aeromonas hydrophilia infection. J Clin Gastroenterol. 2004;38(10):911. [DOI] [PubMed] [Google Scholar]
- 125. Vantrappen G, Geboes K, Ponette E: Yersinia enteritis. Med Clin North Am. 1982;66(3):639–53. [DOI] [PubMed] [Google Scholar]
- 126. Puylaert JB, Van der Zant FM, Mutsaers JA: Infectious ileocecitis caused by Yersinia, Campylobacter, and Salmonella: clinical, radiological and US findings. Eur Radiol. 1997;7(1):3–9. 10.1007/s003300050098 [DOI] [PubMed] [Google Scholar]
- 127. IASR 29-8 food poisoning, foodborne infections, Food Sanitation Law, Listeriosis, E. sakazakii, C. perfringens, V. parahaemolyticus . Reference Source [Google Scholar]
- 128. Yamazaki W, Uemura R, Sekiguchi S, et al. : Campylobacter and Salmonella are prevalent in broiler farms in Kyushu, Japan: results of a 2-year distribution and circulation dynamics audit. J Appl Microbiol. 2016;120(6):1711–22. 10.1111/jam.13141 [DOI] [PubMed] [Google Scholar]
- 129. Mylonaki M, Langmead L, Pantes A, et al. : Enteric infection in relapse of inflammatory bowel disease: importance of microbiological examination of stool. Eur J Gastroenterol Hepatol. 2004;16(8):775–8. [DOI] [PubMed] [Google Scholar]
- 130. Rock K, Brand S, Moir J, et al. : Dynamics of infectious diseases. Rep Prog Phys. 2014;77(2):026602. 10.1088/0034-4885/77/2/026602 [DOI] [PubMed] [Google Scholar]
- 131. Van Kruiningen HJ, Colombel JF, Cartun RW, et al. : An in-depth study of Crohn’s disease in two French families. Gastroenterology. 1993;104(2):351–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Maconi G, Orlandini L, Asthana AK, et al. : The impact of symptoms, irritable bowel syndrome pattern and diagnostic investigations on the diagnostic delay of Crohn’s disease: A prospective study. Dig Liver Dis. 2015;47(8):646–51. 10.1016/j.dld.2015.04.009 [DOI] [PubMed] [Google Scholar]
- 133. Oberc A, Coombes BK: Convergence of External Crohn’s Disease Risk Factors on Intestinal Bacteria. Front Immunol. 2015;6:558. 10.3389/fimmu.2015.00558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Ricanek P, Lothe SM, Frye SA, et al. : Gut bacterial profile in patients newly diagnosed with treatment-naïve Crohn’s disease. Clin Exp Gastroenterol. 2012;5:173–86. 10.2147/CEG.S33858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Liguori G, Lamas B, Richard ML, et al. : Fungal Dysbiosis in Mucosa-associated Microbiota of Crohn’s Disease Patients. J Crohns Colitis. 2016;10(3):296–305. 10.1093/ecco-jcc/jjv209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Naftali T, Reshef L, Kovacs A, et al. : Distinct Microbiotas are Associated with Ileum-Restricted and Colon-Involving Crohn’s Disease. Inflamm Bowel Dis. 2016;22(2):293–302. 10.1097/MIB.0000000000000662 [DOI] [PubMed] [Google Scholar]
- 137. Youmans BP, Ajami NJ, Jiang ZD, et al. : Characterization of the human gut microbiome during travelers’ diarrhea. Gut Microbes. 2015;6(2):110–9. 10.1080/19490976.2015.1019693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Quince C, Ijaz UZ, Loman N, et al. : Extensive Modulation of the Fecal Metagenome in Children With Crohn’s Disease During Exclusive Enteral Nutrition. Am J Gastroenterol. 2015;110(12):1718–29; quiz 1730. 10.1038/ajg.2015.357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Zaura E, Brandt BW, Teixeira de Mattos MJ, et al. : Same Exposure but Two Radically Different Responses to Antibiotics: Resilience of the Salivary Microbiome versus Long-Term Microbial Shifts in Feces. MBio. 2015;6(6):e01693–15. 10.1128/mBio.01693-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Hashash JG, Chintamaneni P, Ramos Rivers CM, et al. : Patterns of Antibiotic Exposure and Clinical Disease Activity in Inflammatory Bowel Disease: A 4-year Prospective Study. Inflamm Bowel Dis. 2015;21(11):2576–82. 10.1097/MIB.0000000000000534 [DOI] [PubMed] [Google Scholar]
- 141. Lee T, Clavel T, Smirnov K, et al. : Oral versus intravenous iron replacement therapy distinctly alters the gut microbiota and metabolome in patients with IBD. Gut. 2016; pii: gutjnl-2015-309940. 10.1136/gutjnl-2015-309940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Bashan A, Gibson TE, Friedman J, et al. : Universality of human microbial dynamics. Nature. 2016;534(7606):259–62. 10.1038/nature18301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Bernstein CN: Antibiotics, probiotics and prebiotics in IBD. Nestle Nutr Inst Workshop Ser. 2014;79:83–100. 10.1159/000360713 [DOI] [PubMed] [Google Scholar]
- 144. Fujiya M, Ueno N, Kohgo Y: Probiotic treatments for induction and maintenance of remission in inflammatory bowel diseases: a meta-analysis of randomized controlled trials. Clin J Gastroenterol. 2014;7(1):1–13. 10.1007/s12328-013-0440-8 [DOI] [PubMed] [Google Scholar]
- 145. Benjamin JL, Hedin CR, Koutsoumpas A, et al. : Randomised, double-blind, placebo-controlled trial of fructo-oligosaccharides in active Crohn’s disease. Gut. 2011;60(7):923–9. 10.1136/gut.2010.232025 [DOI] [PubMed] [Google Scholar]
- 146. Rossen NG, MacDonald JK, de Vries EM, et al. : Fecal microbiota transplantation as novel therapy in gastroenterology: A systematic review. World J Gastroenterol. 2015;21(17):5359–71. 10.3748/wjg.v21.i17.5359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Vermeire S, Joossens M, Verbeke K, et al. : Donor Species Richness Determines Faecal Microbiota Transplantation Success in Inflammatory Bowel Disease. J Crohns Colitis. 2016;10(4):387–94. 10.1093/ecco-jcc/jjv203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Cosnes J, Gower-Rousseau C, Seksik P, et al. : Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140(6):1785–94. 10.1053/j.gastro.2011.01.055 [DOI] [PubMed] [Google Scholar]
- 149. Versini M, Jeandel PY, Bashi T, et al. : Unraveling the Hygiene Hypothesis of helminthes and autoimmunity: origins, pathophysiology, and clinical applications. BMC Med. 2015;13:81. 10.1186/s12916-015-0306-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Leong RW, Mitrev N, Ko Y: Hygiene Hypothesis: Is the Evidence the Same All Over the World? Dig Dis. 2016;34(1–2):35–42. 10.1159/000442922 [DOI] [PubMed] [Google Scholar]
- 151. Strachan DP: Hay fever, hygiene, and household size. BMJ. 1989;299(6710):1259–60. 10.1136/bmj.299.6710.1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Black RE, Cousens S, Johnson HL, et al. : Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375(9730):1969–87. 10.1016/S0140-6736(10)60549-1 [DOI] [PubMed] [Google Scholar]
- 153. Levine MM, Kaper JB, Black RE, et al. : New knowledge on pathogenesis of bacterial enteric infections as applied to vaccine development. Microbiol Rev. 1983;47(4):510–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Ahuja V, Tandon RK: Inflammatory bowel disease in the Asia-Pacific area: a comparison with developed countries and regional differences. J Dig Dis. 2010;11(3):134–47. 10.1111/j.1751-2980.2010.00429.x [DOI] [PubMed] [Google Scholar]
- 155. Victoria CR, Sassak LY, Nunes HR: Incidence and prevalence rates of inflammatory bowel diseases, in midwestern of São Paulo State, Brazil. Arq Gastroenterol. 2009;46(1):20–5. 10.1590/S0004-28032009000100009 [DOI] [PubMed] [Google Scholar]
- 156. Hu D, Ren J, Wang G, et al. : Geographic mapping of Crohn’s disease and its relation to affluence in jiangsu province, an eastern coastal province of China. Gastroenterol Res Pract. 2014;2014: 590467. 10.1155/2014/590467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Mayberry J, Mann R: Inflammatory bowel disease in rural sub-Saharan Africa: rarity of diagnosis in patients attending mission hospitals. Digestion. 1989;44(3):172–6. 10.1159/000199907 [DOI] [PubMed] [Google Scholar]
- 158. Guerrant RL, DeBoer MD, Moore SR, et al. : The impoverished gut--a triple burden of diarrhoea, stunting and chronic disease. Nat Rev Gastroenterol Hepatol. 2013;10(4):220–9. 10.1038/nrgastro.2012.239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Lahariya C, Paul VK: Burden, differentials, and causes of child deaths in India. Indian J Pediatr. 2010;77(11):1312–21. 10.1007/s12098-010-0185-z [DOI] [PubMed] [Google Scholar]
- 160. Kirk MD, Pires SM, Black RE, et al. : World Health Organization Estimates of the Global and Regional Disease Burden of 22 Foodborne Bacterial, Protozoal, and Viral Diseases, 2010: A Data Synthesis. PLoS Med. 2015;12(12):e1001921. 10.1371/journal.pmed.1001921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Ojha SC, Jaide C, Jinawath N, et al. : Geohelminths: public health significance. J Infect Dev Ctries. 2014;8(1):5–16. 10.3855/jidc.3183 [DOI] [PubMed] [Google Scholar]
- 162. Chu KM, Watermeyer G, Shelly L, et al. : Childhood helminth exposure is protective against inflammatory bowel disease: a case control study in South Africa. Inflamm Bowel Dis. 2013;19(3):614–20. 10.1097/MIB.0b013e31827f27f4 [DOI] [PubMed] [Google Scholar]
- 163. Bager P, Vinkel Hansen A, Wohlfahrt J, et al. : Helminth infection does not reduce risk for chronic inflammatory disease in a population-based cohort study. Gastroenterology. 2012;142(1):55–62. 10.1053/j.gastro.2011.09.046 [DOI] [PubMed] [Google Scholar]
- 164. Garg SK, Croft AM, Bager P: Helminth therapy (worms) for induction of remission in inflammatory bowel disease. Cochrane Database Syst Rev. 2014;1:CD009400. 10.1002/14651858.CD009400.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. O’Ryan M, Vidal R, del Canto F, et al. : Vaccines for viral and bacterial pathogens causing acute gastroenteritis: Part I: Overview, vaccines for enteric viruses and Vibrio cholerae. Hum Vaccin Immunother. 2015;11(3):584–600. 10.1080/21645515.2015.1011019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Levine MM, Black RE, Clements ML, et al. : Duration of infection-derived immunity to cholera. J Infect Dis. 1981;143(6):818–20. 10.1093/infdis/143.6.818 [DOI] [PubMed] [Google Scholar]
- 167. Clements ML, Levine MM, Young CR, et al. : Magnitude, kinetics, and duration of vibriocidal antibody responses in North Americans after ingestion of Vibrio cholerae. J Infect Dis. 1982;145(4):465–73. 10.1093/infdis/145.4.465 [DOI] [PubMed] [Google Scholar]
- 168. Levine MM, Nalin DR, Hoover DL, et al. : Immunity to enterotoxigenic Escherichia coli. Infect Immun. 1979;23(3):729–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Nyachuba DG: Foodborne illness: is it on the rise? Nutr Rev. 2010;68(5):257–69. 10.1111/j.1753-4887.2010.00286.x [DOI] [PubMed] [Google Scholar]
- 170. Khabbaz RF, Moseley RR, Steiner RJ, et al. : Challenges of infectious diseases in the USA. Lancet. 2014;384(9937):53–63. 10.1016/S0140-6736(14)60890-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Gormley FJ, Little CL, Rawal N, et al. : A 17-year review of foodborne outbreaks: describing the continuing decline in England and Wales (1992–2008). Epidemiol Infect. 2011;139(5):688–99. 10.1017/S0950268810001858 [DOI] [PubMed] [Google Scholar]
- 172. Kumagai Y, Gilmour S, Ota E, et al. : Estimating the burden of foodborne diseases in Japan. Bull World Health Organ. 2015;93(8):540–9C. 10.2471/BLT.14.148056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Sonnenberg A: Age distribution of IBD hospitalization. Inflamm Bowel Dis. 2010;16(3):452–7. 10.1002/ibd.21058 [DOI] [PubMed] [Google Scholar]
- 174. Loftus EV, Jr, Schoenfeld P, Sandborn WJ: The epidemiology and natural history of Crohn’s disease in population-based patient cohorts from North America: a systematic review. Aliment Pharmacol Ther. 2002;16(1):51–60. 10.1046/j.1365-2036.2002.01140.x [DOI] [PubMed] [Google Scholar]
- 175. Burisch J, Pedersen N, Čuković-Čavka S, et al. : East-West gradient in the incidence of inflammatory bowel disease in Europe: the ECCO-EpiCom inception cohort. Gut. 2014;63(4):588–97. 10.1136/gutjnl-2013-304636 [DOI] [PubMed] [Google Scholar]
- 176. Murray CJ, Ortblad KF, Guinovart C, et al. : Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9947):1005–70. 10.1016/S0140-6736(14)60844-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Butler AJ, Thomas MK, Pintar KD: Expert elicitation as a means to attribute 28 enteric pathogens to foodborne, waterborne, animal contact, and person-to-person transmission routes in Canada. Foodborne Pathog Dis. 2015;12(4):335–44. 10.1089/fpd.2014.1856 [DOI] [PubMed] [Google Scholar]
- 178. Hung CC, Chang SY, Ji DD: Entamoeba histolytica infection in men who have sex with men. Lancet Infect Dis. 2012;12(9):729–36. 10.1016/S1473-3099(12)70147-0 [DOI] [PubMed] [Google Scholar]
- 179. Simms I, Field N, Jenkins C, et al. : Intensified shigellosis epidemic associated with sexual transmission in men who have sex with men-- Shigella flexneri and S. sonnei in England, 2004 to end of February 2015. Euro Surveill. 2015;20(15): pii: 21097. 10.2807/1560-7917.ES2015.20.15.21097 [DOI] [PubMed] [Google Scholar]
- 180. Danila RN, Eikmeier DL, Robinson TJ, et al. : Two concurrent enteric disease outbreaks among men who have sex with men minneapolis-st paul area. Clin Infect Dis. 2014; 59(7):987–9. 10.1093/cid/ciu478 [DOI] [PubMed] [Google Scholar]
- 181. Gaudreau C, Helferty M, Sylvestre JL, et al. : Campylobacter coli outbreak in men who have sex with men, Quebec, Canada, 2010–2011. Emerg Infect Dis. 2013;19(5):764–7. 10.3201/eid1905.121344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Aragón TJ, Vugia DJ, Shallow S, et al. : Case-control study of shigellosis in San Francisco: the role of sexual transmission and HIV infection. Clin Infect Dis. 2007;44(3):327–34. 10.1086/510593 [DOI] [PubMed] [Google Scholar]
- 183. Levine MM, DuPont HL, Formal SB, et al. : Pathogenesis of Shigella dysenteriae 1 (Shiga) dysentery. J Infect Dis. 1973;127(3):261–70. 10.1093/infdis/127.3.261 [DOI] [PubMed] [Google Scholar]
- 184. Cooper F, Barber T: ‘Gay bowel syndrome’: relic or real (and returning) phenomenon? Curr Opin Infect Dis. 2014;27(1):84–9. 10.1097/QCO.0000000000000032 [DOI] [PubMed] [Google Scholar]
- 185. de Vrieze NH, de Vries HJ: Lymphogranuloma venereum among men who have sex with men. An epidemiological and clinical review. Expert Rev Anti Infect Ther. 2014;12(6):697–704. 10.1586/14787210.2014.901169 [DOI] [PubMed] [Google Scholar]
- 186. Salit IE, Khairnar K, Gough K, et al. : A possible cluster of sexually transmitted Entamoeba histolytica: genetic analysis of a highly virulent strain. Clin Infect Dis. 2009;49(3):346–53. 10.1086/600298 [DOI] [PubMed] [Google Scholar]
- 187. Edwards S, Carne C: Oral sex and transmission of non-viral STIs. Sex Transm Infect. 1998;74(2):95–100. 10.1136/sti.74.2.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Yamamoto S, Tsukamoto T, Terai A, et al. : Genetic evidence supporting the fecal-perineal-urethral hypothesis in cystitis caused by Escherichia coli. J Urol. 1997;157(3):1127–9. 10.1016/S0022-5347(01)65154-1 [DOI] [PubMed] [Google Scholar]
- 189. Grüneberg RN: Relationship of infecting urinary organism to the faecal flora in patients with symptomatic urinary infection. Lancet. 1969;2(7624):766–8. 10.1016/S0140-6736(69)90478-4 [DOI] [PubMed] [Google Scholar]
- 190. Mercer CH, Tanton C, Prah P, et al. : Changes in sexual attitudes and lifestyles in Britain through the life course and over time: findings from the National Surveys of Sexual Attitudes and Lifestyles (Natsal). Lancet. 2013;382(9907):1781–94. 10.1016/S0140-6736(13)62035-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Chandra A, Mosher WD, Copen C, et al. : Sexual behavior, sexual attraction, and sexual identity in the United States: data from the 2006–2008 National Survey of Family Growth. Natl Health Stat Report. 2011; (36):1–36. [PubMed] [Google Scholar]
- 192. Benson LS, Martins SL, Whitaker AK: Correlates of Heterosexual Anal Intercourse among Women in the 2006–2010 National Survey of Family Growth. J Sex Med. 2015;12(8):1746–52. 10.1111/jsm.12961 [DOI] [PubMed] [Google Scholar]
- 193. Halperin DT: Heterosexual anal intercourse: prevalence, cultural factors, and HIV infection and other health risks, Part I. AIDS Patient Care STDS. 1999;13(12):717–30. 10.1089/apc.1999.13.717 [DOI] [PubMed] [Google Scholar]
- 194. Cunningham SD, Olthoff G, Burnett P, et al. : Evidence of heterosexual bridging among syphilis-positive men who have sex with men. Sex Transm Infect. 2006;82(6):444–5. 10.1136/sti.2005.019513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Zheng JJ, Zhu XS, Huangfu Z, et al. : Prevalence and incidence rates of Crohn’s disease in mainland China: a meta-analysis of 55 years of research. J Dig Dis. 2010;11(3):161–6. 10.1111/j.1751-2980.2010.00431.x [DOI] [PubMed] [Google Scholar]
- 196. Al-Mofarreh MA, Al-Mofleh IA: Emerging inflammatory bowel disease in saudi outpatients: a report of 693 cases. Saudi J Gastroenterol. 2013;19(1):16–22. 10.4103/1319-3767.105915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Ng JY, Wong ML, Chan RK, et al. : Gender Differences in Factors Associated With Anal Intercourse Among Heterosexual Adolescents in Singapore. AIDS Educ Prev. 2015;27(4):373–85. 10.1521/aeap.2015.27.4.373 [DOI] [PubMed] [Google Scholar]
- 198. Liu CM, Hungate BA, Tobian AA, et al. : Penile Microbiota and Female Partner Bacterial Vaginosis in Rakai, Uganda. MBio. 2015;6(3):e00589. 10.1128/mBio.00589-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Mändar R, Punab M, Borovkova N, et al. : Complementary seminovaginal microbiome in couples. Res Microbiol. 2015;166(5):440–7. 10.1016/j.resmic.2015.03.009 [DOI] [PubMed] [Google Scholar]
- 200. Blaser MJ, LaForce FM, Wilson NA, et al. : Reservoirs for human campylobacteriosis. J Infect Dis. 1980;141(5):665–9. 10.1093/infdis/141.5.665 [DOI] [PubMed] [Google Scholar]
- 201. Gunn JS, Marshall JM, Baker S, et al. : Salmonella chronic carriage: epidemiology, diagnosis, and gallbladder persistence. Trends Microbiol. 2014;22(11):648–55. 10.1016/j.tim.2014.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Todd EC, Greig JD, Bartleson CA, et al. : Outbreaks where food workers have been implicated in the spread of foodborne disease. Part 4. Infective doses and pathogen carriage. J Food Prot. 2008;71(11):2339–73. [DOI] [PubMed] [Google Scholar]
- 203. To N, Gracie DJ, Ford AC: Systematic review with meta-analysis: the adverse effects of tobacco smoking on the natural history of Crohn’s disease. Aliment Pharmacol Ther. 2016;43(5):549–61. 10.1111/apt.13511 [DOI] [PubMed] [Google Scholar]
- 204. Duffy LC, Zielezny MA, Marshall JR, et al. : Cigarette smoking and risk of clinical relapse in patients with Crohn’s disease. Am J Prev Med. 1990;6(3):161–6. [PubMed] [Google Scholar]
- 205. Roos M, Soskic V, Poznanovic S, et al. : Post-translational modifications of endothelin receptor B from bovine lungs analyzed by mass spectrometry. J Biol Chem. 1998;273(2):924–31. 10.1074/jbc.273.2.924 [DOI] [PubMed] [Google Scholar]
- 206. Zimmerman DD, Gosselink MP, Mitalas LE, et al. : Smoking impairs rectal mucosal bloodflow--a pilot study: possible implications for transanal advancement flap repair. Dis Colon Rectum. 2005;48(6):1228–32. 10.1007/s10350-004-0943-y [DOI] [PubMed] [Google Scholar]
- 207. Endoh K, Kauffman GL, Jr, Leung FW: Mechanism of aggravation of mucosal injury by intravenous nicotine in rat stomach. Am J Physiol. 1991;261(6 Pt 1):G1037–42. [DOI] [PubMed] [Google Scholar]
- 208. Sher ME, Bank S, Greenberg R, et al. : The influence of cigarette smoking on cytokine levels in patients with inflammatory bowel disease. Inflamm Bowel Dis. 1999;5(2):73–8. 10.1002/ibd.3780050202 [DOI] [PubMed] [Google Scholar]
- 209. Arnott ID, Williams N, Drummond HE, et al. : Whole gut lavage fluid interleukin-1beta and interleukin-8 in smokers and non-smokers with Crohn’s disease in clinical remission. Dig Liver Dis. 2002;34(6):424–9. [DOI] [PubMed] [Google Scholar]
- 210. Gilat T, Hacohen D, Lilos P, et al. : Childhood factors in ulcerative colitis and Crohn’s disease. An international cooperative study. Scand J Gastroenterol. 1987;22(8):1009–24. 10.3109/00365528708991950 [DOI] [PubMed] [Google Scholar]
- 211. Kantsø B, Simonsen J, Hoffmann S, et al. : Inflammatory Bowel Disease Patients Are at Increased Risk of Invasive Pneumococcal Disease: A Nationwide Danish Cohort Study 1977-2013. Am J Gastroenterol. 2015;110(11):1582–7. 10.1038/ajg.2015.284 [DOI] [PubMed] [Google Scholar]
- 212. Whorwell PJ, Eade OE, Hossenbocus A, et al. : Crohn’s disease in a husband and wife. Lancet. 1978;2(8082):186–7. 10.1016/S0140-6736(78)91923-2 [DOI] [PubMed] [Google Scholar]
- 213. Su JW, Ma JJ, Zhang HJ: Use of antibiotics in patients with Crohn’s disease: a systematic review and meta-analysis. J Dig Dis. 2015;16(2):58–66. 10.1111/1751-2980.12216 [DOI] [PubMed] [Google Scholar]
- 214. Altare F, Durandy A, Lammas D, et al. : Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science. 1998;280(5368):1432–5. 10.1126/science.280.5368.1432 [DOI] [PubMed] [Google Scholar]
- 215. Segal AW, Loewi G: Neutrophil dysfunction in Crohn’s disease. Lancet. 1976;2(7979):219–21. 10.1016/S0140-6736(76)91024-2 [DOI] [PubMed] [Google Scholar]
- 216. Marks DJ, Harbord MW, MacAllister R, et al. : Defective acute inflammation in Crohn’s disease: a clinical investigation. Lancet. 2006;367(9511):668–78. 10.1016/S0140-6736(06)68265-2 [DOI] [PubMed] [Google Scholar]
- 217. De Bruin AF, Schouten SB, de Kort PP, et al. : The impact of chronic smoking on rectal mucosal blood flow. Tech Coloproctol. 2009;13(4):269–72. 10.1007/s10151-009-0529-8 [DOI] [PubMed] [Google Scholar]
- 218. Segal AW, Arnot RN, Thakur ML, et al. : Indium-111-labelled leucocytes for localisation of abscesses. Lancet. 1976;2(7994):1056–8. 10.1016/S0140-6736(76)90969-7 [DOI] [PubMed] [Google Scholar]
- 219. Smith AM, Rahman FZ, Hayee B, et al. : Disordered macrophage cytokine secretion underlies impaired acute inflammation and bacterial clearance in Crohn’s disease. J Exp Med. 2009;206(9):1883–97. 10.1084/jem.20091233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Williams GT, Williams WJ: Granulomatous inflammation--a review. J Clin Pathol. 1983;36(7):723–33. 10.1136/jcp.36.7.723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Zorzi F, Monteleone I, Sarra M, et al. : Distinct profiles of effector cytokines mark the different phases of Crohn’s disease. PLoS One. 2013;8(1):e54562. 10.1371/journal.pone.0054562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Parronchi P, Romagnani P, Annunziato F, et al. : Type 1 T-helper cell predominance and interleukin-12 expression in the gut of patients with Crohn’s disease. Am J Pathol. 1997;150(3):823–32. [PMC free article] [PubMed] [Google Scholar]
- 223. Liu Y, van Kruiningen HJ, West AB, et al. : Immunocytochemical evidence of Listeria, Escherichia coli, and Streptococcus antigens in Crohn’s disease. Gastroenterology. 1995;108(5):1396–404. 10.1016/0016-5085(95)90687-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Ryan P, Kelly RG, Lee G, et al. : Bacterial DNA within granulomas of patients with Crohn’s disease--detection by laser capture microdissection and PCR. Am J Gastroenterol. 2004;99(8):1539–43. 10.1111/j.1572-0241.2004.40103.x [DOI] [PubMed] [Google Scholar]
- 225. Mielke ME, Peters C, Hahn H: Cytokines in the induction and expression of T-cell-mediated granuloma formation and protection in the murine model of listeriosis. Immunol Rev. 1997;158(1):79–93. 10.1111/j.1600-065X.1997.tb00994.x [DOI] [PubMed] [Google Scholar]
- 226. Ozen S, Bilginer Y: A clinical guide to autoinflammatory diseases: familial Mediterranean fever and next-of-kin. Nat Rev Rheumatol. 2014;10(3):135–47. 10.1038/nrrheum.2013.174 [DOI] [PubMed] [Google Scholar]
- 227. Broderick L: Recurrent Fevers for the Pediatric Immunologist: It’s Not All Immunodeficiency. Curr Allergy Asthma Rep. 2016;16(1):2. 10.1007/s11882-015-0578-1 [DOI] [PubMed] [Google Scholar]
- 228. Elson CO, Alexander KL: Host-microbiota interactions in the intestine. Dig Dis. 2015;33(2):131–6. 10.1159/000369534 [DOI] [PubMed] [Google Scholar]
- 229. Hansen JJ: Immune Responses to Intestinal Microbes in Inflammatory Bowel Diseases. Curr Allergy Asthma Rep. 2015;15(10):61. 10.1007/s11882-015-0562-9 [DOI] [PubMed] [Google Scholar]
- 230. Neurath MF: New targets for mucosal healing and therapy in inflammatory bowel diseases. Mucosal Immunol. 2014;7(1):6–19. 10.1038/mi.2013.73 [DOI] [PubMed] [Google Scholar]
- 231. Rieder F, Fiocchi C: Mechanisms of tissue remodeling in inflammatory bowel disease. Dig Dis. 2013;31(2):186–93. 10.1159/000353364 [DOI] [PubMed] [Google Scholar]
- 232. Cardona PJ: A spotlight on liquefaction: evidence from clinical settings and experimental models in tuberculosis. Clin Dev Immunol. 2011;2011: 868246. 10.1155/2011/868246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Scharl M, Rogler G: Pathophysiology of fistula formation in Crohn’s disease. World J Gastrointest Pathophysiol. 2014;5(3):205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Mitchell DN, Dyer NC, Cannon P, et al. : The Kveim test in Crohn’s disease. Postgrad Med J. 1970;46(538):491–4. 10.1136/pgmj.46.538.491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Munro CS, Mitchell DN: The K veim response: still useful, still a puzzle. Thorax. 1987;42(5):321–31. 10.1136/thx.42.5.321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Teirstein AS: Kveim antigen: what does it tell us about causation of sarcoidosis? Semin Respir Infect. 1998;13(3):206–11. [PubMed] [Google Scholar]
- 237. Reich JM: On the nature of sarcoidosis. Eur J Intern Med. 2012;23(2):105–9. 10.1016/j.ejim.2011.09.011 [DOI] [PubMed] [Google Scholar]
- 238. Mitchell DN, Sutherland I, Bradstreet CM, et al. : Validation and standardization of Kveim test suspensions prepared from two human sarcoid spleens. J Clin Pathol. 1976;29(3):203–10. 10.1136/jcp.29.3.203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Chung J, Rosenbach M: Extensive cutaneous sarcoidosis and coexistant Crohn disease with dual response to infliximab: case report and review of the literature. Dermatol Online J. 2014;21(3): pii: 13030/qt6m04m5s3. [PubMed] [Google Scholar]
- 240. Katsanos KH, Torres J, Roda G, et al. : Review article: non-malignant oral manifestations in inflammatory bowel diseases. Aliment Pharmacol Ther. 2015;42(1):40–60. 10.1111/apt.13217 [DOI] [PubMed] [Google Scholar]
- 241. Bartels LE, Jepsen P, Christensen LA, et al. : Diagnosis of Helicobacter Pylori Infection is Associated with Lower Prevalence and Subsequent Incidence of Crohn’s Disease. J Crohns Colitis. 2016;10(4):443–8. 10.1093/ecco-jcc/jjv229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Nugent FW, Richmond M, Park SK: Crohn’s disease of the duodenum. Gut. 1977;18(2):115–20. 10.1136/gut.18.2.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Rahman FZ, Marks DJ, Hayee BH, et al. : Phagocyte dysfunction and inflammatory bowel disease. Inflamm Bowel Dis. 2008;14(10):1443–52. 10.1002/ibd.20449 [DOI] [PubMed] [Google Scholar]
- 244. Movahedi M, Aghamohammadi A, Rezaei N, et al. : Gastrointestinal manifestations of patients with chronic granulomatous disease. Iran J Allergy Asthma Immunol. 2004;3(2):83–7. [PubMed] [Google Scholar]
- 245. Winkelstein JA, Marino MC, Johnston RB, Jr, et al. : Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine (Baltimore). 2000;79(3):155–69. 10.1097/00005792-200005000-00003 [DOI] [PubMed] [Google Scholar]
- 246. Segal AW, Geisow M, Garcia R, et al. : The respiratory burst of phagocytic cells is associated with a rise in vacuolar pH. Nature. 1981;290(5805):406–9. 10.1038/290406a0 [DOI] [PubMed] [Google Scholar]
- 247. Levine AP, Duchen MR, de Villiers S, et al. : Alkalinity of neutrophil phagocytic vacuoles is modulated by HVCN1 and has consequences for myeloperoxidase activity. PLoS One. 2015;10(4):e0125906. 10.1371/journal.pone.0125906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Fournier BM, Parkos CA: The role of neutrophils during intestinal inflammation. Mucosal Immunol. 2012;5(4):354–66. 10.1038/mi.2012.24 [DOI] [PubMed] [Google Scholar]
- 249. Kolaczkowska E, Kubes P: Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13(3):159–75. 10.1038/nri3399 [DOI] [PubMed] [Google Scholar]
- 250. Elliott TR, Hudspith BN, Rayment NB, et al. : Defective macrophage handling of Escherichia coli in Crohn's disease. J Gastroenterol Hepatol. 2015;30(8):1265–74. 10.1111/jgh.12955 [DOI] [PubMed] [Google Scholar]
- 251. Campos N, Magro F, Castro AR, et al. : Macrophages from IBD patients exhibit defective tumour necrosis factor-α secretion but otherwise normal or augmented pro-inflammatory responses to infection. Immunobiology. 2011;216(8):961–70. 10.1016/j.imbio.2011.01.002 [DOI] [PubMed] [Google Scholar]
- 252. Sewell GW, Rahman FZ, Levine AP, et al. : Defective tumor necrosis factor release from Crohn's disease macrophages in response to Toll-like receptor activation: relationship to phenotype and genome-wide association susceptibility loci. Inflamm Bowel Dis. 2012;18(11):2120–7. 10.1002/ibd.22952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Vazeille E, Buisson A, Bringer MA, et al. : Monocyte-derived macrophages from Crohn's disease patients are impaired in the ability to control intracellular adherent-invasive Escherichia coli and exhibit disordered cytokine secretion profile. J Crohns Colitis. 2015;9(5):410–20. 10.1093/ecco-jcc/jjv053 [DOI] [PubMed] [Google Scholar]
- 254. Levine AP, Segal AW: What is wrong with granulocytes in inflammatory bowel diseases? Dig Dis. 2013;31(3–4):321–7. 10.1159/000354686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Wandall JH, Binder V: Leucocyte function in Crohn's disease. Studies on mobilisation using a quantitative skin window technique and on the function of circulating polymorphonuclear leucocytes in vitro. Gut. 1982;23(3):173–80. 10.1136/gut.23.3.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Somasundaram R, Nuij VJ, van der Woude CJ, et al. : Peripheral neutrophil functions and cell signalling in Crohn`s disease. PLoS One. 2013;8(12):e84521. 10.1371/journal.pone.0084521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Geremia A, Biancheri P, Allan P, et al. : Innate and adaptive immunity in inflammatory bowel disease. Autoimmun Rev. 2014;13(1):3–10. 10.1016/j.autrev.2013.06.004 [DOI] [PubMed] [Google Scholar]
- 258. Šedý J, Bekiaris V, Ware CF: Tumor necrosis factor superfamily in innate immunity and inflammation. Cold Spring Harb Perspect Biol. 2014;7(4):a016279. 10.1101/cshperspect.a016279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Holgersen K, Kutlu B, Fox B, et al. : High-resolution gene expression profiling using RNA sequencing in patients with inflammatory bowel disease and in mouse models of colitis. J Crohns Colitis. 2015;9(6):492–506. 10.1093/ecco-jcc/jjv050 [DOI] [PubMed] [Google Scholar]
- 260. O’Toole A, Lucci M, Korzenik J: Inflammatory Bowel Disease Provoked by Etanercept: Report of 443 Possible Cases Combined from an IBD Referral Center and the FDA. Dig Dis Sci. 2016;61(6):1772–4. 10.1007/s10620-015-4007-z [DOI] [PubMed] [Google Scholar]
- 261. Levin AD, Wildenberg ME, van den Brink GR: Mechanism of Action of Anti-TNF Therapy in Inflammatory Bowel Disease. J Crohns Colitis. 2016;10(8):989–97. 10.1093/ecco-jcc/jjw053 [DOI] [PubMed] [Google Scholar]
- 262. Ahmad T, Satsangi J, McGovern D, et al. : Review article: the genetics of inflammatory bowel disease. Aliment Pharmacol Ther. 2001;15(6):731–48. 10.1046/j.1365-2036.2001.00981.x [DOI] [PubMed] [Google Scholar]
- 263. Moller FT, Andersen V, Wohlfahrt J, et al. : Familial risk of inflammatory bowel disease: a population-based cohort study 1977-2011. Am J Gastroenterol. 2015;110(4):564–71. 10.1038/ajg.2015.50 [DOI] [PubMed] [Google Scholar]
- 264. Brant SR: Update on the heritability of inflammatory bowel disease: the importance of twin studies. Inflamm Bowel Dis. 2011;17(1):1–5. 10.1002/ibd.21385 [DOI] [PubMed] [Google Scholar]
- 265. Piitulainen E, Eriksson S: Decline in FEV1 related to smoking status in individuals with severe alpha1-antitrypsin deficiency (PiZZ). Eur Respir J. 1999;13(2):247–51. [DOI] [PubMed] [Google Scholar]
- 266. Dawn Teare M, Barrett JH: Genetic linkage studies. Lancet. 2005;366(9490):1036–44. 10.1016/S0140-6736(05)67382-5 [DOI] [PubMed] [Google Scholar]
- 267. Hugot JP, Chamaillard M, Zouali H, et al. : Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001;411(6837):599–603. 10.1038/35079107 [DOI] [PubMed] [Google Scholar]
- 268. Ogura Y, Bonen DK, Inohara N, et al. : A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature. 2001;411(6837):603–6. 10.1038/35079114 [DOI] [PubMed] [Google Scholar]
- 269. Risch N, Merikangas K: The future of genetic studies of complex human diseases. Science. 1996;273(5281):1516–7. 10.1126/science.273.5281.1516 [DOI] [PubMed] [Google Scholar]
- 270. Pe’er I, Yelensky R, Altshuler D, et al. : Estimation of the multiple testing burden for genomewide association studies of nearly all common variants. Genet Epidemiol. 2008;32(4):381–5. 10.1002/gepi.20303 [DOI] [PubMed] [Google Scholar]
- 271. Spencer CC, Su Z, Donnelly P, et al. : Designing genome-wide association studies: sample size, power, imputation, and the choice of genotyping chip. PLoS Genet. 2009;5(5):e1000477. 10.1371/journal.pgen.1000477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Hirschhorn JN, Daly MJ: Genome-wide association studies for common diseases and complex traits. Nat Rev Genet. 2005;6(2):95–108. 10.1038/nrg1521 [DOI] [PubMed] [Google Scholar]
- 273. Jostins L, Ripke S, Weersma RK, et al. : Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491(7422):119–24. 10.1038/nature11582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Ellinghaus D, Jostins L, Spain SL, et al. : Analysis of five chronic inflammatory diseases identifies 27 new associations and highlights disease-specific patterns at shared loci. Nat Genet. 2016;48(5):510–8. 10.1038/ng.3528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. de Lange KM, Moutsianas L, Lee JC, et al. : Genome-wide association study implicates immune activation of multiple integrin genes in inflammatory bowel disease. BioRxiv. 10.1101/058255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Liu JZ, van Sommeren S, Huang H, et al. : Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet. 2015;47(9):979–86. 10.1038/ng.3359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Molodecky NA, Soon IS, Rabi DM, et al. : Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142(1):46–54.e42; quiz e30. 10.1053/j.gastro.2011.10.001 [DOI] [PubMed] [Google Scholar]
- 278. Brown MA, Pile KD, Kennedy LG, et al. : HLA class I associations of ankylosing spondylitis in the white population in the United Kingdom. Ann Rheum Dis. 1996;55(4):268–70. 10.1136/ard.55.4.268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Chinn S: A simple method for converting an odds ratio to effect size for use in meta-analysis. Stat Med. 2000;19(22):3127–31. [DOI] [PubMed] [Google Scholar]
- 280. Smigoc Schweiger D, Mendez A, Kunilo Jamnik S, et al. : High-risk genotypes HLA-DR3-DQ2/DR3-DQ2 and DR3-DQ2/DR4-DQ8 in co-occurrence of type 1 diabetes and celiac disease. Autoimmunity. 2016;49(4):240–7. 10.3109/08916934.2016.1164144 [DOI] [PubMed] [Google Scholar]
- 281. Schreiber TH, Podack ER: Immunobiology of TNFSF15 and TNFRSF25. Immunol Res. 2013;57(1–3):3–11. 10.1007/s12026-013-8465-0 [DOI] [PubMed] [Google Scholar]
- 282. Zhernakova A, van Diemen CC, Wijmenga C: Detecting shared pathogenesis from the shared genetics of immune-related diseases. Nat Rev Genet. 2009;10(1):43–55. 10.1038/nrg2489 [DOI] [PubMed] [Google Scholar]
- 283. Christophers E: Comorbidities in psoriasis. Clin Dermatol. 2007;25(6):529–34. 10.1016/j.clindermatol.2007.08.006 [DOI] [PubMed] [Google Scholar]
- 284. Najarian DJ, Gottlieb AB: Connections between psoriasis and Crohn’s disease. J Am Acad Dermatol. 2003;48(6):805–21; quiz 822–4. 10.1067/mjd.2003.540 [DOI] [PubMed] [Google Scholar]
- 285. Hsu LN, Armstrong AW: Psoriasis and autoimmune disorders: a review of the literature. J Am Acad Dermatol. 2012;67(5):1076–9. 10.1016/j.jaad.2012.01.029 [DOI] [PubMed] [Google Scholar]
- 286. Lubrano E, Ciacci C, Ames PR, et al. : The arthritis of coeliac disease: prevalence and pattern in 200 adult patients. Br J Rheumatol. 1996;35(12):1314–8. 10.1093/rheumatology/35.12.1314 [DOI] [PubMed] [Google Scholar]
- 287. Selmi C: Diagnosis and classification of autoimmune uveitis. Autoimmun Rev. 2014;13(4–5):591–4. 10.1016/j.autrev.2014.01.006 [DOI] [PubMed] [Google Scholar]
- 288. Rosenbaum JT: Uveitis in spondyloarthritis including psoriatic arthritis, ankylosing spondylitis, and inflammatory bowel disease. Clin Rheumatol. 2015;34(6):999–1002. 10.1007/s10067-015-2960-8 [DOI] [PubMed] [Google Scholar]
- 289. Mielants H, Veys EM, Cuvelier C, et al. : Ileocolonoscopic findings in seronegative spondylarthropathies. Br J Rheumatol. 1988;27(Suppl 2):95–105. 10.1093/rheumatology/XXVII.suppl_2.95 [DOI] [PubMed] [Google Scholar]
- 290. Segal AW, Isenberg DA, Hajirousou V, et al. : Preliminary evidence for gut involvement in the pathogenesis of rheumatoid arthritis? Br J Rheumatol. 1986;25(2):162–6. 10.1093/rheumatology/25.2.162 [DOI] [PubMed] [Google Scholar]
- 291. Nadorra RL, Nakazato Y, Landing BH: Pathologic features of gastrointestinal tract lesions in childhood-onset systemic lupus erythematosus: study of 26 patients, with review of the literature. Pediatr Pathol. 1987;7(3):245–59. 10.1080/15513818709177128 [DOI] [PubMed] [Google Scholar]
- 292. Generali E, Ceribelli A, Massarotti M, et al. : Seronegative reactive spondyloarthritis and the skin. Clin Dermatol. 2015;33(5):531–7. 10.1016/j.clindermatol.2015.05.004 [DOI] [PubMed] [Google Scholar]
- 293. Sekhwal MK, Li P, Lam I, et al. : Disease Resistance Gene Analogs (RGAs) in Plants. Int J Mol Sci. 2015;16(8):19248–90. 10.3390/ijms160819248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Gay NJ, Symmons MF, Gangloff M, et al. : Assembly and localization of Toll-like receptor signalling complexes. Nat Rev Immunol. 2014;14(8):546–58. 10.1038/nri3713 [DOI] [PubMed] [Google Scholar]
- 295. Nakamura N, Lill JR, Phung Q, et al. : Endosomes are specialized platforms for bacterial sensing and NOD2 signalling. Nature. 2014;509(7499):240–4. 10.1038/nature13133 [DOI] [PubMed] [Google Scholar]
- 296. Keestra-Gounder AM, Byndloss MX, Seyffert N, et al. : NOD1 and NOD2 signalling links ER stress with inflammation. Nature. 2016;532(7599):394–7. 10.1038/nature17631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Caruso R, Warner N, Inohara N, et al. : NOD1 and NOD2: signaling, host defense, and inflammatory disease. Immunity. 2014;41(6):898–908. 10.1016/j.immuni.2014.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Maekawa S, Ohto U, Shibata T, et al. : Crystal structure of NOD2 and its implications in human disease. Nat Commun. 2016;7: 11813. 10.1038/ncomms11813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Ogura Y, Inohara N, Benito A, et al. : Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. J Biol Chem. 2001;276(7):4812–8. 10.1074/jbc.M008072200 [DOI] [PubMed] [Google Scholar]
- 300. Ogura Y, Lala S, Xin W, et al. : Expression of NOD2 in Paneth cells: a possible link to Crohn’s ileitis. Gut. 2003;52(11):1591–7. 10.1136/gut.52.11.1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Shi C, Pamer EG: Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11(11):762–74. 10.1038/nri3070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Serbina NV, Jia T, Hohl TM, et al. : Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol. 2008;26:421–52. 10.1146/annurev.immunol.26.021607.090326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Gerhardt T, Ley K: Monocyte trafficking across the vessel wall. Cardiovasc Res. 2015;107(3):321–30. 10.1093/cvr/cvv147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Yona S, Kim KW, Wolf Y, et al. : Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38(1):79–91. 10.1016/j.immuni.2012.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Varol C, Yona S, Jung S: Origins and tissue-context-dependent fates of blood monocytes. Immunol Cell Biol. 2009;87(1):30–8. 10.1038/icb.2008.90 [DOI] [PubMed] [Google Scholar]
- 306. Auffray C, Fogg D, Garfa M, et al. : Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317(5838):666–70. 10.1126/science.1142883 [DOI] [PubMed] [Google Scholar]
- 307. Clark SL, Jr: Cellular differentiation in the kidneys of newborn mice studies with the electron microscope. J Biophys Biochem Cytol. 1957;3(3):349–62. 10.1083/jcb.3.3.349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. De Duve C, Wattiaux R: Functions of lysosomes. Annu Rev Physiol. 1966;28:435–92. 10.1146/annurev.ph.28.030166.002251 [DOI] [PubMed] [Google Scholar]
- 309. Mizushima N, Noda T, Yoshimori T, et al. : A protein conjugation system essential for autophagy. Nature. 1998;395(6700):395–8. 10.1038/26506 [DOI] [PubMed] [Google Scholar]
- 310. Narayanan LA, Edelmann MJ: Ubiquitination as an efficient molecular strategy employed in salmonella infection. Front Immunol. 2014;5:558. 10.3389/fimmu.2014.00558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Tooze SA, Yoshimori T: The origin of the autophagosomal membrane. Nat Cell Biol. 2010;12(9):831–5. 10.1038/ncb0910-831 [DOI] [PubMed] [Google Scholar]
- 312. Kawabata T, Yoshimori T: Beyond starvation: An update on the autophagic machinery and its functions. J Mol Cell Cardiol. 2016;95:2–10. 10.1016/j.yjmcc.2015.12.005 [DOI] [PubMed] [Google Scholar]
- 313. Ohsumi Y: Historical landmarks of autophagy research. Cell Res. 2014;24(1):9–23. 10.1038/cr.2013.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Wilson MI, Dooley HC, Tooze SA: WIPI2b and Atg16L1: setting the stage for autophagosome formation. Biochem Soc Trans. 2014;42(5):1327–34. 10.1042/BST20140177 [DOI] [PubMed] [Google Scholar]
- 315. Dooley HC, Razi M, Polson HE, et al. : WIPI2 links LC3 conjugation with PI3P, autophagosome formation, and pathogen clearance by recruiting Atg12-5-16L1. Mol Cell. 2014;55(2):238–52. 10.1016/j.molcel.2014.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316. Sorbara MT, Girardin SE: Emerging themes in bacterial autophagy. Curr Opin Microbiol. 2015;23:163–70. 10.1016/j.mib.2014.11.020 [DOI] [PubMed] [Google Scholar]
- 317. Kimmey JM, Huynh JP, Weiss LA, et al. : Unique role for ATG5 in neutrophil-mediated immunopathology during M. tuberculosis infection. Nature. 2015;528(7583):565–9. 10.1038/nature16451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Huang J, Brumell JH: Bacteria-autophagy interplay: a battle for survival. Nat Rev Microbiol. 2014;12(2):101–14. 10.1038/nrmicro3160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. Schwerd T, Pandey S, Yang HT, et al. : Impaired antibacterial autophagy links granulomatous intestinal inflammation in Niemann-Pick disease type C1 and XIAP deficiency with NOD2 variants in Crohn’s disease. Gut. 2016; pii: gutjnl-2015-310382. 10.1136/gutjnl-2015-310382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. Verlhac P, Viret C, Faure M: Dual function of CALCOCO2/NDP52 during xenophagy. Autophagy. 2015;11(6):965–6. 10.1080/15548627.2015.1046672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Gardet A, Benita Y, Li C, et al. : LRRK2 is involved in the IFN-gamma response and host response to pathogens. J Immunol. 2010;185(9):5577–85. 10.4049/jimmunol.1000548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Ying H, Yue BY: Cellular and molecular biology of optineurin. Int Rev Cell Mol Biol. 2012;294:223–58. 10.1016/B978-0-12-394305-7.00005-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Tumbarello DA, Kendrick-Jones J, Buss F: Myosin VI and its cargo adaptors - linking endocytosis and autophagy. J Cell Sci. 2013;126(Pt 12):2561–70. 10.1242/jcs.095554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Hampe J, Franke A, Rosenstiel P, et al. : A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat Genet. 2007;39(2):207–11. 10.1038/ng1954 [DOI] [PubMed] [Google Scholar]
- 325. Fujita N, Morita E, Itoh T, et al. : Recruitment of the autophagic machinery to endosomes during infection is mediated by ubiquitin. J Cell Biol. 2013;203(1):115–28. 10.1083/jcb.201304188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326. Conway KL, Kuballa P, Song JH, et al. : Atg16l1 is required for autophagy in intestinal epithelial cells and protection of mice from Salmonella infection. Gastroenterology. 2013;145(6):1347–57. 10.1053/j.gastro.2013.08.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327. Sadaghian Sadabad M, Regeling A, de Goffau MC, et al. : The ATG16L1-T300A allele impairs clearance of pathosymbionts in the inflamed ileal mucosa of Crohn’s disease patients. Gut. 2015;64(10):1546–52. 10.1136/gutjnl-2014-307289 [DOI] [PubMed] [Google Scholar]
- 328. Kuballa P, Huett A, Rioux JD, et al. : Impaired autophagy of an intracellular pathogen induced by a Crohn’s disease associated ATG16L1 variant. PLoS One. 2008;3(10):e3391. 10.1371/journal.pone.0003391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Cadwell K, Liu JY, Brown SL, et al. : A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature. 2008;456(7219):259–63. 10.1038/nature07416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330. Murthy A, Li Y, Peng I, et al. : A Crohn’s disease variant in Atg16l1 enhances its degradation by caspase 3. Nature. 2014;506(7489):456–62. 10.1038/nature13044 [DOI] [PubMed] [Google Scholar]
- 331. Singh SB, Davis AS, Taylor GA, et al. : Human IRGM induces autophagy to eliminate intracellular mycobacteria. Science. 2006;313(5792):1438–41. 10.1126/science.1129577 [DOI] [PubMed] [Google Scholar]
- 332. Matsuda N: Phospho-ubiquitin: upending the PINK-Parkin-ubiquitin cascade. J Biochem. 2016;159(4):379–85. 10.1093/jb/mvv125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. El Chemaly A, Demaurex N: Do Hv1 proton channels regulate the ionic and redox homeostasis of phagosomes? Mol Cell Endocrinol. 2012;353(1–2):82–7. 10.1016/j.mce.2011.10.005 [DOI] [PubMed] [Google Scholar]
- 334. Marshansky V, Futai M: The V-type H +-ATPase in vesicular trafficking: targeting, regulation and function. Curr Opin Cell Biol. 2008;20(4):415–26. 10.1016/j.ceb.2008.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335. Bekpen C, Hunn JP, Rohde C, et al. : The interferon-inducible p47 (IRG) GTPases in vertebrates: loss of the cell autonomous resistance mechanism in the human lineage. Genome Biol. 2005;6(11):R92. 10.1186/gb-2005-6-11-r92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. Wu C, Jin X, Tsueng G, et al. : BioGPS: building your own mash-up of gene annotations and expression profiles. Nucleic Acids Res. 2016;44(D1):D313–6. 10.1093/nar/gkv1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337. Li Q, Singh CR, Ma S, et al. : Label-free proteomics and systems biology analysis of mycobacterial phagosomes in dendritic cells and macrophages. J Proteome Res. 2011;10(5):2425–39. 10.1021/pr101245u [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338. Soldati T, Neyrolles O: Mycobacteria and the Intraphagosomal Environment: Take it with a pinch of salt(s)! Traffic. 2012;13(8):1042–52. 10.1111/j.1600-0854.2012.01358.x [DOI] [PubMed] [Google Scholar]
- 339. Kratochvílová K, Moráň L, Paďourová S, et al. : The role of the endoplasmic reticulum stress in stemness, pluripotency and development. Eur J Cell Biol. 2016;95(3–5):115–23. 10.1016/j.ejcb.2016.02.002 [DOI] [PubMed] [Google Scholar]
- 340. Shaffer AL, Wright G, Yang L, et al. : A library of gene expression signatures to illuminate normal and pathological lymphoid biology. Immunol Rev. 2006;210(1):67–85. 10.1111/j.0105-2896.2006.00373.x [DOI] [PubMed] [Google Scholar]
- 341. Kleizen B, Braakman I: Protein folding and quality control in the endoplasmic reticulum. Curr Opin Cell Biol. 2004;16(4):343–9. 10.1016/j.ceb.2004.06.012 [DOI] [PubMed] [Google Scholar]
- 342. Ogata M, Hino S, Saito A, et al. : Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol. 2006;26(24):9220–31. 10.1128/MCB.01453-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343. Ozcan L, Tabas I: Role of endoplasmic reticulum stress in metabolic disease and other disorders. Annu Rev Med. 2012;63:317–28. 10.1146/annurev-med-043010-144749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344. Díaz-Villanueva JF, Díaz-Molina R, García-González V: Protein Folding and Mechanisms of Proteostasis. Int J Mol Sci. 2015;16(8):17193–230. 10.3390/ijms160817193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345. Adolph TE, Tomczak MF, Niederreiter L, et al. : Paneth cells as a site of origin for intestinal inflammation. Nature. 2013;503(7475):272–6. 10.1038/nature12599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346. Hosomi S, Kaser A, Blumberg RS: Role of endoplasmic reticulum stress and autophagy as interlinking pathways in the pathogenesis of inflammatory bowel disease. Curr Opin Gastroenterol. 2015;31(1):81–8. 10.1097/MOG.0000000000000144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347. Schapansky J, Nardozzi JD, LaVoie MJ: The complex relationships between microglia, alpha-synuclein, and LRRK2 in Parkinson’s disease. Neuroscience. 2015;302:74–88. 10.1016/j.neuroscience.2014.09.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348. Waschbüsch D, Michels H, Strassheim S, et al. : LRRK2 transport is regulated by its novel interacting partner Rab32. PLoS One. 2014;9(10):e111632. 10.1371/journal.pone.0111632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349. Spanò S: Host restriction in Salmonella: insights from Rab GTPases. Cell Microbiol. 2014;16(9):1321–8. 10.1111/cmi.12327 [DOI] [PubMed] [Google Scholar]
- 350. Hoffmann C, Finsel I, Otto A, et al. : Functional analysis of novel Rab GTPases identified in the proteome of purified Legionella-containing vacuoles from macrophages. Cell Microbiol. 2014;16(7):1034–52. 10.1111/cmi.12256 [DOI] [PubMed] [Google Scholar]
- 351. Gerondopoulos A, Langemeyer L, Liang JR, et al. : BLOC-3 mutated in Hermansky-Pudlak syndrome is a Rab32/38 guanine nucleotide exchange factor. Curr Biol. 2012;22(22):2135–9. 10.1016/j.cub.2012.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352. Lin JC, Lin CS, Hsu CW, et al. : Association Between Parkinson’s Disease and Inflammatory Bowel Disease: a Nationwide Taiwanese Retrospective Cohort Study. Inflamm Bowel Dis. 2016;22(5):1049–55. 10.1097/MIB.0000000000000735 [DOI] [PubMed] [Google Scholar]
- 353. Nalls MA, Saad M, Noyce AJ, et al. : Genetic comorbidities in Parkinson’s disease. Hum Mol Genet. 2014;23(3):831–41. 10.1093/hmg/ddt465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354. Kouklakis G, Efremidou EI, Papageorgiou MS, et al. : Complicated Crohn’s-like colitis, associated with Hermansky-Pudlak syndrome, treated with Infliximab: a case report and brief review of the literature. J Med Case Rep. 2007;1:176. 10.1186/1752-1947-1-176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355. Smith AM, Sewell GW, Levine AP, et al. : Disruption of macrophage pro-inflammatory cytokine release in Crohn’s disease is associated with reduced optineurin expression in a subset of patients. Immunology. 2015;144(1):45–55. 10.1111/imm.12338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356. Ying H, Yue BY: Optineurin: The autophagy connection. Exp Eye Res. 2016;144:73–80. 10.1016/j.exer.2015.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357. Chew TS, O’Shea NR, Sewell GW, et al. : Optineurin deficiency in mice contributes to impaired cytokine secretion and neutrophil recruitment in bacteria-driven colitis. Dis Model Mech. 2015;8(8):817–29. 10.1242/dmm.020362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358. Slowicka K, Vereecke L, Mc Guire C, et al. : Optineurin deficiency in mice is associated with increased sensitivity to Salmonella but does not affect proinflammatory NF-κB signaling. Eur J Immunol. 2016;46(4):971–80. 10.1002/eji.201545863 [DOI] [PubMed] [Google Scholar]
- 359. O’Shea NR, Chew TS, Dunne J, et al. : Critical Role of the Disintegrin Metalloprotease ADAM-like Decysin-1 [ADAMDEC1] for Intestinal Immunity and Inflammation. J Crohns Colitis. 2016; pii: jjw111. 10.1093/ecco-jcc/jjw111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360. Sawyer SL, Hartley T, Dyment DA, et al. : Utility of whole-exome sequencing for those near the end of the diagnostic odyssey: time to address gaps in care. Clin Genet. 2016;89(3):275–84. 10.1111/cge.12654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361. Stranneheim H, Wedell A: Exome and genome sequencing: a revolution for the discovery and diagnosis of monogenic disorders. J Intern Med. 2016;279(1):3–15. 10.1111/joim.12399 [DOI] [PubMed] [Google Scholar]
- 362. Prescott NJ, Lehne B, Stone K, et al. : Pooled sequencing of 531 genes in inflammatory bowel disease identifies an associated rare variant in BTNL2 and implicates other immune related genes. PLoS Genet. 2015;11(2):e1004955. 10.1371/journal.pgen.1004955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363. Cardinale CJ, Kelsen JR, Baldassano RN, et al. : Impact of exome sequencing in inflammatory bowel disease. World J Gastroenterol. 2013;19(40):6721–9. 10.3748/wjg.v19.i40.6721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364. Rivas MA, Beaudoin M, Gardet A, et al. : Deep resequencing of GWAS loci identifies independent rare variants associated with inflammatory bowel disease. Nat Genet. 2011;43(11):1066–73. 10.1038/ng.952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365. Momozawa Y, Mni M, Nakamura K, et al. : Resequencing of positional candidates identifies low frequency IL23R coding variants protecting against inflammatory bowel disease. Nat Genet. 2011;43(1):43–7. 10.1038/ng.733 [DOI] [PubMed] [Google Scholar]
- 366. MacArthur DG, Balasubramanian S, Frankish A, et al. : A systematic survey of loss-of-function variants in human protein-coding genes. Science. 2012;335(6070):823–8. 10.1126/science.1215040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367. Luo Y, de Lange KM, Jostins L, et al. : Exploring the genetic architecture of inflammatory bowel disease by whole genome sequencing identifies association at ADCY7. Cold Spring Harbor Labs Journals. 2016. 10.1101/058347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368. Carmi S, Hui KY, Kochav E, et al. : Sequencing an Ashkenazi reference panel supports population-targeted personal genomics and illuminates Jewish and European origins. Nat Commun. 2014;5: 4835. 10.1038/ncomms5835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369. Chuang LS, Villaverde N, Hui KY, et al. : A Frameshift in CSF2RB Predominant Among Ashkenazi Jews Increases Risk for Crohn’s Disease and Reduces Monocyte Signaling via GM-CSF. Gastroenterology. 2016;151(4):710–723.e2. 10.1053/j.gastro.2016.06.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370. Levine AP, Pontikos N, Schiff ER, et al. : Genetic Complexity of Crohn’s Disease in Two Large Ashkenazi Jewish Families. Gastroenterology. 2016;151(4):698–709. 10.1053/j.gastro.2016.06.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371. Gewurz BE, Towfic F, Mar JC, et al. : Genome-wide siRNA screen for mediators of NF-κB activation. Proc Natl Acad Sci U S A. 2012;109(7):2467–72. 10.1073/pnas.1120542109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372. Chakraborty S, Lambie EJ, Bindu S, et al. : Engulfment pathways promote programmed cell death by enhancing the unequal segregation of apoptotic potential. Nat Commun. 2015;6: 10126. 10.1038/ncomms10126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373. Denson LA, Klein C: Granulocyte-Macrophage Colony Stimulating Factor Bioactivity and Mucosal Homeostasis in Crohn’s Disease: A Role for Genetic Variation. Gastroenterology. 2016;151(4):593–6. 10.1053/j.gastro.2016.08.042 [DOI] [PubMed] [Google Scholar]
- 374. Broughton SE, Dhagat U, Hercus TR, et al. : The GM-CSF/IL-3/IL-5 cytokine receptor family: from ligand recognition to initiation of signaling. Immunol Rev. 2012;250(1):277–302. 10.1111/j.1600-065X.2012.01164.x [DOI] [PubMed] [Google Scholar]
- 375. Wicks IP, Roberts AW: Targeting GM-CSF in inflammatory diseases. Nat Rev Rheumatol. 2016;12(1):37–48. 10.1038/nrrheum.2015.161 [DOI] [PubMed] [Google Scholar]
- 376. Metcalf D: Hematopoietic cytokines. Blood. 2008;111(2):485–91. 10.1182/blood-2007-03-079681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377. Sanderson CJ: Eosinophil differentiation factor (interleukin-5). Immunol Ser. 1990;49:231–56. [PubMed] [Google Scholar]
- 378. Bedard K, Krause KH: The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87(1):245–313. 10.1152/physrev.00044.2005 [DOI] [PubMed] [Google Scholar]
- 379. Sommer F, Bäckhed F: The gut microbiota engages different signaling pathways to induce Duox2 expression in the ileum and colon epithelium. Mucosal Immunol. 2015;8(2):372–9. 10.1038/mi.2014.74 [DOI] [PubMed] [Google Scholar]
- 380. Chang S, Linderholm A, Franzi L, et al. : Dual oxidase regulates neutrophil recruitment in allergic airways. Free Radic Biol Med. 2013;65:38–46. 10.1016/j.freeradbiomed.2013.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381. Grasberger H, El-Zaatari M, Dang DT, et al. : Dual oxidases control release of hydrogen peroxide by the gastric epithelium to prevent Helicobacter felis infection and inflammation in mice. Gastroenterology. 2013;145(5):1045–54. 10.1053/j.gastro.2013.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382. Lipinski S, Till A, Sina C, et al. : DUOX2-derived reactive oxygen species are effectors of NOD2-mediated antibacterial responses. J Cell Sci. 2009;122(Pt 19):3522–30. 10.1242/jcs.050690 [DOI] [PubMed] [Google Scholar]
- 383. O’Sullivan M, O’Morain C: Liquid diets for Crohn’s disease. Gut. 2001;48(6):757. 10.1136/gut.48.6.757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384. Sajid MS, Iqbal Z, Muhammad G, et al. : Immunomodulatory effect of various anti-parasitics: a review. Parasitology. 2006;132(Pt 3):301–13. 10.1017/S0031182005009108 [DOI] [PubMed] [Google Scholar]
- 385. Segal AW, Levi AJ, Loewi G: Levamisole in the treatment of Crohn’s disease. Lancet. 1977;2(8034):382–5. 10.1016/S0140-6736(77)90307-5 [DOI] [PubMed] [Google Scholar]
- 386. Sachar DB, Rubin KP, Gumaste V: Levamisole in Crohn’s disease: a randomized, double-blind, placebo-controlled clinical trial. Am J Gastroenterol. 1987;82(6):536–9. [PubMed] [Google Scholar]
- 387. Segal AW, Pugh SF, Levi AJ, et al. : Levamisole-induced arthritis in Crohn’s disease. Br Med J. 1977;2(6086):555. 10.1136/bmj.2.6086.555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388. Benfield GF, Felix-Davies DD, Thompson RA, et al. : Severe acute polyarthropathy associated with levamisole therapy in a patient with Crohn’s disease. Eur J Rheumatol Inflamm. 1984;7(2):63–5. [PubMed] [Google Scholar]
- 389. Ding NS, Hart A, De Cruz P: Systematic review: predicting and optimising response to anti-TNF therapy in Crohn’s disease - algorithm for practical management. Aliment Pharmacol Ther. 2016;43(1):30–51. 10.1111/apt.13445 [DOI] [PubMed] [Google Scholar]
- 390. Wright AV, Nuñez JK, Doudna JA: Biology and Applications of CRISPR Systems: Harnessing Nature’s Toolbox for Genome Engineering. Cell. 2016;164(1–2):29–44. 10.1016/j.cell.2015.12.035 [DOI] [PubMed] [Google Scholar]
- 391. Shui B, Hernandez Matias L, Guo Y, et al. : The Rise of CRISPR/Cas for Genome Editing in Stem Cells. Stem Cells Int. 2016;2016: 8140168. 10.1155/2016/8140168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392. Ghosh S, Thrasher AJ, Gaspar HB: Gene therapy for monogenic disorders of the bone marrow. Br J Haematol. 2015;171(2):155–170. 10.1111/bjh.13520 [DOI] [PubMed] [Google Scholar]