Abstract
Virtually every neuron within the suprachiasmatic nucleus (SCN) communicates via GABAergic signaling. The extracellular levels of GABA within the SCN are determined by a complex interaction of synthesis and transport, as well as synaptic and non-synaptic release. The response to GABA is mediated by GABAA receptors that respond to both phasic and tonic GABA release and that can produce excitatory as well as inhibitory cellular responses. GABA also influences circadian control through the exclusively inhibitory effects of GABAB receptors. Both GABA and neuropeptide signaling occur within the SCN, although the functional consequences of the interactions of these signals are not well understood. This review considers the role of GABA in the circadian pacemaker, in the mechanisms responsible for the generation of circadian rhythms, in the ability of non-photic stimuli to reset the phase of the pacemaker, and in the ability of the day-night cycle to entrain the pacemaker.
Keywords: GABAA receptors, GABAB receptors, Glutamic acid decarboxylase, GABA vesicular transporters, Membrane GABA transporters, Cation chloride cotransporters, Benzodiazepines, Neurosteroids, Ethanol, Entrainment
1. Suprachiasmatic nucleus (SCN): functional, anatomical, and biochemical organization
1.1. The SCN: a primary circadian pacemaker
The SCN has been identified as the location of a circadian pacemaker that generates endogenous rhythmicity and mediates the entrainment of that rhythmicity with the day-night cycle in mammals, including humans (Moore and Eichler, 1972; Stephan and Zucker, 1972; Cohen and Albers, 1991). Remarkable progress has been made over the last few decades in defining the molecular mechanisms that generate circadian rhythmicity (for recent reviews see Hastings et al., 2014; Zhang and Kay, 2010). In brief, mammalian “clock cells” contain transcriptional and translational feedback loops, with the primary loop consisting of two proteins that activate transcription and two proteins that repress transcription. CLOCK and BMAL1 are the proteins that activate transcription and PERIOD (PER1, PER2, PER3) and CRYPTOCHROME (CRY1, CRY2) are the proteins that inhibit transcription. In a simplified model, circadian transcription begins with CLOCK and BMAL1 activating transcription of Per and Cry genes resulting in the translation of PER and CRY proteins over the day. Late in the day these proteins form heterodimers, translocate to the cell nucleus and inhibit their own transcription by repressing CLOCK-BMAL1 activity. As the levels of PER and CRY decline, CLOCK and BMAL1 are disinhibited resulting in reactivation of Per and Cry transcription and initiation of a new cycle. This molecular feedback loop operates in individual SCN neurons that coordinate or couple with other SCN clock cells to form a self-sustained circadian pacemaker. Interestingly, many of these same clock genes and proteins can be found in cellular oscillators throughout the body.
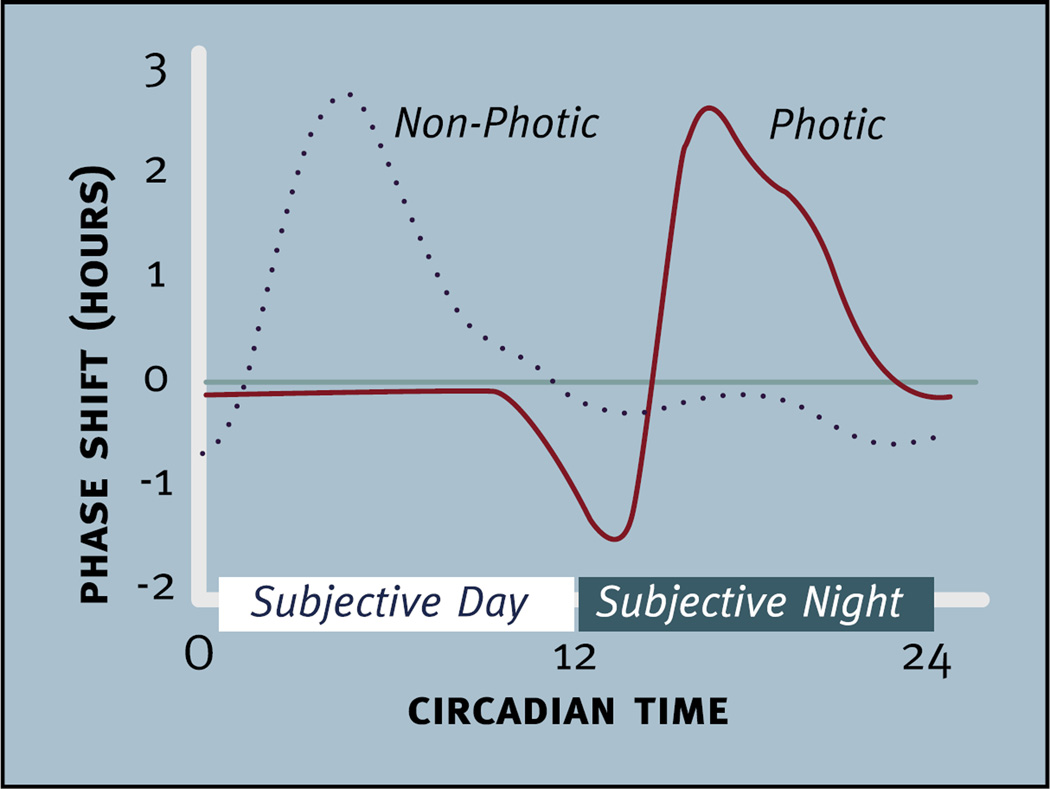
The circadian timing system evolved to enable organisms to synchronize their physiology and behavior with 24 h rhythms in the environment. Because circadian pacemakers exhibit non-24 h rhythms, these clocks must to be reset to 24 h each day (i.e., entrained with the day-night cycle). The ability of light to reset or phase shift the circadian pacemaker is illustrated by the effects of light on circadian phase when the pacemaker is free-running in an environment without time cues (e.g., constant darkness) (Daan and Pittendrigh, 1976b). In nocturnally active rodents, for example, a brief pulse of light delivered in constant darkness after the beginning of locomotor activity (i.e., early in the subjective night) delays the onset of activity on subsequent days. When delivered toward the end of the subjective night, the light advances the daily rhythm. During the subjective day (i.e., the inactive phase of nocturnal animals in constant conditions), pulses of light do not phase shift the pacemaker. The effects of light on circadian phase are summarized in a phase response curve (Fig. 1).
Fig. 1.
Comparison of the phase shifting effects of “photic” (solid red line) and “non-photic” stimuli (dotted black line) presented to nocturnally active rodents housed in constant darkness. Light does not produce phase shifts until the late subjective day and early subjective night when it produces phase delays. Later in the subjective night light produces phase advances. Non-photic stimuli, such as injection of neuropeptide Y into the suprachiasmatic region, induce large phase advances during the subjective day and smaller phase delays in the subjective night. Note: not all “non-photic” phase shifting stimuli produce a pattern of phase shifts like those seen in this figure. In nocturnal rodents the subjective day refers to the inactive phase and the subjective night refers to the active phase of the circadian cycle. Circadian time 12 is designated as the time of locomotor onset (modified from Webb et al., 2014).
Stimuli other than light can also phase shift the circadian pacemaker (see Section 9.1). Most of these stimuli produce a pattern of phase shifts that differ dramatically from those produced by light pulses (Fig. 1). The phase response curve for these stimuli was initially termed a dark-type or neuropeptide Y (NPY)-type phase response curve because these patterns of phase shifts were first observed following brief pulses of darkness or the injection of NPY directly into the SCN (for reviews see Moore and Card, 1990; Morin, 1991). More recently, however, this type of phase response curve has been used to summarize the phase shifting effects of “non-photic” stimuli (for a review see Webb et al., 2014). Although we will use the term “non-photic” phase shifting here, it is important to point out that there are non-photic stimuli that can produce phase shifts in a pattern that differs considerably from the dark- or NPY-type phase response curve seen in Fig. 1. Although the role of “non-photic” phase shifting stimuli in determining entrainment in the natural environment is not well understood, understanding how these stimuli phase shift the clock could be useful for chronotherapy.
There are several major pathways that project to the SCN that allow it to synchronize with the 24 h environment including: the retinohypothalamic tract (RHT), a direct projection from the retina (Hendrickson et al., 1972; Pickard, 1982, 1985; Moore and Lenn, 1972), the geniculohypothalamic tract (GHT), a direct projection from the intergeniculate leaflet (IGL) (Ribak and Peters, 1975; Swanson et al., 1974; Card and Moore, 1982; Harrington et al., 1987; Moore et al., 1984), and a direct serotonergic (5-HT) projection from the median raphe (Meyer-Bernstein and Morin, 1996; Morin, 1999). In addition to these major projections, an estimated 85 distinct brain regions send less prominent projections to the SCN (Morin, 2013).
The RHT appears to be the most important pathway for communicating photic information to the SCN because its destruction eliminates the ability of an animal to entrain to the light-dark (LD) cycle (Johnson et al., 1988a). There is considerable evidence that glutamate serves as the primary neurotransmitter in the RHT thereby communicating environmental lighting information to the SCN (Shibata et al., 1986; Cahill and Menaker, 1989; Colwell and Menaker, 1992; Colwell et al., 1991; Ding et al., 1994; Mintz et al., 1999; Mintz and Albers, 1997; Novak and Albers, 2002; Gamble et al., 2003). Nevertheless, other neurochemical signals such as pituitary adenylate cyclase-activating peptide (Hannibal et al., 1997) may also contribute to the entrainment process (for reviews see Colwell and Menaker, 1995; Ebling, 1996; Golombek and Rosenstein, 2010). The GHT appears to be the most important pathway for communicating non-photic phase shifting information to the SCN and there is considerable evidence that NPY released by the GHT mediates the phase shifting effects of many non-photic stimuli (for reviews see Mrosovsky, 1995; Webb et al., 2014).
1.2. Functional and anatomical heterogeneity within the SCN
The bilateral SCN is composed of a heterogeneous population of 16–20,000 neurons as well as a large number of neuroglia (van den Pol, 1980). One of the very interesting features of the SCN is the high number of neuronal cell bodies that contain γ-aminobutyric acid (GABA) throughout the nucleus. Estimates of the proportion of SCN neurons containing GABA in rodents range from over 50% to virtually all neurons in the nucleus (Buijs et al., 1995; Moore and Speh, 1993; Okamura et al., 1989; Tanaka et al., 1997b; Castel and Morris, 2000; van den Pol, 1986; van den Pol and Tsujimoto, 1985; Francois-Bellan et al., 1990). GABA is found in a large number of local circuit neurons in the SCN as well as in terminals derived from projections originating from just outside the nucleus and from more distant sites (van den Pol, 1986; van den Pol and Gorcs, 1986; Kim and Dudek, 1992; Strecker et al., 1997; Morin and Blanchard, 2001; Jiang et al., 1997a; Buijs et al., 1994). GABA receptors also have a ubiquitous presence throughout the SCN and are well positioned to respond to changing levels of extracellular GABA (see Section 4). All or nearly all SCN neurons exhibit spontaneous inhibitory postsynaptic potentials (IPSPs) across the circadian cycle suggesting that GABA receptors are found on all SCN neurons (De Jeu and Pennartz, 2002; Jiang et al., 1997a; Strecker et al., 1997; Kim and Dudek, 1992; Kononenko and Dudek, 2004; Kretschmannova et al., 2003; Burgoon and Boulant, 1998). Indeed, GABA is the dominant neurochemical signal within the SCN and it plays a major role in circadian timekeeping (Cardinali and Golombek, 1998; Ehlen et al., 2010; Allen et al., 2014).
Before exploring the circadian functions of GABA in the SCN, it is important to briefly review the basic anatomical organization of the nucleus. The SCN has an elongated ovoid shape in rodents, although its gross morphology varies considerably across mammalian orders (Lydic et al., 1982). Historically, the SCN has been partitioned into subdivisions based on a variety of anatomical criteria, such as the sites where afferent projections terminate and the location of cells that produce different neuropeptides. At present, however, there is controversy about how best to define subdivisions of the SCN and even the number of anatomical subregions contained within the SCN (Morin and Allen, 2006; Morin, 2007). This controversy is the result of a variety of factors, including species differences in the anatomy of the SCN as well as the dynamic changes that can occur in the anatomical features of the SCN (e.g., rhythmicity in neuropeptide content). It is important to note that SCN anatomy has only been examined in a limited number of species, so the full extent of species differences in SCN anatomy is not known. The existing data suggest, however, that there is considerable conservation of the neurotransmitter and neuropeptide content of the SCN even though their exact anatomical locations may vary across species. It is also noteworthy that studies of the SCN have been conducted almost exclusively in males. While accurate topographical descriptions of SCN anatomy in discussions of SCN function are quite important (Lee et al., 2003; Morin, 2007), they have not been routinely provided in published reports. Despite the controversial nature of how SCN subdivisions have been defined, these designations have been used extensively over the last 25 years, retain heuristic value, and therefore cannot be ignored in discussions of the functional properties of SCN neurons.
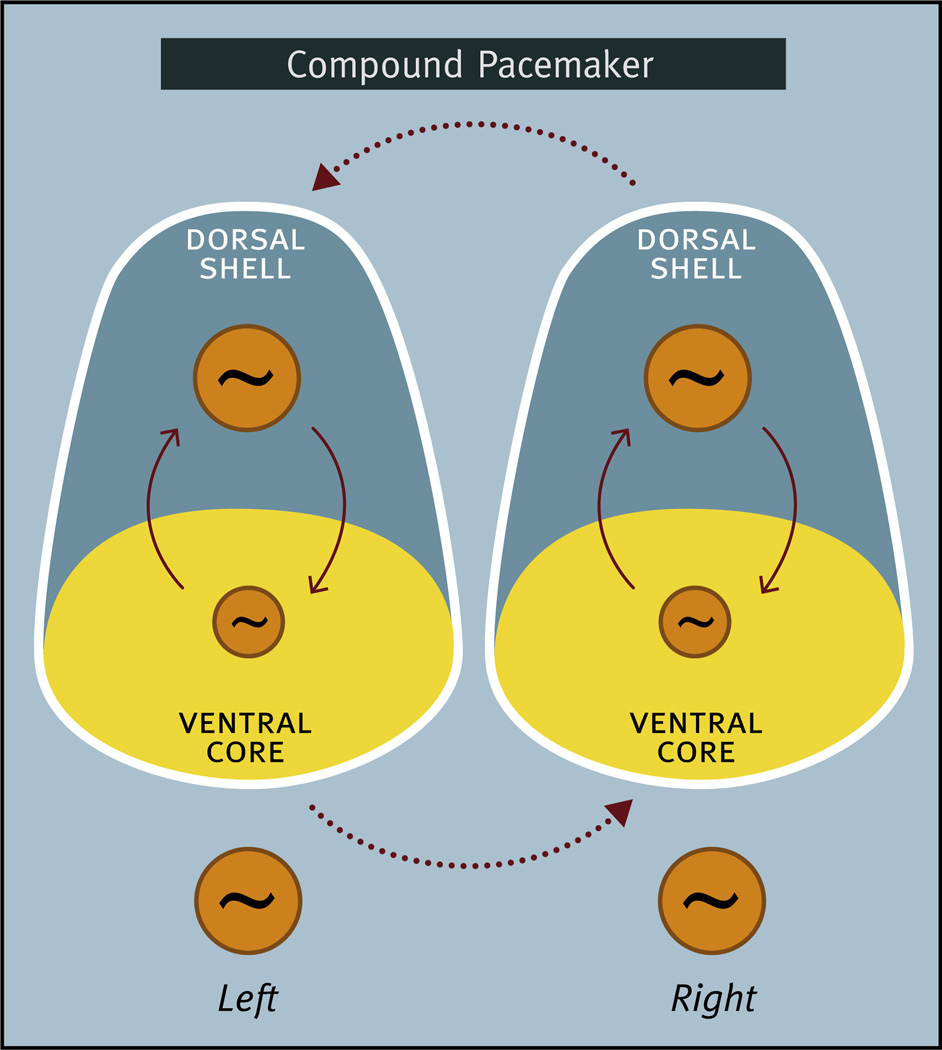
There are a number of excellent reviews of SCN neuroanatomy that discuss the issues related to SCN subdivisions, so we provide only a brief background and discussion here (Moore et al., 2002; Hastings and Herzog, 2004; Lee et al., 2003; Antle et al., 2009; Morin, 2007; Antle and Silver, 2005; Yan et al., 2007; Yan, 2009; Evans, 2016; Evans and Gorman, 2016). Historically, each side of the bilaterally paired SCN has been divided into two regions (although a third division has been described as well) (Morin, 2007). One region has been called the ventral/ventrolateral and/or core, while the other subdivision has been called the dorsal/dorsomedial and/or shell. For consistency we will use the terms ventral or ventral core and dorsal or dorsal shell. Fig. 2 summarizes the general features of the ventral core/dorsal shell conceptualization of SCN organization.
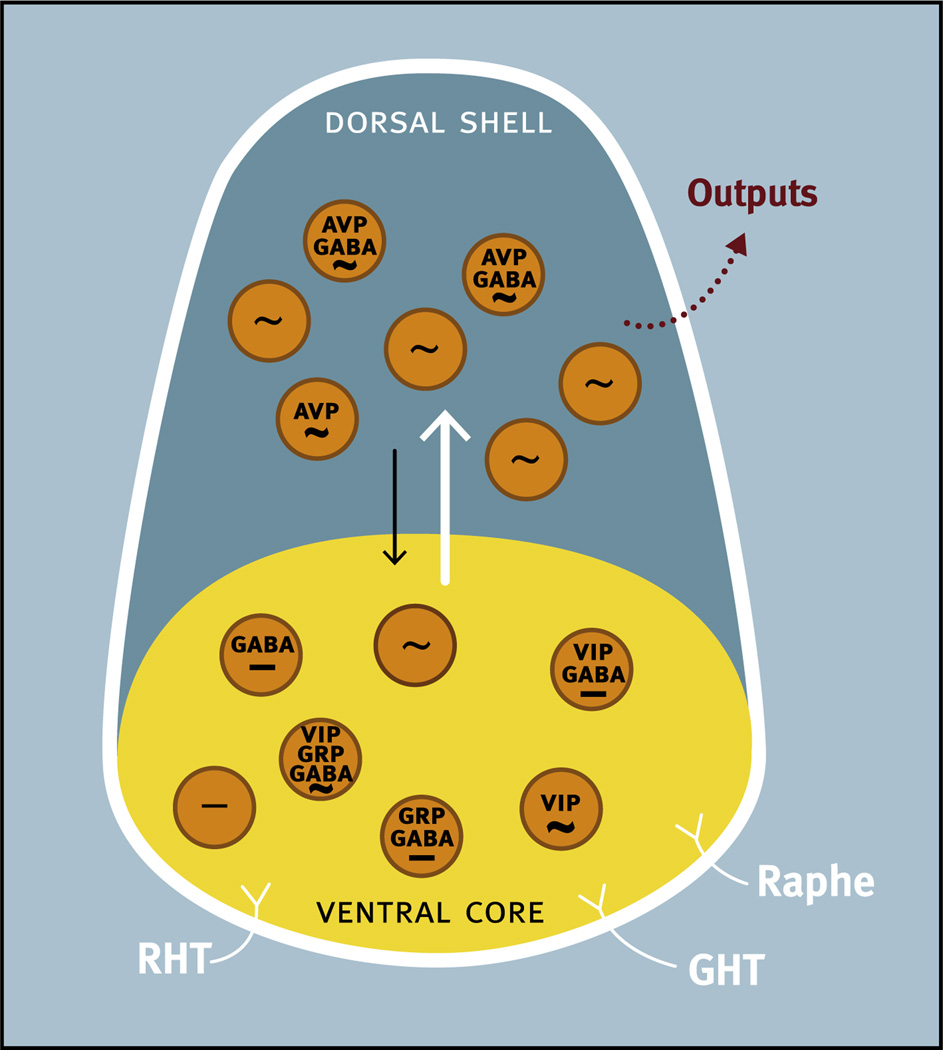
Fig. 2.
Schematic diagram of the ventral core/dorsal shell conceptualization of the organization of the suprachiasmatic nucleus illustrating the anatomical and functional heterogeneity of the nucleus. Major afferent pathways including the retinohypothalamic tract (RHT), geniculohypothalamic tract (GHT), and a projection from the raphe nucleus (Raphe) terminate primarily in the ventral core, although RHT terminals can also be found in the dorsal shell. Neurons in the ventral core contain GABA and a variety of neuropeptides including vasoactive intestinal peptide (VIP) and gastrin releasing peptide (GRP), all of which are frequently colocalized in the same neuron. Some neurons in the ventral core display endogenous rhythmicity (~) while others do not (−). In the dorsal shell, more neurons display endogenous rhythmicity. Neurons in the dorsal shell contain GABA and neuropeptides including arginine-vasopressin (AVP), all of which are frequently colocalized in the same neuron. Neurons in the dorsal shell and ventral core can communicate via GABA and probably other neurochemical signals.
Neurons in what has been called the ventral, ventrolateral, or core region receive direct input from the retina (Morin, 2013). A substantial number of these neurons produce vasoactive intestinal peptide (VIP), peptide histidine isoleucine (PHI), and/or gastrin releasing peptide (GRP) (Okamura et al., 1986; van den Pol and Tsujimoto, 1985; Stopa et al., 1988). VIP and PHI are derived from a common precursor and are therefore consistently found within the same SCN neurons (Romijn et al., 1998; Nishizawa et al., 1985). Some of these VIP/PHI producing SCN neurons also produce GRP (Okamura et al., 1986; Albers et al., 1991; Romijn et al., 1997). Although the pattern of retinal input differs across species, retinal inputs synapse directly on many VIP, PHI, and GRP neurons (Ibata et al., 1989; Tanaka et al., 1997a; Aioun et al., 1998), and environmental lighting influences SCN levels of these neuropeptides (Albers et al., 1987; Shinohara et al., 1993; Zoeller et al., 1992; Albers et al., 1990; Okamoto et al., 1991). Light reduces VIP mRNA and protein and increases GRP mRNA and protein in rodents housed in LD cycles (Takahashi et al., 1989; Albers et al., 1990; Zoeller et al., 1992; Okamoto et al., 1991; Duncan et al., 1995), while in constant lighting conditions no rhythms in the levels of VIP or GRP mRNA and protein are observed (Takahashi et al., 1989; Zoeller et al., 1992; Shinohara et al., 1993). Lighting conditions also influence SCN release of VIP and GRP (Francl et al., 2010a, 2010b). Neurons in the ventral SCN exhibit increased levels of Per1 and Per2 mRNA and protein in response to phase shifting pulses of light. Light induces Per1 expression in the ventral SCN throughout the night, even at times when light does not induce phase shifts (Yan and Silver, 2002). Circadian rhythms in Per1 and Per2 mRNA have also been detected in neurons of the ventral SCN by some methods, but not by others (Karatsoreos et al., 2004; Yan et al., 1999; Hamada et al., 2001; Smyllie et al., 2016).
In the dorsal, dorsomedial, or shell region there is a large population of neurons that produce arginine-vasopressin (AVP) (Vandesande et al., 1975; van den Pol and Tsujimoto, 1985; Card and Moore, 1984). Although originally thought to be devoid of retinal input, more recent evidence indicates that the dorsal region contains RHT terminals (Moore and Card, 1985; Muscat et al., 2003; Morin et al., 2006; Hattar et al., 2006; Fernandez et al., 2016). The dorsal SCN exhibits increased levels of Per mRNA in response to light only after increased mRNA levels are observed in the ventral SCN (Yan and Silver, 2002; Yamamoto et al., 2001; Albrecht et al., 1997; Hamada et al., 2004a). In the dorsal region there are a large number of neurons that exhibit rhythms in Per1 and Per2 mRNA and other immediate early genes (Guido et al., 1999; Yan et al., 1999; Hamada et al., 2001). In response to photic stimuli that induce phase shifts, several important indices of neuronal activity (e.g., peaks in Per expression and spike rate) phase shift more rapidly in the ventral core than in the dorsal shell (Albus et al., 2005; Yan and Silver, 2002, 2004; Nagano et al., 2003).
Several investigators have proposed hypotheses on the mechanisms that mediate entrainment within the SCN (Albers et al., 1992; Hastings and Herzog, 2004; Antle and Silver, 2005; Lee et al., 2003; Yan et al., 2007). One proposition is that there are “non-rhythmic” SCN cells in the ventral core that respond directly to light input and intrinsically “rhythmic” SCN clock cells in the dorsal shell that do not. A light-induced phase shift occurs when non-rhythmic cells in the ventral core communicate the lighting signal to the oscillator in the dorsal shell. An alternative proposition is that there are rhythmic neurons in the ventral core that directly or indirectly respond to light, and interactions between the oscillators in the ventral core and dorsal shell are responsible for light-induced phase shifts. It is clear that endogenously driven circadian oscillations are not entirely restricted to the dorsal shell although it is not known if rhythmic cells in the ventral core are light-responsive (Albus et al., 2005; Myung et al., 2015; Evans et al., 2013). Indeed, studies of cultured SCN neurons have found that both VIP and AVP positive cells can, but do not always, exhibit intrinsic rhythmicity, and that these neuropeptide containing cells represent a surprisingly small percentage of the cells displaying intrinsic rhythmicity (Shinohara et al., 1996; Webb et al., 2009). Although the ventral core/dorsal shell dichotomy is overly simplistic, the concept of rhythmic elements as well as non-rhythmic light-responsive elements provides a simple and testable view of the function of these two distinct groups of SCN neurons. While the distribution of rhythmic and light-responsive elements appears to be a common feature within the SCN, the anatomical distribution of these elements as well as their neurochemical phenotype likely differs across species (Lee et al., 2003; Ramanathan et al., 2009; Yan and Silver, 2002; Yan and Okamura, 2002).
Much remains to be learned about how neurons communicate within and between subdivisions of the SCN (see Section 8.1). There are a number of different types of signaling processes that may be responsible for communication among SCN cells (for reviews see Michel and Colwell, 2001; van den Pol and Dudek, 1993). Synaptic activity is an important form of communication among SCN neurons. Synaptic arrangements in the SCN are complex, and ultrastructural differences can be found across neighboring neurons throughout the nucleus (Guldner and Wolff, 1996; van den Pol, 1980; Lenn et al., 1977; Buijs et al., 1994; Suburo and Pellegrino, 1969; Guldner, 1978). Synaptic activity within the SCN is essential for the expression of overt circadian rhythmicity and for the entrainment of the pacemaker with the LD cycle (Schwartz et al., 1987; Schwartz, 1991). In contrast, several lines of evidence suggest that synaptic activity may have little to no role in the timekeeping ability of the SCN pacemaker (Shirakawa et al., 2000; Schwartz et al., 1987; Bouskila and Dudek, 1993; Honma et al., 2000; Yamaguchi et al., 2003; Reppert and Schwartz, 1984; Schwartz, 1991). There is evidence that non-synaptic release of neurochemical signals may be important in SCN communication. Neurochemical signaling outside of classical synapses has become increasingly recognized as a significant form of inter-neuronal communication (for reviews see Trueta and De Miguel, 2012; Stoop, 2012; Leng and Ludwig, 2008). Inter-dendritic and intersomatic appositions within the SCN have the potential to mediate non-synaptic interactions (Guldner and Wolff, 1996; van den Pol, 1980), and neurochemical signals can be released in non-synaptic regions of SCN neurons (Castel et al., 1996). In addition, SCN neurons are close together, so even small amounts of extrasynaptic GABA release have the potential to significantly raise extracellular GABA levels (van den Pol, 1980). While initially controversial, gap junctions have now also been confirmed in the SCN, indicating that SCN neurons may communicate directly through electrotonic coupling (Jiang et al., 1997b; Shirakawa et al., 2000; Colwell, 2000; Rash et al., 2007; Long et al., 2005). In addition, the potential importance of neuroglia in SCN communication should not be overlooked (Slat et al., 2013; Bosler et al., 2015). One great mystery is how circadian organization is communicated to the vast number of physiological and behavioral variables that display endogenous rhythmicity. It is clear, however, that the circadian timing system employs both neural and humoral signaling as outputs of the pacemaker (see Section 10).
1.3. Summary of SCN organization
Much remains to be learned about how the anatomically and functionally heterogeneous elements within the SCN come together to form an entrainable circadian pacemaker. The nucleus receives numerous afferent projections, but the most important for influencing the phase of the circadian pacemaker appear to be the RHT and GHT. There is a general consensus that there are at least two major subdivisions in the SCN, the ventral core and the dorsal shell, although uncertainty remains about the anatomical and functional boundaries of these two regions. For example, the RHT synapses preferentially in the ventral core in some species but not others. Environmental lighting influences the expression of neuropeptides and clock genes within the ventral core and at least some neurons in this subdivision can exhibit endogenous circadian oscillations. A larger percentage of neurons within the dorsal shell exhibit circadian oscillations and likely represent fundamental components of the circadian pacemaker. Some of the most important neurobiological questions remaining about SCN functioning are how neurochemical signaling serves to communicate entrainment signals to the pacemaker, couple clock cells and subpopulations of clock cells in the SCN to form a pacemaker, and how the pacemaker drives rhythmicity in its diverse outputs.
2. Investigation of GABA function within the SCN
GABA is recognized as the primary inhibitory neurotransmitter in the brain. While much has been learned about GABA’s actions, the study of GABA function has been complicated by its role in so many different circuits and the complexity in so many features of its signaling. The SCN provides an outstanding model system to study the dynamics of GABA signaling because GABA is found in such a large percentage of SCN neurons and, as a circadian pacemaker, the SCN provides a GABAergic network with easily quantifiable and functionally significant inputs (e.g., light) and outputs (e.g., phase shifts). In this review, we will begin by briefly discussing the methodological approaches that have been used to study GABA in the SCN. We will then discuss the current state of knowledge of GABA signaling as revealed by its investigation throughout the brain, but with a special emphasis on the SCN. More specifically, we will review the factors governing GABA release, how GABA interacts with other neurochemical signals, the complex nature of GABA receptors, the regulation of extracellular levels of GABA, and the controversies that have developed surrounding GABA’s ability to evoke excitatory responses. Finally, we will examine the ways that GABA signaling contributes to the formation of a circadian pacemaker within the SCN, the entrainment of circadian rhythms with the day-night cycle, and the imposition of circadian rhythmicity on systems throughout the body.
2.1. Methodological issues
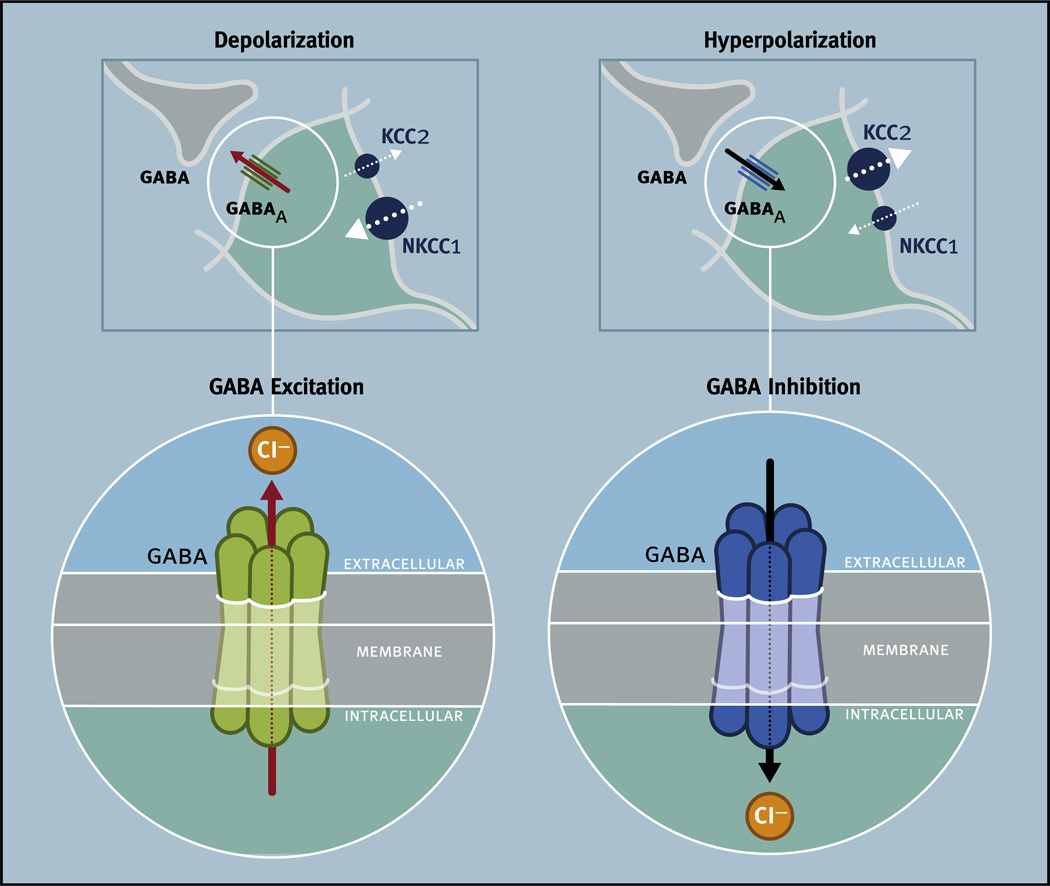
GABA function in the SCN has been investigated using a wide range of powerful and innovative approaches in both in vitro and in vivo systems. In vitro studies have employed primary cultures of SCN neurons to investigate a variety of fundamental questions, such as whether single SCN cells are capable of circadian rhythmicity and whether GABA is involved in communication between rhythmic SCN neurons (Welsh et al., 1995; Liu and Reppert, 2000; Shirakawa et al., 2000). Primary culture is a useful tool to determine single cell contributions to the overall network. It is important, however, to note the animal’s age at the time of culture preparation because of the developmental changes in the polarity of the neuronal responses evoked by GABA (see Section 6). As such, extrapolations on the functions of GABA from data collected in primary culture to the intact circadian system in adults should be interpreted with this transition in mind.
Another in vitro approach that has contributed greatly to our understanding of the cellular actions of GABA in the SCN is the hypothalamic slice preparation (Green and Gillette, 1982; Groos and Hendriks, 1982; Meijer and Michel, 2015). This preparation, typically employing adult SCN tissue, has been used to study the cellular properties of SCN neurons using techniques such as electrophysiological recordings and Ca2+ imaging. Commonly used recording approaches range from multiple unit activity recordings, single unit recordings, and cellular electrophysiology using intracellular and whole-cell patch clamp configurations. Careful attention must be paid to the ionic concentration of extracellular and intracellular solutions (e.g., Cl− concentration) (Wagner et al., 2001) and the recording time after membrane rupture (Schaap et al., 2003). Multiple unit recordings allow measurement of global neurophysiological activity by recording from the same group of neurons over long time intervals. This strategy is relatively noninvasive (i.e., the intracellular ionic regulation is not perturbed) and allows continuous measurement of neuronal activity over several circadian cycles. Single cell recordings can include extracellular single-unit recordings, visually guided loose patch recordings, or whole cell patch clamp recordings. These powerful approaches allow delivery of pharmacological compounds and/or electrical current to specific SCN regions or cells. Furthermore, intracellular or whole-cell recordings permit isolation of GABA-mediated changes in current (i.e., voltage clamp) or voltage (i.e., current clamp). Single cell recordings have also been commonly used to study circadian rhythmicity in firing rates of SCN neurons. This approach employs multiple episodic single unit recordings in which a single neuron is recorded for a few minutes and then the extracellular electrode is moved to record from another neuron. This process is repeated over long intervals resulting in records of neuronal firing that have been obtained from many different neurons at different time-points.
There are other factors that should be considered when interpreting in vitro SCN data. First, the loss of many afferent projections to the SCN is obviously problematic for investigating the function of afferent entrainment pathways. In fact, there is evidence to suggest that extra-SCN oscillators play a powerful role in entrainment and possibly in the generation of circadian rhythmicity (Albers et al., 1984c; Vansteensel et al., 2003b). Another consideration is how SCN neuronal activity is affected by the environmental lighting conditions donor animals are exposed to prior to slice preparation. We do know that these prior lighting conditions can have a significant impact. For example, the electrical properties of SCN neurons from mice entrained to a LD cycle immediately prior to slice preparation differ substantially from slices obtained from mice housed on constant darkness for four days before slice preparation (see Section 5.4). We do not know, however, how long these prior conditions impact SCN neuronal activity in the slice. One approach used to study entrainment in hypothalamic slices has been to expose animals to light pulses immediately prior to the preparation of a slice. This approach has provided some very interesting data indicating that short pulses of light trigger sustained firing of SCN neurons that can last 6–8 h (LeSauter et al., 2011; Kuhlman et al., 2003), and that NPY applied in vitro blocks the phase shift induced by a light pulse administered in vivo (Yannielli and Harrington, 2000). Nevertheless, this approach has obvious limitations for the investigation of the dynamic interactions that may occur among multiple afferent entrainment pathways and SCN interneurons in the time interval between the light pulse and the phase shift of the pacemaker.
Second, the effects of drugs can differ qualitatively as well as quantitatively in the slice preparation versus the intact circadian system. For example, administration of glutamate in the slice preparation produces phase shifts that mimic those produced by light pulses given to intact animals (Ding et al., 1998), while administration of glutamate into the SCN in animals with an intact circadian system mimics the resetting effects of pulses of darkness or administration of NPY (Meijer et al., 1988). The magnitude of the response to neurotransmitter agonists can also be substantially larger in reduced preparations such as the hypothalamic slice. For example, NPY injected into the SCN produces phase advances in locomotor rhythms of approximately 1.5 h in vivo (Albers and Ferris, 1984; Gamble et al., 2004, 2005; Biello et al., 1997) but advances of approximately 4 h in single unit activity rhythms in the hypothalamic slice (Medanic and Gillette, 1993; Golombek et al., 1996). Interestingly, the results from studies of phase shifting using multiple unit recordings more closely mimic the size of phase shifts observed in intact animals (see Gribkoff et al., 1998). The efficacy of antagonists can also differ substantially between in vitro and in vivo situations. For example, the same NPY antagonist potentiates light induced phase shifts in vivo (Yannielli et al., 2004; Gamble et al., 2005) but has no effect in vitro (Yannielli et al., 2004). It has been proposed that these differences are the result of a lower endogenous tone of NPY in vitro than in vivo, because the SCN slice is unlikely to have the circuitry to support regulated release of the neuropeptide (Yannielli and Harrington, 2004). These finding emphasize that there are a complex series of inputs that provide feedback to the SCN that are lost when experiments are conducted in brain slices (Vansteensel et al., 2003b; Meijer and Michel, 2015).
Third, in vivo studies have administered GABA-active drugs both systemically and by direct injection into the SCN region. Of course the effects of GABA-active drugs administered systemically could occur in many different sites within the body. The use of site-specific injections of GABA drugs is a far more discrete approach to manipulating the GABA system in the SCN. However, even with this approach it is not possible to avoid the spread of the drug throughout the SCN, and possibly to surrounding areas given its close proximity to the third ventricle. Comparison of drug effects from systemic versus site-specific injections into the SCN have revealed that these routes of administration can produce dramatically different and sometimes opposite effects on circadian control (Ralph and Menaker, 1985; Gillespie et al., 1996) (see Section 9.2.4).
Finally, it is important to consider the functional significance of the measurements made by the techniques used to study circadian function in the SCN. The period and phase of overt rhythms can be confidently considered to be properties of the underlying circadian pacemaker (Daan and Pittendrigh, 1976b). Measurements of the firing rate of SCN neurons provide important insights into the electrical state of these neurons, although the relationships among firing rate, circadian period, and phase are not fully understood. For example, inhibition of synaptic signaling in the SCN by blocking sodium (Na+)-dependent action potentials does not prevent the pacemaker from keeping time, alter its circadian period, or induce phase shifts (see Section 8.1). On the other hand, when the firing of SCN neurons is increased daily for 1 h with optogenetic manipulation, the phase of molecular rhythms as well as locomotor behavior is entrained to this increased firing in a manner similar to light (Jones et al., 2015). Suppression of the firing of SCN neurons for 1 h with optogenetic manipulation has no effect on circadian phase during the night, but produces large phase delays at circadian time (CT) 0–6 and advances at CT 6–12 (Jones et al., 2015). Although acute suppression of firing rate by inhibitory neurotransmitters (e.g., NPY) does not appear to be correlated with the size of the phase shift (Gribkoff et al., 1998), the induction of persistent inhibition (lasting for 2–4 h after washout) is phase-dependent (Besing et al., 2012). Moreover, potassium induced depolarization several hours after NPY application completely blocks the NPY-induced phase shifts, suggesting that the resting membrane potential is critically important for phase resetting (Besing et al., 2012). NPY and other neurotransmitters that typically produce phase advances during the day, hyperpolarize the resting membrane potential and reduce firing rate through activation of G-protein coupled inwardly rectifying K+ (GIRK) channels (Scott et al., 2010; Hablitz et al., 2014, 2015). Together, these results suggest that sustained changes in membrane potential at specific phases of the circadian cycle are key to resetting the circadian pacemaker.
Studies in the reduced circadian system have made it possible to also examine the period and phase of constituent cells within the pacemaker. Powerful techniques, such as the analysis of the PER2 bioluminescence exhibited by individual neurons within a slice and the analysis of the firing of hundreds of SCN neurons continuously over days, have been used to study how SCN neurons interact. These preparations have provided dramatic new information on how elements within the SCN can interact, although these interactions are sometimes studied following exposure to highly atypical environments (e.g., LD 20:4). As a result, it remains important to verify that these same mechanisms underlie the interactions of SCN neurons in the fully intact circadian system operating in lighting conditions seen by animals in nature.
3. GABA release and its interaction with other neurochemical signals
It is now clear that many neurons, including GABAergic neurons, can release more than one neurochemical signal. Based on the frequency with which neurochemical signals are co-expressed in the brain, it seems likely that co-release is a common phenomenon, although actual demonstrations of co-release from neurons are rare. There is evidence, however, that GABA can be released with other low molecular weight neurotransmitters such as glycine and glutamate (Tritsch et al., 2016). There is also substantial anatomical evidence for the colocalization of GABA with neuropeptides. In most cases, amino acid neurotransmitters like GABA are packaged in small synaptic vesicles (SSV) in presynaptic regions, while neuropeptides are found in large densecore vesicles (LDCV) in pre-synaptic areas as well as nonsynaptic areas. The colocalization of amino acid neurotransmitters and neuropeptides is a common occurrence in the nervous system (for reviews see van den Pol, 2012; Albers, 2015), and the coexistence of GABA and neuropeptides within SCN neurons is strongly indicated by the consistent presence of LDCVs in GABA-containing SCN terminals (Decavel and van den Pol, 1990).
The co-release of amino acid neurotransmitters and neuropeptides occurs as a result of the exocytosis of both SSV and LDCVs induced by an influx of high levels of Ca2+ through voltage-gated ion channels (for reviews see Hokfelt, 1991; Stoop, 2012; Ludwig and Leng, 2006). The frequency of neuronal firing determines the amount and distribution of Ca2+ influx, which in turn determines whether amino acid neurotransmitters and neuropeptides are released. Low frequency firing is sufficient to induce exocytosis of SSVs because they are close to where Ca2+ enters the terminal while high frequency firing is necessary for the exocytosis of LDCVs because they are further away from the ion channels where Ca2+ enters the terminal. As a result, synaptic release of neuropeptides lags behind the release of amino acid neurotransmitters (Fig. 3).
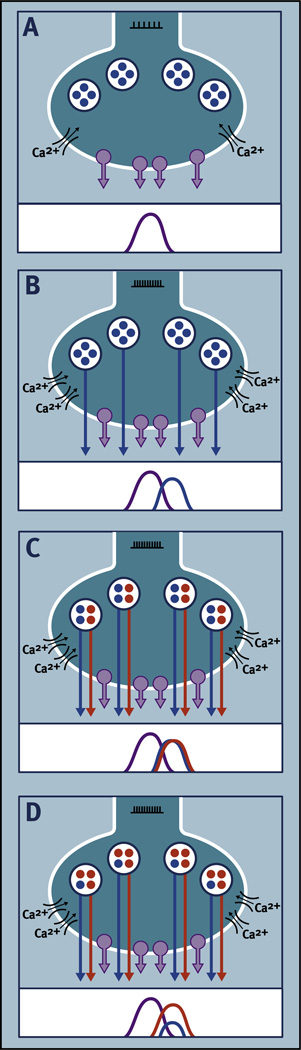
Fig. 3.
Hypothetical illustration of how different patterns of synaptic release of an amino acid neurotransmitter (NT) and colocalized neuropeptides (NP) could result from differences in neuronal firing and neuropeptide biosynthesis. Each panel is an example of differences that might occur in NT and NP release at a specific phase of the circadian cycle. (A) Moderate neuronal firing (l l l l l l) produces moderate levels of Ca2+ influx through voltage-gated ion channels resulting in exocytosis of small synaptic vesicles (SSV; purple circles) and release of NT. (B) High levels of neuronal firing (lllllllllll) produce high levels of Ca2+ influx through voltage-gated ion channels resulting in exocytosis of both SSVs and large dense-core vesicles (LDCV; blue circles) resulting in release of NT and NP. (C) Two different neuropeptides are packaged in LDCVs (red and blue) in a ratio of 1:1. High levels of neuronal firing (lllllllllll) produce high levels of Ca2+ influx through voltage-gated ion channels, resulting in exocytosis of SSVs and release of NT and exocytosis of LDCVs and release of a “cocktail” of neuropeptides in a 1:1 ratio. (D) Two different neuropeptides are packaged in LDCVs (red and blue) in a ratio of 3:1. High levels of neuronal firing (lllllllllll) produce high levels of Ca2+ influx through voltage-gated ion channels resulting in exocytosis of SSVs and release of NT and exocytosis of LDCVs and release of a “cocktail” of neuropeptides in a 3:1 ratio. Differential regulation of the biosynthesis and storage of neuropeptides could result in different ratios of neuropeptide release. If neuropeptide biosynthesis is differentially regulated over the circadian cycle then different ratios of neuropeptide would be released at different times of day (modified from Albers (2015)).
Although GABA is found primarily in SSVs in the SCN, GABA immunoreactivity has been identified in LDCVs as well (Castel and Morris, 2000; van den Pol, 1986). These findings have led to the hypothesis that GABA can be synthesized or taken up in the neuronal cell body and then packaged in LDCVs with and possibly without neuropeptides. GABA containing LDCVs can be transported to synaptic terminals and exocytosed in response to Ca2+ influx through voltage-gated ion channels. Alternatively, LDCVs containing GABA could be exocytosed in non-synaptic regions as the result of Ca2+ influx from membrane bound voltage-gated Ca2+ channels in close proximity to the LDCVs, or voltage-independent Ca2+ release from intracellular stores (e.g., endoplasmic reticulum) (Fig. 4). The exocytosis of LDCVs resulting from the release of intracellular Ca2+ occurs in other hypothalamic regions and can occur in the absence of changes in electrical activity, because Ca2+ influx through voltage-gated ion channels is not required. Once released into the extracellular fluid outside of the synaptic cleft, neurotransmitters and neuropeptides are capable of producing prolonged diffuse signals that can spread as far as 4–5 mm. This mode of communication, called volume transmission, contrasts dramatically with the more rapid and local signaling produced by release within the synapse (Engelmann et al., 2000; Fuxe et al., 2010). Much of what we know about volume transmission comes from studies of non-synaptic neuropeptide release; however, this same signaling mechanism also applies to low molecular weight neurotransmitters, including GABA, released from neurons or astrocytes (Trueta and De Miguel, 2012; Lee et al., 2010; Rossi et al., 2003; Rozsa et al., 2015).
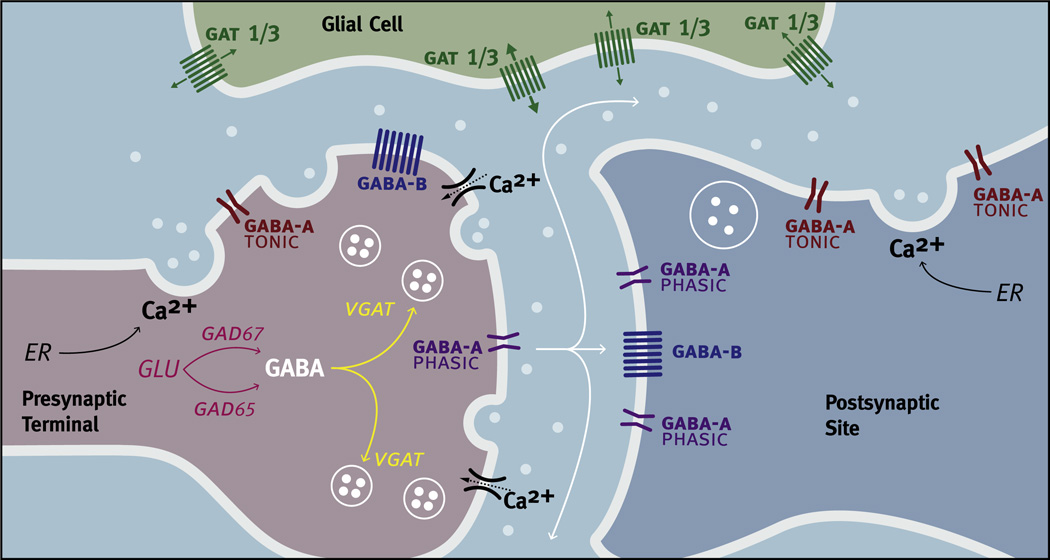
Fig. 4.
Factors regulating GABA signaling. Glutamic acid decarboxylase (GAD) catalyzes glutamic acid (GLU) into GABA in presynaptic neurons (light red). There are two isoforms of GAD. GAD67 synthesizes GABA for tonic release, while GAD65 synthesizes GABA for phasic release. GABA vesicular transporters (VGATs) are responsible for the transport of GABA into synaptic vesicles (yellow). GABA (white circles) can be found both in small synaptic vesicles (SSVs) and in large dense core-vesicles (LDCVs). The exocytosis of SSVs and LDCVs occurs in response to increases in intracellular calcium (Ca2+) resulting in the release of GABA into the extracellular space. Increases in intracellular Ca2+ can result from the influx of Ca2+ through voltage-gated ion channels as the result of an action potential or by the release of intracellular stores of Ca2+ from the endoplasmic reticulum (ER) that do not require changes in electrical activity. GABAA-PHASIC (purple), GABAA-TONIC (dark red) and GABAB (blue) receptors are found on both presynaptic terminals and postsynaptic sites. GABAA-PHASIC receptors are frequently found in synaptic regions while GABAA-TONIC receptors are frequently found in extra-synaptic regions. GABAB receptors can be found in both synaptic and extra-synaptic regions. GABA transporters in the membrane (GATs) remove GABA from the extracellular space by a rapid reuptake of GABA but can also release GABA. In the SCN, GAT1 and GAT3 (Green) are found on astrocytic processes in extra-synaptic regions.
The percentage of SCN neurons that contain both GABA and neuropeptides is quite high, although precise numbers are hard to estimate for a variety of technical reasons. For example, in immunohistochemical studies GABA immunoreactivity can be faint, immunoreactive neurons often overlap, and the density of immunoreactivity for GABA and neuropeptides may change over the circadian cycle. Therefore, quantitation of the percentages of neurons containing GABA and/or neuropeptide immunoreactivity may be underestimated. While some studies report that all VIP/PHI and AVP immunoreactive neurons also contain GABA (Moore and Speh, 1993) other studies have found that GABA is more frequently colocalized with VIP/PHI than with AVP (Castel and Morris, 2000; Buijs et al., 1995; Francois-Bellan et al., 1990). Indeed, counts from duaI-labeled electron micrographs have suggested that GABA may be found in up to 70% of VIP immunoreactive boutons and 35% of AVP immunoreactive boutons (Castel and Morris, 2000). Another study examined the colocalization of the mRNAs encoding glutamic acid decarboxylase (GAD), the rate-limiting step in GABA synthesis, with the mRNAs encoding AVP and VIP/PHI using double-labeled in situ hybridization (Tanaka et al., 1997a, 1997b). Using this approach 91% of AVP mRNA positive neurons and 94% of VIP mRNA positive neurons also contained GAD mRNA.
The number of neuropeptides potentially released from a single GABA containing neuron could be quite large. For example, some GABA and VIP/PHI containing neurons likely also contain GRP (Okamura et al., 1986; Albers et al., 1991; Romijn et al., 1997). The high frequency of GABA colocalization with VIP/PHI combined with the existence of dense, direct projections from neurons in the ventral to the more dorsal SCN suggests that GABA could play an important role in synaptic communication between retinorecipient regions containing non-rhythmic and/or rhythmic neurons and rhythmic neurons in the dorsal SCN (Leak et al., 1999; Romijn et al., 1997).
GABA producing SCN neurons are closely associated with the terminals of the GHT and the serotonergic projection from the raphe. The GHT contains GABA and NPY producing neurons that project primarily to the retino-recipient field within the SCN (Moore and Card, 1994). GABA and NPY terminals frequently converge on the same postsynaptic targets in the ventral SCN thereby mediating the functionally important GABA-NPY interactions to be discussed later (see Section 9.1). Similarly, GABA terminals and 5-HT terminals often converge on the same postsynaptic membranes in the SCN (Bosler, 1989; François-Bellan and Bosler, 1992). A large number of these serotonergic terminals are found within the retino-recipient terminal field. Serotonergic terminals can presynaptically inhibit RHT activity via 5-HT1b receptors (Bramley et al., 2005). Interestingly, however, most of the 5-HT1b receptors are on terminals other than those of the RHT (Pickard et al., 1996; Manrique et al., 1999). Indeed, many of the 5-HT1b receptors are on GABA terminals and their activation reduces GABA release (Bramley et al., 2005).
In summary, GABA is most frequently found packaged in SSVs in pre-synaptic regions, although GABA has also been identified in LDCVs. GABA may be released from SCN neurons alone when low frequency stimulation causes a focal increase in Ca2+ at the presynaptic membrane. High frequency stimulation, however, likely results in the release of neuropeptides as well as GABA. The possibility that GABA can act by volume transmission has the potential to produce a very different type of GABA signaling that could be involved in the coupling of circadian clock cells and/or serve as an efferent signal driving the rhythmicity of output systems (see Sections 8.1 and 10.2). The functional significance of synaptic and possibly non-synaptic co-release of GABA and neuropeptides within the SCN is a major gap in our knowledge, because the dynamic interactions of these signals will likely be critically important in understanding GABA function in circadian timekeeping. Indeed, because of the variety of distinct temporal patterns displayed by different neurochemical signals in the SCN, it is likely that these signals result in different ratios of receptor activation at different times of the circadian cycle (Fig. 3). It has been proposed that the ratio of receptor activation within the SCN provides clear “time of day” information within the nucleus and that the release of different ratios of signals from SCN efferents could serve as an output signal of the pacemaker in animals entrained to a LD cycle (Albers et al., 1992).
4. GABA receptors
Receptors that bind GABA are found on most neurons in the mammalian brain (Decavel and van den Pol, 1990; Mody and Pearce, 2004). The effects of GABA are mediated by two major classes of GABA receptors: GABAA and GABAB (Olsen and Sieghart, 2008, 2009; Benarroch, 2012; Ulrich and Bettler, 2007), both of which are found in the SCN (Table 1) (Francois-Bellan et al., 1989b). GABAA receptors are ligand-gated chloride (Cl−) ion channels and GABAB receptors are metabotropic G-protein-coupled receptors that modulate Ca2+ and potassium (K+) channels (Ulrich and Bettler, 2007). Both GABAA and GABAB receptors are composed of multiple subunits that can determine their subcellular distribution and function, and both classes of receptors can be found presynaptically, postsynaptically, and on extrasynaptic membranes in the brain (Benarroch, 2012).
Table 1.
GABA associated transcripts in the SCN (from SCN 2014 Mouse 1.0OST, CircaDB, http://circadb.hogeneschlab.org/).
| Common name | Symbol | Description | Mid expression rangea | RefSeq |
|---|---|---|---|---|
| Receptors | ||||
| GABAA α1 | Gabra1 | GABA A receptor, subunit alpha 1 | 1044 | NM_010250 |
| GABAA α2 | Gabra2 | GABA A receptor, subunit alpha 2 | 512 | NM_008066 |
| GABAA α3 | Gabra3 | GABA A receptor, subunit alpha 3 | 526 | NM_008067 |
| GABAA α4 | Gabra4 | GABA A receptor, subunit alpha 4 | 192 | NM_010251 |
| GABAA α5 | Gabra5 | GABA A receptor, subunit alpha 5 | 511, 165 | NM_176942 |
| GABAA α6 | Gabra6 | GABA A receptor, subunit alpha 6 | 15 | NM_001099641 |
| GABAA β1 | Gabrb1 | GABA A receptor, subunit beta 1 | 777 | NM_008069 |
| GABAA β2 | Gabrb2 | GABA A receptor, subunit beta 2 | 2025, 1151, 573 | NM_008070 |
| GABAA β3 | Gabrb3 | GABA A receptor, subunit beta 3 | 1345 | NM_008071 |
| GABAA δ | Gabrd | GABA A receptor, subunit delta | 56 | NM_008072 |
| GABAA ε | Gabre | GABA A receptor, subunit epsilon | 274 | NM_017369 |
| GABAA γ1 | Gabrg1 | GABA A receptor, subunit gamma 1 | 1031 | NM_010252 |
| GABAA γ2 | Gabrg2 | GABA A receptor, subunit gamma 2 | 472 | NM_008073 |
| GABAA γ3 | Gabrg3 | GABA A receptor, subunit gamma 3 | 982 | NM_008074 |
| GABAA π | Gabrp | GABA A receptor, pi | 35 | NM_146017 |
| GABAA θ | Gabrq | GABA A receptor, subunit theta | 544 | NM_020488 |
| GABAB1 | Gabbr1 | GABA B receptor, 1 | 1519 | NM_019439 |
| GABAB2 | Gabbr2 | GABA B receptor, 2 | 691 | NM_001081141 |
| GABAA ρ1 | Gabrr1 | GABA C receptor, subunit rho 1 | 33 | NM_008075 |
| GABAA ρ2 | Gabrr2 | GABA C receptor, subunit rho 2 | 66 | NM_008076 |
| GABAA ρ3 | Gabrr3 | GABA C receptor, subunit rho 3 | 35 | NM_001081190 |
| Transporters | ||||
| GAT1 | Slc6a1 | Solute carrier family 6 (neurotransmitter transporter, GABA), member 1 | 937 | NM_178703 |
| GAT3, GAT4 | Slc6a11 | Solute carrier family 6 (neurotransmitter transporter, GABA), member 11 | 1734 | NM_172890 |
| GAT2 | Slc6a12 | Solute carrier family 6 (neurotransmitter transporter, betaine/GABA), member 12 | 137 | NM_133661 |
| GAT2, GAT3 | Slc6a13 | Solute carrier family 6 (neurotransmitter transporter, GABA), member 13 | 198 | NM_144512 |
| NKCC2 | Slc12a1 | Solute carrier family 12, member 1 | 34 | NM_183354 |
| NKCC1 | Slc12a2 | Solute carrier family 12, member 2 | 650 | NM_009194 |
| NCC | Slc12a3 | Solute carrier family 12, member 3 | 73 | NM_019415 |
| KCC1 | Slc12a4 | Solute carrier family 12, member 4 | 87 | NM_009195 |
| KCC2 | Slc12a5 | Solute carrier family 12, member 5 | 518 | NM_020333 |
| KCC3 | Slc12a6 | Solute carrier family 12, member 6 | 1665 | NM_133648 |
| KCC4 | Slc12a7 | Solute carrier family 12, member 7 | 122 | NM_011390 |
| CCC6 | Slc12a9 | Solute carrier family 12 (potassium/chloride transporters), member 9 | 189 | NM_031406 |
| VGAT | Slc32a1 | Solute carrier family 32 (GABA vesicular transporter), member 1 | 807 | NM_009508 |
| Enzymes | ||||
| GABA transaminase | Abat | 4-Aminobutyrate aminotransferase | 1490 | NM_172961 |
| GAD67 | Gad1 | Glutamic acid decarboxylase 1 | 845 | NM_008077 |
| GAD65 | Gad2 | Glutamic acid decarboxylase 2 | 1953 | NM_008078 |
| Associated proteins | ||||
| Gabarapl1 | GABA A receptor-associated protein-like 1 | 950 | NM_020590 | |
| Gabarapl2 | GABA A receptor-associated protein-like 2 | 1351, 785 | NM_026693 |
More than one expression value indicates hybridization to more than one probe set in the microarray.
Investigation of GABA neurotransmission has benefited greatly from the availability of potent and selective GABA-active drugs that discriminate between GABA’s effects on GABAA and GABAB receptors (see discussion in Table 2). Pharmacological tools for studies of the different forms of the GABAA receptor are also emerging, although their actions are not fully understood. GABA-active drugs have been used in a wide range of in vitro and in vivo preparations to investigate GABA function in the SCN.
Table 2.
Pharmacological specificity of commonly used GABA receptor drugs.
| Site of action | Other pharmacological effectsa | |
|---|---|---|
| Agonist | ||
| GABA | GABAA, GABAB | |
| Muscimol | GABAA | Inhibitor: Neuronal and glial GABA uptake, GABA transaminase |
| Does not bind to GABAAρ receptor | ||
| THIP (gaboxadol) | GABAA-TONIC | Antagonist: GABAAρ receptor |
| Baclofen | GABAB | |
| SKF-97541 (CGP 35024, 3-APMPA) | GABAB | Antagonist: GABAAρ |
| Antagonist | ||
| Bicuculline (quaternary salts) | GABAA | Antagonist: nACh receptor, α2 glycine receptor, 5HT(3A) receptor, SK channel |
| Inhibitor: acetylcholinesterase | ||
| Gabazine (SR 95531) | GABAA-TONIC, GABAA-PHASIC | Antagonist: Glycine receptor |
| Picrotoxin | GABAA | Antagonist: Glycine receptor |
| RO15-4513 | GABAA-TONIC | High affinity for all BZD binding sites |
| Phaclofen | GABAB | |
| CGP 55845 | GABAB |
Pharmacological approaches to studying GABAergic neurotransmission in the SCN necessarily rely on receptor-specific drugs affecting both orthosteric and allosteric sites. Recombinant expression systems are widely used to characterize pharmacology of receptors using forced concatenation of GABAA subunits. Yet this method may fail to recapitulate pharmacological profiles of native receptors due to alternative positioning of subunits or altered allosteric conformation of binding sites, which is demonstrated by the enormous amount of variability in sensitivity (greater than an order of magnitude) of recombinant GABAARs to ligands (for a review see Hevers and Luddens, 1998). There are additional issues specific to using recombinant GABAA-TONIC receptors experimentally. Varying amounts of δ subunits are incorporated into recombinant GABAA-TONIC receptors, resulting in receptors that do not share the pharmacological properties of native receptors (Sigel et al., 2009; Meera et al., 2010). Although several studies have investigated the expression of individual GABAA receptor subunits within the SCN, the exact subunit composition and intranuclear location of various native GABAA receptors represents a large gap in our knowledge (see Section 4.1).
Muscimol is used as a GABAA-specific agonist. However it also inhibits GABA uptake potentially resulting in higher extracellular GABA levels (for a review see Johnston, 2014). These additional effects on GABAergic neurotransmission should be taken into consideration when interpreting results based on experimental use of muscimol. Antagonists provide an additional cautionary example of the issues surrounding GABAA receptor pharmacology and the use of recombinant receptor systems to classify pharmacological specificity. For example, bicuculline is widely reported to be a specific antagonist to GABAA receptors. This is true for bicuculline. However bicuculline is not readily soluble in aqueous solutions and degrades rapidly; thus bicuculline base and bicuculline salts are frequently used instead given their aqueous solubility and stability in solution at physiological pH (for a review see Johnston, 2013). These forms of bicuculline have broad pharmacological effects outside of GABAA receptors. Bicuculline salts antagonize Ca2+ activated K+ (SK) channels (Seutin and Johnson, 1999), inhibit acetylcholinesterase activity (Breuker and Johnston, 1975), and also antagonize other ligand gated ion channels in the cys-loop superfamily, such as the nicotinic acetylcholine receptor, the α2 glycine Cl− channel, and the 5HT(3A) cation channel (Sun and Machu, 2000), all of which are expressed in the SCN (Ito et al., 1991; Meredith et al., 2006; Carrillo et al., 2010) (for a review see Seutin and Johnson, 1999). Picrotoxin has been used as a GABAA antagonist, however, it is also a potent antagonist at glycine receptors (Chattipakorn and McMahon, 2002; James et al., 2014). Similarly, strychnine, which is widely reported to be a glycine receptor-specific antagonist, has equal potency at inhibiting GABAA receptors in vitro (Shirasaki et al., 1991).
Another popular GABAA antagonist, gabazine (SR 95531), can differentially affect GABAA-PHASIC and GABAA-TONIC receptors, depending on extracellular GABA concentrations. Under low extracellular GABA conditions, 200 nM gabazine preferentially blocks GABAA-TONIC currents (Cope et al., 2005), whereas under high extracellular GABA, 200 nM gabazine blocks GABAA-PHASIC currents (Stell and Mody, 2002). At higher concentrations (10 lM), gabazine can inhibit both classes of GABAARs (Stell and Mody, 2002) and function as a competitive antagonist at glycine receptors (Wang and Slaughter, 2005). Although gabazine has the potential to discriminate between GABAA-PHASIC and GABAA-TONIC receptors, there is currently not enough data to support its application as a selective antagonist for this purpose, and caution should be used in interpreting its effects on GABAA receptors containing different combinations of subunits.
Furthermore, species- and anatomically-specific differences exist in GABAA receptor pharmacology. In regard to allosteric modulators the “ethanol antagonist” RO15-4513, for example, is used as a GABAA-TONIC antagonist. However, it is effective at antagonizing the effects of ethanol in rats but not mice (for a review see (Lister and Nutt, 1987). Although RO15-4513 only has direct pharmacological effects at extrasynaptic GABAA receptors, it also has high affinity for all GABAA receptors with BZD binding sites, and can antagonize the effects of diazepam, another allosteric modulator that is reported to act exclusively at synaptic (phasic) GABAA receptors (Lister and Nutt, 1988). Furthermore, RO15-4513 can also be displaced from its binding site by both diazepam and the benzodiazepine antagonist flumazenil (RO15-1788) (Korpi et al., 2002). Thus it appears that all GABAA receptor subtypes may have BZD binding sites, but different BZD ligands can have both direct and indirect modulatory effects on these receptors dependent upon subtype.
Thus, when performing studies and interpreting the results of in vivo and in vitro research on GABAA receptors, one must carefully scrutinize the methodology and consider that the pharmacological and behavioral effects being measured. These effects may indeed be a result of actions of the drugs at other GABAA receptor subtypes, or on other receptors in the cys-loop superfamily of ligand gated ion channels that are present in the circadian system. Fortunately, the pharmacology of the GABAA family of receptors is a very active field of research, and thus new drugs with greater specificity are continuously being developed which should facilitate studies both advancing the field and confirming the plethora of data currently available on GABAergic neurotransmission in the circadian system.
See text above for specific citations.
4.1. GABAA receptors
GABAA receptors are pentameric hetero-oligomers with at least 19 possible subunits (see Table 1) (Rudolph et al., 2001; Vicini and Ortinski, 2004; Olsen and Sieghart, 2008). Different combinations of these subunits alter the receptors’ pharmacological properties and subcellular location (Belelli et al., 2009; Farrant and Nusser, 2005; Winsky-Sommerer, 2009). Some subunits are expressed widely throughout the brain, while others have more restricted anatomical distribution (for a review see Lee and Maguire, 2014). Most GABAA receptors contain two copies of a single α subunit, two copies of a single β subunit, and one copy of another subunit. One notable exception is GABAA receptors composed of varying combinations of three ρ subunits; these receptors were formerly classified as GABAC receptors (Olsen and Sieghart, 2008).
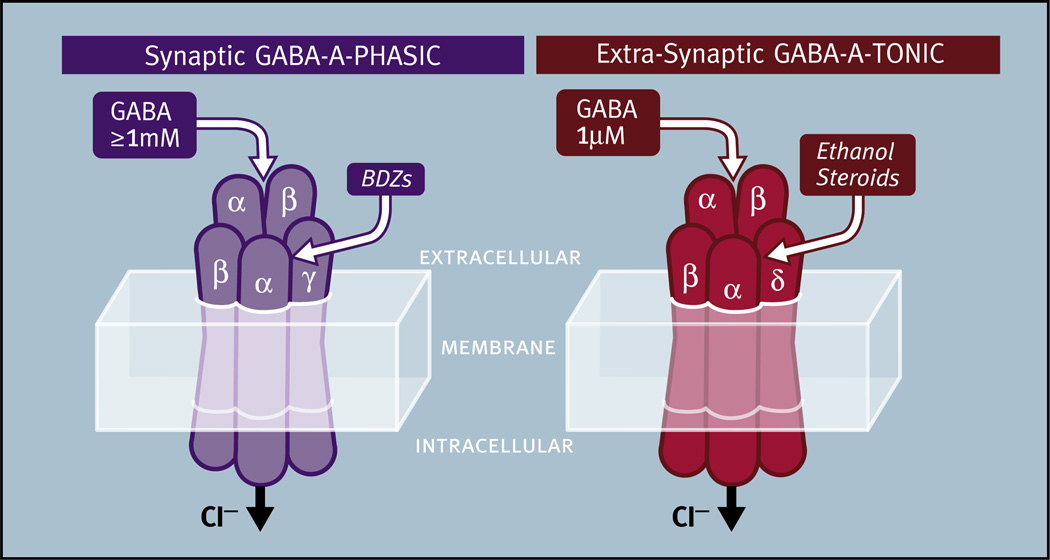
Most of the research on GABAA mediated neurotransmission has focused on receptors containing the γ subunit. The most common GABAA receptor in the brain (>40% of all GABAA receptors) is composed of the α1, β2 and γ2 subunits (i.e., 2 α1; 2 β2 and 1 γ2) (Chang et al., 1996; Baumann et al., 2002). These receptors are found in the synapse and mediate transient ‘phasic’ inhibitory postsynaptic currents (IPSCs) produced in response to presynaptically released GABA. These phasic GABAA receptors respond to GABA release within the synapse in concentrations greater than 1 mM, produce IPSCs which peak and decay within milliseconds, and display rapid desensitization (Mozrzymas et al., 2003). We will refer to these receptors as GABAA-PHASIC (after Stell and Mody, 2002) (Fig. 5). γ subunit-containing GABAA receptors are sensitive to benzodiazepines (BDZs) which bind to GABAA receptors in a pocket formed by adjacent α and γ subunits, a different site from the region that binds GABA (for a review see Tan et al., 2011) (see Table 2 for further discussion).
Fig. 5.
The properties of synaptic GABAA-PHASIC and extra-synaptic GABAA-TONIC receptors. Synaptic GABAA-PHASIC receptors are characterized by the presence of a γ subunit and respond to presynaptically released saturating concentrations of GABA (>1 mM). These receptors can produce inhibitory postsynaptic currents that peak and decay within milliseconds and rapidly desensitize. Benzodiazepines (BDZs) are thought to bind in the pocket formed by the α and γ subunits. Extra-synaptic GABAA-TONIC receptors are characterized by the presence of a δ subunit and respond to non-saturating GABA concentrations (0.5–1.0 µM). These receptors are activated for long intervals because they display low levels of desensitization. Ethanol and steroids are thought to bind in the pocket formed by the αl and δ subunits.
In contrast to GABAA-PHASIC receptors, GABAA receptors found outside of the synapse mediate tonic currents. Tonic GABA currents can control the gain of overall neuronal excitability resulting in stronger excitation as well as stronger inhibition (Glykys and Mody, 2007). Characterization of the magnitude of tonic currents mediated by these receptors has been complicated by a variety of experimental factors (e.g., temperature, pH) (for a review see Bright and Smart, 2013). What is clear is that these extrasynaptic receptors are BDZ insensitive, steroid sensitive, display a longer duration of activity due to low levels of desensitization, and respond to low ambient levels of GABA of around 0.5–1 µM (Semyanov et al., 2004; Santhakumar et al., 2006). Low ambient levels of GABA in the extracellular space outside of synapses can result from a variety of factors such as synaptic spillover or nonsynaptic GABA release from neurons or glia (Lee et al., 2010; Semyanov et al., 2004; Yoon and Lee, 2014). We will refer to these receptors as GABAA-TONIC receptors (after Stell and Mody, 2002) (Fig. 5). Although these receptors frequently contain the δ subunit, some extrasynaptic GABAA receptors that mediate tonic GABAergic currents do not (for a review see Lee and Maguire, 2014). GABAA receptors containing the δ subunit appear to associate exclusively with α1, α4, or α6 subunits in vivo (Jones et al., 1997; Sur et al., 1999; Glykys et al., 2007). It is largely unknown which β subunits associate with the δ subunit to form functional GABAA-TONIC receptors, but α6β2/3δ and α4β2/3δ oligomers have been identified in vivo (Nusser et al., 1999; Peng et al., 2002).
Much remains to be learned about the functions and potential interactions of GABAA receptor subtypes within the SCN and throughout the CNS (Wu et al., 2013). Given that subunit composition dictates GABAA receptor subcellular location and function, characterization of GABAA receptor composition is critical to understanding GABAergic communication within the SCN. Toward this end, a number of experimental approaches have been used to investigate the subunit composition of GABAA receptors in the SCN. Electrophysiological studies have taken advantage of the fact that GABAA receptors composed of αβ subunits are highly sensitive to zinc (Zn2+) while the presence of a γ subunit reduces Zn2+ sensitivity by two-fold (Smart et al., 1991; Draguhn et al., 1990). Results from studies of SCN neurons obtained from early postnatal rats are not consistent. One study found that GABA-induced inward current in the SCN was inhibited by Zn2+ in a dose-dependent manner, and that there was no potentiation of the current response by the BDZ diazepam as would be expected if the γ subunit was present (Kawahara et al., 1993). Another study also using SCN neurons from early postnatal rats found that diazepam potentiates GABA-induced currents, thereby suggesting that the SCN does contain γ subunits (Shimura et al., 1996). More recent studies in SCN neurons from older rats confirm that both Zn2+ and BDZ responses are found in the SCN, but disagree as to whether a significant number of SCN neurons contain receptor subunit configurations that include the γ subunit (Strecker et al., 1999; Kretschmannova et al., 2003, 2005). Interestingly, although Zn2+ is found mainly in the ventrolateral SCN (Huang et al., 1993), Zn2+ has a greater inhibitory effect during the day in the dorsomedial SCN, suggesting there is a rhythm in the number of GABAA receptors that contain the γ subunit, with peak levels occurring at night in this region (Kretschmannova et al., 2003). Consistent with this observation, we have recently observed that γ2 peaks in the SCN at night in hamsters housed in LD cycles, however in hamsters housed in constant darkness for 10 days this pattern is reversed and γ2 peaks during the subjective day (Walton et al., 2016).
Molecular and cellular approaches have also been used to investigate the presence of different GABAA subunits in the SCN. It is important to remember, however, that the presence of subunits does not necessarily indicate the presence of functional GABAA receptor subtypes composed of those subunits (see Olsen and Sieghart, 2008 for a discussion). As can be seen in Table 1, microarray data suggest that the SCN contains transcripts for all 19 GABAA receptor subunits. Although a previous study reported that transcripts for δ and ρ subunits were not detectable using Northern blot analysis (O’Hara et al., 1995), this study did not probe for some subunits that were not yet identified at that time (i.e., α6, γ3, ε, π, or θ). Based on immunohistochemical studies, the SCN appears to contain, at a minimum, protein for the α2, α3, α5, β1, β3, γ2, and δ subunits (Belenky et al., 2003; Naum et al., 2001; Walton et al., 2016). Subunit distribution varies across the ventral-dorsal and rostro-caudal extent of the SCN (Gao et al., 1995; Belenky et al., 2003).
GABAA transcript and protein levels of at least some of these subunits are rhythmic in the SCN, including γ2 mRNA (Pizarro et al., 2013; Walton et al., 2016), δ mRNA (Walton et al., 2016), β1 protein (Naum et al., 2001), and γ2 protein (Walton et al., 2016), suggesting that GABAARs composed of different subunits may have distinct roles in the SCN across the circadian cycle. It is important to remember, however, that mRNA rhythms can be uncoupled from protein rhythms in the SCN (Challet et al., 2013; Chiang et al., 2014), suggesting that rhythms in protein may prove more informative than rhythms in mRNA levels. We recently found that the ratio of the δ subunit (contained in the GABAA-TONIC receptor) to the γ2 subunit (contained in the GABAA-PHASIC receptor) is rhythmic in the SCN. mRNA expression patterns did not predict protein levels, indicating that the regulation of these receptors does not occur at the transcript level. The pattern of the protein rhythm suggests that GABAA-TONIC receptors are in greater abundance during the subjective night while GABAA-PHASIC receptors are in greater abundance during the subjective day (Walton et al., 2016). As discussed in Sections 9.1 and 9.2.2, these data are consistent with the hypothesis that the effects of GABA on circadian phase are mediated primarily by GABAA-PHASIC receptors during the subjective day and GABAA-TONIC receptors during the subjective night.
One study, investigated the subcellular localization of GABAA receptors with electron microscopic immunocytochemistry using a well characterized antibody to the α3 receptor subunit. These studies suggest that GABAA receptors are found on dendritic processes, neuronal perikarya, and axonal fibers and terminals (Belenky et al., 2003). These data also suggest that the majority of GABAA receptors are found on dendrites and are associated with both synaptic and extrasynaptic regions of the plasma membrane. About 25% of GABAergic axonal terminals display immunopositive staining for α3 subunits on extrasynaptic areas of their plasma membrane. Functionally, these presynaptic GABAA receptors can reduce GABA release within the SCN (Belenky et al., 2003). The subcellular localization and subcellular patterns of expression across the circadian cycle of the remainder of the GABAA subunits have yet to be described across the circadian cycle. Additionally, to our knowledge characterization of any complete assembled functional GABAA receptor pentamer has yet to be described in the SCN. Given the central role of GABAergic neurotransmission in the SCN, a thorough characterization of the GABAA receptors within the SCN remains a large gap in our knowledge.
4.2. GABAB receptors
The GABAB receptor was first identified more than three decades ago, after the demonstration that GABAA receptor antagonists were unable to block the late component of inhibitory transmission (Hill and Bowery, 1981; Bowery et al., 1981). GABAB receptors, like other G protein coupled receptors, have a central core domain composed of seven transmembrane helices. The GABAB receptor is composed of two subunits, GABAB1 and GABAB2, and the activation of GABAB receptors results from conformational changes within and across these two subunits. GABAB receptors respond to extracellular GABA concentrations of around 50 nM (Enna and McCarson, 2013).
Immunocytochemical studies of GABAB subunits have provided a great deal of information on the location of GABAB receptors within the SCN (Belenky et al., 2008). Overall, there appear to be more GABAB receptors in the dorsal SCN than in the ventral SCN. Consistent with the GABAB receptor functioning as a heterodimer, protein for both subunits of the GABAB receptor appears to be equally expressed in the SCN (Belenky et al., 2008), despite differences in their mRNA expression levels (Table 1). Although no immunocytochemical studies have directly investigated circadian patterns of GABAB expression in the SCN, the GABAB agonist baclofen has greater potency in inhibiting SCN neuronal activity at night than during the day (Gribkoff et al., 2003), suggesting the possibility that GABAB receptor number also varies across the circadian cycle. In the dorsal SCN, GABAB receptors are numerous on cells bodies and dendrites, but appear in smaller numbers on terminals. Further, many of the GABAB receptors found in the dorsal SCN are located in extrasynaptic regions of the membrane. The identification of postsynaptic GABAB receptors in SCN raises questions, because the GABAA receptor antagonist bicuculline blocks most postsynaptic GABAergic responses in the SCN. These data suggest that GABAB receptors are primarily responsible for pre- and not postsynaptic inhibition (Jiang et al., 1995, 1997a; Chen and van den Pol, 1998; Kim and Dudek, 1992). GABAB receptors in the ventral SCN are highly localized in discrete areas and are closely associated with retinal afferents and terminals. As such, GABAB receptors are well positioned to modulate photic input to the SCN through presynaptic mechanisms (see Sections 5.5 and 9.2.3).
4.3. Summary of GABA receptors
Although the SCN appears to contain transcripts for all 19 GABAA receptor subunits, and both GABAB subunits, the translation of these transcripts into protein has only been confirmed for 7 of the GABAA subunits and both GABAB subunits. Some SCN neurons appear to have only GABAA receptors, others only GABAB receptors, while still others appear to have both GABAA and GABAB receptors (Strecker et al., 1994; Liou et al., 1990). GABAA-PHASIC, GABAA-TONIC, and GABAB receptors can be found both presynaptically and postsynaptically in the SCN (Fig. 4). The sustained effects of GABAA-TONIC receptors are distinct from the transient activation of GABAA-PHASIC receptors, and from the slower, but still transient, response of GABAB receptors.
GABAA receptors are found in greater numbers in the ventral SCN than in the dorsal SCN. While GABAA receptor subunits can be found throughout the neuronal membrane, many are seen on dendrites associated with both synaptic (GABAA-PHASIC receptors) and extrasynaptic (GABAA-TONIC receptors) regions. As such, GABAA receptors have the capacity to mediate a variety of GABA effects within the SCN. Conversely, GABAB receptors occur more frequently in the dorsal than in the ventral SCN, though presynaptic GABAB receptors are well positioned in the ventral SCN to modulate photic input.
The expression of some GABAA subunits is rhythmic across the circadian cycle, whereas rhythmic expression of GABAB receptors has yet to be reported. Because GABA-active drugs can have phase-dependent effects on circadian rhythms it is important to define the variations in GABA receptor composition and expression across the circadian cycle. As discussed below, there is evidence that all three of these GABA receptor subtypes play important roles in regulating the phase of the circadian pacemaker by their actions within the SCN region (see Section 9).
5. Factors regulating extracellular levels of GABA
The amount and distribution of GABA in the intra- and extracellular space depends on a complex interaction among factors controlling GABA release, synthesis, and transport. As discussed above (Section 3), GABA release from neurons depends on the exocytosis of GABA containing vesicles resulting from Ca2+ influx via voltage-gated ion channels or Ca2+ released from intracellular stores. Of course, the amount of GABA release from neurons depends on the amount of GABA contained within vesicles that are ready for release. In the following section, two mechanisms that mediate the amount of GABA available for neuronal release, GABA synthesis and the transport of GABA into vesicles, will be discussed (Fig. 4).
5.1. Regulation of releasable GABA in the SCN: GABA synthesis and GABA vesicular transporters
Glutamic acid decarboxylase (GAD) is the enzyme that catalyzes the reaction converting glutamic acid into GABA and represents the rate-limiting step in GABA synthesis (for reviews see Buddhala et al., 2009; Kaufman et al., 1991). Two distinct isoforms of GAD (i.e., GAD65 and GAD67), both found within the SCN, are encoded by two different genes and differ in sequence, molecular weight, and subcellular distribution. These isoforms of GAD regulate GABA synthesis thus influencing the cellular and vesicular content of GABA (Soghomonian and Martin, 1998; Engel et al., 2001). Studies employing knockout mice indicate that deletion of the GAD67 gene reduces basal levels of GABA in the brain by over 90%, while deletion of the GAD65 gene does not reduce GABA levels (Asada et al., 1996, 1997; Condie et al., 1997). Activity dependent increases in GAD67 lead to increased GABA synthesis, while reduction in activity reduces GABA levels, likely through down regulation of GAD67 (Soghomonian and Martin, 1998; Freichel et al., 2006). Although the regulation of GABA synthesis is not fully understood, GAD67 appears to provide the resting levels of GABA for tonic release, whereas activation of GAD65 synthesizes GABA at the synapse for phasic release during times of elevated synaptic activity (Tian et al., 1999; Patel et al., 2006).
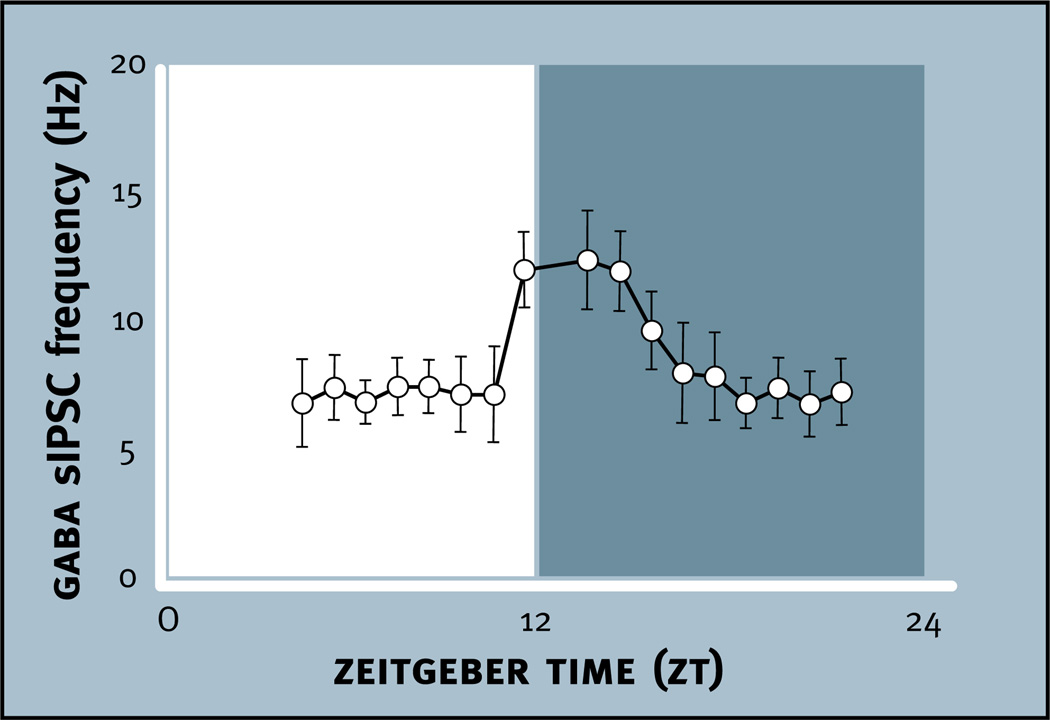
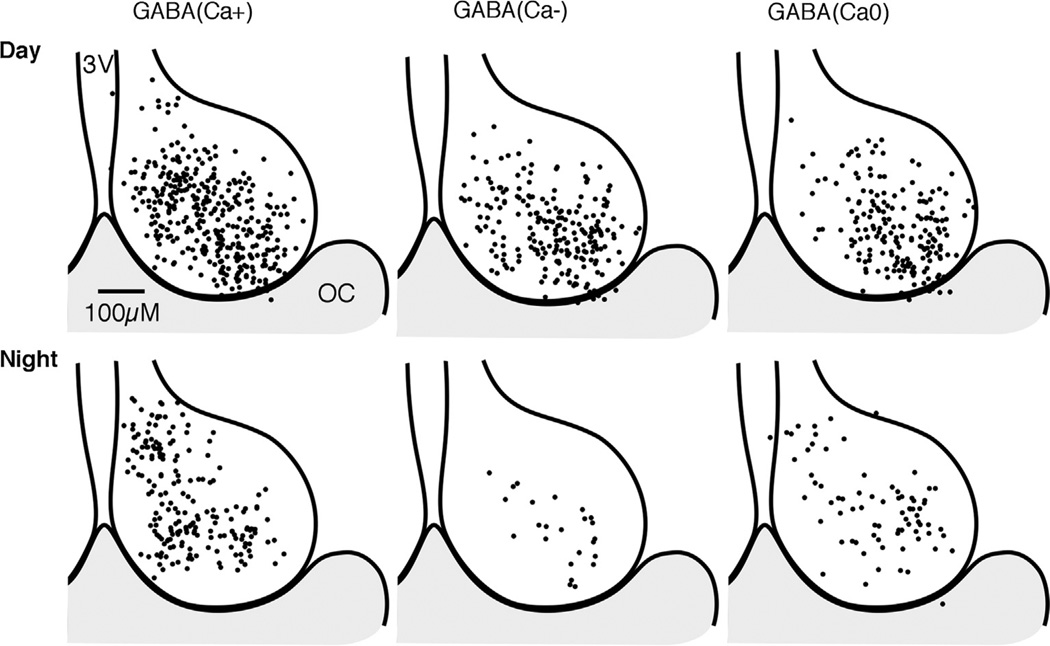
Using an antibody against both GAD65 and GAD67 (GAD65,67), an immunohistochemical study examined where these enzymes are localized in the SCN in tissue obtained from rats during the light phase of the LD cycle (Belenky et al., 2008). GAD65,67 immunoreactivity is found at high levels in the ventral region and in particular in association with VIP immunoreactive neurons and projections. Lower levels of immunoreactivity are found in the dorsal SCN. Measurements of GAD activity and GABA content within dissections of the rat SCN are highly correlated; rhythms in GAD activity and GABA content peak during the early night (Aguilar-Roblero et al., 1993). The rhythm in GABA content can persist in constant conditions for at least three days. SCN levels of GAD65 mRNA, but not GAD67 mRNA have a 24 h rhythm that peaks during the light phase (Huhman et al., 1996). Cellular analysis reveals that GAD65 mRNA is rhythmic in the dorsal SCN but not the ventral SCN. It appears that this rhythm of GAD65 mRNA can persist for at least one day in constant darkness but becomes arrhythmic within nine days in constant darkness (Huhman et al., 1999; Cagampang et al., 1996). Taken together, these data suggest that the highest level of GAD65 occurs in the dorsal and not the ventral SCN during the late light phase and early dark phase, resulting in peak GAD activity and synaptic GABA during this phase of the cycle. Interestingly, peak spontaneous inhibitory postsynaptic currents (sIPSCs) in the dorsal but not the ventral SCN also occur during the late light phase and early dark phase, which would be consistent with GAD65-induced GABA synthesis during this time of elevated synaptic activity (Itri et al., 2004) (see Section 5.4).
GABA vesicular transporters (VGATs) are responsible for the transport of both GABA as well as the neurotransmitter glycine into synaptic vesicles (McIntire et al., 1997; Gasnier, 2004). Due to the ability of VGAT to package both major inhibitory neurotransmitters, these transporters are also referred to as vesicular inhibitory amino acid transporters (VIAAT) (Sagne et al., 1997). The VGAT/VIAAT transporter is a member of the amino acid/polyamine/organocation superfamily and is expressed in GABAergic and glycinergic nerve terminals (Chaudhry et al., 1998; Dumoulin et al., 1999; Takamori et al., 2000). VGAT plays an important functional role in GABAergic synaptic transmission by regulating the loading of GABA into synaptic vesicles. For example, reductions in vesicle loading of GABA leads to reductions in the amplitude and frequency of GABAA receptor-mediated currents, suggesting a reduction in pre- and post-synaptic activation of GABA receptors (Riazanski et al., 2011).
VGATs are expressed in both the ventral and dorsal regions of the SCN, and like GAD, are expressed more highly in VIP-containing neurons of the ventral SCN than in AVP-containing neurons of the dorsal SCN in rats and mice examined in tissue obtained during the early light phase of the LD cycle (Castel and Morris, 2000; Belenky et al., 2008). At the subcellular level, VGATs are found extensively in both neuronal cell bodies as well as in axon terminals in the ventral SCN. Double labeling for VGATs and VIP were identified in some neurons. It is not known if VGAT occurs rhythmically in the SCN. In whole mouse brain the levels of VGAT protein do not vary over the LD cycle, although protein levels are significantly higher in mice housed in LD cycles than in mice housed in constant darkness (Darna et al., 2009). Given that GAD and VGAT are functionally coupled and that the rhythms in GAD mRNA seen in animals entrained to LD cycles appear to damp out in constant darkness, it is possible that both may be regulated at the transcriptional level in the SCN by photic stimuli (Jin et al., 2003; Huhman et al., 1999; Darna et al., 2009).
In summary, GAD and VGAT are essential for the formation of releasable GABA. GAD is found at higher levels in the ventral than in the dorsal SCN, at least during the day. GAD65 mRNA is rhythmic in the dorsal SCN but not the ventral SCN with the highest levels occurring in the light phase. There appears to be a 24-h rhythm in GABA synthesis and content in the SCN that peaks during the early night. Neither GABA synthesis nor the transport of GABA into vesicles appear to be under strong circadian control but rather seem to be regulated by environmental lighting conditions.
5.2. Membrane GABA transporters (GATs)
GATs are high affinity membrane proteins that are members of the solute carrier superfamily 6 (SLC6) (Gadea and Lopez-Colome, 2001; Minelli et al., 1996; Durkin et al., 1995; Conti et al., 2011). GATs use Na+ and Cl− gradients to cotransport GABA across cell membranes (Kristensen et al., 2011) into neurons and astrocytes from the extracellular space (for reviews see Iversen and Neal, 1968; Zhou and Danbolt, 2013) (Fig. 4). Because there is no known extracellular metabolism of GABA, GATs provide the primary mechanism for terminating GABA’s actions in synaptic and extrasynaptic regions. The rapid reuptake of GABA via GATs is also thought to provide a barrier separating the effects of GABA at synaptic regions from GABA’s effects in the extrasynaptic space (Yoon and Lee, 2014).
Four SLC6 transporters have been identified for GABA (GAT-1, GAT-2, GAT-3, and BGT-1). Although all four types are found in the CNS, they are expressed differentially across brain regions and cell types (Gadea and Lopez-Colome, 2001; Moldavan et al., 2015). The most important transporters in the brain are GAT-1 and GAT-3 (Zhou and Danbolt, 2013; Zhou et al., 2012). GAT-1 is found on astrocytic processes as well as on some neurons, and can regulate both phasic and tonic GABA neurotransmission (Ribak et al., 1996; Jensen et al., 2003; Bragina et al., 2008). In contrast, GAT-3 may be localized exclusively on astrocytes (Minelli et al., 1996; Clark et al., 1992). Pharmacological inhibition of GAT-3, in many of the same regions in which GAT-1 mechanisms have been explored, increases tonic GABA inhibition and extracellular concentrations of GABA (Kersante et al., 2013; Jin et al., 2011).
Another important function of astrocytic GATs is the release of neuroactive substances or “gliotransmitters” into the extracellular space (Petrelli and Bezzi, 2016). Gliotransmitters are released from astrocytes when the actions of GATs are reversed, causing an extrusion instead of an uptake of neuroactive substances. Elevations of Ca2+ within astrocytes may also release gliotransmitters, although this possibility is highly controversial (Bazargani and Attwell, 2016). GABA is one of the gliotransmitters that can be released by the reverse action of GATs. This form of GABA release can occur during some pathological states, but also during normal physiological processes (Richerson and Wu, 2003; Yoon and Lee, 2014). As such, the actions of GATs are complex in that they can both remove GABA from, and release GABA into, extracellular spaces. These interactions between neurons and astrocytes have led to the idea that synapses can be “tripartite,” in that their functional activity is dependent upon the actions of three elements: (1) neuronal presynaptic terminals, (2) neuronal postsynaptic membranes, and (3) astrocytic GATs driving both reuptake and release (Fig. 4) (Araque et al., 1999, 2014). In fact, the concept of tripartite functional interactions between neurons and astrocytes likely modulates non-synaptic neurotransmission, such as volume transmission, as well as synaptic GABA neurotransmission.
Immunohistochemical studies have found GAT-1 and GAT-3 to be distributed evenly throughout the major subdivisions of the SCN. Interestingly, their distribution is not highly correlated with the distribution of glial fibrillary acidic protein (GFAP), an essential element within the cytoskeleton of astrocytes (Moldavan et al., 2015). GAT-1- and GAT-3-immunoreactivity has not been identified in SCN neurons but rather in a substantial number of SCN astrocytes (Fig. 4). GAT-1 and GAT-3 appear to be localized in astrocytic processes in extrasynaptic and not synaptic areas around SCN neurons. Neither GAT-1 nor GAT-3 appear to change rhythmically over the day when measured in rats housed in a 24 h LD cycle. There is evidence however, although controversial, that neuroglial synaptic arrangements change rhythmically over the day in the SCN (Elliott and Nunez, 1994; Girardet et al., 2010).
At present, little is known about the specific roles of GATs within the SCN in circadian timekeeping. Administration of the general GAT inhibitors nipecotic acid and riluzole increases AVP in SCN slice preparations several hours after their administration (Isobe and Nishino, 1997). Inhibition of GABA transporters with nipecotic acid in rat hypothalamic slices, results in the activation of GABAB receptors, producing an inhibition of glutamate release from RHT terminals in the ventral SCN (Moldavan and Allen, 2013). Thus, it appears that inhibition of GATs can increase ambient GABA levels in the SCN (see Sections 5.5 and 9.2.3). It seems likely, however, that the role of GATs in determining the levels of extracellular GABA is far more extensive. GATs likely have a substantial role in determining the strength and mode of GABAergic neurotransmission in the SCN by both removing GABA from and releasing GABA into the extracellular space (Moldavan et al., 2015).
5.3. Extracellular levels of GABA within the SCN
As discussed above, the processes that release GABA and remove GABA from the extracellular space determine the extracellular levels of GABA at specific circadian phases and within specific regions of the SCN. When considering the pattern of GABA release within the SCN, it is important to remember that a major source of GABA is the large number of GABAergic synaptic terminals found in the SCN, and that these terminals come from neuronal cell bodies arising from different subdivisions within the SCN as well as from neuronal cell bodies from a variety of sites outside the SCN. Although extracellular concentrations of GABA in the low nanomolar range have been measured in the SCN, it has not yet been feasible to measure the patterns of extracellular GABA in the SCN across the 24-h day (Ehlen et al., 2005). It does seem likely that terminals coming from extra-SCN sites contribute to extracellular levels of GABA in the SCN, given that electrical stimulation of extra-SCN sites can induce IPSPs in a large percentage of SCN neurons (Kim and Dudek, 1992). The factors that regulate the release of GABA from extra-SCN sites, however, are largely unknown. Of the three major afferent projections to the SCN only the GHT contains GABA, but the pattern of GABA release from this pathway is also not known.
Distinct subpopulations of GABA containing terminals within the SCN may display different patterns of synaptic GABA release. Given the higher firing rate of SCN neurons during the day, it would be predicted that the spontaneous synaptic release of GABA would be higher during the day than during the night. It is also likely that non-synaptically released GABA from neurons and/or astrocytes contributes to extracellular levels of GABA. Therefore, the extracellular concentrations of GABA could result from a constitutive presence of GABA in the extracellular space as well as more punctuated and pronounced local release of GABA from synaptic terminals. Indeed, it has been hypothesized that a tonic release of GABA via non-synaptic mechanisms modulates excitability levels in the SCN by their action on GABAA-TONIC receptors (Wagner et al., 2001). An important role of relatively low levels of tonically released GABA is also suggested by the low levels of desensitization of GABAA-TONIC receptors, and the possibility that even small quantities of GABA from densely packed SCN neurons may substantially increase the levels of extracellular GABA.
5.4. GABA-induced currents within the SCN
One approach that has been used to investigate the endogenous pattern of GABA release within the SCN is to measure GABAA-mediated neurotransmission using the hypothalamic slice preparation. Of course this approach limits the analysis to GABA released from cells left intact within the slice preparation itself. Spontaneous GABAA mediated currents have been observed in most SCN neurons using whole-cell, patch clamp recordings (Jiang et al., 1997a; Strecker et al., 1997; Kim and Dudek, 1992). Because this technique relies on the intracellular pipette Cl− concentration, it can be difficult to determine whether observed events are excitatory or inhibitory, although a clear role for GABA in mediating these currents is indicated by their inhibition by GABA antagonists (e.g., bicuculline).
In mice housed in a 12:12 LD cycle prior to slice preparation, the frequency of fast sIPSCs is consistently higher in the dorsal SCN than in the ventral SCN. In addition, there is a rhythm in GABAA-induced sIPSCs in the dorsal SCN that peaks in the late subjective day and early subjective night (Itri et al., 2004; Itri and Colwell, 2003) (Fig. 6). The rhythm is dependent on synaptic activity because it can be blocked by tetrodotoxin (TTX) administration, which inhibits Na+-dependent action potentials. In contrast, no rhythm is observed in GABAA-induced IPSCs in the ventral SCN. As discussed earlier, these data correspond well with the peak of GABA synthesis that occurs during the late day and early night in the dorsal SCN (see Section 5.1).
Fig. 6.
GABAergic spontaneous inhibitory postsynaptic current (sIPSC) frequency peaks between Zeitgeber time (ZT) 11 and ZT 15 in suprachiasmatic neurons of mice housed in a 12:12 light:dark cycle prior to slice preparation. Each data point represents the average frequency of GABAergic sIPSCs during a 1-h time bin ± SE (n = 8–17/bin) (modified from Itri et al., 2004).
This rhythm of GABAA-induced currents appears to depend on activation of VIP VPAC2 receptors. No 24-h rhythms in GABAA-induced IPSCs are observed in slices prepared from VIP/PHI-deficient mice or in VPAC2 receptor antagonist-treated slices obtained from wild-type mice. In addition, administration of VIP within the slice significantly increases GABAA-induced IPSCs, an effect that can be blocked by a VPAC2 receptor antagonist. Consistent with the hypothesis that synaptically released VIP induces the rhythm in GABAA-induced IPSCs is the finding that VIP is synaptically released in the SCN during the middle of the light period in hamsters entrained to a LD cycle (Francl et al., 2010a, 2010b). Further support for this hypothesis comes from the finding that VIP neurons in the SCN have a higher repetitive firing rate during the second half of the light period than at night in slices prepared from mice previously entrained to a LD cycle (Hermanstyne et al., 2016). Thus, there is strong support for the hypothesis that the rhythmicity in GABAA-induced currents, observed in the dorsal SCN, is mediated by rhythmic activation of VPAC2 receptors by VIP during the late day and early night.
In mice housed in constant darkness for four days prior to slice preparation, the pattern of GABAA mediated IPSCs changes from that seen in mice previously housed in LD cycles (Itri et al., 2004). GABAA-mediated currents are enhanced not only during the early subjective night but also during the mid subjective day. Later in the subjective night, however, GABAA IPSCs are significantly lower. Therefore, day-night differences persist for at least four days in constant darkness, and these currents are enhanced for a much larger proportion of the circadian cycle in constant darkness than in LD. Induction of high levels of GABAA-mediated currents requires VIP/PHI because they are absent in VIP/PHI-deficient mice. It will be important to determine whether the persistent rhythmicity in GABAA mediated currents seen in constant darkness represents a damped rhythm like that seen for GAD65 mRNA, because rhythms in extracellular levels of VIP in the SCN rapidly damp out in constant darkness, at least in hamsters (Francl et al., 2010a, 2010b).
5.5. Antagonism of extracellular GABA within the SCN
GABA found in extracellular space can come from synaptic, extrasynaptic or glial release. One approach to investigating the temporal pattern of GABA levels present in the extracellular space is to examine when GABA antagonists are capable of altering the firing of SCN neurons. Presumably, alterations in neuronal firing would result from the antagonist blocking the actions of endogenous levels of extracellular GABA. As discussed below, GABA has predominantly inhibitory effects in the SCN; however, there are conflicting views as to whether GABA can also have excitatory effects on some neurons (see Section 7). Similarly, there are conflicting reports on whether GABA antagonists consistently increase firing (as they would if GABA is exclusively inhibitory) or whether GABA antagonists can reduce firing in some cases (as they would if GABA had excitatory effects on some SCN neurons). For the purposes of the present discussion we will ignore whether GABA antagonists increase or decrease firing for the moment to see if there are consistencies across studies regarding when and where GABA antagonists alter SCN firing rates.
Multiple unit recordings from the hypothalamic slice have found that administration of the GABAA antagonist bicuculline significantly alters spontaneous firing within the SCN during the day and during the night (Gribkoff et al., 1999, 2003; Albus et al., 2005). GABAA antagonism alters the firing rate of SCN neurons in the ventral core and the dorsal shell, even when the ventral core and dorsal shell are surgically separated (Albus et al., 2005). In contrast, GABAB antagonism does not alter spontaneous activity in the SCN at any time point (Gribkoff et al., 2003). Studies employing single unit recordings have also shown that bicuculline alters the firing rate of SCN neurons (Mason et al., 1991; Choi et al., 2008). Evaluation of the effects of bicuculline on the firing recorded from a large number of SCN single units reveals that about 45% do not alter their firing rate in response to bicuculline, and that there does not appear to be a substantial difference in the percentage of units responding during the day versus the night (Choi et al., 2008). The same investigators also employed gramicidin-perforated patch clamp recordings, which do not perturb intracellular ionic concentrations, to show that bicuculline completely blocks GABAAR mediated spontaneous IPSPs and EPSPs. Other studies using gramicidin-perforated patch-clamp recordings found that bicuculline has a robust effect on neuronal firing during the day (Gribkoff et al., 1999; De Jeu and Pennartz, 2002). At night, however, the effects of bicuculline on neuronal firing are more limited and more variable.
In vivo studies in hamsters housed in constant darkness for at least a week suggest that there is a circadian rhythm in extracellular GABA in the SCN. Antagonism of either GABAA or GABAB receptors in the SCN prior to a light pulse provided at circadian time (CT) 13.5, but not at CT 19, significantly increases the phase shifting effects of light, suggesting that extracellular GABA is present at CT 13.5 but not CT 19 (Gillespie et al., 1996, 1997). Thus, this indirect evidence suggests that GABA is high during the early, but not late subjective night. One possible explanation for the presence of extracellular GABA at CT 13.5 but not CT 19 involves the combined activity of GABAergic SCN neurons, extra-SCN inputs to the SCN, and GABA uptake mechanisms (Moldavan and Allen, 2013). Because the firing frequency of SCN neurons is substantially higher during the day, synaptic GABA release and spillover may be higher during the day, and early night (e.g., CT 13.5), than during the late night (e.g., CT 19) (Green and Gillette, 1982; Jobst and Allen, 2002; Meijer et al., 1998; Montgomery et al., 2013). Because of the higher level of GABA release during the day/early night, this endogenous GABA increases the inhibition of release from RHT terminals. Antagonism of GABAB receptors increases evoked postsynaptic current amplitude and increases the magnitude of light-induced phase delays (Moldavan and Allen, 2013). Nevertheless, because of strong GABA uptake mechanisms, the endogenous extracellular GABA around RHT terminals remains less than maximal and, as a result, the GABAB agonist baclofen enhances presynaptic inhibition of RHT terminals and reduces light-induced phase delays.
In summary, several different indirect measures of spontaneous GABA levels in the SCN suggest that extracellular levels of GABA can be found during both the day and night, but most prominently during the late day and early night in animals housed in LD cycles. Some of these measures of spontaneous GABA levels suggest that these rhythms damp out in constant conditions (e.g., GAD65 mRNA levels) while other measures suggest they persist (e.g., GABA-mediated currents). It will be important to more clearly define the pattern of extracellular GABA levels both in animals entrained to LD cycles and free-running in constant conditions.
6. Excitatory and inhibitory effects of GABA: role of cation Cl− cotransporters (CCCs)
While GABA is widely recognized as the primary inhibitory neurotransmitter in the adult brain, GABA acts primarily as an excitatory neurotransmitter during early development (for a review see Ben Ari et al., 2012). The transition from GABA’s depolarizing effects to its hyperpolarizing effects appears to occur around the second postnatal week in rodents (Owens and Kriegstein, 2002; Kilb, 2012). Studies in the SCN are consistent with this timeframe, in that GABA primarily elevates intracellular Ca2+ through approximately postnatal day 10 and subsequently reduces intracellular Ca2+ in most SCN neurons (Obrietan and van den Pol, 1995; Ikeda et al., 2003).
While GABA is the primary inhibitory neurotransmitter in the mature brain, GABA can also have excitatory effects. GABA acts as an inhibitory neurotransmitter when intracellular Cl− concentrations are lower than extracellular Cl− concentrations, resulting in an influx of Cl− and hyperpolarization of the membrane potential. In contrast, GABA acts as an excitatory neurotransmitter when intracellular Cl− concentrations are higher than extracellular Cl− concentrations, resulting in outward current (i.e., efflux of Cl− ions) and depolarization of the membrane potential (Fig. 7). Although the SCN was among the first brain regions in which GABA was reported to have excitatory effects in adults, excitatory effects of GABA have also been seen in other brain regions (e.g., Haam et al., 2012).
Fig. 7.
Mechanisms underlying the excitatory and inhibitory effects of GABA on neuronal activity. In neurons that are depolarized and thus excited by GABA, NKCC1 transporters are more abundant than KCC2 transporters, resulting in higher levels of intracellular chloride (Cl−) than extracellular Cl− (left, top). Upon GABAA channel opening, the efflux of negatively charged Cl− ions produces depolarization of the neuron (left, bottom). In neurons that are hyperpolarized and thus inhibited by GABA, KCC2 transporters are more abundant than NKCC1 transporters resulting in lower Cl− in the neuron relative to Cl− outside the neuron (right, top). Upon GABAA channel opening, negatively charged Cl− ions entering the neuron cause hyperpolarization of the neuron (right, bottom).
Although ion trafficking across membranes is the basis of electrical signaling and depends upon both ion channels and ion transporters, channels have been studied extensively while comparatively little attention has been paid to transporters (for reviews see Blaesse et al., 2009; Benarroch, 2013). More recently, however, it is clear that CCCs maintain the electrochemical Cl− gradient required for classic hyperpolarizing postsynaptic inhibition mediated by GABAA receptors. In fact, the actions of CCCs are critical in determining whether GABA is hyperpolarizing or depolarizing in both immature and mature brains. Two categories of CCCs appear to be particularly important in regulating intracellular Cl− and thereby determining the polarity of GABA’s effects. The Na-K-2Cl cotransporters (NKCC) mediate Cl− uptake and consist of two isoforms, NKCC1 and NKCC2. NKCC1 is widely distributed throughout the nervous system and is found in cell bodies, dendrites, and axons of neurons as well as in glial cells. The K-Cl cotransporters (KCCs) mediate Cl− extrusion and include four isoforms, KCC1-4. KCC2 is found extensively throughout the nervous system and is expressed in cell bodies and dendrites but, generally, not in axons. KCC3 is coexpressed with KCC2 in some neuronal subtypes and in glial cells. At present, there is substantially more information about the function of NKCC1, KCC2 and KCC3 than the other CCC isoforms in regulating Cl− gradients and therefore the polarity of GABAA receptor activity in the brain (Benarroch, 2013).
Detailed anatomical studies reveal that NKCC1 and KCC1-4 are found in the SCN (Kanaka et al., 2001; Belenky et al., 2008, 2010). KCC2 can be colocalized with VIP and GRP neurons within the ventral SCN, and is often closely associated with RHT and GHT input. KCC2 is not expressed in the dorsal SCN or in AVP neurons. Similarly, KCC1 is also found prominently in the ventral SCN but rarely in the dorsal SCN. In contrast, KCC3 is expressed primarily in cell bodies and KCC4 in cell bodies and dendrites of AVP neurons. The relative roles of KCC1-4 in mediating the removal of Cl− from within SCN neurons is not known; however, there is evidence that KCC2 has higher cation affinity than KCC1, 3, or 4 (Mercado et al., 2006). In the SCN, NKCC1 can be found in AVP, VIP, and GRP containing neurons. In rats and mice, NKCC1 expression is significantly higher during the night than during the day in the dorsal, but not the ventral SCN (Choi et al., 2008; Myung et al., 2015). In mice, for example, NKCC1 protein measurements in dissections of the SCN are two-fold higher in the dorsal SCN during the night (i.e., 4 h after lights off) as compared to during the day (i.e., 4 h after lights on) (Choi et al., 2008).
In summary, given the importance of ion trafficking in electrical signaling, CCCs play an important role in determining the electrical activity of SCN neurons by establishing Cl− gradients, which in turn determine whether GABA’s effects are inhibitory or excitatory. The Cl− exporter KCC2 is found primarily in the ventral SCN in association with VIP and GRP neurons. Although the Cl− importer NKCC1 is found in VIP and GRP neurons in the ventral SCN, it appears to be expressed in substantially higher levels in AVP neurons within the dorsal SCN. Therefore, GABA may be more excitatory to more neurons in the dorsal SCN than in the ventral SCN. It remains important, however, to identify the ratio of Cl− importers to Cl− exporters to define the polarity of the response to GABA. Because CCCs play a critical role in determining the polarity of the response to GABA, understanding their functions in the SCN will become increasingly important as the potential circadian functions of the ratio of GABA inhibition:excitation are more clearly defined.
7. Cellular responses to GABA within the SCN
Studies of the cellular actions of GABA have examined the response of SCN neurons to GABA as well as to selective GABAA-and GABAB-active drugs (Tables 3–5). The results of these studies are complex, and as yet no clear consensus of the cellular actions of GABA within the SCN has been reached. At least some of the differences in the results among studies may be due to the use of different techniques to administer GABA as well as differences in the methods used to record the cellular responses to GABA and GABA-active drugs. GABA’s role in the SCN at the cellular level has been investigated primarily using in vitro preparations employing a variety of electrophysiological techniques (e.g., multiple unit recordings, patch clamp, etc.), measurements of intracellular Ca2+ concentrations, and the mRNA and protein products of a variety of different genes. In most cases, these studies have examined the acute but not the sustained changes in cellular activity in response to manipulation of GABA in the SCN.
Table 3.
Cellular effects of GABA on SCN neurons.
| Paper | LD cycle |
Technique | Tissue preparation | GABA administration | Effects on SCN neurons |
|---|---|---|---|---|---|
|
Shibata et al. (1983) PMID 6633950 |
12:12 | Extracellular single unit recording |
Slice preparation; PD11 and adult rats |
GABA (iontophoresis) | GABA inhibited 97% of neurons examined. All recordings done during the day |
|
Shibata et al. (1983) PMID 6668768 |
12:12 | Extracellular single unit recording |
Slice preparation; rats | GABA (iontophoresis) | GABA inhibited 90% and 4% were excited/inhibited; no differences in GABA effects in ventral versus dorsal SCN. All recordings done during the day |
|
Mason (1986) PMID 3795084 |
12:12 | Extracellular single-unit recording |
Urethane anesthetized rats |
GABA (iontophoresis) | No day-night variation in the inhibitory effects of GABA; the inhibitory effects of GABA were reduced in rats housed in constant light |
|
Liou et al. (1990) PMID 1976421 |
12:12 | Extracellular single unit recording |
Slice preparation; rats | GABA (bath application) | Overall GABA inhibited 65% and excited 12%; 61% of neurons in the ventral SCN were inhibited and 69% of neurons in the dorsal SCN were inhibited |
|
Liou and Albers (1990) PMID 2207720 |
14:10 | Extracellular single unit recording |
Slice preparation; hamsters |
GABA (bath application) | GABA inhibited 55% and excited 7%; no differences between ventral and dorsal SCN; GABA altered firing in 48% of neurons during the day and 66% at night |
|
Mason et al. (1991) PMID 1913180 |
12:12 | Extracellular single unit recordings |
Slice preparation; hamsters |
GABA (iontophoresis) | GABA inhibited 92% with no effect in 8%; GABA inhibited firing in 98% during the day and 89% at night |
|
Bos and Mirmiran (1993) PMID 8095843 |
Not noted |
Extracellular single unit recordings |
Cultured organotypic SCN slices prepared from PD12 rats |
GABA (iontophoresis) | GABA inhibited 91% |
|
Kawahara et al. (1993) PMID 8247331 |
Not noted |
Whole-cell voltage- clamp |
Dissociated culture of SCN neurons; PD21 rats |
GABA (bath application) | GABA elicited inward currents in all neurons tested at a holding potential of −60 mV |
|
Kawahara et al. (1994) PMID 7965836 |
Not noted |
Whole-cell voltage- clamp |
Dissociated culture of SCN neurons; PD23–28 rats |
GABA (bath application) | GABA elicited inward currents in all neurons tested at a holding potential of −60 mV |
|
Obrietan and van den Pol (1995) PMID 7623135 |
Not noted |
Fura-2 Ca2+imaging | Dissociated culture of SCN neurons; ED18 rats |
GABA (bath application) | GABA elevated Ca2+in embryonic SCN neurons |
|
Obrietan and van den Pol (1996) PMID 8627385 |
Not noted |
Fura-2 Ca2+imaging | Dissociated culture of SCN neurons; organotypic SCN cultures; ED19–21 rats |
GABA (bath application) | GABA elevated Ca2+in embryonic SCN neurons maintained in dissociated and organotypic cultures |
|
Shimura et al. (1996) PMID 8764156 |
12:12 | Voltage-clamp condition of nystatin- perforated patch clamp recordings |
Dissociated culture of SCN neurons; PD11–14 rats |
GABA (bath application) | GABA induced inward currents at a holding potential of −40 mV |
|
Wagner et al. (1997) PMID 9177347 |
12:12 | Extracellular single unit recordings; whole cell patch clamp recordings |
Slice preparation; PD21– 56 rats |
GABA (bath application) | During the day GABA excited 71%, inhibited 16% and had no effect on 13%; during the night GABA excited 32%, inhibited 56% and had no effect on 12%; GABA increased the membrane conductance up to nine-fold throughout the day |
|
Gribkoff et al. (1999) PMID 10194649 |
12:12 | Multiple unit recordings; whole cell patch clamp recordings |
Slice preparation; rats | GABA (bath application or pressure applied with a Picospritzer) |
Multiple unit recordings found GABA inhibited firing during the day and night. In cell attached recordings during the day GABA inhibited nearly all action currents |
|
Liu and Reppert (2000) PMID 10707977 |
12:12 | Extracellular single unit recordings |
Dissociated culture of SCN neurons; PD0–3 mice |
GABA (bath application) | One or 6 h administration of GABA completely inhibited the firing rate of all neurons at all phases of the circadian cycle. One or 6 h of GABA treatment yielded patterns of phase shifts similar to each other. GABA produced maximal phase delays of 10 h and phase advances of 6 h |
|
Wagner et al. (2001) PMID 11744760 |
12:12 | Whole cell patch clamp recordings |
Slice preparation; PD14– 28 rats |
GABA (bath application and locally from a patch pipette using pressure injection) |
Currents induced by GABA are carried by both chloride and bicarbonate ions; lack slow desensitization processes and are moderately voltage dependent; there are two independent mechanisms regulating chloride – one accumulating intracellular Ca2+and one removing; the mechanism reintroducing Ca2+is less efficient at night |
|
De Jeu and Pennartz (2002) PMID 11826050 |
12:12 | Gramicidin-perforated- patch clamp recordings |
Slice preparation; rats | GABA (bath application) | During the day GABA produced hyperpolarization in 10/11 neurons. During the night GABA produced hyperpolarization in 7/12 and depolarization in 5/12 |
|
Shimura et al. (2002) PMID 11788348 |
12:12 | Gramicidin- or nystatin- perforated-patch clamp recordings |
Dissociated culture of SCN neurons; PD11,12 rats |
GABA (bath application) | GABA induced an inward current at a holding potential of −40 mV |
|
Gribkoff et al. (2003) PMID 12750413 |
12:12 | Multiple unit recordings | Slice preparation; rats | GABA (bath application) | GABA produced exclusively inhibitory responses with no differences between day and night. Continuous administration of GABA inhibited firing rate for more than 1 h |
|
Choi et al. (2008) PMID 18495878 |
12:12 | Extracellular single unit and gramicidin- perforated-patch clamp recordings |
Slice preparation; PD30– 40 rats |
GABA (bath application) | Over the entire circadian cycle, GABA inhibited 72%, excited 15% and produced excitation followed by inhibition in 13%; GABA excitation present in ventral and dorsal SCN, excitatory responses most common in dorsal SCN at night. GABA increased Ca2+in 40% of neurons sampled during both the day and night |
|
Irwin and Allen (2009) PMID 19821838 |
12:12 | Fura-2 Ca2+imaging | Slice preparation; PD28– 42 rats |
GABA (bath application) | GABA increases Ca2+in some neurons, decreases Ca2+in others and has no effect in still others. More neurons in which GABA increased Ca2+and fewer neurons in which GABA reduced Ca2+were found at night than during the day. GABA increased Ca2+more in neurons in the dorsal than the ventral SCN |
|
Moldavan and Allen (2013) PMID 23401614 |
12:12 | Whole cell patch clamp | Slice preparation prepared during the light period; rats |
GABA (bath application) | GABA decreased EPSCs evoked by optic nerve stimulation in bicuculline or picrotoxin treated slices |
|
Farajnia et al. (2014) PMID 24979761 |
8:16 12:12 16:8 |
Fura-2 Ca2+imaging | Slice preparation; PD56– 112 mice |
GABA (focal application) | In LD 16:8 GABA increased Ca2+in 40% of neurons and decreased Ca2+in 36%; in LD 12:12 GABA increased Ca2+ in 32% and decreased Ca2+in 43%; in LD 8:16 GABA increased Ca2+in 28% of neurons and decreased Ca2+in 52%; no differences were observed between ventral and dorsal SCN |
Notes: LD cycle refers to animal housing prior to tissue preparation; day and night refer to subjective day and night; whole cell voltage clamp recordings clamp the intracellular chloride concentrations making determination of the nature (excitatory or inhibitory) of spontaneous synaptic events difficult. Postnatal day (PD), embryonic day (ED), calcium (Ca2+), inhibitory postsynaptic current (IPSC), Pubmed identification number (PMID).
Table 5.
Cellular effects of GABA antagonists on SCN neurons.
| Paper | LD cycle |
Technique | Preparation | GABAA antagonists | Results |
|---|---|---|---|---|---|
|
Shibata et al. (1983) PMID 6668768 |
12:12 | Extracellular single unit recording |
Slice preparation; rats | Bicuculline hydrochloride (iontophoresis) |
Bicuculline blocked the inhibitory effects of GABA but not the inhibitory effects of baclofen; all recordings done during the day |
|
Liou et al. (1990) PMID 1976421 |
12:12 | Extracellular single unit recording |
Slice preparation; rats | Bicuculline (bath application) |
Bicuculline increased, decreased, or had no effect on spontaneous firing; bicuculline blocked the inhibitory effects of GABA and diazepam in the majority of neurons; the inhibitory effects of muscimol, but not baclofen were blocked by bicuculline |
|
Mason et al. (1991) PMID 1913180 |
12:12 | Extracellular single unit recordings |
Slice preparation; hamsters |
Bicuculline methiodide (iontophoresis) |
Bicuculline increased firing in the majority of SCN neurons; blocked the inhibitory effects of GABA and flurazepam |
|
Kawahara et al. (1993) PMID 8247331 |
Not noted |
Whole-cell voltage- clamp |
Dissociated culture of SCN neurons; PD21 rats |
Bicuculline (bath application) |
Bicuculline blocked inward currents induced by GABA |
|
Obrietan and van den Pol (1995) PMID 7623135 |
Not noted |
Fura-2 Ca2+ imaging | Dissociated culture of SCN neurons; ED18 rats |
Bicuculline (bath application) |
Bicuculline depressed Ca2+ in embryonic SCN neurons |
|
Obrietan and van den Pol (1996) PMID 8627385 |
Not noted |
Fura-2 Ca2+ imaging | Dissociated culture of SCN neurons; organotypic SCN cultures; ED19–21 rats |
Bicuculline (bath application) |
Bicuculline depressed Ca2+ in embryonic SCN neurons maintained in dissociated and organotypic cultures |
|
Shimura et al. (1996) PMID 8764156 |
12:12 | Voltage-clamp condition of nystatin- perforated patch clamp recordings |
Dissociated culture of SCN neurons; PD11–14 rats |
Bicuculline (bath application) |
Bicuculline suppressed GABA induced inward currents at a holding potential of −40 mV |
|
Wagner et al. (1997) PMID 9177347 |
12:12 | Extracellular single unit recordings; whole cell patch clamp recordings |
Slice preparation; PD21–56 rats |
Bicuculline, picrotoxin (bath application) |
Bicuculline and picrotoxin blocked GABA-induced inhibition and excitation; bicuculline increases should be increased not increases spontaneous firing in some cases and should be decreased not decreases it in others |
|
Gribkoff et al. (1999) PMID 10194649 |
12:12 | Multiple unit recordings; gramicidin perforated-patch recordings |
Slice preparation; rats | Bicuculline methiodide; picrotoxin (bath application or pressure applied with a Picospritzer) |
Bicuculline and picrotoxin produced significant excitation of multiple unit activity during the day and night; in gramicidin perforated-patch recordings bicuculline increased firing during the day and night although the responses of individual cells was more varied at night. During the day, bicuculline blocked EPSPs induced by hyperpolarizing current injection to ~−80 mV |
|
Shinohara et al. (2000) PMID 10717439 |
14:10 | Optical imaging of voltage sensitive dye |
Cultured organotypic SCN slices prepared from PD6 rats |
Bicuculline (bath application) |
Bicuculline increased the spread of SCN depolarization following electrical stimulation |
|
Wagner et al. (2001) PMID 11744760 |
12:12 | Whole cell patch clamp recordings |
Slice preparation; PD14–28 rats |
Bicuculline (bath application) |
Bicuculline blocked GABA-induced currents |
|
De Jeu and Pennartz (2002) PMID 11826050 |
12:12 | Gramicidin-perforated- patch clamp recordings |
Slice preparation; rats | Bicuculline methochloride (bath application) |
Bicuculline blocked hyperpolarization and depolarization induced by application of GABA during the day and the night; during the day bicuculline increased spontaneous firing in all neurons but at night the effects of bicuculline were variable and not statistically significant |
|
Shimura et al. (2002) PMID 11788348 |
12:12 | Gramicidin- or nystatin-perforated- patch clamp recordings |
Dissociated culture of SCN neurons; PD11, 12 rats |
Bicuculline methiodide (bath application) |
Bicuculline blocked GABA induced inward current at a holding potential of −40 mV |
|
Gribkoff et al. (2003) PMID 12750413 |
12:12 | Multiple unit recordings |
Slice preparation; rats | Bicuculline methiodide, picrotoxin (bath application) |
Bicuculline and picrotoxin increased multiple unit activity when applied during the mid-subjective day and the mid-subjective night |
|
Albus et al. (2005) PMID 15916945 |
12:12 | Multiple unit recordings |
Slice preparation; rats | Bicuculline methochloride | In the ventral SCN bicuculline increased electrical activity by 23%; in the dorsal SCN bicuculline increased electrical activity from early day to mid day, but during the end of the day and beginning of night bicuculline significantly decreased electrical activity; similar results are obtained when the ventral and dorsal are separated |
|
Choi et al. (2008) PMID 18495878 |
12:12 | Extracellular single unit and gramicidin- perforated-patch clamp recordings |
Slice preparation; PD30–40 rats |
Bicuculline methiodide, picrotoxin (bath application) |
Bicuculline blocked both inhibitory and excitatory responses to GABA; bicuculline produced excitation in 54%, inhibition in 11%, and had no effect in 35% of the neurons examined; the inhibitory response to bicuculline was greater at night; bicuculline combined with picrotoxin blocked GABA-induced changes in Ca2+ |
|
Irwin and Allen (2009) PMID 19821838 |
12:12 | Fura-2 Ca2+ imaging | Slice preparation; PD28–42 rats |
Gabazine (bath application) | Gabazine combined with the GABAB antagonist CGP55485 eliminated GABA-induced changes in Ca2+; gabazine inhibited GABA-induced increases in Ca2+ but not all reductions in Ca2+; Ca2+ changes evoked by optic nerve stimulation were blocked by gabazine in 77% of neurons tested; in neurons where optic nerve stimulation increased Ca2+, gabazine reduced Ca2+ in 53%, increased Ca2+ in 21%, and had no effect in 25% |
|
Moldavan and Allen (2013) PMID 23401614 |
12:12 | Whole cell patch clamp | Slice preparation prepared during the light period; rats |
Bicuculline and picrotoxin (bath application) |
GABA decreased excitatory postsynaptic currents evoked by optic nerve stimulation in bicuculline and picrotoxin treated slices |
|
Freeman et al. (2013) PMID 23764285 |
12:12 | Multi-electrode arrays | Cell culture; PD7–9 mice |
Bicuculline methiodide; gabazine (bath application) |
60% of all GABA-dependent connections were inhibitory and 40% were excitatory throughout the circadian day |
|
Farajnia et al. (2014) PMID 24979761 |
8:16 12:12 16:8 |
Gramicidin-perforated- patch clamp recordings |
Slice preparation; PD56–112 mice |
Gabazine (bath application) | In 16:8 gabazine reduced the frequency of EPSPs by 71% |
|
Fan et al. (2015) PMID 25653351 |
12:12 | Whole cell patch clamp | Slice preparation during the light and dark periods; P25–35 mice |
Picrotoxin (bath application) | Picrotoxin blocked 94% of postsynaptic currents induced by optogenetic stimulation of presynaptic VIP + SCN neurons during both day and night. However, the same stimulation was more likely to increase firing of VIP−SCN neurons at night than during the day |
7.1. Cellular effects of GABA on neuronal activity during early development
Studies of the effects of GABA administration on SCN neuronal firing early in development have been limited. In one study where dissociated SCN cells were obtained from postnatal day (PD) 0–3 rats and then recorded for periods ranging from PD19-34, GABA administration for 1 or 6 h completely inhibited all neuronal firing (Liu and Reppert, 2000). In contrast, in another study where dissociated SCN cells were obtained from PD4 rats and then recorded between PD4-109, the response to bicuculline suggested that the firing of a majority of SCN neurons is inhibited by GABA, although direct GABA application excited 20–40% of the neurons (Shirakawa et al., 2000). In dispersed cell culture, the GABAB agonist baclofen produces exclusively inhibitory effects on SCN neurons and does so during the day and night (Liu and Reppert, 2000). Interestingly, activation of GABAB receptors reduces GABA release from the terminals of cultured SCN neurons (Chen and van den Pol, 1998).
7.2. Cellular effects of GABA on SCN neuronal activity in adults
The effects of GABA and its agonists and antagonists on the cellular activity of SCN neurons have been studied extensively for more than 30 years using a variety of techniques. The vast majority of data indicate that application of GABA within the adult SCN in vitro produces primarily inhibitory responses throughout the nucleus during the day and during the night (Fig. 8, Table 3). In fact, some investigators report GABA produces exclusively or nearly exclusively inhibitory effects within the SCN (Mason et al., 1991). Nevertheless, a number of other studies report that GABA can produce excitatory effects in at least some neurons (Liou and Albers, 1990; Liou et al., 1990; Choi et al., 2008; Wagner et al., 1997; De Jeu and Pennartz, 2002; Freeman et al., 2013), and that GABA can also produce an initial increase in firing followed by a decrease in firing in still other neurons (Shibata et al., 1983; Choi et al., 2008). Another area of controversy is whether GABA has different effects on SCN neurons during different phases of the circadian cycle. The firing rate of SCN neurons is collectively higher during the subjective day than during the subjective night. In some studies, no differences are observed in the effects of GABA administered during the subjective day versus the subjective night (Mason et al., 1991; Liou and Albers, 1991; Gribkoff et al., 1999, 2003). Other investigators, however, report GABA to be excitatory during the subjective day in a substantial number of SCN neurons (Wagner et al., 1997, 2001), while yet others report that GABA can be excitatory during certain times of subjective night (De Jeu and Pennartz, 2002). Some studies have found that GABA has similar effects throughout the SCN (Liou and Albers, 1991; Farajnia et al., 2014; Shibata et al., 1983; Liou et al., 1990). The most comprehensive study of the effects of GABA on SCN neuronal firing (i.e., 786 neurons) found that GABA produces excitation in some neurons in both the dorsal and ventral SCN, but that the excitatory effects of GABA were most common in the dorsal SCN at night (Choi et al., 2008).
Fig. 8.
Effects of GABA and the GABAA antagonist bicuculline on integrated firing rate of suprachiasmatic (SCN) neurons in Syrian hamsters. (A) Inhibitory responses of a SCN neuron to different concentrations of GABA. (B) Bicuculline induces an excitatory response at 10−5 M and an inhibitory response at 10−4 M. (C) Bicuculline induces a bursting response at 10−6 M, however, at 10−5 M and 10−4 M excitatory responses are observed (modified from Liou and Albers (1990)).
Like GABA, the GABAA agonist muscimol produces inhibition or excitation followed by inhibition in the hypothalamic slice preparation and dispersed cell culture (Liou et al., 1990; Tominaga et al., 1994; Liu and Reppert, 2000) (Table 4). The response to GABA is inhibited by the GABAA antagonist bicuculline in many SCN neurons (Mason et al., 1991; Shibata et al., 1983; De Jeu and Pennartz, 2002; Wagner et al., 1997) (Table 5). In some studies, bicuculline has been reported to produce only excitatory responses (Mason et al., 1991; Gribkoff et al., 1999), while in others, bicuculline produces both excitation and inhibition (Liou et al., 1990; Wagner et al., 1997; De Jeu and Pennartz, 2002; Albus et al., 2005). The effects of bicuculline have also been reported to be phase dependent (De Jeu and Pennartz, 2002; Albus et al., 2005). The GABAB agonist baclofen administered in the hypothalamic slice preparation produces exclusively inhibitory effects on SCN neurons during both day and night, although its potency is greater at night (Gribkoff et al., 2003; Liou et al., 1990).
Table 4.
Cellular effects of GABA agonists on SCN neurons.
| Paper | LD cycle |
Technique | Preparation | GABA agonists | Effects on SCN neurons |
|---|---|---|---|---|---|
|
Liou et al. (1990) PMID 1976421 |
12:12 | Extracellular single unit recording |
Slice preparation; rats | Baclofen, muscimol, (bath application) |
During the day, muscimol produced excitation followed by inhibition (N = 5); baclofen produced exclusively inhibition (N = 4) |
|
Liou and Albers (1990) PMID 2207720 |
14:10 | Extracellular single unit recording |
Slice preparation; hamsters |
Baclofen (bath application) |
Baclofen produced exclusively inhibition |
|
Kawahara et al. (1993) PMID 8247331 |
Not noted |
Whole-cell voltage-clamp | Dissociated culture of SCN neurons; PD21 rats |
Muscimol, baclofen (bath application) |
Muscimol elicited large inward currents in all neurons tested at a holding potential of −60 mV; baclofen had no effect on current |
|
Tominaga et al. (1994) PMID 8190363 |
12:12 | Extracellular single unit recording |
Slice preparation; rats | Muscimol (bath application) |
Muscimol produced an inhibitory response in 70% of neurons at 10 µM and in 100% at 100 µM when administered between CT 2–10 |
|
Jiang et al. (1995) PMID 7715789 |
12:12 | Whole cell patch clamp | Slice preparation prepared during the light period; rats |
Baclofen (bath application) |
Baclofen reduced amplitude of EPSCs evoked by optic nerve stimulation by a presynaptic action; in a subpopulation of SCN neurons baclofen induced an outward current by postsynaptic action; baclofen had no effect on spontaneous or induced IPSCs with a holding potential of −60 mV |
|
Obrietan and van den Pol (1996) PMID 8627385 |
Not noted |
Fura-2 Ca2+ imaging | Dissociated culture of SCN neurons; organotypic SCN cultures; ED19–21 rats |
Baclofen (bath application) |
GABA, but not baclofen, elevated Ca2+ in embryonic SCN neurons maintained in dissociated and organotypic cultures |
|
Chen and van den Pol (1998) PMID 9465016 |
Not noted |
Whole cell patch clamp | Dissociated culture of SCN neurons; PD1 and PD2 |
Baclofen (bath application) |
Baclofen induced large, dose-dependent inhibition of evoked IPSCs at a holding potential of −60 mV; baclofen reduced the frequency of spontaneous IPSCs but had little effect on miniature IPSCs in the presence of TTX; GABAB receptor activation produced a strong presynaptic inhibition of GABA release from SCN neurons |
|
Gribkoff et al. (1999) PMID 10194649 |
12:12 | Multiple unit recordings | Slice preparation; rats | Muscimol (bath application or pressure applied with a Picospritzer) |
Muscimol inhibited firing during the day and night |
|
Colwell (2000) PMID 10861563 |
12:12 | Whole cell recordings with biocytin-filled patch electrodes |
Slice preparation; P10– 14 rats |
Muscimol (bath application) |
Muscimol decreased dye coupling during the day and the number of cells in a coupled cluster |
|
Liu and Reppert (2000) PMID 10707977 |
12:12 | Extracellular single unit recordings |
Dissociated culture of SCN neurons; PD0–3 mice |
Baclofen, muscimol (bath application) |
Muscimol given between CT 8 and 14 inhibited firing and produced phase delays; baclofen inhibited firing but did not produce phase delays |
|
Shinohara et al. (2000) PMID 10717439 |
14:10 | Optical imaging of voltage sensitive dye; application of Lucifer Yellow, to detect gap junctions |
Cultured organotypic SCN slices prepared from PD6 rats |
Muscimol (bath application) |
Muscimol decreased the spread of SCN depolarization following electrical stimulation; muscimol reduced the spread of the gap junction-permeable dye |
|
Gribkoff et al. (2003) PMID 12750413 |
12:12 | Multiple unit recordings | Slice preparation; rats | Baclofen, muscimol (bath application) |
Muscimol produced exclusively inhibitory responses with no differences between day and night. Baclofen inhibited neurons during the day and night but with greater potency at night |
|
Ikeda et al. (2003) PMID 12534969 |
12:12 | Fura-2 Ca2+ imaging | Slice preparation; PD6– 16 mice |
Muscimol hydrobromide (bath application) |
Muscimol increased Ca2+ in PD6–8 mice; Ca2+ was reduced in PD9–10 mice preferentially at night |
|
Moldavan et al. (2006) PMID 16709723 |
12:12 | Whole cell patch clamp; Fura- 2 Ca2+ imaging |
Slice preparation prepared during the light period; rats |
Baclofen (bath application) |
Baclofen inhibited evoked EPSCs in a dose-dependent manner during day and night; GABAB receptors acted presynaptically to reduce glutamate release |
|
Irwin and Allen (2009) PMID 19821838 |
12:12 | Fura-2 Ca2+ imaging | Slice preparation; PD28–42 rats |
Baclofen; muscimol (bath application) |
Baclofen reduced Ca2+ levels in neurons where GABA reduced Ca2+ and in neurons where GABA increased Ca2+ |
|
Moldavan and Allen (2013) PMID 23401614 |
12:12 | Whole cell patch clamp | Slice preparation prepared during the light period; rats |
Baclofen (bath application) |
55% of RHT inputs on SCN neurons were tonically inhibited during the day and 33% were tonically inhibited at night by baclofen |
Circadian time (CT), excitatory postsynaptic current (EPSC); see other notes in Table 1.
7.3. Effects of GABA on intracellular Ca2+ concentrations
Another approach to studying the cellular effects of GABA in the adult SCN has been to determine how GABA influences intracellular Ca2+ concentrations using Ca2+ imaging techniques (Choi et al., 2008; Irwin and Allen, 2009). GABA-induced increases in Ca2+ are associated with membrane depolarization (i.e., excitation) while GABA-induced decreases in Ca2+ are associated with hyperpolarization (i.e., inhibition). Administration of GABA into the hypothalamic slice preparation increases Ca2+ in some SCN neurons (Ca+), decreases Ca2+ in other SCN neurons (Ca−) and has no effect on Ca2+ levels in still other SCN neurons (Ca0). Significantly more Ca + neurons and fewer Ca− neurons are found at night than during the day. A higher proportion of Ca+ neurons are found in the dorsal SCN than in the ventral SCN. In contrast, there are more Ca− and Ca0 neurons in the ventral SCN than in the dorsal SCN (Fig. 9). The transient responses to RHT stimulation and GABA stimulation are similar across neurons. In some neurons, both GABA and RHT stimulation increase Ca2+ and in other neurons, GABA and RHT stimulation reduces Ca2+. Administration of the GABAA antagonist gabazine combined with the GABAB antagonist CGP55485 eliminates GABA-induced Ca2+ alterations. The GABAA agonist muscimol, however, mimics the ability of GABA to increase Ca2+ in some SCN cells and to reduce Ca2+ in others (Irwin and Allen, 2009). Administration of gabazine eliminates GABA-induced Ca2+ increases but does not eliminate the ability of GABA to reduce Ca2+ in all cases. In the majority of neurons where GABA increases Ca2+ or decreases Ca2+, gabazine has the opposite effect on Ca2+ levels. Activation of GABAB receptors with baclofen in the slice consistently reduces Ca2+ levels in SCN neurons (Irwin and Allen, 2009) suggesting that activation of GABAB receptors consistently results in inhibition.
Fig. 9.
GABA-induced calcium (Ca2+) responses of rat suprachiasmatic (SCN) neurons. The position of each neuron is superimposed on a representative drawing of the SCN, with the 3rd ventricle (3V) on the left and the optic chiasm (OC) on the bottom. Note that while the number of cells in the day and night are not equal, the relative proportions varied between the day and night (modified from Irwin and Allen (2009)).
7.4. GABA and the Cl− equilibrium potential
Another approach to investigating the prevalence of the excitatory and inhibitory effects of GABA has been to evaluate the Cl− equilibrium potential in the SCN. The studies described above have examined the effects of GABA on the firing patterns of SCN neurons. However, it has been argued that analysis of the Cl− equilibrium potential may be a purer and more accurate approach to examining the inhibitory/excitatory effects of GABA (Alamilla et al., 2014). The spatial and temporal patterns of the Cl− equilibrium potential in SCN neurons has been examined using both perforated patch clamp and whole patch clamp experiments (Alamilla et al., 2014). In perforated patch clamp experiments, two populations of SCN neurons have been identified. Approximately 50% of the neurons have an equilibrium potential of GABA postsynaptic currents (EGABA) that are hyperpolarized, as is typically observed in GABAergic neurons, and are therefore inhibitory. The other 50% of SCN neurons exhibit an EGABA that is more depolarized, resulting in an outward flux of Cl− ions and an excitatory response following activation of GABAA receptors. In the dorsal SCN, more neurons have an “excitatory” EGABA during the day than at night, while in the ventral SCN, fewer neurons have an “excitatory” EGABA during the day than at night. Recently, another study evaluated the temporal and spatial distribution of the Cl− equilibrium potential using fluorescence to quantify Cl− concentrations in the SCN. Cl− equilibrium potentials are higher in the dorsal than in the ventral SCN although no differences are observed in Cl− levels over time (DeWoskin et al., 2015).
7.5. Factors regulating the polarity of the cellular response to GABA
As discussed above, the actions of CCCs are critical in determining whether GABA is hyperpolarizing or depolarizing in the immature and mature brain. The NKCC1 inhibitor bumetanide administered in the hypothalamic slice can significantly alter the response of SCN neurons to GABA (Choi et al., 2008; Irwin and Allen, 2009). GABA can produce inhibitory, excitatory, or excitatory followed by inhibitory responses in SCN neurons (Table 3). Bumetanide potently inhibits excitatory responses to GABA in the neurons displaying exclusively excitatory responses to GABA. In SCN neurons where GABA has biphasic effects, bumetanide eliminates the excitatory portion of the responses and enhances the inhibitory responses. Administration of bumetanide onto the slice also blocks GABA-induced increases in Ca2+ and magnifies GABA-induced reductions in Ca2+ (Irwin and Allen, 2009). When taken together with the anatomical data showing greater NKCC1 protein in the dorsal SCN (see Section 6), these data support the proposition that enhanced activity of NKCC1 cotransporters underlies the excitatory effects of GABA found in a substantial portion of dorsal SCN neurons during the night.
Interestingly, a recent study in mice found that the percentage of excitatory and inhibitory responses to GABA in the SCN can be altered by the length of the photoperiod during prior entrainment; the longer the photoperiod, the more excitatory responses to GABA (Farajnia et al., 2014). When previously entrained to an LD cycle containing 16 h of light per day, 40% of SCN neurons display excitatory Ca2+ transients in response to GABA while 32% are inhibitory. When previously entrained to an LD cycle containing 8 h of light per day, 28% of SCN neurons display excitatory Ca2+ transients in response to GABA and 52% are inhibitory. When previously entrained to an LD cycle containing 12 h of light per day the ratio of excitation:inhibition in response to GABA is intermediate. Interestingly, the excitatory effects of GABA do not differ across SCN subregions in this study, despite earlier reports of higher levels of NKCC1 protein in the dorsal than in the ventral SCN in this strain of mouse (see Section 6). The relationship between the excitatory effects of GABA and the duration of light per day appear to be mediated by intracellular Cl− uptake via NKCC1s, because the excitatory effects are blocked by the NKCC1 inhibitor bumetanide. These dramatic photoperiod-induced changes in the excitatory versus inhibitory effects of GABA within the SCN have led to the hypothesis that this ratio may be involved in encoding seasonal changes in photoperiod length and thus may be instrumental in photoperiodism (Myung et al., 2015; Farajnia et al., 2014). It is also interesting to consider what role the ratio of GABA-induced excitation: inhibition might have on circadian pacemaker function in the SCN. Because circadian rhythmicity can be easily entrained to LD cycles with very short or very long photoperiods, it seems unlikely that specific ratios of GABA-induced excitation:inhibition are necessary for light to phase shift the pacemaker. Therefore, the polarity of the response to GABA within the SCN may have a modulatory role on entrainment. Indeed, the magnitude of light-induced phase delays and advances is enhanced in short-day housed animals when compared to animals housed in longer photoperiods (e.g., 14 or 18 h of light per day) (Evans et al., 2004; Pittendrigh et al., 1984). Taken together, these data suggest the hypothesis that the lower the ratio of GABA-induced excitation:inhibition, the more responsive the pacemaker is to the phase shifting effects of photic stimuli during the night. In contrast, prior entrainment to photoperiods of different lengths does not alter the phase shifting response to non-photic stimuli (Evans et al., 2004). Therefore, it can be speculated that the mechanisms underlying the induction of phase shifts by non-photic stimuli are not influenced by the ratio of GABA-induced excitation:inhibition.
7.6. Summary of the cellular effects of GABA within the SCN
The cellular effects of GABA have been highly inconsistent across studies, even in those using nearly identical techniques. The reasons for these inconsistencies are not known. One possibility relates to the effectiveness of different techniques to identify GABA-induced excitation in a large population of cells where GABA is predominately inhibitory. For example, multiple unit recordings might miss a small number of excitatory responses because this technique records the activity of populations of neurons rather than the responses of single cells. This explanation, however, seems unlikely because, although some studies using multiple unit recordings have found GABA to be exclusively inhibitory, others indicate GABA can have substantial excitatory actions (Gribkoff et al., 1999; Albus et al., 2005). Other explanations related to technical differences in the studies have also been advanced. For example, it has been suggested that in studies employing whole cell, patch clamp, excitatory responses to GABA may have been masked by the use of high Cl− concentrations, thereby enforcing inwardly directed Cl− currents (Wagner et al., 1997). Another suggestion is that the excitatory effects of GABA might be masked by a depolarizing shunting that prevents repolarization and recurrent action potentials, and is thus undetectable by multiple unit and cell-attached recordings (De Jeu and Pennartz, 2002). Using perforated-patch recordings, however, where the intracellular contents remain relatively intact membrane potential and input resistance can be determined without altering the intracellular contents, the effects of GABA were also inconsistent (De Jeu and Pennartz, 2002; Gribkoff et al., 1999). Another potential contributing factor may relate to the difficulties in defining responses as excitatory when they reflect post-inhibitory rebound (Slat et al., 2013). Finally, as can be seen in Tables 3–5, it is unlikely that differences in the frequency of GABA inhibition:excitation observed across studies can be accounted for by differences in the duration of the photoperiods used in these studies.
Investigation of the excitatory effects of GABA in the SCN has generated substantial research and controversy. While substantial discrepancies remain in the literature, perhaps the most parsimonious interpretation of the cellular effects of GABA in the SCN is that GABA is predominately inhibitory throughout the SCN during the day; however, at night GABA remains predominately inhibitory in the ventral core but can have substantial excitatory effects in the dorsal shell. The recent finding of large differences in the ratio of GABA-induced excitation:inhibition in the SCN of mice housed in different photoperiods suggests the polarity of the GABA response could have a modulatory effect on the mechanisms responsible for light-induced phase shifting within the SCN. In stark contrast to the inconsistencies reported in activation of GABAA receptors, activation of GABAB receptors consistently produces inhibition in SCN neurons.
8. Role of GABA in the coupling of circadian clock cells into a circadian pacemaker in the SCN
Cells in the SCN exhibit a synchronized circadian rhythm in spontaneous neural activity in vivo (Inouye and Kawamura, 1979). Although individual SCN neurons can retain the ability to generate rhythms of around 24 h even when isolated in culture, these neurons are out of phase with each other and display circadian periods ranging from 20 to 28 h (Welsh et al., 1995; Honma et al., 1998; Herzog et al., 1998). SCN clock cells do not appear to be a specialized or an anatomically localized class of cells and, in fact, many cultured SCN neurons do not display intrinsic rhythmicity (Webb et al., 2009). Because the pacemaker is comprised of multiple clock cells with different free-running periods, the activity of these cells must be coordinated or coupled so that they function as a pacemaker and produce coherent circadian output signals. Theoretically, one clock cell could couple all other clock cells forcing them to adopt its free-running period, much as the LD cycle entrains the circadian pacemaker. It is more likely, however, that individual clock cells with different free-running periods influence each other via mutual coupling. Mutual coupling is mediated by the ability of clock cells to phase shift other clock cells so that a stable pacemaker with a compromise period is formed. The stronger the coupling or phase shifting signal of a clock cell, the more influence it has on other clock cells and the closer the compromise period is to its own free-running period. Despite this simple concept of mutual coupling, the neurobiological mechanisms that underlie the mutual coupling of thousands of cells, as is thought to occur in the SCN, are not well understood.
8.1. Coupling of individual clock cells
One significant question yet to be resolved is whether synaptic activity is required for the coupling of individual clock cells into a functional pacemaker in the SCN. There are several lines of evidence that synaptic activity may not be required for the generation of synchronized molecular circadian rhythms within the SCN (Shirakawa et al., 2000; Bouskila and Dudek, 1993; Honma et al., 2000; Yamaguchi et al., 2003; Reppert and Schwartz, 1984; Schwartz, 1991). One important approach to studying SCN synaptic activity has been the use of TTX, because it is a reversible blocker of voltage-dependent Na+ channels that inhibits action potentials without altering resting membrane potential, K+ currents, the Na+ pump, or the ability of postsynaptic membranes to depolarize (Kao, 1966). In support of the hypothesis that synaptic activity is not required for the coupling of SCN clock cells is the finding that infusion of TTX into the SCN continuously for 14 days does not significantly reduce its timekeeping ability (Schwartz et al., 1987). Not only does the pacemaker keep ticking in the absence of action potentials, the absence of synaptic activity over this period has little to no effect on its period. Further, if synaptic activity is responsible for the coupling of clock cells then its complete inhibition for even short periods of time might be expected to perturb the driven rhythms (e.g., induce a phase shift). Administration of TTX does not, however, alter circadian phase or induce Per1 clock gene activation; although it does prevent the phase shifting effects produced by increasing the firing rate of SCN neurons and the spread of Per1 activation to AVP-expressing cells after a phase shifting pulse of GRP (Shibata and Moore, 1993; Earnest et al., 1991; Huhman et al., 1997; Gamble et al., 2007; Jones et al., 2015). In contrast, recent studies monitoring the rhythmicity of cultured SCN neurons using bioluminescence reporters have found that application of TTX reduces the synchrony of SCN clock cells when applied for 5–7 days, and that removal of TTX restores the synchrony across cells (Yamaguchi et al., 2003; Webb et al., 2009; Evans et al., 2013). These data suggest that synaptic activity plays a critical role in the coupling of clock cells. At present, it is hard to reconcile the differences in the effects of TTX on the coupling of SCN clock cells from in vivo and in vitro studies. One interesting possibility comes from the work of Webb et al., 2009. These investigators propose that network interactions serve to stabilize noisy oscillations, such that larger networks exhibit a more stable circadian oscillation that is resistant to disruption (Webb et al., 2009). Perhaps the substantially larger network present in the intact circadian system (composed of SCN and possibly extra-SCN oscillators) provides more stability than can be achieved in reduced preparations such as cultures or hypothalamic slices. Inhibition of synaptic activity might be predicted to reduce synchrony in smaller, less stable networks like SCN cultures, but to have little effect on the larger more robust circadian networks of intact animals. If synaptic activity has only a minor role in the coupling of clock cells, then it will be important to identify the non-synaptic forms of communication that are involved.
There are several ways that SCN neurons could be coupled non-synaptically. Individual SCN neurons could communicate non-synaptically through electrotonic potentials. Electrotonic potentials rely on gap junctions that produce direct connections between SCN neurons (Jiang et al., 1997b; Colwell, 2000; Shirakawa et al., 2000). There are, however, relatively few gap junctions in the SCN, and thus would likely provide only a relatively weak mechanism for the coupling of SCN clock cells. Further, carbenoxolone, a selective gap-junction blocker, has only minimal effects on Ca2+ rhythms in the SCN (Enoki et al., 2012). Nevertheless, genetic loss of connexin 36, a critical component in electrotonic potentials, reduces the coherence of free-running circadian locomotor rhythms (Long et al., 2005). Interestingly, administration of a GABAA agonist significantly reduces the potentials between SCN neurons in a dose-dependent manner, suggesting that activation of GABAA receptors would reduce the synchrony of SCN clock cells (Colwell, 2000; Shinohara et al., 2000).
Another hypothesis is that SCN clock cells are coupled by the activation of GABAA-TONIC receptors resulting from a constitutive release of low levels of GABA from SCN neurons (Wagner et al., 2001). It was further hypothesized that the excitatory:inhibitory ratio of tonic GABA conductance could vary in a circadian fashion, providing a mechanism for the coupling of clock cells. As discussed earlier, non-synaptic release of neurochemical signals likely occurs in the SCN, and has the potential to mediate the coupling of SCN clock cells.
Neuropeptides are important in the coupling of clock cells in the SCN. Disruption of VIP signaling can reduce the coupling of clock cells and the overall coherence of overt circadian rhythms (for reviews see Harmar et al., 2002; Colwell et al., 2003; Maywood et al., 2006, 2007; Aton et al., 2005; Vosko et al., 2007; Ciarleglio et al., 2009). Although VIP acting on VPAC2 receptors is of substantial importance in neuropeptide coupling of clock cells, GRP and AVP may also play a role (Brown et al., 2005; Maywood et al., 2011). It will be interesting to determine how VIP mediates this cellular coupling, because only a small percentage of VIP containing cells display rhythmicity in culture, and rhythms of VIP mRNA, protein, release, and receptor number in the SCN are absent in intact animals under free-running conditions (Francl et al., 2010a, 2010b; Zoeller et al., 1992; Shinohara et al., 1993; Ajpru et al., 2002).
There is evidence that GABA could also contribute to the coupling of cellular clocks within the SCN. Studies of the effects of GABA and GABA agonists on the phase of the circadian rhythm of neuronal firing in individual SCN neurons in culture provide support for this possibility (Liu and Reppert, 2000). Application of GABA, muscimol, or baclofen to individual SCN neurons acutely inhibits neuronal firing when administered at various phases of the circadian cycle. Longer-term GABA administration provided in pulses of either 1 or 6 h produce phase delays, phase advances, or has no effect on phase depending on when in the circadian cycle GABA is administered. The phase shifting effects of GABA appear to be mediated by GABAA receptors and not GABAB receptors, because muscimol but not baclofen mimics the effects of GABA. Taken together, these data suggest that phase shifts produced by activation of GABAA receptors allows SCN clock cells to become stably synchronized (i.e., mutually coupled).
If GABA receptors play a necessary role in the synchronization of clock cells within the SCN then inhibition of GABA receptors should desynchronize their activity. Long-term inhibition of endogenous GABA activity in the SCN slice, however, does not interfere with the ability of individual neurons to synchronize daily rhythms in clock gene expression, and may actually increase synchrony among SCN neurons (Aton et al., 2006). That is, PER2::LUC bioluminescence, a marker of circadian rhythmicity, exhibited by individual neurons within a slice from PER2::LUC mice, continues to occur at a similar time each day following exposure to a cocktail containing both GABAA and GABAB antagonists for 8 days. These data indicate that, although GABA may be capable of phase shifting the activity of dispersed SCN neurons in culture, it is not necessary for their synchronization when connected even in significantly reduced networks such as the slice preparation. In fact, several studies have found that GABAA signaling in the SCN may actually reduce the cycle-to-cycle precision of networked SCN neurons studied in vitro (Evans et al., 2013; Myung et al., 2015; Freeman et al., 2013; Aton et al., 2006). For example, administration of the GABAA antagonist gabazine increases the precision of circadian rhythms of PER2::LUC in SCN explants (Freeman et al., 2013). Interestingly, gabazine also prevents the damping of circadian rhythms in PER2::LUC that normally occurs in VIP null SCN explants, thus suggesting that some signal other than VIP can couple SCN neurons, at least in the absence of GABAA receptor activation. Much remains to be learned about how SCN neurons are coupled and these data raise a number of interesting questions about the interactions between GABA and VIP. Is synaptically released VIP necessary to induce GABA release (see Section 5.4)? Are GABA and VIP co-released in SCN neurons firing at high frequency (see Section 3)? Are there rhythms in the activation of GABA and VIP receptors in the absence of photic input (see above)? If synaptic activity is necessary for the synchrony of SCN clock cells (Yamaguchi et al., 2003; Webb et al., 2009), how is this achieved when GABA, which reduces synchrony, and VIP, which increases synchrony, are both present in the extracellular space?
8.2. Coupling subpopulations of clock cells
A series of classic studies by Pittendrigh and Daan published in 1976 served to define a model of how multiple circadian oscillators can interact to form a circadian pacemaker capable of entraining to the LD cycle (Pittendrigh and Daan, 1976a, 1976b, 1976c; Daan and Pittendrigh, 1976a). These studies led to the development of a model of two coupled oscillators that has considerable relevance for understanding how subpopulations of oscillators in the SCN may interact to form a circadian pacemaker. The evidence supporting the existence of two coupled oscillators comes primarily from the phenomenon of “splitting”. In hamsters housed in constant light for several weeks, the circadian rhythm of locomotor activity desynchronizes into two free-running components that subsequently develop a stable, coupled state where the two activity bouts occur about 12 h apart. Based on these and other data, Pittendrigh and Daan proposed that (1) the free-running period of one oscillator is a positive function of light intensity while the period of the other oscillator is a negative function of light intensity, (2) when mutually coupled the interaction between these component oscillators results in a compound pacemaker expressing a period different from each of its component oscillators, and (3) the relative influence of each component oscillator on the other depends on the phase relationship between the two oscillators. This qualitative model provides a functional and structural basis for understanding the behavior of the circadian timekeeping system in many different lighting environments. For example, as the photoperiod lengthens, the period of the compound pacemaker changes because of the opposite effects of increased light on the period of the component oscillators. Thus, when the compound pacemaker is entrained to a 24 h cycle, the phase relationship between the two oscillators is a function of photoperiod length. The Pittendrigh and Daan model provides relatively simple principles to understand how oscillating subpopulations of SCN neurons might interact (for a review see Evans and Gorman, 2016).
8.2.1. Component oscillators in the left and right SCN
As can be seen in Fig. 10, the left and right SCN can function independently as component oscillators. Interestingly, each of the two rhythmic bouts of activity seen in the split condition are associated with either the component oscillator in the left or the component oscillator in the right SCN (de la Iglesia et al., 2000; Ohta et al., 2005). While there is evidence that glutamate is involved in communication between the left and right SCN (Michel et al., 2013), it remains possible that GABA could also contribute. There are significant GABA containing projections that connect the bilateral SCN (Buijs et al., 1994). Because GABA may communicate lighting information within the pacemaker (see Section 9.2.6), it is possible that exposure to constant light could modulate GABA signaling and thereby contribute to the splitting of the two oscillators.
Fig. 10.
The compound pacemaker in the suprachiasmatic nuclei is composed of two normally coupled component oscillators in the left and right nuclei and two normally coupled component oscillators in the ventral core and dorsal shell.
8.2.2. Component oscillators in the ventral and dorsal SCN
The ventral core and dorsal shell can also function independently as component oscillators with each oscillator linked to one of the two rhythmic bouts of activity that occur during splitting (Yan et al., 2005; Butler et al., 2012) (Fig. 10). Several studies have examined the role of GABA in the re-synchronization of the ventral and dorsal oscillators following manipulations of the LD cycle that alter their phase relationships. One approach measured rhythms in multiple unit activity in SCN slices obtained from rats that had been exposed to a 6 h phase delay of the LD cycle just before slice preparation. Rhythms in unit activity are rapidly reset in the ventral SCN but require several days to reset in the dorsal SCN (Albus et al., 2005). Rhythms persist in both the ventral and dorsal SCN following their physical separation or administration of bicuculline, although these treatments prevent resetting of the rhythm in the dorsal SCN. Interestingly, administration of pulses of bicuculline suggests that GABAA receptors produce primarily inhibitory effects on firing rate in the ventral SCN and primarily excitatory effects in the dorsal SCN. These data led to a model of asymmetrical coupling of SCN oscillations in the dorsal and ventral SCN. More specifically, GABA released from interneurons projecting from the ventral SCN to the dorsal SCN produce robust GABAA-induced excitation resulting in a strong coupling signal, while GABA released from interneurons projecting from the dorsal to the ventral SCN produce more modest GABAA-induced inhibition resulting in a weaker coupling signal.
In another study, the role of GABAA receptors in the coupling of the component oscillators in the ventral core and dorsal shell was examined by studying PER2::LUC mice entrained to photoperiods of various lengths prior to slice preparation (Evans et al., 2013). As would be predicted by the Pittendrigh and Daan model, the phase relationships between the peaks of the PER2::LUC rhythms in the ventral core and dorsal shell become further apart as the photoperiod lengthens. When housed in extremely long photoperiods (e.g., LD 20:4) prior to slice preparation the peaks are 6–12 h out of phase. Within a week, however, the peaks of the PER2::LUC rhythms in the ventral core and dorsal shell from these mice spontaneously re-synchronize. The role of GABAA activity in the re-synchronization of the ventral core and dorsal shell was studied by incubating the slice with bicuculline. Interestingly, GABAA activity promotes re-synchronization when the ventral core and dorsal shell are 6–12 h out of phase with each other, but inhibits their synchronization when they are in phase with each other under standard lighting conditions. VIP acts in concert with GABAA signaling to promote re-synchronization of the network when it is 6–12 h out of phase. In contrast, VIP promotes resynchronization of the network by counteracting the actions of GABAA signaling when the ventral core and dorsal shell oscillations are in phase. Thus, both VIP and GABAA activity can influence the coupling and/or the period of the component oscillators in the ventral core and dorsal shell of the SCN. Further, GABAA activity can promote or inhibit the re-synchronization of the core and shell depending on the phase relationship between the ventral core and dorsal shell. These data are consistent with the hypothesis that activation of GABAA receptors does not contribute to the synchronization of the ventral core and ventral shell under most common environmental lighting conditions, and in fact may reduce their synchronization. In contrast, however, when very long photoperiods push the phase relationship of the ventral core and dorsal shell far apart, GABAA receptor activation can contribute to their resynchronization. The neurochemical mechanisms mediating the effects of photoperiod length on the phase relationship between the ventral core and dorsal shell are not known. One possibility is suggested by the finding that photoperiod length during prior entrainment determines the ratio of excitatory and inhibitory responses to GABA in the SCN (Farajnia et al., 2014) (see Section 7.5). Perhaps the ratio of GABA-induced excitation:inhibition influences the period length of the component oscillators in the ventral core and dorsal shell and/or their phase relationship.
Another recent study has further examined the role of GABA in the coupling of circadian oscillations in the dorsal and ventral SCN and the possibility that the ratio of GABA-induced excitation:inhibition alters the period and/or coupling of the component oscillators (Myung et al., 2015). Using a Bmal1-luc reporter to monitor circadian oscillations in cultured SCN explants, the length of the photoperiod of prior entrainment was found to determine the phase relationship between the oscillations in the dorsal and ventral SCN. Longer photoperiods produce oscillations in the ventral and dorsal SCN that are more out of phase with each other. The circadian period of the compound pacemaker (i.e., the combined period of the dorsal and the ventral SCN) is also related to the phase relationships of the dorsal and ventral SCN; the period of the compound pacemaker becomes shorter as the dorsal and ventral SCN become more out of phase with each other. In support of the hypothesis that the ratio of GABA excitation:inhibition in the SCN plays a role in the coupling and/or period length of the dorsal and ventral regions, these investigators found that mice previously entrained to long photoperiods have a higher NKCC1/KCC2 ratio in the dorsal SCN (resulting in more GABA-induced excitation) than in the ventral SCN (see Section 6). Further, reducing the excitatory effects of GABA signaling increases the circadian period of the compound pacemaker in the SCN (i.e., dorsal and ventral SCN). Further support for the role of GABAA signaling in altering the period of the dorsal SCN and/or the coupling of the dorsal and ventral SCN is provided by the finding that gabazine disrupts the phase and period changes induced by long photoperiods. Taken together, these data suggest that GABA-induced excitation alters the phase relationship between the coupled dorsal and ventral SCN by shortening the period of the oscillator in the dorsal SCN. It is also possible that alterations in the degree of GABA-induced excitation could change the strength of coupling between the dorsal and ventral oscillators. Therefore GABAA receptor activation contributes to determining the phase relationship between the circadian oscillations in the dorsal and ventral SCN by pushing the phase of the two oscillations apart. These investigators further propose that VIP plays an opposite role to that of GABA in that it pulls the two oscillators more in phase with each other. Thus, the free-running period of the compound pacemaker in the SCN may be determined by the asymmetrically coupled component oscillators in the ventral and dorsal SCN.
8.3. Summary of coupling and rhythm generation
Construction of a circadian pacemaker from many SCN clock cells that generate different free-running periods requires that these clock cells be coupled together so that they produce coherent circadian output signals. It will be important to identify the relative roles of synaptic and non-synaptic communication in the coupling of clock cells, and in particular to determine if non-synaptically released GABA is involved in coupling. There is considerable evidence that neuropeptides, especially VIP, play an important role in the coupling of SCN clock cells. Exactly how these neuropeptides might mediate coupling is unclear, particularly in free-running conditions where their activity may be arrhythmic. Although GABA is capable of phase shifting the activity of dispersed SCN neurons in culture, it is does not appear to be necessary for their synchronization. The compound circadian pacemaker in the SCN is composed of component oscillators that are normally coupled together. The phenomenon of splitting has revealed that the right and left SCN as well as the ventral and dorsal SCN contain component oscillators. While at present there is little evidence that GABA couples the left and right SCN, there is evidence that GABA and VIP can influence the periods of the ventral and dorsal oscillators and/or their coupling. The relationships between the mechanisms that couple SCN clock cells and the mechanisms that couple the component oscillators in the SCN are not known. Understanding both forms of coupling will require a better understanding of the dynamic interactions between synaptically and non-synaptically released neurochemical signals in the SCN, including but probably not limited to GABA and VIP.
9. Role of GABA in entrainment of the circadian pacemaker
Entrainment is the process whereby an endogenous circadian pacemaker is synchronized to an external rhythm (e.g., the LD cycle) so that the endogenous pacemaker adopts the period length of the external rhythm. Photic entrainment of the SCN requires a series of neurochemical events that transduce light into a signal that can ultimately phase shift the pacemaker. Both acute and sustained activation of GABA receptors in the SCN are involved in mediating the effects of photic stimuli on circadian phase. GABA also mediates most, if not all, phase shifts induced by non-photic stimuli, although other neurochemical signals likely participate, e.g., serotonin (for a review see Webb et al., 2014).
9.1. GABA and the phase shifting effects of non-photic stimuli
As discussed above (see Section 1.1), there is a class of stimuli, which have been described as “non-photic”, that produces a pattern of phase shifts that are distinctly different from the pattern produced by light (Fig. 1). The first demonstration of this pattern of phase shifting that did not involve any manipulation of lighting conditions was seen following the direct injection of NPY-like neuropeptides into the SCN region (Albers et al., 1984a; Albers and Ferris, 1984; Huhman and Albers, 1994). A similar, but more variable, pattern of phase shifts is seen across studies when NPY is administered in vitro (Golombek et al., 1996; Medanic and Gillette, 1993; Harrington and Schak, 2000). A short pulse of darkness provided to animals housed in constant light is a “photic” stimulus, but it produces a pattern of phase shifts very similar to those produced by NPY-like neuropeptides (Boulos and Rusak, 1982; Ellis et al., 1982). Subsequent studies have shown that pulses of darkness produce phase shifts, not because they change the photic environment (i.e., interrupt light), but because they induce locomotor activity (Reebs et al., 1989; Van Reeth and Turek, 1989). As such, dark pulses may represent a “non-photic” phase shifting stimulus and not a “photic” phase shifting stimulus in most cases (for a review see Rosenwasser and Dwyer, 2001). In fact, several non-photic stimuli capable of inducing phase shifts appear to do so by increasing locomotor activity and possibly general arousal. Examples of non-photic phase shifting stimuli include spontaneous running on a novel wheel, several hours of gentle handling, and saline injections (Yannielli and Harrington, 2004; Mrosovsky, 1995; Hastings et al., 1998). In contrast to a number of other non-photic phase shifting stimuli, NPY injected into the SCN does not induce phase shifts by increasing arousal (Mrosovsky, 1995; Biello et al., 1994).
A role for GABA-active drugs in non-photic phase shifts was first suggested by the finding that systemic injection of short acting BDZs (i.e., triazolam, midazolam, brotizolam) as well as the GABAA agonist muscimol produced phase shifts similar to those resulting from injection of NPY-like neuropeptides into the SCN (Turek and Losee-Olsen, 1986; Wee and Turek, 1989; Yokota et al., 2000; Ebihara et al., 1988; Vansteensel et al., 2003a). Interestingly, the induction of phase shifts by triazolam also results from its ability to induce locomotor activity. Immobilization of hamsters after triazolam administration blocks the induction of phase shifts (Van Reeth and Turek, 1989). In contrast, the long acting BDZ chlordiazepoxide injected systemically into hamsters induces NPY-like patterns of phase shifts without increasing locomotor activity (Biello and Mrosovsky, 1993; Meyer et al., 1993). Thus, although BDZs can induce phase shifts by increasing locomotor activity, increased activity is not necessary for the phase shifts produced by all BDZs.
There is now considerable evidence that NPY release within the SCN mediates the phase shifting effects of a variety of non-photic stimuli (Mrosovsky, 1995, 1996). Neurons in the IGL that project to the SCN as part of the GHT produce both GABA and NPY; however, there is no compelling evidence that GABA released from the GHT participates in mediating non-photic phase shifting. Lesions of the GHT inhibit the ability of systemically administered short acting and long acting BDZs to induce phase shifts (Johnson et al., 1988b; Meyer et al., 1993; Biello et al., 1991). Not only does injection of NPY into the SCN mimic the phase shifting effects of non-photic stimuli, injection of antiserum to NPY nearly abolishes phase shifts induced by locomotor activity (Biello et al., 1994). Thus, NPY release from the GHT appears to play a principal role in mediating non-photic phase shifts induced by BDZs. Although BDZ antagonists inhibit the phase shifting effects of BDZs, suggesting that the BDZ-GABA receptor complex mediates these effects, the location of these receptors is not known (Van Reeth et al., 1988). BDZ binding studies have revealed moderate levels of binding within the SCN but only low levels of binding in the IGL, suggesting that, although the GHT is important for BDZ-induced phase shifts, the IGL may not be the anatomical site where BDZs act to induce phase shifts (Michels et al., 1990). BDZs can inhibit the neuronal firing of SCN neurons and phase shift the single unit firing rhythm in vitro (Mason et al., 1991; Liou et al., 1990; Strecker et al., 1999; McElroy et al., 2009). Therefore, BZDs may act in the SCN and possibly other sites to induce phase shifts of the circadian system.
In nocturnal animals, muscimol activation of GABAA receptors within the SCN during the subjective day produces large phase advances whether administered in vivo (Smith et al., 1989, 1990; Huhman et al., 1995, 1997; Mintz et al., 2002; Biello, 2009; Ehlen et al., 2006, 2008; Gamble et al., 2003) or in vitro (Bergeron et al., 1999; Tominaga et al., 1994). In contrast, administration of muscimol during the late subjective night consistently produces small phase delays in vivo, whereas muscimol administered in vitro does not. Interestingly, a light pulse or injection of NMDA blocks the ability of muscimol to induce phase advances during the middle of the subjective day (Gamble et al., 2003; Mintz et al., 2002). The ability of NMDA to block muscimol-induced phase advances is also blocked by TTX, suggesting that synaptic activity is required for the communication of photic information within the SCN during the day. Perhaps light is communicated to the pacemaker during the day via NMDA receptors located on interneurons or presynaptic terminals. Interestingly, injection of [4,5,6,7-tetrahy dro-isoxazolo[5,4-c]pyridine-3-ol] (THIP), a drug thought to be a GABAA-TONIC receptor agonist, into the SCN in vivo does not induce phase advances during the subjective day (Ehlen and Paul, 2009). In contrast, diazepam, which enhances GABAA-PHASIC receptor conductance, induces phase advances when given during the subjective day in vitro (McElroy et al., 2009). Further, as discussed in Section 4.1, GABAA-PHASIC receptors appear to be more abundant in the SCN during the subjective day than GABAA-TONIC receptors. Taken together, these data suggest that the phase shifting effects of muscimol in the SCN during the subjective day is mediated primarily by GABAA-PHASIC receptors.
The similarity in the pattern of phase shifts produced by activation of GABAA receptors and NPY receptors suggests that common mechanisms might mediate their effects. In support of this hypothesis, the GABAA antagonist bicuculline blocks the ability of NPY to induce phase advances in locomotor rhythms followings injection into the SCN (Huhman et al., 1995). NPY-induced phase advances in multiple unit activity measured in vitro are also blocked by bicuculline (Gribkoff et al., 1998), although NPY-induced phase advances in extracellular single unit recordings are not (Biello et al., 1997). Bicuculline also inhibits the phase advancing effects of another non-photic phase shifting stimulus, systemic injection of the 5-HT1a/7 receptor agonist 7-(Dipropylamino)-5,6,7,8-tetra hydronaphthalen-1-ol (8-OH-DPAT) (Mintz et al., 1997). The possibility that the phase shifting effects of NPY are mediated by the release of GABA is supported by in vivo studies showing that Na+-dependent action potentials within the SCN are required for NPY-induced, but not muscimol-induced phase advances (Huhman et al., 1997). In in vitro studies, however, neither muscimol- nor NPY-induced phase advances of extracellular single unit recording rhythms require Na+-dependent action potentials (Bergeron et al., 1999; Biello et al., 1997). Although not all the data are in agreement, the most parsimonious hypothesis is that GABAA receptor activation in the SCN mediates the induction of phase shifts by non-photic stimuli.
Considerably less is known about non-photic phase shifting in diurnally active species (Challet, 2007). Of course, the middle of the subjective day represents the active phase in diurnal species and the rest phase in nocturnal species. Scheduled locomotor activity has modest effects in phase shifting the pacemaker, but does appear to produce phase delays during the middle of the subjective day (Glass et al., 2001; Hut et al., 1999; Kas and Edgar, 2001). Thus, the phase response to non-photic stimuli depends on the phase of the pacemaker and not the phase of the activity cycle. In the two diurnal species so far examined, GABAA-active drugs induce phase shifts in the middle of the subjective day as they do in nocturnal species. In the diurnally active squirrel monkey, systemically administered triazolam induces significant sedation, and yet it produces a pattern of phase shifts quite similar to that seen in hamsters (Mistlberger et al., 1991). In contrast, however, injection of muscimol into the SCN of diurnally active Nile grass rats has the opposite effect, inducing phase delays (Novak and Albers, 2004a, 2004b). In both diurnal and nocturnal rodents, the phase shifting effects of muscimol are not blocked by TTX, suggesting that muscimol is acting directly on the pacemaker or on mechanisms that do not require conventional synaptic mechanisms (Huhman et al., 1997; Novak and Albers, 2004b). Interestingly, while the phase advancing effects of muscimol can be inhibited by a light pulse in nocturnal animals (Mintz et al., 2002), the phase delaying effects of muscimol in diurnal animals cannot (Novak and Albers, 2004a). Thus, during the subjective day, activation of GABAA receptors produces the opposite response in a diurnal rodent compared to a nocturnal rodent.
Several studies have examined how GABAA receptor activation during the middle of the subjective day alters the expression of Per1 and Per2. In nocturnal hamsters, injection of muscimol into the SCN during the middle of the subjective day produces phase advances and consistently suppresses Per1 mRNA, and can under certain circumstances suppress Per2 mRNA as well (Ehlen et al., 2006, 2008). The systemically administered BDZ brotizolam also induces phase advances during the subjective day and suppresses both Per1 and Per2 mRNA levels in the hamster SCN (Yokota et al., 2000). In contrast, in the diurnal Nile grass rat, injection of muscimol into the SCN during the middle of the subjective day produces phase delays and suppresses Per2 mRNA but not Per1 mRNA (Novak et al., 2006) (Fig. 11). One possibility is that the suppression of Per1 contributes to the production of phase advances and the suppression of Per2 contributes to the production of phase delays during the subjective day. It is clear, however, that although suppression of Per1 is sufficient to induce phase advances (Hamada et al., 2004b), there is not always a consistent relationship between Per expression and the ability of muscimol to induce phase shifts during the day. Light treatment inhibits muscimol induced phase shifts when given shortly after drug administration in the hamster SCN, but does not reduce muscimol’s ability to suppress Per induction (Ehlen et al., 2008).
Fig. 11.
Effects of muscimol on Period 1 (Per1) and Period 2 (Per2) mRNA in the suprachiasmatic nucleus (SCN) of diurnally active Nile grass rats. Autoradiograms of (A) Per1 and (B) Per2 after microinjection of muscimol or vehicle into the SCN. Muscimol decreases Per2 mRNA levels in the SCN 1 and 2 h after injection at Zeitgeber Time 4. No changes are seen in Per1 mRNA levels. (C) The coronal brain sections processed for Per2 in situ hybridization were photographed after Nissl staining to illustrate the site of injection. (* Indicates area immediately below microinjection site). Third Ventricle (3V); optic chiasm (OC) (from Novak et al. (2006)).
The role of GABAB receptors in the control of non-photic phase shifts has been examined both in vivo and in vitro. The results of these studies are inconsistent. Activation of GABAB receptors produces phase advances in some in vivo and in vitro studies (Smith et al., 1990; Bergeron et al., 1999; Biggs and Prosser, 1998, 1999), but not in others (Novak et al., 2004; Liu and Reppert, 2000).
9.1.1. Summary of the role of GABA in non-photic phase shifting
BDZs have potent phase advancing effects when systemically administered during the subjective day. The phase advancing effects of some BDZs appears to be mediated by an associated increase in locomotor activity, although other BDZs can induce phase shifts without altering locomotor activity. The brain site(s) where BDZs act to induce phase shifts is not clear but may include the SCN. The GHT appears to be necessary for the induction of phase shifts by BDZs, thus suggesting the importance of extra-SCN neurons. Nevertheless, BDZs administered in vitro can phase advance SCN single unit firing rhythms and inhibit spontaneous firing in SCN neurons. Activation of GABAA receptors in the SCN with muscimol during the middle of the subjective day produces large phase advances in nocturnal rodents and large phase delays in the only diurnal rodent so far studied. In nocturnal species, the induction of phase shifts by non-photic stimuli results from NPY released from the GHT and subsequent activation of GABAA receptors in the SCN. In nocturnal rodents synaptic activity appears to be unnecessary for muscimol to induce a phase shift; and interestingly, there is evidence to suggest that muscimol acts on synaptic GABAA-PHASIC receptors to induce phase shifts.
9.2. GABA and the phase shifting effects of light
Over the last decade it has become clear that the SCN response to photic stimuli can be separated into transient responses and sustained responses. Light initiates a series of transient responses, including NMDA receptor activation, that in turn induce a cascade of sustained responses that ultimately result in a phase shift of the circadian pacemaker. The most complete analysis of the sustained response comes from in vivo studies in hamsters given light pulses at times that produce phase advances (Hamada et al., 2001) and in in vitro studies using slices prepared from transgenic mice given light pulses at times that produce phase delays or advances (Kuhlman et al., 2003; LeSauter et al., 2011). The initial transient responses induce neuronal activity in the ventral SCN that begins approximately 30–60 min after the light pulse and persists for at least 4–5 h as indicated by measures of neuronal firing and induction of Per gene expression. Application of other photic-like neurotransmitters (i.e., VIP or GRP) also produces a light-like persistent increase in neural activity (Gamble et al., 2007, 2011; Kudo et al., 2013). Following this initial sustained response, Per expression increases in the dorsal SCN beginning approximately 90 min after the light pulse; this upregulation of Per expression is necessary for long-term increases in excitability (Kuhlman et al., 2003; Gamble et al., 2007). In the following sections we review the effects of the acute administration of GABA-active drugs, given just prior to the transient response, on light-induced phase shifts, as well as the role of GABA in mediating the ability of light to induce phase shifts during the sustained response to light. As discussed below, the effects of GABA-active drugs on entraining stimuli can differ dramatically depending on their route of administration.
9.2.1. Acute effects of GABA-active drugs given systemically
The first evidence that GABA-active drugs could influence the entrainment of circadian rhythmicity was the finding that systemic administration of BDZs could accelerate re-entrainment to phase shifts of the LD cycle (Davies et al., 1974; Childs and Redfern, 1981). Subsequent studies revealed that systemic injections of GABA-active drugs during the subjective night modulate the phase shifting effects of light (Ralph and Menaker, 1985, 1986, 1989; Golombek and Ralph, 1994). The GABAA antagonist bicuculline injected just prior to exposure to a brief light pulse blocks the ability of light to induce phase delays but not advances. Neither THIP nor diazepam reduces the phase delaying effects of light, although both drugs significantly reduce the ability of bicuculline to inhibit the phase delaying effects of light. Light-induced phase delays but not advances are also inhibited by the systemic injection of vigabatrin, a drug that increases GABA levels by inhibiting GABA transaminase. Interestingly, the BDZ diazepam injected systemically before a light pulse significantly inhibits light-induced phase advances but not delays. The ability of diazepam to inhibit light-induced phase advances is blocked or significantly inhibited by pretreatment with bicuculline, picrotoxin, or the competitive diazepam antagonist RO15-1788. Although systemic administration of bicuculline and diazepam potently inhibit light-induced phase delays and phase advances, respectively, it is surprising that neither drug inhibits light induction of Fos protein within the SCN (Colwell et al., 1993). Activation of GABAB receptors by the systemic administration of the GABAB agonist baclofen just prior to exposure to a brief light pulse also significantly inhibits both the phase delaying and phase advancing effects of light, as well as induction of Fos and Per1 in the SCN (Ralph and Menaker, 1989; Colwell et al., 1993; Crosio et al., 2000). These data demonstrate that the systemic administration of GABA-active drugs, delivered acutely just before light exposure, can modulate the ability of light to phase shift the circadian system and may provide new therapeutics for circadian phase disorders.
9.2.2. Acute effects of GABAA-active drugs within the SCN region
The acute administration of GABAA drugs into the SCN just prior to light stimulation dramatically modulates the resetting effects of light (Gillespie et al., 1996, 1997; Novak and Albers, 2004a). Injection of muscimol into the SCN just before the presentation of a brief light pulse inhibits the ability of light to induce phase delays during early subjective night and phase advances the during late subjective night in both hamsters and diurnally active grass rats. Injection of THIP likewise inhibits this photic resetting in hamsters, suggesting the involvement of GABAA-TONIC receptors in mediating these effects (Ehlen and Paul, 2009). Interestingly, GABAA-TONIC receptors appear to be more abundant in the SCN during the subjective night than GABAA-PHASIC receptors (see Section 4.1). Taken together, these data suggest that the inhibition of light-induced phase shifts by muscimol during the subjective night is mediated primarily by GABAA-TONIC receptors.
Also consistent with the light-modulating role of GABAA receptors, bicuculline enhances light-induced phase delays during early night (Gillespie et al., 1996, 1997; Lall and Biello, 2003b). In contrast, bicuculline does not enhance phase advances after photic stimulation during the late night. Interestingly, injection of bicuculline into a region just above the supraoptic nucleus in the early night induces phase delays (Kallingal and Mintz, 2014). The microinjection of GABAA agonists and antagonists into the hamster SCN also modulates the ability of light to induce Fos in a manner similar to their modulatory effects on light’s ability to induce phase shifts (Gillespie et al., 1999). Muscimol and bicuculline also modulate the phase delays produced by the injection of a cocktail containing VIP, PHI, and GRP. Surprisingly, despite the potent inhibitory effects of GABAA receptor activation on light-induced phase shifts, SCN field potentials evoked by optic nerve stimulation are potentiated by muscimol and inhibited by bicuculline (Gannon et al., 1995). In summary, activation of GABAA receptors, likely GABAA-TONIC receptors, just prior to light exposure profoundly inhibits the phase shifting effects of light.
How activation of GABAA receptors inhibits the phase shifting effects of light is not known. GABAA receptors could act presynaptically to inhibit glutamate release from RHT terminals, and/or postsynaptically to inhibit the response to activation of glutamate receptors. Although presynaptic GABAA receptors have been identified in the SCN they appear to occur primarily on neurons that release GABA, and there is no evidence of presynaptic GABAA receptors on RHT terminals (Belenky et al., 2003). Muscimol significantly inhibits the phase shifting effects of NMDA injected into the SCN, providing evidence that GABAA activation can inhibit the phase shifting effects of light by a postsynaptic action (Mintz et al., 2002).
Induction of Per1 and Per2 gene expression within the SCN appears to be required for light-induced phase shifts (Shigeyoshi et al., 1997; Albrecht et al., 1997, 2001; Shearman et al., 1997; Horikawa et al., 2000). Therefore, GABA may inhibit light-induced phase shifts by reducing the expression of Per1 and Per2. In hamsters, muscimol injected into the SCN region just prior to a light pulse in the early subjective night significantly reduces Per1 but not Per2 mRNA levels in the SCN; and muscimol injected into the SCN region just prior to a light pulse in the late subjective night significantly reduces Per2 and Per1 mRNA levels (Ehlen et al., 2008) (Fig. 12). Interestingly, muscimol injected into the SCN region in diurnal grass rats just prior to a light pulse during the early subjective night significantly reduces Per2 but not Per1 mRNA levels in SCN (Novak et al., 2006). Thus, although muscimol significantly reduces light-induced phase delays in both nocturnal hamsters and diurnal grass rats, the effect of muscimol on light-induced changes in Per1 and Per2 mRNA is species specific. In hamsters, the GABAA-TONIC receptor agonist THIP significantly inhibits Per1 and Per2 mRNA when injected before a light pulse given in the early subjective night and Per1 mRNA when injected before a light pulse given in the late subjective night (Ehlen and Paul, 2009).
Fig. 12.
Effects of muscimol on light-induced Period 1 (Per1) and Period 2 (Per2) mRNA in the suprachiasmatic nucleus (SCN) of Syrian hamsters. Autoradiograms illustrate the ability of muscimol to inhibit light-induced increases in Per1 and Per2 mRNA hybridization signal in the SCN at circadian time 13.5 (top) and circadian time 19 (bottom). Light (LIGHT) increases Per1 and Per2 mRNA but a sham pulse (DARK) does not. Muscimol significantly inhibits induction of Per1 and Per2 when injected just prior to the light pulse (modified from Ehlen et al., 2008).
Activation of GABA receptors is not the only way that light-induced phase shifts can be inhibited in the SCN. Injection of NPY into the SCN significantly inhibits light-induced phase shifts (Weber and Rea, 1997; Lall and Biello, 2002, 2003a; Gamble et al., 2005). Interestingly, however, inhibition of GABAA receptor activity does not inhibit the ability of NPY to reduce the phase delaying effects of light (Lall and Biello, 2003b). Thus, the phase advancing effects of NPY during the day are mediated by GABAA receptors, but the inhibitory effects of NPY on light-induced phase advances at night are independent of GABAA activity.
9.2.3. Acute effects of GABAB-active drugs within the SCN region
The acute activation of GABAB receptors within the SCN can completely block the phase shifting effects of light. Microinjection of the GABAB agonist baclofen into the SCN of hamsters (Gillespie et al., 1997) or diurnal grass rats (Novak et al., 2004), just prior to a light pulse in the early subjective night, inhibits light-induced phase delays. Conversely, microinjection of the GABAB antagonist CGP-35348 into the SCN region of hamsters before a light pulse in the early subjective night enhances light-induced phase delays (Gillespie et al., 1997). Although microinjection of baclofen into the SCN region of hamsters reduces the phase advancing effects of light in the late subjective night, microinjection of CGP-35348 does not increase the phase shifting effects of light at this same time. GABAB agonists and antagonists also modulate the ability of light to induce Fos in a manner similar to their modulatory effects on light-induced phase shifts (Gillespie et al., 1999). Interestingly, unlike GABAA receptors, activation or antagonism of GABAB receptors does not alter the phase delaying effects of a cocktail containing VIP, PHI, and GRP administered in the SCN.
Activation of GABAB receptors also has potent inhibitory effects on SCN field potentials and excitatory post-synaptic potentials evoked by optic nerve stimulation during the day and the night (Jiang et al., 1995; Moldavan et al., 2006; Moldavan and Allen, 2013; Gannon et al., 1995; Shibata et al., 1986). Baclofen activates GABAB receptors on presynaptic terminals and reduces glutamate release from RHT terminals by inhibiting voltage-dependent Ca2+ channels. Baclofen also has potent effects on excitatory postsynaptic currents (EPSCs) evoked by optic nerve stimulation, reducing them by around 85%. The GABAB antagonist CGP55845 reduces EPSCs evoked by optic nerve stimulation by 55% during the day and 33% during the night (Moldavan and Allen, 2013). The sources of the extracellular GABA providing this tonic inhibition are not known, but they are not of a sufficient magnitude to maximally activate GABAB receptors and thereby completely block light induced phase shifts. Rather, this tonic inhibition would appear to be of the magnitude to reduce but not eliminate light-induced phase shifts. As discussed earlier (see Sections 5.2 and 5.5), it seems likely that the level of tonic inhibition produced by GABA is regulated by GABA uptake mechanisms that modulate the levels of extracellular GABA in the vicinity of RHT terminals (Moldavan and Allen, 2013). Taken together, the existing data provide strong support for the hypothesis that activation of GABAB receptors inhibits light-induced phase shifts by reducing glutamate release from the RHT.
A functional role for postsynaptic GABAB receptors found within the SCN cannot be ruled out. Baclofen not only significantly inhibits the phase delaying effects of NMDA, but it does so in the presence of TTX, suggesting that GABAB receptors that inhibit the phase shifting effects of NMDA are on the same cells that contain the NMDA receptors responsible for phase shifting the circadian pacemaker (Mintz et al., 2002). Alternatively, it is possible that baclofen is not acting on the same cells as NMDA, but inhibits the phase shifting effects of NMDA by activating GABAB receptors on other cells, possibly clock mechanisms, that communicate through signaling pathways not dependent on action potentials. As noted earlier (see Section 4.2) GABAB receptors are found throughout the dorsal SCN.
GABAB receptors also modulate 5-HT release within the SCN. GABA administered to hypothalamic explants stimulates 5-HT release, and the effects of GABA on 5-HT release are neither blocked by GABAA receptor antagonists nor mimicked by muscimol (Francois-Bellan et al., 1987, 1988). In contrast, baclofen can mimic the effects of GABA on 5-HT release whether given in vitro or administered systemically. Interestingly, the ability of GABAB receptor activation to increase 5-HT in the SCN occurs in males and ovariectomized females but not in ovariectomized females treated with estradiol (Francois-Bellan et al., 1989a, 1989b).
9.2.4. Comparison of the acute effects of GABA-active drugs given systemically with those given within the SCN region
The effects of drugs acting on GABAA receptors, but not GABAB receptors, can be dramatically different depending on the route of administration (Table 6). Indeed, administration of bicuculline has the opposite effects on the expression of light-induced phase delays depending on its route of administration; reducing phase delays when given systemically and increasing phase delays when given into the SCN region. One site where systemically administered bicuculline might act is the eye. Intraocular injections of bicuculline significantly reduce light-induced phase delays but not light-induced phase advances (Della and Ralph, 1999). The effects of GABAA active drugs on light induced Fos expression also differ dramatically depending on the route of administration. Systemic administration of bicuculline inexplicably does not reduce light-induced Fos in the SCN while injection of bicuculline into the SCN region does. These data suggest that modulation of GABAA receptors outside of the SCN as well as inside the SCN can significantly impact mechanisms that alter entrainment. GABAA-active drugs could affect entrainment by acting on elements of the circadian system such as the retina, raphe, IGL, or many other sites (see Gillespie et al., 1997, 1999 for a discussion). In contrast, the similar effects of GABAB-active drugs on phase shifting, whether injected systemically or within the SCN, are consistent with the hypothesis that the SCN is the primary site where GABAB drugs act.
Table 6.
Effects of GABA-active drugs on light-induced phase shifts following acute administration systemically or into the suprachiasmatic nucleus (SCN).
| Drug | Systemic | SCN region | ||
|---|---|---|---|---|
| CT 13–14 | CT 18–20 | CT 13–14 | CT 18–20 | |
| Muscimol | − − | − − | ↓ | ↓ |
| Bicuculline | ↓ | − − | ↑ | − − |
| THIP | − − | − − | ↓ | ↓ |
| Baclofen | ↓ | ↓ | ↓ | ↓ |
9.2.5. Summary of the effects of the acute effects of GABA-active drugs
Acutely administered GABA-active drugs given in the SCN just prior to the transient response to light can have powerful effects on the response to light. The acute activation of GABAA or GABAB receptors in the SCN blocks the ability of light pulses to phase shift the circadian pacemaker in both nocturnal and diurnal rodents. There appears to be a tonic GABA inhibition of light-induced phase delays during early subjective night that can be mediated by either GABAA or GABAB receptors. This tonic GABA inhibition is not present during late subjective night. Several lines of evidence are consistent with the hypothesis that activation of GABAB receptors inhibits light-induced phase shifts by inhibiting presynaptic release of glutamate from RHT terminals. Nevertheless, the large number of GABAB receptors found outside of the retinorecipient zone, combined with the finding that GABAB agonists inhibit the phase delaying effects of NMDA even in the presence of TTX, suggests that GABAB receptors have important postsynaptic functions as well. It seems likely that activation of GABAA receptors on postsynaptic membranes is responsible for the inhibition of the phase shifting effects of light. Inhibition of Na+-dependent action potentials inhibits the phase shifting effects of light but not the phase shifting effects of NMDA receptor activation (Mintz et al., 1999). Therefore, it seems likely that GABAA and glutamate receptors are found on the same cells or on cells that communicate nonsynaptically, and that glutamate receptors are found on pacemaker mechanisms or cells that non-synaptically communicate with the pacemaker. Activation of GABAA receptors reduces light-induced Per in both nocturnal and diurnal species. GABAA-PHASIC receptors may mediate the induction of phase advances by GABA during the day and GABAA-TONIC receptors may mediate the ability of GABA to inhibit of light-induced phase shifts at night.
9.2.6. Sustained effects of GABA within the SCN
In contrast to the inhibition of light-induced phase shifts by acute activation of GABAA receptors, the sustained activation of GABAA receptors appears to actually mediate the phase delaying effects of light. As discussed above, a light pulse triggers a sustained increase in neuronal firing in the SCN that lasts more than 4 h. Because GABA is contained in nearly all SCN neurons it seems likely that a light pulse would induce a sustained synaptic release of GABA. Synaptic release of GABA could therefore activate synaptic GABAA-PHASIC receptors and possibly extrasynaptic GABAA-TONIC receptors via synaptic spillover. We have proposed that sustained activation of GABAA receptors in the SCN mediates the ability of light to phase delay the circadian pacemaker. We tested this hypothesis by determining whether the sustained administration of GABAA agonists and antagonists can mimic and inhibit the phase delaying effects of light, respectively (Hummer et al., 2015). The sustained administration of muscimol into the SCN over four consecutive hours beginning in the early subjective night induces large phase delays, but no phase delays are observed when muscimol is administered over 1, 2 or 3 h intervals (Fig. 13). Additional studies found these effects to be dose-dependent and ruled out the possibility that the phase and not the duration of muscimol administration is critical for the induction of phase delays. Further support for the hypothesis comes from studies demonstrating that the sustained antagonism of GABAA receptors inhibits the ability of light to phase delay the circadian system. Sustained administration of bicuculline for at least 6 h beginning 1 or 4 h after the light pulse significantly reduces light-induced phase delays (Fig. 14). In contrast, sustained administration of bicuculline for 6 h, beginning 10 h after the light pulse, does not inhibit light-induced phase delays. In addition, administration of bicuculline for 3, 4 or 6 h in different temporal patterns between CT 14.5 and CT 21.5 does not inhibit light-induced phase delays, indicating that there is no critical phase of GABAA activation that is necessary for the induction of phase delays. Interestingly, bicuculline administration for 6 h interrupted by a 2-h window between CT 14.5 and CT 21.5 is ineffective in blocking light-induced phase delays, suggesting that the sustained activation of GABAA receptors does not have to occur continuously to induce a phase delay. These data support the hypothesis that sustained activation of GABAA receptors in the SCN is sufficient to induce phase delays and that sustained activation of GABAA receptors is necessary for light to phase delay the circadian pacemaker.
Fig. 13.
The sustained administration of muscimol for at least 4 h is necessary to induce light-like phase delays in Syrian hamsters. (A) Injection regimen used to determine the duration of GABAA receptor activation necessary to induce a phase delay. All groups received a series of 4 hourly injections into the suprachiasmatic nucleus (SCN) region between circadian time CT13.5 and CT16.5. However, the number of consecutive injections containing muscimol (21.9 mM) varied from 0 to 4 (VEH = vehicle; MUS = muscimol). (B) Mean ± SE of phase delays produced by the 5 treatments outlined in A (∗ vs. 0 muscimol injections, p = 0.002). (C and D) Representative activity records demonstrating the effect of 4 hourly injections of vehicle (C) or muscimol (21.9 mM) (D) into the SCN region between CT13.5 and CT16.5 on locomotor rhythms in DD. Bars depict the 4-h injection period (white: saline; red: muscimol) (modified from Hummer et al., 2015).
Fig. 14.
Summary of the effects of over 1700 injections containing muscimol, bicuculline, or vehicle into the SCN region on the phase of circadian locomotor rhythms in Syrian hamsters. (A) Solid red bar indicates the timing of SCN injections in which muscimol induces a significant phase delay in the locomotor rhythm. Open red bars indicate the timing of SCN injections of muscimol that did not produce significant phase delays. (B) Solid blue bars indicate the timing of SCN injections in which bicuculline significantly inhibits light-induced phase delays. Open blue bars indicate the timing of SCN injections of bicuculline that do not significantly inhibit light-induced phase delays. Yellow bar indicates the timing of the 15-min light pulse. (C) Proposed sequence of neurochemical events within the SCN necessary for a light pulse to induce a phase delay. Light induces release of glutamate (GLU) that activates NMDA receptors within the SCN for seconds and possibly minutes (initial transient response). The transient responses to light induce activity in non-rhythmic SCN neurons (or possibly rhythmic SCN neurons, see Fig. 2) that begins 30–60 min after the light pulse resulting in the sustained release of GABA for 6 or more hours. The sustained GABA release from non-rhythmic neurons results in the sustained activation of GABAA receptors on rhythmic SCN neurons, producing a phase delay in the pacemaker (from Hummer et al., 2015).
How the sustained activation of GABAA receptors mediates the phase delaying effects of light within the SCN is not known. We have proposed that light induces the sustained release of GABA for four or more hours from neurons in the ventral SCN and that this sustained release of GABA results in the sustained activation of GABAA receptors in the dorsal SCN, thereby producing a phase delay in the pacemaker (Fig. 15). Support for this hypothesis comes from findings that neurons in the ventral SCN have dense, direct projections to neurons in the dorsal SCN (Leak et al., 1999; Romijn et al., 1997) and that GABA appears to be essential for communicating phase shifting information from the ventral to the dorsal SCN (Han et al., 2012). Reduction of GABA neurotransmission as a result of the targeted deletion of the gene that encodes the voltage-gated sodium channel type 1 significantly reduces light-induced phase delays, and chronic treatment of the SCN with GABA transmission-enhancing drugs restores the ability of light to induce phase delays (Han et al., 2012). Importantly, this gene deletion does not inhibit light induction of Fos or Per in the ventral SCN, but does eliminate the induction of Fos and Per in the dorsal SCN.
Fig. 15.
Proposed regulation of the phase of the circadian clock and Period (Per) gene expression in the SCN by GABAA receptor activation and inactivation. Left Panel: As described in Fig. 14C, light results in glutamate release from the retinohypothalamic tract (RHT). In response, there is a sustained release of GABA from, as well as a sustained induction of Per in, non-rhythmic neurons (or possibly rhythmic SCN neurons, see Fig. 2). In response, there is a sustained activation of GABAA receptors and a sustained induction of Per in rhythmic neurons resulting in a phase delay of the circadian pacemaker. Middle Panel: Acute activation of GABAA receptors by injection of muscimol prior to a light pulse inhibits light induction of the sustained release of GABA from, as well as an inhibition of Per induction in, non-rhythmic neurons. Acute activation of GABAA receptors inhibits NMDA-induced phase delays suggesting that activation of GABAA receptors does not inhibit light-induced phase delays solely by inhibiting light-induced glutamate release (Mintz et al., 2002). Acute activation of GABAA receptors ultimately blocks light-induced phase delays by preventing Per induction in rhythmic neurons. Right Panel: Sustained inhibition of GABAA receptors by at least six hourly injections of bicuculline following a light pulse blocks light-induced phase delays by inhibiting Per induction in rhythmic neurons (from Hummer et al., 2015).
Further support for the role of the sustained release of GABA within the SCN comes from some interesting data initially predicted by modeling studies (DeWoskin et al., 2015). It has been proposed that sustained, tonic GABA release is a function of a neuron’s membrane voltage. More specifically, SCN neurons that become hyperexcited and sufficiently depolarized will release tonic levels of GABA for approximately 5 h even in the absence of action potentials. Indeed, in vitro studies have found that subpopulations of SCN neurons can spontaneously achieve such a hyperexcited state during the subjective day (Belle et al., 2009). It has also been suggested that this hyperexcited state, resulting in a sustained release of GABA could be induced by neurotransmitters like glutamate (DeWoskin et al., 2015). If so, perhaps glutamate release induced by light around CT 13.5 stimulates a hyperexcited state in the intact SCN and the resultant sustained GABA release and activation of GABAA receptors induces a phase delay in the pacemaker.
It is interesting to consider how GABA may influence circadian phase, given the different possibilities on how the ventral and dorsal SCN may interact. One proposition is that GABAA receptors located on intrinsically rhythmic SCN clock cells in the dorsal SCN are activated in response to a sustained release of GABA from non-rhythmic SCN cells in the ventral SCN that respond directly to light input (Fig. 15). Alternatively, it is possible rhythmic neurons in the ventral core respond directly or indirectly to light and that interactions between the component oscillators in the ventral core and dorsal shell are responsible for the light-induced phase delay. The possibility that sustained GABAA signaling could alter the period of the dorsal SCN and/or the coupling of the dorsal and ventral SCN is supported by both empirical and modeling studies (DeWoskin et al., 2015; Evans et al., 2013; Myung et al., 2015; Albus et al., 2005). Although the GABAA receptors that mediate the sustained activation necessary for the phase delaying effects of light are not known, GABAA-TONIC receptors would seem to be a likely candidate because of their low levels of desensitization. While it has been hypothesized that tonic and not phasic GABA signaling shifts the pacemaker (DeWoskin et al., 2015; Myung et al., 2015), further studies will be required to define the GABAA receptor(s) that mediate the phase delaying effects of light.
It will also be important to determine whether the ratio of GABA-induced excitation:inhibition in the SCN plays a role in the entrainment of the pacemaker, particularly in view of the finding that the duration of light per day alters this ratio. As noted earlier, it seems unlikely that there are specific ratios of GABA-induced excitation:inhibition that are necessary for light to induce phase shifts of the pacemaker because circadian rhythmicity can be easily entrained to LD cycles with very short or very long photoperiods. Therefore, it seems most likely that the polarity of the response to GABA within the SCN has a modulatory role on entrainment (see Section 7.5). One way this ratio could modulate entrainment is by altering GABA’s effects on the coupling and/or period length of the component oscillators in the dorsal and ventral SCN.
How the sustained activation of GABAA receptors might cause changes in Per gene expression is not presently known, although alterations in Ca2+ levels are a likely possibility (for a review see Antle et al., 2009). Critical events mediating phase delays include the activation of ryanodine receptors that amplify Ca2+ release from internal stores followed by Ca2+/calmodulin-dependent protein kinase (CaMK) II-dependent signaling, resulting in the induction of Per gene expression. How the sustained activation of GABAA receptors might influence these events is not known. It is clear that GABAA receptor activation can increase, decrease or have no effect on Ca2+ in SCN neurons depending on circadian phase and their location within the nucleus (Irwin and Allen, 2009). In summary, the sustained activation of GABAA receptors within the SCN appears to be necessary and sufficient to mediate light-induced phase delays of the circadian pacemaker. Whether similar mechanisms regulate light-induced phase advances is not known because phase delays and advances are mediated, at least in part, by different neurochemical pathways (Ding et al., 1998).
10. GABA as an SCN output signal
How efferent signals of the SCN impart circadian rhythmicity to the large number of physiological systems controlled by the circadian timing system is not known. It is clear, however, that understanding the efferent system of the SCN requires consideration of the properties of its target tissues. Different models of the organization of the mammalian circadian timing system have been discussed for more than 40 years (Moore-Ede et al., 1976). The possibilities range from a single master circadian pacemaker simply imposing rhythmicity on non-rhythmic tissues to a system composed of multiple, mutually coupled, autonomous oscillators (Fig. 16). The SCN is not the only tissue in the body capable of circadian oscillations. Indeed, the ability of peripheral mammalian tissues to display circadian rhythmicity has been known since the classic studies of Bünning more than 50 years ago (Bünning, 1958) (Fig. 17). There is also evidence that circadian rhythms can persist in the absence of the SCN (Fuller et al., 1981; Reppert et al., 1981; Stephan, 1983; Albers et al., 1984b; Moore-Ede et al., 1982; Honma et al., 1989; Stephan, 2002; Tataroglu et al., 2006). More recently, however, with the discovery of clock genes in many tissues outside of the SCN, the recognition that the circadian timing system functions as a multi-oscillator system has reemerged (Yamazaki et al., 2000; Abe et al., 2002; Yoo et al., 2004; Guilding and Piggins, 2007; Dibner et al., 2010). Indeed, the circadian timing system appears to be a multi-oscillator, hierarchical system containing central and peripheral oscillators. The organization of the system emphasizes the importance of studying the interactions among the many timing elements within the system, particularly the number, location, and interactions of oscillators outside of the SCN. It is clear, however, that the outputs of the SCN that contribute to internal synchronization and induction of circadian rhythmicity can be mediated by both neural and humoral signaling.
Fig. 16.
Alternative models of the mammalian circadian timing system. Model A is a single pacemaker (DO) system whereas the other models are multi-oscillator systems. Model B is hierarchical and Model C is non-hierarchical. Circles containing ~ indicate units capable of generating a self-sustained or damped circadian oscillations. Boxes indicate units that are driven to produce rhythms. Black ~ indicate the oscillating concentration of a chemical or electrical mediator. White dotted lines and arrows indicate entrainment of a self-sustained pacemaker with environmental stimuli by a phase response mechanism. Red dotted lines and arrows indicate entrainment of internal oscillators by a chemical or electrical mediator by a phase shifting mechanism. Solid white arrows and lines indicate the direction and flow of passive responses to an oscillating driving force (modified from Moore-Ede and Sulzman (1977)).
Fig. 17.
Oscillators in peripheral tissues. Persistence of diurnal periodicity of contractions in excised segments of Syrian hamster intestine. These rhythms in motor activity continue for three days under suitable conditions (modified from Bünning (1958)).
10.1. Neural outputs of the SCN
The efferent projections of the SCN have been studied extensively (Watts et al., 1987; Watts and Swanson, 1987; Stephan et al., 1981; Berk and Finkelstein, 1981; Swanson and Cowan, 1975; Novak et al., 2000; Bamshad et al., 1999). Primary SCN efferents include projections to pre-autonomic (i.e., paraventricular nucleus (PVN)), intermediate (i.e., dorsomedial hypothalamus (DMH), medial preoptic area (mPOA), and subparaventricular zone (SPZ)), as well as to neuroendocrine cells (i.e., PVN, PVN/DMH, and ventrolateral preoptic area (VLPO)) in the medial hypothalamus (Kalsbeek et al., 2006). Classical GABAergic synaptic release from terminals of SCN efferents plays an important role in regulating at least some SCN outputs. Surprisingly few projections from the SCN have been reported to contain GABA; only 20–30% of efferent terminals received by major SCN targets in the medial hypothalamus are GABAergic (Buijs et al., 1994). Yet studies employing electrophysiology, pharmacological manipulations, and lesions suggest that direct, inhibitory GABAergic pathways from the SCN to downstream targets, especially those in the medial hypothalamus, play a conspicuous role in regulating a variety of circadian outputs.
The PVN plays a central role in regulating endocrine and autonomic functions, including the secretion of neurohypophysial hormones, sympathetic input to the liver and pineal gland, and parasympathetic input to the pancreas. Although direct input from the SCN is sparse (Watts et al., 1987; Buijs et al., 1994), electrophysiological data indicate a remarkably powerful monosynaptic projection from SCN to PVN. Electrical stimulation of the SCN, both in vivo (Hermes et al., 1996b; Hermes and Renaud, 1993) and in vitro (Hermes et al., 1996b), produces rapid, monosynaptic glutamate receptor-mediated excitatory responses as well as GABAA receptor-mediated inhibitory responses in PVN neurons.
The SCN regulates the daily rhythm in plasma melatonin levels via both excitatory glutamatergic and inhibitory GABAergic projections to the PVN (Perreau-Lenz et al., 2004, 2005). SCN lesions eliminate the circadian rhythms in plasma melatonin and mRNA of its synthesizing enzyme, arylalkylamine N-acetyltransferase (AA-NAT) in the pineal gland, which is required for melatonin synthesis (Kalsbeek et al., 1996, 2000; Perreau-Lenz et al., 2003). SCN-lesioned animals exhibit elevated levels of melatonin and AA-NAT mRNA during the day compared to controls, but lower levels than controls at night. These data suggest that pre-sympathetic neurons in the PVN receive inhibitory input from the SCN during the day and excitatory input at night (Perreau-Lenz et al., 2003). This possibility is supported by data showing that intra-PVN administration of bicuculline during the subjective day results in the daytime release of melatonin (Kalsbeek et al., 2000). Increased GABAergic inhibition from the SCN to PVN alone, however, is not adequate to explain the drop in melatonin levels during the day. It has been proposed that the daytime decline in melatonin results from increased inhibitory GABAergic signaling combined with decreased excitatory glutamatergic signaling from the SCN to PVN (Perreau-Lenz et al., 2004, 2005). This hypothesis is supported by the findings that: (1) intra-PVN administration of a GABAA antagonist during the transition from late night to early morning does not prevent the drop in plasma melatonin at dawn (Perreau-Lenz et al., 2005), (2) shutting down synaptic activity in the SCN during the day with TTX does not cause a rise in plasma melatonin (Perreau-Lenz et al., 2004), and (3) blocking GABAA activity in the PVN during the day results in an immediate rise in melatonin to nighttime levels (Kalsbeek et al., 2000). Together, these data provide evidence that an excitatory pathway between the SCN and PVN exists and is active during the day (Perreau-Lenz et al., 2004). A concomitant daytime increase in inhibitory GABAergic signaling and withdrawal of excitatory glutamatergic signaling from the SCN to PVN appears to result in decreased sympathetic input to the pineal and subsequent drop in plasma melatonin levels during the day (Perreau-Lenz et al., 2004, 2005). In contrast, the nighttime release of melatonin results from increased glutamatergic signaling combined with decreased GABAergic signaling from SCN to PVN. TTX-induced inhibition of the SCN during the middle of subjective night causes a drop in plasma melatonin (Perreau-Lenz et al., 2004). Infusion of a NMDA receptor antagonist into the PVN during the subjective night produces the same result, indicating that glutamate release from SCN afferents in the PVN is required for the nighttime release of melatonin (Perreau-Lenz et al., 2004). Together, these data indicate that the SCN regulates the circadian cycle in melatonin by sending timed glutamatergic and GABAergic signals to pre-sympathetic neurons in the PVN, which ultimately regulate the synthesis and release of melatonin by the pineal gland (Perreau-Lenz et al., 2005).
The SCN is connected to the liver and modulates its activity through a multi-synaptic pathway that includes both branches of the autonomic nervous system (La Fleur et al., 2000; Kalsbeek et al., 2004). The daily rhythm in hepatic glucose production results primarily from rhythmic GABAergic input from the SCN to pre-autonomic sympathetic neurons in the PVN (Kalsbeek et al., 2008). Intra-PVN infusions of bicuculline increase plasma glucose concentrations when administered during mid-day, but not during mid-night (Kalsbeek et al., 2008). The hyperglycemic response to GABAA receptor blockade in the PVN is not exhibited by animals lacking intact sympathetic innervation of the liver (Kalsbeek et al., 2004), or by those with lesions to the SCN (Kalsbeek et al., 2008). Together these data indicate that the SCN sends a rhythmic GABA-mediated inhibitory signal to pre-autonomic sympathetic neurons in the PVN, which is heightened during the daytime (Kalsbeek et al., 2008). The fact that glucose concentrations increase rapidly when GABAA receptor-mediated inhibition of the PVN is removed, and intra-PVN infusion of NMDA increases plasma glucose concentrations, whether administered during mid-day or mid-night (Kalsbeek et al., 2008), suggests that pre-autonomic neurons in the PVN also receive tonic glutamatergic input. However, infusions of TTX into the SCN during mid-day mimic the hyperglycemic response to intra-PVN infusion of a GABAA receptor antagonist (Kalsbeek et al., 2004). These data are not consistent with a glutamatergic signal coming from the SCN. So while the SCN appears to be the source of rhythmic inhibitory input to preautonomic sympathetic neurons in the PVN, chronic excitatory, glutamatergic input must be derived from a source outside the SCN. Thus, the daily rhythm in hepatic glucose production is mediated by a continuously active, non-SCN glutamatergic signal, and rhythmic GABAergic signals from the SCN, which together regulate the activity of pre-sympathetic neurons in the PVN. The removal of inhibitory signaling from the SCN to PVN drives sympathetic input to the liver, resulting in the daily peak in plasma glucose concentrations at dusk (Kalsbeek et al., 2004).
GABAergic efferents from the SCN also modulate the activity of pre-autonomic neurons in the PVN that control parasympathetic input to, and insulin release from, the pancreas. In order to assess the effects of PVN activity on the parasympathetic regulation of the pancreas, insulin responses to scheduled feeding during the middle of both the day and the night have been examined (Kalsbeek et al., 2008). The administration of a GABAA agonist into the PVN attenuated feeding-induced increases in plasma insulin concentrations during the nighttime, but not during the daytime. Intra-PVN administration of NMDA receptor antagonists produced the same result. SCN lesions, however, enhance feeding-induced insulin responses during the daytime (Strubbe et al., 1987), indicating that the SCN is the source of GABA-mediated inhibitory input, but not glutamate-mediated excitatory input to pre-autonomic parasympathetic neurons in the PVN (Kalsbeek et al., 2008). In addition to regulating the activity of pre-autonomic sympathetic neurons in the PVN involved in hepatic glucose production, it appears that rhythmic GABAergic signaling from the SCN also regulates the activity of pre-autonomic parasympathetic neurons in the PVN that drive the release of insulin from the pancreas.
The SCN likely influences circadian rhythms in oxytocin (OT) and AVP release, in part, via direct projections to magnocellular neurosecretory neurons in the PVN and supraoptic nucleus of the hypothalamus (SON). Although SCN afferents in the magnocellular area of the PVN and the SON are limited (Watts et al., 1987; Watts and Swanson, 1987; Buijs et al., 1994), data from electrophysiology experiments indicate a remarkably strong connection. Most OT- and AVP-synthesizing cells in the magnocellular area of the PVN exhibit a GABAA receptor-mediated reduction in excitability following electrical stimulation of the SCN (Hermes et al., 1996a, 1996b; Hermes and Renaud, 1993). This direct inhibitory signal from the SCN to magnocellular neurons in the PVN is further modulated by presynaptic GABAB receptors on the terminals of SCN afferents (Cui et al., 2000). Thus, activation of GABA efferents from SCN to PVN likely results in competing responses, simultaneously inhibiting the responsiveness of magnocellular neurons in the PVN via activation of postsynaptic GABAA receptors and reducing subsequent GABAergic inhibition of these neurons by activating presynaptic GABAB receptors (Cui et al., 2000). Although the majority of magnocellular neurons in the SON also reduce their excitability in response to SCN stimulation, the cellular response is less uniform. In fact, while the vast majority of AVP-synthesizing cells in the SON reduced their excitability in response to electrical stimulation of the SCN, OT-synthesizing cells were more likely to show increased excitability (Cui et al., 1997). In any case, excitatory responses to SCN stimulation are mediated by glutamate receptors, and inhibitory responses are mediated by GABAA receptors (Cui et al., 1997).
The SCN communicates both directly and indirectly with the ventromedial preoptic area (VMPO) and VLPO of the hypothalamus, two “sleep active” areas that play a central role in the neural sleep/wake mechanism (Mistlberger, 2005). Direct projections from the SCN to VMPO and VLPO include glutamate-mediated excitatory signals and GABA-mediated inhibitory signals (Sun et al., 2000, 2001); but direct projections are scarce (Novak and Nunez, 2000). The SCN projects to the VMPO and VLPO indirectly as well, by way of the SPZ, DMH, lateral hypothalamus, and mPOA (Deurveilher et al., 2002). The SPZ may serve as an especially important relay through which the SCN modulates the VMPO and VLPO (Novak and Nunez, 2000; Mistlberger, 2005). The SPZ receives dense GABAA receptor-mediated inhibitory input from the SCN (Watts et al., 1987; Watts and Swanson, 1987; Buijs et al., 1994; Hermes et al., 2009). The SPZ in turn, sends dense projections to the VLPO (Novak and Nunez, 2000). The efferent projections of the SPZ overlap almost entirely with the efferent projections of the SCN (Watts, 1991) and may, in fact, be a site through which the SCN is able to indirectly communicate with a variety of hypothalamic areas that comprise sleep-wake circuits (Mistlberger, 2005).
10.2. Humoral outputs of the SCN
In addition to classical synaptic activity, the SCN can communicate efferent information via humoral signals. The possibility of functional humoral outputs of the SCN was first suggested by the restoration of circadian rhythms by the transplant of fetal SCN tissue into SCN-lesioned rodents (Drucker-Colin et al., 1984; Sawaki et al., 1984). Even small amounts of SCN tissue are capable of rescuing circadian locomotor rhythms (Silver et al., 1990; LeSauter et al., 1996), but circadian input to several neuroendocrine systems is not restored by the SCN transplants (Lehman et al., 1987; Meyer-Bernstein et al., 1999). A possible role for humoral signaling in SCN output was most convincingly demonstrated by the finding that SCN tissue placed in semipermeable capsules, thereby blocking neural outgrowth but allowing the extrusion of humoral signals, could restore circadian locomotor rhythms in SCN-lesioned hamsters (Silver et al., 1996). At present, the nature of the humoral signal that restores circadian rhythms in locomotor rhythms of SCN-lesion rodents is not known. GABA would seem to be one possibility, given its low molecular weight. It is also possible that GABA could modulate the release of other humoral signals that might serve as an SCN output signal. For example, activation of GABAA but not GABAB receptors in SCN slices modulates AVP release in the SCN (Isobe and Nishino, 1997).
11. Modulation of GABA neurotransmission
A variety of substances other than endogenous GABA can act on GABA receptors. Indeed, GABA-active drugs are used extensively for therapeutic purposes ranging from the treatment of psychiatric disease to sleep disorders. The extent to which these therapeutic actions occur at the level of the SCN is not known. It is clear, however, that disruptions of the circadian timing system have major health consequences (Zelinski et al., 2014; Baron and Reid, 2014). Not only does modulation of GABA neurotransmission have the potential for significant therapeutic value (e.g., Buxton et al., 2000), it also has the potential to produce deleterious effects on health by disrupting circadian functioning.
11.1. Steroids
Gonadal steroid hormones have long been recognized to modulate the period of circadian rhythms (Morin et al., 1977; Albers et al., 1981) and notable sex differences in the ability of ovarian hormones to alter circadian function have been identified (Zucker et al., 1980; Albers, 1981). While these hormonal effects could be mediated by classical steroid hormone receptors in the SCN or other sites (Karatsoreos et al., 2007; Clancy et al., 1994), they could also influence circadian rhythmicity by altering neural activity following their metabolism to neurosteroids in the brain. Neurosteroids are neuroactive steroids synthesized within the brain or metabolized from peripheral hormones (MacKenzie and Maguire, 2014). The actions of neurosteroids are region-specific, as cells must either synthesize the neurosteroid or express the steroidogenic enzymes required for conversion from peripheral hormones that have crossed the blood-brain barrier (MacKenzie and Maguire, 2014). Circulating levels of neurosteroids in the brain are typically low, but increase dramatically with stress, pregnancy, and at specific stages of the ovarian cycle (MacKenzie and Maguire, 2013). The GABAA receptor is a primary target for neurosteroids (Belelli and Lambert, 2005). At low levels, neurosteroids facilitate GABA binding at GABAA receptors, while at high concentrations they are capable of activating GABAA receptors directly (Majewska et al., 1986). GABAA-TONIC receptors are particularly sensitive to low, physiological concentrations of neurosteroids (Stell et al., 2003). For example, low concentrations of the naturally occurring neurosteroid allotetrahydroxy-corticosterone (THDOC) enhances tonic GABAergic conductance mediated by GABAA-TONIC receptors, thereby suppressing the excitability of cell populations (Stell et al., 2003). Further support for the role of GABAA-TONIC receptors in mediating the effects of neurosteroids is the failure of THDOC to enhance tonic GABAA conductance in mice lacking the GABAA receptor δ subunit and decreased sensitivity to neurosteroids in these Gabrd−/− mice (Mihalek et al., 1999).
Neurosteroids not only modulate GABAergic activity directly by modulating GABAA-receptor mediated activation, but also indirectly, by influencing the expression of GABAA receptor subunits (MacKenzie and Maguire, 2013). Estrous cycle, puberty, and pregnancy-dependent changes in the expression of various GABAA receptor subunits are believed to serve as a compensatory mechanism that result in steady neuronal inhibition despite fluctuating levels of neurosteroids. Progesterone is synthesized and P450scc activity (one of the enzymes responsible for its synthesis) is detectable within the SCN of hamsters, suggesting that the SCN contains the substrates required for the formation of neurosteroids (Pinto and Golombek, 1999). Although neither the endogenous activity of neurosteroids in the SCN nor their impact on GABAergic activity in the SCN has been investigated, there are data indicating that neurosteroid treatment alters neural activity in the SCN and circadian rhythmicity. THDOC reduces the neural response of the ventral SCN to optic chiasm stimulation in rats in vitro likely through the inhibition of glutamate release from the RHT resulting from the activation of GABAA-TONIC receptors (Trachsel et al., 1996). Another neuroactive steroid that inhibits GABAA activity, dehydroepiandrosterone sulfate (DHEAS), can modulate circadian phase following its systemic administration in hamsters (Pinto and Golombek, 1999). Injection of DHEAS during mid-subjective day produces phase advances similar to non-photic stimuli; whereas DHEAS administration during late subjective night inhibits light-induced phase advances (Pinto and Golombek, 1999). In contrast, systemic administration of androsterone during late subjective night actually produces light-like phase advances (Pinto and Golombek, 1999). It is also clear, however, that steroids can alter neuronal excitability of SCN neurons through actions unrelated to GABAA receptors because estradiol can alter SCN neuronal activity in slices in the presence of picrotoxin (Fatehi and Fatehi-Hassanabad, 2008).
Although steroids can produce significant changes in circadian period and phase, whether these effects are mediated by the modulation of GABAergic receptors in the SCN is not clear. Further investigation of the effects of steroids in the SCN, particularly on GABAA-TONIC receptors, is important in light of the fact that rhythms in neurosteroid secretion could modulate pacemaker functioning.
11.2. Ethanol
Ethanol consumption and withdrawal are differentially regulated by the circadian timing system (for a review see Damaggio and Gorman, 2014). Ethanol can modulate the phase shifting effects of photic and non-photic stimuli (for a review see Prosser and Glass, 2015), although chronic consumption of ethanol in a light-dark cycle does not alter the phase of the circadian pacemaker in the SCN (Filiano et al., 2013). In hamsters, systemic administration of ethanol attenuates light-induced phase advances without affecting phase delays (Seggio et al., 2007; Ruby et al., 2009a, 2009b). In contrast, ethanol administered either systemically or directly into the SCN of mice via microdialysis attenuates light-induced phase delays without affecting photic phase advances (Brager et al., 2010, 2011; Seggio et al., 2009). Systemically administered ethanol also modulates phase shifts induced by non-photic stimuli in hamsters. Acute administration of ethanol attenuates the ability of the 5-HT1a/7 agonist 8-OH-DPAT to induce phase advances during the subjective day (Ruby et al., 2009b), although studies conducted in the hypothalamic slice have yielded different results. In contrast to systemic ethanol administration, ethanol applied in vitro to mouse SCN slices attenuates both phase delays and phase advances induced by glutamate, and ethanol enhances phase advances stimulated by administration of 8-OH-DPAT (Prosser et al., 2008; McElroy et al., 2009). Thus, while ethanol produces substantial effects on the ability of photic and non-photic stimuli to induce phase shifts, the effects differ considerably among species and between studies conducted in vivo and in vitro.
While there is substantial evidence that GABAA-TONIC receptors are sensitive to ethanol, and that tonic Cl− conductance through these channels is enhanced by ethanol (for a review see Wallner and Olsen, 2008), there is only one study that has examined the roles of GABAA-PHASIC and GABAA-TONIC receptors in the SCN in mediating the effects of ethanol on phase resetting (McElroy et al., 2009). In hypothalamic slices from mice, the GABAA-TONIC receptor antagonist RO15-4513 blocked ethanol’s inhibition of glutamate-induced phase delays, whereas the GABAA-PHASIC-active BDZ diazepam had no effect. In addition, RO15-4513 blocked ethanol’s enhancement of phase advances induced by the 5-HT1a/7 agonist 8-OH-DPAT. Taken together, these in vitro studies suggest that GABAA-TONIC receptors are involved in the effects of ethanol on both photic and non-photic phase resetting within the SCN.
12. Conclusions
Despite substantial progress in understanding the role of GABA in circadian timing, fundamental questions about GABA neurotransmission within the SCN remain unanswered. Although anatomical evidence indicates that GABA is frequently colocalized with multiple neuropeptides (e.g. VIP and GRP) we know very little about the functional consequences of their corelease. Only a few studies have examined the circadian effects of the interaction of multiple signals in the SCN (Albers et al., 1991; Peters et al., 1994; Chan et al., 2016). Similarly, GABA is likely released both synaptically and non-synaptically in the SCN. And yet we do not have a clear understanding of the relative contributions of these forms of signaling to the coupling of SCN clock cells into a circadian pacemaker and the entrainment of that pacemaker with the day-night cycle. While we know that intra- and extracellular levels of GABA are the result of a complex interaction of release, synthesis, and transport, we have only limited knowledge about the temporal patterning and distribution of extracellular GABA in the SCN. Existing evidence suggests, however, that there is a 24-h rhythm in extracellular levels of GABA that peaks in the late morning and early night. Several of the mechanisms regulating the levels of extracellular GABA (e.g., GAD mRNA) are influenced by lighting conditions and display a 24-h rhythm that damps out in constant lighting conditions. In contrast, other evidence suggests that rhythms in extracellular GABA may be truly circadian and persist in constant conditions (e.g., GABA-mediated currents).
GABA receptors are found throughout all regions of the SCN. A greater understanding of GABA neurotransmission awaits a better understanding of the complex nature of the many potential forms and actions of GABAA receptors. In this review we have focused on what we have termed GABAA-PHASIC and GABAA-TONIC receptors that are involved in synaptic and non-synaptic responses, respectively. The rhythmic pattern of GABAA subunits in the SCN suggests that GABAA-TONIC receptors are in greater abundance during the subjective night while GABAA-PHASIC receptors are in greater abundance during the subjective day. It is likely, however, that GABAA receptors containing different combinations of subunits resulting in different functional properties will be identified in the SCN. GABAB receptors in the SCN consistently inhibit neuronal firing and can significantly inhibit the phase shifting effects of light by presynaptic and possibly postsynaptic actions.
The most perplexing results that have come from the investigation of GABA in the SCN relate to the polarity of the cellular responses to GABA. Some studies have found that GABA consistently reduces neuronal firing, while other studies have found GABA to reduce firing in some neurons and to increase firing in others. Additional controversies include whether GABA has different effects at different phases of the circadian cycle and whether GABA has different effects in different subregions of the SCN. Unfortunately, no resolution to these different outcomes is evident. Nevertheless, the most parsimonious interpretation may be that GABA is primarily inhibitory throughout the SCN during the day, while at night GABA remains primarily inhibitory in the ventral core but can have substantial excitatory effects in the dorsal shell. This possibility is supported by recent anatomical evidence that the transporters underlying the excitatory effects of GABA are found in greater number in the dorsal SCN at night. Interestingly, recent data suggest that the percentage of excitatory responses to GABA depends on the photoperiod length of the entraining LD cycle. A larger proportion of excitatory responses are observed in SCN neurons obtained from animals that have been exposed to longer photoperiods.
One of the key functions of the pacemaker in the SCN is to generate circadian rhythms. Individual clock cells contain the molecular machinery to generate circadian rhythms but must be coupled together to form a functional pacemaker. VIP acting on VPAC2 receptors is important in the coupling of clock cells, although how VIP mediates coupling is unclear. While VIP activity is rhythmic in animals entrained to LD cycles, it appears to be non-rhythmic under free-running conditions. GABA has the potential to influence the coupling of individual clock cells because activation of GABAA receptors can phase shift the clocks, thereby providing a mechanism for their mutual synchronization. In contrast, clock cells do not become desynchronized when GABA activity is blocked for days, suggesting that GABA is not a critical coupling agent. Another major question is whether synaptic activity is necessary for the coupling of clock cells. Inhibition of synaptic activity within the SCN of intact animals for two weeks does not stop the pacemaker or dramatically change its circadian period. In addition, briefer periods of synaptic inhibition do not perturb the pacemaker in intact animals (e.g., produce phase shifts). On the other hand, inhibition of synaptic activity for 5–7 days desynchronizes cultured SCN clock cells. The very different effects of inhibiting synaptic transmission on coupling in the SCN in whole animals versus reduced preparations are difficult to reconcile. One possibility relates to the size of the circadian network being studied. If network interactions serve to stabilize noisy circadian oscillations, then circadian oscillations within larger networks should be more resistant to disruption. Perhaps the substantially larger network present in the intact circadian system (composed of SCN and possibly extra-SCN oscillators) provides more stability than can be achieved in reduced preparations such as SCN cultures or slices. As a result, inhibition of synaptic activity should reduce synchrony in smaller, less stable networks like SCN cultures, but have little effect on larger circadian networks of intact animals. If this view is correct, and synaptic activity has only a minor role in the coupling of clock cells in the intact circadian system, it will be important to identify the non-synaptic forms of communication that are involved.
GABA’s most important role in the SCN is to modulate the phase of the circadian pacemaker. Activation of GABA receptors can both induce phase shifts and block phase shifts. The acute activation of GABAA receptors within the SCN during the middle of the day mediates the ability of non-photic stimuli to phase shift the pacemaker. Interestingly, however, systemic administration of BDZs that, like other non-photic stimuli, produce phase advances during the middle of the subjective day in nocturnal rodents, may act within as well as outside of the SCN to produce phase shifts. It is also noteworthy that during the day, activation of GABAA receptors in the SCN of diurnal rodents produces phase delays, not phase advances like it does in nocturnal rodents. During the night, acute activation of either GABAA or GABAB receptors in the SCN just prior to exposure to a phase delaying or phase advancing pulse of light profoundly inhibits the ability of the pacemaker to phase shift. In contrast, during the night the sustained activation of GABAA receptors in the SCN is both necessary and sufficient to induce phase delays in response to light. Several lines of evidence suggest that the effects of GABA on circadian phase are mediated primarily by GABAA-PHASIC receptors during the subjective day and GABAA-TONIC receptors during the subjective night. Taken together these data suggest that GABA plays a major role in determining the phase of the circadian pacemaker in all circumstances.
It has been proposed that the entrainment of the compound pacemaker in the SCN is the result of light’s effects on component SCN oscillators that can function semi-independently from each other. Together these component oscillators form the entrainable, compound circadian pacemaker in the SCN. Because these component oscillators respond differently to light, their interactions are key to understanding the entrainment of the compound pacemaker. The left and right SCN, as well as the ventral and dorsal SCN, are component oscillators. While there is little evidence that GABA or VIP are involved in the coupling of the left and right SCN, there is evidence that GABA and VIP can influence the periods of the component oscillators in the ventral and dorsal SCN and/or the coupling between these oscillators. Indeed, several lines of evidence support the hypothesis that the sustained activation of GABAA receptors mediates the phase delaying effects of light on the pacemaker by acting on these component oscillators and/or their coupling. It also appears likely that the periods of the ventral and dorsal oscillators and/or their coupling are influenced by the ratio of excitation:inhibition evoked by GABA.
A great deal has been learned about the role of GABA neurotransmission in the regulation of circadian rhythmicity over the last 40 years. GABA plays a fundamental, yet at times a seemingly paradoxical, role in the single most important adaptive trait mediated by the circadian timing system: photic entrainment. GABA neurotransmission can block the ability of the pacemaker in the SCN to phase shift in response to light and can mediate the ability of the pacemaker to phase delay in response to light. We have, however, studied GABA function in a very limited number of species, primarily in nocturnally active rodents. It will be important to expand the number of species examined to fully understand the role of GABA in circadian timekeeping, because the circadian effects of GABA drugs can differ substantially even in different strains of the same species (Ebihara et al., 1988). It is also important to recognize that the SCN is but one component in a hierarchical, multi-oscillator system where feedback from other components of the system likely play a critical role in its circadian timekeeping mechanisms. Indeed, the SCN receives neural projections from approximately 85 other brain structures and contains hormone receptors. It seems likely that GABA has many important circadian functions in elements of the system found outside of the SCN. Therefore, while studies of the isolated SCN have provided a large body of important data, understanding the neurobiology of circadian timekeeping will require a thorough analysis of the entire circadian system.
Acknowledgments
The original research discussed in this paper was supported by NIH grants GM31199, GM34798, NS30022, NS34586, MH58789, NS0780220, MH067420, MH12956, MH12683, NS09927 and ONR N00014-87-K0172 to HEA; NIH grant NS092545 to JCW; NIH grants NS082413, NS051183, GM086683, GM086683 and NS084683 to KLG; and NSF grant IOS-1022050 to DLH. The authors thank Drs. Charles Allen, Chistopher Colwell, Ralph Mistleberger, and Martin Moore-Ede for permission to adapt figures. The authors also thank Drs. Jennifer Evans, Daniel Forger and Lauren Hablitz for comments on the manuscript and Jason Snape for the completion of the original figures.
Abbreviations
- 5HT
serotonin
- AA-NAT
arylalkylamine N-acetyltransferase
- AVP
arginine vasopressin
- BZD
benzodiazepine
- CCC
cation chloride cotransporter
- CNS
central nervous system
- Cry
cryptochrome
- CT
circadian time
- DHEAS
dehydroepiandrosterone sulfate
- DMH
dorsomedial hypothalamus
- EPSC
excitatory post-synaptic current
- GABA
γ-aminobutyric acid
- GAD
glutamic acid decarboxylase
- GAT
GABA transporter
- GFAP
glial fibrillary acidic protein
- GHT
geniculohypothalamic tract
- GIRK
G-protein coupled inwardly rectifying K+ channel
- GRP
gastrin releasing peptide
- IGL
intergeniculate leaflet
- IPSC
inhibitory post-synaptic current
- KCC
potassium chloride cotransporter
- LD
light:dark cycle
- LDCV
large dense-core vesicle
- mPOA
medial preoptic area
- NKCC
sodium potassium chloride cotransporter
- NPY
neuropeptide Y
- OT
oxytocin
- PD
postnatal day
- Per
period
- PER:LUC
period:luciferase
- PHI
peptide histidine isoleucine
- PVN
paraventricular nucleus
- RHT
retinohypothalamic tract
- SCN
suprachiasmatic nucleus
- sIPSC
spontaneous inhibitory post-synaptic current
- SON
supraoptic nucleus
- SPZ
subparaventricular zone
- SSV
small synaptic vesicle
- THDOC
tetrahydrodeoxycorticosterone
- TTX
tetrodotoxin
- VGAT
vesicular GABA transporter
- VIAAT
vesicular inhibitory amino acid transporter
- VIP
vasoactive intestinal peptide
- VLPO
ventrolateral preoptic area
- VMPO
ventromedial preoptic area
References
- Abe M, Herzog ED, Yamazaki S, Straume M, Tei H, Sakaki Y, Menaker M, Block GD. Circadian rhythms in isolated brain regions. J. Neurosci. 2002;22:350–356. doi: 10.1523/JNEUROSCI.22-01-00350.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar-Roblero R, Verduzco-Carbajal L, Rodriguez C, Mendez-Franco J, Moran J, Perez de la Mora M. Circadian rhythmicity in the GABAergic system in the suprachiasmatic nuclei of the rat. Neurosci. Lett. 1993;157:199–202. doi: 10.1016/0304-3940(93)90736-5. [DOI] [PubMed] [Google Scholar]
- Aioun J, Chambille I, Peytevin J, Martinet L. Neurons containing gastrin-releasing peptide and vasoactive intestinal polypeptide are involved in the reception of the photic signal in the suprachiasmatic nucleus of the Syrian hamster: an immunocytochemical ultrastructural study. Cell Tissue Res. 1998;291:239–253. doi: 10.1007/s004410050994. [DOI] [PubMed] [Google Scholar]
- Ajpru S, McArthur AJ, Piggins HD, Sugden D. Identification of PAC1 receptor isoform mRNAs by real-time PCR in rat suprachiasmatic nucleus. Brain Res. Mol. Brain Res. 2002;105:29–37. doi: 10.1016/s0169-328x(02)00387-x. [DOI] [PubMed] [Google Scholar]
- Alamilla J, Perez-Burgos A, Quinto D, Aguilar-Roblero R. Circadian modulation of the Cl(−) equilibrium potential in the rat suprachiasmatic nuclei. Biomed. Res. Int. 2014;2014:424982. doi: 10.1155/2014/424982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers HE. Gonadal hormones organize and modulate the circadian system of the rat. Am. J. Physiol. 1981;241:R62–R66. doi: 10.1152/ajpregu.1981.241.1.R62. [DOI] [PubMed] [Google Scholar]
- Albers HE. Species, sex and individual differences in the vasotocin/vasopressin system: relationship to neurochemical signaling in the social behavior neural network. Front. Neuroendocrinol. 2015;36:49–71. doi: 10.1016/j.yfrne.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers HE, Ferris CF. Neuropeptide Y: role in light-dark cycle entrainment of hamster circadian rhythm. Neurosci. Lett. 1984;50:163–168. doi: 10.1016/0304-3940(84)90480-4. [DOI] [PubMed] [Google Scholar]
- Albers HE, Ferris CF, Leeman SE, Goldman BD. Avian pancreatic polypeptide phase shifts hamster circadian rhythms when microinjected into the suprachiasmatic region. Science. 1984a;223:833–835. doi: 10.1126/science.6546454. [DOI] [PubMed] [Google Scholar]
- Albers HE, Gerall AA, Axelson JF. Effect of reproductive state on circadian periodicity in the rat. Physiol. Behav. 1981;26:21–25. doi: 10.1016/0031-9384(81)90073-1. [DOI] [PubMed] [Google Scholar]
- Albers HE, Liou SY, Stopa EG, Zoeller RT. Interaction of co-localized neuropeptides: functional significance in the circadian timing system. J. Neurosci. 1991;11:846–851. doi: 10.1523/JNEUROSCI.11-03-00846.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers HE, Liou SY, Stopa EG, Zoeller RT. Neurotransmitter colocalization and circadian rhythms. In: Joosse J, Buijs RM, Tilders FJH, editors. Progress in Brain Research. Amsterdam: Elsevier Science Publishers; 1992. pp. 289–307. [DOI] [PubMed] [Google Scholar]
- Albers HE, Lydic R, Gander PH, Moore-Ede MC. Role of the suprachiasmatic nuclei in the circadian timing system of the squirrel monkey. I. The generation of rhythmicity. Brain Res. 1984b;300:275–284. doi: 10.1016/0006-8993(84)90837-0. [DOI] [PubMed] [Google Scholar]
- Albers HE, Lydic R, Moore-Ede MC. Role of the suprachiasmatic nuclei in the circadian timing system of the squirrel monkey. II. Light-dark cycle entrainment. Brain Res. 1984c;300:285–293. doi: 10.1016/0006-8993(84)90838-2. [DOI] [PubMed] [Google Scholar]
- Albers HE, Minamitani N, Stopa E, Ferris CF. Light selectively alters vasoactive intestinal peptide and peptide histidine isoleucine immunoreactivity within the rat suprachiasmatic nucleus. Brain Res. 1987;437:189–192. doi: 10.1016/0006-8993(87)91544-7. [DOI] [PubMed] [Google Scholar]
- Albers HE, Stopa EG, Zoeller RT, Kauer JS, King JC, Fink JS, Mobtaker H, Wolfe H. Day-night variation in prepro vasoactive intestinal peptide/peptide histidine isoleucine mRNA within the rat suprachiasmatic nucleus. Mol. Brain Res. 1990;7:85–89. doi: 10.1016/0169-328x(90)90077-q. [DOI] [PubMed] [Google Scholar]
- Albrecht U, Sun ZS, Eichele G, Lee CC. A differential response of two putative mammalian circadian regulators, mper1 and mper2, to light. Cell. 1997;91:1055–1064. doi: 10.1016/s0092-8674(00)80495-x. [DOI] [PubMed] [Google Scholar]
- Albrecht U, Zheng B, Larkin D, Sun ZS, Lee CC. mPer1 and mPer2 are essential for normal resetting of the circadian clock. J. Biol. Rhythms. 2001;16:100–104. doi: 10.1177/074873001129001791. [DOI] [PubMed] [Google Scholar]
- Albus H, Vansteensel MJ, Michel S, Block GD, Meijer JH. A GABAergic mechanism is necessary for coupling dissociable ventral and dorsal regional oscillators within the circadian clock. Curr. Biol. 2005;15:886–893. doi: 10.1016/j.cub.2005.03.051. [DOI] [PubMed] [Google Scholar]
- Allen CN, Klett NJ, Irwin RP, Moldavan MG. GABAA receptor-mediated neurotransmission in the suprachiasmatic nucleus. In: Aguilar-Roblero R, et al., editors. Mechanisms of Circadian Systems in Animals and Their Clinical Relevance. Basil: Springer Verlag; 2014. pp. 133–148. [Google Scholar]
- Antle MC, Silver R. Orchestrating time: arrangements of the brain circadian clock. Trends Neurosci. 2005;28:145–151. doi: 10.1016/j.tins.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Antle MC, Smith VM, Sterniczuk R, Yamakawa GR, Rakai BD. Physiological responses of the circadian clock to acute light exposure at night. Rev. Endocr. Metab. Disord. 2009;10:279–291. doi: 10.1007/s11154-009-9116-6. [DOI] [PubMed] [Google Scholar]
- Araque A, Carmignoto G, Haydon PG, Oliet SH, Robitaille R, Volterra A. Gliotransmitters travel in time and space. Neuron. 2014;81:728–739. doi: 10.1016/j.neuron.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22:208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- Asada H, Kawamura Y, Maruyama K, Kume H, Ding R, Ji FY, Kanbara N, Kuzume H, Sanbo M, Yagi T, Obata K. Mice lacking the 65 kDa isoform of glutamic acid decarboxylase (GAD65) maintain normal levels of GAD67 and GABA in their brains but are susceptible to seizures. Biochem. Biophys. Res. Commun. 1996;229:891–895. doi: 10.1006/bbrc.1996.1898. [DOI] [PubMed] [Google Scholar]
- Asada H, Kawamura Y, Maruyama K, Kume H, Ding RG, Kanbara N, Kuzume H, Sanbo M, Yagi T, Obata K. Cleft palate and decreased brain gamma-aminobutyric acid in mice lacking the 67-kDa isoform of glutamic acid decarboxylase. Proc. Natl. Acad. Sci. U.S.A. 1997;94:6496–6499. doi: 10.1073/pnas.94.12.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aton SJ, Colwell CS, Harmar AJ, Waschek J, Herzog ED. Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat. Neurosci. 2005;8:476–483. doi: 10.1038/nn1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aton SJ, Huettner JE, Straume M, Herzog ED. GABA and Gi/o differentially control circadian rhythms and synchrony in clock neurons. Proc. Natl. Acad. Sci. U.S.A. 2006;103:19188–19193. doi: 10.1073/pnas.0607466103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamshad M, Song CK, Bartness TJ. CNS origins of the sympathetic nervous system outflow to brown adipose tissue. Am. J. Physiol. 1999;276:R1569–R1578. doi: 10.1152/ajpregu.1999.276.6.R1569. [DOI] [PubMed] [Google Scholar]
- Baron KG, Reid KJ. Circadian misalignment and health. Int. Rev. Psychiatry. 2014;26:139–154. doi: 10.3109/09540261.2014.911149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann SW, Baur R, Sigel E. Forced subunit assembly in alpha1beta2gamma2 GABAA receptors. Insight into the absolute arrangement. J. Biol. Chem. 2002;277:46020–46025. doi: 10.1074/jbc.M207663200. [DOI] [PubMed] [Google Scholar]
- Bazargani N, Attwell D. Astrocyte calcium signaling: the third wave. Nat. Neurosci. 2016;19:182–189. doi: 10.1038/nn.4201. [DOI] [PubMed] [Google Scholar]
- Belelli D, Harrison NL, Maguire J, Macdonald RL, Walker MC, Cope DW. Extrasynaptic GABAA receptors: form, pharmacology, and function. J. Neurosci. 2009;29:12757–12763. doi: 10.1523/JNEUROSCI.3340-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat. Rev. Neurosci. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- Belenky MA, Sagiv N, Fritschy JM, Yarom Y. Presynaptic and postsynaptic GABAA receptors in rat suprachiasmatic nucleus. Neuroscience. 2003;118:909–923. doi: 10.1016/s0306-4522(03)00062-9. [DOI] [PubMed] [Google Scholar]
- Belenky MA, Sollars PJ, Mount DB, Alper SL, Yarom Y, Pickard GE. Cell-type specific distribution of chloride transporters in the rat suprachiasmatic nucleus. Neuroscience. 2010;165:1519–1537. doi: 10.1016/j.neuroscience.2009.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belenky MA, Yarom Y, Pickard GE. Heterogeneous expression of gamma-aminobutyric acid and gamma-aminobutyric acid-associated receptors and transporters in the rat suprachiasmatic nucleus. J. Comp. Neurol. 2008;506:708–732. doi: 10.1002/cne.21553. [DOI] [PubMed] [Google Scholar]
- Belle MD, Diekman CO, Forger DB, Piggins HD. Daily electrical silencing in the mammalian circadian clock. Science. 2009;326:281–284. doi: 10.1126/science.1169657. [DOI] [PubMed] [Google Scholar]
- Ben Ari Y, Woodin MA, Sernagor E, Cancedda L, Vinay L, Rivera C, Legendre P, Luhmann HJ, Bordey A, Wenner P, Fukuda A, van den Pol AN, Gaiarsa JL, Cherubini E. Refuting the challenges of the developmental shift of polarity of GABA actions: GABA more exciting than ever! Front. Cell Neurosci. 2012;6:35. doi: 10.3389/fncel.2012.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch EE. GABAB receptors: structure, functions, and clinical implications. Neurology. 2012;78:578–584. doi: 10.1212/WNL.0b013e318247cd03. [DOI] [PubMed] [Google Scholar]
- Benarroch EE. Cation-chloride cotransporters in the nervous system: general features and clinical correlations. Neurology. 2013;80:756–763. doi: 10.1212/WNL.0b013e318283bb1c. [DOI] [PubMed] [Google Scholar]
- Bergeron HE, Danielson B, Biggs KR, Prosser RA. TTX blocks baclofen-induced phase shifts of the mammalian circadian pacemaker in vitro. Brain Res. 1999;841:193–196. doi: 10.1016/s0006-8993(99)01791-6. [DOI] [PubMed] [Google Scholar]
- Berk ML, Finkelstein JA. An autoradiographic determination of the efferent projections of the suprachiasmatic nucleus of the hypothalamus. Brain Res. 1981;226:1–13. doi: 10.1016/0006-8993(81)91079-9. [DOI] [PubMed] [Google Scholar]
- Besing RC, Hablitz LM, Paul JR, Johnson RL, Prosser RA, Gamble KL. Neuropeptide Y-induced phase shifts of PER2::LUC rhythms are mediated by long-term suppression of neuronal excitability in a phase-specific manner. Chronobiol. Int. 2012;29:91–102. doi: 10.3109/07420528.2011.649382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biello SM. Circadian clock resetting in the mouse changes with age. Age (Dordr) 2009;31:293–303. doi: 10.1007/s11357-009-9102-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biello SM, Golombek DA, Schak KM, Harrington ME. Circadian phase shifts to neuropeptide Y in vitro: cellular communication and signal transduction. J. Neurosci. 1997;17:8468–8475. doi: 10.1523/JNEUROSCI.17-21-08468.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biello SM, Harrington ME, Mason R. Geniculo-hypothalamic tract lesions block chlordiazepoxide-induced phase advances in Syrian hamsters. Brain Res. 1991;552:47–52. doi: 10.1016/0006-8993(91)90658-i. [DOI] [PubMed] [Google Scholar]
- Biello SM, Janik D, Mrosovsky N. Neuropeptide Y and behaviorally induced phase shifts. Neuroscience. 1994;62:273–279. doi: 10.1016/0306-4522(94)90331-x. [DOI] [PubMed] [Google Scholar]
- Biello SM, Mrosovsky N. Circadian phase-shifts induced by chlordiazepoxide without increased locomotor activity. Brain Res. 1993;622:58–62. doi: 10.1016/0006-8993(93)90801-s. [DOI] [PubMed] [Google Scholar]
- Biggs KR, Prosser RA. Neuropeptide Y blocks GABAB-induced phase-shifts of the suprachiasmatic circadian clock in vitro. Brain Res. 1999;821:461–466. doi: 10.1016/s0006-8993(99)01104-x. [DOI] [PubMed] [Google Scholar]
- Biggs KR, Prosser RA. GABAB-receptor stimulation phase-shifts the mammalian circadian clock in vitro. Brain Res. 1998;807:250–254. doi: 10.1016/s0006-8993(98)00820-8. [DOI] [PubMed] [Google Scholar]
- Blaesse P, Airaksinen MS, Rivera C, Kaila K. Cation-chloride cotransporters and neuronal function. Neuron. 2009;61:820–838. doi: 10.1016/j.neuron.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Bos NP, Mirmiran M. Effects of excitatory and inhititory amino acids on neuronal discharges in the cultured suprachiasmatic nucleus. Brain Res. Bull. 1993;31:67–72. doi: 10.1016/0361-9230(93)90012-z. [DOI] [PubMed] [Google Scholar]
- Bosler O. Ultrastructural relationships of serotonin and GABA terminals in the rat suprachiasmatic nucleus. Evidence for a close interconnection between the two afferent systems. J. Neurocytol. 1989;18:105–113. doi: 10.1007/BF01188429. [DOI] [PubMed] [Google Scholar]
- Bosler O, Girardet C, Franc JL, Becquet D, François-Bellan AM. Structural plasticity of the circadian timing system. An overview from flies to mammals. Front. Neuroendocrinol. 2015;38:50–65. doi: 10.1016/j.yfrne.2015.02.001. [DOI] [PubMed] [Google Scholar]
- Boulos Z, Rusak B. Circadian phase response curves for dark pulses in the hamster. J. Comp. Physiol. 1982;146:411–417. [Google Scholar]
- Bouskila Y, Dudek FE. Neuronal synchronization without active calcium-dependent synaptic transmission in the hypothalamus. Proc. Natl. Acad. Sci. U.S.A. 1993;15:3207–3210. doi: 10.1073/pnas.90.8.3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowery NG, Doble A, Hill DR, Hudson AL, Shaw JS, Turnbull MJ, Warrington R. Bicuculline-insensitive GABA receptors on peripheral autonomic nerve terminals. Eur. J. Pharm. 1981;71:53–70. doi: 10.1016/0014-2999(81)90386-1. [DOI] [PubMed] [Google Scholar]
- Brager AJ, Ruby CL, Prosser RA, Glass JD. Chronic ethanol disrupts circadian photic entrainment and daily locomotor activity in the mouse. Alcohol. Clin. Exp. Res. 2010;34:1266–1273. doi: 10.1111/j.1530-0277.2010.01204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brager AJ, Ruby CL, Prosser RA, Glass JD. Acute ethanol disrupts photic and serotonergic circadian clock phase-resetting in the mouse. Alcohol. Clin. Exp. Res. 2011;35:1467–1474. doi: 10.1111/j.1530-0277.2011.01483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragina L, Marchionni I, Omrani A, Cozzi A, Pellegrini-Giampietro DE, Cherubini E, Conti F. GAT-1 regulates both tonic and phasic GABA(A) receptor-mediated inhibition in the cerebral cortex. J. Neurochem. 2008;105:1781–1793. doi: 10.1111/j.1471-4159.2008.05273.x. [DOI] [PubMed] [Google Scholar]
- Bramley JR, Sollars PJ, Pickard GE, Dudek FE. 5-HT1B receptor-mediated presynaptic inhibition of GABA release in the suprachiasmatic nucleus. J. Neurophysiol. 2005;93:3157–3164. doi: 10.1152/jn.00770.2004. [DOI] [PubMed] [Google Scholar]
- Breuker E, Johnston GA. Inhibition of acetylcholinesterase by bicuculline and related alkaloids. J. Neurochem. 1975;25:903–904. doi: 10.1111/j.1471-4159.1975.tb04427.x. [DOI] [PubMed] [Google Scholar]
- Bright DP, Smart TG. Methods for recording and measuring tonic GABAA receptor-mediated inhibition. Front. Neural Circ. 2013;7:193. doi: 10.3389/fncir.2013.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TM, Hughes AT, Piggins HD. Gastrin-releasing peptide promotes suprachiasmatic nuclei cellular rhythmicity in the absence of vasoactive intestinal polypeptide-VPAC2 receptor signaling. J. Neurosci. 2005;25:11155–11164. doi: 10.1523/JNEUROSCI.3821-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buddhala C, Hsu CC, Wu JY. A novel mechanism for GABA synthesis and packaging into synaptic vesicles. Neurochem. Int. 2009;55:9–12. doi: 10.1016/j.neuint.2009.01.020. [DOI] [PubMed] [Google Scholar]
- Buijs RM, Hou Y, Shinn S, Renaud LP. Ultrastructural evidence for intra- and extranuclear projections of GABAergic neurons of the suprachiasmatic nucleus. J. Comp. Neurol. 1994;340:381–391. doi: 10.1002/cne.903400308. [DOI] [PubMed] [Google Scholar]
- Buijs RM, Wortel J, Hou Y. Colocalization of y-aminobutyric acid with vasopressin, vasoactive intestinal peptide, and somatostatin in the rat suprachiasmatic nucleus. J. Comp. Neurol. 1995;358:343–352. doi: 10.1002/cne.903580304. [DOI] [PubMed] [Google Scholar]
- Bünning E. Das Weiterlaufen der physiologischen Uhr im Saugerdarm ohne zentrale Steuerung. Naturwissenschaften. 1958;45:68. [Google Scholar]
- Burgoon PW, Boulant JA. Synaptic inhibition: its role in suprachiasmatic nucleus neuronal thermosensitivity and temperature compensation in the rat. J. Physiol. 1998;512:793–807. doi: 10.1111/j.1469-7793.1998.793bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MP, Rainbow MN, Rodriguez E, Lyon SM, Silver R. Twelve-hour days in the brain and behavior of split hamsters. Eur. J. Neurosci. 2012;36:2556–2566. doi: 10.1111/j.1460-9568.2012.08166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton OM, Copinshi G, Van Onderbergen A, Karrison TG, Van Cauter E. A benzodiapepine hypnotic facilitates adaptation of circadian rhythms and sleep-wake homeostasis to an eight hour delay shift stimulating westward jet lag. Sleep. 2000;23:915–927. [PubMed] [Google Scholar]
- Cagampang FRA, Rattray M, Powell JF, Campbell IC, Coen CW. Circadian changes of glutamate decarboxylase 65 and 67 mRNA in the rat suprachiasmatic nuclei. NeuroReport. 1996;12:1925–1928. doi: 10.1097/00001756-199608120-00011. [DOI] [PubMed] [Google Scholar]
- Cahill GM, Menaker M. Effects of excitatory amino acid receptor antagonists and agonists on suprachiasmatic nucleus responses to retinohypothalamic tract volleys. Brain Res. 1989;479:76–82. doi: 10.1016/0006-8993(89)91337-1. [DOI] [PubMed] [Google Scholar]
- Card JP, Moore RY. Ventral lateral geniculate nucleus efferents to the rat suprachiasmatic nucleus exhibit avian pancreatic polypeptide-like immunoreactivity. J. Comp. Neurol. 1982;206:390–396. doi: 10.1002/cne.902060407. [DOI] [PubMed] [Google Scholar]
- Card JP, Moore RY. The suprachiasmatic nucleus of the golden hamster: immunohistochemical analysis of cell and fiber distribution. Neuroscience. 1984;13:415–431. doi: 10.1016/0306-4522(84)90240-9. [DOI] [PubMed] [Google Scholar]
- Cardinali DP, Golombek DA. The rhythmic GABAergic system. Neurochem. Res. 1998;23:607–614. doi: 10.1023/a:1022426519297. [DOI] [PubMed] [Google Scholar]
- Carrillo M, Ricci LA, Schwartzer JJ, Melloni RH. Immunohistochemical characterization of 5-HT(3A) receptors in the Syrian hamster forebrain. Brain Res. 2010;1329:67–81. doi: 10.1016/j.brainres.2010.02.038. [DOI] [PubMed] [Google Scholar]
- Castel M, Morris J, Belenky M. Non-synaptic and dendritic exocytosis from dense-core vesicles in the suprachiasmatic nucleus. NeuroReport. 1996;7:543–547. doi: 10.1097/00001756-199601310-00040. [DOI] [PubMed] [Google Scholar]
- Castel M, Morris JF. Morphological heterogeneity of the GABAergic network in the suprachiasmatic nucleus, the brain’s circadian pacemaker. J. Anat. 2000;196:1–13. doi: 10.1046/j.1469-7580.2000.19610001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challet E. Minireview: entrainment of the suprachiasmatic clockwork in diurnal and nocturnal mammals. Endocrinology. 2007;148:5648–5655. doi: 10.1210/en.2007-0804. [DOI] [PubMed] [Google Scholar]
- Challet E, Denis I, Rochet V, Aioun J, Gourmelen S, Lacroix H, Goustard-Langelier B, Papillon C, Alessandri JM, Lavialle M. The role of PPARbeta/delta in the regulation of glutamatergic signaling in the hamster suprachiasmatic nucleus. Cell. Mol. Life Sci. 2013;70:2003–2014. doi: 10.1007/s00018-012-1241-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan RK, Sterniczuk R, Enkhbold Y, Jeffers RT, Basu P, Duong B, Chow SL, Smith VM, Antle MC. Phase shifts to light are altered by antagonists to neuropeptide receptors. Neuroscience. 2016;327:115–124. doi: 10.1016/j.neuroscience.2016.04.010. [DOI] [PubMed] [Google Scholar]
- Chang Y, Wang R, Barot S, Weiss DS. Stoichiometry of a recombinant GABAA receptor. J. Neurosci. 1996;16:5415–5424. doi: 10.1523/JNEUROSCI.16-17-05415.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattipakorn SC, McMahon LL. Pharmacological characterization of glycine-gated chloride currents recorded in rat hippocampal slices. J. Neurophysiol. 2002;87:1515–1525. doi: 10.1152/jn.00365.2001. [DOI] [PubMed] [Google Scholar]
- Chaudhry FA, Reimer RJ, Bellocchio EE, Danbolt NC, Osen KK, Edwards RH, Storm-Mathisen J. The vesicular GABA transporter, VGAT, localizes to synaptic vesicles in sets of glycinergic as well as GABAergic neurons. J. Neurosci. 1998;18:9733–9750. doi: 10.1523/JNEUROSCI.18-23-09733.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, van den Pol AN. Presynaptic GABAB autoreceptor modulation of P/Q-type calcium channels and GABA release in rat suprachiasmatic nucleus neurons. J. Neurosci. 1998;18:1913–1922. doi: 10.1523/JNEUROSCI.18-05-01913.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang CK, Mehta N, Patel A, Zhang P, Ning Z, Mayne J, Sun WY, Cheng HY, Figeys D. The proteomic landscape of the suprachiasmatic nucleus clock reveals large-scale coordination of key biological processes. PLoS Genet. 2014;10:e1004695. doi: 10.1371/journal.pgen.1004695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs G, Redfern PD. A circadian rhythm in passive avoidance behavior: the effect of phase shift and the benzodiazepines. Neuropharmacology. 1981;20:1365–1366. [PubMed] [Google Scholar]
- Choi HJ, Lee CJ, Schroeder A, Kim YS, Jung SH, Kim JS, Kim DY, Son EJ, Han HC, Hong SK, Colwell CS, Kim YI. Excitatory actions of GABA in the suprachiasmatic nucleus. J. Neurosci. 2008;28:5450–5459. doi: 10.1523/JNEUROSCI.5750-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciarleglio CM, Gamble KL, Axley JC, Strauss BR, Cohen JY, Colwell CS, McMahon DG. Population encoding by circadian clock neurons organizes circadian behavior. J. Neurosci. 2009;29:1670–1676. doi: 10.1523/JNEUROSCI.3801-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy AN, Whitman DC, Michael RP, Albers HE. Distribution of androgen receptor-like immunoreactivity in the brains of intact and castrated male hamsters. Brain Res. Bull. 1994;33:325–332. doi: 10.1016/0361-9230(94)90201-1. [DOI] [PubMed] [Google Scholar]
- Clark JA, Deutch AY, Gallipoli PZ, Amara SG. Functional expression and CNS distribution of a beta-alanine-sensitive neuronal GABA transporter. Neuron. 1992;9:337–348. doi: 10.1016/0896-6273(92)90172-a. [DOI] [PubMed] [Google Scholar]
- Cohen R, Albers HE. Disruption of human circadian and cognitive regulation following a discrete hypothalamic lesion: a case study. Neurology. 1991;41:726–729. doi: 10.1212/wnl.41.5.726. [DOI] [PubMed] [Google Scholar]
- Colwell CS. Rhythmic coupling among cells in the suprachiasmatic nucleus. J. Neurobiol. 2000;43:379–388. doi: 10.1002/1097-4695(20000615)43:4<379::aid-neu6>3.0.co;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell CS, Foster RG, Menaker M. NMDA receptor antagonists block the effects of light on circadian behavior in the mouse. Brain Res. 1991;554:105–110. doi: 10.1016/0006-8993(91)90177-w. [DOI] [PubMed] [Google Scholar]
- Colwell CS, Kaufman CM, Menaker M. Photic induction of Fos in the hamster suprachiasmatic nucleus is inhibited by baclofen but not by diazepam or bicuculline. Neurosci. Lett. 1993;163:177–181. doi: 10.1016/0304-3940(93)90376-v. [DOI] [PubMed] [Google Scholar]
- Colwell CS, Menaker M. NMDA as well as non-NMDA receptor antagonists can prevent the phase shifting effects of light on the circadian system of the golden hamster. J. Biol. Rhythms. 1992;7:125–136. doi: 10.1177/074873049200700204. [DOI] [PubMed] [Google Scholar]
- Colwell CS, Menaker M. Regulation of circadian rhythms by excitatory amino acids. In: Brann DW, Mahesh VB, editors. Excitatory Amino Acids: Their Role in Neuroendocrine Function. New York: CRC Press; 1995. pp. 223–252. [Google Scholar]
- Colwell CS, Michel S, Itri J, Rodriguez W, Tam J, Lelievre V, Hu Z, Liu X, Waschek JA. Disrupted circadian rhythms in VIP- and PHI-deficient mice. Am. J. Physiol. 2003;285:R939–R949. doi: 10.1152/ajpregu.00200.2003. [DOI] [PubMed] [Google Scholar]
- Condie BG, Bain G, Gottlieb DI, Capecchi MR. Cleft palate in mice with a targeted mutation in the gamma-aminobutyric acid-producing enzyme glutamic acid decarboxylase 67. Proc. Natl. Acad. Sci. U.S.A. 1997;94:11451–11455. doi: 10.1073/pnas.94.21.11451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti F, Melone M, Fattorini G, Bragina L, Ciappelloni S. A role for GAT-1 in presynaptic GABA homeostasis? Front. Cell Neurosci. 2011;5:2. doi: 10.3389/fncel.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope DW, Hughes SW, Crunelli V. GABAA receptor-mediated tonic inhibition in thalamic neurons. J. Neurosci. 2005;25:11553–11563. doi: 10.1523/JNEUROSCI.3362-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosio C, Cermakian N, Allis CD, Sassone-Corsi P. Light induces chromatin modification in cells of the mammalian circadian clock. Nat. Neurosci. 2000;3:1241–1247. doi: 10.1038/81767. [DOI] [PubMed] [Google Scholar]
- Cui LN, Coderre E, Renaud LP. GABA(B) presynaptically modulates suprachiasmatic input to hypothalamic paraventricular magnocellular neurons. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;278:R1210–R1216. doi: 10.1152/ajpregu.2000.278.5.R1210. [DOI] [PubMed] [Google Scholar]
- Cui LN, Saeb-Parsy K, Dyball RE. Neurones in the supraoptic nucleus of the rat are regulated by a projection from the suprachiasmatic nucleus. J. Physiol. 1997;502(Pt 1):149–159. doi: 10.1111/j.1469-7793.1997.149bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daan S, Pittendrigh CS. A functional analysis of circadian pacemakers in nocturnal rodents. III. Heavy water and constant light: homeostasis of frequency? J. Comp. Physiol. 1976a;106:267–290. [Google Scholar]
- Daan S, Pittendrigh CS. A functional analysis of circadian pacemakers in nocturnal rodents. II. The variability of phase response curves. J. Comp. Physiol. 1976b;106:253–266. [Google Scholar]
- Damaggio AS, Gorman MR. The circadian timing system in ethanol consumption and dependence. Behav. Neurosci. 2014;128:371–386. doi: 10.1037/a0036408. [DOI] [PubMed] [Google Scholar]
- Darna M, Schmutz I, Richter K, Yelamanchili SV, Pendyala G, Holtje M, Albrecht U, Ahnert-Hilger G. Time of day-dependent sorting of the vesicular glutamate transporter to the plasma membrane. J. Biol. Chem. 2009;284:4300–4307. doi: 10.1074/jbc.M805480200. [DOI] [PubMed] [Google Scholar]
- Davies JA, Navarathnam V, Redfern PH. The effect of phase-shift on the passive avoidance response in rats and the modifying action of chlordiazepoxide. Br. J. Pharmacol. 1974;51:447–451. doi: 10.1111/j.1476-5381.1974.tb10681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jeu M, Pennartz C. Circadian modulation of GABA function in the rat suprachiasmatic nucleus: excitatory effects during the night phase. J. Neurophysiol. 2002;87:834–844. doi: 10.1152/jn.00241.2001. [DOI] [PubMed] [Google Scholar]
- de la Iglesia HO, Meyer J, Carpino A, Jr, Schwartz WJ. Antiphase oscillation of the left and right suprachiasmatic nuclei. Science. 2000;290:799–801. doi: 10.1126/science.290.5492.799. [DOI] [PubMed] [Google Scholar]
- Decavel C, van den Pol AN. GABA: a dominant neurotransmitter in the hypothalamus. J. Comp. Neurol. 1990;302:1019–1037. doi: 10.1002/cne.903020423. [DOI] [PubMed] [Google Scholar]
- Della MV, Ralph MR. Retinal GABA(A) receptors participate in the regulation of circadian responses to light. J. Biol. Rhythms. 1999;14:47–53. doi: 10.1177/074873049901400107. [DOI] [PubMed] [Google Scholar]
- Deurveilher S, Burns J, Semba K. Indirect projections from the suprachiasmatic nucleus to the ventrolateral preoptic nucleus: a dual tract-tracing study in rat. Eur. J. Neurosci. 2002;16:1195–1213. doi: 10.1046/j.1460-9568.2002.02196.x. [DOI] [PubMed] [Google Scholar]
- DeWoskin D, Myung J, Belle MD, Piggins HD, Takumi T, Forger DB. Distinct roles for GABA across multiple timescales in mammalian circadian timekeeping. Proc. Natl. Acad. Sci. U.S.A. 2015;112:E3911–E3919. doi: 10.1073/pnas.1420753112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu. Rev. Physiol. 2010;72:517–549. doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- Ding JM, Buchanan GF, Tischkau SA, Chen D, Kuriashkina L, Faiman LE, Alster JM, McPherson PS, Campbell KP, Gillette MU. A neuronal ryanodine receptor mediates light-induced phase delays of the circadian clock. Nature. 1998;394:381–384. doi: 10.1038/28639. [DOI] [PubMed] [Google Scholar]
- Ding JM, Chen D, Wever ET, Faiman LE, Rea MA, Gillette MU. Resetting the biological clock: mediation of nocturnal circadian shifts by glutamate and NO. Science. 1994;266:1713–1717. doi: 10.1126/science.7527589. [DOI] [PubMed] [Google Scholar]
- Draguhn A, Verdorn TA, Ewert M, Seeburg PH, Sakmann B. Functional and molecular distinction between recombinant rat GABAA receptor subtypes by Zn2+ . Neuron. 1990;5:781–788. doi: 10.1016/0896-6273(90)90337-f. [DOI] [PubMed] [Google Scholar]
- Drucker-Colin R, Aguilar-Roblero R, Fernandez-Cancino F, Rattoni FB. Fetal suprachiasmatic nucleus transplants: diurnal rhythm recovery of lesioned rats. Brain Res. 1984;311:353–357. doi: 10.1016/0006-8993(84)90099-4. [DOI] [PubMed] [Google Scholar]
- Dumoulin A, Rostaing P, Bedet C, Levi S, Isambert MF, Henry JP, Triller A, Gasnier B. Presence of the vesicular inhibitory amino acid transporter in GABAergic and glycinergic synaptic terminal boutons. J. Cell Sci. 1999;112(Pt 6):811–823. doi: 10.1242/jcs.112.6.811. [DOI] [PubMed] [Google Scholar]
- Duncan MJ, Cheng X, Heller KS. Photoperiodic exposure and time of day modulate the expression of arginine vasopressin mRNA and vasoactive intestinal peptide mRNA in the suprachiasmatic nuclei of Siberian hamsters. Mol. Brain Res. 1995;32:181–186. doi: 10.1016/0169-328x(95)00072-z. [DOI] [PubMed] [Google Scholar]
- Durkin MM, Smith KE, Borden LA, Weinshank RL, Branchek TA, Gustafson EL. Localization of messenger RNAs encoding three GABA transporters in rat brain: an in situ hybridization study. Mol. Brain Res. 1995;33:7–21. doi: 10.1016/0169-328x(95)00101-w. [DOI] [PubMed] [Google Scholar]
- Earnest DJ, Digiorgio SM, Sladek CD. Effects of tetrodotoxin on the circadian pacemaker mechanism in suprachiasmatic explants in vitro. Brain Res. Bull. 1991;26:677–682. doi: 10.1016/0361-9230(91)90160-l. [DOI] [PubMed] [Google Scholar]
- Ebihara S, Goto M, Oshima I. Different responses of the circadian system to GABA-active drugs in two strains of mice. J. Biol. Rhythms. 1988;3:357–364. doi: 10.1177/074873048800300405. [DOI] [PubMed] [Google Scholar]
- Ebling FJP. The role of glutamate in the photic regulation of the suprachiasmatic nucleus. Prog. Neurobiol. 1996;50:109–132. doi: 10.1016/s0301-0082(96)00032-9. [DOI] [PubMed] [Google Scholar]
- Ehlen JC, Albers HE, Breyer ED. MEKC-LIF of gamma-amino butyric acid in microdialysate: systematic optimization of the separation conditions by factorial analysis. J. Neurosci. Methods. 2005;147:36–47. doi: 10.1016/j.jneumeth.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Ehlen JC, Hummer DL, Paul KN, Albers HE. GABA involvement in the circadian regulation of sleep. In: Monti JM, et al., editors. GABA and Sleep. Basil: Springer Verlag; 2010. pp. 303–321. [Google Scholar]
- Ehlen JC, Novak CM, Karom MC, Gamble KL, Albers HE. Interactions of GABA A receptor activation and light on period mRNA expression in the suprachiasmatic nucleus. J. Biol. Rhythms. 2008;23:16–25. doi: 10.1177/0748730407310785. [DOI] [PubMed] [Google Scholar]
- Ehlen JC, Novak CM, Karom MC, Gamble KL, Paul KN, Albers HE. GABAA receptor activation suppresses Period 1 mRNA and Period 2 mRNA in the suprachiasmatic nucleus during the mid-subjective day. Eur. J. Neurosci. 2006;23:3328–3336. doi: 10.1111/j.1460-9568.2006.04857.x. [DOI] [PubMed] [Google Scholar]
- Ehlen JC, Paul KN. Regulation of light’s action in the mammalian circadian clock: role of the extrasynaptic GABAA receptor. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;296:R1606–R1612. doi: 10.1152/ajpregu.90878.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott AS, Nunez AA. An ultrastructural study of somal appositions in the suprachiasmatic nucleus and anterior hypothalamus of the rat. Brain Res. 1994;662:278–282. doi: 10.1016/0006-8993(94)90826-5. [DOI] [PubMed] [Google Scholar]
- Ellis GB, McKlveen RE, Turek FW. Dark pulses affect the circadian rhythm of activity in hamsters kept in constant light. Am. J. Physiol. 1982;242:R44–R50. doi: 10.1152/ajpregu.1982.242.1.R44. [DOI] [PubMed] [Google Scholar]
- Engel D, Pahner I, Schulze K, Frahm C, Jarry H, Ahnert-Hilger G, Draguhn A. Plasticity of rat central inhibitory synapses through GABA metabolism. J. Physiol. 2001;535:473–482. doi: 10.1111/j.1469-7793.2001.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann M, Wotjak CT, Ebner K, Landgraf R. Behavioural impact of intraseptally released vasopressin and oxytocin in rats. Exp. Physiol. 2000;85(Spec No):125S–130S. doi: 10.1111/j.1469-445x.2000.tb00015.x. [DOI] [PubMed] [Google Scholar]
- Enna SJ, McCarson KE. Characterization of GABA receptors. Curr. Protoc. Pharmacol. 2013;63(Unit) doi: 10.1002/0471141755.ph0107s63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoki R, Kuroda S, Ono D, Hasan MT, Ueda T, Honma S, Honma K. Topological specificity and hierarchical network of the circadian calcium rhythm in the suprachiasmatic nucleus. Proc. Natl. Acad. Sci. U.S.A. 2012;109:21498–21503. doi: 10.1073/pnas.1214415110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JA. Collective timekeeping among cells of the master circadian clock. J. Endocrinol. 2016;230:R27–R49. doi: 10.1530/JOE-16-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JA, Elliott JA, Gorman MR. Photoperiod differentially modulates photic and nonphotic phase response curves of hamsters. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;286:R539–R546. doi: 10.1152/ajpregu.00456.2003. [DOI] [PubMed] [Google Scholar]
- Evans JA, Gorman MR. In synch but not in step: circadian clock circuits regulating plasticity in daily rhythms. Neuroscience. 2016;320:259–280. doi: 10.1016/j.neuroscience.2016.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JA, Leise TL, Castanon-Cervantes O, Davidson AJ. Dynamic interactions mediated by nonredundant signaling mechanisms couple circadian clock neurons. Neuron. 2013;80:973–983. doi: 10.1016/j.neuron.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Zeng H, Olson DP, Huber KM, Gibson JR, Takahashi JS. Vasoactive intestinal peptide (VIP)-expressing neurons in the suprachiasmatic nucleus provide sparse GABAergic outputs to local neurons with circadian regulation occurring distal to the opening of postsynaptic GABAA ionotropic receptors. J. Neurosci. 2015;35:1905–1920. doi: 10.1523/JNEUROSCI.2661-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farajnia S, van Westering TL, Meijer JH, Michel S. Seasonal induction of GABAergic excitation in the central mammalian clock. Proc. Natl. Acad. Sci. U.S.A. 2014;111:9627–9632. doi: 10.1073/pnas.1319820111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat. Rev. Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Fatehi M, Fatehi-Hassanabad Z. Effects of 17beta-estradiol on neuronal cell excitability and neurotransmission in the suprachiasmatic nucleus of rat. Neuropsychopharmacology. 2008;33:1354–1364. doi: 10.1038/sj.npp.1301523. [DOI] [PubMed] [Google Scholar]
- Fernandez DC, Chang YT, Hattar S, Chen SK. Architecture of retinal projections to the central circadian pacemaker. Proc. Natl. Acad. Sci. U.S.A. 2016;113:6047–6052. doi: 10.1073/pnas.1523629113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filiano AN, Millender-Swain T, Johnson R, Jr, Young ME, Gamble KL, Bailey SM. Chronic ethanol consumption disrupts the core molecular clock and diurnal rhythms of metabolic genes in the liver without affecting the suprachiasmatic nucleus. PLoS ONE. 2013;8:e71684. doi: 10.1371/journal.pone.0071684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francl JM, Kaur G, Glass JD. Regulation of vasoactive intestinal polypeptide release in the suprachiasmatic nucleus circadian clock. NeuroReport. 2010a;21:1055–1059. doi: 10.1097/WNR.0b013e32833fcba4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francl JM, Kaur G, Glass JD. Roles of light and serotonin in the regulations of gastrin-releasing peptide and arginine vasopressin output in the hamster SCN circadian clock. Eur. J. Neurosci. 2010b;32:1170–1179. doi: 10.1111/j.1460-9568.2010.07374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- François-Bellan AM, Bosler O. Convergent serotonin and GABA innervation of VIP neurons in the suprachiasmatic nucleus demonstrated by triple labeling in the rat. Brain Res. 1992;595:149–153. doi: 10.1016/0006-8993(92)91466-r. [DOI] [PubMed] [Google Scholar]
- Francois-Bellan AM, Hery M, Barrit MC, Faudon M, Hery F. The stimulation of GABAB receptors increases serotonin release in the rat suprachiasmatic area. Neurochem. Int. 1987;11:55–62. doi: 10.1016/0197-0186(87)90148-3. [DOI] [PubMed] [Google Scholar]
- Francois-Bellan AM, Hery M, Faudon M, Hery F. Evidence for GABA control of serotonin metabolism in the rat suprachiasmatic area. Neurochem. Int. 1988;13:455–462. doi: 10.1016/0197-0186(88)90074-5. [DOI] [PubMed] [Google Scholar]
- Francois-Bellan AM, Hery M, Faudon M, Hery F. Analysis of the inhibitory effect of oestradiol on functional GABA/5-HT relationship in the rat suprachiasmatic area. J. Neuroendocrinol. 1989a;1:415–422. doi: 10.1111/j.1365-2826.1989.tb00140.x. [DOI] [PubMed] [Google Scholar]
- Francois-Bellan AM, Kachidian P, Dusticier G, Tonon MC, Vaudry H, Bosler O. GABA neurons in the rat suprachiasmatic nucleus: involvement in chemospecific synaptic circuitry and evidence for GAD-peptide colocalization. J. Neurocytol. 1990;19:937–947. doi: 10.1007/BF01186821. [DOI] [PubMed] [Google Scholar]
- Francois-Bellan AM, Segu L, Hery M. Regulation by estradiol of GABAA and GABAB binding sites in the diencephalon of the rat: an autoradiographic study. Brain Res. 1989b;503:144–147. doi: 10.1016/0006-8993(89)91715-0. [DOI] [PubMed] [Google Scholar]
- Freeman GM, Jr, Krock RM, Aton SJ, Thaben P, Herzog ED. GABA networks destabilize genetic oscillations in the circadian pacemaker. Neuron. 2013;78:799–806. doi: 10.1016/j.neuron.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freichel C, Potschka H, Ebert U, Brandt C, Loscher W. Acute changes in the neuronal expression of GABA and glutamate decarboxylase isoforms in the rat piriform cortex following status epilepticus. Neuroscience. 2006;141:2177–2194. doi: 10.1016/j.neuroscience.2006.05.040. [DOI] [PubMed] [Google Scholar]
- Fuller CA, Lydic R, Sulzman FM, Albers HE, Tepper B, Moore-Ede MC. Circadian rhythm of body temperature persists after suprachiasmatic lesions in the squirrel monkey. Am. J. Physiol. 1981;241:R385–R391. doi: 10.1152/ajpregu.1981.241.5.R385. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Dahlstrom AB, Jonsson G, Marcellino D, Guescini M, Dam M, Manger P, Agnati L. The discovery of central monoamine neurons gave volume transmission to the wired brain. Prog. Neurobiol. 2010;90:82–100. doi: 10.1016/j.pneurobio.2009.10.012. [DOI] [PubMed] [Google Scholar]
- Gadea A, Lopez-Colome AM. Glial transporters for glutamate, glycine, and GABA: II. GABA transporters. J. Neurosci. Res. 2001;63:461–468. doi: 10.1002/jnr.1040. [DOI] [PubMed] [Google Scholar]
- Gamble KL, Allen GC, Zhou T, McMahon DG. Gastrin-releasing peptide mediates light-like resetting of the suprachiasmatic nucleus circadian pacemaker through cAMP response element-binding protein and Per1 activation. J. Neurosci. 2007;44:12078–12087. doi: 10.1523/JNEUROSCI.1109-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble KL, Ehlen JC, Albers HE. Circadian control during the day and night: role of neuropeptide Y Y5 receptors in the suprachiasmatic nucleus. Brain Res. Bull. 2005;65:513–519. doi: 10.1016/j.brainresbull.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Gamble KL, Kudo T, Colwell CS, McMahon DG. Gastrin-releasing peptide modulates fast delayed rectifier potassium current in Per1-expressing SCN neurons. J. Biol. Rhythms. 2011;26:99–106. doi: 10.1177/0748730410396678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble KL, Novak CM, Albers HE. Neuropeptide y and n-methyl-d-aspartic acid interact within the suprachiasmatic nuclei to alter circadian phase. Neuroscience. 2004;126:559–565. doi: 10.1016/j.neuroscience.2004.04.018. [DOI] [PubMed] [Google Scholar]
- Gamble KL, Novak CM, Paul KN, Albers HE. Tetrodotoxin blocks the circadian effects of NMDA during the day but not at night. NeuroReport. 2003;14:641–644. doi: 10.1097/00001756-200303240-00024. [DOI] [PubMed] [Google Scholar]
- Gannon RL, Cato MJ, Hart Kelley K, Armstrong DL, Rea MA. GABAergic modulation of optic nerve-evoked field potentials in the rat suprachiasmatic nucleus. Brain Res. 1995;694:264–270. doi: 10.1016/0006-8993(95)00854-j. [DOI] [PubMed] [Google Scholar]
- Gao B, Fritschy JM, Moore RY. GABAA receptor subunit composition in the circadian timing system. Brain Res. 1995;700:142–156. doi: 10.1016/0006-8993(95)00944-l. [DOI] [PubMed] [Google Scholar]
- Gasnier B. The SLC32 transporter, a key protein for the synaptic release of inhibitory amino acids. Pflugers Arch. 2004;447:756–759. doi: 10.1007/s00424-003-1091-2. [DOI] [PubMed] [Google Scholar]
- Gillespie CF, Babagbemi TO, Huhman KL, Albers HE. Bicuculline increases and muscimol reduces the phase-delaying effects of light and VIP/PHI/GRP in the suprachiasmatic region. J. Biol. Rhythms. 1996;11:137–144. doi: 10.1177/074873049601100206. [DOI] [PubMed] [Google Scholar]
- Gillespie CF, Mintz EM, Marvel CL, Huhman KL, Albers HE. GABAA and GABAB agonists and antagonists alter the phase-shifting effects of light when microinjected into the suprachiasmatic region. Brain Res. 1997;759:181–189. doi: 10.1016/s0006-8993(97)00235-7. [DOI] [PubMed] [Google Scholar]
- Gillespie CF, van der Beek EM, Mintz EM, Mickley NC, Jasnow AM, Huhman KL, Albers HE. GABAergic regulation of light-Induced c-Fos immunoreactivity within the suprachiasmatic nucleus. J. Comp. Neurol. 1999;411:683–692. [PubMed] [Google Scholar]
- Girardet C, Becquet D, Blanchard MP, Francois-Bellan AM, Bosler O. Neuroglial and synaptic rearrangements associated with photic entrainment of the circadian clock in the suprachiasmatic nucleus. Eur. J. Neurosci. 2010;32:2133–2142. doi: 10.1111/j.1460-9568.2010.07520.x. [DOI] [PubMed] [Google Scholar]
- Glass JD, Tardif SD, Clements R, Mrosovsky N. Photic and nonphotic circadian phase resetting in a diurnal primate, the common marmoset. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;280:R191–R197. doi: 10.1152/ajpregu.2001.280.1.R191. [DOI] [PubMed] [Google Scholar]
- Glykys J, Mody I. Activation of GABAA receptors: view from outside the synaptic cleft. Neuron. 2007;56:763–770. doi: 10.1016/j.neuron.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Glykys J, Peng Z, Chandra D, Homanics GE, Houser CR, Mody I. A new naturally occurring GABA(A) receptor subunit partnership with high sensitivity to ethanol. Nat. Neurosci. 2007;10:40–48. doi: 10.1038/nn1813. [DOI] [PubMed] [Google Scholar]
- Golombek DA, Biello SM, Rendon RA, Harrington ME. Neuropeptide Y phase shifts the circadian clock in vitro via a Y2 receptor. NeuroReport. 1996;7:1315–1319. doi: 10.1097/00001756-199605170-00020. [DOI] [PubMed] [Google Scholar]
- Golombek DA, Ralph MR. Inhibition of GABA transaminase enhances light-induced circadian phase delays but not advances. J. Biol. Rhythms. 1994;9:251–261. doi: 10.1177/074873049400900306. [DOI] [PubMed] [Google Scholar]
- Golombek DA, Rosenstein RE. Physiology of circadian entrainment. Physiol. Rev. 2010;90:1063–1102. doi: 10.1152/physrev.00009.2009. [DOI] [PubMed] [Google Scholar]
- Green DJ, Gillette R. Circadian rhythm of firing rate recorded from single cells in the rat suprachiasmatic brain slice. Brain Res. 1982;245:198–200. doi: 10.1016/0006-8993(82)90361-4. [DOI] [PubMed] [Google Scholar]
- Gribkoff VK, Pieschl RL, Dudek FE. GABA receptor-mediated inhibition of neuronal activity in rat SCN in vitro: pharmacology and influence of circadian phase. J. Neurophysiol. 2003;90:1438–1448. doi: 10.1152/jn.01082.2002. [DOI] [PubMed] [Google Scholar]
- Gribkoff VK, Pieschl RL, Wisialowski TA, Park WK, Strecker GJ, de Jeu MTG, Pennartz CMA, Dudek FE. A reexamination of the role of GABA in the mammalian suprachiasmatic nucleus. J. Biol. Rhythms. 1999;14:126–130. doi: 10.1177/074873099129000515. [DOI] [PubMed] [Google Scholar]
- Gribkoff VK, Pieschl RL, Wisialowski TA, van den Pol AN, Yocca FD. Phase shifting of circadian rhythms and depression of neuronal activity in the rat suprachiasmatic nucleus by neuropeptide Y: mediation by different receptor subtypes. J. Neurosci. 1998;18:3014–3022. doi: 10.1523/JNEUROSCI.18-08-03014.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groos G, Hendriks J. Circadian rhythms in electrical discharge of rat suprachiasmatic neurones recorded in vitro. Neurosci. Lett. 1982;34:283–288. doi: 10.1016/0304-3940(82)90189-6. [DOI] [PubMed] [Google Scholar]
- Guido ME, Goguen D, de Guido L, Robertson HA, Rusak B. Circadian and photic regulation of immediate-early gene expression in the hamster suprachiasmatic nucleus. Neuroscience. 1999;90:555–571. doi: 10.1016/s0306-4522(98)00467-9. [DOI] [PubMed] [Google Scholar]
- Guilding C, Piggins HD. Challenging the omnipotence of the suprachiasmatic timekeeper: are circadian oscillators present throughout the mammalian brain? Eur. J. Neurosci. 2007;25:3195–3216. doi: 10.1111/j.1460-9568.2007.05581.x. [DOI] [PubMed] [Google Scholar]
- Guldner FH. Synapses of optic nerve afferents in the rat suprachiasmatic nucleus. II. Structural variability as revealed by morphometric examination. Cell Tissue Res. 1978;194:37–54. doi: 10.1007/BF00209232. [DOI] [PubMed] [Google Scholar]
- Guldner FH, Wolff JR. Complex synaptic arrangements in the rat suprachiasmatic nucleus: a possible basis for the “Zeitgeber” and non-synaptic synchronization of neuronal activity. Cell Tissue Res. 1996;284:203–214. doi: 10.1007/s004410050580. [DOI] [PubMed] [Google Scholar]
- Haam J, Popescu IR, Morton LA, Halmos KC, Teruyama R, Ueta Y, Tasker JG. GABA is excitatory in adult vasopressinergic neuroendocrine cells. J. Neurosci. 2012;32:572–582. doi: 10.1523/JNEUROSCI.3826-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hablitz LM, Molzof HE, Abrahamsson KE, Cooper JM, Prosser RA, Gamble KL. GIRK channels mediate the nonphotic effects of exogenous melatonin. J. Neurosci. 2015;45:14957–14965. doi: 10.1523/JNEUROSCI.1597-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hablitz LM, Molzof HE, Paul JR, Johnson RL, Gamble KL. Suprachiasmatic nucleus function and circadian entrainment are modulated by G protein-coupled inwardly rectifying (GIRK) channels. J. Physiol. 2014;22:5079–5092. doi: 10.1113/jphysiol.2014.282079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada T, Antle MC, Silver R. Temporal and spatial expression patterns of canonical clock genes and clock-controlled genes in the suprachiasmatic nucleus. Eur. J. Neurosci. 2004a;19:1741–1748. doi: 10.1111/j.1460-9568.2004.03275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada T, Antle MC, Silver R. The role of Period1 in non-photic resetting of the hamster circadian pacemaker in the suprachiasmatic nucleus. Neurosci. Lett. 2004b;362:87–90. doi: 10.1016/j.neulet.2004.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada T, LeSauter J, Venuti JM, Silver R. Expression of Period genes: rhythmic and nonrhythmic compartments of the suprachiasmatic nucleus pacemaker. J. Neurosci. 2001;21:7742–7750. doi: 10.1523/JNEUROSCI.21-19-07742.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Yu FH, Schwartz MD, Linton JD, Bosma MM, Hurley JB, Catterall WA, de la Iglesia HO. Na(V)1.1 channels are critical for intercellular communication in the suprachiasmatic nucleus and for normal circadian rhythms. Proc. Natl. Acad. Sci. U.S.A. 2012;109:E368–E377. doi: 10.1073/pnas.1115729109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannibal J, Ding JM, Chen D, Fahrenkrug J, Larsen PJ, Gillette MU, Mikkelsen JD. Pituitary adenylate cyclase-activating peptide (PACAP) in the retinohypothalamic tract: a potential regulator of the biological clock. J. Neurosci. 1997;17:2637–2644. doi: 10.1523/JNEUROSCI.17-07-02637.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmar AJ, Marston HM, Shen S, Spratt C, West KM, Sheward WJ, Morrison CF, Dorin JR, Piggins HD, Reubi JC, Kelly JS, Maywood ES, Hastings MH. The VPAC(2) receptor is essential for circadian function in the mouse suprachiasmatic nuclei. Cell. 2002;109:497–508. doi: 10.1016/s0092-8674(02)00736-5. [DOI] [PubMed] [Google Scholar]
- Harrington ME, Nance DM, Rusak B. Double-labeling of neuropeptide Y-immunoreactive neurons which project from the geniculate to the suprachiasmatic nuclei. Brain Res. 1987;410:275–282. doi: 10.1016/0006-8993(87)90325-8. [DOI] [PubMed] [Google Scholar]
- Harrington ME, Schak KM. Neuropeptide Y phase advances the in vitro hamster circadian clock during the subjective day with no effect on phase during the subjective night. Can. J. Physiol. Pharmacol. 2000;78:87–92. [PubMed] [Google Scholar]
- Hastings MH, Brancaccio M, Maywood ES. Circadian pacemaking in cells and circuits of the suprachiasmatic nucleus. J. Neuroendocrinol. 2014;26:2–10. doi: 10.1111/jne.12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings MH, Duffield GE, Smith EJ, Maywood ES, Ebling FJ. Entrainment of the circadian system of mammals by nonphotic cues. Chronobiol. Int. 1998;15:425–445. doi: 10.3109/07420529808998700. [DOI] [PubMed] [Google Scholar]
- Hastings MH, Herzog ED. Clock genes, oscillators, and cellular networks in the suprachiasmatic nuclei. J. Biol. Rhythms. 2004;19:400–413. doi: 10.1177/0748730404268786. [DOI] [PubMed] [Google Scholar]
- Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW, Berson DM. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J. Comp. Neurol. 2006;497:326–349. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson AE, Wagoner N, Cowan WM. An autoradiographic and electron microscopic study of retino-hypothalamic connections. Z. Zell. 1972;135:1–26. doi: 10.1007/BF00307084. [DOI] [PubMed] [Google Scholar]
- Hermanstyne TO, Simms CI, Carrasquillo Y, Herzog ED. Distance firing properties of vasoactive intestinal peptide-expressing neurons in the suprachiasmatic nucleus. J. Biol. Rhythms. 2016;31:57–67. doi: 10.1177/0748730415619745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermes ML, Buijs RM, Renaud LP. Electrophysiology of suprachiasmatic nucleus projections to hypothalamic paraventricular nucleus neurons. Prog. Brain Res. 1996a;111:241–252. doi: 10.1016/s0079-6123(08)60412-4. [DOI] [PubMed] [Google Scholar]
- Hermes ML, Coderre EM, Buijs RM, Renaud LP. GABA and glutamate mediate rapid neurotransmission from suprachiasmatic nucleus to hypothalamic paraventricular nucleus in rat. J. Physiol. 1996b;496(Pt 3):749–757. doi: 10.1113/jphysiol.1996.sp021724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermes ML, Kolaj M, Doroshenko P, Coderre E, Renaud LP. Effects of VPAC2 receptor activation on membrane excitability and GABAergic transmission in subparaventricular zone neurons targeted by suprachiasmatic nucleus. J. Neurophysiol. 2009;102:1834–1842. doi: 10.1152/jn.91261.2008. [DOI] [PubMed] [Google Scholar]
- Hermes MLHJ, Renaud LP. Differential responses of identified rat hypothalamic paraventricular neurons to suprachiasmatic nucleus stimulation. Neuroscience. 1993;56:823–832. doi: 10.1016/0306-4522(93)90130-8. [DOI] [PubMed] [Google Scholar]
- Herzog ED, Takahashi JS, Block GD. Clock controls circadian period in isolated suprachiasmatic nucleus neurons. Nat. Neurosci. 1998;1:708–713. doi: 10.1038/3708. [DOI] [PubMed] [Google Scholar]
- Hevers W, Luddens H. The diversity of GABAA receptors. Pharmacological and electrophysiological properties of GABAA channel subtypes. Mol. Neurobiol. 1998;18:35–86. doi: 10.1007/BF02741459. [DOI] [PubMed] [Google Scholar]
- Hill DR, Bowery NG. 3H-baclofen and 3H-GABA bind to bicuculline-insensitive GABAB sites in rat brain. Nature. 1981;290:149–152. doi: 10.1038/290149a0. [DOI] [PubMed] [Google Scholar]
- Hokfelt T. Neuropeptides in perspective: the last ten years. Neuron. 1991;7:867–879. doi: 10.1016/0896-6273(91)90333-u. [DOI] [PubMed] [Google Scholar]
- Honma S, Honma k, Hiroshige T. Methamphetamine induced locomotor rhythm entrains to restricted daily feeding in SCN lesioned rats. Physiol. Behav. 1989;45:1057–1065. doi: 10.1016/0031-9384(89)90237-0. [DOI] [PubMed] [Google Scholar]
- Honma S, Shirakawa T, Katsuno Y, Namihira M, Honma KI. Circadian periods of single suprachiasmatic neurons in rats. Neurosci. Lett. 1998;250:157–160. doi: 10.1016/s0304-3940(98)00464-9. [DOI] [PubMed] [Google Scholar]
- Honma S, Shirakawa T, Nakamura W, Honma K. Synaptic communication of cellular oscillations in the rat suprachiasmatic neurons. Neurosci. Lett. 2000;294:113–116. doi: 10.1016/s0304-3940(00)01558-5. [DOI] [PubMed] [Google Scholar]
- Horikawa K, Yokota S, Fuji K, Akiyama M, Moriya T, Okamura H, Shibata S. Nonphotic entrainment by 5-HT1A/7 receptor agonists accompanied by reduced Per1 and Per2 mRNA levels in the suprachiasmatic nucleus. J. Neurosci. 2000;20:5867–5873. doi: 10.1523/JNEUROSCI.20-15-05867.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang RC, Peng YW, Yau KW. Zinc modulation of a transient potassium current and histochemical localization of the metal in neurons of the suprachiasmatic nucleus. Proc. Natl. Acad. Sci. U.S.A. 1993;90:11806–11810. doi: 10.1073/pnas.90.24.11806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhman KL, Albers HE. Neuropeptide Y microinjected into the suprachiasmatic region phase shifts circadian rhythms in constant darkness. Peptides. 1994;15:1475–1478. doi: 10.1016/0196-9781(94)90126-0. [DOI] [PubMed] [Google Scholar]
- Huhman KL, Babagbemi TO, Albers HE. Bicuculline blocks neuropeptide Y-induced phase advances when microinjected in the suprachiasmatic nucleus of Syrian hamsters. Brain Res. 1995;675:333–336. doi: 10.1016/0006-8993(95)00018-l. [DOI] [PubMed] [Google Scholar]
- Huhman KL, Hennessey AC, Albers HE. Rhythms of glutamic acid decarboxylase mRNA in the suprachiasmatic nucleus. J. Biol. Rhythms. 1996;11:311–316. doi: 10.1177/074873049601100404. [DOI] [PubMed] [Google Scholar]
- Huhman KL, Jasnow AM, Sisitsky AK, Albers HE. Glutamic acid decarboxylase mRNA in the suprachiasmatic nucleus of rats housed in constant darkness. Brain Res. 1999;851:266–269. doi: 10.1016/s0006-8993(99)02167-8. [DOI] [PubMed] [Google Scholar]
- Huhman KL, Marvel CL, Gillespie CF, Mintz EM, Albers HE. Tetrodotoxin blocks NPY-induced but not muscimol-induced phase advances of wheel-running activity in Syrian hamsters. Brain Res. 1997;772:176–180. doi: 10.1016/s0006-8993(97)00831-7. [DOI] [PubMed] [Google Scholar]
- Hummer DL, Ehlen JC, Larkin TE, 2nd, McNeill JK, 4th, Pamplin JR, 2nd, Walker CA, Walker PV, 2nd, Dhanraj DR, Albers HE. Sustained activation of GABAA receptors in the suprachiasmatic nucleus mediates light-induced phase delays of the circadian clock: a novel function of ionotropic receptors. Eur. J. Neurosci. 2015;42:1830–1830. doi: 10.1111/ejn.12918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hut RA, Mrosovsky N, Daan S. Nonphotic entrainment in a diurnal mammal, the European ground squirrel (Spermophilus citellus) J. Biol. Rhythms. 1999;14:409–419. doi: 10.1177/074873099129000812. [DOI] [PubMed] [Google Scholar]
- Ibata Y, Takahashi Y, Okamura H, Fumio K, Terubayashi H, Kubo T, Yanaihara N. Vasoactive intestinal peptide (VIP)-like immunoreactive neurons located in the rat suprachiasmatic nucleus receive a direct retinal projection. Neurosci. Lett. 1989;97:1–5. doi: 10.1016/0304-3940(89)90129-8. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Yoshioka T, Allen CN. Developmental and circadian changes in Ca2+ mobilization mediated by GABAA and NMDA receptors in the suprachiasmatic nucleus. Eur. J. Neurosci. 2003;17:58–70. doi: 10.1046/j.1460-9568.2003.02427.x. [DOI] [PubMed] [Google Scholar]
- Inouye ST, Kawamura H. Persistance of circadian rhythmicity in a mammalian hypothalamic “island” containing the suprachiasmatic nucleus. Proc. Natl. Acad. Sci. U.S.A. 1979;76:5962–5966. doi: 10.1073/pnas.76.11.5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin RP, Allen CN. GABAergic signaling induces divergent neuronal Ca2+ responses in the suprachiasmatic nucleus network. Eur. J. Neurosci. 2009;30:1462–1475. doi: 10.1111/j.1460-9568.2009.06944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isobe Y, Nishino H. GABAergic control of Arg-vasopressin release from suprachiasmatic nucleus slice culture. Brain Res. 1997;755:213–220. doi: 10.1016/s0006-8993(97)00099-1. [DOI] [PubMed] [Google Scholar]
- Ito C, Wakamori M, Akaike N. Dual effect of glycine on isolated rat suprachiasmatic neurons. Am. J. Physiol. 1991;260:C213–C218. doi: 10.1152/ajpcell.1991.260.2.C213. [DOI] [PubMed] [Google Scholar]
- Itri J, Colwell CS. Regulation of inhibitory synaptic transmission by vasoactive intestinal peptide (VIP) in the mouse suprachiasmatic nucleus. J. Neurophysiol. 2003;90:1589–1597. doi: 10.1152/jn.00332.2003. [DOI] [PubMed] [Google Scholar]
- Itri J, Michel S, Waschek JA, Colwell CS. Circadian rhythm in inhibitory synaptic transmission in the mouse suprachiasmatic nucleus. J. Neurophysiol. 2004;92:311–319. doi: 10.1152/jn.01078.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen LL, Neal MJ. The uptake of [3H]GABA by slices of rat cerebral cortex. J. Neurochem. 1968;15:1141–1149. doi: 10.1111/j.1471-4159.1968.tb06831.x. [DOI] [PubMed] [Google Scholar]
- James OT, Livesey MR, Qiu J, Dando O, Bilican B, Haghi G, Rajan R, Burr K, Hardingham GE, Chandran S, Kind PC, Wyllie DJ. Ionotropic GABA and glycine receptor subunit composition in human pluripotent stem cell-derived excitatory cortical neurones. J. Physiol. 2014;592:4353–4363. doi: 10.1113/jphysiol.2014.278994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen K, Chiu CS, Sokolova I, Lester HA, Mody I. GABA transporter-1 (GAT1)-deficient mice: differential tonic activation of GABAA versus GABAB receptors in the hippocampus. J. Neurophysiol. 2003;90:2690–2701. doi: 10.1152/jn.00240.2003. [DOI] [PubMed] [Google Scholar]
- Jiang ZG, Allen CN, North RA. Presynaptic inhibition by baclofen of retinohypothalamic excitatory synaptic transmission in rat suprachiasmatic nucleus. Neuroscience. 1995;64:813–819. doi: 10.1016/0306-4522(94)00429-9. [DOI] [PubMed] [Google Scholar]
- Jiang ZG, Yang Y, Lui ZP, Allen CN. Membrane properties and synaptic inputs of suprachiasmatic nucleus neurons in rat brain slices. J. Physiol. 1997a;499(1):141–159. doi: 10.1113/jphysiol.1997.sp021917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang ZG, Yang YQ, Allen CN. Tracer and electrical coupling of rat suprachiasmatic nucleus neurons. Neuroscience. 1997b;77:1059–1066. doi: 10.1016/s0306-4522(96)00539-8. [DOI] [PubMed] [Google Scholar]
- Jin H, Wu H, Osterhaus G, Wei J, Davis K, Sha D, Floor E, Hsu CC, Kopke RD, Wu JY. Demonstration of functional coupling between gamma-aminobutyric acid (GABA) synthesis and vesicular GABA transport into synaptic vesicles. Proc. Natl. Acad. Sci. U.S.A. 2003;100:4293–4298. doi: 10.1073/pnas.0730698100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin XT, Pare JF, Smith Y. Differential localization and function of GABA transporters, GAT-1 and GAT-3, in the rat globus pallidus. Eur. J. Neurosci. 2011;33:1504–1518. doi: 10.1111/j.1460-9568.2011.07636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobst EE, Allen CN. Calbindin neurons in the hamster suprachiasmatic nucleus do not exhibit a circadian variation in spontaneous firing rate. Eur. J. Neurosci. 2002;16:2469–2474. doi: 10.1046/j.1460-9568.2002.02309.x. [DOI] [PubMed] [Google Scholar]
- Johnson RF, Moore RY, Morin LP. Loss of entrainment and anatomical plasticity after lesions of the hamster retinohypothalamic tract. Brain Res. 1988a;460:297–313. doi: 10.1016/0006-8993(88)90374-5. [DOI] [PubMed] [Google Scholar]
- Johnson RF, Smale L, Moore RY, Morin LP. Lateral geniculate lesions block circadian phase-shift responses to a benzodiazepine. Proc. Natl. Acad. Sci. U.S.A. 1988b;85:5301–5304. doi: 10.1073/pnas.85.14.5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston GA. Advantages of an antagonist: bicuculline and other GABA antagonists. Br. J. Pharmacol. 2013;169:328–336. doi: 10.1111/bph.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston GA. Muscimol as an ionotropic GABA receptor agonist. Neurochem. Res. 2014;39:1942–1947. doi: 10.1007/s11064-014-1245-y. [DOI] [PubMed] [Google Scholar]
- Jones A, Korpi ER, McKernan RM, Pelz R, Nusser Z, Makela R, Mellor JR, Pollard S, Bahn S, Stephenson FA, Randall AD, Sieghart W, Somogyi P, Smith AJ, Wisden W. Ligand-gated ion channel subunit partnerships: GABAA receptor alpha6 subunit gene inactivation inhibits delta subunit expression. J. Neurosci. 1997;17:1350–1362. doi: 10.1523/JNEUROSCI.17-04-01350.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JR, Tackenberg MC, McMahon DG. Manipulating circadian clock neuron firing rate resets molecular circadian rhythms and behavior. Nat. Neurosci. 2015;18:373–375. doi: 10.1038/nn.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallingal GJ, Mintz EM. Site-specific effects of gastrin-releasing peptide in the suprachiasmatic nucleus. Eur. J. Neurosci. 2014;39:630–639. doi: 10.1111/ejn.12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsbeek A, Drijfhout WJ, Westerink BH, van Heerikhuize JJ, van der Woude TP, Van DV, Buijs RM. GABA receptors in the region of the dorsomedial hypothalamus of rats are implicated in the control of melatonin and corticosterone release. Neuroendocrinology. 1996;63:69–78. doi: 10.1159/000126937. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Foppen E, Schalij I, van Heijningen C, Van DV, Fliers E, Buijs RM. Circadian control of the daily plasma glucose rhythm: an interplay of GABA and glutamate. PLoS ONE. 2008;3:e3194. doi: 10.1371/journal.pone.0003194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsbeek A, Garidou ML, Palm IF, Van DV, Simonneaux V, Pevet P, Buijs RM. Melatonin sees the light: blocking GABA-ergic transmission in the paraventricular nucleus induces daytime secretion of melatonin. Eur. J. Neurosci. 2000;12:3146–3154. doi: 10.1046/j.1460-9568.2000.00202.x. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, La Fleur S, van Heijningen C, Buijs RM. Suprachiasmatic GABAergic inputs to the paraventricular nucleus control plasma glucose concentrations in the rat via sympathetic innervation of the liver. J. Neurosci. 2004;24:7604–7613. doi: 10.1523/JNEUROSCI.5328-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsbeek A, Palm IF, La Fleur SE, Scheer FA, Perreau-Lenz S, Ruiter M, Kreier F, Cailotto C, Buijs RM. SCN outputs and the hypothalamic balance of life. J. Biol. Rhythms. 2006;21:458–469. doi: 10.1177/0748730406293854. [DOI] [PubMed] [Google Scholar]
- Kanaka C, Ohno K, Okabe A, Kuriyama K, Itoh T, Fukuda A, Sato K. The differential expression patterns of messenger RNAs encoding K-Cl cotransporters (KCC1,2) and Na-K-2Cl cotransporter (NKCC1) in the rat nervous system. Neuroscience. 2001;104:933–946. doi: 10.1016/s0306-4522(01)00149-x. [DOI] [PubMed] [Google Scholar]
- Kao CY. Tetrodotoxin, saxitoxin and their significance in the study of excitation phenomena. Pharmacol. Rev. 1966;18:997–1049. [PubMed] [Google Scholar]
- Karatsoreos IN, Wang A, Sasanian J, Silver R. A role for androgens in regulating circadian behavior and the suprachiasmatic nucleus. Endocrinology. 2007;148:5487–5495. doi: 10.1210/en.2007-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatsoreos IN, Yan L, LeSauter J, Silver R. Phenotype matters: identification of light-responsive cells in the mouse suprachiasmatic nucleus. J. Neurosci. 2004;24:68–75. doi: 10.1523/JNEUROSCI.1666-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kas MJ, Edgar DM. Scheduled voluntary wheel running activity modulates free-running circadian body temperature rhythms in Octodon degus. J. Biol. Rhythms. 2001;16:66–75. doi: 10.1177/074873040101600108. [DOI] [PubMed] [Google Scholar]
- Kaufman DL, Houser CR, Tobin AJ. Two forms of the gamma-aminobutyric acid synthetic enzyme glutamate decarboxylase have distinct intraneuronal distributions and cofactor interactions. J. Neurochem. 1991;56:720–723. doi: 10.1111/j.1471-4159.1991.tb08211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara F, Saito H, Katsuki H. Pharmacological characteristics of GABAA responses in postnatal suprachiasmatic neurons in culture. Neurosci. Lett. 1993;160:45–48. doi: 10.1016/0304-3940(93)90913-6. [DOI] [PubMed] [Google Scholar]
- Kawahara F, Saito H, Katsuki H. Inhibition by 5-HT7 receptor stimulation of GABAA receptor-activated current in cultured rat suprachiasmatic neurones. J. Physiol. 1994;478:67–73. doi: 10.1113/jphysiol.1994.sp020230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersante F, Rowley SC, Pavlov I, Gutierrez-Mecinas M, Semyanov A, Reul JM, Walker MC, Linthorst AC. A functional role for both -aminobutyric acid (GABA) transporter-1 and GABA transporter-3 in the modulation of extracellular GABA and GABAergic tonic conductances in the rat hippocampus. J. Physiol. 2013;591:2429–2441. doi: 10.1113/jphysiol.2012.246298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilb W. Development of the GABAergic system from birth to adolescence. Neuroscientist. 2012;18:613–630. doi: 10.1177/1073858411422114. [DOI] [PubMed] [Google Scholar]
- Kim YI, Dudek FE. Intracellular electrophysiological study of suprachiasmatic nucleus neurones in rodents: inhibitory synaptic mechanisms. J. Physiol. 1992;458:247–260. doi: 10.1113/jphysiol.1992.sp019416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kononenko NI, Dudek FE. Mechanism of irregular firing of suprachiasmatic nucleus neurons in rat hypothalamic slices. J. Neurophysiol. 2004;91:267–273. doi: 10.1152/jn.00314.2003. [DOI] [PubMed] [Google Scholar]
- Korpi ER, Mihalek RM, Sinkkonen ST, Hauer B, Hevers W, Homanics GE, Sieghart W, Luddens H. Altered receptor subtypes in the forebrain of GABA(A) receptor delta subunit-deficient mice: recruitment of gamma 2 subunits. Neuroscience. 2002;109:733–743. doi: 10.1016/s0306-4522(01)00527-9. [DOI] [PubMed] [Google Scholar]
- Kretschmannova K, Svobodova I, Balik A, Mazna P, Zemkova H. Circadian rhythmicity in AVP secretion and GABAergic synaptic transmission in the rat suprachiasmatic nucleus. Ann. N. Y. Acad. Sci. 2005;1048:103–115. doi: 10.1196/annals.1342.010. [DOI] [PubMed] [Google Scholar]
- Kretschmannova K, Svobodova I, Zemkova H. Day-night variations in zinc sensitivity of GABAA receptor-channels in rat suprachiasmatic nucleus. Mol. Brain Res. 2003;120:46–51. doi: 10.1016/j.molbrainres.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Kristensen AS, Andersen J, Jorgensen TN, Sorensen L, Eriksen J, Loland CJ, Stromgaard K, Gether U. SLC6 neurotransmitter transporters: structure, function, and regulation. Pharmacol. Rev. 2011;63:585–640. doi: 10.1124/pr.108.000869. [DOI] [PubMed] [Google Scholar]
- Kudo 1T, Tahara Y, Gamble KL, McMahon DG, Block GD, Colwell CS. Vasoactive intestinal peptide produces long-lasting changes in neural activity in the suprachiasmatic nucleus. J. Neurophysiol. 2013;110:1097–1106. doi: 10.1152/jn.00114.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman SJ, Silver R, Le Sauter J, Bult-Ito A, McMahon DG. Phase resetting light pulses induce Per1 and persistent spike activity in a subpopulation of biological clock neurons. J. Neurosci. 2003;23:1441–1450. doi: 10.1523/JNEUROSCI.23-04-01441.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Fleur SE, Kalsbeek A, Wortel J, Buijs RM. Polysynaptic neural pathways between the hypothalamus, including the suprachiasmatic nucleus, and the liver. Brain Res. 2000;871:50–56. doi: 10.1016/s0006-8993(00)02423-9. [DOI] [PubMed] [Google Scholar]
- Lall GS, Biello SM. Attenuation of phase shifts to light by activity or neuropeptide Y: a time course study. Brain Res. 2002;957:109–116. doi: 10.1016/s0006-8993(02)03610-7. [DOI] [PubMed] [Google Scholar]
- Lall GS, Biello SM. Attenuation of circadian light induced phase advances and delays by neuropeptide Y and a neuropeptide Y Y1/Y5 receptor agonist. Neuroscience. 2003a;119:611–618. doi: 10.1016/s0306-4522(02)00811-4. [DOI] [PubMed] [Google Scholar]
- Lall GS, Biello SM. Neuropeptide Y, GABA and circadian phase shifts to photic stimuli. Neuroscience. 2003b;120:915–921. doi: 10.1016/s0306-4522(03)00396-8. [DOI] [PubMed] [Google Scholar]
- Leak RK, Card JP, Moore RY. Suprachiasmatic pacemaker organization analyzed by viral transynaptic transport. Brain Res. 1999;819:23–32. doi: 10.1016/s0006-8993(98)01317-1. [DOI] [PubMed] [Google Scholar]
- Lee HS, Billings HJ, Lehman MN. The suprachiasmatic nucleus: a clock of multiple components. J. Biol. Rhythms. 2003;18:435–449. doi: 10.1177/0748730403259106. [DOI] [PubMed] [Google Scholar]
- Lee S, Yoon BE, Berglund K, Oh SJ, Park H, Shin HS, Augustine GJ, Lee CJ. Channel-mediated tonic GABA release from glia. Science. 2010;330:790–796. doi: 10.1126/science.1184334. [DOI] [PubMed] [Google Scholar]
- Lee V, Maguire J. The impact of tonic GABAA receptor-mediated inhibition on neuronal excitability varies across brain region and cell type. Front. Neural Circ. 2014;8:3. doi: 10.3389/fncir.2014.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman MN, Silver R, Gladstone WR, Khan RM, Gibson M, Bittman EL. Circadian rhythmicity restored by neural transplant immunocytochemical characterization of the graft and its integration with the host. J. Neurosci. 1987;7:1626–1638. doi: 10.1523/JNEUROSCI.07-06-01626.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng G, Ludwig M. Neurotransmitters and peptides: whispered secrets and public announcements. J. Physiol. 2008;586:5625–5632. doi: 10.1113/jphysiol.2008.159103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenn NJ, Beebe B, Moore RY. Postnatal development of the suprachiasmatic hypothalamic nucleus of the rat. Cell Tissue Res. 1977;178:463–475. doi: 10.1007/BF00219568. [DOI] [PubMed] [Google Scholar]
- LeSauter J, Lehman MN, Silver R. Restoration of circadian rhythmicity by transplants of SCN “micropunches”. J. Biol. Rhythms. 1996;11:163–171. doi: 10.1177/074873049601100208. [DOI] [PubMed] [Google Scholar]
- LeSauter J, Silver R, Cloues R, Witkovsky P. Light exposure induces short- and long-term changes in the excitability of retinorecipient neurons in suprachiasmatic nucleus. J. Neurophysiol. 2011;106:576–588. doi: 10.1152/jn.00060.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou SY, Albers HE. Single unit response of neurons within the suprachiasmatic nucleus to GABA and low chloride perfusate during the day and night. Brain Res. Bull. 1990;25:93–98. doi: 10.1016/0361-9230(90)90257-z. [DOI] [PubMed] [Google Scholar]
- Liou SY, Albers HE. Single unit response of neurons within the hamster suprachiasmatic nucleus to neuropeptide Y. Brain Res. Bull. 1991;27:825–828. doi: 10.1016/0361-9230(91)90216-7. [DOI] [PubMed] [Google Scholar]
- Liou SY, Shibata S, Albers HE, Ueki S. Effects of GABA and anxiolytics on the single unit discharge of suprachiasmatic neurons in rat hypothalamic slices. Brain Res. Bull. 1990;25:103–107. doi: 10.1016/0361-9230(90)90259-3. [DOI] [PubMed] [Google Scholar]
- Liu C, Reppert SM. GABA synchronizes clock cells within the suprachiasmatic circadian clock. Neuron. 2000;25:123–128. doi: 10.1016/s0896-6273(00)80876-4. [DOI] [PubMed] [Google Scholar]
- Lister RG, Nutt DJ. Is RO 15–4513 a specific alcohol antagonist? Trends Neurosci. 1987;10:223–225. [Google Scholar]
- Lister RG, Nutt DJ. RO 15–4513 and its interaction with ethanol. Adv. Alcohol Subst. Abuse. 1988;7:119–123. doi: 10.1300/J251v07n03_19. [DOI] [PubMed] [Google Scholar]
- Long MA, Jutras MJ, Connors BW, Burwell RD. Electrical synapses coordinate activity in the suprachiasmatic nucleus. Nat. Neurosci. 2005;8:61–66. doi: 10.1038/nn1361. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nat. Rev. Neurosci. 2006;7:126–136. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- Lydic R, Albers HE, Tepper B, Moore-Ede MC. Three-dimensional structure of the mammalian suprachiasmatic nuclei: a comparative study of five species. J. Comp. Neurol. 1982;204:225–239. doi: 10.1002/cne.902040303. [DOI] [PubMed] [Google Scholar]
- MacKenzie G, Maguire J. Neurosteroids and GABAergic signaling in health and disease. Biomol. Concepts. 2013;4:29–42. doi: 10.1515/bmc-2012-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie G, Maguire J. The role of ovarian hormone-derived neurosteroids on the regulation of GABAA receptors in affective disorders. Psychopharmacology. 2014;231:3333–3342. doi: 10.1007/s00213-013-3423-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- Manrique C, Hery F, Faudon M, Francois-Bellan AM. Indirect evidence for an association of 5-HT(1B) binding sites with retinal and geniculate axon terminals in the rat suprachiasmatic nucleus. Synapse. 1999;33:314–323. doi: 10.1002/(SICI)1098-2396(19990915)33:4<314::AID-SYN8>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Mason R. Circadian variation in sensitivity of suprachiasmatic and lateral geniculate neurones to 5-hydroxytryptamine in the rat. J. Physiol. 1986;377:1–13. doi: 10.1113/jphysiol.1986.sp016172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R, Biello SM, Harrington ME. The effects of GABA and benzodiazepenes on neurones in the suprachiasmatic nucleus (SCN) of Syrian hamsters. Brain Res. 1991;552:53–57. doi: 10.1016/0006-8993(91)90659-j. [DOI] [PubMed] [Google Scholar]
- Maywood ES, Chesham JE, O’Brien JA, Hastings MH. A diversity of paracrine signals sustains molecular circadian cycling in suprachiasmatic nucleus circuits. Proc. Natl. Acad. Sci. U.S.A. 2011;108:14306–14311. doi: 10.1073/pnas.1101767108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maywood ES, O’Neill JS, Chesham JE, Hastings MH. Minireview: the circadian clockwork of the suprachiasmatic nuclei–analysis of a cellular oscillator that drives endocrine rhythms. Endocrinology. 2007;148:5624–5634. doi: 10.1210/en.2007-0660. [DOI] [PubMed] [Google Scholar]
- Maywood ES, Reddy AB, Wong GK, O’Neill JS, O’Brien JA, McMahon DG, Harmar AJ, Okamura H, Hastings MH. Synchronization and maintenance of timekeeping in suprachiasmatic circadian clock cells by neuropeptidergic signaling. Curr. Biol. 2006;16:599–605. doi: 10.1016/j.cub.2006.02.023. [DOI] [PubMed] [Google Scholar]
- McElroy B, Zakaria A, Glass JD, Prosser RA. Ethanol modulates mammalian circadian clock phase resetting through extrasynaptic GABA receptor activation. Neuroscience. 2009;164:842–848. doi: 10.1016/j.neuroscience.2009.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire SL, Reimer RJ, Schuske K, Edwards RH, Jorgensen EM. Identification and characterization of the vesicular GABA transporter. Nature. 1997;389:870–876. doi: 10.1038/39908. [DOI] [PubMed] [Google Scholar]
- Medanic M, Gillette MU. Suprachiasmatic circadian pacemaker of rat shows two windows of sensitivity to neuropeptide Y in vitro. Brain Res. 1993;620:281–286. doi: 10.1016/0006-8993(93)90166-k. [DOI] [PubMed] [Google Scholar]
- Meera P, Olsen RW, Otis TS, Wallner M. Alcohol- and alcohol antagonist-sensitive human GABAA receptors: tracking delta subunit incorporation into functional receptors. Mol. Pharmacol. 2010;78:918–924. doi: 10.1124/mol.109.062687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer JH, Michel S. Neurophysiological analysis of the suprachiasmatic nucleus: a challenge at multiple levels. Methods Enzymol. 2015;552:75–102. doi: 10.1016/bs.mie.2014.11.001. [DOI] [PubMed] [Google Scholar]
- Meijer JH, van der Zee EA, Dietz M. Glutamate phase shifts circadian activity rhythms in hamsters. Neurosci. Lett. 1988;86:177–183. doi: 10.1016/0304-3940(88)90567-8. [DOI] [PubMed] [Google Scholar]
- Meijer JH, Watanabe K, Schaap J, Albus H, Detari L. Light responsiveness of the suprachiasmatic nucleus: long-term multiunit and single-unit recordings in freely moving rats. J. Neurosci. 1998;18:9078–9087. doi: 10.1523/JNEUROSCI.18-21-09078.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercado A, Broumand V, Zandi-Nejad K, Enck AH, Mount DB. A C-terminal domain in KCC2 confers constitutive K+-Cl− cotransport. J. Biol. Chem. 2006;281:1016–1026. doi: 10.1074/jbc.M509972200. [DOI] [PubMed] [Google Scholar]
- Meredith AL, Wiler SW, Miller BH, Takahashi JS, Fodor AA, Ruby NF, Aldrich RW. BK calcium-activated potassium channels regulate circadian behavioral rhythms and pacemaker output. Nat. Neurosci. 2006;9:1041–1049. doi: 10.1038/nn1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer EL, Harrington ME, Rahmani T. A phase-response curve to the benzodiazepine chlordiazepoxide and the effect of geniculo-hypothalamic tract ablation. Physiol. Behav. 1993;53:237–243. doi: 10.1016/0031-9384(93)90199-p. [DOI] [PubMed] [Google Scholar]
- Meyer-Bernstein EL, Jetton AE, Matsumoto SI, Markuns JF, Lehman MN, Bittman EL. Effects of suprachiasmatic transplants on circadian rhythms of neuroendocrine function in golden hamsters. Endocrinology. 1999;140:207–218. doi: 10.1210/endo.140.1.6428. [DOI] [PubMed] [Google Scholar]
- Meyer-Bernstein EL, Morin LP. Differential serotonergic innervation of the suprachiasmatic nucleus and the intergeniculate leaflet and its role in circadian rhythm modulation. J. Neurosci. 1996;16:2097–2111. doi: 10.1523/JNEUROSCI.16-06-02097.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel S, Colwell CS. Cellular communication and coupling within the suprachiasmatic nucleus. Chronobiol. Int. 2001;18:579–600. doi: 10.1081/cbi-100106074. [DOI] [PubMed] [Google Scholar]
- Michel S, Marek R, Vanderleest HT, Vansteensel MJ, Schwartz WJ, Colwell CS, Meijer JH. Mechanism of bilateral communication in the suprachiasmatic nucleus. Eur. J. Neurosci. 2013;37:964–971. doi: 10.1111/ejn.12109. [DOI] [PubMed] [Google Scholar]
- Michels KM, Morin LP, Moore RY. GABA-A/benzodizepine receptor localization in the circadian timing system. Brain Res. 1990;531:16–24. doi: 10.1016/0006-8993(90)90753-x. [DOI] [PubMed] [Google Scholar]
- Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone LL, Mi ZP, Lagenaur C, Tretter V, Sieghart W, Anagnostaras SG, Sage JR, Fanselow MS, Guidotti A, Spigelman I, Li Z, DeLorey TM, Olsen RW, Homanics GE. Attenuated sensitivity to neuroactive steroids in gamma-aminobutyrate type A receptor delta subunit knockout mice. Proc. Natl. Acad. Sci. U.S.A. 1999;96:12905–12910. doi: 10.1073/pnas.96.22.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minelli A, DeBiasi S, Brecha NC, Zuccarello LV, Conti F. GAT-3, a high-affinity GABA plasma membrane transporter, is localized to astrocytic processes, and it is not confined to the vicinity of GABAergic synapses in the cerebral cortex. J. Neurosci. 1996;16:6255–6264. doi: 10.1523/JNEUROSCI.16-19-06255.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz EM, Albers HE. Microinjection of NMDA into the SCN region mimics the phase shifting effect of light in hamsters. Brain Res. 1997;758:245–249. doi: 10.1016/s0006-8993(97)00022-x. [DOI] [PubMed] [Google Scholar]
- Mintz EM, Gillespie CF, Marvel CL, Huhman KL, Albers HE. Serotonergic regulation of circadian rhythms. Neuroscience. 1997;79:563–569. doi: 10.1016/s0306-4522(96)00696-3. [DOI] [PubMed] [Google Scholar]
- Mintz EM, Jasnow AM, Gillespie CF, Huhman KL, Albers HE. GABA interacts with photic signaling in the suprachiasmatic nucleus to regulate circadian phase shifts. Neuroscience. 2002;109:773–778. doi: 10.1016/s0306-4522(01)00519-x. [DOI] [PubMed] [Google Scholar]
- Mintz EM, Marvel CL, Gillespie CF, Price KM, Albers HE. Activation of NMDA receptors in the suprachiasmatic nucleus produces light-like phase shifts of the circadian clock in vivo. J. Neurosci. 1999;19:5124–5130. doi: 10.1523/JNEUROSCI.19-12-05124.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistlberger RE. Circadian regulation of sleep in mammals: role of the suprachiasmatic nucleus. Brain Res. Brain Res. Rev. 2005;49:429–454. doi: 10.1016/j.brainresrev.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE, Houpt TA, Moore-Ede MC. The benzodiazepine triazolam phase-shifts circadian activity rhythms in a diurnal primate, the squirrel monkey (Saimiri sciureus) Neurosci. Lett. 1991;124:27–30. doi: 10.1016/0304-3940(91)90814-a. [DOI] [PubMed] [Google Scholar]
- Mody I, Pearce RA. Diversity of inhibitory neurotransmission through GABA (A) receptors. Trends Neurosci. 2004;27:569–575. doi: 10.1016/j.tins.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Moldavan M, Cravetchi O, Williams M, Irwin RP, Aicher SA, Allen CN. Localization and expression of GABA transporters in the suprachiasmatic nucleus. Eur. J. Neurosci. 2015;42:3018–3032. doi: 10.1111/ejn.13083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldavan MG, Allen CN. GABAB receptor-mediated frequency-dependent and circadian changes in synaptic plasticity modulate retinal input to the suprachiasmatic nucleus. J. Physiol. 2013;591:2475–2490. doi: 10.1113/jphysiol.2012.248047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldavan MG, Irwin RP, Allen CN. Presynaptic GABA(B) receptors regulate retinohypothalamic tract synaptic transmission by inhibiting voltage-gated Ca2+ channels. J. Neurophysiol. 2006;95:3727–3741. doi: 10.1152/jn.00909.2005. [DOI] [PubMed] [Google Scholar]
- Montgomery JR, Whitt JP, Wright BN, Lai MH, Meredith AL. Mis-expression of the BK K(+) channel disrupts suprachiasmatic nucleus circuit rhythmicity and alters clock-controlled behavior. Am. J. Physiol. Cell Physiol. 2013;304:C299–C311. doi: 10.1152/ajpcell.00302.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RY, Card JP. Visual pathways and the entrainment of circadian rhythms. Ann. N.Y. Acad. Sci. 1985;453:123–133. doi: 10.1111/j.1749-6632.1985.tb11805.x. [DOI] [PubMed] [Google Scholar]
- Moore RY, Card JP. Neuropeptide Y in the circadian timing system. Ann. N. Y. Acad. Sci. 1990;611:247–257. doi: 10.1111/j.1749-6632.1990.tb48936.x. [DOI] [PubMed] [Google Scholar]
- Moore RY, Card JP. Intergeniculate leaflet: an anatomically and functionally distinct subdivision of the lateral geniculate complex. J. Comp. Neurol. 1994;344:403–430. doi: 10.1002/cne.903440306. [DOI] [PubMed] [Google Scholar]
- Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic nuclear lesions in the rat. Brain Res. 1972;42:201–206. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- Moore RY, Gustafson EL, Card JP. Identical immunoreactivity of afferents to the rat suprachiasmatic nucleus with antisera against avian pancreatic polypeptide, molluscan cardioexcitatory peptide and neuropeptide Y. Cell Tissue Res. 1984;236:41–46. doi: 10.1007/BF00216511. [DOI] [PubMed] [Google Scholar]
- Moore RY, Lenn NJ. A retinohypothalamic projection in the rat. J. Comp. Neurol. 1972;146:1–14. doi: 10.1002/cne.901460102. [DOI] [PubMed] [Google Scholar]
- Moore RY, Speh JC. GABA is the principal neurotransmitter of the circadian system. Neurosci. Lett. 1993;150:112–116. doi: 10.1016/0304-3940(93)90120-a. [DOI] [PubMed] [Google Scholar]
- Moore RY, Speh JC, Leak RK. Suprachiasmatic nucleus organization. Cell Tissue Res. 2002;309:89–98. doi: 10.1007/s00441-002-0575-2. [DOI] [PubMed] [Google Scholar]
- Moore-Ede MC, Schmelzer WS, Kass DA, Herd JA. Internal organization of the circadian timing system in multicellular animals. Fed Proc. 1976;35:2333–2338. [PubMed] [Google Scholar]
- Moore-Ede MC, Sulzman FM. The physiological basis of circadian timekeeping in primates. Physiologist. 1977;20:17–25. [PubMed] [Google Scholar]
- Moore-Ede MC, Sulzman FM, Fuller CA. The Clocks That Time Us. Cambridge: Harvard University Press; 1982. [Google Scholar]
- Morin LP. Neural control of circadian rhythms as revealed through the use of benzodiazepines. In: Klein DC, Moore RY, Reppert SM, editors. Suprachiasmatic Nucleus: The Mind’s Clock. 1991. pp. 324–338. [Google Scholar]
- Morin LP. Serotonin and the regulation of mammalian circadian rhythmicity. Ann. Med. 1999;31:12–33. doi: 10.3109/07853899909019259. [DOI] [PubMed] [Google Scholar]
- Morin LP. SCN organization reconsidered. J. Biol. Rhythms. 2007;22:3–13. doi: 10.1177/0748730406296749. [DOI] [PubMed] [Google Scholar]
- Morin LP. Neuroanatomy of the extended circadian rhythm system. Exp. Neurol. 2013;243:4–20. doi: 10.1016/j.expneurol.2012.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin LP, Allen CN. The circadian visual system, 2005. Brain Res. Rev. 2006;51:1–60. doi: 10.1016/j.brainresrev.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Morin LP, Blanchard JH. Neuromodulator content of hamster intergeniculate leaflet neurons and their projection to the suprachiasmatic nucleus or visual midbrain. J. Comp. Neurol. 2001;437:79–90. doi: 10.1002/cne.1271. [DOI] [PubMed] [Google Scholar]
- Morin LP, Fitzgerald KM, Zucker I. Estradiol shortens the period of hamster circadian rhythms. Science. 1977;196:305–307. doi: 10.1126/science.557840. [DOI] [PubMed] [Google Scholar]
- Morin LP, Shivers KY, Blanchard JH, Muscat L. Complex organization of mouse and rat suprachiasmatic nucleus. Neuroscience. 2006;137:1285–1297. doi: 10.1016/j.neuroscience.2005.10.030. [DOI] [PubMed] [Google Scholar]
- Mozrzymas JW, Zarnowska ED, Pytel M, Mercik K. Modulation of GABA (A) receptors by hydrogen ions reveals synaptic GABA transient and a crucial role of the desensitization process. J. Neurosci. 2003;23:7981–7992. doi: 10.1523/JNEUROSCI.23-22-07981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrosovsky N. A non-photic gateway to the circadian clock of hamsters. Ciba Found. Symp. 1995;183:154–167. doi: 10.1002/9780470514597.ch9. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N. Locomotor activity and non-photic influences on circadian clocks. Biol. Rev. Camb. Philos. Soc. 1996;71:343–372. doi: 10.1111/j.1469-185x.1996.tb01278.x. [DOI] [PubMed] [Google Scholar]
- Muscat L, Huberman AD, Jordan CL, Morin LP. Crossed and uncrossed retinal projections to the hamster circadian system. J. Comp. Neurol. 2003;466:513–524. doi: 10.1002/cne.10894. [DOI] [PubMed] [Google Scholar]
- Myung J, Hong S, DeWoskin D, De Schutter E, Forger DB, Takumi T. GABA-mediated repulsive coupling between circadian clock neurons in the SCN encodes seasonal time. Proc. Natl. Acad. Sci. U.S.A. 2015;112:E3920–E3929. doi: 10.1073/pnas.1421200112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano M, Adachi A, Nakahama K, Nakamura T, Tamada M, Meyer-Bernstein E, Sehgal A, Shigeyoshi Y. An abrupt shift in the day/night cycle causes desynchrony in the mammalian circadian center. J. Neurosci. 2003;23:6141–6151. doi: 10.1523/JNEUROSCI.23-14-06141.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naum OG, Fernanda RM, Golombek DA. Rhythmic variation in gamma-aminobutyric acid(A)-receptor subunit composition in the circadian system and median eminence of Syrian hamsters. Neurosci. Lett. 2001;310:178–182. doi: 10.1016/s0304-3940(01)02129-2. [DOI] [PubMed] [Google Scholar]
- Nishizawa M, Hayakawa Y, Yanaihara N, Okamoto H. Nucleotide sequence divergence and functional constraint in VIP precursor mRNA evolution between human and rat. FEBS. 1985;183:55–59. doi: 10.1016/0014-5793(85)80953-4. [DOI] [PubMed] [Google Scholar]
- Novak CM, Albers HE. N-methyl-d-aspartate microinjected into the suprachiasmatic nucleus mimics the phase-shifting effects of light in the diurnal Nile grass rat (Arvicanthis niloticus) Brain Res. 2002;951:255–263. doi: 10.1016/s0006-8993(02)03168-2. [DOI] [PubMed] [Google Scholar]
- Novak CM, Albers HE. Circadian phase alteration by GABA and light differs in diurnal and nocturnal rodents during the day. Behav. Neurosci. 2004a;118:498–504. doi: 10.1037/0735-7044.118.3.498. [DOI] [PubMed] [Google Scholar]
- Novak CM, Albers HE. Novel phase-shifting effects of GABAA receptor activation in the suprachiasmatic nucleus of a diurnal rodent. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004b;286:R820–R825. doi: 10.1152/ajpregu.00575.2003. [DOI] [PubMed] [Google Scholar]
- Novak CM, Ehlen JC, Huhman KL, Albers HE. GABA(B) receptor activation in the suprachiasmatic nucleus of diurnal and nocturnal rodents. Brain Res. Bull. 2004;63:531–535. doi: 10.1016/j.brainresbull.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Novak CM, Ehlen JC, Paul KN, Fukuhara C, Albers HE. Light and GABA) (A) receptor activation alter period mRNA levels in the SCN of diurnal Nile grass rats. Eur. J. Neurosci. 2006;24:2843–2852. doi: 10.1111/j.1460-9568.2006.05166.x. [DOI] [PubMed] [Google Scholar]
- Novak CM, Harris JA, Smale L, Nunez AA. Suprachiasmatic nucleus projections to the paraventricular thalamic nucleus in nocturnal rats (Rattus norvegicus) and diurnal Nile grass rats (Arviacanthis niloticus) Brain Res. 2000;874:147–157. doi: 10.1016/s0006-8993(00)02572-5. [DOI] [PubMed] [Google Scholar]
- Novak CM, Nunez AA. A sparse projection from the suprachiasmatic nucleus to the sleep active ventrolateral preoptic area in the rat. NeuroReport. 2000;11:93–96. doi: 10.1097/00001756-200001170-00019. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Ahmad Z, Tretter V, Fuchs K, Wisden W, Sieghart W, Somogyi P. Alterations in the expression of GABAA receptor subunits in cerebellar granule cells after the disruption of the alpha6 subunit gene. Eur. J. Neurosci. 1999;11:1685–1697. doi: 10.1046/j.1460-9568.1999.00581.x. [DOI] [PubMed] [Google Scholar]
- O’Hara BF, Andretic R, Heller HC, Carter DB, Kilduff TS. GABAA, GABAC, and NMDA receptor subunit expression in the suprachiasmatic nucleus and other brain regions. Mol. Brain Res. 1995;28:239–250. doi: 10.1016/0169-328x(94)00212-w. [DOI] [PubMed] [Google Scholar]
- Obrietan K, van den Pol AN. GABA neurotransmission in the hypothalamus: developmental reversal from Ca2+ elevating to depressing. J. Neurosci. 1995;15:5065–5077. doi: 10.1523/JNEUROSCI.15-07-05065.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrietan K, van den Pol AN. Neuropeptide Y depresses GABA-mediated calcium transients in developing suprachiasmatic nucleus: a novel form of calcium long-term depression. J. Neurosci. 1996;16:3521–3533. doi: 10.1523/JNEUROSCI.16-10-03521.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta H, Yamazaki S, McMahon DG. Constant light desynchronizes mammalian clock neurons. Nat. Neurosci. 2005;8:267–269. doi: 10.1038/nn1395. [DOI] [PubMed] [Google Scholar]
- Okamoto S, Okamura H, Miyake M, Takahashi Y, Takagi S, Akagi Y, Fukui K, Okamoto H, Ibata Y. A diurnal variation of vasoactive intestinal peptide (VIP) mRNA under a daily light-dark cycle in the rat suprachiasmatic nucleus. Histochemistry. 1991;95:525–528. doi: 10.1007/BF00315750. [DOI] [PubMed] [Google Scholar]
- Okamura H, Berod A, Julien JF, Geffard M, Kitahama K, Mallet J, Bobillier P. Demonstration of GABAergic cell bodies in the suprachiasmatic nucleus: in situ hybridization of glutamic acid decarboxylase (GAD) mRNA and immunocytochemistry of GAD and GABA. Neurosci. Lett. 1989;102:131–136. doi: 10.1016/0304-3940(89)90067-0. [DOI] [PubMed] [Google Scholar]
- Okamura H, Murakami S, Uda K, Sugano T, Takahashi Y, Yanaihara C, Yanaihara N, Ibata Y. Coexistence of vasoactive intestinal peptide (VIP)-, peptide histidine isoleucine amide (PHI)-, and gastrin releasing peptide (GRP)-like immunoreactivity in neurons of the rat suprachiasmatic nucleus. Biomed. Res. 1986;7:295–299. [Google Scholar]
- Olsen RW, Sieghart W. International Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid(A) receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol. Rev. 2008;60:243–260. doi: 10.1124/pr.108.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. GABA A receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology. 2009;56:141–148. doi: 10.1016/j.neuropharm.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens DF, Kriegstein AR. Is there more to GABA than synaptic inhibition? Nat. Rev. Neurosci. 2002;3:715–727. doi: 10.1038/nrn919. [DOI] [PubMed] [Google Scholar]
- Patel AB, de Graaf RA, Martin DL, Battaglioli G, Behar KL. Evidence that GAD65 mediates increased GABA synthesis during intense neuronal activity in vivo. J. Neurochem. 2006;97:385–396. doi: 10.1111/j.1471-4159.2006.03741.x. [DOI] [PubMed] [Google Scholar]
- Peng Z, Hauer B, Mihalek RM, Homanics GE, Sieghart W, Olsen RW, Houser CR. GABA(A) receptor changes in delta subunit-deficient mice: altered expression of alpha4 and gamma2 subunits in the forebrain. J. Comp. Neurol. 2002;446:179–197. doi: 10.1002/cne.10210. [DOI] [PubMed] [Google Scholar]
- Perreau-Lenz S, Kalsbeek A, Garidou ML, Wortel J, Van DV, van Heijningen C, Simonneaux V, Pevet P, Buijs RM. Suprachiasmatic control of melatonin synthesis in rats: inhibitory and stimulatory mechanisms. Eur. J. Neurosci. 2003;17:221–228. doi: 10.1046/j.1460-9568.2003.02442.x. [DOI] [PubMed] [Google Scholar]
- Perreau-Lenz S, Kalsbeek A, Pevet P, Buijs RM. Glutamatergic clock output stimulates melatonin synthesis at night. Eur. J. Neurosci. 2004;19:318–324. doi: 10.1111/j.0953-816x.2003.03132.x. [DOI] [PubMed] [Google Scholar]
- Perreau-Lenz S, Kalsbeek A, Van DV, Pevet P, Buijs RM. In vivo evidence for a controlled offset of melatonin synthesis at dawn by the suprachiasmatic nucleus in the rat. Neuroscience. 2005;130:797–803. doi: 10.1016/j.neuroscience.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Petrelli F, Bezzi P. Novel insights into gliotransmitters. Curr. Opin. Pharmacol. 2016;26:138–145. doi: 10.1016/j.coph.2015.11.010. [DOI] [PubMed] [Google Scholar]
- Peters RV, Zoeller RT, Hennessey AC, Stopa EG, Anderson G, Albers HE. The control of circadian rhythms and the levels of vasoactive intestinal peptide mRNA in the suprachiasmatic nucleus are altered in spontaneously hypertensive rats. Brain Res. 1994;14:217–227. doi: 10.1016/0006-8993(94)91733-7. [DOI] [PubMed] [Google Scholar]
- Pickard GE. The afferent connections of the suprachiasmatic nucleus of the golden hamster with emphasis on the retinohypothalamic projection. J. Comp. Neurol. 1982;211:65–83. doi: 10.1002/cne.902110107. [DOI] [PubMed] [Google Scholar]
- Pickard GE. Bifurcating axons of retinal ganglion cells terminate in the hypothalamic suprachiasmatic nucleus and the intergeniculate leaflet of the thalamus. Neurosci. Lett. 1985;55:211–217. doi: 10.1016/0304-3940(85)90022-9. [DOI] [PubMed] [Google Scholar]
- Pickard GE, Weber ET, Scott PA, Riberdy AF, Rea MA. 5-HT1B receptor agonists inhibit light-induced phase shifts of behavioral circadian rhythms and expression of the immediate-early gene c-fos in the suprachiasmatic nucleus. Neuroscience. 1996;16:8208–8220. doi: 10.1523/JNEUROSCI.16-24-08208.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto FT, Golombek DA. Neuroactive steroids alter the circadian system of the Syrian hamster in a phase-dependent manner. Life Sci. 1999;65:2497–2504. doi: 10.1016/s0024-3205(99)00516-0. [DOI] [PubMed] [Google Scholar]
- Pittendrigh CS, Daan S. A functional analysis of circadian pacemakers in nocturnal rodents I. The stability and lability of spontaneous frequency. J. Comp. Physiol. 1976a;106:223–252. [Google Scholar]
- Pittendrigh CS, Daan S. A functional analysis of circadian pacemakers in nocturnal rodents. IV. Entrainment: pacemaker as clock. J. Comp. Physiol. 1976b;106:291–331. [Google Scholar]
- Pittendrigh CS, Daan S. A functional analysis of circadian pacemakers in nocturnal rodents. V. Pacemaker structure: a clock for all seasons. J. Comp. Physiol. 1976c;106:333–355. [Google Scholar]
- Pittendrigh CS, Elliott J, Takamura T. The circadian component in photoperiodic induction. Ciba Found. Symp. 1984;104:26–47. [Google Scholar]
- Pizarro A, Hayer K, Lahens NF, Hogenesch JB. CircaDB: a database of mammalian circadian gene expression profiles. Nucleic Acids Res. 2013;41:D1009–D1013. doi: 10.1093/nar/gks1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser RA, Glass JD. Assessing ethanol’s actions in the suprachiasmatic circadian clock using in vivo and in vitro approaches. Alcohol. 2015;49:321–339. doi: 10.1016/j.alcohol.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser RA, Mangrum CA, Glass JD. Acute ethanol modulates glutamatergic and serotonergic phase shifts of the mouse circadian clock in vitro. Neuroscience. 2008;152:837–848. doi: 10.1016/j.neuroscience.2007.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph MR, Menaker M. Bicuculline blocks circadian phase delays but not advances. Brain Res. 1985;325:362–365. doi: 10.1016/0006-8993(85)90341-5. [DOI] [PubMed] [Google Scholar]
- Ralph MR, Menaker M. Effects of diazepam on circadian phase advances and delays. Brain Res. 1986;372:405–408. doi: 10.1016/0006-8993(86)91154-6. [DOI] [PubMed] [Google Scholar]
- Ralph MR, Menaker M. GABA regulation of circadian responses to light. I. Involvement of GABAA-benzodiazepine and GABAB receptors. J. Neurosci. 1989;9:2858–2865. doi: 10.1523/JNEUROSCI.09-08-02858.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan C, Campbell A, Tomczak A, Nunez AA, Smale L, Yan L. Compartmentalized expression of light-induced clock genes in the suprachiasmatic nucleus of the diurnal grass rat (Arvicanthis niloticus) Neuroscience. 2009;161:960–969. doi: 10.1016/j.neuroscience.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash JE, Olson CO, Pouliot WA, Davidson KG, Yasumura T, Furman CS, Royer S, Kamasawa N, Nagy JI, Dudek FE. Connexin36 vs. connexin32, “miniature” neuronal gap junctions, and limited electrotonic coupling in rodent suprachiasmatic nucleus. Neuroscience. 2007;149:350–371. doi: 10.1016/j.neuroscience.2007.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reebs SG, Lavery RJ, Mrosovsky N. Running activity mediates the phase-advancing effects of dark pulses on hamster circadian rhythms. J. Comp. Physiol. 1989;165:811–818. doi: 10.1007/BF00610879. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Perlow MJ, Ungerleider LG, Mishkin M, Tamarkin L, Orloff DG, Hoffman HJ, Klein DC. Effects of damage to the suprachiasmatic area of the anterior hypothalamus on the daily melatonin and cortisol rhythms in the rhesus monkey. J. Neurosci. 1981;1:1414–1425. doi: 10.1523/JNEUROSCI.01-12-01414.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppert SM, Schwartz WJ. The suprachiasmatic nucleus of the fetal rat: characterization of a functional circadian clock using 14C-labeled deoxyglucose. J. Neurosci. 1984;4:1677–1682. doi: 10.1523/JNEUROSCI.04-07-01677.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riazanski V, Deriy LV, Shevchenko PD, Le B, Gomez EA, Nelson DJ. Presynaptic CLC-3 determines quantal size of inhibitory transmission in the hippocampus. Nat. Neurosci. 2011;14:487–494. doi: 10.1038/nn.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribak CE, Peters A. An autoradiographic study of the projections from the lateral geniculate body of the rat. Brain Res. 1975;92:261–294. doi: 10.1016/0006-8993(75)90322-4. [DOI] [PubMed] [Google Scholar]
- Ribak CE, Tong WM, Brecha NC. GABA plasma membrane transporters, GAT-1 and GAT-3, display different distributions in the rat hippocampus. J. Comp. Neurol. 1996;367:595–606. doi: 10.1002/(SICI)1096-9861(19960415)367:4<595::AID-CNE9>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Richerson GB, Wu Y. Dynamic equilibrium of neurotransmitter transporters: not just for reuptake anymore. J. Neurophysiol. 2003;90:1363–1374. doi: 10.1152/jn.00317.2003. [DOI] [PubMed] [Google Scholar]
- Romijn HJ, Sluiter AA, Pool CW, Wortel J, Buijs RM. Evidence from confocal fluorescence microscopy for a dense, reciprocal innervation between AVP-, somatostatin-, VIP/PHI-, GRP-, and VIP/PHI/GRP-immunoreactive neurons in the rat suprachiasmatic nucleus. Eur. J. Neurosci. 1997;9:2613–2623. doi: 10.1111/j.1460-9568.1997.tb01691.x. [DOI] [PubMed] [Google Scholar]
- Romijn HJ, Sluiter AA, Wortel J, Van Uum JF, Buijs RM. Immunocytochemical evidence for a diurnal rhythm of neurons showing colocalization of VIP with GRP in the rat suprachiasmatic nucleus. J. Comp. Neurol. 1998;391:397–405. [PubMed] [Google Scholar]
- Rosenwasser AM, Dwyer SM. Circadian phase shifting: relationships between photic and nonphotic phase-response curves. Physiol. Behav. 2001;73:175–183. doi: 10.1016/s0031-9384(01)00466-8. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Hamann M, Attwell D. Multiple modes of GABAergic inhibition of rat cerebellar granule cells. J. Physiol. 2003;548:97–110. doi: 10.1113/jphysiol.2002.036459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozsa M, Baka J, Borde S, Rozsa B, Katona G, Tamas G. Unitary GABAergic volume transmission from individual interneurons to astrocytes in the cerebral cortex. Brain Struct. Funct. 2015 doi: 10.1007/s00429-015-1166-9. http://dx.doi.org/10.1007/s00429-015-1166-9. [DOI] [PubMed] [Google Scholar]
- Ruby CL, Brager AJ, DePaul MA, Prosser RA, Glass JD. Chronic ethanol attenuates circadian photic phase resetting and alters nocturnal activity patterns in the hamster. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009a;297:R729–R737. doi: 10.1152/ajpregu.00268.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby CL, Prosser RA, DePaul MA, Roberts RJ, Glass JD. Acute ethanol impairs photic and nonphotic circadian phase resetting in the Syrian hamster. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009b;296:R411–R418. doi: 10.1152/ajpregu.90782.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Mohler H. GABA(A) receptor subtypes: dissecting their pharmacological functions. Trends Pharmacol. Sci. 2001;22:188–194. doi: 10.1016/s0165-6147(00)01646-1. [DOI] [PubMed] [Google Scholar]
- Sagne C, El Mestikawy S, Isambert MF, Hamon M, Henry JP, Giros B, Gasnier B. Cloning of a functional vesicular GABA and glycine transporter by screening of genome databases. FEBS Lett. 1997;417:177–183. doi: 10.1016/s0014-5793(97)01279-9. [DOI] [PubMed] [Google Scholar]
- Santhakumar V, Hanchar HJ, Wallner M, Olsen RW, Otis TS. Contributions of the GABAA receptor alpha6 subunit to phasic and tonic inhibition revealed by a naturally occurring polymorphism in the alpha6 gene. J. Neurosci. 2006;26:3357–3364. doi: 10.1523/JNEUROSCI.4799-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaki Y, Nihonmatsu I, Kawamura H. Transplantation of the neonatal suprachiasmatic nuclei into rats with complete bilateral suprachiasmatic lesions. Neurosci. Res. 1984;1:67–72. doi: 10.1016/0168-0102(84)90031-2. [DOI] [PubMed] [Google Scholar]
- Schaap J, Pennartz CM, Meijer JH. Electrophysiology of the circadian pacemaker in mammals. Chronobiol. Int. 2003;20:171–188. doi: 10.1081/cbi-120019311. [DOI] [PubMed] [Google Scholar]
- Schwartz WJ. Further evaluation of the tetrodotoxin-resistant circadian pacemaker in the suprachiasmatic nuclei. J. Biol. Rhythms. 1991;6:149–158. doi: 10.1177/074873049100600205. [DOI] [PubMed] [Google Scholar]
- Schwartz WJ, Gross RA, Morton MT. The suprachiasmatic nuclei contain a tetrodotoxin-resistant circadian pacemaker. Proc. Natl. Acad. Sci. U.S.A. 1987;84:1694–1698. doi: 10.1073/pnas.84.6.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott FF, Belle MD, Delagrange P, Piggins HD. Electrophysiological effects of melatonin on mouse Per1 and non-Per1 suprachiasmatic nuclei neurones in vitro. J. Neuroendocrinol. 2010;22:1148–1156. doi: 10.1111/j.1365-2826.2010.02063.x. [DOI] [PubMed] [Google Scholar]
- Seggio JA, Fixaris MC, Reed JD, Logan RW, Rosenwasser AM. Chronic ethanol intake alters circadian phase shifting and free-running period in mice. J. Biol. Rhythms. 2009;24:304–312. doi: 10.1177/0748730409338449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seggio JA, Logan RW, Rosenwasser AM. Chronic ethanol intake modulates photic and non-photic circadian phase responses in the Syrian hamster. Pharmacol. Biochem. Behav. 2007;87:297–305. doi: 10.1016/j.pbb.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABAA receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Seutin V, Johnson SW. Recent advances in the pharmacology of quaternary salts of bicuculline. Trends Pharmacol. Sci. 1999;20:268–270. doi: 10.1016/s0165-6147(99)01334-6. [DOI] [PubMed] [Google Scholar]
- Shearman LP, Zylka MJ, Weaver DR, Kolakowski LF, Jr, Reppert SM. Two period homologs: circadian expression and photic regulation in the suprachiasmatic nuclei. Neuron. 1997;19:1261–1269. doi: 10.1016/s0896-6273(00)80417-1. [DOI] [PubMed] [Google Scholar]
- Shibata S, Liou SY, Ueki S. Effect of amino acids and monoamines on the neuronal activity of suprachiasmatic nucleus in hypothalamic slice preparations. Jpn. J. Pharmacol. 1983;33:1225–1231. doi: 10.1254/jjp.33.1225. [DOI] [PubMed] [Google Scholar]
- Shibata S, Liou SY, Ueki S. Influence of excitatory amino acid receptor antagonists and of baclofen on synaptic transmission in the optic nerve to the suprachiasmatic nucleus in slices of rat hypothalamus. Neuropharmacology. 1986;25:403–409. doi: 10.1016/0028-3908(86)90235-2. [DOI] [PubMed] [Google Scholar]
- Shibata S, Moore RY. Tetrodotoxin does not affect circadian neuronal activity or metabolic rhythms in suprachiasmatic nucleus in vitro. Brain Res. 1993;606:259–266. doi: 10.1016/0006-8993(93)90993-w. [DOI] [PubMed] [Google Scholar]
- Shigeyoshi Y, Taguchi K, Yamamota S, Takekida S, Yan L, Tei H, Moriya T, Shibata S, Loros JJ, Dunlap J, Okamura H. Light-induced resetting of a mammalian circadian clock is associated with rapid induction of the mPer1 transcript. Cell. 1997;91:1043–1053. doi: 10.1016/s0092-8674(00)80494-8. [DOI] [PubMed] [Google Scholar]
- Shimura M, Akaike N, Harata N. Circadian rhythm in intracellular Cl(−) activity of acutely dissociated neurons of suprachaismatic nucleus. Am. J. Physiol. 2002;282:C366–C373. doi: 10.1152/ajpcell.00187.2000. [DOI] [PubMed] [Google Scholar]
- Shimura M, Harata N, Tamai M, Akaike N. Allosteric modulation of GABAA receptors in acutely dissociated neurons of the suprachiasmatic nucleus. Am. J. Physiol. 1996;270:C1726–C1734. doi: 10.1152/ajpcell.1996.270.6.C1726. [DOI] [PubMed] [Google Scholar]
- Shinohara K, Hiruma H, Funabashi T, Kimura F. GABAergic modulation of gap junction communication in slice cultures of the rat suprachiasmatic nucleus. Neuroscience. 2000;96:591–596. doi: 10.1016/s0306-4522(99)00556-4. [DOI] [PubMed] [Google Scholar]
- Shinohara K, Honma S, Katsuno Y, Abe H, Honma K. Two distinct oscillators in the rat suprachiasmatic nucleus. Proc. Natl. Acad. Sci. U.S.A. 1996;92:7396–7400. doi: 10.1073/pnas.92.16.7396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara K, Tominaga K, Isobe Y, Inouye ST. Photic regulation of peptides located in the ventrolateral subdivision of the suprachiasmatic nucleus of the rat: daily variations of vasoactive intestinal polypeptide, gastrin-releasing peptide, and neuropeptide Y. J. Neurosci. 1993;13:793–800. doi: 10.1523/JNEUROSCI.13-02-00793.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa T, Honma S, Katsuno Y, Oguchi H, Honma KI. Synchornization of circadian firing rhythms in cultured rat suprachiasmatic neurons. Eur. J. Neurosci. 2000;12:2833–2838. doi: 10.1046/j.1460-9568.2000.00170.x. [DOI] [PubMed] [Google Scholar]
- Shirasaki T, Klee MR, Nakaye T, Akaike N. Differential blockade of bicuculline and strychnine on GABA- and glycine-induced responses in dissociated rat hippocampal pyramidal cells. Brain Res. 1991;561:77–83. doi: 10.1016/0006-8993(91)90751-g. [DOI] [PubMed] [Google Scholar]
- Sigel E, Kaur KH, Luscher BP, Baur R. Use of concatamers to study GABAA receptor architecture and function: application to delta-subunit-containing receptors and possible pitfalls. Biochem. Soc. Trans. 2009;37:1338–1342. doi: 10.1042/BST0371338. [DOI] [PubMed] [Google Scholar]
- Silver R, Lehman MN, Gibson M, Gladstone WR, Bittman EL. Dispersed cell suspensions of fetal SCN restore circadian rhythmicity in SCN-lesioned adult hamsters. Brain Res. 1990;525:45–58. doi: 10.1016/0006-8993(90)91319-c. [DOI] [PubMed] [Google Scholar]
- Silver R, LeSauter J, Tresco PA, Lehman MN. A diffusible coupling signal from the transplanted suprachiasmatic nucleus controlling circadian locomotor rhythms. Nature. 1996;382:810–813. doi: 10.1038/382810a0. [DOI] [PubMed] [Google Scholar]
- Slat E, Freeman M, Herzog ED. The clock in the brain: neurons, glia, and networks in daily rhythms. Handb. Exp. Pharmacol. 2013;217:105–123. doi: 10.1007/978-3-642-25950-0_5. [DOI] [PubMed] [Google Scholar]
- Smart TG, Moss SJ, Xie X, Huganir RL. GABAA receptors are differentially sensitive to zinc: dependence on subunit composition. Br. J. Pharmacol. 1991;103:1837–1839. doi: 10.1111/j.1476-5381.1991.tb12337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RD, Inouye SIT, Turek FW. Central administration of muscimol phase-shifts the mammalian circadian clock. J. Comp. Physiol. 1989;164:805–814. doi: 10.1007/BF00616752. [DOI] [PubMed] [Google Scholar]
- Smith RD, Turek FW, Slater NT. Bicuculline and picrotoxin block phase advances induced by GABA agonists in the circadian rhythm of locomotor activity in the golden hamster by a phaclofen-insensitive mechanism. Brain Res. 1990;530:275–282. doi: 10.1016/0006-8993(90)91295-r. [DOI] [PubMed] [Google Scholar]
- Smyllie NJ, Pilorz V, Boyd J, Meng AJ, Saer B, Chesham JE, Maywood ES, Krogager TP, Spiller DG, Boot-Handford R, White MRH, Hastings MH, Loudon ASI. Visualizing and quantifying intracellular behavior and abundance of the core circadian clock protein PERIOD2. Curr. Biol. 2016;26:1880–1886. doi: 10.1016/j.cub.2016.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soghomonian JJ, Martin DL. Two isoforms of glutamate decarboxylase: why? Trends Pharmacol. Sci. 1998;19:500–505. doi: 10.1016/s0165-6147(98)01270-x. [DOI] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABAA receptors. Proc. Natl. Acad. Sci. U.S.A. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell BM, Mody I. Receptors with different affinities mediate phasic and tonic GABA(A) conductances in hippocampal neurons. J. Neurosci. 2002;22:RC223. doi: 10.1523/JNEUROSCI.22-10-j0003.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan FK. Circadian rhythm dissociation induced by periodic feeding in rats with suprachiasmatic lesions. Behav. Brain Res. 1983;7:81–98. doi: 10.1016/0166-4328(83)90006-2. [DOI] [PubMed] [Google Scholar]
- Stephan FK. The “other” circadian system: food as a Zeitgeber. J. Biol. Rhythms. 2002;17:284–292. doi: 10.1177/074873040201700402. [DOI] [PubMed] [Google Scholar]
- Stephan FK, Berkley KJ, Moss RL. Efferent connections of the rat suprachiasmatic nucleus. Neuroscience. 1981;6:2626–2641. doi: 10.1016/0306-4522(81)90108-1. [DOI] [PubMed] [Google Scholar]
- Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc. Nat. Acad. Sci. U.S.A. 1972;69:1583–1586. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoop R. Neuromodulation by oxytocin and vasopressin. Neuron. 2012;76:142–159. doi: 10.1016/j.neuron.2012.09.025. [DOI] [PubMed] [Google Scholar]
- Stopa EG, Minamitani N, Jonassen JA, King JC, Wolfe H, Mobtaker H, Albers HE. Localization of vasoactive intestinal peptide and peptide histidine isoleucine immunoreactivity and mRNA within the rat suprachiasmatic nucleus. Mol. Brain Res. 1988;4:319–325. doi: 10.1016/0169-328x(88)90041-1. [DOI] [PubMed] [Google Scholar]
- Strecker GJ, Jackson MB, Dudek FE. Blockade of NMDA-activated channels by magnesium in the immature rat hippocampus. J. Neurophysiol. 1994;72:1538–1548. doi: 10.1152/jn.1994.72.4.1538. [DOI] [PubMed] [Google Scholar]
- Strecker GJ, Park WK, Dudek FE. Zinc and flunitrazepam modulation of GABA-mediated currents in rat suprachiasmatic neurons. J. Neurophysiol. 1999;81:184–191. doi: 10.1152/jn.1999.81.1.184. [DOI] [PubMed] [Google Scholar]
- Strecker GJ, Wuarin JP, Dudek FE. GABAA-mediated local synaptic pathways connect neurons in the rat suprachiasmatic nucleus. J. Neurophysiol. 1997;78:2217–2220. doi: 10.1152/jn.1997.78.4.2217. [DOI] [PubMed] [Google Scholar]
- Strubbe JH, Prins AJ, Bruggink J, Steffens AB. Daily variation of food-induced changes in blood glucose and insulin in the rat and the control by the suprachiasmatic nucleus and the vagus nerve. J. Auton. Nerv. Syst. 1987;20:113–119. doi: 10.1016/0165-1838(87)90108-1. [DOI] [PubMed] [Google Scholar]
- Suburo AM, Pellegrino dl. An ultrastructural study of the rat’s suprachiasmatic nucleus. J. Anat. 1969;105:439–446. [PMC free article] [PubMed] [Google Scholar]
- Sun H, Machu TK. Bicuculline antagonizes 5-HT(3A) and alpha2 glycine receptors expressed in Xenopus oocytes. Eur. J. Pharmacol. 2000;391:243–249. doi: 10.1016/s0014-2999(00)00083-2. [DOI] [PubMed] [Google Scholar]
- Sun X, Rusak B, Semba K. Electrophysiology and pharmacology of projections from the suprachiasmatic nucleus to the ventromedial preoptic area in rat. Neuroscience. 2000;98:715–728. doi: 10.1016/s0306-4522(00)00170-6. [DOI] [PubMed] [Google Scholar]
- Sun X, Whitefield S, Rusak B, Semba K. Electrophysiological analysis of suprachiasmatic nucleus projections to the ventrolateral preoptic area in the rat. Eur. J. Neurosci. 2001;14:1257–1274. doi: 10.1046/j.0953-816x.2001.0001755.x. [DOI] [PubMed] [Google Scholar]
- Sur C, Farrar SJ, Kerby J, Whiting PJ, Atack JR, McKernan RM. Preferential coassembly of alpha4 and delta subunits of the gamma-aminobutyric acid A receptor in rat thalamus. Mol. Pharmacol. 1999;56:110–115. doi: 10.1124/mol.56.1.110. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Cowan WM. The efferent connections of the suprachiasmatic nucleus of the hypothalamus. J. Comp. Neurol. 1975;160:1–12. doi: 10.1002/cne.901600102. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Cowan WM, Jones EG. An autoradiographic study of the efferent connections of the ventral lateral geniculate nucleus in the albino rat and cat. J. Comp. Neurol. 1974;156:143–163. doi: 10.1002/cne.901560203. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Okamura H, Yanaihara N, Hamada S, Fujita S, Ibata Y. Vasoactive intestinal peptide immunoreactive neurons in the rat suprachiasmatic nucleus demonstrates diurnal variation. Brain Res. 1989;497:374–377. doi: 10.1016/0006-8993(89)90283-7. [DOI] [PubMed] [Google Scholar]
- Takamori S, Riedel D, Jahn R. Immunoisolation of GABA-specific synaptic vesicles defines a functionally distinct subset of synaptic vesicles. J. Neurosci. 2000;20:4904–4911. doi: 10.1523/JNEUROSCI.20-13-04904.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan KR, Rudolph U, Luscher C. Hooked on benzodiazepines: GABAA receptor subtypes and addiction. Trends Neurosci. 2011;34:188–197. doi: 10.1016/j.tins.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Hayashi S, Tamada Y, Ikeda T, Hisa Y, Takamatsu T, Ibata Y. Direct retinal projections to GRP neurons in the suprachiasmatic nucleus of the rat. NeuroReport. 1997a;8:2187–2191. doi: 10.1097/00001756-199707070-00020. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Matsuda T, Shigeyoshi Y, Ibata Y, Okamura H. Peptide expression in GABAergic neurons in rat suprachiasmatic nucleus in comparison with other forebrain structures: a double labeling in situ hybridization study. J. Histochem. Cytochem. 1997b;45:1231–1237. doi: 10.1177/002215549704500906. [DOI] [PubMed] [Google Scholar]
- Tataroglu O, Davidson AJ, Benvenuto LJ, Menaker M. The methamphetamine-sensitive circadian oscillator (MASCO) in mice. J. Biol. Rhythms. 2006;21:185–194. doi: 10.1177/0748730406287529. [DOI] [PubMed] [Google Scholar]
- Tian N, Petersen C, Kash S, Baekkeskov S, Copenhagen D, Nicoll R. The role of the synthetic enzyme GAD65 in the control of neuronal gamma-aminobutyric acid release. Proc. Natl. Acad. Sci. U.S.A. 1999;96:12911–12916. doi: 10.1073/pnas.96.22.12911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga K, Shibata S, Hamada T, Watanabe S. GABAA receptor agonist muscimol can reset the phase of neural activity rhythm in the rat suprachiasmatic nucleus in vitro. Neurosci. Lett. 1994;166:81–84. doi: 10.1016/0304-3940(94)90845-1. [DOI] [PubMed] [Google Scholar]
- Trachsel L, Dodt HU, Zieglgänsberger W. The intrinsic optical signalevoked by chiasm stimulation in the rat suprachiasmatic nuclei exhibits GABAergic day-night variation. Eur. J. Neurosci. 1996;8:319–328. doi: 10.1111/j.1460-9568.1996.tb01216.x. [DOI] [PubMed] [Google Scholar]
- Tritsch NX, Granger AJ, Sabatini BL. Mechanisms and functions of GABA co-release. Nat. Rev. Neurosci. 2016;17:139–145. doi: 10.1038/nrn.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trueta C, De Miguel FF. Extrasynaptic exocytosis and its mechanisms: a source of molecules mediating volume transmission in the nervous system. Front. Physiol. 2012;3:319. doi: 10.3389/fphys.2012.00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turek FW, Losee-Olsen S. A benzodiazepine used in treatment of insomnia phase-shifts the mammalian circadian clock. Nature. 1986;321:167–168. doi: 10.1038/321167a0. [DOI] [PubMed] [Google Scholar]
- Ulrich D, Bettler B. GABA(B) receptors: synaptic functions and mechanisms of diversity. Curr. Opin. Neurobiol. 2007;17:298–303. doi: 10.1016/j.conb.2007.04.001. [DOI] [PubMed] [Google Scholar]
- van den Pol AN. The hypothalamic suprachiasmatic nucleus of rat: intrinsic anatomy. J. Comp. Neurol. 1980;191:661–702. doi: 10.1002/cne.901910410. [DOI] [PubMed] [Google Scholar]
- van den Pol AN. Gamma-aminobutyrate, gastrin releasing peptide, serotinin, somatostatin, and vasopressin: ultrastructural immunocytochemical localization in presynaptic axons in the suprachiasmatic nucleus. Neuroscience. 1986;17:643–659. doi: 10.1016/0306-4522(86)90037-0. [DOI] [PubMed] [Google Scholar]
- van den Pol AN. Neuropeptide transmission in brain circuits. Neuron. 2012;76:98–115. doi: 10.1016/j.neuron.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN, Dudek FE. Cellular communication in the biological clock, the suprachiasmatic nucleus. Neuroscience. 1993;56:793–811. doi: 10.1016/0306-4522(93)90128-3. [DOI] [PubMed] [Google Scholar]
- van den Pol AN, Gorcs T. Synaptic relationships between neurons containing vasopressin, gastrin-releasing peptide, vasoactive intestinal polypeptide, and glutamate decarboxylase immunoreactivity in the suprachiasmatic nucleus: dual ultrastructural immunocytochemistry with gold-substituted silver peroxidase. J. Comp. Neurol. 1986;252:507–521. doi: 10.1002/cne.902520407. [DOI] [PubMed] [Google Scholar]
- van den Pol AN, Tsujimoto KL. Neurotransmitters of the hypothalamic suprachiasmatic nucleus: immunocytochemical analysis of 25 neuronal antigens. Neuroscience. 1985;15:1049–1086. doi: 10.1016/0306-4522(85)90254-4. [DOI] [PubMed] [Google Scholar]
- Van Reeth O, Turek FW. Stimulated activity mediates phase shifts in the hamster circadian clock induced by dark pulses or benzodiazepenes. Nature. 1989;339:49–51. doi: 10.1038/339049a0. [DOI] [PubMed] [Google Scholar]
- Van Reeth O, Vanderhaeghen JJ, Turek FW. A benzodiazepine antagonist, Ro 15–1788, can block the phase-shifting effects of triazolam on the mammalian circadian clock. Brain Res. 1988;444:333–339. doi: 10.1016/0006-8993(88)90942-0. [DOI] [PubMed] [Google Scholar]
- Vandesande F, Dierickx K, Demey J. Identification of the vasopressin-neurophysin producing neurons of the rat suprachiasmatic nuclei. Cell Tissue Res. 1975;156:377–380. doi: 10.1007/BF00225365. [DOI] [PubMed] [Google Scholar]
- Vansteensel MJ, Deboer T, Dahan A, Meijer JH. Differential responses of circadian activity onset and offset following GABA-ergic and opioid receptor activation. J. Biol. Rhythms. 2003a;18:297–306. doi: 10.1177/0748730403254283. [DOI] [PubMed] [Google Scholar]
- Vansteensel MJ, Yamazaki S, Albus H, Deboer T, Block GD, Meijer JH. Dissociation between circadian Per1 and neuronal and behavioral rhythms following a shifted environmental cycle. Curr. Biol. 2003b;13:1538–1542. doi: 10.1016/s0960-9822(03)00560-8. [DOI] [PubMed] [Google Scholar]
- Vicini S, Ortinski P. Genetic manipulations of GABAA receptor in mice make inhibition exciting. Pharmacol. Ther. 2004;103:109–120. doi: 10.1016/j.pharmthera.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Vosko AM, Schroeder A, Loh DH, Colwell CS. Vasoactive intestinal peptide and the mammalian circadian system. Gen. Comp. Endocrinol. 2007;152:165–175. doi: 10.1016/j.ygcen.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner S, Castel M, Gainer H, Yarom Y. GABA in the mammalian suprachiasmatic nucleus and its role in diurnal rhythmicity. Nature. 1997;387:598–603. doi: 10.1038/42468. [DOI] [PubMed] [Google Scholar]
- Wagner S, Sagiv N, Yarom Y. GABA-induced current and circadian regulation of chloride in neurones of the rat suprachiasmatic nucleus. J. Physiol. 2001;537:853–869. doi: 10.1111/j.1469-7793.2001.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner M, Olsen RW. Physiology and pharmacology of alcohol: the imidazobenzodiazepine alcohol antagonist site on subtypes of GABAA receptors as an opportunity for drug development? Br. J. Pharmacol. 2008;154:288–298. doi: 10.1038/bjp.2008.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton JC, McNeill JK, IV, Albers HE. Circadian rhythms in the expression and function of GABAA receptors in the suprachiasmatic nucleus; Society for Research on Biological Rhythms Biennial Meeting; 2016. [Google Scholar]
- Wang P, Slaughter MM. Effects of GABA receptor antagonists on retinal glycine receptors and on homomeric glycine receptor alpha subunits. J. Neurophysiol. 2005;93:3120–3126. doi: 10.1152/jn.01228.2004. [DOI] [PubMed] [Google Scholar]
- Watts AG. The efferent projections of the suprachiasmatic nucleus. Anatomical insights into the control of circadian rhythms. In: Klein DC, Moore RY, Reppert SM, editors. Suprachiasmatic Nucleus: The Mind’s Clock. New York: Oxford University Press; 1991. [Google Scholar]
- Watts AG, Swanson LW. Efferent projections of the suprachiasmatic nucleus: II. Studies using retrograde transport of fluorescent dyes and simultaneous peptide immunohistochemistry in the rat. J. Comp. Neurol. 1987;258:230–252. doi: 10.1002/cne.902580205. [DOI] [PubMed] [Google Scholar]
- Watts AG, Swanson LW, Sanchez-Watts G. Efferent projections of the suprachiasmatic nucleus: I. Studies using anterograde transport of Phaseolus vulgaris leucoagglutinin in the rat. J. Comp. Neurol. 1987;258:204–229. doi: 10.1002/cne.902580204. [DOI] [PubMed] [Google Scholar]
- Webb AB, Angelo N, Huettner JE, Herzog ED. Intrinsic, nondeterministic circadian rhythm generation in identified mammalian neurons. Proc. Natl. Acad. Sci. U.S.A. 2009;106:16493–16498. doi: 10.1073/pnas.0902768106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb IC, Antle MC, Mistlberger RE. Regulation of circadian rhythms in mammals by behavioral arousal. Behav. Neurosci. 2014;128:304–325. doi: 10.1037/a0035885. [DOI] [PubMed] [Google Scholar]
- Weber TE, Rea MA. Neuropeptide Y blocks light-induced phase advances but not delays of the circadian activity rhythm in hamsters. Neurosci. Lett. 1997;231:159–162. doi: 10.1016/s0304-3940(97)00559-4. [DOI] [PubMed] [Google Scholar]
- Wee BE, Turek FW. Midazolam, a short-acting benzodiazepine, resets the circadian clock of the hamster. Pharmacol. Biochem. Behav. 1989;32:901–906. doi: 10.1016/0091-3057(89)90056-7. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Logothetis DE, Meister M, Reppert SM. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron. 1995;14:697–706. doi: 10.1016/0896-6273(95)90214-7. [DOI] [PubMed] [Google Scholar]
- Winsky-Sommerer R. Role of GABAA receptors in the physiology and pharmacology of sleep. Eur. J. Neurosci. 2009;29:1779–1794. doi: 10.1111/j.1460-9568.2009.06716.x. [DOI] [PubMed] [Google Scholar]
- Wu X, Huang L, Wu Z, Zhang C, Jiang D, Bai Y, Wang Y, Chen G. Homeostatic competition between phasic and tonic inhibition. J. Biol. Chem. 2013 doi: 10.1074/jbc.M113.491464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Isejima H, Matsuo T, Okura R, Yagita K, Kobayashi M, Okamura H. Synchronization of cellular clocks in the suprachiasmatic nucleus. Science. 2003;302:1408–1412. doi: 10.1126/science.1089287. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Shigeyoshi Y, Ishida Y, Fukuyama T, Yamaguchi S, Yagita K, Moriya T, Shibata S, Takashima N, Okamura H. Expression of the Per1 gene in the hamster: brain atlas and circadian characteristics in the suprachiasmatic nucleus. J. Comp. Neurol. 2001;430:518–532. [PubMed] [Google Scholar]
- Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- Yan L. Expression of clock genes in the suprachiasmatic nucleus: effect of environmental lighting conditions. Rev. Endocr. Metab. Disord. 2009;10:301–310. doi: 10.1007/s11154-009-9121-9. [DOI] [PubMed] [Google Scholar]
- Yan L, Foley NC, Bobula JM, Kriegsfeld LJ, Silver R. Two antiphase oscillations occur in each suprachiasmatic nucleus of behaviorally split hamsters. J. Neurosci. 2005;25:9017–9026. doi: 10.1523/JNEUROSCI.2538-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Karatsoreos I, LeSauter J, Welsh DK, Kay S, Foley D, Silver R. Exploring spatiotemporal organization of SCN circuits. Cold Spring Harb. Symp. Quant. Biol. 2007;72:527–541. doi: 10.1101/sqb.2007.72.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Okamura H. Gradients in the circadian expression of Per1 and Per2 genes in the rat suprachiasmatic nucleus. Eur. J. Neurosci. 2002;15:1153–1162. doi: 10.1046/j.1460-9568.2002.01955.x. [DOI] [PubMed] [Google Scholar]
- Yan L, Silver R. Differential induction and localization of mPer1 and mPer2 during advancing and delaying phase shifts. Eur. J. Neurosci. 2002;16:1531–1540. doi: 10.1046/j.1460-9568.2002.02224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Silver R. Resetting the brain clock: time course and localization of mPER1 and mPER2 protein expression in suprachiasmatic nuclei during phase shifts. Eur. J. Neurosci. 2004;19:1105–1109. doi: 10.1111/j.1460-9568.2004.03189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Takekida S, Shigeyoshi Y, Okamura H. Per1 and Per2 gene expression in the rat suprachiasmatic nucleus: circadian profile and the compartment-specific response to light. Neuroscience. 1999;94:141–150. doi: 10.1016/s0306-4522(99)00223-7. [DOI] [PubMed] [Google Scholar]
- Yannielli P, Harrington ME. Let there be “more” light: enhancement of light actions on the circadian system through non-photic pathways. Prog. Neurobiol. 2004;74:59–76. doi: 10.1016/j.pneurobio.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Yannielli PC, Brewer JM, Harrington ME. Blockade of the NPY Y5 receptor potentiates circadian responses to light: complementary in vivo and in vitro studies. Eur. J. Neurosci. 2004;19:891–897. doi: 10.1111/j.0953-816x.2004.03098.x. [DOI] [PubMed] [Google Scholar]
- Yannielli PC, Harrington ME. Neuropeptide Y applied in vitro can block the phase shifts induced by light in vivo. NeuroReport. 2000;11:1587–1591. [PubMed] [Google Scholar]
- Yokota SI, Horikawa K, Akiyama M, Moriya T, Ebihara S, Komuro G, Ohta T, Shibata S. Inhibitory action of brotizolam on circadian and light-induced per1 and per2 expression in the hamster suprachiasmatic nucleus. Br. J. Pharmacol. 2000;131:1739–1747. doi: 10.1038/sj.bjp.0703735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, Takahashi JS. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc. Natl. Acad. Sci. U.S.A. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon BE, Lee CJ. GABA as a rising gliotransmitter. Front. Neural Circ. 2014;8:141. doi: 10.3389/fncir.2014.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelinski EL, Deibel SH, McDonald RJ. The trouble with circadian clock dysfunction: multiple deleterious effects on the brain and body. Neurosci. Biobehav. Rev. 2014;40:80–101. doi: 10.1016/j.neubiorev.2014.01.007. [DOI] [PubMed] [Google Scholar]
- Zhang EE, Kay SA. Clocks not winding down: unravelling circadian networks. Nat. Rev. Mol. Cell. 2010;11:764–776. doi: 10.1038/nrm2995. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Danbolt NC. GABA and glutamate transporters in brain. Front Endocrinol. (Lausanne) 2013;4:165. doi: 10.3389/fendo.2013.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Holmseth S, Hua R, Lehre AC, Olofsson AM, Poblete-Naredo I, Kempson SA, Danbolt NC. The betaine-GABA transporter (BGT1, slc6a12) is predominantly expressed in the liver and at lower levels in the kidneys and at the brain surface. Am. J. Physiol. Renal. Physiol. 2012;302:F316–F328. doi: 10.1152/ajprenal.00464.2011. [DOI] [PubMed] [Google Scholar]
- Zoeller RT, Broyles B, Earley J, Anderson ER, Albers HE. Cellular levels of messenger ribonucleic acids encoding vasoactive intestinal Peptide and gastrin-releasing Peptide in neurons of the suprachiasmatic nucleus exhibit distinct 24-hour rhythms. J. Neuroendocrinol. 1992;4:119–124. doi: 10.1111/j.1365-2826.1992.tb00354.x. [DOI] [PubMed] [Google Scholar]
- Zucker I, Fitzgerald KM, Morin LP. Sex differentiation of the circadian system in the golden hamster. Am. J. Physiol. 1980;238:R97–R101. doi: 10.1152/ajpregu.1980.238.1.R97. [DOI] [PubMed] [Google Scholar]