Abstract
This study examined differences between adults with autism spectrum disorder (ASD; N=40) and typical community volunteers (N=25) on measures of stressful life events, perceived stress, and biological stress response (cardiovascular and cortisol reactivity) during a novel social stress task. Additional analyses examined the relationship between stress and social functioning as measured by the Social Adjustment Scale-II and the Waisman Activities of Daily Living scale. Results indicated that adults with ASD experienced significantly more stressful life events and perceived stress, and greater systolic blood pressure reactivity than typical community volunteers. Results also indicated that perceived stress and stressful life events were significantly associated with social disability. Interventions targeting stress management might improve social function in adults with ASD.
Keywords: cortisol, cardiovascular reactivity, ASD, Asperger’s, high-functioning autism, stress, intervention
Introduction
Recent estimates indicate that as many as 50% of individuals with autism spectrum disorder (ASD) have IQ scores that are within the normal range (CDC 2014), yet much historical work on interventions and available services for people with ASD has been targeted to individuals with intellectual disabilities (e.g., Koegel et al. 1977; Lovaas et al. 1974; Strain and Odom 1986). There is also a growing awareness of the need for interventions for adults with ASD as the first large wave of individuals diagnosed with ASD as children are rapidly aging into adulthood (Gerhardt and Lainer 2011) when current evidence-based treatments and services largely come to an end (Shattuck et al. 2012). The emerging interventions for adults with ASD and without intellectual disability focus on promoting growth of social and nonsocial cognitive skills through secondary socialization methods (e.g., Eack et al. 2013; Smith et al. 2012a; Smith et al. 2014). Such interventions emphasize the development of cognitive control over various aspects of behavior that limit function in life roles. However, there is also substantial evidence that individuals with ASD have greater stress and distress (Hirvikoski and Blomqvist 2015; Bishop-Fitzpatrick et al. 2015; Baron et al. 2006) which might compromise their capacity to employ cognitive strategies taught in these interventions. As such, ASD may be characterized by both a need for greater cognitive control over behavior and difficulties employing specific cognitive control strategies as a result of greater stress.
Reports of stress in individuals with ASD have become common (Baron et al. 2006), as has the frequent comorbidity of anxiety disorder (Bejerot et al. 2014; Chalfant et al. 2007; Gillott and Standen 2007; Volkmar et al. 1999) and depression (Gotham et al. 2014; Ghaziuddin et al. 2002). Emerging literature on stress in children with ASD indicates that a maladaptive pattern of response to stress starts early and worsens over time (Lydon et al. 2014). In individuals with ASD, preliminary research suggests that hypothalamic-pituitary-adrenal (HPA) axis regulation (Nir et al. 1995; Richdale and Prior 1992), sympathetic-adrenal-medullary (SAM) axis regulation (Goodwin et al. 2006; Groden et al. 2005; Lydon et al. 2014), and emotion regulation (Mazefsky et al. 2013) may be inherently disturbed in ASD. Recent findings indicate that children with ASD experience heightened biological response patterns to stressors compared to typical community volunteers, although results vary across studies (for review, see: Lydon et al. 2014). Of note, studies that use naturalistic stressor tasks in children with ASD (e.g., conversation; Klusek et al. 2013) and studies that focus on purely adult samples with ASD (e.g., Maras and Bowler 2012; Maras et al. 2012; Mathersul et al. 2013) have found similar biological responses to stress in individuals with ASD and typical community volunteers.
Although the literature on biological response to stress in adults indicates a comparable response between those with ASD and controls, evidence from a small body of recent research indicates that adults with ASD consistently report greater perceived stress and stressful life events than do typical community volunteers. Findings from one recent study indicated that adults with ASD experienced greater perceived and interviewer-observed stress than did typical community volunteers (Bishop-Fitzpatrick et al. 2015). In another recent study, adults with ASD reported significantly higher perceived stress and poorer ability to cope effectively with stress in everyday life than did typical community volunteers (Hirvikoski and Blomqvist 2015). In addition, adults with ASD experience more stress and anxiety than adults with intellectual disabilities (Gillott and Standen 2007). This research also identified associations between higher levels of perceived stress and lower levels of social functioning (Bishop-Fitzpatrick et al. 2015), between higher levels of perceived stress and lower levels of quality of life (Hong et al. 2016), and between higher levels of perceived stress and greater levels of autism symptomatology (Maras et al. 2012). Taken together, this research on stress in ASD suggests that impaired stress mechanisms may be intrinsic deficits in ASD that have not been previously considered. Additional research is needed to understand how this relates to physiological arousal, and to connect variation in stress response patterns, perceived stress, and stressful life events to social disability and daily living skills in ASD, particularly concurrently in the same sample of adults with ASD.
The overall objective of the present study was to investigate whether poor response to stress further compromises social functioning in adults with ASD, since stress response constitutes a modifiable factor that can be readily remediated with focused treatment. This study hypothesized that adults with ASD would report greater perceived stress and more stressful life events than typical community volunteers. It also hypothesized that adults with ASD would experience greater cardiovascular and cortisol reactivity than typical community volunteers. Finally, this study hypothesized that greater perceived stress, stressful life events, and biological stress response would be associated with greater social disability and poorer daily living skills in adults with ASD.
Method
Power Analysis and Sample Size
Estimates of the sample size requirements for the proposed study were calculated using standard procedures (Cohen 1988), assuming that the criterion for statistical significance is set at alpha = .05 and for statistical power (1-beta) = .80. Sample size was set to detect a large effect size (d = .80). All power analyses were conducted a priori using with G*Power 3.1 (Faul et al. 2007). Based on this criteria, approximately 25 participants would be needed per group in order to detect a large effect (d = .80) for the difference in perceived stress and stress reactivity between adults with ASD and typical community volunteers. Based on a four predictor model using multiple regression and set at the aforementioned criteria, 40 participants would be required to detect a large effect size for the relationship between stress and adult outcomes (f2 = .35, R2 = .35).
Participants
Forty participants with ASD were recruited from an active, federally funded, comparative effectiveness clinical trial of two psychosocial treatments for adults with ASD. All participants with ASD enrolled in this trial were sent letters with information about the current study, and the first forty who responded to the letter and who met eligibility criteria were enrolled. Eligibility criteria included meeting expert clinical opinion and research criteria for ASD based on the Autism Diagnostic Observation Schedule (Lord et al. 2000) or Autism Diagnostic Interview-R (Lord et al. 1994), age 18–55, an IQ > 80 as assessed by the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler 2008), and no history of cardiovascular disease or diabetes.
An additional cohort of 25 typical community volunteers matched to the ASD group for age, biological sex, and race were recruited. These participants had no current psychiatric disability, as confirmed through the Structured Clinical Interview for the DSM-IV (First et al. 2002) in the weeks immediately proceeding this study and had no history of cardiovascular disease or other major medical disorders. These participants were recruited through a database of participants who had served as typical community volunteers in previous autism and schizophrenia studies.
This study was approved as a separate study from the larger intervention study by the [university name removed for blind review] Institutional Review Board, and all data and information collected were treated confidentially and complied with the policies and procedures governing research set forth by the University. All participants completed informed consent procedures prior to testing.
Stress Measures
The Social Stress Recall Task
Measures of biological stress response were collected before (salivary cortisol), during (heart rate, systolic blood pressure, and diastolic blood pressure), and after (salivary cortisol) the Social Stress Recall Task. The Social Stress Recall Task consisted of three segments: (1) a 10-minute rest condition where participants were asked to sit quietly with both feet on the floor; (2) a stressor condition in which subjects were asked to speak for five minutes using a prompt of “describe the three most challenging aspects of your life on a day-to-day basis”; and (3) a 5-minute recovery condition where participants were again asked to sit quietly with both feet on the floor. If, during the stressor condition, the participant was unable to recall items that were stressful, standardized probes were used to assist participants in recalling events. These included probes such as: “can you tell me more about how that experience made you feel?” and “tell me about the physical sensations you were aware of when you were experiencing stress.” Most individuals in both the ASD and typical community volunteer groups needed one or more prompts in order to discuss a stressful situation for five minutes, although these data were not systematically recorded.
The Social Stress Recall Task (Richman et al. 2007) is a social stress challenge task that has elicited reliable and statistically significant changes in biological stress response in caregivers of family members with Alzheimer’s disease (Williams et al. 2010), healthy adults experiencing psychosocial distress (Kirby et al. 2006), and adults who have experienced stress as a result of discrimination (Richman et al. 2007). The development of this task was based on research indicating that activities which elicit a strong emotional reaction to a stressor during recall of the event also elicit cardiovascular and neuroendocrine reactivity (Krantz and Manuck 1984). This task was chosen over the Trier Social Stress Test (TSST), which is the most commonly used social stress task in studies of individuals with ASD, because previous research has noted either an absence of response or a dampened response among individuals with ASD in response to the TSST, particularly among those individuals without co-occurring intellectual disabilities (Taylor and Corbett 2014). Because the Social Stress Recall Task involves discussing challenging aspects of day-to-day life rather than speaking publically in front of strangers, it thus provides a more ecologically valid assessment of social stress (Krantz and Manuck 1984), which may be particularly appropriate for individuals with ASD who may be unlikely to engage in public speaking on a regular basis (Taylor and Corbett 2014).
Cardiovascular reactivity
Systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate (HR) were measured at one-minute intervals using a portable Datascope Accutor Plus Monitor during the Social Stress Recall Task. The Datascope Accutor Plus Monitor has an interval mode, which enables the unit to take automatic non-invasive blood pressure measurements at specified time intervals (here, one-minute). The Datascope Accutor Plus Monitor is not able to assess cardiovascular reactivity continuously, thus precluding the use of measures of continuous heart rate variability. Participants were instructed to sit in a chair with their feet on the floor and to not move for the duration of the Social Stress Recall Task in order to control experimentally for motor movement. After selection of the appropriate cuff size, the blood pressure cuff was attached to the participant’s upper arm, consistent with recommended practices (Pickering et al. 2005). All participants complied fully with instructions and completed all SBP, DBP, and HR measures.
Data produced during the Social Stress Recall Task were 10 measures of SBP, DBP, and HR during the rest condition, five measures of SBP, DBP, and HR during the stressor condition, and five measures of SBP, DBP, and HR during the recovery condition. For the purpose of this research, the first five minutes of the rest condition was treated as a habituation condition where participants adjusted to the pressure of repeated measures with the blood pressure cuff before the rest condition was assessed. Data collected during the five-minute recovery condition after the Social Stress Recall Task were not analyzed in this research because they represent recovery from, not reactivity to, the stressor (Kirby et al. 2006; Williams et al. 2010). Thus, analysis of cardiovascular reactivity in this research includes a total of 5 measures of SBP, DBP, and HR during the rest condition and 5 measures of SBP, DBP, and HR during the stressor condition. Individual linear growth curve coefficients were calculated for SBP, DBP, and HR using hierarchical linear modeling to assess cardiovascular reactivity, which created cardiovascular reactivity scores for SBP, DBP, and HR that represent each participant’s individual linear growth coefficient (slope) for each cardiovascular reactivity measure. A high positive score (greater slope) on SBP reactivity, DBP reactivity, and HR reactivity measures represents greater cardiovascular reactivity to the Social Stress Recall Task.
Cortisol reactivity
Salivary “free” cortisol has been shown to track closely with plasma levels in both ambulatory and laboratory challenge paradigms, and collection of cortisol levels through saliva samples has been shown to be superior to blood sampling in psychoneuroendocrine research settings (Kirschbaum and Hellhammer 1994). For the purposes of this study, salivary cortisol was assayed using radioimmunoassay (RIA) techniques. Salivary cortisol samples were collected with commercially available Salivette tubes before the Social Stress Recall Task and at the end of testing after participants filled out the questionnaires described below. Participants were instructed to fully wet a cotton swab contained within the Salivette tube by chewing on the swab. Participants did not eat, drink, or smoke 30 minutes prior to collection of the sample to ensure the quality and potency of the sample, which was ensured by a 30-minute wait time between arrival at the clinic and collection of the first cortisol sample. Salivettes containing saliva samples were stored in a −20°C freezer based on established guidelines (Robins et al. 2009) until shipment to the assay laboratory (Dr. Clemens Kirschbaum’s laboratory at the Technical University of Dresden, Dresden, Germany). Assays were centrifuged by the assay laboratory after defrosting and assayed in batches of 65, balanced for ASD versus typical community volunteer group in order to avoid introduction of error due to assay batch variation. Cortisol levels were measured in micrograms/deciliter (µg/dl). In order to determine cortisol reactivity, the difference between cortisol levels collected before the Social Stress Recall Task and at the end of testing was calculated.
Stressful Life Events
The degree to which individuals perceive life events to be stressful was measured with the Stress Survey Schedule (SSS) for Persons with Autism and Other Developmental Disabilities (Groden et al. 2001), an instrument developed for measuring stress in the lives of people with ASD and other developmental disabilities. The SSS is a 48-item questionnaire, scored on a 5-point Likert scale where higher scores indicate more stressful life events. Questions include items such as: “having a change in environment from familiar to unfamiliar”; “being unable to assert oneself with others”; and “someone else making mistakes.” The SSS has been found to be ecologically valid and to track closely with the clinical presentation of response to specific stressors, indicating high construct validity (Goodwin et al. 2007). Cronbach’s alpha reliability ranges from .81 to .90, indicating high internal consistency (Groden et al. 2001). Total raw scores for all questions related to specific stressors (questions 1 through 49) were used, and Cronbach’s alpha in the current study was .96 for participants with ASD and .96 for typical community volunteers.
Perceived Stress
Perceived stress was measured with the Perceived Stress Scale (PSS; Cohen and Janicki-Deverts 2012; Cohen et al. 1983; Cohen and Williamson 1988) which consists of 10 items that are rated on a 5-point Likert scale where higher scores indicated greater perceived stress. Questions include items such as: “in the last month, how often have you been upset because of something that happened unexpectedly?”; “in the last month, how often have you felt confident about your ability to handle your personal problems?”; and “in the last month, how often have you felt that things were going your way?” Comparisons of the 10-item version with the 14-item version reveal that the shorter version is psychometrically superior. Cronbach’s alpha reliability ranges from .78 to .91 in numerous national surveys (Cohen and Janicki-Deverts 2012; Cohen and Williamson 1988). Cronbach’s alpha in the current study was .87 for participants with ASD and .89 for typical community volunteers.
Social Functioning Measures
Social Disability
Disability with regard to social adjustment was assessed using the Social Adjustment Scale-II (SAS-II; Schooler et al. 1979), a structured interview-based measure that assesses social disability, and relative level of social functioning, in the domains of work, household life, family life outside of the household, social leisure, and personal well-being. The SAS-II contains 45 items covering the aforementioned domains, and scores on individual items range from 0 to 4, with higher scores representing more social disability. The SAS-II has high internal consistency (α = .92 to .99) in psychiatric populations (Bellack et al. 1990; Davies et al. 1989; Glazer et al. 1980; Schooler et al. 1979). Cronbach’s alpha in the current study was .81 for participants with ASD.
Daily Living Skills
Independence in activities of daily living were assessed with the Waisman Activities of Daily Living Scale (W-ADL; Maenner et al. 2013; Smith et al. 2012b), a 17-item measure designed specifically for adults with developmental disabilities, including ASD, which assesses daily living skills. Higher total scores represent greater independence. Cronbach’s alpha for total scores range from .90 to .94 across multiple measurement occasions, indicating high internal consistency (Maenner et al. 2013; Smith et al. 2012b). Cronbach’s alpha in the current study was .81 for participants with ASD.
Demographic Measures
Age was recorded on the day of data collection. Full-scale IQ was determined using the Wechsler Abbreviated Scale of Intelligence (Wechsler 2008). Participants were asked to report their biological sex on the day of data collection. Because all individuals with ASD enrolled in this study were participants in a clinical intervention trial, length of treatment exposure was recorded to control for the potential influence of intervention on findings. Thus, treatment exposure was broken down by the intervention time point as 0 (baseline), 9 months, 18 months, and 30 months (18 months of treatment plus 12 months of follow-up) of treatment.
Procedure
When participants arrived at the clinic for data collection, they were greeted and brought back to the testing room. After the consent process, all participants provided the first of two saliva samples. Then, for both adults with ASD and typical community volunteers, a blood pressure cuff was attached to the participant’s arm, and the participant’s blood pressure and heart rate were then measured a total of 20 times for a total of 20 minutes (once per minute for 20 minutes), during which the Social Stress Recall Task was administered. Next, all participants filled out a series of questionnaires. Finally, participants supplied the second of the two saliva samples. At the end of the study visit, participants were debriefed about their experiences, and any questions were discussed in detail. The clinic visit took between 45 minutes and 2.5 hours, and typical community volunteers generally completed the questionnaire assessments more quickly than adults with ASD.
Data Analysis
Prior to investigating the primary hypotheses of this research, preliminary analyses were conducted to verify internal consistency among study measures, check assumptions associated with the statistical tests, inform the primary analyses about the potential effects of demographic heterogeneity, and ensure that the Social Stress Recall Task elicited statistically significant changes in biological stress response. Winsorization procedures were used to move outlier cases to within 2.0 times the interquartile range of the data distribution by setting the value of the outlier to the next closest value within 2.0 times the interquartile range (Dixon and Tukey 1968). Missing data were imputed using the expectation-maximization (EM) algorithm, which results in less biased parameter estimates than mean or regression imputation (Honaker et al. 2011; Schafer and Graham 2002). Descriptive statistics were reported for age, race, biological sex, IQ, employment, education, and independent living by group.
Analyses were conducted with R version 3.2.0 (R Core Team 2015), with packages nlme (Pinheiro et al. 2015), Amelia (Honaker et al. 2011), Hmisc (Harrell 2008), psych (Revelle 2014), car (Fox and Weisberg 2011), and lsmeans (Lenth 2013).
In order to assess for group differences in stress variables, SBP reactivity, DBP reactivity, HR reactivity, cortisol reactivity, stressful life events, and perceived stress were analyzed using ANOVA procedures, adjusting for salient demographic factors.
Separate hierarchical multiple regression models were created for all combinations of stress (i.e., SBP reactivity, DBP reactivity, HR reactivity, cortisol reactivity, perceived stress, stressful life events) and social functioning (i.e., social impairment, social disability, daily living skills) variables for the ASD sample only. Salient demographic variables were entered into the model in step one, and the stress variable was entered into the model in step two.
Results
Preliminary Analyses
Demographic characteristics of participants with ASD and typical community volunteers are presented in Table 1. Most participants were male, of European American descent, and in their mid-twenties. Of the participants with ASD, few had completed college or were employed, although they were, on average, of normal intelligence. Adults with ASD and typical community volunteers did not differ significantly with regard to sex, race, age, or IQ, suggesting that group matching was successful. As expected, adults with ASD and typical community volunteers did differ significantly in terms of employment, education, and independent living.
Table 1.
Participant Demographics
| M (SD) / % (N) |
||||
|---|---|---|---|---|
| ASD | Control | Combined | ||
| Variable | (N = 40) | (N = 25) | (N = 65) | pa |
| Age | 24.20 (6.95) range = 18–44 |
24.84 (3.69) range = 18–32 |
24.45 (5.89) range = 18–44 |
.673 |
| IQb | 106.53 (15.33) range = 80–132 |
110.60 (14.67) range = 82–138 |
108.09 (15.10) range = 80–138 |
.293 |
| Male | 90.00 (36) | 84.00 (21) | 87.69 (57) | .743 |
| European American | 82.50 (33) | 68.00 (17) | 76.92 (50) | .295 |
| Employedc | 47.50 (19) | 84.00 (21) | 61.54 (40) | .007 |
| College Graduate | 22.50 (9) | 60.00 (15) | 36.92 (24) | <.001 |
| Lives Independentlyd | 17.5 (7) | 88.00 (22) | 44.62 (29) | .005 |
Note. ASD = autism spectrum disorder, M = mean, SD = standard deviation, N = number, IQ = intelligence quotient
Fisher’s exact test or independent t-test, two-tailed, for significant differences between adults with ASD or typical community volunteers.
Based on the full Wechsler Adult Intelligence Scale-Revised
Based on any paid employment
Lives either alone or with non-relatives
p < .05,
p < .01,
p < .001
Analyses were conducted to ensure that measures met assumptions for parametric testing. Age was positively skewed (skewness statistic = 1.31), and was thus transformed using winsorization procedures for two data points (both individuals with ASD) at the top of the distribution. Missing data were imputed using the EM algorithm for pre-test cortisol (1.5% missing) and SAS-II (12.5% missing). Results of tests of the between-group demographic differences revealed no confounding covariates. However, a significant relationship between age and daily living skills (W-ADL) and a trend-level relationship between cortisol reactivity and perceived stress (PSS) and treatment exposure were found. Thus, treatment exposure and age were included in step one in regression analyses. Biological sex was also included as a covariate in regression analyses.
Validation of Stress Task Effects
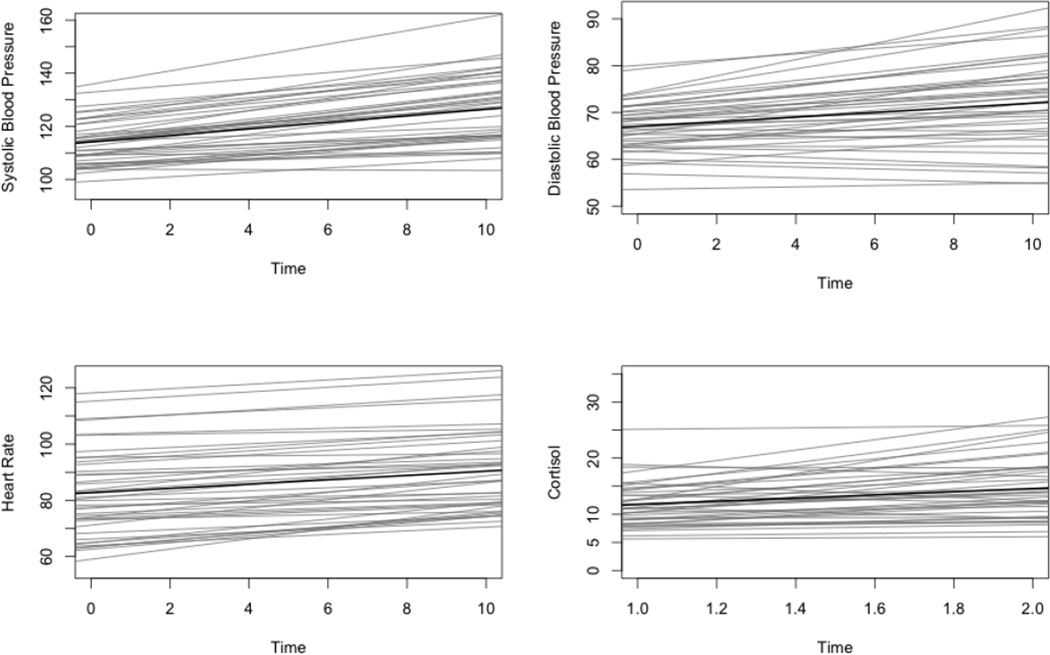
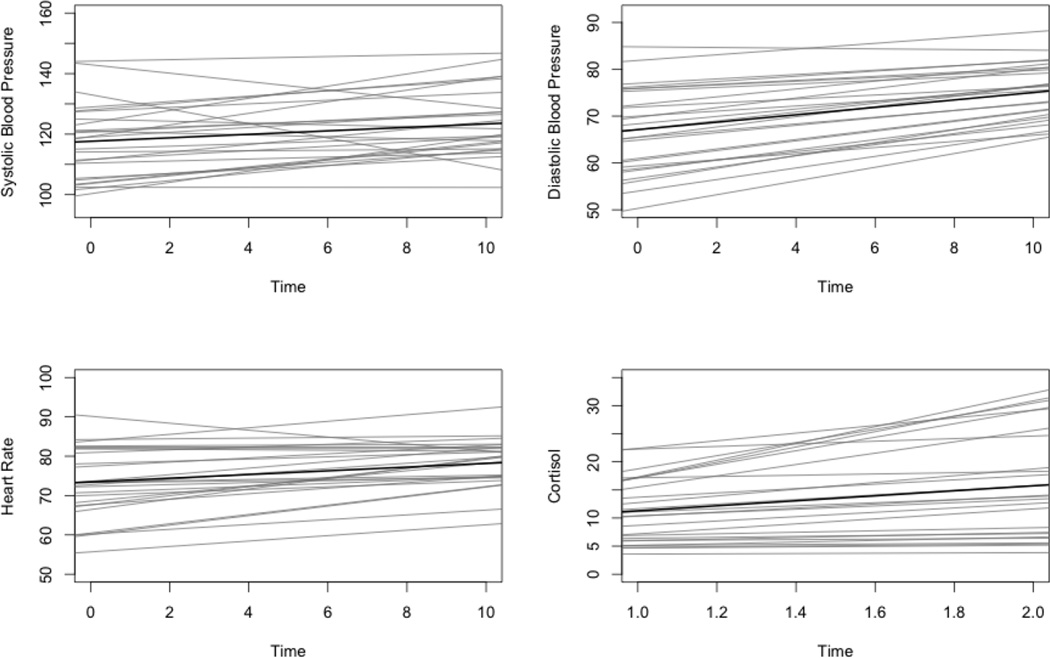
The effects of the social stress challenge task were examined in order to ensure that the task contributed to statistically significant changes in cardiovascular and cortisol reactivity measures over time, first for the entire sample and then separately for adults with ASD and for typical community volunteers. Results of individual growth curve models revealed statistically significant, positive linear growth from the rest to stressor condition for SBP (β10 = .982, t = 6.76, p < .001), DBP (β10 = .612, t = 4.76, p < .001), and HR (β10 = .654, t = 6.86, p < .001) across all participants, confirming that this stress task produced increased cardiovascular response over time. Results were also significant when run separately for adults with ASD and typical community volunteers for SBP (ASD: β10 = 1.24, t = 8.24, p < .001; control: β10 = .576, t = 2.08, p = .03), DBP (ASD: β10 = .50, t = 3.13, p = .002; control: β10 = .793, t = 3.69, p < .001), and HR (ASD: β10 = .77, t = 7.22, p < .001; control: β10 = .472, t = 2.66, p = .008).
A paired sample t-test was used to confirm differences between pre-test and post-test cortisol levels. This analysis revealed a statistically significant increase, t(64) = 3.296, p < .001, in cortisol levels from pre-test to post-test, confirming that the stress task produced increased cortisol over time. Similar to results of analyses for cardiovascular reactivity measures, results also revealed a statistically significant increase in cortisol when conducted separately for adults with ASD, t(39) = 2.21, p = 0.03, and typical community volunteers, t(24) = 2.46, p = 0.02. Task effects are displayed graphically in Figure 1 for adults with ASD and in Figure 2 for typical community volunteers. These trajectories based on individual linear growth coefficients and cortisol reactivity scores were then used in subsequent analyses to represent SBP reactivity, DBP reactivity, HR reactivity, and cortisol reactivity.
Figure 1.
Task Effects for Adults with Autism Spectrum Disorder (N = 40)
Figure 2.
Task Effects for Typical Community Volunteers
Group Differences on Stress Measures
Descriptive statistics for SBP, DBP, HR, and cortisol are displayed in Table 2. Descriptively, adults with ASD and typical community volunteers had statistically similar mean SBP, DBP, and cortisol during both the stressor and rest conditions. Adults with ASD did have significantly higher HR during both the stressor and rest conditions than typical community volunteers. This means that adults with ASD had HR that was, on average, 10.04 beats per minute higher during the rest condition and 11.10 points higher during the stress condition than typical community volunteers.
Table 2.
Descriptive Statistics for Biological Stress Response Variables
| Variable | ASD | Control | p | ||
|---|---|---|---|---|---|
| Mean | S.D. | Mean | S.D. | ||
| SBP Rest | 115.45 | 9.75 | 117.92 | 12.46 | .376 |
| SBP Stressor | 123.87 | 12.33 | 122.53 | 11.40 | .663 |
| DBP Rest | 67.63 | 7.34 | 68.34 | 9.37 | .734 |
| DBP Stressor | 70.93 | 9.12 | 73.04 | 9.07 | .364 |
| HR Rest | 83.66 | 15.36 | 73.62 | 8.69 | .004 |
| HR Stressor | 88.65 | 15.03 | 77.55 | 7.84 | .001 |
| Cortisol Pre-Test | 11.76 | 6.75 | 11.29 | 8.19 | .801 |
| Cortisol Post-Test | 14.59 | 7.87 | 15.74 | 11.34 | .632 |
Note. ASD = autism spectrum disorder; S.D. = standard deviation; SBP = systolic blood pressure; DBP = diastolic blood pressure; HR = heart rate.
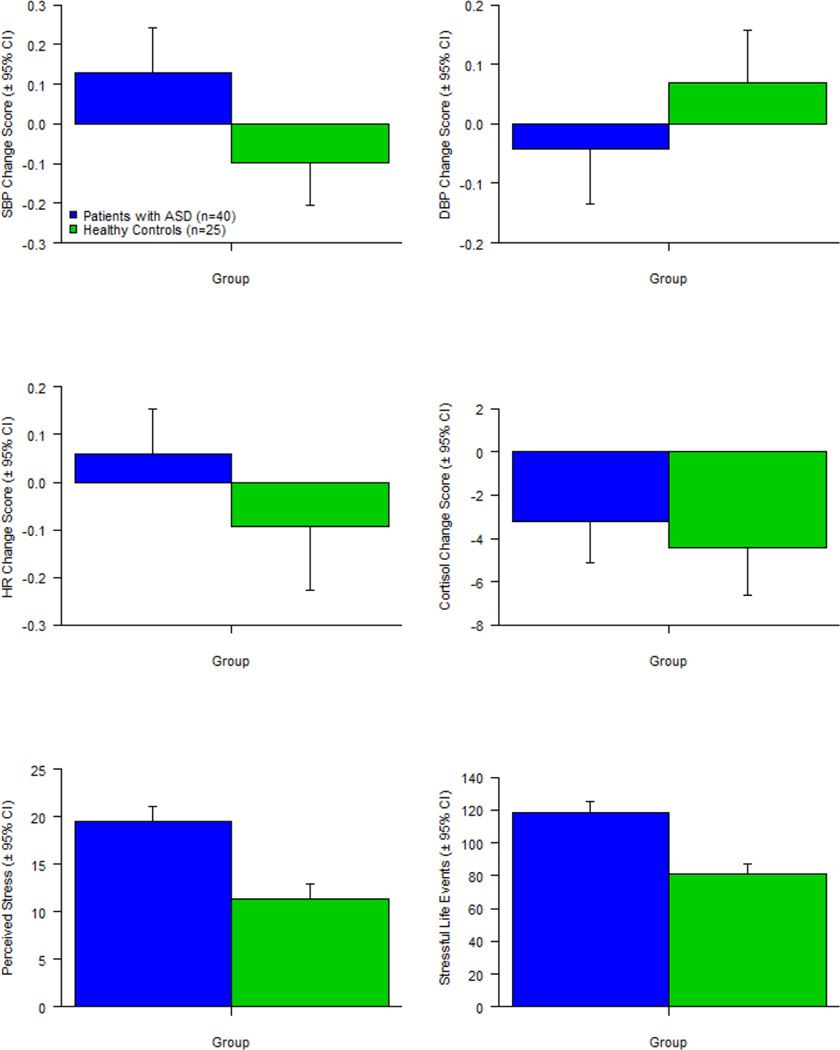
Using ANOVA procedures, data were queried to determine if adults with ASD and typical community volunteers differed significantly in terms of SBP reactivity, DBP reactivity, HR reactivity, cortisol reactivity, perceived stress, and stressful life events. As noted in Table 3, adults with ASD had significantly greater SBP reactivity than typical community volunteers, F(1, 63) = 4.95, p = .030, but did not differ from typical community volunteers on DBP reactivity, F(1, 63) = 1.46, p = .232, HR reactivity, F(1, 63) = 1.72, p = .194, or cortisol reactivity, F(1, 63) = .34, p = .564, scores. Participants with ASD also reported significantly greater perceived stress, F(1, 63) = 23.13, p < .001, and more stressful life events, F(1, 63) = 25.90, p < .001, than the typical community volunteers. Mean scores, and their corresponding standard deviations, as well as Cohen’s d effect size of the magnitude of between-group differences, are presented in Figure 3.
Table 3.
One-Way Analysis of Variance for Stress Variables by Group
| Source | df | SS | MS | F | p | Cohen’s da |
|---|---|---|---|---|---|---|
| Variable: SBP reactivity; ASD: M = 0.21, SD = 0.84; Control: M = −0.34, SD = 1.16 | ||||||
| Between Groups | 1 | 2.96 | 2.96 | 4.95 | 0.030* | 0.54 |
| Total | 63 | 37.65 | 0.60 | |||
| Variable: DBP reactivity; ASD: M = −0.12, SD = 1.01; Control: M = 0.19, SD = 0.97 | ||||||
| Between Groups | 1 | 0.20 | 0.20 | 1.46 | 0.232 | 0.31 |
| Total | 63 | 8.49 | 0.13 | |||
| Variable: HR reactivity; ASD: M = 0.13, SD = 0.88; Control: M = −0.20, SD = 1.16 | ||||||
| Between Groups | 1 | 0.35 | 0.35 | 1.72 | 0.194 | 0.32 |
| Total | 63 | 12.70 | 0.20 | |||
| Variable: Cortisol reactivity; ASD: M = 0.07, SD = 0.96; Control: M = −0.12, SD = 1.07 | ||||||
| Between Groups | 1 | 24.00 | 23.53 | 0.34 | 0.564 | 0.19 |
| Total | 63 | 4414.00 | 70.07 | |||
| Variable: PSS; ASD: M = 19.45, SD = 6.68; Control: M = 11.40, SD = 6.37 | ||||||
| Between Groups | 1 | 997.00 | 997.00 | 23.13 | < 0.001*** | 1.23 |
| Total | 63 | 2716.00 | 43.1 | |||
| Variable: SSS; ASD: M = 118.00, SD = 31.14; Control: M = 81.28, SD = 22.95 | ||||||
| Between Groups | 1 | 20744.00 | 20744.00 | 25.90 | < 0.001*** | 1.34 |
| Total | 63 | 50453.00 | 801.00 | |||
Note. df = degrees of freedom, SS = sum of squares, MS = mean square, SBP = systolic blood pressure, DBP = diastolic blood pressure, HR = heart rate, PSS = Perceived Stress Scale, SSS = Stress Survey Schedule, M = mean, SD = standard deviation.
p < .05,
p < .01,
p < .001
Cohen’s d is presented here for all stress variables but should only be interpreted for SBP reactivity, PSS, and SSS.
Figure 3.
Group Differences on Stress Measures
The Relationship between Stress and Social Functioning in ASD
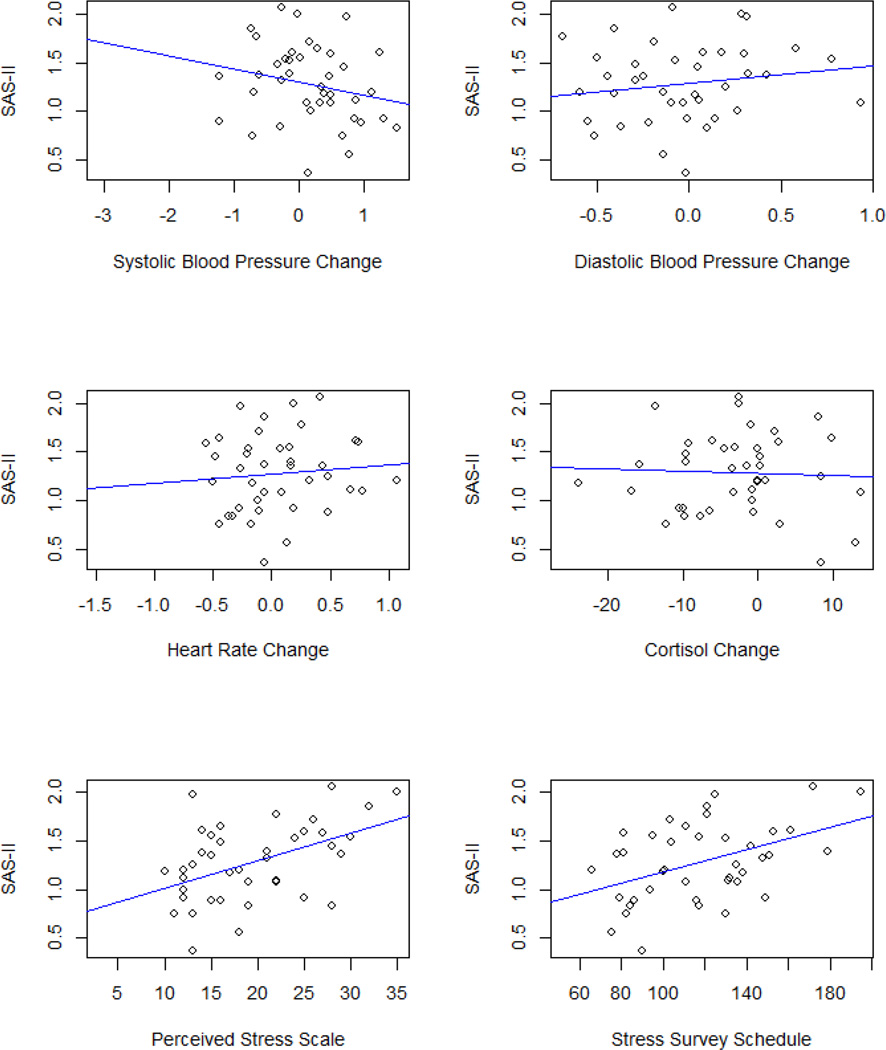
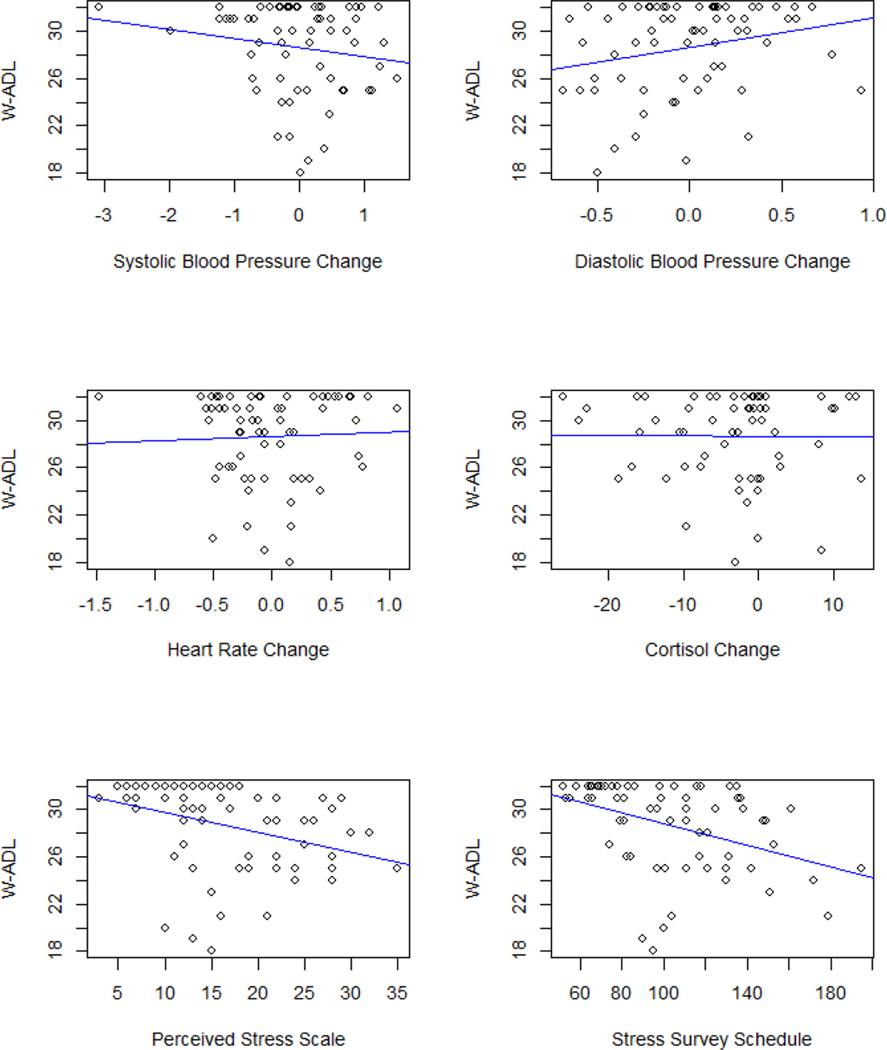
Results of hierarchical regression analyses revealed significant, positive associations between perceived stress and social disability, B = 0.073, t(35) = 3.155, p = 0.003, R2 Change = 0.217, and between stressful life events and social disability, B = 0.016, t(35) = 3.199, p = 0.003, R2 Change = 0.221, in adults with ASD, when controlling for age, biological sex, and treatment exposure. There were no other significant associations between stress and social functioning variables in adults with ASD (see Table 4, and Figures 4 and 5).
Table 4.
Relationship between Stress Variables and Social Functioning Variables
| Variable/Step | B | SE | β | t | p |
|---|---|---|---|---|---|
| SAS-II | |||||
| Step 1a | |||||
| Age | 0.012 | 0.024 | 0.080 | 0.475 | 0.638 |
| Sex | 0.293 | 0.544 | 0.089 | 0.540 | 0.593 |
| Treatment Exposure | 0.004 | 0.014 | 0.053 | 0.318 | 0.753 |
| Step 2: SBP Change (ΔR2 = 0.045) | |||||
| SBP Change | −0.321 | 0.247 | −0.214 | −1.302 | 0.201 |
| Step 2: DBP Change (ΔR2 = 0.043) | |||||
| DBP Change | 0.584 | 0.458 | 0.218 | 1.275 | 0.211 |
| Step 2: HR Change (ΔR2 = 0.009) | |||||
| HR Change | 0.243 | 0.421 | 0.096 | 0.577 | 0.568 |
| Step 2: Cortisol Change (ΔR2 = 0.002) | |||||
| Cortisol Change | −0.006 | 0.022 | −0.050 | −0.282 | 0.780 |
| Step 2: PSS (ΔR2 = 0.217**) | |||||
| PSS | 0.073 | 0.023 | 0.486 | 3.155 | 0.003** |
| Step 2: SSS (ΔR2 = 0.221**) | |||||
| SSS | 0.016 | 0.005 | 0.491 | 3.199 | 0.003** |
| W-ADL | |||||
| Step 1a | |||||
| Age | 0.015 | 0.023 | 0.101 | 0.640 | 0.507 |
| Sex | 1.015 | 0.510 | 0.336 | 2.169 | 0.037* |
| Treatment Exposure | 0.007 | 0.013 | 0.081 | 0.514 | 0.611 |
| Step 2: SBP Change (ΔR2 = 0.005) | |||||
| SBP Change | 0.105 | 0.238 | 0.070 | 0.441 | 0.662 |
| Step 2: DBP Change (ΔR2 = 0.079†) | |||||
| DBP Change | 0.786 | 0.419 | 0.293 | 1.877 | 0.069† |
| Step 2: HR Change (ΔR2 = 0.040) | |||||
| HR Change | 0.505 | 0.387 | 0.201 | 1.304 | 0.201 |
| Step 2: Cortisol Change (ΔR2 = 0.005) | |||||
| Cortisol Change | −0.001 | 0.021 | −0.010 | −0.057 | 0.955 |
| Step 2: PSS (ΔR2 = 0.002) | |||||
| PSS | −0.007 | 0.024 | −0.043 | −0.265 | 0.793 |
| Step 2: SSS (ΔR2 = 0.017) | |||||
| SSS | −0.004 | 0.005 | −0.135 | −0.836 | 0.409 |
Note. SBP = systolic blood pressure, DBP = diastolic blood pressure, HR = heart rate, PSS = Perceived Stress Scale, SSS = Stress Survey Schedule;
p < .10,
p < .05,
p < .01,
p < .001
Values for Step 1 remain consistent across analyses for each independent variable
Figure 4.
The Association between Stress Measures and Social Disability (SAS-II)
Figure 5.
The Association between Stress Measures and Daily Living Skills (W-ADL)
Post-Hoc Analyses
All regression analyses with significant findings were re-run for a sub-sample of adults with ASD (n = 34) to control for short- and long-term working memory using the Weschler Memory Scale (Wechsler 1945) logical memory I and II indices, which assess short-term (logical I) and long-term (logical II) working memory, in order to account for individual differences that may influence one’s ability to understand conversation and identify, experience, and report stress irrespective of autonomic response. Data on short- and long-term working memory were not available for typical community volunteers. Findings of these analyses revealed that, indeed, both better short-term, B = −0.041, t(30) = −2.133, p = 0.041, and better long-term, B = −0.054, t(30) = −2.784, p = 0.009, working memory are significantly associated with lesser social disability in adults with ASD. However, findings from our main analyses that both higher perceived stress (short-term: B = 0.060, t(29) = 2.320, p = 0.028, R2 Change = 0.126; long-term: B = 0.061, t(29) = 2.484, p = 0.019, R2 Change = 0.130) and more stressful life events (short-term: B = 0.012, t(29) = 2.122, p = 0.043, R2 Change = 0.108; long-term: B = 0.010, t(29) = 1.859, p = 0.073, R2 Change = 0.079) are associated with greater social disability in adults with ASD remain when controlling for short-term or long-term working memory, even though processing speed issues related to short- and long-term working memory were significantly associated with social disability in the adults with ASD in our sample.
Discussion
This research sought to examine the role of stress in social functioning in adults with ASD. It first explored group differences in survey measures of stressful life events and perceived stress and measures of change in biological stress response before and during a social stress challenge task in adults with ASD compared to typical community volunteers. It then examined the relationship between the aforementioned stress measures and measures of social functioning (social disability and daily living skills) in adults with ASD.
As expected, findings from survey measures revealed that adults with ASD reported significantly greater perceived stress and more stressful life events than did typical community volunteers. The effect sizes for perceived stress and stressful life events were large (Cohen 1988), indicating that stress is a clinically significant factor that leads to significant distress in adults with ASD and thus warrants intervention. This finding is not surprising, but it has not received much explicit research attention in previous studies. Stress is often overlooked in the assessment of individuals with ASD, and it likely has been referred to by other labels or contributed to other concepts (e.g., quality of life, mood symptoms, emotion regulation, etc.) that have greater foundations within the ASD literature.
The findings of this study also indicate that adults with ASD experienced greater SBP reactivity than typical community volunteers but similar DBP reactivity, HR reactivity, and cortisol reactivity as typical community volunteers in response to stress induced by recall of a stressful event, an indication of an acute response to stress. An examination of the scatter cloud of DBP, HR, and cortisol reactivity confirmed that both the magnitude and distribution of reactivity patterns between adults with ASD and typical community volunteers were remarkably similar, even though significant group differences in SBP reactivity were identified. Notably, these finding emerge despite clear task effects that demonstrate that the Social Stress Recall Task elicits statistically significant increases in SBP, DBP, HR, and cortisol both among adults with ASD and typical community volunteers. The greater increase in SBP reactivity is consistent with the increased perception of stress given high correlations between systolic blood pressure and perceived stress identified in extant literature (Manuck 1994; Cohen et al. 2000). The remainder of the findings suggests that some aspects of biological stress response of adults with ASD is comparable to typical adult community volunteers (e.g., Maras & Bowler, 2012; Maras et al., 2012; Mathersul et al., 2013). However, of note, descriptive data adds to the body of research that indicates that individuals with ASD have resting heart rates that are significantly higher than typical community volunteers.
The findings of the current research with respect to differences in self-reported stress and biological response to recall of stress indicate that adults with ASD who do not have co-occurring intellectual disabilities experience a great deal of perceived stress and stressful life events despite exhibiting similar patterns of biological stress response to typical community volunteers. Thus, the emotional and psychological experience of stress is substantially greater in higher-functioning adults with ASD than in typical community volunteers, even though patterns of biological response to stress are similar.
The second major finding of this research was that more perceived stress and more stressful life events were associated with greater social disability in adults with ASD, and this association persisted after controlling for treatment exposure, age, biological sex, and short- and long-term working memory. This suggests that the experience of distress itself contributes to social disability. Alternatively, it is plausible that because of their social impairments, adults with ASD are less capable of coping with life events, making life more stressful. However, this study strongly suggests that adults with ASD perceive day-to-day life as distressing and stressful, and this perception is associated with greater social disability. This explanation is informed by emerging evidence that indicates that an individual with ASD’s perception of the extent to which he is socially impaired, but not the actual extent of their social impairment, predicts depressive symptomatology in adults with ASD (Gotham et al. 2014). In other words, much in the same way that perception drives the relationship between stress and social disability, it may be a primary contributing factor in the emergence of depressive symptoms in individuals with ASD.
This study has a number of limitations, the first of which is the modest sample size. The specific aims of this research were geared towards identifying a modifiable predictor of social functioning in adults with ASD that can be addressed with targeted treatment in future research. Thus, power estimates were set to detect large effects and not small or small to medium effects. Given the heterogeneity of ASD, it is possible that a larger sample would reveal subgroups of individuals that are lower or higher than the present sample in terms of perceived stress, stressful life events, and biological stress response. Second, these findings are based on the study of adults with ASD without co-occurring intellectual disabilities who were capable of participating in a group-based longitudinal intervention. The findings could be even more relevant to those who were unable to participate in the intervention trial due to challenging behavior but who were not part of the available sample. Third, data related to continuous heart rate variability and number of prompts used for each individual during the Social Stress Recall Task were not collected. Finally, the use of a novel stress task in ASD may be a limitation. Because the task is novel, previous evidence supporting its use in ASD is limited. However, the presence of robust task-related effects in the expected direction in the whole sample, as well as both typical community volunteer and ASD groups, suggest it provides a valid experimental manipulation of stress. Because of these limitations, the results of this study should be interpreted as observational and descriptive but not mechanistic or causal.
Of note, there were large differences between adults with ASD and typical community volunteers in regard to employment, education, and independent living in that a significantly larger proportion of typical community volunteers than adults with ASD were employed, had graduated from college, and were living independently. While these findings were expected based on previous research on adult outcome in ASD (e.g., Howlin et al. 2004; Seltzer et al. 2004), this lack of community participation and integration in the ASD sample may contribute to higher stress levels. Thus, adults with ASD may not have benefitted from factors, such as social integration and social support garnered through work or school settings (Koegel et al 2013), that might mitigate the impact of stress on functioning. However, the stress buffering effects of social support have not yet been studied in adults with ASD, which is a prime opportunity for future research. Nonetheless, group differences in employment, education, and independent living signal the continued need for support for employment, vocational training, post-secondary education, and independent living even for adults with ASD without co-occurring intellectual disabilities.
This study, as well as previous preliminary work, suggests that adults with ASD experience more perceived stress than typical community volunteers. The body of research implies that heightened perceived stress is a problem in its own right in ASD (Bishop-Fitzpatrick et al. 2015; Hirvikoski and Blomqvist 2015) and may further compromise social functioning.
The importance of collecting behavioral data that help researchers to understand levels of normative outcome (i.e., employment, independent living, social engagement) and quality of life (for reference, see Bishop-Fitzpatrick et al. 2016) in order to more holistically characterize outcomes and functioning is highlighted, and data on normative outcomes and quality of life could be compared to stress levels to assess whether stress causes behavioral problems in ASD, or vice versa. However, although the hypotheses of this research framed higher stress as a predictor of poorer social functioning in ASD, the design of this study precluded a test of bidirectional effects or the inclusion of other potential correlates of social functioning in adults with ASD. However, we know that other factors, including processing speed, language comprehension, and executive function, may serve as mediators or moderators of the relationship between stress and social functioning, and this is an important area for future work.
The development and testing of psychosocial interventions in adults with ASD (Bishop-Fitzpatrick et al. 2013; Gerhardt and Lainer 2011) has been limited by a lack of modifiable predictors of social functioning identified by extant research. Notably, most interventions focus on cognitive strategies for managing behavior, and stress interferes with the capacity to consider and apply such strategies when under distress. Such interventions may be more effective if stress is managed. Perceived stress has been shown to be modifiable with targeted stress management interventions in individuals not affected by ASD (Kirby et al. 2006; Williams et al. 2010). In addition, research has shown that targeting social skills in individuals with ASD can result in benefits to untreated comorbidities such as depression and anxiety (e.g., Gantman et al. 2012; Koegel el al. in press; Laugeson et al. 2012). There is thus sufficient evidence to add an intervention specifically focused on stress management to broader cognitive behavioral interventions. Interventions that teach generalizable skills to help people with ASD better handle stress have the potential to create more durable change because their effects can be applied to a wide and varied set of situations and not simply a prescribed set of rehearsed situations. Systematic interventions to improve stress management through mindfulness (e.g., Spek et al. 2013; Singh et al. 2011) or cognitive behavioral therapy (e.g., Sze and Wood 2007; Wood et al. 2009; Wood et al 2011) might hold considerable utility for stress reduction, and in turn, improvement in social functioning in adults with ASD.
Acknowledgments
This study was supported by grants from the NIH (MH-85851, MH-95783, RR-24154, HD-55748, P30HD003352, T32HD007489); Autism Speaks, (5703 and 8568); Department of Defense (AR100344); and Pennsylvania Department of Health. We are extremely grateful to the individuals with autism spectrum disorder who participated in this study; without their generous support and commitment, our research would not be possible.
Footnotes
Dr. Bishop-Fitzpatrick, Dr. Minshew, Dr. Mazefsky, and Dr. Eack declare that they have no conflict of interest.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- Baron-Cohen S, Ring HA, Bullmore ET, Wheelwright S, Ashwin C, Williams S. The amygdala theory of autism. Neuroscience & Biobehavioral Reviews. 2000;24(3):355–364. doi: 10.1016/s0149-7634(00)00011-7. [DOI] [PubMed] [Google Scholar]
- Baron MG, Groden J, Groden G, Lipsitt LP. Stress and coping in autism. New York: Oxford University Press; 2006. [Google Scholar]
- Bejerot S, Eriksson JM, Mörtberg E. Social anxiety in adult autism spectrum disorder. Psychiatry research. 2014;220(1):705–707. doi: 10.1016/j.psychres.2014.08.030. [DOI] [PubMed] [Google Scholar]
- Bellack AS, Morrison RL, Mueser KT, Wade JH, Sayers SL. Role play for assessing the social competence of psychiatric patients. Psychological Assessment: A Journal of Consulting and Clinical Psychology. 1990;2(3):248. [Google Scholar]
- Bishop-Fitzpatrick L, Hong J, Smith LE, Makuch R, Greenberg JS, Mailick MR. Characterizing objective quality of life and normative outcomes in adults with autism spectrum disorder: An exploratory latent class analysis. Journal of Autism & Developmental Disorders. 2016;46(8):2707–2719. doi: 10.1007/s10803-016-2816-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop-Fitzpatrick L, Mazefsky C, Minshew NJ, Eack SM. The relationship between stress and social functioning in adults with autism spectrum disorder and without intellectual disability. Autism Research. 2015;8(2):164–173. doi: 10.1002/aur.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop-Fitzpatrick L, Minshew NJ, Eack SM. A systematic review of psychosocial interventions for adults with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2013;43(3):687–694. doi: 10.1007/s10803-012-1615-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Prevalence of autism spectrum disorder among children aged 8 years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2010. Surveillance Summaries, MMWR. 2012;63(SS02):1–21. [PubMed] [Google Scholar]
- Chalfant AM, Rapee R, Carroll L. Treating anxiety disorders in children with high functioning autism spectrum disorders: A controlled trial. Journal of Autism and Developmental Disorders. 2007;37(10):1842–1857. doi: 10.1007/s10803-006-0318-4. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciencies. 2nd. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- Cohen S, Hamrick NM, Rodriguez MS, Feldman PJ, Rabin BS, Manuck SB. The stability of and intercorrelations among cardiovascular, immune, endocrine, and psychological reactivity. Annals of Behavioral Medicine. 2000;22(3):171–179. doi: 10.1007/BF02895111. [DOI] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D. Who's Stressed? Distributions of Psychological Stress in the United States in Probability Samples from 1983, 2006, and 20091. Journal of Applied Social Psychology. 2012;42(6):1320–1334. [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24(4):385–396. [PubMed] [Google Scholar]
- Cohen S, Williamson GM. Perceived stress in a probability sample of the United States. In: S S, O S, editors. The Social Psychology of Health. Newbury Park: Sage Publications; 1988. pp. 31–67. [Google Scholar]
- Davies MA, Bromet EJ, Schulz SC, Dunn LO. Community adjustment of chronic schizophrenic patients in urban and rural settings. Hospital & Community Psychiatry. 1989 doi: 10.1176/ps.40.8.824. [DOI] [PubMed] [Google Scholar]
- Dixon WJ, Tukey JW. Approximate behavior of the distribution of Winsorized t (Trimming/Winsorization 2) Technometrics. 1968;10(1):83–98. [Google Scholar]
- Eack SM, Greenwald DP, Hogarty SS, Bahorik AL, Litschge MY, Mazefsky CA, et al. Cognitive Enhancement Therapy for adults with autism spectrum disorder: Results of an 18-month feasibility study. Journal of Autism & Developmental Disorders. 2013;43(12):2866–2877. doi: 10.1007/s10803-013-1834-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior research methods. 2007;39(2):175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for the DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Fox J, Weisberg S. An {R} Companion to Applied Regression. Second. Thousand Oaks, CA: Sage; 2011. URL: http://socserv.socsci.mcmaster.ca/jfox/Books/Companion. [Google Scholar]
- Gantman A, Kapp SK, Orenski K, Laugeson EA. Social Skills Training for Young Adults with High-Functioning Autism Spectrum Disorders: A Randomized Controlled Pilot Study. Journal of Autism and Developmental Disorders. 2012;42(6):1094–1103. doi: 10.1007/s10803-011-1350-6. [DOI] [PubMed] [Google Scholar]
- Gerhardt P, Lainer I. Addressing the needs of adolescents and adults with autism: A crisis on the horizon. Journal of Contemporary Psychotherapy. 2011;41(1):37–45. [Google Scholar]
- Gillott A, Standen PJ. Levels of anxiety and sources of stress in adults with autism. Journal of Intellectual Disabilities. 2007;11(4):359–370. doi: 10.1177/1744629507083585. [DOI] [PubMed] [Google Scholar]
- Glazer WM, Aaronson HS, Prusoff BA, Williams DH. Assessment of social adjustment in chronic ambulatory schizophrenics. The Journal of Nervous and Mental Disease. 1980;168(8):493–497. doi: 10.1097/00005053-198008000-00008. [DOI] [PubMed] [Google Scholar]
- Goodwin MS, Groden J, Velicer WF, Diller A. Brief report: Validating the Stress Survey Schedule for persons with autism and other developmental disabilities. Focus on Autism and Other Developmental Disabilities. 2007;22(3):183–189. [Google Scholar]
- Goodwin MS, Groden J, Velicer WF, Lipsitt LP, Baron MG, Hofmann SG, et al. Cardiovascular arousal in individuals with autism. Focus on Autism and Other Developmental Disabilities. 2006;21(2):100–123. [Google Scholar]
- Gotham K, Bishop SL, Brunwasser S, Lord C. Rumination and Perceived Impairment Associated With Depressive Symptoms in a Verbal Adolescent-Adult ASD Sample. Autism Research. 2014;7(3):381–391. doi: 10.1002/aur.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groden J, Diller A, Bausman M, Velicer W, Norman G, Cautela J. The development of a stress survey schedule for persons with autism and other developmental disabilities. Journal of Autism and Developmental Disorders. 2001;31(2):207–217. doi: 10.1023/a:1010755300436. [DOI] [PubMed] [Google Scholar]
- Groden J, Goodwin MS, Baron MG, Groden G, Velicer WF, Lipsitt LP, et al. Assessing cardiovascular responses to stressors in individuals with autism spectrum disorders. Focus on Autism and Other Developmental Disabilities. 2005;20(4):244–252. [Google Scholar]
- Harrell FE. Hmisc: Harrell miscellaneous. R package version 3.16–10. 2008 http://CRAN.R-project.org/package=Hmisc.
- Hirvikoski T, Blomqvist M. High self-perceived stress and poor coping in intellectually able adults with autism spectrum disorder. Autism. 2015;19(6):752–757. doi: 10.1177/1362361314543530. [DOI] [PubMed] [Google Scholar]
- Honaker J, King G, Blackwell M. Amelia II: A program for missing data. Journal of Statistical Software. 2011;45(7):1–47. [Google Scholar]
- Hong J, Bishop-Fitzpatrick L, Smith LE, Greenberg JS, Mailick MR. Factors associated with subjective quality of life of adults with autism spectrum disorder: Self-report versus maternal reports. Journal of Autism and Developmental Disorders. 2016;46(4):1368–1378. doi: 10.1007/s10803-015-2678-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlin P, Goode S, Hutton J, Rutter M. Adult outcome for children with autism. Journal of Child Psychology and Psychiatry. 2004;45(2):212–229. doi: 10.1111/j.1469-7610.2004.00215.x. [DOI] [PubMed] [Google Scholar]
- Kirby ED, Williams VP, Hocking MC, Lane JD, Williams RB. Psychosocial benefits of three formats of a standardized behavioral stress management program. Psychosomatic medicine. 2006;68(6):816–823. doi: 10.1097/01.psy.0000238452.81926.d3. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DH. Salivary cortisol in psychoneuroendocrine research: Recent developments and applications. Psychoneuroendocrinology. 1994;19(4):313–333. doi: 10.1016/0306-4530(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Klusek J, Martin GE, Losh M. Physiological arousal in autism and fragile X syndrome: Group comparisons and links with pragmatic language. American Journal on Intellectual and Developmental Disabilities. 2013;118(6):475–495. doi: 10.1352/1944.7558-118.6.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koegel LK, Ashbaugh K, Koegel RL, Detar WJ, Regester A. Increasing socialization in adults with Asperger's syndrome. Psychology in the Schools. 2013;50(9):899–909. [Google Scholar]
- Koegel LK, Navab A, Ashbaugh K, Koegel RL. Using Reframing to Reduce Negative Statements in Social Conversation for Adults With Autism Spectrum Disorder. Journal of Positive Behavior Interventions. in press. [Google Scholar]
- Koegel RL, Russo DC, Rincover A. Assessing and training teachers in the generalized use of behavior modification with autistic children. Journal of Applied Behavior Analysis. 1977;10(2):197–205. doi: 10.1901/jaba.1977.10-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krantz DS, Manuck SB. Acute psychophysiologic reactivity and risk of cardiovascular disease: A review and methodologic critique. Psychological Bulletin. 1984;96(3):435–464. [PubMed] [Google Scholar]
- Laugeson EA, Frankel F, Gantman A, Dillon AR, Mogil C. Evidence-Based Social Skills Training for Adolescents with Autism Spectrum Disorders: The UCLA PEERS Program. Journal of Autism and Developmental Disorders. 2012;42(6):1025–1036. doi: 10.1007/s10803-011-1339-1. [DOI] [PubMed] [Google Scholar]
- Lenth RV. lsmeans: Least-squares means. R package version 1.06–05. 2013 [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, et al. The Autism Diagnostic Observation Schedule-Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Lecouteur A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lovaas OI, Schreibman L, Koegel RL. A behavior modification approach to the treatment of autistic children. Journal of Autism and Childhood Schizophrenia. 1974;4(2):111–129. doi: 10.1007/BF02105365. [DOI] [PubMed] [Google Scholar]
- Lydon S, Healy O, Reed P, Mulhern T, Hughes BM, Goodwin MS. A systematic review of physiological reactivity to stimuli in autism. Developmental neurorehabilitation. 2014;(0):1–21. doi: 10.3109/17518423.2014.971975. [DOI] [PubMed] [Google Scholar]
- Maenner MJ, Smith LE, Hong J, Makuch R, Greenberg JS, Mailick MR. Evaluation of an activities of daily living scale for adolescents and adults with developmental disabilities. Disability and Health Journal. 2013;6(1):8–17. doi: 10.1016/j.dhjo.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuck SB. Cardiovascular reactivity in cardiovascular disease: "Once more unto the breach". International Journal of Behavioral Medicine. 1994;1(1):4–31. doi: 10.1207/s15327558ijbm0101_2. [DOI] [PubMed] [Google Scholar]
- Maras KL, Bowler DM. Context reinstatement effects on eyewitness memory in autism spectrum disorder. British Journal of Psychology. 2012;103(3):330–342. doi: 10.1111/j.2044-8295.2011.02077.x. [DOI] [PubMed] [Google Scholar]
- Maras KL, Gaigg SB, Bowler DM. Memory for emotionally arousing events over time in autism spectrum disorder. Emotion. 2012;12(5):1118. doi: 10.1037/a0026679. [DOI] [PubMed] [Google Scholar]
- Mathersul D, McDonald S, Rushby JA. Psychophysiological correlates of social judgement in high-functioning adults with autism spectrum disorder. International Journal of Psychophysiology. 2013;87(1):88–94. doi: 10.1016/j.ijpsycho.2012.11.005. [DOI] [PubMed] [Google Scholar]
- Mazefsky C, Herrington J, Siegel M, Scarpa A, Maddox BB, Scahill L, et al. The role of emotion regulation in autism spectrum disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2013;52(7):679–688. doi: 10.1016/j.jaac.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir I, Meir D, Zilber N, Knobler H, Hadjez J, Lerner Y. Brief report: Circadian melatonin, thyroid-stimulating hormone, prolactin, and cortisol levels in serum of young adults with autism. Journal of Autism and Developmental Disorders. 1995;25(6):641–654. doi: 10.1007/BF02178193. [DOI] [PubMed] [Google Scholar]
- Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, et al. Recommendations for blood pressure measurement in humans and experimental animals part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45(1):142–161. doi: 10.1161/01.HYP.0000150859.47929.8e. [DOI] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, Core Team nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1–120. 2015 URL: http://CRAN.R-project.org/package=nlme.
- R Core Team (Ed.) R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2015. [Google Scholar]
- Revelle W. Northwestern University, Evanston. R package version 1.5.4. Evanston, IL: Northwestern University; 2014. psych: Procedures for personality and psychological research. [Google Scholar]
- Richdale A, Prior M. Urinary cortisol circadian rhythm in a group of high-functioning children with autism. Journal of Autism and Developmental Disorders. 1992;22(3):433–447. doi: 10.1007/BF01048245. [DOI] [PubMed] [Google Scholar]
- Richman LS, Bennett GG, Pek J, Siegler I, Williams RB. Discrimination, dispositions, and cardiovascular responses to stress. Health Psychology. 2007;26(6):675. doi: 10.1037/0278-6133.26.6.675. [DOI] [PubMed] [Google Scholar]
- Robins RW, Fraley RC, Krueger RF. Handbook of Research Methods in Personality Psychology. New York: Guilford Press; 2009. [Google Scholar]
- Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychological methods. 2002;7(2):147–177. [PubMed] [Google Scholar]
- Schooler N, Weissman M, Hogarty GE. Social adjustment scale for schizophrenics. In: Hargreaves WA, Attkisson CC, Sorenson J, editors. Resource material for community mental health program evaluators. 1979. pp. 290–303. DHHS Pub. No. (ADM) [Google Scholar]
- Seltzer MM, Shattuck PT, Abbeduto L, Greenberg JS. Trajectory of development in adolescents and adults with autism. Mental Retardation and Developmental Disabilities Research Reviews. 2004;10(4):234–247. doi: 10.1002/mrdd.20038. [DOI] [PubMed] [Google Scholar]
- Shattuck PT, Roux AM, Hudson LE, Taylor JL, Maenner MJ, Trani JF. Services for adults with an autism spectrum disorder. Canadian Journal of Psychiatry. 2012;57(5):284–291. doi: 10.1177/070674371205700503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NN, Lancioni GE, Manikam R, Winton AS, Singh AN, Singh J, Singh AD. A mindfulness-based strategy for self-management of aggressive behavior in adolescents with autism. Research in Autism Spectrum Disorders. 2011;5(3):1153–1158. [Google Scholar]
- Spek AA, Van Ham NC, Nyklíček I. Mindfulness-based therapy in adults with an autism spectrum disorder: A randomized controlled trial. Research in Developmental Disabilities. 2013;34(1):246–253. doi: 10.1016/j.ridd.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Smith LE, Greenberg JS, Mailick MR. Adults with autism: Outcomes, family effects, and the multi-family group psychoeducation model. Current Psychiatry Reports. 2012a;14(6):732–738. doi: 10.1007/s11920-012-0328-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LE, Maenner MJ, Seltzer MM. Developmental trajectories in adolescents and adults with autism: The case of daily living skills. Journal of the American Academy of Child & Adolescent Psychiatry. 2012b;51(6):622–631. doi: 10.1016/j.jaac.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MJ, Ginger EJ, Wright K, Wright MA, Taylor JL, Humm LB, et al. Virtual reality job interview training in adults with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2014;44(10):2450–2463. doi: 10.1007/s10803-014-2113-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strain PS, Odom SL. Peer social initiations: Effective intervention for social skills development of exceptional children. Exceptional Children. 1986;52(6):543–551. doi: 10.1177/001440298605200607. [DOI] [PubMed] [Google Scholar]
- Sze KM, Wood JJ. Cognitive behavioral treatment of comorbid anxiety disorders and social difficulties in children with high-functioning autism: A case report. Journal of Contemporary Psychotherapy. 2007;37(3):133–143. [Google Scholar]
- Taylor JL, Corbett BA. A review of rhythm and responsiveness of cortisol in individuals with autism spectrum disorders. Psychoneuroendocrinology. 2014;49(0):207–228. doi: 10.1016/j.psyneuen.2014.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmar F, Cook EH, Pomeroy J, Realmuto G, Tanguay P. Practice parameters for the assessment and treatment of children, adolescents, and adults with autism and other pervasive developmental disorders. Journal of the American Academy of Child & Adolescent Psychiatry. 1999;38(12):32S–54S. doi: 10.1016/s0890-8567(99)80003-3. [DOI] [PubMed] [Google Scholar]
- Wechsler D. A standardized memory scale for clinical use. The Journal of Psychology: Interdisciplinary and Applied. 1945;19(1):87–95. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale® 4th Edition (WAIS®-IV) San Antonio, TX: Harcourt Assessment; 2008. [Google Scholar]
- Williams VP, Bishop-Fitzpatrick L, Lane JD, Gwyther LP, Ballard EL, Vendittelli AP, et al. Video-based coping skills to reduce health risk and improve psychological and physical well-being in Alzheimer's disease family caregivers. Psychosomatic medicine. 2010;72(9):897–904. doi: 10.1097/PSY.0b013e3181fc2d09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JJ, Drahota A, Sze K, Har K, Chiu A, Langer DA. Cognitive behavioral therapy for anxiety in children with autism spectrum disorders: A randomized, controlled trial. Journal of Child Psychology and Psychiatry. 2009;50(3):224–234. doi: 10.1111/j.1469-7610.2008.01948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JJ, Drahota A, Sze K, Van Dyke M, Decker K, Fujii C, Bahng C, Renno P, Hwang W, Spiker M. Brief report: Effects of cognitive behavioral therapy on parent-reported autism symptoms in school-age children with high-functioning autism. Journal of Autism and Developmental Sisorders. 2009;39(11):1608–1612. doi: 10.1007/s10803-009-0791-7. [DOI] [PMC free article] [PubMed] [Google Scholar]