Abstract
Heart failure, a leading cause of death in humans, can emanate from myocarditis. Although most individuals with myocarditis recover spontaneously, some develop chronic dilated cardiomyopathy. Myocarditis may result from both infectious and noninfectious causes, including autoimmune responses to cardiac antigens. In support of this notion, intracellular cardiac antigens, like cardiac myosin heavy chain-α, cardiac troponin-I, and adenine nucleotide translocator 1 (ANT1), have been identified as autoantigens in cardiac autoimmunity. Herein, we demonstrate that ANT1 can induce autoimmune myocarditis in A/J mice by generating autoreactive T cells. We show that ANT1 encompasses multiple immunodominant epitopes (namely, ANT1 21-40, ANT1 31-50, ANT1 171-190, and ANT1 181-200). Although all four peptides induce comparable T-cell responses, only ANT1 21-40 was found to be a major myocarditogenic epitope in immunized animals. The myocarditis-inducing ability of ANT1 21-40 was associated with the generation of T cells producing predominantly IL-17A, and the antigen-sensitized T cells could transfer the disease to naïve recipients. These data indicate that cardiac mitochondrial proteins can be target autoantigens in myocarditis, supporting the notion that the antigens released as a result of primary damage may contribute to the persistence of chronic inflammation through autoimmunity.
Myocarditis can occur as a result of exposure to various infectious and noninfectious insults, but does not generally lead to a fatal outcome (ie, most affected individuals can recover spontaneously). However, a proportion of those affected can develop dilated cardiomyopathy (DCM). Estimates indicate that approximately half of DCM patients undergo heart transplantation because of a lack of alternative therapeutic options.1, 2, 3 Furthermore, several clinical studies suggest that DCM patients can have autoantibodies to several cardiac antigens, including adenine nucleotide translocator (ANT).4, 5, 6 Because DCM can arise as a sequel to myocarditis, it has been postulated that autoimmune response may be an underlying mechanism in its pathogenesis.7
ANT exists in multiple isoforms, all four of which are expressed in humans (ANT1, ANT2, ANT3, and ANT4), but only three in mice (ANT1, ANT2, and ANT4). ANT1 is expressed in muscle tissues (heart and skeletal) and the brain, ANT2 can be expressed in liver, kidney, and heart, and ANT4 expression is restricted to the testes in mice.8, 9 Nonetheless, all isoforms are encoded by nuclear DNA, and after transcription and translation, proteins are imported into the mitochondria and finally inserted into the inner mitochondrial membrane.10 The solute carrier family 25, member 4 (Slc25a4) gene encoding for ANT1 protein plays a critical role in energy metabolism in that it shuttles ADP from the cytoplasm to the mitochondria in exchange for ATP; also, it has been proposed to be involved in the formation of mitochondrial permeability transition pore complex.11, 12 Pathologically, ANT1 dysfunction has been found to be associated with several diseases, such as autosomal dominant progressive external ophthalmoplegia, Sengers syndrome, and autosomal dominant facioscapulohumeral muscular dystrophy.13 More important, mice deficient in Slc25a4 show DCM/myocardial hypertrophy, ventricular dilation, and reduced cardiac function in association with enhanced cytochrome c release and caspase 3 activation, and myopathy involving ragged-red muscle fibers and hyper-proliferation of mitochondria but with no significant changes in retinal function.14, 15, 16 Conversely, transgenic overexpression of Slc25a4 in the cardiomyocytes of rodents leads to improved contractile function/myocardial remodeling and calcium transport, significant reduction in cardiac fibrosis, including the prevention of diabetic cardiomyopathy, and apoptotic response to transforming growth factor-β1.17, 18, 19, 20 These observations suggest that cardiomyocytes may be more sensitive than skeletal myocytes to alterations in ANT1 functions, like muscle contractibility and apoptosis. This notion is further supported by observations that autoantibodies to human ANT1 (hANT1) epitopes, like hANT1 139-148 and hANT1 234-243, were identified in sera from idiopathic DCM patients.21 Likewise, ANT antibodies were found to be associated with impairment in myocardial calcium homeostasis, and development of idiopathic DCM in mice immunized with human ANT peptides.22 Mechanistically, how these autoantibodies can alter the functions of ANT1 is not clear. Similarly, it is not known whether autoreactive T cells for ANT1 have any role in the pathogenesis of myocardial damage, given that T-cell help is critical for the production of antibodies to protein antigens like ANT1.
To define a role for putative autoantigens, various experimental models of autoimmune diseases have been successfully used. One such model for cardiac autoimmunity is experimental autoimmune myocarditis (EAM), which involves induction of disease in susceptible rodent strains by immunizing animals with cardiac antigens or their peptide fragments.23, 24, 25 In this study, we used the EAM model in A/J mice to demonstrate that ANT1 possesses multiple immunodominant epitopes, and one of these, ANT1 21-40, was found to induce myocarditis in the immunized animals. Furthermore, myocarditogenicity of ANT1 21-40 was associated with the generation of T cells capable of producing predominantly IL-17A, and the antigen-sensitized T cells can transfer the disease to naïve recipients, suggesting that ANT1 can be a potential autoantigen in the heart autoimmunity.
Materials and Methods
Mice
A/J mice (6 to 8 weeks old, H-2a) obtained from the Jackson Laboratory (Bar Harbor, ME) were maintained in accordance with the guidelines of the University of Nebraska-Lincoln. All experiments involved the use of female mice unless otherwise indicated. The animals were maintained according to the guidelines of the Institutional Animal Care and Use Committee.
Peptide Synthesis
A total of 29 overlapping peptides of 20-mers, except ANT1 281-298 (18-mer), spanning the entire amino acid sequence of mouse ANT1 (298 residues), bovine ribonuclease (RNase) 43-56 (VNTFVHESLADVQA), and biotinylated hen egg lysozyme (HEL) 46-61 (YNTDGSTDYGILQINSR) were synthesized on 9-fluorenylmethyloxycarbonyl chemistry (Neopeptide, Cambridge, MA). All peptides were high-performance liquid chromatography purified (>90%), and identity was confirmed by mass spectroscopy. The peptides were dissolved in ultrapure water, aliquoted, and stored at −20°C.
Induction of EAM by Active Immunization
Peptide emulsions were prepared in complete Freund adjuvant (CFA; Sigma-Aldrich, St. Louis, MO) containing Mycobacterium tuberculosis H37RA extract (Difco Laboratories, Detroit, MI) to a final concentration of 5 mg/mL. The disease was induced by twice administering peptide/CFA emulsions (100 μg per mouse) s.c., in inguinal and sternal regions, with an interval of 7 days. In addition, each animal received pertussis toxin (PT; List Biological Laboratories, Campbell, CA; 100 ng per mouse) i.p., on days 0 and 2 after the first immunization.23, 24, 25 In some experiments, animals were administered with CFA emulsions with no peptides, but all received PT as described above.
Histology
Hearts and noncardiac tissues (brain and liver) were collected at termination on day 21 after immunization, and the tissues were fixed by immersion in 10% phosphate-buffered formalin.24 Four to six longitudinal tissue layers were cut from each heart, and up to two layers were also cut from noncardiac tissues (namely, brain and liver). Two to six serial histological sections were then cut to a 5 μm thickness from each layer, skipping 25 μm between sections. All sections were stained with hematoxylin and eosin and examined by a board-certified pathologist (D.S.) blinded to treatment. After ascertaining the inflammatory changes, the total number of inflammatory foci was determined by sections with the largest number of foci or by adding nonoverlapping foci across sections, as reported previously.26 The disease severity was assessed as follows: heart: 0 foci, normal; 1 to 5 foci, mild multifocal; 6 to 25 foci, moderate multifocal to coalescing; and 26 or more foci, diffuse24, 27; and liver: 0 to 6 foci, background; and >6 foci, multiple scattered.
Immunohistochemistry
Hearts collected on day 21 from animals immunized with ANT1 21-40 were examined for the presence of T cells and non-T cells, and also for apoptosis. T cells were identified by immunostaining with anti-mouse CD3 (Abcam, Cambridge, MA); CD11b (BD Biosciences, San Jose, CA) and Iba1 (Abcam) antibodies, which recognize both macrophages and dendritic cells, were used to detect non-T cells.28, 29, 30 To evaluate apoptosis, anti-mouse, cleaved caspase 3 (Cell Signaling Technology, Danvers, MA) antibody was used. All the primary antibodies were raised in rabbits. Briefly, paraffin-embedded heart sections were deparaffinized and rehydrated, and endogenous peroxidase activity was blocked with 3% hydrogen peroxide for 30 minutes. To retrieve antigens, sections were treated with 10 mmol/L sodium citrate buffer (pH 6.0) in a water bath at 98°C for 15 minutes. After blocking with 5% nonfat dry milk for 30 minutes, sections were incubated with primary antibodies at 4°C overnight. After incubating with goat anti-rabbit IgG conjugated with horseradish peroxidase (Vector Laboratories, Burlingame, CA; and Abcam) as a secondary antibody for 2 hours at room temperature, sections were incubated with diaminobenzidine as a substrate, fixed, counterstained with hematoxylin, and examined as described above.
MRM Imaging
For the assessment of structural and functional abnormalities of hearts in ANT1 21-40–immunized mice, magnetic resonance microscopy (MRM) imaging was used as a noninvasive tool.31 Briefly, animals were anesthetized using 2% isoflurane and transferred onto animal holder, and the body temperature was monitored using a rectal thermometer. Respirometry and pulse oximetry were used to gate the respiratory and cardiac signals, respectively. MRM imaging was performed using a wide-bore (89-mm) 9.4-T vertical-bore magnet (Varian, Inc., Walnut Creek, CA) equipped with triple axis gradients of 100 G/cm and a 4-cm radiofrequency imaging coil, as we have described previously.31 Short axis slices of hearts were captured in eight time frames using an echo-based cine pulse sequence, and the images were analyzed using Segment software version 1.8 R1430 (Segment, Medviso, Sweden) to assess the structural (left ventricular wall thickness) and functional parameters [end diastolic volume, end systolic volume, stroke volume (SV), and ejection fraction (EF)].
T-Cell Proliferation Assay
Draining lymph nodes (maxillary, mandibular, axillary, and inguinal) were harvested from EAM animals at day 21 after immunization. Single-cell suspensions were prepared by lysing the erythrocytes using 1× ammonium chloride potassium buffer (Lonza, Walkersville, MD). After washing, cell pellets were suspended in RPMI 1640 medium supplemented with 10% fetal bovine serum, 1 mmol/L sodium pyruvate, 4 mmol/L l-glutamine, 1× each of nonessential amino acids and vitamin mixture, and 100 U/mL penicillin-streptomycin (Lonza; hereafter called growth medium). Lymph node cells (LNCs) were stimulated at a density of 5 × 106 cells/mL in triplicate with the indicated peptides (0 to 100 μg/mL) in growth medium; cells cultured with no peptides were used as medium controls. After 48 hours, cells were pulsed with tritiated 3[H]-thymidine (1 μCi/well; MP Biomedicals, Santa Ana, CA) for 16 hours, and proliferative responses were then measured as counts per minute using a Wallac liquid scintillation counter (Perkin Elmer, Waltham, MA). In some experiments, recall responses were tested in animals immunized with a single dose of peptide/CFA emulsion (100 μg per mouse).
IAk-Binding Assay
To determine the affinities of peptides binding to major histocompatibility complex (MHC) class II (IAk), soluble IAk molecules were expressed as described previously.32, 33 Briefly, IAk constructs representing the extracellular domains of IAk-α and IAk-β were designed with the IAk-β construct engineered to contain class II–associated invariant-chain peptide 88-102 (VSQMRMATPLLMRPM) in the N-terminus, followed by a thrombin cleavage site to facilitate the release of class II–associated invariant-chain peptide. After expressing the protein using baculovirus vector in sf9 insect cells, the soluble IAk molecules were purified on an anti-IAk affinity column (clone 10-2.16; BioXcell, West Lebanon, NH), and the empty IAk molecules were finally obtained by cleaving the class II–associated invariant-chain peptide with thrombin (20 IU/mg; Novagen, Madison, WI). The reaction mixtures were prepared for individual peptides to include thrombin-cleaved IAk monomers (0.35 μg), competitor peptides [ANT1 21-40, ANT1 171-190, and ANT1 181-200 (0.00001 to 100 μmol/L)], and a constant amount of biotinylated reference peptide, HEL 46-61 (1 μmol/L), in a buffer containing 50 mmol/L sodium phosphate, pH 7.0, 100 mmol/L sodium chloride, 1 mmol/L EDTA, and 1× protease inhibitor (Sigma-Aldrich). The mixtures were incubated at room temperature overnight. In addition, anti-IAk (10 μg/mL) was coated onto 96-well white fluorescence plates (Nunc, Rochester, NY) in 0.2 mol/L sodium phosphate, pH 6.8, and the plates were incubated overnight at 4°C. After washing five times with 1× wash buffer (Perkin Elmer), wells were blocked with 2% casein for 2 hours at room temperature, and then washed. The reaction mixtures described above were added in duplicate and incubated on a rocker at room temperature for 1 hour, followed by washing as above. Finally, europium-labeled streptavidin (0.1 μg/mL) was added in dissociation-enhanced lanthanide fluoroimmunoassay buffer, followed by dissociation-enhanced lanthanide fluoroimmunoassay enhancement solution (Perkin Elmer). Fluorescence intensity was measured at excitation/emission wavelengths of 340/615 nm using a Victor Multilabel Plate Reader (Perkin Elmer). The 50% inhibitory concentration (IC50) for each peptide was calculated based on the concentrations of competitor peptides, which prevented 50% binding of the reference peptide (HEL 46-61), as described previously.34, 35
Cytokine Bead Array Analysis
LNCs obtained from mice immunized with ANT1 peptides were stimulated with or without immunizing ANT1 peptide/RNase 43-56 (50 μg/mL). Culture supernatants were collected on day 3 after stimulation and analyzed for cytokines using beads conjugated with capture and detection antibodies, according to the manufacturer's recommendations (BD Biosciences). Briefly, standard curves were obtained by serially diluting (1:2) the lyophilized mouse cytokine standard mix containing IL-2, interferon (IFN)-γ, IL-4, IL-6, IL-10, IL-17A, and tumor necrosis factor (TNF)-α, in concentrations ranging from 20 to 5000 pg/mL, whereas assay diluent alone served as a zero standard. Capture beads coated with antibodies representing the above cytokines were mixed together and added to a tube containing diluted standards or test samples. After adding detection antibodies, tubes were incubated at room temperature in the dark for 2 hours. All samples were washed by centrifugation at 200 × g for 5 minutes, and the pellets were suspended in wash buffer. Finally, the samples were acquired by flow cytometer, and data analyzed by FCAP Array software version 3.0 (BD Biosciences).
Induction of EAM by Adoptive Transfer of Antigen-Sensitized T Cells
LNCs were obtained from animals immunized with ANT1 21-40 in CFA once or twice, and the cells were stimulated with concanavalin-A (2.5 μg/mL; Sigma-Aldrich) for 2 days. Viable cells were then harvested and administered retro-orbitally or i.p. (1.6 to 6.0 × 107 cells per animal in 1× PBS/1% fetal bovine serum) into naïve mice primed with lipopolysaccharide (25 μg per mouse; Sigma-Aldrich), which was administered i.p., on day −4 and on the day of infusion, as previously described.26, 36 Naïve mice that received 1× PBS or lipopolysaccharide alone served as controls. Each animal also received PT (100 ng per mouse) i.p., on day 0 and day 2 after transfer; at termination on day 14, hearts were collected for histology.26, 36
Determination of Antibody Titers by ELISA
A/J mice were immunized with ANT1 21-40 in CFA for induction of EAM, as described above, and serum samples were collected on indicated days for measurement of ANT1 21-40–reactive antibodies by enzyme-linked immunosorbent assay (ELISA). ANT1 21-40 (10 μg/mL) and RNase 43-56 (negative control; 10 μg/mL) peptides in 1× coating buffer (eBioscience, San Diego, CA) were coated onto 96-well polystyrene microtiter plates, which were incubated at 4°C overnight. After washing with 1× PBS/0.05% Tween-20, wells were blocked with a blocking buffer (1× PBS/2% bovine serum albumin/3% normal goat serum) for 1.5 hours at room temperature. Plates were washed again, and 1:10- to 1:100-diluted serum samples were added in duplicate and incubated at 37°C for 1 hour.37 After washing as above, horseradish peroxidase–labeled donkey anti-mouse IgG secondary antibody (Santa Cruz, Dallas, TX) was added and incubated at room temperature for 2 hours, followed by addition of 1× tetramethylbenzidine solution as substrate (eBioscience). Finally, the reactions were stopped using 1 mol/L phosphoric acid. Plates were read at 450 nm using an automated ELISA reader (BioTek Instruments, Winooski, VT), and OD values were then obtained.
Measurement of Serum cTnI and AST
Cardiac troponin I (cTnI) levels were measured in the sera from mice immunized with or without ANT1 21-40 using a high-sensitivity cTnI ELISA kit, according to manufacturer's recommendations (Life Diagnostics Inc., West Chester, PA). Briefly, 100 μL of cTnI horseradish peroxidase conjugate and 100 μL of standards/samples were added to anti–cTnI-coated wells. After mixing, plates were incubated on an orbital shaker for 1 hour at room temperature, followed by addition of tetramethylbenzidine as substrate, and the reactions were stopped by adding 1N HCl. Plates were read at 405 nm using an automated ELISA reader (BioTek Instruments) to obtain the OD values. Aspartate aminotransferase (AST) concentrations were measured by using Vitros 250 Chemistry Analyzer (Ortho-Clinical Diagnostics, Rochester, NY).
RT-PCR Analysis
Total RNA was extracted from lymphoid (thymus and spleen) and nonlymphoid (heart, thigh muscle, brain, and liver) tissues harvested from naïve A/J mice using the Trizol method (Invitrogen, Carlsbad, CA). To remove genomic DNA contamination, 1 μg of total RNA was treated with amplification grade DNase I (Invitrogen), and cDNAs were synthesized using M-MLV Reverse Transcriptase kit, as recommended (Invitrogen). PCR amplifications were performed using gene-specific primers, as previously described: ANT1, 5′-AAAAATATGTGTAATACCCAAGCTCACA-3′ (forward) and 5′-TGTTTTCTTTCCTCAAGAATAGTCTGTTAAAC-3′ (reverse)38; ANT2, 5′-AGGGCGCATGGTCCAA-3′ (forward) and 5′-ATCTCATACAAGACAAGCACAAAC-3′ (reverse)38; ANT4, 5′-TGGAGCAACATCCTTGTGTG-3′ (forward) and 5′-AGAAATGGGGTTTCCTTTGG-3′ (reverse)9; and glyceraldehyde-3-phosphate dehydrogenase, 5′-CGGCAAATTCAACGGCACAGTCAA-3′ (forward) and 5′-CTTTCCAGAGGGGCCATCCACAG-3′ (reverse).39 The PCR products were stained with ethidium bromide and resolved on 1.5% agarose gel electrophoretic analysis.
Statistical Analysis
t-test was used to determine differences between groups for cardiac abnormalities, heart and body weight ratio, T-cell responses, IAk-binding affinity, antibody reactivity, cytokine secretion, inflammatory foci, and AST concentrations. In T-cell responses, for some peptides with varied background levels, the count per minute values were scaled within the replicates and doses using a constant multiplier determined by the average count per minute values. P ≤ 0.05 was considered significant.
Results
Identification of Myocarditogenic Epitopes of ANT1 in A/J Mice
To identify the disease-inducing epitopes of ANT1, we used A/J mice bearing the MHC class II haplotype H-2a; these mice are highly susceptible to the induction of EAM by active immunizations.23 We first generated an overlapping peptide library to identify the disease-inducing epitopes by generating 29 peptides of 20-mers with 10 overlapping amino acids between them, except ANT1 281-298 (18-mer) (Table 1). These peptides encompassed the complete coding sequence (298 amino acids) of ANT1, and, for reasons described later, some peptides were acetylated at the N-terminal end. We designate these as acetylated peptides, whereas routinely synthesized (ie, amidated) peptides are termed nonacetylated peptides.
Table 1.
List of Overlapping Peptides of ANT1 Used to Determine Their Myocarditogenicity and Immunogenicity
| ANT1 Peptide | Sequence |
|---|---|
| 1-20 | MGDQALSFLKDFLAGGIAAA |
| 11-30 | DFLAGGIAAAVSKTAVAPIE |
| 21-40 | VSKTAVAPIERVKLLLQVQH |
| 31-50 | RVKLLLQVQHASKQISAEKQ |
| 41-60 | ASKQISAEKQYKGIIDCVVR |
| 51-70 | YKGIIDCVVRIPKEQGFLSF |
| 61-80 | IPKEQGFLSFWRGNLANVIR |
| 71-90 | WRGNLANVIRYFPTQALNFA |
| 81-100 | YFPTQALNFAFKDKYKQIFL |
| 91-110 | FKDKYKQIFLGGVDRHKQFW |
| 101-120 | GGVDRHKQFWRYFAGNLASG |
| 111-130 | RYFAGNLASGGAAGATSLCF |
| 121-140 | GAAGATSLCFVYPLDFARTR |
| 131-150 | VYPLDFARTRLAADVGKGSS |
| 141-160 | LAADVGKGSSQREFNGLGDC |
| 151-170 | QREFNGLGDCLTKIFKSDGL |
| 161-180 | LTKIFKSDGLKGLYQGFSVS |
| 171-190 | KGLYQGFSVSVQGIIIYRAA |
| 181-200 | VQGIIIYRAAYFGVYDTAKG |
| 191-210 | YFGVYDTAKGMLPDPKNVHI |
| 201-220 | MLPDPKNVHIIVSWMIAQSV |
| 211-230 | IVSWMIAQSVTAVAGLVSYP |
| 221-240 | TAVAGLVSYPFDTVRRRMMM |
| 231-250 | FDTVRRRMMMQSGRKGADIM |
| 241-260 | QSGRKGADIMYTGTLDCWRK |
| 251-270 | YTGTLDCWRKIAKDEGANAF |
| 261-280 | IAKDEGANAFFKGAWSNVLR |
| 271-290 | FKGAWSNVLRGMGGAFVLVL |
| 281-298 | GMGGAFVLVLYDEIKKYV |
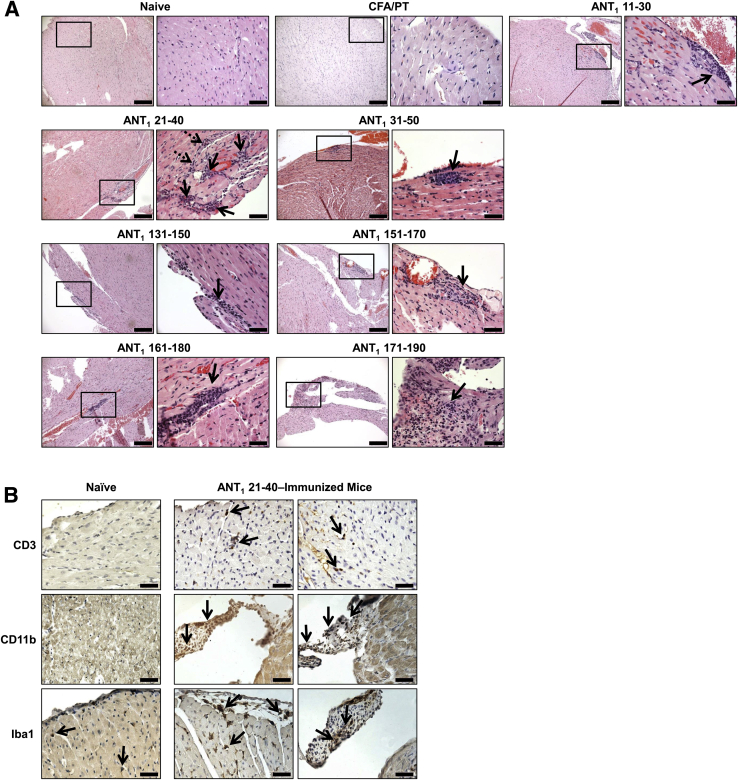
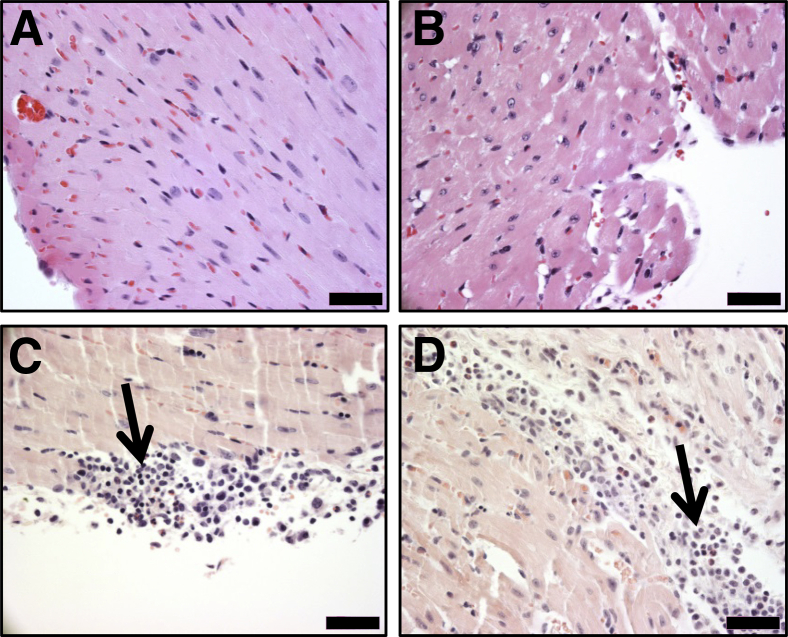
In our initial screening, to determine the pathogenicity of ANT1 peptides, we made seven pools of nonacetylated peptides (pools I to VII) with each containing four to five overlapping peptides. Groups of mice were immunized with peptide/CFA emulsions (50 μg of each peptide), and the animals were euthanized on day 21 after immunization to harvest hearts for histology and lymph nodes for T-cell proliferation assay. Histological analysis of hearts by hematoxylin and eosin staining from pools I, IV, and V revealed inflammatory infiltrates composed of lymphocytes and macrophages. The disease prevalence ranged from 25% to 50%, and the mean foci in the respective pools were 3.50 ± 0.50, 5.00, and 1.55 ± 0.50 (Supplemental Table S1). This moderate number of foci was expected because all the peptides in a given pool might not be immunogenic or myocarditogenic. In support of this proposition, T-cell proliferative responses were evident only in ANT1 21-40 and ANT1 31-50 in pool I, none in pool IV, and only in ANT1 171-190 and ANT1 181-200 in pool V (data not shown). Thus, the possibility existed that animals immunized with the individual peptides could show more pronounced disease in tandem with T-cell responses. We then chose a total of 12 peptides, four from each pool (I, IV, and V), and immunized groups of mice with the individual peptide/CFA emulsions. As shown in Table 2, ANT1 21-40 induced myocarditis in the immunized animals with an incidence of 50%. In most of those affected, mild multifocal inflammatory foci were evident (3.40 ± 1.03). In contrast, animals immunized with other peptides (namely, ANT1 11-30, ANT1 31-50, ANT1 131-150, ANT1 151-170, and ANT1 161-180) showed only isolated lesions and the disease incidences were also low. Interestingly, one animal immunized with ANT1 171-190 showed widespread lesions, but the reason for such an isolated occurrence is not known. Figure 1A shows the distribution of lesions, and as expected, sections derived from the ANT1 21-40–immunized group showed multiple foci composed of lymphocytes and macrophages as opposed to isolated lesions seen in other groups, whereas no lesions were detected in the naïve or CFA/PT groups. No necrosis or fibrosis was evident in any of the groups. Further characterization of infiltrates revealed the detection of both T cells and non-T cells (macrophages and dendritic cells), as ascertained by staining respectively with CD3 and CD11b and Iba1 antibodies (Figure 1B). We also verified that the disease process induced with ANT1 21-40 was not associated with apoptosis of cardiac myocytes nor were there any alterations in the levels of cTnI, which was used as a marker of cardiac damage (data not shown).
Table 2.
Induction of Myocarditis by ANT1 Peptides
| ANT1 Peptides | Peptide modifications |
|||
|---|---|---|---|---|
| Nonacetylated |
Acetylated |
|||
| Incidence (%) | Inflammatory foci∗ | Incidence (%) | Inflammatory foci∗ | |
| 1-20 | 0/5 (0) | 0 | 1/5 (20) | 1.00 |
| 11-30 | 1/5 (20) | 1.00 | 2/5 (40) | 1.00 |
| 21-40 | 5/10 (50) | 3.40 ± 1.03 | 4/10 (40) | 3.25 ± 1.44 |
| 31-50 | 2/8 (25) | 1.00 | Not tested | |
| 121-140 | 0/5 (0) | 0 | Not tested | |
| 131-150 | 1/5 (20) | 2.00 | 0/5 (0) | 0 |
| 141-160 | 0/5 (0) | 0 | Not tested | |
| 151-170 | 1/5 (20) | 1.00 | 0/5 (0) | 0 |
| 161-180 | 1/5 (20) | 3.00 | 1/5 (20) | 1.00 |
| 171-190 | 1/10 (10) | 16.00 | 1/5 (20) | 2.00 |
| 181-200 | 0/10 (0) | 0 | 0/5 (0) | 0 |
| 191-210 | 0/5 (0) | 0 | Not tested | |
Inflammatory foci were absent in hearts from both naïve (n = 10) and CFA/PT (n = 5) groups.
CFA, complete Freund adjuvant; PT, pertussis toxin.
Represents means ± SEM derived from myocarditic animals.
Figure 1.
Induction of myocarditis by ANT1 peptides in A/J mice. Groups of mice were immunized with or without peptide/complete Freund adjuvant (CFA) emulsions pertussis toxin (PT) twice with an interval of 7 days, and PT was administered on day 0 and day 2 after the first immunization. After 21 days, animals were euthanized and hearts were collected for histological evaluation of inflammation. A: Hematoxylin and eosin staining. Representative sections are shown for each peptide [naïve and CFA/PT groups: normal cardiac tissue with intact myocytes; ANT1 11-30: moderate inflammatory focus along the epicardium; ANT1 21-40: multiple foci near the epicardium (solid arrows) and in the myocardium (dotted arrows); ANT1 31-50 and ANT1 131-150: small focus in the endocardium; ANT1 151-170: widespread inflammatory focus along the epicardium; ANT1 161-180: isolated focus in the myocardium; and ANT1 171-190: small focus in the myocardium, all shown with solid arrows]. Boxed areas are shown at higher magnification to the right. B: Immunohistochemistry. Heart sections obtained from animals immunized with or without ANT1 21-40 were stained for the presence of CD3+ T cells, and non-T cells (CD11b+ and Iba1+) using the corresponding antibodies. After washing, sections were stained with horseradish peroxidase–conjugated, secondary antibodies to detect cells positive for each marker, shown with arrows. No CD3+ T cells are seen in the control; and rare dendritic cells were CD11b+ and Iba1+ in the controls. Inflammatory foci were also present in the cardiac valves in mice immunized with ANT1 21-40. n = 5 to 10 mice per group (A). Scale bars: 200 μm (A, main images); 50 μm (A, magnifications); 40 μm (B).
Previous reports suggest that the disease-inducing abilities of some autoantigens can be increased when peptides are modified to include an acetyl group at the N-terminal end.40 One such epitope is cardiac myosin heavy chain-α acetylated 614-643, which can induce myocarditis in Balb/c mice only in the acetylated form, and the acetylated peptides are expected to prevent from intracellular degradation, leading to their better loading with MHC class II molecules.25 Thus, we reasoned that the acetylated forms of poor disease-inducing ANT1 peptides could induce more severe disease than their counterparts. Contrary to our expectations, none of the acetylated peptides resulted in significant change in myocarditis severity (Table 2), suggesting that the pathogenic potential is unlikely to be affected for all peptides with acetylation. Nevertheless, the disease incidence and severity induced with the acetylated form of ANT1 21-40 was comparable to that induced by the nonacetylated form (Table 2). Thus, we have identified ANT1 21-40 as the myocarditogenic peptide that induces EAM in immunized animals.
MRM Imaging of Animals Immunized with ANT1 21-40 Revealed Cardiac Abnormalities in Live Animals
We had previously established a MRM technique that permitted us to determine cardiac functions as a noninvasive procedure in mice affected with autoimmune myocarditis.31 Groups of mice were immunized with or without ANT1 21-40, and the animals were subjected for MRM imaging to measure end diastolic volume, end systolic volume, SV, EF, and left ventricular wall thickness. Expectedly, both SV and EF were significantly reduced in immunized animals on different days as compared to healthy group [day 30 to 35: SV, 11.33 ± 2.17 μL versus 25.67 ± 4.67 μL; EF, 30.17 ± 2.52% versus 65.67 ± 0.88% (P < 0.05); and day 46: SV, 9.00 ± 2.52 μL versus 29.00 ± 0.58 μL; EF, 24.00 ± 4.51% versus 60.00 ± 1.15% (P < 0.01)]. Likewise, increase in end systolic volume was apparent on day 46, whereas reduction in end diastolic volume was not significant (Table 3). Nevertheless, increase in left ventricular wall thickness was noted on all the days (Table 3 and Supplemental Figure S1A), suggesting that ANT1 21-40 may be a candidate autoantigen that can trigger dilated cardiomyopathy. This finding was further supported by an observation that the heart weight/body weight ratios were significantly elevated in the ANT1 21-40–immunized animals (P = 0.041) (Supplemental Figure S1B).
Table 3.
Assessment of Cardiac Abnormalities of Hearts in Mice Immunized with ANT1 21-40
| Parameters | Day 30 to 35 |
Day 46 |
||
|---|---|---|---|---|
| Naïve | ANT1 21-40 | Naïve | ANT1 21-40 | |
| End diastolic volume, μL | 37.33 ± 6.17 | 30.33 ± 3.90 | 42.67 ± 2.91 | 33.00 ± 4.51 |
| End systolic volume, μL | 11.67 ± 2.33 | 18.83 ± 2.37 | 13.67 ± 2.33 | 24.33 ± 1.86∗ |
| Stroke volume, μL | 25.67 ± 4.67 | 11.33 ± 2.17∗ | 29.00 ± 0.58 | 9.00 ± 2.52∗∗ |
| Ejection fraction, % | 65.67 ± 0.88 | 30.17 ± 2.52∗∗∗ | 60.00 ± 1.15 | 24.00 ± 4.51∗∗ |
| LV wall thickness, mm | 0.87 ± 0.09 | 1.45 ± 0.06∗∗∗ | 0.83 ± 0.03 | 1.43 ± 0.03∗∗∗ |
Data are expressed as means ± SEM.
∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 versus naïve group.
LV, left ventricular.
Comparison of T-Cell Responses between Various ANT1 Peptides Revealed No Direct Correlation with Myocarditogenicity
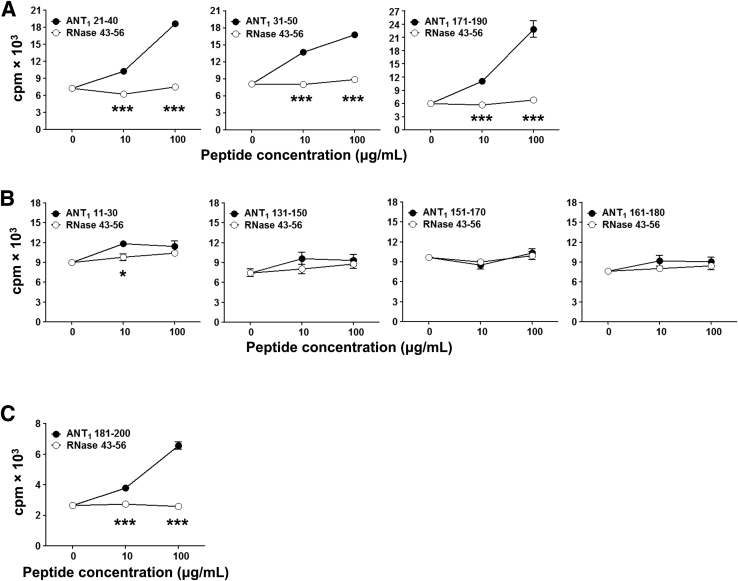
We sought to determine a relationship, if any, between myocarditogenicity and immunogenicity by evaluating T-cell responses. LNCs derived from immunized mice were used to measure recall responses based on 3[H]-thymidine incorporation assay. These analyses resulted in the identification of three patterns of peptides (Figure 2): i) Peptides that induce both myocarditis and T-cell responses. The strong candidate in this category is ANT1 21-40, to which the LNCs generated from immunized mice responded with a greater magnitude, but had no response to the control, RNase 43-56, suggesting that the responses were specific to ANT1 21-40 (Figure 2A). Although two other peptides, ANT1 31-50 and ANT1 171-190 (Figure 2A), induced T-cell responses comparable to ANT1 21-40, their ability to induce disease was low (Table 2). ii) Peptides that induce negligible myocarditis but mild or no T-cell responses. This scenario was apparent for the following peptides: ANT1 11-30, ANT1 131-150, ANT1 151-170, and ANT1 161-180 (Figure 2B). iii) Peptides that do not induce myocarditis but generate T-cell responses. The peptide ANT1 181-200 (Figure 2C) qualifies for this feature. Overall, because ANT1 21-40 induced both T-cell responses and myocarditis to a greater severity than other peptides, we decided to follow this peptide for further characterization.
Figure 2.
ANT1 peptides induce T-cell responses in A/J mice. Experimental autoimmune myocarditis was induced in A/J mice by immunizing with peptide/complete Freund adjuvant (CFA) emulsions, as described in Materials and Methods. Animals were euthanized on day 21 after immunization to harvest lymph nodes, and single-cell suspensions were prepared. Recall responses were tested by stimulating lymph node cells (LNCs) with immunizing peptides or RNase 43-56 as control. After 2 days, cells were pulsed with 3[H]-thymidine for 16 hours, and proliferative responses were then measured as counts per minute (cpm). A: Peptides that induce both myocarditis and T-cell responses. B: Peptides that induce negligible myocarditis but mild or no T-cell responses. C: Peptides that do not induce myocarditis but generate T-cell responses. For some peptides (ANT1 21-40, ANT1 171-190, and ANT1 181-200), proliferative responses also were measured in animals immunized with only one dose of peptide/CFA emulsions and euthanized 10 days later for preparation of LNCs. Because there were no differences between animals immunized once or twice, data were pooled. Values representing two to five individual experiments each involving three to five mice are shown, and each experiment contained three replicates. Data are expressed as means ± SEM (A–C). ∗P < 0.05, ∗∗∗P < 0.001 between indicated doses (10 μg/mL and 100 μg/mL of ANT1 peptides versus RNase 43-56).
Binding of ANT1 21-40 to MHC Class II Molecule, IAk, Is Poor Compared to Other Peptides
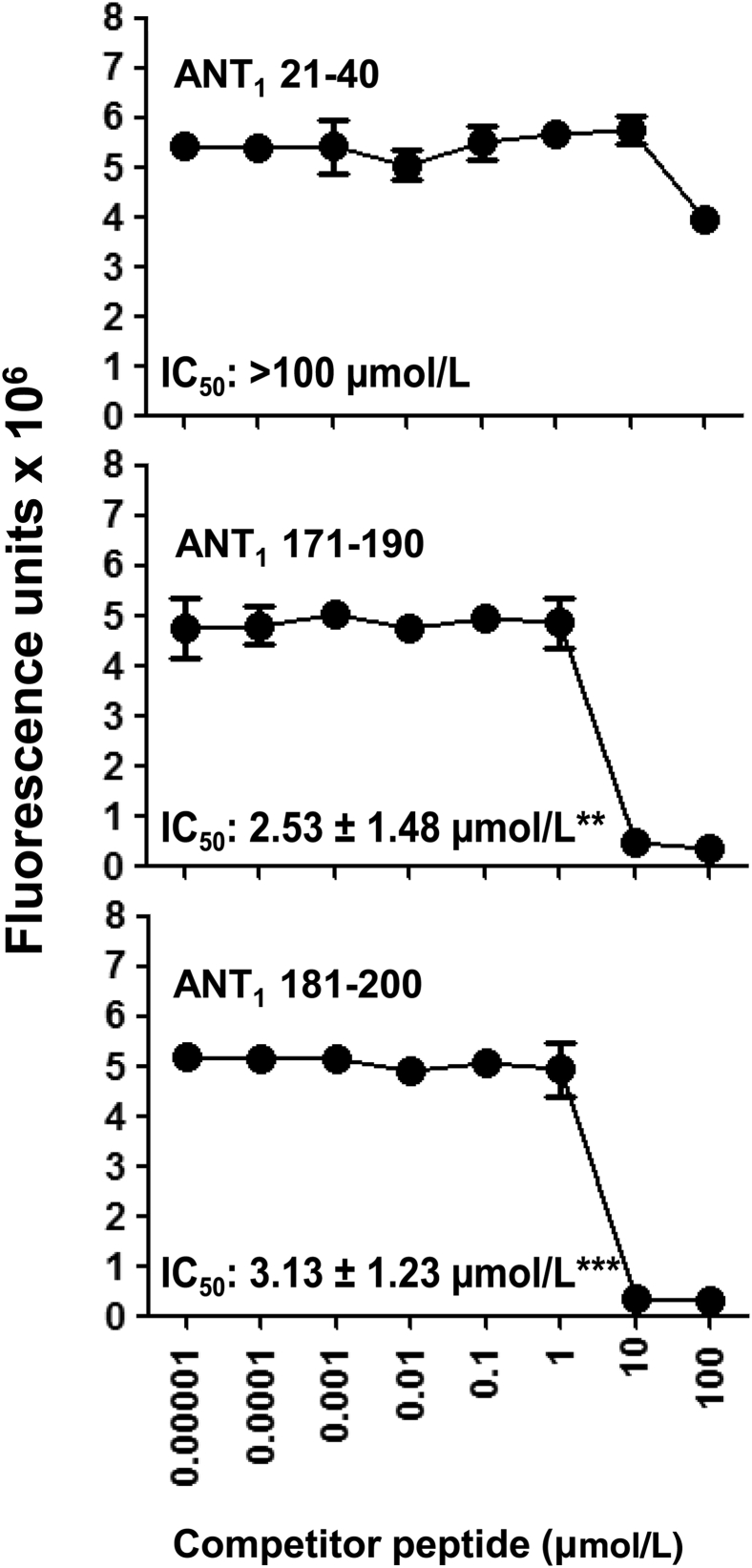
One characteristic feature of immunodominant epitopes that induce T-cell responses is their ability to bind MHC class II molecules. To evaluate whether the ANT1 peptides that we have identified can bind MHC molecules with similar affinity, we used IAk-binding assay.24 In brief, we used HEL 46-61, a well-characterized IAk-binder,41 as a reference peptide to derive the binding affinities of ANT1 peptides. We chose three peptides that induce T-cell responses but varying degrees of myocarditis: ANT1 21-40, an inducer of both myocarditis and T-cell response, and ANT1 171-190 and ANT1 181-200, both of which induce T-cell responses but with mild or no disease (Table 2). Unexpectedly, we found ANT1 21-40 to be a poor binder of IAk (IC50, >100 μmol/L) compared to ANT1 171-190 (IC50, 2.53 ± 1.48 μmol/L) and ANT1 181-200 (IC50, 3.13 ± 1.23 μmol/L) (Figure 3). It is possible that ANT1 21-40 may bind to the IEk molecule, which also is expressed in A/J mice42; however, because of the lack of reagents for the IEk-binding assay, we did not address this possibility.
Figure 3.
Binding of ANT1 peptides to IAk molecules. Reaction mixtures containing thrombin-cleaved IAk monomers (0.35 μg), competitor peptides [ANT1 21-40, ANT1 171-190, and ANT1 181-200 (0.00001 to 100 μmol/L)], and biotinylated HEL 46-61 (1 μmol/L) were individually prepared and added to fluorescence plates coated with anti-IAk in duplicate. After washing, europium-labeled streptavidin was added in dissociation-enhanced lanthanide fluoroimmunoassay (DELFIA) buffer, followed by DELFIA enhancer. Fluorescence intensity was measured at excitation/emission wavelengths of 340/615 nm, and 50% inhibitory concentration (IC50) values were calculated. Representative data sets from three individual experiments with two replicates in each are shown. Data are means ± SEM. ∗∗P < 0.01, ∗∗∗P < 0.001 versus ANT1 21-40 IC50.
ANT1 21-40–Sensitized T Cells Produce Predominantly Cytokines of Th17 Phenotype That Transfer Disease to Naïve Recipients
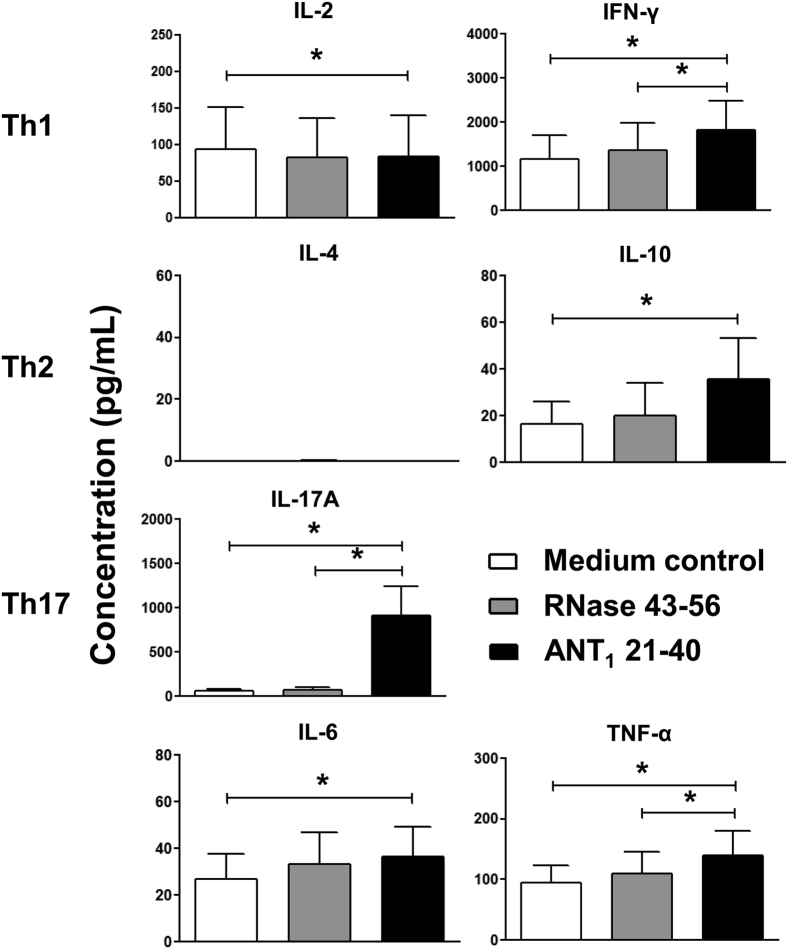
To evaluate the pathogenic potential of ANT1 21-40–sensitized T cells, we used two readouts. First, we evaluated cytokine secretion in the supernatants obtained from cells stimulated with ANT1 21-40, whereas supernatants obtained from RNase 43-56 (irrelevant peptide)–stimulated cultures and medium alone served as controls. As shown in Figure 4, cytokine analysis was performed for a panel of cytokines: T helper (Th) 1 (IL-2 and IFN-γ), Th2 (IL-4 and IL-10), Th17 (IL-17A), IL-6, and TNF-α. Evidently, excluding IL-4, supernatants from all groups were positive for all cytokines tested and the magnitude of cytokine responses to be higher for IFN-γ, and IL-17A, followed by IL-2 and TNF-α, and IL-6 and IL-10. Comparisons between groups revealed that the levels of the above cytokines were significantly higher in ANT1 21-40–sensitized culture supernatants in relation to control groups (medium alone or RNase 43-56; P ≤ 0.05) (Figure 4). Correspondingly, however, secretion of IL-2 was lower in ANT1 21-40 group as compared to controls (P = 0.052), suggesting that the proliferating cells may continuously consume IL-2. The findings that the total amount of inflammatory cytokines (IFN-γ, IL-6, IL-17A, and TNF-α) produced in response to ANT1 21-40 was more than IL-10 suggest that the inflammatory microenvironment can possibly negate the effects of anti-inflammatory cytokines, like IL-10. However, among the inflammatory cytokines, IL-17A may be a key contributor for disease pathology as its levels were strikingly elevated versus others. We next asked whether ANT1 21-40–sensitized T cells could transfer the disease to naïve mice. Briefly, LNCs obtained from ANT1 21-40–immunized mice were stimulated with concanavalin-A for 2 days. Viable cells were administered into naïve mice primed with lipopolysaccharide, and 14 days later, hearts were collected for histological evaluation of inflammatory changes. Figure 5 shows that heart sections from recipients of ANT1 21-40–reactive T cells (Figure 5, C and D), but neither those from the PBS group (Figure 5A) nor lipopolysaccharide-primed (Figure 5B) animals, showed inflammatory infiltrates, suggesting that ANT1 21-40–sensitized T cells are pathogenic. Thus, the histological data obtained with both active immunization (Figure 1 and Table 2) and adoptive transfer EAM (Figure 5) complement each other and indicate that ANT1 21-40 is a pathogenic epitope that can act as a target autoantigen in cardiac autoimmunity.
Figure 4.
Cytokine analysis in the supernatants derived from ANT1 21-40–stimulated cultures. Lymph node cells obtained from ANT1 21-40–immunized mice were stimulated with or without ANT1 21-40 or RNase 43-56 (control). Supernatants collected on day 3 after stimulation were analyzed using beads conjugated with cytokine-capture antibodies and detection antibodies by cytometric bead array, as described in Materials and Methods. Values obtained from four individual experiments are shown. Data are expressed as means ± SEM. ∗P ≤ 0.05. IFN-γ, interferon-γ; Th, T helper; TNF-α, tumor necrosis factor-α.
Figure 5.
Induction of myocarditis by ANT1 21-40–sensitized T cells in naïve mice. Lymph node cells were prepared from animals immunized for experimental autoimmune myocarditis induction or those immunized with peptide/complete Freund adjuvant emulsions only once, and the cells were stimulated with concanavalin-A for 2 days. Viable cells were then harvested and administered i.p. or retro-orbitally into naïve mice primed with lipopolysaccharide (LPS), where LPS-primed alone, and phosphate-buffered saline (PBS) only recipients were used as controls. All animals received pertussis toxin i.p., on days 0 and 2 after transfer. At termination on day 14, hearts were collected and examined for inflammatory changes by hematoxylin and eosin staining. Representative sections are shown from two individual experiments, each involving two mice per group: normal cardiac tissues in A (PBS control) and B (LPS control), as opposed to inflammatory foci (arrows) containing infiltration by mononuclear cells in C and D (recipients of ANT1 21-40–sensitized T cells). Scale bars = 40 μm (A–D).
Naïve Peripheral Repertoire in A/J Mice Does Not Contain ANT1 21-40–Reactive T cells
We asked whether preexisting autoreactive T-cell repertoire is a contributing factor for disease occurrence such that their expansion may occur in the immunized animals, leading to the development of myocarditis. The presence of such a repertoire of cells has previously shown to be correlated with the lack of expression of autoantigens, like cardiac myosin and proteolipid protein in the thymus.43, 44 Splenocytes harvested from naïve mice were used to assess their reactivity to ANT1 21-40. Using RNase 43-56 as a control peptide, we noted that the splenocytes did not respond to ANT1 21-40 (Supplemental Figure S2), suggesting the absence of preexisting endogenously derived repertoire of ANT-reactive T cells in the naïve animals.
Evidence that ANT1 21-40 Can Act as a Target Antigen for the Production of Autoantibodies
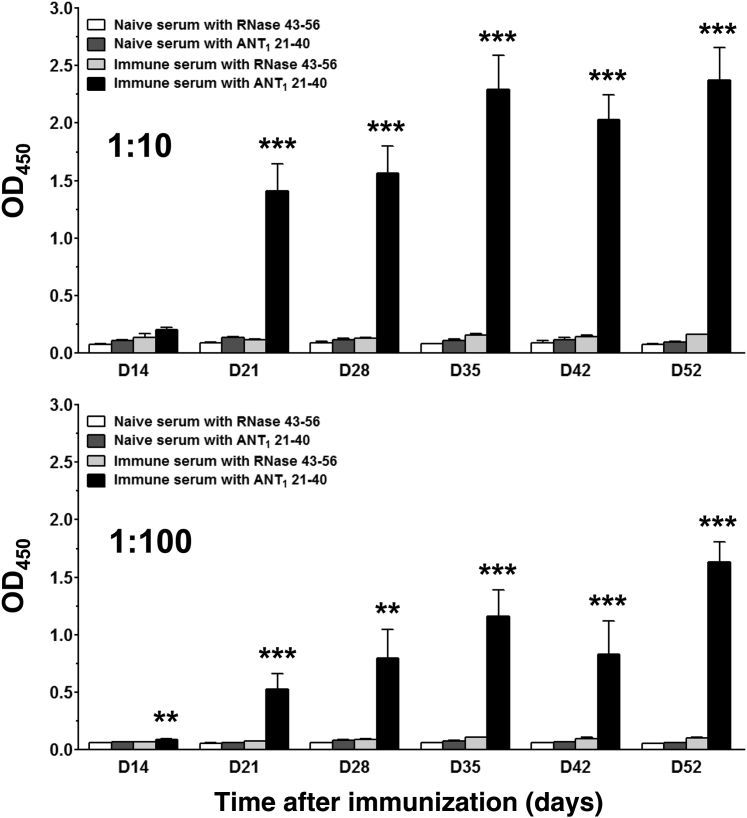
It was previously found that some ANT1 antibodies impair myocardial calcium homeostasis by cross-reacting with myolemmal calcium channels.22 ANT1 antibodies also were detected in patients with idiopathic DCM.21 In addition, mice immunized with human ANT1 peptides showed varied amounts of autoantibodies in association with DCM. In our model, by testing for antibodies in the serum samples collected from immunized animals, we noted a significant increase in the production of ANT1 21-40–reactive antibodies by at least 12- to 20-fold (day 21 to 52 after immunization) as compared to the control groups (P < 0.001), and the response was specific as the sera from ANT1 21-40–immunized animals did not react with the control peptide (RNase 43-56) (Figure 6). Similarly, sera from naïve animals also showed no reactivity to ANT1 21-40 (Figure 6). Because ANT1 21-40–immunized animals showed autoantibodies and also T-cell responses, the data suggest that ANT1 21-40 may act as a common epitope for both B cells and T cells.
Figure 6.
Evaluation of ANT1 21-40–reactive antibodies by enzyme-linked immunosorbent assay. Groups of A/J mice were immunized with or without ANT1 21-40 twice with an interval of 7 days, and pertussis toxin was administered on days 0 and 2 after the first immunization. Serum samples were collected for measurement of ANT1 21-40–reactive antibodies on indicated days. Plates were coated with ANT1 21-40 or RNase 43-56 (negative control) overnight at 4°C, and after washing and blocking, 1:10 (top panel) and 1:100 (bottom panel) diluted serum samples were added. Plates were further incubated for 1 hour at 37°C, followed by addition of secondary antibody. After washing and incubating with the substrate, OD values were measured at 450 nm. Data are means ± SEM. n = 3 to 10 individual mice. ∗∗P < 0.01, ∗∗∗P < 0.001 versus control groups.
Animals Immunized with ANT1 21-40 Showed Inflammatory Lesions in the Liver, but Are Not Specific to Antigen
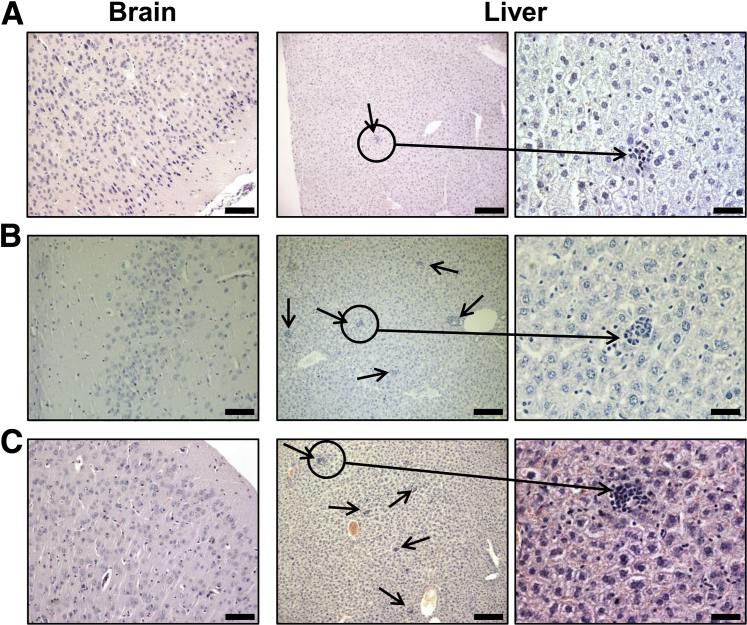
It has been reported that localization of different ANT isoforms varies by tissue, and our focused isoform ANT1 is highly expressed in heart and skeletal muscles, and brain.8 Therefore, we sought to determine whether noncardiac tissues from immunized animals show inflammatory infiltrates. We first verified the expression of all the three ANT isoforms (ANT1, ANT2, and ANT4) in A/J mice by RT-PCR analysis. The analysis revealed that ANT1 was expressed strongly in the heart, thigh muscle, and brain, but not in other organs (liver, thymus, and spleen) (Supplemental Figure S3). We could not provide histological data on thigh muscles as the tissues were not available, but brains from ANT1 21-40–immunized mice remained unaffected (Figure 7). Next, we compared the sequences of various ANT1 epitopes with other isoforms, leading us to note the sequence of ANT1 21-40 to be 95% identical with sequences of both ANT2 and ANT4 (Supplemental Table S2). Based on this observation, and also because of the finding that ANT1 21-40 was found to be a disease-inducing epitope, we envisioned that ANT1 21-40–reactive T cells would affect the organs in which other isoforms are highly expressed (ANT2: liver; and ANT4: testes). By evaluating livers for inflammatory changes, we noted that the livers obtained from mice immunized with ANT1 21-40 showed multiple scattered lesions containing aggregates of lymphocytes as opposed to scanty inflammatory foci in the naïve animals (20.86 ± 3.58 versus 2.10 ± 0.69; P = 0.0002) (Figure 7 and Table 4). In addition, by using AST as a biochemical readout of liver damage, we noted that serum from animals immunized with ANT1 21-40 showed elevated AST levels during the chronic phase of disease (beyond 30 days after immunization) as compared to naïve mice (Supplemental Figure S4). However, livers, but not brains, from animals that received CFA/PT with no peptide also showed relatively more inflammatory foci as compared to ANT1 21-40–immunized group (34.93 ± 2.82 versus 20.86 ± 3.58; P = 0.039) (Figure 7 and Table 4), suggesting that the occurrence of inflammation in livers from the ANT1 21-40 group is not specific to antigen. Thus, the data do not point to a possibility that the inflammatory changes might have resulted from ANT1 21-40 immunizations in the liver through cross-reaction with an equivalent epitope that exists in ANT2 (Supplemental Table S2).
Figure 7.
Histological evaluation of livers obtained from mice immunized with or without ANT1 21-40. Groups of A/J mice were immunized with or without ANT1 21-40 or complete Freund adjuvant (CFA) alone twice with an interval of 7 days, and the animals also received pertussis toxin (PT) on day 0 and day 2 after the first immunization. After 21 days, the indicated tissues harvested from both naive and immunized animals were evaluated for inflammatory changes by hematoxylin and eosin staining. A: Sections from naive group representing normal brain, and a small aggregate of lymphocytes in liver. Sections from CFA/PT (B), and sections from ANT1 21-40–immunized groups (C) showing unaffected brain; and liver with multiple scattered aggregates of lymphocytes (arrows). n = 5 to 14 mice per group (A–C). Scale bars: 80 μm (brain); 160 μm (liver, left column); 40 μm (liver, right column).
Table 4.
Histological Evaluation of Liver for Inflammatory Lesions
| Group | Incidence (%) | Inflammatory foci |
|---|---|---|
| Naïve | 8/10 (80) | 2.10 ± 0.69 |
| CFA/PT | 5/5 (100) | 34.93 ± 2.82∗∗∗ |
| ANT1 21-40 | 14/14 (100) | 20.86 ± 3.58∗∗∗ |
Data are expressed as means ± SEM. The ANT1 21-40 group represents data obtained from animals immunized with both nonacetylated and acetylated peptides.
∗∗∗P < 0.001 versus naïve group.
CFA, complete Freund adjuvant; PT, pertussis toxin.
Discussion
We described a new EAM model in which we demonstrated that ANT1 can act as a target autoantigen in the development of myocarditis. Clinically, myocarditis can lead to DCM, and antibodies to several cardiac antigens, including ANT1, have been detected in patients with DCM, suggesting that an autoimmune response can be one potential underlying mechanism of DCM pathogenesis. In support of this proposition, experimentally, it has been shown that mice deficient for ANT1 show DCM/myocardial hypertrophy and transgenic overexpression of ANT1 can improve myocardial contractile function.14, 18 Furthermore, in various experimental settings, formation of autoantibodies has been demonstrated in association with DCM in animals immunized with ANT peptides.4, 5, 6, 7, 21 Because CD4 T cells occupy a pivotal role in the induction of various effector responses, including antibody production for protein antigens, we made efforts to identify the T-cell epitopes of ANT to gain new insights into the pathogenesis of myocardial injuries. To this end, we focused on ANT1, because this isoform is highly expressed in muscle tissues than others.8
For identification of myocarditogenic epitopes of ANT1, we used pools of peptides for initial screening, and after identifying the pools that resulted in myocarditis, individual peptides were tested for their myocarditogenicity. These analyses led us to choose ANT1 21-40 as the major epitope that can induce myocarditis in A/J mice that are routinely being used to study cardiac autoimmunity.23 Although disease incidence induced with ANT1 21-40 was only 50%, our efforts to enhance its myocarditogenicity using the acetylated form of this peptide were not successful. A similar degree of myocarditis severity has been noted previously with cardiac myosin 614-643–induced myocarditis in rats.25 Pathologically, inflammation was a consistent finding noted in all the immunized animals. By verifying immunogenicity, ANT1 21-40 was found to induce T-cell response and T-cell sensitization might not have occurred through the ability of ANT1 21-40 to bind IAk (MHC class II molecule), as its IAk-binding affinity was estimated to be poor (Figure 3). Such an observation was previously made with another autoantigen, myelin basic protein acetylated 1-11, which also induces severe experimental autoimmune encephalomyelitis despite being a poor IAu-binder.34 It may be that in a polyclonal population, some T-cell clones can recognize such low-affinity peptides displayed by the MHC molecules in vivo. Alternatively, ANT1 21-40 might induce a T-cell response by binding to another MHC molecule, IEk, which is also expressed in A/J mice. We could not address this possibility because of the lack of reagents for the IEk molecule. In contrast, however, we noted three peptides that can induce T-cell responses, but with little (ANT1 31-50 and ANT1 171-190) or no disease (ANT1 181-200) (Figure 2 and Table 2). Similar observations were also previously noted for other self-antigens, like myelin oligodendrocyte glycoprotein 181-195 and 186-200,45 and proteolipid protein 50-69 and 60-79,46 supporting the notion that generation of autoreactive T cells does not necessarily mean that autoimmune responses ensue.
Next, we addressed the mechanistic basis for the induction of myocarditis induced with ANT1 21-40. Unlike other autoimmune disease models, it was initially held that Th2 cytokines, like IL-4, favor myocarditis development,27 whereas Th1 cytokines, more important, IFN-γ can dampen such an outcome.47 However, with the discovery of Th17 cytokines, the current theory is that both Th1 and Th17 cytokines promote myocardial damage and that Th2 cytokines mediate anti-inflammatory functions, much as they do in other autoimmune disease models, such as cardiac myosin heavy chain-α 334-352–induced myocarditis, experimental autoimmune encephalomyelitis induced with myelin oligodendrocyte glycoprotein 35-55 or proteolipid protein 139-151, and experimental autoimmune uveitis induced with interphotoreceptor retinoid-binding protein 161-180.7, 48, 49, 50, 51, 52 By evaluating cytokine production in ANT1 21-40–sensitized T-cell cultures, we noted increased production of all inflammatory cytokines (IFN-γ, IL-17A, IL-6, and TNF-α), including anti-inflammatory cytokine, IL-10. But the finding that the amount of secreted IL-10 was negligible relative to cytokines that promote autoimmunity, more important, IFN-γ and IL-17A, suggests that the disease-modifying effects of IL-10 are expected to be insignificant in the microenvironment in vivo. Furthermore, we asked whether variations, if any, in cytokine production can differentiate peptides that induce T-cell responses and disease, from those that induce only T-cell responses, but no disease. We addressed this possibility by evaluating cytokine secretion in response to one such epitope, ANT1 181-200, that induces T-cell response but not myocarditis. Expectedly, none of the cytokines was found significantly increased in peptide-stimulated cultures as compared to control groups (Supplemental Figure S5). Together, the data suggest that the T-cell–derived inflammatory cytokines, in particular IL-17A, appear to be key mediators of tissue damage in the target organs, as we and others have seen with other animal models, as indicated above.
Our data may have a translational significance to support an observation made in humans in that the severe combined immunodeficient mice receiving peripheral blood lymphocytes from patients with myocarditis carrying autoantibodies for ANT showed infiltration of lymphocytes in the hearts, and impairment of left ventricular function.53, 54 Furthermore, antibody reactivity has been noted for two ANT epitopes (namely, hANT1 27-36 and hANT1 290-297) in 62% of myocarditis/DCM patients.55 In addition, Balb/c mice immunized with hANT1 27-36 and hANT1 290-297 showed autoantibodies in association with DCM without affecting other organs, such as lung, liver, and kidney.37 In our model, we also noted antibodies to ANT1 21-40 in the immunized animals. Because the sequence of the mouse peptide, ANT1 21-40, is identical with the human counterpart (100%), which also encompasses the hANT1 27-36 sequence, the ANT1 21-40–reactive antibodies may be pathologically relevant. Likewise, antibodies for other epitopes, like ANT1 11-30 and ANT1 131-150, may be relevant to cardiac pathology because these peptides can induce myocarditis, albeit low (10% to 30% of animals) in the absence of T-cell reactivity (Table 2 and Figure 2B). In all of these scenarios, there exists a possibility that autoantibodies can alter ANT1 functions, like energy metabolism or calcium homeostasis, but whether such antibodies can also mediate tissue destruction through immune complex–driven complement activation has not been shown. Addressing the latter also requires a mechanistic understanding of how autoantibodies can reach mitochondria across the cell membrane in cardiac cells.
Additional investigations in our model revealed two more observations. Unlike experimental autoimmune encephalomyelitis induced with proteolipid protein 139-151,43, 56 preexistence of endogenous repertoire of ANT1-reactive T cells appears not critical for the induction of myocarditis with ANT1 21-40 as the naïve animals did not show reactivity to ANT1 21-40. The occurrence of inflammation in the liver in ANT1 21-40–immunized animals as well as in those that received CFA/PT with no peptide suggests that ANT1 21-40 cannot be a target antigen for induction of hepatitis. Although we did not examine skeletal muscles, we had expected inflammation in the brain where ANT1 is also highly expressed, but it was not the case. Impermeability of autoreactive T cells into the brain by being an immune-privileged organ is unlikely because the immunized animals had received pertussis toxin, which is expected to break the blood-brain barrier.57 It is, however, possible that ANT1 21-40–sensitized T cells may reach the brain, but may not gain access to ANT. Nonetheless, inflammatory lesions in the liver were expected because the sequence of ANT1 21-40 is 95% identical to an equivalent epitope within ANT2. However, livers in naïve animals also can show infiltrations as a consequence of immune reactions to gut microflora.58 More important, animals that received only CFA/PT also showed relatively more inflammatory foci than the ANT1 21-40–immunized group. Although the reason for such an elevation is unknown, occurrence of non-specific inflammation in livers from animals immunized with CFA has been described previously in C57Bl/6 mice, and it was speculated to be because of adjuvant oil.59
Although the data presented in this report support the notion that mitochondrial proteins, like ANT1, can become targets for autoreactive T cells, it is still unclear how the T cells can recognize such proteins. A similar scenario has been described for one other mitochondrial protein, the E2 subunit of pyruvate dehydrogenase, a target antigen for the generation of autoreactive T cells in the mediation of primary biliary cirrhosis.60, 61 In this system, it is proposed that the epithelial cells in the bile duct can aberrantly express MHC class II molecules and that mitochondrial protein may find a way to assemble with the MHC molecules. In addition, surface expression of pyruvate dehydrogenase also has been demonstrated.62 Whether such mechanisms are also relevant to ANT1 remains to be investigated. However, reports suggest that resident antigen-presenting cells in heart tissue can carry the peptide fragments of intracellular cardiac proteins, like myosin heavy chain-α.23 It is possible that resident antigen-presenting cells, like dendritic cells or macrophages, can present the proteins from cardiac cells acquired by phagocytosis or autophagy through cross-presentation pathway. Speculations have been made that mitochondrial membrane proteins may escape into the cytoplasm because of faulty leader sequences due to possible mutations, leading to their eventual assembly with MHC molecules.63 All of these observations suggest that mitochondrial proteins may gain access by some means to MHC presentation pathways. In addition, a possibility exists that ANT1 expression may occur aberrantly at the cell surface in the hearts of A/J mice. Previous demonstration of the expression of ANT1 or ANT2 in the plasma membranes of cerebellar neurons in C57Bl/6 mice supports this possibility.64
In summary, we have demonstrated that the mitochondrial protein ANT1 can be a source of target antigen for immune attack by autoreactive T cells in the mediation of autoimmune myocarditis. We have identified ANT1 21-40 as a myocarditogenic epitope, and the disease induced by this peptide resembles the features of inflammatory cardiomyopathy that can occur with or without myocyte necrosis.65 Furthermore, we demonstrate that ANT1 21-40 cannot act as an autoantigen in the mediation of hepatitis as the animals immunized with CFA/PT also showed lesions in the liver. Based on antibody detection, ANT1 has been shown to be relevant to DCM pathogenesis that can emanate from myocarditis as a chronic sequela.5 Because we show that ANT1 21-40 can induce myocarditis by generating autoreactive T cells, and T-cell help is critical for the production of antibodies to protein antigens, evaluation of both autoreactive T cells and ANT1 antibodies may prove useful to find strong correlations with DCM in clinical settings. The finding that ANT being a mitochondrial protein acting as a target antigen in the development of myocarditis, the disease induced with ANT1 21-40 can be used to address the autoimmune pathology in the heart. Our data also raise questions as to how self-tolerance for mitochondrial proteins can be attained, and how such a tolerance can be broken. Mechanistic delineation of this phenomenon may provide new insights into the development of heart autoimmunity.
Acknowledgments
R.H.B., C.M., and J.R. conceived and designed the experiments; R.H.B. and C.M. performed the experiments; R.H.B., B.K., A.G., J.-J.R., R.A.S., and D.S. analyzed the data; G.K., V.K.-S., Z.H., S.O., and Q.L. contributed reagents/materials/analysis tools; and R.H.B. and J.R wrote the manuscript.
Footnotes
Supported by NIH grant HL114669 (J.R.), and the National Institute of General Medical Science grant P20GM104320, Nebraska Center for the Prevention of Obesity Diseases, University of Nebraska-Lincoln.
Disclosures: None declared.
Current address of A.G., Laboratory of Immunology, National Institute of Allergy and Infectious Diseases, NIH, Bethesda, MD.
Supplemental material for this article can be found at http://dx.doi.org/10.1016/j.ajpath.2016.08.005.
Supplemental Data
Evaluation of cardiac abnormalities in animals immunized with ANT1 21-40 by magnetic resonance microscopy (MRM) imaging. A: Left ventricular (LV) wall thickness. Groups of mice were immunized with or without ANT1 21-40 in complete Freund adjuvant on day 0 and day 7, and pertussis toxin was administered on day 0 and day 2 after the first immunization. Unimmunized (healthy) and ANT1 21-40–immunized animals were subjected for MRM imaging on days 30 to 35 to evaluate LV wall thickness, as described in Materials and Methods (double-headed arrows indicate LV wall thickness). Images from three individual mice from each group are shown. B: Body weight/heart weight ratios. Body weights of the healthy and ANT1 21-40–immunized mice were determined on the indicated days and plotted against days after immunization (left panel). At the termination on day 52, hearts were weighed and the heart weight (wt)/body wt ratios were then determined. Values representing heart weights (middle panel) and heart wt/body wt ratio (right panel) for a group of mice are shown. Data are expressed as means ± SEM (B). n = 3 to 6 mice per group (A and B). ∗P < 0.05 versus naïve group.
Proliferative response of splenocytes to ANT1 21-40 in naïve mice. Splenocytes harvested from naïve mice were stimulated with ANT1 21-40 or RNase 43-56 for 2 days; after pulsing with 3[H]-thymidine for 16 hours, proliferative responses were measured as counts per minute (cpm). Data are expressed as means ± SEM. n = 3 individual mice.
RT-PCR analysis of ANT isoforms in A/J mice. Lymphoid (thymus and spleen) and nonlymphoid (heart, thigh muscle, brain, and liver) tissues were collected from 6- to 8-week-old A/J mice, and total RNA was isolated. After synthesizing the cDNA, expression levels of ANT1, ANT2, and ANT4 were examined by PCR using gene-specific primers. The PCR amplicons were resolved on 1.5% ethidium bromide–stained agarose gel electrophoretic analysis. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH), loading control. n = 3 mice.
Estimation of serum aspartate aminotransferase (AST) in mice immunized with ANT1 21-40. Groups of mice were immunized with or without ANT1 21-40 in complete Freund adjuvant on day 0 and day 7, and pertussis toxin was administered on day 0 and day 2 after the first immunization. Serum samples collected on indicated days after immunization were estimated for AST levels. Data are expressed as means ± SEM. n = 3 to 5 mice per group. ∗P < 0.05 versus naïve group.
Cytokine analysis in the supernatants derived from ANT1 181-200–stimulated cultures. Lymph node cells obtained from ANT1 181-200–immunized mice were stimulated with or without ANT1 181-200 or RNase 43-56 (control). Supernatants collected on day 3 after stimulation were analyzed using beads conjugated with cytokine-capture antibodies and detection antibodies by cytometric bead array, as described in Materials and Methods. Data are expressed as means ± SEM. n = 3 experiments. IFN-γ, interferon-γ; Th, T helper; TNF-α, tumor necrosis factor-α.
References
- 1.Brown C.A., O'Connell J.B. Myocarditis and idiopathic dilated cardiomyopathy. Am J Med. 1995;99:309–314. doi: 10.1016/S0002-9343(99)80164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fujinami R.S., von Herrath M.G., Christen U., Whitton J.L. Molecular mimicry, bystander activation, or viral persistence: infections and autoimmune disease. Clin Microbiol Rev. 2006;19:80–94. doi: 10.1128/CMR.19.1.80-94.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor D.O., Edwards L.B., Boucek M.M., Trulock E.P., Aurora P., Christie J., Dobbels F., Rahmel A.O., Keck B.M., Hertz M.I. Registry of the International Society for Heart and Lung Transplantation: twenty-fourth official adult heart transplant report–2007. J Heart Lung Transplant. 2007;26:769–781. doi: 10.1016/j.healun.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Liao Y., Tu Y., Chen L., Dai S., Peng Y., Li S., Zhang J. Cardiac cytotoxic mechanism mediated by antibodies against myocardial mitochondrial ADP/ATP carrier in patients with dilated cardiomyopathy. Chin Med J. 1996;109:193–196. [PubMed] [Google Scholar]
- 5.Schultheiss H.P., Bolte H.D. Immunological analysis of auto-antibodies against the adenine nucleotide translocator in dilated cardiomyopathy. J Mol Cell Cardiol. 1985;17:603–617. doi: 10.1016/s0022-2828(85)80029-8. [DOI] [PubMed] [Google Scholar]
- 6.Schulze K., Becker B.F., Schauer R., Schultheiss H.P. Antibodies to ADP-ATP carrier–an autoantigen in myocarditis and dilated cardiomyopathy–impair cardiac function. Circulation. 1990;81:959–969. doi: 10.1161/01.cir.81.3.959. [DOI] [PubMed] [Google Scholar]
- 7.Cihakova D., Rose N.R. Pathogenesis of myocarditis and dilated cardiomyopathy. Adv Immunol. 2008;99:95–114. doi: 10.1016/S0065-2776(08)00604-4. [DOI] [PubMed] [Google Scholar]
- 8.Levy S.E., Chen Y.S., Graham B.H., Wallace D.C. Expression and sequence analysis of the mouse adenine nucleotide translocase 1 and 2 genes. Gene. 2000;254:57–66. doi: 10.1016/s0378-1119(00)00252-3. [DOI] [PubMed] [Google Scholar]
- 9.Rodic N., Oka M., Hamazaki T., Murawski M.R., Jorgensen M., Maatouk D.M., Resnick J.L., Li E., Terada N. DNA methylation is required for silencing of ant4, an adenine nucleotide translocase selectively expressed in mouse embryonic stem cells and germ cells. Stem Cells. 2005;23:1314–1323. doi: 10.1634/stemcells.2005-0119. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y., Chen X.J. Adenine nucleotide translocase, mitochondrial stress, and degenerative cell death. Oxid Med Cell Longev. 2013;2013:146860. doi: 10.1155/2013/146860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Portman M.A. The adenine nucleotide translocator: regulation and function during myocardial development and hypertrophy. Clin Exp Pharmacol Physiol. 2002;29:334–338. doi: 10.1046/j.1440-1681.2002.03654.x. [DOI] [PubMed] [Google Scholar]
- 12.Belzacq A.S., Vieira H.L., Kroemer G., Brenner C. The adenine nucleotide translocator in apoptosis. Biochimie. 2002;84:167–176. doi: 10.1016/s0300-9084(02)01366-4. [DOI] [PubMed] [Google Scholar]
- 13.Sharer J.D. The adenine nucleotide translocase type 1 (ANT1): a new factor in mitochondrial disease. IUBMB Life. 2005;57:607–614. doi: 10.1080/15216540500217735. [DOI] [PubMed] [Google Scholar]
- 14.Narula N., Zaragoza M.V., Sengupta P.P., Li P., Haider N., Verjans J., Waymire K., Vannan M., Wallace D.C. Adenine nucleotide translocase 1 deficiency results in dilated cardiomyopathy with defects in myocardial mechanics, histopathological alterations, and activation of apoptosis. JACC Cardiovasc Imaging. 2011;4:1–10. doi: 10.1016/j.jcmg.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phillips M.J., Webb-Wood S., Faulkner A.E., Jabbar S.B., Biousse V., Newman N.J., Do V.T., Boatright J.H., Wallace D.C., Pardue M.T. Retinal function and structure in Ant1-deficient mice. Invest Ophthalmol Vis Sci. 2010;51:6744–6752. doi: 10.1167/iovs.10-5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graham B.H., Waymire K.G., Cottrell B., Trounce I.A., MacGregor G.R., Wallace D.C. A mouse model for mitochondrial myopathy and cardiomyopathy resulting from a deficiency in the heart/muscle isoform of the adenine nucleotide translocator. Nat Genet. 1997;16:226–234. doi: 10.1038/ng0797-226. [DOI] [PubMed] [Google Scholar]
- 17.Heger J., Abdallah Y., Shahzad T., Klumpe I., Piper H.M., Schultheiss H.P., Schluter K.D., Schulz R., Euler G., Dorner A. Transgenic overexpression of the adenine nucleotide translocase 1 protects cardiomyocytes against TGFbeta1-induced apoptosis by stabilization of the mitochondrial permeability transition pore. J Mol Cell Cardiol. 2012;53:73–81. doi: 10.1016/j.yjmcc.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 18.Vogelpohl I., Vetter R., Heger J., Ebermann L., Euler G., Schultheiss H.P., Dorner A. Transgenic overexpression of heart-specific adenine nucleotide translocase 1 positively affects contractile function in cardiomyocytes. Cell Physiol Biochem. 2011;27:121–128. doi: 10.1159/000325214. [DOI] [PubMed] [Google Scholar]
- 19.Walther T., Tschope C., Sterner-Kock A., Westermann D., Heringer-Walther S., Riad A., Nikolic A., Wang Y., Ebermann L., Siems W.E., Bader M., Shakibaei M., Schultheiss H.P., Dorner A. Accelerated mitochondrial adenosine diphosphate/adenosine triphosphate transport improves hypertension-induced heart disease. Circulation. 2007;115:333–344. doi: 10.1161/CIRCULATIONAHA.106.643296. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y., Ebermann L., Sterner-Kock A., Wika S., Schultheiss H.P., Dorner A., Walther T. Myocardial overexpression of adenine nucleotide translocase 1 ameliorates diabetic cardiomyopathy in mice. Exp Physiol. 2009;94:220–227. doi: 10.1113/expphysiol.2008.044800. [DOI] [PubMed] [Google Scholar]
- 21.Manchado C., Orus J., Villarroya F., Roig E., Heras M., Giralt M., Iglesias R., Sanz G., Mampel T., Vinas O. Epitope mapping of mitochondrial adenine nucleotide translocase-1 in idiopathic dilated cardiomyopathy. J Mol Cell Cardiol. 2002;34:571–582. doi: 10.1006/jmcc.2002.1538. [DOI] [PubMed] [Google Scholar]
- 22.Schulze K., Heineman F.W., Schultheiss H.P., Balaban R.S. Impairment of myocardial calcium homeostasis by antibodies against the adenine nucleotide translocator. Cell Calcium. 1999;25:361–370. doi: 10.1054/ceca.1999.0039. [DOI] [PubMed] [Google Scholar]
- 23.Donermeyer D.L., Beisel K.W., Allen P.M., Smith S.C. Myocarditis-inducing epitope of myosin binds constitutively and stably to I-Ak on antigen-presenting cells in the heart. J Exp Med. 1995;182:1291–1300. doi: 10.1084/jem.182.5.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Massilamany C., Gangaplara A., Steffen D., Reddy J. Identification of novel mimicry epitopes for cardiac myosin heavy chain-alpha that induce autoimmune myocarditis in A/J mice. Cell Immunol. 2011;271:438–449. doi: 10.1016/j.cellimm.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 25.Pummerer C.L., Luze K., Grassl G., Bachmaier K., Offner F., Burrell S.K., Lenz D.M., Zamborelli T.J., Penninger J.M., Neu N. Identification of cardiac myosin peptides capable of inducing autoimmune myocarditis in BALB/c mice. J Clin Invest. 1996;97:2057–2062. doi: 10.1172/JCI118642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Massilamany C., Gangaplara A., Basavalingappa R.H., Rajasekaran R.A., Khalilzad-Sharghi V., Han Z., Othman S., Steffen D., Reddy J. Localization of CD8 T cell epitope within cardiac myosin heavy chain-alpha334-352 that induces autoimmune myocarditis in A/J mice. Int J Cardiol. 2016;202:311–321. doi: 10.1016/j.ijcard.2015.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Afanasyeva M., Wang Y., Kaya Z., Park S., Zilliox M.J., Schofield B.H., Hill S.L., Rose N.R. Experimental autoimmune myocarditis in A/J mice is an interleukin-4-dependent disease with a Th2 phenotype. Am J Pathol. 2001;159:193–203. doi: 10.1016/S0002-9440(10)61685-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Z.W., Ahren B., Ostenson C.G., Cintra A., Bergman T., Moller C., Fuxe K., Mutt V., Jornvall H., Efendic S. Identification, isolation, and characterization of daintain (allograft inflammatory factor 1), a macrophage polypeptide with effects on insulin secretion and abundantly present in the pancreas of prediabetic BB rats. Proc Natl Acad Sci U S A. 1997;94:13879–13884. doi: 10.1073/pnas.94.25.13879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kohler C. Allograft inflammatory factor-1/ionized calcium-binding adapter molecule 1 is specifically expressed by most subpopulations of macrophages and spermatids in testis. Cell Tissue Res. 2007;330:291–302. doi: 10.1007/s00441-007-0474-7. [DOI] [PubMed] [Google Scholar]
- 30.Lai L., Alaverdi N., Maltais L., Morse H.C., 3rd Mouse cell surface antigens: nomenclature and immunophenotyping. J Immunol. 1998;160:3861–3868. [PubMed] [Google Scholar]
- 31.Massilamany C., Khalilzad-Sharghi V., Gangaplara A., Steffen D., Othman S.F., Reddy J. Noninvasive assessment of cardiac abnormalities in experimental autoimmune myocarditis by magnetic resonance microscopy imaging in the mouse. J Vis Exp. 2014;88:e51654. doi: 10.3791/51654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Massilamany C., Steffen D., Reddy J. An epitope from Acanthamoeba castellanii that cross-react with proteolipid protein 139-151-reactive T cells induces autoimmune encephalomyelitis in SJL mice. J Neuroimmunol. 2010;219:17–24. doi: 10.1016/j.jneuroim.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 33.Reddy J., Bettelli E., Nicholson L., Waldner H., Jang M.H., Wucherpfennig K.W., Kuchroo V.K. Detection of autoreactive myelin proteolipid protein 139-151-specific T cells by using MHC II (IAs) tetramers. J Immunol. 2003;170:870–877. doi: 10.4049/jimmunol.170.2.870. [DOI] [PubMed] [Google Scholar]
- 34.Fugger L., Liang J., Gautam A., Rothbard J.B., McDevitt H.O. Quantitative analysis of peptides from myelin basic protein binding to the MHC class II protein, I-Au, which confers susceptibility to experimental allergic encephalomyelitis. Mol Med. 1996;2:181–188. [PMC free article] [PubMed] [Google Scholar]
- 35.Hausmann D.H., Yu B., Hausmann S., Wucherpfennig K.W. pH-dependent peptide binding properties of the type I diabetes-associated I-Ag7 molecule: rapid release of CLIP at an endosomal pH. J Exp Med. 1999;189:1723–1734. doi: 10.1084/jem.189.11.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamada Y., Takata M., Kiyoku H., Enzan H., Doi Y., Fujimoto S. Monomethoxypolyethylene glycol-modified cardiac myosin treatment blocks the active and passive induction of experimental autoimmune myocarditis. Circ J. 2004;68:149–155. doi: 10.1253/circj.68.149. [DOI] [PubMed] [Google Scholar]
- 37.Liao Y.H., Yuan J., Wang Z.H., Cheng X., Zhang J.H., Tian Y., Dong J.H., Guo H.P., Wang M. Infectious tolerance to ADP/ATP carrier peptides induced by anti-L3T4 monoclonal antibody in dilated cardiomyopathy mice. J Clin Immunol. 2005;25:376–384. doi: 10.1007/s10875-005-4187-y. [DOI] [PubMed] [Google Scholar]
- 38.Shabalina I.G., Kramarova T.V., Nedergaard J., Cannon B. Carboxyatractyloside effects on brown-fat mitochondria imply that the adenine nucleotide translocator isoforms ANT1 and ANT2 may be responsible for basal and fatty-acid-induced uncoupling respectively. Biochem J. 2006;399:405–414. doi: 10.1042/BJ20060706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Massilamany C., Gangaplara A., Gardner D.J., Musser J.M., Steffen D., Somerville G.A., Reddy J. TCA cycle inactivation in Staphylococcus aureus alters nitric oxide production in RAW 264.7 cells. Mol Cell Biochem. 2011;355:75–82. doi: 10.1007/s11010-011-0840-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao W., Wegmann K.W., Trotter J.L., Ueno K., Hickey W.F. Identification of an N-terminally acetylated encephalitogenic epitope in myelin proteolipid apoprotein for the Lewis rat. J Immunol. 1994;153:901–909. [PubMed] [Google Scholar]
- 41.Babbitt B.P., Allen P.M., Matsueda G., Haber E., Unanue E.R. Binding of immunogenic peptides to Ia histocompatibility molecules. Nature. 1985;317:359–361. doi: 10.1038/317359a0. [DOI] [PubMed] [Google Scholar]
- 42.Hirayama M., Azuma E., Jiang Q., Kobayashi M., Iwamoto S., Kumamoto T., Kisenge R., Yamamoto H., Komada Y. The reconstitution of CD45RBhiCD4+ naive T cells is inversely correlated with donor age in murine allogeneic haematopoietic stem cell transplantation. Br J Haematol. 2000;111:700–707. doi: 10.1046/j.1365-2141.2000.02391.x. [DOI] [PubMed] [Google Scholar]
- 43.Klein L., Klugmann M., Nave K.A., Tuohy V.K., Kyewski B. Shaping of the autoreactive T-cell repertoire by a splice variant of self protein expressed in thymic epithelial cells. Nat Med. 2000;6:56–61. doi: 10.1038/71540. [DOI] [PubMed] [Google Scholar]
- 44.Lv H., Havari E., Pinto S., Gottumukkala R.V., Cornivelli L., Raddassi K., Matsui T., Rosenzweig A., Bronson R.T., Smith R., Fletcher A.L., Turley S.J., Wucherpfennig K., Kyewski B., Lipes M.A. Impaired thymic tolerance to alpha-myosin directs autoimmunity to the heart in mice and humans. J Clin Invest. 2011;121:1561–1573. doi: 10.1172/JCI44583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shetty A., Gupta S.G., Varrin-Doyer M., Weber M.S., Prod'homme T., Molnarfi N., Ji N., Nelson P.A., Patarroyo J.C., Schulze-Topphoff U., Fogal S.E., Forsthuber T., Sobel R.A., Bernard C.C., Slavin A.J., Zamvil S.S. Immunodominant T-cell epitopes of MOG reside in its transmembrane and cytoplasmic domains in EAE. Neurol Neuroimmunol Neuroinflamm. 2014;1:e22. doi: 10.1212/NXI.0000000000000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Greer J.M., Sobel R.A., Sette A., Southwood S., Lees M.B., Kuchroo V.K. Immunogenic and encephalitogenic epitope clusters of myelin proteolipid protein. J Immunol. 1996;156:371–379. [PubMed] [Google Scholar]
- 47.Fairweather D., Frisancho-Kiss S., Yusung S.A., Barrett M.A., Davis S.E., Steele R.A., Gatewood S.J., Rose N.R. IL-12 protects against coxsackievirus B3-induced myocarditis by increasing IFN-gamma and macrophage and neutrophil populations in the heart. J Immunol. 2005;174:261–269. doi: 10.4049/jimmunol.174.1.261. [DOI] [PubMed] [Google Scholar]
- 48.Bettelli E., Oukka M., Kuchroo V.K. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 49.Eriksson U. Springer; Basel: 2013. The Role of IL-17 in Experimental Autoimmune Myocarditis. IL-17, IL-22 and Their Producing Cells: Role in Inflammation and Autoimmunity; pp. 165–175. [Google Scholar]
- 50.Jager A., Dardalhon V., Sobel R.A., Bettelli E., Kuchroo V.K. Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. J Immunol. 2009;183:7169–7177. doi: 10.4049/jimmunol.0901906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luger D., Silver P.B., Tang J., Cua D., Chen Z., Iwakura Y., Bowman E.P., Sgambellone N.M., Chan C.C., Caspi R.R. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J Exp Med. 2008;205:799–810. doi: 10.1084/jem.20071258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stromnes I.M., Cerretti L.M., Liggitt D., Harris R.A., Goverman J.M. Differential regulation of central nervous system autoimmunity by T(H)1 and T(H)17 cells. Nat Med. 2008;14:337–342. doi: 10.1038/nm1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schwimmbeck P.L., Badorff C., Schultheiss H.P., Strauer B.E. Transfer of human myocarditis into severe combined immunodeficiency mice. Circ Res. 1994;75:156–164. doi: 10.1161/01.res.75.1.156. [DOI] [PubMed] [Google Scholar]
- 54.Schwimmbeck P.L., Badorff C., Rohn G., Schulze K., Schultheiss H.P. Impairment of left ventricular function in combined immune deficiency mice after transfer of peripheral blood leukocytes from patients with myocarditis. Eur Heart J. 1995;16 Suppl O:59–63. doi: 10.1093/eurheartj/16.suppl_o.59. [DOI] [PubMed] [Google Scholar]
- 55.Schwimmbeck P.L., Bland N.K., Schultheiss H.P., Strauer B.E. The possible value of synthetic peptides in the diagnosis and therapy of myocarditis and dilated cardiomyopathy. Eur Heart J. 1991;12 Suppl D:76–80. doi: 10.1093/eurheartj/12.suppl_d.76. [DOI] [PubMed] [Google Scholar]
- 56.Anderson A.C., Nicholson L.B., Legge K.L., Turchin V., Zaghouani H., Kuchroo V.K. High frequency of autoreactive myelin proteolipid protein-specific T cells in the periphery of naive mice: mechanisms of selection of the self-reactive repertoire. J Exp Med. 2000;191:761–770. doi: 10.1084/jem.191.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Linthicum D.S., Munoz J.J., Blaskett A. Acute experimental autoimmune encephalomyelitis in mice, I: adjuvant action of Bordetella pertussis is due to vasoactive amine sensitization and increased vascular permeability of the central nervous system. Cell Immunol. 1982;73:299–310. doi: 10.1016/0008-8749(82)90457-9. [DOI] [PubMed] [Google Scholar]
- 58.Scudamore C.L. Wiley-Blackwell; Hoboken, NJ: 2014. A practical guide to the histology of the mouse; pp. 43–61. [Google Scholar]
- 59.Howell C.D., Yoder T.D. Murine experimental autoimmune hepatitis: nonspecific inflammation due to adjuvant oil. Clin Immunol Immunopathol. 1994;72:76–82. doi: 10.1006/clin.1994.1109. [DOI] [PubMed] [Google Scholar]
- 60.Nishio A., Keeffe E.B., Gershwin M.E. Primary biliary cirrhosis: lessons learned from an organ-specific disease. Clin Exp Med. 2001;1:165–178. doi: 10.1007/s102380100000. [DOI] [PubMed] [Google Scholar]
- 61.Van de Water J., Ansari A.A., Surh C.D., Coppel R., Roche T., Bonkovsky H., Kaplan M., Gershwin M.E. Evidence for the targeting by 2-oxo-dehydrogenase enzymes in the T cell response of primary biliary cirrhosis. J Immunol. 1991;146:89–94. [PubMed] [Google Scholar]
- 62.Joplin R., Lindsay J.G., Johnson G.D., Strain A., Neuberger J. Membrane dihydrolipoamide acetyltransferase (E2) on human biliary epithelial cells in primary biliary cirrhosis. Lancet. 1992;339:93–94. doi: 10.1016/0140-6736(92)91001-o. [DOI] [PubMed] [Google Scholar]
- 63.Mackay I.R., Gershwin M.E. Molecular basis of mitochondrial autoreactivity in primary biliary cirrhosis. Immunol Today. 1989;10:315–318. doi: 10.1016/0167-5699(89)90088-1. [DOI] [PubMed] [Google Scholar]
- 64.Loers G., Makhina T., Bork U., Dorner A., Schachner M., Kleene R. The interaction between cell adhesion molecule L1, matrix metalloproteinase 14, and adenine nucleotide translocator at the plasma membrane regulates L1-mediated neurite outgrowth of murine cerebellar neurons. J Neurosci. 2012;32:3917–3930. doi: 10.1523/JNEUROSCI.6165-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Calabrese F., Thiene G. Myocarditis and inflammatory cardiomyopathy: microbiological and molecular biological aspects. Cardiovasc Res. 2003;60:11–25. doi: 10.1016/s0008-6363(03)00475-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Evaluation of cardiac abnormalities in animals immunized with ANT1 21-40 by magnetic resonance microscopy (MRM) imaging. A: Left ventricular (LV) wall thickness. Groups of mice were immunized with or without ANT1 21-40 in complete Freund adjuvant on day 0 and day 7, and pertussis toxin was administered on day 0 and day 2 after the first immunization. Unimmunized (healthy) and ANT1 21-40–immunized animals were subjected for MRM imaging on days 30 to 35 to evaluate LV wall thickness, as described in Materials and Methods (double-headed arrows indicate LV wall thickness). Images from three individual mice from each group are shown. B: Body weight/heart weight ratios. Body weights of the healthy and ANT1 21-40–immunized mice were determined on the indicated days and plotted against days after immunization (left panel). At the termination on day 52, hearts were weighed and the heart weight (wt)/body wt ratios were then determined. Values representing heart weights (middle panel) and heart wt/body wt ratio (right panel) for a group of mice are shown. Data are expressed as means ± SEM (B). n = 3 to 6 mice per group (A and B). ∗P < 0.05 versus naïve group.
Proliferative response of splenocytes to ANT1 21-40 in naïve mice. Splenocytes harvested from naïve mice were stimulated with ANT1 21-40 or RNase 43-56 for 2 days; after pulsing with 3[H]-thymidine for 16 hours, proliferative responses were measured as counts per minute (cpm). Data are expressed as means ± SEM. n = 3 individual mice.
RT-PCR analysis of ANT isoforms in A/J mice. Lymphoid (thymus and spleen) and nonlymphoid (heart, thigh muscle, brain, and liver) tissues were collected from 6- to 8-week-old A/J mice, and total RNA was isolated. After synthesizing the cDNA, expression levels of ANT1, ANT2, and ANT4 were examined by PCR using gene-specific primers. The PCR amplicons were resolved on 1.5% ethidium bromide–stained agarose gel electrophoretic analysis. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH), loading control. n = 3 mice.
Estimation of serum aspartate aminotransferase (AST) in mice immunized with ANT1 21-40. Groups of mice were immunized with or without ANT1 21-40 in complete Freund adjuvant on day 0 and day 7, and pertussis toxin was administered on day 0 and day 2 after the first immunization. Serum samples collected on indicated days after immunization were estimated for AST levels. Data are expressed as means ± SEM. n = 3 to 5 mice per group. ∗P < 0.05 versus naïve group.
Cytokine analysis in the supernatants derived from ANT1 181-200–stimulated cultures. Lymph node cells obtained from ANT1 181-200–immunized mice were stimulated with or without ANT1 181-200 or RNase 43-56 (control). Supernatants collected on day 3 after stimulation were analyzed using beads conjugated with cytokine-capture antibodies and detection antibodies by cytometric bead array, as described in Materials and Methods. Data are expressed as means ± SEM. n = 3 experiments. IFN-γ, interferon-γ; Th, T helper; TNF-α, tumor necrosis factor-α.