Abstract
Cyclophilins (CYPs) belong to the immunophilin superfamily with peptidyl-prolyl cis-trans isomerase (PPIase) activity. They catalyze the interconversion of the cis- and trans-rotamers of the peptidyl-prolyl amide bond of peptides. A yeast-two-hybrid screening using the isoflavonoid regulator GmMYB176 as bait identified GmCYP1 as one of the interacting proteins in soybean embryos. GmCYP1 localizes both in the nucleus and cytoplasm, and interacts in planta with GmMYB176, in the nucleus, and with SGF14l (a soybean 14-3-3 protein) in the nucleus and the cytoplasm. GmCYP1 contains a single cyclophilin-like domain and displays a high sequence identity with other plant CYPs that are known to have stress-specific function. Tissue-specific expression of GmCYP1 revealed higher expression in developing seeds compared to other vegetative tissues, suggesting their seed-specific role. Furthermore, GmCYP1 transcript level was reduced in response to stress. Since isoflavonoids are involved in plant stress resistance against biotic and abiotic factors, the interaction of GmCYP1 with the isoflavonoid regulators GmMYB176 and 14-3-3 protein suggests its role in defense in soybean.
Cyclophilins (CYPs) are a group of proteins that possess peptidyl prolyl cis/trans isomerase (PPIase) activity. They are involved in protein folding by interconverting the cis- and trans-rotamers of the peptidyl prolyl amide bond of peptides, and are broadly classified into three major classes: parvulins, FK506 binding proteins (FKBP) and cyclophilins1. FKBP and CYPs are collectively called immunophilins as they were originally identified as receptors for immunosuppressive drugs, FK506 and cyclosporine A, respectively2,3,4. CYPs are present in a wide range of organisms, from archaea, bacteria to plants and animals5,6,7. Genome-wide analyses of CYP genes in various organisms revealed disparity in the number of genes, ranging from 8 to 16 in organisms such as Drosophila8, Caenorhabditis elegans9, Saccharomyces cerevisiae10, and human8. A large number of studies focussed on human CYP, hCYPA, have shown its crucial role in protein folding, signal transduction, cell signaling, regulation of gene expression, immune response, and disease conditions11,12,13.
The first plant CYPs were identified concomitantly in 1990, from tomato (Lycopersicon esculentum), maize (Zea mays), and oilseed rape (Brassica napus)14. With the advancement in genome sequencing technology, and availability of plant genome sequence data in public domain, the identification and characterization of plant CYPs has progressed significantly in the recent years. Compared to other organisms, photosynthetic organisms contain significantly higher number of CYPs; 62 in soybean15, 35 in Arabidopsis16,17, 28 in rice17,18, and 26 in Chlamydomonas19.
In order to combat biotic and abiotic stress, sessile organisms like plants have developed several sophisticated mechanisms at the cellular and molecular levels20,21. One of the consequences of abiotic stress is the denaturation and aggregation of cellular proteins leading to cell death. The chaperone-like activity of CYPs and their role in the rate-limiting step of protein folding by peptidyl prolyl bond isomerization22 is associated with their involvement in stress responses. Expression of many plant CYPs is induced in response to stress suggesting their possible function in stress tolerance. For example, expression of the Arabidopsis CYP, ROTAMASE CYCLOPHILIN 1 (ROC1), increases upon wounding23. Similarly, maize and bean CYP gene expression increases in response to heat stress, wounding, high salinity, or low temperature24. Solanum commersonii CYP gene expression is also up-regulated by low temperature, abscisic acid, drought, or wounding25. Pepper CYPs are differentially regulated during abiotic stress and pathogen infection26. Ectopic expression of Thellungiella halophile CYP, ThCYP1, in fission yeast and tobacco cells increased salt tolerance27. Transgenic Arabidopsis plants overexpressing pigeon pea CYP (CcCYP1) showed enhanced PPIase activity under stressed conditions, which correlated with their increased tolerance against drought, salinity and high temperature28. Similarly, overexpression of cotton CYP (GhCYP1) in tobacco plants conferred tolerance against salt stress and fire-blight disease29. Together, these findings clearly demonstrate a role for plant CYPs in stress tolerance.
Soybean (Glycine max) is a grain legume belonging to the family Fabaceae. Soybean seeds provide a major supply of oil, protein and beneficial plant natural compounds such as isoflavonoids and saponins. The soybean genome contains 88,647 predicted transcripts and 56,044 protein coding loci located on 20 different chromosomes30. Previously, we performed a genome-wide analysis of soybean CYPs and identified 62 CYP genes15. Among these, GmCYP1 has been shown to act as a “helper” to Phytophthora sojae RXLR effector Avr3b by activating its hydrolase activity in plant cells31. The protein-protein interaction between GmCYP1 and Avr3b was shown to be isoform-specific since GmCYP1 paralogs failed to interact with Avr3b. Here we present a molecular characterization of GmCYP1 covering its sequence analysis, phylogeny, temporal and spatial expression, subcellular localization, and provide the evidence for its possible role in isoflavonoid biosynthesis and stress response in soybean.
Results and Discussion
Isolation, sequence analysis and phylogeny of GmCYP1
GmCYP1 was identified in our Y2H screening as a protein that demonstrated protein-protein interaction with the isoflavonoid regulator GmMYB176. The Y2H assay was performed to identify GmMYB176-interacting proteins using GmMYB176 as the bait protein and proteins from soybean embryos (50–60 days after pollination) as prey. Of the several hundred yeast colonies screened, 6.5% contained a sequence corresponding to GmCYP1 (accession #AF456323, locus Glyma.11G098700). GmCYP1 is predicted to contain only one exon (519 bp), and is located on the long arm (q arm) of chromosome 11, approximately 16 Mb from the centromere. It encodes a single domain protein of 172 amino acid residues with a calculated molecular mass of 18.22 kDa and a pI of 8.69. The cyclophilin-like domain in GmCYP1 is predicted in between the amino acid residues 7 and 169.
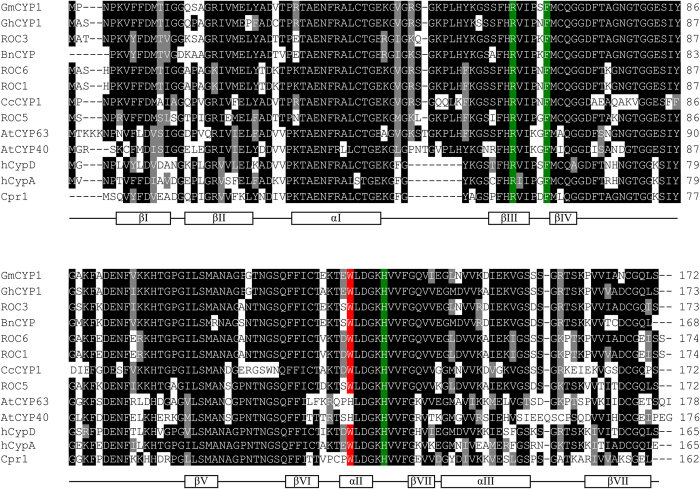
In order to find sequences closely related to GmCYP1, a protein-protein BLAST (BLASTP) was performed using GmCYP1 as a query against the NCBI non-redundant protein database. A list of 12 high-scoring and previously characterized CYPs is shown in Table S1. Alignment of the deduced sequence of GmCYP1 with previously characterised CYPs from several different plant species, human, yeast, and two Arabidopsis multi-domain CYPs (AtCYP40 and AtCYP63) revealed two general features (Fig. 1). First, three amino acid residues that critically affect PPIase activity (R55, F60 and H126)32 are conserved in all CYPs aligned. Second, the tryptophan residue (W121) implicated in substrate cyclosporinA binding32,33 is present in all of the CYPs studied except in the multi domain CYPs.
Figure 1. Multiple sequence alignment of deduced amino acid sequence of GmCYP1 with CYPs from other species.
Amino acid sequences of ROC3, BnCYP, GhCYP1, CcCYP1, ROC6, ROC1, ROC5, Cpr1, hCYP-D, hCYP-A, AtCYP40, AtCYP63 and GmCYP1 (refer to Table S1 for accession numbers) were aligned by ClustalW, and imported into BOXSHADE 3.21 for shading. Identical amino acids are shown in the dark box and similar amino acids are indicated by the grey box. Amino acid residues involved in PPIase activity (R55, F60 and H126) (Zydowsky et al.32) and CsA binding (W121) (Liu et al.33; Zydowsky et al.32) are highlighted with green and red, respectively. Secondary structure is shown below the alignment. The relative positions of amino acids indicated for PPIase activity and CsA binding sites, and the secondary structure features are based on hCYP-A (Kallen et al., 1991).
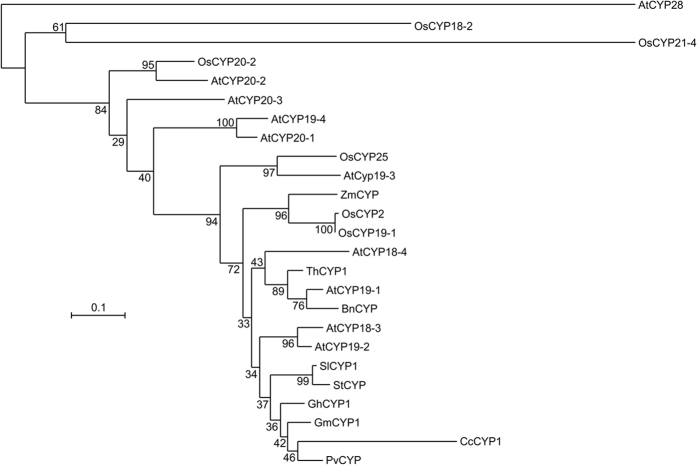
A phylogenetic analysis of GmCYP1 and other functionally characterized plant CYPs clustered GmCYP1 close to GhCYP1, CcCYP1 and PvCYP from cotton, pigeon pea and common bean, respectively. Both GhCYP1 and CcCYP1 are known to have stress-specific function (Fig. 2). Overexpression of GhCYP1 in tobacco conferred increased tolerance to biotic and abiotic stress29. Similarly, Arabidopsis plants overexpressing CcCYP1 showed higher PPIase activity during stress and increased tolerance against multiple abiotic stresses as compared to control28. Differential accumulation of PvCYP transcripts in response to various external stimuli suggested that it may possess a stress-related function24. The high amino acid sequence identity of GmCYP1 with GhCYP1 (96%), CcCYP1 (81%) and PvCYP (96%), suggests similar possible functions of GmCYP1 in stress response.
Figure 2. Phylogenetic analysis of GmCYP1 with other known plant CYPs.
Deduced amino acid sequence of GmCYP1 was aligned with other plant CYPs using Clustal omega, and the alignment was imported to MEGA 6.0 to create a phylogenetic tree using Maximum Likelihood method. Numbers indicate bootstrap value in percentage. The tree was exported into Interactive Tree of Life (http://itol.embl.de/) for annotation and manipulation. Accession numbers used for the alignment are: OsCYP2 (AAA57046, O. sativa), OsCYP20-2 (XP_015640756, O. sativa), OsCYP18-2 (XP_015649749, O. sativa), OsCYP25 (XP_015610625, O. sativa), OsCYP21-4 (XP_015647974, O. sativa), AtCYP19-3 (NP_191166, A. thaliana), AtCYP18-3 (NP_195585, A. thaliana), AtCYP18-4 (NP_195213, A. thaliana), AtCYP19-1 (NP_179251, A. thaliana), AtCYP19-2 (NP_179709, A. thaliana), AtCYP19-4 (NP_180557, A. thaliana), AtCYP20-1 (NP_191166, A. thaliana), AtCYP20-2 (NP_196816, A. thaliana), AtCYP20-3 (NP_001154684, A. thaliana), AtCYP28 (NP_198360, A. thaliana), AtCYP37 (NP_188171, A. thaliana), AtCYP38 (NP_186797, A. thaliana), AtCYP40 (NP_565381, A. thaliana), GhCYP1 (ACT63839, G. hirsutum), SlCYP1 (AAA63543, S. lycopersicum), BnCYP (AAA62706, B. napus), ZmCYP (CAA48638, Z. mays), CcCYP1 (ADB04247, C. cajan), ThCYP1 (AAR27291, T. halophila), StCYP (AAD22975, S.tuberosum), PvCYP (CAA52414, P. vulgaris).
GmCYP1 localizes in the nucleus and the cytoplasm
To further study the subcellular localization of GmCYP1, a translation fusion of GmCYP1 with YFP was created under the control of 35S promoter, and transiently expressed in N. benthamiana leaves. Although there was no predicted nuclear localization sequence in GmCYP1, confocal imaging of the GmCYP1-YFP-infiltrated tobacco leaves showed both nuclear and cytoplasmic localization (Fig. 3a). Nuclear localization of GmCYP1 was confirmed by co-expression of GmCYP1-YFP and NLS-CFP.
Figure 3. Subcellular localization of GmCYP1.
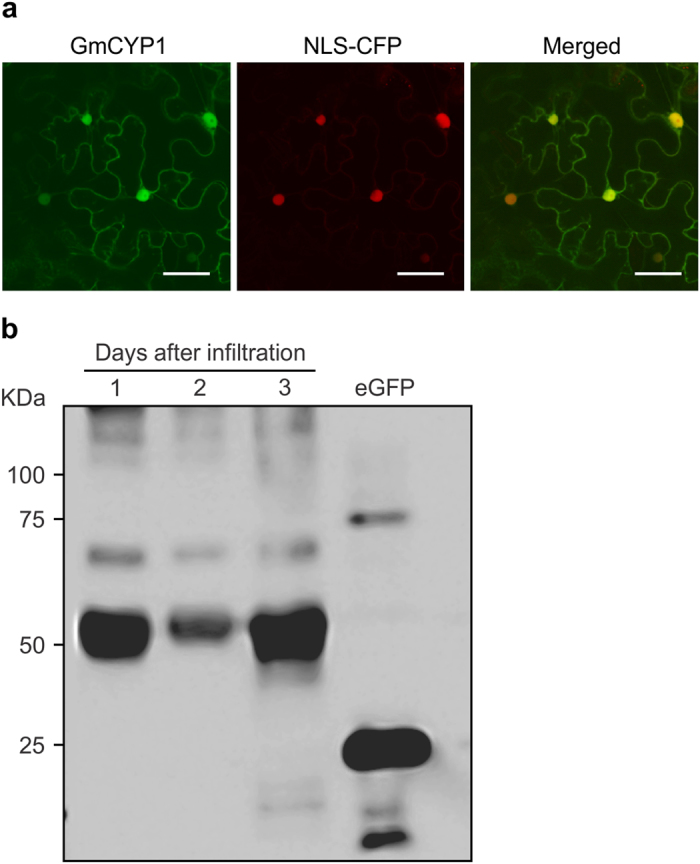
A. tumefaciens GV3101 carrying the plasmids with GmCYP1-YFP and nuclear localizing CFP (NLS-CFP) constructs were co-infiltrated into N. benthamiana leaves and visualized by confocal microscopy. Expression of (a) GmCYP1-YFP, (b) NLS-CFP, (c) images of A and B merged to confirm nuclear localization of GmCYP1. Scale bars indicate 50 μm. (d) Western blot analysis of translational fusion of GmCYP1-YFP proteins. GmCYP1-YFP was transiently expressed in N. benthamiana leaves for 1, 2 or 3 days, and protein accumulation was measured. Proteins (30 μg) were separated on a SDS-PAGE and transferred to PVDF membrane by electroblotting. GmCYP1-YFP protein was detected by sequential incubation of the blot with anti-GFP antibody and anti-mouse IgG conjugated with HRP, followed by chemiluminescent reaction. eGFP is shown as a positive control.
Molecules of size smaller than 20–40 kDa, such as ions, water, and small proteins, can pass through the nuclear pore complex by diffusion34, whereas movement of larger molecules (70 kDa or higher) entails an active transport system35, mediated by transport receptors and signal peptides36. The size of GmCYP1-YFP (45.22 kDa) is not considerably larger than the size of molecules that can pass through the nuclear pore complex by diffusion. It is also possible that heterologous protein GmCYP1-YFP, expressed in N. benthamiana is cleaved by the endogenous host proteases and only the cleaved YFP fragments are localized to the nucleus. The confirmation of the YFP signal in the nucleus arising from the intact GmCYP1-YFP and not from the cleaved product of a fusion protein was performed by Western blot analysis (Fig. 3b). Therefore, it is not clear whether GmCYP1-YFP localization in the nucleus was due to passive diffusion or to active transport. Regardless, its nuclear localization indicates a possible role in the regulation of gene expression.
GmCYP1 interacts with GmMYB176 in planta
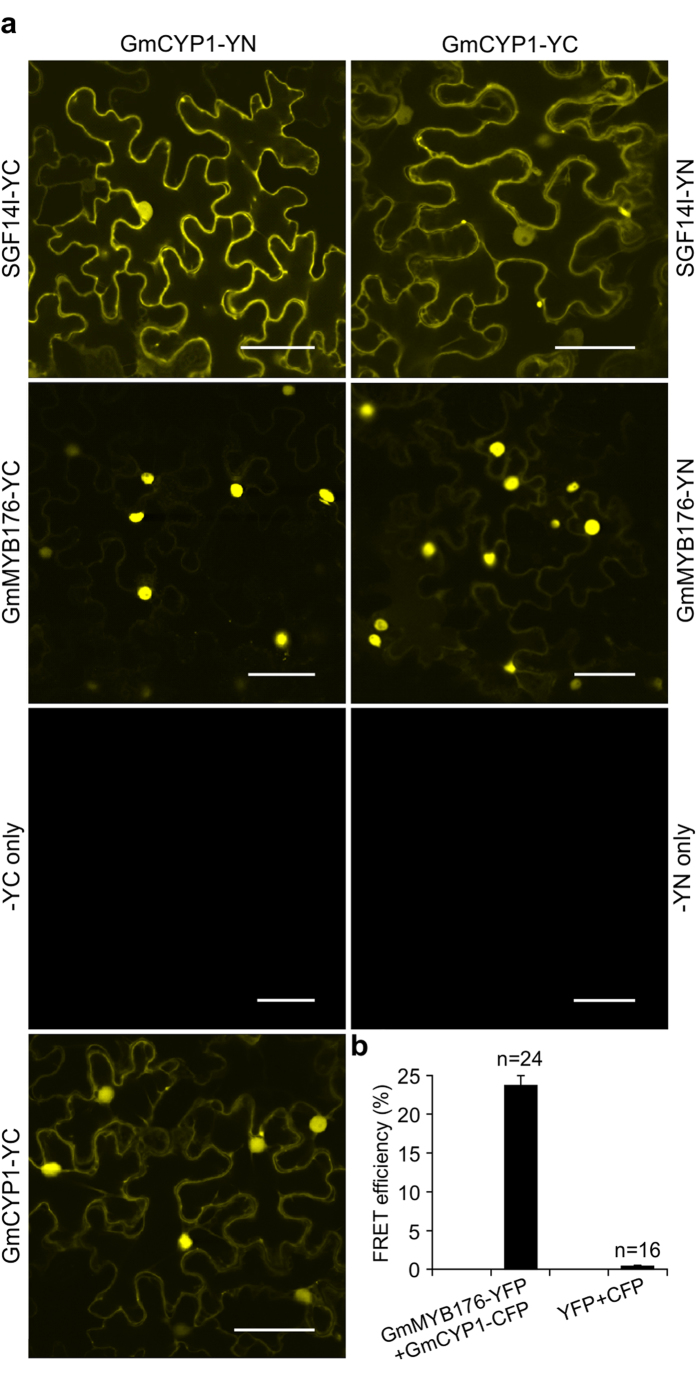
GmCYP1 was identified as one of the interacting proteins of GmMYB176 in the Y2H assay. Protein chaperones often bind with misfolded bait proteins when a protein is overexpressed or heterologously expressed. To confirm that GmCYP1 is a true GmMYB176-interacting protein, we performed a targeted Y2H assay using GmCYP1 and GmMYB176. However, our targeted Y2H assay failed to verify the interaction between GmCYP1 with GmMYB176. This result led us to hypothesize that there may be an indirect interaction between GmCYP1 and GmMYB176 via involvement of protein (s) that may not be conserved between the species. Therefore, a BiFC assay37 was carried to further investigate in planta interaction between GmCYP1 and GmMYB176. Translational fusions of GmMYB176 and GmCYP1 were created with N-terminal (YN) or C-terminal (YC) halves of YFP38 and co-expressed in N. benthamiana leaf epidermal cells in the following combinations: (A) GmCYP1-YN and GmMYB176-YC, (B) GmMYB176-YN and GmCYP1-YC. The negative controls used for the experiment were co-expression of GmCYP1-YN and -YC only or GmCYP1-YC and -YN only. As shown in Fig. 4a, GmCYP1 interacts with GmMYB176 in planta, and the interaction between GmCYP1 and GmMYB176 was strong in the nucleus. Similar results were obtained for the reciprocal combinations. No YFP signal was detected during co-expression of control constructs -YC or -YN with GmCYP1-YN or GmCYP1-YC, respectively, confirming that YFP signal was due to the protein-protein interaction in planta. Furthermore, we measured the strength of interaction by using FRET approach. It is a powerful tool for non-invasive monitoring of protein-protein interactions that involves transfer of energy between two closely positioned fluorophores39, and has been widely used to determine the interactions between proteins in living plant cells40,41,42. Translational fusions of GmMYB176 with YFP and GmCYP1 with CFP were co-expressed in N. benthamiana leaf epidermal cells and analysed for FRET efficiency between the reporters in vivo. The results revealed a FRET efficiency of 23.8%, indicating a close proximity of GmMYB176 and GmCYP1 and their co-existence in a complex (Fig. 4b). Empty vectors containing YFP only and CFP only were used as negative control which showed a FRET efficiency of 0.5%. A FRET value of 8–10% was considered as the background level in previous studies43,44.
Figure 4. GmCYP1 interacts with GmMYB176 and SGF14l in planta.
N. benthamiana leaves were co-transformed with A. tumefaciens carrying (a) GmCYP1-YN and SGF14l-YC or their reciprocal combination, GmCYP1-YN and GmMYB176-YC or their reciprocal combination, GmCYP1-YN and GmCYP1-YC with vector only control (-YN or –YC only), GmCYP1-YN and GmCYP1-YC, and observed by confocal microscopy. Protein-protein interactions were visualized by a strong yellow fluorescence. Scale bars indicate 50 μm. (b) FRET analysis demonstrating protein-protein interactions between GmMYB176 and GmCYP1. The CFP and YFP channels were excited with 458 nm and 514 nm lasers respectively, and FRET efficiencies were calculated in multiple samples (n > 15). The empty pEG101 (YFP) and pEG102 (CFP) vector pairs were used as a FRET signal control.
When A. tumefaciens GV3101 strains carrying BiFC plasmids containing GmCYP1-YN or GmCYP1-YC were co-infiltrated into N. benthamiana leaves and visualized by confocal microscope, a strong yellow fluorescence was observed in the nucleus and relatively weaker fluorescence in the cytoplasm, suggesting that GmCYP1 forms a homodimer in both cellular compartments in planta (Fig. 4a). Even though recombinant hCYP-A has been reported to form monomers, dimers, and trimers when expressed in E. coli45, there is no published literature on homo-dimerization of plant CYPs. Search for predicted motifs in GmCYP1 identified a putative phosphorylation (pST binding) site within the GmCYP1 sequence (97-ENFVKKHTGPGILSM-112), where T105 is potentially phosphorylated. The pST binding motifs are binding sites for 14-3-3 family of proteins. 14-3-3 protein functions as a dimer to bind with its client proteins where each monomer in the dimer is capable of interacting with a separate client protein. The dimeric nature of 14-3-3 proteins allows them to serve as scaffolds by bringing two regions of the same proteins into proximity or two different proteins together46. We have previously demonstrated that GmMYB176 interacts with 14-3-3 proteins, thereby affecting its subcellular localization38. The interactions between GmCYP1 and GmMYB176 in the Y2H screen and in BiFC assay where soybean and N. benthamiana 14-3-3 s possibly bring the two proteins together could be explained if GmCYP1 is a true client of 14-3-3 protein. Therefore, we performed a BiFC assay between SGF14l (a soybean 14-3-3) and GmCYP1. Indeed, GmCYP1 interacted with SGF14l in planta (Fig. 4), suggesting that 14-3-3 proteins may act as a scaffold to facilitate binding of GmCYP1 and GmMYB176. Despite that the mechanism and consequence of GmCYP1 dimerization are not yet known, it is possible that binding of 14-3-3 with GmCYP1 could bring two GmCYP1 monomers together to produce fluorescence in the BiFC assay. Further, it is not clear whether the binding of GmCYP1 and GmMYB176 mediated by SGF14l is involved in the CHS8 gene regulation, and subsequent isoflavonoid biosynthesis in soybean, or to some other, as-yet unknown function.
GmCYP1 is expressed ubiquitously in soybean tissues
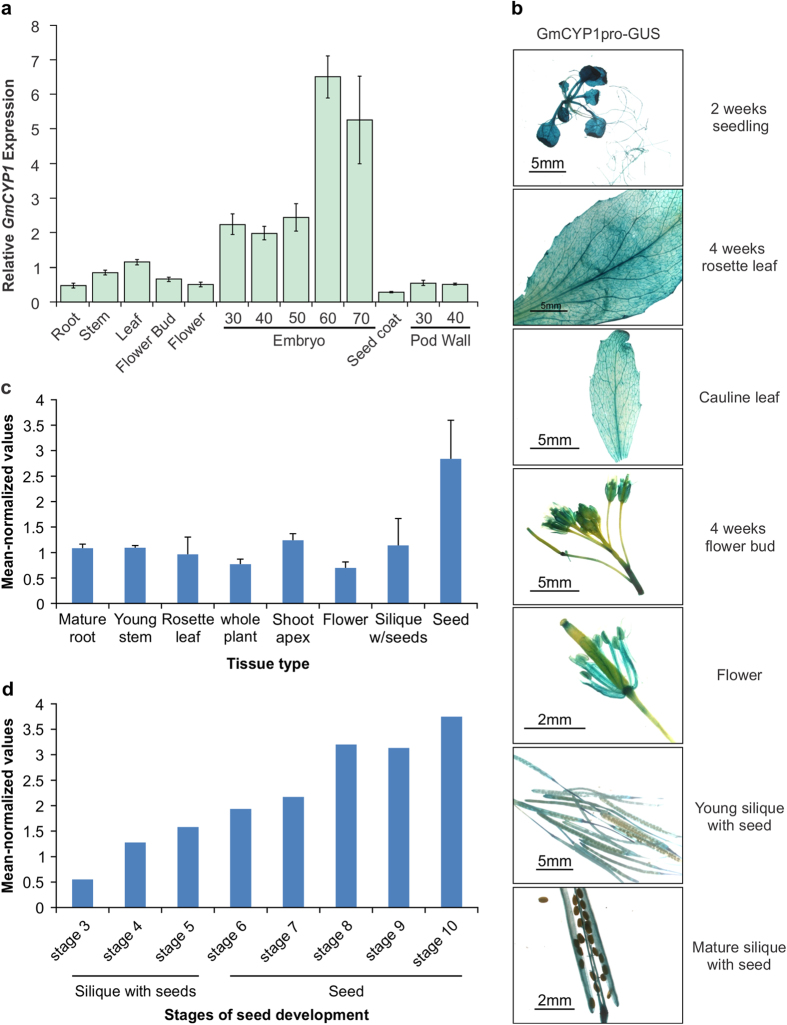
To study the temporal and spatial expression of GmCYP1 in soybean, a detailed transcript analysis using quantitative PCR was performed. Total RNA isolated from different tissues of soybean cultivar Harosoy63, at several different developing stages, was used in the analysis. As shown in Fig. 5a, GmCYP1 was expressed in all soybean tissues, albeit at various levels. Transcript accumulation was higher in embryos compared to that in other tissues. The level of GmCYP1 transcript increased in soybean embryos during the late developmental stages, showing highest levels (a 3-fold increase) in the embryos at 60 and 70 days after pollination compared to that in embryos at 30, 40 or 50 days after pollination (mid developmental stage) (Fig. 5a). No GmCYP1 transcripts were detected at early embryo developmental stages or in mature seeds15. Seed coat tissues accumulated 23- and 18-fold less GmCYP1 transcripts compared to the embryos at 60 and 70 days after pollination, respectively. The seed coat is rich in defensive and pathogen-related proteins47. Among all the soybean tissues and organs tested for the expression of GmCYP1, the level of expression was lowest in the seed coat.
Figure 5. Expression analysis of GmCYP1 in soybean.
(a) Total RNA extracted from soybean root, stem, leaf, flower bud, flower, embryo (30, 40, 50, 60, and 70 days after pollination), seed coat and pod wall (30 and 40 days after pollination) were used for quantitative RT-PCR analysis of GmCYP1. Two biological replicates and three technical replicates for each biological replicate were carried out. The standard error of the mean is represented by an error bar. The data were normalized against SUBI-3 gene. (b) Histochemical analysis of GmCYP1promoter-GUS activity in vegetative and reproductive tissues during various stages of development in Arabidopsis. Construct containing a GmCYP1 promoter driven GUS gene was transformed into Arabidopsis and selected T2 transgenic plants were used for analysis. (c) The mean normalized expression values of ROC1 in different Arabidopsis tissues and (d) stages of seed development were obtained from AtGenExpress Visualization Tool (http://jsp.weigelworld.org/expviz/expviz.jsp). Error bars indicate the standard deviation of the mean. The stages of seed developments are: stage 3, mid globular to early heart embryos; stage 4, early to late heart embryos; stage 5, late heart to mid torpedo embryos; stage 6, mid to late torpedo embryos; stage 7, late torpedo to early walking-stick embryos; stage 8, walking-stick to early curled cotyledons embryos; stage 9, curled cotyledons to early green cotyledon embryos; and stage 10, green cotyledon embryos.
For an in-depth analysis of GmCYP1 expression, we constructed a reporter vector containing the 1,148 bp GmCYP1 promoter fragment (upstream of the translation start site) to drive GUS gene expression. Agrobacterium strain containing the pGmCYP1pro-GUS was transformed into wild type Arabidopsis. Transgenic lines (8–10 independent T2 progenies) carrying GmCYP1pro-GUS were selected for measuring GUS activity in different tissues during development. As observed in soybean tissues, the GmCYP1 promoter was active in most tissues of Arabidopsis (Fig. 5b). Strong GUS activity was observed in leaves and roots of young seedling while relatively less activity was found in the hypocotyl. This study revealed additional information on tissue-specific expression of GmCYP1. For example, in leaves, GUS staining was more pronounced in the vascular tissues. Similarly, GmCYP1 promoter was active in flower buds, stamen, stigma and silique walls but not in flower stem, style, petal or seeds. Failure to observe GUS activity in seeds could be due to weaker activity of the GmCYP1 promoter in the seed coat, and is supported by GmCYP1 expression in seed coat as shown in Fig. 5a. Similar results were observed for soybean chalcone synthase (CHS) gene promoters. Both CHS7 and CHS8 promoter driven GUS activities were absent in Arabidopsis seeds, despite the fact that CHS7 and CHS8 transcripts were present in the seed coat in soybean48. It is possible that the observed differences of gene expression in soybean and Arabidopsis may be caused by the presence or absence of the required regulatory factors in the specific tissue or developmental stage. Like GmCYP1, the Arabidopsis ortholog of GmCYP1, ROC1 (AGI:At4g38740), also exhibited higher transcript accumulation in seeds than in other tissues (Fig. 5c). The normalized mean expression data of ROC1 was compiled from AtGenExpress Visualization Tool (http://jsp.weigelworld.org/expviz/expviz.jsp). The expression of ROC1 increased gradually, and approx. 7-fold throughout seed development, from a relatively low value (0.55) at the mid globular stage (stage 3), to values of 3.2–3.75 during the later developmental stages (early curled cotyledon embryos, stage 8) to green cotyledon embryos (stage 10) (Fig. 5d). The similar expression pattern during embryo development of GmCYP1 and its Arabidopsis ortholog ROC1 suggests a conserved role for GmCYP1 and ROC1 in seed development.
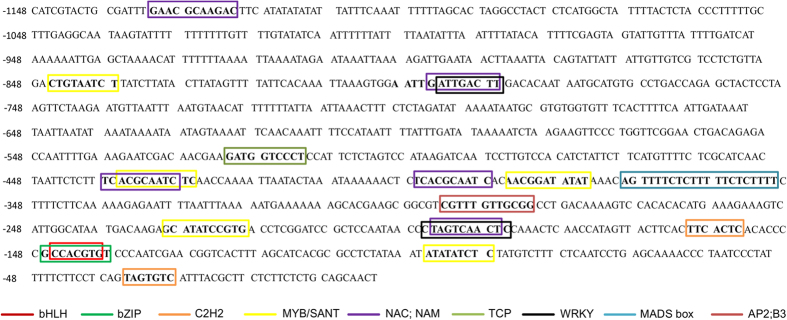
To identify putative cis-elements that regulate the expression of GmCYP1 gene in soybean, we performed in silico motif analysis of 1148 bp upstream of translational start site using PlantPan2.0 database (http://plantpan2.itps.ncku.edu.tw/promoter.php). The transcription factors specific to soybean was selected during the analysis. Transcription factors that are known for their role in stress and hormonal pathways are shown in Fig. 6. The analysis identified several sequence motifs that are recognized by a number of key factors such as bZIP, MYB, WRKY, bHLH, AP2, NAC factors have been identified. Besides their role in normal plant growth and development, the stress-specific roles of these factors have been well documented in crop plant and model species49,50,51.
Figure 6. Promoter analysis of GmCYP1.
A 1148 bp upstream of translation start site of GmCYP1 was used for analysis using PlantPan2.0 database (http://plantpan2.itps.ncku.edu.tw/promoter.php). The transcription factors that are known for their role in stress and hormonal pathways are indicated.
GmCYP1 expression is reduced in response to stress
P. sojae effector Avr3b contains Nudix hydrolase activity in planta that is required for the virulence of the pathogen in soybean52. Recently, it has been shown that GmCYP1 acts as a ‘helper’ by directly interacting with Avr3b, and modulating the hydrolase activity of it in soybean31. This GmCYP1-Avr3b interaction is required for the virulence and avirulence functions of Avr3b in soybean. Treatment of soybean hypocotyls with AgNO3 is known to induce defense response and phytoalexin accumulation53. Here we measured the accumulation of GmCYP1 transcripts in response to stress by treating etiolated soybean hypocotyls with AgNO3 and monitoring GmCYP1 expression 24, 48 or 72 h post-treatment. The results revealed that AgNO3 treated soybean hypocotyls accumulate reduced level of GmCYP1 transcripts compared to the control hypocotyls at all the time points under the study (Fig. 7a). The difference in the level of GmCYP1 transcript accumulation between AgNO3 treated and control hypocotyls was more pronounced at 24 h compared to 48 or 72 h post-treatment.
Figure 7. Expression of GmCYP1 and isoflavonoid biosynthetic genes in response to stress.
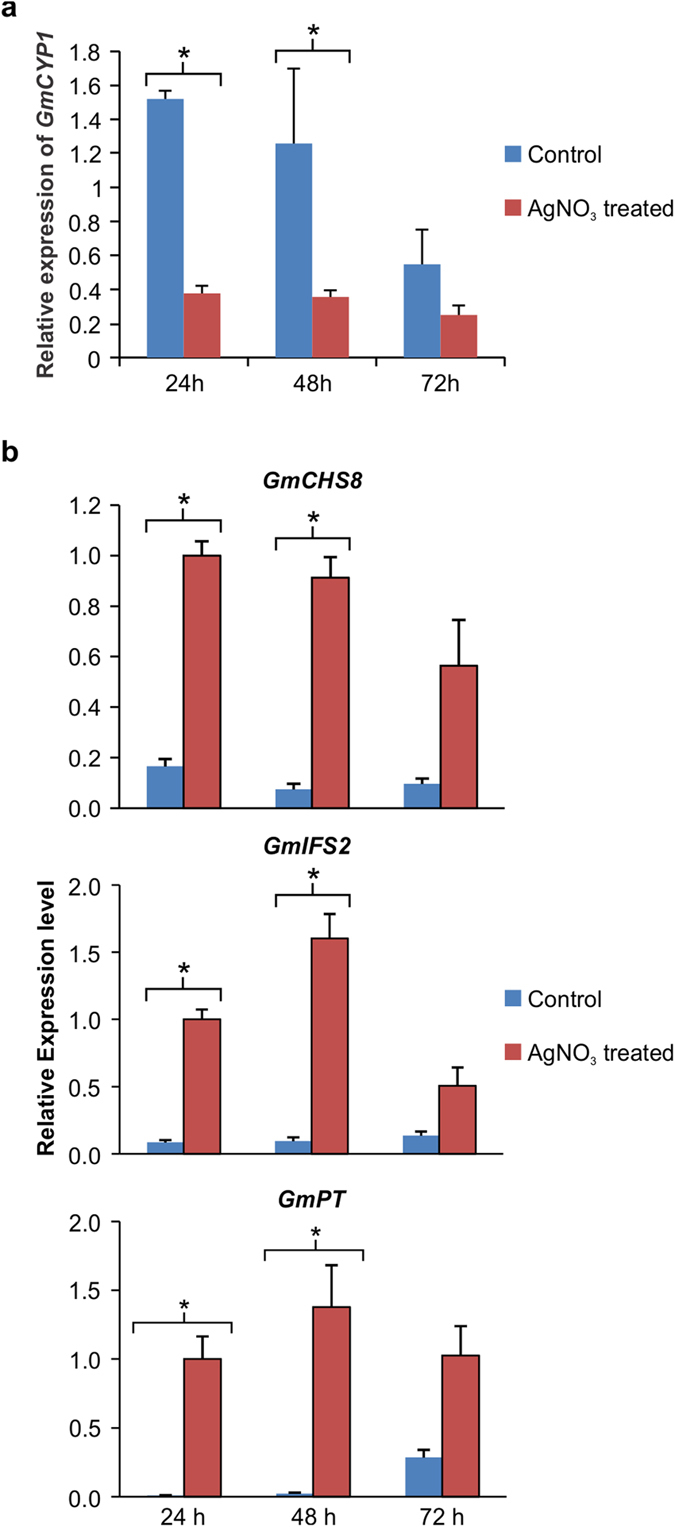
Etiolated soybean hypocotyls were treated with either 1 mM AgNO3 or water (control) for 24, 48 or 72 h, and tissues were used to evaluate (a) GmCYP1 (b) isoflavonoid biosynthetic gene (GmCHS8, GmCHI1B1, GmIFS2, GmPT) transcript accumulation using quantitative RT-PCR. Error bars indicate SEM of two biological replicates and three technical replicates for each biological replicate. The data were normalized against the SUBI-3 gene for GmCYP1 and CON4 gene for isoflavonoid genes. Asterisk (*) indicate significant difference between the samples using Student’s t-test.
Isoflavonoid phytoalexins are host-produced antimicrobial compounds that are massively induced by pathogen attack or any other stress54,55. GmMYB176 regulates isoflavonoid biosynthesis by regulating CHS8 gene expression56. Since GmCYP1 interacts with GmMYB176, and its expression is down-regulated upon stress, whereas phytoalexin biosynthesis is induced upon stress, we measured the expression levels of isoflavonoid biosynthetic genes GmCHS8, isoflavone synthase (GmIFS2) and a isoflavonoid-specific prenyltransferase (GmPT) in response to AgNO3 treatment. Our results revealed that expression of all these genes were induced upon AgNO3 treatment albeit at different levels (Fig. 7b). GmCHS8 is the first enzyme in the flavonoid pathway, and as compared to control, its transcripts were accumulated at 6, 12 and 5.8 fold higher at 24, 48 and 72 hours, respectively after AgNO3 treatment. GmIFS2 is a key legume-specific enzyme that introduces the isoflavonoid branch in the flavonoid pathway in legumes. Transcript levels of GmIFS2 were 11.6, 17 and 3.7 fold higher after 24, 48 and 72 hours, respectively in AgNO3 treated samples compared to control. To confirm if downstream phytoalexin biosynthetic genes are induced upon stress, we measured the transcript levels of GmPT. Our results demonstrated that GmPT transcripts accumulated at 121, 64 and 3.5 fold greater than control at 24, 48 and 72 hours, respectively after AgNO3 treatment (Fig. 7b). A significantly higher difference in the expression of isoflavonoid genes were observed only when there was a significant reduction of GmCYP1 gene expression (Fig. 7).
Several studies have shown that pathogen effectors interact with plant helper proteins for their activation and proper function57,58. Activated effector proteins bind with plant targets to suppress plant defenses and otherwise enable pathogen growth. For effectors encoded by Avr genes, activation can also result in detection by an immune receptor encoded by a resistance (R) gene. Thus, activation of Avr3b by GmCYP1 triggers immunity in soybean cultivars containing Rps3b, whereas activation of Avr3b in soybean cultivars lacking Rps3b enables pathogen growth31. Unlike many other plant CYPs that function in protecting plants during biotic and abiotic stress29,59, GmCYP1 acts as a susceptibility factor in soybean, at least in instances when Avr3b does not trigger immunity31. The expression of susceptibility proteins is generally induced during infection in susceptible plants60. However, our results show that GmCYP1 transcripts are reduced in stressed plants compared to controls. This finding, together with the results that show GmCYP1 interacts with GmMYB176, suggests a role for GmCYP1 as a negative regulator of plant defense and isoflavonoid biosynthesis in soybean. The high expression of GmCYP1 in the late stage developing embryos cannot be explained by this hypothesis because isoflavonoid biosynthesis is active in seed tissues, albeit this is seed isoflavonoid and not phytoalexin isoflavonoid biosynthesis.
Overall, this study presents a detailed analysis of GmCYP1. The presence of predicted cyclophilin domain, subcellular localization and its sequence homology with other identified CYPs from other organisms provides insights into its putative function. The interaction of GmCYP1 and GmMYB176 is particularly intriguing because of the conditional functionality of GmCYP1 in effector-triggered immunity or susceptibility to the pathogen P. sojae. It seems more than coincidence that the biosynthesis of isoflavonoid phytoalexins, being necessary for resistance to P. sojae, is also connected to GmCYP1. Since isoflavonoids are involved in plant stress resistance against biotic and abiotic factors61,62,63, the interaction of GmCYP1 with isoflavonoid regulators and its potential role as a suppressor of plant defense merits further investigation.
Methods
Plant growth conditions
Soybean (Glycine max [L.] Merr) cultivar Harosoy 63 was grown in AAFC-London field plots during 2011 and 2012 for tissue collection. Nicotiana benthamiana plants were grown in pots under 16 h light at 25 °C and 8 h dark 20 °C cycle with 70–80% relative humidity and 100–150 μmol m2/s light intensity.
RNA extraction and quantitative RT-PCR analysis
Total RNA was extracted from soybean tissues according to Wang and Vodkin64. The RNA samples were quantified using a NanoDrop spectrophotometer (Thermo Scientific, USA), and their integrity was checked. Total RNA (1 μg) from each sample was used for cDNA synthesis using the QuantiTect® Reverse Transcription Kit (Qiagen, USA). For quantitative RT-PCR, SsoFastTM EvaGreen® Supermix (Bio-Rad, USA) was used with the CFX96 real-time PCR detection system (Bio-Rad, USA). Quantitative analysis of GmCYP1 and isoflavonoid biosynthetic gene expression was carried out using the primers listed in Table S2. The amplicons were cloned into a pGEM-T Easy vector (Promega, USA), and its sequence verified. SOYBEAN UBIQUITIN-3 (SUBI-3) or CON4 was used as a reference gene for data normalization and to calculate the relative mRNA levels. The data were analyzed using CFX manager (Bio-Rad, USA).
Plasmid constructions
Full length GmCYP1 was amplified from soybean cDNA constructed from mature embryo (60 and 70 DAP) using the primers GmCYP1-Gate-F: 5′- GGGGACAAGTTTGTACAAAAAAGCAGGCTTCATGCCTAACCCTAAGGTCTTCTTC-3′ and GmCYP1-Gate-R: 5′-GGGGACCACTTTGTACAAGAAAGCTGGGTCAGAGGGTTGACCGCAGTTG- 3′. The PCR product was recombined into pDONR-Zeo (Invitrogen, USA) using the BP clonase reaction mix (Invitrogen, USA), transformed into Escherichia coli DH5α, and grown on LB media supplemented with zeocin (50 μg/mL). The E. coli colonies containing recombinant plasmids were screened by colony PCR using gene-specific primers to identify pDONR-Zeo-GmCYP1. For subcellular localization study, the pDONR-Zeo-GmCYP1 was recombined with the destination vector pEarlyGate10165 using the LR clonase reaction mix (Invitrogen, USA). The LR reaction was transformed into E. coli DH5α, PCR screened, then transformed into Agrobacterium tumefaciens GV3101 for plant transformation.
For in planta protein-protein interaction study, pDONR-Zeo-GmCYP1 was recombined separately with pEarlyGate201-YN and pEarlyGate202-YC to obtain pEG201-GmCYP1-YN and pEG202-GmCYP1-YC, respectively. The recombinant plasmids were transformed into E. coli DH5α, PCR screened, and then transformed into A. tumefacians GV3101. For FRET analysis, pDONR221-GmMYB176 and pDONR-Zeo-GmCYP1 were recombined into the pEG101 or pEG102 upstream of the YFP or CFP sequence under the control of the CaMV 35 S promoter. The recombinant plasmids were transformed into E. coli DH5α, PCR screened, and then transformed into A. tumefacians GV3101.
The promoter fragment of GmCYP1 (1148 bp) was amplified using the primers GmCYP1-P-F: 5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTCATCGTACTGCGATTTGAACGCAAGACTTC-3′, and GmCYP1-P-R: 5′- GGGGACCACTTTGTACAAGAAAGCTGGGTAGTTGCTGCAGAGAAGAGAAGCGTAAATGAC-3′, cloned into pDONR-Zeo (Invitrogen, USA), as described previously, followed by the recombination in the destination vector pMDC162 to obtain pGmCYP1pro-GUS. The pGmCYP1pro-GUS was transformed into A. tumefaciens GV3101 by electroporation, and then into wild-type Arabidopsis Col-0 by floral dip method66.
Subcellular localization and bimolecular fluorescent complementation assay
The subcellular localization of GmCYP1 was studied by infiltrating A. tumefaciens GV3101 carrying pEG101-GmCYP1 into N. benthamiana leaves, as described by Sparkes et al.67. For co-expression, equal volumes of two construct-bearing strains, suspended in Gamborg’s solution, were mixed together and then infiltrated into N. benthamiana leaf epidermal cells. The protein expression was visualized by confocal microscopy using a Leica TCS SP2 inverted confocal microscope. An excitation wavelength of 514 nm was used for YFP imaging, and 525–545 nm emissions were collected. For visualization of CFP, an excitation wavelength of 458 nm was used, and emissions were collected between 465–495 nm.
Fluorescence resonance energy transfer (FRET) assay
Equal volumes of two construct-bearing strains containing YFP and CFP fusions (in Gamborg’s solution), were mixed together and then infiltrated into N. benthamiana leaf epidermal cells. The protein expression was visualized by confocal microscopy using a Leica TCS SP2 inverted confocal microscope. An excitation wavelength of 458 nm and 514 nm were used for CFP and YFP imaging respectively. FRET acceptor bleaching, with CFP as donor and YFP as acceptor, was carried out by following Leica confocal application manual. The average of the FRET efficiency was calculated from multiple samples (n > 15).
Histochemical GUS assay
For histochemical GUS staining, T2 transgenic Arabidopsis tissues were used. Tissues were incubated in dark in a solution containing 100 mM sodium phosphate buffer pH 7.0, 10 mM EDTA, 0.05% Triton X-100, 1 mM potassium ferricyanide, 1 mM potassium ferrocyanide and 1 mM X-Gluc (5-Bromo-4-chloro-3-indolyl-β-D-glucuronide) for 16 h at 37 °C with gentle shaking. De-staining was carried out with 95% ethanol for 4 to 6 times. A LeicaM2 FLIIITM microscope with a QImaging Retiga 2000 R camera was used to take the photographs. For staining of silique and seeds, improved clearing method was used68.
Yeast two-hybrid assay
Yeast two-hybrid assay (Y2H) was performed using the Matchmaker® Gold Two-Hybrid System (Clontech Laboratories, Inc., USA). Briefly, GmMYB176 was cloned into the vector pGBKT7 as the bait. The cDNA library as prey was generated by SMART™ cDNA Synthesis technology (Clontech Laboratories, Inc., USA) from soybean embryos (50–60 days after pollination) and fused to GAL4 activation domain. Screening was performed by co-transformation of bait and prey using Mate & PlateTM library system (Clontech Laboratories, Inc., USA). After transformation, yeast cells were spread on SD/Ade/-His/-Leu/-Trp agar plates, and incubated at 30 °C for 5 days.
Additional Information
How to cite this article: Mainali, H. R. et al. Soybean cyclophilin GmCYP1 interacts with an isoflavonoid regulator GmMYB176. Sci. Rep. 7, 39550; doi: 10.1038/srep39550 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
The authors thank Alex Molnar for graphic designs, and Ling Chen, Arjun Sukumaran and Dorothy Drew for technical assistance.
Footnotes
Author Contributions H.R.M. performed experiments, analysed data and prepared draft manuscript. A.K.A.V. and X.L. performed experiments. M.G. contributed to data interpretation and manuscript preparation. S.D. concieved and designed experiments, prepared manuscript.
References
- Dilworth D., Gudavicius G., Leung A. & Nelson C. J. The roles of peptidyl-proline isomerases in gene regulation. Biochem. Cell Biol. 90, 55–69, doi: 10.1139/o11-045 (2012). [DOI] [PubMed] [Google Scholar]
- Harding M. W., Galat A., Uehling D. E. & Schreiber S. L. A receptor for the immunosuppressant FK506 is a cis-trans peptidyl-prolyl isomerase. Nature 341, 758–760, doi: 10.1038/341758a0 (1989). [DOI] [PubMed] [Google Scholar]
- Siekierka J. J., Hung S. H., Poe M., Lin C. S. & Sigal N. H. A cytosolic binding protein for the immunosuppressant FK506 has peptidyl-prolyl isomerase activity but is distinct from cyclophilin. Nature 341, 755–757, doi: 10.1038/341755a0 (1989). [DOI] [PubMed] [Google Scholar]
- Takahashi N., Hayano T. & Suzuki M. Peptidyl-prolyl cis-trans isomerase is the cyclosporin A-binding protein cyclophilin. Nature 337, 473–475, doi: 10.1038/337473a0 (1989). [DOI] [PubMed] [Google Scholar]
- Galat A. Variations of sequences and amino acid compositions of proteins that sustain their biological functions: An analysis of the cyclophilin family of proteins. Arch. Biochem. Biophys. 371, 149–162, doi: 10.1006/abbi.1999.1434 (1999). [DOI] [PubMed] [Google Scholar]
- Maruyama T., Suzuki R. & Furutani M. Archaeal peptidyl prolyl cis-trans isomerases (PPIases) update 2004. Front. Biosci. 9, 1680–1720 (2004). [DOI] [PubMed] [Google Scholar]
- Kumari S., Roy S., Singh P., Singla-Pareek S. & Pareek A. Cyclophilins: Proteins in search of function. Plant Signaling & Behavior 8, e22734, doi: 10.4161/psb.22734 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galat A. Peptidylprolyl cis/trans isomerases (immunophilins): biological diversity-targets-functions. Curr Top Med Chem 3, 1315–1347 (2003). [DOI] [PubMed] [Google Scholar]
- Page A. P., MacNiven K. & Hengartner M. O. Cloning and biochemical characterization of the cyclophilin homologues from the free-living nematode Caenorhabditis elegans. Biochem. J. 317, 179–185 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arevalo-Rodriguez M., Wu X., Hanes S. D. & Heitman J. Prolyl isomerases in yeast. Front. Biosci. 9, 2420–2446 (2004). [DOI] [PubMed] [Google Scholar]
- Braaten D. & Luban J. Cyclophilin A regulates HIV-1 infectivity, as demonstrated by gene targeting in human T cells. EMBO J. 20, 1300–1309, doi: 10.1093/emboj/20.6.1300 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu K. P., Finn G., Lee T. H. & Nicholson L. K. Prolyl cis-trans isomerization as a molecular timer. Nat. Chem. Biol. 3, 619–629, doi: 10.1038/nchembio.2007.35 (2007). [DOI] [PubMed] [Google Scholar]
- Nigro P., Pompilio G. & Capogrossi M. C. Cyclophilin A: A key player for human disease. Cell Death Dis. 4, doi: 10.1038/cddis.2013.410 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser C. S., Gunning D. A., Budelier K. A. & Brown S. M. Structure and expression of cytosolic cyclophilin/peptidyl-prolyl cis-trans isomerase of higher plants and production of active tomato cyclophilin in Escherichia coli. Proc. Natl. Acad. Sci. USA 87, 9519–9523 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainali H., Chapman P. & Dhaubhadel S. Genome-wide analysis of Cyclophilin gene family in soybean (Glycine max). BMC Plant Biol. 14, 282, doi: 10.1186/s12870-014-0282-7 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano P. G., Horton P. & Gray J. E. The Arabidopsis cyclophilin gene family. Plant Physiol. 134, 1268–1282, doi: 10.1104/pp.103.022160 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi D., Yadav S., Vaid N. & Tuteja N. Genome wide analysis of Cyclophilin gene family from rice and Arabidopsis and its comparison with yeast. Plant Signaling & Behavior 7, 1653–1666 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn J. C. et al. Classification of rice (Oryza sativa L. Japonica nipponbare) immunophilins (FKBPs, CYPs) and expression patterns under water stress. BMC Plant Biol. 10, 253, doi: 10.1186/1471-2229-10-253 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallon O. Chlamydomonas Immunophilins and Parvulins: Survey and Critical Assessment of Gene Models. Eukaryotic Cell 4, 230–241, doi: 10.1128/ec.4.2.230-241.2005 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M. et al. Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Curr. Opin. Plant Biol. 9, 436–442 (2006). [DOI] [PubMed] [Google Scholar]
- Atkinson N. J. & Urwin P. E. The interaction of plant biotic and abiotic stresses: from genes to the field. J. Exp. Bot. 63, 3523–3543 (2012). [DOI] [PubMed] [Google Scholar]
- Brandts J. F., Halvorson H. R. & Brennan M. Consideration of the Possibility that the slow step in protein denaturation reactions is due to cis-trans isomerism of proline residues. Biochemistry 14, 4953–4963 (1975). [DOI] [PubMed] [Google Scholar]
- Chou I. T. & Gasser C. S. Characterization of the cyclophilin gene family of Arabidopsis thaliana and phylogenetic analysis of known cyclophilin proteins. Plant Mol. Biol. 35, 873–892 (1997). [DOI] [PubMed] [Google Scholar]
- Marivet J., Margis-Pinheiro M., Frendo P. & Burkard G. Bean cyclophilin gene expression during plant development and stress conditions. Plant Mol. Biol. 26, 1181–1189, doi: 10.1007/bf00040698 (1994). [DOI] [PubMed] [Google Scholar]
- Meza-Zepeda L. A., Baudo M. M., Palva E. T. & Heino P. Isolation and characterization of a cDNA corresponding to a stress-activated cyclophilin gene in Solanum commersonii. J. Exp. Bot. 49, 1451–1452, doi: 10.1093/jxb/49.325.1451 (1998). [DOI] [Google Scholar]
- Kong H. Y., Lee S. C. & Hwang B. K. Expression of pepper cyclophilin gene is differentially regulated during the pathogen infection and abiotic stress conditions. Physiol. Mol. Plant Pathol. 59, 189–199, doi: 10.1006/pmpp.2001.0356 (2001). [DOI] [Google Scholar]
- Chen A. P. et al. Ectopic expression of ThCYP1, a stress-responsive cyclophilin gene from Thellungiella halophila, confers salt tolerance in fission yeast and tobacco cells. Plant Cell Rep. 26, 237–245, doi: 10.1007/s00299-006-0238-y (2007). [DOI] [PubMed] [Google Scholar]
- Sekhar K., Priyanka B., Reddy V. D. & Rao K. V. Isolation and characterization of a pigeonpea cyclophilin (CcCYP) gene, and its over-expression in Arabidopsis confers multiple abiotic stress tolerance. Plant, Cell Environ. 33, 1324–1338, doi: 10.1111/j.1365-3040.2010.02151.x (2010). [DOI] [PubMed] [Google Scholar]
- Zhu C. et al. Overexpression of a cotton cyclophilin gene (GhCyp1) in transgenic tobacco plants confers dual tolerance to salt stress and Pseudomonas syringae pv. tabaci infection. Plant Physiol. Biochem. 49, 1264–1271, doi: 10.1016/j.plaphy.2011.09.001 (2011). [DOI] [PubMed] [Google Scholar]
- Schmutz J. et al. Genome sequence of the palaeopolyploid soybean. Nature 463, 178–183, doi: 10.1038/nature08670 (2010). [DOI] [PubMed] [Google Scholar]
- Kong G. et al. The Activation of Phytophthora Effector Avr3b by Plant Cyclophilin is Required for the Nudix Hydrolase Activity of Avr3b. PLoS Pathog. 11, e1005139, doi: 10.1371/journal.ppat.1005139 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zydowsky L. D. et al. Active site mutants of human cyclophilin A separate peptidyl-prolyl isomerase activity from cyclosporin A binding and calcineurin inhibition. Protein Sci. 1, 1092–1099, doi: 10.1002/pro.5560010903 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Chen C. M. & Walsh C. T. Human and Escherichia coli cyclophilins: sensitivity to inhibition by the immunosuppressant cyclosporin A correlates with a specific tryptophan residue. Biochemistry 30, 2306–2310 (1991). [DOI] [PubMed] [Google Scholar]
- Fried H. & Kutay U. Nucleocytoplasmic transport: taking an inventory. Cellular and Molecular Life Sciences CMLS 60, 1659–1688 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Angelo M. A., Raices M., Panowski S. H. & Hetzer M. W. Age-dependent deterioration of nuclear pore complexes causes a loss of nuclear integrity in postmitotic cells. Cell 136, 284–295 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel A. & Forbes D. J. Importin beta: conducting a much larger cellular symphony. Mol. Cell 16, 319–330, doi: 10.1016/j.molcel.2004.10.026 (2004). [DOI] [PubMed] [Google Scholar]
- Ohad N., Shichrur K. & Yalovsky S. The analysis of protein-protein interactions in plants by bimolecular fluorescence complementation. Plant Physiol. 145, 1090–1099 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Chen L. & Dhaubhadel S. 14-3-3 proteins regulate the intracellular localization of the transcriptional activator GmMYB176 and affect isoflavonoid synthesis in soybean. Plant J. 71, 239–250 (2012). [DOI] [PubMed] [Google Scholar]
- Day R. N., Periasamy A. & Schaufele F. Fluorescence Resonance Energy Transfer Microscopy of Localized Protein Interactions in the Living Cell Nucleus. Methods 25, 4–18, doi: http://dx.doi.org/10.1006/meth.2001.1211 (2001). [DOI] [PubMed] [Google Scholar]
- Immink R. G. H., Gadella T. W. J., Ferrario S., Busscher M. & Angenent G. C. Analysis of MADS box protein–protein interactions in living plant cells. Proc. Natl. Acad. Sci. USA 99, 2416–2421, doi: 10.1073/pnas.042677699 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato N., Pontier D. & Lam E. Spectral Profiling for the Simultaneous Observation of Four Distinct Fluorescent Proteins and Detection of Protein-Protein Interaction via Fluorescence Resonance Energy Transfer in Tobacco Leaf Nuclei. Plant Physiol. 129, 931–942, doi: 10.1104/pp.005496 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achnine L., Blancaflor E. B., Rasmussen S. & Dixon R. A. Colocalization of L-phenylalanine ammonia-lyase and cinnamate 4-hydroxylase for metabolic channeling in phenylpropanoid biosynthesis. Plant Cell 16, 3098–3109, doi: 10.1105/tpc.104.024406 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel T. et al. Colocalization and FRET-analysis of subunits c and a of the vacuolar H+ -ATPase in living plant cells. J. Biotechnol. 112, 165–175, doi: 10.1016/j.jbiotec.2004.04.027 (2004). [DOI] [PubMed] [Google Scholar]
- Seidel T., Golldack D. & Dietz K.-J. Mapping of C-termini of V-ATPase subunits by in vivo-FRET measurements. FEBS Lett. 579, 4374–4382, doi: 10.1016/j.febslet.2005.06.077 (2005). [DOI] [PubMed] [Google Scholar]
- Zhang X. C., Wang W. D., Wang J. S., Pan J. C. & Zou G. L. Evidences of monomer, dimer and trimer of recombinant human cyclophilin A. Protein Pept Lett 18, 1188–1193 (2011). [DOI] [PubMed] [Google Scholar]
- Tzivion G., Shen Y. H. & Zhu J. 14-3-3 proteins; bringing new definitions to scaffolding. Oncogene 20, 6331–6338 (2001). [DOI] [PubMed] [Google Scholar]
- Gijzen M., Kuflu K., Qutob D. & Chernys J. T. A class I chitinase from soybean seed coat. J. Exp. Bot. 52, 2283–2289, doi: 10.1093/jexbot/52.365.2283 (2001). [DOI] [PubMed] [Google Scholar]
- Yi J., Derynck M. R., Chen L. & Dhaubhadel S. Differential expression of CHS7 and CHS8 genes in soybean. Planta 231, 741–753, doi: 10.1007/s00425-009-1079-z (2010). [DOI] [PubMed] [Google Scholar]
- Puranik S., Sahu P. P., Srivastava P. S. & Prasad M. NAC proteins: regulation and role in stress tolerance. Trends Plant Sci. 17, 369–381, doi: http://dx.doi.org/10.1016/j.tplants.2012.02.004 (2012). [DOI] [PubMed] [Google Scholar]
- Gutterson N. & Reuber T. L. Regulation of disease resistance pathways by AP2/ERF transcription factors. Curr. Opin. Plant Biol. 7, 465–471, doi: 10.1016/j.pbi.2004.04.007 (2004). [DOI] [PubMed] [Google Scholar]
- Dong J., Chen C. & Chen Z. Expression profiles of the Arabidopsis WRKY gene superfamily during plant defense response. Plant Mol. Biol. 51, 21–37, doi: 10.1023/a:1020780022549 (2003). [DOI] [PubMed] [Google Scholar]
- Dong S. et al. Phytophthora sojae Avirulence Effector Avr3b is a Secreted NADH and ADP-ribose Pyrophosphorylase that Modulates Plant Immunity. PLoS Pathog. 7, e1002353, doi: 10.1371/journal.ppat.1002353 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya M. K. & Ward E. W. B. Resistance, susceptibility and accumulation of glyceollins I–III in soybean organs inoculated with Phytophthora megasperma f. sp. glycinea. Physiol. Mol. Plant Pathol. 29, 227–237, doi: http://dx.doi.org/10.1016/S0048-4059(86)80023-6 (1986). [Google Scholar]
- Phillips D. A. & Kapulnik Y. Plant isoflavonoids, pathogens and symbionts. Trends Microbiol. 3, 58–64, doi: 10.1016/s0966-842x(00)88876-9 (1995). [DOI] [PubMed] [Google Scholar]
- Subramanian S., Graham M. A., Yu O. & Graham T. L. RNA interference of soybean isoflavone synthase genes leads to silencing in tissues distal to the transformation site and to enhanced susceptibility to Phytophthora sojae. Plant Physiol. 137, 1345–1353 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi J. et al. A single-repeat MYB transcription factor, GmMYB176, regulates CHS8 gene expression and affects isoflavonoid biosynthesis in soybean. Plant J. 62, 1019–1034, doi: 10.1111/j.1365-313X.2010.04214.x (2010). [DOI] [PubMed] [Google Scholar]
- Win J. et al. Effector Biology of Plant-Associated Organisms: Concepts and Perspectives. Cold Spring Harbor Symp. Quant. Biol. 77, 235–247, doi: 10.1101/sqb.2012.77.015933 (2012). [DOI] [PubMed] [Google Scholar]
- Lapin D. & Van den Ackerveken G. Susceptibility to plant disease: more than a failure of host immunity. Trends Plant Sci. 18, 546–554, doi: http://dx.doi.org/10.1016/j.tplants.2013.05.005 (2013). [DOI] [PubMed] [Google Scholar]
- Coaker G., Falick A. & Staskawicz B. Activation of a Phytopathogenic Bacterial Effector Protein by a Eukaryotic Cyclophilin. Science 308, 548–550, doi: 10.1126/science.1108633 (2005). [DOI] [PubMed] [Google Scholar]
- Huibers R. P., de Jong M., Dekter R. W. & Van den Ackerveken G. Disease-Specific Expression of Host Genes During Downy Mildew Infection of Arabidopsis. Mol. Plant-Microbe Interact. 22, 1104–1115, doi: 10.1094/MPMI-22-9-1104 (2009). [DOI] [PubMed] [Google Scholar]
- Graham T. L., Graham M. Y., Subramanian S. & Yu O. RNAi silencing of genes for elicitation or biosynthesis of 5-deoxyisoflavonoids suppresses race-specific resistance and hypersensitive cell death in phytophthora sojae infected tissues. Plant Physiol. 144, 728–740 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Gonzalez J. J. et al. Differential Expression of Isoflavone Biosynthetic Genes in Soybean During Water Deficits. Plant Cell Physiol. 51, 936–948, doi: 10.1093/pcp/pcq065 (2010). [DOI] [PubMed] [Google Scholar]
- Chennupati P., Seguin P., Chamoun R. & Jabaji S. Effects of High-Temperature Stress on Soybean Isoflavone Concentration and Expression of Key Genes Involved in Isoflavone Synthesis. J. Agric. Food Chem. 60, 12421–12427, doi: 10.1021/jf3036319 (2012). [DOI] [PubMed] [Google Scholar]
- Wang C. S. & Vodkin L. O. Extraction of RNA from tissues containing high levels of procyanidins that bind RNA. Plant Mol. Biol. Rep. 12, 132–145, doi: 10.1007/BF02668374 (1994). [DOI] [Google Scholar]
- Earley K. W. et al. Gateway-compatible vectors for plant functional genomics and proteomics. Plant J. 45, 616–629, doi: 10.1111/j.1365-313X.2005.02617.x (2006). [DOI] [PubMed] [Google Scholar]
- Zhang X., Henriques R., Lin S. S., Niu Q. W. & Chua N. H. Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nature Prot. 1, 641–646, doi: 10.1038/nprot.2006.97 (2006). [DOI] [PubMed] [Google Scholar]
- Sparkes I. A., Runions J., Kearns A. & Hawes C. Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nature Prot. 1, 2019–2025 (2006). [DOI] [PubMed] [Google Scholar]
- Stangeland B. & Salehian Z. An improved clearing method for GUS assay in Arabidopsis endosperm and seeds. Plant Mol. Biol. Rep. 20, 107–114 (2002). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.