Abstract
In Saccharomyces cerevisiae, the (C2H2)2 zinc finger transcription factors Msn2 and Msn4 play central roles in responses to a range of stresses by activating gene transcription via the stress response element (STRE; CCCCT). The pathogen Candida albicans displays stress responses that are thought to help it survive adverse environmental conditions encountered within its human host. However, these responses differ from those in S. cerevisiae, and hence we predicted that the roles of Msn2- and Msn4-like proteins might have been functionally reassigned in C. albicans. C. albicans has two such proteins: CaMsn4 and Mnl1 (for Msn2- and Msn4-like). CaMSN4, but not MNL1, weakly complemented the inability of an S. cerevisiae msn2 msn4 mutant to activate a STRE-lacZ reporter. Also, the disruption of CaMsn4 and Mnl1 had no discernible effect upon the resistance of C. albicans to heat, osmotic, ethanol, nutrient, oxidative, or heavy-metal stress or upon the stress-activated transcriptome in C. albicans. Furthermore, although Cap1-dependent activation of a Yap response element-luciferase reporter was observed, a STRE reporter was not activated in response to stresses in C. albicans. Ectopic expression of CaMsn4 or Mnl1 did not affect the cellular or molecular responses of C. albicans to stress. Under the conditions tested, the putative activation and DNA binding domains of CaMsn4 did not appear to be functional. These data suggest that CaMsn4 and Mnl1 do not contribute significantly to stress responses in C. albicans. The data are consistent with the idea that stress signaling in this fungus has diverged significantly from that in budding yeast.
All living organisms have evolved mechanisms to detect and respond to adverse environmental conditions. In particular, pathogenic microbes must adapt efficiently to stresses imposed by their microenvironments during disease establishment and progression. For example, microbial pathogens must evade or counteract host immune defenses, and they must adapt to changes in pH or nutrient deprivation, depending upon the site of infection.
Candida albicans is the major systemic fungal pathogen of humans (6, 44, 45). This fungus is carried as a commensal in the oral and gastrointestinal tracts of many individuals but often causes oral and vaginal infections when fungus-host interactions are disturbed. C. albicans also causes systemic infections of internal organs in immunocompromised patients (44), sometimes escaping phagocytic killing, even following engulfment (33). The fact that C. albicans is relatively resistant to oxidative stresses (27) might contribute to this. It is likely that C. albicans has evolved to counter host defenses in a range of distinct niches within the host, and presumably this is dependent upon specific stress responses.
The relatively benign budding yeast Saccharomyces cerevisiae adapts to stress by using several distinct signaling pathways (36). Responses to oxidative and heavy-metal stresses are dependent upon the bZIP transcription factor Yap1 (56), which activates stress-responsive genes via sequences closely related to the Yap response element (YRE; TTA[G/C]TAA) (10). C. albicans Cap1, which is a functional homologue of Yap1, mediates responses to oxidative, heavy-metal, and drug-induced stresses (1, 68).
In S. cerevisiae, general responses to stresses, including mild heat shock, starvation, osmotic stress, alcohol, and weak acids, are dependent upon the closely related, functionally redundant (C2H2)2 zinc finger transcription factors Msn2 and Msn4 (17, 37). There is a third Msn2- and Msn4-like protein in S. cerevisiae, Yer130c, but its cellular function remains obscure (http://db.yeastgenome.org/cgi-bin/SGD). In response to stresses, Msn2 and Msn4 accumulate in the nucleus (23, 26). This leads to the transcriptional activation of stress-responsive genes via stress response elements (STRE; CCCCT) in their promoters (36, 38, 39). Msn2 and Msn4 appear to interact directly with the STRE element (38), and this interaction is thought to be enhanced by yeast glycogen synthase kinase 3 (25). Msn2- and Msn4-mediated stress responses are down-regulated by the Ras-cyclic AMP pathway (20). Activation of this pathway leads to the phosphorylation of Msn2 by protein kinase A, which causes cytoplasmic accumulation of Msn2 and hence inhibition of the general stress response (23, 24).
Transcript profiling has revealed that C. albicans does not display a general stress response under conditions that stimulate such a response in S. cerevisiae (15). Hence, our working hypothesis was that the functions of Msn2- and Msn4-like proteins have diverged in C. albicans. In this study, we tested this hypothesis by examining Msn2- and Msn4-like proteins in C. albicans using a range of approaches, including reverse genetics and genomics. We show that in contrast to S. cerevisiae, C. albicans Msn2- and Msn4-like proteins do not play significant roles in responses to heat, osmotic, ethanol, or nutrient stress. This indicates that the functions of Msn2- and Msn4-like proteins have been lost in C. albicans or that they play differing roles in these yeasts. Our data reinforce the notion that stress responses in C. albicans and S. cerevisiae have diverged significantly.
MATERIALS AND METHODS
Strains and growth conditions.
The S. cerevisiae strains used were W303-1A (MATa SUC2 ade2 can1 his3 leu2 trp1 ura3 [62]) and the congenic strain Wmsn2msn4 (MATa SUC2 ade2 can1 his3 leu2 trp1 ura3 msn2::HIS3 msn4::TRP1) (a generous gift from J. Thevelein). C. albicans strains are listed in Table 1. The strains were grown in yeast-peptone-dextrose (YPD) medium (57), yeast-peptone medium containing 3% raffinose or 3% galactose, YPD medium containing 10% fetal calf serum (61), synthetic complete (SC) medium (29), or SD minimal medium (57). Stress phenotypes were assayed by plating 10-fold dilutions of C. albicans strains under the conditions specified.
TABLE 1.
C. albicans strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| SC5314 | Wild type | 22 |
| CAF2-1 | URA3/ura3::λ imm434 | 19 |
| CA14 | ura3::λ imm434/ura3::λ imm434 | 19 |
| CA18 | ura3::λ imm434/ura3::λ imm434 ade2::hisG/ade2::hisG | 19 |
| MMY301 | ura3::λ imm434/ura3::λ imm434 cap1::hisG/cap1::hisG-URA3-hisG | 68 |
| MMC4 | ura3::λ imm434/ura3::λ imm434 nrg1::hisG/nrg1::hisG | 41 |
| MSC1 | ura3::λ imm434/ura3::λ imm434 ade2::hisG/ade2::hisG MNL1/mnl1::hisG-URA3-hisG | This study |
| MSC2 | ura3::λ imm434/ura3::λ imm434 ade2::hisG/ade2::hisG MNL1/mnl1::hisG | This study |
| MSC3 | ura3::λ imm434/ura3::λ imm434 ade2::hisG/ade2::hisG mnl1::hisG-URA3-hisG/mnl1::hisG | This study |
| MSC4 | ura3::λ imm434/ura3::λ imm434 ade2::hisG/ade2::hisG mnl1::hisG/mnl1::hisG | This study |
| MSC5 | ura3::λ imm434/ura3::λ imm434 ade2::hisG/ade2::hisG MSN4/msn4::hisG-URA3-hisG | This study |
| MSC6 | ura3::λ imm434/ura3::λ imm434 ade2::hisG/ade2::hisG MSN4/msn4::hisG | This study |
| MSC7 | ura3::λ imm434/ura3::λ imm434 ade2::hisG/ade2::hisG msn4::hisG-URA3-hisG/msn4::hisG | This study |
| MSC8 | ura3::λ imm434/ura3::λ imm434 ade2::hisG/ade2::hisG msn4::hisG/msn4::hisG | This study |
| MSC9 | ura3::λ imm434/ura3::λ imm434 ade2::hisG/ade2::hisG mnl1::hisG/mnl1::hisG MSN4/msn4::hisG-URA3-hisG | This study |
| MSC10 | ura3::λ imm434/ura3::λ imm434 ade2::hisG/ade2::hisG mnl1::hisG/mnl1::hisG MSN4/msn4::hisG | This study |
| MSC11 | ura3::λ imm434/ura3::λ imm434 ade2::hisG/ade2::hisG mnl1::hisG/mnl1::hisG msn4::hisG-URA3-hisG/msn4::hisG | This study |
| MSC12 | ura3::λ imm434/ura3::λ imm434 ade2::hisG/ade2::hisG mnl1::hisG/mnl1::hisG msn4::hisG/msn4::hisG | This study |
| MSC13 | ura3::λ imm434/ura3::λ imm434 ade2::hisG/ade2::hisG-ADE2 mnl1::hisG/mnl1::hisG msn4::hisG/msn4::hisG pCRW3 (ADE2) Clp10 (URA3) | This study |
| MSC14 | ura3::λ imm434/ura3::λ imm434 ade2::hisG/ade2::hisG-ADE2 mnl1::hisG/mnl1::hisG msn4::hisG/msn4::hisG pCRW3 (ADE2) pMNL1 (URA3) | This study |
| MSC15 | ura3::λ imm434/ura3::λ imm434 ade2::hisG/ade2::hisG-ADE2 mnl1::hisG/mnl1::hisG msn4::hisG/msn4::hisG pCRW3 (ADE2) pMSN4 (URA3) | This study |
| MSC16 | ura3::λ imm434/ura3::λ imm434 ade2::hisG/ade2::hisG pCRW3 (ADE2) pACT1 (URA3) | This study |
| MSC17 | ura3::λ imm434/ura3::λ imm434 ade2::hisG/ade2::hisG pCRW3 (ADE2), pACT1-MNL1 (URA3) | This study |
| MSC18 | ura3::λ imm434/ura3::λ imm434 ade2::hisG/ade2::hisG pCRW3 (ADE2), pACT1-MSN4 (URA3) | This study |
| SNC7 | ura3::λ imm434/ura3::λ imm434 ade2::hisG/ade2::hisG/mnl1::hisG/mnl1::hisG msn4::hisG/msn4::hisG NRG1/nrg1::hisG-URA3-hisG | This study |
| SNC8 | ura3::λ imm434/ura3::λ imm434 ade2::hisG/ade2::hisG mnl1::hisG/mnl1::hisG msn4::hisG/msn4::hisG NRG1/nrg1::hisG | This study |
| SNC9 | ura3::λ imm434/ura3::λ imm434 ade2::hisG/ade2::hisG mnl1::hisG/mnl1::hisG msn4::hisG/msn4::hisG nrg1::hisG-URA3-hisG/nrg1::hisG | This study |
| SNC10 | ura3::λ imm434/ura3::λ imm434 ade2::hisG/ade2::hisG mnl1::hisG/mnl1::hisG msn4::hisG/msn4::hisG nrg1::hisG/nrg1::hisG | This study |
| SNC11 | ura3::λ imm434/ura3::λ imm434 pMET3-VP16 (URA3) | This study |
| SNC12 | ura3::λ imm434/ura3::λ imm434 pMET3-VP16-MNL1DBD (URA3) | This study |
| SNC13 | ura3::λ imm434/ura3::λ imm434 pMET3-VP16-MSN4DBD (URA3) | This study |
| CRC116 | ura3::λ imm434/ura3::λ imm434 ade2::hisG/ade2::hisG pCR-lacZ (ADE2) Clp-LexA (URA3) | 53 |
| CRC110 | ura3::λ imm434/ura3::λ imm434 ade2::hisG/ade2::hisG pCR-OPlacZ (ADE2) Clp-LexA (URA3) | 53 |
| CRC122 | ura3::λ imm434/ura3::λ imm434 ade2::hisG/ade2::hisG pCR-lacZ (ADE2) Clp-LexA-GCN4 (URA3) | 53 |
| CRC121 | ura3::λ imm434/ura3::λ imm434 ade2::hisG/ade2::hisG pCR-OPlacZ (ADE2) Clp-LexA-GCN4 (URA3) | 53 |
| SNC14 | ura3::λ imm434/ura3::λ imm434 ade2::hisG/ade2::hisG pCR-lacZ (ADE2) Clp-LexA-MSN4 (URA3) | This study |
| SNC15 | ura3::λ imm434/ura3::λ imm434 ade2::hisG/ade2::hisG pCR-OPlacZ (ADE2) Clp-LexA-MSN4 (URA3) | This study |
Strain construction.
The CaMSN4 and MNL1 loci were disrupted by Ura blasting, as described previously (19), to generate homozygous single mutants (MSC4 and MSC8) (Table 1). The msn4::hisG-URA3-hisG disruption cassette deleted codons 2 to 903 of the 906-codon CaMSN4 open reading frame (ORF), and the mnl1::hisG-URA3-hisG disruption cassette deleted codons 3 to 757 of the 759-codon MNL1 ORF. The homozygous mnl1/mnl1 msn4/msn4 double mutant (MSC12) was created by disrupting the CaMSN4 locus in the mnl1/mnl1 mutant. The CaMSN4 and MNL1 loci were PCR amplified and cloned into a URA3-containing integrating plasmid, CIp10 (40), to create pMSN4 and pMNL1. These plasmids and the control, CIp10, were integrated at the RPS10 locus in MSC12 to create the strains MSC13 to MSC15 (Table 1). Finally, to generate the homozygous mnl1/mnl1 msn4/msn4 nrg1/nrg1 triple mutant (SNC10), the CaNRG1 locus was disrupted in the double mutant (MSC12) using a previously described nrg1::hisG-URA3-hisG disruption cassette (41). At each stage of this process, the genotype of each strain was confirmed by both PCR diagnosis and Southern analysis (not shown).
To achieve ectopic expression of CaMSN4 and MNL1 in C. albicans, these ORFs were PCR amplified using primers MSN4-F, MSN4-R, MNL1-F, and MNL1-R (Table 2); resequenced; and cloned between the CaACT1 promoter and the ScCYC1 terminator in pACT1 (63). The resultant plasmids, pACT1, pACT1-MSN4, and pACT1-MNL1, were integrated at the RPS10 locus in CAI8 (40) to create the strains MSC16 to MSC18 (Table 1).
TABLE 2.
Oligonucleotides used in this study
| Name | Sequence (5′ → 3′) | Use |
|---|---|---|
| MSN4-F | CCACCAAGTCGACCAAATATGTC | Amplification of CaMSN4 ORF for pACT1 fusion |
| MSN4-R | CCCAAAAACGCGTTGATATAG | Amplification of CaMSN4 ORF for pACT1 fusion |
| MNL1-F | CGAAGGAGGGTCGACCAATATGG | Amplification of MNL1 ORF for pACT1 fusion |
| MNL1-R | GCAATAGACGCGTGCCACAAG | Amplification of MNL1 ORF for pACT1 fusion |
| VP16-1T | GGGAGATCTATAATGGCTCCACCAACCGATGTTTCTTTGGGTGATGAGCTCCACTTGGATGGTGAAGATGTTGC | Construction of VP16 activation domain |
| VP16-1B | TTCACCATCCAAGTGGAGCTCATCACCCAAAGAAACATCGGTTGGTGGAGCCATTATAGATCTCCC | Construction of VP16 activation domain |
| VP16-2T | TATGGCTCACGCTGATGCTTTGGATGATTTCGATTTGGATATGTTGGGTGATGGTGATTCTCCAGGGCCCGGTTTCACCCCACACGATTCTG | Construction of VP16 activation domain |
| VP16-2B | TGTGGGGTGAAACCGGGCCCTGGAGAATCACCATCACCCAACATATCCAAATCGAAATCATCCAAAGCATCAGCGTGAGCCATAGCAACATC | Construction of VP16 activation domain |
| VP16-3T | CTCCATACGGTGCTTTGGATATGGCTGATTTCGAATTCGAACAAATGTTCACCGATGCTTTGGGTATTGATGAATACGG | Construction of VP16 activation domain |
| VP16-3B | ATCAATACCCAAAGCATCGGTGAACATTTGTTCGAATTCGAAATCAGCCATATCCAAAGCACCGTATGGAGCAGAATCG | Construction of VP16 activation domain |
| VP16-4T | TGGTATAACGCGTATAACTAGTTATGCTAGCATATCTAGAATAGGATCCTAACTCGAGATACTGCAGGGGG | Construction of VP16 activation domain |
| VP16-4B | CCCCCTGCAGTATCTCGAGTTAGGATCCTATTCTAGATATGCTAGCATAACTAGTTATACGCGTTATACCACCGTATTC | Construction of VP16 activation domain |
| VP16-T | GGGAGATCTATAATGGCTCCACC | Amplification of VP16 activation domain |
| VP16-B | CCCCCTGCAGTATCTCGAGTTAGG | Amplification of VP16 activation domain |
| CYC1-T | GATCCATATATGTCGACGTCCCTATTTATTTTTTTATAGTTATGTTAGTATTAAGAACGTTATTTATATTTCAAATCCATGGG | ScCYC1 transcriptional terminator |
| CYC1-B | TCGACCCATGGATTTGAAATATAAATAACGTTCTTAATACTAACATAACTATAAAAAAATAAATAGGGACGTCGACATATATG | ScCYC1 transcriptional terminator |
| Linker-T | CTAGCATATGGTACCATATGATATCATATACGCGTATATACTAGTTATACTCGAGATATG | Polylinker |
| Linker-B | GATCCATATCTCGAGTATAACTAGTATATACGCGTATATGATATCATATGGTACCATATG | Polyliker |
| NLS-T | CTAGCCCAAAGAAAAAGAGAAAAGTTGCGGCCGCTATATTCGATATAGGTAC | SV40 T-antigen NLS |
| NLS-B | CTATATCGAATATAGCGGCCGCAACTTTTCTCTTTTTCTTTGGG | SV40 T-antigen NLS |
| MSNDBD-F | TGATGGTACCAGCAATCCC | Amplification of CaMsn4DBD |
| MSNDBD-R | CAGCAAGGGATCCAACAAAC | Amplification of CaMsn4DBD |
| MNLDBD-F | GCTGGTACCGCTAGTG | Amplification of Mn11DBD |
| MNLDBD-R | GTGGAGGATCCAACAA | Amplification of Mn11DBD |
| MSN4lex-F | GGTCCACGCGTTGGTGGAGGTCCAGGTGGATCTCAAGAATTCCAACCTTTATTTGAAAC | Amplification of CaMsn4 for lexA fusion |
| MSN4lex-R | CAAAATCTGCAGGGTAAACACCATAC | Amplification of CaMsn4 for lexA fusion |
| STRE-T | GTCACCACCCCTAACAGCCCCTGTATACCCCTGGATCCCCCCTGTAAGCCCCTA | Introduction of STRE into reporter |
| STRE-B | CTAGTAGGGGCTTACAGGGGGGATCCAGGGGTATACAGGGGCTGTTAGGGGTG | Introduction of STRE into reporter |
| YRE-T | GTCACCTTAGTAAGAGCCTTAGTAAGGATCCTTAGTAATCGGATTAGTAATAGCGTTAGTAAA | Introduction of YRE into reporter |
| YRE-B | CTAGTTTACTAACGCTATTACTAATCCGATTACTAAGGATCCTTACTAAGGCTCTTACTAAG | Introduction of YRE into reporter |
To perform the complementation tests in S. cerevisiae, the CaMSN4 and MNL1 ORFs from pACT1-MSN4 and pACT1-MNL1 were subcloned into the centromeric plasmid pRS315 (58) under the control of the S. cerevisiae GAL1 promoter. This generated the plasmids pGAL10-MSN4 and pGAL10-MNL1, which were transformed separately into the S. cerevisiae msn2 msn4 mutant alongside the empty pGAL10 control.
To generate the SalexA-MSN4 fusion, the CaMSN4 ORF was PCR amplified and cloned into CIp-LexA (53) using the primers MSN4lex-F and MSN4lex-R (Table 2). The forward primer introduced a (Gly)3-Pro-(Gly)2 linker between the amino-terminal LexA domain and the carboxy-terminal CaMsn4 domain (Table 2). The CIp-LexA-MSN4 plasmid was then transformed into C. albicans CAI8 (Table 1) via integration at the RPS10 locus, selecting for the plasmid-borne URA3 marker (53).
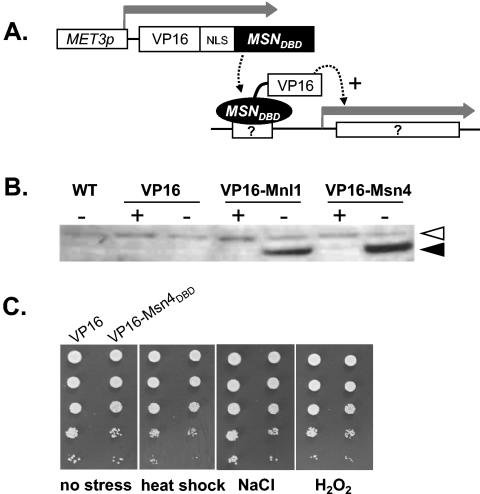
To generate a synthetic, codon-optimized VP16 activation domain, oligonucleotides VP16-1T to VP16-4B were sequentially annealed and ligated together (Table 2). Codon optimization was performed using published tables of preferred C. albicans codons (5). The full-length VP16 fragment was then PCR amplified using primers VP16-T and VP16-B; the product was cloned into pGEM-T EASY (Promega, Southampton, United Kingdom) and sequenced to create pGEM-VP16. The S. cerevisiae CYC1 transcriptional terminator was then cloned downstream of the VP16 domain by inserting the annealed oligonucleotides CYC1-T and CYC1-B into the XhoI site of pGEM-VP16. The resultant BglII-KpnI VP16-CYC1t fragment was subcloned between the BamHI and KpnI sites of the MET3 expression vector, pCaEXPa (7). A new linker was then inserted between the VP16 and CYC1t sequences to introduce additional restriction sites (oligonucleotides Linker-T and Linker-B [Table 2]), and the simian virus 40 (SV40) T-antigen nuclear localization signal (NLS) was inserted between the new NheI and KpnI sites (oligonucleotides NLS-T and NLS-B [Table 2]). This generated the control VP16 expression plasmid, pMET3-VP16 (see Fig. 9).
FIG. 9.
Expression of a VP16-CaMsn4DBD fusion in C. albicans causes no obvious stress phenotype. (A) Cartoon illustrating the experimental rationale to identify gene targets of CaMsn4. The CaMET3 promoter is repressed by methionine and cysteine (7). (B) Western blot with anti-VP16 antibody showing regulated expression of MET3-VP16-MSN4DBD in C. albicans. Wild type, CAI4 (WT; Table 1); SNC11 (VP16); SNC12 (VP16-Mnl1); SNC13 (VP16-Msn4); no methionine or cysteine (−); 10 mM methionine and 10 mM cysteine (+); open arrow, nonspecific band; closed arrow, VP16 fusion protein. (C) Phenotype of C. albicans CAI4 cells expressing VP16-CaMsn4DBD or the VP16 control on plates lacking methionine and cysteine. No stress (SC; 30°C), mild heat shock (25 to 37°C), 1.0 M NaCl, and 2.5 mM H2O2.
To express VP16-CaMsn4DBD and VP16-Mnl1DBD fusions in C. albicans (see Fig. 9), the zinc finger regions of MSN4 and MNL1 were PCR amplified (primers MSNDBD-F, MSNDBD-R, MNLDBD-F, and MNLDBD-R [Table 2]) and cloned between the KpnI and BamHI sites of pMET3-VP16. The resultant plasmids were transformed into C. albicans CAI8 (Table 1) via integration at RPS10, selecting for the URA3 marker (40).
Transcript profiling.
Transcript profiling of the congenic C. albicans strains CAI8, MSC12, MSC16, MSC17, and MSC18 was performed on cells growing exponentially in YPD medium. The cells were exposed to the appropriate stress (mild heat shock [23 to 37°C], osmotic stress [0.3 M NaCl], or oxidative stress [0.4 mM H2O2]) and analyzed 0, 10, 30, and 60 min thereafter. At each time point, MSC12 was compared to its control (CAI8), and MSC17 and MSC18 were compared to their control (MSC16).
Transcript profiling was performed as described previously (15, 43). Briefly, RNA was isolated, Cy3 and Cy5-labeled cDNAs were prepared, and the probes were hybridized with arrays comprising ∼95% of C. albicans ORFs (43). Slides were scanned at 10-μm resolution with a ScanArray 5000 scanner (version 2.11; Packard Bioscience) and quantified using QuantArray software (version 2.0; Packard Bioscience), and data normalization and analysis were performed using GeneSpring software (Silicon Genetics, Redwood City, Calif.) and significance analysis of microarrays (64). Data from at least three independent experiments were used in the analysis. The data are accessible at http://www.cbr.nrc.ca/genetics/stress/.
Sequence analyses.
DNA sequences were analyzed using CandidaDB (http://genolist.pasteur.fr/CandidaDB) and the Stanford Genome Database (http://genome-www.stanford.edu/).
Southern, Northern, and Western analyses.
Published methods were used for RNA and DNA preparation, Southern blotting, and Northern analysis (41). Western blotting was performed as described previously (11).
Reporter assays.
Renilla reniformis LUC constructs were made using the basal ADH1b-RLUC reporter (63), created by introducing a basal CaADH1 promoter into a derivative of pCRW3 (60). STRE-RLUC and YRE-RLUC reporters were made by cloning STRE- and YRE-containing oligonucleotides between BstEII and SpeI sites upstream of the basal promoter (Table 2). pADH1b-RLUC, pSTRE-RLUC, and pYRE-RLUC were transformed into C. albicans CAI8, and luciferase assays were performed in quadruplicate on independent transformants as described previously (63). An equivalent STRE-lacZ fusion was created by cloning a STRE-containing oligonucleotide upstream of the basal S. cerevisiae CYC1 promoter (46) and StlacZ reporter (65) in CIp10 (40). The activities of SaLexA fusion proteins in C. albicans CAI8 were assayed using a StlacZ reporter containing the SaLexA operator, as described previously (53). Triplicate β-galactosidase assays were performed on independent transformants using plate overlays and in liquid media (52).
RESULTS
C. albicans has two MSN2- and MSN4-like loci.
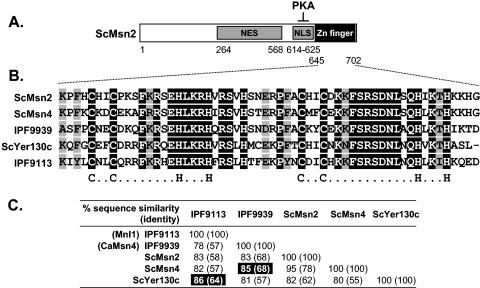
Our first aim was to identify MSN2- and MSN4-like loci in C. albicans. Detailed searches of the genome sequence (http://www-sequence.stanford.edu/group/candida) and the C. albicans genome database (http://genolist.pasteur.fr/CandidaDB) revealed two loci with significant sequence similarity to S. cerevisiae MSN2, MSN4, or YER130c: IPF9113 (orf19.6121) and IPF9939 (orf19.4752). These C. albicans proteins display significant sequence similarity in their putative DNA binding domains to each other, as well as to S. cerevisiae Msn2, Msn4, and Yer130c (Fig. 1). The sequence similarities between the C. albicans and S. cerevisiae proteins were limited to the putative DNA binding domains. This is also the case for other functionally related transcription factors in these fungi, such as Gcn4, Nrg1, and Rox1/Rfg1 (2, 28, 41, 63).
FIG. 1.
Comparison of Msn2- and Msn4-like sequences in C. albicans and S. cerevisiae. (A) Cartoon of S. cerevisiae Msn2 (ScMsn2) illustrating the coordinates of the (C2H2)2 DNA binding domain (Zn finger), the nuclear export signal (NES), the NLS, and the repression of the NLS by protein kinase A (PKA) (24). (B) Sequence alignments for the (C2H2)2 regions of the Msn2- and Msn4-like proteins in C. albicans and S. cerevisiae. Residues conserved in all proteins, black; conservative substitutions, grey. (C) Percentage sequence similarities (identities) in these regions. IPF9113 and IPF9939 are most similar to the S. cerevisiae proteins ScYer130c and ScMsn4, respectively.
IPF9113 is related to Msn2 but is most similar to Yer130c. However, C. albicans genes should not be named using the formal gene names of S. cerevisiae orthologues. Hence, IPF9113 was provisionally named MNL1 (for Msn2- and Msn4-like protein). IPF9939 is most similar to Msn4 and therefore was provisionally named CaMsn4. To avoid confusion, we use the prefixes “Ca” and “Sc” to distinguish C. albicans and S. cerevisiae orthologues.
CaMSN4 weakly complements an S. cerevisiae msn2 msn4 double mutation.
The next objective was to test whether CaMsn4 and Mnl1 are functional homologues of ScMsn2, ScMsn4, or ScYer130c. Unfortunately, S. cerevisiae yer130c mutants display no obvious phenotype (http://db.yeastgenome.org/cgi-bin/SGD), and therefore it was not possible to perform a complementation test in such strains. However, we were able to exploit the transcriptional defect of S. cerevisiae msn2 msn4 cells with respect to STRE-lacZ activation (38).
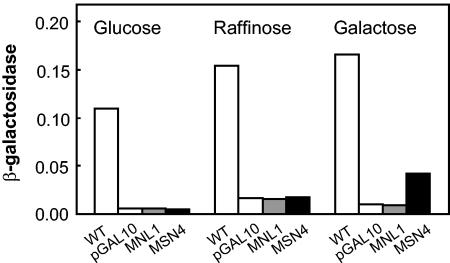
GAL1-MSN4 and GAL1-MNL1 fusions were constructed to drive the expression of these C. albicans ORFs in S. cerevisiae using the ScGAL1 promoter. These centromeric plasmids, and the empty pGAL10 control, were transformed into S. cerevisiae wild-type and msn2 msn4 cells. These cells were exposed to osmotic stress during exponential growth on glucose (to repress the ScGAL1 promoter), raffinose (to derepress the ScGAL1 promoter), or galactose (to activate the ScGAL1 promoter). As expected (38), wild-type S. cerevisiae cells displayed STRE-lacZ induction in response to the stress, and the S. cerevisiae msn2 msn4 cells containing the empty pGAL10 plasmid showed no significant induction (Fig. 2). The STRE-lacZ reporter was not induced in cells containing pGAL10-MNL1, indicating that MNL1 is unable to complement the double msn2 msn4 mutation in S. cerevisiae. This was consistent with the idea that IPF9113 is most closely related to S. cerevisiae Yer130c (Fig. 1). In contrast, cells containing pGAL10-MSN4 displayed weak STRE-lacZ activation during growth on galactose. This activation was not apparent during growth on raffinose. This suggested that CaMSN4 is able to complement the double msn2 msn4 mutation, but only weakly.
FIG. 2.
Ability of CaMSN4 and MNL1 to complement an S. cerevisiae msn2 msn4 double mutation. CaMSN4 and MNL1 were expressed using the ScGAL1 promoter in S. cerevisiae strain Wmsn2msn4 containing a STRE-lacZ reporter. Cells were grown to mid-exponential phase on glucose, raffinose, or galactose and then exposed to 0.3 M NaCl for 30 min, and β-galactosidase activities were measured (Miller units). S. cerevisiae W303-1A (WT); S. cerevisiae Wmsn2msn4 containing the empty expression vector pGAL10 (pGAL10); pGAL10-MNL1 (MNL1), or; pGAL10-MSN4 (MSN4). Errors were <10%, and similar data were obtained in three independent experiments.
Cellular phenotypes of C. albicans msn4 and mnl1 mutants.
Isogenic homozygous null mutants were generated to examine the roles of CaMsn4 and Mnl1 in C. albicans (Table 1). This involved the sequential deletion of both alleles for each locus in this diploid fungus using standard Ura-blasting procedures (19). Each CaMSN4 allele was inactivated by deleting essentially all of the 759-codon ORF and inserting the Ura blaster cassette (see Materials and Methods). Similarly, MNL1 was disrupted by replacing all of the 906-codon ORF with the Ura blaster cassette. A double mutant was then created by disrupting the CaMSN4 locus in the homozygous mnl1/mnl1 null mutant (Table 1). The loss of CaMSN4 and MNL1 mRNAs in the corresponding mutants was confirmed by Northern analysis (not shown).
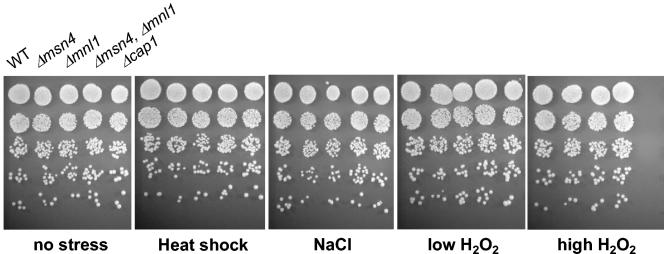
An S. cerevisiae msn2 msn4 double mutant displays sensitivity to general stresses (38), but S. cerevisiae yer130c mutants display no obvious phenotype. If CaMSN4 and MNL1 have been functionally reassigned in C. albicans, the corresponding mutants might not be expected to display stress phenotypes. Hence, we examined the sensitivities of the C. albicans msn4/msn4 mutants, the mnl1/mnl1 mutants, and double mutants under a wide range of stress conditions. These included mild heat shock (25 to 37°C), osmotic stress (0.3 to 2 M NaCl), oxidative stress (0.4 to 2.5 mM H2O2) (Fig. 3), heavy-metal stress (0.1 to 1.0 mM CdSO4), ethanol stress (7%), and carbon starvation (growth to stationary phase and on minimal medium lacking glucose and containing glycerol) (not shown). As predicted, neither the msn4/msn4 and mnl1/mnl1 single mutants nor the double msn4/msn4 mnl1/mnl1 mutant displayed any obvious phenotype under any of these conditions. As a control, we examined the behavior of a cap1/cap1 mutant. As expected (1), this mutant displayed sensitivity to H2O2 but not to the other stresses tested (Fig. 3).
FIG. 3.
Inactivation of CaMsn4 and Mnl1 does not alter stress resistance in C. albicans. C. albicans strains were exposed to a wide range of stresses, including no stress (YPD; 30°C), mild heat shock (25 to 37°C), 1.0 M NaCl, 0.4 mM H2O2, and 2.5 mM H2O2. Strains: CAI8 (WT), MSC8 (Δmsn4), MSC4 (Δmnl1), MSC12 (Δmsn4, Δmnl1), and MMY301 (Δcap1) (Table 1). Where necessary, strains were transformed with CIp10 (URA3) and pCRW3 (ADE2) to make them prototrophic.
The observation that C. albicans msn4/msn4 mnl1/mnl1 mutants lacked an obvious stress phenotype was consistent with our working hypothesis that the functions of CaMsn4 and Mnl1 have been reassigned in C. albicans. However, the data did not exclude the possibility that these factors play a nonessential role in stress responses in this fungus. It remained a formal possibility that functional redundancy might exist between CaMsn4/Mnl1 and some other unidentified factor(s) in C. albicans. We reasoned that some hidden role in stress responses might be revealed by ectopic expression of CaMsn4 or Mnl1. Hence, the C. albicans ACT1 promoter was used to drive ectopic expression of CaMSN4 and MNL1. This promoter has been used successfully to drive ectopic expression of other transcription factors in C. albicans. For example, ACT1-CaNRG1 and ACT1-CaGCN4 fusions have been shown to confer morphological and metabolic phenotypes upon C. albicans (2, 63). However, C. albicans strains carrying ACT1-CaMSN4 or ACT1-MNL1 fusions displayed no significant elevation in resistance to heat shock, osmotic stress, heavy-metal stress, or oxidative stress (not shown). This was consistent with the idea that CaMsn4 and Mnl1 play no significant roles in the stress responses examined.
Molecular phenotypes of C. albicans msn4 and mnl1 mutants.
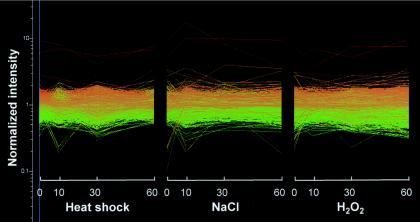
It was possible that by examining the cellular phenotypes of msn4 and mnl1 mutants we might have missed subtle influences of CaMsn4 and Mnl1 upon C. albicans stress responses. However, we reasoned that subtle roles for these proteins would be revealed by analyzing the molecular phenotypes of the corresponding null mutants. Hence, transcript profiling was used to compare the double msn4/msn4 mnl1/mnl1 mutant with its isogenic wild-type parent during exposure to a mild heat shock (23 to 37°C), an osmotic shock (0.3 M NaCl), or an oxidative stress (0.4 mM H2O2). These conditions were chosen because they had been shown previously to generate specific stress responses in the C. albicans transcriptome (15). The strains were compared at 0, 10, 30, and 60 min after exposure to each stress. A high degree of statistical reproducibility was observed for the three independent experiments that were performed for each time point. However, inactivation of CaMsn4 and Mnl1 had no significant effect upon the transcriptional responses of C. albicans to these stresses (Fig. 4). Wild-type C. albicans cells display a well-defined transcriptional response to the osmotic, oxidative, and heat stresses examined in this study (15). When the transcript profiles of msn4/msn4 mnl1/mnl1 and wild-type cells were compared, the expression ratios for most genes approximated to 1 at each time point under each condition (Fig. 4 and Table 3). This indicates that the inactivation of CaMsn4 and Mnl1 had no significant effect upon the expression of almost all C. albicans genes. Hence, all stress-induced transcription was retained in the msn4/msn4 mnl1/mnl1 cells under the stress conditions examined.
FIG. 4.
Inactivation of CaMsn4 and Mnl1 does not significantly affect the stress transcriptomes of C. albicans. Strains CAI8 (wild type) and MSC12 (Δmsn4Δmnl1) were transformed with CIp10 (URA3) and pCRW3 (ADE2) to make them prototrophic. The cells were exposed to a mild heat shock (25 to 37°C), an osmotic stress (0.3 M NaCl), or an oxidative stress (0.4 mM H2O2); RNA was isolated after 0, 10, 30, and 60 min; and transcript profiling was performed (15). Mean ratios (Δmsn4 Δmnl1 mutant versus wild type) of the normalized signal intensities for each gene under each experimental condition were calculated using data from three independent experiments. Each line represents the effect of the Δmsn4 Δmnl1 double mutation upon the expression of a gene under the conditions shown; a ratio of 1.0 indicates that any gene regulation observed in wild-type cells is not affected by the inactivation of CaMsn4 and Mnl1.
TABLE 3.
Effects of inactivating CaMsn4 and Mn11 upon gene induction in response to osmotic stress
| Gene | Expression ratio
|
Functiond | |||||||
|---|---|---|---|---|---|---|---|---|---|
| WT stress/WT no stressa,b at time (min):
|
msn4 mnl1 stress/WT stressc at time (min):
|
||||||||
| 0 | 10 | 30 | 60 | 0 | 10 | 30 | 60 | ||
| orf19.7284 | 0.9 | 20.2 | 55.3 | 1.4 | 0.8 | 1.1 | 0.8 | 0.7 | Unknown function |
| orf19.5302 | 0.9 | 14.8 | 27.8 | 4.4 | 1.1 | 2.0 | 1.3 | 0.8 | Unknown function |
| MSC1 | 1.0 | 14.1 | 23.2 | 1.7 | 0.8 | 0.9 | 0.9 | 0.7 | Meiotic sister chromatid recombination |
| orf19.5070 | 0.9 | 13.7 | 1.3 | 1.1 | 1.2 | 1.1 | 0.9 | 1.1 | Unknown function |
| CTA1 | 1.0 | 11.8 | 7.7 | 1.2 | 1.0 | 1.0 | 1.1 | 0.8 | Peroxisomal catalase A |
| orf19.2048 | 0.9 | 10.8 | 3.2 | 0.7 | 1.5 | 1.1 | 1.1 | 1.1 | Unknown function |
| DDR48 | 1.1 | 9.9 | 12.2 | 5.0 | 1.0 | 1.1 | 1.3 | 1.1 | DNA damage-responsive protein |
| orf19.2344 | 1.0 | 9.7 | 14.2 | 1.8 | 1.1 | 0.8 | 1.2 | 0.8 | Unknown function |
| orf19.7350 | 1.1 | 9.3 | 5.1 | 2.3 | 0.5 | 0.5 | 0.8 | 0.6 | Unknown function |
| CEX16 | 0.8 | 8.8 | 1.3 | 2.4 | 1.4 | 1.5 | 1.8 | 1.5 | Putative interaction with heat shock proteins and chaperones |
| orf19.3932 | 1.1 | 8.3 | 7.5 | 1.2 | 1.0 | 0.9 | 0.7 | 0.8 | Unknown function |
| orf19.2737 | 0.9 | 8.3 | 8.0 | 0.9 | 1.0 | 0.9 | 1.0 | 1.0 | FGGY family of carbohydrate kinases |
| orf19.7296 | 0.9 | 8.1 | 0.6 | 0.7 | 0.8 | 0.9 | 1.0 | 0.6 | Unknown function |
| ADH6 | 1.1 | 7.5 | 6.1 | 1.0 | 0.9 | 0.9 | 1.0 | 1.0 | Alcohol dehydrogenase |
| DCW2 | 1.0 | 7.1 | 1.9 | 1.3 | 1.1 | 1.1 | 1.0 | 1.0 | GP1-anchored protein with cell wall role |
| SGA1 | 0.9 | 7.0 | 0.9 | 1.3 | 1.1 | 1.2 | 1.0 | 1.2 | Glucoamylase |
| orf19.692 | 0.9 | 6.1 | 0.6 | 0.9 | 1.1 | 1.2 | 1.0 | 1.0 | Unknown function |
| YBR56 | 1.0 | 5.9 | 6.9 | 1.0 | 1.0 | 0.8 | 1.0 | 0.8 | Putative exo-1,3-beta-glucanase |
| AGP2 | 1.0 | 5.8 | 1.7 | 2.2 | 1.0 | 1.0 | 1.0 | 1.0 | Putative amino acid permease |
| orf19.3007.2 | 1.0 | 5.5 | 2.7 | 0.8 | 1.2 | 1.0 | 1.0 | 0.9 | Unknown function |
| LRR | 0.9 | 5.1 | 1.1 | 1.0 | 0.6 | 0.8 | 1.3 | 0.7 | Leucine-rich repeat protein |
Data from Enjalbert et al. (15).
The osmotic stress was 0.3 M NaCl. Similar observations were obtained for the heat and oxidative stresses (not shown). WT, wild type.
Data from this study (Fig. 4).
Gene information from http://candida.bri.nrc.ca/candida/index.cfm and http://genolis1.pasteur.fr//CandidaDB/.
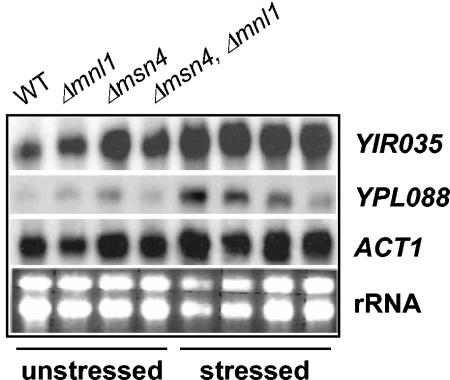
The expression of only one stress-induced gene was consistently reduced more than twofold in the msn4/msn4 mnl1/mnl1 mutant: CaYPL088. Northern analysis confirmed that this transcript was induced moderately by osmotic stress in wild-type cells and that this increase was blocked in msn4/msn4 mnl1/mnl1 cells (Fig. 5). This gene encodes a protein with sequence similarity to Agrobacterium tumefaciens MocA, but the biological significance of this change, if any, is not clear.
FIG. 5.
Confirmation of the responses of the CaYPL088 and CaYIR035 transcripts by Northern analysis. Northern blotting was used to compare the levels of the CaYPL088 and CaYIR035 transcripts in unstressed C. albicans cells and in cells exposed to an osmotic stress (0.3 M NaCl) for 10 min. CAI8 (WT), MSC4 (Δmnl1), MSC8 (Δmsn4), and MSC12 (Δmsn4, Δmnl1). The ACT1 mRNA and rRNAs were used as internal loading controls.
Signals for two probes on the microarray were constitutively elevated in msn4/msn4 mnl1/mnl1 cells. However, both probes corresponded to the same transcript (CaYIR035). The elevation in CaYIR035 mRNA levels in the unstressed double mutant was confirmed by Northern analysis (Fig. 5). Northern analysis of the single msn4/msn4 and mnl1/mnl1 mutants suggested that CaMsn4 plays a greater role than Mnl1 in the regulation of CaYIR035, which is predicted to encode a short-chain dehydrogenase. Again, the biological significance of this change is not clear.
The CaMSN4 transcript was constitutively reduced in msn4/msn4 mnl1/mnl1 cells, confirming our earlier Northern analyses, which indicated that msn4/msn4 cells lacked CaMSN4 mRNA (not shown). The transcript profiling data of Enjalbert and coworkers (15) indicate that CaMSN4 and MNL1 are expressed under the heat, osmotic, and oxidative stress conditions examined and that their transcript levels do not change significantly under these conditions.
Although unlikely, it was possible that the effects of the msn4/msn4 mnl1/mnl1 mutations upon the transcriptome might have been masked by functional redundancy with some unknown factor. Therefore, we examined the effects of the ACT1-CaMSN4 and ACT1-MNL1 fusions upon the C. albicans transcriptome. Again, the three independent transcript-profiling experiments performed for each experimental condition were highly reproducible. However, the ectopic expression of CaMsn4 or Mnl1 had no significant effect upon the transcript profile relative to the pACT1 control, even following exposure of the C. albicans cells to heat, osmotic, or oxidative stress (not shown). Therefore, transcript profiling revealed no obvious roles for CaMsn4 or Mnl1 during the C. albicans stress responses tested or during exponential growth on glucose. This was consistent with our working hypothesis.
The STRE in C. albicans.
S. cerevisiae Msn2 and Msn4 activate transcription via the STRE (38), and the role of the STRE in mediating transcriptional responses to stress is conserved in other fungi (47). Many C. albicans genes that respond to stress contain STRE-like sequences in their promoters. Furthermore, we have shown that other S. cerevisiae regulatory elements, such as GCRE and YRE, are conserved in C. albicans (34, 63). However, if CaMsn4 and Mnl1 have been functionally reassigned, we predicted that STRE-like sequences might not mediate transcriptional responses to general stresses in C. albicans.
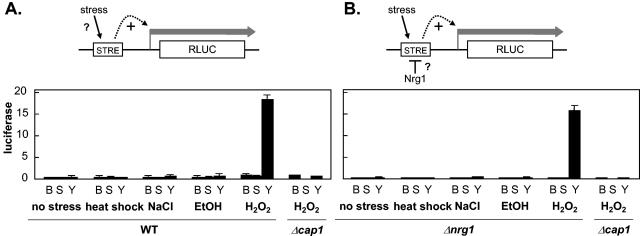
To test this, we introduced four tandem STRE upstream of the basal C. albicans reporter, ADH1b-RLUC (63), to create STRE-RLUC. As a positive control, we constructed an analogous YRE-RLUC reporter containing four tandem YRE. As expected, the YRE-RLUC reporter did not respond to a mild heat shock, 0.3 M NaCl, or 7% ethanol but was activated in response to 2.5 mM H2O2 in a Cap1-dependent fashion (Fig. 6A). In contrast, the STRE reporter displayed no significant activation above basal levels following exposure to these heat, osmotic, ethanol, or oxidative stresses.
FIG. 6.
The STRE does not mediate stress-activated transcription in C. albicans. (A) To test the working model that some stresses activate transcription via the STRE in C. albicans, the expression of basal RLUC (B), STRE-RLUC (S), and YRE-RLUC (Y) fusions were monitored in CAI8 (WT) following exposure to a range of stresses: no stress (YPD; 30°C), mild heat shock (25 to 37°C), 0.3 M NaCl, 7% ethanol (EtOH), and 2.5 mM H2O2. The effect of Cap1 inactivation upon the response to 2.5 mM H2O2 was measured as a control using strain MMY301 (Table 1). Luciferase levels were measured in triplicate for three independent transformants (105 relative light units). (B) To test the hypothesis that CaNrg1 might repress STRE activation, the same experiment was performed using strain MMC4 (Δnrg1) (Table 1).
It was possible that, although the YRE-RLUC reporter had responded appropriately to an oxidative stress, something about the design of the STRE-RLUC reporter had inhibited STRE-mediated transcriptional activation. Therefore, we generated a second reporter in which the STRE were provided with alternative flanking nucleotides, an alternative basal promoter region (ScCYC1), and an alternative reporter (StlacZ) (65). However, no STRE-mediated transcriptional activation was observed using this alternative reporter (not shown). Therefore, no obvious role was observed for the STRE with respect to stress-mediated transcription in C. albicans. This was consistent with the lack of involvement of CaMsn4 and Mnl1 in general stress responses.
Potential overlap between CaMsn4, Mnl1, and CaNrg1 regulons in C. albicans.
Previously, CaNrg1 was identified as a transcriptional repressor that mediates its effects in C. albicans via the Nrg1 response element (NRE) (41, 42). The consensus sequence for the NRE [(A/C)(A/C/G)C3T] (41) is closely related to the STRE (CCCCT), so that STREs appear to be a subset of potential NREs. Therefore, in principle, CaNrg1 might repress STRE-mediated transcriptional activation in C. albicans. It follows, therefore, that there might be some overlap between CaMsn4, Mnl1, and CaNrg1 regulons in C. albicans and that this overlap might have masked the activities of CaMsn4, Mnl1, and STRE in the above-mentioned experiments. A prediction of this working hypothesis was that these activities would be unmasked by inactivating CaNrg1.
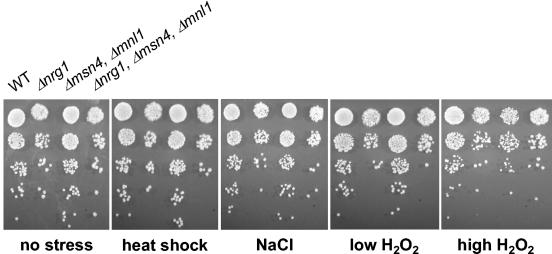
To test this, we generated a C. albicans msn4/msn4 mnl1/mnl1 nrg1/nrg1 triple mutant and compared its phenotype to those of wild-type, nrg1/nrg1, and msn4/msn4 mnl1/mnl1 cells (Fig. 7). Inactivation of CaNrg1 derepresses filamentous growth in C. albicans (2, 41), and therefore, cells carrying the nrg1/nrg1 mutation formed wrinkly colonies. However, the triple mutant displayed no significant difference from nrg1/nrg1 cells with respect to stress sensitivity (Fig. 7). Again, this reinforced the idea that CaMsn4 and Mnl1 do not have significant roles in the C. albicans stress responses examined, even in the absence of CaNrg1.
FIG. 7.
CaNrg1, CaMsn4, and Mnl1 do not display synthetic stress phenotypes. C. albicans cells were exposed to a wide range of stresses, including no stress (YPD; 30°C), mild heat shock (25 to 37°C), 1.0 M NaCl, 0.4 mM H2O2, and 2.5 mM H2O2. Strains: CAI8 (WT), MMC4 (Δnrg1), MSC12 (Δmsn4, Δmnl1), and SNC10 (Δnrg1, Δmsn4, Δmnl1) (Table 1). The strains were transformed with CIp10 (URA3) and/or pCRW3 (ADE2) to make them prototrophic.
Does CaNrg1 repress STRE-mediated transcriptional activation in response to stresses? This was tested by assaying the activity of the STRE-RLUC reporter in nrg1/nrg1 cells (Fig. 6B). The inactivation of CaNrg1 did not release any significant activation of the reporter following exposure to mild heat, osmotic, ethanol, or oxidative stress. Therefore, no significant overlap between the CaMsn4, Mnl1, and CaNrg1 regulons was observed in this study.
Functionality of CaMsn4 in C. albicans.
Northern analysis and transcript profiling had indicated that the CaMSN4 gene is expressed, and the complementation experiment suggested that CaMsn4 might have some transcriptional activity at least in S. cerevisiae (Fig. 2). However, our cellular and molecular analyses had revealed no obvious function for this protein in C. albicans. Therefore, we assayed the activities of the putative transcriptional activation and DNA binding domains of CaMsn4 in C. albicans.
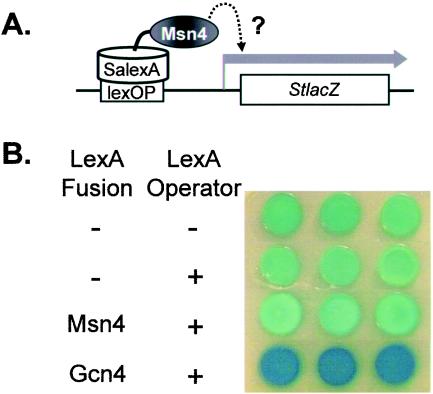
LexA fusions have been used to examine the activities of specific transcription factors in S. cerevisiae (3, 30). Hence, we expressed CaMsn4 as a LexA fusion protein in C. albicans and asked whether it could activate the expression of a reporter gene carrying the corresponding lexA operator in its promoter (Fig. 8A). Staphylococcus aureus lexA was used because it lacks CUG codons (53), which are decoded as serine, not leucine, in C. albicans (55). Hence, a SalexA-CaMSN4 fusion was introduced into a C. albicans strain containing a Streptococcus thermophilus lacZ reporter gene under the control of a SalexA operator sequence (53). Control strains contained a StlacZ reporter lacking the SalexA operator, and these generated basal levels of β-galactosidase in C. albicans (Fig. 8B). These levels were not affected significantly by the introduction of the S. aureus lexA operator. As expected (53), a control LexA-Gcn4 fusion showed significant StlacZ activation. However, the LexA-CaMsn4 fusion displayed no significant activation, even following exposure to osmotic (1.0 M NaCl) or ethanol (7%) stress. Therefore, we were unable to detect any significant transcriptional activation by CaMsn4.
FIG. 8.
Transcriptional activity of the putative activation domain of CaMsn4. The activity of a SaLexA-Msn4 fusion protein was compared to that of a SaLexA-Gcn4 fusion (positive control) in C. albicans using a StlacZ reporter carrying the SaLexA operator. (A) Cartoon illustrating the experimental rationale. (B) β-Galactosidase activities were assayed in strains CRC116, CRC110, CRC121, and SNC15 (Table 1) with three independent transformants using an X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) overlay assay developed for 1 h. −, absent; +, present.
To examine the functionality of the zinc finger domain of CaMsn4, codons 554 to 759 of CaMSN4 were fused to a synthetic codon-optimized VP16 transcriptional activation domain (see Materials and Methods). This VP16 domain has been shown to activate transcription in S. cerevisiae (12, 50). The VP16-CaMsn4DBD fusion was expressed in C. albicans using the methionine-conditional MET3 promoter (7) and targeted to the nucleus using a synthetic SV40 T-antigen nuclear localization signal (Fig. 9A). Control cells contained the empty expression plasmid pMET3-VP16 or pMET3-VP16-MNL1. Methionine-conditional expression of the VP16-CaMsn4DBD fusion and the control VP16-Mnl1DBD protein in C. albicans was confirmed by Western blotting (Fig. 9B). However, no obvious growth or stress phenotype was observed for cells expressing the VP16-CaMsn4DBD fusion compared with control cells (Fig. 9C).
We reasoned that C. albicans genes containing the CaMsn4 DNA binding site would be activated artificially following expression of this VP16-CaMsn4DBD fusion. The intention was to identify these CaMsn4 target genes, and hence the CaMsn4 DNA binding site. Therefore, transcript profiling was performed to compare the molecular responses of C. albicans cells to the VP16-CaMsn4DBD fusion and the VP16 control. Highly reproducible signals were obtained for four independent hybridizations, but no significant differences were observed between the transcript profiles of pMET3-VP16-CaMSN4DBD and pMET3-VP16 cells. This reinforced the idea that CaMsn4 no longer retains activity as a transcriptional activator in C. albicans.
DISCUSSION
In general, there appears to be a high degree of conservation between S. cerevisiae and C. albicans with respect to their signal transduction modules. These include the MAP kinase module involved in mating responses, the adenylyl cyclase-protein kinase A module that regulates cellular morphogenesis, the Rim101 module involved in pH signaling, the Gcn4 module that activates general amino acid control, the Yap1 module that mediates oxidative stress response, and the Hog1 module involved in osmotic stress responses (1, 4, 13, 14, 16, 18, 31, 32, 35, 48, 51, 54, 59, 63, 66, 68). However, transcript profiling has highlighted significant differences between the general stress responses of C. albicans and those of S. cerevisiae (15). Hence, we anticipated significant differences between the pathogenic fungus and the relatively benign fungus with respect to their Msn2- and Msn4-like signaling modules.
C. albicans has two Msn2- and Msn4-like proteins. IPF9939 (orf19.4752) was called CaMSN4 on the basis that its product is most similar to S. cerevisiae Msn4 (Fig. 1), and it was able to complement an S. cerevisiae msn2 msn4 double mutation, albeit weakly (Fig. 2). IPF9113 (orf19.6121), which was most similar to S. cerevisiae YER130c, was called MNL1 on the basis of its similarity to Msn2- and Msn4-like proteins (Fig. 1). Furthermore, MNL1, like S. cerevisiae YER130c, was unable to complement an S. cerevisiae msn2 msn4 double mutation (Fig. 2). The existence of a single C. albicans orthologue (CaMSN4) of the functionally redundant MSN2-MSN4 gene pair in S. cerevisiae is consistent with the idea that, during fungal evolution, genome duplication occurred after the divergence of C. albicans and S. cerevisiae (67).
To test our working model that CaMsn2- and Msn4-like proteins in C. albicans have been functionally reassigned, we examined their roles in detail. As expected, neither CaMsn4 nor Mnl1 appears to play an obvious role in stress responses. This conclusion was based on numerous complementary observations. (i) Inactivation of CaMSN4 and MNL1 did not increase the sensitivity of C. albicans to any of the numerous stresses tested (Fig. 3). This differs from the situation in S. cerevisiae, where an msn2 msn4 mutant is more sensitive to general stresses (38). It also contrasts with CAP1, the inactivation of which renders C. albicans more sensitive to oxidative stresses (Fig. 3) (1). (ii) Inactivation of CaMSN4 and MNL1 did not affect the C. albicans transcriptome during responses to mild heat shock or osmotic or oxidative stress (Fig. 4 and Table 3). Again, this contrasts with S. cerevisiae, in which the inactivation of Msn2 and Msn4 inhibits transcriptional responses to many stresses (8, 21). Subtle effects of CaMsn4 or Mnl1 upon stress responses might have been missed in our analyses of cellular stress responses. However, such effects are unlikely to have been missed by transcript profiling, which is exquisitely sensitive to environmental change (8, 9, 15, 21, 43). (iii) Ectopic expression of CaMSN4 or MNL1 did not increase the tolerance of C. albicans to stresses and did not affect the C. albicans transcriptome significantly during responses to stress (not shown). The ACT1 promoter has been used successfully to generate overexpression phenotypes for at least two other transcription factors in C. albicans (CaNrg1 and CaGcn4) (2, 63). Nevertheless, we are unable to exclude the possibility that the absence of cellular and molecular phenotypes was due to a lack of overexpression, improper folding, or mislocalization of CaMsn4 and Mnl1. (iv) The STRE did not mediate transcriptional activation in response to stresses in C. albicans (Fig. 6), although CaMsn4 was capable of activating the transcription of a STRE reporter in S. cerevisiae in a stress-dependent fashion, albeit weakly (Fig. 2). Therefore, CaMsn4 and Mnl1 do not appear to play significant roles in responses to cellular stresses in C. albicans. (v) Expression of a protein fusion containing the Msn4DBD domain linked to the VP16 transcriptional activation domain (Fig. 9) did not lead to the activation of any stress-related functions in C. albicans (not shown). Indeed, no significant CaMsn4 targets were observed using this approach. Also, a SaLexA-CaMsn4 fusion showed no transcriptional activation in C. albicans. We are unable to exclude the possibility that the SaLexA-CaMsn4 fusion was aberrantly expressed, folded, or localized. However, the positive control, SaLexA-Gcn4, did activate transcription in this experimental system (Fig. 8). Hence, if CaMsn4 does retain functionality as a transcription factor in C. albicans, this functionality presumably depends on other factors not examined in this study.
Two potential targets of CaMsn4 were identified by transcript profiling and confirmed by Northern blotting (Fig. 5). The induction of CaYPL088 in response to stress appeared to be dependent upon CaMsn4 (and, to a lesser extent, upon CaMnl1). In contrast, CaYIR035 mRNA levels were constitutively elevated in cells lacking CaMsn4. CaMsn4 might act indirectly upon these genes. Nevertheless, these represent the first identified gene targets for CaMsn4.
On the basis of the above observations, we conclude that CaMsn4 and Mnl1 do not play significant roles in the stress responses examined. This conclusion is consistent with the view that there has been significant evolutionary divergence between S. cerevisiae and C. albicans with respect to their stress responses. Such divergence has probably been driven by the evolution of niche-specific environmental responses, because the environmental challenges posed to a fungal pathogen of humans are likely to be quite distinct from those posed to a saprophytic fungus.
It is not surprising, therefore, that recent transcript-profiling experiments have revealed significant differences in the molecular responses of different fungi to stress. Schizosaccharomyces pombe and S. cerevisiae exhibit core transcriptional responses to a variety of different stresses, including heat, acid and alkali shifts, and osmotic and oxidative stresses (8, 9, 21). This core transcriptional response is reflected at the cellular level by the phenomenon of “cross-protection,” in which exposure to a mild dose of one form of stress protects the fungus against more severe doses of a quite different type of stress. Interestingly, these core transcriptional responses are regulated in different ways. S. pombe appears to exploit a common SAPK signaling pathway in which Sty1 activates its common set of stress genes (9), whereas S. cerevisiae uses different signaling pathways to activate its common set of stress genes (8, 9, 21). C. albicans differs from these benign fungi in that it did not display a common core transcriptional response to sublethal heat, osmotic, and oxidative stresses that induce such responses in S. pombe and S. cerevisiae (15). Instead, specific molecular responses to each stress were observed, and this was consistent with the lack of cross-protection provided by mild heat, osmotic, or oxidative stress (15). Hence, these three fungi clearly display specialized stress responses that presumably reflect their contrasting niches.
The situation is complicated by the potential involvement of dose-dependent stress-signaling networks in each fungus. For example, in S. pombe, the Sty1 pathway is activated by H2O2 in a dose-dependent fashion via two distinct sensing mechanisms (49). Hence, it could be argued that CaMsn4 (and possibly Mnl1) might be required for responses to only certain doses of a particular stress. However, we observed no phenotypic effects of the msn4/msn4 and mnl1/mnl1 mutations following exposure to a wide range of salt, nutrient, or oxidative stresses. Therefore, stress signaling in C. albicans appears to have diverged to the extent that Msn2- and Msn4-like proteins no longer play significant roles.
Acknowledgments
We thank the BRI microarray and informatics facilities, in particular, D. Tessier, T. Rigby, and F. Benoit, for arrays and advice. We also thank Janet Quinn for helpful comments, Johan Thevelein and Dominique Sanglard for strains, and the Stanford DNA Sequencing and Technology Center for access to their C. albicans genome sequence data (http://www-sequence.stanford.edu/group/candida).
S.N., M.W., and A.J.P.B. were supported by a grant from the National Research Council of Canada and the British Council (CRP004). M.S., S.M., and A.J.P.B. were supported by the BBSRC (1/P11585, 97/B1/P/03008). A.J.P.B. was also supported by the Wellcome Trust (055015, 063204) and the EC (QLK2CT-2000-00795). M.W. was supported by the Genomics and Health Initiative of the National Research Council of Canada and by a CIHR grant (MOP-42516). B.E. was a NSERC visiting fellow funded by the GHI.
Footnotes
This is NRC publication no. 46183.
REFERENCES
- 1.Alarco, A. M., and M. Raymond. 1999. The bZip transcription factor Cap1p is involved in multidrug resistance and oxidative stress response in Candida albicans. J. Bacteriol. 181:700-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braun, B. R., D. Kadosh and A. D. Johnson. 2001. NRG1, a repressor of filamentous growth in Candida albicans, is down-regulated during filament induction. EMBO. J. 20:4753-4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brent, R., and M. Ptashne. 1985. A eukaryotic transcriptional activator bearing the DNA specificity of a prokaryotic repressor. Cell 43:729-736. [DOI] [PubMed] [Google Scholar]
- 4.Brown, A. J. P. 2002. Expression of growth form-specific factors during morphogenesis in Candida albicans, p. 87-93. In R. Calderone (ed.), Candida and candidiasis. ASM Press, Washington, D.C.
- 5.Brown, A. J. P., G. Bertram, P. J. Feldmann, M. W. Peggie, and R. K. Swoboda. 1991. Codon utilisation in the pathogenic yeast, Candida albicans. Nucleic Acids Res. 19:4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calderone, R. A. 2002. Candida and candidiasis. ASM Press, Washington, D.C.
- 7.Care, R. S., J. Trevethick, K. M. Binley, and P. E. Sudbery. 1999. The MET3 promoter: a new tool for Candida albicans molecular genetics. Mol. Microbiol. 43:792-798. [DOI] [PubMed] [Google Scholar]
- 8.Causton, H. C., B. Ren, S. S. Koh, C. T. Harbison, E. Kanin, E. G. Jennings, T. I. Lee, H. L. True, E. S. Lander, and R. A. Young. 2001. Remodeling of yeast genome expression in response to environmental changes. Mol. Biol. Cell 12:323-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, D., W. M. Toone, J. Mata, R. Lyne, G. Burns, K. Kivinen, A. Brazma, N. Jones, and J. Bahler. 2003. Global transcriptional responses of fission yeast to environmental stress. Mol. Biol. Cell 14:214-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen, B. A., Y. Pilpel, R. Mitra, and G. M. Church. 2002. Discrimination between paralogs using microarray analysis: application to the Yap1p and Yap2p transcription networks. Mol. Biol. Cell 13:1608-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cormack, B., G. Bertram, M. Egerton, N. A. R. Gow, S. Falkow, and A. J. P. Brown. 1997. Yeast enhanced green fluorescent protein yEGFP: a reporter of gene expression in Candida albicans. Microbiology 143:303-311. [DOI] [PubMed] [Google Scholar]
- 12.Cress, W. D., and S. J. Triezenberg. 1991. Critical structural elements of the VP16 transcriptional activation domain. Science 251:87-90. [DOI] [PubMed] [Google Scholar]
- 13.Csank, C., K. Schroppel, E. Leberer, D. Harcus, O. Mohamed, S. Meloche, D. Y. Thomas, and M. Whiteway. 1998. Roles of the Candida albicans mitogen-activated protein kinase homolog, Cek1p, in hyphal development and systemic candidiasis. Infect. Immun. 66:2713-2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis, D., R. B. Wilson, and A. P. Mitchell. 2000. RIM101-dependent and -independent pathways govern pH responses in Candida albicans. Mol. Cell. Biol. 20:971-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Enjalbert, B., A. Nantel, and M. Whiteway. 2003. Stress induced gene expression in Candida albicans: absence of a general stress response. Mol. Biol. Cell 14:1460-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ernst, J. F. 2000. Transcription factors in Candida albicans—environmental control of morphogenesis. Microbiology 146:1763-1774. [DOI] [PubMed] [Google Scholar]
- 17.Estruch, F., and M. Carlson. 1993. Two homologous zinc finger genes identified by multicopy suppression in a SNF1 protein kinase mutant of Saccharomyces cerevisiae. Mol. Cell. Biol. 13:3872-3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng, Q., E. Summers, B. Guo, and G. R. Fink. 1999. Ras signaling is required for serum-induced hyphal differentiation in Candida albicans. J. Bacteriol. 181:6339-6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fonzi, W. A., and M. Y. Irwin. 1993. Isogenic strain construction and gene mapping in Candida albicans. Genetics 134:717-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garreau, H., R. N. Hasa, G. Renault, F. Estruch, E. Boy-Marcotte, and M. Jacquet. 2000. Hyperphosphorylation of Msn2 and Msn4 in response to heat shock and the diauxic shift is inhibited by cAMP in Saccharomyces cerevisiae. Microbiology 146:2113-2120. [DOI] [PubMed] [Google Scholar]
- 21.Gasch, A. P., P. T. Spellman, C. M. Kao, O. Carmel-Harel, M. B. Eisen, G. Storz, D. Botstein, and P. O. Brown. 2000. Genomic expression programs in the response of yeast cells to environmental changes. Mol. Biol. Cell 11:4241-4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gillum, A. M., E. Y. Tsay, and D. R. Kirsch. 1984. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol. Gen. Genet. 198:179-182. [DOI] [PubMed] [Google Scholar]
- 23.Gorner, W., E. Durchschlag, M. T. Martinez-Pastor, F. Estruch, G. Ammerer, B. Hamilton, H. Ruis, and C. Schuller. 1998. Nuclear localisation of the C2H2 zinc finger protein Msn2p is regulated by stress and protein kinase A activity. Genes Dev. 12:586-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gorner, W., E. Durchschlag, J. Wolf, E. L. Brown, G. Ammerer, B. Hamilton, H. Ruis, and C. Schuller. 2002. Acute glucose starvation activates the nuclear localization signal of a stress-specific yeast transcription factor. EMBO J. 21:135-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirata, Y., T. Andoh, T. Asahara, and A. Kikuchi. 2003. Yeast glycogen synthase kinase-3 activates Msn2p-dependent transcription of stress responsive genes. Mol. Biol. Cell 14:302-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacquet, M., G. Renault, S. Lallet, J. De Mey, and A. Goldbeter. 2003. Oscillatory nucleocytoplasmic shuttling of the general stress response transcriptional activators Msn2 and Msn4 in Saccharomyces cerevisiae. J. Cell Biol. 161:497-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jamieson, D. J., D. W. S. Stephen, and E. C. Terriere. 1996. Analysis of the adaptive oxidative stress response of Candida albicans. FEMS Microbiol. Lett. 138:83-88. [DOI] [PubMed] [Google Scholar]
- 28.Kadosh, D., and A. D. Johnson. 2001. Rfg1, a protein related to the S. cerevisiae hypoxic regulator Rox1, controls filamentous growth and virulence in C. albicans. Mol. Cell. Biol. 21:2496-2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaiser, C., S. Michaelis, and A. Mitchell. 1994. Methods in yeast genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 30.Keleher, C. A., M. J. Redd, J. Schultz, M. Carlson, and A. D. Johnson. 1992. Ssn6-Tup1 is a general repressor of transcription in yeast. Cell 68:709-719. [DOI] [PubMed] [Google Scholar]
- 31.Leberer, E., D. Harcus, I. D. Broadbent, K. L. Clark, D. Dignard, K. Ziegelbauer, A. Schmit, N. A. R. Gow, A. J. P. Brown, and D. Y. Thomas. 1996. Homologs of the Ste20p and Ste7p protein kinases are involved in hyphal formation of Candida albicans. Proc. Natl. Acad. Sci. USA 93:13217-13222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu, H., J. R. Kohler, and G. R. Fink. 1994. Suppression of hyphal formation in Candida albicans by mutation of a STE12 homolog. Science 266:1723-1726. [DOI] [PubMed] [Google Scholar]
- 33.Lo, H. J., J. R. Kohler, B. DiDomenico, D. Loebenberg, A. Cacciapuoti, and G. R. Fink. 1997. Nonfilamentous C. albicans mutants are avirulent. Cell 90:939-949. [DOI] [PubMed] [Google Scholar]
- 34.Macaskill, S. 2003. Functional analysis of specific promoter elements involved in the control of Candida albicans transcription. Ph.D. thesis. University of Aberdeen, Aberdeen, United Kingdom.
- 35.Magee, B. B., M. Legrand, A.-M. Alarco, M. Raymond, and P. T. Magee. 2002. Many of the genes required for mating in Saccharomyces cerevisiae are also required for mating in Candida albicans. Mol. Microbiol. 46:1345-1351. [DOI] [PubMed] [Google Scholar]
- 36.Mager, W. H., and A. J. J. de Kruijff. 1995. Stress-induced transcriptional activation. Microbiol. Rev. 59:506-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marchler, G., C. Schuller, G. Adam, and H. Ruis. 1993. A Saccharomyces cerevisiae UAS element controlled by protein kinase A activates transcription in response to a variety of stress conditions. EMBO J. 12:1997-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martinez-Pastor, M. T., G. Marchler, C. Schuller, A. Marchler-Bauer, H. Ruis, and F. Estruch. 1996. The Saccharomyces cerevisiae zinc finger proteins Msn2p and Msn4p are required for transcriptional induction through the stress-response element. EMBO J. 15:2227-2235. [PMC free article] [PubMed] [Google Scholar]
- 39.Moskvina, E., C. Schuller, C. T. C. Maurer, W. H. Mager, and H. Ruis. 1998. A search in the genome of Saccharomyces cerevisiae for genes regulated via stress response elements. Yeast 14:1041-1050. [DOI] [PubMed] [Google Scholar]
- 40.Murad, A. M. A., P. R. Lee, I. D. Broadbent, C. J. Barelle, and A. J. P. Brown. 2000. CIp10, an efficient and convenient integrating vector for Candida albicans. Yeast 16:325-327. [DOI] [PubMed] [Google Scholar]
- 41.Murad, A. M. A., P. Leng, M. Straffon, J. Wishart, S. Macaskill, D. MacCallum, N. Schnell, D. Talibi, D. Marechal, F. Tekaia, C. d'Enfert, C. Gaillardin, F. C. Odds, and A. J. P. Brown. 2001. NRG1 represses yeast-hypha morphogenesis and hypha-specific gene expression in Candida albicans. EMBO J. 20:4742-4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murad, A. M. A., C. d'Enfert, C. Gaillardin, H. Tournu, F. Tekaia, D. Talibi, D. Marechal, V. Marchais, J. Cottin, and A. J. P. Brown. 2001. Transcript profiling in Candida albicans reveals new cellular functions for the transcriptional repressors, CaTup1, CaMig1 and CaNrg1. Mol. Microbiol. 42:981-993. [DOI] [PubMed] [Google Scholar]
- 43.Nantel, A., D. Dignard, C. Bachewich, D. Harcus, A. Marcil, A.-P. Bouin, C. W. Sensen, H. Hogues, M. van het Hoog, P. Gordon, T. Rigby, F. Benoit, D. C. Tessier, D. Y. Thomas, and M. Whiteway. 2002. Transcript profiling of Candida albicans cells undergoing the yeast-to-hyphal transition. Mol. Biol. Cell 13:3452-3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Odds, F. C. 1988. Candida and candidosis, 2nd ed. Bailliere Tindall, London, United Kingdom.
- 45.Odds, F. C. 1994. Candida species and virulence. ASM News 60:313-318.
- 46.Osborne, B. I., and L. Guarente. 1989. Mutational analysis of a yeast transcriptional terminator. Proc. Natl. Acad. Sci. USA 86:4097-4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peterbauer, C. K., D. Litscher, and C. P. Kubicek. 2002. The Trichoderma atroviride seb1 stress response element binding gene encodes an AGGGG-binding protein which is involved in the response to high osmolarity stress. Mol. Genet. Genom. 268:223-231. [DOI] [PubMed] [Google Scholar]
- 48.Porta, A., A. M. Ramon, and W. A. Fonzi. 1999. PRR1, a homolog of Aspergillus nidulans palF controls pH-dependent gene expression and filamentation in Candida albicans. J. Bacteriol. 181:7516-7523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quinn, J., V. J. Findlay, K. Dawson, J. B. A. Millar, N. Jones, B. A. Morgan, and W. M. Toone. 2002. Distinct regulatory proteins control the graded transcriptional response to increasing H2O2 levels in fission yeast Schizosaccharomyces pombe. Mol. Biol. Cell 13:805-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Regier, J. L., F. Shen, and S. J. Triezenberg. 1993. Pattern of aromatic and hydrophobic amino acids critical for one of two subdomains of the VP16 transcriptional activator. Proc. Natl. Acad. Sci. USA 90:883-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rocha, C. R. C., K. Schroppel, D. Harcus, A. Marcil, D. Dignard, B. N. Taylor, D. Y. Thomas, M. Whiteway, and E. Leberer. 2001. Signaling through adenyl cyclase is essential for hyphal growth and virulence in the pathogenic fungus, Candida albicans. Mol. Biol. Cell 12:3631-3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rupp, S. 2002. lacZ assays in yeast. Methods Enzymol. 350:112-131. [DOI] [PubMed] [Google Scholar]
- 53.Russell, C. L., and A. J. P. Brown. Expression of Staphylococcus aureus LexA fusions in Candida albicans confirm that CaNrg1 is a transcriptional repressor and that CaGcn4 is a transcriptional activator. Submitted for publication. [DOI] [PubMed]
- 54.San Jose, C., R. Alonso, R. M. Perez-Diaz, J. Pla, and C. Nombela. 1996. The mitogen activated protein kinase homolog HOG1 gene controls glycerol accumulation in the pathogenic fungus Candida albicans. J. Bacteriol. 178:5850-5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Santos, M. A. S., G. Keith, and M. F. Tuite. 1993. Non-standard translational events in Candida albicans mediated by an unusual seryl-tRNA with a 5′-CAG-3′ leucine anticodon. EMBO J. 12:607-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schnell, N., B. Krems and K.-D. Entian. 1992. The PAR1 YAP1/SNQ3 gene of Saccharomyces cerevisiae, a c-jun homolog, is involved in oxygen metabolism. Curr. Genet. 21:269-273. [DOI] [PubMed] [Google Scholar]
- 57.Sherman, F. 1991. Getting started with yeast. Methods Enzymol. 194:3-21. [DOI] [PubMed] [Google Scholar]
- 58.Sikorski, R. S., and P. Hieter. 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 122:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sonneborn, A., D. P. Bockmuhl, M. Gerads, K. Kurpanek, D. Sanglard, and J. F. Ernst. 2000. Protein kinase A encoded by TPK2 regulates dimorphism of Candida albicans. Mol. Microbiol. 35:386-396. [DOI] [PubMed] [Google Scholar]
- 60.Srikantha, T., A. Klapach, W. W. Lorenz, L. K. Tsai, L. A. Laughlin, J. A. Gorman, and D. R. Soll. 1996. The sea pansey Renilla reniformis luciferase serves as a sensitive bioluminescent reporter for differential gene expression in Candida albicans. J. Bacteriol. 178:121-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Swoboda, R. K., G. Bertram, S. Delbruck, J. F. Ernst, N. A. R. Gow. G. W. Gooday, and A. J. P. Brown. 1994. Fluctuations in glycolytic mRNA levels during the yeast-to-hyphal transition in Candida albicans reflect underlying changes in growth rather than a response to cellular dimorphism. Mol. Microbiol. 13:663-672. [DOI] [PubMed] [Google Scholar]
- 62.Thomas, B. J., and R. Rothstein. 1989. The genetic control of direct-repeat recombination in Saccharomyces: the effect of rad52 and rad1 on mitotic recombination at GAL10, a transcriptional regulation gene. Genetics 123:725-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tripathi, G., C. Wiltshire, S. Macaskill, H. Tournu, S. Budge, and A. J. P. Brown. 2002. CaGcn4 co-ordinates morphogenetic and metabolic responses to amino acid starvation in Candida albicans. EMBO J. 21:5448-5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tusher, V. G., R. Tibshirani, and G. Chu. 2001. Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl. Acad. Sci. USA 98:5116-5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Uhl, M. A., and A. D. Johnson. 2001. Development of a Streptococcus thermophilus lacZ as a reporter gene for Candida albicans. Microbiology 147:1189-1195. [DOI] [PubMed] [Google Scholar]
- 66.Whiteway, M. 2000. Transcriptional control of cell type and morphogenesis in Candida albicans. Curr. Opin. Microbiol. 3:582-588. [DOI] [PubMed] [Google Scholar]
- 67.Wolfe, K. H., and D. C. Shields. 1997. Molecular evidence for an ancient duplication of the entire yeast genome. Nature 387:708-713. [DOI] [PubMed] [Google Scholar]
- 68.Zhang, X., M. de Micheli, S. Coleman, D. Sanglard, and W. S. Moye-Rowley. 2000. Analysis of the oxidative stress regulation of the Candida albicans transcription factor, Cap1p. Mol. Microbiol. 36:618-629. [DOI] [PubMed] [Google Scholar]