Abstract
BACKGROUND
In nonhypertensive individuals, lower levels of 25-hydroxyvitamin D (25[OH]D) have been associated with an increased risk of hypertension, and vitamin D deficiency has been associated with endothelial dysfunction in such individuals. However, the effect of vitamin D supplementation on endothelial dysfunction in nonhypertensive individuals has not been examined in a rigorous fashion.
METHODS
In this randomized, double-blind, placebo-controlled trial of nonhypertensive, nondiabetic overweight, or obese individuals with vitamin D deficiency (body mass index ≥25 and 25[OH]D ≤ 20 ng/ml), we assigned subjects to receive either ergocalciferol (50,000 units) or matching placebo, once a week for 8 weeks. Our primary outcome was endothelial-dependent vasodilation (EDV) measured by brachial artery ultrasound at baseline and 8 weeks postrandomization.
RESULTS
By the end of the trial, 46 and 47 participants were allocated to receive ergocalciferol and placebo, respectively. Mean 25(OH)D levels increased from 14.9 to 30.3 in the vitamin D group and 14.4 to 17.4 in the placebo. EDV did not change significantly with either vitamin D repletion (from 6.3 ± 3.6% at baseline to 6.1 ± 4.6% at 8 weeks; P value = 0.78) or placebo (7.9 ± 4.7% to 6.8 ± 4.7%; P = 0.17). The treatment effect P value (comparing the 8-week change with ergocalciferol to the change with placebo) was 0.35.
CONCLUSIONS
In this randomized, double-blind, placebo-controlled trial, there was no improvement in endothelial function (measured as EDV) after repletion of vitamin D in overweight/obese nonhypertensive individuals.
Keywords: blood pressure, endothelial function, hypertension, randomized controlled trial, vitamin D deficiency
Several observational studies have reported an increased risk of cardiovascular disease (CVD) and its risk factors, such as hypertension, with 25-hydroxyvitamin D (25[OH]D) deficiency.1–4 Also, patients with vitamin D deficiency are more likely to have known risk factors for hypertension and CVD, including endothelial dysfunction.5–9 Despite observational studies identifying vitamin D deficiency as a risk factor for hypertension and CVD, it is not clear whether these associations are causal. The effect of vitamin D repletion on endothelial function in humans is controversial. Several interventional studies have examined the effect of vitamin D supplementation on endothelial function in various populations, yielding conflicting results.6,10–14 Of these, 2 were performed in nonhypertensive individuals; 1 study found that a single dose of vitamin D did not improve endothelial function in South Asian women, while the other found that monthly vitamin D for 4 months improved brachial reactivity in African Americans.11,12
In addition to vitamin D deficiency, overweight and obesity are well-established risk factors for the development of hypertension and CVD15,16; adiposity is also associated with a higher prevalence of vitamin D deficiency, possibly through adipose sequestration of 25[OH]D.17,18 Furthermore, overweight and obesity are associated with endothelial dysfunction, a precursor in the development of hypertension.8,9 Taken together, vitamin D deficiency may be an important factor underlying the association of adiposity with hypertension and CVD, potentially mediated by endothelial dysfunction.
Thus, we conducted a randomized, double-blind, placebo-controlled trial to determine the effect of vitamin D supplementation on endothelial function among overweight and obese individuals who were vitamin D deficient.
METHODS
Subjects
The study population consisted of overweight or obese adults (aged 18 years or older who had a body mass index ≥25 kg/m2) with vitamin D deficiency (defined as a 25[OH]D level <20 ng/ml). Potential subjects were excluded if they had any of the following: hypertension (any known prior history of hypertension, a systolic blood pressure ≥140 mm Hg or a diastolic blood pressure ≥90 mm Hg as measured by 2 automated blood pressures 5 minutes apart at the screening visit, or use of antihypertensive drugs); diabetes mellitus; coronary heart disease; chronic kidney disease (defined as an estimated glomerular filtration rate <60ml/min/1.73m2)19; active malignancy (except nonmelanoma skin cancer); history of kidney stones; and osteoporosis. In addition, we excluded pregnant women, those with hypocalcemia or hypercalcemia, hypophosphatemia or hyperphosphatemia, and those taking vitamin D supplements if they were unwilling to stop for the period of the trial.
Potential subjects were recruited from the metropolitan Boston area using electronic and print advertisements and also using recruitment systems set up by Brigham and Women’s Hospital and Partners Healthcare. Interested individuals first underwent a screening phone call, and those with no obvious exclusion criteria were invited for a screening visit. All subjects provided written informed consent. The study was approved by the Institutional Review Board at Brigham and Women’s Hospital and is registered at ClinicalTrials.gov (NCT01320722).
Study design
The study design is depicted in Figure 1. Willing and eligible subjects underwent 2 identical inpatient visits, including a baseline visit and a follow-up visit at 8 weeks. Starting 3 days prior to admission to both visits, subjects commenced a high-sodium diet by supplementing their normal diet with 150 mmol/day of sodium in the form of dry bouillon. High-sodium balance was confirmed upon admission using a timed urine specimen, and the high-sodium diet was continued until the time of discharge. Assessments of endothelial function were performed (as described below) on the morning following admission, after an 8-hour fast.
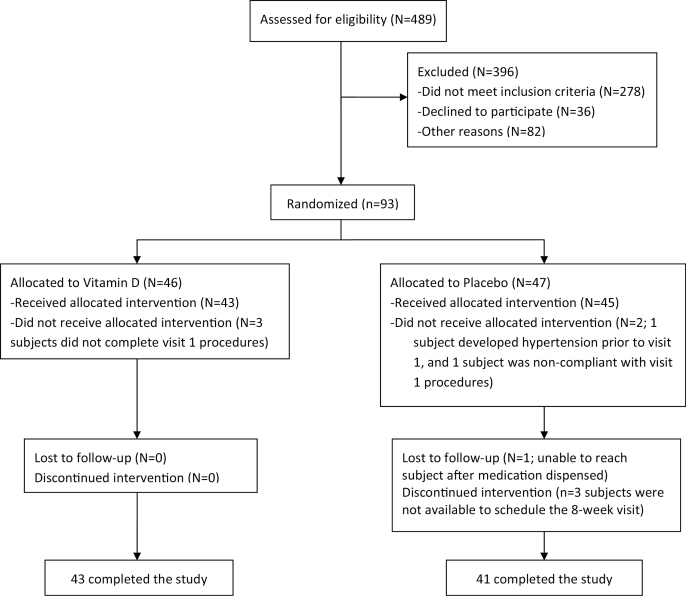
Figure 1.
Randomization and follow-up of the study participants.
Subjects were randomly assigned to receive either ergocalciferol (50,000 unit gel tablets to be taken by mouth once weekly) or matching placebo to be initiated after the baseline visit, and to be taken for 8 weeks until the follow-up visit. Ergocalciferol and placebo tablets were indistinguishable from each other to preserve blinding, and were provided by the Brigham and Women’s Hospital Investigational Drug Service (IDS). Randomization was performed by the IDS using stratification (by race and sex) and was computer generated. Investigators, subjects, research staff, and outcome assessors were all blinded to study assignment until completion of the study.
Between the baseline and the 8-week inpatient visit, subjects returned for an outpatient checkup at 4 weeks to assess potential adverse effects, including laboratory abnormalities (e.g., calcium and phosphorous were measured), and to assess compliance using pill counts. In addition, study staff contacted subjects weekly to ascertain side effects and compliance with study medication.
Plasma vitamin D levels
25(OH)D was measured by radioimmunoassay, as described elsewhere.20 This assay, which uses reagents from the DiaSorin Corporation (Stillwater, NM), recognizes and quantitates 25(OH)D2 and 25(OH)D3 equally. This assay has been in widespread use for nearly 20 years, and has been employed for research use in numerous studies. We analyzed the intraassay and interassay coefficients of variation of this assay using blinded split samples from a subset of subjects at the beginning of the trial; coefficient of variations were <10%.
Endothelial function
In the morning of the baseline and 8-week inpatient visits, subjects had endothelial function assessed by ultrasound. Caffeine and alcohol were withheld for at least 12 hours before the procedure. One experienced technician measured brachial artery diameter under basal conditions and during reactive hyperemia following 5 minutes of an ischemic stimulus, using a methodology recommended by the International Brachial Artery Reactivity Task Force.21 This flow-mediated, endothelium-dependent vasodilation (EDV) of the brachial artery, expressed as a percent change of brachial luminal diameter, occurs at 1 minute of reactive hyperemia and is mediated by nitric oxide. Endothelial-independent vasodilation (EIV) was assessed by measuring brachial artery diameter under basal conditions and 3 minutes following administration of sublingual nitroglycerin (0.4 mg). This measurement serves as a control for underlying vascular smooth muscle cell function. Acquisition and analysis of the stored images were performed by the Brigham and Women’s Ultrasound Core Laboratory using software designed for this purpose by Medical Imaging Applications (Coralville, IA).
Statistical analysis
To assess the balance of potential confounders achieved by the randomization procedure, we first compared participant’s baseline characteristics by randomized treatment assignment. After confirming a normal distribution in endothelial function assessments, we evaluated the change of EDV and EIV from baseline to 8 weeks as a continuous variable using a paired t-test and compared the treatment groups using a 2-group t-test. As sensitivity analyses, we assessed whether the change in EDV between groups differed according to sex, age, body mass index, or race. In addition, we evaluated the association of the 8-week change in 25(OH)D level with corresponding changes in EDV and EIV using Pearson correlation coefficients. Statistical significance was set for a 2-tailed P <0.05. All analyses were performed using the SAS statistical package (version 9.4; SAS Institute, Cary, NC).
Power calculations
Data from available literature was used to estimate the sample size used in our trial to detect changes in EDV. Tarcin et al. reported a 10.4% improvement with a SD of 3.3% in EDV after vitamin D supplementation.7 Furthermore, another study reported a 2.3% improvement in EDV with vitamin D supplementation compared with placebo (SD = 3.1%).6 Thus, with 40 individuals in each treatment group (after a potential 10% drop out), we anticipated a 99% and 91% power to detect effects similar to these 2 studies, respectively.
RESULTS
A total of 489 individuals were screened. Of these, 93 individuals underwent randomized assignment, including 46 to receive ergocalciferol and 47 to receive placebo (Figure 1). Five subjects discontinued the study prior to receiving study medication (3 and 1, respectively, in the ergocalciferol and placebo groups, who did not complete the baseline visit, and 1 in the placebo group who developed hypertension prior to the baseline visit). A total of 84 subjects completed the study and were analyzed (43 assigned ergocalciferol and 41 assigned placebo).
The mean age of the study population was 37 years (SD ± 12.3) and the mean body mass index was 33.9 (SD ± 5.6) kg/m2. The median 25(OH)D level was 15.4 [interquartile range: 11.4–17.5] ng/ml. Baseline characteristics of all randomized participants according to study group are depicted in Table 1. There were no significant differences in any of the variables.
Table 1.
Baseline characteristics of randomized participants
| Vitamin D arm (N = 46) | Placebo (N = 47) | P value | |
|---|---|---|---|
| Age (years) | 39 (13) | 35 (11) | 0.07 |
| Men (%) | 33 | 34 | 0.88 |
| Body mass index (kg/m2) | 33.5 (5) | 34.3 (6.6) | 0.48 |
| White (%) | 48 | 34 | 0.56 |
| Systolic BP (mm Hg) | 118 (10) | 118 (11) | 0.98 |
| Diastolic BP (mm Hg) | 78 (7) | 77 (8) | 0.68 |
| Serum calcium (mg/dl) | 9.5 (0.3) | 9.5 (0.4) | 0.98 |
| Serum phosphate (mg/dl) | 3.4 (0.5) | 3.5 (0.5) | 0.79 |
| eGFR (ml/1.73m2) | 105 (19) | 112 (20) | 0.08 |
Values are mean (SD) unless stated otherwise. Abbreviations: BP, blood pressure; eGFR, estimated glomerular filtration rate.
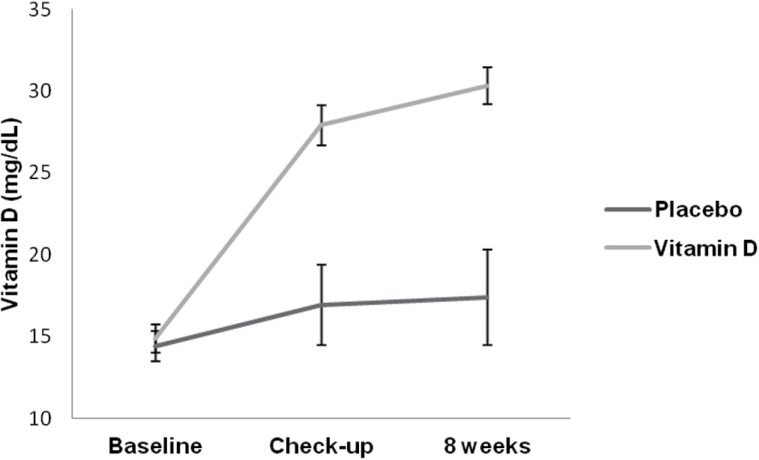
Plasma levels of 25(OH)D substantially increased with ergocalciferol. Baseline 25(OH)D increased from a median of 15 (11.2–16.9) ng/ml at baseline to 26.5 (23–32.7) ng/ml at the 4 week checkup visit, and to 28.7 (25.4–36.3) ng/ml at 8 weeks (Figure 2). In contrast, median 25(OH)D in the placebo group was 15.8 ng/ml at baseline, 13.9 ng/ml at 4 weeks, and 14.5 ng/ml at 8 weeks. Correction of vitamin D deficiency (to a level above 20 ng/ml) was achieved in 90% of subjects assigned ergocalciferol, and 19% of subjects assigned placebo.
Figure 2.
Change in vitamin D level from baseline to 8 weeks.
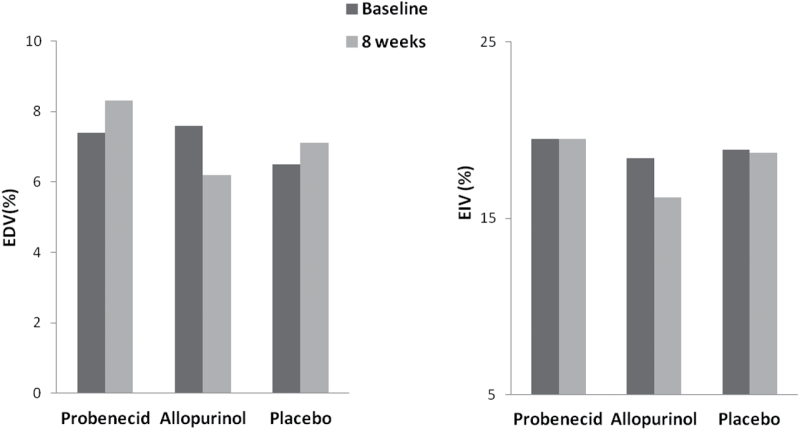
There was no significant change in EDV after either vitamin D repletion or placebo (Figure 3). EDV in the ergocalciferol group was 6.3% (SD = 3.6%) at baseline and 6.1% (SD = 4.6%) at 8 weeks; the corresponding values in the placebo group were 7.9% (SD = 4.7%) and 6.8% (SD = 4.7%).
Figure 3.
Changes in endothelial-dependent vasodilation (EDV) or endothelial-independent vasodilation (EIV) with vitamin D repletion as compared with placebo.
There was no statistical difference in the 8-week change in EDV comparing ergocalciferol with placebo (P = 0.35).
EIV significantly decreased (from 18.5% to 16.7%, P value = 0.03) after vitamin D but not after placebo (Figure 3). However, there was no significant difference in the 8-week EIV changes comparing ergocalciferol with placebo (P = 0.93). In sensitivity analyses, we did not find any consistent interaction between treatment group and sex, age, body mass index, or race with respect to effects on endothelial function. Also, there were no associations between the 8-week changes in 25(OH)D levels with the corresponding changes in EDV and EIV (Pearson correlation coefficients of −0.1 and 0.05, respectively).
Of 93 randomized participants, 43 and 45 subjects in the ergocalciferol and placebo groups, respectively, underwent at least 1 study visit; adverse events among these individuals are presented in Table 2. Overall, there were no serious adverse events, and 6 minor adverse events occurred in each group. Elevated potassium of 5.5 mmol/l was observed in 1 participant in the intervention group; the level was re-checked within 3 days and found to be normal.
Table 2:
Adverse events of randomized participants
| Vitamin D arm (N = 43) | Placebo (N = 45) | |
|---|---|---|
| Serious adverse events | 0 | 0 |
| All | 0 | 0 |
| Minor adverse events | 6 | 6 |
| All | 6 | 6 |
| Abdominal discomfort | 4 | 4 |
| Dry mouth | 0 | 1 |
| Elevated phosphorus (mg/dl) | 0 | 1 |
| Elevated potassium (mmol/l) | 1 | 0 |
DISCUSSION
In this randomized, double-blind, placebo-controlled trial of overweight and obese vitamin D deficient individuals, we found that vitamin D supplementation had no effect on endothelial function.
The effect of vitamin D on endothelial function has so far been controversial. Several studies have found salutary effects. In one double-blind, parallel group, placebo-controlled trial, for example, vitamin D deficient patients with type 2 diabetes mellitus were randomized to a single dose of 100,000 IU of oral vitamin D2 or placebo; EDV was significantly improved compared with the placebo group (2.35 vs. 0.06%; P value = 0.048).6 In another randomized trial, 45 nonhypertensive African Americans with 25(OH)D deficiency were randomized to 60,000 IU monthly of oral vitamin D3 or placebo for 16 weeks. After treatment and normalization of 25(OH)D levels in the intervention group, significant improvements were noted in EDV as compared to the placebo group (1.8 ± 1.3 and −1.3 ± 0.6%; P value = 0.047).12
However, as in our study, more recent trials found no benefit on endothelial function when vitamin D was repleted.10,11,13,14 As an example, 100 participants with type 2 diabetes mellitus were randomized to receive 5,000 IU per day of vitamin D or placebo for 12 weeks. EDV did not change significantly after repletion (3.36 ± 2.67 vs. 3.40 ± 1.99%; P value = 0.99).10 In another randomized trial of 50 vitamin D deficient South Asian women, there was no improvement in EDV when vitamin D was supplemented with a single dose of 100,000 units of oral vitamin D3, as compared with placebo (0.1%, P value = 0.84 at 4 weeks or 0.0%, P value = 0.98 at 8 weeks).11
We found no effect of vitamin D supplementation for 8 weeks on endothelial function, despite effective treatment of vitamin D deficiency, and despite the fact that our participants had endothelial dysfunction at baseline. Baseline endothelial function in our study was similar to that in other obese individuals without hypertension and was lower than has been reported in lean subjects. In a study of 73 individuals, for example, obese participants with no known CVD were found to have lower EDV as compared with lean individuals (6.7 and 9.7%, respectively).22 Thus, our findings cannot be explained by either inadequate vitamin D dosing or by the absence of baseline dysfunction that could be ameliorated. Our findings therefore do not support the hypothesis that vitamin D deficiency is the sole cause of endothelial dysfunction.
Previous studies have shown a direct association between lower 25(OH)D levels and hypertension incidence.1,2,23,24 In addition, an observational study found that 25(OH)D deficiency and insufficiency are associated with endothelial dysfunction in middle-aged to older individuals with no clinical disease.5 Thus, the observational literature supports the hypothesis that vitamin D deficiency may be a risk factor for hypertension and for endothelial dysfunction (a potential mechanism of increased blood pressure), suggesting that vitamin D could be an important modifiable factor for prevention of hypertension.
However, the effect of vitamin D supplementation on blood pressure remains in question, and most randomized trials thus far have found no beneficial effect.25–29 In the DAYLIGHT trial, for example, 383 participants with vitamin D deficiency and systolic blood pressure of 120 to 159 mm Hg received either high dose (4,000 IU/day) or low dose (400 IU/day) cholecalciferol for 6 months.26 There was no effect on blood pressure, either in participants with prehypertension or stage I hypertension. In addition, a recent meta-analysis including 46 randomized controlled trials of vitamin D supplementation for at least 1 month found no effect on blood pressure.29
Our study has several potential limitations. First, our participants were all overweight or obese, so our findings do not necessarily apply to a lean population. However, by enrolling overweight and obese subjects, we studied a population that was at high risk for developing hypertension and other disorders, and therefore one in which any beneficial effects of vitamin D supplementation would be critically important. Second, because all of our participants were in high-sodium balance (which is similar to most Western diets), the findings may not apply to individuals who are sodium restricted.
The strengths of our study include its double-blind, placebo-controlled design, low drop-out rates, and rigorous evaluation of endothelial function using gold-standard techniques. Also, our study was the first, to our knowledge, to analyze the effects of vitamin D supplementation in an overweight or obese population without any CVD.
In conclusion, we found no effect of vitamin D supplementation on endothelial function in vitamin D deficiency. Taken together with prior studies evaluating the effects of supplementation on blood pressure, our findings are consistent with vitamin D not being a modifiable risk factor for hypertension.
DISCLOSURE
The authors declared no conflict of interest.
ACKNOWLEDGMENTS
Borgi, McMullan, Fisher, and Forman contributed to the conception and design of the study. All authors were involved in the analysis and interpretation of the data. Borgi designed and conducted the statistical analysis. Borgi worked on the drafting of the manuscript, which was thoroughly reviewed and approved by all authors. This work was funded by the National Institute of Health’s grant: 1R01HL105440-01, as well as grant numbers 1 UL1 TR 00110 and 8 UL1 TR000170-05 Harvard Clinical and Translational Science Center from the National Center for Advancing Translational Science, and 1 UL1 RR025758-04 Harvard Clinical and Translational Science Center, from the National Center for Research Resources.
REFERENCES:
- 1. Forman JP, Giovannucci E, Holmes MD, Bischoff-Ferrari HA, Tworoger SS, Willett WC, Curhan GC. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension 2007; 49:1063–1069. [DOI] [PubMed] [Google Scholar]
- 2. Forman JP, Curhan GC, Taylor EN. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension among young women. Hypertension 2008; 52:828–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, Benjamin EJ, D’Agostino RB, Wolf M, Vasan RS. Vitamin D deficiency and risk of cardiovascular disease. Circulation 2008; 117:503–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Song Y, Wang L, Pittas AG, Del Gobbo LC, Zhang C, Manson JE, Hu FB. Blood 25-hydroxy vitamin D levels and incident type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care 2013; 36:1422–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jablonski KL, Chonchol M, Pierce GL, Walker AE, Seals DR. 25-Hydroxyvitamin D deficiency is associated with inflammation-linked vascular endothelial dysfunction in middle-aged and older adults. Hypertension 2011; 57:63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sugden JA, Davies JI, Witham MD, Morris AD, Struthers AD. Vitamin D improves endothelial function in patients with type 2 diabetes mellitus and low vitamin D levels. Diabet Med 2008; 25:320–325. [DOI] [PubMed] [Google Scholar]
- 7. Tarcin O, Yavuz DG, Ozben B, Telli A, Ogunc AV, Yuksel M, Toprak A, Yazici D, Sancak S, Deyneli O, Akalin S. Effect of vitamin D deficiency and replacement on endothelial function in asymptomatic subjects. J Clin Endocrinol Metab 2009; 94:4023–4030. [DOI] [PubMed] [Google Scholar]
- 8. Landmesser U, Drexler H. Endothelial function and hypertension. Curr Opin Cardiol 2007; 22:316–320. [DOI] [PubMed] [Google Scholar]
- 9. Paravicini TM, Touyz RM. NADPH oxidases, reactive oxygen species, and hypertension: clinical implications and therapeutic possibilities. Diabetes Care 2008; 31(suppl 2):S170–S180. [DOI] [PubMed] [Google Scholar]
- 10. Yiu YF, Yiu KH, Siu CW, Chan YH, Li SW, Wong LY, Lee SW, Tam S, Wong EW, Lau CP, Cheung BM, Tse HF. Randomized controlled trial of vitamin D supplement on endothelial function in patients with type 2 diabetes. Atherosclerosis 2013; 227:140–146. [DOI] [PubMed] [Google Scholar]
- 11. Witham MD, Adams F, Kabir G, Kennedy G, Belch JJ, Khan F. Effect of short-term vitamin D supplementation on markers of vascular health in South Asian women living in the UK–a randomised controlled trial. Atherosclerosis 2013; 230:293–299. [DOI] [PubMed] [Google Scholar]
- 12. Harris RA, Pedersen-White J, Guo DH, Stallmann-Jorgensen IS, Keeton D, Huang Y, Shah Y, Zhu H, Dong Y. Vitamin D3 supplementation for 16 weeks improves flow-mediated dilation in overweight African-American adults. Am J Hypertens 2011; 24:557–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Witham MD, Dove FJ, Dryburgh M, Sugden JA, Morris AD, Struthers AD. The effect of different doses of vitamin D(3) on markers of vascular health in patients with type 2 diabetes: a randomised controlled trial. Diabetologia 2010; 53:2112–2119. [DOI] [PubMed] [Google Scholar]
- 14. Longenecker CT, Hileman CO, Carman TL, Ross AC, Seydafkan S, Brown TT, Labbato DE, Storer N, Tangpricha V, McComsey GA. Vitamin D supplementation and endothelial function in vitamin D deficient HIV-infected patients: a randomized placebo-controlled trial. Antivir Ther 2012; 17:613–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dickey RA, Janick JJ. Lifestyle modifications in the prevention and treatment of hypertension. Endocr Pract 2001; 7:392–399. [DOI] [PubMed] [Google Scholar]
- 16. Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, Abraham JP, Abu-Rmeileh NM, Achoki T, AlBuhairan FS, Alemu ZA, Alfonso R, Ali MK, Ali R, Guzman NA, Ammar W, Anwari P, Banerjee A, Barquera S, Basu S, Bennett DA, Bhutta Z, Blore J, Cabral N, Nonato IC, Chang JC, Chowdhury R, Courville KJ, Criqui MH, Cundiff DK, Dabhadkar KC, Dandona L, Davis A, Dayama A, Dharmaratne SD, Ding EL, Durrani AM, Esteghamati A, Farzadfar F, Fay DF, Feigin VL, Flaxman A, Forouzanfar MH, Goto A, Green MA, Gupta R, Hafezi-Nejad N, Hankey GJ, Harewood HC, Havmoeller R, Hay S, Hernandez L, Husseini A, Idrisov BT, Ikeda N, Islami F, Jahangir E, Jassal SK, Jee SH, Jeffreys M, Jonas JB, Kabagambe EK, Khalifa SE, Kengne AP, Khader YS, Khang YH, Kim D, Kimokoti RW, Kinge JM, Kokubo Y, Kosen S, Kwan G, Lai T, Leinsalu M, Li Y, Liang X, Liu S, Logroscino G, Lotufo PA, Lu Y, Ma J, Mainoo NK, Mensah GA, Merriman TR, Mokdad AH, Moschandreas J, Naghavi M, Naheed A, Nand D, Narayan KM, Nelson EL, Neuhouser ML, Nisar MI, Ohkubo T, Oti SO, Pedroza A, Prabhakaran D, Roy N, Sampson U, Seo H, Sepanlou SG, Shibuya K, Shiri R, Shiue I, Singh GM, Singh JA, Skirbekk V, Stapelberg NJ, Sturua L, Sykes BL, Tobias M, Tran BX, Trasande L, Toyoshima H, van de Vijver S, Vasankari TJ, Veerman JL, Velasquez-Melendez G, Vlassov VV, Vollset SE, Vos T, Wang C, Wang X, Weiderpass E, Werdecker A, Wright JL, Yang YC, Yatsuya H, Yoon J, Yoon SJ, Zhao Y, Zhou M, Zhu S, Lopez AD, Murray CJ, Gakidou E. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet (London, England) 2014; 384:766–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang CJ, McAllister MJ, Slusher AL, Webb HE, Mock JT, Acevedo EO. Obesity-related oxidative stress: the impact of physical activity and diet manipulation. Sports Med Open 2015; 1:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr 2000; 72:690–693. [DOI] [PubMed] [Google Scholar]
- 19. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999; 130:461–470. [DOI] [PubMed] [Google Scholar]
- 20. Hollis BW. Comparison of commercially available (125)I-based RIA methods for the determination of circulating 25-hydroxyvitamin D. Clin Chem 2000; 46:1657–1661. [PubMed] [Google Scholar]
- 21. Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R; International Brachial Artery Reactivity Task Force Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 2002; 39:257–265. [DOI] [PubMed] [Google Scholar]
- 22. Williams IL, Chowienczyk PJ, Wheatcroft SB, Patel AG, Sherwood RA, Momin A, Shah AM, Kearney MT. Endothelial function and weight loss in obese humans. Obes Surg 2005; 15:1055–1060. [DOI] [PubMed] [Google Scholar]
- 23. Wang L, Manson JE, Buring JE, Lee IM, Sesso HD. Dietary intake of dairy products, calcium, and vitamin D and the risk of hypertension in middle-aged and older women. Hypertension 2008; 51:1073–1079. [DOI] [PubMed] [Google Scholar]
- 24. Burgaz A, Orsini N, Larsson SC, Wolk A. Blood 25-hydroxyvitamin D concentration and hypertension: a meta-analysis. J Hypertens 2011; 29:636–645. [DOI] [PubMed] [Google Scholar]
- 25. Pilz S, Gaksch M, Kienreich K, Grübler M, Verheyen N, Fahrleitner-Pammer A, Treiber G, Drechsler C, Ó Hartaigh B, Obermayer-Pietsch B, Schwetz V, Aberer F, Mader J, Scharnagl H, Meinitzer A, Lerchbaum E, Dekker JM, Zittermann A, März W, Tomaschitz A. Effects of vitamin D on blood pressure and cardiovascular risk factors: a randomized controlled trial. Hypertension 2015; 65:1195–1201. [DOI] [PubMed] [Google Scholar]
- 26. Arora P, Song Y, Dusek J, Plotnikoff G, Sabatine MS, Cheng S, Valcour A, Swales H, Taylor B, Carney E, Guanaga D, Young JR, Karol C, Torre M, Azzahir A, Strachan SM, O’Neill DC, Wolf M, Harrell F, Newton-Cheh C, Wang TJ. Vitamin D therapy in individuals with prehypertension or hypertension: the DAYLIGHT trial. Circulation 2015; 131:254–262. [DOI] [PubMed] [Google Scholar]
- 27. Witham MD, Price RJ, Struthers AD, Donnan PT, Messow CM, Ford I, McMurdo ME. Cholecalciferol treatment to reduce blood pressure in older patients with isolated systolic hypertension: the VitDISH randomized controlled trial. JAMA Intern Med 2013; 173:1672–1679. [DOI] [PubMed] [Google Scholar]
- 28. Scragg R, Slow S, Stewart AW, Jennings LC, Chambers ST, Priest PC, Florkowski CM, Camargo CA, Jr, Murdoch DR. Long-term high-dose vitamin D3 supplementation and blood pressure in healthy adults: a randomized controlled trial. Hypertension 2014; 64:725–730. [DOI] [PubMed] [Google Scholar]
- 29. Beveridge LA, Struthers AD, Khan F, Jorde R, Scragg R, Macdonald HM, Alvarez JA, Boxer RS, Dalbeni A, Gepner AD, Isbel NM, Larsen T, Nagpal J, Petchey WG, Stricker H, Strobel F, Tangpricha V, Toxqui L, Vaquero MP, Wamberg L, Zittermann A, Witham MD; D-PRESSURE Collaboration Effect of vitamin D supplementation on blood pressure: a systematic review and meta-analysis incorporating individual patient data. JAMA Intern Med 2015; 175:745–754. [DOI] [PMC free article] [PubMed] [Google Scholar]