Abstract
Osteosarcoma (OS) is a common primary malignant bone tumor with high morbidity and mortality in children and young adults. How to improve poor prognosis of OS due to resistance to chemotherapy remains a challenge. Recently, growing findings show activation of mammalian target of rapamycin (mTOR), is associated with OS cell growth, proliferation, metastasis. Targeting mTOR may be a promising therapeutic approach for treating OS. This review summarizes the roles of mTOR pathway in OS and present research status of mTOR inhibitors in the context of OS. In addition, we have attempted to discuss how to design a better treatment project for OS by combining mTOR inhibitor with other drugs.
Keywords: osteosarcoma, mTOR, target, autophagy, apoptosis
INTRODUCTION
Osteosarcoma (OS) is the most common primary bone malignant neoplasm in children and young adults which is featured with high local aggressiveness and distant organic metastasize [1]. Despite great advances in treatments, comprising neoadjuvant chemotherapy and surgical technology, a notable number of relapse or metastasis still occur [2, 3]. The cure rate of OS is approximately 25 % when accompanied with metastasis at the time of diagnosis, which remains almost stagnant over the past 20 years [4, 5]. Thus, novel chemotherapy drugs are urgently needed.
Mammalian target of rapamycin (mTOR), a downstream mediator in the phosphatidylinositol 3-kinase(PI3K) signaling pathway, is an essential serine/threonine kinase [6]. It involves in regulating important cellular functions including survival, cell growth, proliferation, migration and angiogenesis [7, 8]. Recently, growing researches show aberrant activation of mTOR in many cancer including human osteosarcoma [9]. Notably, the inhibitors of mTOR can demonstrate anti-tumor effect in OS by inhibiting cell growth and proliferation, which raises great interesting in exploring available drug targeting mTOR to improve survival rate of OS [8].
In this review, the role of mTOR pathway and present inhibitors targeting on mTOR in OS are summarized. In addition, we also discuss the strategy reversing resistance to chemotherapeutics for OS patients.
OVERVIEW OF THE MTOR PATHWAY
mTOR is a serine/threonine kinase, which acts as a central controller in regulating important cellular functions [6]. It exists in two multiprotein complexes, mTOR complex 1(mTORC1) and mTOR complex 2(mTORC2). mTORC1 consists of mTOR, regulatory associated protein of mTOR (Raptor), mLST8(mammalian lethal with SEC13 protein 8)/G-protein α-subunit like protein (GαL), RAS40 and Deptor [10]. While mTORC2 is composed of rapamycin-insensitive companion of mTOR (Rictor), mTOR, mLST8/GαL, proline-rich repeat protein-5 (PRR-5)/protein observed with Rictor-1 (Protor-1), stress-activated-protein-kinase-interacting protein 1 (Sin1), and Deptor [11]. Despite both mTORC1 and mTORC2 can be restrained by rapamycin, mTORC1 seem to be relatively sensitive to it [12].
The main upstream signals of mTORC1 are adenosine 5′-monophosphate (AMP)-activated protein kinase (AMPK) and PI3K pathway [13, 14]. PI3Ks constitute a lipid kinase family. Once activated, its catalytic subunit activates AKT. Subsequently mTORC1 is activated. Another upstream effector, AMPK, is a key energy sensor [15], which can regulate cellular metabolism. Activation of AMPK by nutrient deprivation promotes mTORC1 inactivation. The downstream mediators of mTORC1 include ribosomal S6 protein kinase 1 (S6K1) and eIF4E-binding protein 1 (4E-BP1), cyclin dependent kinases (CDKs) and the hypoxia-inducible factor 1α (HIF1α), which promote the expression of a wide range of glycolytic genes [16]. Thus, in the nutrient rich environment, mTORC1 is stimulated and promotes protein synthesis, cellular growth as well as the inhibition of autophagy, a saving program to survive starvation [17].
Compared with mTORC1, the upstream pathways of mTORC2 are less known. PI3K is regarded as a direct upstream effector of mTORC2 [18], while AKT is the main target. Stimulation of AKT by mTORC2 activates mTORC1, thus forming a positive feedback to enhance the signal (Figure 1). Besides, mTORC2 is related to insulin sensitivity and cytoskeletal reorganization [19, 20].
Figure 1. Overview of mTOR signaling pathway.
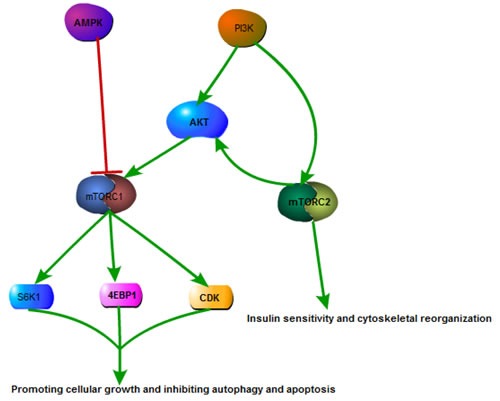
Activation of PI3K/AKT pathway can stimulate mTORC1, meanwhile mTORC1are negatively regulated by AMPK. Activation of mTORC1 upregulates CDK and phosphorylates S6K1 and 4EBP1, modulating cellular growth, autophagy, and apoptosis process. Additionally, PI3K is also the upstream controller of mTORC2, activation of which phosphorylates AKT, forming a positive feedback to enhance the signal. Moreover, activation of mTOR2 is involved in insulin sensitivity and cytoskeletal reorganization.
ROLES OF THE MTOR PATHWAY IN OS
Promoting cellular growth and proliferation
Activation of mTOR pathway is a important signaling pathway stimulating cell growth and proliferation [17]. Aberrant activation of mTOR has been detected in OS [9]. Rapamycin is a common mTOR inhibitor. Treatment with rapamycin suppressing OS cell growth and proliferation has been well documented [21]. Moreover, Rapamycin can effectively inhibit osteosarcoma stem cells proliferation [22]. Additionally, some moleculars and drugs, such as lupeol, Oleanolic acid, metformin, p53, icariside II, capsaicin, phosphorus-containing sirolimus, heat shock protein 90B1, inhibit OS cell growth and proliferation by targeting AMPK/mTOR and PI3K/AKT/mTOR signaling and down-regulating cyclin D1 and phosphorylation of S6K1 and 4EBP1, which are regarded as downstream target of mTORC1 [23-30]. Besides, overexpression of miR-101 can down-regulate the expression of mTOR, contributing to the inhibition of OS cell proliferation [31]. Moreover, activation of PI3K/mTOR signaling by X-Box Binding Protein 1 correlates to Poor Prognosis [32]. Taken together, mTOR play a vital role in promoting growth and proliferation in OS.
Inducing cellular metastasis
Distant organic metastasize remains the predominant lethal for cancer patients. Thus, how to prevent metastas presents a great challenge. It has been proved that mTOR has potential function on facilitating metastasis. Notablely, rapamycin reduces tumor cell metastasis in a murine model of osteosarcoma via blocking the mTOR/S6K1/4E-BP1 pathway [33, 34]. Metformin exerts markedly anti-metastatic potentials by downregulating matrix metalloproteinases, which have an ability of degrading extracellular matrix to facilitate tumor cell metastasis [25, 35-36]. In addition, the histone deacetylase inhibitor and P53 can also downregulate mTOR to restrain metastasis [26, 37]. Another pathway by which activation of mTOR pathway promotes OS cell metastasis is angiogenesis. P53 and phosphorus-containing sirolimus suppresses OS cell angiogenesis through inhibition of mTOR [26, 30]. Thus, inhibition of mTOR may be a novel effective candidate therapeutic strategy against OS cell metastasis.
Inhibition of apoptosis
Apoptosis is refered to a process of programmed cell death which occurs in multicellular organisms [38, 39]. Chemotherapy kills cancer cell mainly by inducing apoptosis. Therefore, developing an effective proapoptotic drug seemed to be a good therapeutic candidate for OS. Interestingly, many findings demonstrate that inhibition of mTOR pathway can induce apoptosis of OS cell [26, 27, 29, 31, 40-44]. At the same time, α-Elemene, isolated from herbs and plants, upregulates HIF-1αprotein via PI3K/Akt/mTor signaling pathway, contributing to inhibition of apoptosis [45]. Moreover, overexpression of miR-101 can suppress the expression of mTOR, inducing the apoptosis of OS cell [31]. Therefore, drug suppressing mTOR pathway has pro-apoptotic effect, which may be a useful therapeutic option for OS.
Suppression of autophagy
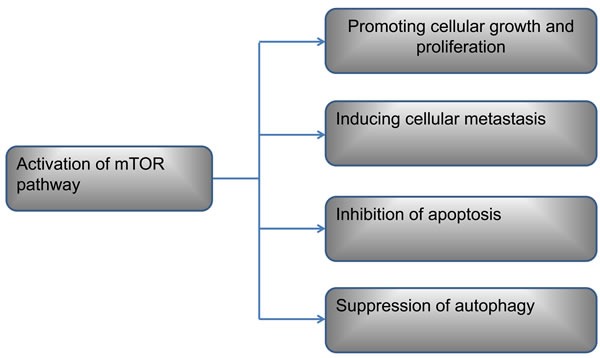
Autophagy is a cellular physiological process which delivers cytoplasmic material to the lysosome to provide energy and nutrients [46, 47]. It occurs as a strategic survival mechanism that reuses energy and nutrients under special conditions [48, 49]. Thus, autophagy is regarded as an emergency pathway of protecting cells from adverse microenvironment. Surprisedly, autophagy is also detected in OS cell [50]. Inhibition of mTOR in OS cell leads to autophagy which has advantage effect on cell [51-52]. Meanwhile, inhibition of autophagy has a negative impact on osteosarcoma tumors [50]. Therefore, activation of mTOR induces autophagy, which is regared as a prosurvival response contributing to drug resistance. Moreover, treating with autophagy inhibitors may lead OS cell apotosis [53]. Nevertheless, activation of autophagy by rapamycin also leads to OS cell death. This mechanism may be due to the extent of autophagy activation beyond the reversibility of cell viability, contributing to out of control of autophagy process [54-58]. Taken together, the signaling pathways involved in autophagy are still little known. In addition, in view of the mTOR is the mutual upstream controller of apoptosis and autophagy process, breaking the balance between apoptosis and autophagy and shifting to apotosis after activation of mTOR pathway may be a promising strategy for facing the challenges of OS. Further investigations are needed to help us understand completely about the roles of mTOR pathway in OS (Figure 2).
Figure 2. The roles of mTOR pathway in OS cell.
INHIBITORS OF MTOR
Despite great advances in treating OS, significant improvement in survival rate and survival time is not acquired. The reason is that cancer cell exerts resistance to chemotherapy drug in clinical application, even it shows promising anti-tumor activity in pre-clinical test. Surprisingly, overactivation of mTOR pathway may relate to resistance to chemotherapy drug [59]. Therefore, the combination of chemotherapy drugs and mTOR inhibitors may demonstrate synergistic effects. Consistent with this notion, C6 ceramide can sensitize pemetrexed-induced apoptosis and cytotoxicity via inactivation of AKT-mTOR signaling in OS [59]. Moreover, specil inhibition of mTORC2 but not mTORC1 can promote cisplatin-induced apoptosis [60]. Thus, exploring novel mTOR inhibitors raise great interest treating OS.
Table 1 lists present research status of mTOR in the context of OS.
Table 1. Research status of mTOR in the context of OS.
| Publication | Name | Main Findings | Ref |
|---|---|---|---|
| 2005,2009 2013,2015 | Rapamycin | Rapamycin can inhibit OS cell proliferation, metastasis, and induce autophagy. | [22][33][34] [44][61] |
| 2010 | Everolimus | Combination with ZOL(zoledronate, an anti-osteoporotic drug) augments the inhibition of Everolimus in cell proliferation. | [65] |
| 2011 | Oleanolic acid (OA) | OA exhibits potent anti-tumor activity against osteosarcoma cells | [23] |
| 2011 | Cucurbitacin B | Cucurbitacin B alone or in combination with methotrexate(MTX) exerts anti-tumor effects on human OS | [66] |
| 2012 | Ridaforolimus | In Phase II study, ridaforolimus shows promising anti-proliferative activity against OS | [62] |
| 2013 | Everolimus | Sorafenib in combined with everolimus contributes to an increasing antitumor activity | [67] |
| 2014 | NVP-BEZ235 | NVP-BEZ235, a dual PI3K/mTOR inhibitor, shows promising antitumor activity in OS. | [72] |
| 2014 | Temsirolimus | Temsirolimus combined with cisplatin or bevacizumab exerts synergistic effects for treatment of OS. | [68] |
| 2014 | PP242 | Inhibition of mTORC2 effectively promotes cisplatin-induced apoptosis | [60] |
| 2014 | Temsirolimus, LY294.002 and PP242 | mTOR inhibitors can blunt the p53 response to nucleolar stress in OS. | [79] |
| 2015 | Rapamycin | JQ1 and rapamycin synergistically inhibite the growthl of OS cells in vitro and in vivo. | [69] |
| 2015 | Temsirolimus | In this phase II trial the combination of cixutumumab and temsirolimus does not show objective result. | [78] |
| 2015 | Everolimus | The combination of sorafenib and everolimusdoes not attain the prespecified target of 6 month PFS in a non-randomised phase 2 clinical trial | [70] |
| 2015 | MLN0128 | MLN0128 exerts anti-tumor activity in in vitro and in vivo model of OS. | [63] |
| 2015 | NVP-BEZ235 | NVP-BEZ235 shows promising anti-tumor activity, which is enhanced by MEK/Erk inhibitors | [73] |
| 2015 | INK-128 | INK-128 exibit potent anti-OS activity in vitro and in vivo. | [64] |
| 2016 | Rapamycin | The combination of rapamycin and an autophagy inhibitor exerts synergistic effects for treatment of OS byeffectively promoting the apoptotic pathway. | [71] |
mTOR inhibitor suppresses OS cell growth solely in vivo and in vitro and phase II study [22, 23, 33, 34, 44, 61-64]. Besides, Some reports find mTOR inhibitor achieves an increasing anti-tumor effect when combining with other forms of drugs, such as anti-osteoporotic drug, extra terminal domain protein inhibitor, conventional chemotherapy drugs [65-71]. In addition, a dual PI3K/mTOR inhibitor shows an promising result in treating OS cell, and this anti-tumor activity can be enhanced by MEK/Erk inhibitors [72, 73].
The roles of autophagy in OS cell survival and death are paradoxical and complex [74] just as we talk above. Notablely, some researchers pay attention to inhibiting both mTOR and antophagy process for treating OS. Heat shock protein 90 (Hsp90), an abundant molecular chaperone, is involved in cell growth, differentiation and survival [75, 76]. Hsp90 inhibitor suppresses mTOR, contributing to autophagy. However, in combination with antophagy inhibitor, hsp90 exerts a much greater extent apoptosis [77]. Another finding also shows that rapamycin induces the apoptosis of OS cells, which is enhanced by antophagy inhibitor [71]. Thus, treating OS cell with mTOR inhibitor alone may inhibit the proliferation and promotion of OS cell by targeting mTOR pathaway. However, as the ability of pro-apoptosis is growing, the escape pathway of autophagy is triggered, counteracting the anti-tumor effect of mTOR inhibitor and contributing resistence to mTOR inhibitor, which is consistent with the modest anti-tumor effect of mTOR inhibitor in clinical application. Autophagy inhibitor can elevate efficiency of mTOR inhibitor by blocking autophagy process in treating OS. Owing to partly understand in the autophagy pathway in OS, further investigations are needed.
Overall, mTOR inhibitor combined with other drugs may provide a novel therapeutic strategy against OS. However, the combination of the anti-insulin-growth factor type 1 receptor antibody and mTOR inhibitor does not show a objective result in an phase II trial [78]. The different conditions of cell living in between pre-clincal test and clinical study and the distrinct type of drug combined with mTOR inhibitor may lead to dissatisfied result. Moreover, nucleolar stress, induced by chemotherapeutic drugs, stimulates p53-dependent signaling pathways which contribute to cell cycle arrest, apoptosis, and mTOR inhibitor can alleviate this p53 response to nucleolar stress [79-85]. The cross-linking of p53-dependent signaling pathways and mTOR pathway may explain this inconsistent result. Thus, we should take the complexity and potential problems into consideration when mTOR inhibitor combined with other cytotoxic compounds is applied in treating OS.
Taken together, the combination of mTOR inhibitor and other drugs may provide an efficient therapeutic strategy against OS. However, the mTOR signaling pathway is complexity in OS, and its roles in OS are still not completely understood. Further studies will help us design a combinatorial chemotherapy regimen against OS.
CONCLUSIONS
Activation of mTOR pathway promotes OS cell proliferation, metastasis, and inhibits the intracellular processes of apoptosis and autophagy. mTOR inhibitor used alone exerts a promising anti-tumor activity, which is enhenced by combining with other drugs for OS. Thus, exploring a better combinatorial chemotherapy regimen provide a novel therapeutic approach for OS. However, the detail mechanism of mTOR pathway and synergistical effect of mTOR inhibitor and other drugs in OS are still not fully understood. Therefore, future further researches are required to gain a better understanding.
Acknowledgments
The present study is supported by the National Natural Science Foundation of China (No. 81372180) and thanks for advice from the fellows in Metabolic Syndrome Research Center of Central South University.
Footnotes
CONFLICTS OF INTEREST
The authors declare that they have no competing interests.
REFERENCES
- 1.Arndt CA, Rose PS, Folpe AL, Laack NN. Common musculoskeletal tumors of childhood and adolescence. Mayo Clin Proc. 2012;87:475–487. doi: 10.1016/j.mayocp.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bacci G, Ferrari S, Longhi A, Perin S, Forni C, Fabbri N, Salduca N, Versari M, Smith KV. Pattern of relapse in patients with osteosarcoma of the extremities treated with neoadjuvant chemotherapy. Eur J Cancer. 2001;37:32–38. doi: 10.1016/s0959-8049(00)00361-0. [DOI] [PubMed] [Google Scholar]
- 3.Kempf-Bielack B, Bielack SS, Jurgens H, Branscheid D, Berdel WE, Exner GU, Gobel U, Helmke K, Jundt G, Kabisch H, Kevric M, Klingebiel T, Kotz R, et al. Osteosarcoma relapse after combined modality therapy: an analysis of unselected patients in the Cooperative Osteosarcoma Study Group (COSS) J Clin Oncol. 2005;23:559–568. doi: 10.1200/JCO.2005.04.063. [DOI] [PubMed] [Google Scholar]
- 4.Gorlick R, Anderson P, Andrulis I, Arndt C, Beardsley GP, Bernstein M, Bridge J, Cheung NK, Dome JS, Ebb D, Gardner T, Gebhardt M, Grier H, et al. Biology of childhood osteogenic sarcoma and potential targets for therapeutic development: meeting summary. Clin Cancer Res. 2003;9:5442–5453. [PubMed] [Google Scholar]
- 5.Allison DC, Carney SC, Ahlmann ER, Hendifar A, Chawla S, Fedenko A, Angeles C, Menendez LR. A meta-analysis of osteosarcoma outcomes in the modern medical era. Sarcoma. 2012;2012:704872. doi: 10.1155/2012/704872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 7.Gridelli C, Maione P, Rossi A. The potential role of mTOR inhibitors in non-small cell lung cancer. Oncologist. 2008;13:139–147. doi: 10.1634/theoncologist.2007-0171. [DOI] [PubMed] [Google Scholar]
- 8.Mita MM, Tolcher AW. The role of mTOR inhibitors for treatment of sarcomas. Curr Oncol Rep. 2007;9:316–322. doi: 10.1007/s11912-007-0039-7. [DOI] [PubMed] [Google Scholar]
- 9.Porta C, Paglino C, Mosca A. Targeting PI3K/Akt/mTOR Signaling in Cancer. Front Oncol. 2014;4:64. doi: 10.3389/fonc.2014.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ballou LM, Lin RZ. Rapamycin and mTOR kinase inhibitors. J Chem Biol. 2008;1:27–36. doi: 10.1007/s12154-008-0003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 14.Kimura N, Tokunaga C, Dalal S, Richardson C, Yoshino K, Hara K, Kemp BE, Witters LA, Mimura O, Yonezawa K. A possible linkage between AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) signalling pathway. Genes Cells. 2003;8:65–79. doi: 10.1046/j.1365-2443.2003.00615.x. [DOI] [PubMed] [Google Scholar]
- 15.Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- 16.Martinet W, De Loof H, De Meyer GR. mTOR inhibition: a promising strategy for stabilization of atherosclerotic plaques. Atherosclerosis. 2014;233:601–607. doi: 10.1016/j.atherosclerosis.2014.01.040. [DOI] [PubMed] [Google Scholar]
- 17.Hosokawa N, Hara T, Kaizuka T, Kishi C, Takamura A, Miura Y, Iemura S, Natsume T, Takehana K, Yamada N, Guan JL, Oshiro N, Mizushima N. Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol Biol Cell. 2009;20:1981–1991. doi: 10.1091/mbc.E08-12-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu P, Gan W, Chin YR, Ogura K, Guo J, Zhang J, Wang B, Blenis J, Cantley LC, Toker A, Su B, Wei W. PtdIns(3,4,5)P3-Dependent Activation of the mTORC2 Kinase Complex. Cancer Discov. 2015;5:1194–1209. doi: 10.1158/2159-8290.CD-15-0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu L, Parent CA. Review series: TOR kinase complexes and cell migration. J Cell Biol. 2011;194:815–824. doi: 10.1083/jcb.201102090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oh WJ, Jacinto E. mTOR complex 2 signaling and functions. Cell Cycle. 2011;10:2305–2316. doi: 10.4161/cc.10.14.16586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gordon IK, Ye F, Kent MS. Evaluation of the mammalian target of rapamycin pathway and the effect of rapamycin on target expression and cellular proliferation in osteosarcoma cells from dogs. Am J Vet Res. 2008;69:1079–1084. doi: 10.2460/ajvr.69.8.1079. [DOI] [PubMed] [Google Scholar]
- 22.Liu PY, Zhang WB, Wang J, Shen YH, Wei YY. [Inhibitory effect and significance of rapamycin on the mammalian target of rapamycin signaling pathway in osteosarcoma stem cells and osteosarcoma cells]. [Article in Chinese] Zhonghua Zhong Liu Za Zhi. 2013;35:175–180. doi: 10.3760/cma.j.issn.0253-3766.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Hua Y, Zhang Z, Li J, Li Q, Hu S, Li J, Sun M, Cai Z. Oleanolic acid derivative Dex-OA has potent anti-tumor and anti-metastatic activity on osteosarcoma cells in vitro and in vivo. Invest New Drugs. 2011;29:258–265. doi: 10.1007/s10637-009-9354-1. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Bi T, Dai W, Wang G, Qian L, Shen G, Gao Q. Lupeol Induces Apoptosis and Cell Cycle Arrest of Human Osteosarcoma Cells Through PI3K/AKT/mTOR Pathway. Technol Cancer Res Treat. 2015 doi: 10.1177/1533034615609014. [DOI] [PubMed] [Google Scholar]
- 25.Chen X, Hu C, Zhang W, Shen Y, Wang J, Hu F, Yu P. Metformin inhibits the proliferation, metastasis, and cancer stem-like sphere formation in osteosarcoma MG63 cells in vitro. Tumour Biol. 2015;36:9873–9883. doi: 10.1007/s13277-015-3751-1. [DOI] [PubMed] [Google Scholar]
- 26.Song R, Tian K, Wang W, Wang L. P53 suppresses cell proliferation, metastasis, and angiogenesis of osteosarcoma through inhibition of the PI3K/AKT/mTOR pathway. Int J Surg. 2015;20:80–87. doi: 10.1016/j.ijsu.2015.04.050. [DOI] [PubMed] [Google Scholar]
- 27.Li G, Cai M, Fu D, Chen K, Sun M, Cai Z, Cheng B. Heat shock protein 90B1 plays an oncogenic role and is a target of microRNA-223 in human osteosarcoma. Cell Physiol Biochem. 2012;30:1481–1490. doi: 10.1159/000343336. [DOI] [PubMed] [Google Scholar]
- 28.Geng YD, Yang L, Zhang C, Kong LY. Blockade of epidermal growth factor receptor/mammalian target of rapamycin pathway by Icariside II results in reduced cell proliferation of osteosarcoma cells. Food Chem Toxicol. 2014;73:7–16. doi: 10.1016/j.fct.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Ying H, Wang Z, Zhang Y, Yang TY, Ding ZH, Liu SY, Shao J, Liu Y, Fan XB. Capsaicin induces apoptosis in human osteosarcoma cells through AMPK-dependent and AMPK-independent signaling pathways. Mol Cell Biochem. 2013;384:229–237. doi: 10.1007/s11010-013-1802-8. [DOI] [PubMed] [Google Scholar]
- 30.Liu WN, Lin JH, Cheng YR, Zhang L, Huang J, Wu ZY, Wang FS, Xu SG, Lin WP, Lan WB, Yang GX. FIM-A, a phosphorus-containing sirolimus, inhibits the angiogenesis and proliferation of osteosarcomas. Oncol Res. 2013;20:319–326. doi: 10.3727/096504013x13644751511888. [DOI] [PubMed] [Google Scholar]
- 31.Lin S, Shao NN, Fan L, Ma XC, Pu FF, Shao ZW. Effect of microRNA-101 on proliferation and apoptosis of human osteosarcoma cells by targeting mTOR. J Huazhong Univ Sci Technolog Med Sci. 2014;34:889–895. doi: 10.1007/s11596-014-1369-y. [DOI] [PubMed] [Google Scholar]
- 32.Yang J, Cheng D, Zhou S, Zhu B, Hu T, Yang Q. Overexpression of X-Box Binding Protein 1 (XBP1) Correlates to Poor Prognosis and Up-Regulation of PI3K/mTOR in Human Osteosarcoma. Int J Mol Sci. 2015;16:28635–28646. doi: 10.3390/ijms161226123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wan X, Mendoza A, Khanna C, Helman LJ. Rapamycin inhibits ezrin-mediated metastatic behavior in a murine model of osteosarcoma. Cancer Res. 2005;65:2406–2411. doi: 10.1158/0008-5472.CAN-04-3135. [DOI] [PubMed] [Google Scholar]
- 34.Mu X, Isaac C, Schott T, Huard J, Weiss K. Rapamycin Inhibits ALDH Activity, Resistance to Oxidative Stress, and Metastatic Potential in Murine Osteosarcoma Cells. Sarcoma. 2013;2013:480713. doi: 10.1155/2013/480713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stamenkovic I. Matrix metalloproteinases in tumor invasion and metastasis. Semin Cancer Biol. 2000;10:415–433. doi: 10.1006/scbi.2000.0379. [DOI] [PubMed] [Google Scholar]
- 36.Lekstan A, Lampe P, Lewin-Kowalik J, Olakowski M, Jablonska B, Labuzek K, Jedrzejowska-Szypulka H, Olakowska E, Gorka D, Filip I, Dranka-Bojarowska D. Concentrations and activities of metalloproteinases 2 and 9 and their inhibitors (TIMPS) in chronic pancreatitis and pancreatic adenocarcinoma. J Physiol Pharmacol. 2012;63:589–599. [PubMed] [Google Scholar]
- 37.Mu X, Brynien D, Weiss KR. The HDAC inhibitor Vorinostat diminishes the in vitro metastatic behavior of Osteosarcoma cells. Biomed Res Int. 2015;2015:290368. doi: 10.1155/2015/290368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kerr JF, Winterford CM, Harmon BV. Apoptosis. Its significance in cancer and cancer therapy. Cancer. 1994;73:2013–2026. doi: 10.1002/1097-0142(19940415)73:8<2013::aid-cncr2820730802>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 39.Boehm I. Apoptosis in physiological and pathological skin: implications for therapy. Curr Mol Med. 2006;6:375–394. doi: 10.2174/156652406777435390. [DOI] [PubMed] [Google Scholar]
- 40.Wang X, Lai P, Zhang Z, Huang M, Wang L, Yin M, Jin D, Zhou R, Bai X. Targeted inhibition of mTORC2 prevents osteosarcoma cell migration and promotes apoptosis. Oncol Rep. 2014;32:382–388. doi: 10.3892/or.2014.3182. [DOI] [PubMed] [Google Scholar]
- 41.Yao C, Wei JJ, Wang ZY, Ding HM, Li D, Yan SC, Yang YJ, Gu ZP. Perifosine induces cell apoptosis in human osteosarcoma cells: new implication for osteosarcoma therapy? Cell Biochem Biophys. 2013;65:217–227. doi: 10.1007/s12013-012-9423-5. [DOI] [PubMed] [Google Scholar]
- 42.Miwa S, Sugimoto N, Yamamoto N, Shirai T, Nishida H, Hayashi K, Kimura H, Takeuchi A, Igarashi K, Yachie A, Tsuchiya H. Caffeine induces apoptosis of osteosarcoma cells by inhibiting AKT/mTOR/S6K, NF-kappaB and MAPK pathways. Anticancer Res. 2012;32:3643–3649. [PubMed] [Google Scholar]
- 43.Zhou R, Zhang Z, Zhao L, Jia C, Xu S, Mai Q, Lu M, Huang M, Wang L, Wang X, Jin D, Bai X. Inhibition of mTOR signaling by oleanolic acid contributes to its anti-tumor activity in osteosarcoma cells. J Orthop Res. 2011;29:846–852. doi: 10.1002/jor.21311. [DOI] [PubMed] [Google Scholar]
- 44.Gazitt Y, Kolaparthi V, Moncada K, Thomas C, Freeman J. Targeted therapy of human osteosarcoma with 17AAG or rapamycin: characterization of induced apoptosis and inhibition of mTOR and Akt/MAPK/Wnt pathways. Int J Oncol. 2009;34:551–561. [PubMed] [Google Scholar]
- 45.Liang D, Yang M, Guo B, Yang L, Cao J, Zhang X. HIF-1alpha induced by beta-elemene protects human osteosarcoma cells from undergoing apoptosis. J Cancer Res Clin Oncol. 2012;138:1865–1877. doi: 10.1007/s00432-012-1256-5. [DOI] [PubMed] [Google Scholar]
- 46.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klionsky DJ, Abdalla FC, Abeliovich H, Abraham RT, Acevedo-Arozena A, Adeli K, Agholme L, Agnello M, Agostinis P, Aguirre-Ghiso JA, Ahn HJ, Ait-Mohamed O, Ait-Si-Ali S, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8:445–544. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burada F, Nicoli ER, Ciurea ME, Uscatu DC, Ioana M, Gheonea DI. Autophagy in colorectal cancer: An important switch from physiology to pathology. World J Gastrointest Oncol. 2015;7:271–284. doi: 10.4251/wjgo.v7.i11.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lindqvist LM, Simon AK, Baehrecke EH. Current questions and possible controversies in autophagy. Cell Death Discov. 2015:1. doi: 10.1038/cddiscovery.2015.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akin D, Wang SK, Habibzadegah-Tari P, Law B, Ostrov D, Li M, Yin XM, Kim JS, Horenstein N, Dunn WJ. A novel ATG4B antagonist inhibits autophagy and has a negative impact on osteosarcoma tumors. Autophagy. 2014;10:2021–2035. doi: 10.4161/auto.32229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mori M, Hitora T, Nakamura O, Yamagami Y, Horie R, Nishimura H, Yamamoto T. Hsp90 inhibitor induces autophagy and apoptosis in osteosarcoma cells. Int J Oncol. 2015;46:47–54. doi: 10.3892/ijo.2014.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pan J, Chen B, Su CH, Zhao R, Xu ZX, Sun L, Lee MH, Yeung SC. Autophagy induced by farnesyltransferase inhibitors in cancer cells. Cancer Biol Ther. 2008;7:1679–1684. doi: 10.4161/cbt.7.10.6661. [DOI] [PubMed] [Google Scholar]
- 53.Xie Z, Xie Y, Xu Y, Zhou H, Xu W, Dong Q. Bafilomycin A1 inhibits autophagy and induces apoptosis in MG63 osteosarcoma cells. Mol Med Rep. 2014;10:1103–1107. doi: 10.3892/mmr.2014.2281. [DOI] [PubMed] [Google Scholar]
- 54.Xie ZG, Xie Y, Dong QR. Inhibition of the mammalian target of rapamycin leads to autophagy activation and cell death of MG63 osteosarcoma cells. Oncol Lett. 2013;6:1465–1469. doi: 10.3892/ol.2013.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Al-Ejeh F, Kumar R, Wiegmans A, Lakhani SR, Brown MP, Khanna KK. Harnessing the complexity of DNA-damage response pathways to improve cancer treatment outcomes. Oncogene. 2010;29:6085–6098. doi: 10.1038/onc.2010.407. [DOI] [PubMed] [Google Scholar]
- 56.Gewirtz DA. Autophagy as a mechanism of radiation sensitization in breast tumor cells. Autophagy. 2007;3:249–250. doi: 10.4161/auto.3723. [DOI] [PubMed] [Google Scholar]
- 57.John S, Nayvelt I, Hsu HC, Yang P, Liu W, Das GM, Thomas T, Thomas TJ. Regulation of estrogenic effects by beclin 1 in breast cancer cells. Cancer Res. 2008;68:7855–7863. doi: 10.1158/0008-5472.CAN-07-5875. [DOI] [PubMed] [Google Scholar]
- 58.Buytaert E, Callewaert G, Vandenheede JR, Agostinis P. Deficiency in apoptotic effectors Bax and Bak reveals an autophagic cell death pathway initiated by photodamage to the endoplasmic reticulum. Autophagy. 2006;2:238–240. doi: 10.4161/auto.2730. [DOI] [PubMed] [Google Scholar]
- 59.Zhu X, Du X, Deng X, Yi H, Cui S, Liu W, Shen A, Cui Z. C6 ceramide sensitizes pemetrexed-induced apoptosis and cytotoxicity in osteosarcoma cells. Biochem Biophys Res Commun. 2014;452:72–78. doi: 10.1016/j.bbrc.2014.08.065. [DOI] [PubMed] [Google Scholar]
- 60.Wang X, Lai P, Zhang Z, Huang M, Wang L, Yin M, Jin D, Zhou R, Bai X. Targeted inhibition of mTORC2 prevents osteosarcoma cell migration and promotes apoptosis. Oncol Rep. 2014;32:382–388. doi: 10.3892/or.2014.3182. [DOI] [PubMed] [Google Scholar]
- 61.Zhao S, Lu N, Chai Y, Yu X. Rapamycin inhibits tumor growth of human osteosarcomas. J Buon. 2015;20:588–594. [PubMed] [Google Scholar]
- 62.Chawla SP, Staddon AP, Baker LH, Schuetze SM, Tolcher AW, D'Amato GZ, Blay JY, Mita MM, Sankhala KK, Berk L, Rivera VM, Clackson T, Loewy JW, et al. Phase II study of the mammalian target of rapamycin inhibitor ridaforolimus in patients with advanced bone and soft tissue sarcomas. J Clin Oncol. 2012;30:78–84. doi: 10.1200/JCO.2011.35.6329. [DOI] [PubMed] [Google Scholar]
- 63.Slotkin EK, Patwardhan PP, Vasudeva SD, de Stanchina E, Tap WD, Schwartz GK. MLN0128, an ATP-competitive mTOR kinase inhibitor with potent in vitro and in vivo antitumor activity, as potential therapy for bone and soft-tissue sarcoma. Mol Cancer Ther. 2015;14:395–406. doi: 10.1158/1535-7163.MCT-14-0711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jiang H, Zeng Z. Dual mTORC1/2 inhibition by INK-128 results in antitumor activity in preclinical models of osteosarcoma. Biochem Biophys Res Commun. 2015;468:255–261. doi: 10.1016/j.bbrc.2015.10.119. [DOI] [PubMed] [Google Scholar]
- 65.Moriceau G, Ory B, Mitrofan L, Riganti C, Blanchard F, Brion R, Charrier C, Battaglia S, Pilet P, Denis MG, Shultz LD, Monkkonen J, Redini F, et al. Zoledronic acid potentiates mTOR inhibition and abolishes the resistance of osteosarcoma cells to RAD001 (Everolimus): pivotal role of the prenylation process. Cancer Res. 2010;70:10329–10339. doi: 10.1158/0008-5472.CAN-10-0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee DH, Thoennissen NH, Goff C, Iwanski GB, Forscher C, Doan NB, Said JW, Koeffler HP. Synergistic effect of low-dose cucurbitacin B and low-dose methotrexate for treatment of human osteosarcoma. Cancer Lett. 2011;306:161–170. doi: 10.1016/j.canlet.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pignochino Y, Dell'Aglio C, Basirico M, Capozzi F, Soster M, Marchio S, Bruno S, Gammaitoni L, Sangiolo D, Torchiaro E, D'Ambrosio L, Fagioli F, Ferrari S, et al. The Combination of Sorafenib and Everolimus Abrogates mTORC1 and mTORC2 upregulation in osteosarcoma preclinical models. Clin Cancer Res. 2013;19:2117–2131. doi: 10.1158/1078-0432.CCR-12-2293. [DOI] [PubMed] [Google Scholar]
- 68.Fleuren ED, Versleijen-Jonkers YM, Roeffen MH, Franssen GM, Flucke UE, Houghton PJ, Oyen WJ, Boerman OC, Van der Graaf WT. Temsirolimus combined with cisplatin or bevacizumab is active in osteosarcoma models. Int J Cancer. 2014;135:2770–2782. doi: 10.1002/ijc.28933. [DOI] [PubMed] [Google Scholar]
- 69.Lee DH, Qi J, Bradner JE, Said JW, Doan NB, Forscher C, Yang H, Koeffler HP. Synergistic effect of JQ1 and rapamycin for treatment of human osteosarcoma. Int J Cancer. 2015;136:2055–2064. doi: 10.1002/ijc.29269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grignani G, Palmerini E, Ferraresi V, D'Ambrosio L, Bertulli R, Asaftei SD, Tamburini A, Pignochino Y, Sangiolo D, Marchesi E, Capozzi F, Biagini R, Gambarotti M, et al. Sorafenib and everolimus for patients with unresectable high-grade osteosarcoma progressing after standard treatment: a non-randomised phase 2 clinical trial. Lancet Oncol. 2015;16:98–107. doi: 10.1016/S1470-2045(14)71136-2. [DOI] [PubMed] [Google Scholar]
- 71.Grignani G, Palmerini E, Ferraresi V, D'Ambrosio L, Bertulli R, Asaftei SD, Tamburini A, Pignochino Y, Sangiolo D, Marchesi E, Capozzi F, Biagini R, Gambarotti M, et al. Sorafenib and everolimus for patients with unresectable high-grade osteosarcoma progressing after standard treatment: a non-randomised phase 2 clinical trial. Lancet Oncol. 2015;16:98–107. doi: 10.1016/S1470-2045(14)71136-2. [DOI] [PubMed] [Google Scholar]
- 72.Gobin B, Battaglia S, Lanel R, Chesneau J, Amiaud J, Redini F, Ory B, Heymann D. NVP-BEZ235, a dual PI3K/mTOR inhibitor, inhibits osteosarcoma cell proliferation and tumor development in vivo with an improved survival rate. Cancer Lett. 2014;344:291–298. doi: 10.1016/j.canlet.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 73.Zhu YR, Min H, Fang JF, Zhou F, Deng XW, Zhang YQ. Activity of the novel dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor NVP-BEZ235 against osteosarcoma. Cancer Biol Ther. 2015;16:602–609. doi: 10.1080/15384047.2015.1017155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Amaravadi RK, Thompson CB. The roles of therapy-induced autophagy and necrosis in cancer treatment. Clin Cancer Res. 2007;13:7271–7279. doi: 10.1158/1078-0432.CCR-07-1595. [DOI] [PubMed] [Google Scholar]
- 75.Bishop SC, Burlison JA, Blagg BS. Hsp90: a novel target for the disruption of multiple signaling cascades. Curr Cancer Drug Targets. 2007;7:369–388. doi: 10.2174/156800907780809778. [DOI] [PubMed] [Google Scholar]
- 76.Zuehlke A, Johnson JL. Hsp90 and co-chaperones twist the functions of diverse client proteins. Biopolymers. 2010;93:211–217. doi: 10.1002/bip.21292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mori M, Hitora T, Nakamura O, Yamagami Y, Horie R, Nishimura H, Yamamoto T. Hsp90 inhibitor induces autophagy and apoptosis in osteosarcoma cells. Int J Oncol. 2015;46:47–54. doi: 10.3892/ijo.2014.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wagner LM, Fouladi M, Ahmed A, Krailo MD, Weigel B, DuBois SG, Doyle LA, Chen H, Blaney SM. Phase II study of cixutumumab in combination with temsirolimus in pediatric patients and young adults with recurrent or refractory sarcoma: a report from the Children's Oncology Group. Pediatr Blood Cancer. 2015;62:440–444. doi: 10.1002/pbc.25334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Goudarzi KM, Nister M, Lindstrom MS. mTOR inhibitors blunt the p53 response to nucleolar stress by regulating RPL11 and MDM2 levels. Cancer Biol Ther. 2014;15:1499–1514. doi: 10.4161/15384047.2014.955743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Miliani DMP, Zhang Y. The RP-Mdm2-p53 pathway and tumorigenesis. Oncotarget. 2011;2:234–238. doi: 10.18632/oncotarget.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang Y, Lu H. Signaling to p53: ribosomal proteins find their way. Cancer Cell. 2009;16:369–377. doi: 10.1016/j.ccr.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhou X, Liao JM, Liao WJ, Lu H. Scission of the p53-MDM2 Loop by Ribosomal Proteins. Genes Cancer. 2012;3:298–310. doi: 10.1177/1947601912455200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Avitabile D, Bailey B, Cottage CT, Sundararaman B, Joyo A, McGregor M, Gude N, Truffa S, Zarrabi A, Konstandin M, Khan M, Mohsin S, Volkers M, et al. Nucleolar stress is an early response to myocardial damage involving nucleolar proteins nucleostemin and nucleophosmin. Proc Natl Acad Sci U S A. 2011;108:6145–6150. doi: 10.1073/pnas.1017935108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sun XX, Dai MS, Lu H. 5-fluorouracil activation of p53 involves an MDM2-ribosomal protein interaction. J Biol Chem. 2007;282:8052–8059. doi: 10.1074/jbc.M610621200. [DOI] [PubMed] [Google Scholar]
- 85.Sun XX, Dai MS, Lu H. Mycophenolic acid activation of p53 requires ribosomal proteins L5 and L11. J Biol Chem. 2008;283:12387–12392. doi: 10.1074/jbc.M801387200. [DOI] [PMC free article] [PubMed] [Google Scholar]


