Abstract
Background
Food allergy is an important public health problem because it affects children and adults, it may be severe and even life-threatening, and it may be increasing in prevalence. Beginning in 2008, the National Institute of Allergy and Infectious Diseases (NIAID), working with other organizations and advocacy groups, led the development of the first clinical guidelines for the diagnosis and management of food allergy. A recent landmark clinical trial and other emerging data suggest that peanut allergy can be prevented through introduction of peanut-containing foods beginning in infancy.
Objectives
Prompted by these findings, along with 25 professional organizations, federal agencies, and patient advocacy groups, NIAID facilitated development of Addendum Guidelines to specifically address the prevention of peanut allergy.
Results
The Addendum provides three separate guidelines for infants at various risk levels for development of peanut allergy and is intended for use by a wide variety of health care providers. Topics addressed include the definition of risk categories, appropriate use of testing (specific IgE, skin prick testing, and oral food challenge) and the timing and approaches for introduction of peanut-containing foods in the health care provider’s office or at home. The Addendum Guidelines provide the background, rationale, and strength of evidence for each recommendation.
Conclusions
Guidelines have been developed for early introduction of peanut-containing foods into the diets of infants at various risk levels for peanut allergy.
Keywords: Food, peanut, allergy, prevention, guidelines
INTRODUCTION
Peanut allergy is a growing public health problem. In 1999, peanut allergy was estimated to affect 0.4% of children in the United States and 0.7% of adults1; and by 2010, peanut allergy prevalence had increased to ~2% among children in a national survey2 with similar results reported in a regional cohort3. Peanut allergy is the leading cause of death related to food-induced anaphylaxis in the United States4,5 and although overall mortality is low, the fear of life-threatening anaphylactic reactions contributes significantly to the medical and psychosocial burden of disease. In the majority of patients, peanut allergy begins early in life and persists as a lifelong problem. Cost effective measures to prevent peanut allergy would, therefore, have high impact in terms of improving public health, reducing personal suffering and lowering health care utilization and costs.
Guidelines for the Diagnosis and Management of Food Allergy in the United States6 were published in December 2010 by an Expert Panel (EP) and a Coordinating Committee (CC) convened by the National Institute of Allergy and Infectious Diseases (NIAID). These Guidelines did not offer strategies for the prevention of food allergy and, particularly, peanut allergy, due to a lack of definitive studies at the time. The Guidelines indicated that “insufficient evidence exists for delaying introduction of solid foods, including potentially allergenic foods, beyond 4 to 6 months of age, even in infants at risk of developing allergic disease.” This statement differed from previous clinical practice guidelines in the United Kingdom7 and in the United States8, which recommended the exclusion of allergenic foods from the diets of infants at high risk for allergy, and is consistent with more recent recommendations regarding primary allergy prevention9–12.
In February 2015, the New England Journal of Medicine published the results of the “Learning Early about Peanut Allergy” (LEAP) trial13. This trial was based on a prior observation14 that the prevalence of peanut allergy was 10-fold higher among Jewish children in the United Kingdom compared to Israeli children of similar ancestry. In Israel, peanut-containing foods are usually introduced in the diet when infants are approximately 7 months of age, and consumed in substantial amounts, whereas in the United Kingdom children do not typically consume any peanut-containing foods during their first year of life. The LEAP trial randomized 640 children, between 4 and 11 months of age, with severe eczema and/or egg allergy to consume or avoid peanut-containing foods until 60 months of age, when a peanut oral food challenge was conducted to determine the prevalence of peanut allergy. LEAP participants were stratified at study entry into 2 separate study cohorts on the basis of pre-existing sensitization to peanut, determined by skin prick testing: one cohort consisted of infants with no measureable skin test wheal to peanut (negative skin test) and the other of those with measurable wheal (1 to 4 mm in diameter). Infants with 5 mm wheal diameter or higher were not randomized because the majority of infants at this level of sensitization were presumed to already be allergic to peanut. Among the 530 participants in the intention-to-treat population with negative baseline skin test to peanut, the prevalence of peanut allergy at 60 months of age was 13.7% in the peanut avoidance group and 1.9% in the consumption group (P<0.001; an 86.1% relative reduction in the prevalence of peanut allergy). Among the 98 participants with a measurable peanut skin test at entry, the prevalence of peanut allergy was 35.3% in the avoidance group and 10.6% in the consumption group (P=0.004; a 70% relative reduction in the prevalence of peanut allergy).
The LEAP trial was the first randomized trial to study early allergen introduction as a preventive strategy. Because of the size of the observed effect and the large number of study participants, its outcome received wide publicity in both the medical community and the press. This raised the need to operationalize the LEAP findings by developing clinical recommendations focusing on peanut allergy prevention. To achieve this goal and its wide implementation, NIAID invited the members of the 2010 Guidelines Coordinating Committee and other stakeholder organizations to develop this Addendum on peanut allergy prevention to the 2010 Guidelines for the Diagnosis and Management of Food Allergy in the United States. Twenty-six stakeholder organizations participated in this 2015–2016 Coordinating Committee. Of note, unrelated to this effort, a consensus statement on behalf of nine international professional societies regarding the implications and implementation of the LEAP findings also was published15.
Additional evidence on early introduction of allergenic foods comes from the LEAP-On study16, which demonstrated the durability of oral tolerance to peanut achieved in the LEAP study, and the Enquiring About Tolerance (EAT) study17, which assessed the potential benefits of early introduction of six allergenic foods in a non-high risk cohort.
DEVELOPMENT OF THE 2017 ADDENDUM TO THE 2010 GUIDELINES FOR THE DIAGNOSIS AND MANAGEMENT OF FOOD ALLERGY
The process to develop the 2017 Addendum closely followed that used in the 2010 Guidelines6.
The Coordinating Committee (CC)
NIAID established a CC, whose members are listed in Appendix A, to oversee the development of the Addendum; review drafts of the Addendum for accuracy, practicality, clarity, and broad utility of the recommendations in clinical practice; review and approve the final Addendum; and disseminate the Addendum. The CC members represented 26 professional organizations, advocacy groups, and federal agencies.
The Expert Panel (EP)
The CC convened an EP in June 2015 that was chaired by Joshua Boyce, MD. The 26 panel members, listed in Appendix B, were specialists from a variety of relevant clinical, scientific, and public health areas. Panel members were nominated by the CC organizations and NIAID, and the composition of the panel received unanimous approval by the CC member organizations.
The charge to the EP was to use the literature review prepared by NIAID (see next section), in conjunction with consensus expert opinion and EP-identified supplementary documents, to: 1) develop evidence-based recommendations for the early introduction of dietary peanut to prevent peanut allergy; 2) agree on principles for grading the evidence; 3) achieve consensus while allowing ample opportunity for consideration of divergent opinions; 4) determine if the recommendations could extend beyond peanut to other food allergens; and 5) keep patient and societal interests at the forefront. The new recommendations are intended to supplement and modify Guidelines 37 to 40 in Section 5.3.4 of the 2010 Guidelines: “Prevention of Food Allergy.”
The Literature Review
NIAID staff conducted a literature search of PubMed, limited to the years 2010 (January) to 2016 (June). Using the following specific search terms [(food allergy or milk allergy or egg allergy or peanut allergy) OR (eczema or atopic dermatitis)] AND prevention], PubMed returned >1,500 articles. NIAID staff reviewed 1,506 abstracts and assessed each for relevance to the topic of food allergy prevention with an emphasis on peanut allergy. Sixty four publications (original research articles, editorials/letters, and systematic reviews) were deemed relevant and placed into two tiers: Tier 1 contained 18 items considered highly relevant to the early introduction of peanut or other allergenic foods (see Appendix C) and Tier 2 contained 46 items on related topics such as food allergy or eczema prevention.
Assessing the Quality of the Body of Evidence
For each of the 18 Tier 1 references, the EP assessed the quality using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach18. GRADE provides a comprehensive and transparent methodology to develop recommendations for the diagnosis, treatment, and management of patients. In assessing the body of evidence of a group of relevant papers or of a single paper, GRADE considers study design and other factors, such as the precision, consistency, and directness of the data. Using this approach, GRADE then provides a categorical assessment of the contribution of individual publications and the overall quality and strength of the body of evidence.
Each publication was assigned a grade according to the following criteria19–20:
High—Further research is very unlikely to have an impact on the quality of the body of evidence, and therefore the confidence in the recommendation is high and unlikely to change.
Moderate—Further research is likely to have an impact on the quality of the body of evidence and may change the recommendation.
Low—Further research is very likely to have an important impact on the body of evidence and is likely to change the recommendation.
A GRADE designation of “Low” for the quality of evidence does not imply that an article is not factually correct or lacks scientific merit. For example, a well-designed and executed single-site study of a treatment in a small cohort of highly selected subjects might still yield an overall GRADE of “Low.” This is because such a study is characterized as providing “sparse” data, and the patient population may not be representative of the at-risk population. Each of these factors reduces the level of evidence from “High,” which is the initial designation for evidence from randomized controlled trials. It is worth emphasizing that these two limitations are not of the study per se, but of the body of evidence.
Preparation of the Draft Addendum
The draft version of the Addendum, prepared by NIAID, contained 3 new guidelines and was reviewed, modified and endorsed by the EP members. The EP-approved document was forwarded to the CC members for review.
Public Comment Period, Addendum Revision and Final Approval
Concurrent with CC member review, the draft Addendum was posted to the NIAID Web site in March 2016 for a period of 45 days to allow for public review and comment. One hundred and four comments were received. All comments were reviewed by the EP and the CC and some contributed to the final revision of the Addendum.
The final Addendum was reviewed and approved by the EP and the CC.
Dissemination of the Addendum to the Guidelines
The final Addendum is published herein and available via the Internet.
Defining the strength of each clinical guideline
The EP has used the verb “recommends” or “suggests” for each clinical recommendation. These words convey the strength of the recommendation, defined as follows:
Recommend is used when the EP strongly recommended for or against a particular course of action.
Suggest is used when the EP weakly recommended for or against a particular course of action.
ADDENDUM TO GUIDELINES
Table I provides a summary of the three addendum guidelines to be used as a quick reference.
Table I.
Summary of Addendum Guidelines 1, 2, and 3
| Addendum Guideline | Infant Criteria | Recommendations | Earliest Age of Peanut Introduction |
|---|---|---|---|
| 1 | Severe eczema, egg allergy or both | Strongly consider evaluation by sIgE and/or SPT, and if necessary an oral food challenge. Based on test results, introduce peanut containing foods | 4 to 6 months |
| 2 | Mild to moderate eczema | Introduce peanut-containing foods | Around 6 months |
| 3 | No eczema or any food allergy | Introduce peanut-containing foods | Age appropriate and in accordance with family preferences and cultural practices |
The EP came to consensus on the following three definitions used throughout the Addendum Guidelines.
Severe eczema is defined as persistent or frequently recurring eczema with typical morphology and distribution, assessed as severe by a health care provider and requiring frequent need for prescription-strength topical corticosteroids, calcineurin inhibitors or other anti-inflammatory agents despite appropriate use of emollients.
Egg allergy is defined as a history of an allergic reaction to egg and a skin prick test wheal diameter of ≥3 mm with egg white extract; or a positive oral egg food challenge.
A specialist is defined as a health care provider with the training and experience to: 1) perform and interpret skin prick testing and oral food challenges; and 2) know and manage their risks. Such individuals must have appropriate medications and equipment on site.
Addendum Guideline 1
The EP recommends that infants with severe eczema, egg allergy or both have introduction of age-appropriate peanut-containing food as early as 4 to 6 months of age to reduce the risk of peanut allergy. Other solid food(s) should be introduced before peanut-containing foods to show that the infant is developmentally ready. The EP recommends that evaluation with peanut-specific IgE and/or skin prick testing be strongly considered before introduction of peanut to determine if peanut should be introduced, and if so, the preferred method of introduction. To minimize a delay in peanut introduction for children who may test negative, testing for peanut-specific IgE may be the preferred initial approach in certain health care settings, e.g., family medicine, pediatrics, or dermatology practices where skin prick testing is not routine. Alternatively, referral for assessment by a specialist may be an option if desired by the health care provider and when available in a timely manner.
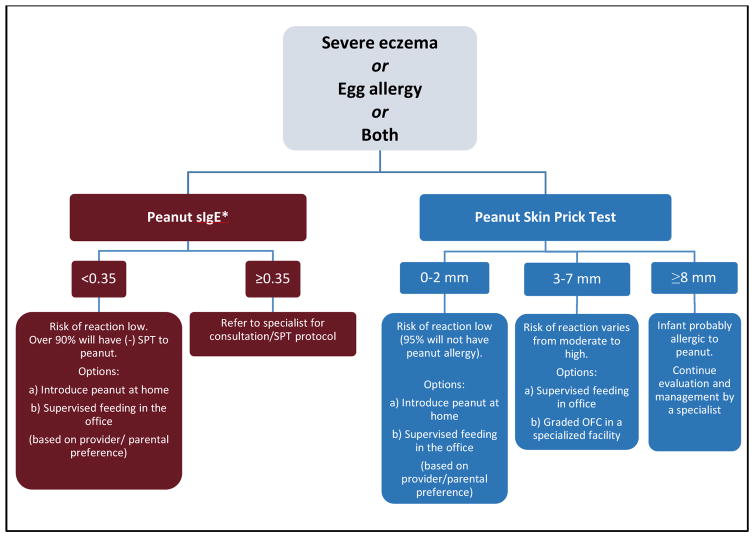
Figure 1 provides recommended approaches for evaluation of children with severe eczema and/or egg allergy before peanut introduction.
Figure 1.
Recommended approaches for evaluation of children with severe eczema and/or egg allergy before peanut introduction
* To minimize a delay in peanut introduction for children who may test negative, testing for peanut-specific IgE may be the preferred initial approach in certain health care settings. Food allergen panel testing or the addition of sIgE testing for foods other than peanut is not recommended due to poor positive predictive value.
Peanut-specific IgE <0.35 kUA/L has strong negative predictive value for the diagnosis of peanut allergy21. Therefore, peanut-specific IgE testing may help in certain health care settings (e.g., family medicine, pediatric, or dermatology practices where skin prick testing is not routine) to reduce unnecessary referrals of children with severe eczema and/or egg allergy and to minimize a delay in peanut introduction for children who may test negative. However, the EP emphasizes that peanut-specific IgE ≥ 0.35 kUA/L lacks adequate positive predictive value for the diagnosis of peanut allergy and an infant with a value ≥ 0.35 kUA/L should be referred to a specialist.
Thus, peanut-specific IgE testing can place an infant into one of 2 categories (Figure 1):
sIgE Category A: If peanut-specific IgE is <0.35 kUA/L (ImmunoCAP), the EP recommends that peanut should be introduced in the diet soon thereafter, with a cumulative first dose of ~2 grams of peanut protein given in this feeding. This can be given as a feeding at home (Appendix D), considering the low likelihood of a severe allergic reaction. If the caregiver or health care provider has concerns, a supervised feeding can be offered at the health care provider’s office (Appendix E).
sIgE Category B: If peanut-specific IgE is ≥0.35 kUA/L (ImmunoCAP), the EP recommends that the child be referred to a specialist for further consultation and possible skin prick testing.
The EP does not recommend food allergen panel testing or the addition of sIgE testing for foods other than peanut because of their poor positive predictive value, which could lead to misinterpretation, over diagnosis of food allergy, and unnecessary dietary restrictions6.
Skin prick testing with peanut extract can place an infant into one of 3 categories (Figure 1):
SPT Category A: If skin prick testing to peanut extract produces a wheal diameter ≤2 mm above saline control, the EP recommends that peanut be introduced in the diet soon after testing, with a cumulative first dose of ~2 grams of peanut protein given in this feeding. This can be given at home (Appendix D), considering the low likelihood of a severe allergic reaction. If the caregiver or health care provider has concerns, a supervised feeding can be offered at the health care provider’s office (Appendix E).
SPT Category B: If skin prick testing to peanut extract produces a wheal diameter of 3 to 7 mm above saline control, the EP suggests that a supervised peanut feeding or a graded oral food challenge (OFC) be undertaken at a specialist’s office or a specialized facility (see Appendices E and G, respectively). Infants in this category can be sensitized without being allergic to peanut and might benefit from early peanut consumption. If the supervised peanut feeding or graded OFC yields no reaction, the EP recommends that peanut should be added to the child’s diet. If the supervised peanut feeding or the graded OFC results in an allergic reaction, the EP recommends that the child should strictly avoid dietary peanut and the family should be counseled regarding food allergy management.
SPT Category C: If skin prick testing produces a wheal diameter ≥8 mm above saline control, the likelihood of peanut allergy is high. Children in this category should continue to be evaluated and managed by a specialist.
It is important to note that the skin prick testing reagents, testing devices, and methodology may differ significantly among health care providers in the United States or elsewhere22. The EP recommends that specialists adjust their skin prick testing categorization criteria according to their own training and experience.
Health care providers conducting oral food challenges in infants with ≥3 mm skin prick tests should be aware that the probability of a positive challenge increases with wheal size. These data come from the HealthNuts Study in children 12 to 18 months of age; of note, the severity of these reactions was relatively mild21,23.
If the decision is made to introduce dietary peanut based on the recommendations of Addendum Guideline 1, the total amount of peanut protein to be regularly consumed per week should be approximately 6 to 7 grams, over 3 or more feedings (see Appendix F). In the LEAP study, at evaluations conducted at 12 and 30 months of age, 75% of children in the peanut consumption group reported eating at least this amount of peanut, based on analysis of a 3-day food diary recorded just prior to the evaluation.
Rationale
Infants with severe eczema and/or egg allergy are at high risk for development of peanut allergy. Significant evidence on this group is available from the infants who participated in the LEAP trial or were screened for the LEAP trial but were not enrolled because of a large SPT (> 4 mm). At 60 months of age, ~23% of peanut avoiders and those infants not enrolled had food allergy24.
Balance of benefits and harms
In the LEAP trial, among the 530 participants in the intention-to-treat population with negative baseline SPT to peanut, 13.7% of the avoidance group and 1.9% of the consumption group had peanut allergy at 60 months of age (P<0.001; a 12.6% absolute risk reduction and an 86.1% relative risk reduction in the prevalence of peanut allergy, resulting in a number needed to treat of 8.5 [number of infants needed to have early introduction of peanut to prevent peanut allergy in one child]). Among the 98 participants with positive peanut SPT at entry, 35.3% of the avoidance group and 10.6% of the consumption group had peanut allergy at 60 months of age (P=0.004; a 24.7% absolute risk reduction and a 70% relative risk reduction in the prevalence of peanut allergy, resulting in a number needed to treat of 4). The LEAP-On study24 demonstrated that the benefits achieved in LEAP persisted when LEAP peanut consumers subsequently avoided peanut for 1 year, from 60 to 72 months of age. This indicates that the oral tolerance achieved in LEAP was durable.
The LEAP trial did not include infants with SPT wheals > 4 mm and, therefore, no data are available on the potential effectiveness of peanut consumption in preventing peanut allergy in this group. However, EP members believe that it is possible that some of these infants may benefit from early introduction of peanut provided that they tolerate oral peanut.
As shown in Figure 1, the EP recommends that infants with peanut-specific IgE < 0.35 kUA/L or with a peanut SPT ≤2mm have dietary peanut introduced as early as 4 to 6 months of age without a need for further evaluation. This recommendation is supported by expert opinion and analysis of the LEAP population findings. In the LEAP trial, infants consuming peanut in this post-hoc defined category had a relative risk reduction of 79% of having peanut allergy at 60 months of age compared to infants who avoided peanut.
In the LEAP trial, at study entry, all infants randomly assigned to the consuming group had a baseline peanut OFC. Out of the 272 infants with no wheal to peanut SPT who received a baseline oral peanut challenge, only one infant had a reaction presenting as an erythematous urticarial rash that was graded as a “moderate” adverse event and was treated successfully with chlorpheniramine. Among the 29 infants with a wheal diameter of 1–2 mm who received a baseline oral peanut challenge, 2 had reactions, which also presented with mild symptoms not requiring treatment with epinephrine. Therefore, for the SPT Category A children, the risk of a severe reaction to peanut at first introduction is low and introduction of peanut at home is an option. It is understandable, however, that some caregivers of infants with severe eczema and/or egg allergy may be uncomfortable introducing dietary peanut at home. In such cases, the health care provider should offer the option of a supervised feeding of a peanut-containing food taking place in the office.
The rate of positive peanut OFC at baseline for infants with 3–4 mm wheal diameter (4 of 17 infants) was higher than in infants with 0–2 mm wheal diameters (3 of 301 infants), but the elicited symptoms were mild. Infants with larger wheal diameters (>4mm) were not included in the LEAP study and, therefore, no safety data are available from this group. However, based on the Australian HealthNuts study, which conducted peanut OFCs on a large number of older (12 to 18 month old) children from the general Australian population, the rate of reactions to peanut is expected to be substantially higher with increasing SPT wheal diameter 21,23. In the HealthNuts study23, a skin prick test wheal diameter of ≥8 mm had a 95% positive predictive value for peanut allergy (positive oral peanut challenge). Therefore, the EP recommends that for SPT Category B infants (3–7 mm SPT wheal diameter) a supervised feeding or a graded peanut OFC be conducted in a specialist’s office or a specialized facility (Appendix G). SPT Category C infants are considered high risk for established allergy to peanut and should not receive peanut-containing foods in their diet, unless such foods are recommended by a specialist after further evaluation.
Quality of evidence
Moderate.
The designation of the Quality of Evidence as “Moderate” (as opposed to “High”) is based on the fact that this recommendation derives primarily from a single randomized, open-label study, the LEAP study. However, it should be noted that the assessment of the LEAP study primary outcome was based on a double blind, placebo controlled, oral food challenge. Furthermore, confidence in this recommendation is bolstered by the large effect size demonstrated in the LEAP study and prior epidemiological data that peanut allergy is relatively infrequent in Israel, where early childhood consumption is common.
Contribution of expert opinion
Significant.
Additional Comments
1) Breastfeeding recommendations
The EP recognizes that early introduction of peanut may seem to depart from recommendations for exclusive breastfeeding through six months of age25,26. However, it should be noted that data from the nutrition analysis of the LEAP cohort27 indicate that introduction of peanut did not affect the duration or frequency of breastfeeding, and did not influence growth or nutrition.
2) Age of peanut introduction
For children with severe eczema and/or egg allergy, the EP recommends that introduction of solid foods begin at 4 to 6 months of age, starting with solid food other than peanut, so that the child can demonstrate the ability to consume solid food without evidence of non-specific signs and symptoms that could be confused with IgE-mediated food allergy. However, it is important to note that the infants in LEAP were enrolled between 4 and 11 months of age and benefitted from peanut consumption regardless of age at entry. Therefore, if the 4 to 6 month time window is missed for any reason including developmental delay, infants may still benefit from early peanut introduction. On the other hand, older age at screening is associated with larger wheal diameter peanut SPT and hence a higher likelihood of established peanut allergy28.
A practical consideration for applying this guideline at 4 to 6 months of age is that infants visit their health care provider for well-child evaluations and infant immunizations at this time. This provides a fortuitous opportunity for eczema evaluation, caregiver reporting of egg allergy, and, if needed, referral to a specialist for peanut allergy evaluation prior to dietary introduction of peanut.
3) Considerations for family members with established peanut allergy
The EP recognizes that many infants eligible for early peanut introduction under this guideline will have older siblings or caregivers with established peanut allergy. The EP recommends that in this situation caregivers discuss with their health care providers the overall benefit (reduced risk of peanut allergy in the infant) vs. risks (potential for further sensitization and accidental exposure of the family member to peanut) of adding peanut to the infant’s diet.
4) Children identified as allergic to peanut
For children who have been identified as allergic to peanut, the EP recommends strict peanut avoidance. This may include those children in SPT Category B who fail the supervised peanut feeding or the OFC, or those children in SPT Category C who, upon further evaluation by a specialist, are confirmed as being peanut allergic. These children should be under long-term management by a specialist.
Addendum Guideline 2
The EP suggests that infants with mild to moderate eczema should have introduction of age-appropriate peanut-containing food around 6 months of age, in accordance with family preferences and cultural practices, to reduce the risk of peanut allergy. Other solid food(s) should be introduced before peanut containing foods to show that the infant is developmentally ready. The EP recommends that infants in this category may have dietary peanut introduced at home without an in-office evaluation. However, the EP recognizes that some caregivers and health care providers may desire an in-office supervised feeding and/or evaluation.
Rationale
The LEAP study did not target infants with mild or moderate eczema. The EP considered the potential risk to benefit ratio of early dietary peanut introduction in infants with mild to moderate eczema and concluded that the individual and societal benefits of introducing peanut in this population would be significant. The EP has no reason to believe that the mechanisms of protection of early dietary peanut differ in infants with mild to moderate eczema from those that lead to protection in infants at higher risk of peanut allergy.
Balance of benefits and harms
The LEAP trial included only infants with severe eczema or egg allergy based on careful medical history. Therefore, some infants who participated in the LEAP trial based on the presence of egg allergy had atopic dermatitis severity scores (SCOring Atopic Dermatitis – SCORADs29) at screen that would have placed them in a moderate or mild eczema category. The EP considered the outcomes of these children and concluded that infants with mild to moderate eczema would likely benefit from early peanut introduction.
Quality of evidence
Low.
The quality of evidence is low because this recommendation is based on extrapolation of data from a single study.
Contribution of expert opinion
Significant.
Additional Comment
Additional support for early introduction of peanut in infants who do not have severe eczema comes from the EAT study17 that enrolled infants from the general population at 3 months of age, and sequentially introduced 6 allergenic beginning at the time of enrollment. These children were not intentionally selected based on increased risk of food allergy or atopy. Although the intention-to-treat group did not show benefit, most likely due to relatively poor compliance with feeding recommendations, the children in the per protocol group who had peanut introduced early in infancy showed a significant reduction in peanut sensitization and peanut allergy at age 3. This study also provides support for Guideline 3 below.
Addendum Guideline 3
The EP suggests that infants without eczema or any food allergy have age-appropriate peanut-containing foods freely introduced in the diet, together with other solid foods, and in accordance with family preferences and cultural practices.
Rationale
No evidence exists for restricting allergenic foods in infants without known risks for food allergy. The probability for development of peanut allergy in such children is very low. However, approximately 14% of all children with peanut allergy at age 12 to 18 months in the HealthNuts Study lacked known risk factors for food allergy.16 Consequently, because such children constitute a significant majority of any birth cohort, they contribute substantially to the overall societal burden of peanut allergy. The EP finds no evidence to suggest that mechanisms of oral tolerance induction would differ in these infants from the immunological mechanisms that are protective in infants at higher risk of peanut allergy. Thus, the early introduction of dietary peanut in children without risk factors for peanut allergy is generally anticipated to be safe and to contribute modestly to an overall reduction in the prevalence of peanut allergy. Furthermore, in countries such as Israel, where peanuts are a popular component of the diet and where they are introduced early in life, the prevalence of peanut allergy is low14.
Balance of benefits and harms
The EP acknowledges that any analysis of benefit and harm in this population relies primarily on expert opinion and is subject to current differences in regional/societal rates of peanut consumption and peanut sensitization. In countries where peanut products are not widely consumed by adults, early dietary introduction of peanut could lead to an increase in sensitization and allergic manifestations. Hence, the EP cautions that this Guideline be implemented in the context of societal routines/norms.
Quality of evidence
Low.
Contribution of expert opinion
Significant.
Clinical Implications.
These guidelines will help health care providers with early introduction of peanut containing foods in infants at various risk levels for peanut allergy. Early introduction of peanut will result in the prevention of peanut allergy in a large number of infants.
Abbreviations used
- CC
Coordinating Committee
- EAT
Enquiring About Tolerance
- EP
Expert Panel
- GRADE
Grading of Recommendations Assessment, Development and Evaluation
- LEAP
Learning Early about Peanut Allergy
- NIAID
National Institute of Allergy and Infectious Diseases
- OFC
Oral food challenge
- SCORAD
SCOring Atopic Dermatitis
- sIgE
Specific Immunoglobulin E
- SPT
Skin prick test
APPENDIX A. COORDINATING COMMITTEE MEMBER ORGANIZATIONS AND REPRESENTATIVES
-
Academy of Nutrition and Dietetics
http://www.eatright.org/ Alison Steiber PhD, RD
-
Allergy & Asthma Network Mothers of Asthmatics (AANMA)
http://www.allergyasthmanetwork.org/main/ Tonya A. Winders, MBA
-
American Academy of Allergy, Asthma & Immunology (AAAAI)
https://www.aaaai.org/home.aspx Hugh A. Sampson, MD
David Fleischer, MD
-
American Academy of Family Physicians (AAFP)
http://www.aafp.org/home.html Jason Matuszak, MD
-
American Academy of Dermatology (AAD)
https://www.aad.org/ Lawrence F. Eichenfield, MD, FAAD
Jon Hanifin, MD
-
American Academy of Emergency Medicine (AAEM)
http://www.aaem.org/ Joseph P. Wood, MD, JD
-
American Academy of Pediatrics (AAP)
https://www.aap.org Scott H. Sicherer, MD, FAAP
-
American Academy of Physician Assistants (AAPA)
https://www.aapa.org/ Gabriel Ortiz, MPAS, PA-C, DFAAPA
-
American College of Allergy, Asthma and Immunology (ACAAI)
http://acaai.org/ Amal Assa’ad, MD
-
American College of Gastroenterology (ACG)
http://gi.org/ Steven J. Czinn, MD, FACG
-
American Partnership for Eosinophilic Disorders (APFED)
http://apfed.org/ Wendy Book, MD
-
American Society for Nutrition (ASN)
http://www.nutrition.org/ George J. Fuchs, III, MD
-
Asthma and Allergy Foundation of America (AAFA)
http://www.aafa.org/ Meryl Bloomrosen, MBA, MBI
David R. Stukus, MD
-
Canadian Society of Allergy and Clinical Immunology (CSACI)
http://www.csaci.ca/ Edmond Chan, MD, FRCPC
-
Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD)
https://www.nichd.nih.gov Gilman Grave, MD
-
European Academy of Allergy and Clinical Immunology (EAACI)
http://www.eaaci.org/ Antonella Muraro, MD, PhD
-
Food Allergy Research & Education (FARE)
https://www.foodallergy.org/ James R. Baker, MD
Mary Jane Marchisotto
-
National Eczema Association
http://nationaleczema.org/ Julie Block
-
National Heart, Lung, and Blood Institute (NHLBI)
http://www.nhlbi.nih.gov/ Janet M. de Jesus, MS, RD
-
National Institute of Allergy and Infectious Diseases (NIAID)
http://www.niaid.nih.gov/ Daniel Rotrosen, MD
Alkis Togias, MD
Marshall Plaut, MD
-
National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS)
http://www.niams.nih.gov/ Ricardo Cibotti, PhD
-
National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
www.niddk.nih.gov Frank Hamilton, MD, MPH
Margaret A. McDowell, PhD, MPH, RD (retired)
Rachel Fisher, MS, MPH, RD
-
North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN)
http://www.naspghan.org/ Glenn Furuta, MD
-
Society of Pediatric Nurses (SPN)
http://www.pedsnurses.org/ Michele Habich, DNP, APN/CNS, CPN
-
United States Department of Agriculture (USDA)
http://www.usda.gov/ Soheila J. Maleki, PhD
-
World Allergy Organization
http://www.worldallergy.org/ Lanny J. Rosenwasser, MD
APPENDIX B: EXPERT PANEL, JUNE 2015
Chair
-
Joshua A. Boyce, MD
Professor of Medicine and Pediatrics
Harvard Medical School
Director, Inflammation and Allergic Disease Research Section
Director, Jeff and Penny Vinik Center for Allergic Disease Research
Specialty: Allergy/pediatric pulmonology
Panelists
-
Maria Acebal, JD
Board of Directors, Food Allergy Research & Education
Member of NIAID Advisory Council
Former CEO of Food Allergy and Anaphylaxis Network
Specialty: Advocacy
-
Amal Assa’ad, MD
Professor, University of Cincinnati Department of Pediatrics
Director, FARE Center of Excellence in Food Allergy
Director of Clinical Services, Division of Allergy and Immunology
Associate Director, Division of Allergy and Immunology
Cincinnati Children’s Hospital Medical Center
Specialty: Allergy/pediatrics
-
James R. Baker Jr, MD
CEO and Chief Medical Officer
Food Allergy Research & Education, McLean VA
Founding Director, Mary H. Weiser Food Allergy Center, University of Michigan
Professor of Internal Medicine, Division of Allergy and Clinical Immunology
University of Michigan Health System
Specialty: Allergy/advocacy/education
-
Lisa A. Beck, MD
Professor, Department of Dermatology
University of Rochester Medical Center
School of Medicine and Dentistry
Specialty: Dermatology
-
Julie Block
President and CEO
National Eczema Association
Specialty: Advocacy/education
-
Carol Byrd-Bredbenner, PhD, RD, FAND
Professor of Nutrition/Extension Specialist
Rutgers University, School of Environmental and Biological Sciences
Specialty: Nutrition/health communication/behavior science
-
Edmond S. Chan, MD, FRCPC
Clinical Associate Professor
Head, Division of Allergy and Immunology
Department of Pediatrics
BC Children’s Hospital
University of British Columbia
Specialty: Allergy/pediatrics
-
Lawrence F. Eichenfield, MD
Professor of Pediatrics and Dermatology
Chief, Pediatric and Adolescent Dermatology
Rady Children’s Hospital, San Diego
University of California, San Diego School of Medicine
Specialty: Dermatology/pediatrics
-
David M. Fleischer, MD
Associate Professor of Pediatrics
University of Colorado School of Medicine
Children’s Hospital Colorado, Aurora, CO
Specialty: Allergy/pediatrics
-
George J. Fuchs III, MD
Professor of Pediatrics
University of Kentucky College of Medicine
Chief, Gastroenterology, Nutrition & Hepatology
Kentucky Children’s Hospital
Specialty: Gastroenterology/pediatrics
-
Glenn T. Furuta, MD
Professor of Pediatrics
Director, Gastrointestinal Eosinophilic Diseases Program
University of Colorado School of Medicine
Children’s Hospital Colorado, Aurora, CO
Specialty: Gastroenterology/pediatrics
-
Matthew J. Greenhawt, MD MBA, MSc
Assistant Professor of Pediatrics
Allergy Section
University of Colorado School of Medicine
Children’s Hospital Colorado, Aurora, CO
Specialty: Allergy/pediatrics
-
Ruchi Gupta, MD, MPH
Associate Professor of Pediatrics and Medicine
Director, Food Allergy Outcomes Research Program
Ann and Robert H. Lurie Children’s Hospital of Chicago
Northwestern Medicine, Northwestern University
Specialty: Pediatrics
-
Michele Habich, DNP, APN/CNS, CPN
Advanced Practice Nurse
Northwestern Medicine, Central DuPage Hospital
Specialty: Nursing/pediatrics/education
-
Stacie M. Jones, MD
Professor of Pediatrics
University of Arkansas for Medical Sciences
Chief, Allergy and Immunology
Arkansas Children’s Hospital
Specialty: Allergy/pediatrics
-
Kari Keaton
Facilitator, Metro DC Food Allergy Support Group
Specialty: Advocacy/education
-
Antonella Muraro, MD, PhD
President of European Academy of Allergy and Clinical Immunology (EAACI)
Professor of Allergy and Pediatric Allergy
Head of the Veneto Region Food Allergy Centre of Excellence for Research and Treatment
University Hospital of Padua, Italy
Specialty: Allergy/pediatrics
-
Lanny J. Rosenwasser, MD
Immediate Past President, World Allergy Organization
Professor of Medicine
University of Missouri-Kansas City-School of Medicine
Specialty: Allergy/pediatrics
-
Hugh A. Sampson, MD
Professor of Pediatrics, Allergy and Immunology
Icahn School of Medicine at Mount Sinai
Director, Jaffe Food Allergy Institute
Specialty: Allergy/pediatrics
-
Lynda C. Schneider, MD
Professor of Pediatrics
Harvard Medical School
Director, Allergy Program
Boston Children’s Hospital
Specialty: Allergy/pediatrics
-
Scott H. Sicherer, MD
Professor Pediatrics, Allergy and Immunology
Icahn School of Medicine at Mount Sinai
Division Chief, Pediatric Allergy and Immunology
Specialty: Allergy/pediatrics
-
Robert Sidbury, MD, MPH
Professor
Department of Pediatrics
Chief, Division of Dermatology
Seattle Children’s Hospital
University of Washington School of Medicine
Specialty: Dermatology/pediatrics
-
Jonathan Spergel, MD, PhD
Stuart Starr Professor of Pediatrics
Chief, Allergy Section
Director, Center for Pediatric Eosinophilic Disorders
The Children’s Hospital of Philadelphia
Perelman School of Medicine, University of Pennsylvania
Specialty: Allergy/pediatrics
-
David R. Stukus, MD
Assistant Professor of Pediatrics
Section of Allergy/Immunology
Nationwide Children’s Hospital
Columbus, OH
Specialty: Allergy/pediatrics
-
Carina Venter, PhD, RD
Allergy Specialist, Dietitian
Cincinnati Children’s Hospital Medical Center
University of Cincinnati College of Medicine
Specialty: Allergy/dietitian/pediatrics
APPENDIX C: TIER 1 REFERENCES
- Feeney M, Du Toit G, Roberts R, Sayre PH, Lawson K, Bahnson HT, et al. Impact of peanut consumption in The LEAP Study: feasibility, growth and nutrition. J Allergy Clin Immunol. 2016 Jun 8; doi: 10.1016/j.jaci.2016.04.016. pii: S0091-6749(16)30262-7. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koplin JJ, Peters RL, Dharmage SC, Gurrin L, Tang MLK, Ponsonby AL, et al. Understanding the feasibility and implications of implementing early peanut introduction for prevention of peanut allergy. J Allergy Clin Immunol. 2016 May 2; doi: 10.1016/j.jaci.2016.04.011. pii: S0091-6749(16)30195-6. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Perkin MR, Logan K, Tseng A, Raji B, Ayis S, Peacock J, et al. Randomized Trial of Introduction of Allergenic Foods in Breast-Fed Infants. N Engl J Med. 2016 May 5;374(18):1733–43. doi: 10.1056/NEJMoa1514210. Epub 2016 Mar 4. [DOI] [PubMed] [Google Scholar]
- Du Toit G, Sayre PH, Roberts G, Sever ML, Lawson K, Bahnson HT, et al. Effect of Avoidance on Peanut Allergy after Early Peanut Consumption. N Engl J Med. 2016 Apr 14;374(15):1435–43. doi: 10.1056/NEJMoa1514209. Epub 2016 Mar 4. [DOI] [PubMed] [Google Scholar]
- Chang YS, Trivedi MK, Jha A, Lin YF, Dimaano L, García-Romero MT. Synbiotics for Prevention and Treatment of Atopic Dermatitis: A Meta-analysis of Randomized Clinical Trials. JAMA Pediatr. 2016 Mar 1;170(3):236–42. doi: 10.1001/jamapediatrics.2015.3943. [DOI] [PubMed] [Google Scholar]
- O’Connor C, Kelleher M, O’B Hourihane J. Calculating the effect of population-level implementation of the Learning Early About Peanut Allergy (LEAP) protocol to prevent peanut allergy. J Allergy Clin Immunol. 2016 Apr;137(4):1263–1264. e2. doi: 10.1016/j.jaci.2015.11.029. Epub 2016 Feb 10. [DOI] [PubMed] [Google Scholar]
- Grimshaw KE, Bryant T, Oliver EM, Martin J, Maskell J, Kemp T, et al. Incidence and risk factors for food hypersensitivity in UK infants: results from a birth cohort study. Clin Transl Allergy. 2016 Jan 26;6:1. doi: 10.1186/s13601-016-0089-8. eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovitch N, Shah D, Lanser BJ. Look before you LEAP: Risk of anaphylaxis in high-risk infants with early introduction of peanut. J Allergy Clin Immunol. 2015 Sep;136(3):822. doi: 10.1016/j.jaci.2015.07.002. Epub 2015 Aug 5. [DOI] [PubMed] [Google Scholar]
- Peters RL, Allen KJ, Dharmage SC, Lodge CJ, Koplin JJ, Ponsonby AL, et al. Differential factors associated with challenge-proven food allergy phenotypes in a population cohort of infants: a latent class analysis. Clin Exp Allergy. 2015 May;45(5):953–63. doi: 10.1111/cea.12478. [DOI] [PubMed] [Google Scholar]
- Peters RL, Allen KJ, Dharmage SC, Koplin JJ, Dang T, Tilbrook KP, et al. Natural history of peanut allergy and predictors of resolution in the first 4 years of life: A population-based assessment. J Allergy Clin Immunol. 2015 May;135(5):1257–1266. e2. doi: 10.1016/j.jaci.2015.01.002. Epub 2015 Feb 26. [DOI] [PubMed] [Google Scholar]
- Du Toit G, Roberts G, Sayre PH, Bahnson HT, Radulovic S, Santos AF, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015 Feb 26;372(9):803–13. doi: 10.1056/NEJMoa1414850. Epub 2015 Feb 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PE, Eckert JK, Koplin JJ, Lowe AJ, Gurrin LC, Dharmage SC, et al. Which infants with eczema are at risk of food allergy? Results from a population-based cohort. Clin Exp Allergy. 2015 Jan;45(1):255–64. doi: 10.1111/cea.12406. [DOI] [PubMed] [Google Scholar]
- Grimshaw KE, Maskell J, Oliver EM, Morris RC, Foote KD, Mills EN, et al. Introduction of complementary foods and the relationship to food allergy. Pediatrics. 2013 Dec;132(6):e1529–38. doi: 10.1542/peds.2012-3692. Epub 2013 Nov 18. [DOI] [PubMed] [Google Scholar]
- Palmer DJ, Metcalfe J, Makrides M, Gold MS, Quinn P, West CE, et al. Early regular egg exposure in infants with eczema: A randomized controlled trial. J Allergy Clin Immunol. 2013 Aug;132(2):387–92. e1. doi: 10.1016/j.jaci.2013.05.002. Epub 2013 Jun 26. [DOI] [PubMed] [Google Scholar]
- Du Toit G, Roberts G, Sayre PH, Plaut M, Bahnson HT, Mitchell H, et al. Identifying infants at high risk of peanut allergy: the Learning Early About Peanut Allergy (LEAP) screening study. J Allergy Clin Immunol. 2013 Jan;131(1):135–43. e1–12. doi: 10.1016/j.jaci.2012.09.015. Epub 2012 Nov 19. [DOI] [PubMed] [Google Scholar]
- Joseph CL, Ownby DR, Havstad SL, Woodcroft KJ, Wegienka G, MacKechnie H, et al. Early complementary feeding and risk of food sensitization in a birth cohort. J Allergy Clin Immunol. 2011 May;127(5):1203–10. e5. doi: 10.1016/j.jaci.2011.02.018. Epub 2011 Apr 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koplin JJ, Osborne NJ, Wake M, Martin PE, Gurrin LC, Robinson MN, et al. Can early introduction of egg prevent egg allergy in infants? A population-based study. J Allergy Clin Immunol. 2010 Oct;126(4):807–13. doi: 10.1016/j.jaci.2010.07.028. [DOI] [PubMed] [Google Scholar]
- Katz Y, Rajuan N, Goldberg MR, Eisenberg E, Heyman E, Cohen A, Leshno M. Early exposure to cow’s milk protein is protective against IgE-mediated cow’s milk protein allergy. J Allergy Clin Immunol. 2010 Jul;126(1):77–82. e1. doi: 10.1016/j.jaci.2010.04.020. Epub 2010 Jun 11. [DOI] [PubMed] [Google Scholar]
- Du Toit G, Katz Y, Sasieni P, Mesher D, Maleki SJ, Fisher HR, et al. Early consumption of peanuts in infancy is associated with a low prevalence of peanut allergy. J Allergy Clin Immunol. 2008 Nov;122(5):984–91. doi: 10.1016/j.jaci.2008.08.039. [DOI] [PubMed] [Google Scholar]
APPENDIX D. INSTRUCTIONS FOR HOME FEEDING OF PEANUT PROTEIN FOR INFANTS AT LOW RISK OF AN ALLERGIC REACTION TO PEANUT
These instructions for home feeding of peanut protein are provided by your doctor. You should discuss any questions that you have with your doctor before starting. These instructions are meant for feeding infants who have severe eczema or egg allergy and were allergy tested (blood test and/or skin test) with results that your doctor considers safe for you to introduce peanut protein at home (low risk of allergy).
GENERAL INSTRUCTIONS
Feed your infant only when he/she is healthy; do not do the feeding if he or she has a cold, vomiting, diarrhea, or other illness.
Give the first peanut feeding at home, not at a day care facility or a restaurant.
Make sure at least one adult will be able to focus all of his or her attention on the infant, without distractions from other children or household activities.
Make sure that you will be able to spend at least 2 hours with your infant after the feeding to watch for any signs of an allergic reaction.
FEEDING YOUR INFANT
Prepare a full portion of one of the peanut-containing food from the recipe options below.
Offer your infant a small part of the peanut serving on the tip of a spoon.
Wait 10 minutes.
If there is no allergic reaction after this small taste, then slowly give the remainder of the peanut-containing food at the infant’s usual eating speed.
WHAT ARE SYMPTOMS OF AN ALLERGIC REACTION? WHAT SHOULD I LOOK FOR?
-
Mild symptoms may include
-
a new rash
or
a few hives around the mouth or face
-
-
More severe symptoms may include any of the following alone or in combination:
lip swelling
vomiting
widespread hives (welts) over the body
face or tongue swelling,
any difficulty breathing
wheeze
repetitive coughing,
change in skin color (pale, blue)
sudden tiredness/lethargy/seeming limp
If you have any concerns about your infant’s response to peanut, seek immediate medical attention/call 911.
FOUR RECIPE OPTIONS, EACH CONTAINING ~2 GRAMS OF PEANUT PROTEIN
Note: Teaspoons and tablespoons are US measures (5 ml and 15 ml, for a level teaspoon or tablespoon, respectively)
Option 1: Bamba®, 21 pieces (~2 g of peanut protein):
Note: Bamba® is named because it was the product used in the LEAP study and therefore has proven efficacy and safety. Other peanut puffs products with similar peanut protein content may be substituted.
For infants less than seven months of age, soften the Bamba® with 4–6 teaspoons of water.
For older infants who can manage dissolvable textures, unmodified Bamba® may be fed. If dissolvable textures are not yet part of the infant’s diet, softened Bamba® should be provided.
Option 2: Thinned Smooth Peanut Butter:
Measure 2 teaspoons (9–10g peanut butter; ~2 g peanut protein) of peanut butter and slowly add 2–3 teaspoons hot water.
Stir until peanut butter is dissolved and thinned and well blended.
Let cool.
Increase water amount if necessary (or add previously tolerated infant cereal) to achieve consistency comfortable for the infant.
Option 3: Smooth Peanut Butter Puree:
Measure 2 teaspoons (9–10g peanut butter; ~2g peanut protein) of peanut butter.
Add 2–3 tablespoons of pureed tolerated fruit or vegetables to peanut butter. You may increase or reduce volume of puree to achieve desired consistency.
Option 4: Peanut Flour and Peanut Butter Powder
Note: Peanut flour and peanut butter powder are two distinct products that may be interchanged because they have very similar peanut protein content per 10g.
Measure 2 teaspoons (4g peanut flour or 4 g of peanut butter powder; ~2g peanut protein) of peanut flour or peanut butter powder.
Add ~2 tablespoons (6 to 7 teaspoons) of pureed tolerated fruit or vegetables to flour or powder. You may increase or reduce volume of puree to achieve desired consistency.
APPENDIX E. FOR HEALTH CARE PROVIDERS: IN OFFICE SUPERVISED FEEDING PROTOCOL USING 2 G OF PEANUT PROTEIN
GENERAL INSTRUCTIONS
-
These recommendations are reserved for an infant defined in Guideline 1 as one with severe eczema and/or egg allergy and with negative or minimally reactive (1 to 2 mm) SPTs and/or peanut-specific IgE <0.35 kUA/L. They also may apply to the infant with a 3 to 7mm SPT if the specialist care provider decides to conduct a supervised feeding in the office (as opposed to a graded oral food challenge in a specialized facility.) (See Figure 1).
These recommendations may also be followed for infants with mild to moderate eczema, as defined in Guideline 2, when caregivers and health care providers may desire an in-office supervised feeding.
-
Proceed only if the infant shows no evidence of any concomitant illness such as an upper respiratory infection.
Start with a small portion of the initial peanut serving, e.g., tip of teaspoon of peanut butter puree/softened Bamba.
Wait 10 minutes; if there is no sign of reaction after this small portion is given, then continue gradually feeding the remaining serving of peanut-containing food (see options below) at the infant’s typical feeding pace.
Observe the infant for 30 minutes after 2 g peanut protein ingestion for signs/symptoms of an allergic reaction.
FOUR RECIPE OPTIONS, EACH CONTAINING ~2 GRAMS OF PEANUT PROTEIN
NOTE: Teaspoons and tablespoons are US measures (5 ml and 15 ml, for a level teaspoon or tablespoon, respectively)
Option 1: Bamba®, 21 pieces (~2 g of peanut protein):
Note: Bamba® is named because it was the product used in the LEAP study and therefore has known peanut protein content and proven efficacy and safety. Other peanut puffs products with similar peanut protein content may be substituted for Bamba®.
For infants less than seven months of age, soften the Bamba® with 4 to 6 teaspoons of water.
For older infants who can manage dissolvable textures, unmodified Bamba® may be fed. If dissolvable textures are not yet part of the infant’s diet, softened Bamba® should be provided.
Option 2: Thinned Smooth Peanut Butter:
Measure 2 teaspoons (9 to 10 g peanut butter; ~2 g peanut protein) of peanut butter and slowly add 2 to 3 teaspoons hot water.
Stir until peanut butter is dissolved and thinned and well blended.
Let cool.
Increase water amount if necessary (or add previously tolerated infant cereal) to achieve consistency comfortable for the infant.
Option 3: Smooth Peanut Butter Puree:
Measure 2 teaspoons (9 to 10g peanut butter; ~2g peanut protein) of peanut butter.
Add 2 to 3 tablespoons of previously tolerated pureed fruit or vegetables to peanut butter. You may increase or reduce volume of puree to achieve desired consistency.
Option 4: Peanut Flour and Peanut Butter Powder
Note: Peanut flour and peanut butter powder are two distinct products that may be interchanged because they have, on average, a similar peanut protein content per 10g.
Measure 2 teaspoons (4g peanut flour or 4 g of peanut butter powder; ~2g peanut protein) of peanut flour or peanut butter powder.
Add ~2 tablespoons (6 to 7 teaspoons) of pureed tolerated fruit or vegetables to flour or powder. You may increase or reduce volume of puree to achieve desired consistency.
APPENDIX F. PEANUT PROTEIN IN PEANUT-CONTAINING FOODS
If the decision is made to introduce dietary peanut in the infant’s diet, the total amount of peanut protein to be regularly consumed per week should be approximately 6 to 7 grams, over 3 or more feedings. In the LEAP study, at evaluations conducted at 12 and 24 months of age, 75% of children in the peanut consumption group reported eating at least this amount of peanut.
BE AWARE OF CHOKING RISKS
Whole nuts should not be given to children under 5 years of age.
Peanut butter directly from a spoon or in lumps/dollops should not be given to children under 4 years.
If, after a week or more eating peanut, your infant or child displays mild allergic symptoms within 2 hours of eating peanut, you should contact your healthcare provider.
Typical peanut-containing foods, their peanut protein content, and feeding tips for infants are provided in Table S-I, and their nutritional content is found in Table S-I
Notes:
Bamba® is named because it was the product used in the LEAP study and therefore has known peanut protein content and proven efficacy and safety. Other peanut puffs products with similar peanut protein content may be substituted for Bamba®.
Teaspoons and tablespoons are US measures (5 ml and 15 ml, for a level teaspoon or tablespoon, respectively)
TABLE S-I.
TYPICAL PEANUT-CONTAINING FOODS, THEIR PEANUT PROTEIN CONTENT, AND FEEDING TIPS FOR INFANTS
| Bamba® | Peanut Butter | Peanuts | Peanut Flour or Peanut Butter Powder | |
|---|---|---|---|---|
| Amount containing approximately 2 grams peanut protein | 17 g or 2/3 of a 28 g (1oz) bag or 21 ‘sticks’ |
9 to10 g or 2 teaspoons |
8 g or ~10 whole peanuts (2 ½ teaspoons of grounded |
4 g or 2 teaspoons |
| Typical serving size | 1 bag (28 g) | Spread on a slice of bread or toast (16 g) | 2 ½ teaspoons ground peanuts (8 g) | No typical serving size |
| Peanut protein per typical serving | 3.2 g | 3.4 g | 2.1 g | No typical serving size |
| Feeding Tips | For a smooth texture, mix with warm water (then let cool) or the infant’s milk and mash well. Pureed or mashed fruit or vegetables can be added. Older children can be offered sticks of Bamba®. |
For a smooth texture, mix with warm water (then let cool) or breastmilk or infant formula. For older children, mix with pureed or mashed fruit or vegetables or any suitable family foods such as yoghurt or mashed potatoes. |
Use blender to create a powder or paste. 2 – 2 ½ teaspoons ground peanuts can be added to a portion of yogurt or pureed fruit or savory meal. |
Mix with yogurt or apple sauce. |
TABLE S-II. NUTRITIONAL CONTENT OF PEANUT CONTAINING FOODS.
Note: The nutritional content of peanut puff products (other than Bamba®) can be obtained from their manufacturers.
| Per approx. 2 g peanut protein: | Bamba® (17 g) | Peanut butter (10 g) | Peanuts (8 g) | Peanut butter powder (4 g) | Peanut flour (4 g) |
|---|---|---|---|---|---|
| kcal | 93 | 59 | 45 | 15 | 13 |
| Sugar (g) | 0.4 | 0.65 | 0.38 | 0.4 | 0.33 |
| Salt (mg) | 68 | 48 | 1 | 31 | 7 |
| Fat (g) | 6.1 | 4.95 | 3.94 | 0.49 | 0.02 |
APPENDIX G. GRADED ORAL FOOD CHALLENGE PROTOCOL
From “Conducting an Oral Food Challenge to Peanut in an Infant: A Work Group Report30.”
GENERAL INSTRUCTIONS
A graded oral food challenge (OFC) should be performed only by a specialist (a health care provider with the training and experience to: 1) perform and interpret skin prick testing and oral food challenges; and 2) know and manage their risks; such individuals must have appropriate medications, and equipment on site).
-
Four peanut preparations are provided:
Option 1: Smooth peanut butter mixed with either a previously tolerated pureed fruit or vegetable.
Option 2: Smooth peanut butter dissolved carefully with hot water and cooled.
Option 3: Peanut flour mixed with either a previously tolerated pureed fruit or vegetable. Peanut butter powder may be used instead of the peanut flour.
-
Option 4: Bamba® peanut snack (Osem Group) dissolved in hot water and cooled or even as a solid (i.e. as a stick).
Note: Bamba® is named because it was the product used in the LEAP study and therefore has known peanut protein content and proven efficacy and safety. Other peanut puffs products with similar peanut protein content may be substituted for Bamba®.
The peanut protein content of the graded OFC protocol is identical for all peanut preparations, except that the volume of food ingested per dose is different. The protocol consists of 5 incremental doses, given 15 to 20 minutes apart, with a cumulative peanut protein total of ~4g as per the 3.9 g total in the LEAP trial.
Refer to Table S-III and direct parents to discontinue specific medications for the prescribed amount of time prior to the graded OFC. Note that certain medications are allowed.
BE PREPARED IN CASE OF A SEVERE REACTION
SEE TABLE S-IV
Note: Teaspoons and tablespoons are US measures (5 ml and 15 ml, for a level teaspoon or tablespoon, respectively)
OPTION 1: MEASURES FOR SMOOTH PEANUT BUTTER PUREE
| Dose | Peanut butter volume* | Equivalent weight peanut butter (g) (Peanut protein content (g))** | Pureed fruit or vegetable volume | Total volume |
|---|---|---|---|---|
| 1 | 1/8 teaspoon | 0.67 (0.15) | ½ teaspoon | 5/8 teaspoon |
| 2 | ¼ teaspoon | 1.33 (0.29) | 3/4 teaspoon | 1 teaspoons |
| 3 | ½ teaspoon | 2.67 (0.59) | 1 teaspoons | 1 ½ teaspoons |
| 4 | 1 teaspoon | 5.33 (1.17) | 2 teaspoons | 3 teaspoons*** |
| 5 | 1 ½ teaspoons | 8 (1.6) | 4 teaspoons | 5 ½ teaspoons |
| Total protein: 3.96g |
The amounts (volume) of peanut butter measured as teaspoons are approximate measures to keep the dosing as practical as possible
The peanut protein content is calculated on the average amount of protein for a range of butters using “Report: 16167, USDA Commodity, Peanut Butter, smooth,” from the USDA Nutrition Database (http://ndb.nal.usda.gov/ndb/foods)
3 teaspoons = 1 tablespoon
OPTION 2: MEASURES FOR SMOOTH THINNED PEANUT BUTTER
| Dose | Peanut butter volume * | Equivalent weight peanut butter (g) (Peanut protein content (g))** | Volume of hot water | Total volume |
|---|---|---|---|---|
| 1 | 1/8 teaspoon | 0.67 (0.15) | 1/8 teaspoon | ¼ teaspoon |
| 2 | ¼ teaspoon | 1.33 (0.29) | ¼ teaspoon | ½ teaspoon |
| 3 | ½ teaspoon | 2.67 (0.59) | ½ teaspoon | 1 teaspoon |
| 4 | 1 teaspoon | 5.33 (1.17) | 1 teaspoon | 2 teaspoons |
| 5 | 1 ½ teaspoons | 8 (1.76) | 1 ½ teaspoons | 3 teaspoons*** |
| Total protein: 3.96g |
The amounts (volume) of peanut butter measured as teaspoons are approximate measures to keep the dosing as practical as possible.
The peanut protein content is calculated on the average amount of protein for a range of butters using “Report: 16167, USDA Commodity, Peanut Butter, smooth,” from the USDA Nutrition Database (http://ndb.nal.usda.gov/ndb/foods).
3 teaspoons = 1 tablespoon
OPTION 3: MEASURES FOR PEANUT FLOUR OR PEANUT BUTTER POWDER
| Dose | Peanut flour or peanut butter powder volume* | Equivalent weight peanut flour or peanut butter powder** (g) (Peanut protein content (g) | Pureed fruit or vegetable volume | Total volume |
|---|---|---|---|---|
| 1 | 1/8 teaspoon | 0.25 (0.13) | ½ teaspoon | 3/4 teaspoon |
| 2 | 1/4 teaspoon | 0.5 (0.25) | 1 teaspoon | 1 1/4 teaspoons |
| 3 | 1/2 teaspoon | 1.0 (0.5) | 2 teaspoons | 2 1/2 teaspoons |
| 4 | 1 teaspoon | 2.0 (1.0) | 3 teaspoons *** | 4 teaspoons |
| 5 | 2 teaspoons | 4.0 (2.0) | 6 teaspoons **** | 8 teaspoons |
| Total protein: 3.88g |
The amounts (volume) of peanut flour or peanut butter powder measured as teaspoons are approximate measures to keep the dosing as practical as possible
The information regarding peanut powder and flour reflects averages obtained from the producers. Most brands of peanut flour/peanut butter powder are approximately 50% peanut protein by weight. However, weight may vary based on the fat content and also the brand chosen. Therefore a weight measurement may be more accurate than household measurements.
3 teaspoons = 1 tablespoon
6 teaspoons = 2 tablespoons
PROTOCOL INSTRUCTIONS FOR OPTIONS 1, 2, AND 3
Measure peanut butter, peanut flour, or peanut butter powder for Dose 1.
-
Prepare the first dose:
If using Option 1, add previously tolerated pureed fruit or vegetable to measured Dose 1 peanut butter and stir until well-blended. You may increase or reduce volume of puree to achieve desired consistency. Note: increasing the volume may increase the difficulty of getting through entire protocol with a young baby.
If using Option 2, slowly add hot water to measured Dose 1 peanut butter and stir until peanut butter is dissolved, thinned, and well-blended. Let the mixture cool. You may increase water volume (or add previously tolerated infant cereal) to achieve desired consistency.
If using Option 3, add previously tolerated pureed fruit or vegetable to measured Dose 1 peanut flour or peanut butter powder and stir until well-blended. You may increase or reduce volume of puree to achieve desired consistency. Note: increasing the volume may increase the difficulty of getting through entire protocol with a young baby.
Label Dose 1.
Repeat steps 1 to 3 for the remaining Doses 2 through 5, labeling each dose appropriately and before proceeding to the preparation of the next dose.
Feed Dose 1 to infant and observe for symptoms of reactivity for 15 to 20 minutes.
If no symptoms appear, repeat with Dose 2 and observe for 15 to 20 minutes
Continue in this manner with Doses 3, 4, and 5.
OPTION 4: BAMBA® PEANUT SNACK (OSEM GROUP)
Note: Other peanut puffs products with equivalent peanut protein content may be substituted for Bamba®.
| Dose | Bamba® # sticks | Equivalent weight (Peanut protein content (g)***) | Volume of hot water (approximate, will need to be adjusted for each child) | Approximate final volume |
|---|---|---|---|---|
| 1 | 1 stick | 0.81 (0.1) | 1/2 teaspoon | 3/4 teaspoons |
| 2 | 3 sticks | 2.43 (0.3) | 1 teaspoon | 1 ½ teaspoons |
| 3 | 5 sticks | 4.05 (0.5) | 1 ½ teaspoons | 2 ¼ teaspoons |
| 4 | 10 sticks | 8.1 (1.0) | 3 teaspoons | 4 teaspoons |
| 5 | 21 sticks | 17.01 (2.0) | 6 teaspoons | 7 ½ teaspoons |
| TOTAL protein: 3.9g |
The amount of Bamba® sticks are approximate measures looking at a range of Bamba® products i.e., we have found that Bamba® snacks from different parts of the world have a varied peanut protein content. The peanut protein content of Bamba® was calculated according to the publication by Du Toit et al.13
PROTOCOL INSTRUCTIONS FOR OPTION 4
Count Bamba® sticks for Dose 1.
-
Prepare the first dose:
Slowly add hot water to measured Bamba® and stir until Bamba® is dissolved, thinned, well-blended, and cooled. You may increase water volume to achieve desired consistency. Note: increasing the volume may increase the difficulty of getting through entire protocol with a young baby.
Label Dose 1.
Repeat steps 1 to 3 for the remaining Doses 2 through 5, labeling each dose appropriately and before proceeding to the preparation of the next dose.
Feed Dose 1 to infant and observe for symptoms of reactivity for 15 to 20 minutes.
If no symptoms appear, repeat with Dose 2 and observe for 15 to 20 minutes
Continue in this manner with Doses 3, 4, and 5.
TABLE S-III.
MEDICATION DISCONTINUATION CONSIDERATIONS PRIOR TO OFC
| Medication | Last dose before OFC |
|---|---|
| Cetirizine | 5 days |
| Cyproheptadine | 10 days |
| Diphenhydramine | 3 days |
| Fexofenadine | 3 days |
| Loratadine | 7 days |
| Short-acting bronchodilator (e.g. albuterol) | 8 hours |
| MEDICATIONS THAT MAY BE CONTINUED | |
| Antihistamine eye drops | |
| Inhaled/intranasal corticosteroids | |
| Topical (cutaneous) steroids | |
| Topical (cutaneous) pimecrolimus, tacrolimus |
TABLE S-IV.
EMERGENCY MEDICATIONS FOR A SEVERE REACTION DURING AN OFFICE-BASED INFANT ORAL FOOD CHALLENGE
| MEDICATION | DOSE | |
|---|---|---|
| First-line treatment | Epinephrine (1:1000 concentration) | 0.01 mg/kg IM in the mid- outer thigh in health care settings or 0.15 mg auto-injector IM in the mid-outer thigh in community settings |
| Adjunctive treatment | Albuterol nebulization | 0.15 mg/kg every 20 minutes × 3 doses (minimum of 2.5 mg per dose) over 5 to 15 minutes |
| Albuterol MDI inhalation | 2 puffs, 90 mcg/puff, with face mask | |
| Oxygen | 8 – 10 L/min via face mask | |
| Diphenhydramine | 1.25 mg/kg/dose orally | |
| Cetirizine | 2.5 mg orally | |
| Normal saline (0.9% isotonic solution) or lactated ringers | 20 ml/kg/dose administered over 5 minutes intravenously | |
| Steroids | Prednisolone 1 mg/kg orally or Solumedrol 1 mg/kg intravenously |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sicherer SH, Muñoz-Furlong A, Burks AW, Sampson HA. Prevalence of peanut and tree nut allergy in the US determined by a random digit dial telephone survey. J Allergy Clin Immunol. 1999 Apr;103(4):559–62. doi: 10.1016/s0091-6749(99)70224-1. [DOI] [PubMed] [Google Scholar]
- 2.Gupta RS, Springston EE, Warrier MR, Smith B, Kumar R, Pongracic J, Holl JL. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. 2011 Jul;128(1):e9–17. doi: 10.1542/peds.2011-0204. [DOI] [PubMed] [Google Scholar]
- 3.Bunyavanich S, Rifas-Shiman SL, Platts-Mills TA, Workman L, Sordillo JE, Gillman MW, et al. Peanut allergy prevalence among school-age children in a US cohort not selected for any disease. J Allergy Clin Immunol. 2014 Sep;134(3):753–5. doi: 10.1016/j.jaci.2014.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bock SA, Muñoz-Furlong A, Sampson HA. Fatalities due to anaphylactic reactions to foods. J Allergy Clin Immunol. 2001 Jan;107(1):191–3. doi: 10.1067/mai.2001.112031. [DOI] [PubMed] [Google Scholar]
- 5.Bock SA, Muñoz-Furlong A, Sampson HA. Further fatalities caused by anaphylactic reactions to food, 2001–2006. J Allergy Clin Immunol. 2007 Apr;119(4):1016–8. doi: 10.1016/j.jaci.2006.12.622. [DOI] [PubMed] [Google Scholar]
- 6.Boyce JA, Assa’ad A, Burks AW, Jones SM, Sampson HA, Wood RA, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010 Dec;126(6 Suppl):S1–58. doi: 10.1016/j.jaci.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment. Peanut Allergy. 1998 http://cot.food.gov.uk/sites/default/files/cot/cotstatement200807peanut.pdf.
- 8.American Academy of Pediatrics, Committee on Nutrition. Hypoallergenic infant formulas. Pediatrics. 2000 Aug;106(2):346–349. [PubMed] [Google Scholar]
- 9.Greer FR, Sicherer SH, Burks AW American Academy of Pediatrics Committee on Nutrition; American Academy of Pediatrics Section on Allergy and Immunology. Effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, timing of introduction of solid foods, and hydrolyzed formulas Pediatrics. 2008 Jan;121(1):183–91. doi: 10.1542/peds.2007-3022. [DOI] [PubMed] [Google Scholar]
- 10.Chan ES, Cummings C Canadian Paediatric Society, Community Paediatrics Committee and Allergy Section. Dietary exposures and allergy prevention in high-risk infants: A joint statement with the Canadian Society of Allergy and Clinical Immunology. Paediatr Child Health. 2013 Dec;18(10):545–54. doi: 10.1093/pch/18.10.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleischer DM, Spergel JM, Assa’ad AH, Pongracic JA. Primary prevention of allergic disease through nutritional interventions. J Allergy Clin Immunol Pract. 2013 Jan;1(1):29–36. doi: 10.1016/j.jaip.2012.09.003. Epub 2012 Nov 22. [DOI] [PubMed] [Google Scholar]
- 12.Muraro A, Halken S, Arshad SH, Beyer K, Dubois AE, Du Toit G, et al. Allergy. 2014 May;69(5):590–601. doi: 10.1111/all.12398. Epub 2014 Apr 3. [DOI] [PubMed] [Google Scholar]
- 13.Du Toit G, Roberts G, Sayre PH, Bahnson HT, Radulovic S, Santos AF, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015 Feb 26;372(9):803–13. doi: 10.1056/NEJMoa1414850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du Toit G, Katz Y, Sasieni P, Mesher D, Maleki SJ, Fisher HR, et al. Early consumption of peanuts in infancy is associated with a low prevalence of peanut allergy. J Allergy Clin Immunol. 2008 Nov;122(5):984–91. doi: 10.1016/j.jaci.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 15.Fleischer DM, Sicherer S, Greenhawt M, Campbell D, Chan E, Muraro A, et al. Consensus Communication on Early Peanut Introduction and Prevention of Peanut Allergy in High-Risk Infants. J Allergy Clin Immunol. 2015 Aug;136(2):258–61. doi: 10.1016/j.jaci.2015.06.001. Epub 2015 Jun 20. [DOI] [PubMed] [Google Scholar]
- 16.Du Toit G, Sayre PH, Roberts G, Sever ML, Lawson K, Bahnson HT, et al. Effect of Avoidance on Peanut Allergy after Early Peanut Consumption. N Engl J Med. 2016 Apr 14;374(15):1435–43. doi: 10.1056/NEJMoa1514209. Epub 2016 Mar 4. [DOI] [PubMed] [Google Scholar]
- 17.Perkin MR, Logan K, Tseng A, Raji B, Ayis S, Peacock J, et al. Randomized Trial of Introduction of Allergenic Foods in Breast-Fed Infants. N Engl J Med. 2016 May 5;374(18):1733–43. doi: 10.1056/NEJMoa1514210. Epub 2016 Mar 4. [DOI] [PubMed] [Google Scholar]
- 18.GRADE working group [Internet 2000-present] http://www.gradeworkinggroup.org.
- 19.Schünemann HJ, Oxman AD, Brozek J, Glasziou P, Jaeschke R, Vist GE, et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ. 2008 May 17;336(7653):1106–10. doi: 10.1136/bmj.39500.677199.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brozek JL, Akl EA, Alonso-Coello P, Lang D, Jaeschke R, Williams JW, et al. Grading quality of evidence and strength of recommendations in clinical practice guidelines. Part 1 of 3. An overview of the GRADE approach and grading quality of evidence about interventions. Allergy. 2009 May;64(5):669–77. doi: 10.1111/j.1398-9995.2009.01973.x. [DOI] [PubMed] [Google Scholar]
- 21.Koplin JJ, Peters RL, Dharmage SC, Gurrin L, Tang MLK, Ponsonby AL, et al. Understanding the feasibility and implications of implementing early peanut introduction for prevention of peanut allergy. J Allergy Clin Immunol. 2016 May 2; doi: 10.1016/j.jaci.2016.04.011. pii: S0091-6749(16)30195–6. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 22.Tversky JR, Chelladurai Y, McGready J, Hamilton RG. Performance and Pain Tolerability of Current Diagnostic Allergy Skin Prick Test Devices. J Allergy Clin Immunol Pract. 2015 Nov-Dec;3(6):888–93. doi: 10.1016/j.jaip.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 23.Peters RL, Allen KJ, Dharmage SC, Tang ML, Koplin JJ, Ponsonby AL, et al. Skin prick test responses and allergen-specific IgE levels as predictors of peanut, egg, and sesame allergy in infants. J Allergy Clin Immunol. 2013 Oct;132(4):874–80. doi: 10.1016/j.jaci.2013.05.038. Epub 2013 Jul 24. [DOI] [PubMed] [Google Scholar]
- 24.Du Toit G, Tsakok T, Lack S, Lack G. Prevention of food allergy. J Allergy Clin Immunol. 2016 Apr;137(4):998–1010. doi: 10.1016/j.jaci.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 25.American Academy of Pediatrics. Breastfeeding and the use of human milk. Policy statement. Pediatrics. 2012 Mar;129(3) http://pediatrics.aappublications.org/content/129/3/e827.full.pdf+html. [Google Scholar]
- 26.The World Health Organization’s infant feeding recommendation. 2001 May 1; http://www.who.int/nutrition/topics/infantfeeding_recommendation/en/index.html.
- 27.Feeney M, Du Toit G, Roberts R, Sayre PH, Lawson K, Bahnson HT, et al. Impact of peanut consumption in The LEAP Study: feasibility, growth and nutrition. J Allergy Clin Immunol. 2016 Jun 8; doi: 10.1016/j.jaci.2016.04.016. pii: S0091-6749(16)30262–7. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Du Toit G, Roberts G, Sayre PH, Plaut M, Bahnson HT, et al. Identifying infants at high risk of peanut allergy: the Learning Early About Peanut Allergy (LEAP) screening study. J Allergy Clin Immunol. 2013 Jan;131(1):135–43. e1–12. doi: 10.1016/j.jaci.2012.09.015. Epub 2012 Nov 19. [DOI] [PubMed] [Google Scholar]
- 29.Severity scoring of atopic dermatitis: the SCORAD index. Consensus Report of the European Task Force on Atopic Dermatitis. Dermatology. 1993;186(1):23–31. doi: 10.1159/000247298. [DOI] [PubMed] [Google Scholar]
- 30.Bird JA, Groetch M, Allen KJ, Bock SA, Leonard A, Nowak-Wegrzyn AH, et al. Conducting an Oral Food Challenge to Peanut in an Infant: A Work Group Report. American Academy of Allergy, Asthma and Immunology. 2016 doi: 10.1016/j.jaip.2016.07.019. Accepted for publication. (Note to publisher - To be published in JACI In Practice and will be published before the Addendum. Will send the eventual citation) [DOI] [PubMed] [Google Scholar]