Abstract
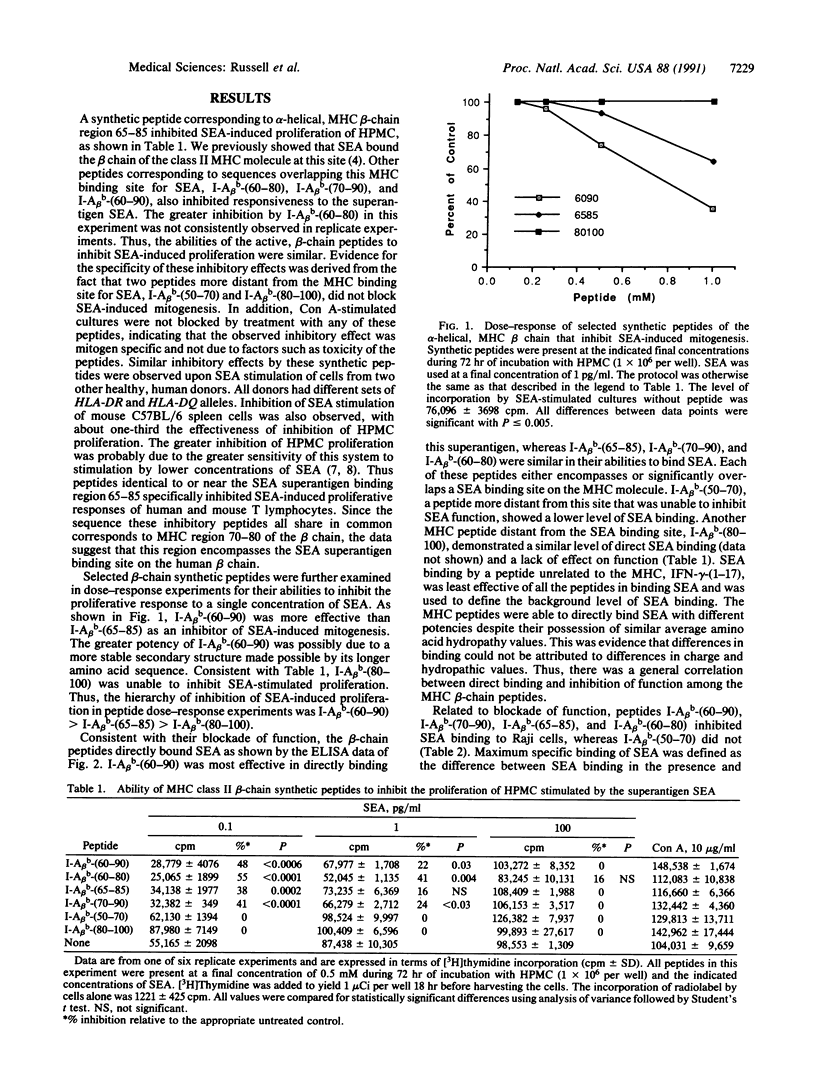
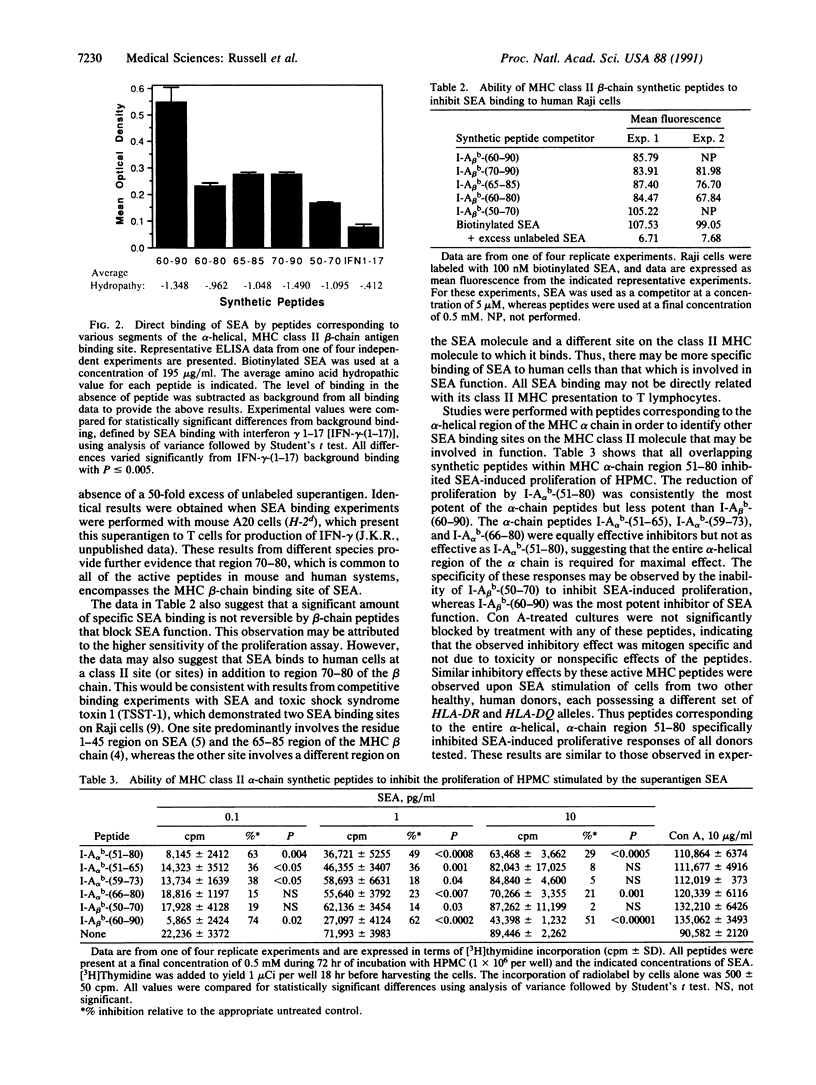
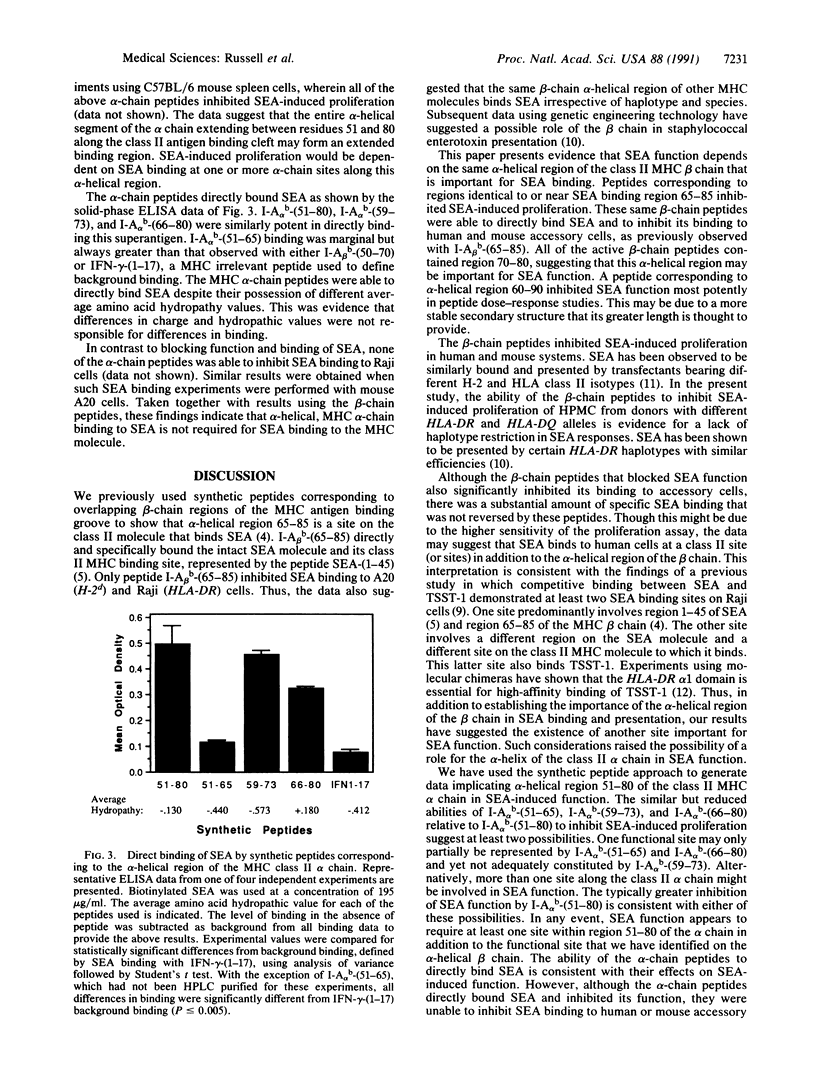
The superantigen staphylococcal enterotoxin A (SEA) requires interaction with class II major histocompatibility complex (MHC) molecules to activate T cells. We have previously used the synthetic peptide approach to establish one side of the hypothetical class II foreign-antigen binding cleft, alpha-helical region 65-85 of the beta chain, as a binding site involved in accessory cell presentation of SEA to T cells. To further characterize the structural basis for MHC-SEA interaction we have examined the role of the alpha-helical regions of the class II alpha and beta chains in SEA function. Using the synthetic peptide approach, we have found that both alpha-helical regions are required for SEA-induced proliferation. Their corresponding peptides directly bound SEA. Although the beta-chain peptides were able to inhibit SEA binding to human and mouse cells, the alpha-chain peptides were not. The data suggest that the alpha-helices along both sides of the hypothetical class II MHC molecule binding cleft are required for SEA-induced function, whereas the beta-chain alpha-helix is sufficient for SEA binding. A model of superantigen presentation is proposed wherein the MHC beta chain, possibly region 70-80, interacts with SEA region 1-45, whereas another region of SEA binds region 51-80 of the alpha chain.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987 Oct 8;329(6139):506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. The foreign antigen binding site and T cell recognition regions of class I histocompatibility antigens. Nature. 1987 Oct 8;329(6139):512–518. doi: 10.1038/329512a0. [DOI] [PubMed] [Google Scholar]
- Brown J. H., Jardetzky T., Saper M. A., Samraoui B., Bjorkman P. J., Wiley D. C. A hypothetical model of the foreign antigen binding site of class II histocompatibility molecules. Nature. 1988 Apr 28;332(6167):845–850. doi: 10.1038/332845a0. [DOI] [PubMed] [Google Scholar]
- Chang C. D., Meienhofer J. Solid-phase peptide synthesis using mild base cleavage of N alpha-fluorenylmethyloxycarbonylamino acids, exemplified by a synthesis of dihydrosomatostatin. Int J Pept Protein Res. 1978 Mar;11(3):246–249. doi: 10.1111/j.1399-3011.1978.tb02845.x. [DOI] [PubMed] [Google Scholar]
- Dellabona P., Peccoud J., Kappler J., Marrack P., Benoist C., Mathis D. Superantigens interact with MHC class II molecules outside of the antigen groove. Cell. 1990 Sep 21;62(6):1115–1121. doi: 10.1016/0092-8674(90)90388-u. [DOI] [PubMed] [Google Scholar]
- Herman A., Croteau G., Sekaly R. P., Kappler J., Marrack P. HLA-DR alleles differ in their ability to present staphylococcal enterotoxins to T cells. J Exp Med. 1990 Sep 1;172(3):709–717. doi: 10.1084/jem.172.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp D. R., Teletski C. L., Scholl P., Geha R., Long E. O. The alpha 1 domain of the HLA-DR molecule is essential for high-affinity binding of the toxic shock syndrome toxin-1. Nature. 1990 Aug 2;346(6283):474–476. doi: 10.1038/346474a0. [DOI] [PubMed] [Google Scholar]
- Langford M. P., Stanton G. J., Johnson H. M. Biological effects of staphylococcal enterotoxin A on human peripheral lymphocytes. Infect Immun. 1978 Oct;22(1):62–68. doi: 10.1128/iai.22.1.62-68.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollick J. A., Chintagumpala M., Cook R. G., Rich R. R. Staphylococcal exotoxin activation of T cells. Role of exotoxin-MHC class II binding affinity and class II isotype. J Immunol. 1991 Jan 15;146(2):463–468. [PubMed] [Google Scholar]
- Pontzer C. H., Russell J. K., Jarpe M. A., Johnson H. M. Site of nonrestrictive binding of SEA to class II MHC antigens. Int Arch Allergy Appl Immunol. 1990;93(2-3):107–112. doi: 10.1159/000235288. [DOI] [PubMed] [Google Scholar]
- Pontzer C. H., Russell J. K., Johnson H. M. Structural basis for differential binding of staphylococcal enterotoxin A and toxic shock syndrome toxin 1 to class II major histocompatibility molecules. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):125–128. doi: 10.1073/pnas.88.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. K., Pontzer C. H., Johnson H. M. The I-A beta b region (65-85) is a binding site for the superantigen, staphylococcal enterotoxin A. Biochem Biophys Res Commun. 1990 Apr 30;168(2):696–701. doi: 10.1016/0006-291x(90)92377-c. [DOI] [PubMed] [Google Scholar]