Abstract
Objective
The antioxidant N-Acetylcysteine (NAC) is being increasingly investigated as a therapeutic agent in the treatment of substance use disorders. Preclinical and clinical findings suggest that NAC normalizes extracellular glutamate by restoring the activity of glutamate transporters and antiporters in the nucleus accumbens. This study explored the efficacy of NAC in the treatment of post-traumatic stress disorder (PTSD), which frequently co-occurs with substance use disorders (SUD) and shares impaired prefrontal cortex regulation of basal ganglia circuitry, in particular at glutamate synapses in the nucleus accumbens.
Method
Veterans with current PTSD and SUD (N=35) were randomly assigned to receive a double-blind, 8-week course of NAC (2400 mg/day) or placebo plus outpatient group cognitive-behavioral therapy for SUD. Primary outcome measures included PTSD symptoms (Clinician Administered PTSD Scale, PTSD Checklist-Military) and craving (Visual Analogue Scale). Depression (Beck Depression Inventory-II) and substance use (Timeline Follow Back, urine drug screens) were also assessed.
Results
Participants treated with NAC, as compared to placebo, evidenced significant improvements in PTSD symptoms, craving, and depression. Substance use at the start of treatment was low for both the NAC and placebo groups and no significant between-group differences were observed. NAC was well tolerated and retention was high.
Conclusions
This is the first randomized controlled trial to investigate NAC as a pharmacological treatment for PTSD. The findings show a significant treatment effect on symptoms of PTSD and drug craving, and provide initial support for the use of NAC in combination with cognitive-behavioral therapy among individuals with co-occurring PTSD and SUD.
Keywords: N-acetylcysteine, PTSD, posttraumatic stress disorder, substance use disorders, military, Veterans
Introduction
Posttraumatic stress disorder (PTSD) is the most common mental health disorder among Veterans presenting for treatment at Veterans Affairs (VA) hospitals (1) and 45.7% of military personnel with PTSD remain symptomatic three years after discontinuing service (2). Data from the Department of Veterans Affairs indicate that, among Veterans serving in the Vietnam era or later (N=1,001,996), 41.4% with a substance use disorder (SUD) meet criteria for current PTSD (3). Veterans with co-occurring PTSD/SUD have poorer treatment outcomes on multiple indices of functioning, including more social and legal problems, suicide attempts, and violence, and they undergo more episodes of substance abuse treatment (4).
Pharmacological treatment for co-occurring PTSD/SUD is not well explored. Only serotonin specific reuptake inhibitors (SSRIs) have received Food and Drug Administration (FDA) approval for treatment of PTSD, and SSRIs are of limited efficacy for Veteran populations with comorbid PTSD/SUD (5–6). Investigations of other pharmacologic treatments of co-occurring PTSD/SUD have had suboptimal results (7–10). The antioxidant N-acetylcysteine (NAC) has been used in the treatment of acetaminophen overdose for over three decades (11), and is being increasingly investigated as a therapeutic agent in the treatment of a variety of psychiatric disorders that share impaired executive functioning, impulse control, and top-down regulation, including addiction (i.e., cannabis, cocaine, nicotine), pathological gambling, and trichotillomania (11–13). Preclinical and clinical research suggests that NAC normalizes extracellular glutamate by restoring the activity of glutamate transporters and antiporters in the nucleus accumbens (14). Animal models demonstrate that chronic SUD down-regulates the glial glutamate transporter (GLT-1, EAAT-2) in the nucleus accumbens, and treatment with NAC restores this transporter thereby normalizing synaptic glutamate transmission (12).
PTSD and SUD share impaired prefrontal cortex regulation of basal ganglia circuitry, in particular at glutamate synapses in the nucleus accumbens (15–18). Neuroimaging studies demonstrate that individuals with PTSD exhibit hypoactive executive functioning (prefrontal cortex) and hyperactive fear circuitry (amygdala) activity (19–21), and significant uncoupling between the prefrontal cortex and amygdala at rest and during symptom provocation (22–23). Individuals with SUD also demonstrate attenuated prefrontal-amygdala functional connectivity (18, 24), which is a marker of early relapse risk (25). This converging evidence suggests that the prefrontal cortex of individuals with PTSD/SUD is less able to disrupt compulsive anxiety-related and drug-seeking behaviors that are driven, in part, by intrusive thoughts about stressful events and/or cravings to use drugs (18, 26).
In light of the capacity of NAC to restore normal glutamatergic synaptic physiology in prefrontal cortex projections and to inhibit drug use in animal models and human addiction, as well as the shared prefrontal impairments identified in PTSD and SUD, we examined NAC as a candidate pharmacotherapy for comorbid PTSD/SUD. We conducted a double-blind, randomized placebo-controlled pilot trial of NAC plus group cognitive-behavioral therapy for SUD among U.S. military Veterans with PTSD and SUD.
Method
Trial Design
Treatment-seeking Veterans with PTSD and SUD were randomly assigned, in a 1:1 parallel group allocation, to receive a double-blind, 8-week course of NAC or placebo, along with group cognitive behavioral therapy for SUD. A one-month assessment following medication discontinuation was conducted. The Investigational New Drug application for this study was approved by the FDA. All study procedures were approved by the institutional review boards and were in accordance with the Helsinki Declaration of 1975.
Participants
The sample was comprised of 35 Veterans enrolled in the outpatient Substance Abuse Treatment Clinic (SATC) at the Ralph H. Johnson VA Medical Center in Charleston, SC. Participants were 18–65 year old; U.S. military Veteran, Reservist, or member of the National Guard; met DSM-IV (27) diagnostic criteria for current (past 6 months) SUD, and PTSD or subthreshold PTSD (i.e., met criteria for cluster B (re-experiencing) and either cluster C (avoidance) or D (hyperarousal), as well as duration of one month and clinically significant impairment); score > 21 on the Mini-Mental State Exam (MMSE) (28). Participants were excluded for unstable medical conditions, diagnosis of bipolar or psychotic disorders, history of seizures or asthma, prior treatment with NAC, suicidality, or if they were enrolled in ongoing PTSD treatment (pharmacotherapy or psychosocial). Female participants could not be pregnant or lactating, and had to use adequate contraception during the trial.
General Procedures
After receiving a complete description of the study, participants provided written informed consent. Veterans were recruited through clinical referral and local advertisements. After being screened for initial eligibility, interested Veterans completed a comprehensive baseline assessment that involved diagnostic interviews, history and physical examination, assessment of concomitant medications, self-report questionnaires, breathalyzer and urine drug screen (UDS) tests, and pregnancy test for women. Eligible participants were randomized after at least one week of abstinence from alcohol and drugs, as verified by self-report, UDS test, and breathalyzer test. Participants came into the office twice weekly for visits. Vital signs and concomitant medications were assessed weekly. Study medication was dispensed each week and participants were asked to return the prior week’s medication container in order to assess medication compliance. After the 8-week trial, participants completed a one-month follow-up visit (Week 12). Participants received $510 for completing all visits.
Interventions
Medication
Eligible participants were randomized to either NAC (2400 mg/day) or placebo for 8 weeks. The starting dose of NAC was 1200 mg twice daily (2400 mg/day). One participant achieved target dose at week 4 (1200 mg/day for first 4 weeks). Equivalent numbers of identical appearing placebo capsules were dispensed. All NAC and placebo capsules contained riboflavin 25 mg, which was used as a biomarker for medication compliance. Participants who wished to take a multivitamin during the trial were given a multivitamin (Tri-Vi-Sol) that does not contain riboflavin. Weekly pill counts and documentation of missed doses were recorded each week.
Study medications (USP-grade NAC and matched placebo capsules) were compounded and packaged by Pitt Street Pharmacy in Charleston, South Carolina, and dispensed to participants by the medical clinician or study staff. Treatment assignment followed a pre-arranged randomization scheme and was carried out by study personnel at the pharmacy (i.e., personnel not involved in clinical management of participants to preserve the double-blind design).
Cognitive Behavioral Therapy for SUD
Participants were enrolled in the SATC and received weekly, structured group therapy targeting addiction. Topics included, for example, cognitive restructuring, coping with cravings, drink/drug refusal, and relaxation skills. Enrollment in SATC ensured that all participants received adequate psychosocial support and monitoring, regardless of medication arm. It also provided a standardized behavioral platform, which has been shown to enhance statistical power and precision of analyses by restricting variability resulting from varied types of interventions (29). Importantly, participants did not receive any psychosocial interventions targeting PTSD during the trial.
Measures
Participants completed a demographic form, which included information about their service history, and the MMSE (28) at baseline. The Mini International Neuropsychiatric Interview (MINI) (30) assessed SUD and other psychiatric diagnoses at baseline.
The Clinician Administered PTSD Scale (CAPS) assessed traumatic life events, PTSD diagnosis and symptom severity (31). The CAPS was administered at baseline, mid- and post-treatment, and follow up. The PTSD Checklist-Military (PCL-M), a 17-item self-report measure of PTSD symptoms, was administered weekly (32). The Timeline Follow Back (TLFB), a self-report, calendar-based instrument, measured the frequency of alcohol and drug use (i.e., percent of day using) (33). It was administered at baseline (past 60 days) and weekly during the study. Visual Analog Scales (VAS), which consist of 100-mm lines with anchoring statements at both ends, were used to assess craving over the past week. Participants rated 3 items (amount, intensity and frequency) using anchors of 0 mm representing “not at all” and 100 mm representing “extreme” or “all the time.” UDS tests (CLIAwaived 6 Panel), for THC/marijuana, cocaine, opiates, methamphetamines, amphetamines and benzodiazepines were collected weekly. Alcohol breathalyzer tests measured blood alcohol concentration each week.
The Beck Depression Inventory (BDI)-II, a 21-item self-report questionnaire, measured depressive symptoms weekly (34). The Columbia Suicide Severity Rating Scale (C-SSRS) was conducted at baseline for study eligibility (35). Adverse events (AEs) were assessed weekly using a 6-item checklist measuring the most common AEs associated with NAC as indicated on the FDA-approved label for NAC (Anjinomoto North America Inc.).
Statistical Analyses
The primary hypothesis was that NAC treatment would significantly decrease PTSD symptoms and drug craving (from baseline to week 8, adjusting for baseline). Secondary analyses examined the effect of NAC on depression symptoms and substance use). Analyses were conducted using SPSS Version 22 (IBM Corp.). Demographic characteristics were compared using chi-square tests for categorical variables and t-tests for continuous variables. A series of hierarchical linear regression analyses were conducted to examine the effects of treatment group (NAC or placebo) on PTSD symptomatology (PCL-M, CAPS), craving (VAS variables), depression (BDI-II), and substance use (TLFB, UDS). The primary outcome measure was entered as the criterion variable. Baseline levels of the respective outcome measures were entered in Step 1 of the model in order to adjust for baseline differences. Treatment group was entered as the predictor in Step 2 of the model. Changes in the primary outcome measures were examined from baseline to end of treatment (Week 8), and baseline to one-month follow-up (Week 12). This was a pilot study with adequate power to detect within-group changes on key outcome measures. Thus, we conducted a series of paired samples t-tests to examine within-group changes in PTSD symptoms, depressive symptoms, craving, and substance use. The significance threshold was set at a p-value of .05 (two-sided) for all statistical tests.
Results
Study Retention and Medication Compliance
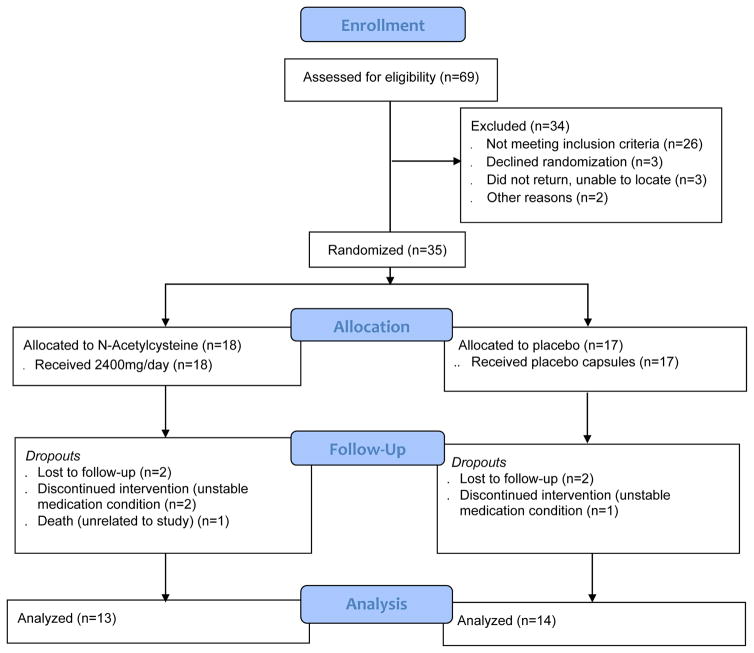
Participants were enrolled between March, 2013 and April, 2014. Figure 1 illustrates the CONSORT flow diagram. Sixty-nine participants completed the baseline assessment for eligibility and 34 were excluded. Thus, 35 participants were randomized (18 to NAC, 17 to placebo). The majority of participants (77%) completed the 8-week treatment phase and there were no group differences in retention (13/18 NAC, 14/17 placebo). Similarly, 12/18 (67%) in the NAC group and 12/17 (71%) in the placebo group completed the one month follow-up visit. Of the eight participants that did not complete the study, five were lost to follow-up, two were excluded due to unstable medical conditions, and one participant died of cardiovascular collapse, judged to be unrelated to the study. No participants dropped out secondary to adverse events related to study medication. Medication compliance (i.e., ≥1000 ng/ml of riboflavin and greater than baseline at ≥ 2/3 assessment time points) was 82.9% and did not differ significantly by group. This resulted in 13 participants in the NAC treated group and 14 in the placebo group who were included in the final per-protocol analyses of treatment effect.
Figure 1.
Enrollment and Study Flow of Veterans with PTSD and SUD assigned to N-Acetylcysteine or placebo
Demographics
Table 1 presents the demographic characteristics for the sample. Participants were primarily male (96.3%) with an average age of 49 years old. The most common service branches were the Army (44.4%) and Navy (22.2%), and the average length of service was 80.9 months. No significant group differences were identified.
Table 1. Baseline Demographic and Clinical Characteristics among Participants in the N-Acetylcysteine and Placebo Groups.
| Placebo (n=14) | NAC (n=13) | Total (N=27) | |
|---|---|---|---|
|
| |||
| Variable | % | % | % |
| Gender (Male) | 100.0% | 92.3% | 96.3% |
| Race (Caucasian) | 28.6% | 30.8% | 29.6% |
| Race (African-American) | 71.4% | 69.2% | 70.4% |
| Relationship (Married) | 21.4% | 23.1% | 22.2% |
| Education (At Least Some College) | 42.8% | 69.2% | 55.5% |
| Unemployed/Retired/Disabled | 64.3% | 92.3% | 77.7% |
| Trauma Type | |||
| Military, combat | 14.3% | 23.1% | 18.5% |
| Military, non-combat | 28.6% | 38.5% | 33.3% |
| Civilian-related event(s) | 57.1% | 38.5% | 48.1% |
| Substance Use Disorders | |||
| Alcohol Use Disorder | 85.7% | 76.9% | 81.5% |
| Drug Use Disorder | 71.4% | 84.6% | 77.8% |
|
| |||
| M (SD) | M (SD) | M (SD) | |
|
| |||
| Age (Years) | 49.9 (8.1) | 48.2 (8.6) | 49.0 (8.2) |
| PCL-M Total Score | 43.4 (18.6) | 45.7 (14.6) | 44.5 (16.5) |
| CAPS Total Score | 68.6 (23.7) | 58.8 (21.2) | 63.8 (22.6) |
| Re-experiencing Subscale | 21.9 (7.3) | 18.8 (9.5) | 20.4 (8.4) |
| Avoidance Subscale | 25.5 (12.5) | 18.3 (8.3) | 22.0 (11.1) |
| Hyperarousal Subscale | 21.2 (6.97) | 21.8 (7.5) | 21.5 (7.1) |
| BDI-II Total Score | 22.8 (13.1) | 19.1(6.7) | 21.0 (10.5) |
Note. NAC = N-Acetylcysteine; PCL-M = PTSD Checklist, Military; CAPS = Clinician Administered PTSD Scale; BDI-II = Beck Depression Inventory Second Edition.
PTSD Symptoms
PTSD symptomatology was quantified using the PCL-M total score, CAPS total score, and the CAPS subscale scores (re-experiencing, CAPS-R; avoidance, CAPS-A; hyperarousal, CAPS-H). Table 2 includes the means and standard deviations for these measures.
Table 2. Efficacy of N-Acetylcysteine Treatment in Symptoms of PTSD, Depression and Craving.
| Placebo (n = 14)Within-Group Outcomes | NAC (n = 13) Within-Group Outcomes | Between Groups β | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Weeks | Weeks | Weeks | |||||||
| Measure | 0 | 4 | 8 | 0 | 4 | 8 | 0 – 8 | ||
| PCL-M | 43.4 (18.6) | 41.9 (21.7) | 41.9 (22.8) | 45.7 (14.6) | 33.8 (10.6)a | 31.2 (9.7)a | −.355* | ||
| CAPS | 68.6 (23.7) | 52.8 (36.9)a | 51.5 (43.1) | 58.8 (21.2) | 38.7 (20.0)a | 32.0 (23.5)a | −.127 | ||
| CAPS-R | 21.9 (7.3) | 15.6 (10.2)a | 12.4 (13.1)a | 18.8 (9.5) | 12.6 (6.9)a | 10.1 (8.1)a | −.119 | ||
| CAPS-A | 25.5 (12.5) | 21.6 (16.1) | 20.4 (19.6) | 18.3 (8.3) | 10.5 (9.1)a | 10.7 (9.2)a | −.330 | ||
| CAPS-H | 21.2 (6.9) | 15.6 (11.9) | 13.6 (13.0) | 21.8 (7.5) | 15.6 (8.3)a | 11.8 (9.5)a | −.194 | ||
| BDI -II | 22.8 (13.1) | 18.5 (14.8) | 19.3 (15.8) | 19.1 (6.7) | 10.9 (6.4) | 9.9 (6.7)a | −.325* | ||
| Craving- A | 4.1 (3.1) | 2.8 (2.6) | 2.8 (2.8) | 3.7 (3.4) | 1.8 (1.9)a | 0.7 (0.7)a | −.413* | ||
| Craving- F | 4.2 (3.2) | 2.4 (2.3) | 3.0 (2.9) | 3.6 (3.0) | 1.8 (2.0)a | 1.0 (0.9)a | −.387* | ||
| Craving-I | 3.7 (3.0) | 2.9 (2.8) | 2.8 (3.1) | 3.7 (3.1) | 1.8 (2.1) | 1.3 (1.9)a | −.288 | ||
Note. Table includes means (standard deviation). NAC = N-Acetylcysteine; PCL-M = PTSD Checklist, Military; CAPS = Clinician Administered PTSD Scale total score; CAPS-R = CAPS re-experiencing subscale; CAPS-A = CAPS avoidance subscale; CAPS-H = CAPS hyperarousal subscale; BDI-II = Beck Depressive Inventory; Craving-A = amount of craving; Craving-F = frequency of craving; Craving-I = intensity of craving.
Superscript indicates a significant (p<.05) within-group difference as compared to baseline (week 0).
p<.05 indicates a between-group difference at the end of treatment (week 8) as compared to baseline (week 0).
Self-Report PTSD Symptoms
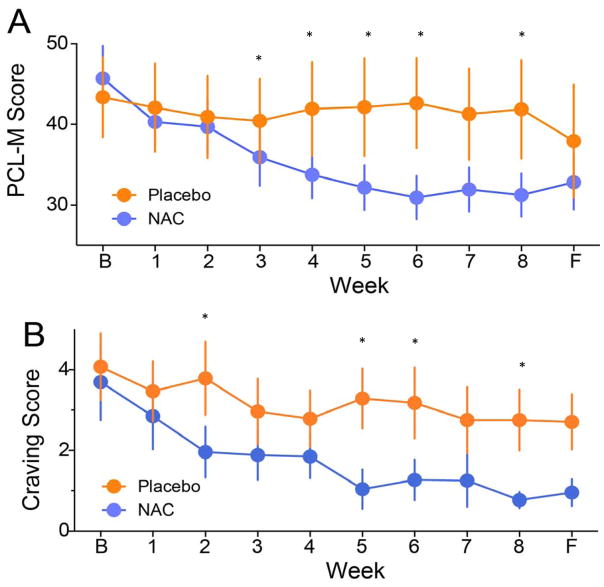
As shown in Figure 2A, the PCL-M total score was reduced by 32% in the NAC group from baseline to Week 8 (45.7 to 31.2) as compared to 3% in the placebo group (43.4 to 41.9). The adjusted regression model examining the efficacy of NAC as compared to placebo on reduction of PCL-M total scores at the end of treatment (Week 8) accounted for 73.8% of the variance (F (2, 24) = 33.89, p < .001), with the NAC group having significantly lower scores on the PCL-M at Week 8 than the placebo group (β = −.36, p < .01). Examination of follow-up (Week 12) PCL-M total score revealed a trend toward a significant reduction by NAC (β = −.70, p = .06).
Figure 2. Change in PTSD Symptoms and Drug Craving Over Time by Treatment Condition.
A) Weekly total score on the PTSD Military Checklist (PCL-M). NAC (N-acetylcysteine) showed a significant treatment effect to reduce PTSD symptoms over the 8-week treatment period. Follow-up measure was obtained 4 weeks after discontinuing NAC or placebo (i.e., week 12 of the study) B) Weekly subjective craving score measured using a Visual Analog Scale (VAS). NAC showed a significant treatment effect to reduce drug craving over the 8-week treatment period. *p<.05. B = Baseline, F = Follow-Up.
A series of paired-sample t-tests were performed to evaluate within-group effects on the PCL-M total score. Results revealed significant within-group effects in the NAC group on the PCL-M from baseline to Week 4 (d = 1.84, p < .01) and baseline to Week 8 (d = 1.30, p < .001), reflecting large effect sizes (36). In contrast, no significant within-group effects on the PCL-M were observed in the placebo group.
Clinician-Rated PTSD Symptoms
The CAPS total score was reduced by 46% in the NAC treated group from baseline to Week 8 (58.8 to 32.0), as compared to 25% in the placebo group (68.6 to 51.5). Examination of between-group differences in the CAPS total and subscales scores revealed no statistically significant differences. Significant within-group effects in the NAC group on the CAPS total score from baseline to week 8 (d = 1.27, p < .001) and baseline to Week 12 (d = 1.48, p < .05) were observed. Further, significant within-group effects on the CAPS-R, CAPS-A, and CAPS-H subscales were observed in the NAC group from baseline to Week 8 (d = 1.01, d = 1.03, and d = 1.16, respectively, p’s < .01) and baseline to Week 12 (d = 1.04, d = 1.34, and d = 1.57, respectively, p’s < .01). Among the placebo group, no significant within-group effects on the CAPS total was observed at Week 8 or 12, but significant within-group effects on the CAPS-R subscale score were observed from baseline to Week 8 (d = .73, p < .05) and Week 12 (d = .67, p < .05).
Craving and Substance Use
Craving
The effect of treatment group on subjective craving for substances was tested using the VAS. As illustrated in Figure 2B, amount of craving was reduced 81% from baseline to Week 8 in the NAC group (3.7 to 0.7), as compared to 32% in the placebo group (4.1 to 2.8). The adjusted regression equation model examining the efficacy of NAC as compared to placebo on reduction of amount of craving from baseline to Week 8 accounted for 32.7% of the variance (F (2, 24) = 18.34, p < .001), with the NAC group having significantly lower amount of craving at Week 8 than the placebo group (β = −.41, p < .05). No significant group difference was observed at follow-up (β = −.41, p = ns). Tests of within-group effects revealed a significant effect for amount of craving in the NAC, but not the placebo, group from baseline to Week 8 (d = 1.03, p < .05) and Week 12 (d = .75, p < .05).
Frequency of craving was reduced by 72% from baseline to Week 8 in the NAC group (3.6 to 1.0), as compared to 29% in the placebo group (4.2 to 3.0). The adjusted regression equation model examining the efficacy of NAC as compared to placebo on reduction of frequency of craving from baseline to Week 8 accounted for 30.3% of the variance (F (2, 24) = 6.65, p < .001), with the NAC group having significantly lower level of frequency of craving at Week 8 than the placebo group (β = −.39, p < .05). There was a significant group differences in frequency of craving at follow-up with the adjusted regression equation model accounting for 27.9% of the variance (F (2, 21) = 4.057, p < .05), with the NAC group having significantly lower level of frequency of craving at Week 12 than the placebo group (β = −.40, p < .05). Tests of within-group effects revealed a significant effect for frequency of craving in the NAC, but not the placebo, group from baseline to Week 8 (d = 1.03, p < .05) and Week 12 (d = .79, p < .05).
Treatment condition was not a significant predictor of intensity of craving at Week 8 (β = −.28, p < .05) or Week 12 (β = −.51, p < .05) in the between-group analyses. However, significant within-group effects from baseline to Week 8 (d = 1.06) and to Week 12 were revealed in the NAC (d = .82) but not placebo group.
Substance Use
Treatment group differences in alcohol and drug use were examined using the TLFB (percent days using any substance and total days used) and no significant between-group differences were revealed. Substance use was low in both groups. We also examined differences in positive UDS tests. A linear regression was computed to examine the efficacy of NAC as compared to placebo and a trend toward significance was observed (F (1, 24) = 3.60, β = −.36, p = .07), with the NAC group having slightly fewer positive UDS tests during the course of treatment as compared to the placebo group.
Depressive Symptoms
The BDI-II total score was reduced by 48% in the NAC group from baseline to Week 8 (19.1 to 9.9) as compared to 15% in the placebo group (22.8 to 19.3). The adjusted regression equation examining the impact of treatment group on BDI-II total score at Week 8 accounted for 57.1% of the variance (F (2, 24) = 18.34, p < .001), with the NAC group having significantly lower symptoms at Week 8 than the placebo group (β = −.33, p < .05). Change from baseline to follow-up was examined and no significant between-group differences were observed (β = −.22, p = ns). Significant within-group effects in the NAC group on the BDI from baseline to Week 8 (d = 1.62, p < .001) and baseline to Week 12 (d = .71, p < .05) were observed. Among the placebo group, no significant within-group effects were observed from baseline to Week 8; however, a significant within-group effect was found at Week 12 (d = .71, p < .05).
Adverse Events
There were 31 total AEs, with the most common being dry mouth and heartburn. AEs were reported in 66.7% of participants in the NAC group and 47.1% in the placebo group. Three different participants experienced a serious adverse event (SAE) during the trial, which involved cardiac arrhythmia, hospitalization due to pancreatitis, syncopal episode, and hospitalization for suicidality. Of these SAEs, one was conservatively estimated to be possibly related to the study (syncopal episode).
Discussion
This is the first double-blind, randomized placebo-controlled trial to investigate NAC as a pharmacological treatment for comorbid PTSD/SUD. Results support the primary hypothesis that NAC significantly reduced pre-to-post treatment PTSD symptoms and craving. On average, participants who received NAC experienced a significant reduction in self-reported and clinician-rated PTSD symptoms from pre-to-post treatment. Furthermore, clinician-rated reductions in PTSD symptoms remained significantly reduced at follow-up for participants in the NAC condition. Participants receiving NAC also reported significant pre-to-post treatment decreases in craving. NAC produced average reductions in the amount and frequency of craving that were more than 2.5 times the magnitude of placebo effects. Secondary hypotheses were partially supported. NAC participants reported significant pre-to-post treatment reductions in depression symptoms that were sustained at one-month follow-up. Substance use was low for both the NAC and placebo groups and no significant differences were observed.
Five prior randomized clinical trials have examined the efficacy of pharmacotherapies for comorbid PTSD/SUD and all focused on comorbid alcohol dependence (7–9). These trials of sertraline, disulfiram, naltrexone, paroxetine, topiramate and desipramine produced mixed results (10). Preclinical studies show that NAC normalizes corticostriatal glutamate transmission and reduces levels of inflammatory cytokines, two mechanisms associated with PTSD and addiction. Previous clinical trials demonstrate some efficacy for NAC in treating SUD and it is currently under investigation for treating PTSD (NCT01664260; clinicaltrials.gov), but the current study is the first to assess NAC’s effects on comorbid PTSD and a broad range of SUD. The 8-week treatment with NAC significantly reduced PTSD symptoms, and led to a qualitative shift to sub-diagnostic PTSD symptom presentation (<50 CAPS score) for many participants, which was particularly remarkable given that participants were not receiving psychotherapy addressing PTSD.
Although significant group differences in drug craving were observed, there was a lack of treatment group differences in substance use behaviors. This may be due, in part, to the fact that all participants were required to demonstrate abstinence from alcohol and drugs for at least 7 days prior to study entry, and were receiving cognitive-behavioral therapy targeting SUD. The current trial offers additional support to the extant literature that suggests the effects of NAC may not be specific to a particular psychosocial platform, but rather generalizable to disorders involving impaired regulation of compulsive, intrusive thinking that promotes anxiety, drug craving, gambling or trichotillomania (18, 37–40). In combination with animal research and human imaging studies showing impaired corticostriatal regulation in SUD and PTSD, we postulate that by normalizing corticostriatal transmission NAC is reducing intrusive thinking and restoring top-down control in disorders sharing this endophenotype.
Conclusion
In summary, the current data provide encouraging preliminary support for combining NAC and cognitive-behavioral therapy to reduce PTSD symptoms, craving, and depression over 8 weeks. The wide availability, low cost, and low side-effect profile of NAC make it a potentially promising pharmacologic intervention for PTSD/SUD and our results support a future full-scale trial to investigate the synergistic effects of combining NAC with evidence-based psychosocial platforms that concurrently address PTSD/SUD. Additional preclinical research is also warranted to disentangle specific mechanisms through which NAC promotes symptom reduction among individuals with comorbid PTSD/SUD.
Acknowledgments
The authors would like to acknowledge support from the Department of Defense grant number W81XWH-11-1-0245 (PK). There are no conflicts of interest to disclose.
References
- 1.Seal KH, Metzler TJ, Gima KS, Bertenthal D, Maguen S, Marmar CR. Trends and risk factors for mental health diagnoses among Iraq and Afghanistan veterans using Department of Veterans Affairs health care, 2002–2008. Am J Pub Heal. 2009;99:1651–1658. doi: 10.2105/AJPH.2008.150284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith TC, Ryan MAK, Wingard DL, Slymen DJ, Sallis JF, Kritz-Silverstein D. New onset and persistent symptoms of post-traumatic stress disorder self reported after deployment and combat exposures: prospective population based US military cohort study. BMJ. 2008;336:366. doi: 10.1136/bmj.39430.638241.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petrakis IL, Rosenheck R, Desai R. Substance use comorbidity among veterans with posttraumatic stress disorder and other psychiatric illness. Am J Addict. 2011;20:185–189. doi: 10.1111/j.1521-0391.2011.00126.x. [DOI] [PubMed] [Google Scholar]
- 4.Young HE, Rosen CS, Finney JW. A survey of PTSD screening and referral practices in VA addiction treatment programs. J Substan Abus Treat. 2005;28:313–319. doi: 10.1016/j.jsat.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Hertzberg MA, Feldman ME, Beckham JC, Kudler HS, Davidson JRT. Lack of efficacy for fluoxetine in PTSD: a placebo controlled trial in combat veterans. Ann Clin Psychiat. 2000;12:101–105. doi: 10.1023/a:1009076231175. [DOI] [PubMed] [Google Scholar]
- 6.Friedman MJ, Marmar CR, Baker DG, Sikes CR, Farel GM. Randomized double-blind comparison of sertraline and placebo for posttraumatic stress disorder in a Department of Veterans Affairs setting. J Clin Psychiat. 2007;68:711–720. doi: 10.4088/jcp.v68n0508. [DOI] [PubMed] [Google Scholar]
- 7.Batki SL, Pennington DL, Lasher B, Neylan TC, Metzler T, Waldrop A, Delucchi K, Herbst E. Topiramate treatment of alcohol use disorder in veterans with posttraumatic stress disorder: a randomized controlled pilot trial. 2014;38:2169–2177. doi: 10.1111/acer.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brady KT, Sonne S, Anton RF, Randall CL, Back SE, Simpson K. Sertaline in the treatment of co-occurring alcohol dependence and posttraumatic stress disorder. Alcoholism Clin Exper Res. 2005;29:395–401. doi: 10.1097/01.alc.0000156129.98265.57. [DOI] [PubMed] [Google Scholar]
- 9.Foa EB, Yusko DA, McLean CP, Suvak MK, Bux DA, Oslin D, O’Brien CP, Imms P, Riggs DS, Volpicelli Concurrent naltrexone and prolonged exposure therapy for patients with comorbid alcohol dependence and PTSD: a randomized clinical trial. JAMA. 2013;310:488–495. doi: 10.1001/jama.2013.8268. [DOI] [PubMed] [Google Scholar]
- 10.Sofuoglu M, Rosenheck R, Petrakis I. Pharmacological treatment of comorbid PTSD and substance use disorder: Recent progress. Addict Behav. 2014;39:428–433. doi: 10.1016/j.addbeh.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dean O, Biorlando F, Berk M. N-acetylcysteine in psychiatry: current therapeutic evidence and potential mechanisms of action. J Psychiat Neurosci. 2011;36:78–86. doi: 10.1503/jpn.100057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalivas PW, Volkow ND. New medications for drug addiction hiding in glutamatergic neuroplasticity. Molecular psychiatry. 2011;16:974–986. doi: 10.1038/mp.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grant JE, Odlaug BL, Chamberlain SR, Potenza MN, Schreiber LR, Donahue CB, Kim SW. A randomized, placebo-controlled trial of N-acetylcysteine plus imaginal desensitization for nicotine-dependent pathological gamblers. J Clin Psychiat. 2014;75:39–45. doi: 10.4088/JCP.13m08411. [DOI] [PubMed] [Google Scholar]
- 14.Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW. Neuroadaptations in cysteine-glutamate exchange underlie cocaine relapse. Nature Neurosci. 2003;6:743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- 15.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aupperle RL, Melrose AJ, Stein MB, Paulus MP. Executive function and PTSD: disengaging from trauma. Neuropharmacology. 2012;62:686–694. doi: 10.1016/j.neuropharm.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bremner JD, Elzinga B, Schmahl C, Vermetten E. Structural and functional plasticity of the human brain in posttraumatic stress disorder. Progress in brain research. 2008;167:171–186. doi: 10.1016/S0079-6123(07)67012-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nature reviews Neuroscience. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang MX, Yurgil KA, Robb A, Angeles A, Diwakar M, Risbrough VB, Nichols SL, McLay R, Theilmann RJ, Song T, Huang CW, Lee RR, Baker DG. Voxel-wise resting-state MEG source magnitude imaging reveals neurocircuitry abnormality in active-duty service members and veterans with PTSD. NeuroImage Clin. 2014;5:408–419. doi: 10.1016/j.nicl.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, Milad MR, Liberzon I. Biological studies of post-traumatic stress disorder. Nature Rev Neurosci. 2012;13:769–787. doi: 10.1038/nrn3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, Orr SP, Pitman RK. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a funcational MRI study. Biolog Psychiat. 2000;47:769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- 22.Osuch EA, Willis MW, Bluhm R, Ursano RJ, Drevets WC. Neurophysiological responses to traumatic reminders in the acute aftermath of serious motor vehicle collisions using [15O}-H2O Positron Emission Tomography. Biolog Psychiat. 2008;64:327–335. doi: 10.1016/j.biopsych.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sripada RK, King AP, Garfinkel SN, Wang X, Sripada CS, Welsh RC, Liberzon I. Altered resting-state amygdala functional connectivity in men with posttraumatic stress disorder. J Psychiat Neurosci. 2012;37:241–249. doi: 10.1503/jpn.110069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gu H, Salmeron BJ, Ross TJ, Geng X, Zhan W, Stein EA, Yang Y. Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. Neuroimag. 2010;53:593–601. doi: 10.1016/j.neuroimage.2010.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McHugh MJ, Demers CH, Salmeron BJ, Devous MD, Sr, Stein EA, Adinoff B. Cortico-amygdala coupling as a marker of early relapse risk in cocaine-addicted individuals. Front Psychiatry. 2014;5:16. doi: 10.3389/fpsyt.2014.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feil J, Sheppard D, Fitzgerald PB, Yucel M, Lubman DI, Bradshaw JL. Addiction, compulsive drug seeking, and the role of frontostriatal mechanisms in regulating inhibitory control. Neurosci Biobehav Rev. 2010;35:248–275. doi: 10.1016/j.neubiorev.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 27.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington DC: American Psychiatric Association; 2000. text revision. [Google Scholar]
- 28.Folstein MF, Folstein SE, McHugh PR. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiat Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 29.Carroll KM, Kosten TR, Rounsaville BJ. Choosing a behavioral therapy platform for pharmacotherapy of substance users. Drug Alc Depend. 2004;75:123–134. doi: 10.1016/j.drugalcdep.2004.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiat. 1998;59:22–33. [PubMed] [Google Scholar]
- 31.Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a clinician-administered PTSD scale. J Traumat Stres. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 32.Weathers FW, Litz BT, Herman DS, Huska JA, Keane TM. The PTSD Checklist: reliability, validity and diagnostic utility. Annual meeting of the International Society for Traumatic Stress Studies; San Antonio, TX. 1993. [Google Scholar]
- 33.Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring alcohol consumption: psychosocial and biochemical methods. Totowa, NJ: Humana Press; 1992. pp. 41–73. [Google Scholar]
- 34.Beck A, Steer R, Brown G. Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 35.Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, Currier GW, Melvin GA, Greenhill L, Shen S, Mann JJ. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiat. 2011;168:1266–1277. doi: 10.1176/appi.ajp.2011.10111704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cohen J. Statistical power for the social sciences. Hillsdale, NJ: Laurence Erlbaum and Associates; 1988. [Google Scholar]
- 37.Goldstein RZ, Craig AD, Bechara A, Garavan H, Childress AR, Paulus MP, Volkow ND. The neurocircuitry of impaired insight in drug addiction. Trends Cogn Sci. 2009;13:372–380. doi: 10.1016/j.tics.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grant JE, Odlaug BL, Kim SW. N-acetylcysteine, a glutamate modulator, in the treatment of trichotillomania: a double-blind, placebo-controlled study. Arch Gen Psychiatry. 2009;66:756–763. doi: 10.1001/archgenpsychiatry.2009.60. [DOI] [PubMed] [Google Scholar]
- 39.Gray KM, Carpenter MJ, Baker NL, DeSantis SM, Kryway E, Hartwell KJ, McRae-Clark AL, Brady KT. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiat. 2012;169:805–812. doi: 10.1176/appi.ajp.2012.12010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Asevedo E, Mendes AC, Berk M, Brietzke E. Systematic review of N-acetylcysteine in the treatment of addictions. Rev Bras Psiquiatr. 2014;36:168–175. doi: 10.1590/1516-4446-2013-1244. [DOI] [PubMed] [Google Scholar]