Abstract
Purpose
Dietary calorie restriction (CR) and exercise (EX) promote weight loss and may have additive effects for improving insulin sensitivity, independent of weight loss. It is not known if these effects are attributable to changes in circulating cytokines. We evaluated the hypothesis that modest, matched weight loss induced by CR and EX have additive effects on circulating cytokines and these changes correlate with improvements in insulin sensitivity.
Methods
Overweight and sedentary women and men (n=52, 45–65y) were randomized to undergo 7% weight loss by using 3–6 months of CR, EX, or a combination of both (CREX). Concentrations of cytokines and hormones were measured in fasting and oral glucose tolerance test (OGTT) blood samples. Insulin sensitivity was estimated based on OGTT glucose and insulin.
Results
With all groups combined fasting leptin (p<0.0001) and HMW adiponectin (p=0.04) decreased and pentraxin-3 increased (p<0.0001), in a manner that correlated with improvements in insulin sensitivity (all p≤0.0002). These changes, combined with decreases in glucose-dependent insulinotropic polypeptide from the OGTT, explained 63% of the variance (p<0.0001) in insulin sensitivity improvements. EX and CR had additive effects on leptin, with a similar trend for HMW adiponectin. MCP-1 and CRP concentrations did not change. CR and EX had opposite effects on soluble TNF receptor-1.
Conclusions
Modest weight loss in overweight adults decreases serum leptin and HMW adiponectin, and increases pentraxin-3 concentrations in a manner that correlates with increased insulin sensitivity. Exercise has additive effects to those induced by CR for reductions in leptin and possibly adiponectin. These changes may contribute to the additive effects of CR and exercise for improving insulin sensitivity.
Keywords: adiposity, diet modification, aerobic exercise, adipokine, insulin resistance
INTRODUCTION
Weight loss improves insulin action and reduces the risk of developing type 2 diabetes in overweight and obese individuals [1]. When obese individuals undergo major weight loss, changes in circulating cytokines may contribute to these beneficial effects [2]. Although more modest weight loss in less severe states of adiposity (i.e. overweight) also improves insulin action [3], the role of circulating cytokines in these adaptations is not clear. Furthermore, although dietary calorie restriction and exercise are both recommended for weight management and diabetes prevention [4], their independent and additive effects on circulating cytokines is not known.
The purpose of this study was to evaluate the effects of matched, modest (7%) weight loss, induced by calorie restriction (CR), endurance exercise training (EX), or both (CREX) on circulating cytokines, and to evaluate the association of cytokine changes with improvements in insulin sensitivity. We previously reported the insulin sensitivity data, which showed two-fold greater increases from CREX, as compared to the same magnitude weight loss using either CR or EX alone [5]. For the present study we hypothesized that CR and EX also have additive effects on circulating cytokines and that these changes partly explain the additive effects of CR and EX on insulin sensitivity.
MATERIALS AND METHODS
Subjects
Healthy men and postmenopausal women were recruited by using local advertisements. Eligible participants were overweight (BMI 25.0–29.9 kg/m2) and weight stable (<3% weight change over 6 months), 45–65 year of age, and did not exercise regularly (defined as ≥20 min/session and ≥3 d/wk). A medical screening, including ECG stress test, was used to identify and exclude individuals with major chronic diseases and conditions that would be contraindicated and/or interfere with exercise. Use of medications to control blood glucose was exclusionary. For other medications, stable dosages were required for ≥6 months prior to baseline testing and the participants were advised to maintain stable dosages during the study. Additional details about medical screening have been published previously [5]. The study was approved by the Institutional Review Boards at Saint Louis University and Washington University. Written informed consent was obtained from each subject. The trial was registered at ClinicalTrials.gov (identifier: NCT00777621).
Study Design and Randomization
Subjects were randomized, with stratification for sex, to calorie restriction (CR), exercise (EX), or a combination of both (CREX). The allocation ratio was initially 1:1:1 but was later revised to 2:2:1 to compensate for more withdrawals from CR and EX. Outcomes were assessed at baseline and after a 2-week weight stability period at the end of the intervention.
Interventions
The interventions were designed to reduce body weight by 6–8% over 12–14 weeks. The duration was adjusted as needed for participants to reach the weight loss goal. The energy deficit goal was equal to 20% of baseline total energy expenditure (TEE) and energy intake, which was evaluated as described previously [5]. Diet and exercise prescriptions were adjusted periodically to achieve the targeted rate of weight loss. After the active weight loss portion of the study, the diet and exercise prescriptions were adjusted to stabilize body weight (≤0.5 kg change in 3-day rolling average weight) for ≥2 weeks before follow-up testing. The participants recorded daily fasted morning body weights at home and visited our facility each week for weigh-ins, to turn in home weight logs, and for other intervention-specific requirements.
Calorie restriction
The CR intervention was designed to reduce energy intake by 20% and not change physical activity. Participants attended weekly one-on-one meetings for dietary counseling. Personalized recommendations were based on 3-day food diaries, which were recorded weekly for 3 weeks and periodically thereafter. The intervention strategy was to reduce portion sizes and to replace high energy density foods with lower energy density foods. The goals for macronutrient balance were consistent with recommendations [6]. As needed to promote intervention compliance, weeklong periods of full food provision were implemented.
Exercise intervention
The EX intervention goal was to use exercise to increase total energy expenditure by 20%, while not changing energy intake. The types of exercise included cardiovascular exercise and functional physical activities (e.g. active transportation). Weekly exercise energy expenditure prescriptions were calculated as described elsewhere [3]. Heart rate (HR) monitors (Polar, Kempele, Finland) were used to monitor exercise energy expenditure and other exercise metrics, which were retrieved from the monitors by study personnel during weekly meetings. Exercise frequency and intensity prescriptions were not provided but participants were encouraged to exercise daily and to use moderate- and high-intensity exercise to maximize energy expenditure. Access to the on-campus exercise facilities was provided; however, participants were encouraged to exercise outdoors, at home, and at other convenient locations.
Calorie restriction + exercise intervention
The goal in the CREX intervention was create a 20% energy deficit by using CR and EX with each contributing to half of the total energy deficit.
Body weight and composition
Fasted, gowned body weight was measured on two mornings and averaged. Stature was measured without shoes and using a wall-mounted stadiometer. Body composition was measured by using dual-energy X-ray absorptiometry (Lunar iDXA, software version 13.31, GE Healthcare, Madison, WI).
Intervention compliance (energy intake and expenditure)
Energy intake and expenditure were evaluated at baseline and just prior to the weight stability period at the end of the intervention. Energy intake was quantified by using 3-day food diaries (2 weekdays and 1 weekend day) and nutrient analyses (Food Processor SQL, ESHA Research, Inc., Salem, OR). Physical activity recall interviews (modified Stanford 7-day PAR [7]) and accelerometry (RT3 triaxial accelerometers, StayHealthy, Inc. Monrovia, CA) were used to estimate total energy expenditure. Physical activity recall and accelerometry results were averaged.
Aerobic capacity
Maximal oxygen uptake (VO2max) was measured with indirect calorimetry (MedGraphics CardiO2; Medical Graphics Corporation, St. Paul, MN) during a graded treadmill test to exhaustion (modified Balke protocol).
Blood collection and analyses
After an overnight fast and between 5:00 and 9:00 am (just prior to the oral glucose tolerance test described below), venous blood was collected from an arm vein. Serum was isolated by using centrifugation and stored at −80°C for later batch analyses of baseline and follow-up samples. Cytokine and dipeptidyl peptidase-IV (DPP-IV) concentrations were measured by using ELISA assays with commercially available reagents (R&D Systems, Minneapolis MN). Samples were run in duplicate; if the coefficient of variation for duplicates was >10%, the sample was re-analyzed. Leptin was measured as a sensor of adipose tissue energy status and because it may induce adiponectin [8]. Total and high molecular weight (HMW) adiponectin were measured because of their effects on insulin sensitivity [9,10]. Monocyte chemoattractant protein-1 (MCP1) was measured as a marker of adipose tissue inflammation [11]. Pentraxin-3 is an acute phase protein secreted by adipose and other tissues; its circulating concentrations increase with weight loss [12] and exercise training [13] and may be correlated with adiponectin [12]. C-reactive protein (CRP) was measured as a marker of systemic inflammation. The soluble tumor necrosis factor receptor-1 (sTNFR1) was measured as a marker of TNF system activity.
Oral glucose tolerance tests (OGTTs) were performed after an overnight fast and were initiated between 5:00 and 9:00 am. For 81% of the participants, the time of day for the baseline and follow-up tests did not differ by more than 2 hr. As recommended by the World Health Organization [14] and American Diabetes Association [15] for OGTT preparation, the participants were advised to consume ≥150 g/d carbohydrate for 3 days prior to the OGTT. Based the carbohydrate intake data from the 3-day food diaries, this did not represent an increase in habitual carbohydrate intake at baseline or follow-up. For follow-up testing, EX and CREX group participants continued exercise until the day before testing. Venous blood was collected before and 10, 20, 30, 60, 90, and 120 min after a 75-g oral glucose load. Samples to be analyzed for incretin hormones (glucagon-like peptide 1, GLP-1; and glucose-dependent insulinotropic polypeptide, GIP) were collected into tubes containing a DPP-IV inhibitor (DPP4–010; Millipore, Billerica MA) to prevent the degradation of active forms of GLP-1 and GIP. Plasma was isolated by centrifugation and glucose concentrations were analyzed immediately with an automated analyzer (2300 Stat Plus; YSI Inc, Yellow Springs, OH). Additional plasma and serum was stored at −80°C for later batch analysis of baseline and follow-up samples for insulin concentrations (IMMULITE Chemiluminescence Kit; Diagnostics Products Corporation, Los Angeles, CA), and the active forms of GLP-1 (7–36 and 7–37 amides) and GIP (1–42 amide) (ELISA assay kits from IBL International, Toronto, ON, Canada). Glucose, insulin, GLP-1, and GIP total areas under the curve (AUCs) were calculated by using the trapezoidal rule [16]. Insulin sensitivity index (ISI) was calculated as described by Matsuda and DeFronzo [17] and Stumvoll [18].
Statistical analyses
Statistical analyses included data from subjects who provided follow-up data and were adherent to the intervention (lack of adherence was defined as <1% weight loss). Among-group comparisons of participant characteristics were performed with Fisher’s exact tests and ANOVAs. Outcomes were analyzed by using ANCOVA, in which the study group was the independent variable, change in the outcome was the dependent variable, and the baseline value was included as a covariate. Post-hoc comparisons were performed using the protected f-test principle and least significant difference tests. Baseline-adjusted means were used to evaluate the significance of within-group changes. Bivariate associations were evaluated with Pearson correlations. Multivariate analyses used the forward selection method.
All tests were two-tailed with p≤0.05 considered significant. Data are presented as arithmetic means ± SE except for mean change values, which were adjusted for baseline values. Analyses were performed using SAS for Windows (version 9.3, SAS Institute Inc., Cary, NC).
RESULTS
Participants
Sixty-nine men and women enrolled, underwent baseline testing, and were randomized. Twelve participants withdrew before providing follow-up data; 5 participants were non-adherent (weight loss range: 0.5% loss to 1.3% gain). Therefore, the analyses for the present report are based on data from 52 participants. Demographic and baseline characteristics did not differ between groups (Table 1). Baseline BMI was in the middle of the overweight range. Body fat percentage was ~45% for women and ~35% for men (Table 1). Based on fasting and 2-hr OGTT plasma glucose values and hemoglobin A1C levels, 54% (n=28) of the participants were classified as having prediabetes and 6% (n=3) had provisional diabetes; these frequencies did not differ among groups (p=1.00 and p=0.51, respectively).
Table 1.
Baseline characteristics of study participants.
| All | CR | EX | CREX | Among group p-value | |
|---|---|---|---|---|---|
|
|
|||||
| Participants, n | 52 | 17 | 16 | 19 | ---- |
| Sex, women | 39 (75%) | 13 (76%) | 11 (69%) | 15 (79%) | 0.85 |
| Age, y | 57 ± 5 | 57 ± 5 | 56 ± 6 | 57 ± 7 | 0.86 |
| Race | |||||
| Caucasian | 42 (81%) | 16 (94%) | 12 (75%) | 14 (74%) | |
| African-American | 7 (13%) | 0 (0%) | 3 (19%) | 4 (21%) | |
| Other or not specified | 3 (6%) | 1 (6%) | 1 (6%) | 1 (5%) | 0.26 |
| BMI, kg/m2 | 27.7 ± 1.7 | 27.7 ± 1.7 | 27.0 ± 1.5 | 28.3 ± 1.8 | 0.08 |
| Body weight, kg | |||||
| Women | 75.5 ± 6.9 | 73.2 ± 5.1 | 74.2 ± 5.5 | 77.2 ± 8.6 | 0.26 |
| Men | 92.0 ± 11.5 | 92.4 ± 7.9 | 86.3 ± 11.4 | 98.7 ± 13.5 | 0.30 |
| Body fat, % of total mass | |||||
| Women | 44.6 ± 3.4 | 46.8 ± 2.1 | 43.3 ± 3.0 | 43.6 ± 3.7 | 0.26 |
| Men | 34.7 ± 5.2 | 32.7 ± 7.1 | 34.3 ± 5.1 | 37.1 ± 3.1 | 0.30 |
| Body fat, kg | |||||
| Women | 32.4 ± 4.0 | 33.1 ± 2.7 | 31.2 ± 4.0 | 32.8 ± 5.0 | 0.26 |
| Men | 31.1 ± 7.8 | 29.5 ± 8.3 | 28.8 ± 7.7 | 35.5 ± 7.5 | 0.30 |
| Fat free mass, kg | |||||
| Women | 42.6 ± 4.5 | 39.8 ± 3.4 | 42.9 ± 2.7 | 44.8 ± 5.3 | 0.26 |
| Men | 60.7 ± 5.0 | 62.7 ± 2.8 | 57.4 ± 4.8 | 62.7 ± 5.6 | 0.30 |
Values are means ± SD for quantitative data and counts (%) for categorical data. Among group p-values for quantitative data are from ANOVAs and for categorical data are from Fisher’s Exact Tests.
Intervention compliance
Energy intake, energy expenditure, and VO2max data have been reported previously as indicators of intervention compliance [5]. Energy intake decreased in the CR (−32±4%, p<0.0001) and CREX (−27±4%, p<0.0001) groups and remained unchanged in the EX group (−7±4%, p=0.11) (among group p=0.0002). Net exercise energy expenditure was 412±26 kcal/d in the EX group and 217±23 kcal/d in the CREX group (between group p<0.0001). Corresponding exercise frequencies in the EX and CREX groups were 8±1 and 6±1 sessions/wk (p=0.08), duration was 7.4±0.5 and 4.4±0.5 hr/week (p=0.0002), and intensity was 77±1% and 74±1% of maximum heart rate (p=0.17). Total energy expenditure increased in the EX (185±53 kcal/d, p=0.001) and CREX (126±48 kcal/d, p=0.01) groups and did not change in the CR group (−22±51 kcal/d, p=0.66) (among-group p=0.02). VO2max increased in the CREX (11±3%, p=0.0006) and EX (22±3%, p<0.0001) groups and did not change in the CR group (−1±3%, p=0.85) (among-group p<0.0001).
Weight loss and body composition
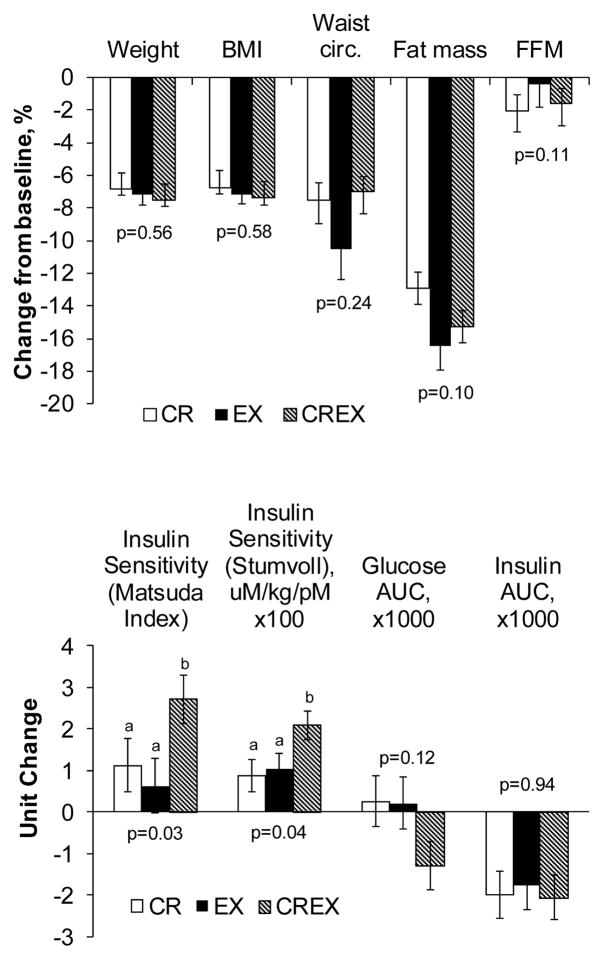
Weight loss was ~7% in all three study groups, with no difference (p=0.43) among groups (Figure 1), which was intended by study design. Fat mass decreased by ~15% with a tendency (p=0.10) for greater reductions in the EX and CREX groups (Figure 1). Body weight did not change during the 2-week weight stability period at the end of the intervention (all p≥0.28).
Figure 1.
Changes in body composition and measures of glucoregulation. Values are least squares means ± SE that have been adjusted for differences in baseline values among groups. P-values reflect the significance of the among-group differences in change values after adjustment for baseline values using ANCOVA. For each outcome, means with unlike superscripts are significantly different (P<0.05). These data have been presented previously [5] but are included in the present report to assist in interpreting the cytokine data.
Oral glucose tolerance test
As previously reported [5], insulin sensitivity index increased 2-fold more in response to the CREX intervention than it did in response to similar weight loss induced by CR alone or EX alone (Figure 1). There was a tendency for greater reductions in OGTT glucose AUC in the CREX group (among group p=0.12). OGTT insulin AUC decreased 22% (p<0.0001) with no difference among groups (p=0.94) (Figure 1). Changes in GLP-1 OGTT incretin hormone responses have been previously reported in detail [5]. In brief, GLP-1 AUC decreased in the CR group only (within group p=0.04; among group p=0.05). GIP AUC tended to decrease with all groups combined (p=0.06), with no differences among groups (p=0.0.76). DPP-IV did not change in any group (all p≥0.19).
Serum cytokines
Leptin concentrations decreased significantly with all groups combined; however, the decrease was 2- to 3-fold greater in the EX and CREX groups compared to the CR group (Table 2). Total and HMW adiponectin concentrations decreased in the CREX group only (p=0.04 and p=0.008, respectively) but the among-group comparison of changes was not significant (p=0.07 and p=0.18, respectively). No change was observed for MCP-1 and the reductions in CRP were only marginally significant within all groups combined (p=0.06, Table 2). Pentraxin-3 increased in all three groups (~50% with all groups combined, p<0.0001), with no difference among groups (p=0.62). Changes in sTNFR1 concentrations differed among groups (p=0.04) with a 6% increase in the EX group (p=0.05), a non-significant 5% decrease in the CR group (p=0.10) and no change (p=0.74) in the CREX group.
Table 2.
Changes in serum cytokine concentrations in response to weight loss induced by calorie restriction (CR), endurance exercise training (EX), or a combination of CR and EX (CREX).
| All | CR | EX | CREX | Among group p-value | |
|---|---|---|---|---|---|
|
|
|||||
| Leptin, pg/mL | |||||
| Baseline | 9.2 ± 0.3 | 9.0 ± 0.6 | 9.0 ± 0.5 | 9.6 ± 0.3 | 0.71 |
| Final | 7.9 ± 0.4 | 8.4 ± 0.7 | 7.4 ± 0.8 | 7.8 ± 0.5 | |
| Change | −1.3 ± 0.2 | −0.6 ± 0.4a | −1.5 ± 0.4a,b | −1.8 ± 0.4b | 0.03 |
| Within-group P | <0.0001 | 0.13 | 0.0004 | <0.0001 | |
| Total adiponectin, μg/mL | |||||
| Baseline | 13.5 ± 0.6 | 13.6 ± 0.9 | 12.6 ± 1.1 | 14.2 ± 1.1 | 0.54 |
| Final | 13.2 ± 0.5 | 13.5 ± 0.8 | 13.5 ± 0.8 | 12.8 ± 1.1 | |
| Change | −0.3 ± 0.4 | −0.1 ± 0.6 | 0.6 ± 0.6 | −1.1 ± 0.6 | 0.07 |
| Within-group P | 0.55 | 0.96 | 0.24 | 0.04 | |
| HMW adiponectin, μg/mL | |||||
| Baseline | 6.1 ± 0.5 | 6.2 ± 0.8 | 5.4 ± 0.7 | 6.6 ± 1.1 | 0.73 |
| Final | 5.6 ± 0.4 | 6.0 ± 0.6 | 5.1 ± 0.6 | 5.7 ± 0.9 | |
| Change | −0.5 ± 0.2 | −0.2 ± 0.3 | −0.5 ± 0.3 | −0.8 ± 0.3 | 0.18 |
| Within-group P | 0.04 | 0.95 | 0.40 | 0.008 | |
| Adiponectin ratio (HMW:total) | |||||
| Baseline | 0.42 ± 0.02 | 0.41 ± 0.03 | 0.41 ± 0.03 | 0.43 ± 0.04 | 0.91 |
| Final | 0.40 ± 0.02 | 0.41 ± 0.02 | 0.36 ± 0.03 | 0.41 ± 0.04 | |
| Change | −0.02 ± 0.01 | 0.00 ± 0.02 | −0.05 ± 0.02 | −0.02 ± 0.02 | 0.36 |
| Within-group P | 0.11 | 0.84 | 0.04 | 0.45 | |
| MCP-1, pg/mL | |||||
| Baseline | 410 ± 34 | 381 ± 36 | 390 ± 50 | 452 ± 77 | 0.92 |
| Final | 407 ± 22 | 423 ± 31 | 383 ± 26 | 415 ± 48 | |
| Change | −3 ± 33 | 19 ± 37 | −22 ± 37 | −5 ± 34 | 0.68 |
| Within-group P | 0.32 | 0.14 | 0.51 | 0.74 | |
| Pentraxin 3, ng/mL | |||||
| Baseline | 0.63 ± 0.03 | 0.68 ± 0.07 | 0.63 ± 0.07 | 0.59 ± 0.04 | 0.67 |
| Final | 0.82 ± 0.05 | 0.84 ± 0.08 | 0.81 ± 0.10 | 0.83 ± 0.09 | |
| Change | 0.20 ± 0.03 | 0.16 ± 0.06 | 0.17 ± 0.06 | 0.25 ±0.06 | 0.62 |
| Within-group P | <0.0001 | 0.0007 | 0.002 | <0.0001 | |
| C-reactive protein, mg/L | |||||
| Baseline | 1.98 ± 0.22 | 2.47 ± 0.46 | 1.72 ± 0.30 | 1.81 ± 0.37 | 0.20 |
| Final | 1.64 ± 0.19 | 1.96 ± 0.32 | 1.38 ± 0.32 | 1.59 ± 0.33 | |
| Change | −0.35 ± 0.20 | −0.22 ± 0.31 | −0.50 ± 0.30 | −0.32 ± 0.28 | 0.71 |
| Within-group P | 0.06 | 0.45 | 0.07 | 0.29 | |
| sTNFR1, ng/mL | |||||
| Baseline | 1.05 ± 0.03 | 1.02 ± 0.05 | 1.06 ± 0.05 | 1.06 ± 0.03 | 0.80 |
| Final | 1.05 ± 0.03 | 0.98 ± 0.04 | 1.12 ± 0.06 | 1.05 ± 0.03 | |
| Change | 0.00 ± 0.02 | −0.05±0.03a | 0.06 ± 0.03b | −0.01±0.03a | 0.04 |
| Within-group P | 0.97 | 0.10 | 0.05 | 0.75 | |
Values are arithmetic means ± SE except for group-specific change values, which are least squares means ± SE that have been adjusted for differences in baseline values among groups. Means within each row with unlike superscripts are significantly different (P<0.05). Statistical analyses and p-values were based on transformed data (log or power transformations, as appropriate for each analyte) to normalize data distributions and ensure homogeneity of variances. Among group p-values reflect the significance of the among-group differences in change values after adjustment for baseline values using ANCOVA. Exclusion of two participants who had changes in anti-inflammatory medications altered the significance of the results as follows: the among-group comparison of sTNFR changes became non-significant (p=0.07) and the within group sTNFR change in the EX group became non-significant (p=0.10); the within-group change in CRP in the EX group became significant (p=0.04); the significance of all other results was unaffected.
Baseline associations between cytokine concentrations and glucoregulation
At baseline, higher values for insulin sensitivity index and lower values for OGTT insulin AUC were associated with higher serum concentrations of HMW adiponectin and the HMW:total adiponectin ratio (Table 3). A negative association existed between insulin AUC and total adiponectin and a positive association existed between OGTT glucose AUC and pentraxin-3. All of these associations remained significant after accounting for variability in baseline BMI, except for the association between Stumvoll insulin sensitivity index and adiponectin ratio, which became non-significant (p=0.08). No other associations were observed between cytokines or incretin hormones and indices of glucoregulation at baseline (Table 3).
Table 3.
Correlations between baseline values for serum cytokine concentrations and baseline values for glucoregulation indices.
| Pearson correlation coefficient (P value) | ||||
|---|---|---|---|---|
| ISI (Matsuda & DeFronzo index) | ISI (Stumvoll index) | OGTT Glucose AUC | OGTT Insulin AUC | |
| Leptin, pg/mL | −0.16 (0.27) | 0.05 (0.73) | −0.11 (0.45) | −0.02 (0.91) |
| Total adiponectin, μg/mL | 0.27 (0.06) | 0.24 (0.09) | −0.18 (0.20) | −0.28 (0.04) |
| HMW adiponectin, μ/mL | 0.35 (0.01) | 0.26 (0.06) | −0.14 (0.31) | −0.36 (0.008) |
| Adiponectin ratio (HMW:total) | 0.35 (0.01) | 0.32 (0.02) | −0.15 (0.31) | −0.38 (0.006) |
| MCP-1, pg/mL | −0.10 (0.49) | 0.10 (0.46) | −0.17 (0.24) | 0.06 (0.69) |
| Pentraxin 3, ng/mL | 0.05 (0.74) | −0.09 (0.53) | 0.31 (0.02) | −0.06 (0.68) |
| C-reactive protein, mg/L | 0.03 (0.84) | 0.02 (0.91) | 0.01 (0.92) | 0.02 (0.89) |
| sTNFR1, ng/mL | 0.15 (0.29) | 0.04 (0.78) | −0.06 (0.68) | −0.03 (0.81) |
| GLP-1 AUC, pmol/L·min | 0.20 (0.15) | 0.09 (0.51) | −0.15 (0.28) | 0.03 (0.81) |
| GIP AUC, pmol/L·min | 0.19 (0.17) | 0.13 (0.37) | −0.17 (0.24) | −0.19 (0.17) |
| DPP-IV, ng/mL | −0.19 (0.19) | −0.11 (0.45) | −0.12 (0.38) | 0.14 (0.32) |
Values are Pearson correlation coefficients, with p-values in parentheses. Bold font indicates significant correlations based on P≤0.05. Analyses were performed on transformed data (log or power transformations, as appropriate for each variable) to normalize data distributions.
Associations between changes in cytokine concentrations and changes in glucoregulation
Weight loss-induced increases in the Masuda and DeFronzo insulin sensitivity index were associated with decreases in serum leptin, decreases in HMW adiponectin, and increases in serum pentraxin-3 (Table 4). These associations remained significant after accounting for changes in BMI and fat mass. None of the changes in cytokine concentrations were associated with changes in Stumvoll insulin sensitivity index or with changes in the OGTT insulin and glucose AUCs (Table 4). Because insulin and leptin increase the expression of adiponectin [19], we evaluated the correlation between changes in these analytes and adiponectin. Reductions in leptin were associated with reductions in HMW adiponectin (r=0.36, p=0.01) and the HMW-to-total adiponectin ratio (r=0.29, p=0.04) but not with total adiponectin (r=0.11, p=0.46). Fasting insulin and insulin AUC were not correlated with any measures of adiponectin (all p>0.23)
Table 4.
Correlations between changes in serum cytokine concentrations and changes in indices of glucoregulation.
| Pearson correlation coefficient (P value) | ||||
|---|---|---|---|---|
| ISI (Matsuda & DeFronzo index) | ISI (Stumvoll index) | OGTT Glucose AUC | OGTT Insulin AUC | |
| Leptin, pg/mL | −0.52 (<0.0001) | 0.06 (0.69) | 0.08 (0.56) | −0.02 (0.86) |
| Total adiponectin, μg/mL | −0.27 (0.06) | −0.01 (0.94) | 0.18 (0.20) | −0.09 (0.51) |
| HMW adiponectin, μ/mL | −0.50 (0.0002) | −0.04 (0.80) | 0.11 (0.43) | 0.14 (0.31) |
| Adiponectin ratio (HMW:total) | −0.25 (0.08) | 0.03 (0.82) | −0.11 (0.43) | 0.16 (0.27) |
| MCP-1, pg/mL | −0.20 (0.16) | 0.00 (0.99) | 0.09 (0.52) | −0.03 (0.84) |
| Pentraxin 3, ng/mL | 0.53 (<0.0001) | −0.08 (0.57) | −0.15 (0.28) | −0.24 (0.09) |
| C-reactive protein, mg/L | 0.01 (0.94) | 0.17 (0.22) | −0.11 (0.43) | −0.24 (0.09) |
| sTNFR1, ng/mL | 0.08 (0.58) | −0.08 (0.56) | 0.12 (0.40) | −0.12 (0.39) |
| GLP-1 AUC, pmol/L·min | −0.21 (0.14) | 0.001 (0.99) | 0.009 (0.95) | 0.09 (0.50) |
| GIP AUC, pmol/L·min | −0.54 (<0.0001) | −0.24 (0.08) | 0.47 (0.0004) | 0.45 (0.0009) |
| DPP-IV, ng/mL | −0.07 (0.63) | 0.03 (0.84) | 0.04 (0.78) | 0.08 (0.58) |
Values are Pearson correlation coefficients, with p-values in parentheses. Bold font indicates significant correlations based on P≤0.05. Analyses were performed on transformed data (log or power transformations, as appropriate for each variable) to normalize data distributions.
Changes in incretin hormones (GLP-1 and GIP) from the OGTT and DPP-IV have been reported previously [5]; however, their associations with changes in insulin sensitivity were not previously reported. Weight loss-induced decreases in GIP AUC from the OGTT were associated with increases in insulin sensitivity and decreases in OGTT glucose and insulin (Table 4). Changes in GLP-1 and DPP-IV were not associated with changes in insulin sensitivity or other OGTT outcomes.
A multivariate regression analysis including changes in leptin, HMW adiponectin, pentraxin-3, and GIP AUC as independent variables indicated that all four were significant independent predictors of the improvements in insulin sensitivity. Change in pentraxin-3 was the single strongest predictor and entered the model first, followed by leptin, GIP AUC, and HMW adiponectin (Table 5). The full model accounted for 63% of the variance in ISI improvements (p<0.0001).
Table 5.
Multiple regression analysis to evaluate changes in serum cytokine concentrations as determinants of the changes in insulin sensitivity resulting from weight loss.
| Variable | Entry into model | Coefficient | SE | P-value | R2 |
|---|---|---|---|---|---|
| Full model | ---- | ---- | ---- | <0.0001 | 0.63 |
| Intercept | ---- | −0.373 | 0.39 | 0.34 | ---- |
| Pentraxin-3 change, ng/mL | 1st | 2.776 | 1.067 | 0.01 | 0.28 |
| Leptin change, pg/mL | 2nd | −0.023 | 0.006 | 0.001 | 0.17 |
| GIP AUC, pmol/L·min | 3rd | −0.001 | 0.0003 | 0.0002 | 0.14 |
| HMW adiponectin change, μ/mL | 4th | −2.404 | 1.132 | 0.039 | 0.04 |
Regression analysis was performed using a forward selection approach, only including cytokines that were significant predictors of changes in insulin sensitivity, based on bivariate correlations as shown in Table 4. Analyses were performed on transformed data (log or power transformations, as appropriate for each variable) to normalize data distributions.
DISCUSSION
The objectives of the present study were to evaluate the effects of modest weight loss on circulating cytokines, to compare the responses to matched weight loss from calorie restriction and/or exercise interventions, and to evaluate associations between changes in circulating cytokines and changes in indices of glucoregulation. Weight loss reduced serum leptin concentrations in all groups in a manner that was correlated with improvements in insulin sensitivity. However, the reductions in leptin were 2- to 3-fold greater in the EX and CREX groups suggesting that EX has additive effects to those from CR. It also suggests that adipose tissue perceived a larger energy deficit in the EX and CREX groups, even though weight losses were matched. HMW adiponectin concentrations decreased, which is opposite to that which occurs with weight loss in obesity [20,21], and these reductions were associated with improvements in insulin sensitivity. Although the among-group comparisons of changes in HMW adiponectin were not significant (p=0.18), the reductions appeared to be mainly driven by the CREX group (p=0.008), which suggests additive effects of CR and EX. Large, significant increases in pentraxin-3 were observed, with no difference among groups (p=0.62); these increases were associated with improvements in insulin sensitivity. In a multiple regression analysis, weight loss-induced changes in serum leptin, HMW adiponectin, and pentraxin-3, combined with the changes in GIP concentrations from our previous report [5], accounted for 63% of the variability in insulin sensitivity improvements.
At baseline, higher HMW adiponectin concentrations were associated with greater insulin sensitivity and lower insulin AUC. However, HMW adiponectin concentrations decreased in response to weight loss in a manner that was correlated with improvements in insulin action. This finding in our overweight study participants was unexpected because in obese individuals adiponectin concentrations increase with weight loss [20–22] and because adiponectin enhances insulin action on skeletal muscle [9] and liver [10]. However, some studies have reported no change in adiponectin levels despite improvements in glucoregulation [23–25]. Furthermore, in healthy weight subjects, adiponectin decreases with weight loss [26] and increases with weight gain [27], which is consistent with our findings.
Findings from our study and previous studies suggest that weight loss-induced changes in adiponectin may depend on the degree of adiposity present at baseline. In obesity, adipose tissue adiponectin secretion is suppressed [28], resulting in hypoadiponectinemia and insulin resistance. Then, with weight loss, adiponectin secretion increases [20,21] and consequently improves insulin sensitivity [22]. Although speculative, we propose that in non-obese individuals, adiponectin secretion is not inhibited and can be secreted in sufficient quantities to compensate for insulin resistance. Then, during weight loss insulin sensitivity improves through mechanisms that do not involve adiponectin, which “permits” reductions in adiponectin. Because leptin and insulin increase adiponectin expression [27,19], reductions in these hormones with weight loss (as was observed in the present study) may be partly responsible for the decrease in HMW adiponectin. From a broader perspective, it may be biologically prudent to minimize circulating adiponectin concentrations, provided that insulin sensitivity is adequate, because elevated adiponectin concentrations have been consistently associated with earlier mortality [29–32]. Therefore, improvements in insulin sensitivity paired with reductions in adiponectin, as was observed in our study, might be viewed as an optimal adaptation.
Another important finding from the present study is that pentraxin-3 concentrations increased with weight loss in a manner that correlated with improvements in insulin sensitivity. Furthermore, pentraxin-3 was the strongest predictor of the improvement in insulin sensitivity when compared to the other significant predictors of ISI (i.e. GIP AUC, leptin, and HMW adiponectin). Although pentraxin-3 has been reported to have both pro- and anti-inflammatory effects [33], our finding suggests that it had beneficial effects on insulin action in our subjects. Cross-sectional studies have reported low pentraxin-3 concentrations with increasing adiposity [12,34,35] and insulin resistance [35] and in agreement with our findings, other studies showed that weight loss increases pentraxin-3 concentrations [12,36]. However, to our knowledge, ours is the only study to evaluate the association between pentraxin-3 and insulin sensitivity in response to weight loss. Additional research is needed to determine if pentraxin-3 is mechanistically linked with insulin sensitivity.
Two inflammatory markers showed little or no change with weight loss. A marginally significant decrease in CRP concentrations was observed (p=0.06). This finding is similar to what we previously observed in non-obese individuals undergoing weight loss [37]. We also measured serum levels of MCP-1, which has been mechanistically linked with propagating adipose tissue inflammation by recruiting macrophages [38]. However, no changes were observed. Neither the changes in changes in CRP or MCP-1 were associated with changes in insulin sensitivity. These findings are consistent with the notion that our participants did not have chronic inflammation at baseline and that the improvements in insulin sensitivity may not have been mediated by reductions in inflammation.
We previously measured serum concentrations of TNFα in response to modest weight loss in non-obese adults and found no change [39]. However, TNFα concentrations are very low (~1 pg/mL) and subject to diurnal variation [40], which raises the possibility that measurement error may have masked modest changes. Therefore, we measured sTNFR1 as a more abundant (~1 ng/mL) and stable indicator of TNF system activity. Small and opposing effects were observed in response to CR (5% decrease) and exercise (6% increase), with no change in the CREX group. These findings are suggestive of opposing effects of CR and EX on TNF system activity.
This study has limitations. First, although circulating cytokines have important physiologic roles, they do not necessarily reflect tissue concentrations. This is especially important in light of the autocrine and paracrine roles of many cytokines. Therefore, future studies are needed in which adipose, muscle, and other tissues are analyzed for cytokine content. Another limitation is that we did not include normal weight and obese subjects, which would have allowed us to determine more definitively whether these populations have different HMW adiponectin responses to weight loss. Lastly, to eliminate the potentially confounding effects of negative energy balance on the outcomes [41], we included a weight stability (i.e. energy balance) period of ≥2 weeks after weight loss and prior to follow-up testing. To our knowledge, the time course of changes in insulin sensitivity and cytokines during weight stability after weight loss is not known. Therefore, it is possible that this time frame may not have been sufficient for the outcome measures to stabilize after active weight loss. Other studies have used weight stability periods of 5 days [42,43], 2 weeks [23,24], or 4 weeks [44] or did not describe a weight stability period [45,26].
In conclusion, modest weight loss in overweight and sedentary women and men decreased circulating concentrations of leptin, HMW adiponectin, and pentraxin-3 and that all of these were correlated with improvements in insulin sensitivity. Additionally, exercise had additive effects to those from CR for reductions in leptin and possibly HMW adiponectin, even in the absence of greater weight loss. Finally, CR and matched weight loss induced by exercise had opposite effects on sTNFR1, as an indicator of TNF system activation. Taken together, these findings provide novel insights about changes in circulating cytokines that may contribute to improvements in insulin sensitivity in overweight men and women undergoing weight loss. The findings also indicate that CR and exercise training may each have unique beneficial effects, as evidence by differential effects on leptin, HMW adiponectin, and sTNFR1. Thus, modest weight loss using both calorie restriction and exercise should be encouraged for overweight women and men for the purpose of improving insulin sensitivity and potentially decreasing type 2 diabetes risk.
Acknowledgments
We are grateful to the study participants for their cooperation and to the staff of the NIH/ICTS Clinical Research Unit for their skilled assistance. We thank the Saint Louis University Department of Campus Recreation for providing our study participants with access to the campus fitness facilities.
Grants: This work was supported by National Institutes of Health grants K01 DK080886, DK56341 (Nutrition and Obesity Research Center), and UL1 RR024992 (Clinical Translational Science Award) and by a grant from the Saint Louis University President’s Research Fund.
Footnotes
Conflict of Interest. The authors declare that they have no conflict of interest.
Ethical approval. All procedures performed were in accordance with the ethical standards of the institutional review boards at Saint Louis University and at Washington University, and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent. Informed consent was obtained from all individual participants included in the study.
Trial registration: clinicaltrials.gov identifier NCT00777621
References
- 1.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller GD, Isom S, Morgan TM, Vitolins MZ, Blackwell C, Brosnihan KB, Diz DI, Katula J, Goff D. Effects of a community-based weight loss intervention on adipose tissue circulating factors. Diabetes Metab Syndr. 2014;8(4):205–211. doi: 10.1016/j.dsx.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiss EP, Racette SB, Villareal DT, Fontana L, Steger-May K, Schechtman KB, Klein S, Holloszy JO Washington University School of Medicine, C.G. Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: a randomized controlled trial. Am J Clin Nutr. 2006;84(5):1033–1042. doi: 10.1093/ajcn/84.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Diabetes Association. Prevention or Delay of Type 2 Diabetes. Diabetes Care. 2016;39(Suppl 1):S36–38. doi: 10.2337/dc16-S007. [DOI] [PubMed] [Google Scholar]
- 5.Weiss EP, Albert SG, Reeds DN, Kress KS, Ezekiel UR, McDaniel JL, Patterson BW, Klein S, Villareal DT. Calorie Restriction and Matched Weight Loss From Exercise: Independent and Additive Effects on Glucoregulation and the Incretin System in Overweight Women and Men. Diabetes Care. 2015;38(7):1253–1262. doi: 10.2337/dc14-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat , Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients) The National Academies Press; Washington D.C: 2005. [Google Scholar]
- 7.Sallis JF. Seven-Day Physical Activity Recall. Med Sci Sports Exerc. 1997;29(6 Supplement):S89–S103. [Google Scholar]
- 8.Delporte ML, El Mkadem SA, Quisquater M, Brichard SM. Leptin treatment markedly increased plasma adiponectin but barely decreased plasma resistin of ob/ob mice. Am J Physiol Endocrinol Metab. 2004;287(3):E446–453. doi: 10.1152/ajpendo.00488.2003. [DOI] [PubMed] [Google Scholar]
- 9.Bruce CR, Mertz VA, Heigenhauser GJ, Dyck DJ. The stimulatory effect of globular adiponectin on insulin-stimulated glucose uptake and fatty acid oxidation is impaired in skeletal muscle from obese subjects. Diabetes. 2005;54(11):3154–3160. doi: 10.2337/diabetes.54.11.3154. [DOI] [PubMed] [Google Scholar]
- 10.Magkos F, Fabbrini E, Patterson BW, Eagon JC, Klein S. Portal vein and systemic adiponectin concentrations are closely linked with hepatic glucose and lipoprotein kinetics in extremely obese subjects. Metabolism. 2011;60(11):1641–1648. doi: 10.1016/j.metabol.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116(6):1494–1505. doi: 10.1172/jci26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Witasp A, Carrero JJ, Michaelsson K, Ahlstrom H, Kullberg J, Adamsson V, Riserus U, Larsson A, Helmersson-Karlqvist J, Lind L, Stenvinkel P, Arnlov J. Inflammatory biomarker pentraxin 3 (PTX3) in relation to obesity, body fat depots and weight loss. Obesity (Silver Spring) 2014;22(5):1373–1379. doi: 10.1002/oby.20695. [DOI] [PubMed] [Google Scholar]
- 13.Miyaki A, Maeda S, Choi Y, Akazawa N, Tanabe Y, Ajisaka R. Habitual aerobic exercise increases plasma pentraxin 3 levels in middle-aged and elderly women. Appl Physiol Nutr Metab. 2012;37(5):907–911. doi: 10.1139/h2012-069. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. Report of a WHO Consultation. Part 1. Diagnosis and Classification of Diabetes Mellitus. Geneva: 1999. Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications. [Google Scholar]
- 15.American Diabetes Association. 2. Classification and Diagnosis of Diabetes. Diabetes Care. 2016;39(Suppl 1):S13–22. doi: 10.2337/dc16-S005. [DOI] [PubMed] [Google Scholar]
- 16.Allison DB, Paultre F, Maggio C, Mezzitis N, Pi-Sunyer FX. The use of areas under curves in diabetes research. Diabetes Care. 1995;18(2):245–250. doi: 10.2337/diacare.18.2.245. [DOI] [PubMed] [Google Scholar]
- 17.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 18.Stumvoll M, Mitrakou A, Pimenta W, Jenssen T, Yki-Jarvinen H, Van Haeften T, Renn W, Gerich J. Use of the oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care. 2000;23(3):295–301. doi: 10.2337/diacare.23.3.295. [DOI] [PubMed] [Google Scholar]
- 19.Hernandez-Morante JJ, Milagro FI, Lujan JA, Martinez JA, Zamora S, Garaulet M. Insulin effect on adipose tissue (AT) adiponectin expression is regulated by the insulin resistance status of the patients. Clin Endocrinol (Oxf) 2008;69(3):412–417. doi: 10.1111/j.1365-2265.2008.03185.x. [DOI] [PubMed] [Google Scholar]
- 20.Kelly KR, Navaneethan SD, Solomon TP, Haus JM, Cook M, Barkoukis H, Kirwan JP. Lifestyle-induced decrease in fat mass improves adiponectin secretion in obese adults. Med Sci Sports Exerc. 2014;46(5):920–926. doi: 10.1249/mss.0000000000000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mai S, Walker GE, Brunani A, Guzzaloni G, Grossi G, Oldani A, Aimaretti G, Scacchi M, Marzullo P. Inherent insulin sensitivity is a major determinant of multimeric adiponectin responsiveness to short-term weight loss in extreme obesity. Sci Rep. 2014;4:5803. doi: 10.1038/srep05803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang WS, Lee WJ, Funahashi T, Tanaka S, Matsuzawa Y, Chao CL, Chen CL, Tai TY, Chuang LM. Weight reduction increases plasma levels of an adipose-derived anti-inflammatory protein, adiponectin. J Clin Endocrinol Metab. 2001;86(8):3815–3819. doi: 10.1210/jcem.86.8.7741. [DOI] [PubMed] [Google Scholar]
- 23.Ryan AS, Nicklas BJ, Berman DM, Elahi D. Adiponectin levels do not change with moderate dietary induced weight loss and exercise in obese postmenopausal women. Int J Obes Relat Metab Disord. 2003;27(9):1066–1071. doi: 10.1038/sj.ijo.0802387. [DOI] [PubMed] [Google Scholar]
- 24.Abbasi F, Chang SA, Chu JW, Ciaraldi TP, Lamendola C, McLaughlin T, Reaven GM, Reaven PD. Improvements in insulin resistance with weight loss, in contrast to rosiglitazone, are not associated with changes in plasma adiponectin or adiponectin multimeric complexes. Am J Physiol Regul Integr Comp Physiol. 2006;290(1):R139–R144. doi: 10.1152/ajpregu.00287.2005. [DOI] [PubMed] [Google Scholar]
- 25.Abbasi F, Lamendola C, McLaughlin T, Hayden J, Reaven GM, Reaven PD. Plasma adiponectin concentrations do not increase in association with moderate weight loss in insulin-resistant, obese women. Metabolism. 2004;53(3):280–283. doi: 10.1016/j.metabol.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 26.Wolfe BE, Jimerson DC, Orlova C, Mantzoros CS. Effect of dieting on plasma leptin, soluble leptin receptor, adiponectin and resistin levels in healthy volunteers. Clin Endocrinol (Oxf) 2004;61(3):332–338. doi: 10.1111/j.1365-2265.2004.02101.x. [DOI] [PubMed] [Google Scholar]
- 27.Singh P, Sharma P, Sahakyan KR, Davison DE, Sert-Kuniyoshi FH, Romero-Corral A, Swain JM, Jensen MD, Lopez-Jimenez F, Kara T, Somers VK. Differential effects of leptin on adiponectin expression with weight gain versus obesity. Int J Obes (Lond) 2015 doi: 10.1038/ijo.2015.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hector J, Schwarzloh B, Goehring J, Strate TG, Hess UF, Deuretzbacher G, Hansen-Algenstaedt N, Beil FU, Algenstaedt P. TNF-alpha alters visfatin and adiponectin levels in human fat. Horm Metab Res. 2007;39(4):250–255. doi: 10.1055/s-2007-973075. [DOI] [PubMed] [Google Scholar]
- 29.Ortega Moreno L, Copetti M, Fontana A, De Bonis C, Salvemini L, Trischitta V, Menzaghi C. Evidence of a causal relationship between high serum adiponectin levels and increased cardiovascular mortality rate in patients with type 2 diabetes. Cardiovasc Diabetol. 2016;15(1):17. doi: 10.1186/s12933-016-0339-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Witberg G, Ayers CR, Turer AT, Lev E, Kornowski R, de Lemos J, Neeland IJ. Relation of Adiponectin to All-Cause Mortality, Cardiovascular Mortality, and Major Adverse Cardiovascular Events (from the Dallas Heart Study) Am J Cardiol. 2016;117(4):574–579. doi: 10.1016/j.amjcard.2015.11.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi SH, Ku EJ, Hong ES, Lim S, Kim KW, Moon JH, Kim KM, Park YJ, Park KS, Jang HC. High serum adiponectin concentration and low body mass index are significantly associated with increased all-cause and cardiovascular mortality in an elderly cohort, “adiponectin paradox”: the Korean Longitudinal Study on Health and Aging (KLoSHA) Int J Cardiol. 2015;183:91–97. doi: 10.1016/j.ijcard.2015.01.057. [DOI] [PubMed] [Google Scholar]
- 32.Sook Lee E, Park SS, Kim E, Sook Yoon Y, Ahn HY, Park CY, Ho Yun Y, Woo Oh S. Association between adiponectin levels and coronary heart disease and mortality: a systematic review and meta-analysis. Int J Epidemiol. 2013;42(4):1029–1039. doi: 10.1093/ije/dyt087. [DOI] [PubMed] [Google Scholar]
- 33.Deban L, Russo RC, Sironi M, Moalli F, Scanziani M, Zambelli V, Cuccovillo I, Bastone A, Gobbi M, Valentino S, Doni A, Garlanda C, Danese S, Salvatori G, Sassano M, Evangelista V, Rossi B, Zenaro E, Constantin G, Laudanna C, Bottazzi B, Mantovani A. Regulation of leukocyte recruitment by the long pentraxin PTX3. Nat Immunol. 2010;11(4):328–334. doi: 10.1038/ni.1854. [DOI] [PubMed] [Google Scholar]
- 34.Abu Seman N, Witasp A, Wan Mohamud WN, Anderstam B, Brismar K, Stenvinkel P, Gu HF. Evaluation of the association of plasma pentraxin 3 levels with type 2 diabetes and diabetic nephropathy in a Malay population. J Diabetes Res. 2013;2013:298019. doi: 10.1155/2013/298019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chu SH, Park JH, Lee MK, Jekal Y, Ahn KY, Chung JY, Lee DH, Kim ES, Naruse M, Im JA, Kong ID, Chung CH, Lee JW, Chung KM, Kim YB, Jeon JY. The association between pentraxin 3 and insulin resistance in obese children at baseline and after physical activity intervention. Clin Chim Acta. 2012;413(19–20):1430–1437. doi: 10.1016/j.cca.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 36.Barazzoni R, Palmisano S, Gortan Cappellari G, Giuricin M, Moretti E, Vinci P, Semolic A, Guarnieri G, Zanetti M, Manzini N. Gastric bypass-induced weight loss alters obesity-associated patterns of plasma pentraxin-3 and systemic inflammatory markers. Surg Obes Relat Dis. 2016;12(1):23–32. doi: 10.1016/j.soard.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 37.Fontana L, Villareal DT, Weiss EP, Racette SB, Steger-May K, Klein S, Holloszy JO Washington University School of Medicine, C.G. Calorie restriction or exercise. effects on coronary heart disease risk factors. A randomized, controlled trial. Am J Physiol Endocrinol Metab. 2007;293(1):E197–202. doi: 10.1152/ajpendo.00102.2007. [DOI] [PubMed] [Google Scholar]
- 38.Yu R, Kim CS, Kwon BS, Kawada T. Mesenteric adipose tissue-derived monocyte chemoattractant protein-1 plays a crucial role in adipose tissue macrophage migration and activation in obese mice. Obesity (Silver Spring) 2006;14(8):1353–1362. doi: 10.1038/oby.2006.153. [DOI] [PubMed] [Google Scholar]
- 39.Weiss EP, Racette SB, Villareal DT, Fontana L, Steger-May K, Schechtman KB, Klein S, Holloszy JO. Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: a randomized controlled trial. Am J Clin Nutr. 2006;84(5):1033–1042. doi: 10.1093/ajcn/84.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petrovsky N, Harrison LC. The chronobiology of human cytokine production. Int Rev Immunol. 1998;16(5–6):635–649. doi: 10.3109/08830189809043012. [DOI] [PubMed] [Google Scholar]
- 41.Kelley DE, Wing R, Buonocore C, Sturis J, Polonsky K, Fitzsimmons M. Relative effects of calorie restriction and weight loss in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1993;77(5):1287–1293. doi: 10.1210/jcem.77.5.8077323. [DOI] [PubMed] [Google Scholar]
- 42.Joseph LJ, Trappe TA, Farrell PA, Campbell WW, Yarasheski KE, Lambert CP, Evans WJ. Short-term moderate weight loss and resistance training do not affect insulin-stimulated glucose disposal in postmenopausal women. Diabetes Care. 2001;24(11):1863–1869. doi: 10.2337/diacare.24.11.1863. [DOI] [PubMed] [Google Scholar]
- 43.Joseph LJ, Prigeon RL, Blumenthal JB, Ryan AS, Goldberg AP. Weight loss and low-intensity exercise for the treatment of metabolic syndrome in obese postmenopausal women. J Gerontol A Biol Sci Med Sci. 2011;66(9):1022–1029. doi: 10.1093/gerona/glr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kirk E, Reeds DN, Finck BN, Mayurranjan SM, Patterson BW, Klein S. Dietary fat and carbohydrates differentially alter insulin sensitivity during caloric restriction. Gastroenterology. 2009;136(5):1552–1560. doi: 10.1053/j.gastro.2009.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jazet IM, Schaart G, Gastaldelli A, Ferrannini E, Hesselink MK, Schrauwen P, Romijn JA, Maassen JA, Pijl H, Ouwens DM, Meinders AE. Loss of 50% of excess weight using a very low energy diet improves insulin-stimulated glucose disposal and skeletal muscle insulin signalling in obese insulin-treated type 2 diabetic patients. Diabetologia. 2008;51(2):309–319. doi: 10.1007/s00125-007-0862-2. [DOI] [PubMed] [Google Scholar]