Abstract
Intermittently administered parathyroid hormone (PTH 1-34) has been shown to promote bone formation in both human and animal studies. The hormone and its analogues stimulate both bone formation and resorption, and as such at low doses are now in clinical use for the treatment of severe osteoporosis. By varying the duration of exposure, parathyroid hormone can modulate genes leading to increased bone formation within a so-called ‘anabolic window’. The osteogenic mechanisms involved are multiple, affecting the stimulation of osteoprogenitor cells, osteoblasts, osteocytes and the stem cell niche, and ultimately leading to increased osteoblast activation, reduced osteoblast apoptosis, upregulation of Wnt/β-catenin signalling, increased stem cell mobilisation, and mediation of the RANKL/OPG pathway. Ongoing investigation into their effect on bone formation through ‘coupled’ and ‘uncoupled’ mechanisms further underlines the impact of intermittent PTH on both cortical and cancellous bone. Given the principally catabolic actions of continuous PTH, this article reviews the skeletal actions of intermittent PTH 1-34 and the mechanisms underlying its effect.
Cite this article: L. Osagie-Clouard, A. Sanghani, M. Coathup, T. Briggs, M. Bostrom, G. Blunn. Parathyroid hormone 1-34 and skeletal anabolic action: The use of parathyroid hormone in bone formation. Bone Joint Res 2017;6:14–21. DOI: 10.1302/2046-3758.61.BJR-2016-0085.R1.
Keywords: Parathyroid hormone, Bone formation, Anabolic
Introduction
Human parathyroid hormone (PTH) is an 84-amino acid polypeptide secreted from the parathyroid gland. Acting predominantly on the skeletal system and renal tubules, the hormone modulates serum calcium and phosphate.1-3 Teriparatide (PTH 1-34) is the N-terminal fragment of the intact hormone and is approved for use in the treatment of osteoporosis in both the United States and Europe.4 During normal homeostatic conditions bone resorption is equal to bone formation,5,6 yet PTH, when administered in both a continuous and intermittent (iPTH) fashion, alters this balance leading to increased bone turnover. Moreover, when administered intermittently, there is an early stimulation in bone formation without an increase in bone resorption, termed the ‘anabolic window’.7,8 Both animal and human studies have supported these findings in young and old subjects. Studies, including those conducted on postmenopausal women, demonstrate a profound anabolic effect of PTH in areas of increased bone turnover such as at fracture sites and at the acute bone-implant interface.9-12
Both PTH 1-34 and PTH 1-84 are used in the treatment of osteoporosis in postmenopausal women, with both having been shown to reduce the risk of new vertebral fractures.13-16 In addition to these agents, parathyroid hormone-related protein (PTHrP) has gained more attention in the last decade. This factor is isolated from tumours associated with the paraneoplastic syndrome of humoral hypercalcaemia of malignancy, and shares homology with PTH 1-34.17 Shown to be integral to bone formation, PTHrP acts via both PTH-Receptor1 (PTHR1) and an unrelated PTHrP-specific osteoblast surface receptor.17,18 PTHrP mediates osteoblast differentiation and proliferation via a number of pathways shared with PTH (extracellular signal related kinases, cyclin dependent kinase inhibitor (ERK,P27) ERK, P27) and receptor activator of nuclear kappa B ligand/osteoprotegerin (RANKL/OPG); though unlike teriparatide, its use remains off-label. Nonetheless, the clinical applications of PTHrP may be further reaching than those of teriparatide alone, with rodent and canine studies outlining the regulatory role of PTHrP on cartilaginous proliferation at the growth plate.19-20
The so-called ‘anabolic window’ synonymous with intermittent PTH 1-34 is a result of multiple mechanisms, the net effect being a positive balance between formation and resorption within the bone remodelling unit (BMU). Intermittent dosing has been shown to have an effect on both remodelling- and modelling-based bone formation at cortical and cancellous surfaces.21-22 Although highlighting the differences between continuous and intermittent administration, this review focuses on the mechanisms of pulsatile PTH 1-34.
Anabolic role of PTH
Osteoblast activation and stem cell differentiation
Both continuous and intermittent administration of PTH lead to increased bone turnover at trabecular and cortical sites.21-24 However, continuous dosing results in increased osteoclast activation and lifespan, and thus enhanced endosteal resorption. In contrast, intermittent dosing results in increased trabecular bone volume. A significant proportion of this anabolism is mediated by the intermittent PTH effect on the osteoblast. The primary receptor for PTH and PTHrP is the G-protein-coupled receptor PTH1R, known to be expressed on the surface of osteoblasts, osteocytes, stromal cells, T cells and macrophages.25-27 At low concentrations, PTH binds preferentially to PTH1R on cells of the osteoblastic lineage, thus driving osteoblastic bone formation.28,29 Moreover, rapid degradation of the hormone ensures that osteoclastic bone resorption is not activated via this mechanism. Stimulation of PTH1R activates a multitude of G-proteins including the Gαs-mediated cascade, which leads to the conversion of adenylyl cyclase to cyclic-adenosine monophosphate and the activation of protein kinase a (cAMP/PKA pathway).30,31 The resultant subunits translocate into the cell nucleus, leading to transcription factor phosphorylation and the expression of messenger ribonucleic acid (mRNA) responsible for the anabolic effects.
The transient upregulation of mRNAs encoding for transcription factors, cytokines and growth factors via cAMP/PKA signalling mediates a variety of the anabolic actions of intermittent PTH. Upregulation has been demonstrated to occur within the first six to nine hours following administration.32-33 As such, intermittent PTH may lead to repeated cycles of upregulation and thus to an overall net anabolic effect. Of these multiple transcription factors, both the Notch ligand jagged-1 and the proto-oncogene c-fos play substantial roles in the proliferation of osteoblasts as a result of PTH treatment.34-36 In vivo studies have shown that c-fos expression is greatest from osteoblasts following the administration of intermittent PTH in the first 90 minutes, after which gene production was a result only of the osteoclast population.35 Intermittent PTH has also been shown to induce the activation of runt related transcription factor 2 (RUNX2). This transcription factor not only drives the differentiation of stem cells down the osteoblastic lineage, but it also maintains osteoblast maturity. However, continuous administration leads to rapid degradation of RUNX2 and thus reduced bone formation.37-38 These changes to RUNX2 levels are thought to be due to alterations of the genes’ stability through changes mediated by the cell cycle regulator cyclin D1.39-40
The role of insulin-like growth factors (IGF) on bone anabolism can also not be understated; these proteins have been shown to not only induce osteoblast differentiation of stem cells, but also to increase the activity of mature osteoblasts.41 Intermittent PTH dosing increases the expression of IGF-1 in rodent experiments, with knockout IGF-1 animals, showing no change in bone formation when treated with intermittent PTH,42 while human studies in postmenopausal women demonstrated similar effects with concurrent increases in IGF-2 and bone formation following short courses of intermittent PTH.43 Similar to RUNX2, IGF not only affects osteoblast differentiation and activity, but also plays a role in cell survival via an anti-apoptotic effect.44
Osteoblast apoptosis
A large body of work has demonstrated that increased osteoblast number is driven by an anti-apoptotic effect and thus, increased cell survival rates.36 Osteoporotic murine models are known to have increased osteoblast apoptosis in cancellous regions, yet this phenomenon is reversed following in vivo administration of intermittent TH, leading to an overall increase in bone formation.36,45 Of note, the anti-apoptotic effect of PTH is mediated via actions on the PTHR1 at the earlier stages of osteoblast differentiation, with very little effect seen with mature cells, resulting in a net ‘clearance’ of older osteoblasts in preference for younger cells.45
In vitro work has demonstrated that the cAMP/PKA pathway is the underlying mode for this anti-apoptotic effect, leading to phosphorylation of the cellular transcription factor cAMP response element binding protein (CREB), the transcription of anti-apoptotic genes Bcl-2 and P21, and the inactivation of pro-apoptotic genes such as Bad and the apoptosis inducer Cell Cycle Apoptosis Regulator Protein (CARP-1).36,45-48 Conversely, continuous administration results in an inhibition of RUNX2 through proteasomal degradation by Smad, thus reducing osteoblast survival.45
Wnt signalling
One of the major effects of intermittent PTH is via activation of the canonical Wnt pathway and, in turn, Wnt/β-catenin signalling in osteoblasts. Essential for normal bone formation and cartilage repair, the pathway not only mediates the differentiation of stem cells to osteoblasts but it also regulates the maturation, proliferation and anti-apoptosis of osteoblast precursors.44,49-50 Canonical Wnt is imperative in PTH anabolism, whereby Wnt ligands activate frizzled receptors (Fzd) and low-density receptor proteins 5 and 6 (LRP5, LRP6). This dimeric receptor complex reduces the proteolysis of β-catenin, resulting in its increased stability and accumulation within the nucleus. Intermittent PTH is a pathway agonist, thus, the accumulated β-catenin binds to T-cell-specific transcription factor (TCF) and lymphoid enhancing factor (LEF), displacing the Groucho repressor gene, and thereby allowing the transcription of Wnt-specific osteoblast differentiation genes.51
Sclerostin is a glycoprotein primarily secreted from osteocytes, and acts to antagonise the canonical Wnt pathway.52-53 Mutations in the SOST gene and thus reductions in sclerostin levels lead to Van Buchem disease and sclerosteosis, phenotypically characterised by bone overgrowth and skeletal sclerosis. Sclerostin can occupy the Wnt ligand binding sites on LRP5 and LRP6, and therefore reduce Wnt signalling, leading to reduced bone formation. Intermittent PTH reduces SOST mRNA levels and increases bone mineral density in vivo via the cAMP/PKA signalling pathway downstream to PTHR1.54,55 Moreover, transgenic mice overexpressing PTHR1 have shown reduced SOST levels and concurrently increased bone mass.54 As such, in a mouse model with osteocytes ablated of PTH1R, intermittent PTH failed to suppress SOST expression, with an undetectable effect on bone formation.56 Similarly, serum sclerostin levels in healthy women are inversely correlated to PTH serum levels, while postmenopausal women treated with teriparatide also demonstrate reduced serum sclerostin levels. In addition to the effects of PTH on sclerostin levels, a large body of work has investigated the role of PTH on canonical Wnt antagonists Dickkopf-1 (Dkk1). Unlike sclerostin, Dkk1 is expressed in cells of the osteoblast lineage, though similarly acts on LRP5/6 to inhibit Wnt signalling.57 Intermittent PTH has been found to reduce Dkk1 mRNA levels, leading to the functional activation of the canonical Wnt pathway.58,59 These in vitro findings were supported by in vivo work on transgenic mice overexpressing Dkk1, which, when dosed with intermittent PTH, demonstrated a blunting of the anabolic effect. There remains some controversy on the importance of Dkk1 specifically, as opposing studies have demonstrated no effect of increased Dkk1 levels on Wnt signalling, with variable results on the actions of PTH.60,61
A growing body of work has investigated the effect of PTH on LRP5 and LRP6. Inactivation of LRP5 has been shown to result in osteoporosis-pseudoglioma syndrome62 associated with premature generalised osteoporosis, while LRP6 mutation leads to early coronary disease in addition to severe osteoporosis.63 Significantly, intermittent PTH still exerts a bone-forming effect in LRP5-deficient mice, yet, as the PTHR1/LRP6 complex leads to the upregulation of β-catenin signalling, and thus TCF/LEF-mediated stimulation of bone formation,64,65 LRP6 is specifically required for differentiation and survival of osteoblasts during bone remodelling.65 Resultantly, the anabolic effects of intermittent PTH are significantly blunted in LRP6 knockout mice.63-66
The activation of Wnt signalling is dependent on a multitude of factors, one of which is osteoimmunity and the role of T cells. In the absence of T cells, it has been demonstrated in vivo that intermittent PTH does not induce increased proliferation or differentiation of osteoblasts, nor does it reduce apoptosis. Both CD4 and CD8 T cells are thought to be particularly vital, with in vivo studies outlining the ability of CD8 T cells to potentiate intermittent PTH anabolism through its provision of Wnt-10b.67,68 Similarly, human studies have demonstrated that in the context of teriparatide treatment, again, CD8 T cells were the main source of increased levels of Wnt-10b; this finding was not replicated in patients with primary hyperparathyroidism.68
Stromal cell activation and mobilisation
Studies have identified the pivotal action of intermittent PTH on the reactivation of quiescent periosteal lining cells into active osteoblasts, subsequently leading to an increase in overall osteoblast number and net bone formation.69-71 In vivo lineage tracing has demonstrated increased osteoblast number on the periosteal surface of animals treated with intermittent PTH. This is in the context of reduced bone lining cell fraction, reactivated traced lining cells and the absence of increased osteoblast proliferation overall,70,71 all of which led to significant increases in osteoblast proliferation.
The effect of intermittent PTH on the bone marrow stromal cell niche has also been elucidated, identifying actions on perivascular niches created in part by mesenchymal stromal cells and often located near trabecular bone. Calvi et al72 outlined the pivotal role of intermittent PTH on the stem cell niche microenvironment, demonstrating the regulatory role of osteoblasts on the haematopoietic stem cell niche using transgenic mice with activated PTH/PTHrP receptors (PPRs). The receptor-specific osteoblasts were found to produce increased levels of the Notch ligand Jagged 1, resulting in an increased volume of haematopoietic stem cells with Notch ligand activation. Their further work assessed this effect in vivo, whereby intermittent PTH was administered to mice undergoing myeloablative bone marrow transplantation.73 Results showed a 73% improvement in animal survival after 28 days, with an expansion of bone marrow cellularity and reduced adipocytes when treated with PTH.
The ability of intermittent PTH to mobilise or increase the migration of cells from the haematopoietic niche is particularly significant in the context of sites of increased bone turnover (fractures and peri-implant). Cells are initially ‘mobilised’ from their niche into the circulation, migrate across the tissue endothelium and mature into active cell types, eventually ‘modulating’ the local environment. The SDF-1/CXCR4 axis has been found to be an important regulator of stem cell migration.74-77 Stromal derived factor-1/CXCL12 (SDF-1) is produced by a multitude of tissue types including fracture endosteum, and in its active form is bound to the chemokine receptor type 4 (CXCR4) r found on mesenchymal stem cells. Granero-Moltó et al78 demonstrated that dynamic stem cell migration to the fracture site in a stabilised tibial osteotomy model was CXCR4-dependent. The clinical significance of the SDF-1/CXCR4 axis has further been alluded to, whereby the overexpression of CXCR4 on mesenchymal stem cells leads to significant increases in bone mineral density, thus having implications in the treatment of osteoporosis.78 In addition to a body of work from haematology and cardiologists,79-81 Kitaori et al82 demonstrated increased osteoblast expression of SDF-1 following intermittent PTH administration and thus upregulation of the stem cell homing axis SDF-1/CXCR4, with significant implications for endochondral repair and increased bone volume fraction.82
Catabolic role of PTH
As discussed, many of the effects of intermittent PTH are mediated via actions on the PTHR1 receptor, affecting both aspects of skeletal remodelling, with net anabolic or catabolic effects dependent upon duration of exposure and dosage. In vivo and in vitro studies have demonstrated that these resorptive effects are a result of direct and indirect osteoclast activation, and of cascades mediated by both osteoblast and osteocyte actions.
The RANKL/OPG axis
Unlike the multiple mediators of the anabolic actions of intermittent PTH, it is the Receptor activator of nuclear factor kappa-B ligand/osteoprotegerin ligand (RANKL/OPG) pathway that predominantly regulates the effects on osteoclastogenesis of intermittent PTH. RANKL produced by osteoblasts binds to RANK on the surface of osteoclast precursors, leading to nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB )activation, and ultimately maturation and terminal differentiation of these cells into mature osteoclasts.83-84 Conversely, OPG secreted by stromal cells is a soluble decoy receptor, binding to RANKL, inhibiting RANK activation and in turn reducing bone resorption.83-84 The varying balance between resorption and formation, as seen with continuous and intermittent PTH, can in part be explained through the regulation of the RANKL/OPG pathway. The mRNA encoding for RANKL is increased and for OPG decreased in the presence of continuous PTH, leading to increased osteoclastogenesis and thus bone resorption.85,86 As such, human studies also confirm the effects of PTH exposure on serum RANKL levels, with these markers correlating with femoral bone loss and increased bone resorption markers87 in patients with primary hyperparathyroidism, demonstrating increased serum RANKL and the RANKL/OPG ratio.88 In addition, recent work has further highlighted the role of osteocytes whereby transgenic mice, either overexpressing or ablated of PPR/RANKL, demonstrated increased osteocyte production of RANKL following continuous PTH and subsequently had increased bone loss.89
Although not yet fully understood, there is physiological coupling between osteoclasts and osteoblasts mediated by systemic hormones.90 Intermittent PTH mimics this trend, coupling osteoclasts to osteoblasts and highlighting the pertinent role of active resorption. Evidence has demonstrated the direct resorptive effects of PTH, by activation of a PTHR1 receptor identified on osteoclasts, with a number of studies identifying upregulation of the receptor in pathological states.91,92 Such findings suggest a dual mechanism whereby PTH acts not only directly on osteoclasts but also indirectly via osteoblasts.
Similar to the pro-osteoclast activity of upregulating RANKL levels, PTH has also been shown to affect cell response and production of macrophage colony-stimulating factor (M-CSF). M-CSF is a cytokine involved in the regulation of both cell proliferation and differentiation from the bone marrow niche; in vivo stimulation by PTH leads to its release from osteoblasts and subsequent effects on osteoclasts.93 Indeed, murine studies have demonstrated that increased RANKL and M-CSF levels stimulated osteoclast formation and bone resorption following intermittent PTH treatment, though this effect was ameliorated after 14 days.94 The importance of increased osteoclast number, through increases in the RANKL/OPG ratio and M-CSF mediated by intermittent PTH, has an unclear role in the overall bone anabolism.95,96 The relationship between osteoclast and osteoblast number may further underpin bone formation, and, as such, may be integral to coupled bone formation.88,89
Monocyte chemoattractant protein-1 (MCP- is one of the key chemokines that regulate migration and infiltration of monocytes or macrophages. MCP-1 is produced by many cell types, including osteoblasts, endothelial cells, fibroblasts, epithelial, smooth muscle cells, mesangial cells, astrocytes, monocytes, and microglial cells. Chemokines selectively recruit monocytes, neutrophils, and lymphocytes, and induce chemotaxis through the activation of G-protein-coupled receptors. MCP-1 specifically plays an active role in PTH-induced bone resorption. Via the cAMP/PKA pathway, in vitro studies demonstrated increased expression of MCP-1 in rat osteoblasts following exposure to both intermittent and continuous PTH, leading to bone resorption through chemoattraction of RANKL-activated osteoclastogenesis and pre-osteoclasts. Importantly, although sustained during continuous infusion, intermittent PTH administration led to an initial spike followed by a rapid degradation in MCP-1 levels.97 As would be expected, patients with primary hyperparathyroidism demonstrated increased serum MCP-1 levels which, like RANKL, fell following parathyroidectomy.98
Modelling versus remodelling
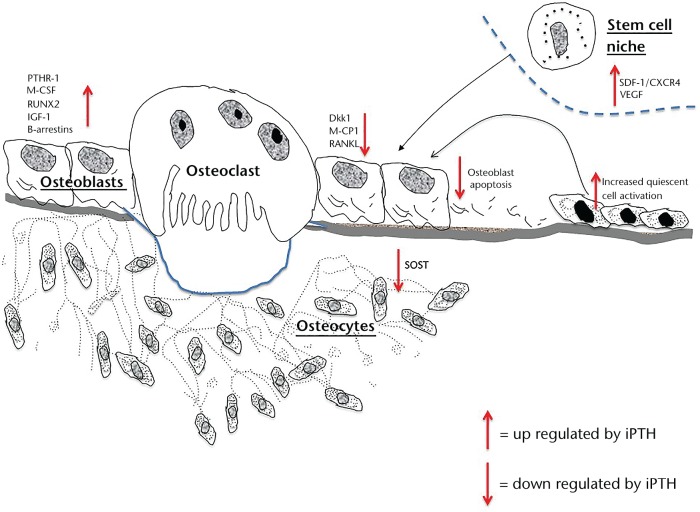
The modalities discussed thus far pertain to a balance of bone formation and resorption; intermittent PTH is known to rapidly increase markers of formation prior to also having an effect on resorption. Yet within this window, as previously discussed, intermittent PTH also activates quiescent lining cells on the modelling surface to further induce bone formation (Fig. 1). This ‘modelling’ mode of formation was initially identified with the use of double tetracycline labelling in osteoporotic women.99 Rodent data suggested that modelling accounted for only 20% of bone formation, with this figure further decreasing with age, while human studies indicate between 5% and 30% of formation occurs at modelling surfaces.42,100 Rhee et al100 further demonstrated the role of intermittent PTH on modelling-based bone formation, whereby RANK deficient mice were treated with intermittent PTH, and thus remodelling was ablated in the absence of active osteoclasts. Resultantly, short-term high-dose PTH led to increases in serum osteocalcin, and trabecular and cortical bone mineral density. More recent evidence has suggested that the effect of intermittent PTH on modelling is most significant in the early stages of treatment in human populations, whereby the proportion of modelling-based bone formation reduces in the steady state situation following long-term intermittent PTH treatment in osteoporotic populations.101,102
Fig. 1.
Illustration of the regulatory actions of intermittent PTH 1-34
In both modelling- and remodelling-based bone formation, the actions of PTH receptor signalling on osteocytes are pivotal. DMP1–8kb-caPTHR1 transgenic mice express a constitutively active PTH osteocyte receptor and, in conjunction with anti-resorptive agents, have been used to investigate the role of modelling and remodelling within various bony compartments.100 Subsequently, on the periosteal cortical surface, inhibiting remodelling-based bone formation had no effect on overall bone formation or bone mineral density. Importantly, when SOST was overexpressed and thus the Wnt pathway blocked, this modelling-based bone formation at the periosteal surface was ablated.54 Ultimately, in animal studies, bone formation induced by osteocytic PTH receptor signalling on the periosteal surface appears to be Wnt pathway-dependent and independent of bone resorption; as such, periosteal bone formation enhanced by intermittent PTH may be predominantly a result of modelling effects.
Recently, increased investigation into PTHR1 activation has led to the development of abaloparatide, a synthetic analogue of PTHrP 1-34, which has demonstrated a lower catabolic profile than teriparatide, and thus clinically would have fewer hypercalcaemia-related side effects.99 Work has demonstrated that structurally distinct ligands can bind to differing receptor conformations; the R0 receptor conformation is G-protein-independent and thus unaffected by analogues that act to dissociate G protein-receptor complexes. Consequently, when activated with long-acting ligands like long-acting -PTH, the net effect is an increase in catabolism and net resorption. Conversely, abaloparatide and short-acting ligands have been found to have an affinity for the G-protein-sensitive receptor conformation RG.103-105 Subsequently, activation of the receptor occurs only briefly due to rapid dissociation of the ligand receptor complex upon G protein activation, thus inducing a net anabolic response in vivo. Phase 2 trials suggest that abaloparatide has a smaller stimulatory effect on remodelling than does teriparatide. As such, in clinical osteoporotic populations, this analogue may lead to a reduction in non-vertebral fractures with a concurrent improvement in cortical porosity and bone strength. Importantly, PTH 1-34 and 1-84 both led to abnormal bone architecture in murine studies, with a reported incidence of osteosarcomas following lifetime treatment,106-108 though this has not been reported with the use of abaloparatide.
Parathyroid hormone has profound and complex effects on the skeleton; its elevation in the circulation can generate both catabolic and anabolic effects depending on the temporal profile of its increase, and, as such, is used clinically in short bursts (12- to 18-month cycles). At the cellular level, intermittent PTH directly stimulates bone formation via osteoblasts, increasing number and activity; concurrently, intermittent PTH stimulates bone resorption by also increasing the recruitment and activation of osteoclasts. In conclusion, crosstalk between modelling- and remodelling-based bone formation is driven by PTH receptor signalling in osteocytes, osteoclasts, osteoblasts and undifferentiated cells. Ultimately there is no ‘common’ intermittent PTH pathway; instead, the hormone acts via multiple mechanisms to exert its anabolic effect. By understanding both the anabolic and catabolic actions of PTH 1-34, one can hope to enhance its clinical utility as a mode of increasing bone formation in both the osteoporotic and fracture-healing contexts.
Footnotes
Author Contribution: L. Osagie-Clouard: Writing body of text, Literature review, Article structure
A. Sanghani: Literature review
M. Coathup: Literature review and article structure
T. Briggs: Article structure and manuscript review
M. Bostrom: Manuscript concept and review
G. Blunn: Manuscript concept and review
ICMJE Conflicts of Intrest: None declared
Funding Statement
L. Osagie-Clouard has received a grant from the Rosetrees Trust, Gwen Fish Orthopaedic Fund and the Stoneygate trust, all of which is related to this article.
M. Bostrom carries out consultancy for Smith & Nephew and has an NIH grant unrelated to this work currently pending.
G. Blunn is a board member of OCUK, has provided expert testimony for Leigh Day, has received or has grants pending with Smith & Nephew, Lima and OCUK, and holds 12 patents for which royalties are received from Stryker, Biomet and Zimmer; none of which is related to this article.
References
- 1. Hanley DA, Watson PH, Hodsman AB, Dempster DW. Pharmacological Mechanisms of Therapeutics: Parathyroid Hormone. In: Bilezikian JP, Raisz LG, Martin TJ, eds. Principles of Bone Biology Vol. 2 Third ed. San Diego: Elsevier Inc., 2008:1661-1695. [Google Scholar]
- 2. Civitelli R, Ziambaras K. Calcium and phosphate homeostasis: concerted interplay of new regulators. J Endocrinol Invest 2011;34:3-7. [PubMed] [Google Scholar]
- 3. Egbuna OI, Brown EM. Hypercalcaemic and hypocalcaemic conditions due to calcium-sensing receptor mutations. Best Pract Res Clin Rheumatol 2008;22:129-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 2001;344:1434-1441. [DOI] [PubMed] [Google Scholar]
- 5. Tam CS, Heersche JN, Murray TM, Parsons JA. Parathyroid hormone stimulates the bone apposition rate independently of its resorptive action: differential effects of intermittent and continuous administration. Endocrinology 1982;110:506-512. [DOI] [PubMed] [Google Scholar]
- 6. Greenfield EM. Anabolic effects of intermittent PTH on osteoblasts. Curr Mol Pharmacol 2012;5:127-134. [PubMed] [Google Scholar]
- 7. Canalis E, Giustina A, Bilezikian JP. Mechanisms of anabolic therapies for osteoporosis. N Engl J Med 2007;357:905-916. [DOI] [PubMed] [Google Scholar]
- 8. Rubin MR, Bilezikian JP. The anabolic effects of parathyroid hormone therapy. Clin Geriatr Med 2003;19:415-432. [DOI] [PubMed] [Google Scholar]
- 9. Leder BZ, Tsai JN, Uihlein AV, et al. Two years of Denosumab and teriparatide administration in postmenopausal women with osteoporosis (The DATA Extension Study): a randomized controlled trial. J Clin Endocrinol Metab 2014;99:1694-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aspenberg P, Genant HK, Johansson T, et al. Teriparatide for acceleration of fracture repair in humans: a prospective, randomized, double-blind study of 102 postmenopausal women with distal radial fractures. J Bone Miner Res 2010;25:404-414. [DOI] [PubMed] [Google Scholar]
- 11. Skripitz R, Andreassen TT, Aspenberg P. Strong effect of PTH (1-34) on regenerating bone: a time sequence study in rats. Acta Orthop Scand 2000;71:619-624. [DOI] [PubMed] [Google Scholar]
- 12. Kim HW, Jahng JS. Effect of intermittent administration of parathyroid hormone on fracture healing in ovariectomized rats. Iowa Orthop J 1999;19:71-77. [PMC free article] [PubMed] [Google Scholar]
- 13. Nakajima A, Shimoji N, Shiomi K, et al. Mechanisms for the enhancement of fracture healing in rats treated with intermittent low-dose human parathyroid hormone (1-34). J Bone Miner Res 2002;17:2038-2047. [DOI] [PubMed] [Google Scholar]
- 14. Goldhahn J, Scheele WH, Mitlak BH, et al. Clinical evaluation of medicinal products for acceleration of fracture healing in patients with osteoporosis. Bone 2008;43:343-347. [DOI] [PubMed] [Google Scholar]
- 15. Moen MD, Scott LJ. Recombinant full-length parathyroid hormone (1-84). Drugs 2006;66:2371-2381. [DOI] [PubMed] [Google Scholar]
- 16. Greenspan SL, Bone HG, Ettinger MP, et al. Effect of recombinant human parathyroid hormone (1-84) on vertebral fracture and bone mineral density in postmenopausal women with osteoporosis: a randomized trial. Ann Intern Med 2007;146:326-339. [DOI] [PubMed] [Google Scholar]
- 17. Strewler GJ, Williams RD, Nissenson RA. Human renal carcinoma cells produce hypercalcemia in the nude mouse and a novel protein recognized by parathyroid hormone receptors. J Clin Invest 1983;71:769-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xu JL, Rong H., Ji H, et al. Effects of different dosages of parathyroid hormone related -1-34 on the bone metabolism of the overiectomized rat model of osteoporosis. Calcif Tissue Int 2013;93:276-287. [DOI] [PubMed] [Google Scholar]
- 19. de Castro LF, Lozano D, Dapía S, et al. Role of the N- and C-terminal fragments of parathyroid-hormone-related protein as putative therapies to improve bone regeneration under high glucocorticoid treatment. Tissue Eng Part A 2010;16:1157-1168. [DOI] [PubMed] [Google Scholar]
- 20. de Castro LF, Lozano D, Portal-Núñez S, et al. Comparison of the skeletal effects induced by daily administration of PTHrP (1-36) and PTHrP (107-139) to ovariectomized mice. J Cell Physiol 2012;227:1752-1760. [DOI] [PubMed] [Google Scholar]
- 21. Grosso MJ, Courtland HW, Yang X, et al. Intermittent PTH administration and mechanical loading are anabolic for periprosthetic cancellous bone. J Orthop Res 2015;33:163-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Amugongo SK, Yao W, Jia J, et al. Effect of sequential treatments with alendronate, parathyroid hormone (1-34) and raloxifene on cortical bone mass and strength in ovariectomized rats. Bone 2014;67:257-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhou H, Iida-Klein A, Lu SS, et al. Anabolic action of parathyroid hormone on cortical and cancellous bone differs between axial and appendicular skeletal sites in mice. Bone 2003;32:513-520. [DOI] [PubMed] [Google Scholar]
- 24. Jiang Y, Zhao JJ, Mitlak BH, et al. Recombinant human parathyroid hormone (1-34) [teriparatide] improves both cortical and cancellous bone structure. J Bone Miner Res 2003;18:1932-1941. [DOI] [PubMed] [Google Scholar]
- 25. Calvi LM, Sims NA, Hunzelman JL, et al. Activated parathyroid hormone/parathyroid hormone-related protein receptor in osteoblastic cells differentially affects cortical and trabecular bone. J Clin Invest 2001;107:277-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Powell WF, Jr, Barry KJ, Tulum I, et al. Targeted ablation of the PTH/PTHrP receptor in osteocytes impairs bone structure and homeostatic calcemic responses. J Endocrinol 2011;209:21-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Terauchi M, Li JY, Bedi B, et al. T lymphocytes amplify the anabolic activity of parathyroid hormone through Wnt10b signaling. Cell Metab 2009;10:229-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fermor B, Skerry TM. PTH/PTHrP receptor expression on osteoblasts and osteocytes but not resorbing bone surfaces in growing rats. J Bone Miner Res 1995;10:1935-1943. [DOI] [PubMed] [Google Scholar]
- 29. Datta NS, Abou-Samra AB. PTH and PTHrP signaling in osteoblasts. Cell Signal 2009;21:1245-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gardella TJ. Interactions of PTH with Receptors and Signaling. In: Bilezikian JP, Marcus R, Levine MA, et al., eds. The Parathyroids: Basic and Clinical Concepts. Third ed. Elsevier Inc., 2015:65–80. [Google Scholar]
- 31. Aslan D, Andersen MD, Gede LB, et al. Mechanisms for the bone anabolic effect of parathyroid hormone treatment in humans. Scand J Clin Lab Invest 2012;72:14-22. [DOI] [PubMed] [Google Scholar]
- 32. Brunner S, Zaruba MM, Huber B, et al. Parathyroid hormone effectively induces mobilization of progenitor cells without depletion of bone marrow. Exp Hematol 2008;36:1157-1166. [DOI] [PubMed] [Google Scholar]
- 33. Brunner S, Theiss HD, Murr A, Negele T, Franz WM. Primary hyperparathyroidism is associated with increased circulating bone marrow-derived progenitor cells. Am J Physiol Endocrinol Metab 2007;293:E1670-E1675. [DOI] [PubMed] [Google Scholar]
- 34. McCauley LK, Koh AJ, Beecher CA, Rosol TJ. Proto-oncogene c-fos is transcriptionally regulated by parathyroid hormone (PTH) and PTH-related protein in a cyclic adenosine monophosphate-dependent manner in osteoblastic cells. Endocrinology 1997;138:5427-5433. [DOI] [PubMed] [Google Scholar]
- 35. Nobta M, Tsukazaki T, Shibata Y, et al. Critical regulation of bone morphogenetic protein-induced osteoblastic differentiation by Delta1/Jagged1-activated Notch1 signaling. J Biol Chem 2005;280:15842-15848. [DOI] [PubMed] [Google Scholar]
- 36. Bellido T, Ali AA, Plotkin LI, et al. Proteasomal degradation of Runx2 shortens parathyroid hormone-induced anti-apoptotic signaling in osteoblasts. A putative explanation for why intermittent administration is needed for bone anabolism. J Biol Chem 2003;278:50259-50272. [DOI] [PubMed] [Google Scholar]
- 37. Tintut Y, Parhami F, Le V, Karsenty G, Demer LL. Inhibition of osteoblast-specific transcription factor Cbfa1 by the cAMP pathway in osteoblastic cells. Ubiquitin/proteasome-dependent regulation. J Biol Chem 1999;274:28875-28879. [DOI] [PubMed] [Google Scholar]
- 38. Krishnan V, Moore TL, Ma YL, et al. Parathyroid hormone bone anabolic action requires Cbfa1/Runx2-dependent signaling. Mol Endocrinol 2003;17:423-435. [DOI] [PubMed] [Google Scholar]
- 39. Datta NS, Pettway GJ, Chen C, Koh AJ, McCauley LK. Cyclin D1 as a target for the proliferative effects of PTH and PTHrP in early osteoblastic cells. J Bone Miner Res 2007;22:951-964. [DOI] [PubMed] [Google Scholar]
- 40. Linkhart TA, Mohan S. Parathyroid hormone stimulates release of insulin-like growth factor-I (IGF-I) and IGF-II from neonatal mouse calvaria in organ culture. Endocrinology 1989;125:1484-1491. [DOI] [PubMed] [Google Scholar]
- 41. Miyakoshi N, Kasukawa Y, Linkhart TA, Baylink DJ, Mohan S. Evidence that anabolic effects of PTH on bone require IGF-I in growing mice. Endocrinology 2001;142:4349-4356. [DOI] [PubMed] [Google Scholar]
- 42. Ma YL, Zeng Q, Donley DW, et al. Teriparatide increases bone formation in modeling and remodeling osteons and enhances IGF-II immunoreactivity in postmenopausal women with osteoporosis. J Bone Miner Res 2006;21:855-864. [DOI] [PubMed] [Google Scholar]
- 43. Dong Y, Canalis E. Insulin-like growth factor (IGF) I and retinoic acid induce the synthesis of IGF-binding protein 5 in rat osteoblastic cells. Endocrinology 1995;136:2000-2006. [DOI] [PubMed] [Google Scholar]
- 44. Jilka RL. Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone 2007;40:1434-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chen HL, Demiralp B, Schneider A, et al. Parathyroid hormone and parathyroid hormone-related protein exert both pro- and anti-apoptotic effects in mesenchymal cells. J Biol Chem 2002;277:19374-19381. [DOI] [PubMed] [Google Scholar]
- 46. Sharma S, Mahalingam CD, Das V, et al. Cell cycle and apoptosis regulatory protein (CARP)-1 is expressed in osteoblasts and regulated by PTH. Biochem Biophys Res Commun 2013;436:607-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schnoke M, Midura SB, Midura RJ. Parathyroid hormone suppresses osteoblast apoptosis by augmenting DNA repair. Bone 2009;45:590-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kramer I, Halleux C, Keller H, et al. Osteocyte Wnt/beta-catenin signaling is required for normal bone homeostasis. Mol Cell Biol 2010;30:3071-3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Glass DA, II, Bialek P, Ahn JD, et al. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell 2005;8:751-764. [DOI] [PubMed] [Google Scholar]
- 50. Alberts B, Johnson A, Lewis J, et al. Molecular Biology of The Cell. 5th ed. New York, USA: Garland Science; 2008. [Google Scholar]
- 51. Poole KE, van Bezooijen RL, Loveridge N, et al. Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J 2005;19:1842-1844. [DOI] [PubMed] [Google Scholar]
- 52. Portal-Núñez S, Lozano D, de Castro LF, et al. Alterations of the Wnt/beta-catenin pathway and its target genes for the N- and C-terminal domains of parathyroid hormone-related protein in bone from diabetic mice. FEBS Lett 2010;584:3095-3100. [DOI] [PubMed] [Google Scholar]
- 53. Rhee Y, Allen MR, Condon K, et al. PTH receptor signalling in osteocytes governs periosteal bone formation and intracortical remodeling. J Bone Miner Res 2011;26:1035-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Weinstein RS, Jilka RL, Almeida M, Roberson PK, Manolagas SC. Intermittent parathyroid hormone administration counteracts the adverse effects of glucocorticoids on osteoblast and osteocyte viability, bone formation, and strength in mice. Endocrinology 2010;151:2641-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Powell WF, Jr, Barry KJ, Tulum I, et al. Targeted ablation of the PTH/PTHrP receptor in osteocytes impairs bone structure and homeostatic calcemic responses. J Endocrinol 2011;209:21-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mason JJ, Williams BO. SOST and DKK: Antagonists of LRP Family Signaling as Targets for Treating Bone Disease. J Osteoporos 2010;2010:460120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Guo J, Liu M, Yang D, et al. Suppression of Wnt signaling by Dkk1 attenuates PTH-mediated stromal cell response and new bone formation. Cell Metab 2010;11:161-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kulkarni NH, Halladay DL, Miles RR, et al. Effects of parathyroid hormone on Wnt signaling pathway in bone. J Cell Biochem 2005;95:1178-1190. [DOI] [PubMed] [Google Scholar]
- 59. Yao GQ, Wu JJ, Troiano N, Insogna K. Targeted overexpression of Dkk1 in osteoblasts reduces bone mass but does not impair the anabolic response to intermittent PTH treatment in mice. J Bone Miner Metab 2011;29:141-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Viapiana O, Fracassi E, Troplini S, et al. Sclerostin and DKK1 in primary hyperparathyroidism. Calcif Tissue Int 2013;92:324-329. [DOI] [PubMed] [Google Scholar]
- 61. Khosla S. Minireview: the OPG/RANKL/RANK system. Endocrinology 2001;142:5050-5055. [DOI] [PubMed] [Google Scholar]
- 62. Li C, Wang W, Xie L, et al. Lipoprotein receptor-related protein 6 is required for parathyroid hormone-induced Sost suppression. Ann N Y Acad Sci 2016;1364:62-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yu B, Zhao X, Yang C, et al. Parathyroid hormone induces differentiation of mesenchymal stromal/stem cells by enhancing bone morphogenetic protein signaling. J Bone Miner Res 2012;27:2001-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Revollo L, Kading J, Jeong SY, et al. N-cadherin restrains PTH activation of Lrp6/β-catenin signaling and osteoanabolic action. J Bone Miner Res 2015;30:274-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Li C, Xing Q, Yu B, et al. Disruption of LRP6 in osteoblasts blunts the bone anabolic activity of PTH. J Bone Miner Res 2013;28:2094-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Shi C, Li J, Wang W, et al. Antagonists of LRP6 regulate PTH-induced cAMP generation. Ann N Y Acad Sci 2011;1237:39-46. [DOI] [PubMed] [Google Scholar]
- 67. Bedi B, Li JY, Tawfeek H, et al. Silencing of parathyroid hormone (PTH) receptor 1 in T cells blunts the bone anabolic activity of PTH. Proc Natl Acad Sci U S A 2012;109:E725-E733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bianchi ML, Duca P, Vai S, et al. Improving adherence to and persistence with oral therapy of osteoporosis. Osteoporos Int 2015;26:1629-1638. [DOI] [PubMed] [Google Scholar]
- 69. Dobnig H, Turner RT. Evidence that intermittent treatment with parathyroid hormone increases bone formation in adult rats by activation of bone lining cells. Endocrinology 1995;136:3632-3638. [DOI] [PubMed] [Google Scholar]
- 70. Leaffer D, Sweeney M, Kellerman LA, et al. Modulation of osteogenic cell ultrastructure by RS-23581, an analog of human parathyroid hormone (PTH)-related peptide-(1-34), and bovine PTH-(1-34). Endocrinology 1995;136:3624-3631. [DOI] [PubMed] [Google Scholar]
- 71. Kim SW, Pajevic PD, Selig M, et al. Intermittent parathyroid hormone administration converts quiescent lining cells to active osteoblasts. J Bone Miner Res 2012;27:2075-2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Calvi LM, Adams GB, Weibrecht KW, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 2003;425:841-846. [DOI] [PubMed] [Google Scholar]
- 73. Bromberg O, Frisch BJ, Weber JM, et al. Osteoblastic N-cadherin is not required for microenvironmental support and regulation of hematopoietic stem and progenitor cells. Blood 2012;120:303-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Peled A, Petit I, Kollet O, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science 1999;283:845-848. [DOI] [PubMed] [Google Scholar]
- 75. Lapidot T, Kollet O. The essential roles of the chemokine SDF-1 and its receptor CXCR4 in human stem cell homing and repopulation of transplanted immune-deficient NOD/SCID and NOD/SCID/B2m(null) mice. Leukemia 2002;16:1992-2003. [DOI] [PubMed] [Google Scholar]
- 76. Wynn RF, Hart CA, Corradi-Perini C, et al. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood 2004;104:2643-2645. [DOI] [PubMed] [Google Scholar]
- 77. Fong EL, Chan CK, Goodman SB. Stem cell homing in musculoskeletal injury. Biomaterials 2011;32:395-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Granero-Moltó F, Weis JA, Miga MI, et al. Regenerative effects of transplanted mesenchymal stem cells in fracture healing. Stem Cells 2009;27:1887-1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Huber BC, Fischer R, Brunner S, et al. Comparison of parathyroid hormone and G-CSF treatment after myocardial infarction on perfusion and stem cell homing. Am J Physiol Heart Circ Physiol 2010;298:H1466-H1471. [DOI] [PubMed] [Google Scholar]
- 80. Zaruba MM, Huber BC, Brunner S, et al. Parathyroid hormone treatment after myocardial infarction promotes cardiac repair by enhanced neovascularization and cell survival. Cardiovasc Res 2008;77:722-731. [DOI] [PubMed] [Google Scholar]
- 81. Huber BC, Grabmaier U, Brunner S. Impact of parathyroid hormone on bone marrow-derived stem cell mobilization and migration. World J Stem Cells 2014;6:637-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kitaori T, Ito H, Schwarz EM, et al. Stromal cell-derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis Rheum 2009;60:813-823. [DOI] [PubMed] [Google Scholar]
- 83. Simonet WS, Lacey DL, Dunstan CR, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 1997;89:309-319. [DOI] [PubMed] [Google Scholar]
- 84. O’Brien CA, Nakashima T, Takayanagi H. Osteocyte control of osteoclastogenesis. Bone 2013;54:258-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lee SK, Lorenzo JA. Parathyroid hormone stimulates TRANCE and inhibits osteoprotegerin messenger ribonucleic acid expression in murine bone marrow cultures: correlation with osteoclast-like cell formation. Endocrinology 1999;140:3552-3561. [DOI] [PubMed] [Google Scholar]
- 86. Lee SK, Lorenzo JA. Regulation of receptor activator of nuclear factor-kappa B ligand and osteoprotegerin mRNA expression by parathyroid hormone is predominantly mediated by the protein kinase a pathway in murine bone marrow cultures. Bone 2002;31:252-259. [DOI] [PubMed] [Google Scholar]
- 87. Ma YL, Cain RL, Halladay DL, et al. Catabolic effects of continuous human PTH (1–38) in vivo is associated with sustained stimulation of RANKL and inhibition of osteoprotegerin and gene-associated bone formation. Endocrinology 2001;142:4047-4054. [DOI] [PubMed] [Google Scholar]
- 88. Nakchbandi IA, Lang R, Kinder B, Insogna KL. The role of the receptor activator of nuclear factor-kappaB ligand/osteoprotegerin cytokine system in primary hyperparathyroidism. J Clin Endocrinol Metab 2008;93:967-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Szymczak J, Bohdanowicz-Pawlak A. Osteoprotegerin, RANKL, and bone turnover in primary hyperparathyroidism: the effect of parathyroidectomy and treatment with alendronate. Horm Metab Res 2013;45:759-764. [DOI] [PubMed] [Google Scholar]
- 90. O’Brien CA, Plotkin LI, Galli C, et al. Control of bone mass and remodeling by PTH receptor signaling in osteocytes. PLoS One 2008;3:e2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Xiong J, Piemontese M, Thostenson JD, et al. Osteocyte-derived RANKL is a critical mediator of the increased bone resorption caused by dietary calcium deficiency. Bone 2014;66:146-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wang M, Nasiri AR, Broadus AE, Tommasini SM. Periosteal PTHrP Regulates Cortical Bone Remodeling During Fracture Healing. Bone 2015;81:104-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Jacome-Galarza CE, Lee SK, Lorenzo JA, Aguila HL. Parathyroid hormone regulates the distribution and osteoclastogenic potential of hematopoietic progenitors in the bone marrow. J Bone Miner Res 2011;26:1207-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Jacquin C, Koczon-Jaremko B, Aguila HL, et al. Macrophage migration inhibitory factor inhibits osteoclastogenesis. Bone 2009;45:640-649. [DOI] [PubMed] [Google Scholar]
- 95. Patel H, Trooskin S, Shapses S, Sun W, Wang X. Serum monocyte chemokine protein-1 levels before and after parathyroidectomy in patients with primary hyperparathyroidism. Endocr Pract 2014;20:1165-1169. [DOI] [PubMed] [Google Scholar]
- 96. Tsai JN, Uihlein AV, Lee H, et al. Teriparatide and denosumab, alone or combined, in women with postmenopausal osteoporosis: the DATA study randomised trial. Lancet 2013;382:50-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Gesty-Palmer D, Luttrell LM. ‘Biasing’ the parathyroid hormone receptor: a novel anabolic approach to increasing bone mass? Br J Pharmacol 2011;164:59-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Lindsay R, Cosman F, Zhou H, et al. A novel tetracycline labeling schedule for longitudinal evaluation of the short-term effects of anabolic therapy with a single iliac crest bone biopsy: early actions of teriparatide. J Bone Miner Res 2006;21:366-373. [DOI] [PubMed] [Google Scholar]
- 99. Hattersley G, Dean T, Corbin BA, Bahar H, Gardella TJ. Binding Selectivity of Abaloparatide for PTH-Type-1-Receptor Conformations and Effects on Downstream Signaling. Endocrinology 2016;157:141-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Rhee Y, Lee EY, Lezcano V, et al. Resorption controls bone anabolism driven by parathyroid hormone (PTH) receptor signaling in osteocytes. J Biol Chem 2013;288:29809-29820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Cosman F, Dempster DW, Nieves JW, et al. Effect of teriparatide on bone formation in the human femoral neck. J Clin Endocrinol Metab 2016;101:1498-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Ma YL, Zeng QQ, Chiang AY, et al. Effects of teriparatide on cortical histomorphometric variables in postmenopausal women with or without prior alendronate treatment. Bone 2014;59:139-147. [DOI] [PubMed] [Google Scholar]
- 103. Cosman F. Abaloparatide: a new anabolic therapy on the horizon. Bonekey Rep 2015;4:661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Leder BZ, O’Dea LS, Zanchetta JR, et al. Effects of abaloparatide, a human parathyroid hormone-related peptide analog, on bone mineral density in postmenopausal women with osteoporosis. J Clin Endocrinol Metab 2015;100:697-706. [DOI] [PubMed] [Google Scholar]
- 105. Eriksen EF, Brown JP. Commentary: Concurrent administration of PTH and antiresorptives: Additive effects or DXA cosmetics. Bone 2016;86:139-142. [DOI] [PubMed] [Google Scholar]
- 106. Vahle JL, Sato M, Long GG, et al. Skeletal changes in rats given daily subcutaneous injections of recombinant human parathyroid hormone (1-34) for 2 years and relevance to human safety. Toxicol Pathol 2002;30:312-321. [DOI] [PubMed] [Google Scholar]
- 107. Sato M, Vahle J, Schmidt A, et al. Abnormal bone architecture and biomechanical properties with near-lifetime treatment of rats with PTH. Endocrinology 2002;143:3230-3242. [DOI] [PubMed] [Google Scholar]
- 108. Jolette J, Wilker CE, Smith SY, et al. Defining a noncarcinogenic dose of recombinant human parathyroid hormone 1-84 in a 2-year study in Fischer 344 rats. Toxicol Pathol 2006;34:929-940. [DOI] [PubMed] [Google Scholar]