Abstract
Some herbivore-induced-plant volatiles (HIPVs) compounds are vital for the functioning of an ecosystem, by triggering multi-trophic interactions for natural enemies, plants and herbivores. However, the effect of these chemicals, which play a crucial role in regulating the multi-trophic interactions between plant-herbivore-entomopathogenic fungi, is still unknown. To fill this scientific gap, we therefore investigated how these chemicals influence the entomopathogenic fungi growth and efficacy. In this study, Lipaphis erysimi induced Arabidopsis thaliana HIPVs were collected using headspace system and detected with GC-MS, and then analyzed the effects of these HIPVs chemicals on Lecanicillium lecanii strain V3450. We found that the HIPVs menthol and methyl salicylate at 1 and 10 nmol·ml−1 improved many performance aspects of the fungus, such as germination, sporulation, appressorial formation as well as its pathogenicity and virulence. These findings are not only important for understanding the multi-trophic interactions in an ecosystem, but also would contribute for developing new and easier procedures for conidial mass production as well as improve the pathogenicity and virulence of entomopathogenic fungi in biological pest management strategies.
Herbivore-induced-plant-volatiles (HIPVs) are emitted from plants after infestation by arthropods, and these volatiles are composed by many organic compounds which are involved in plant communication with natural enemies of the insect herbivores, neighboring plants, and different parts of the damaged plant. For instance, green leaf volatiles (GLVs) are comprised of C6 aldehydes, alcohols as well as esters, and terpenoids1,2.
It was well known that HIPVs play a significant role in attracting natural enemies of herbivores when plants become infested by herbivorous insects. Likewise, Arabidopsis thaliana (L) Heynh (Brassicales: Brassicaceae) attracted more parasitic wasp Cotesia glomerata (Linnaeus) (Hymenoptera: Braconidae), when the plant emitted a high amount of GLVs3. Recently, it has been reported that (Z)-3-hexenol, a unique compound of GLVs is the most important info-chemical for the natural enemy attraction4. A blend of six HIPVs compounds, such as beta-myrcene, n-octanal, and alpha-phellandrene, along with other host-nonspecific (E)-beta-ocimene, gamma-terpinene, and linalool played a role in the communication between plant and parasitic wasps Aphidius ervi Haliday (Hymenoptera: Aphidiidae) at a minimal quantity of 0.001 ng to 5 ng5. In addition, it also reported that predators respond to transgenic plant volatiles, like (E, E)-4, 8,12-trimethyltrideca-1, 3, 7, 11-tetraene, which is produced endogenously6. Furthermore, plant roots also emit HIPVs to attract natural enemies, such as; (E)-beta-caryophyllene. Furthermore, maize roots have been shown to attract entomopathogenic nematodes by using (E)-beta-caryophyllene after being attacked by the western corn rootworm, Diabrotica virgifera LeConte (Coleoptera: Chrysomelidae)7. It was also observed that when herbivorous insects oviposited on plants, they emitted electroantennographic-active compounds, such as (E)-4,8-dimethyl-1, 3, 7-nonatriene8, cis-3-hexen-1-ol, linalool and cis-alpha-bergamotene, which induced high egg feeding by generalist predators9.
Some studies have revealed the effect of HIPVs on pathogens, especially insect pathogen. Hountondji, et al.10 found that HIPVs promote the conidial growth of entomopathogenic fungus, Neozygites tanajoae. In another study, allyl isothiocyanate emitted by macerated wasabi plant (Wasabia japonica Matsumura) inhibited the growth of two entomopathogenic fungi Beauveria bassiana and Isaria fumosorosea11. Our previous study demonstrated that A. thaliana plant infested by Lipaphis erysimi Kaltenbach, emitted HIPVs compounds that promoted the performance of the entomopathogenic fungus (EPF) Lecanicillium lecanii, which was used for biocontrol of L. erysimi12. However, the role of each chemical component of the HIPVs induced by L. erysimi, is still unknown. It is therefore important to know how these chemicals influence the entomopathogenic fungi.
To fill in this gap, this study was designed and conducted at the College of Plant Protection, Fujian Agriculture and Forestry University, Fuzhou, China, where we collected HIPVs using headspace system and detected with GC-MS, and then treated the L. lecanii strain V3450 with similar synthetic chemical of HIPVs to analyze their biological function. Arabidopsis thaliana and L. erysimi were used to stimulate HIPVs production and collection. We therefore investigated and identified the components of these volatiles induced by L. erysimi in A. thaliana and synthetic chemicals from identified HIPVs were used to treat conidia of L. lecanii. The activity and effects of chemicals from HIPVs on the performance of L. lecanii were evaluated and the validity of various synthetic chemical’s concentrations to induce L. lecanii conidia was assessed.
Results
Headspace volatiles collected and detected by GC-MS
Nine HIPVs compounds notably limonene, 2-methyl-6-heptene, menthol, methyl salicylate, 1-octen-3-ol benzaldehyde, phenylacetaldehyde, decan-3-ol and terpineols were in total collected and identified from aphid-induced A. thaliana. Limonene and 2-methyl-6-heptene were detected in all the treatments (I, II, III, IV and V) i.e. independent of the different densities of L. erysimi apterous adults (1, 2, 5, 10 and 20) that infested A. thaliana plants, and with no significant differences (F4, 10 = 1.79, P = 0.23; F2, 4 = 3.99, P = 0.31) between treatments as regards to the quantities emitted (Table 1). Similarly, Menthol and 1-octen-3-ol were also collected from all treatments, but the quantities of Menthol produced in treatments III-V were significantly higher than those emitted in treatments I -II (F4, 10 = 50.89, P = 0.04); whereas the quantity of 1-octen-3-ol in treatment III was significantly higher than all the rest of the treatments (F4, 10 = 5.38, P = 0.009) (Table 1). Methyl salicylate was absent in treatment I but detected in all the other treatments II-V, and with the lowest quantity collected in treatment I compared to treatments II - V (F3, 8 = 30.74, P = 0.01) (Table 1). Likewise, benzaldehyde, phenylacetaldehyde and decan-3-ol were only detected in treatment III-V and completely absent in I and II. However, the benzaldehyde quantities collected from the three treatments were not significantly different (F2, 6 = 28.27, P = 0.1). The phenylacetaldehyde quantity was much higher in treatment V, compared to the other two treatments (F2, 6 = 84.81, P = 0.001), while the quantity of decan-3-ol emitted was significantly lower in treatment IV compared to treatments III and V (F2, 6 = 64.08, P = 0.03). In addition, Terpineols were only detected in treatment V (Table 1).
Table 1. Quantities of major compounds in headspace volatiles released from A. thaliana infested by L. erysimi.
| Compounds | Quantities ± SD (nmol) |
||||
|---|---|---|---|---|---|
| I | II | III | IV | V | |
| Limonene | 3.10 ± 0.06a | 3.80 ± 0.18a | 3.23 ± 0.09a | 3.05 ± 0.26a | 2.82 ± 0.52a |
| 2-Methyl-6-heptene | 4.98 ± 0.13a | 4.87 ± 0.23a | 5.10 ± 0.34a | 4.76 ± 0.29a | 4.94 ± 0.39a |
| 1-Octen-3-ol | 1.44 ± 0.06a | 1.33 ± 0.08ab | 1.78 ± 0.15b | 1.46 ± 0.14ab | 1.57 ± 0.14ab |
| Menthol | 0.41 ± 0.02a | 0.47 ± 0.11a | 3.26 ± 0.17b | 3.68 ± 0.39b | 2.17 ± 0.19c |
| Methyl salicylate | Nd | 1.26 ± 0.05a | 3.23 ± 0.26b | 3.61 ± 0.35a | 4.52 ± 0.56b |
| Benzaldehyde | Nd | nd | 1.32 ± 0.25a | 1.38 ± 1.05a | 1.43 ± 0.12a |
| Phenylacetaldehyde | Nd | nd | 1.01 ± 0.04a | 1.07 ± 0.06a | 1.46 ± 0.13b |
| Decan-3-ol | Nd | nd | 1.06 ± 0.06a | 0.67 ± 0.08b | 1.29 ± 0.13a |
| Terpineols | Nd | nd | nd | nd | 0.77 ± 0.06 |
Data show mean values of quantities ± SD (nmol) of main compounds in headspace which were collected for 12 h. There were 0, 2, 5, 10, 20 L. erysimi in treatment I, II, III, IV, V, respectively. Means followed by a different letter per compound emitted across each row per treatment are significantly different (Tukey’s HSD test, P < 0.05). ‘nd’ means not detected.
Influence of aphid density on conidial germination and appressorial formation rate
The conidial germination and appressorial formation rate after exposure of L. lecanii strain V3450 to different concentrations of HIPVs induced by different densities’ number of aphids varied greatly after 12 h exposure (Table 2). For instance, the germination rate of conidia in treatments IV and V were significantly high (F4, 4 = 6.06; P < 0.05) compared to treatments I (control) (Table 2). Similar trend was observed for appressorial development where the highest rate of appressorial formation was obtained in treatment IV compared to other treatments (F4, 4 = 20.32; P < 0.0001). In addition, the rates have significantly increased (P < 0.0001) in treatment III and V compared with control (Table 2).
Table 2. The influence of headspace to conidial germination and appressorial rate.
| Treatments | Insect density | Germination rate % |
Appressorial formation rate % |
||
|---|---|---|---|---|---|
| mean value ± SE | confidence limited | mean value ± SE | confidence limited | ||
| I | 0 | 54.86 ± 1.61a | 57.59–65.79 | 5.06 ± 1.11a | 3.68-0.64 |
| II | 2 | 59.33 ± 2.16ab | 53.33–65.32 | 7.08 ± 1.87ab | 4.76-9.41 |
| III | 5 | 61.69 ± 1.48ab | 58.40–71.06 | 10.15–1.21b | 8.65–11.66 |
| IV | 10 | 67.22 ± 2.08bc | 61.44–73.01 | 13.97–1.20c | 12.48–15.47 |
| V | 20 | 64.7 ± 2.28bc | 50.38–59.33 | 8.49 ± 2.51b | 5.37–11.61 |
Data shows germination and appressorial formation rate mean mortality ± SE of 5 replicates 12 h exposure to headspace at 25 °C. The HIPVs was emitted from Aradopsis was induced by different densities of aphids. Means followed by the different letter are significantly different (Tukey’s HSD test, P < 0.05).
Correlation analysis for HIPVs chemical compounds and conidial performance
The correlation between HIPVs chemicals and conidial performance of L. lecanii showed that the germination and appressorial formation rates were influenced by multiple HIPVs compounds as summarized in Table 3. For conidial germination, the correlation coefficient for menthol was higher than 0.5, indicating a significant correlation trend. Similarly, for appressorial formation, the correlation coefficients for menthol, methyl salicylate (MeSA), benzaldehyde and phenylacetaldehyde were all higher than 0.5, indicating a significantly strong correlation between these HIPVs compounds and the conidial performance (Table 3).
Table 3. Analysis of correlation between the single HIPVs chemical and performance of conidia as influenced by HIPVs.
| Correlation analysis for germination rate and chemicals | Correlation analysis for appressorial formation rate and chemicals | |
|---|---|---|
| Limonene | 0.049 | −0.265 |
| 2-Methyl-6-heptene | −0.144 | −0.431 |
| 1-Octen-3-ol | 0.173 | 0.285 |
| Menthol | 0.532 | 0.919 |
| Methyl salicylate | −0.070 | 0.705 |
| Benzaldehyde | 0.162 | 0.772 |
| Phenylacetaldehyde | −0.042 | 0.515 |
| Decan-3-ol | −0.132 | 0.504 |
| Terpineols | −0.782 | −0.077 |
Conidial performance after exposure to synthetic compounds
L. lecanii were exposed to synthetic menthol, methyl salicylate, decan-3-ol, benzaldehyde and phenylacetaldehyde according to the results of GC-MS. When L. lecanii conidia were treated with menthol, no significant impact on their germination (F4, 20 = 0.41, P = 0.24; Fig. 1a) was observed, while conidial germination was significantly lower in the case of 1, 100 and 1000 nmol·ml−1 methyl salicylate application as compared to 10 nmol·ml−1 and the control (F4, 20 = 6.26, P = 0.03; Fig. 1b). Appressorial formation in the menthol treatment was boosted in the case of 10 and 100 nmol·ml−1, and same trend was observed with the 10 nmol·ml−1 methyl salicylate treatment (F4, 20 = 6.23, P = 0.022; F4, 20 = 2.03, P = 0.034; Fig. 1a,b). However, all decan-3-ol treatments had a negative impact on both the germination and appressorial formation of L. lecanii (F4, 20 = 17.89, P = 0.027; F4, 20 = 3.23, P = 0.033; Fig. 1c). In addition, conidia did not germinate in the treatment of benzaldehyde and phenylacetaldehyde.
Figure 1. Germination and appressorial formation rate of L. lecanii treated with the different chemicals at different quantities for 12 h.
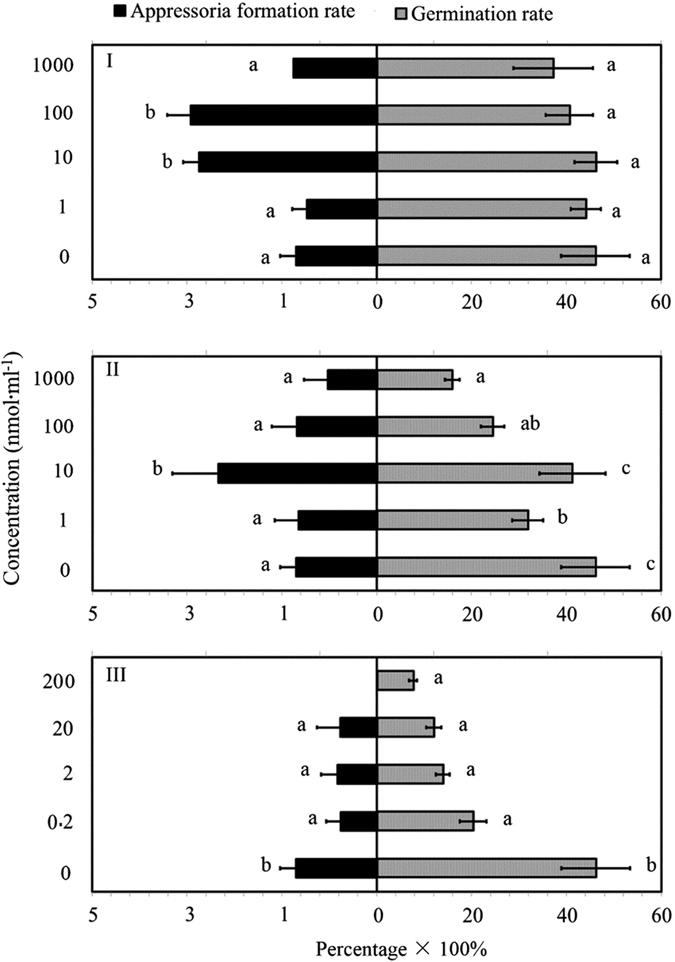
Chemicals used in the test were (I) menthol, (II) methyl salicylate and (III) decan-3-ol. The number ‘0’ on the y-axis is the control treatment. The bars indicate mean value of germination and appressorial formation rate and the error bars indicate ± standard error (n = 5). The different letters above the error bars on each side indicates significance (Tukey’s test, P < 0.05).
Hyphal growth
Impacts of synthetic chemicals on colony extension of L. lecanii after 15 days are summarized (Table 4). Growth speed of the fungal colony was faster when treated with 1 and 10 nmol·ml−1 menthol, and 1 nmol methyl salicylate (0.89, 1.09 and 0.92 cm·day−1, respectively), as compared to the control treatment (0 nmol·ml−1 treatment, 0.74 cm·day−1). Similarly, the diameter of the colony growth was also observed to be significantly larger after exposure to 1 and 10 nmol·ml−1 menthol (F4, 20 = 162.33, P = 0.0002), as well as 1 nmol·ml−1 methyl salicylate (F4, 20 = 34.83, P = 0.012) compared to the control, respectively (Table 4). However, the other chemical treatments (decan-3-ol, benzaldehyde, and phenylacetaldehyde) not only had a negative impact on the diameter of the fungal colony but also on the fungal growth speed (Table 4).
Table 4. The hyphal growth of L. lecanii when exposed to the different chemical compounds at different concentrations.
| Series | Treatments |
Fungal colony extension |
||
|---|---|---|---|---|
| Compounds | Quantities (nmol·ml−1) | Speed (cm·d−1)* | Diameter (cm)*** | |
| 0 | 0.74 ± 0.05 | 4.29 ± 0.21d | ||
| 1 | 0.89 ± 0.05 | 5.39 ± 0.24a | ||
| I | Menthol | 10 | 1.09 ± 0.03 | 6.27 ± 0.11b |
| 100 | 0.71 ± 0.14 | 4.14 ± 0.09c | ||
| 1000 | 0.68 ± 0.04 | 4.19 ± 0.06c | ||
| 0 | 0.74 ± 0.05 | 4.29 ± 0.21d | ||
| 1 | 0.92 ± 0.04 | 5.37 ± 0.13a | ||
| II | Methyl salicylate | 10 | 0.53 ± 0.02 | 3.17 ± 0.08b |
| 100 | 0.24 ± 0.03 | 1.58 ± 0.16c | ||
| 1000 | 0.14 ± 0.02 | 1.14 ± 0.05c | ||
| 0 | 0.74 ± 0.05 | 4.29 ± 0.21d | ||
| 1 | 0.62 ± 0.04 | 3.73 ± 0.12a | ||
| III | Decan-3-ol | 10 | 0.58 ± 0.03 | 2.89 ± 0.07b |
| 100 | 0.25 ± 0.02 | 1.62 ± 0.08c | ||
| 1000 | 0.20 ± 0.01 | 1.37 ± 0.09c | ||
| 0 | 0.74 ± 0.05 | 4.29 ± 0.21b | ||
| IV | Benzaldehye | 0.2 | 0.03 ± 0.02 | 1.09 ± 0.04a |
| 2 | 0.06 ± 0.01 | 0.77 ± 0.05a | ||
| V | Phenylacetaldehyde | 0 | 0.74 ± 0.05 | 4.29 ± 0.21b |
| 0.2 | 0.05 ± 0.01 | 0.76 ± 0.04a | ||
Data show mean value ± of the fungal colony of 5 replicates which cultured for 15 d. The number ‘0’ in all series are controlled treatments. Means followed by the different letter in a column are significantly different (Tukey’s HSD test, P < 0.05).
Conidial production
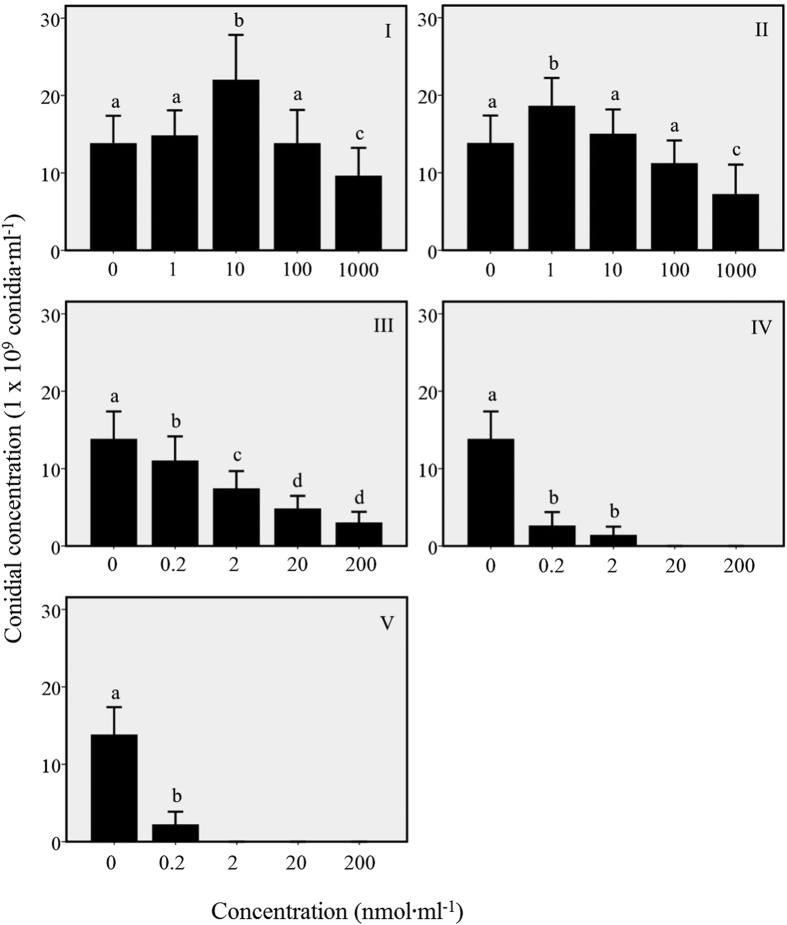
The effects of synthetic chemical compounds on the conidial production of L. lecanii after 15 days exposure are summarized in Fig. 2. Conidial production was enhanced by applications of 10 nmol·ml−1 menthol (F4, 20 = 22.57, P = 0.0003) and 1 nmol·ml−1 methyl salicylate (F4, 20 = 30.52, P = 0.0002), with significantly higher conidia yield than the control and other defined concentrations, respectively (Fig. 2a,b). However, conidial production was significantly inhibited under the tested concentrations in the treatments of decan-3-ol (F4, 20 = 59.63, P = 0.017), benzaldehyde (F4, 20 = 197.37, P = 0.0001) and phenylacetaldehyde (F4, 20 = 230.90, P = 0.0001) as compared to the controls (Fig. 2c–e). The controls therefore, yielded high conidia concentrations as compared to the decan-3-ol, benzaldehyde and phenylacetaldehyde treatments unlike in the cases of menthol and methyl salicylate (Fig. 2).
Figure 2. Sporulation of L. lecanii after exposure to compounds for 15 days.
The chemicals which were used in the analysis are (I) menthol, (II) methyl salicylate, (III) decan-3-ol, (IV) benzaldehyde and (V) phenylacetaldehyde. The number ‘0’ on the x-axis is the control treatment. The bars indicate mean value of conidial concentration and error bars indicate a standard error (n = 5). The different letters above the error bars indicate significance (Tukey’s test, P < 0.05).
Pathogenicity of Lecanicillium lecanii
The pathogenicity of L. lecanii to the adult aphids after being pre-treated with the chemicals was assessed (Table 5). Lecanicillium lecanii fungus exposed to menthol had a lowest LT50 (4.57 d) at the concentration of 10 nmol·ml−1 after 15 days post-exposure and with percent mortality significantly higher after 7 days compared to other chemical treatments as well as the control (F4, 20 = 19.36, P = 0.0021) (Table 5). A similar trend was observed for 1 nmol·ml−1 methyl salicylate treated fungus (F4, 20 = 24.34, P = 0.0001). For the other chemicals tested, the lethal time of the fungus as well as percent mortality was similar compared to the control (Table 5).
Table 5. Pathogenicity of L. lecanii pre-treated by the different chemical in different quantities.
| Series | Treatments | Fungal pathogenicity to L. erysimi | |||
|---|---|---|---|---|---|
| Compounds | Quantities (nmol·ml−1) | LT50 | 95% Confidence limits (lower-upper) | Mortality (%) | |
| 0 | 5.94 | 5.63–6.30 | 46.00 ± 2.67a | ||
| 1 | 4.94 | 4.68–5.23 | 49.33 ± 2.45a | ||
| I | Menthol | 10 | 4.57 | 4.34–4.81 | 73.33 ± 4.35b |
| 100 | 6.04 | 5.69–6.42 | 46.00 ± 3.23a | ||
| 1000 | 5.87 | 5.53–6.29 | 38.00 ± 2.00a | ||
| 0 | 5.94 | 5.63–6.30 | 46.00 ± 2.67ab | ||
| 1 | 5.05 | 4.80–5.34 | 62.00 ± 2.71c | ||
| II | Methyl salicylate | 10 | 6.30 | 5.91–6.80 | 50.0 0 ± 2.36ad |
| 100 | 7.08 | 6.55–7.79 | 37.32 ± 2.21be | ||
| 1000 | 7.60 | 6.95–8.53 | 32.00 ± 1.70e | ||
| 0 | 5.94 | 5.63–6.30 | 46.00 ± 2.67a | ||
| 1 | 6.05 | 5.71–6.46 | 36.67 ± 3.33a | ||
| III | Decan-3-ol | 10 | 6.62 | 6.24–7.12 | 35.33 ± 4.55a |
| 100 | 7.05 | 6.54–7.73 | 40.66 ± 3.71a | ||
| 1000 | 7.52 | 6.93–8.36 | 34.66 ± 3.42a | ||
| 0 | 5.94 | 5.63–6.30 | 46.00 ± 2.67a | ||
| IV | Benzaldehye | 0.2 | 7.32 | 6.79–8.04 | 42.67 ± 4.14a |
| 2 | 7.90 | 7.22–8.88 | 34.66 ± 2.67a | ||
| V | Phenylacetaldehyde | 0 | 5.94 | 5.63–6.30 | 46.00 ± 2.67a |
| 0.2 | 7.80 | 7.13–8.77 | 38.67 ± 3.43a | ||
Data shows LT50 value and percent mean mortality ± SE of 3 replicates 15 d post-exposure at 25 °C. LT50 were analyzed by linear regression. The number ‘0’ in the quantities column represent control treatments. Means followed by the different letter are significantly different (Tukey’s HSD test, P < 0.05).
Discussion
From the volatiles trapping, nine different compounds were emitted and identified as HIPVs when A. thaliana was infested with L. erysimi. Limonene, 2-methyl-6-heptene, menthol and 1-octen-3-ol were detected in all the aphid-induced A. thaliana plants. However, equal quantities of Limonene and 2-methyl-6-heptene were emitted independent of the aphid densities, while high quantities of menthol and 1-octen-3-ol were produced where aphid densities were increased (from 5 to 20 aphids). These results indicate that the quantities of some HIPVs (aphid-induced A. thaliana) compounds production depend on the densities of the herbivores. In addtition, our results indicated that, benzaldehyde, phenylacetaldehyde and decan-3-ol were produced only if the densities of aphids were between 5 and 20 adult aphids, while Terpineols were produced only when de density reached 20 aphids. This shows that some HIPVs could only be emitted if the pest population is high on the host plants as observed for Terpineols in aphid-induced A. thaliana. Furthermore, decan-3-ol, benzaldehyde, phenylacetaldehyde and salicylate acid were recorded in the headspace when A. thaliana was damaged by L. erysimi, and the quantity of menthol emitted from infested plants was significantly higher than control and the other HIPVs. Since insect herbivores are the key factor that induces HIPVs, it could be deduced from these results that the quantity and the composition of the emitted HIPVs might be influenced by the density of the pest population present at a particular time and stage of the host plants. Similar results were reported by Rodriguez-Saona, et al.13 through a gradual perennial shrub Vaccinium corymbosum infestation by gypsy moth caterpillars Lymantria dispar over time. The authors indicated that, the plant VOC emission rate also increased concomitantly as regards to the level of infestations. Our results also showed similar effects where the composition in terms of the number of HIPVs emitted from aphid-induced A. thaliana and their quantities increased with the subsequent increase in the number of aphids on the A. thaliana plant. In our previous finding and in this present study, HIPVs emitted from aphids-induced Arabidopsis increased performance of L. lecanii, and the rates of conidial germination as well as appressorial formation varied depending on the exposed aphid densities12. The results of this study showed that, decan-3-ol, benzaldehyde, phenylacetaldehyde, salicylate acid and menthol were considered as key synergetic compounds that enhanced conidial performance and consequently, improved the pathogenicity and virulence of L. lecanii strain V3450. Similar results were also reported by Lin, et al.12 using the same host plant and different aphid densities. In addition, we collected volatiles from 20 adult aphids in the glass jar of diagrammatic sketch of headspace collecting system, and analyzed the volatiles profile with GC-MS in a supplementary experiment (Supplementary experiment 3). The results showed that there were not menthol, methyl salicylate, decan-3-ol, benzaldehyde and phenylacetaldehyde in the headspace volatiles from aphids (Supplementary Fig. 1). These results suggested that the above mentioned chemical compounds (benzaldehyde, menthol, phenylacetaldehyde, decan-3-ol and methyl salicylate) found in the headspace volatiles were not emitted by L. erysimi but rather produced by the L. erysimi-induced Arabidopsis host plants.
When applied to L. lecanii, the five identified compounds produced different influences as regards to the level of fungal performance vis-a-vis aphid densities. Methyl salicylate and menthol did not provoke significant changes to the germination of the fungus, while decan-3-ol, benzaldehyde, phenylacetaldehyde inhibited the conidial germination. But when focusing on the appressorium formation, methyl salicylate (1 nmol·ml−1) and menthol (1 and 10 nmol·ml−1) showed their inducing function on L. lecanii performance, while the other compounds showed their inhibition function vis-a-vis to the fungus. This finding is significant because the formation of the appressoria is a precursor to the infection process of pathogenic fungi, which are swollen, dome-shaped cells12,14,15. These two compounds, menthol and methyl salicylate might therefore contribute to enhancing the pathogenicity and virulence of the entomopathogenic fungus L. lecanii with a synergetic effect. These results are similar to the previous research, which reported that salicylate acid indirectly promoted arbuscular mycorrhizal fungus in clover roots16. Spence, et al.17 also found that appressorial formation in Magnaporthe oryzae infecting rice was negatively impacted by hydrogen cyanide, a volatile produced by rhizospheric bacteria, had suppressed the rice blast infections.
In addition, when also applying the five identified compounds to the culture of L. lecanii, we found that methyl salicylate (10 nmol·ml−1) and menthol (1 and 1 nmol·ml−1) promoted hyphal growth and the toxicity of the entomopathogenic fungus increased significantly, while other compounds inhibited significantly the growth speed (sporulation) and consequently the pathogenicity of the fungus. Similarly, it was also found that methyl salicylate (10 nmol·ml−1) and menthol (10 nmol·ml−1) promoted sporulation significantly, while others inhibited significantly the fungal sporulation. These results indicated that methyl salicylate and menthol may increase or enhance the toxicity by up regulating the appressorial formation, hyphal growth and sporlulation of L. lecanii.
Entomopathogenic fungi (EPFs) are satisfactory candidates which have the potential to replace synthetic broad spectrum insecticides without having a major impact on the environment18,19. EPFs showed their potential and prospects of controlling insect vectors20,21,22, pests23,24,25,26,27 under laboratory and field conditions through inundative and autodissemination devices applications. Some of them, such as Metarhizium anisopliae and Beauveria bassiana had been used in the field with significant effects on their target pests28,29. EPFs infect insect pests and are not harmful to other participants of the particular agroecosystem. However, these EPFs encountered low pathogenicity, conidia viability and instability due to the effect of UVB irradiance, and as consequence, there are only few EPF products being used in the field30,31. In this regards, to overcome these challenges, many methods of molecular biology32,33, chemical biology34,35,36, and biophysics37,38 have been investigated to increase the use of EPFs under adverse field conditions and some studies look promising39. Therefore, our findings in this study are important for understanding the multi-trophic interactions in ecosystems as well as for developing new and easier means for increasing conidial production and improving the pathogenicity of EPFs. Further molecular studies are warranted to determine, which gene locus or loci is responsible for the production of these chemicals. Once identified, it might possibly be overexpressed as the gene segment to increase the efficacy of L. lecanii or promote its use in other EPFs.
Methods
Biological materials
Arabidopsis thaliana ecotype Colombia (Col-0) was used for all experiments. The plant was grown according to the methods of Hirao, et al.40 with slight modification. Instead of the 100-ml glass tubes used in reference, the 7-day-old seeding was transported into a 50 ml glass cup ( mm diameter, 8 mm length) (one seedling/cup), and incubated for 14 days in the growth chamber (23 °C, 70e80 mmol.m−2. s−1 fluorescent light, 16 h light/8 h dark, 65 RH). Plants visually healthy and not showed any damage for any other plant pest that could influence the volatile emissions were sampled or selected and used in the experiments.
Lipaphis erysimi were collected from cabbage fields at Fujian Agriculture and Forestry University (FAFU) campus and bred in mesh cages (50 cm × 50 cm × 50 cm) under laboratory conditions of 25 °C temperature, 75% RH, under a photoperiod of 16 L: 8D on A. thaliana for 5 generations prior to the experiments. Two-day-old apterous adults were collected from the established colony and used for the experiments.
The entomopathogenic fungus, L. lecanii strain V3450, was initially isolated from Siphoninus phillyreae Hasiday. This strain was obtained from China General Microbiological Culture Collection Center (CGMCC) in 2015, and cultured in the Laboratory of Insect Ecology in Fujian Agriculture and Forestry University. The fungus was identified according to morphological characteristics using the taxonomic keys for the genus Lecanicillium41,42.
Headspace collection and volatiles analysis
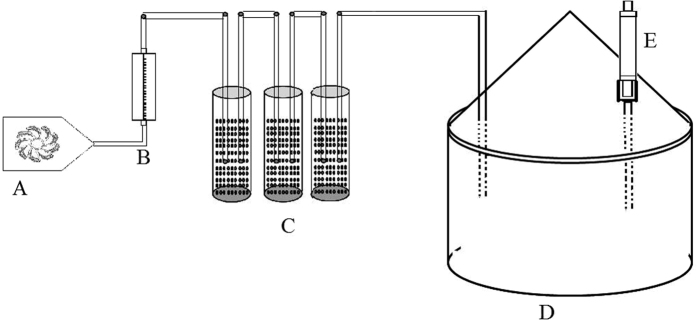
The aphids-induced-Aradopsis plant volatiles were collected in the first 12 h using a headspace collecting system as shown in Fig. 3. According to the supplementary experiment 1, different densities of L. erysimi apterous adults (1, 2, 5, 10 and 20 which corresponded to five different defined treatments I, II, III, IV and V respectively) were released on A. thaliana for infestation for one hour. And then, each four infested plants with the same density of L. erysimi were transferred into a clean glass jar (12 cm diameter, 30 cm high). The air was blown into the jar at the rate of 300 ml·min−1 and flowed out of the headspace through a glass tube filled with 200 mg Tenax TA (60/80 mesh; Grace-Alltech, Deerfield, USA). After collection, the HIPVs were eluted into 1 ml methenyl trichloride and stored at −20 °C immediately. Four clean plants without aphid infestation were used as a control, and the experiment was repeated 3 times.
Figure 3. Diagrammatic sketch of headspace collecting system.
(A) pump; (B) flower meter; (C) air-purify cup; (D) glass jar; (E) absorbing column.
HIPVs were analyzed with a gas chromatograph (Agilent Technologies 7890B GC System)-mass spectrometer (Agilent Technologies 5977 A MSD) (GC-MS) via a HP-5 column (30 mm × 0.25 mm/1.0 μm film thickness, Agilent). The GC oven temperature was programmed from 40 °C (5-min hold), which was increased at a rate of 10 °C·min−1 regularly until it reached a temperature of 280 °C. The column effluent was ionized by electron impact ionization at 70 eV. Mass spectra were acquired by scanning from 35 to 350 m/z with the scanning rate of 5.38 scans·sec−1.
Compounds were identified by using the deconvolution software AMDIS (version 2.64, NIST, USA) in combination with NIST 05 and Wiley 7th edition spectral libraries and by comparing their retention indices with those from the literature. Quantities of headspace compounds were also compared with a standard sample quantity and by the detected peak area from GC-MS results.
Influence of aphid density on conidial germination and appressorial formation
HIPVs were collected as described above based on the various aphid densities on the host plant. The air from the headspace was then allowed to flow into another jar (incubator jar) instead of absorbent column. There was 10 ml sterile water in the incubator jar to keep humidity for conidial germination. A concave slide was plastered onto the top of the incubator jar. Ten microliter of L. lecanii conidial suspension with a concentration of 1 × 108 conidia·ml−1 was printed on the wall of the concave slide. Both conidial germination and appressorial formation of L. lecanii were determined after 12 h under a compound light microscope at 400X magnification for all the aphid density levels. The criterion used to assess conidial germination was that the length of germ tube > 50% the length of the conidia12. The treatment of 0 or no L. lecanii adults was used as control. Each test was conducted four times with 5 replicates.
Efficiency of HIPVs compounds on fungal germination and appressorial formation
The results of GC-MS test and bioassay for conidial performance were analyzed by SPSS software 21.0 version to determine the correlation between single HIPVs chemical and germination rate or appressorial formation rate. Major compounds menthol, methyl salicylate, decan-3-ol, benzaldehyde, and phenylacetaldehyde identified in HIPVs of A. thaliana induced plant by the aphids revealed significantly high correlation with appressorial formation rate when different aphids’ densities were used as treatments (Table 3). Prior to the experiment, synthetic of similar identified compounds were dissolved in triethyl citrate (TEC) to achieve slow volatilization as described in Uefune, et al.43. A supplementary experiment was conducted to determine the experimental quantities of synthetic chemical (Supplementary experiment 2). All the synthetic chemicals previously listed above were purchased from Macklin Biochemical Co. Ltd. (Shanghai, China).
To assess germination and appressorial formation of conidia, a 2.5 μl conidial suspension (1 × 108 conidia·ml−1) was prepared in Czapek’s liquid medium and placed onto the wall of a concave glass slide, and allowed to dry for 5 min44. A sterilized cotton roll was put into the jar of headspace collecting system (Fig. 3). One milliliter of each synthetic compound solution was applied on the cotton ball. The concentrations of menthol and methyl salicylate (MeSA) were 1, 10, 100 and 1000 nmol·ml−1 TEC, respectively; while the ones of decan-3-ol, benzaldehyde, and phenylacetaldehyde were 0.2, 2, 20 and 200 nmol·ml−1 TEC, respectively (Supplementary Table 2). The air from the headspace was then allowed to flow into another jar (incubator jar) instead of absorbent column as described above in the aphid density section. There was 10 ml sterile water in the incubator jar to keep humidity for conidial germination. A concave slides were plastered onto the top of the incubator jar. Ten microliter of L. lecanii conidial suspension with the concentration of 1 × 108 conidia·ml−1 was printed on the wall of the concave slide. Both conidial germination and appressorial formation of L. lecanii were determined after 12 h under a compound light microscope at 400X magnification for all the defined concentrations. The criterion used to assess conidial germination was the same as describes above where the length of germ tube > 50% the length of the conidia12. Each test was conducted four times with 5 replicates per synthetic compound.
Activity of synthetic compounds on fungal sporulation and growth
The fungus L. lecanii V3450 was cultured on Czapek’s solid medium and incubated at 25 °C, 75% RH and a photoperiod of 12:12 L:D for 14 days. Conidia were harvested by scraping the surface of sporulating cultures with an inoculating loop. Conidial suspension adjusted at the concentration of 1 × 108 conidia·ml−1 was prepared with sterile water containing 0.05% Triton X-8045. Ten microliters of the fungal suspension were transferred to the center of round cellophane membranes (80 mm diameter) which covered the surface of the Czapek’s medium in a Petri dish (90 mm diameter). Sterilized tiny cotton wool ball was placed on the bottom of the Petri dish which was inoculated with L. lecanii V3450, and then amended with 1 ml of serial dilutions of each synthetic compound solution (1, 10, 100 and 1000 nmol·ml−1 TEC for menthol and MeSA; 0.2, 2, 20 and 200 nmol·ml−1 TEC for decan-3-ol, benzaldehyde, and phenylacetaldehyde). All the dishes were sealed tightly with Parafilm and kept in the incubator at 25 °C, at 75% RH, and with 12:12 L:D photoperiod. The range of dilutions used for the bioassay was based on the pre-experimental outputs mentioned above (Supplementary Table 2). Control petri dishes were treated with 0.1% Tween X-80, and each dilution was repeated 5 times for each synthetic compounds.
To access the growth rate, diameter of colony in each Petri dish was determined by vernier caliper every 24 h, and the diameters were measured for 14 days46.
To quantify conidial production, colonies and cellophane membranes were transferred into a 50 ml-centrifuge tube containing 10 ml sterile solution of 0.1% Tween-80 and filtered by three layers of sterile gauze to make conidial suspensions after 14-days incubation47. The concentration of suspensions was determined by vortexing for about 5 min to produce homogenous conidial suspensions. Conidial counts were then made using an improved Neubauer Hemacytometer to determine the right concentration of conidia.
In vivo pathogenicity and virulence determination
The pathogenicity and virulence of L. lecanii to the adult aphids were assessed after the fungus was pre-treated with the HIPVs synthetic chemicals. Conidia from multi treated by synthetic chemicals was collected from previous experiment and made into conidial suspensions with distilled water respectively. Conidial suspensions were diluted to 5 × 107 conidia·ml−1 with sterilized solution of 0.1% Tween-80 for infection bioassays. Fifty apterous adult L. erysimi per treatment were immersed in conidial suspension for 30 sec in a 50 ml beaker to inoculate them with fungal conidia. After inoculation, all L. erysimi were air dried for 5 min and transferred to cauliflower leaves (5 × 5 cm) with wetted cotton to keep them fresh48.
Each treatment in this experiment was repeated three times, while the control were treated with sterilized solution of 0.1% Tween X-80. The lethal time (LT50) and mortality were assessed where LT50 was analyzed by linear regression. The whole experiment was conducted in an illumination incubator under constant conditions of 25 °C, 75% RH, and 12:12 L:D photoperiod.
Statistical analysis
Mortality of L. erysimi in treated groups was corrected by that observed in controlled conditions following the method described by Jones, et al.49. The median lethal time (LT50) was estimated using probit analysis in the statistical software SPSS 20.0. The means of compound quantities, fungal colony growth, conidial production, corrected mortality, germination rate and appressorial formation rate were compared by Tukey’s HSD test (α = 0.05) following an analysis of variance using the SPSS 20.0.
Additional Information
How to cite this article: Lin, Y. et al. The Herbivore-Induced Plant Volatiles Methyl Salicylate and Menthol Positively affect Growth and Pathogenicity of Entomopathogenic Fungi. Sci. Rep. 7, 40494; doi: 10.1038/srep40494 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
We are grateful to J. Liu for offering assistance in evaluating the headspace absorbance, and Y. Lu and J. Zhu for their analytical assistance, to M. Wu for help with maintaining the fungal culture, and to D. Fang for supplying the plant material. This project was funded by the National Natural Science Foundation of China (31371998) and Key projects of Science and Technology of Fujian Province (2016N0005).
Footnotes
Author Contributions Y.L. and L.W. conceived and designed the experiments. Y.L. conducted the experiments, Y.L., M.Q. and M.H. analyzed the data. Y.L., M.Q., M.H., K.A., P.A., C.D. and L.W. authors reviewed the manuscript.
References
- Arimura G. I., Kost C. & Boland W. Herbivore-induced, indirect plant defences. Biochimica Et Biophysica Acta 1734, 91–111 (2005). [DOI] [PubMed] [Google Scholar]
- Arimura G., Matsui K. & Takabayashi J. Chemical and molecular ecology of herbivore-induced plant volatiles: proximate factors and their ultimate functions. Plant Cell Physiol 50, 911–923, doi: 10.1093/pcp/pcp030 (2009). [DOI] [PubMed] [Google Scholar]
- Kaori S. et al. Changing green leaf volatile biosynthesis in plants: an approach for improving plant resistance against both herbivores and pathogens. Proceedings of the National Academy of Sciences 103, 16672–16676 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J. & Kang L. Roles of (Z)-3-hexenol in plant-insect interactions. Plant signaling & behavior 6, 369–371 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto H. & Takabayashi J. Parasitic wasps Aphidius ervi are more attracted to a blend of host-induced plant volatiles than to the independent compounds. Journal of chemical ecology 41, 801–807, doi: (2015). [DOI] [PubMed] [Google Scholar]
- Brillada C. et al. Metabolic engineering of the C 16 homoterpene TMTT in Lotus japonicus through overexpression of (E, E)-geranyllinalool synthase attracts generalist and specialist predators in different manners. New Phytologist 200, 1200–1211 (2013). [DOI] [PubMed] [Google Scholar]
- Degenhardt J. & Iii J. H. T. Restoring a maize root signal that attracts insect-killing nematodes to control a major pest. Proceedings of the National Academy of Sciences of the United States of America 106, 13213–13218 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutyambai D. M. et al. Responses of parasitoids to volatiles induced by Chilo partellus oviposition on teosinte, a wild ancestor of maize. Journal of chemical ecology 41, 323–329, doi: 10.1007/s10886-015-0570-1 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler A. & Baldwin I. T. Defensive function of herbivore-induced plant volatile emissions in nature. Science 291, 2141–2144, doi: 10.1126/science.291.5511.2141 (2001). [DOI] [PubMed] [Google Scholar]
- Hountondji F. C., Sabelis M. W., Hanna R. & Janssen A. Herbivore-induced plant volatiles trigger sporulation in entomopathogenic fungi: the case of Neozygites tanajoae infecting the cassava green mite. Journal of chemical ecology 31, 1003–1021 (2005). [DOI] [PubMed] [Google Scholar]
- Atsumi A. & Saito T. Volatiles from wasabi inhibit entomopathogenic fungi: implications for tritrophic interactions and biological control. Journal of Plant Interactions 10, 152–157, doi: 10.1080/17429145.2015.1039613 (2015). [DOI] [Google Scholar]
- Lin Y. et al. Volatiles from plants induced by multiple aphid attacks promote conidial performance of Lecanicillium lecanii. PLoS ONE 11 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Saona C. R., Rodriguez-Saona L. E. & Frost C. J. Herbivore-induced volatiles in the perennial shrub, Vaccinium corymbosum, and their role in inter-branch signaling. Journal of Chemical Ecology 35, 163–175 (2009). [DOI] [PubMed] [Google Scholar]
- Chengshu W. & Leger R. J. St. The Metarhizium anisopliae perilipin homolog mpl1 regulates lipid metabolism, appressorial turgor pressure, and virulence. Journal of Biological Chemistry 282, 21110–21115 (2007). [DOI] [PubMed] [Google Scholar]
- Gachomo E. W., Seufferheld M. J. & Kotchoni S. O. Melanization of appressoria is critical for the pathogenicity of Diplocarpon rosae. Molecular Biology Reports 37, 3583–3591 (2010). [DOI] [PubMed] [Google Scholar]
- Zhu H. et al. The possible involvement of salicylic acid and hydrogen peroxide in the systemic promotion of phenolic biosynthesis in clover roots colonized by arbuscular mycorrhizal fungus. Journal of Plant Physiology 178, 27–34 (2015). [DOI] [PubMed] [Google Scholar]
- Spence C. et al. Natural rice rhizospheric microbes suppress rice blast infections. Bmc Plant Biology 14, 1–17 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain A., Rizwan-Ul-Haq M., Al-Ayedh H. & Al-Jabr A. M. Mycoinsecticides: potential and future perspective. Recent Patents on Food 6, 45–53 (49) (2014). [DOI] [PubMed] [Google Scholar]
- Zimmermann G. Review on safety of the entomopathogenic fungus Metarhizium anisopliae. Biocontrol Science & Technology 17, 879–920 (2007). [Google Scholar]
- Polar P. et al. Thermal characteristics of Metarhizium anisopliae isolates important for the development of biological pesticides for the control of cattle ticks. Veterinary Parasitology 134, 159–167 (2005). [DOI] [PubMed] [Google Scholar]
- Benjamin M. A., Zhioua E. & Ostfeld R. S. Laboratory and field evaluation of the entomopathogenic fungus Metarhizium anisopliae (Deuteromycetes) for controlling questing adult Ixodes scapularis (Acari: Ixodidae). Journal of Medical Entomology 39, 723–728 (2002). [DOI] [PubMed] [Google Scholar]
- Scholte E. J., Knols B. G. J. & Takken W. Infection of the malaria mosquito Anopheles gambiae with the entomopathogenic fungus Metarhizium anisopliae reduces blood feeding and fecundity. Journal of Invertebrate Pathology 91, 43–49 (2006). [DOI] [PubMed] [Google Scholar]
- Wright M. S., Raina A. K. & Lax A. R. A strain of the fungus Metarhizium anisopliae for controlling subterranean termites. Journal of Economic Entomology 98, 1451–1458 (2014). [DOI] [PubMed] [Google Scholar]
- Kanga L. H. B., Jones W. A. & James R. R. Field trials using the fungal pathogen, Metarhizium anisopliae (Deuteromycetes: Hyphomycetes) to control the ectoparasitic mite, Varroa destructor (Acari: Varroidae) in honey bee, Apis mellifera (Hymenoptera: Apidae) colonies. Journal of Economic Entomology 96, 1091–1099 (2003). [DOI] [PubMed] [Google Scholar]
- Bruck D. J. Ecology of Metarhizium anisopliae in soilless potting media and the rhizosphere: implications for pest management. Biological Control 32, 155–163 (2005). [Google Scholar]
- Dimbi S., Maniania N. K., Lux S. A. & Mueke J. M. Effect of constant temperatures on germination, radial growth and virulence of Metarhizium anisopliae to three species of African tephritid fruit flies. Biocontrol 49, 83–94 (2004). [Google Scholar]
- Wraight S. P. et al. Evaluation of the entomopathogenic fungi Beauveria bassiana and Paecilomyces fumosoroseus for microbial control of the silverleaf whitefly, Bemisia argentifolii. Biological Control 17, 203–217 (2000). [Google Scholar]
- Feng M. G., Poprawski T. J. & Khachatourians G. G. Production, formulation and application of the entomopathogenic fungus Beauveria bassiana for insect control: current status. Biocontrol Science & Technology 4, 3–34 (1994). [Google Scholar]
- Farenhorst M. et al. African water storage pots for the delivery of the entomopathogenic fungus Metarhizium anisopliae to the malaria vectors Anopheles gambiae s.s. and Anopheles funestus. American Journal of Tropical Medicine & Hygiene 78, 910–916 (2008). [PubMed] [Google Scholar]
- Fernandes É. K. K., Rangel D. E. N., Braga G. U. L. & Roberts D. W. Tolerance of entomopathogenic fungi to ultraviolet radiation: a review on screening of strains and their formulation. Current Genetics 61, 1–14 (2015). [DOI] [PubMed] [Google Scholar]
- Braga G. U. L., Flint S. D., Messias C. L., Anderson A. J. & Roberts D. W. Effects of UVB irradiance on conidia and germinants of the entomopathogenic Hyphomycete Metarhizium anisopliae: a study of reciprocity and recovery. Photochemistry & Photobiology 73, 140–146 (2001). [DOI] [PubMed] [Google Scholar]
- Linzhi Y. et al. Expression of a toll signaling regulator serpin in a mycoinsecticide for increased virulence. Applied & Environmental Microbiology 80, 4531–4539 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuqi Q., Almudena O.-U. & Nemat O., K. A putative methyltransferase, mtrA, contributes to development, spore viability, protein secretion and virulence in the entomopathogenic fungus Beauveria bassiana. Microbiology 160, 2526–2537 (2014). [DOI] [PubMed] [Google Scholar]
- Safavi S. A. et al. Effect of nutrition on growth and virulence of the entomopathogenic fungus Beauveria bassiana. Fems Microbiology Letters 270, 116–123 (2007). [DOI] [PubMed] [Google Scholar]
- St Leger R. J., Nelson J. O. & Screen S. E. The entomopathogenic fungus Metarhizium anisopliae alters ambient pH, allowing extracellular protease production and activity. Microbiology 145 (Pt 10), 2691–2699 (1999). [DOI] [PubMed] [Google Scholar]
- Hallsworth J. E. & Magan N. Effects of KCl concentration on accumulation of acyclic sugar alcohols and trehalose in conidia of three entomopathogenic fungi. Letters in Applied Microbiology 18, 8–11 (1994). [Google Scholar]
- Yeo H., Pell J. K., Alderson P. G., Clark S. J. & Pye B. J. Laboratory evaluation of temperature effects on the germination and growth of entomopathogenic fungi and on their pathogenicity to two aphid species. Pest Management Science 59, 156–165 (2003). [DOI] [PubMed] [Google Scholar]
- Devi K. U., Sridevi V., Mohan C. M. & Padmavathi J. Effect of high temperature and water stress on in vitro germination and growth in isolates of the entomopathogenic fungus Beauveria bassiana (Bals.) Vuillemin. Journal of Invertebrate Pathology 88, 181–189 (2005). [DOI] [PubMed] [Google Scholar]
- Batta Y. A. Recent advances in formulation and application of entomopathogenic fungi for biocontrol of stored-grain insects. Biocontrol Science & Technology, 1–28 (2016). [Google Scholar]
- Hirao T. et al. Green leaf volatiles enhance methyl jasmonate response in Arabidopsis. Journal of Bioscience & Bioengineering 114, 540–545 (2012). [DOI] [PubMed] [Google Scholar]
- Zare R. & Gams W. A revision of Verticillium section Prostrata. IV. The genera Lecanicillium and Simplicillium gen. nov. Nova Hedwigia 73, 1–50 (2001). [Google Scholar]
- Zare R. & Gams W. A revision of the Verticillium fungicola species complex and its affinity with the genus Lecanicillium. Mycological research 112, 811–824, doi: http://dx.doi.org/10.1016/j.mycres.2008.01.019 (2008). [DOI] [PubMed] [Google Scholar]
- Uefune M., Kugimiya S., Shimoda T. & Takabayashi J. Starvation and herbivore-induced plant volatiles affect the color preferences of parasitic wasps. Biocontrol 58, 187–193 (2012). [Google Scholar]
- González L. C. et al. Effect of six fungicides on Lecanicillium (Verticillium) lecanii (Zimm.) Zare & Gams. Journal of Food Agriculture & Environment 2012, 1142–1145 (2013). [Google Scholar]
- Ganassi S., Grazioso P., Moretti A. & Sabatini M. A. Effects of the fungus Lecanicillium lecanii on survival and reproduction of the aphid Schizaphis graminum. Biocontrol 55, 299–312 (2010). [Google Scholar]
- Gao Liu & XZ. A novel two-stage cultivation method to optimize carbon concentration and carbon-to-nitrogen ratio for sporulation of biocontrol. Folia Microbiologica 54.0 (2009). [DOI] [PubMed] [Google Scholar]
- Manhong S. & Xingzhong L. Carbon requirements of some nematophagous, entomopathogenic and mycoparasitic hyphomycetes as fungal biocontrol agents. Mycopathologia 161.0, 295–305 (2006). [DOI] [PubMed] [Google Scholar]
- Lin Y. W., Wu G. & Miyata T. Insecticide susceptibility of surviving Cotesia plutellae (Hym: Braconidae) and Diaeretiella rapae (M’Intosh) (Hym: Aphidiidae) as affected by sublethal insecticide dosages on host insects. Pest Management Science 63, 841–850 (2007). [DOI] [PubMed] [Google Scholar]
- Jones W. E., Grace J. K. & Tamashiro M. Virulence of seven isolates of Beauveria bassiana and Metarhizium anisopliae to Coptotermes formosanus (Isoptera: Rhinoterrnitidae). Environmental Entomology 25, 481–487 (1996). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.