Abstract
The monounsaturated fatty acid palmitoleate (palmitoleic acid) is one of the most abundant fatty acids in serum and tissues, particularly adipose tissue and liver. Its endogenous production by stearoyl-CoA desaturase 1 gives rise to its cis isoform, cis-palmitoleate. Although trans-palmitoleate is also synthesized in humans, it is mainly found as an exogenous source in ruminant fat and dairy products. Recently, palmitoleate was considered to be a lipokine based on evidence demonstrating its release from adipose tissue and its metabolic effects on distant organs. After this finding, research has been performed to determine whether palmitoleate has beneficial effects on metabolism and to elucidate the underlying mechanisms. Thus, the aim of this work was to review the current status of knowledge about palmitoleate, its metabolism, and its influence on metabolic abnormalities. Results have shown mixed cardiovascular effects, direct or inverse correlations with obesity, and hepatosteatosis, but a significant amelioration or prevention of insulin resistance and diabetes. Finally, the induction of palmitoleate release from adipose tissue, dietary intake, and its supplementation are all interventions with a potential impact on certain metabolic diseases.
Keywords: fatty acids, palmitoleate, lipokine, diet, obesity, diabetes, insulin resistance, cardiovascular disease
Introduction
Metabolic syndrome is characterized by the accumulation of visceral adipose tissue, dyslipidemias, hypertension, and high concentrations of fasting plasma glucose, as well as low-grade inflammation (1, 2). Dietary interventions that promote lifestyle changes can be used as an appropriate alternative to reduce metabolic syndrome. Such interventions include modifications of the type and concentration of dietary FAs, which influence metabolism through various pathways. For instance, dietary fat saturation plays a role in lipoprotein metabolism and insulin action. SFAs are associated with a higher risk of ischemic heart disease and insulin resistance, whereas unsaturated FAs have multiple beneficial effects on cardiovascular health (3–5) and insulin sensitivity (6–8) in clinical and animal studies. MUFAs promote a healthy blood lipid profile and improved blood pressure, insulin sensitivity, and glycemic control (9–12). PUFAs positively affect insulin sensitivity, cardiovascular and mental health, and development, and reduce hypertension and inflammation (13, 14).
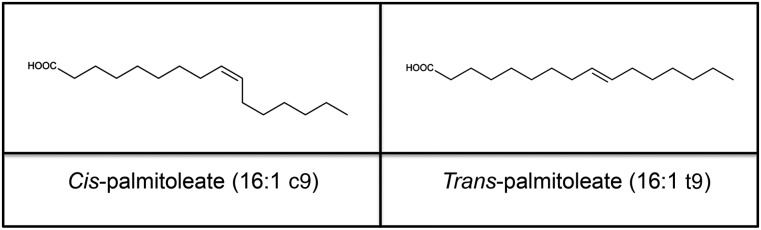
The MUFA palmitoleic acid or palmitoleate (16:1n–7 or 16:1D9) has received a lot of attention in recent years, even though its metabolism was described in the 1960s (15). Palmitoleate can be found as a cis (16:1c9) or a trans (16:1t9) isomer (Figure 1). The cis isoform (cis-palmitoleate) has been associated with increased insulin sensitivity and decreased lipid accumulation in the liver (16). In animal models, cis-palmitoleate decreased the expression of proinflammatory markers and adipokines, which are related to the establishment of metabolic abnormalities (16–18). Trans-palmitoleate (1) is found in dairy products and partially hydrogenated oils and may be associated with favorable metabolic profiles and decreased incident diabetes (19). Typically, trans FAs, which are produced for commercial cooking and frying, are related to a higher risk of endothelial dysfunction and coronary heart disease (20). Nevertheless, prospective cohorts have denied the association of plasma trans-palmitoleate with stroke (21), and further research is needed to determine whether the proinflammatory effects of trans-linoleic acid and trans-oleic acid also are attributed to trans FAs other than trans-palmitoleate.
FIGURE 1.
Chemical structure of palmitoleate. Palmitoleate (16:1n–7 or 16:1Δ9) is a MUFA with 16 carbons. The double bond is located in the n–7 or Δ9 carbon, counting from the methyl-terminal carbon or the carboxylic acid end, respectively. The 2 isoforms of palmitoleate, cis and trans, are shown here.
Therefore, considering the evidence of its beneficial effects, it is important to evaluate whether palmitoleate could be used as a strategy for preventing or treating metabolic diseases. Here, we review the current status of knowledge of palmitoleate, including its sources, metabolism, effects on metabolic abnormalities, and underlying mechanisms.
Endogenous Synthesis of Palmitoleate
Palmitoleic acid mainly originates from de novo lipogenesis in humans. Lipogenesis is mediated by stearoyl-CoA desaturase 1 (SCD1)6, the rate-limiting enzyme catalyzing the synthesis of MUFAs, mainly oleate (18:1c9) and palmitoleate (16:1c9) (22). In addition to palmitate, oleate also can be converted into palmitoleate by chain shortening in human hepatocellular carcinoma cells (23). In humans, cis-palmitoleate biosynthesis occurs primarily in the liver, and secondarily in adipose tissue, where it is later incorporated into phospholipids, TGs, waxes, and cholesterol esters.
Because SCD1 is the main pathway for palmitoleate generation, researchers have assumed before that endogenous production of palmitoleate is represented only by cis-palmitoleate. However, recent findings show that trans-palmitoleate also could be generated endogenously by the shortening of dietary vaccenic acid (18:1t11), with a conversion rate of 17% (24). Because dietary concentrations of palmitoleate are relatively low, and it is rapidly oxidized (15, 25), it is possible that palmitoleate plasma concentrations are affected by endogenous production from dietary vaccenic acid. Vaccenic acid is the most predominant ruminant trans FA, and its metabolic effects are either neutral or suggest protection against atherosclerosis and activation of PPARs (23, 26–29). Thus, because vaccenic acid is a precursor of trans-palmitoleate, the chance of increased metabolic benefits through dairy consumption arises.
Dietary Palmitoleate
Dietary sources with high palmitoleate content include salmon, cod liver oil, and macadamia oil (6%, 7%, and 17% or g/100 g total FAs, respectively). Currently, the highest reported concentration of palmitoleate in foods corresponds to the shrub sea buckthorn, which is native to Asia and Europe. The oil of its pulp contains palmitoleic acid at 32–42% (30, 31). Other natural sources of palmitoleate are olive oil, chocolate, and eggs.
trans-Palmitoleate can be found in partially hydrogenated oils, but it is principally derived from dairy and ruminant trans fats (32). In humans, serum trans-palmitoleate concentration ranges from 0.02% to 0.55% of total FAs (33), which means that <1% of FAs are represented by palmitoleate. trans-Palmitoleate plasma concentrations are positively correlated with self-reported consumption of whole-fat dairy, butter, margarine, and baked desserts (33). Thus, it has been considered to be a dairy fat biomarker. In a prospective cohort study in 3736 adults [the Cardiovascular Health Study (CHS)], circulating trans-palmitoleate concentrations correlated strongly with FA biomarkers of dairy fat intake, but weakly with markers of partially hydrogenated oils (33, 34), suggesting that trans-palmitoleate is found mainly in naturally occurring fats.
It has been shown that palmitoleic acid in plasma TG and cholesteryl ester is positively related to carbohydrate intake (35). It is known that carbohydrate intake increases SCD1 expression (36) and, therefore, a direct relation between dietary carbohydrate and palmitoleate concentrations was expected. Various other studies in humans also have exposed a direct association between carbohydrate intake and plasma palmitoleate, indicating upregulation of de novo lipogenesis (37–39). Because cis-palmitoleate is synthesized endogenously, diet components modifying SCD1 activity as carbohydrate necessarily change serum cis-palmitoleate concentrations, which range approximately from 0.19% to 0.5% of FAs (40). An intervention with distinct groups of carbohydrate content in the diet showed that highest carbohydrate consumption increases cis-palmitoleate 1.3 times (35). In addition to carbohydrate consumption, protein intake also was associated with plasma palmitoleate (34). Nevertheless, neither alanine nor arginine supplementation modified plasma or adipose tissue palmitoleic acid in rats (41). Natural sources of cis-palmitoleate as fish and macadamia oil could increase the blood concentration of this isoform (42, 43). For instance, a 1.3-fold increase in circulating palmitoleate was achieved in hypercholesterolemic men after they consumed macadamia nuts (43). Controversy exists in terms of serum palmitoleate modifications after fish oil intake because of its distribution in different lipids. The content of palmitoleate in lysophophatidic acid, phopsholipids, and other fractions results increased or decreased in different studies and species (44–46), with no general conclusions.
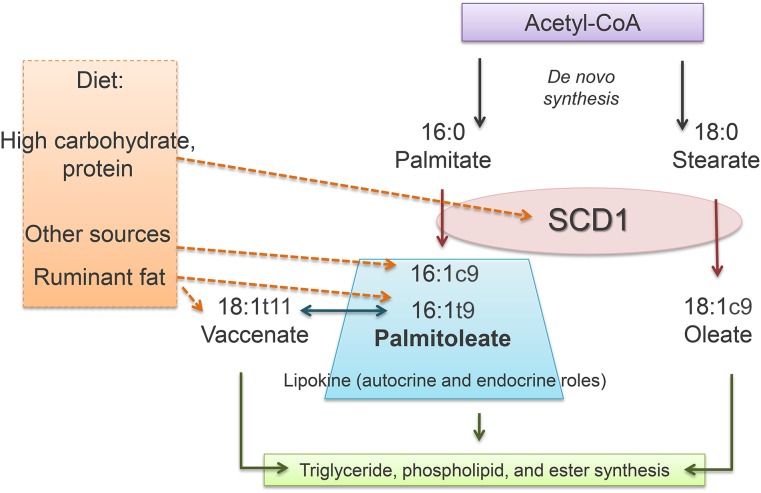
Thus, although the evidence shows that some dietary components modify palmitoleate concentrations, nutrient distribution and specific nutrients need to be investigated further in order to clarify whether diet and lifestyle changes induce metabolic improvements through palmitoleic acid. Palmitoleate sources and metabolism are summarized in Figure 2.
FIGURE 2.
Palmitoleate metabolism. De novo lipogenesis leads to FA synthesis and desaturation via SCD1, with consequent production of 18:1c9 (oleate) and 16:1c9 (cis-palmitoleate). Dietary carbohydrate and protein increase SCD1 activity and the generation of both oleate and cis-palmitoleate. Natural sources of cis-palmitoleate include macadamia nuts and fish oil. Dietary sources of palmitoleate are represented mainly by ruminant fat, which contains 16:1t9 (trans-palmitoleate) and18:1t11 (vaccenate), a precursor of trans-palmitoleate. Palmitoleate, oleate, and vaccenate can be stored in adipose tissue as TGs, cholesteryl esters, or phospholipids. Moreover, palmitoleate is now considered to be a lipokine because of its bioactivity and effects in the liver and muscle. SCD1, stearoyl-CoA desaturase 1.
Where Is Palmitoleate Hidden?
Palmitoleate is a major constituent of human muscle, liver, and adipose tissue among miristic, palmitic, stearic, oleic, and linoleic acids (47, 48). In humans, palmitoleic acid is site-specific. For example, the calf adipose depot contains more palmitoleic acid (6%) than the trapezius (3%), perirenal (4%), and mesenteric (4%) depots (49). Also, palmitoleate in subcutaneous adipose tissue in the upper arm and thigh is more abundant than in abdominal subcutaneous fat (50–55). Interestingly, the content of palmitoleic acid in adipose tissue decreases with age. Higher concentrations of palmitoleate are found in the adipose tissue of infants (>10%), lower concentrations are found in children, and the lowest are found in adults (3–7%) (47, 48, 56). Women tend to have more adipose palmitoleate than men, although the meaning of such sexual differences remains to be determined (57).
Serum palmitoleate correlates with human adipose tissue palmitoleate under fasting conditions (58). However, palmitoleate is relatively more abundant in adipose tissue than it is in serum. In animals, infusion of cis-palmitoleate increases the content of cis-palmitoleate in subcutaneous and mesenteric adipose tissue, longissimus and semitendinosus muscles, and the liver (59). This means that increasing circulating palmitoleate influences tissue abundance. FA composition in tissues has been evaluated extensively and, in many cases, dietary FAs influence its concentration in tissues. Accordingly, the content of palmitoleate in tissues is reflected by diet. In human skeletal muscle, a saturated fat–enriched diet increased palmitoleate phospholipids in comparison with a diet with a high proportion of MUFAs (60). An increase in palmitoleate concentration in white and brown adipose tissues was associated with ethanol consumption in rats (61). In the aorta of obese rats, treatment with vitamin E and selenium decreased the concentration of palmitoleate (62). The liver of rodents was more abundant in palmitoleate after supplementation with live Lactobacillus rhamnosus LA68 bacteria (63) and ubiquinone (64). Therefore, the presence of palmitoleate in distinct compartments partly depends on environmental stimuli that modify tissue and systemic concentrations.
Is Palmitoleate a Lipokine?
With the use of a mouse model that lacks the adipose chaperones fatty acid–binding proteins (FABPs) 4 and 5, an increased activation of SCD1 and a consequent rise in palmitoleate concentration in adipose tissue were observed. Augmented palmitoleate concentration significantly reduced hepatosteatosis in one study, and promoted glucose transport in skeletal muscle (16). Because palmitoleate was released from adipose tissue and induced these metabolic effects on distant organs, it was referred to as a “lipokine.” This study revealed that mobilization of bioactive lipids from adipose tissue regulates systemic metabolic homeostasis. Other works have confirmed that increased palmitoleate production influences metabolism in the absence of FABP and through activation of SCD1 (65, 66).
Because animal models are not always translated to humans, the verity of palmitoleic acid’s behaving as a lipokine was questioned in the work of Gong et al. (67). The authors of this paper concluded that palmitoleate is associated with increased SCD1 activity and human obesity. Also, they stratified this association by carbohydrate intake as a way to disentagle liver-derived palmitoleate from adipose palmitoleate. High carbohydrate increases palmitoleate generation in adipose tissue, but decreases de novo lipogenesis in the liver, according to the animal model by Cao et al. (16). However, the connection of palmitoleate and obesity was attenuated when stratifying by carbohydrate consumption. Therefore, the association between adipose-specific palmitoleate and human obesity and lipogenesis was not confirmed in this study (67).
Recently, several works have examined palmitoleate release in humans, Relative release of palmitoleate was higher than that of other FAs, and its release from gluteofemoral adipose tissue was increased compared with that of abdominal adipose tissue, mainly because of greater tissue abundance (55, 57). If palmitoleate is readily mobilized from peripheral subcutaneous adipose tissue, then its role as a lipokine would be consistent with the insulin-sensitizing action of peripheral fat. Other roles of palmitoleate that suggest that it may be a lipokine are cardiac growth (68), endothelial function (69), inhibition of cell gap junction (70), β cell proliferation (71), prevention of endoplasmic reticulum stress (72), reduction of lipogenesis and FA desaturation in adipocytes (73), and suppression of cytokine production (16). The latest studies suggest that palmitoleate could attenuate inflammation in metabolically active tissues. Palmitoleate reverses proinflammatory gene expression in bone marrow cells and macrophage polarization through the activation of AMP-activated protein kinase (74).
Finally, the reasons why palmitoleate fulfills the requirements for being lipokine are explained as follows. Circulating concentrations are changed in response to distinct metabolic states, concentrations in tissues and cells are low unless biosynthesis is initiated, and bioactivity is achieved at low concentrations. The possibility that palmitoleate may act in an autocrine or endocrine fashion has been proposed because endogenous and exogenous palmitoleate can be converted into phospholipids that contain palmitoleate (palmitoleoyl-phosphatidylinositol), and both palmitoleate and its phospholipids could be a modulating metabolism (75).
Palmitoleate in Health and Disease
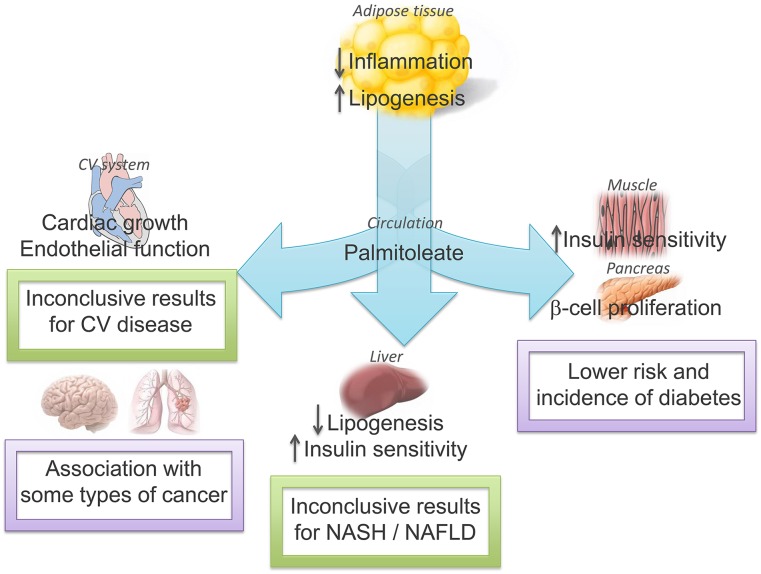
Controversial results have been found with respect to the potential metabolic benefits of palmitoleate in humans. Plasma and adipose tissue concentrations of palmitoleate have been associated with an increased risk of obesity, dyslipidemia, and insulin resistance (34, 76–78). In addition, some studies have established an association between circulating and dietary palmitoleate and decreased diabetes incidence, lower cardiovascular disease risk, and inflammation status (33, 79, 80). However, mixed cardiovascular and insulin-sensitizing effects might be explained by sex, because greater insulin resistance was shown in men but not in women (34). Moreover, different ethnic groups, mean ages, and lifestyle confounders could have influenced metabolic outcomes, because some studies include only white men, mean ages range from 50 to 75 y, and results are not always adjusted for carbohydrate or energy intake. Laboratory techniques were also different between studies, which could lead to diverse FA concentrations. Finally, palmitoleate has been measured in phospholipids, cholesteryl esters, TGs, and nonesterified FAs with different interpretations of its metabolic action. For instance, the abundance of palmitoleate in cholesteryl esters was inversely associated with insulin sensitivity in one study (81), but plasma or VLDL palmitoleate was not different between insulin-resistant and insulin-sensitive individuals in another study (82). Although the concentration of the nonesterified FA palmitoleate in plasma is increased by adipose tissue release, which is consistent with its role as a lipokine, the measurement of the FFA in plasma or its esters in plasma or tissues should be considered and related to its metabolic effects. The role of palmitoleate as a lipokine and its association with metabolic diseases are summarized in Figure 3.
FIGURE 3.
The autocrine and endocrine role of palmitoleate and its influence on metabolic diseases. Palmitoleate is produced in adipose tissue and exerts its lipokine actions in adipose tissue, the cardiovascular system, the liver, muscle, the pancreas, and other organs. These effects are associated with health or disease, or are as yet inconclusive with no connection to improvement in metabolic abnormalities. CV, cardiovascular; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; ↓, decreased; ↑, increased.
Obesity
Some studies have evaluated palmitoleate concentration in plasma or in tissues of obese compared with nonobese subjects. Results in children (77) and adults (78) showed significantly higher palmitoleate content in the plasma of the obese group. Also, a correlation was found between abdominal adiposity and palmitoleate abundance, which was consistent with increased SCD1 activity. Therefore, endogenous lipogenesis causing an increase in palmitoleate could be an important factor involved in the pathogenesis of obesity. In the Diet, Obesity, and Genes study group of subjects, an association between baseline content of palmitoleate and total weight loss was observed, i.e., the lower the initial percentage of palmitoleate, the greater was the weight loss (83). Comparing overweight and morbidly obese individuals, dietary and adipose tissue palmitoleate was higher in the overweight group (84). However, in the prospective cohort of the CHS, circulating trans-palmitoleate was associated with slightly lower BMI and waist circumference (33). These contrasting results may be due to the divergent adiposity in the groups of the latter studies. Apparently, obesity is associated with increased palmitoleate, which has been considered to be a negative predictor of weight loss. Therefore, the metabolic beneficial effects of palmitoleate could be lost during obesity despite increased circulating concentrations. This is frequently observed with some adipokines, which are exacerbated in obese conditions, but resistance to its actions has been associated with poor metabolic outcomes (85).
In young adults, a higher intake of palmitoleic acid for 1 d was inversely associated with gastric half-emptying time, which affects gastrointestinal transit and appetite (86). In male rats, administration of palmitoleic acid and a TG form of palmitoleate decreased food intake via increased concentrations of cholecystokinin, but independently of the PPAR-α pathway (87). Thus, it is possible that the short-term effects of exogenous palmitoleate include satiety, although this affirmation and the fact that obese subjects are resistant to its effects should be confirmed in future investigations.
Cardiovascular Disease
Epidemiologic studies suggest that circulating palmitoleate is involved in cholesterol metabolism and hemostasis, although net cardiovascular effects are not clear yet. In the CHS cohort, plasma phospholipid palmitoleate was associated with lower LDL cholesterol and fibrinogen and higher HDL cholesterol, but increased TGs (34, 78). Furthermore, palmitoleic acid has been correlated with multiple cardiometabolic risk factors, including high blood pressure, total cholesterol, TGs, apoA-I, apoB, and endothelial dysfunction (69, 88). In a middle-aged and elderly Chinese population, the content of palmitoleate in erythrocytes correlated with higher retinol-binding protein 4, hypertriglyceridemia, reduced HDL cholesterol, and elevated blood pressure, and inversely correlated with adiponectin (89). In a group of healthy male physicians, cis-palmitoleate concentration in the membrane of erythrocytes was positively associated with coronary heart disease, whereas vaccenic acid in the same compartment was inversely related to coronary heart disease (90). However, FA concentration in red blood cells does not necessarily represent circulating or tissue concentrations in the short term, which would reflect its synthesis and its contribution to metabolism (91). Also, the effects of palmitoleate as a lipokine would be attained as free palmitoleate and not esterified or linked to the membrane of an organelle.
Supplementation with palmitoleate or enriched diets shows decreased plasma cholesterol and plasma TG concentrations (92, 93). When dyslipidemic subjects received capsules with 220.5 mg cis-palmitoleate for 30 d, there were significant reductions in C-reactive protein, TGs, and LDL cholesterol and a significant increase in HDL cholesterol (79). Also, the consumption of trans-palmitoleate from dairy was associated with lower amounts of inflammation, but mixed relations with serum lipids (19, 33). In various studies (42, 43, 92), but not all studies (93), the consumption of macadamia nuts (which contain high cis-palmitoleate concentrations) was related to favorable serum lipid profiles.
Overall, contrasting results in humans could be due to heterogeneous populations, which include healthy subjects and patients with pre-existing dyslipidemia. Apparently, healthy subjects have no metabolic advantages with increased circulating palmitoleate, whereas supplementation in dyslipidemic subjects could be a strategy for the improvement of the serum lipid profile, although further research is needed to support this statement.
Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis
The role of palmitoleate in nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH) is controversial. In animals, increased serum palmitoleate has been associated with reduced hepatosteatosis and adipokine expression (16), Also, dietary or orally administered palmitoleate decreased plasma cholesterol, atherogenic risk, and total lipids in the liver (94, 95). In KK-Ay mice (a model of obesity and type 2 diabetes), palmitoleate consumption reduced body weight, ameliorated development of hypertriglyceridemia, and decreased lipogenesis in the liver (96). Antidiabetic thiazolidinediones changed the liver lipid profile by increasing palmitoleate due to the upregulation of SCD1 in this tissue, which was related to the insulin-sensitizing effects of thiazolidinediones in mice (97). In human and mouse primary hepatocytes, palmitoleate was cytoprotective, preventing endoplasmic reticulum stress–induced apoptosis (72). However, other studies showed that hepatic steatosis was caused by palmitoleate in mice, even though liver inflammation was attenuated (17). In rats fed sucrose, which is typically associated with the development of NASH, palmitoleic acid was increased in the plasma and liver (98). In humans, plasma palmitoleate was found to be increased in patients with either NAFLD or NASH (99, 100).
Taken together, these results indicate that liver and serum concentrations of palmitoleate change inconsistently in NAFLD and NASH, possibly due to study design, i.e., dietary palmitoleate, supplementation, or endogenously produced, time of exposure, existing metabolic abnormalities, or different conclusions for distinct species.
Diabetes and Insulin Resistance
After results indicating that palmitoleate could be acting as a lipokine, several groups examined this FA in the context of insulin resistance and type 2 diabetes. A substantial decreased risk of diabetes in the CHS and Multi-Ethnic Study of Atherosclerosis was associated with increased serum trans-palmitoleate. Each 0.05% higher trans-palmitoleate concentration was related to a 32% and 28% lower risk of diabetes in the Multi-Ethnic Study of Atherosclerosis and the CHS, respectively (33). In a study that recruited 100 Caucasian subjects, circulating palmitoleate predicted insulin sensitivity, estimated by euglycemic–hyperinsulinemic clamp and oral glucose tolerance tests, independent of age, sex, and adiposity (80).
The abundance of palmitoleate in cholesteryl esters has been associated with insulin sensitivity in most studies (101, 102). For instance, palmitoleate in this same fraction was greater in women living in the Amazonian rain forest than in urbanized women, and less body fat and HOMA-IR also was found in the former group (81). Similarly, nonesterified palmitoleate was associated with lower HOMA-IR in healthy subjects (103). Relative release of palmitoleate was markedly higher from gluteofemoral adipose tissue than from abdominal subcutaneous adipose tissue (57). This implies that lower body adipose tissue is the major source of palmitoleate that serves as a lipokine (nonesterified form) and protects against metabolic disease risk. This is consistent with findings showing that central adiposity, but not peripheral fat, is related to metabolic abnormalities (1, 104). One work confirms that increased production of palmitoleate is associated with insulin sensitivity. Here, the authors concluded that rosiglitazone improved insulin sensitivity by increasing adipose SCD1 activity measured by the desaturation index 16:1/16:0, and that this process was dependent on PPAR-γ transcriptional activity (105).
Only 2 early investigations from the Uppsala Longitudinal Study of Adult Men associated adipose tissue palmitoleic acid with insulin resistance assessed by the clamp. In these studies, only elderly white men with a mean age of 71 were included, which limits external validity (81, 106).
In summary, palmitoleic acid has been associated with increased insulin sensitivity in most studies and consistently with lower incident diabetes in humans. Animal models have been useful to explore mechanisms by which palmitoleate induces insulin sensitivity. Palmitoleic acid from adipose tissue promotes insulin sensitivity in muscles and suppresses the expression of monocyte chemoattractant protein 1 (MCP-1) and TNF-α in adipose tissue (16). Other studies corroborate the favorable effects of palmitoleic acid on insulin action (71, 107, 108) through translocation of glucose transporter 4 (GLUT-4) to the plasma membrane (109). Cellular studies found that palmitoleate induces β cell proliferation and secretory function (110, 111), regulates the expression and degradation of metabolic enzymes (16), and prevents endoplasmatic reticulum stress and apoptosis mediated by palmitate (65, 72). Thus, endogenously produced or dietary palmitoleate has been related to a reduced onset of diabetes; therefore, it could be a potential strategy for preventing this disease in humans.
Cancer
Palmitoleate concentrations have been found to be associated with some cancer types. In clinical studies, breast and prostate cancer risk and incidence were increased with augmented palmitoleate concentration in blood and tissues (51, 112–117). In addition, gallbladder carcinoma and brain tumors have been associated with increased palmitoleate content in erythrocyte membranes and membrane phospholipids of the tumors, respectively (118, 119). The proportion of palmitoleate in serum and variance of the SCD1 gene was associated with cancer mortality in Swedish men (120). In 3T3-L1 cells, SCD1 promotes cell survival through palmitoleate production (121). In cancer cells, inhibition of SCD1 blocks cell cycle progression, and this is reversed by palmitoleate, which is linked to cancer cell proliferation (122). Thus, mechanistically, SCD1-augmented activity and the consequent production of palmitoleate is apparently connected with cancer. It is plausible that the endogenous production of palmitoleate is the underlying cause of cell proliferation and survival in cancer progression. However, dietary palmitoleate and supplementation should be considered and investigated in order to rule out its possible association with cancer.
Conclusions
Palmitoleate is considered to be a lipokine, because it is released from adipose tissue and exerts its actions on distant organs. In humans, subcutaneous lower body fat releases significantly more palmitoleate than other fat depots linking beneficial effects of palmitoleate with fat distribution. Although its role in obesity development and its contribution to liver or cardiovascular health is not clear, decreased incident diabetes is certainly associated with higher palmitoleate concentrations. In animals, an improved lipid profile and increased insulin sensitivity are explained through increased transcriptional activity, improved insulin signaling, and modulation of enzymes and cytokines. The rationale of inconsistencies observed between animals and humans is unknown. However, free palmitoleate concentrations or its incorporation in phospholipids or TGs for metabolic actions or for storage possibly could explain different metabolic outcomes among species. Further research is needed to clarify the effects of palmitoleate in humans with distinct ethnicities, ages, and pre-existing diseases, and the molecular mechanisms that explain such effects. Clinical studies that reveal such information will help to resolve whether palmitoleate represents a valid strategy to prevent or treat diabetes, along with other metabolic abnormalities integrated in metabolic syndrome.
Acknowledgments
Both authors read and approved the final manuscript.
Footnotes
Abbreviations used: CHS, Cardiovascular Health Study; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; SCD1, stearoyl-CoA desaturase 1.
References
- 1.Westphal SA. Obesity, abdominal obesity, and insulin resistance. Clin Cornerstone 2008;9:23–9, discussion 30–1. [DOI] [PubMed] [Google Scholar]
- 2.Grundy SM. Metabolic syndrome: a multiplex cardiovascular risk factor. J Clin Endocrinol Metab 2007;92:399–404. [DOI] [PubMed] [Google Scholar]
- 3.Kris-Etherton PM, Yu S, Etherton TD, Morgan R, Moriarty K, Shaffer D. Fatty acids and progression of coronary artery disease. Am J Clin Nutr 1997;65:1088–90. [DOI] [PubMed] [Google Scholar]
- 4.Illingworth DR, Schmidt EB. The influence of dietary n-3 fatty acids on plasma lipids and lipoproteins. Ann N Y Acad Sci 1993;676:60–9. [DOI] [PubMed] [Google Scholar]
- 5.Temme EH, Mensink RP, Hornstra G. Comparison of the effects of diets enriched in lauric, palmitic, or oleic acids on serum lipids and lipoproteins in healthy women and men. Am J Clin Nutr 1996;63:897–903. [DOI] [PubMed] [Google Scholar]
- 6.Ellis BA, Poynten A, Lowy AJ, Furler SM, Chisholm DJ, Kraegen EW, Cooney GJ. Long-chain acyl-CoA esters as indicators of lipid metabolism and insulin sensitivity in rat and human muscle. Am J Physiol Endocrinol Metab 2000;279:E554–60. [DOI] [PubMed] [Google Scholar]
- 7.Turinsky J, O’Sullivan DM, Bayly BP. 1,2-Diacylglycerol and ceramide levels in insulin-resistant tissues of the rat in vivo. J Biol Chem 1990;265:16880–5. [PubMed] [Google Scholar]
- 8.Idris I, Gray S, Donnelly R. Protein kinase C activation: isozyme-specific effects on metabolism and cardiovascular complications in diabetes. Diabetologia 2001;44:659–73. [DOI] [PubMed] [Google Scholar]
- 9.Ros E. Dietary cis-monounsaturated fatty acids and metabolic control in type 2 diabetes. Am J Clin Nutr 2003; 78(3, Suppl)617S–25S. [DOI] [PubMed] [Google Scholar]
- 10.Kris-Etherton PM. AHA Science Advisory. Monounsaturated fatty acids and risk of cardiovascular disease. American Heart Association. Nutrition Committee. Circulation 1999;100:1253–8. [DOI] [PubMed] [Google Scholar]
- 11.Garg A. High-monounsaturated-fat diets for patients with diabetes mellitus: a meta-analysis. Am J Clin Nutr 1998; 67(3, Suppl)577S–82S. [DOI] [PubMed] [Google Scholar]
- 12.Gillingham LG, Harris-Janz S, Jones PJ. Dietary monounsaturated fatty acids are protective against metabolic syndrome and cardiovascular disease risk factors. Lipids 2011;46:209–28. [DOI] [PubMed] [Google Scholar]
- 13.Siriwardhana N, Kalupahana NS, Moustaid-Moussa N. Health benefits of n-3 polyunsaturated fatty acids: eicosapentaenoic acid and docosahexaenoic acid. Adv Food Nutr Res 2012;65:211–22. [DOI] [PubMed] [Google Scholar]
- 14.Ruxton CH, Reed SC, Simpson MJ, Millington KJ. The health benefits of omega-3 polyunsaturated fatty acids: a review of the evidence. J Hum Nutr Diet 2004;17:449–59. [DOI] [PubMed] [Google Scholar]
- 15.Goeransson G. The metabolism of fatty acids in the rat. V. Palmitoleic acid. Acta Physiol Scand 1965;63:428–33. [DOI] [PubMed] [Google Scholar]
- 16.Cao H, Gerhold K, Mayers JR, Wiest MM, Watkins SM, Hotamisligil GS. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell 2008;134:933–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo X, Li H, Xu H, Halim V, Zhang W, Wang H, Ong KT, Woo SL, Walzem RL, Mashek DG, et al. Palmitoleate induces hepatic steatosis but suppresses liver inflammatory response in mice. PLoS One 2012;7:e39286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol 2011;11:85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mozaffarian D, de Oliveira Otto MC, Lemaitre RN, Fretts AM, Hotamisligil G, Tsai MY, Siscovick DS, Nettleton JA. trans-Palmitoleic acid, other dairy fat biomarkers, and incident diabetes: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr 2013;97:854–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mozaffarian D. Trans fatty acids—effects on systemic inflammation and endothelial function. Atheroscler Suppl 2006;7:29–32. [DOI] [PubMed] [Google Scholar]
- 21.Yakoob MY, Shi P, Hu FB, Campos H, Rexrode KM, Orav EJ, Willett WC, Mozaffarian D. Circulating biomarkers of dairy fat and risk of incident stroke in U.S. men and women in 2 large prospective cohorts. Am J Clin Nutr 2014;100:1437–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ntambi JM, Miyazaki M. Regulation of stearoyl-CoA desaturases and role in metabolism. Prog Lipid Res 2004;43:91–104. [DOI] [PubMed] [Google Scholar]
- 23.Lee WN, Lim S, Bassilian S, Bergner EA, Edmond J. Fatty acid cycling in human hepatoma cells and the effects of troglitazone. J Biol Chem 1998;273:20929–34. [DOI] [PubMed] [Google Scholar]
- 24.Jaudszus A, Kramer R, Pfeuffer M, Roth A, Jahreis G, Kuhnt K. trans-Palmitoleic acid arises endogenously from dietary vaccenic acid. Am J Clin Nutr 2014;99:431–5. [DOI] [PubMed] [Google Scholar]
- 25.Hagenfeldt L, Wahren J, Pernow B, Cronestrand R, Ekestrom S. Free fatty acid metabolism of leg muscles during exercise in patients with obliterative iliac and femoral artery disease before and after reconstructive surgery. J Clin Invest 1972;51:3061–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Motard-Bélanger A, Charest A, Grenier G, Paquin P, Chouinard Y, Lemieux S, Couture P, Lamarche B. Study of the effect of trans fatty acids from ruminants on blood lipids and other risk factors for cardiovascular disease. Am J Clin Nutr 2008;87:593–9. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Jacome-Sosa MM, Ruth MR, Lu Y, Shen J, Reaney MJ, Scott SL, Dugan ME, Anderson HD, Field CJ, et al. The intestinal bioavailability of vaccenic acid and activation of peroxisome proliferator-activated receptor-alpha and -gamma in a rodent model of dyslipidemia and the metabolic syndrome. Mol Nutr Food Res 2012;56:1234–46. [DOI] [PubMed] [Google Scholar]
- 28.Bassett CM, Edel AL, Patenaude AF, McCullough RS, Blackwood DP, Chouinard PY, Paquin P, Lamarche B, Pierce GN. Dietary vaccenic acid has antiatherogenic effects in LDLr−/− mice. J Nutr 2010;140:18–24. [DOI] [PubMed] [Google Scholar]
- 29.Chardigny JM, Destaillats F, Malpuech-Brugere C, Moulin J, Bauman DE, Lock AL, Barbano DM, Mensink RP, Bezelgues JB, Chaumont P, et al. Do trans fatty acids from industrially produced sources and from natural sources have the same effect on cardiovascular disease risk factors in healthy subjects? Results of the trans Fatty Acids Collaboration (TRANSFACT) study. Am J Clin Nutr 2008;87:558–66. [DOI] [PubMed] [Google Scholar]
- 30.Fatima T, Snyder CL, Schroeder WR, Cram D, Datla R, Wishart D, Weselake RJ, Krishna P. Fatty acid composition of developing sea buckthorn (Hippophae rhamnoides L.) berry and the transcriptome of the mature seed. PLoS One 2012;7:e34099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maguire LS, O’Sullivan SM, Galvin K, O’Connor TP, O’Brien NM. Fatty acid profile, tocopherol, squalene and phytosterol content of walnuts, almonds, peanuts, hazelnuts and the macadamia nut. Int J Food Sci Nutr 2004;55:171–8. [DOI] [PubMed] [Google Scholar]
- 32.Micha R, King IB, Lemaitre RN, Rimm EB, Sacks F, Song X, Siscovick DS, Mozaffarian D. Food sources of individual plasma phospholipid trans fatty acid isomers: the Cardiovascular Health Study. Am J Clin Nutr 2010;91:883–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mozaffarian D, Cao H, King IB, Lemaitre RN, Song X, Siscovick DS, Hotamisligil GS. Trans-palmitoleic acid, metabolic risk factors, and new-onset diabetes in U.S. adults: a cohort study. Ann Intern Med 2010;153:790–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mozaffarian D, Cao H, King IB, Lemaitre RN, Song X, Siscovick DS, Hotamisligil GS. Circulating palmitoleic acid and risk of metabolic abnormalities and new-onset diabetes. Am J Clin Nutr 2010;92:1350–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Volk BM, Kunces LJ, Freidenreich DJ, Kupchak BR, Saenz C, Artistizabal JC, Fernandez ML, Bruno RS, Maresh CM, Kraemer WJ, et al. Effects of step-wise increases in dietary carbohydrate on circulating saturated datty acids and palmitoleic acid in adults with metabolic syndrome. PLoS One 2014;9:e113605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mangravite LM, Dawson K, Davis RR, Gregg JP, Krauss RM. Fatty acid desaturase regulation in adipose tissue by dietary composition is independent of weight loss and is correlated with the plasma triacylglycerol response. Am J Clin Nutr 2007;86:759–67. [DOI] [PubMed] [Google Scholar]
- 37.Forsythe CE, Phinney SD, Feinman RD, Volk BM, Freidenreich D, Quann E, Ballard K, Puglisi MJ, Maresh CM, Kraemer WJ, et al. Limited effect of dietary saturated fat on plasma saturated fat in the context of a low carbohydrate diet. Lipids 2010;45:947–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chong MF, Hodson L, Bickerton AS, Roberts R, Neville M, Karpe F, Frayn KN, Fielding BA. Parallel activation of de novo lipogenesis and stearoyl-CoA desaturase activity after 3 d of high-carbohydrate feeding. Am J Clin Nutr 2008;87:817–23. [DOI] [PubMed] [Google Scholar]
- 39.Aarsland A, Wolfe RR. Hepatic secretion of VLDL fatty acids during stimulated lipogenesis in men. J Lipid Res 1998;39:1280–6. [PubMed] [Google Scholar]
- 40.Djoussé L, Weir NL, Hanson NQ, Tsai MY, Gaziano JM. Plasma phospholipid concentration of cis-palmitoleic acid and risk of heart failure. Circ Heart Fail 2012;5:703–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nall JL, Wu G, Kim KH, Choi CW, Smith SB. Dietary supplementation of L-arginine and conjugated linoleic acid reduces retroperitoneal fat mass and increases lean body mass in rats. J Nutr 2009;139:1279–85. [DOI] [PubMed] [Google Scholar]
- 42.Hiraoka-Yamamoto J, Ikeda K, Negishi H, Mori M, Hirose A, Sawada S, Onobayashi Y, Kitamori K, Kitano S, Tashiro M, et al. Serum lipid effects of a monounsaturated (palmitoleic) fatty acid-rich diet based on macadamia nuts in healthy, young Japanese women. Clin Exp Pharmacol Physiol 2004;31 Suppl 2:S37–8. [DOI] [PubMed] [Google Scholar]
- 43.Garg ML, Blake RJ, Wills RB. Macadamia nut consumption lowers plasma total and LDL cholesterol levels in hypercholesterolemic men. J Nutr 2003;133:1060–3. [DOI] [PubMed] [Google Scholar]
- 44.Zulyniak MA, Perreault M, Gerling C, Spriet LL, Mutch DM. Fish oil supplementation alters circulating eicosanoid concentrations in young healthy men. Metabolism 2013;62:1107–13. [DOI] [PubMed] [Google Scholar]
- 45.Stenson WF, Seetharam B, Talkad V, Pickett W, Dudeja P, Brasitus TA. Effects of dietary fish oil supplementation on membrane fluidity and enzyme activity in rat small intestine. Biochem J 1989;263:41–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abdolahi A, Georas SN, Brenna JT, Cai X, Thevenet-Morrison K, Phipps RP, Lawrence P, Mousa SA, Block RC. The effects of aspirin and fish oil consumption on lysophosphatidylcholines and lysophosphatidic acids and their correlates with platelet aggregation in adults with diabetes mellitus. Prostaglandins Leukot Essent Fatty Acids 2014;90:61–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hirsch J, Farquhar JW, Ahrens EH Jr, Peterson ML, Stoffel W. Studies of adipose tissue in man. A microtechnic for sampling and analysis. Am J Clin Nutr 1960;8:499–511. [DOI] [PubMed] [Google Scholar]
- 48.McLaren DS, Ajans ZA, Awdeh Z. Composition of human adipose tissue, with special reference to site and age differences. Am J Clin Nutr 1965;17:171–6. [DOI] [PubMed] [Google Scholar]
- 49.Calder PC, Harvey DJ, Pond CM, Newsholme EA. Site-specific differences in the fatty acid composition of human adipose tissue. Lipids 1992;27:716–20. [DOI] [PubMed] [Google Scholar]
- 50.Malcom GT, Bhattacharyya AK, Velez-Duran M, Guzman MA, Oalmann MC, Strong JP. Fatty acid composition of adipose tissue in humans: differences between subcutaneous sites. Am J Clin Nutr 1989;50:288–91. [DOI] [PubMed] [Google Scholar]
- 51.Mamalakis G, Kafatos A, Manios Y, Kalogeropoulos N, Andrikopoulos N. Abdominal vs buttock adipose fat: relationships with children’s serum lipid levels. Eur J Clin Nutr 2002;56:1081–6. [DOI] [PubMed] [Google Scholar]
- 52.Phinney SD, Stern JS, Burke KE, Tang AB, Miller G, Holman RT. Human subcutaneous adipose tissue shows site-specific differences in fatty acid composition. Am J Clin Nutr 1994;60:725–9. [DOI] [PubMed] [Google Scholar]
- 53.Pittet PG, Halliday D, Bateman PE. Site differences in the fatty acid composition of subcutaneous adipose tissue of obese women. Br J Nutr 1979;42:57–61. [DOI] [PubMed] [Google Scholar]
- 54.Field CJ, Angel A, Clandinin MT. Relationship of diet to the fatty acid composition of human adipose tissue structural and stored lipids. Am J Clin Nutr 1985;42:1206–20. [DOI] [PubMed] [Google Scholar]
- 55.Halliwell KJ, Fielding BA, Samra JS, Humphreys SM, Frayn KN. Release of individual fatty acids from human adipose tissue in vivo after an overnight fast. J Lipid Res 1996;37:1842–8. [PubMed] [Google Scholar]
- 56.Hodson L, Karpe F. Is there something special about palmitoleate? Curr Opin Clin Nutr Metab Care 2013;16:225–31. [DOI] [PubMed] [Google Scholar]
- 57.Pinnick KE, Neville MJ, Fielding BA, Frayn KN, Karpe F, Hodson L. Gluteofemoral adipose tissue plays a major role in production of the lipokine palmitoleate in humans. Diabetes 2012;61:1399–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yli-Jama P, Haugen TS, Rebnord HM, Ringstad J, Pedersen JI. Selective mobilisation of fatty acids from human adipose tissue. Eur J Intern Med 2001;12:107–15. [DOI] [PubMed] [Google Scholar]
- 59.Duckett SK, Volpi-Lagreca G, Alende M, Long NM. Palmitoleic acid reduces intramuscular lipid and restores insulin sensitivity in obese sheep. Diabetes Metab Syndr Obes 2014;7:553–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Andersson A, Nalsen C, Tengblad S, Vessby B. Fatty acid composition of skeletal muscle reflects dietary fat composition in humans. Am J Clin Nutr 2002;76:1222–9. [DOI] [PubMed] [Google Scholar]
- 61.Tavares E, Gomez-Tubio A, Murillo ML, Carreras O. Brown and white adipose tissue lipid composition in three successive progenies of rats: effects of ethanol exposure. Arch Tierernahr 2001;55:53–67. [DOI] [PubMed] [Google Scholar]
- 62.Douillet C, Bost M, Accominotti M, Borson-Chazot F, Ciavatti M. Effect of selenium and vitamin E supplementation on lipid abnormalities in plasma, aorta, and adipose tissue of Zucker rats. Biol Trace Elem Res 1998;65:221–36. [DOI] [PubMed] [Google Scholar]
- 63.Ivanovic N, Minic R, Djuricic I, Dimitrijevic L, Sobajic S, Zivkovic I, Djordjevic B. Brain and liver fatty acid composition changes upon consumption of Lactobacillus rhamnosus LA68. Int J Food Sci Nutr 2015;66:93–7. [DOI] [PubMed] [Google Scholar]
- 64.Celik S, Akarcay H, Yilmaz O, Ozkaya A. Effects of intraperitoneally administered ubiquinone on the level of total lipid and fatty acids in rat liver. Cell Biochem Funct 2006;24:561–4. [DOI] [PubMed] [Google Scholar]
- 65.Erbay E, Babaev VR, Mayers JR, Makowski L, Charles KN, Snitow ME, Fazio S, Wiest MM, Watkins SM, Linton MF, et al. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat Med 2009;15:1383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Coleman SL, Park YK, Lee JY. Unsaturated fatty acids repress the expression of adipocyte fatty acid binding protein via the modulation of histone deacetylation in RAW 264.7 macrophages. Eur J Nutr 2011;50:323–30. [DOI] [PubMed] [Google Scholar]
- 67.Gong J, Campos H, McGarvey S, Wu Z, Goldberg R, Baylin A. Adipose tissue palmitoleic acid and obesity in humans: does it behave as a lipokine? Am J Clin Nutr 2011;93:186–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Foryst-Ludwig A, Kreissl MC, Benz V, Brix S, Smeir E, Ban Z, Januszewicz E, Salatzki J, Grune J, Schwanstecher AK, et al. adipose tissue lipolysis promotes exercise-induced cardiac hypertrophy involving the lipokine C16:1n7-palmitoleate. J Biol Chem 2015;290:23603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sarabi M, Vessby B, Millgard J, Lind L. Endothelium-dependent vasodilation is related to the fatty acid composition of serum lipids in healthy subjects. Atherosclerosis 2001;156:349–55. [DOI] [PubMed] [Google Scholar]
- 70.Kenny LC, Baker PN, Kendall DA, Randall MD, Dunn WR. The role of gap junctions in mediating endothelium-dependent responses to bradykinin in myometrial small arteries isolated from pregnant women. Br J Pharmacol 2002;136:1085–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maedler K, Oberholzer J, Bucher P, Spinas GA, Donath MY. Monounsaturated fatty acids prevent the deleterious effects of palmitate and high glucose on human pancreatic beta-cell turnover and function. Diabetes 2003;52:726–33. [DOI] [PubMed] [Google Scholar]
- 72.Akazawa Y, Cazanave S, Mott JL, Elmi N, Bronk SF, Kohno S, Charlton MR, Gores GJ. Palmitoleate attenuates palmitate-induced Bim and PUMA up-regulation and hepatocyte lipoapoptosis. J Hepatol 2010;52:586–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Burns TA, Kadegowda AK, Duckett SK, Pratt SL, Jenkins TC. Palmitoleic (16:1 cis-9) and cis-vaccenic (18:1 cis-11) acid alter lipogenesis in bovine adipocyte cultures. Lipids 2012;47:1143–53. [DOI] [PubMed] [Google Scholar]
- 74.Chan KL, Pillon NJ, Sivaloganathan DM, Costford SR, Liu Z, Theret M, Chazaud B, Klip A. Palmitoleate reverses high fat-induced proinflammatory macrophage polarization via AMP-activated protein kinase (AMPK). J Biol Chem 2015;290:16979–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Koeberle A, Shindou H, Harayama T, Shimizu T. Palmitoleate is a mitogen, formed upon stimulation with growth factors, and converted to palmitoleoyl-phosphatidylinositol. J Biol Chem 2012;287:27244–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Warensjö E, Ohrvall M, Vessby B. Fatty acid composition and estimated desaturase activities are associated with obesity and lifestyle variables in men and women. Nutr Metab Cardiovasc Dis 2006;16:128–36. [DOI] [PubMed] [Google Scholar]
- 77.Okada T, Furuhashi N, Kuromori Y, Miyashita M, Iwata F, Harada K. Plasma palmitoleic acid content and obesity in children. Am J Clin Nutr 2005;82:747–50. [DOI] [PubMed] [Google Scholar]
- 78.Paillard F, Catheline D, Duff FL, Bouriel M, Deugnier Y, Pouchard M, Daubert JC, Legrand P. Plasma palmitoleic acid, a product of stearoyl-coA desaturase activity, is an independent marker of triglyceridemia and abdominal adiposity. Nutr Metab Cardiovasc Dis 2008;18:436–40. [DOI] [PubMed] [Google Scholar]
- 79.Bernstein AM, Roizen MF, Martinez L. Purified palmitoleic acid for the reduction of high-sensitivity C-reactive protein and serum lipids: a double-blinded, randomized, placebo controlled study. J Clin Lipidol 2014;8:612–7. [DOI] [PubMed] [Google Scholar]
- 80.Stefan N, Kantartzis K, Celebi N, Staiger H, Machann J, Schick F, Cegan A, Elcnerova M, Schleicher E, Fritsche A, et al. Circulating palmitoleate strongly and independently predicts insulin sensitivity in humans. Diabetes Care 2010;33:405–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vessby B, Tengblad S, Lithell H. Insulin sensitivity is related to the fatty acid composition of serum lipids and skeletal muscle phospholipids in 70-year-old men. Diabetologia 1994;37:1044–50. [DOI] [PubMed] [Google Scholar]
- 82.Fabbrini E, Magkos F, Su X, Abumrad NA, Nejedly N, Coughlin CC, Okunade AL, Patterson BW, Klein S. Insulin sensitivity is not associated with palmitoleate availability in obese humans. J Lipid Res 2011;52:808–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kunešová M, Hlavaty P, Tvrzicka E, Stankova B, Kalouskova P, Viguerie N, Larsen TM, van Baak MA, Jebb SA, Martinez JA, et al. Fatty acid composition of adipose tissue triglycerides after weight loss and weight maintenance: the DIOGENES study. Physiol Res 2012;61:597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Garaulet M, Hernandez-Morante JJ, Tebar FJ, Zamora S. Relation between degree of obesity and site-specific adipose tissue fatty acid composition in a Mediterranean population. Nutrition 2011;27:170–6. [DOI] [PubMed] [Google Scholar]
- 85.Vázquez-Vela ME, Torres N, Tovar AR. White adipose tissue as endocrine organ and its role in obesity. Arch Med Res 2008;39:715–28. [DOI] [PubMed] [Google Scholar]
- 86.Markey O, McClean CM, Medlow P, Davison GW, Trinick TR, Duly E, Shafat A. Effect of cinnamon on gastric emptying, arterial stiffness, postprandial lipemia, glycemia, and appetite responses to high-fat breakfast. Cardiovasc Diabetol 2011;10:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang ZH, Takeo J, Katayama M. Oral administration of omega-7 palmitoleic acid induces satiety and the release of appetite-related hormones in male rats. Appetite 2013;65:1–7. [DOI] [PubMed] [Google Scholar]
- 88.Cambien F, Warnet JM, Vernier V, Ducimetiere P, Jacqueson A, Flament C, Orssaud G, Richard JL, Claude JR. An epidemiologic appraisal of the associations between the fatty acids esterifying serum cholesterol and some cardiovascular risk factors in middle-aged men. Am J Epidemiol 1988;127:75–86. [DOI] [PubMed] [Google Scholar]
- 89.Zong G, Ye X, Sun L, Li H, Yu Z, Hu FB, Sun Q, Lin X. Associations of erythrocyte palmitoleic acid with adipokines, inflammatory markers, and the metabolic syndrome in middle-aged and older Chinese. Am J Clin Nutr 2012;96:970–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Djoussé L, Matthan NR, Lichtenstein AH, Gaziano JM. Red blood cell membrane concentration of cis-palmitoleic and cis-vaccenic acids and risk of coronary heart disease. Am J Cardiol 2012;110:539–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Arab L. Biomarkers of fat and fatty acid intake. J Nutr 2003;133 Suppl 3:925S–32S. [DOI] [PubMed] [Google Scholar]
- 92.Curb JD, Wergowske G, Dobbs JC, Abbott RD, Huang B. Serum lipid effects of a high-monounsaturated fat diet based on macadamia nuts. Arch Intern Med 2000;160:1154–8. [DOI] [PubMed] [Google Scholar]
- 93.Nestel P, Clifton P, Noakes M. Effects of increasing dietary palmitoleic acid compared with palmitic and oleic acids on plasma lipids of hypercholesterolemic men. J Lipid Res 1994;35:656–62. [PubMed] [Google Scholar]
- 94.Shiba S, Tsunoda N, Wakutsu M, Muraki E, Sonoda M, Tam PS, Fujiwara Y, Ikemoto S, Kasono K. Regulation of lipid metabolism by palmitoleate and eicosapentaenoic acid (EPA) in mice fed a high-fat diet. Biosci Biotechnol Biochem 2011;75:2401–3. [DOI] [PubMed] [Google Scholar]
- 95.Matthan NR, Dillard A, Lecker JL, Ip B, Lichtenstein AH. Effects of dietary palmitoleic acid on plasma lipoprotein profile and aortic cholesterol accumulation are similar to those of other unsaturated fatty acids in the F1B golden Syrian hamster. J Nutr 2009;139:215–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang ZH, Miyahara H, Hatanaka A. Chronic administration of palmitoleic acid reduces insulin resistance and hepatic lipid accumulation in KK-Ay Mice with genetic type 2 diabetes. Lipids Health Dis 2011;10:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kuda O, Stankova B, Tvrzicka E, Hensler M, Jelenik T, Rossmeisl M, Flachs P, Kopecky J. Prominent role of liver in elevated plasma palmitoleate levels in response to rosiglitazone in mice fed high-fat diet. J Physiol Pharmacol 2009;60:135–40. [PubMed] [Google Scholar]
- 98.Fukuchi S, Hamaguchi K, Seike M, Himeno K, Sakata T, Yoshimatsu H. Role of fatty acid composition in the development of metabolic disorders in sucrose-induced obese rats. Exp Biol Med (Maywood) 2004;229:486–93. [DOI] [PubMed] [Google Scholar]
- 99.Puri P, Wiest MM, Cheung O, Mirshahi F, Sargeant C, Min HK, Contos MJ, Sterling RK, Fuchs M, Zhou H, et al. The plasma lipidomic signature of nonalcoholic steatohepatitis. Hepatology 2009;50:1827–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tomita K, Teratani T, Yokoyama H, Suzuki T, Irie R, Ebinuma H, Saito H, Hokari R, Miura S, Hibi T. Plasma free myristic acid proportion is a predictor of nonalcoholic steatohepatitis. Dig Dis Sci 2011;56:3045–52. [DOI] [PubMed] [Google Scholar]
- 101.Lindgärde F, Vessby B, Ahren B. Serum cholesteryl fatty acid composition and plasma glucose concentrations in Amerindian women. Am J Clin Nutr 2006;84:1009–13. [DOI] [PubMed] [Google Scholar]
- 102.Salomaa V, Ahola I, Tuomilehto J, Aro A, Pietinen P, Korhonen HJ, Penttila I. Fatty acid composition of serum cholesterol esters in different degrees of glucose intolerance: a population-based study. Metabolism 1990;39:1285–91. [DOI] [PubMed] [Google Scholar]
- 103.Gertow K, Rosell M, Sjogren P, Eriksson P, Vessby B, de Faire U, Hamsten A, Hellenius ML, Fisher RM. Fatty acid handling protein expression in adipose tissue, fatty acid composition of adipose tissue and serum, and markers of insulin resistance. Eur J Clin Nutr 2006;60:1406–13. [DOI] [PubMed] [Google Scholar]
- 104.Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature 2006;444:881–7. [DOI] [PubMed] [Google Scholar]
- 105.Risérus U, Tan GD, Fielding BA, Neville MJ, Currie J, Savage DB, Chatterjee VK, Frayn KN, O’Rahilly S, Karpe F. Rosiglitazone increases indexes of stearoyl-CoA desaturase activity in humans: link to insulin sensitization and the role of dominant-negative mutation in peroxisome proliferator-activated receptor-gamma. Diabetes 2005;54:1379–84. [DOI] [PubMed] [Google Scholar]
- 106.Iggman D, Arnlov J, Vessby B, Cederholm T, Sjogren P, Riserus U. Adipose tissue fatty acids and insulin sensitivity in elderly men. Diabetologia 2010;53:850–7. [DOI] [PubMed] [Google Scholar]
- 107.Sauma L, Stenkula KG, Kjolhede P, Stralfors P, Soderstrom M, Nystrom FH. PPAR-gamma response element activity in intact primary human adipocytes: effects of fatty acids. Nutrition 2006;22:60–8. [DOI] [PubMed] [Google Scholar]
- 108.Tsuchiya Y, Hatakeyama H, Emoto N, Wagatsuma F, Matsushita S, Kanzaki M. Palmitate-induced down-regulation of sortilin and impaired GLUT4 trafficking in C2C12 myotubes. J Biol Chem 2010;285:34371–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dimopoulos N, Watson M, Sakamoto K, Hundal HS. Differential effects of palmitate and palmitoleate on insulin action and glucose utilization in rat L6 skeletal muscle cells. Biochem J 2006;399:473–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Welters HJ, Diakogiannaki E, Mordue JM, Tadayyon M, Smith SA, Morgan NG. Differential protective effects of palmitoleic acid and cAMP on caspase activation and cell viability in pancreatic beta-cells exposed to palmitate. Apoptosis 2006;11:1231–8. [DOI] [PubMed] [Google Scholar]
- 111.Diakogiannaki E, Dhayal S, Childs CE, Calder PC, Welters HJ, Morgan NG. Mechanisms involved in the cytotoxic and cytoprotective actions of saturated versus monounsaturated long-chain fatty acids in pancreatic beta-cells. J Endocrinol 2007;194:283–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pouchieu C, Chajes V, Laporte F, Kesse-Guyot E, Galan P, Hercberg S, Latino-Martel P, Touvier M. Prospective associations between plasma saturated, monounsaturated and polyunsaturated fatty acids and overall and breast cancer risk—modulation by antioxidants: a nested case-control study. PLoS One 2014;9:e90442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chajès V, Thiebaut AC, Rotival M, Gauthier E, Maillard V, Boutron-Ruault MC, Joulin V, Lenoir GM, Clavel-Chapelon F. Association between serum trans-monounsaturated fatty acids and breast cancer risk in the E3N-EPIC Study. Am J Epidemiol 2008;167:1312–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shannon J, King IB, Moshofsky R, Lampe JW, Gao DL, Ray RM, Thomas DB. Erythrocyte fatty acids and breast cancer risk: a case-control study in Shanghai, China. Am J Clin Nutr 2007;85:1090–7. [DOI] [PubMed] [Google Scholar]
- 115.Mamalakis G, Kafatos A, Kalogeropoulos N, Andrikopoulos N, Daskalopulos G, Kranidis A. Prostate cancer vs hyperplasia: relationships with prostatic and adipose tissue fatty acid composition. Prostaglandins Leukot Essent Fatty Acids 2002;66:467–77. [DOI] [PubMed] [Google Scholar]
- 116.Simonsen NR, Fernandez-Crehuet Navajas J, Martin-Moreno JM, Strain JJ, Huttunen JK, Martin BC, Thamm M, Kardinaal AF, van’t Veer P, Kok FJ, et al. Tissue stores of individual monounsaturated fatty acids and breast cancer: the EURAMIC study. European Community Multicenter Study on Antioxidants, Myocardial Infarction, and Breast Cancer. Am J Clin Nutr 1998;68:134–41. [DOI] [PubMed] [Google Scholar]
- 117.Chavarro JE, Kenfield SA, Stampfer MJ, Loda M, Campos H, Sesso HD, Ma J. Blood levels of saturated and monounsaturated fatty acids as markers of de novo lipogenesis and risk of prostate cancer. Am J Epidemiol 2013;178:1246–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pandey M, Khatri AK, Dubey SS, Gautam A, Shukla VK. Erythrocyte membrane fatty acid profile in patients with primary carcinoma of the gallbladder. J Surg Oncol 1995;59:31–4. [DOI] [PubMed] [Google Scholar]
- 119.Hattori T, Andoh T, Sakai N, Yamada H, Kameyama Y, Ohki K, Nozawa Y. Membrane phospholipid composition and membrane fluidity of human brain tumour: a spin label study. Neurol Res 1987;9:38–43. [DOI] [PubMed] [Google Scholar]
- 120.Byberg L, Kilander L, Warensjo Lemming E, Michaelsson K, Vessby B. Cancer death is related to high palmitoleic acid in serum and to polymorphisms in the SCD-1 gene in healthy Swedish men. Am J Clin Nutr 2014;99:551–8. [DOI] [PubMed] [Google Scholar]
- 121.Yee JK, Wahjudi PN, Vega J, Lim S, Martin A, Patterson ME, Cohen JN, Mao CS, Lee WN. Stearoyl-CoA desaturase enzyme 1 inhibition reduces glucose utilization for de novo fatty acid synthesis and cell proliferation in 3T3–L1 adipocytes. Metabolomics 2013;9:809–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hess D, Chisholm JW, Igal RA. Inhibition of stearoylCoA desaturase activity blocks cell cycle progression and induces programmed cell death in lung cancer cells. PLoS One 2010;5:e11394. [DOI] [PMC free article] [PubMed] [Google Scholar]