Abstract
Osteoporosis is induced by the reduction in bone mass through decreased osteoblastic osteogenesis and increased osteoclastic bone resorption, and it is associated with obesity and diabetes. Osteoblasts and adipocytes are derived from bone marrow mesenchymal stem cells. The prevention of osteoporosis is an important public health concern in aging populations. β-caryophyllene, a component of various essential oils, is a selective agonist of the cannabinoid receptor type 2 and exerts cannabimimetic anti-inflammatory effects in animals. The present study aimed to identify the effect of β-caryophyllene on adipogenesis, osteoblastic mineralization and osteoclastogenesis in mouse bone marrow cell cultures in vitro. Bone marrow cells obtained from mouse femoral tissues were cultured in the presence of β-caryophyllene (0.1–100 µM) in vitro. The results revealed that β-caryophyllene stimulated osteoblastic mineralization, and suppressed adipogenesis and osteoclastogenesis. Thus, β-caryophyllene may be used as a therapeutic agent for the prevention and treatment of osteoporosis.
Keywords: β-caryophyllene, adipogenesis, osteoblastogenesis, osteoclastogenesis, bone marrow cells
Introduction
Bone homeostasis is maintained through osteocytes, osteoclasts and osteoblasts that are present in bone tissues (1,2). Osteoblasts, which develop from bone marrow mesenchymal stem cells, promote bone formation and mineralization. By contrast, osteoclasts are derived from hematopoietic progenitor cells and stimulate bone resorption (1,2). Bone marrow mesenchymal stem cells (MSCs) are multipotent stromal cells that can differentiate into a number of different cell types, including adipocytes, myoblasts, osteoblasts and chondrocytes (3,4). MSC differentiation is triggered by crosstalk between complex signaling pathways involving numerous components, such as Wnt, delta/jagged, bone morphogenic, Wnt and hedgehog proteins, as well as insulin, insulin-like growth factors, fibroblastic growth factors, and transcriptional regulators of adipocyte and osteoblast differentiation, including peroxisome proliferators-activated receptor-γ (PPARγ) and runt-related transcription factor 2 (5–7). Bone marrow MSC differentiation is crucial for the homeostasis of bone remodeling.
Osteoporosis is associated with a deterioration of bone mass through the suppression of osteoblastic osteogenesis and promotion of osteoclastic bone resorption, and may result in bone fractures (8). Osteoporosis is widely recognized as a major public health problem worldwide and the incidence is increasing in countries with ageing populations (8). Fractures of the proximal femur represent the most serious complication of this disease (8). Bone mass decrease in females is primarily due to reduced secretion of estrogen following the beginning of menopause (8); thus, osteoporosis is an important cause of morbidity and mortality. Furthermore, there is growing evidence that osteoporosis is associated with obesity and diabetes, which are increasingly becoming a major public health concern (9,10). Notably, osteoporosis and obesity are implicated with a number of features (3,4,11,12); osteoblasts and adipocytes develop from bone marrow MSCs and there is a reverse association between the differentiation of MSCs into osteoblasts and adipocytes. Since MSCs differentiate osteoblasts, the differentiation of osteoblasts into adipocytes is reduced (3,4). In addition, a previous study identified that obesity, diabetes and osteoporosis were associated with bone marrow adiposity, which increases production of the inflammatory cytokine tumor necrosis factor-α (TNF-α) (13). TNF-α has been shown to suppress osteoblastogenesis and bone mineralization (14,15). These previous findings suggest that agents inhibiting adipogenesis and stimulating osteoblastogenesis will be useful in the prevention and treatment of osteoporosis.
Numerous constituents of herbs are known to possess anti-inflammatory and analgesic effects (16–19). The sesquiterpene β-caryophyllene is present in various essential oils, particularly in clove oil derived from the stems and flowers of Syzygium aromaticum, rosemary oil from Rosmarinus officinalis, hemp oil from Cannabis sativa, and in cinnamon and hop oils (16–19). β-caryophyllene is found in numerous edible plants that are ingested daily, and it is approved as a food additive by the Food and Drug Administration (FDA). It is a selective agonist of cannabinoid receptor type 2 (CB2) and exerts anti-inflammatory effects in animals (17,19). In addition, β-caryophyllene reduces acute and chronic pain associated with inflammation (19–21). The anti-inflammatory effects of β-caryophyllene have been implicated with reduced TNF-α and interleukin (IL)-1β production, which is associated with opioid receptors (22).
Plant-derived molecules that inhibit adipogenesis and stimulate osteoblastic bone mineralization are poorly understood. The present study aimed to determine whether β-caryophyllene regulates the differentiation of bone marrow cells that are associated with adipogenesis and osteoblastogenesis. β-caryophyllene was demonstrated to enhance osteoblastogenesis, and suppress adipogenesis and osteoclastogenesis in mouse bone marrow cells in vitro. To the best of our knowledge, this is the first time that results concerning the role of β-caryophyllene in the differentiation of bone marrow MSCs have been reported.
Materials and methods
Reagents
Dulbecco's modified Eagle's medium (DMEM) and antibiotics (penicillin and streptomycin) were purchased from Thermo Fisher Scientific, Inc. (Waltham, MA, USA). Fetal bovine serum (FBS) was obtained from HyClone (GE Healthcare Life Sciences, Logan, UT, USA). β-caryophyllene was purchased from Cayman Chemical Company (Ann Arbor, MI, USA). TNF-α, tartrate-resistant acid phosphatase (TRAP), insulin, dexamethasone, 3-isobutyl-1-methylxanthine (IBMX), Alizarin Red S, Oil Red O and other reagents were purchased from Sigma-Aldrich (Merck Millipore, Darmstadt, Germany). Insulin was dissolved in diluted acetic acid solution and other reagents were dissolved in 100% ethanol.
Experimental animals and bone marrow cell isolation
Female C57BL6 mice (n=8; age, 2 months; weight, 18–20 g), purchased from Charles River Laboratories (Wilmington, MA, USA), were housed in a pathogen-free facility, with a 12 h light/dark cycle, temperature/atomosphere and ad libitum access to feed and water. All protocols used in the current study were approved by the Institutional Animal Care and Use Committee at Emory University School of Medicine (Atlanta, GA, USA). Tissue from the femur and tibia was removed immediately following sacrifice with exposure to CO2 in chamber box, and bone marrow cells were isolated from these tissues with needle flush under sterile conditions in safety cabinet (23,24).
In vitro adipogenesis assay
This experiment was based on the methods described in our previous studies (23,24). Bone marrow cells (1×106 cells/well; 2 ml medium added per well using 12-well plates) were cultured in a water-saturated atmosphere containing 5% CO2 and 95% air at 37°C for 3 days in culture medium, consisting of DMEM supplemented with 10% FBS and 1% penicillin-streptomycin (10,000 units/l). The cells were cultured in the presence or absence of differentiation medium (DM) (23,24), which consisted of dexamethasone (1 µM/ml) and IBMX (0.5 mM/ml). Cells were treated with culture medium only, DM plus ethanol (final concentration, 0.1%) or DM plus β-caryophyllene (0.1–100 µM). Subsequently, the medium was replaced with fresh culture medium containing insulin (10 µg/ml) without dexamethasone and IBMX, and cells were cultured for a further 4 days in the presence or absence of β-caryophyllene (0.1–100 µM). In other experimental groups, the cells were cultured in culture medium only, DM plus vehicle (0.1% ethanol as a final concentration) or DM plus β-caryophyllene (0.1–100 µM) for 3 days, then the medium was replaced with fresh culture medium containing insulin (10 µg/ml) and cultured for a further 4 days. The medium was then removed, and adipocytes were stained with Oil Red O. Cell numbers were counted under a light microscope (Olympus MTV-3; Olympus, Tokyo, Japan) using a Hemocytometer plate (23). Quantification was performed by extracting the dye with 0.2 ml of isopropanol for 1 min and measuring the absorbance at 490 nm with a Spectracount microplate photometer. Results are presented as the mean ± standard deviation of 8 replicate samples per data set using different dishes and cell preparations.
In vitro mineralization assay
Bone marrow cells (1×106 cells/ml/well) were cultured in 12-well plates in the presence or absence of DMEM-mineralization medium [DMEM-MM; culture medium plus ascorbic acid (100 µg/ml) and β-glycerophosphate (4 mM)] along with the vehicle or β-caryophyllene (0.1–100 µM) (23,24), for 7 or 18 days at 37°C and 5% CO2. The medium was changed every 3 days. After 18 days of culture, the cells were washed with phosphate-buffered saline (PBS) and stained with Alizarin Red S. For quantification, the dye was eluted with 10% cetylpyridinium chloride solution and the absorbance of the eluted solution at 570 nm was measured using a plate reader. Results were presented as the mean ± standard deviation of 8 replicate samples per data set using different dishes and cell preparation.
In vitro osteoclastogenesis assay
Bone marrow cells (2×105 cells/ml/well) were plated in 24-well plates with culture media (1 ml/well) in an atmosphere containing 5% CO2 at 37°C. Cells were cultured in medium only (containing 0.1% ethanol as a final concentration), β-caryophyllene (0.1–100 µM) only, TNF-α (5 ng/ml medium) or TNF-α (5 ng/ml medium) plus β-caryophyllene (0.1–100 µM) for 3 days. Next, 0.5 ml of the old medium was replaced with fresh culture medium with or without TNF-α (5 ng/ml), and in the presence or absence of β-caryophyllene (0.1–100 µM). The cultures were then maintained for a further 4 days (25,26). After a total of 7 days of culture, adherent cells were stained for tartrate-resistant acid phosphatase (TRAP; Sigma-Aldrich; Merck Millipore), a marker of osteoclasts (26). Briefly, cells were washed with PBS, fixed with 10% neutralized formalin-phosphate (pH 7.2) for 10 min, dried and then stained with acetate buffer (pH 5.0) containing Naphthol AS-MX phosphate (Sigma-Aldrich; Merck Millipore) in the presence of sodium tartrate (10 mM) for 90 min at room temperature. TRAP-positive multinucleated cells (MNCs with ≥3 nuclei) were considered to be osteoclast-like cells, and the cells were counted using light microscopy. MNC scores are expressed as the mean ± standard deviation of six cultures with 2 replicate wells per data set using different dishes and cell preparation.
Statistical analysis
Statistical analysis was performed using GraphPad InStat software (version 3; GraphPad Software, Inc., La Jolla, CA, USA). Multiple comparisons were performed by one-way analysis of variance, followed by a post-hoc Tukey's range test for parametric data as indicated. P<0.05 was considered to indicate a a statistically significant difference.
Results
β-caryophyllene suppresses bone marrow adipogenesis in vitro
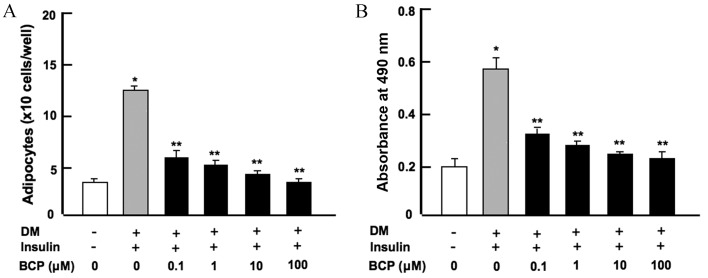
Bone marrow MSCs are known to differentiate into adipocytes (3,4). In order to determine the effect of β-caryophyllene on bone marrow adipogenesis, mouse bone marrow cells were cultured in either medium without DM and insulin or with DM plus insulin, along with β-caryophyllene (0, 0.1, 1, 10 or 100 µM) for 7 days. Mouse bone marrow cells cultured without DM, insulin or β-caryophyllene acted as a control. As shown in Fig. 1, cells treated with DM + insulin alone significantly increased adipogenesis when compared with the control group (without DM or insulin; P<0.001). The addition of β-caryophyllene at any concentration was observed to significantly suppress the differentiation of bone marrow cells into adipocytes (P<0.001 vs. DM + insulin only) in a dose-dependent manner, as determined by cell counting (Fig. 1A) and spectrophotometry (Fig. 1B) following Oil O Red staining. Furthermore, reduced differentiation into adipocytes was found in bone marrow cells cultured in media containing β-caryophyllene for 3 days and then in media without β-caryophyllene for a further 4 days (data not shown).
Figure 1.
β-caryophyllene suppresses adipogenesis in mouse bone marrow cell cultures in vitro. (A) Number of adipocytes per cell, determined by counting under a light microscope. (B) Quantification of adipocytes based on the absorbance at 490 nm measured by spectrophotometry. Symbols indicate the presence (+) and (−) absence of the listed reagents. Statistical analysis was performed by one-way way analysis of the variance and post-hoc Tukey's range test. *P<0.001 vs. control; **P<0.001 vs. DM + insulin only. DM, differentiation medium; BCP, β-caryophyllene.
β-caryophyllene stimulates osteoblastic mineralization in vitro
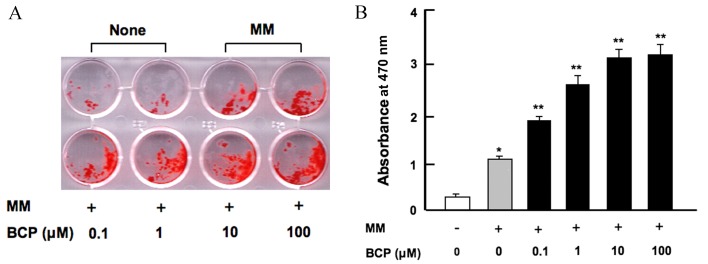
Osteoblasts develop from bone marrow MSCs (3,4). To investigate the effect of β-caryophyllene on osteoblastogenesis and mineralization in the bone marrow, cells were cultured in MM with or without β-caryophyllene (0.1–100 µM) for 18 days. As shown in Fig. 2, MM alone significantly increased bone marrow mineralization compared with the control group without MM (P<0.001). The addition of β-caryophyllene at all doses significantly increased osteoblastic mineralization in a dose-dependent manner when compared with cells treated with MM alone (P<0.001; Fig. 2). Furthermore, increased osteoblastic mineralization was observed when bone marrow cells were cultured in the presence of β-caryophyllene for 7 days (data not shown).
Figure 2.
β-caryophyllene stimulates osteoblastic mineralization in mouse bone marrow cell cultures in vitro. (A) Representative image of cells cultured in none (without MM and β-caryophyllene) or MM in the presence or absence of increasing concentrations of β-caryophyllene (0.1–100 µM) for 18 days. (B) Quantification of mineralization based on the absorbance at 570 nm, determined by spectrophotometry. Symbols indicate the presence (+) and (−) absence of the listed reagents. The white bar represents the control cells grown in regular culture medium without MM or BCP. Statistical analysis was performed by one-way way analysis of the variance and post-hoc Tukey's range test. *P<0.001 vs. control; **P<0.001 vs. MM only. MM, mineralization medium; BCP, β-caryophyllene.
β-caryophyllene reduces osteoclastogenesis in vitro
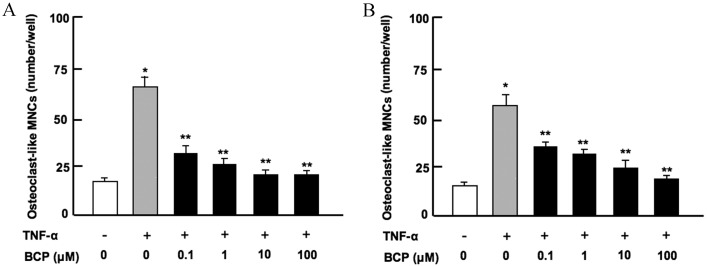
Osteoclasts are differentiated from monocytes and macrophages in the bone marrow (26). To assess the effect of β-caryophyllene on osteoclastogenesis in bone marrow in vitro, bone marrow cells were cultured in the presence of TNF-α, which stimulates osteoclastogenesis by activating nuclear factor-κB (NF-κB) in preosteoclasts (13), and with or without β-caryophyllene for 7 days. The results revealed that osteoclastogenesis was significantly enhanced in the presence of TNF-α alone when compared with the untreated control group (P<0.001; Fig. 3). This increase was significantly suppressed following culture with β-caryophyllene at all doses for 7 days (P<0.001 vs. TNF-α only; Fig. 3A). Furthermore, β-caryophyllene (0.1–100 µM) did not have a significant effect on osteoclastogenesis in the absence of TNF-α (data not shown).
Figure 3.
β-caryophyllene suppresses osteoclastogenesis in mouse bone marrow cell cultures in vitro. (A) Number of osteoclast-like MNCs, when bone marrow cells were cultured with TNF-α and increasing concentrations of β-caryophyllene (0–100 µM) for 7 days. (B) Number of osteoclast-like MNCs, when bone marrow cells were cultured with TNF-α and β-caryophyllene (0–100 µm) for 3 days, and then with TNF-α but without β-caryophyllene for a further 4 days. The white bar indicates the control and represents cells grown in regular culture medium without TNF-α or BCP. Statistical analysis was performed by one-way way analysis of the variance and post-hoc Tukey's range test. *P<0.001 vs. control; **P<0.001 vs. TNF-α alone. TNF-α; tumor necrosis factor-α; BCP, β-caryophyllene; MNCs, multinucleated cells.
Discussion
In the present study, β-caryophyllene was demonstrated to enhance osteoblastic mineralization, and to suppress adipogenesis and osteoclastogenesis in mouse bone marrow cell cultures in vitro. To the best of our knowledge, this is the first time that these effects of β-caryophyllene are reported. The effects of β-caryophyllene on adipogenesis and osteoblastic mineralization were observed at an early stage of culture following 7–8 days. This indicates that β-caryophyllene strongly stimulates the differentiation of bone marrow MSCs into osteoblasts, and strongly suppresses the differentiation to adipocytes.
Bone marrow MSCs are multipotent cells that can differentiate into adipocytes and osteoblasts (3,4). This process is mediated through numerous complex signaling pathways, including those involving PPARγ (5–7). For instance, enhanced mitogen-activated protein kinase/extracellular signal-regulated kinase signaling during adipogenesis potentiates the activity of factors that regulate the expression of CCAAT/enhancer-binding protein a and PPARγ (6,7). Similarly, β-caryophyllene may exhibit a specific regulatory effect on signaling pathways involved in the differentiation of bone marrow MSCs to adipocytes.
Osteoclasts, which promote bone resorption, are derived from hematopoietic progenitors (1,2,26). The current study demonstrated that β-caryophyllene suppressed TNF-α-enhanced osteoclastogenesis in mouse bone marrow in vitro, mediated through the activation of NF-κB signaling in preosteoclasts. This suppressive effect was observed following 7 days culture with bone marrow cells. Therefore, the results of the present study suggest that β-caryophyllene inhibited osteoclastogenesis at the stage of differentiation into preosteoclasts in bone marrow culture.
The sesquiterpene β-caryophyllene is present in various essential oils, particularly in clove, hemp, rosemary and hop oil (16–22). In addition, β-caryophyllene is found in plants that are ingested daily and has been approved as a food additive by the FDA (16–18). β-caryophyllene is a selective agonist of CB2 and has been shown to have anti-inflammatory effects in animals, which have been implicated with reduced TNF-α and IL-1β production associated with opioid receptors (17,19,22). Furthermore, this molecule reduces acute and chronic pain associated with inflammation (19–21). In the current study, β-caryophyllene was demonstrated to enhance osteoblastic mineralization, and suppress adipogenesis and osteoclastogenesis in mouse bone marrow cells in vitro. These results indicate that β-caryophyllene stimulates osteoblastic bone formation and suppresses osteoclastic bone resorption, which may provide a means to prevent and treat osteoporosis.
Osteoporosis has been associated with obesity and diabetes (9,10), which are increasingly prevalent public health concerns. Osteoporosis and obesity share a number of similar features (3,4,11,12); bone marrow MSCs can differentiate into adipocytes and osteoblasts and enhanced adipogenesis may suppress osteoblastogenesis in bone marrow cells (3,4). In the present study, β-caryophyllene was found to stimulate osteoblastogenesis and suppress adipogenesis in mouse bone marrow cell cultures in vitro. Therefore, β-caryophyllene may serve an important role in the prevention and treatment of osteoporosis associated with obesity and diabetes.
In conclusion, the present study demonstrated that β-caryophyllene promotes osteoblastic mineralization, and reduces adipogenesis and osteoclastogenesis in mouse bone marrow cell cultures in vitro. These results indicate that β-caryophyllene may be a useful tool in the treatment of osteoporosis. Further studies into the effects of β-caryophyllene on bone remodeling should be performed to validate these effects in an in vivo environment and in models of osteoporosis.
References
- 1.Raggatt LJ, Partridge NC. Cellular and molecular mechanisms of bone modeling. J Biol Chem. 2010;285:25103–25108. doi: 10.1074/jbc.R109.041087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chambers TJ, Fuller K. How are osteoclasts induced to resorb bone? Ann N Y Acad Sci. 2011;1240:1–6. doi: 10.1111/j.1749-6632.2011.06249.x. [DOI] [PubMed] [Google Scholar]
- 3.Minguell JJ, Erices A, Conget P. Mesenchymal stem cells. Exp Biol Med (Maywood) 2001;226:507–520. doi: 10.1177/153537020122600603. [DOI] [PubMed] [Google Scholar]
- 4.Muruganandan S, Roman AA, Sinal CJ. Adipocyte differentiation of bone marrow-derived mesenchymal stem cells: Cross talk with the osteoblastogenesis program. Cell Mol Life Sci. 2009;66:236–253. doi: 10.1007/s00018-008-8429-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laudes M. Role of WNT signaling in the determination of human mesenchymal stem cells into preadipocytes. J Mol Endocrinol. 2011;46:R65–R72. doi: 10.1530/JME-10-0169. [DOI] [PubMed] [Google Scholar]
- 6.Gharibi B, Abraham AA, Ham J, Evans BA. Adenosine receptor subtype expression and activation influence the differentiation of mesenchymal stem cells to osteoblasts and adipocytes. J Bone Miner Res. 2011;26:2112–2124. doi: 10.1002/jbmr.424. [DOI] [PubMed] [Google Scholar]
- 7.Kawai M, Rosen CJ. PPARγ: A circadian transcription factor in adipogenesis and osteogenesis. Nat Rev Endocrinol. 2010;6:629–636. doi: 10.1038/nrendo.2010.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weitzmann MN, Pacifici R. Estrogen deficiency and bone loss: An inflammatory tale. J Clin Invest. 2006;116:1186–1194. doi: 10.1172/JCI28550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leslie WD, Rubin MR, Schwartz AV, Kanis JA. Type 2 diabetes and bone. J Bone Miner Res. 2012;27:2231–2237. doi: 10.1002/jbmr.1759. [DOI] [PubMed] [Google Scholar]
- 10.Nielson CM, Srikanth P, Orwoll ES. Obesity and fracture in men and women: An epidemiologic perspective. J Bone Miner Res. 2012;27:1–10. doi: 10.1002/jbmr.1486. [DOI] [PubMed] [Google Scholar]
- 11.Gharibi B, Abraham AA, Ham J, Evans BA. Adenosine receptor subtype expression and activation influence the differentiation of mesenchymal stem cells to osteoblasts and adipocytes. J Bone Miner Res. 2011;26:2112–2124. doi: 10.1002/jbmr.424. [DOI] [PubMed] [Google Scholar]
- 12.Pirih F, Lu J, Ye F, Bezouglaia O, Atti E, Ascenzi MG, Tetradis S, Demer L, Aghaloo T, Tintut Y. Adverse effects of hyperlipidemia on bone regeneration and strength. J Bone Miner Res. 2012;27:309–318. doi: 10.1002/jbmr.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y, Li A, Strait K, Zhang H, Nanes MS, Weitzmann MN. Endogenous TNFalpha lowers maximum peak bone mass and inhibits osteoblastic Smad activation through NF-kappaB. J Bone Miner Res. 2007;22:646–655. doi: 10.1359/jbmr.070121. [DOI] [PubMed] [Google Scholar]
- 14.Chang J, Wang Z, Tang E, Fan Z, McCauley L, Franceschi R, Guan K, Krebsbach PH, Wang CY. Inhibition of osteoblastic bone formation by nuclear factor-kappaB. Nat Med. 2009;15:682–689. doi: 10.1038/nm.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cortez M, Carmo LS, Rogero MM, Borelli P, Fock RA. A high-fat diet increases IL-1, IL-6, and TNF-α production by increasing NF-κB and attenuating PPAR-γ expression in bone marrow mesenchymal stem cells. Inflammation. 2013;36:379–386. doi: 10.1007/s10753-012-9557-z. [DOI] [PubMed] [Google Scholar]
- 16.Ghelardini C, Galeotti N, Di Cesare Mannelli L, Mazzanti G, Bartolini A. Local anaesthetic activity of beta-caryophyllene. Farmaco. 2001;56:387–389. doi: 10.1016/S0014-827X(01)01092-8. [DOI] [PubMed] [Google Scholar]
- 17.Gertsch J, Leonti M, Raduner S, Racz I, Chen JZ, Xie XQ, Altmann KH, Karsak M, Zimmer A. Beta-caryophyllene is a dietary cannabinoid. Proc Nat Acad Sci USA. 2008;105:9099–9104. doi: 10.1073/pnas.0803601105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ormeño E, Baldy V, Ballini C, Fernandez C. Production and diversity of volatile terpenes from plants on calcareous and siliceous soils: Effect of soil nutrients. J Chem Ecol. 2008;34:1219–1229. doi: 10.1007/s10886-008-9515-2. [DOI] [PubMed] [Google Scholar]
- 19.Katsuyama S, Mizoguchi H, Kuwahata H, Komatsu T, Nagaoka K, Nakamura H, Bagetta G, Sakurada T, Sakurada S. Involvement of peripheral cannabinoid and opioid receptors in β-caryophyllene-induced antinociception. Eur J Pain. 2013;17:664–675. doi: 10.1002/j.1532-2149.2012.00242.x. [DOI] [PubMed] [Google Scholar]
- 20.Paula-Freile LI, Andersen ML, Gama VS, Molska GR, Carlini EL. The oral administration of trans-caryophyllene attenuates acute and chronic pain in mice. Phytomedicine. 2015;21:356–362. doi: 10.1016/j.phymed.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 21.Chavan MJ, Wakte PS, Shinde DB. Analgestic and anti-inflammatory activity of caryophyllene oxide from Annona squamosa L. Bark. Phytomedicine. 2010;17:149–151. doi: 10.1016/j.phymed.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 22.Martinez RM, Zarpelon AC, Cardoso RD, Vicentini FT, Georgetti SR, Baracat MM, Andrel CC, Moreira IC, Verri WA, Jr, Casagrande R. Tephrosia sinapou ethyl acetate extract inhibits inflammatory pain in mice: Opioid receptor dependent inhibition of TNFα and IL-1β production. Pharm Biol. 2013;51:1262–1271. doi: 10.3109/13880209.2013.786099. [DOI] [PubMed] [Google Scholar]
- 23.Yamaguchi M, Weitzmann MN, Baile CA, Murata T. Exogenous regucalcin suppresses osteoblastogenesis and stimulates adipogenesis in mouse bone marrow culture. Integr Biol (Camb) 2012;4:1215–1222. doi: 10.1039/c2ib20118f. [DOI] [PubMed] [Google Scholar]
- 24.Yamaguchi M, Zhu S, Zhang S, Wu D, Moore TM, Snyder JP, Shoji M. Curcumin analogue UBS109 prevents bone loss in breast cancer bone metastasis mouse model: Involvement in osteoblastogenesis and osteoclastogenesis. Cell Tissue Res. 2014;357:245–252. doi: 10.1007/s00441-014-1846-4. [DOI] [PubMed] [Google Scholar]
- 25.Minkin C. Bone acid phosphatase: Tartrate-resistant acid phosphatase as a marker of osteoclast function. Calcif Tissue Int. 1982;34:285–290. doi: 10.1007/BF02411252. [DOI] [PubMed] [Google Scholar]
- 26.Zaidi M, Blair HC, Moonga BS, Abe E, Huang CL. Osteoclastogenesis, bone resorption, and osteoblast-based therapeutics. J Bone Miner Res. 2003;18:599–609. doi: 10.1359/jbmr.2003.18.4.599. [DOI] [PubMed] [Google Scholar]