Abstract
Rationale:
We report on a patient with hypersomnia who showed injury of the lower ascending reticular activating system (ARAS) following cerebellar herniation due to a cerebellar infarct, detected on diffusion tensor tractography (DTT).
Patient concerns:
A 53-year-old male patient was diagnosed as a left cerebellar infarct, and underwent decompressive suboccipital craniectomy due to brain edema at 2 days after the onset of a cerebellar infarct. Three weeks after onset when the patient started rehabilitation, he showed hypersomnia without impairment of consciousness; he fell asleep most of daytime without external stimulation and showed an abnormal score on the Epworth Sleepiness Scale: 15 (full score: 24, cut off for hypersomnia: 10).
Diagnoses and Outcomes:
On 3-week DTT, narrowing of the upper portion of the lower ventral ARAS between the pontine reticular formation and the hypothalamus was observed on both sides. In addition, partial tearing was observed in the middle portion of the right lower ventral ARAS.
Lessons:
In conclusion, we found injury of the lower ventral ARAS in a patient with hypersomnia following cerebellar herniation due to a cerebellar infarct.
Keywords: ascending reticular activating system, cerebellar herniation, diffusion tensor imaging, hypersomnia, stroke
1. Introduction
Hypersomnia is a common sequela in stroke patients: 1 study reported that 5.6% of stroke patients showed persistent hypersomnia.[1] Hypersomnia has been mainly reported in patients with lesions in the thalamus, hypothalamus, or pons.[2–9] Several recent studies using diffusion tensor tractography (DTT) for the ascending reticular activating system (ARAS) have demonstrated the association of hypersomnia with injury of the ARAS in patients with brain injury.[10–13] However, the pathogenesis of hypersomnia has not been clearly elucidated.
In the present study, using DTT, we report on a patient with hypersomnia who showed injury of the lower ventral ARAS following cerebellar herniation due to a cerebellar infarct.
2. Methods
2.1. Case report
A 53-year-old male patient was diagnosed with a left cerebellar infarct, and underwent decompressive suboccipital craniectomy due to brain edema at 2 days after onset of a cerebellar infarct at the neurosurgery department of a university hospital (Fig. 1A). A leukomalactic lesion was observed in the left cerebellum on brain magnetic resonance imaging taken at 3 weeks after onset, however, no abnormal lesion was observed in the midbrain (Fig. 1A). Three weeks after onset, he was transferred to the rehabilitation department of a university hospital in order to undergo rehabilitation. He showed hypersomnia without impairment of consciousness; he fell asleep most of daytime without external stimulation. At the time of diffusion tensor imaging (DTI) scanning (3 weeks after onset), he showed an abnormal score on the Epworth Sleepiness Scale: 15 (full score: 24, cut off for hypersomnia: 10).[14] Epworth Sleepiness Scale as a questionnaire was made once by the patient's own. The patient signed an informed consent statement, and the study protocol was approved by the Yeungnam University hospital Institutional Review Board of a university hospital (Table 1).
Figure 1.
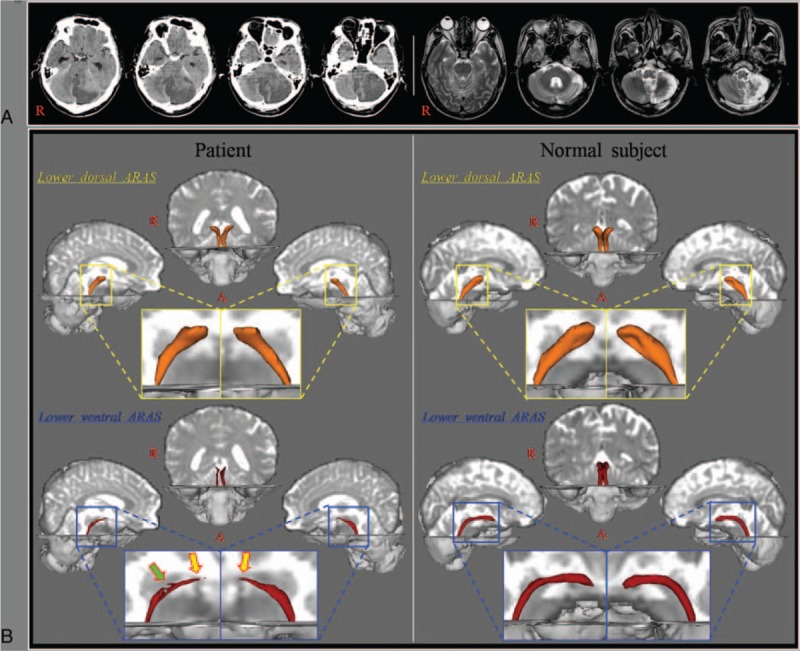
(A) Brain CT images at onset show the left cerebellar infarct and cerebellar herniation and T2-weighted brain MR images at 3 weeks after onset show a leukomalactic lesion in the left cerebellum. (B) Results of diffusion tensor tractography: narrowing of the upper portion (yellow arrows) of the lower ventral ascending reticular activating system is observed on both sides. In addition, partial tearing (green arrow) is observed in the middle portion of the right lower ventral ascending reticular activating system.
Table 1.
Epworth Sleepiness Scale for the patient.

2.2. Diffusion tensor tractography
DTI data were acquired at 3 weeks after onset of the cerebellar infarct using a 1.5T Philips Gyroscan Intera (Philips, Ltd, Best, The Netherlands) with 32 gradients. Imaging parameters were as follows: acquisition matrix = 96 × 96; reconstructed to matrix = 192 × 192; field of view = 240 × 240 mm2; repetition time = 10,398 milliseconds; echo time = 72 milliseconds; parallel imaging reduction factor = 2; echo-planar imaging factor = 59; b = 1000 seconds/mm2; and a slice thickness of 2.5 mm. Before the fiber tracking, eddy current correction was applied for correction of the head motion effect and image distortion using the Oxford Centre for Functional Magnetic Resonance Imaging of the Brain (FMRIB) Diffusion Software. Fiber tracking was performed using probabilistic tractography with default tractography option in the FMRIB Diffusion Software (5000 streamline samples, 0.5 mm step lengths, curvature thresholds = 0.2).[15] Two portions of the ARAS were reconstructed by selection of fibers passing through regions of interest (ROI) as follows[16,17]: the lower dorsal ARAS, between the pontine reticular formation (RF, ROI 1) and the intralaminar thalamic nucleus (ROI 2),[16] the lower ventral ARAS, between the pontine reticular formation (ROI 1) and the hypothalamus (ROI 2).[17] The threshold of 2 streamlines was applied for the results of fiber tracking.
3. Results
Narrowing of the upper portion of the lower ventral ARAS between the pontine RF and the hypothalamus was observed on both sides and partial tearing was observed in the middle portion of the right lower ventral ARAS (Fig. 1B).
4. Discussion
In the present study, the 2 lower portions of the ARAS were evaluated using DTT: the lower dorsal ARAS between the pontine RF and the intralaminar thalamic nucleus, and the lower ventral ARAS between the pontine RF and the hypothalamus. Narrowing of the upper portion of both the lower ventral ARAS and partial tearing of the middle portion of the right lower ventral ARAS were observed. These findings indicated injury of the lower ventral ARAS on both sides. It appeared that hypersomnia of this patient was ascribed to the injury of the lower ventral ARAS. Our results appeared to coincide with the results of previous studies reporting close association of the hypothalamus with hypersomnia.[7–9]
Several recent studies using DTT have reported on the association of hypersomnia with injury of the lower ARAS.[10–13] In 2006, Jang et al[10] reported on a patient who showed hypersomnia following spontaneous pontine hemorrhage. Although this patient showed injury of both the lower dorsal and ventral ARAS, the injury of the lower ventral ARAS was more severe. Subsequently, Jang et al[11] demonstrated injury of the lower ventral ARAS in a patient who showed hypersomnia and narcolepsy following mild traumatic brain injury. During the same year, Jang et al[12] reported on a patient who showed recovery of hypersomnia concurrent with the recovery of an injured lower dorsal and ventral ARAS following spontaneous subarachnoid hemorrhage. This patient also showed more severe injury and more recovery of the lower ventral ARAS than the lower dorsal ARAS. Jang and Kwon[13] recently reported on 2 patients who showed hypersomnia and fatigue with injury of the lower dorsal and ventral ARAS following mild traumatic brain injury. Considering the results of the above-mentioned studies, it appeared that the lower ventral ARAS was more responsible for hypersomnia and it is also consistent with our results.[10–13]
In conclusion, we found injury of the lower ventral ARAS in a patient with hypersomnia following cerebellar herniation due to a cerebellar infarct and we think that injury of the lower ventral ARAS could be a probable cause for hypersomnia in this patient. Since the introduction of DTT for the ARAS, a few studies have reported on injury of the ARAS due to brain herniation including transtentorial herniation, subfalcine herniation, and Kernohan phenomenon.[18–20] However, in terms of cerebellar herniation, to the best of our knowledge, this is the first study to demonstrate injury of the ARAS. Nevertheless, limitations of this study should be considered. First, because it is a case report, this study is limited; therefore, conduct of further studies comprising a large number of patients would be necessary. Second, despite being a powerful anatomic imaging tool, DTI may underestimate or overestimate the fiber tracts because regions of fiber complexity and crossing can prevent full reflection of the underlying fiber architecture by DTI.[21]
Footnotes
Abbreviations: ARAS = ascending reticular activating system, DTI = diffusion tensor imaging, DTT = diffusion tensor tractography, RF = reticular formation, ROI = region of interest.
Funding: This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (NRF-2015R1A2A2A01004073).
The authors have no conflicts of interest to disclose.
References
- [1].Harris AL, Elder J, Schiff ND, et al. Post-stroke apathy and hypersomnia lead to worse outcomes from acute rehabilitation. Transl Stroke Res 2014;5:292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Forcadas MI, Zarranz JJ. Hypersomnia after tegmental pontine hematoma. Neurologia 1995;10:307–10. [PubMed] [Google Scholar]
- [3].Arpa J, Rodriguez-Albarino A, Izal E, et al. Hypersomnia after tegmental pontine hematoma: case report. Neurologia 1995;10:140–4. [PubMed] [Google Scholar]
- [4].Blanco M, Espinosa M, Arpa J, et al. Hypersomnia and thalamic and brain stem stroke: a study of seven patients. Neurologia 1999;14:307–14. [PubMed] [Google Scholar]
- [5].Tosato M, Aquila S, Della Marca G, et al. Sleep disruption following paramedian pontine stroke. BMJ Case Rep 2009;Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Goyal MK, Kumar G, Sahota PK. Isolated hypersomnia due to bilateral thalamic infarcts. J Stroke Cerebrovasc Dis 2012;21:146–7. [DOI] [PubMed] [Google Scholar]
- [7].Menzler K, Belke M, Unger MM, et al. DTI reveals hypothalamic and brainstem white matter lesions in patients with idiopathic narcolepsy. Sleep Med 2012;13:736–42. [DOI] [PubMed] [Google Scholar]
- [8].Tezer FI, Pektezel MY, Gocmen R, et al. Unusual presentation of hypothalamic hamartoma with hypersomnia in an adult patient. Epileptic Disord 2014;16:366–9. [DOI] [PubMed] [Google Scholar]
- [9].Yassin W, Sugihara G, Oishi N, et al. Hypothalamic-amygdalar-brainstem volume reduction in a patient with narcolepsy secondary to diffuse axonal injury. J Clin Sleep Med 2015;11:581–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jang SH, Chang CH, Jung YJ, et al. Post-stroke hypersomnia. Int J Stroke 2016;11:N5–6. [DOI] [PubMed] [Google Scholar]
- [11].Jang SH, Seo WS, Kwon HG. Post-traumatic narcolepsy and injury of the ascending reticular activating system. Sleep Med 2016;17:124–5. [DOI] [PubMed] [Google Scholar]
- [12].Jang SH, Lee HD, Chang CH, et al. Recovery of hypersomnia concurrent with recovery of an injured ascending reticular activating system in a stroke patient: a case report. Medicine (Baltimore) 2016;95:e2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jang SH, Kwon HG. Injury of the ascending reticular activating system in patients with fatigue and hypersomnia following mild traumatic brain injury: two case report. Medicine (Baltimore) 2016;95:e2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bloch KE, Schoch OD, Zhang JN, et al. German version of the Epworth Sleepiness Scale. Respiration 1999;66:440–7. [DOI] [PubMed] [Google Scholar]
- [15].Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004;23(suppl 1):S208–19. [DOI] [PubMed] [Google Scholar]
- [16].Yeo SS, Chang PH, Jang SH. The ascending reticular activating system from pontine reticular formation to the thalamus in the human brain. Front Hum Neurosci 2013;7:416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jang SH, Kwon HG. The ascending reticular activating system from pontine reticular formation to the hypothalamus in the human brain: a diffusion tensor imaging study. Neurosci Lett 2015;590:58–61. [DOI] [PubMed] [Google Scholar]
- [18].Jang SH, Lim HW, Yeo SS. Injury of the ascending reticular activating system by transtentorial herniation in a patient with intracerebral haemorrhage: a diffusion tensor tractography study. J Neurol Neurosurg Psychiatry 2015;86:1164–6. [DOI] [PubMed] [Google Scholar]
- [19].Jang SH, Seo YS. Injury of the contralateral lower ascending reticular activating system by an intracerebral hemorrhage. AJNR Am J Neuroradiol 2015;36:E58–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jang SH, Kim SH, Seo YS. Injury of the ascending reticular activating system by subfalcine herniation after subdural hematoma: a case report. Am J Phys Med Rehabil 2016;95:129–30. [DOI] [PubMed] [Google Scholar]
- [21].Yamada K, Sakai K, Akazawa K, et al. MR tractography: a review of its clinical applications. Magn Reson Med Sci 2009;8:165–74. [DOI] [PubMed] [Google Scholar]


