Abstract
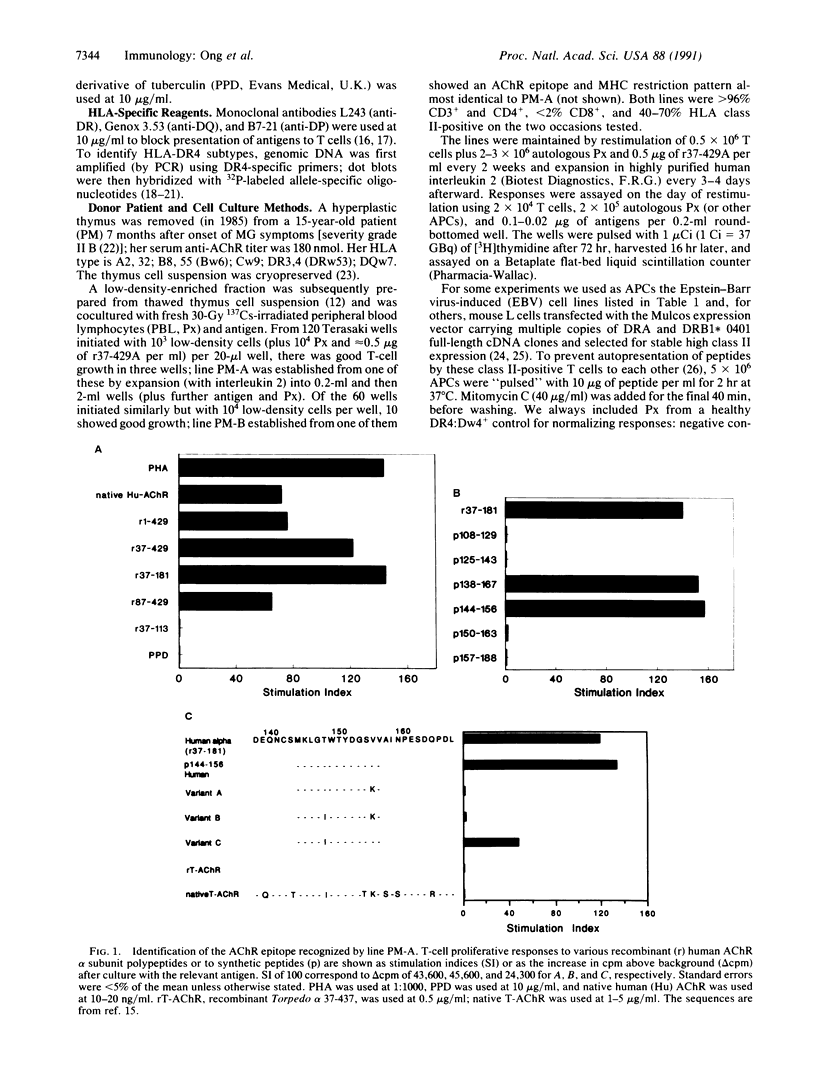
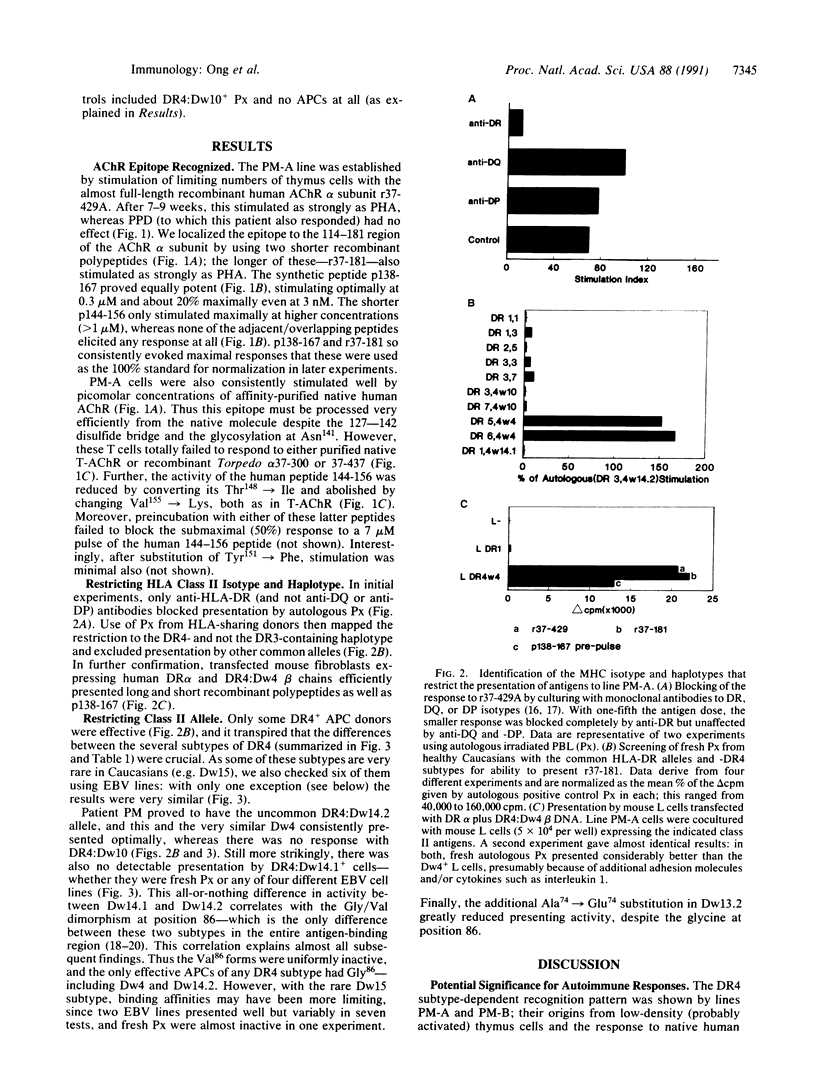
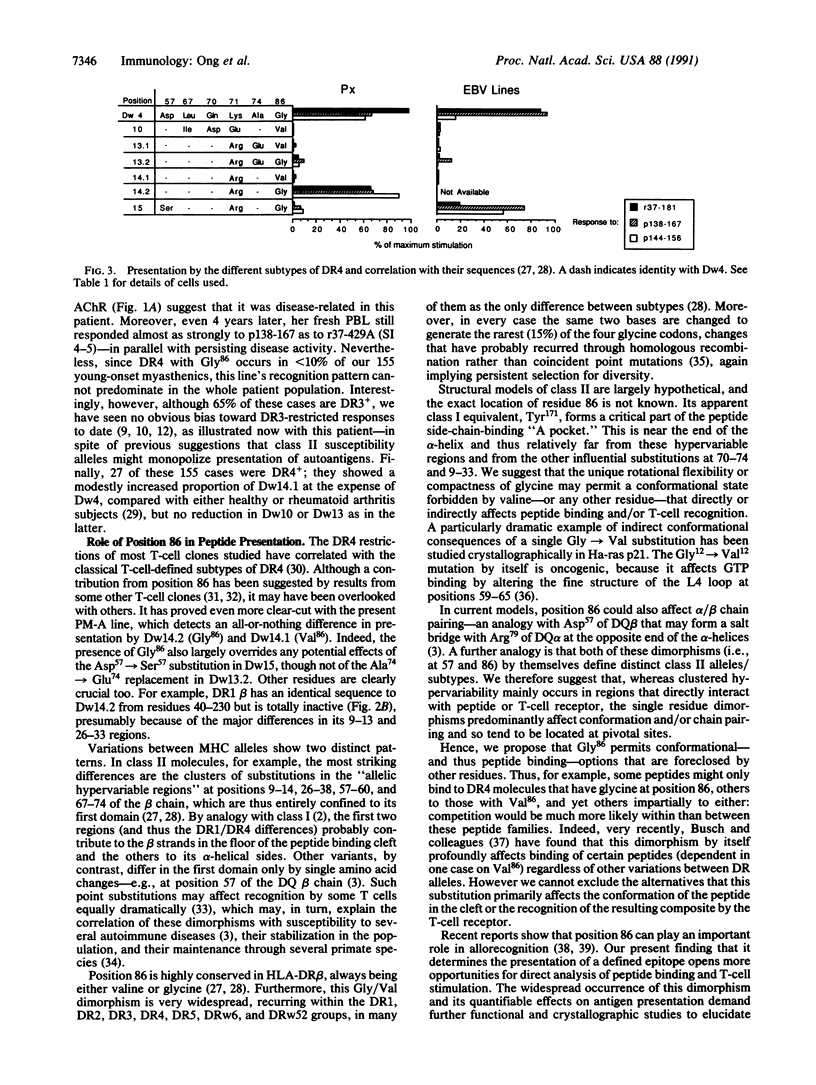
Helper T lymphocytes recognize fragments of foreign (or self) antigens in the peptide-binding clefts of major histocompatibility complex class II molecules; their activation is a crucial step in the induction of many immune and autoimmune responses. While studying the latter, we raised a T-cell line from the thymus of a myasthenia gravis patient against recombinant alpha subunit of the human acetylcholine receptor, the target of this autoimmune disease. The line responds to the 144-156 region of the human sequence and not to the same region of the electric fish homolog, which differs by only three residues. These CD4+ T cells recognize this epitope only in the context of HLA-DR4 class II molecules, of which the variants with Gly86 are absolutely required. Thus the naturally occurring alternatives Dw14.2 (Gly86) and Dw14.1 (Val86)--which differ only at this one position in the entire antigen-binding region--show an all-or-nothing difference in presenting activity. This dimorphism at position 86 is widespread, occurring in subtypes of DR1, DR2, DR3, DR5, and DR6 alleles as well as DR4. Since other DR4 subtypes with substitutions at positions 70-74 also fail to present this peptide, and glycine residues can be uniquely flexible, we suggest that this replacement at position 86 acts locally or at a distance by altering the conformation of the peptide-binding cleft. Such profound functional consequences for T-cell recognition as we report here may explain this example of conserved major histocompatibility complex diversity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altmann D. M., Heyes J. M., Ikeda H., Sadler A. M., Wilkinson D., Madrigal J. A., Bodmer J. G., Trowsdale J. Fine mapping of HLA class II monoclonal antibody specificities using transfected L cells. Immunogenetics. 1990;32(1):51–55. doi: 10.1007/BF01787329. [DOI] [PubMed] [Google Scholar]
- Beeson D., Morris A., Vincent A., Newsom-Davis J. The human muscle nicotinic acetylcholine receptor alpha-subunit exist as two isoforms: a novel exon. EMBO J. 1990 Jul;9(7):2101–2106. doi: 10.1002/j.1460-2075.1990.tb07378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J. I., Denney D., Jr, Foster L., Belt T., Todd J. A., McDevitt H. O. Allelic variation in the DR subregion of the human major histocompatibility complex. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6234–6238. doi: 10.1073/pnas.84.17.6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J. I., Todd J. A., McDevitt H. O. The molecular basis of HLA-disease association. Adv Hum Genet. 1989;18:1–41. [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. The foreign antigen binding site and T cell recognition regions of class I histocompatibility antigens. Nature. 1987 Oct 8;329(6139):512–518. doi: 10.1038/329512a0. [DOI] [PubMed] [Google Scholar]
- Brodsky F. M. A matrix approach to human class II histocompatibility antigens: reactions of four monoclonal antibodies with the products of nine haplotypes. Immunogenetics. 1984;19(3):179–194. doi: 10.1007/BF00364762. [DOI] [PubMed] [Google Scholar]
- Brown J. H., Jardetzky T., Saper M. A., Samraoui B., Bjorkman P. J., Wiley D. C. A hypothetical model of the foreign antigen binding site of class II histocompatibility molecules. Nature. 1988 Apr 28;332(6167):845–850. doi: 10.1038/332845a0. [DOI] [PubMed] [Google Scholar]
- Compston D. A., Vincent A., Newsom-Davis J., Batchelor J. R. Clinical, pathological, HLA antigen and immunological evidence for disease heterogeneity in myasthenia gravis. Brain. 1980 Sep;103(3):579–601. doi: 10.1093/brain/103.3.579. [DOI] [PubMed] [Google Scholar]
- Eckels D. D., Geiger M. J., Sell T. W., Gorski J. A. Involvement of class II beta-chain amino acid residues 85 and 86 in T-cell allorecognition. Hum Immunol. 1990 Mar;27(3):240–253. doi: 10.1016/0198-8859(90)90054-s. [DOI] [PubMed] [Google Scholar]
- Gao X. J., Fernandez-Vina M., Shumway W., Stastny P. DNA typing for class II HLA antigens with allele-specific or group-specific amplification. I. Typing for subsets of HLA-DR4. Hum Immunol. 1990 Jan;27(1):40–50. doi: 10.1016/0198-8859(90)90094-6. [DOI] [PubMed] [Google Scholar]
- Gerrard T. L., Volkman D. J., Jurgensen C. H., Fauci A. S. Activated human T cells can present denatured antigen. Hum Immunol. 1986 Dec;17(4):416–425. doi: 10.1016/0198-8859(86)90301-0. [DOI] [PubMed] [Google Scholar]
- Gregersen P. K., Shen M., Song Q. L., Merryman P., Degar S., Seki T., Maccari J., Goldberg D., Murphy H., Schwenzer J. Molecular diversity of HLA-DR4 haplotypes. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2642–2646. doi: 10.1073/pnas.83.8.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyllensten U. B., Lashkari D., Erlich H. A. Allelic diversification at the class II DQB locus of the mammalian major histocompatibility complex. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1835–1839. doi: 10.1073/pnas.87.5.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohlfeld R., Toyka K. V. Strategies for the modulation of neuroimmunological disease at the level of autoreactive T-lymphocytes. J Neuroimmunol. 1985 Aug;9(3-4):193–204. doi: 10.1016/s0165-5728(85)80018-7. [DOI] [PubMed] [Google Scholar]
- Krengel U., Schlichting I., Scherer A., Schumann R., Frech M., John J., Kabsch W., Pai E. F., Wittinghofer A. Three-dimensional structures of H-ras p21 mutants: molecular basis for their inability to function as signal switch molecules. Cell. 1990 Aug 10;62(3):539–548. doi: 10.1016/0092-8674(90)90018-a. [DOI] [PubMed] [Google Scholar]
- Kubo T., Noda M., Takai T., Tanabe T., Kayano T., Shimizu S., Tanaka K., Takahashi H., Hirose T., Inayama S. Primary structure of delta subunit precursor of calf muscle acetylcholine receptor deduced from cDNA sequence. Eur J Biochem. 1985 May 15;149(1):5–13. doi: 10.1111/j.1432-1033.1985.tb08885.x. [DOI] [PubMed] [Google Scholar]
- Lanchbury J. S., Hall M. A., Welsh K. I., Panayi G. S. Sequence analysis of HLA-DR4B1 subtypes: additional first domain variability is detected by oligonucleotide hybridization and nucleotide sequencing. Hum Immunol. 1990 Feb;27(2):136–144. doi: 10.1016/0198-8859(90)90110-b. [DOI] [PubMed] [Google Scholar]
- Lang B., Navarrete C., LoGalbo P. R., Nepom G. T., Silver J., Winchester R. J., Gregersen P. K. Further DNA sequence microheterogeneity of the HLA-DR4/Dw13 haplotype group: importance of amino acid position 86 of the DR beta 1 chain for T-cell recognition. Hum Immunol. 1990 Apr;27(4):378–389. doi: 10.1016/0198-8859(90)90088-7. [DOI] [PubMed] [Google Scholar]
- Lennon V. A., Lindstrom J. M., Seybold M. E. Experimental autoimmune myasthenia gravis: cellular and humoral immune responses. Ann N Y Acad Sci. 1976;274:283–299. doi: 10.1111/j.1749-6632.1976.tb47693.x. [DOI] [PubMed] [Google Scholar]
- Lundin K. E., Gaudernack G., Qvigstad E., Sollid L. M., Thorsby E. T lymphocyte clones recognizing an HLA-DQw3.2-associated epitope involving residue 57 on the DQ beta chain. Hum Immunol. 1988 Aug;22(4):235–246. doi: 10.1016/0198-8859(88)90003-1. [DOI] [PubMed] [Google Scholar]
- Marsh S. G., Bodmer J. G. HLA-DRB nucleotide sequences, 1990. Immunogenetics. 1990;31(3):141–144. doi: 10.1007/BF00211548. [DOI] [PubMed] [Google Scholar]
- Melms A., Chrestel S., Schalke B. C., Wekerle H., Mauron A., Ballivet M., Barkas T. Autoimmune T lymphocytes in myasthenia gravis. Determination of target epitopes using T lines and recombinant products of the mouse nicotinic acetylcholine receptor gene. J Clin Invest. 1989 Mar;83(3):785–790. doi: 10.1172/JCI113958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osserman K. E., Genkins G. Studies in myasthenia gravis: review of a twenty-year experience in over 1200 patients. Mt Sinai J Med. 1971 Nov-Dec;38(6):497–537. [PubMed] [Google Scholar]
- Rees A. D., Lombardi G., Scoging A., Barber L., Mitchell D., Lamb J., Lechler R. Functional evidence for the recognition of endogenous peptides by autoreactive T cell clones. Int Immunol. 1989;1(6):624–630. doi: 10.1093/intimm/1.6.624. [DOI] [PubMed] [Google Scholar]
- Robbins P. A., Evans E. L., Ding A. H., Warner N. L., Brodsky F. M. Monoclonal antibodies that distinguish between class II antigens (HLA-DP, DQ, and DR) in 14 haplotypes. Hum Immunol. 1987 Apr;18(4):301–313. doi: 10.1016/0198-8859(87)90077-2. [DOI] [PubMed] [Google Scholar]
- Rothbard J. B., Busch R., Bal V., Trowsdale J., Lechler R. I., Lamb J. R. Reversal of HLA restriction by a point mutation in an antigenic peptide. Int Immunol. 1989;1(5):487–495. doi: 10.1093/intimm/1.5.487. [DOI] [PubMed] [Google Scholar]
- Sommer N., Willcox N., Harcourt G. C., Newsom-Davis J. Myasthenic thymus and thymoma are selectively enriched in acetylcholine receptor-reactive T cells. Ann Neurol. 1990 Sep;28(3):312–319. doi: 10.1002/ana.410280303. [DOI] [PubMed] [Google Scholar]
- Weyand C. M., Goronzy J. J. Disease-associated human histocompatibility leukocyte antigen determinants in patients with seropositive rheumatoid arthritis. Functional role in antigen-specific and allogeneic T cell recognition. J Clin Invest. 1990 Apr;85(4):1051–1057. doi: 10.1172/JCI114535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson D., de Vries R. R., Madrigal J. A., Lock C. B., Morgenstern J. P., Trowsdale J., Altmann D. M. Analysis of HLA-DR glycoproteins by DNA-mediated gene transfer. Definition of DR2 beta gene products and antigen presentation to T cell clones from leprosy patients. J Exp Med. 1988 Apr 1;167(4):1442–1458. doi: 10.1084/jem.167.4.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcox H. N., Newsom-Davis J., Calder L. R. Greatly increased autoantibody production in myasthenia gravis by thymocyte suspensions prepared with proteolytic enzymes. Clin Exp Immunol. 1983 Nov;54(2):378–386. [PMC free article] [PubMed] [Google Scholar]
- Wordsworth B. P., Allsopp C. E., Young R. P., Bell J. I. HLA-DR typing using DNA amplification by the polymerase chain reaction and sequential hybridization to sequence-specific oligonucleotide probes. Immunogenetics. 1990;32(6):413–418. doi: 10.1007/BF00241635. [DOI] [PubMed] [Google Scholar]
- Wordsworth B. P., Lanchbury J. S., Sakkas L. I., Welsh K. I., Panayi G. S., Bell J. I. HLA-DR4 subtype frequencies in rheumatoid arthritis indicate that DRB1 is the major susceptibility locus within the HLA class II region. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10049–10053. doi: 10.1073/pnas.86.24.10049. [DOI] [PMC free article] [PubMed] [Google Scholar]


