Abstract
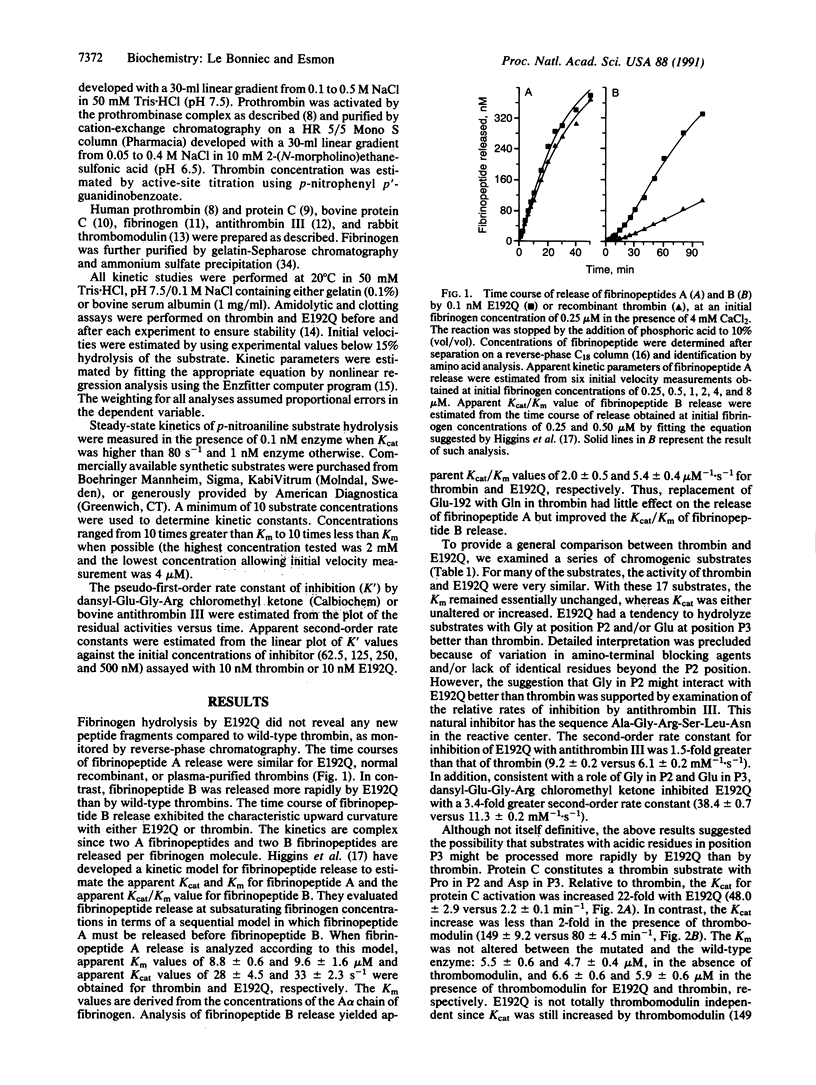
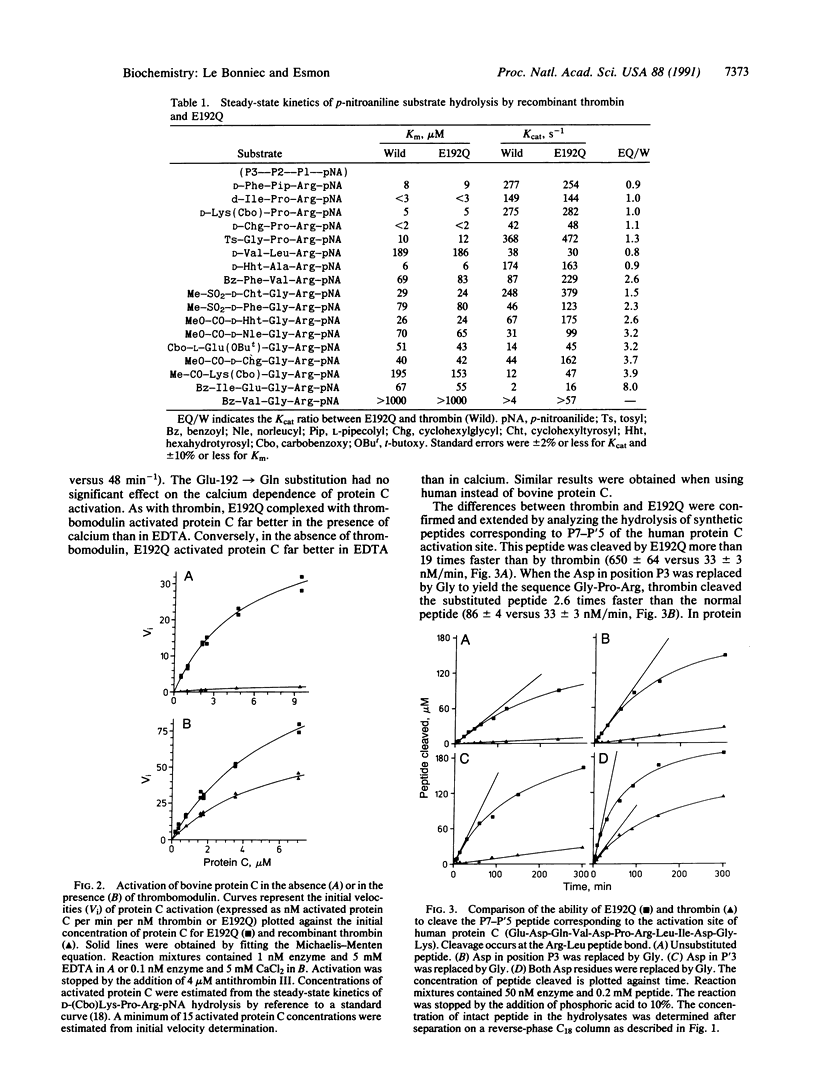
In serine proteases, residue 192, three residues prior to the active site Ser-195, plays an important role in determining substrate specificity. In trypsin (EC 3.4.21.4) and most trypsin-like enzymes with relatively broad specificity, this position is occupied by Gln. In thrombin (EC 3.4.21.5), an enzyme with restricted specificity, position 192 is occupied by Glu. The potential importance of Glu-192 in restricting the specificity of thrombin was investigated by isosterically replacing Glu-192 with Gln. Unlike trypsin, thrombin cleavage of peptides with acidic residues in positions P3 and P'3 [where P3 and P'3 refer to three residues removed from the Arg (P1) cleavage site on the amino and carboxyl side, respectively] is inefficient. Protein C, an anticoagulant zymogen, has Asp residues in positions P3 and P'3. Thrombomodulin, an endothelial cell protein, complexes with thrombin to activate protein C rapidly thus altering the specificity of thrombin. Compared to thrombin, the Glu-192----Gln mutant thrombin activates protein C 22 times more rapidly and cleaves the P7-P'5 peptide from the protein C activation site 19 times faster. Enhanced protein C activation results primarily from an increase in the catalytic rate constant rather than an improved Michaelis constant, a property that is shared by the thrombin-thrombomodulin complex. The Glu-192----Gln mutation does not influence fibrinopeptide A release and only increases the rate of fibrinopeptide B release 2.7-fold. These results demonstrate that Glu-192 plays a critical role in restricting the specificity of thrombin and suggest that thrombomodulin may function in part by altering the enzyme-substrate interaction near residue 192 in thrombin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando B., Wiedmer T., Hamilton K. K., Sims P. J. Complement proteins C5b-9 initiate secretion of platelet storage granules without increased binding of fibrinogen or von Willebrand factor to newly expressed cell surface GPIIb-IIIa. J Biol Chem. 1988 Aug 25;263(24):11907–11914. [PubMed] [Google Scholar]
- Bode W., Mayr I., Baumann U., Huber R., Stone S. R., Hofsteenge J. The refined 1.9 A crystal structure of human alpha-thrombin: interaction with D-Phe-Pro-Arg chloromethylketone and significance of the Tyr-Pro-Pro-Trp insertion segment. EMBO J. 1989 Nov;8(11):3467–3475. doi: 10.1002/j.1460-2075.1989.tb08511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode W., Meyer E., Jr, Powers J. C. Human leukocyte and porcine pancreatic elastase: X-ray crystal structures, mechanism, substrate specificity, and mechanism-based inhibitors. Biochemistry. 1989 Mar 7;28(5):1951–1963. doi: 10.1021/bi00431a001. [DOI] [PubMed] [Google Scholar]
- Bode W., Wei A. Z., Huber R., Meyer E., Travis J., Neumann S. X-ray crystal structure of the complex of human leukocyte elastase (PMN elastase) and the third domain of the turkey ovomucoid inhibitor. EMBO J. 1986 Oct;5(10):2453–2458. doi: 10.1002/j.1460-2075.1986.tb04521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissel J. P., Le Bonniec B., Rabiet M. J., Labie D., Elion J. Covalent structures of beta and gamma autolytic derivatives of human alpha-thrombin. J Biol Chem. 1984 May 10;259(9):5691–5697. [PubMed] [Google Scholar]
- Chang J. Y., Alkan S. S., Hilschmann N., Braun D. G. Thrombin specificity. Selective cleavage of antibody light chains at the joints of variable with joining regions and joining with constant regions. Eur J Biochem. 1985 Sep 2;151(2):225–230. doi: 10.1111/j.1432-1033.1985.tb09092.x. [DOI] [PubMed] [Google Scholar]
- Chang J. Y. Thrombin specificity. Requirement for apolar amino acids adjacent to the thrombin cleavage site of polypeptide substrate. Eur J Biochem. 1985 Sep 2;151(2):217–224. doi: 10.1111/j.1432-1033.1985.tb09091.x. [DOI] [PubMed] [Google Scholar]
- Chen Z., Bode W. Refined 2.5 A X-ray crystal structure of the complex formed by porcine kallikrein A and the bovine pancreatic trypsin inhibitor. Crystallization, Patterson search, structure determination, refinement, structure and comparison with its components and with the bovine trypsin-pancreatic trypsin inhibitor complex. J Mol Biol. 1983 Feb 25;164(2):283–311. doi: 10.1016/0022-2836(83)90078-5. [DOI] [PubMed] [Google Scholar]
- Ehrlich H. J., Grinnell B. W., Jaskunas S. R., Esmon C. T., Yan S. B., Bang N. U. Recombinant human protein C derivatives: altered response to calcium resulting in enhanced activation by thrombin. EMBO J. 1990 Aug;9(8):2367–2373. doi: 10.1002/j.1460-2075.1990.tb07411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmon C. T., Taylor F. B., Jr, Hinshaw L. B., Chang A., Comp P. C., Ferrell G., Esmon N. L. Protein C, isolation and potential use in prevention of thrombosis. Dev Biol Stand. 1987;67:51–57. [PubMed] [Google Scholar]
- Esmon C. T. The roles of protein C and thrombomodulin in the regulation of blood coagulation. J Biol Chem. 1989 Mar 25;264(9):4743–4746. [PubMed] [Google Scholar]
- Esmon N. L., DeBault L. E., Esmon C. T. Proteolytic formation and properties of gamma-carboxyglutamic acid-domainless protein C. J Biol Chem. 1983 May 10;258(9):5548–5553. [PubMed] [Google Scholar]
- Fujinaga M., James M. N. Rat submaxillary gland serine protease, tonin. Structure solution and refinement at 1.8 A resolution. J Mol Biol. 1987 May 20;195(2):373–396. doi: 10.1016/0022-2836(87)90658-9. [DOI] [PubMed] [Google Scholar]
- Fujinaga M., Sielecki A. R., Read R. J., Ardelt W., Laskowski M., Jr, James M. N. Crystal and molecular structures of the complex of alpha-chymotrypsin with its inhibitor turkey ovomucoid third domain at 1.8 A resolution. J Mol Biol. 1987 May 20;195(2):397–418. doi: 10.1016/0022-2836(87)90659-0. [DOI] [PubMed] [Google Scholar]
- Galvin J. B., Kurosawa S., Moore K., Esmon C. T., Esmon N. L. Reconstitution of rabbit thrombomodulin into phospholipid vesicles. J Biol Chem. 1987 Feb 15;262(5):2199–2205. [PubMed] [Google Scholar]
- Grütter M. G., Priestle J. P., Rahuel J., Grossenbacher H., Bode W., Hofsteenge J., Stone S. R. Crystal structure of the thrombin-hirudin complex: a novel mode of serine protease inhibition. EMBO J. 1990 Aug;9(8):2361–2365. doi: 10.1002/j.1460-2075.1990.tb07410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins D. L., Lewis S. D., Shafer J. A. Steady state kinetic parameters for the thrombin-catalyzed conversion of human fibrinogen to fibrin. J Biol Chem. 1983 Aug 10;258(15):9276–9282. [PubMed] [Google Scholar]
- Hofsteenge J., Taguchi H., Stone S. R. Effect of thrombomodulin on the kinetics of the interaction of thrombin with substrates and inhibitors. Biochem J. 1986 Jul 1;237(1):243–251. doi: 10.1042/bj2370243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janin J., Chothia C. Stability and specificity of protein-protein interactions: the case of the trypsin-trypsin inhibitor complexes. J Mol Biol. 1976 Jan 15;100(2):197–211. doi: 10.1016/s0022-2836(76)80148-9. [DOI] [PubMed] [Google Scholar]
- Kraut J. How do enzymes work? Science. 1988 Oct 28;242(4878):533–540. doi: 10.1126/science.3051385. [DOI] [PubMed] [Google Scholar]
- Mann K. G., Jenny R. J., Krishnaswamy S. Cofactor proteins in the assembly and expression of blood clotting enzyme complexes. Annu Rev Biochem. 1988;57:915–956. doi: 10.1146/annurev.bi.57.070188.004411. [DOI] [PubMed] [Google Scholar]
- Musci G., Berliner L. J., Esmon C. T. Evidence for multiple conformational changes in the active center of thrombin induced by complex formation with thrombomodulin: an analysis employing nitroxide spin-labels. Biochemistry. 1988 Jan 26;27(2):769–773. doi: 10.1021/bi00402a042. [DOI] [PubMed] [Google Scholar]
- Navia M. A., McKeever B. M., Springer J. P., Lin T. Y., Williams H. R., Fluder E. M., Dorn C. P., Hoogsteen K. Structure of human neutrophil elastase in complex with a peptide chloromethyl ketone inhibitor at 1.84-A resolution. Proc Natl Acad Sci U S A. 1989 Jan;86(1):7–11. doi: 10.1073/pnas.86.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni F., Konishi Y., Frazier R. B., Scheraga H. A., Lord S. T. High-resolution NMR studies of fibrinogen-like peptides in solution: interaction of thrombin with residues 1-23 of the A alpha chain of human fibrinogen. Biochemistry. 1989 Apr 4;28(7):3082–3094. doi: 10.1021/bi00433a052. [DOI] [PubMed] [Google Scholar]
- Owen W. G., Esmon C. T., Jackson C. M. The conversion of prothrombin to thrombin. I. Characterization of the reaction products formed during the activation of bovine prothrombin. J Biol Chem. 1974 Jan 25;249(2):594–605. [PubMed] [Google Scholar]
- Owen W. G. Evidence for the formation of an ester between thrombin and heparin cofactor. Biochim Biophys Acta. 1975 Oct 20;405(2):380–387. doi: 10.1016/0005-2795(75)90103-8. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Behringer R. R., Quaife C. J., Maxwell F., Maxwell I. H., Brinster R. L. Cell lineage ablation in transgenic mice by cell-specific expression of a toxin gene. Cell. 1987 Jul 31;50(3):435–443. doi: 10.1016/0092-8674(87)90497-1. [DOI] [PubMed] [Google Scholar]
- Rydel T. J., Ravichandran K. G., Tulinsky A., Bode W., Huber R., Roitsch C., Fenton J. W., 2nd The structure of a complex of recombinant hirudin and human alpha-thrombin. Science. 1990 Jul 20;249(4966):277–280. doi: 10.1126/science.2374926. [DOI] [PubMed] [Google Scholar]
- Steitz T. A., Henderson R., Blow D. M. Structure of crystalline alpha-chymotrypsin. 3. Crystallographic studies of substrates and inhibitors bound to the active site of alpha-chymotrypsin. J Mol Biol. 1969 Dec 14;46(2):337–348. doi: 10.1016/0022-2836(69)90426-4. [DOI] [PubMed] [Google Scholar]
- Straughn W., 3rd, Wagner R. H. A simple method for preparing fibrinogen. Thromb Diath Haemorrh. 1966 Jul 31;16(1):198–206. [PubMed] [Google Scholar]
- Tsiang M., Lentz S. R., Dittman W. A., Wen D., Scarpati E. M., Sadler J. E. Equilibrium binding of thrombin to recombinant human thrombomodulin: effect of hirudin, fibrinogen, factor Va, and peptide analogues. Biochemistry. 1990 Nov 27;29(47):10602–10612. doi: 10.1021/bi00499a005. [DOI] [PubMed] [Google Scholar]
- Walker F. J., Sexton P. W., Esmon C. T. The inhibition of blood coagulation by activated Protein C through the selective inactivation of activated Factor V. Biochim Biophys Acta. 1979 Dec 7;571(2):333–342. doi: 10.1016/0005-2744(79)90103-7. [DOI] [PubMed] [Google Scholar]


