Abstract
Major research efforts are directed towards the development of a better antimycobacterial vaccine. But progress in the field of tuberculosis vaccine development has been hampered by the lack of human in vitro models to assess vaccine immunogenicity and efficacy. New candidate vaccines will have to be evaluated against the existing Mycobacterium bovis BCG “gold standard.” It is therefore important to understand the type of immune responses elicited by BCG vaccination to enable comparisons with potential new candidates. We used a novel human in vitro whole-blood model, which measures immune responses to mycobacteria by use of reporter gene-tagged BCG (BCG lux), to study immune responses to BCG vaccination in 50 neonates in a setting in Cape Town, Republic of South Africa, where tuberculosis is endemic. BCG vaccination significantly reduced growth of BCG lux in whole blood (prevaccination median growth ratio [GR], 9.6; range, 1.3 to 24; postvaccination median GR, 3.9; range, 0.6 to 12.2 [P < 0.0001]). Growth of BCG lux was better restricted in vaccinated infants than in unvaccinated age-matched controls (n = 4). BCG vaccination induced significantly higher gamma interferon production in response to BCG lux (P < 0.0001) and to purified protein derivative (P = 0.0001). No significant changes in either growth of BCG lux or cytokine production occurred in an adult control group (n = 6) over the study period. The whole-blood luminescence model detects changes in cellular immune responses to mycobacteria induced by BCG vaccination. It is therefore a useful new tool in studying the immunogenicity of newly developed vaccine candidates prior to large field trials assessing efficacy.
A major research program conducted over the last 10 years has focused on the construction of new tuberculosis vaccine candidates and their evaluation in experimental model systems (36). The most promising candidates are now being tested in phase I clinical trials to determine their safety in humans. A major roadblock to the development of new tuberculosis vaccines is the design of phase II immunogenicity trials to identify those candidates that are suitable for evaluation in costly efficacy trials (23). The complexity of efficacy trials is illustrated by the history of the current tuberculosis vaccine, Mycobacterium bovis bacillus Calmette-Guérin (BCG), an attenuated variant of M. bovis (34). Since the vaccine's introduction in 1921, a series of BCG efficacy trials have recorded a wide variation in protective efficacy (9). Although BCG vaccination confers little or no protection against the common adult forms of pulmonary tuberculosis in countries where the disease is endemic, its ability to reduce severe childhood forms of the disease has resulted in BCG becoming a major component of childhood vaccination programs (7). New tuberculosis vaccines will be tested against a background of prior BCG vaccination.
The immune response to BCG vaccination has traditionally been monitored by the tuberculin skin test, measuring delayed-type hypersensitivity (DTH) to intradermal inoculation of purified protein derivative (PPD), a crude mixture of antigenic proteins from Mycobacterium tuberculosis (2). Tuberculin sensitivity is also induced by exposure to M. tuberculosis itself, and previously sensitized individuals are generally excluded from BCG vaccination programs. The tuberculin reaction is mediated by recruitment of antigen-specific T lymphocytes (3, 35). Although T cells are clearly important mediators of the immune response to tuberculosis, conversion to PPD positivity does not correlate with induction of protective immunity (10). In well-characterized trials of the BCG vaccine in the United Kingdom, for example, the incidence of disease was similar for groups showing a positive or a negative PPD response after BCG vaccination (12). More recently, production of gamma interferon (IFN-γ) by peripheral blood T cells stimulated by mycobacterial antigens in vitro has been used as a measure of exposure to M. tuberculosis infection or BCG vaccination (4, 20, 29). With the selection of strain-specific antigens, this approach provides an important increase in specificity in comparison to that of the tuberculin test (8), but while there is extensive evidence that IFN-γ-secreting T cells are an essential component of immunity, it remains to be determined whether the ability of a vaccine to prime such cells is correlated with its protective efficacy. It is therefore important to include additional measures of immunogenicity in clinical trials of new tuberculosis vaccines.
Mycobacteria reside and multiply within macrophages. The ability of macrophages to upregulate mycobactericidal mechanisms appears to be fundamental to the successful containment of these organisms, since individuals with genetically determined defects in macrophage function are highly susceptible to overwhelming mycobacterial infections (5, 17, 26). We reasoned that the ability to limit the growth of mycobacteria represents a key functional component of a successful immune response.
We have developed a simple whole-blood model that measures the response to in vitro challenge with a reporter strain of BCG (18). The main output of this assay is the luminescence signal provided by a recombinant luciferase enzyme which is directly related to the numbers and viability of the bacteria. Luminescence is proportional to the number of CFU in exponentially growing cultures, and it declines when metabolic activity is reduced in response to signals induced by environmental stress or antibiotic treatment. In the whole-blood assay, changes in luminescence over 4 days of incubation vary between individuals; reduced luminescence is observed in samples from individuals who have previously been exposed to mycobacteria (as judged by a positive PPD or IFN-γ response). T-cell-depletion experiments and assays in human immunodeficiency virus (HIV)-positive cohorts demonstrate that IFN-γ-secreting CD4+ T cells are a major determinant of the fate of the reporter bacteria in the whole-blood assay (33). In earlier works by Cheon et al. and Hoft et al., it has previously been shown, using peripheral blood mononuclear cell-based assays, whole blood, and BACTEC cultures (6, 15), that vaccination with BCG leads to reduction of in vitro growth of mycobacteria in adults. So far, no studies have been published assessing the use of these assays in the context of BCG vaccination in children, who are the primary recipients of BCG vaccinations worldwide.
The aim of the present study was to assess the feasibility of utilizing the whole-blood luminescence assay in future phase II trials of the new generation of tuberculosis vaccines. We describe the use of the assay to monitor the effect of BCG vaccination in a cohort of 50 infants in a setting where tuberculosis is endemic, and we compare the results with those of tuberculin testing and measurements of IFN-γ responses.
MATERIALS AND METHODS
Human subjects. (i) Healthy newborns.
Fifty full-term, healthy infants (29 males and 21 females) of HIV-negative mothers were recruited within 12 h of birth at the Vanguard Maternity Obstetric Unit in Cape Town, Republic of South Africa. All mothers had undergone antenatal HIV testing during pregnancy as part of the local policy, and only HIV-negative mothers were enrolled in the study. Other exclusion criteria were an infant birth weight below 2.5 kg, a recent history of tuberculosis in the mother, occurrence of fever during delivery, and chronic underlying illness of the mother, such as diabetes.
Venous blood (4 ml) from enrolled healthy babies was collected in sterile, heparinized tubes prior to BCG vaccination. The clinic staff administered 0.05 ml of the BCG vaccine SSI (Danish strain 1331, 0.75 mg/ml; Staten Serum Institute, Copenhagen, Denmark) via intradermal injection to each baby on the first day of life, prior to discharge from the Maternity Obstetric Unit.
At 3 to 6 months of age, a follow-up blood sample was taken, and tuberculin skin testing was carried out in the homes of the enrolled infants by intradermally injecting 5 U of PPD (RTI; Staten Serum Institute) into the volar aspect of the left lower forearm of each infant. Induration was assessed 48 to 72 h later by the research nurse (S.M.) Scar formation in response to BCG vaccination, as well as any recent exposure to tuberculosis, was recorded.
(ii) Control groups. (a) Infant control group.
South Africa, the Western Cape in particular, is an area with an extremely high incidence of tuberculosis (630 of 100,000 population [6a]). We deemed it unethical to deliberately withhold BCG vaccination from the infants from this area in order to obtain a sizeable control group, as these infants would be at increased risk of contracting tuberculosis at a very early age. However, within the study group, two infants missed their BCG vaccination for logistical reasons. We recruited two more age-matched infants from the same community who had also missed their BCG vaccination. This group is referred to as the unvaccinated control group (n = 4). As this control group is significantly smaller than the vaccinated study group, we have not conducted a statistical analysis of differences in growth of luciferase-tagged BCG (BCG lux) in whole blood and production of IFN-γ in response to BCG and PPD but have simply displayed the data in the relevant graphs.
(b) Adult control group.
Whole blood from a healthy adult volunteer was used with each assay conducted in the laboratory to monitor potential changes in the assays over time. The individuals involved are collectively referred to as the adult control group. All had received BCG vaccination during infancy, two were PPD negative (induration less than 5 mm in diameter), and four were PPD positive (induration greater than 10 mm) but without any signs of active tuberculosis (n = 6; ages, 23 to 40; female-to-male ratio, 4:2).
Whole-blood assays.
For whole-blood assays, all blood samples were processed in the laboratory within 4 h.
(i) Whole-blood luminescence assay.
The whole-blood assay utilizing BCG lux, along with the construction and properties of the reporter strain, has previously been described (18). Briefly, BCG (Montreal strain) was transformed with the shuttle plasmid pSMT1 carrying the luxA and luxB genes from Vibrio harveyi under control of the mycobacterial hsp60 promoter (13). Luminescence was measured in serial 10-fold dilutions over a 20-s period, and results are expressed as relative light units (RLU). A reading of 10 RLU was found to correspond to 1 CFU, as previously shown (18, 32). This reporter strain was used for infection of whole-blood cultures as described below.
Triplicate samples of heparinized venous blood (0.5 ml), diluted with an equal volume of RPMI medium, were infected with 107 RLU/ml (equal to 106 CFU/ml) of BCG lux and incubated for 96 h, with continuous mixing of the samples. Following lysis of red blood cells, luminescence was measured at the time of inoculation (T0) and at 96 h (T96) with a luminometer (Berthold), as previously described, and a growth ratio (GR) was calculated as follows: GR = RLU of BCG lux at T96/RLU of BCG lux at T0.
All whole-blood luminescence assays were conducted immediately before BCG vaccination and again 3 to 6 months following vaccination. Blood samples from healthy adult volunteers were set up for testing at the same time as the infant samples in order to assess variability in all assays over the period of the study.
Supernatants for cytokine assays were harvested from whole-blood cultures inoculated with BCG lux at 24 and 96 h prior to lysis of red blood cells and stored at −70°C.
The 24-h supernatants were used for measurements of tumor necrosis factor alpha (TNF-α), and the 96-h supernatants were used for measurements of IFN-γ produced during incubation of BCG lux in whole blood.
(ii) Whole-blood assay to measure production of IFN-γ in response to PHA and PPD.
Venous blood samples (0.25 ml) were diluted 1:10 with RPMI medium (Life Technologies, Ltd., Paisley, United Kingdom) and stimulated with phytohemagglutinin (PHA) (5 μg/ml; Sigma, Poole, United Kingdom) or PPD (10 μg/ml; Evans Medical Limited, Leatherhead, United Kingdom) in 96-well tissue culture plates in a CO2 incubator set at 37°C. Supernatants were harvested at 3 and 6 days, immediately frozen at −70°C, and subsequently used for measurements of cytokine production as described below.
Cytokine measurement.
Aliquots of supernatants for cytokine assays were thawed in batches, and serial dilutions were prepared in phosphate-buffered saline-10% fetal calf serum (Life Technologies). IFN-γ and TNF-α were measured by enzyme-linked immunosorbent assay (ELISA) with antibody pairs purchased from Pharmingen (Becton Dickinson, Oxford, United Kingdom). Measurements were performed according to the manufacturer's recommended protocols. Standard curves were generated with reference cytokines provided by Pharmingen. For each of the cytokines, the detection limit was 50 pg/ml, and a linear response was observed for amounts up to 5,000 pg/ml. Results are expressed as the mean of duplicate readings for each sample.
Statistical methods.
All statistical analyses were performed with the PRISM program (GraphPad Software, San Diego, Calif.). Nonparametric paired and unpaired statistical analyses (Wilcoxon matched pairs test and the Mann-Whitney test) were used to compare medians of GRs and cytokine levels between groups of vaccinated infants and adult controls. Spearman's rank correlation coefficients were calculated for correlation analysis. A P value of less than 0.05 was considered statistically significant.
RESULTS
Follow-up.
Of the 50 babies initially enrolled in the study, 35 (66%) could be traced at the follow-up time point. Two babies had missed BCG vaccination and were enrolled in the infant control group; all other infants had a demonstrable scar. There were no adverse effects of BCG vaccination in this study group. PPD skin testing revealed that two babies had indurations of less than 5 mm; the rest remained skin test negative despite BCG vaccination. To the best of our knowledge, no infant had been exposed to acute tuberculosis in his or her household between birth and the follow-up visit.
Growth of BCG lux in the blood of infants pre- and post-BCG vaccination.
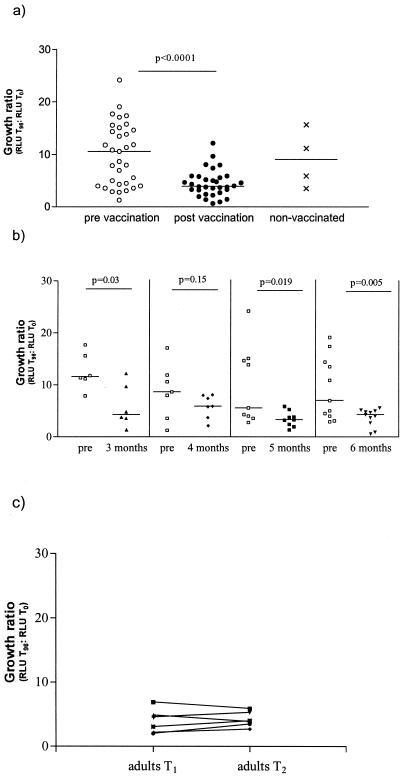
Significant differences in luminescence output were observed in the whole-blood assay of 33 infants pre- and post-BCG vaccination. The median GR prevaccination was 9.6 (range, 1.3 to 24). A significantly reduced GR was observed for blood samples obtained after vaccination; the median GR was 3.9 (range, 0.6 to 12.2 [P < 0.0001, Wilcoxon matched pairs test]). Figure 1a illustrates these findings. We further stratified our results depending on the interval between pre- and postvaccination in order to establish whether any age-related differences of in vitro control of growth of BCG could be found. The second blood samples were collected from 6 infants at 3 months, from 7 at 4 months, from 9 at 5 months, and from 11 at 6 months following the initial BCG vaccination. There was no statistically significant difference between the different age groups in either GRs at baseline or in GRs following BCG vaccination. Apart from the group studied at 4 months following BCG vaccination, all other groups of infants showed a significant reduction in GRs compared to baseline values, as shown in Fig. 1b.
FIG. 1.
Growth of BCG lux in whole blood from infants pre- and post-BCG vaccination and from unvaccinated infant controls (a), in vaccinated infants according to age at the time of the second blood sampling (b), and in adult controls over the entire study period (c). Three groups of individuals were studied: infants tested prevaccination (n = 33) (○) and again 3 to 6 months postvaccination (n = 33) (•), unvaccinated control infants (n = 4) (×) tested at time of postvaccination assessment of the vaccinated infants, and adult controls (n = 6) tested at time points T1 (▴) and T2 (▾). Triplicate whole-blood samples were infected with BCG lux. Growth of BCG lux was measured at 96 h and expressed as a GR of RLU of BCG lux at 96 h to RLU of BCG lux in inoculum.
We tested a group of six adult controls in parallel with the infants to establish that the changes in growth of BCG lux in whole blood were induced by BCG vaccination and were not secondary to variations within the assay over time. There was no significant change in GR among the adult controls at the two assay time points (T1 and T2) (median GR at T1, 3.8; range, 2 to 6.9; median GR at T2, 3.95; range, 2.7 to 5.9 [P = 0.55]), as shown in Fig. 1c. The postvaccination GR of the infant group was similar to that of the group of the adult controls.
Our results are consistent with an increased ability in infants for growth of the challenge strain to be controlled in the postvaccination samples. We were unable to fully evaluate the possible effect of age-related maturation of the immune response, since the increased risk of disease made it unethical to withhold vaccination from a control group of children. However, two infants from the original trial group missed vaccination, and two additional age-matched nonvaccinated children from the same community were available for study at the follow-up time point. The median GR in this group of four infants was 9.0 (range, 3.5 to 15.7), which is higher than that of the vaccinated group at the same age (median, 3.9; range, 0.6 to 12.2), but there clearly is overlap between the data sets. Nevertheless, these results suggest that changes in the whole-blood assay are predominantly the effect of vaccination rather than of ageing, since no differences in GRs depending on age were demonstrable in the vaccinated group.
Production of IFN-γ in whole blood during stimulation with BCG lux.
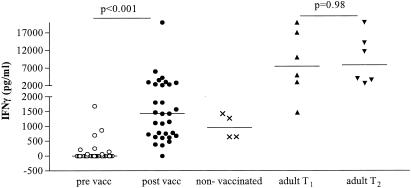
Supernatants harvested from whole blood inoculated with BCG lux were tested for cytokine production. The production levels of IFN-γ after 96 h of in vitro challenge with BCG lux differed significantly (P < 0.0001) pre- and postvaccination (Fig. 2). For infants, IFN-γ was undetectable in most samples before vaccination (median, 0 pg/ml; range, 0 to 1,681 pg/ml) but increased to a median of 1,427 pg/ml postvaccination (range, 0 to 20,000 pg/ml). There was no significant difference in the levels of IFN-γ in response to BCG lux in infants between the ages of 3 and 6 months post-BCG vaccination (data not shown). In the unvaccinated infants, lower levels of IFN-γ production were observed at the follow-up time point (median, 953 pg/ml; range, 636 to 1,344 pg/ml). In adults, levels of IFN-γ did not differ significantly between the two time points (median at T1, 7,472 pg/ml; range, 1,478 to 20,000 pg/ml; median at T2, 9,807 pg/ml; range, 2,702 to 20,000 pg/ml [P = 0.97]) but were significantly higher than the levels in the vaccinated infants (P = 0.003, Mann-Whitney test).
FIG. 2.
Production of IFN-γ in whole blood during growth of BCG lux in infants pre- and post-BCG vaccination, unvaccinated infants, and adult controls. Triplicate whole-blood samples of infants pre (○)- and post (•)-BCG vaccination (n = 33), unvaccinated control infants (×) (n = 4), and adult controls (T1 [▴], n = 6; T2 [▾], n = 6) were infected with BCG lux, and supernatants were harvested at 96 h. IFN-γ was measured in these supernatants by ELISA.
Production of TNF-α in whole blood during stimulation with BCG lux.
The production of TNF-α at 24 h in response to stimulation with BCG lux in whole blood also increased following vaccination (prevaccination median, 2,269 pg/ml; range, 323 to 13,228 pg/ml; postvaccination median, 3,955 pg/ml; range, 80 to 14,744 pg/ml), but the difference was not significant (P = 0.09). There were no differences between TNF-α production levels in vaccinated and unvaccinated infants (unvaccinated control group median at T2, 5,908 pg/ml; range, 3,621 to 8,660 pg/ml) or in TNF-α levels between the infant cohort and the adult controls (P = 0.54) (adult group median at T1, 2,868 pg/ml; range, 1,066 to 4,546 pg/ml; median at T2, 3,224 pg/ml; range, 1,478 to 20,000 pg/ml [P = 0.16]).
Production of IFN-γ in response to PHA.
IFN-γ production levels in response to PHA (a nonspecific T-cell stimulus) and to PPD were also measured in whole-blood samples harvested at days 3 and 6, respectively. Levels of IFN-γ produced by infants in response to PHA stimulation increased during the time of the study, but this increase did not reach significance (prevaccination median, 1,137 pg/ml; range, 0 to 20,000 pg/ml; postvaccination median, 3,952 pg/ml; range, 228 to 30,203 pg/ml [P = 0.53, Wilcoxon matched pairs]). Levels of IFN-γ produced in response to PHA were significantly higher in the adult control group (median, 20,249 pg/ml; range, 12,947-35,000 pg/ml [P = 0.002]) than in the infant group, but these levels did not change significantly during the course of the study (P = 0.31). Levels of IFN-γ in response to PHA in unvaccinated control infants did not differ from those of their vaccinated peers (median at T2, 6,697 pg/ml; range, 3,876 to 9,594 pg/ml).
Production of IFN-γ in response to PPD.
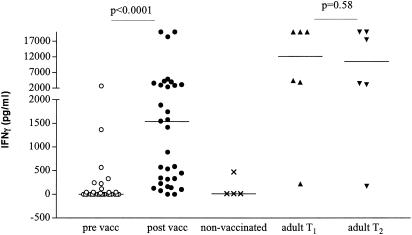
BCG vaccination induced a significant increase of production of IFN-γ in response to PPD in infants (prevaccination median, 0 pg/ml; range, 0 to 2,740 pg/ml; postvaccination median, 1,155 pg/ml; range, 274 to 20,000 pg/ml [P < 0.001, Wilcoxon matched pairs]) (Fig. 3). There was no significant difference in the levels of IFN-γ in response to PPD in infants between the ages of 3 and 6 months post-BCG vaccination (data not shown). Three of the four unvaccinated control children produced no IFN-γ in response to PPD, and the fourth child showed a response of 530 pg/ml. We cannot exclude the possibility that this infant was sensitized to mycobacteria at this early age, although the skin test was negative. The lack of IFN-γ production in the other three unvaccinated children is indicative of an antigen-specific effect of BCG in the vaccinated group. In adults, the IFN-γ levels measured at the two time points did not differ significantly (P = 0.58) but were significantly higher than the levels measured in infants (median, 10,514 pg/ml; range, 179 to 20,000 pg/ml [P = 0.039]).
FIG. 3.
Production of IFN-γ in response to PPD in whole blood of infants pre- and post-BCG vaccination, unvaccinated infants, and adult controls. Triplicate whole-blood samples of infants pre (○)- and post (•)-BCG vaccination (n = 33), unvaccinated control infants (×) (n = 4), and adult controls (T1 [▴], n = 6; T2 [▾], n = 6) were diluted 1:10 with RPMI medium and stimulated with PPD (10 μg/ml). Supernatants were harvested at day 6, and IFN-γ was measured by ELISA.
Correlation between assays.
BCG vaccination was associated with increased production of IFN-γ in response to PPD stimulation or BCG challenge and with an increased ability to restrict growth of the BCG reporter strain. To test for correlations between these parameters, Spearman's rank correlation coefficients were calculated between GRs for individual children and levels of IFN-γ production. No significant correlations were observed between GRs and IFN-γ production in response to both BCG (r = 0.09, P = 0.64) and PPD (r = −0.045, P = 0.8). In order to study a potential influence of age and immune response maturation on correlations between the assays, we also analyzed correlations between GRs and production of IFN-γ in response to BCG and PPD according to age groups. There were no significant correlations between GRs and IFN-γ production levels in response to BCG at 3 (r = −0.9, P = 0.08), 4 (r = −0.1, P = 0.83,), 5 (r = 0.5, P = 0.177), or 6 (r = −0.006, P = 1.0, Spearman's rank correlation coefficients) months. Similarly, no significant correlations existed when GRs and the production levels of IFN-γ in response to PPD at 3 (r = −0.3, P = 0.68), 4 (r = −0.21, P = 0.66), 5 (r = 0.66, P = 0.059), and 6 (r = −0.2, P = 0.58) months were compared. This finding suggests that IFN-γ production is not the sole determinant of the luminescence signal measured in the whole-blood assay for young children, and no significant correlations can be observed independent of the age of the infants studied between 3 and 6 months following BCG vaccination. In contrast, and in accordance with our previous data from studying healthy adults, a significant negative correlation exists between GRs and production of IFN-γ in response to BCG lux (r = −0.94, P = 0.01) and, to a lesser extent, to PPD (r = −0.7, P = 0.13) in the adult control group.
DISCUSSION
In our study, we demonstrated the immunogenicity of BCG vaccine in neonates by measuring restriction of mycobacterial growth in vitro, as well as by measuring antigen-specific production of IFN-γ.
The findings of the present study are consistent with previous reports showing that neonatal vaccination with BCG primes an antigen-specific population of IFN-γ-producing T cells (22). For ethical reasons, we did not include a control group in which BCG vaccination was purposely withheld, and it is possible that the increase in IFN-γ production postvaccination reflects in part some age-related maturation of the immune response. We did not, however, observe an age-related trend in the production of IFN-γ between 3 and 6 months, and lower IFN-γ responses were observed in the small, age-matched group of unvaccinated children living in the same community as our vaccine trial cohort. No age-related trend in IFN-γ production was described in the vaccine studies of Marchant et al. (22), in which a similar age group was investigated. In our study, no significant differences were observed pre- and post-BCG vaccination in terms of antigen-nonspecific responses elicited by PHA, further suggesting an antigen-specific response rather than a maturation effect. Immune responses to BCG vaccination are poorly characterized in newborn children, but several recent publications have described involvement of Th1-type and cytotoxic T cells (16), as well as the successful induction of T-cell memory (14), even in newborns (28). It appears, therefore, that despite its immaturity, the neonatal immune system is capable of mounting potentially protective immune responses. In recent publications, the levels of IFN-γ in response to mycobacterial or other antigens measured in young children are widely scattered, similar to our own observations. It has been suggested that prenatal sensitization might also play a role in this phenomenon (21, 25).
Interestingly, the majority of infants in our study remained tuberculin skin test negative 3 to 6 months postvaccination, despite the presence of a good scar and demonstrable antigen-specific IFN-γ production in whole blood. This finding is in contrast to skin test results in older children or adults, where the majority of individuals show positive PPD responses following BCG vaccination (4). Very few studies have systematically evaluated DTH responses over different age groups in young children, and generally all report low responses at an early age (19, 27, 30). Poor correlation with protective efficacy has also been demonstrated (1). It is possible that the known immaturity of dendritic cells in young infants prevents the development of DTH responses at this early age (11, 31). Therefore, skin test responses may not be a good marker for studying the immunogenicity of new tuberculosis vaccines in young children.
Neonatal BCG vaccination was also associated with a significant increase in the ability to control in vitro growth of a challenge strain of BCG assessed by the decreased GR in the whole-blood assay. Prevaccination GRs were significantly higher in infants than in adults, possibly because of the absence of a T-cell memory pool in response to mycobacterial antigens in the infants. This is in keeping with previous publications, which have shown higher GRs in adults who have not been previously exposed to mycobacterial antigens, as assessed by tuberculin reactivity (18), and also in HIV-infected children, who lack immunocompetent CD4+ T cells by the nature of their underlying immunodeficiency (33). Clinically, these children are highly susceptible to tuberculosis. As discussed above for the IFN-γ responses, the design of the present study does not allow us to completely exclude a possible contribution of age-related effect independent of the vaccination. However, stratification of our data according to age in the second set of assays did not show an age-related effect between 3 and 6 months. Theoretically, the possibility still exists that by 3 months of age, maximal immune maturation and/or background priming from environmental antigens has already occurred. Results from studies in our laboratory assessing the effect of age on the ability to restrict growth of BCG lux in vitro did not show significant differences for cohorts of BCG-vaccinated children above and below 1 year of age either, but few children in this study group were less than 3 months of age (B. Kampmann, unpublished data).
This study is important because it demonstrates the feasibility of using the whole-blood luminescence assay to monitor the effect of vaccination in a setting where tuberculosis is highly endemic and that is suitable for trials of new tuberculosis vaccines. The assay format is suitable for the use of BCG or virulent M. tuberculosis as a reporter strain, though the BCG format used here is simpler in terms of safety and in its independence from the requirement for containment facilities. Significant correlations between IFN-γ production and GRs were found only in the adult control group, not in the infant group. This confirms our previous results, which showed that IFN-γ-producing T cells are important mediators of the responses measured by luminescence output in adults. However, the absence of a simple correlation between GR and levels of IFN-γ in children from the ages of 3 to 6 months following BCG vaccination suggests that the whole-blood luminescence assay provides more information about immune status than can be obtained by measurement of antigen-specific production of IFN-γ. The ability to restrict mycobacterial growth represents a functional component of the complex host-pathogen interactions and is a potential surrogate marker of protective immune responses to mycobacteria. The assay described here may therefore represent an important addition to the set of tests used to evaluate the immunogenicity of the new generation of tuberculosis vaccine candidates currently entering clinical trials (24). The ability to reduce GRs below the level achieved by BCG alone would represent an encouraging criterion in evaluation of new vaccine candidates.
Acknowledgments
B. Kampmann is supported by a Wellcome Trust Training Fellowship (056608). Wellcome Trust Program Grants to M. Levin (059141) and to D. Young (038997) also contributed to this work.
We thank the mothers who allowed us to enroll their babies into a study on the first day of their lives.
Informed consent was obtained from all healthy volunteers and parents of children enrolled in these studies, and the human experimentation guidelines of the Imperial College School of Medicine and the University of Cape Town were followed in the conduct of this research.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.al-Kassimi, F. A., M. S. al-Hajjaj, I. O. al-Orainey, E. A. Bamgboye. 1995. Does the protective effect of neonatal BCG correlate with vaccine-induced tuberculin reaction? Am. J. Respir. Crit. Care Med. 152:1575-1578. [DOI] [PubMed] [Google Scholar]
- 2.American Thoracic Society. 1981. The tuberculin skin test. Am. Rev. Respir. Dis. 124:356-363. [Google Scholar]
- 3.Beck, J. S., S. M. Morley, and J. H. Gibbs. 1986. The cellular responses of tuberculosis and leprosy patients and of healthy controls in skin tests to new tuberculin and leprosin. Clin. Exp. Immunol. 64:484-494. [PMC free article] [PubMed] [Google Scholar]
- 4.Black, G. F., R. E. Weir, S. Floyd, L. Bliss, D. K. Warndorff, A. C. Crampin, B. Ngwira, L. Sichali, B. Nazareth, J. M. Blackwell, K. Branson, S. D. Chaguluka, L. Donovan, E. Jarman, E. King, P. E. Fine, and H. M. Dockrell. 2002. BCG-induced increase in interferon-gamma response to mycobacteria antigens and efficacy of BCG vaccination in Malawi and the UK: two randomised controlled studies. Lancet 359:1393-1401. [DOI] [PubMed] [Google Scholar]
- 5.Casanova, J. L., and L. Abel. 2002. Genetic dissection of immunity to mycobacteria: the human model. Annu. Rev. Immunol. 20:581-620. [DOI] [PubMed] [Google Scholar]
- 6.Cheon, S. H., B. Kampmann, A. G. Hise, M. Phillips, H.-Y. Song, K. Landen, Q. Li, R. Larkin, J. J. Ellner, R. F. Silver, D. F. Hoft, and R. S. Wallis. 2002. Bactericidal activity in whole blood as a potential surrogate marker of immunity after vaccination against tuberculosis. Clin. Diagn. Lab. Immunol. 9:901-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6a.City of Cape Town. City of Cape Town TB Control Programme Report 1997-2002. [Online.] www.who.int/gtb/TBHIV/Durban_feb03/mon/mon6_naidoo_wcape_sa.ppt.
- 7.Colditz, G. A., C. S. Berkey, F. Mosteller, M. Brewer, M. E. Wilson, E. Burdick, and H. V. Fineberg. 1995. The efficacy of bacillus Calmette-Guerin vaccination of newborns and infants in the prevention of tuberculosis: meta-analyses of the published literature. Pediatrics 96:29-35. [PubMed] [Google Scholar]
- 8.Ewer, K., J. Deeks, L. Alvarez, G. Bryant, S. Waller, P. Andersen, P. Monk, and A. Lalvani. 2003. Comparison of T-cell-based assay with tuberculin skin test for diagnosis of Mycobacterium tuberculosis infection in a school outbreak. Lancet 361:1168-1173. [DOI] [PubMed] [Google Scholar]
- 9.Fine, P. E. M. 1995. Variations in protection by BCG: implications of and for protective immunity. Lancet 346:1339-1345. [DOI] [PubMed] [Google Scholar]
- 10.Fine, P. E. M., J. A. C. Sterne, J. M. Ponninghaus, and R. J. Rees 1994. Delayed-type hypersensitivity, mycobacterial vaccines and protective immunity. Lancet 344:1245-1249. [DOI] [PubMed] [Google Scholar]
- 11.Goriely, S., B. Vincart, P. Stordeur, F. Vekemans, F. Willems, M. Goldman, and D. de Wit. 2001. Deficient IL-12 (p35) gene expression by dendritic cells derived from neonatal monocytes. J. Immunol. 166:2141-2146. [DOI] [PubMed] [Google Scholar]
- 12.Hart, P. D. A., and I. Sutherland. 1977. BCG and vole bacillus in the prevention of tuberculosis in adolescence and early adult life: final report to the Medical Research Council. Br. Med. J. 2:293-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hickey, M. J., T. M. Arain, R. M. Shawar, D. J. Humble, M. H. Langhorne, J. N. Morgenroth, and C. K. Stover. 1996. Luciferase in vivo expression technology: use of recombinant mycobacterial reporter strains to evaluate antimycobacterial activity in mice. Antimicrob. Agents Chemother. 40:400-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoft, D. F., R. M. Brown, and S. T. Rodman. 1997. Bacille Calmette-Guerin vaccination enhances human γ/δ T cell responsiveness to mycobacteria suggestive of a memory-like phenotype. J. Immunol. 161:1045-1054. [PubMed] [Google Scholar]
- 15.Hoft, D. F, S. Worku, B. Kampmann, C. Whalen, J. J. Ellner, C. S. Hirsch, R. B. Brown, R. Larkin, Q. Li, H. Yun, and R. F. Silver. 2002. Human BCG vaccination induces immune memory with enhanced inhibitory activity against intracellular mycobacteria. J. Infect. Dis. 186:1448-1457. [DOI] [PubMed] [Google Scholar]
- 16.Hussey, G. D., M. L. Watkins, S. Gottschalk, E. J. Hughes, K. Iloni, M. Kibel, and S. R. Ress. 2002. Neonatal mycobacterial specific cytotoxic T-lymphocyte and cytokine profiles in response to distinct BCG vaccination strategies. Immunology 105:314-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jouanguy E., F. Altare, S. Lamhamedi, P. Revy, J. F. Emile, M. Newport, M. Levin, S. Blanche, E. Seboun, A. Fischer, and J. L. Casanova. 1996. Interferon-gamma-receptor deficiency in an infant with fatal bacille Calmette-Guerin infection. N. Engl. J. Med. 335:1956-1961. [DOI] [PubMed] [Google Scholar]
- 18.Kampmann, B., P. O. Gaora, V. A. Snewin, M. P. Gares, D. B. Young, and M. Levin. 2000. Evaluation of human antimycobacterial immunity using recombinant reporter mycobacteria. J. Infect. Dis. 182:895-901. [DOI] [PubMed] [Google Scholar]
- 19.Karalliedde, S., L. P. Katugaha, and C. G. Uragoda. 1987. Tuberculin response of Sri Lankan children after BCG vaccination at birth. Tubercle 68:33-38. [DOI] [PubMed] [Google Scholar]
- 20.Lalvani, A., R. Brookes, R. J. Wilkinson, A. S. Malin, A. A. Pathan, P. Andersen, H. Dockrell, G. Pasvol, and A. V. Hill. 1998. Human cytolytic and interferon gamma-secreting CD8+ T lymphocytes specific for Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 5:270-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malhotra, I., P. Mungani, A. Wamachi, J. Kioko, J. H. Ouma, J. W. Kazura, and C. L. King. 1999. Helminth- and Bacillus Calmette-Guerin-induced immunity in children sensitized in utero to filariasis and schistosomiasis. J. Immunol. 162:6843-6848. [PubMed] [Google Scholar]
- 22.Marchant, A., T. Goettghebuer, M. O. Ota, I. Wolfe, S. J. Ceesay, D. De Groote, et al. 1999. Newborns develop a Th1-type immune response to Mycobacterium bovis Bacillus Calmette-Guerin vaccination. J. Immunol. 163:2249-2255. [PubMed] [Google Scholar]
- 23.McMurray, D. N. 2003. Recent progress in the development and testing of vaccines against human tuberculosis. Int. J. Parasitol. 33:547-554. [DOI] [PubMed] [Google Scholar]
- 24.Medaglini, D., and A. Hoeveler. 2003. The European research effort for HIV/AIDS, malaria and tuberculosis. Vaccine 21(Suppl. 2):S116-S120. [DOI] [PubMed] [Google Scholar]
- 25.Miles, E. A., J. A. Warner, A. C. Lane, A. C. Jones, B. M. Colwell, and J. O. Warner. 1994. Altered T lymphocyte phenotype at birth in babies born to atopic parents. Pediatr. Allergy Immunol. 5:202-208. [DOI] [PubMed] [Google Scholar]
- 26.Newport, M. J., C. M. Huxley, S. Huston, C. M. Hawrylowicz, B. A. Oostra, R. Williamson, and M. Levin. 1996. A mutation in the interferon-gamma-receptor gene and susceptibility to mycobacterial infection. N. Engl. J. Med. 26:1941-1949. [DOI] [PubMed] [Google Scholar]
- 27.Odujinrin, O. M., and D. A. Ogumekan. 1992. Assessment of post-vaccination tuberculin sensitivity in Lagos-Nigeria. Eur. J. Epidemiol. 8:128-131. [DOI] [PubMed] [Google Scholar]
- 28.Ota, M. O. C., J. Vekemans, S. E. Schlegel-Haueter, K. Fielding, M. Sanneh, M. Kidd, et al. 2002. Influence of Mycobacterium bovis Bacillus Calmette-Guerin on antibody and cytokine responses to human neonatal vaccination. J. Immunol. 168:919-925. [DOI] [PubMed] [Google Scholar]
- 29.Ravn, P., H. Boesen, B. K. Pedersen, and P. Andersen. 1997. Human T cell responses induced by vaccination with Mycobacterium bovis bacillus Calmette-Guerin. J. Immunol. 158:1949-1955. [PubMed] [Google Scholar]
- 30.Santiago, E. M., E. Lawson, K. Gillenwater, S. Kalangi, A. G. Lescano, G. Du Quella, K. Cummings, L. Cabrera, C. Torres, and R. H. Gilman. 2003. A prospective study of bacillus Calmette-Guerin scar formation and tuberculin skin test reactivity in infants in Lima, Peru. Pediatrics 112:e298. [Online.] htpp://pediatrics.aapublications.org/cgi/content/full/112/4/e298. [DOI] [PubMed] [Google Scholar]
- 31.Smith, S., R. F. Jacobs, and C. B. Wilson. 1997. Immunobiology of childhood tuberculosis: a window on the ontogeny of cellular immunity. J. Pediatr. 131:16-26. [DOI] [PubMed] [Google Scholar]
- 32.Snewin, V. A., M.-P. Gares, P. Ó. Gaora, Z. Hasan, I. Brown, and D. B. Young. 1999. Assessment of immunity to mycobacterial infection using luciferase reporter constructs. Infect. Immun. 67:4586-4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tena, G. B., D. B. Young, B. Eley, H. Henderson, M. P. Nicol, M. Levin, and B. Kampmann. 2003. Failure to control growth of mycobacteria in blood from children infected with HIV and its relationship to T cell function. J. Infect. Dis. 187:1544-1551. [DOI] [PubMed] [Google Scholar]
- 34.Wang, J., and Z. Xing. 2002. Tuberculosis vaccines: the past, present and future. Expert Rev. Vaccines 1:341-354. [DOI] [PubMed] [Google Scholar]
- 35.Youmans, G. P. 1975. Relationship between delayed hypersensitivity and immunity in tuberculosis. Am. Rev. Respir. Dis. 111:109. [DOI] [PubMed] [Google Scholar]
- 36.Young, D. 2003. Ten years of research progress and what's to come. Tuberculosis 83:77-78. [DOI] [PubMed] [Google Scholar]