Abstract
Shiga toxin (Stx) binding sites in porcine tissues and leukocytes were identified by the use of Stx overlay and anti-CD77/Gb3 immunoassays. Stx1 and Stx2 bound to similar tissue locations and leukocytes, although some differences were noted. Previously unreported Stx binding sites were identified in kidney tubules, intestinal lymphoid aggregates, sinusoidal liver cells, alveolar macrophages, and peripheral blood leukocytes.
Shiga toxin (Stx)-producing Escherichia coli (STEC) strains, including STEC O157:H7, cause diarrhea, hemorrhagic colitis, and hemolytic-uremic syndrome in humans (26). All STEC strains produce one or more Shiga toxins (Stx1 or Stx2) which mediate damage in the kidney, colon, and brain (26). All Stx types can bind to glycolipid receptors called globotriaosylceramides (Gb3) (26). Stx2-producing strains are more frequently associated with severe disease than are strains that produce only Stx1 or both Stx1 and Stx2 (2, 8). The mechanisms of Stx-mediated tissue damage are not fully understood.
A primary mechanism of Stx-mediated damage is direct Stx cytotoxicity for vascular endothelial (9, 24) and renal epithelial (16) cells. Proposed indirect mechanisms include Stx-induced vascular damage and ischemia (26), Stx-induced immunomodulation (5), Stx-induced activation of platelets (17), and Stx transport by leukocytes (32, 34). Several lines of evidence indicate that leukocytes are involved in Stx-mediated damage. Stx1 binds to isolated human (28, 32, 34) and bovine (30) leukocytes, and Stx binding suppresses the activation and proliferation of lymphocytes from some cattle (10, 20) and induces the production of proinflammatory mediators by human monocytes (28, 34). These mediators may contribute to endothelial cell damage by inducing the localization and activation of polymorphonuclear cells (PMN) (11) or by sensitizing endothelial cells to Stx cytotoxicity (31). Stx also binds to isolated human PMN, which may deliver Stx to target cells (32).
The identification of Stx binding sites is a key step toward elucidating the mechanisms of Stx-induced damage. Pigs are an ideal model for Stx binding studies because they are susceptible to natural (23) and experimental (12, 18, 33) Stx-mediated disease and develop typical Stx-mediated vascular lesions (6, 7, 14, 33). Pig tissues contain Gb3 and bind Stx (3, 14), and Stx binding correlates with gross tissue levels of Gb3 (3).
Information about the locations of Stx binding sites in porcine tissues is limited mostly to vascular and endothelial sites (14, 27). The only direct comparison of Stx1 and Stx2 binding to porcine tissues was done with ileal tissues (27). Stx binding to mononuclear cells in the ileal lamina propria (27), Stx toxicity for alveolar macrophages (21), and STEC-induced changes in lymphoid tissue in gnotobiotic pigs (5) provide indirect evidence that Stx also binds to porcine leukocytes. The objectives of this study were to (i) identify Stx1 and Stx2 binding sites in porcine tissues and (ii) determine if Stx binds to porcine leukocytes.
Stx binding sites and Gb3 were identified by immunoassays with frozen tissues (kidney, ileum, cerebellum, liver, spiral colon, and cecum from three pigs and jejunum from two pigs) obtained from <4-day-old cesarean section-derived, colostrum-deprived or naturally farrowed, colostrum-fed pigs. These tissues were available from pigs used for other studies at the National Animal Disease Center (NADC), Ames, Iowa. None of the pigs had been infected with Stx-producing E. coli. Frozen tissue sections were cut and fixed in undiluted acetone and then cryopreserved (27).
Stx1 and Stx2 binding sites in frozen porcine tissues were identified by use of an Stx overlay assay (27) which was modified for porcine tissues as noted below. Tissue sections were sequentially incubated with affinity-purified Stx1 or Stx2 (provided by Alison O'Brien, Uniformed Services University of the Health Sciences, Bethesda, Md.) diluted to 3.5 and 3.0 μg/ml, respectively, with monoclonal (anti-Stx1 Mab13C4 or anti-Stx2 Mab11E10) (provided by James Samuel, Texas A&M University, College Station, Tex.) or polyclonal rabbit anti-Stx2 (Alison O'Brien) antibodies diluted in normal goat serum (Kirkegaard & Perry Laboratories [KPL], Gaithersburg, Md.), and with biotin-streptavidin-alkaline phosphatase detection reagents. The following modifications were incorporated: (i) Tris-buffered saline (TBS; 0.1 M Tris, 120 mM NaCl, pH 7.6) was used instead of 0.1 M Tris, (ii) hybridization covers (Grace Bio-Labs, Bend, Oreg.) were used for all incubations, except for those with the substrate, and (iii) biotinylated reagents were supplemented with normal swine serum (NSS; in-house reagent, used at a 10% [vol/vol] final concentration). Stx2 binding was also assayed by use of a 10-fold higher concentration of Stx2 (30 μg/ml) and polyclonal anti-Stx2. Negative control sections were incubated with TBS instead of Stx or with heterologous primary antibodies. Bound Stx appeared as a red precipitate against the blue counterstained background.
The distribution of Gb3 in frozen porcine tissues was determined by immunostaining with anti-CD77/Gb3 (14). Tissue sections were sequentially incubated (1 h at 37°C) with rat monoclonal anti-CD77/Gb3 (diluted 1:10 in TBS) (Accurate Chemical, Westbury, N.Y.), biotinylated goat anti-rat immunoglobulin M (IgM; diluted 1:200 in TBS with 10% [vol/vol] NSS) (KPL), alkaline phosphatase conjugate (KPL), and HistoMark Red substrate (KPL). Control sections were incubated with a rat IgM isotypic control (1:10; Accurate Chemical) instead of anti-CD77/Gb3.
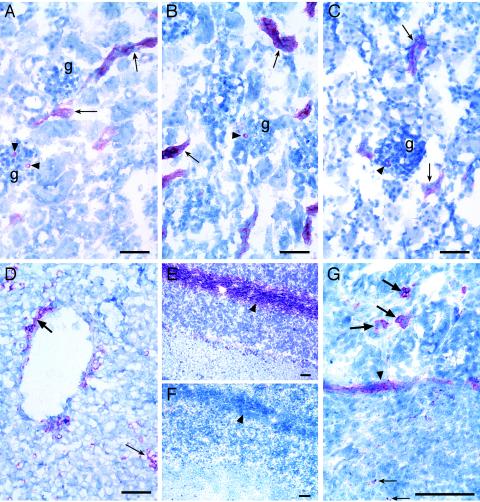
Stx binding was detected in all tissues tested, and the results were similar for tissues from colostrum-deprived and colostrum-fed pigs. Although staining reactions with polyclonal anti-Stx2 were more intense than those with monoclonal anti-Stx2, both antibodies stained similar sites. Both Stx1 and Stx2 bound to immature glomeruli in the outer cortex; to tubules, mature glomeruli, vessels, and arterioles in the inner cortex (Fig. 1B and C); to capillaries and meninges in the cerebellum; to vascular cells surrounding ducts and sinusoidal cells (most likely Kupffer cells) within the parenchyma in the liver (Fig. 1D); to cells in the lamina muscularis mucosae, muscle cells, and individual cells within the lamina propria of all intestinal sites examined; to Peyer's patches and smooth muscle near the Peyer's patches in the ileum (Fig. 1G); and to lymphoid aggregates in the jejunum, spiral colon, and cecum. In addition, Stx1 bound to some sites where Stx2 did not bind, namely, mucosal and serosal blood vessels in the ileum and spiral colon and small blood vessels in the liver. Anti-CD77/Gb3 bound to all sites where Stx bound (e.g., compare Fig. 1A to 1B and C) and also to nerve fibrils in the cerebellar white matter (Fig. 1E), which was a site where neither Stx toxin bound (Fig. 1F).
FIG. 1.
Immunohistochemical identification of Stx binding sites and Gb3 in neonatal porcine tissues. Anti-CD77/Gb3 (A), Stx1 (B), and Stx2 (C) binding to tubules (arrows) and glomeruli (arrowheads) in sections of the kidney (g, glomerulus) are shown. (D) Stx2 binding to endothelial cells lining a vessel (thick arrow) and sinusoidal cells within the parenchyma (thin arrow) of the liver. Anti-CD77/Gb3 (E), but not Stx1 (F), binding to nerve fibrils in the white matter (arrowheads) of the cerebellum was observed. (G) Stx2 binding within a Peyer's patch (thin arrow), to smooth muscle (arrowhead), and to cells within the villous lamina propria (thick arrows) in the ileum. Digital images were captured directly with a Digital Spot RT Slider camera (Diagnostic Instruments, Inc., Sterling Heights, Mich.) using MetaVue, version 5.0.7, imaging software (Universal Imaging Corp., Downingtown, Pa.). Bar = 50 μm.
The Stx overlay and anti-CD77/Gb3 assays were modified for indirect immunofluorescence detection and were used to determine if Stx can bind to isolated porcine tissue leukocytes and if these cells contain Gb3. For these studies, alveolar macrophages obtained from three 4- to 12-week-old pigs by lung lavage (22) (provided by A. Vorwald and S. Brockmeier, NADC) were seeded onto eight-chambered slides (Nalgene Nunc, Rochester, N.Y.) at a density of 4 × 104 cells/slide chamber, incubated (2 h, 37°C, 5% CO2), rinsed with phosphate-buffered saline (PBS), fixed by a brief (∼1 s) immersion in 60% (vol/vol) acetone-PBS, and stored at −80°C.
For Stx binding, alveolar macrophage slides were incubated with Stx2, rabbit anti-Stx2 (as described above), and fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit IgG (1:80; Sigma, St. Louis, Mo.). For Gb3, the slides were incubated with anti-CD77/Gb3 (as described above) and FITC-conjugated goat anti-rat IgM (1:20; Southern Biotechnology, Birmingham, Ala.). Hanks' balanced salt solution without Ca2+ or Mg2+ (HBSS) and HBSS containing 1% fetal bovine serum (HyClone, Logan, Utah) were used for wash steps and reagent dilutions, respectively. Negative controls were incubated with dilution buffer instead of Stx2 or with heterologous antibodies instead of anti-Stx2 or anti-CD77/Gb3. Slides were examined under an Eclipse E800 microscope (Nikon Instruments, Inc., Melville, N.Y.) with FITC filters (excitation, 465 to 495 nm; emission, 515 to 555 nm). Cells with bound Stx2 or anti-CD77/Gb3 fluoresced green. Stx2 and anti-CD77/Gb3 bound to ≥80% of the alveolar macrophages on all evaluated slides. No green fluorescence was seen on negative control slides.
Indirect immunofluorescence assays were also used to determine if Stx bound to peripheral blood leukocytes (PBL) and if PBL contained Gb3. Blood samples were collected in heparin from three adult pigs (≥6 months old) on 2 days. Granulocyte and mononuclear leukocyte fractions were isolated (within 2 h of sample collection) by density gradient centrifugation with Ficoll-sodium diatrizoate (Amersham Pharmacia Biotech, Piscataway, N.J.) (13). Red blood cells were removed from granulocyte fractions by hypotonic lysis. Fractions were adjusted to 107 cells/ml in RPMI 1640 (Life Technologies, Gaithersburg, Md.) containing 4% autologous serum (mononuclear leukocytes) or 10% fetal bovine serum (granulocytes). Direct smears (10 to 15 μl/slide; ∼105 cells) were prepared on glass slides, fixed in 60% acetone-PBS, 1.5% formalin, or 100% methanol or by air drying, and stored (for up to 1 year) at the ambient temperature. In preliminary studies, Stx2 bound similarly to PBL fixed in 60% acetone-PBS or 1.5% formalin or by air drying, but it did not bind to cells that were fixed in 100% methanol. The cellular morphology was maintained better on air-dried smears, so these were used for all studies reported here. Differential staining showed that mononuclear leukocyte fractions contained ≥95% mononuclear cells (≤4% monocytes) and that granulocyte fractions contained ≥90% granulocytes (64 to 86% PMN and 8 to 35% eosinophils).
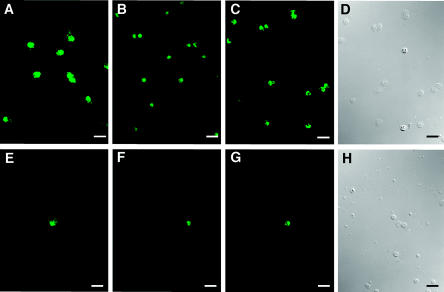
Stx2 and anti-CD77/Gb3 binding was evaluated for duplicate PBL smears from each of six blood draws by use of the immunofluorescence assays described above for alveolar macrophages. Stx1 binding was evaluated for PBL smears from a single blood draw by the use of crude Stx1 (29) (provided by Nancy Cornick, Iowa State University, Ames, Iowa), anti-Stx1 Mab13C4, and FITC-conjugated goat anti-mouse IgG (Vector, Burlingame, Calif.) (diluted 1:10 in HBSS containing 10% [vol/vol] NSS). At least 600 cells per granulocyte smear and 20 high-power fields (HPFs; each containing 30 to 50 cells) per mononuclear leukocyte smear were examined for fluorescence. The results were calculated as the percentages of green fluorescent cells on granulocyte smears or the total numbers of fluorescent cells/20 HPFs on mononuclear leukocyte smears. The numbers of cells and cellular morphologies in the fields examined for fluorescence were determined by differential interference contrast microscopy (Fig. 2). Eosinophils were differentiated from PMN by their red autofluorescence when the slides were examined with tetramethyl rhodamine isothiocyanate filters (excitation, 528 to 563 nm; emission, 600 to 660 nm) (1).
FIG. 2.
Indirect immunofluorescent detection of Stx binding and Gb3 on porcine peripheral blood leukocytes. Anti-CD77/Gb3 (A), Stx1 (B), and Stx2 (C) binding to cells on granulocyte smears are shown. Anti-CD77/Gb3 (E), Stx1 (F), and Stx2 (G) binding to cells on mononuclear leukocyte smears are also shown. (D and H) Differential interference contrast illumination of the same fields as those in panels C and G, respectively. Images were obtained as described in the legend to Fig. 1. Bar = 25 μm.
As shown in Fig. 2, Stx1, Stx2, and anti-CD77/Gb3 bound to similar numbers of cells on PBL smears. On granulocyte smears, 58 to 91% of the cells examined were positive. All positive granulocytes were PMN (i.e., none of the positive granulocytes fluoresced red when examined with tetramethyl rhodamine isothiocyanate filters), and for each sample the percentage of positive cells correlated with the percentage of PMN, as determined by differential counts. On mononuclear leukocyte smears, ≤10 cells/HPF were positive, and positive cells were morphologically identified as monocytes. The number of positive cells correlated with the number of monocytes by differential counts, providing further evidence that Stx and anti-CD77/Gb3 bound to monocytes but not to lymphocytes. No green fluorescence was observed on negative control slides.
In this study, we showed that Stx1 and Stx2 bound to similar locations in multiple porcine tissues, although some differences were noted. We identified some novel porcine Stx binding sites, including kidney tubules, intestinal lymphoid aggregates, sinusoidal cells in the liver, and isolated leukocytes. The observation that Stx bound to porcine kidney tubules, as it does to bovine (15, 27) and human (19) kidney tubules, is consistent with the presence of kidney tubule damage in pigs that are experimentally infected with STEC (6, 14) and with the sensitivity of human kidney proximal tubule epithelial cells to Stx (16). Although intestinal lymphoid aggregates and liver cells are not recognized as targets for Stx-mediated damage, these tissues might be involved in Stx-induced immunomodulation or transport or in the clearance of Stx. We did not identify any unique Stx2 binding sites that correlated with the enhanced virulence associated with Stx2. In fact, Stx1 bound to more vascular sites than Stx2 did (even when Stx2 was tested at 10-fold higher concentrations and detected with polyclonal anti-Stx2 antibodies). Nonetheless, it cannot be ruled out that discrepancies between Stx1 and Stx2 binding were due to differences in the sensitivities of the immunohistochemical assays because of differences in the relative binding affinities of the reagents (which were not assessed in this study) or differences in the form of Stx receptors in the various tissues (4).
This study provided direct evidence that Stx can bind to porcine leukocytes. Stx bound to PBL (monocytes and granulocytes), to tissue leukocytes (alveolar macrophages), and to tissue locations where leukocytes reside (i.e., the intestinal lamina propria, intestinal lymphoid aggregates, and the liver). These results are consistent with reports that Stx binds to human PMN and monocytes (28, 32, 34) and to bovine intestinal lymphoid aggregates (15) and with the demonstration that liver and splenic macrophages are involved in Stx2 cytotoxicity in mice (25). We hypothesize that Stx binding to leukocytes is involved in STEC disease pathogenesis in pigs.
Our results extend the evidence that Stx binds to Gb3 receptors in porcine tissues (3) and that not all Gb3 molecules are Stx receptors (4). The distribution of Stx binding sites correlated with the distribution of Gb3 in porcine tissues and leukocytes. The immunohistochemical detection of Gb3 at all sites where Stx bound confirmed earlier reports of Gb3 detection by high-performance liquid chromatography (HPLC) in the porcine liver, colon, and kidney (3) and by thin-layer chromatography in the kidney (14). The detection of Gb3 in the cerebellum, a site at which Gb3 was not detected by HPLC (3), suggests that the anti-CD77/Gb3 immunoassay may be more sensitive than HPLC for detecting Gb3 and disputes the notion that acetone fixation removes Gb3 from porcine tissues (15). To our knowledge, this is the first report of anti-CD77/Gb3 binding to cerebellar nerve fibrils. Although we did not detect Stx binding to nerve fibrils, Stx may have bound at levels below the detection limit of our assay.
Porcine peripheral blood leukocytes and the Stx binding assays described in this report will be useful for studying Stx binding mechanisms and for evaluating the effects of Stx binding on leukocyte functions. An advantage of using porcine leukocytes for these studies is that porcine STEC infection models are available for testing the relevance of the in vitro findings.
Acknowledgments
We thank B. K. Wheeler for technical assistance; J. F. Pohlenz for consultation, advice, and a critical review of the manuscript; and NADC Visual Services and S. L. Johnson for preparation of the manuscript.
The mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
Editor: J. D. Clements
REFERENCES
- 1.Barnes, D., S. Aggarwal, S. Thomsen, M. Fitzmaurice, and R. Richards-Kortum. 1993. A characterization of the fluorescent properties of circulating human eosinophils. Photochem. Photobiol. 58:297-303. [DOI] [PubMed] [Google Scholar]
- 2.Boerlin, P., S. A. McEwen, F. Boerlin-Petzold, J. B. Wilson, R. P. Johnson, and C. L. Gyles. 1999. Associations between virulence factors of Shiga toxin-producing Escherichia coli and disease in humans. J. Clin. Microbiol. 37:497-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyd, B., G. Tyrrell, M. Maloney, C. Gyles, J. Brunton, and C. Lingwood. 1993. Alteration of the glycolipid binding specificity of the pig edema toxin from globotetraosyl to globotriaosyl ceramide alters in vivo tissue targeting and results in a verotoxin 1-like disease in pigs. J. Exp. Med. 177:1745-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chark, D., A. Nutikka, N. Trusevych, J. Kuzmina, and C. Lingwood. 2004. Differential carbohydrate epitope recognition of globotriaosyl ceramide by verotoxins and a monoclonal antibody. Eur. J. Biochem. 271:405-417. [DOI] [PubMed] [Google Scholar]
- 5.Christopher-Hennings, J., J. A. Willgohs, D. H. Francis, U. A. K. Raman, R. A. Moxley, and D. J. Hurley. 1993. Immunocompromise in gnotobiotic pigs induced by verotoxin-producing Escherichia coli (O111:NM). Infect. Immun. 61:2304-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dean-Nystrom, E. A., A. R. Melton-Celsa, J. F. Pohlenz, H. W. Moon, and A. D. O'Brien. 2003. Comparative pathogenicity of Escherichia coli O157 and intimin-negative non-O157 Shiga toxin-producing E. coli strains in neonatal pigs. Infect. Immun. 71:6526-6533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dean-Nystrom, E. A., J. F. Pohlenz, H. W. Moon, and A. D. O'Brien. 2000. Escherichia coli O157:H7 causes more severe systemic disease in suckling piglets than in colostrum-deprived neonatal piglets. Infect. Immun. 68:2356-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donohue-Rolfe, A., I. Kondova, S. Oswald, D. Hutto, and S. Tzipori. 2000. Escherichia coli O157:H7 strains that express Shiga toxin (Stx) 2 alone are more neurotropic for gnotobiotic piglets than are isotypes producing only Stx1 or both Stx1 and Stx2. J. Infect. Dis. 181:1825-1829. [DOI] [PubMed] [Google Scholar]
- 9.Ergonul, Z., A. K. Hughes, and D. E. Kohan. 2003. Induction of apoptosis of human brain microvascular endothelial cells by Shiga toxin 1. J. Infect. Dis. 187:154-158. [DOI] [PubMed] [Google Scholar]
- 10.Ferens, W. A., and C. J. Hovde. 2000. Antiviral activity of Shiga toxin 1: suppression of bovine leukemia virus-related spontaneous lymphocyte proliferation. Infect. Immun. 68:4462-4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fitzpatrick, M. M., V. Shah, R. S. Trompeter, M. J. Dillon, and T. M. Barratt. 1992. Interleukin-8 and polymorphoneutrophil leucocyte activation in hemolytic uremic syndrome of childhood. Kidney Int. 42:951-956. [DOI] [PubMed] [Google Scholar]
- 12.Francis, D. H., R. A. Moxley, and C. Y. Andraos. 1989. Edema disease-like brain lesions in gnotobiotic piglets infected with Escherichia coli serotype O157:H7. Infect. Immun. 57:1339-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gray, J. T., T. J. Stabel, and P. J. Fedorka-Cray. 1996. Effect of dose on the immune response and persistence of Salmonella choleraesuis infection in swine. Am. J. Vet. Res. 57:313-319. [PubMed] [Google Scholar]
- 14.Gunzer, F., I. Hennig-Pauka, K.-H. Waldmann, R. Sandoff, H.-J. Gröne, H.-H. Kreipe, A. Matussek, and M. Mengel. 2002. Gnotobiotic piglets develop thrombotic microangiopathy after oral infection with enterohemorrhagic Escherichia coli. Am. J. Clin. Pathol. 118:364-375. [DOI] [PubMed] [Google Scholar]
- 15.Hoey, D. E. E., C. Currie, R. W. Else, A. Nutikka, C. A. Lingwood, D. L. Gally, and D. G. E. Smith. 2002. Expression of receptors for verotoxin 1 from Escherichia coli O157 on bovine intestinal epithelium. J. Med. Microbiol. 51:143-149. [DOI] [PubMed] [Google Scholar]
- 16.Hughes, A. K., D. Schmid, and D. E. Kohan. 2002. Sex steroids do not affect shigatoxin cytotoxicity on human renal tubular or glomerular cells. BMC Nephrol. 3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karpman, D., D. Papadopoulou, K. Nilsson, A.-C. Sjögren, C. Mikaelsson, and S. Lethagen. 2001. Platelet activation by Shiga toxin and circulatory factors as a pathogenetic mechanism in the hemolytic uremic syndrome. Blood 97:3100-3108. [DOI] [PubMed] [Google Scholar]
- 18.Kausche, F. M., E. A. Dean, L. H. Arp, J. E. Samuel, and H. W. Moon. 1992. An experimental model for subclinical edema disease (Escherichia coli enterotoxemia) manifests as vascular necrosis in pigs. Am. J. Vet. Res. 53:281-287. [PubMed] [Google Scholar]
- 19.Lingwood, C. A. 1994. Verotoxin-binding in human renal sections. Nephron 66:21-28. [DOI] [PubMed] [Google Scholar]
- 20.Menge, C., L. H. Wieler, T. Schlapp, and G. Baljer. 1999. Shiga toxin 1 from Escherichia coli blocks activation and proliferation of bovine lymphocyte subpopulations in vitro. Infect. Immun. 67:2209-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mengeling, W. L., A. C. Vorwald, N. A. Cornick, K. M. Lager, and H. W. Moon. 2001. In vitro detection of Shiga toxin using porcine alveolar macrophages. J. Vet. Diagn. Investig. 13:421-424. [DOI] [PubMed] [Google Scholar]
- 22.Mengeling, W. L., K. M. Lager, and A. C. Vorwald. 1995. Diagnosis of porcine reproductive and respiratory syndrome. J. Vet. Diagn. Investig. 7:3-16. [DOI] [PubMed] [Google Scholar]
- 23.Moxley, R. A. 2000. Edema disease. Vet. Clin. N. Am. Food Anim. Pract. 16:175-185. [DOI] [PubMed] [Google Scholar]
- 24.Obrig, T. G., R. M. Seaner, M. Bentz, C. A. Lingwood, B. S. A. Boyd, and W. Narrow. 2003. Induction of sphingomyelinase of Shiga toxin receptor and Shiga toxin 2 sensitivity in human microvascular endothelial cells. Infect. Immun. 71:845-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palermo, M. S., M. F. Alves Rosa, N. Van Rooijen, and M. A. Isturiz. 1999. Depletion of liver and splenic macrophages reduces the lethality of Shiga toxin-2 in a mouse model. Clin. Exp. Immunol. 116:462-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paton, J. C., and A. W. Paton. 1998. Pathogenesis and diagnosis of Shiga toxin-producing Escherichia coli infections. Clin. Microbiol. Rev. Infect. Immun. 11:450-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pruimboom-Brees, I. M., T. W. Morgan, M. R. Ackermann, E. D. Nystrom, J. E. Samuel, N. A. Cornick, and H. W. Moon. 2000. Cattle lack vascular receptors for Escherichia coli O157:H7 Shiga toxins. Proc. Natl. Acad. Sci. USA 97:10325-10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramegowda, B., and V. L. Tesh. 1996. Differentiation-associated toxin receptor modulation, cytokine production, and sensitivity to Shiga-like toxins in human monocytes and monocytic cell lines. Infect. Immun. 64:1173-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Samuel, J. E., L. P. Perera, S. Ward, A. D. O'Brien, V. Ginsburg, and H. C. Krivan. 1990. Comparison of the glycolipid receptor specificities of Shiga-like toxin type II and Shiga-like toxin type II variants. Infect. Immun. 58:611-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stamm, I., M. Wuhrer, R. Geyer, G. Baljer, and C. Menge. 2002. Bovine lymphocytes express functional receptors for Escherichia coli Shiga toxin 1. Microb. Pathog. 33:251-264. [DOI] [PubMed] [Google Scholar]
- 31.Stricklett, P. K., A. K. Hughes, Z. Ergonul, and D. E. Kohan. 2002. Molecular basis for up-regulation by inflammatory cytokines of Shiga toxin 1 cytotoxicity and globotriaosylceramide expression. J. Infect. Dis. 186:976-982. [DOI] [PubMed] [Google Scholar]
- 32.te Loo, D. M. W. M., L. A. H. Monnens, T. J. A. M. van der Velden, M. A. Vermeer, F. Preyers, P. N. M. Demacker, L. P. W. J. van den Heuvel, and V. W. M. van Hinsbergh. 2000. Binding and transfer of verocytotoxin by polymorphonuclear leukocytes in hemolytic uremic syndrome. Blood 95:3396-3402. [PubMed] [Google Scholar]
- 33.Tzipori, S., C. W. Chow, and H. R. Powell. 1988. Cerebral infection with Escherichia coli O157:H7 in humans and gnotobiotic piglets. J. Clin. Pathol. 41:1099-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Setten, P. A., L. A. H. Monnens, R. G. G. Verstraten, L. P. W. J. van den Heuvel, and V. W. M. van Hinsbergh. 1996. Effects of verocytotoxin-1 on nonadherent human monocytes: binding characteristics, protein synthesis, and induction of cytokine release. Blood 88:174-183. [PubMed] [Google Scholar]