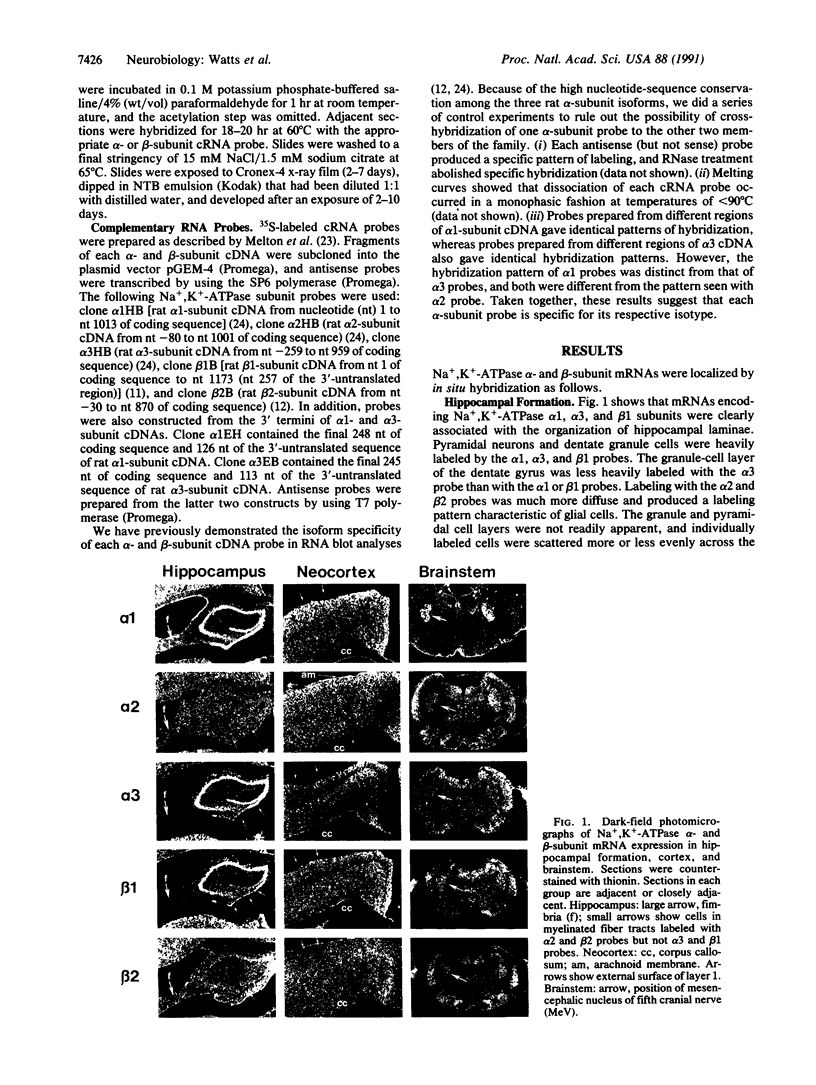
Abstract
We have used in situ hybridization histochemistry to analyze the subcellular distribution of mRNAs encoding Na,K-ATPase alpha- and beta-subunit isoforms in the rat central nervous system. Substantial differences in the cell-specific pattern of expression were found for the genes encoding three isoforms of the alpha subunit. Transcripts of alpha 1-subunit gene were detected in virtually all cell types and structures examined. Expression of alpha 2-subunit mRNA was characteristic of glia, whereas alpha 3-subunit transcripts were predominant in neurons. Transcripts encoding the beta 1 subunit were detected in neurons, whereas beta 2-subunit mRNA expression was characteristic of glia. mRNA encoding both beta-subunit isoforms was present in choroidal epithelial cells. The distribution pattern of alpha- and beta-subunit mRNAs in structures throughout the central nervous system is consistent with the possibility of six structurally distinct Na+,K(+)-ATPase isoenzymes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dictus W. J., van Zoelen E. J., Tetteroo P. A., Tertoolen L. G., de Laat S. W., Bluemink J. G. Lateral mobility of plasma membrane lipids in Xenopus eggs: regional differences related to animal/vegetal polarity become extreme upon fertilization. Dev Biol. 1984 Jan;101(1):201–211. doi: 10.1016/0012-1606(84)90130-1. [DOI] [PubMed] [Google Scholar]
- Emanuel J. R., Garetz S., Stone L., Levenson R. Differential expression of Na+,K+-ATPase alpha- and beta-subunit mRNAs in rat tissues and cell lines. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9030–9034. doi: 10.1073/pnas.84.24.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futamachi K. J., Pedley T. A. Glial cells and extracellular potassium: their relationship in mammalian cortex. Brain Res. 1976 Jun 11;109(2):311–322. doi: 10.1016/0006-8993(76)90532-1. [DOI] [PubMed] [Google Scholar]
- Gloor S., Antonicek H., Sweadner K. J., Pagliusi S., Frank R., Moos M., Schachner M. The adhesion molecule on glia (AMOG) is a homologue of the beta subunit of the Na,K-ATPase. J Cell Biol. 1990 Jan;110(1):165–174. doi: 10.1083/jcb.110.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisar T., Frere J. M., Franck G. Effect of K+ ions on kinetic properties of the (Na+, K+)-ATPase (EC 3.6.1.3) of bulk isolated glial cells, perikarya and synaptosomes from rabbit brain cortex. Brain Res. 1979 Apr 6;165(1):87–103. doi: 10.1016/0006-8993(79)90047-7. [DOI] [PubMed] [Google Scholar]
- HELD D., FENCL V., PAPPENHEIMER J. R. ELECTRICAL POTENTIAL OF CEREBROSPINAL FLUID. J Neurophysiol. 1964 Sep;27:942–959. doi: 10.1152/jn.1964.27.5.942. [DOI] [PubMed] [Google Scholar]
- Henn F. A., Haljamäe H., Hamberger A. Glial cell function: active control of extracellular K + concentration. Brain Res. 1972 Aug 25;43(2):437–443. doi: 10.1016/0006-8993(72)90399-x. [DOI] [PubMed] [Google Scholar]
- Herrera V. L., Emanuel J. R., Ruiz-Opazo N., Levenson R., Nadal-Ginard B. Three differentially expressed Na,K-ATPase alpha subunit isoforms: structural and functional implications. J Cell Biol. 1987 Oct;105(4):1855–1865. doi: 10.1083/jcb.105.4.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen L. L., Kelly J. S. Uptake and metabolism of gamma-aminobutyric acid by neurones and glial cells. Biochem Pharmacol. 1975 May 1;24(9):933–938. doi: 10.1016/0006-2952(75)90422-0. [DOI] [PubMed] [Google Scholar]
- Johanson C. E., Reed D. J., Woodbury D. M. Active transport of sodium and potassium by the choroid plexus of the rat. J Physiol. 1974 Sep;241(2):359–372. doi: 10.1113/jphysiol.1974.sp010660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., Orlowitz L., Hitzig B. M. Differences between CSF and plasma Na+ and K+ activities and concentrations. Am J Physiol. 1985 May;248(5 Pt 2):R621–R626. doi: 10.1152/ajpregu.1985.248.5.R621. [DOI] [PubMed] [Google Scholar]
- Kent R. B., Fallows D. A., Geissler E., Glaser T., Emanuel J. R., Lalley P. A., Levenson R., Housman D. E. Genes encoding alpha and beta subunits of Na,K-ATPase are located on three different chromosomes in the mouse. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5369–5373. doi: 10.1073/pnas.84.15.5369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malo D., Schurr E., Levenson R., Gros P. Assignment of Na,K-ATPase beta 2-subunit gene (Atpb-2) to mouse chromosome 11. Genomics. 1990 Apr;6(4):697–699. doi: 10.1016/0888-7543(90)90507-q. [DOI] [PubMed] [Google Scholar]
- Martin-Vasallo P., Dackowski W., Emanuel J. R., Levenson R. Identification of a putative isoform of the Na,K-ATPase beta subunit. Primary structure and tissue-specific expression. J Biol Chem. 1989 Mar 15;264(8):4613–4618. [PubMed] [Google Scholar]
- Masuzawa T., Saito T., Sato F. Cytochemical study on enzyme activity associated with cerebrospinal fluid secretion in the choroid plexus and ventricular ependyma. Brain Res. 1981 Oct 19;222(2):309–322. doi: 10.1016/0006-8993(81)91035-0. [DOI] [PubMed] [Google Scholar]
- McDonough A. A., Geering K., Farley R. A. The sodium pump needs its beta subunit. FASEB J. 1990 Apr 1;4(6):1598–1605. doi: 10.1096/fasebj.4.6.2156741. [DOI] [PubMed] [Google Scholar]
- McGrail K. M., Phillips J. M., Sweadner K. J. Immunofluorescent localization of three Na,K-ATPase isozymes in the rat central nervous system: both neurons and glia can express more than one Na,K-ATPase. J Neurosci. 1991 Feb;11(2):381–391. doi: 10.1523/JNEUROSCI.11-02-00381.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer R. W., Schneider J. W., Savitz A., Emanuel J., Benz E. J., Jr, Levenson R. Rat-brain Na,K-ATPase beta-chain gene: primary structure, tissue-specific expression, and amplification in ouabain-resistant HeLa C+ cells. Mol Cell Biol. 1986 Nov;6(11):3884–3890. doi: 10.1128/mcb.6.11.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider B. G., Shyjan A. W., Levenson R. Co-localization and polarized distribution of Na,K-ATPase alpha 3 and beta 2 subunits in photoreceptor cells. J Histochem Cytochem. 1991 Apr;39(4):507–517. doi: 10.1177/39.4.1848572. [DOI] [PubMed] [Google Scholar]
- Schneider J. W., Mercer R. W., Gilmore-Hebert M., Utset M. F., Lai C., Greene A., Benz E. J., Jr Tissue specificity, localization in brain, and cell-free translation of mRNA encoding the A3 isoform of Na+,K+-ATPase. Proc Natl Acad Sci U S A. 1988 Jan;85(1):284–288. doi: 10.1073/pnas.85.1.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shull G. E., Greeb J., Lingrel J. B. Molecular cloning of three distinct forms of the Na+,K+-ATPase alpha-subunit from rat brain. Biochemistry. 1986 Dec 16;25(25):8125–8132. doi: 10.1021/bi00373a001. [DOI] [PubMed] [Google Scholar]
- Shyjan A. W., Ceña V., Klein D. C., Levenson R. Differential expression and enzymatic properties of the Na+,K(+)-ATPase alpha 3 isoenzyme in rat pineal glands. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1178–1182. doi: 10.1073/pnas.87.3.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyjan A. W., Gottardi C., Levenson R. The Na,K-ATPase beta 2 subunit is expressed in rat brain and copurifies with Na,K-ATPase activity. J Biol Chem. 1990 Mar 25;265(9):5166–5169. [PubMed] [Google Scholar]
- Shyjan A. W., Levenson R. Antisera specific for the alpha 1, alpha 2, alpha 3, and beta subunits of the Na,K-ATPase: differential expression of alpha and beta subunits in rat tissue membranes. Biochemistry. 1989 May 30;28(11):4531–4535. doi: 10.1021/bi00437a002. [DOI] [PubMed] [Google Scholar]
- Somjen G. G. Electrophysiology of neuroglia. Annu Rev Physiol. 1975;37:163–190. doi: 10.1146/annurev.ph.37.030175.001115. [DOI] [PubMed] [Google Scholar]
- Somjen G. G. Extracellular potassium in the mammalian central nervous system. Annu Rev Physiol. 1979;41:159–177. doi: 10.1146/annurev.ph.41.030179.001111. [DOI] [PubMed] [Google Scholar]
- Stahl W. L., Baskin D. G. Histochemistry of ATPases. J Histochem Cytochem. 1990 Aug;38(8):1099–1122. doi: 10.1177/38.8.2164057. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. Electrogenic sodium pump in nerve and muscle cells. Physiol Rev. 1972 Jul;52(3):563–594. doi: 10.1152/physrev.1972.52.3.563. [DOI] [PubMed] [Google Scholar]
- Wright E. M. Mechanisms of ion transport across the choroid plexus. J Physiol. 1972 Oct;226(2):545–571. doi: 10.1113/jphysiol.1972.sp009997. [DOI] [PMC free article] [PubMed] [Google Scholar]