Abstract
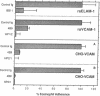
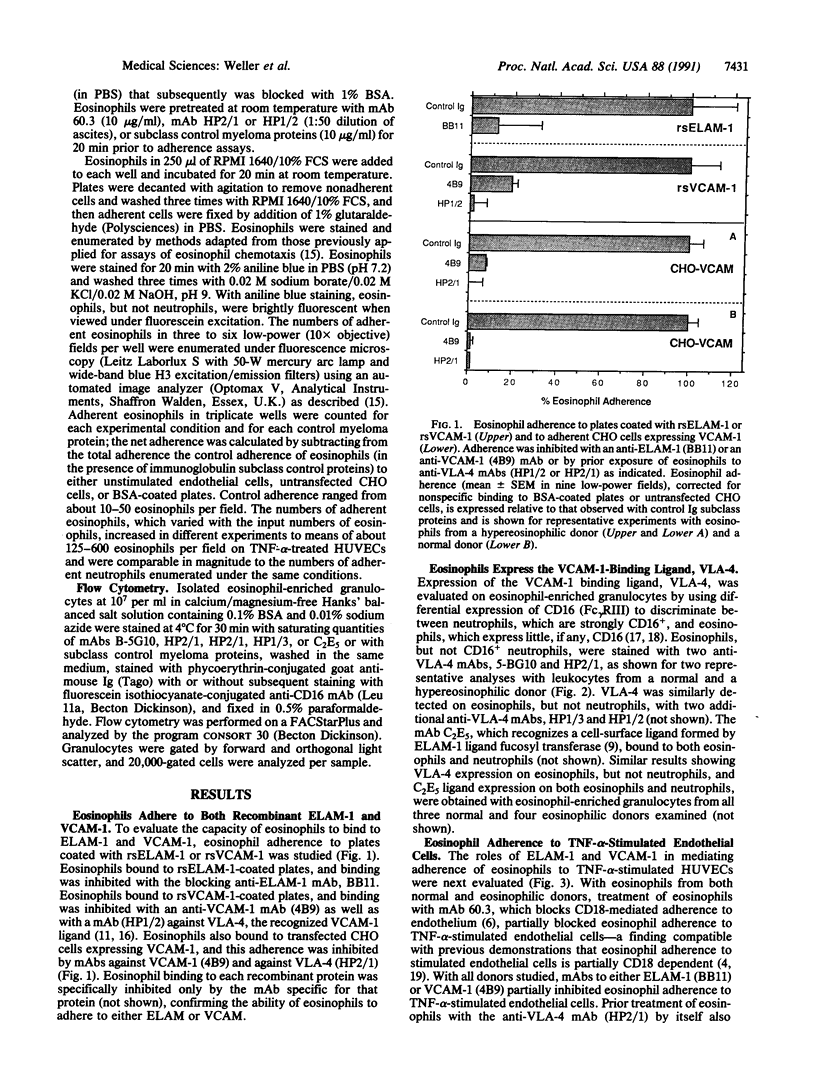
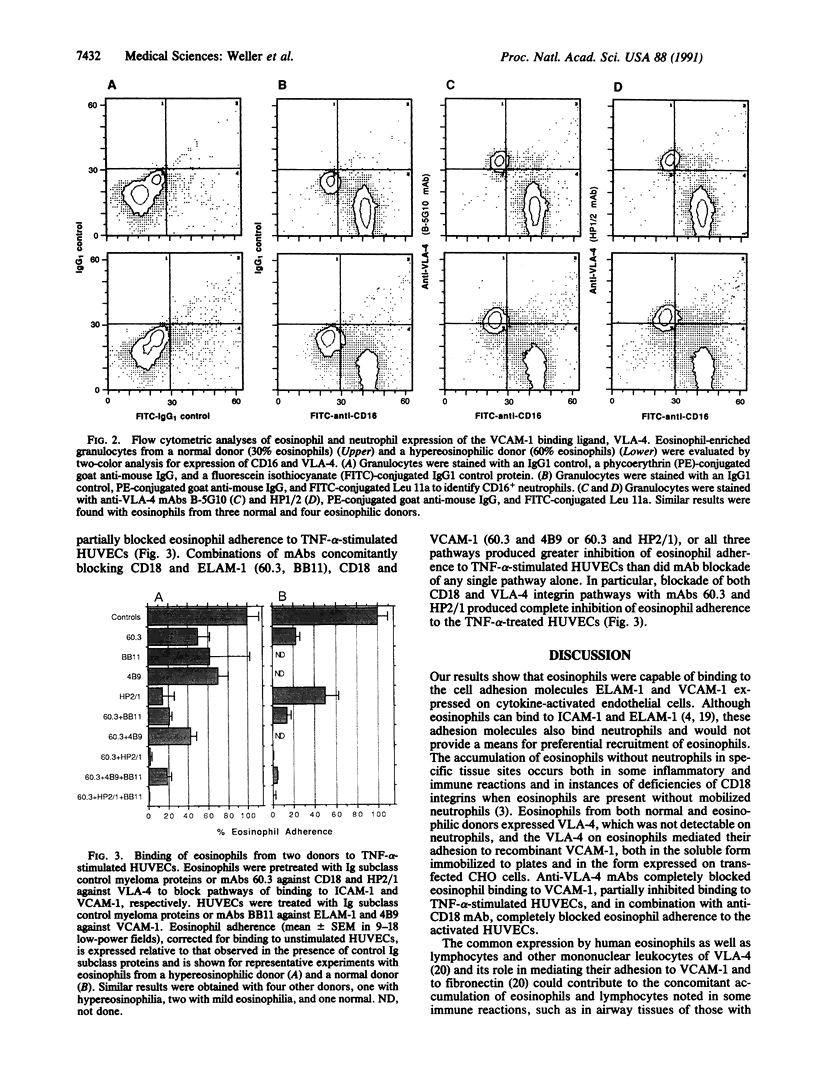
Adherence of human eosinophils to cytokine-stimulated endothelial cells, which was only partially due to CD18-dependent pathways, was also mediated by binding to endothelial leukocyte adhesion molecule 1 (ELAM-1) and vascular cell adhesion molecule 1 (VCAM-1). Eosinophils bound specifically to both recombinant soluble ELAM-1 and recombinant soluble VCAM-1. Eosinophil binding to recombinant soluble VCAM-1 and to transfected CHO cells expressing VCAM-1 was inhibited with anti-VCAM-1 (4B9) and anti-very late activation antigen 4 (anti-VLA-4; HP1/2 or HP2/1) monoclonal antibodies. Eosinophils, but not neutrophils, expressed VLA-4 detected by cytofluorography. Eosinophil adherence to tumor necrosis factor alpha-stimulated human umbilical vein endothelial cells was partially blocked by monoclonal antibodies against ELAM-1 (BB11) and VCAM-1 (4B9) and against VLA-4 (HP2/1). Thus, while both eosinophils and neutrophils can bind to activated endothelial cells by adherence to ICAM-1 and ELAM-1, only eosinophils expressed VLA-4 and adhered to VCAM-1 on activated endothelial cells. Eosinophil adherence to VCAM-1 might provide a mechanism contributing to the selective recruitment of eosinophils into tissue sites of inflammation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. C., Schmalsteig F. C., Finegold M. J., Hughes B. J., Rothlein R., Miller L. J., Kohl S., Tosi M. F., Jacobs R. L., Waldrop T. C. The severe and moderate phenotypes of heritable Mac-1, LFA-1 deficiency: their quantitative definition and relation to leukocyte dysfunction and clinical features. J Infect Dis. 1985 Oct;152(4):668–689. doi: 10.1093/infdis/152.4.668. [DOI] [PubMed] [Google Scholar]
- Azzawi M., Bradley B., Jeffery P. K., Frew A. J., Wardlaw A. J., Knowles G., Assoufi B., Collins J. V., Durham S., Kay A. B. Identification of activated T lymphocytes and eosinophils in bronchial biopsies in stable atopic asthma. Am Rev Respir Dis. 1990 Dec;142(6 Pt 1):1407–1413. doi: 10.1164/ajrccm/142.6_Pt_1.1407. [DOI] [PubMed] [Google Scholar]
- Beatty P. G., Ledbetter J. A., Martin P. J., Price T. H., Hansen J. A. Definition of a common leukocyte cell-surface antigen (Lp95-150) associated with diverse cell-mediated immune functions. J Immunol. 1983 Dec;131(6):2913–2918. [PubMed] [Google Scholar]
- Benjamin C., Dougas I., Chi-Rosso G., Luhowskyj S., Rosa M., Newman B., Osborn L., Vassallo C., Hession C., Goelz S. A blocking monoclonal antibody to endothelial-leukocyte adhesion molecule-1 (ELAM1). Biochem Biophys Res Commun. 1990 Aug 31;171(1):348–353. doi: 10.1016/0006-291x(90)91400-m. [DOI] [PubMed] [Google Scholar]
- Carlos T. M., Dobrina A., Ross R., Harlan J. M. Multiple receptors on human monocytes are involved in adhesion to cultured human endothelial cells. J Leukoc Biol. 1990 Nov;48(5):451–456. doi: 10.1002/jlb.48.5.451. [DOI] [PubMed] [Google Scholar]
- Carlos T. M., Schwartz B. R., Kovach N. L., Yee E., Rosa M., Osborn L., Chi-Rosso G., Newman B., Lobb R., Rosso M. Vascular cell adhesion molecule-1 mediates lymphocyte adherence to cytokine-activated cultured human endothelial cells. Blood. 1990 Sep 1;76(5):965–970. [PubMed] [Google Scholar]
- Diaz P., Gonzalez M. C., Galleguillos F. R., Ancic P., Cromwell O., Shepherd D., Durham S. R., Gleich G. J., Kay A. B. Leukocytes and mediators in bronchoalveolar lavage during allergen-induced late-phase asthmatic reactions. Am Rev Respir Dis. 1989 Jun;139(6):1383–1389. doi: 10.1164/ajrccm/139.6.1383. [DOI] [PubMed] [Google Scholar]
- Elices M. J., Osborn L., Takada Y., Crouse C., Luhowskyj S., Hemler M. E., Lobb R. R. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell. 1990 Feb 23;60(4):577–584. doi: 10.1016/0092-8674(90)90661-w. [DOI] [PubMed] [Google Scholar]
- Freedman A. S., Munro J. M., Rice G. E., Bevilacqua M. P., Morimoto C., McIntyre B. W., Rhynhart K., Pober J. S., Nadler L. M. Adhesion of human B cells to germinal centers in vitro involves VLA-4 and INCAM-110. Science. 1990 Aug 31;249(4972):1030–1033. doi: 10.1126/science.1697696. [DOI] [PubMed] [Google Scholar]
- Frew A. J., Kay A. B. The relationship between infiltrating CD4+ lymphocytes, activated eosinophils, and the magnitude of the allergen-induced late phase cutaneous reaction in man. J Immunol. 1988 Dec 15;141(12):4158–4164. [PubMed] [Google Scholar]
- Goelz S. E., Hession C., Goff D., Griffiths B., Tizard R., Newman B., Chi-Rosso G., Lobb R. ELFT: a gene that directs the expression of an ELAM-1 ligand. Cell. 1990 Dec 21;63(6):1349–1356. doi: 10.1016/0092-8674(90)90430-m. [DOI] [PubMed] [Google Scholar]
- Hartnell A., Moqbel R., Walsh G. M., Bradley B., Kay A. B. Fc gamma and CD11/CD18 receptor expression on normal density and low density human eosinophils. Immunology. 1990 Feb;69(2):264–270. [PMC free article] [PubMed] [Google Scholar]
- Haskard D., Cavender D., Beatty P., Springer T., Ziff M. T lymphocyte adhesion to endothelial cells: mechanisms demonstrated by anti-LFA-1 monoclonal antibodies. J Immunol. 1986 Nov 1;137(9):2901–2906. [PubMed] [Google Scholar]
- Hemler M. E., Huang C., Takada Y., Schwarz L., Strominger J. L., Clabby M. L. Characterization of the cell surface heterodimer VLA-4 and related peptides. J Biol Chem. 1987 Aug 25;262(24):11478–11485. [PubMed] [Google Scholar]
- Hemler M. E. VLA proteins in the integrin family: structures, functions, and their role on leukocytes. Annu Rev Immunol. 1990;8:365–400. doi: 10.1146/annurev.iy.08.040190.002053. [DOI] [PubMed] [Google Scholar]
- Kyan-Aung U., Haskard D. O., Poston R. N., Thornhill M. H., Lee T. H. Endothelial leukocyte adhesion molecule-1 and intercellular adhesion molecule-1 mediate the adhesion of eosinophils to endothelial cells in vitro and are expressed by endothelium in allergic cutaneous inflammation in vivo. J Immunol. 1991 Jan 15;146(2):521–528. [PubMed] [Google Scholar]
- Lamas A. M., Mulroney C. M., Schleimer R. P. Studies on the adhesive interaction between purified human eosinophils and cultured vascular endothelial cells. J Immunol. 1988 Mar 1;140(5):1500–1505. [PubMed] [Google Scholar]
- Lobb R. R., Chi-Rosso G., Leone D. R., Rosa M. D., Bixler S., Newman B. M., Luhowskyj S., Benjamin C. D., Dougas I. G., Goelz S. E. Expression and functional characterization of a soluble form of endothelial-leukocyte adhesion molecule 1. J Immunol. 1991 Jul 1;147(1):124–129. [PubMed] [Google Scholar]
- Masinovsky B., Urdal D., Gallatin W. M. IL-4 acts synergistically with IL-1 beta to promote lymphocyte adhesion to microvascular endothelium by induction of vascular cell adhesion molecule-1. J Immunol. 1990 Nov 1;145(9):2886–2895. [PubMed] [Google Scholar]
- McCrone E. L., Lucey D. R., Weller P. F. Fluorescent staining for leukocyte chemotaxis. Eosinophil-specific fluorescence with aniline blue. J Immunol Methods. 1988 Nov 10;114(1-2):79–88. doi: 10.1016/0022-1759(88)90157-3. [DOI] [PubMed] [Google Scholar]
- Osborn L., Hession C., Tizard R., Vassallo C., Luhowskyj S., Chi-Rosso G., Lobb R. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989 Dec 22;59(6):1203–1211. doi: 10.1016/0092-8674(89)90775-7. [DOI] [PubMed] [Google Scholar]
- Rand T. H., Cruikshank W. W., Center D. M., Weller P. F. CD4-mediated stimulation of human eosinophils: lymphocyte chemoattractant factor and other CD4-binding ligands elicit eosinophil migration. J Exp Med. 1991 Jun 1;173(6):1521–1528. doi: 10.1084/jem.173.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice G. E., Munro J. M., Corless C., Bevilacqua M. P. Vascular and nonvascular expression of INCAM-110. A target for mononuclear leukocyte adhesion in normal and inflamed human tissues. Am J Pathol. 1991 Feb;138(2):385–393. [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Madrid F., De Landázuri M. O., Morago G., Cebrián M., Acevedo A., Bernabeu C. VLA-3: a novel polypeptide association within the VLA molecular complex: cell distribution and biochemical characterization. Eur J Immunol. 1986 Nov;16(11):1343–1349. doi: 10.1002/eji.1830161106. [DOI] [PubMed] [Google Scholar]
- Tepper R. I., Levinson D. A., Stanger B. Z., Campos-Torres J., Abbas A. K., Leder P. IL-4 induces allergic-like inflammatory disease and alters T cell development in transgenic mice. Cell. 1990 Aug 10;62(3):457–467. doi: 10.1016/0092-8674(90)90011-3. [DOI] [PubMed] [Google Scholar]
- Tepper R. I., Pattengale P. K., Leder P. Murine interleukin-4 displays potent anti-tumor activity in vivo. Cell. 1989 May 5;57(3):503–512. doi: 10.1016/0092-8674(89)90925-2. [DOI] [PubMed] [Google Scholar]
- Terstappen L. W., Hollander Z., Meiners H., Loken M. R. Quantitative comparison of myeloid antigens on five lineages of mature peripheral blood cells. J Leukoc Biol. 1990 Aug;48(2):138–148. doi: 10.1002/jlb.48.2.138. [DOI] [PubMed] [Google Scholar]
- Thornhill M. H., Haskard D. O. IL-4 regulates endothelial cell activation by IL-1, tumor necrosis factor, or IFN-gamma. J Immunol. 1990 Aug 1;145(3):865–872. [PubMed] [Google Scholar]
- Thornhill M. H., Wellicome S. M., Mahiouz D. L., Lanchbury J. S., Kyan-Aung U., Haskard D. O. Tumor necrosis factor combines with IL-4 or IFN-gamma to selectively enhance endothelial cell adhesiveness for T cells. The contribution of vascular cell adhesion molecule-1-dependent and -independent binding mechanisms. J Immunol. 1991 Jan 15;146(2):592–598. [PubMed] [Google Scholar]
- Wegner C. D., Gundel R. H., Reilly P., Haynes N., Letts L. G., Rothlein R. Intercellular adhesion molecule-1 (ICAM-1) in the pathogenesis of asthma. Science. 1990 Jan 26;247(4941):456–459. doi: 10.1126/science.1967851. [DOI] [PubMed] [Google Scholar]