Abstract
A transformation analogous in simplicity and functional group tolerance to the venerable Suzuki cross-coupling between alkyl-carboxylic acids and boronic acids is described. This Ni-catalyzed reaction relies upon the activation of alkyl carboxylic acids as their redox-active ester derivatives, specifically N-hydroxy-tetrachlorophthalimide (TCNHPI), and proceeds in a practical and scalable fashion. The inexpensive nature of the reaction components (NiCl2•6H2O – $9.5/mol, Et3N) coupled to the virtually unlimited commercial catalog of available starting materials bodes well for its rapid adoption.
Keywords: nickel catalysis, redox-active, esters, Suzuki, decarboxylation
Graphical abstract
A cross-coupling between alkyl carboxylic acids and aryl boronic acids is enabled by a new activation/cross-coupling strategy under Nickel catalysis. The operational simplicity and the wide range of heterocyclic compounds make a convenient strategy for obtaining aryl-alkyl cross-coupling products.
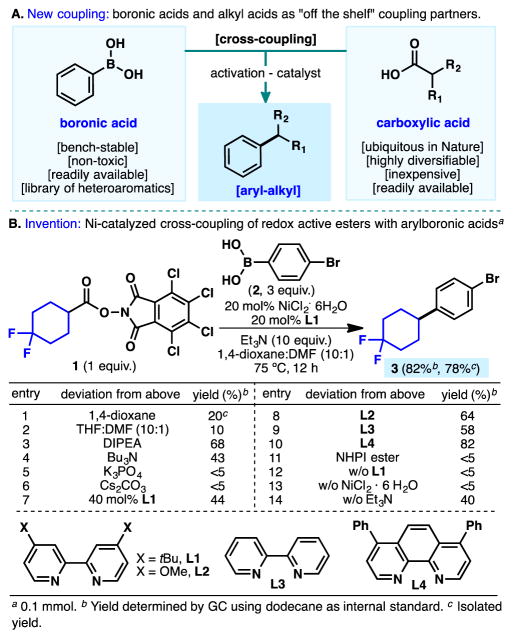
Alkyl carboxylic acids and boronic acids are amongst the most widely available building blocks for modern organic synthesis (Figure 1A). Recent surveys and informatic approaches to mining chemical reaction space within drug discovery explain this phenomenon: Amide bond formation and Suzuki couplings are the most often employed reactions.[1],[2] A reaction that could therefore marry these ubiquitous and convenient building blocks in one diversifying step would likely be of great utility in all areas of chemical science. Earlier this year we reported that common activated esters (HOAt, HOBt, NHPI, etc.) normally employed in amide-bond formation could also engage in Negishi-type couplings with aryl- and alkylzinc reagents under nickel-based catalysis.[3],[4] The fact that all of these activated esters not only prime the carboxyl for nucleophilic attack but also accept an electron from a low-valent metal in an SET-based thermal process was previously unrecognized.[5],[6] Whereas the former process allows for ester and amide formation, the latter enables a simple thermal decarboxylative radical formation with immediate capture by that transition metal (Ni).[3–5],[7] In this Communication, a reaction analogous to the venerable Suzuki coupling for the cross-coupling of activated alkyl carboxylic acids with aryl and vinyl boronic acids is reported exhibiting broad substrate scope, scalability, functional group tolerance, and inherent accessibility.[8]
Figure 1.
(A) Boronic acids and carboxylic acids as desired coupling partners in a new cross-coupling reaction. (B) Optimization of a Ni-catalyzed cross-coupling between boronic acids and redox-active esters.
Although conceptually simple, the execution of this coupling relied on extensive experimentation and some empirical observations that are at this time poorly understood.
Figure 1B presents an abbreviated picture of the optimization conducted on ester 1 and boronic acid 2, culminating in 78% isolated yield of 3. Solvent and concentration played a crucial role in this chemistry, with a mixture of 1,4-dioxane and DMF (0.023 M) emerging as the optimal.[9] For example, exclusion of DMF diminished the yield (entry 1) and when 1,4-dioxane was substituted for THF this seemingly trivial change essentially shut down the reaction (entry 2). The choice of base was also unusually important with excess triethylamine furnishing the best results (entries 3–6).
Next, numerous ligands were screened and it was found that a 1:1 ratio of Ni/L performed the best with L1 and L4 providing the most reliable outcomes (entries 7–10). The choice of ester was also critical as the more electron-rich redox-active NHPI derivative was an incompetent coupling partner under these conditions (entry 11). TCNHPI, an activating agent used for the first time in synthesis as a catalyst for hydrogen atom transfer,[10] proved to be ideal for this coupling. Control experiments pointed to the essential roles of the catalyst and ligand (entries 12–13). Curiously, even in the absence of base the reaction proceeds to some extent, possibly due to the basic nature of the ligands employed (entry 14). It is worth mentioning that a preformed Ni-ligand complex in DMF added to a 1,4-dioxane solution of boronic acid, base, and activated ester gave the most robust results.[9]
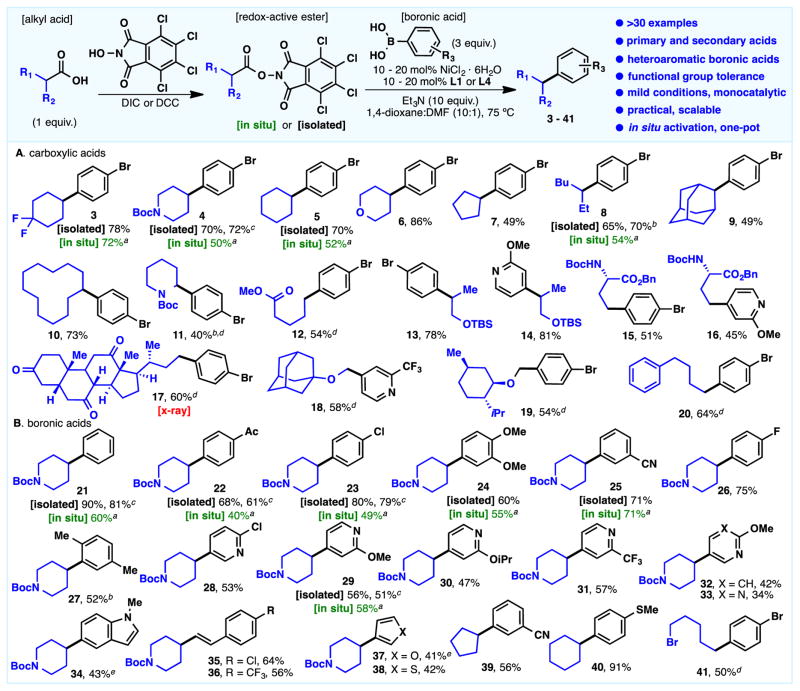
Over thirty examples of this cross-coupling are depicted in Scheme 1. The reaction appears to be general for both coupling partners and largely undeterred by a wide variety of functional groups such as aryl halides (3–13, 15, 17, 19, 20, 23, 26, 28, 35, 41), common protecting groups (4, 11, 13–16, 21–38), esters (12, 15, 16), nitriles (25, 39), ketones (17, 22), carbamates (15–16), and even medicinally relevant heterocycles (14, 16, 18, 28–34). Both primary and secondary alkyl carboxylic acids with or without a radical stabilizing groups are viable (11, 18–19) and N-Boc protected amino acid side chains show no erosion of optical activity (15–16). Both electron-rich (24, 27, 34, 40) and electron-poor (22–23, 25–26, 28–33, 39) aryl boronic acids can be employed as well as vinyl boronic acids (35–36).
Scheme 1.
Initial scope of the Ni-catalyzed cross-coupling of TCNHPI redox-active esters with boronic acids. Reaction conditions: Redox-active ester (1 equiv), NiCl2·6H2O (20 mol%), L1 (20 mol%), Et3N (10 equiv), boronic acid (3 equiv) in 1,4-dioxane:DMF (10:1) at 75 °C for 12 h. [Isolated] refers to yields from the isolated redox-active ester, and [in situ] refers to yields from the carboxylic acid. a for in situ preparation, see SI. b Using L4 instead of L1. c Using 10 mol% NiCl2·6H2O, 10 mol% L1, at 85 °C. d Reaction at 85 °C. e 5 equiv of boronic acid.
An important feature of this new cross-coupling hinges on the simplicity of its setup and the development of a new one-pot protocol. Hence, the transformation could be conducted without the need for isolation of the activated esters, as exemplified by 10 representative substrates in Sheme 1 (3–5, 8, 21–25, 29). A simple activation of the carboxylic acid by DCC, followed by the Suzuki protocol, afforded comparable yields as starting from the isolated redox-active ester. This rapid access coupled with the experimental ease of the union might have a tangible impact on the speed with which many organic compounds can be made. For example, the preparation of p-bromophenyl substituted species such as 5 has previously required as many as five steps to procure.[11] Most routes to compounds such as 3–10 involve organometallic additions to ketones followed by cationic or radical-based reductions.[12] The simple ester 12 has previously required more than 5 steps to prepare.[13] The unnatural amino acids 15 and 16 have required multistep pathways involving homologation of aspartic acid rather than a direct coupling with glutamic acid as in the present work.[14] A direct comparison to reported substrates for Ni-catalyzed protocols using alkyl bromides and arylboronic species resulted similar yields of the cross-coupling product when the redox-active ester is utilized (39–40).[8b] Albeit the apparent similarities of both protocols, we hypothesized that a different mechanism could be operating under our optimized conditions. To test the hypothesis, we embedded an alkyl bromide in a pending chain of a linear redox-active ester and subject it to the optimized conditions. Interestingly, complete chemoselectivity favoring the redox-active ester was obtained for substrate 41, which highlights the orthogonal compatibility with other protocols in the area of Suzuki arylations. It is worth mentioning that the catalyst loading was standardized to 20 mol% across the examples in Scheme 1 for optimal yields. However, we demonstrated that comparable yields could also be obtained when the catalyst loading was lowered to 10% (4, 21–23, 29).
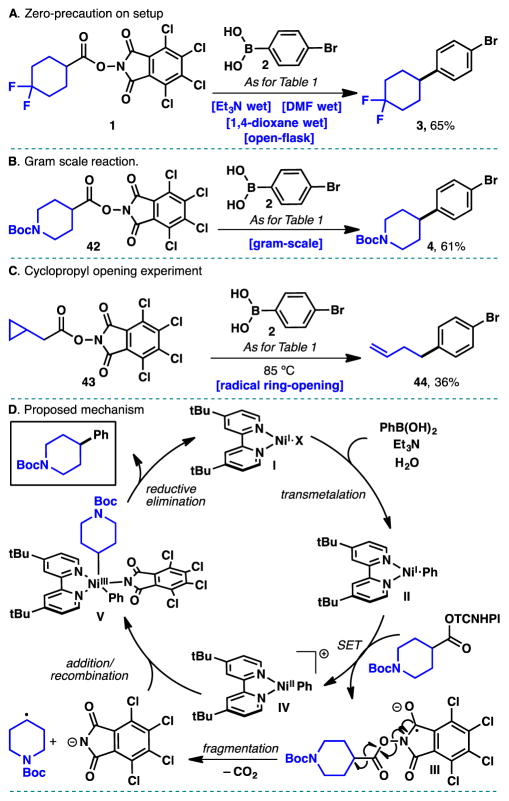
The experimental ease of this transformation cannot be overemphasized as the demanding environment of medicinal chemistry prioritizes those reactions that require minimal experimental precaution. Thus, as depicted in Scheme 2A, a reaction of 1 was conducted using wet solvents without any precaution to exclude air or moisture (open-flask) to produce adduct 3 in 65% isolated yield. The scalability was also evaluated using 42 and it was found to proceed smoothly to deliver 3 on a gram-scale (Scheme 2B). In accord with our previous findings,[3,4] the reaction of redox-active esters with in situ generated low valent Ni species generates radical intermediates as evidenced by the cyclopropane-opened product 44 derived from 43 (Scheme 2C).
Scheme 2.
(A) Reaction carried out with wet solvents and opened to air. (B) Reaction performed at gram-scale. (C) Ring-opening of a cyclopropylmethylene redox-active ester. (D) Working hypothesis for the redox-active ester cross-coupling with aryl- and vinylboronic acids.
A postulated mechanism for this transformation is depicted in Scheme 2D. By analogy to prior mechanistic investigations of Ni-catalyzed cross-coupling reactions with alkyl halides,[7] and our previous studies using organozinc reagents,[3,4] a related pathway is postulated for the coupling of redox-active esters with boronic acids. Initially, a Ni(I) complex I undergoes transmetalation with an arylboronic acid aided by Et3N and H2O delivering aryl–Ni(I) complex II.[15] Reduction of the redox-ester by complex II affords species III which, upon fragmentation, ultimately leads to the alkyl radical and the parent phthalimide anion.[3–5] Subsequent recombination[7,16] with complex IV delivers intermediate complex V which, after reductive elimination, affords the desired product and regenerates the catalytically active species I.
Although it is perhaps too early to cite specific limitations of this transformation, currently ortho-methoxy groups on the boronic acid lead to diminished yield. Acids for which the activated ester is hydrolytically labile leads to lower yields due to competing hydrolysis.[9] Aryl boronic acids that have difficulty in transmetallation, such as 4-pyrimidyl, are also low-yielding. It is possible that optimization of the ligands and redox-active ester might improve the yield of such substrates. Our lab is currently involved in the study of such drawbacks as well as the investigation of the mechanism, in particular the origin on the chemoselectivity with alkyl halides.
A surprisingly simple union of the two most popular building blocks in modern organic chemistry has been enabled through the exclusive use of a redox-active ester (TCNHPI). Numerous examples have been presented to demonstrate the broad scope of this transformation and it has already found use in the context of drug development.[17] It is likely that many other branches of chemistry will benefit from this cross-coupling as well. Studies to expand the scope of coupling to alkyl boronic acids, develop asymmetric variants, and deeply probe the mechanism of this fascinating transformation are underway and will be reported in due course.
Supplementary Material
Acknowledgments
Financial support for this work was provided by NIH (GM-097444), Bristol-Myers Squibb, the Shanghai Institute of Organic Chemistry, Zhejiang Medicine Co., and Pharmaron (postdoctoral fellowship for J. W.), Austrian Science Fund (FWF, Schrödinger Fellowship to L. W.), Department of Defense (NDSEG fellowship to J. T. E.) and Catalan Government (Beatriu de Pinos Postdoctoral Fellowship to J. C.). We thank David E. Hill for help in HPLC measurements. We also thank Ke Chen, Michael Schmidt and Martin D. Eastgate for helpful discussions. We are grateful to D.-H. Huang and L. Pasternack (The Scripps Research Institute) for assistance with nuclear magnetic resonance (NMR) spectroscopy. We also thank A. L. Rheingold, C. E. Moore and M. A. Galella for X-ray crystallographic analysis.[18]
References
- 1.Schneider N, Lowe DM, Sayle RA, Tarselli MA, Landrum GA. J Med Chem. 2016;59:4385. doi: 10.1021/acs.jmedchem.6b00153. [DOI] [PubMed] [Google Scholar]
- 2.Brown DG, Boström J. J Med Chem. 2016;59:4443. doi: 10.1021/acs.jmedchem.5b01409. [DOI] [PubMed] [Google Scholar]
- 3.Cornella J, Edwards JT, Kawamura S, Wang J, Pan C-M, Gianatassio R, Schmidt M, Eastgate MD, Baran PS. J Am Chem Soc. 2016;138:2174. doi: 10.1021/jacs.6b00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qin T, Cornella J, Li C, Malins L, Edwards JT, Kawamura S, Maxwell BD, Eastgate MD, Baran PS. Science. 2016;352:801. doi: 10.1126/science.aaf6123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shortly after ref. 3 was published, D. J. Weix reported that NHPI esters could undergo reductive cross-coupling with aryl iodides using Zn0 as the reducing agent: Huihui KMM, Caputo JA, Melchor Z, Olivares AM, Spiewak AM, Jonhson KA, DiBenedetto TA, Kim S, Ackerman LKG, Weix DJ. J Am Chem Soc. 2016;138:5016. doi: 10.1021/jacs.6b01533.
- 6.For the use of NHPI esters in photochemically initiated SET processes, see: Schnermann MJ, Overman LE. Angew Chem Int Ed. 2012;51:9576. doi: 10.1002/anie.201204977.Angew Chem. 2012;124:9714.Pratsch G, Lackner GL, Overman LE. J Org Chem. 2015;80:6025. doi: 10.1021/acs.joc.5b00795.Okada K, Okamoto K, Oda M. J Am Chem Soc. 1988;110:8736.Okada K, Okamoto K, Morita N, Okubo K, Oda M. J Am Chem Soc. 1991;113:9401.
- 7.As mentioned in ref. 3, redox-active esters are analogous to alkyl halides. Thus, a mechanistic parallel can be directly drawn to pioneering work in the area of Ni-catalyzed single electron transfer cross-coupling of those species, see: Anderson TJ, Jones GD, Vicic DA. J Am Chem Soc. 2004;126:8100. doi: 10.1021/ja0483903.Jones GD, Martin JL, McFarland C, Allen OR, Hall RE, Haley AD, Brandon RJ, Konovalova T, Desrochers PJ, Pulay P, Vicic DA. J Am Chem Soc. 2006;128:13175. doi: 10.1021/ja063334i.Schley ND, Fu GC. J Am Chem Soc. 2014;136:16588. doi: 10.1021/ja508718m.Phapale VB, Buñuel E, García-Iglesias M, Cárdenas DJ. Angew Chem Int Ed. 2007;46:8790. doi: 10.1002/anie.200702528.Angew Chem. 2007;119:8946–8951.Gutierrez O, Tellis JC, Primer DN, Molander GA. J Am Chem Soc. 2015;137:4896. doi: 10.1021/ja513079r.Vedernikov AN. ChemCatChem. 2014;6:2490.Zheng B, Tang F, Luo J, Schultz JW, Rath NP, Mirica LM. J Am Chem Soc. 2014;136:6499. doi: 10.1021/ja5024749.Breitenfeld J, Ruiz J, Wodrich MD, Hu X. J Am Chem Soc. 2013;135:12004. doi: 10.1021/ja4051923.Biswas S, Weix DJ. J Am Chem Soc. 2013;135:16192. doi: 10.1021/ja407589e.
- 8.For selected examples of Ni-catalyzed alkyl-aryl Suzuki-Miyaura cross-couplings, see: Zhou J, Fu GC. J Am Chem Soc. 2004;126:1340. doi: 10.1021/ja039889k.González-Bobes F, Fu GC. J Am Chem Soc. 2006;128:5360. doi: 10.1021/ja0613761.Lundin P, Fu GC. J Am Chem Soc. 2010;132:11027. doi: 10.1021/ja105148g.Wilsily A, Tramutola F, Owston NA, Fu GC. J Am Chem Soc. 2012;134:5794. doi: 10.1021/ja301612y.Zultanski S, Fu GC. J Am Chem Soc. 2013;135:624. doi: 10.1021/ja311669p.Jana R, Pathak TP, Sigman MS. Chem Rev. 2011;111:1417. doi: 10.1021/cr100327p.
- 9.See Supporting Information for details.
- 10.Horn EJ, Rosen BR, Chen Y, Tang J, Chen K, Eastgate MD, Baran PS. Nature. 2016;533:78. doi: 10.1038/nature17431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ueda K, Umihara H, Yokoshima S, Fukuyama T. Org Lett. 2015;17:3191. doi: 10.1021/acs.orglett.5b01351.For an older route involving Friedel-Crafts of benzene with cyclohexene followed by bromination, see: Corson BB, Ipatieff VN. Org Synth. 1939;19:36.
- 12.a) Papanastasiou I, Riganas S, Foscolos GB, Tsotinis A, Akhtar SA, Khan MA, Rahman KM, Thurston DE. Lett Org Chem. 2015;12:319. [Google Scholar]; b) Allwood DM, Blakemore DC, Brown AD, Ley SV. J Org Chem. 2014;79:328. doi: 10.1021/jo402526z. [DOI] [PubMed] [Google Scholar]
- 13.Zaidlewicz M, Wolan A. J Organomet Chem. 2002;657:129. [Google Scholar]
- 14.Jackson RFW, Frase JL, Wishart N, Porter B, Wythes MJ. J Chem Soc Perkin Trans 1. 1998:1903. [Google Scholar]
- 15.Recently, the formation of hydroxy-bridged species between a boronate and the metal center have disclosed as key step for transmetalation in Suzuki cross-couplings with Pd: Thomas AA, Denmark SE. Science. 2016;352:329. doi: 10.1126/science.aad6981.
- 16.The capture of similar radicals was also invoked during the decarboxylative coupling of α-heteroatom stabilized acids with aryl halides: Zuo Z, Ahneman DT, Chu L, Terrett JA, Doyle AG, MacMillan DWC. Science. 2014;345:437. doi: 10.1126/science.1255525.
- 17.This reaction is currently being enlisted at Bristol-Myers Squibb in several ongoing programs, including one involving the co-authors (B. V. and S. A. S.).
- 18.X-ray information for compound 17 can be obtained free of charge from The Cambridge Crystallographic Data center with number CCDC 1477360.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.