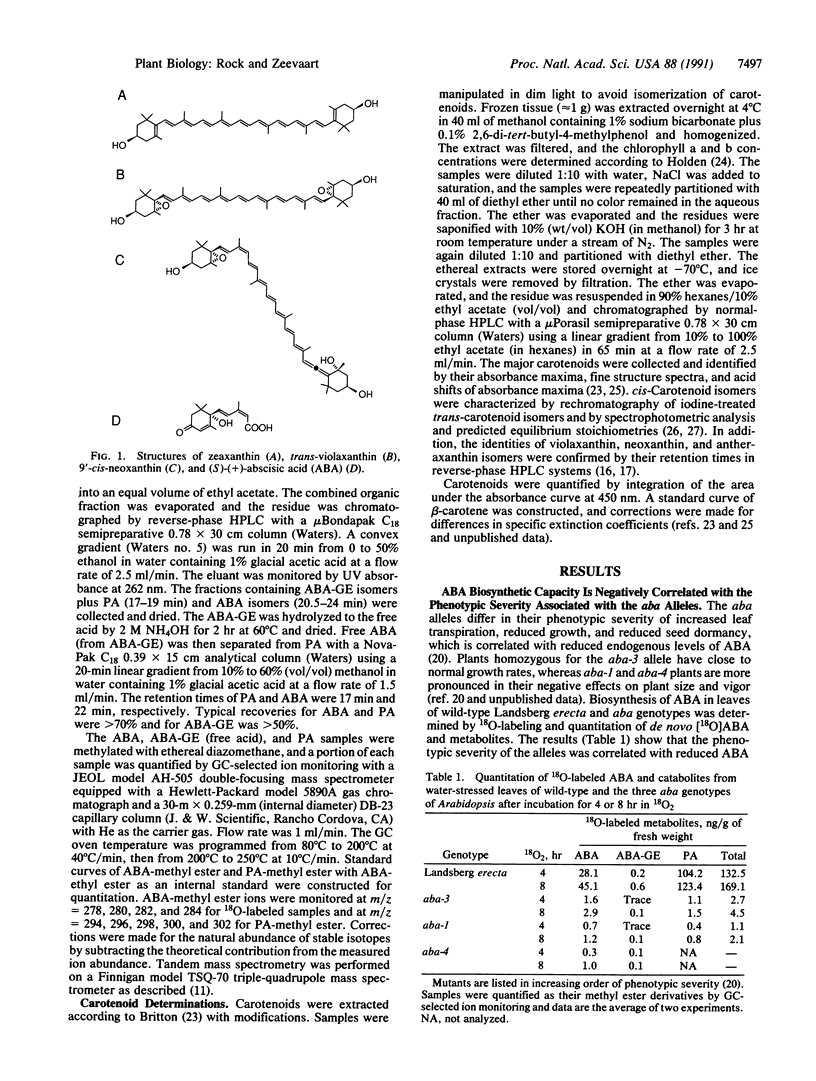
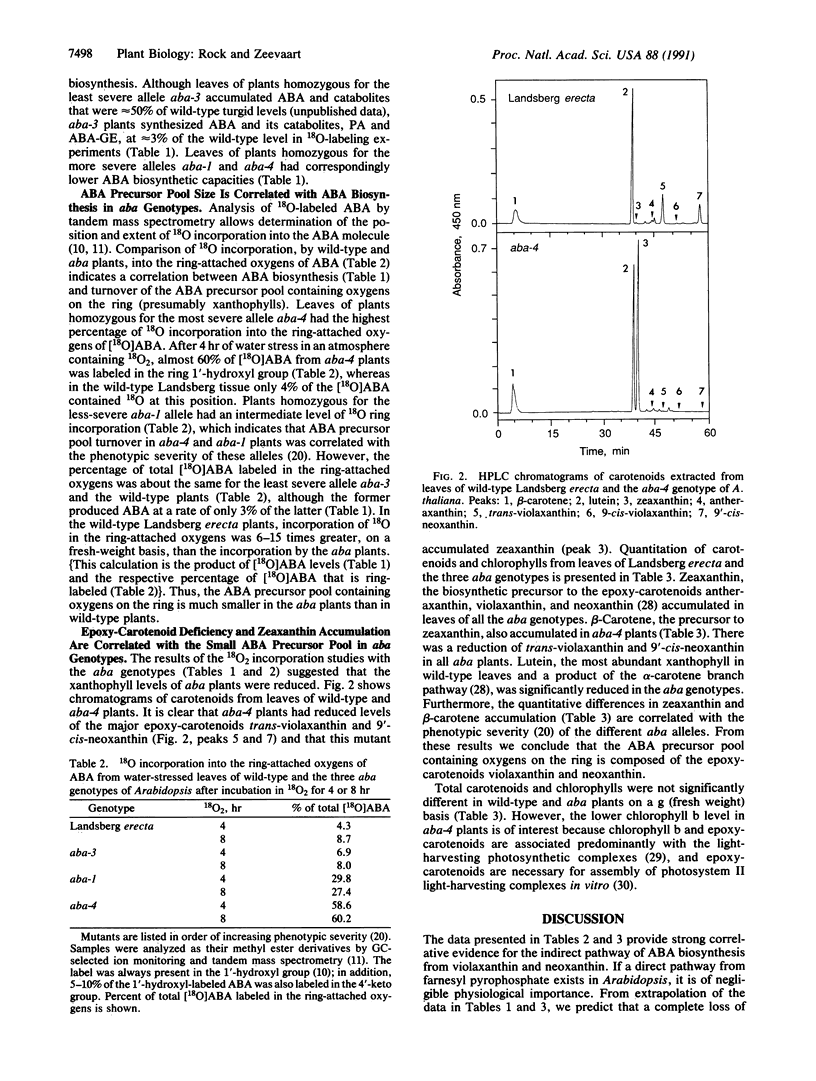
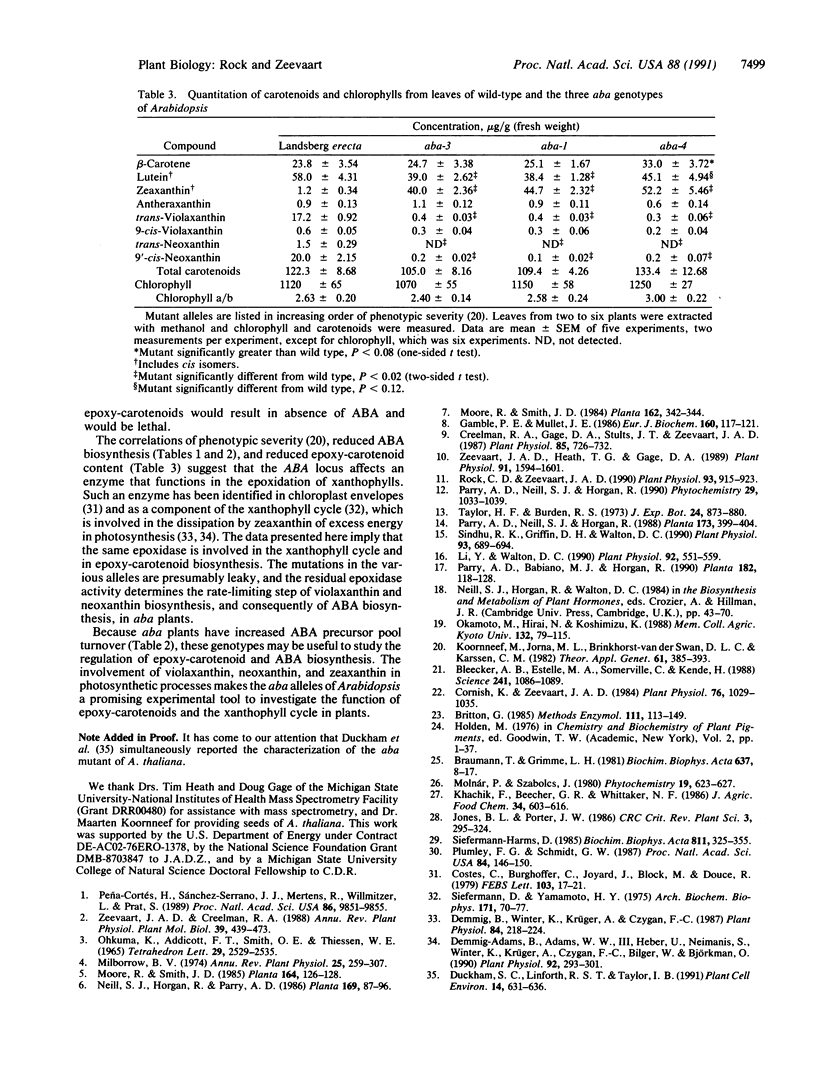
Abstract
The three mutant alleles of the ABA locus of Arabidopsis thaliana result in plants that are deficient in the plant growth regulator abscisic acid (ABA). We have used 18O2 to label ABA in water-stressed leaves of mutant and wild-type Arabidopsis. Analysis by selected ion monitoring and tandem mass spectrometry of [18O]ABA and its catabolites, phaseic acid and ABA-glucose ester (beta-D-glucopyranosyl abscisate), indicates that the aba genotypes are impaired in ABA biosynthesis and have a small ABA precursor pool of compounds that contain oxygens on the ring, presumably oxygenated carotenoids (xanthophylls). Quantitation of the carotenoids from mutant and wild-type leaves establishes that the aba alleles cause a deficiency of the epoxy-carotenoids violaxanthin and neoxanthin and an accumulation of their biosynthetic precursor, zeaxanthin. These results provide evidence that ABA is synthesized by oxidative cleavage of epoxy-carotenoids (the "indirect pathway"). Furthermore the carotenoid mutant we describe undergoes normal greening. Thus the aba alleles provide an opportunity to study the physiological roles of epoxy-carotenoids in photosynthesis in a higher plant.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bleecker A. B., Estelle M. A., Somerville C., Kende H. Insensitivity to Ethylene Conferred by a Dominant Mutation in Arabidopsis thaliana. Science. 1988 Aug 26;241(4869):1086–1089. doi: 10.1126/science.241.4869.1086. [DOI] [PubMed] [Google Scholar]
- Britton G. General carotenoid methods. Methods Enzymol. 1985;111:113–149. doi: 10.1016/s0076-6879(85)11007-4. [DOI] [PubMed] [Google Scholar]
- Cornish K., Zeevaart J. A. Abscisic Acid Metabolism in Relation to Water Stress and Leaf Age in Xanthium strumarium. Plant Physiol. 1984 Dec;76(4):1029–1035. doi: 10.1104/pp.76.4.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creelman R. A., Gage D. A., Stults J. T., Zeevaart J. A. Abscisic Acid Biosynthesis in Leaves and Roots of Xanthium strumarium. Plant Physiol. 1987 Nov;85(3):726–732. doi: 10.1104/pp.85.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmig-Adams B., Adams W. W., Heber U., Neimanis S., Winter K., Krüger A., Czygan F. C., Bilger W., Björkman O. Inhibition of zeaxanthin formation and of rapid changes in radiationless energy dissipation by dithiothreitol in spinach leaves and chloroplasts. Plant Physiol. 1990 Feb;92(2):293–301. doi: 10.1104/pp.92.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmig B., Winter K., Krüger A., Czygan F. C. Photoinhibition and zeaxanthin formation in intact leaves : a possible role of the xanthophyll cycle in the dissipation of excess light energy. Plant Physiol. 1987 Jun;84(2):218–224. doi: 10.1104/pp.84.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble P. E., Mullet J. E. Inhibition of carotenoid accumulation and abscisic acid biosynthesis in fluridone-treated dark-grown barley. Eur J Biochem. 1986 Oct 1;160(1):117–121. doi: 10.1111/j.1432-1033.1986.tb09947.x. [DOI] [PubMed] [Google Scholar]
- Li Y., Walton D. C. Violaxanthin is an abscisic Acid precursor in water-stressed dark-grown bean leaves. Plant Physiol. 1990 Mar;92(3):551–559. doi: 10.1104/pp.92.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R., Smith J. D. Graviresponsiveness and abscisic-acid content of roots of carotenoid-deficient mutants of Zea mays L. Planta. 1985;164:126–128. [PubMed] [Google Scholar]
- Moore R., Smith J. D. Growth, graviresponsiveness and abscisic-acid content of Zea mays seedlings treated with fluridone. Planta. 1984;162:342–344. [PubMed] [Google Scholar]
- Plumley F. G., Schmidt G. W. Reconstitution of chlorophyll a/b light-harvesting complexes: Xanthophyll-dependent assembly and energy transfer. Proc Natl Acad Sci U S A. 1987 Jan;84(1):146–150. doi: 10.1073/pnas.84.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pēna-Cortés H., Sánchez-Serrano J. J., Mertens R., Willmitzer L., Prat S. Abscisic acid is involved in the wound-induced expression of the proteinase inhibitor II gene in potato and tomato. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9851–9855. doi: 10.1073/pnas.86.24.9851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock C. D., Zeevaart J. A. Abscisic (ABA)-Aldehyde Is a Precursor to, and 1',4'-trans-ABA-Diol a Catabolite of, ABA in Apple. Plant Physiol. 1990 Jul;93(3):915–923. doi: 10.1104/pp.93.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siefermann D., Yamamoto H. Y. Properties of NADPH and oxygen-dependent zeaxanthin epoxidation in isolated chloroplasts. A transmembrane model for the violaxanthin cycle. Arch Biochem Biophys. 1975 Nov;171(1):70–77. doi: 10.1016/0003-9861(75)90008-9. [DOI] [PubMed] [Google Scholar]
- Sindhu R. K., Griffin D. H., Walton D. C. Abscisic Aldehyde Is an Intermediate in the Enzymatic Conversion of Xanthoxin to Abscisic Acid in Phaseolus vulgaris L. Leaves. Plant Physiol. 1990 Jun;93(2):689–694. doi: 10.1104/pp.93.2.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevaart J. A., Heath T. G., Gage D. A. Evidence for a universal pathway of abscisic Acid biosynthesis in higher plants from o incorporation patterns. Plant Physiol. 1989 Dec;91(4):1594–1601. doi: 10.1104/pp.91.4.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]


