Abstract
Earlier we reported that NF-κB is activated by protein kinase R (PKR) in herpes simplex virus 1-infected cells. Here we report that in PKR−/− cells the yields of wild-type virus are 10-fold higher than in PKR+/+ cells. In cells lacking NF-κB p50 (nfkb1), p65 (relA), or both p50 and p65, the yields of virus were reduced 10-fold. Neither wild-type nor mutant cells undergo apoptosis following infection with wild-type virus. Whereas PKR+/+ and NF-κB+/+ control cell lines undergo apoptosis induced by the d120 (Δα4) mutant of HSV-1, the mutant PKR−/− and NF-κB−/− cell lines were resistant. The evidence suggests that the stress-induced apoptosis resulting from d120 infection requires activation of NF-κB and that this proapoptotic pathway is blocked in cells in which NF-κB is not activated or absent. Activation of NF-κB in the course of viral infection may have dual roles of attempting to curtain viral replication by rendering the cell susceptible to apoptosis induced by the virus and by inducing the synthesis of proteins that enhance viral replication.
In uninduced cells, the normal constituents of NF-κB are present in the cytosol as an inactive complex with its inhibitor protein, IκBα. Activation of NF-κB usually involves proteasome-dependent degradation of IκBα after it has been phosphorylated by the IκB kinase (IKK). A rich and extensive literature has documented the induction and a role of NF-κB in the course of viral infection of eukaryotic cells. The evidence for the role of NF-κB is based for the most part on experiments with mutated forms of IκBα. The results suggest that activation of NF-κB enhances viral replication. The relationship between the activated NF-κB and apoptosis appears to vary. In cells infected with some viruses, apoptosis is directly related to activation of NF-κB (27, 29). In others, activated NF-κB confers resistance to stress-induced apoptosis (6). In the case of cells infected with herpes simplex virus 1 (HSV-1), the results of the studies done to date are less clear and raise a number of questions that need to be resolved.
Earlier studies noted that HSV-1 induces the translocation of NF-κB from the cytoplasm to the nucleus (35) as a result of the activation of IKK and degradation of IκBα early in infection (2). HSV-1 was reported to activate NF-κB in a biphasic way (39). At first, the activation was rapid, transient, and UV insensitive and was reported to be triggered by the binding of the gD envelope glycoprotein to the cellular receptor (30, 31, 35). The massive and persistent activation of NF-κB was reported to occur later, between 3 and 4 h after infection, concomitant with and dependent on de novo synthesis of viral proteins (2). In particular, two α proteins, ICP4 and ICP27, were reported to be required for NF-κB nuclear translocation (28, 35). Since both ICP4 and ICP27 are proteins that regulate the cascade of viral protein synthesis in infected cells, the requirement for ICP4 or ICP27 could reflect intrinsic properties of the two proteins or a requirement for viral gene expression that was dependent on the presence of functional ICP4 and ICP27 (reviewed in reference 36). A recent study also reported that activation of NF-κB is also required to block apoptosis induced by proapoptotic mutant viruses (14).
We recently reported that the activation of NF-κB in cells productively infected with HSV-1 is dependent on activated protein kinase R (PKR) (42). Activation of PKR results in phosphorylation of the α subunit of translation initiation factor 2 (eIF-2α), causing inhibition of all protein synthesis. The activation of PKR is dependent on the accumulation of complementary RNAs capable of self-annealing to form double-stranded RNA. In infected cells, significant accumulation of complementary RNAs occurs after the onset of viral DNA synthesis (19, 24), and indeed, activation of PKR in infected cells is blocked by phosphonoacetate, a potent inhibitor of viral DNA synthesis (8). In wild-type (WT) virus-infected cells, a viral protein, γ134.5, circumvents the effect of activated PKR by recruiting protein phosphatase 1α to dephosphorylated eIF2-α (15-17). Curiously, HSV-1 also encodes a late, γ2 protein (US11), which when expressed early in infection blocks the activation of PKR (7, 32). A central unresolved question is why HSV-1 selected γ134.5, a protein that merely circumvents the effect of PKR, rather than US11, a protein that blocks its activation.
One hypothesis that could account for the evolutionary selection is that HSV-1 required for its replication a function of activated PKR. To date, the only such activity noted in infected cells is the activation of NF-κB (42). Activated NF-κB could have two roles: to enhance viral replication by inducing the synthesis of NF-κB-dependent cellular proteins and to block apoptosis induced by the stress associated with viral gene expression. In an earlier study (42), we have shown that NF-κB is not required to block apoptosis in productively infected cells, since no evidence of apoptosis was detected in cells infected with a replication-competent mutant (R5104) that does not induce NF-κB activation by blocking PKR early in infection. The objective of the studies reported here was twofold. The first was to examine the role of PKR and NF-κB in the replication of WT virus. In this instance, to avoid methodological errors resulting from tests based solely on IκBα mutant cell lines, we measured viral replication in PKR-null cells as well as in cells from which the major components of NF-κB, p50/nf-kb1 and p65/relA, were knocked out singly or together. Moreover, we also examined the apoptotic activity of both the WT and a proapoptotic virus mutant in these cells to establish unambiguously the role of NF-κB in blocking virally induced apoptosis.
In this article, we report that whereas in PKR−/− cells the yields of WT virus were 10-fold higher than in PKR+/+ cells, in cells lacking NF-κB p50 (nfkb1), p65 (relA), or both p50 and p65, the yields of virus were reduced 10-fold. Neither WT nor mutant cells undergo apoptosis following infection with WT virus. Moreover, whereas PKR+/+ and NF-κB+/+ control cell lines undergo apoptosis induced by the d120 (Δα4) mutant of HSV-1, the mutant PKR−/− and NF-κB−/− cell lines were resistant. We conclude on the basis of the evidence presented in this report that stress-induced apoptosis resulting from infection with the d120 mutant virus requires activation of NF-κB and that in cells in which NF-κB is not activated or absent, this proapoptotic pathway is blocked.
MATERIALS AND METHODS
Cells and viruses.
Immortalized murine fibroblasts nf-kb1-deficient (p50−/−), relA-deficient (p65−/−) and nf-kb1/relA doubly deficient (p50/p65−/−) and WT control cells kindly provided by A. Hoffman and D. Baltimore (University of California, San Diego, and Caltech, Pasadena, Calif., respectively) were propagated in RPMI 1640 supplemented with 10% fetal calf serum. The cells were produced as described elsewhere (18). PKR−/− and PKR+/+ mouse embryo fibroblasts (MEFs) were kindly provided by B. R. G. Williams (Cleveland Clinic Foundation, Cleveland, Ohio) and they were propagated in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum. HSV-1(F) is the prototype HSV-1 strain used in this laboratory (11). The d120 mutant lacking both copies of the α4 gene (9) and the Δα27 mutant virus (27LacZ) (41) were the kind gifts of S. J. Silverstein (Columbia University) and Neal A. DeLuca (University of Pittsburgh), respectively. Cell monolayers were infected with the indicated viruses for 1 h at 37°C at a multiplicity of infection of 10 PFU/cell.
Infectious virus yield titration.
Confluent cell monolayers were exposed to 0.5, 1, or 5 PFU of HSV-1(F) per cell in 199V medium (Sigma) supplemented with 1% calf serum for 1 h at 37°C. The inoculum was then removed, and the cell monolayers were rinsed with 199V medium to remove the unadsorbed virus. The cells were overlaid with complete medium and incubated at 37°C for an additional 24 h. The cells and medium were subjected to 3 cycles of freeze-thawing and then briefly sonicated, and the titers on confluent monolayers of Vero cells were determined.
Immunoblots.
Cells were collected by scraping directly into the medium, rinsed once with cold phosphate-buffered saline (PBS), transferred to a 1.5-ml Eppendorf tube, and lysed in radioimmunoprecipitation assay buffer (PBS containing 1% Nonidet P-40 [NP-40], 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 1 mM sodium orthovanadate, 5 mM EDTA, protease inhibitor mixture [Complete protease mixture; Roche Diagnostics, Indianapolis, Ind.]). Samples were kept on ice for 1 h, and insoluble material was pelleted by centrifugation at maximum speed in Eppendorf centrifuge 5415 C for 10 min at 4°C. The protein concentration was measured with a Bio-Rad protein assay (Bio-Rad, Hercules, Calif.) according to directions provided by the manufacturer. Approximately 50 μg of total proteins was separated on a 10% denaturing polyacrylamide gel and electrically transferred to a nitrocellulose membrane at 300 mA (constant) for 4 h in Tris-glycine-methanol buffer at 4°C. The membranes were blocked for 2 h with 5% nonfat dry milk in PBS and reacted with the appropriate primary antibody overnight at 4°C, rinsed, and exposed to secondary antibody alkaline phosphatase (AP) conjugated at room temperature for 1 h. The antibodies were diluted in PBS containing 1% bovine serum albumin, and 0.05% Tween 20. All rinses were done in PBS containing 0.05% Tween 20. To develop AP-conjugated secondary antibodies, the immunoblots were reacted with AP buffer (100 mM Tris-HCl, pH 9.5, 100 mM NaCl, 5 mM MgCl2), followed by AP buffer containing 5-bromo-4-chloro-3-indolyl phosphate and nitroblue tetrazolium. The HSV-1 proteins were detected with the anti-US11 monoclonal antibody (37), anti-UL38 polyclonal antibody (43), anti-ICP27 monoclonal antibody (1), anti-UL42 monoclonal antibody (40), and anti-thymidine kinase (TK) polyclonal antibody reported elsewhere. Mouse monoclonal antibody LP1 tot α-transinducing factor (α-TIF; VP16) was a kind gift from A. Minson. The rabbit polyclonal anti-PARP antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.).
Measurement of DEVDase activity.
Caspase-3 activity in cellular extracts was assayed by using a tetrapeptide (Asp-Glu-Val-Asp) conjugated to phenylnitraniline (DEVD-pNA) (Biomol, Plymouth Meeting, Pa.) as described elsewhere (4). Briefly, cells grown in 25-cm2 flask cultures were either mock infected or infected with 10 PFU of HSV-1(F) or d120 mutant virus per cell. As a control, the cells were treated with 1 M sorbitol for 5 h or exposed for 16 h to different concentrations of tumor necrosis factor alpha (TNF-α) (Roche Diagnostics) in the presence of 50-ng/ml actinomycin D (Sigma, St. Louis, Mo.). As previously reported, in most fibroblast cell types treated with TNF-α, the apoptotic effects are fully apparent only in the presence of actinomycin D (10). The cells were scraped, rinsed twice with PBS, resuspended in 150 μl of lysis solution (0.1% CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate}, 50 mM HEPES [pH 7.4], 1 mM dithiothreitol, 0.1 mM EDTA), and incubated on ice for 10 min. Lysates were then centrifuged at maximum speed in Eppendorf centrifuge 5415 C for 10 min at 4°C. Supernatant fluids were collected, the protein content was measured, and equal amounts of protein were tested for DEVDase activity according to the manufacturer's instructions (BioMol). Chromophore release was quantified by measuring the A405 with a spectrophotometer after 2 h of incubation at 37°C. The results are expressed as fold increase in activity compared to mock-infected or untreated cells.
Quantification of viral DNA by real-time PCR.
Cells grown in 25-cm2 flask cultures were infected with 10 PFU of HSV-1, collected 18 h after infection, rinsed twice with cold PBS, and resuspended in 200 μl of PBS. Total DNA was extracted with the aid of QIAamp DNA blood kit (QIAGEN, Valencia, Calif.) and eluted in 200 μl of water. HSV-1 DNA polymerase Taqman primers and probe were designed with Primer Express software (Applied Biosystems, Foster City, Calif.). The forward primer was ACCGCCGAACTGAGCAGAC, the reverse primer was TGAGCTTGTAATACACCGTCAGGT, and the probe was 6FAM-CGCGTACACCAACAAGCGCCTG-TAMRA. The assay conditions were optimized in an ABI Prism 7700 SDS (Applied Biosystems). Five microliters of the 200 μl of DNA extract were added to 25 μl of reaction mix, containing 900 nM each primer, 200 nM dual-labeled fluorogenic probe, and 1× Taqman universal master mix. Samples were run in triplicate. Thermal cycling was performed with an initial step of 50°C for 2 min, activation of AmpliTaq Gold DNA polymerase at 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 15 s, and a combined annealing-extension step at 60°C for 1 min. Fluorescence data were collected during each annealing-extension step and analyzed by using ABI Prism SDS software, version 1.6.3. A plasmid containing HSV DNA polymerase kindly provided by B. I. Rosen (New York State Department of Health, Albany, N.Y.) was used to construct the standard curve. A linear six-point standard curve ranging from 10 to 106 copies/5 μl resulted in Ct values of 32.7 to 15.7, respectively.
RESULTS
Virus yield is reduced in cells lacking NF-κB.
It has been reported that in cell expressing a constitutive-repressor version of IκBα, there was a reduced nuclear localization of NF-κB, concomitant with an 80 to 90% decrease in virus yield. The expression of the modified version of IκBα had no effect on the accumulation of representative α, β, and γ viral proteins (2, 35). Several putative NF-κB binding sites in the ICP0 and VP16 HSV-1 genes have been identified (38). It has been proposed, but not proven, that the virus growth-enhancing effects of activated NF-κB were the result of enhanced transcription of α0 and UL48 genes that contained NF-κB consensus sequences in their promoters (2, 39).
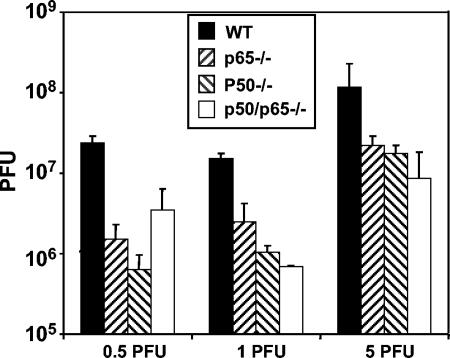
To complete the analysis of the role of NF-κB in the course of HSV-1 infection, we examined the properties of mouse cells from which the major components of NF-κB, p50/nf-κb1 and p65/relA were knocked out singly or together. Confluent cell monolayers of murine fibroblasts nf-kb1-deficient (p50−/−), relA-deficient (p65−/−), and nf-kb1/relA doubly deficient (p50/p65−/−) cells as well as WT control cells were infected with 0.5, 1, or 5 PFU of HSV-1(F) per cell at 37°C. After 1 h of exposure, the virus inoculum was replaced with fresh medium and the cells were incubated at 37°C for an additional 24 h. Virus yields were determined by plaque assay on confluent Vero cell monolayers. The results, representing the average of the three different experiments, are shown in Fig. 1. Irrespective of the initial virus input, the yield of virus was consistently 10-fold lower in mutant cells than in infected WT NF-κB+/+ cells.
FIG. 1.
Virus yield is reduced in cells lacking NF-κB. Confluent cell monolayers of WT, p50−/−, p65−/−, or p50/p65−/− cells were infected with 0.5, 1, or 5 PFU of HSV-1(F) per cell at 37°C. After 1 h of exposure, the virus inoculum was replaced with fresh medium and the cells were incubated at 37°C for an additional 24 h. Virus yields were measured by plaque assay on confluent Vero cell monolayers. The bars represent the average of three different experiments. The standard deviation is also shown. Note that the p50/p65−/− cells infected with 0.5 PFU/cell yielded titers of 7 × 106, 1 × 106, and 8 × 105. Although the high yield obtained in one experiment could not be reproduced, it was nevertheless averaged with the other data.
The accumulation of viral DNA is not affected by the absence of NF-κB.
In the attempt to clarify the role of NF-κB in HSV-1 replication, viral DNA accumulation was analyzed with the aid of real-time PCR. Confluent cell monolayers of p50−/−, p65−/−, p50/p65−/−, and WT cells were mock infected or exposed to 5 PFU of HSV-1(F) per cell at 37°C. After 1 h of exposure, the virus inoculum was replaced with fresh medium and the cells were incubated at 34°C for an additional 18 h. Total DNA was extracted and processed as described in Materials and Methods. The amounts of viral DNA accumulating in infected mutant NF-κB−/− cells could not be differentiated from that accumulating in infected NF-κB+/+ cells. The HSV-1(F) DNA copy numbers were as follows: WT, 4.8 × 107; p50−/−, 6.9 × 107; p65−/−, 6.1 × 107; and p50/p65−/−, 4.3 × 107. The results represent the average of two experiments.
Viral protein synthesis and viral morphology were not affected by the absence of NF-κB.
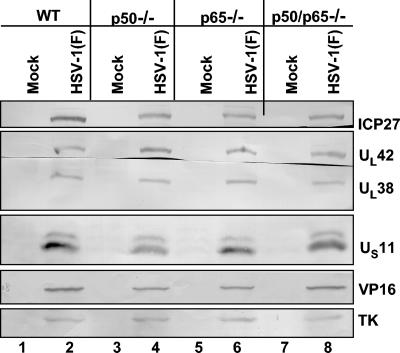
In order to assess if the reduced virus yield reflected defects in the pattern of viral protein synthesis, confluent cell monolayers of p50−/−, p65−/−, p50/p65−/− as well as of WT cells were mock infected or infected with 10 PFU of HSV-1(F) per cell. The cells were harvested at 16 h after infection and processed as described in Materials and Methods. Equal amounts of proteins were electrophoretically separated on a 10% denaturing polyacrylamide gel, transferred to a nitrocellulose sheet, and reacted with the antibodies made against representative α, β, or γ proteins as shown in Fig. 2. As shown in that figure, the accumulation of the α protein ICP27, β proteins UL42 and TK, γ1 protein VP16, and γ2 proteins UL38 and US11 were comparable in extracts prepared from p50−/−, p65−/−, and p50/p65−/− cells and in control WT cells (compare lanes 4, 6, and 8 with lane 2). In a separate experiment, electron microscopic images of infected mutant cells could not be differentiated from those of NF-κB+/+ cells (data not shown).
FIG. 2.
Viral protein synthesis is not affected by the absence of NF-κB. Confluent cell monolayers of WT, p50−/−, p65−/−, and p50/p65−/− cells were mock infected (lanes 1, 3, 5, and 7, respectively) or infected with 10 PFU of HSV-1(F) per cell (lanes 2, 4, 6, and 8, respectively) for 16 h. The cells were then harvested and processed as described in Materials and Methods. Equal amount of proteins were electrophoretically separated on a 10% denaturing polyacrylamide gel, transferred to a nitrocellulose sheet, and reacted with the antibodies made against representative α (ICP27), β (UL42 and TK) or γ (γ1, VP16, γ2, UL38, and US11) proteins.
Activation of NF-κB does not play a role in blocking apoptosis after HSV-1 infection.
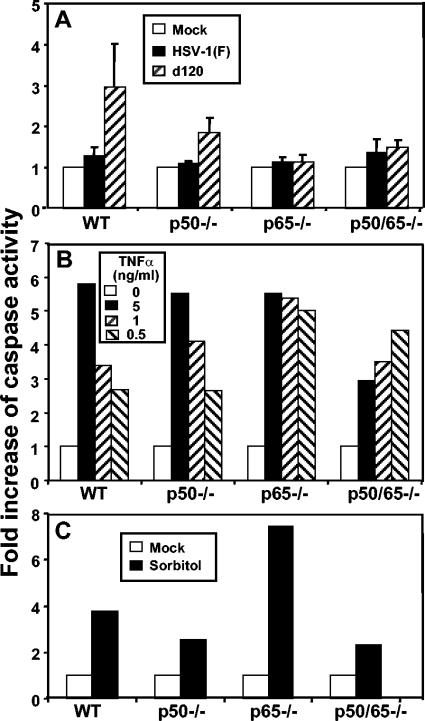
In an earlier study (42), we showed that NF-κB is not required to block apoptosis in productively infected cells, since no evidence of apoptosis was detected in cells infected with a replication-competent mutant (R5104) that does not induce NF-κB nuclear translocation by blocking PKR early in infection. To extend these studies, we also tested p50−/−, p65−/−, p50/p65−/−, or WT control cells that were either mock infected or infected with WT virus or a replication-incompetent mutant lacking both copies of the α4 genes (d120) (9) and known to induce classical apoptosis (chromatin condensation, fragmentation of viral DNA, and cytoplasmic vacuolization and blebbing) in a variety of cell lines (13, 25, 26). In these experiments, confluent cell monolayers were infected with 10 PFU of HSV-1(F) or d120 mutant virus per cell and whole-cell extracts were prepared 18 h after infection. Equal amounts of proteins were assayed for caspase-3 activity as described in Materials and Methods. The caspase-3 activities of the cell lysates were normalized with respect to the baseline activity of mock-infected cells. The results, representing the average of three different experiments, are shown in Fig. 3A and may be summarized as follows. (i) HSV-1(F) did not induce caspase-3 activation in either NF-κB+/+ or mutant cells. The caspase-3 activity of all type of cells infected with WT HSV-1 was equal to or less than 1.4-fold relative to that of the mock-infected cells. (ii) To our surprise, the d120 mutant reproducibly induced caspase-3 activation in WT cells but not in the NF-κB mutant cells. As expected, the caspase-3 activity of the lysates of WT cells infected with d120 mutant virus was an average of threefold higher than that of the mock-infected cells, while those of d120-infected p65−/− and p50/65−/− cells were comparable to those measured in the HSV-1(F)-infected counterparts. In some experiments, we detected caspase-3 activity in d120-infected p50−/− cells that was severalfold lower than that observed in WT cells. To determine whether the NF-κB mutant cells are generally resistant to apoptosis-inducing agents, the cells were incubated with different concentration of TNF-α in the presence of actinomycin D, as described in Materials and Methods. The results shown in Fig. 3B may be summarized as follows: All of the cell lines reproducibly showed induction of caspase-3 activity after exposure to TNF-α. TNF-α induced a dose-dependent apoptotic response in WT and p50−/− mutant cells. p65−/− cells produced higher caspase-3 activity than either WT or p50−/− cells. In contrast, the p50/65−/− mutant cells reproducibly showed higher caspase-3 activation at lower doses of TNF-α and appeared to be resistant to high doses of the drug. We can speculate that a high dose of TNF-α induces the expression of some genes that counteract the proapoptotic pathway in cells lacking both canonical subunits of NF-κB. To further extend our analysis, the NF-κB mutant cells as well as WT control cells were exposed to sorbitol (12, 23). As shown in Fig. 3C, all of the cell lines showed an increased caspase-3 activity after exposure to sorbitol, although the levels of induction varied among the mutant cells. Furthermore, we verified by immunoblotting that the d120 mutant-infected NF-κB mutant cells expressed levels of the α proteins ICP0 and ICP27 comparable to those observed in d120-infected WT control cells (data not shown). Taken together, these results showed that the resistance to d120-induced apoptosis of NF-κB mutant cells is not related to an intrinsic resistance of these cells to apoptosis-inducing agents nor due to resistance of the mutant cells to d120 infection.
FIG. 3.
Caspase-3 activity in infected and TNF-α- or sorbitol-treated WT and NF-κB mutant cells. Caspase-3 activity in cellular extracts was assayed as described in Materials and Methods. (A) Confluent cell monolayers of WT, p50−/−, p65−/−, or p50/p65−/− cells were mock infected or infected with 10 PFU of HSV-1(F) or d120 per cell and collected 18 h after infection. The bars represent the average of three different experiments. The standard deviation is also shown. (B) Confluent cell monolayers of WT, p50−/−, p65−/−, or p50/p65−/− cells were either mock treated or treated with 0.5, 1, and 5 ng of TNF-α per ml in the presence of actinomycin D (50 ng/ml) for 16 h. (C) Confluent cell monolayers of WT, p50−/−, p65−/−, or p50/p65−/− cells were either mock treated or treated with 1 M sorbitol for 5 h.
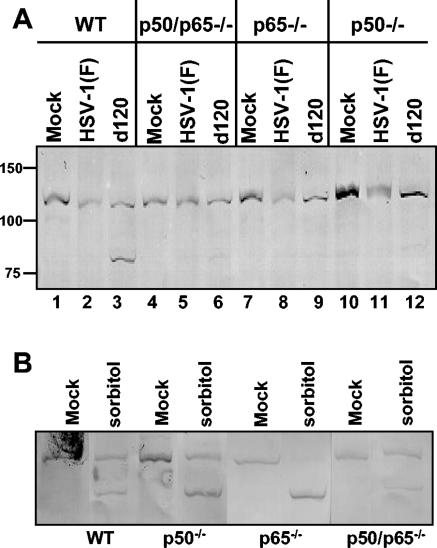
To further verify these results, the experiments were repeated, except that the infected cells were examined for the status of the poly(ADP-ribose) polymerase (PARP) as an indicator of apoptosis. The results shown in Fig. 4 were as follows. (i) The product p85 of PARP cleavage was absent, as expected from lysates of cells infected with HSV-1(F) virus (Fig. 4A, lanes 2, 5, 8, and 11). As anticipated by the caspase-3 assay results, the p85 cleavage product was readily detected only in lysates of wild-type cells infected with d120 mutant virus, but not in d120 mutant-infected NF-κB mutant cells (Fig. 4A, compare lane 3 with lanes 6, 9, and 12), concordant with the evidence that the latter are resistant to d120 mutant-induced apoptosis. (ii) The product p85 of PARP cleavage was present, as expected, in all of the cell lines exposed to sorbitol (Fig. 4B).
FIG. 4.
PARP cleavage activity in infected WT and NF-κB-null cells. (A) Confluent cell monolayers of WT, p50/p65−/−, p50−/−, or p65−/− cells were mock infected (lanes 1, 4, 7, and 10) or infected with 10 PFU of HSV-1(F) (lanes 2, 5, 8, and 11) or d120 mutant virus (lanes 3, 6, 9, and 12) per cell for 18 h. (B) Confluent cell monolayers of WT, p50/p65−/−, p50−/−, or p65−/− cells were mock treated or exposed to 1 M sorbitol for 5 h. The cells were then harvested and processed as described in Materials and Methods. Equal amounts of proteins were electrophoretically separated on a 10% denaturing polyacrylamide gel, transferred to a nitrocellulose sheet, and reacted with the rabbit polyclonal anti-PARP antibody.
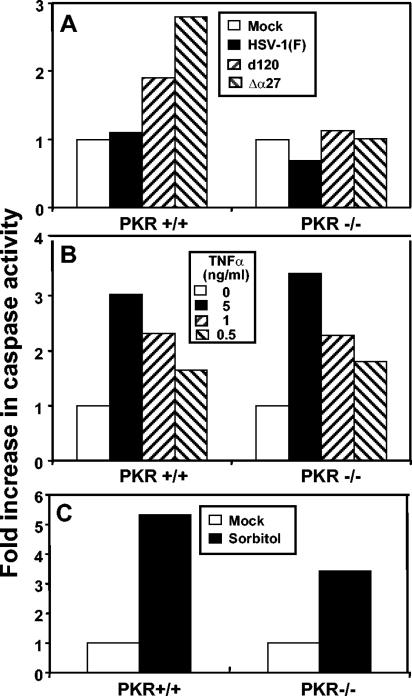
The failure of d120 mutant to induce apoptosis in NF-κB mutant cells was surprising and raised the question of whether this was a general feature of cells in which NF-κB was not activated or a novel property of the null mutants. To resolve this question, we infected PKR−/− and PKR+/+ MEFs with either HSV-1(F) or the d120 mutant virus or another HSV-1 mutant lacking the α27 gene (27lacZ) (41) and also shown to induce apoptosis in several human cell lines (3). Whole-cell extracts were prepared 18 h after infection and assayed for caspase-3 activity as described above. The results shown in Fig. 5 may be summarized as follows. (i) PKR−/− cells were resistant to d120 or to Δα27 mutant virus-induced apoptosis (Fig. 5A). The caspase-3 activities of d120- or Δα27-infected PKR−/− cells were equal to those of the mock-infected cells, while those measured in d120- or Δα27-infected PKR+/+ cells were at least twofold higher. (ii) PKR−/− cells responded to TNF-α treatment by the activation of caspase-3 to levels comparable to those observed in PKR+/+ cells (Fig. 5B). (iii) Finally, PKR−/− cells were susceptible to apoptosis induced by exposure to sorbitol (Fig. 5C).
FIG. 5.
Caspase-3 activity in infected and TNF-α- or sorbitol-treated PKR+/+ and PKR−/− MEFs. Caspase-3 activity in cellular extracts was assayed as described Materials and Methods. (A) Confluent cell monolayers of PKR+/+ and PKR−/− cells were either mock infected or infected with 10 PFU of HSV-1(F), d120, or Δα27 mutant viruses per cell and collected 18 h after infection. (B) Confluent cell monolayers of PKR+/+ and PKR−/− cells were either mock treated or treated with 0.5, 1, and 5 ng of TNF-α per ml in the presence of 50 ng of actinomycin D per ml for 16 h. (C) Confluent cell monolayers of PKR+/+ and PKR−/− cells were either mock treated or treated with 1 M sorbitol for 5 h.
Our data are consistent with the hypothesis that the apoptosis resulting from the intracellular stress induced by the d120 mutant requires activation of an NF-κB-dependent metabolic pathway.
Virus yield is increased in PKR−/− cells.
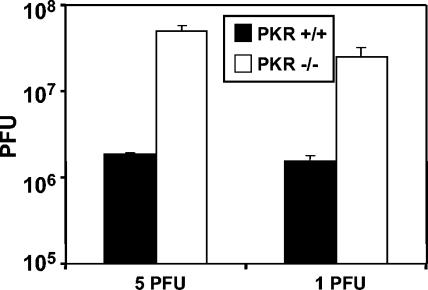
It has been reported that the absence of PKR greatly increases replication of HSV-1 at high multiplicity of infection (22). Since we observed that the PKR−/− and NF-κB−/− cells share the apoptosis resistance phenotype described above, it was of interest to determine whether, at the same low multiplicities of infection used in the experiments with NF-κB mutant cells, PKR−/− cells were also defective in their ability to produced infectious virus. Confluent cell monolayers of null and WT PKR MEFs were infected with 1 and 5 PFU of HSV-1(F) per cell for 1 h at 37°C. The cells were harvested and the titer of virus was determined 24 h after infection. The results, representing the average of two different experiments, are shown in Fig. 6. In contrast to NF-κB mutant cells, PKR−/− cells produce >10-fold more virus than the PKR+/+ cells.
FIG. 6.
Virus yield is increased in PKR−/− cells. Confluent cell monolayers of PKR+/+ and PKR−/− cells were infected with 1 or 5 PFU of HSV-1(F) per cell at 37°C. After 1 h of exposure, the virus inoculum was replaced with fresh medium and the cells were incubated at 37°C for an additional 24 h. The bars represent the average of two different experiments. The standard deviation is also shown.
DISCUSSION
The studies described in this report stemmed from the observations that HSV-1 activates PKR but evades the shutoff of protein synthesis due to phosphorylation of eIF-2α by recruiting and diverting phosphatase 1α to dephosphorylate eIF-2α, thus effectively ignoring the activated PKR. At the same time, HSV-1 encodes a protein, US11, that blocks the activation of PKR as an early protein but has no effect on PKR when expressed as a γ2 protein at late times after infection (7, 15-17). The expectation that PKR may have a potentially deleterious effect on viral replication independently of the phosphorylation of eIF-2α is borne out in the studies presented in this report showing that PKR−/− cells yielded >10-fold more virus than the sibling, PKR+/+ cells. One hypothesis and the focus of this and of an earlier study is that HSV-1 chose to evolve the γ134.5 protein that binds and diverts phosphatase 1α to US11, which could block activation of PKR, because the virus needs the activated PKR. In an earlier report (42), we showed that in HSV-1-infected cells activated PKR induces NF-κB and that in the absence of activated PKR or in PKR−/− cells NF-κB is not activated. This report centers on the role of PKR and NF-κB on viral replication and on the induction of apoptosis resulting from the stress of viral replication. There are two fundamental conclusions to be drawn from the results presented in this report.
The first conclusion concerns the requirements for viral replication. In these studies, we have examined immortalized cells that were PKR+/+/NF-κB+/+ or PKR−/−/NF-κB+/+ and three versions of PKR+/+/NF-κB−/− cells. PKR is activated by WT virus in all PKR+/+ cells, whereas NF-κB is activated only in PKR+/+/NF-κB+/+ cells. Our results show that viral replication was enhanced in PKR−/− cells, in which NF-κB proteins are present but not activated but actually decreased more than 10-fold in cells that were PKR+/+/NF-κB−/−. In PKR−/−/NF-κB+/+ cells, p50 and p65 were present but not translocated to the nucleus, whereas in PKR+/+/NF-κB−/− cells, either one or both p50 and p65 were absent. The conclusion to be drawn from this set of data is that HSV-1 requires for its replication at least the nonactivated NF-κB proteins and possibly both activated and nonactivated constituents of the NF-κB complex.
The role of NF-κB in viral replication remains to be elucidated. It is of interest to note that accumulation of viral DNA in NF-κB−/− cells was significantly and reproducibly higher than in WT cells. This observation suggests that a role of NF-κB is to switch the cells from producing DNA to DNA packaging and viral assembly.
The second conclusion concerns the role of NF-κB in cells exposed to proapoptotic stimuli. The results presented in this and an earlier report (42) show that WT virus does not induce apoptosis in cells in which NF-κB was not activated or absent. Regardless of the role of NF-κB, the data are not surprising, since WT HSV-1 blocks apoptosis induced by numerous exogenous proapoptotic agents. At least three HSV-1 genes—those coding for the glycoproteins D and J and the protein kinase US3—effectively block apoptosis induced by exogenous agents or viral mutants (4, 12, 20, 21, 33, 34, 44, 45). The cells lacking activated NF-κB have not lost their capacity to undergo apoptosis, as illustrated by the observation of their sensitivity to the action of either TNF-α or sorbitol. The selective failure of d120 to induce apoptosis in either PKR−/− or NF-κB−/− cells could be the result of two possible mechanisms: (i) activation of a specific pathway blocking d120-induced apoptosis or (ii) impairment of the proapoptotic pathway induced by d120 infection. Recent studies suggest that both mechanisms may be operative. Thus, recent studies indicate that the US3 protein kinase activates protein kinase A and phosphorylates the regulatory subunit IIα and other substrates of protein kinase A. Moreover, activation of protein kinase A in the absence of the US3 protein kinase blocks apoptosis induced by the d120 mutant (5). It is conceivable that protein kinase A is activated in PKR−/− or NF-κB−/− cells.
We should note in contrast to the studies reported by Der et al. (10) that the PKR−/− cells were susceptible to apoptosis on exposure to 1-ng/ml TNF-α. Τhe discrepancy in the results may reflect the fact that we used caspase-3 activation and PARP cleavage, whereas Der et al. relied primarily on cell viability assays.
At this point, several lines of evidence indicate that failure to activate NF-κB does not lead to apoptosis in HSV-1(F)-infected cells. Thus, a replication-competent mutant virus in which US11 is expressed early in infection does not activate PKR or NF-κB and does not induce apoptosis. Apoptosis is not a consequence of infection with either WT or d120 mutant virus of PKR−/− cells in which NF-κB is not activated or of NF-κB single-knockout (p50 or p65) or double-knockout (p50 and p65) cells. Our results are therefore totally inconsistent with those of Goodkin et al. (14). The discrepancy may well be due to different methodologies used in these studies, including the use of cycloheximide early in infection, which, while inducing apoptosis, precludes the synthesis of viral antiapoptotic proteins. The studies described in this and earlier studies are based on sensitive techniques that do not rely on the use of drugs.
The observations that PKR−/− or NF-κB−/− cells are resistant to apoptosis induced by the d120 mutant raise an interesting possibility that HSV-1 made an evolutionary choice to activate PKR and NF-κB and thus meet a requirement for its application rather than block the activation of both proteins. As noted above, to counteract the activated PKR, HSV-1 evolved the γ134.5 protein to mediate the immediate threat of total shutoff of protein synthesis. In the case of activated NF-κB that appears to render cells more susceptible to apoptosis induced by stress, the virus evolved at least three antiapoptotic proteins.
Acknowledgments
We thank B. R. G. Williams, A. Hoffman, and D. Baltimore for invaluable reagents and Audrey Esclatine for helpful discussion.
These studies were aided by grants from the National Cancer Institute (CA78766, CA71933, CA83939, CA87661, and CA88860) and the Public Health Service.
REFERENCES
- 1.Ackermann, M., D. K. Braun, L. Pereira, and B. Roizman. 1984. Characterization of herpes simplex virus 1 α proteins 0, 4, and 27 with monoclonal antibodies. J. Virol. 52:108-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amici, C., G. Belardo, A. Rossi, and M. G. Santoro. 2001. Activation of I kappa b kinase by herpes simplex virus type 1. A novel target for anti-herpetic therapy. J. Biol. Chem. 276:28759-28766. [DOI] [PubMed] [Google Scholar]
- 3.Aubert, M., and J. A. Blaho. 1999. The herpes simplex virus type 1 regulatory protein ICP27 is required for the prevention of apoptosis in infected human cells. J. Virol. 73:2803-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benetti, L., J. Munger, and B. Roizman. 2003. The herpes simplex virus 1 US3 protein kinase blocks caspase-dependent double cleavage and activation of the proapoptotic protein BAD. J. Virol. 77:6567-6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benetti, L., and B. Roizman. 2004. Herpes simplex virus protein kinase Us3 activates and functionally overlaps protein kinase A to block apoptosis. Proc. Natl. Acad. Sci. USA 101:9411-9416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cahir-McFarland, E. D., K. Carter, A. Rosenwald, J. M. Giltnane, S. E. Henrickson, L. M. Staudt, and E. Kieff. 2004. Role of NF-κB in cell survival and transcription of latent membrane protein 1-expressing or Epstein-Barr virus latency III-infected cells. J. Virol. 78:4108-4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cassady, K. A., M. Gross, and B. Roizman. 1998. The second-site mutation in the herpes simplex virus recombinants lacking the γ134.5 gene precludes shutoff of protein synthesis by blocking the phosphorylation of eIF-2α. J. Virol. 72:7005-7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chou, J., and B. Roizman. 1992. The gamma 1(34.5) gene of herpes simplex virus 1 precludes neuroblastoma cells from triggering total shutoff of protein synthesis characteristic of programmed cell death in neuronal cells. Proc. Natl. Acad. Sci. USA 89:3266-3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeLuca, N. A., A. McCarthy, and P. A. Schaffer. 1985. Isolation and characterization of deletion mutants of herpes simplex virus type 1 in the gene encoding immediate-early regulatory protein ICP4. J. Virol. 56:558-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Der, S. D., Y. L. Yang, C. Weissmann, and B. R. G. Williams. 1997. A double-stranded RNA-activated protein kinase-dependent pathway mediating stress-induced apoptosis. Proc. Natl. Acad. Sci. USA 94:3279-3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ejercito, P. M., E. D. Kieff, and B. Roizman. 1968. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J. Gen. Virol. 2:357-364. [DOI] [PubMed] [Google Scholar]
- 12.Galvan, V., and B. Roizman. 1997. Herpes simplex virus 1 induces and blocks apoptosis at multiple steps during infection and protects cells from exogenous inducers in a cell-type-dependent manner. Proc. Natl. Acad. Sci. USA 95:3931-3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galvan, V., R. Brandimarti, and B. Roizman. 1999. Herpes simplex virus 1 blocks caspase-3-independent and caspase-dependent pathways to cell death. J. Virol. 73:3219-3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodkin, M. L., A. T. Ting, and J. A. Blaho. 2003. NF-κB is required for apoptosis prevention during herpes simplex virus type 1 infection. J. Virol. 77:7261-7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He, B., M. Gross, and B. Roizman. 1997. The gamma(1)34.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1alpha to dephosphorylate the alpha subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc. Natl. Acad. Sci. USA 94:843-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He, B., J. Chou, R. Brandimarti, I. Mohr, Y. Gluzman, and B. Roizman. 1997. Suppression of the phenotype of γ134.5− herpes simplex virus 1: failure of activated RNA-dependent protein kinase to shut off protein synthesis is associated with a deletion in the domain of the α47 gene. J. Virol. 71:6049-6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He, B., M. Gross, and B. Roizman. 1998. The gamma(1) 34.5 protein of herpes simplex virus 1 has the structural and functional attributes of a protein phosphatase 1 regulatory subunit and is present in a high molecular weight complex with the enzyme in infected cells. J. Biol. Chem. 273:20737-20743. [DOI] [PubMed] [Google Scholar]
- 18.Hoffmann, A., T. H. Leung, and D. Baltimore. 2003. Genetic analysis of NF-κB/Rel transcription factors defines functional specificities. EMBO J. 22:5530-5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacquemont, B., and B. Roizman. 1975. RNA synthesis in cells infected with herpes simplex virus. X. Properties of viral symmetric transcripts and of double-stranded RNA prepared from them. J. Virol. 15:707-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jerome, K. R., R. Fox, Z. Chen, A.E. Sears, H.-Y. Lee, and L. Corey. 1999. Herpes simplex virus inhibits apoptosis through the action of two genes, Us5 and Us3. J. Virol. 73:8950-8957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jerome, K. R., Z. Chen, R. Lang, M. R. Torres, J. Hofmeister, S. Smith, R. Fox, C. J. Froelich, and L. Corey. 2001. HSV and glycoprotein J inhibit caspase activation and apoptosis induced by granzyme B or Fas. J. Immunol. 167:3928-3935. [DOI] [PubMed] [Google Scholar]
- 22.Khabar, K. S. A., M. Dhalla, Y. Siddiqui, A. Zhou, M. N. Al-Ahdal, S. D. Der, R. H. Silverman, and B. R. G. Williams. 2000. Effect of deficiency of the double stranded RNA-dependent protein kinase R, PKR, on antiviral resistance in the presence or absence of ribonuclease L: HSV-1 replication is particularly sensitive to deficiency of the major IFN-mediated enzymes. J. Interferon Cytokine Res. 20:653-659. [DOI] [PubMed] [Google Scholar]
- 23.Koyama, A. H., and Y. Miwa. 1997. Suppression of apoptotic DNA fragmentation in herpes simplex virus type 1-infected cells. J. Virol. 71:2567-2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kozak, M., and B. Roizman. 1975. RNA synthesis in cells infected with herpes simplex virus. IX. Evidence for accumulation of abundant symmetric transcripts in nuclei. J. Virol. 15:36-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leopardi, R., and B. Roizman. 1996. The herpes simplex virus major regulatory protein ICP4 blocks apoptosis induced by the virus or by hyperthermia. Proc. Natl. Acad. Sci. USA 93:9583-9587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leopardi, R., C. Van Sant, and B. Roizman. 1997. The herpes simplex virus 1 protein kinase US3 is required for protection from apoptosis induced by the virus. Proc. Natl. Acad. Sci. USA 94:7891-7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin, K. I., J. DiDonato, A. Hoffmann, J. M. Hardwick, and R. R. Ratan. 1998. Suppression of steady-state, but not stimulus-induced NF-kB activity inhibits Alphavirus-induced apoptosis. J. Cell Biol. 141:1479-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Margolis, D. M., A. B. Rabson, S. Straus, and J. M. Ostrove. 1992. Transactivation of the HIV-1 LTR by HSV-1 immediate-early genes. Virology 186:788-791. [DOI] [PubMed] [Google Scholar]
- 29.Marianneau, P., A. Cardona, L. Edelman, V. Deubel, and P. Despres. 1997. Dengue virus replication in human hepatoma cells activates NF-κB which in turn induces apoptotic cell death. J. Virol. 71:3244-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Medici, M. A., M. T. Sciortino, D. Perri, C. Amici, E. Avitabile, M. Ciotti, E. Balestrieri, E. De Smaele, G. Franzoso, and A. Mastino. 2003. Protection by herpes simplex virus glycoprotein D against Fas-mediated apoptosis: role of nuclear factor kappaB. J. Biol. Chem. 278:36059-36067. [DOI] [PubMed] [Google Scholar]
- 31.Mogensen, T. H., and S. R. Paludan. 2001. Molecular pathways in virus-induced cytokine production. Microbiol. Mol. Biol. Rev. 65:131-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohr, I., and Y. Gluzman. 1996. A herpesvirus genetic element which affects translation in the absence of the viral GADD34 function. EMBO J. 15:4759-4766. [PMC free article] [PubMed] [Google Scholar]
- 33.Munger, J., A. V. Chee, and B. Roizman. 2001. The US3 protein kinase blocks apoptosis induced by the d120 mutant of herpes simplex virus 1 at a premitochondrial stage. J. Virol. 75:5491-5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Munger, J., and B. Roizman. 2001. The US3 protein kinase of herpes simplex virus 1 mediates the posttranslational modification of BAD and prevents BAD-induced programmed cell death in the absence of other viral proteins. Proc. Natl. Acad. Sci. USA 98:10410-10415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel, A., J. Hanson, T. I. McLean, J. Olgiate, M. Hilton, W. E. Miller, and S. L. Bachenheimer. 1998. Herpes simplex type 1 induction of persistent NF-kappa B nuclear translocation increases the efficiency of virus replication. Virology 247:212-222. [DOI] [PubMed] [Google Scholar]
- 36.Roizman, B., and D. M. Knipe. 2001. Herpes simplex viruses and their replication, p. 2399-2459. In D. M. Knipe and P. M. Howley (ed.), Fields' virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 37.Roller, R. J., and B. Roizman. 1992. The herpes simplex virus 1 RNA binding protein US11 is a virion component and associates with ribosomal 60S subunits. J. Virol. 66:3624-3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rong, B. L., T. A. Libermann, K. Kogawa, S. Ghosh, L. X. Cao, D. Pavan-Langston, and E. C. Dunkel. 1992. HSV-1-inducible proteins bind to NF-kappa B-like sites in the HSV-1 genome. Virology 189:750-756. [DOI] [PubMed] [Google Scholar]
- 39.Santoro, M. G., A. Rossi, and C. Amici. 2003. NF-kB and virus infection: who controls whom. EMBO J. 22:2552-2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sheaffer, A. K., W. W. Hurlburt, J. T. Stevens, M. Bifano, R. K. Hamatake, R. J. Colonno, and D. J. Tenney. 1995. Characterization of monoclonal antibodies recognizing amino- and carboxy-terminal epitopes of the herpes simplex virus UL42 protein. Virus Res. 38:305-314. [DOI] [PubMed] [Google Scholar]
- 41.Soliman, T. M., R. M. Sandri-Goldin, and S. J. Silverstein. 1997. Shuttling of the herpes simplex virus type 1 regulatory protein ICP27 between the nucleus and cytoplasm mediates the expression of late proteins. J. Virol. 71:9188-9197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taddeo, B., T. R. Luo, W. Zhang, and B. Roizman. 2003. Activation of NF-kappaB in cells productively infected with HSV-1 depends on activated protein kinase R and plays no apparent role in blocking apoptosis. Proc. Natl. Acad. Sci. USA 100:12408-12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ward, P. L., W. O. Ogle, and B. Roizman. 1996. Assemblons: nuclear structures defined by aggregation of immature capsids and some tegument proteins of herpes simplex virus 1. J. Virol. 70:4623-4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou, G., V. Galvan, G. Campadelli-Fiume, and B. Roizman. 2000. Glycoprotein D or J delivered in trans blocks apoptosis in SK-N-SH cells induced by a herpes simplex virus 1 mutant lacking intact genes expressing both glycoproteins. J. Virol. 74:11782-11791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou, G., and B. Roizman. 2001. The domains of glycoprotein D required to block apoptosis depend on whether glycoprotein D is present in the virions carrying herpes simplex virus 1 genome lacking the gene encoding the glycoprotein. J. Virol. 75:6166-6172. [DOI] [PMC free article] [PubMed] [Google Scholar]