Abstract
Seeds of many plant species are green during embryogenesis. To directly assess the influence of light on the physiological status of green oilseeds in planta, Brassica napus and soybean (Glycine max) seeds were rapidly dissected from plants growing in the light or dark. The activation state of malate dehydrogenase, which reflects reduced thioredoxin and NADP/NADPH ratios, was found to be as high in seeds exposed to light as in leaves and to decrease in the dark. Rubisco was highly activated (carbamylated) in both light and dark, most likely reflecting high seed CO2 concentrations. Activities of Rubisco and phosphoribulokinase were sufficient to account for significant refixation of CO2 produced during B. napus oil biosynthesis. To determine the influence of light on oil synthesis in planta, siliques on intact plants in full sunlight or detached siliques fed 3H2O were partly covered with aluminum foil. Seeds from light and dark sections were analyzed, and fatty acid accumulation was found to be higher in seeds exposed to light than seeds from dark sections. The spectrum of light filtering through silique walls and the pigment composition of developing B. napus embryos were determined. In addition to a low chlorophyll a/b ratio, the carotenoid pigments of seeds can provide additional capture of the green light that filters through siliques. Together, these results demonstrate that even the low level of light reaching seeds plays a substantial role in activating light-regulated enzymes, increasing fatty acid synthesis, and potentially powering refixation of CO2.
Oilseeds provide a major source of calories for human consumption and are an increasingly significant source of renewable industrial materials. Interest in engineering enhanced seed oil quantity and quality has prompted efforts to better understand the biosynthesis of oil and other storage products of seeds. Several of the major oilseed crops (e.g. soybean [Glycine max], rapeseed, cotton, and linseed) produce seeds that are green during their development, and this fact has prompted questions regarding the contributions of seed photosynthesis to oilseed metabolism.
In leaf chloroplasts, fatty acid synthesis (FAS) is light dependent and utilizes ATP and reducing power generated by photosynthesis (Browse et al., 1986; Roughan and Ohlrogge, 1996). By contrast, plastids isolated from heterotrophic tissues, such as pea roots and cauliflower floral buds, require externally supplied ATP and/or reducing power for FAS (Möhlmann et al., 1994; Xue et al., 1997). Similarly, plastids isolated from developing chlorophylless seeds of safflower and castor bean are heterotrophic in nature and require added ATP or reducing equivalents (Browse and Slack, 1985; Smith et al., 1992). The situation is more complicated in photosynthetic oilseeds that are green during development. Although these seeds are predominantly sink tissues, they contain chloroplasts with the thylakoid structures and enzymes of typical photosynthetic machinery. However, rather than producing carbon for export as in source tissues, photosynthesis in seeds could participate in metabolism in at least three ways. First, light reactions could produce NADPH and ATP for energetically expensive FAS; second, O2 evolution may help prevent anoxia inside seeds (Rolletschek et al., 2002); and third, the dark reactions of photosynthesis could improve the biosynthetic efficiency of seeds by refixing respiratory CO2 and providing intermediates for metabolism.
Several previous studies on the role of light and photosynthesis in green oilseeds have not reached a consensus. Browse and Slack (1985) examined plastids from green (linseed) and nongreen (safflower) seeds and concluded that light could provide cofactors for FAS in green plastids but was unlikely to contribute to the carbon economy of seeds. Asokanthan et al. (1997) studied plastid structure in Brassica napus embryos as well as measured O2 evolution and CO2 incorporation rates. Their conclusion was that embryo chloroplasts are shade-like, photoheterotrophic, and use light to produce some NADPH and ATP for FAS. Eastmond et al. (1996) concluded that B. napus embryo plastids may have some capacity for photosynthesis, but because of the low-light environment inside the siliques, this makes no net contribution to carbon economy in vivo. By contrast, in a comprehensive study, King et al. (1998) evaluated both light and dark reactions of photosynthesis in B. napus seeds and suggested that embryos use light reactions mainly to drive CO2 fixation by Rubisco. Based on differences in CO2 and O2 gas exchange by soybean fruits in light or dark, Willms et al. (1999) concluded that light stimulates soybean fruit and seed FAS, although diffusion barriers or internal recycling of gases made quantitative conclusions about seeds inside the fruits difficult.
In addition to these studies of photosynthetic or gas-exchange capacity, there are reports that indicate that light stimulates FAS in developing green oilseeds. For example, after removing B. napus seeds from siliques, the incorporation of 14C from different substrates into lipids increased significantly in light (Aach and Heise, 1997) and decreased when photosynthesis was inhibited by 3-(3,4-dichlorophenyl)-1,1-dimethylurea (Fuhrmann et al., 1994). Similarly, after labeling seeds with 14C-Suc, 14C-acetate, or 3H2O, Bao et al. (1998) reported that light enhanced B. napus de novo FAS in plastids but not the elongation of fatty acids (FA) in the cytosol.
Most studies cited above were conducted in vitro, using excised embryos exposed to light, and, therefore, the situation of the seeds in planta within siliques could not be assessed. Conclusions from such studies may be limited because dissecting plant tissues is known to impair metabolism (e.g. Geigenberger et al., 1994). In addition, one argument against a significant role of seed photosynthesis in vivo is that the low transmittance of light through silique walls and seed coats does not provide sufficient energy to produce substantial amounts of cofactors (Eastmond et al., 1996). To address these limitations, we took an in vivo approach to study the role of light and photosynthesis in developing green seeds of B. napus and soybean. We harvested seeds from natural light conditions or from the dark and assessed the influence of light on the seed physiological status and on FA accumulation. In addition, we have assessed the activation state and capacity of Rubisco to refix CO2.
RESULTS
Pigment Composition of B. napus Developing Seeds Reflects Shade Adaptation
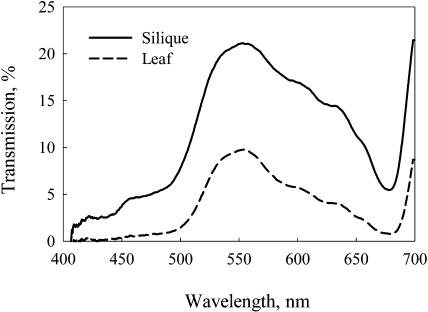
The utilization of light by green oilseeds is determined by two major factors. First, the transmittance of light through the silique or pod wall is fairly low, on average between 20% and 30% (Eastmond et al., 1996; King et al., 1998). Second, the transmitted light available for a seed is enriched with green wavelengths and thus may not be effectively absorbed by chlorophylls (Chl). This is illustrated in Figure 1, where the light transmission spectra of a B. napus leaf and silique wall are presented. Light that has passed through Chl-containing tissue has peak transmission around 550 nm in the green region.
Figure 1.
Transmission spectra of a B. napus leaf and about a 3-week-old green silique. Each spectrum is an average of two measurements over different areas.
We analyzed the pigment composition of developing B. napus seeds to evaluate their capacity to effectively use light that penetrates through the silique wall. Plants acclimated to deep-shade conditions adapt to the low-light environment by increasing the ratio of Chl b to Chl a. In addition, carotenoids (especially violaxanthin and lutein) are significant for low-light harvesting by absorbing light energy mainly in the blue-green spectral range and transferring the excitation energy to Chl a (Siefermann-Harms, 1985; Frank and Cogdell, 1996). Midstage developing B. napus seeds had abundant Chl, on average 3 nmol (about 2.6 μg) seed−1, and, as reported previously (Eastmond et al., 1996; King et al., 1998), the seeds had a lower Chl a/b ratio than leaves, indicating their adaptation to deep shade (Table I). HPLC analysis indicated that B. napus developing seeds also contained all major carotenoid pigments (violaxanthin, antheraxanthin, zeaxanthin, lutein, neoxanthin, and β-carotene), and the ratio between carotenoids and Chl was similar in both seeds and leaves (Table I). The xanthophyll conversion state (Z + A/VAZ; %) in dark-adapted B. napus leaves was low (9%) and higher in leaves harvested from a sunny greenhouse (range 13%–44%; average 27%). In seeds, the conversion state was around 20% regardless of the light conditions (Table I). In summary, both the carotenoid and Chl a/b analysis indicated that developing B. napus seed chloroplasts are well equipped to utilize weak, green light that has filtered through the silique wall.
Table I.
Biochemical characteristics of B. napus leaves and seeds and developing soybean seeds
| B. napus Leaves | B. napus Seeds | Soybean Seeds | |
|---|---|---|---|
| Chl a/b ratio | 2.4 ± 0.2 | 1.9 ± 0.2 | n.d. |
| Carotenoids/Chl (mmol mol−1) | 313 ± 29 | 371 ± 17 | n.d. |
| Carotenoids: A + Z/VAZ in dark (%) | 9.7 ± 2.7 | 20 ± 1.5 | n.d. |
| Carotenoids: A + Z/VAZ in light (%) | 27 ± 16 | 24 ± 1 | n.d. |
| Rubisco/Chl (mmol mol−1) | 5.9 ± 1.2 | 2.0 ± 0.3 | 2.4 ± 0.6 |
| Rubisco (μg seed−1) | – | 3.2 ± 1.3 | 35 ± 4 |
| PRK activity (μmol min−1 mg−1 Chl) | 49 ± 3 | 23 ± 1 | n.d. |
The values are an average ± sd of three to six determinations. Developing seeds were analyzed at the stage of maximum lipid accumulation (see “Materials and Methods”). A, Antheraxanthin; Z, zeaxanthin; V, violaxanthin. n.d., Not determined.
Light Causes Activation of NADP-MDH in Developing Seeds
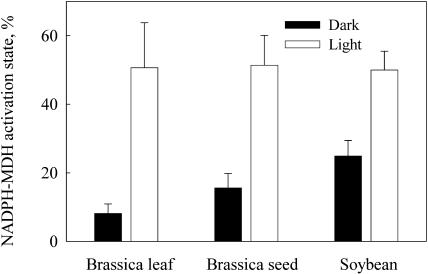
To provide an in planta measure of how light influences the physiological status of developing B. napus seeds, we measured the activation state of chloroplast NADPH-dependent malate dehydrogenase (MDH), an enzyme that is inactive in darkness or at low light and activates upon illumination. This light activation is mediated by the ferredoxin-thioredoxin (Fd/Tx) system, which signals the onset of light to several chloroplast enzymes (Scheibe et al., 1986). However, this reductive activation of MDH is inhibited by NADP+, making the enzyme also sensitive to the NADP+/NADPH ratio (Scheibe and Jacquot, 1983), and, therefore, the activation state of MDH also reflects the stromal NADP pool reduction state (Foyer, 1993). NADPH-MDH activity was measured from B. napus seeds and leaves that were rapidly harvested and quick frozen from light, or after 1- to 2-h dark adaptation. Developing soybean seeds collected from similar conditions were also analyzed. As shown in Figure 2, the activation state for MDH was low (<20%) in dark and increased to an average of 50% in material harvested from light. Thus, MDH is activated similarly by light in both seeds and leaves implicating photosynthetic electron transport in seed NADPH synthesis and thioredoxin-mediated enzyme activation.
Figure 2.
The activation state of chloroplast NADP-dependent MDH in B. napus leaves and developing seeds and in soybean seeds measured from dark-adapted (2 h) material and samples harvested from sunlight. In all samples, the activation state is significantly higher in light, demonstrating the presence of photosynthetic electron transport in light. The values are an average ± sd of three to four independent determinations.
Seeds Contain Highly Active Rubisco
Expressed sequence tag sequencing of Arabidopsis and soybean developing seeds indicates that transcripts encoding Rubisco's small subunit and photosystem components are relatively abundant seed mRNA species (White et al., 2000; http://www.tigr.org/tdb/tgi/gmgi). Furthermore, Rubisco and photosystem gene expression are correlated temporally with expression of transcripts for enzymes of FAS (Ruuska et al., 2002). King et al. (1998) previously measured in vitro activities of phosphoenolpyruvate (PEP) carboxylase (PEPC) and Rubisco in developing B. napus seeds, but these experiments did not reveal whether Rubisco in the seeds is in an active form. Many enzymes of the Calvin cycle are regulated by the Fd/Tx system, but the activation of Rubisco is more complex, involving Rubisco activase. Although the main influencing factor in leaves is light, activation is also determined by available CO2 because the activation requires carbamylation of the active site (Lorimer et al., 1976). Full activation in leaves often requires light intensities close to 1,000 μmol quanta m−2 s−1 (von Caemmerer and Edmondson, 1986). Considering that only 20% to 30% of the ambient light reaches the seeds, this could restrict the activation state of seed Rubisco.
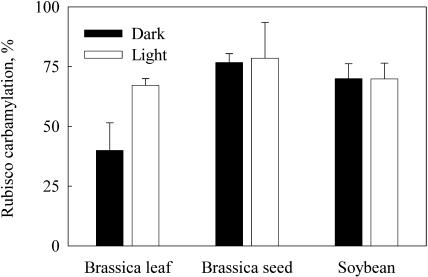
The total amount and carbamylation status of Rubisco were determined in developing B. napus and soybean seeds using the [14C]CPBP-binding method (Collatz, 1978). At midstage development, the average Rubisco contents in B. napus and soybean seeds were 3.2 and 35 μg, respectively (Table I). The ratio of Rubisco to total Chl was low in seeds, as is typical for low-light-adapted plants. Transfer from dark to sunlight caused the expected increase in Rubisco carbamylation status in leaves, from about 40% to almost 80% (Fig. 3). By contrast, in developing B. napus and soybean seeds, carbamylation was equally high in both light and dark (approximately 80%), and even removing soybean seeds from pods and exposing them to full sunlight did not further increase carbamylation (data not shown). The activation states were similar when Rubisco activation was measured using a rapid spectrophotometric assay for Rubisco initial and total activity (Kane et al., 1998). This confirmed that the high carbamylation levels in seeds were not artifacts of the CPBP assay method, where it is crucial that the assay mixture for determination of the initially carbamylated Rubisco sites is essentially CO2 free.
Figure 3.
Carbamylation state of Rubisco in B. napus leaves and seeds harvested from dark or light. For comparison, carbamylation ratios were also measured from soybeans. The values are an average ± sd of three to four independent determinations.
An explanation for high Rubisco carbamylation levels in developing seeds in the absence of high light is most likely the high concentration of dissolved CO2 in cells, resulting from high CO2 release during FAS. King et al. (1998) measured 1% to 2% gaseous CO2 inside developing B. napus siliques (which would result in dissolved CO2 of 350–700 μm). Recent direct measurements of total CO2 in seeds indicate levels over 10 mm accumulate in B. napus and soybean seeds (Goffman et al., 2004). The Kact (CO2) for spontaneous carbamylation of Rubisco is approximately 30 μm (Lorimer et al., 1976) and, therefore, in seeds, spontaneous carbamylation would be sufficient to keep Rubisco highly active in light or dark. Thus, Rubisco should function in seeds even under low-light conditions, providing that a ribulose 1,5-bisphosphate (RuBP) regeneration pathway is active.
Developing Seeds Also Contain Phosphoribulokinase
In the presence of saturating CO2 and with a high carbamylation state, Rubisco activity in developing B. napus and soybean seeds would be limited by the availability of RuBP. To assess the capacity for RuBP production, the activity of phosphoribulokinase (PRK) was measured after rapid extraction from B. napus leaf and seeds harvested from light. In addition, the presence of PRK protein was confirmed from soybean seeds with immunoblotting.
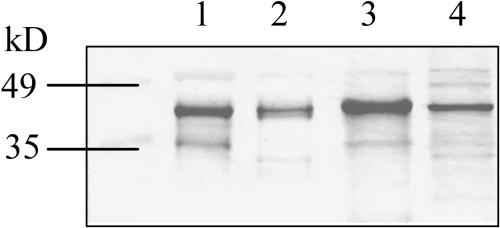
PRK activity in B. napus seeds was on average 36 nmol min−1 seed−1. Similar to the Rubisco to Chl ratio, PRK activity expressed on a Chl basis was about 50% lower in developing B. napus seeds than in leaves (Table I). Because PRK is activated by the same Fd/Tx system as NADP-MDH (Fig. 2), PRK is likely to be highly active in seeds under light conditions. Considering that the average Rubisco initial site concentration in B. napus seeds was 0.04 nmol seed−1 (approximately 3 μg of 80% carbamylated Rubisco), with in vivo Kcat of 3 s−1 site−1 (von Caemmerer et al., 1994), this equals a potential CO2-fixing capacity of Rubisco of about 7.2 nmol min−1 seed−1. Accordingly, the measured PRK activity (36 nmol min−1 seed−1) is about 5-fold higher than Rubisco activity, a ratio comparable to that found from tobacco leaves (Paul et al., 1995). Although we could not reliably measure the activity of PRK from developing soybean seeds due to a high background consumption of NADH in the spectrophotometric assay, immunoblot analysis confirmed the existence of PRK in developing soybean seeds (Fig. 4).
Figure 4.
Immunoblot of protein extracts from leaves and developing seeds of B. napus and soybean. Analysis was performed using antibody against soybean PRK. Lanes 1 and 2 contained 5 μg of protein extracted from B. napus and soybean leaf, respectively, and lanes 3 and 4 contained 20 μg of protein extracted from developing B. napus and soybean seeds. PRK size is about 43 kD.
Seeds Inside Siliques Exposed to Light Produce More FA Than Seeds in the Dark
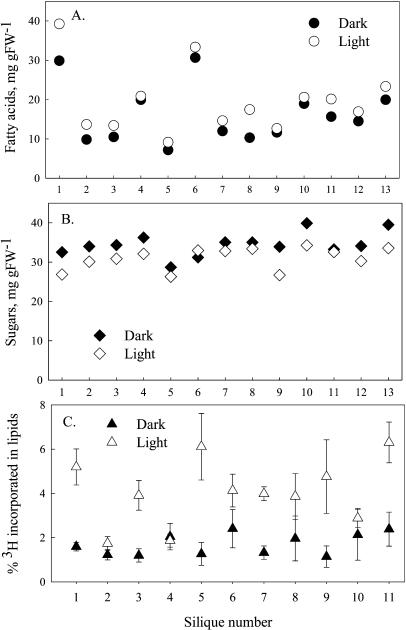
Seed slices have higher rates of FAS when incubated in light compared to dark (e.g. Aach and Heise, 1997; Bao et al., 1998). In addition, when intact embryos are cultured as described by Schwender et al. (2003), rates of fresh-weight accumulation and 14C-Suc incorporation into lipids are approximately 2-fold higher in the light than in the dark (data not shown). To extend these studies to seeds growing within siliques, we performed two types of experiments. The first experiment involved intact B. napus plants growing outdoors on a sunny day. In the morning, an opaque, reflective aluminum foil tube was slipped over 13 siliques such that either the distal or basal half was covered. After 10 h of sunlight, the siliques were removed from plants, the seeds dissected, and the weight, FA, and Suc content of seeds from the dark and light were determined. As shown in Figure 5A, although biological variability between siliques resulted in a wide range of values, the FA content was higher in the seeds exposed to light than to dark. On average, seeds in the light had 28 μg more FA than seeds in the dark. This difference was 23%, whether expressed as milligrams FA per seed or milligrams FA per gram fresh weight, and statistically significant (paired Student's t test; P < 0.001). Suc content was slightly higher in seeds exposed to the dark, as might be expected if its utilization by the seeds was slowed (Fig. 5B). Thus, any differences in FA production were not due to limitations of carbon supply to the seeds. For this experiment, we selected seeds at the early stage of oil accumulation (about 20–25 d after flowering [DAF], with an average weight of 5.5 mg), so that a large background of preformed oil was not present, and the increase in oil content over 10 h could be more easily observed. Based on data from field-grown B. napus (Turnham and Northcote, 1983; Murphy and Cummins, 1989), seeds at this stage would be expected to produce approximately 20 to 50 μg of FA in 1 d. The average difference between the light and dark seeds after the 10-h period was 28 μg of FA. The simplest interpretation of this comparison is that seeds in the field produce a major portion of their FA during the light portion of the diurnal cycle.
Figure 5.
Influence of light on lipid synthesis in seeds inside siliques. A, Comparison of FA content of B. napus seeds exposed to light or dark. Either the basal or the top half of 13 siliques on plants growing outside was covered with aluminum foil so that one-half of the seeds was shaded. After a 10-h period of sunlight, the seeds of each half of the 13 siliques were analyzed. The difference between the light treatments was statistically significant (paired Student's t test; P < 0.001). For siliques 2, 3, and 8, the tip was dark, for others the base. B, Sugar contents of same seeds analyzed in A. C, Incorporation of 3H from 3H2O into lipids of individual seeds from sections of a silique either exposed to light or shaded with foil tubes. Results are expressed as 100 × (dpm in lipid extract/dpm in aqueous extract of seeds). The mean and sd for five seeds is shown. For odd-number siliques, the tip was dark; for even-number siliques, the base was covered.
In the second experiment, 3H2O was fed to siliques and the incorporation of 3H into lipid was determined in light- and dark-exposed seeds. 3H2O is advantageous as a lipid precursor because labeled water equilibrates with endogenous pools to a uniform and easily measurable specific activity, and therefore total FAS activity can be measured without assumptions about internal pool dilutions. Eleven siliques at approximately 30 DAF were detached from plants and fed 3H2O via the transpiration stream. After 24 h (to allow distribution of label throughout the silique and seeds), foil tubes were again used to cover either the distal or basal half of the silique. After an additional 24 h in the light, five seeds were harvested from each light and dark section, and 3H incorporation into lipids of each seed was determined. As shown in Figure 5C, for 10 of the 11 siliques, lipid synthesis from 3H2O was higher in seeds that were exposed to light. The average difference between light and dark seeds from the same silique was 2.5-fold. We note that this value underestimates the difference in rates because some 3H-lipid was synthesized during the initial 24-h light period.
DISCUSSION
In this study, we have examined how light influences the metabolism of green seeds. Because earlier studies were performed with isolated plastids, excised seeds, detached siliques, or theoretical calculations based on gas exchange of tissues, our objective in this study was to use methods that reflect metabolism in intact seeds on the plant.
The spectrum of light transmitted through the silique wall (Fig. 1) indicates that B. napus seeds will be exposed primarily to green light. Thus, not surprisingly, both the contents and compositions of Chls and carotenoids of developing seeds were similar to those characteristic of shade plants that are adapted to both low light and green-enriched light. This situation inside the silique may be analogous to the inner cells of many green leaves. In fact, although blue and red light are better captured by Chl, even in leaves, a large portion of total photosynthesis is dependent on green light (Sun et al., 1998; Evans, 1999).
In both leaves and seeds, exposure to high light led to an increase in NADPH-MDH activation from <20% to around 50% of total activity. This result provides direct evidence that light leads to biochemical responses for plastid enzymes in green seeds that are similar to those in leaves. The increase in NADP-MDH activation state also implies that the chloroplast redox state increases, and, consequently, we can assume that other enzymes that are under regulation by the Fd/Tx pathway, such as acetyl-CoA carboxylase, Fru bisphosphatase, seduheptulose bisphosphatase, PRK, and NADP-GAPDH, are also activated. The latter enzymes may be important in terms of functioning of a CO2 assimilation pathway (see below). Also, NADPH production in plastids is consistent with O2 evolution by PSII as measured for isolated embryos (Eastmond et al., 1996; King et al., 1998). Photosystem activity in green seeds may play a dual role of supplying cofactors and also of avoiding hypoxia. Recent studies have shown that even at ambient O2 levels, the supply of O2 may limit metabolism in developing B. napus seeds (Vigeolas et al., 2003), and seeds may experience hypoxia, as observed in developing legumes (Rolletschek et al., 2002). It has been reported that photosynthesis by B. napus pod walls increases the O2 concentration in the silique locule (Porterfield et al., 1999). However, as the developing seeds themselves are mostly impermeable to gases (King et al., 1998), O2 evolution by the seed itself may be important to avoid anoxia.
Calculations for B. napus FAS and Reductant Production and CO2 Exchange
To provide a quantitative context to the above experiments, in the following paragraphs we calculate the potential gas and reductant fluxes in developing B. napus seeds based on our biochemical measurements and literature values.
During the maximal oil accumulation period (3–5 weeks after flowering), a developing B. napus embryo synthesizes up to 75 μg FA per day (Murphy and Cummins, 1989; Bao et al., 1998; S.A. Ruuska, unpublished data). Assuming that this takes place predominantly during a 16-h light period, the FAS rate is 4.7 μg h−1 seed−1 or 16.7 nmol h−1 seed−1 for 18 carbon FAs. During plastidic FAS, each elongation of the growing chain by 2 carbons requires 1 NADH and 1 NADPH or 8 mol each per 18 carbons, or 135 nmol h−1 seed−1. Pyruvate dehydrogenase (PDH) produces NADH and acetyl-CoA in equimolar amounts. However, the source(s) of NADPH for FAS is uncertain. Several different mechanisms could deliver cytosolic reducing equivalents to the plastid, including the oxaloacetate (OAA)/malate shuttle (Scheibe, 1987) or exchange of dihydroxyacetonphosphate with 3-phosphoglyceraldehyde (PGA) by the triose phosphate transporter (Heineke et al., 1991), combined with cytosolic reduction of 3-PGA and plastidic oxidation of glyceraldehyde-3-phosphate (GAP) by GAPDH. In these two scenarios, net fluxes of carbon and reductant move in the opposite direction to those in source leaf tissues. In addition, NADP(+)-malic activity has been described in B. napus embryos (Kang and Rawsthorne, 1994; Singal et al., 1995) and proposed as a source of reductant. However, analysis of in vivo labeling patterns from 13C-precursors indicates that malate makes a very low contribution to B. napus plastid FAS (Schwender and Ohlrogge, 2002). Finally, NADPH could be produced by the plastidic oxidative pentose phosphate pathway (OPPP; Eastmond and Rawsthorne, 2000). Recent in vivo labeling results with B. napus embryos, however, indicate that flux of carbon through the OPPP during storage oil synthesis can account for only 25% to 45% of seed NADPH demand (Schwender et al., 2003). Furthermore, plastid Glc-6-P dehydrogenase (G6PDH), the first enzyme of the OPPP, is inactivated by reductive modification via the Fd/Tx system (Scheibe and Anderson, 1981). Therefore, seed plastid G6PDH presumably has low activity in light when the chloroplast redox state is high (Fig. 2).
An alternative for NADPH production within plastids of green seeds is photosynthesis, provided that enough light is available. Full sunlight in summer can reach an intensity of 2,000 μmol photons m−2 s−1, with an average of >1,000 over 12 h. Considering that 20% to 30% of this light may penetrate the B. napus silique wall (Fig. 1; Eastmond et al., 1996; King et al., 1998), 200 to 600 μmol photons m−2 s−1 could reach the seed surface. Since only minor amounts of Chl are present in seed coats (King et al., 1998), most of the light will reach and be absorbed by the green embryo. The reported B. napus embryo gross O2 evolution rate at 200 to 400 μmol photons m−2 s−1 is about 180 nmol h−1 (Eastmond et al., 1996; King et al., 1998). This can provide 360 nmol NADPH h−1 embryo−1, which is at least 2-fold above the demand for FAS. Therefore, we conclude that seed photosynthesis contributions quantitatively match the major demand for NADPH for seed FAS and other plastid metabolism. In addition, the photosynthetic electron transport is coupled to proton translocation and photophosphorylation, and, thus, the reported O2 evolution rate corresponds to an ATP synthesis rate of about 240 nmol ATP h−1. Since FAS requires equimolar amounts of ATP and NADPH, the photosynthetic light reactions can produce almost 2 times more ATP than required for FAS.
CO2 and the Carbon Economy of Seeds
In terms of carbon economy, FA biosynthesis is an inefficient process. During the conversion of pyruvate to acetyl-CoA by the plastid PDH, one carbon is lost as CO2. Thus, in oilseeds such as B. napus, with approximately 50% oil content, it can be calculated that more than 70% of the carbon entering the embryo as sugars is processed via pyruvate to acetyl-CoA, and CO2 release could represent up to 20% or more of the seed carbon economy. This high CO2 release rate may cause specific problems in seeds, such as cellular acidification and repression of photosynthetic rates as observed in leaves at high CO2 partial pressures (Sage et al., 1990; Ögren and Evans, 1993; Ruuska et al., 2000). Because of the thickness of the seed tissue and the presence of the seed coat, CO2 formed inside seeds may not be able to easily escape to the silique locule. Moreover, low O2 concentration further promotes cytosolic acidification (Ratcliffe, 1995; Savchenko et al., 2000).
Two enzymes of developing B. napus seeds have potential for refixation of this respiratory CO2. PEPC uses dissolved bicarbonate to produce OAA from PEP. King et al. (1998) suggested that OAA could be used to replenish Krebs cycle intermediates or to make pyruvate and acetyl-CoA for FAS in developing B. napus seeds. However, reformation of pyruvate (via malic enzyme) would again release CO2. In order to store the fixed carbon in a stable form rather than releasing it again during conversion to pyruvate, incorporation of the carbon into storage proteins can be considered. However, based on the amino acid composition of storage proteins (Norton, 1989), the amount of CO2 potentially stored in Asp, Thr, Met, Ile, and Lys in seed protein is only about 4% of the CO2 produced by FAS, and, therefore, CO2 cannot be accommodated quantitatively into seed protein.
The expression of Rubisco provides a second potential route to CO2 fixation, provided sufficient reductant and cofactors are available. Synthesis of an 18-C FA from pyruvate produces 9 mol CO2 mol−1 FA, which, in midstage B. napus, is 150 nmol CO2 h−1 seed−1. The CO2 concentration in the silique cavity is 1% to 2% and much higher in seeds, and, therefore, Rubisco in seeds functions at CO2 saturation and without photorespiration. In B. napus seeds, an average of 3 μg of 80% carbamylated Rubisco per seed (Table I) amounts to a catalytic site concentration of 0.04 nmol seed−1. The Rubisco Kcat (in vivo) is about 3 s−1 site−1, and, therefore, the maximum flux through Rubisco would be approximately 430 nmol CO2 h−1. Although these calculations assume RuBP saturation of Rubisco, which is not likely to be the case, even if only 50% of the Rubisco catalytic sites have RuBP available, the CO2 fixation rate can still be over 200 nmol h−1. These calculations indicate that Rubisco levels present in B. napus seeds are sufficient to accommodate CO2 produced by FAS and therefore could represent a major carbon flux in the oil seed.
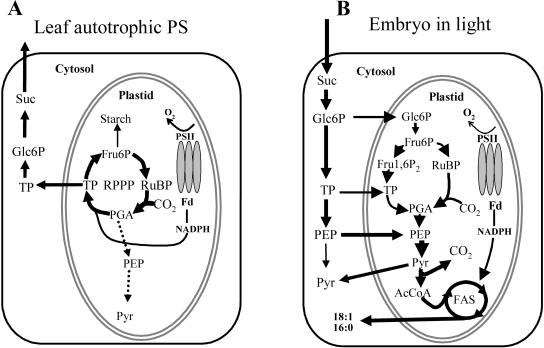
Based on the above considerations, we outline a comparative overview of photosynthesis and FAS in developing green oilseeds versus leaves, as summarized in Figure 6. This model proposes that photosynthesis in green oilseeds can be defined as follows. (1) The nonoxidative pentose phosphate pathway transforms Glc-6-P to Ru-5-P, which is activated to RuPB by PRK. (2) Rubisco produces 3-PGA, which is further transformed to acetyl-CoA. (3) Photosynthetic NADPH and ATP are used to incorporate acetyl-CoA into FAs. Obviously, this is a simplified and speculative scheme and requires more detailed flux analysis. Nevertheless, our calculations demonstrate that the overall demand and production capacity for reductant, as well as CO2 evolution and fixation capacity, in whole B. napus seeds are of the same order of magnitude.
Figure 6.
Simplified comparison of leaf photosynthesis with the metabolism of green oilseeds in light. Only the major reactions involved in photosynthesis or conversion of Suc to oil are shown. For cofactors, only the fate of NADPH, produced by photosynthesis, is indicated. A, In an autotrophic leaf, photosynthetic CO2 is incorporated by Rubisco into PGA, which is reduced to TP. Altogether, five-sixths of the produced TP is used to recycle RuBP, the acceptor molecule for CO2. B, In B. napus embryos, the major pathway of carbon flow during oil synthesis, based on biochemical analyses, stable isotope-labeling patterns, expressed sequence tag, and microarray analysis, is indicated by thick arrows. Embryo chloroplasts contain the full enzymatic set to catalyze glycolysis. The Rubisco reaction is added to the scheme by considering synthesis of RuBP from imported hexose and considering PGA metabolism to pyruvate rather than recycling. OPPP production of NADPH contributes approximately 38% of NADPH (Schwender et al., 2003) but is omitted from the figure for simplicity. Suc, Sucrose; Glc, glucose; Glc-6-P, glucose-6-phosphate; TP, triose phosphate; Pyr, pyruvate; AcCoA, acetyl-CoA; RPPP, reductive pentose phosphate pathway.
CONCLUSION
In this study, we have shown that developing B. napus seeds are adapted to utilize low light in photosynthesis. We have also shown that, under normal growth conditions, light filtered through the silique wall is sufficient to activate seed enzymes that depend on photosynthetic electron transport. Moreover, seed Rubisco is highly active, and there is considerable PRK activity present to provide potential to refix CO2 released by PDH. Our calculations also show that photosynthesis has the capacity to produce enough reductant for FAS and for a large portion of CO2 refixation as well. This notion is reinforced by our finding that in planta seeds exposed to light produce more FAs than seeds that are shaded. Our conclusion is that light harvesting provides several advantages to the seeds, including reductant and O2 generation, and CO2 fixation. The net result is activation of enzymes and increased rates of oil synthesis.
MATERIALS AND METHODS
Growth and Sampling of the Plant Material
Plants were grown in an air-conditioned greenhouse under natural light supplemented with lamps to provide 16/8-h photoperiod and an approximate light intensity of 800 μmol photons m2 s−1. Developing seeds were collected at the time of maximal lipid accumulation. Brassica napus L. cv Reston seeds were used at the age of 3 to 5 weeks after flowering, and soybean (Glycine max L. cv NKS-1990) seeds at 4 to 5 weeks after flowering (Ohlrogge and Kuo, 1984). Whole seeds were used for experiments. Seeds or leaf discs were harvested either from light on a sunny day, or, after 1 to 2 h of dark adaptation, seeds were removed from the siliques and immediately frozen with liquid nitrogen for storage at −80°C before the biochemical assays.
Light Transmission and Pigment Analysis
Seed Chl and carotenoid composition were analyzed by HPLC as outlined in Tian and DellaPenna (2001). The total amount of Chl in samples used for enzyme assays was routinely analyzed in 80% acetone buffered with 25 mm HEPES, pH 7.5 (Porra et al., 1989). Leaf and silique transmission spectra were recorded with a S2000 Fiber Optic Spectrometer (Ocean Optics, Dunedin, FL), using a 50-μm fiber-optic tungsten source for illumination. A collimating lens (f/2; Ocean Optics) was placed onto the fiber-optic source to increase the sampling angle.
NADP-MDH Assays
The activation state of chloroplast NADP-dependent MDH was assayed as outlined in Scheibe and Stitt (1998). The extraction buffer (100 mm Na-acetate, pH 6, 4 mm dithiothreitol [DTT], 0.5% bovine serum albumin, 0.25% Triton X-100, 0.25% CHAPS, 2% polyvinylpolypyrrolidone [PVPP], and 0.5 mm of each benzamidin, γ-aminocaproic acid, and AEBSF) was sparged with humidified nitrogen to maintain low oxygen concentration. Seeds and leaf discs were extracted with ice-cold buffer using a glass homogenizer, the crude extract was centrifuged for 5 min at 10,000g, and the supernatant was used for duplicate assays. Initial activity was assayed immediately in 1 mL volume containing 100 mm Tris-HCl, pH 8, 1 mm EDTA, 1 mm DTT, 0.2 mm NADPH, 2 mm OAA, and 30 to 50 μL of supernatant. The decline in A340 nm was monitored. Reductive activation of MDH was achieved by incubating an aliquot of the supernatant in 250 mm Tris-HCl, pH 9 (sparged with nitrogen), and 125 mm DTT under nitrogen atmosphere at room temperature for 15 to 20 min.
Rubisco Assays
The total amount and carbamylation status of Rubisco were measured by the [14C]CPBP-binding method (Mate et al., 1993) after rapid extraction in ice-cold CO2-free buffer containing 50 mm HEPES-NaOH, pH 7.8, 1 mm EDTA, 5 mm MgCl2, 10 mm DTT, 1 mm each of benzamidin, γ-aminocaproic acid, and AEBSF, 0.1% Triton X-100, and 1% PVPP. Both radiolabeled and unlabeled CPBP (an unresolved mixture of 2′-carboxy d-arabinitol-1,5 bisphosphate and 2′-carboxy d-ribitol-1,5 bisphosphate) was synthesized as described in Collatz (1978). For comparison, the activation status of Rubisco in B. napus seeds was also measured by comparing initial and total activities using an NADPH-linked spectrophotometric assay (Kane et al., 1998). Activation of Rubisco was achieved by incubating an aliquot of the supernatant in the assay mixture for 10 min prior to adding RuBP (Sigma-Aldrich, St. Louis).
PRK Assays
The activity of PRK was measured as outlined in Wara-Aswapati et al. (1980). Samples were extracted in a buffer containing 100 mm HEPES-NaOH, pH 8, 10 mm DTT, 10 mm MgCl2, 2 mm EDTA, 0.25% Triton X-100, 0.25% bovine serum albumin, 1% PVPP, 1 mm each of AEBSF, benzamidin, and γ-aminocaproic acid, and centrifuged for 3 min at 10,000g. Supernatant was used for PRK activity assays immediately. The assay mixture (volume of 1 mL) consisted of 100 mm HEPES-NaOH, pH 7.8, 10 mm MgCl2, 20 mm KCl, 10 mm DTT, 1 mm ATP, 1.5 mm PEP, 0.2 mm NADH, 3 mm ribose-5-phosphate, 3 units ribose-5-phosphate isomerase, 3 units lactate dehydrogenase, and 2 units pyruvate kinase. The mixture minus NADH was incubated for 5 min before the assays to allow for the synthesis of Ru-5-P by the isomerase, and the duplicate assays were started by adding 10 to 25 μL of supernatant to a final volume of 1 mL. For each sample, a control reaction without added Rib-5-P was carried out to correct for background rates of NADH consumption, originating from an ADP impurity in ATP and other endogenous ATPase activities.
Immunoblots
Soluble proteins were extracted from leaf and seed material with a buffer containing 50 mm HEPES-NaOH, pH 7.8, 100 mm NaCl, 0.05% SDS, and 1 mm each of AEBSF, benzamidin, and γ-aminocaproic acid. Extracts were centrifuged for 5 min at 10,000g, and the protein content of the supernatant was measured. Soluble proteins were separated by SDS-PAGE for immunoblot analyses as described in Thelen et al. (2001). Primary antibody against soybean PRK was kindly provided by Dr. Steven Crafts-Brandner (U.S. Department of Agriculture-Agricultural Research Service, Western Cotton Research Lab, Phoenix).
Estimate of Oil Synthesis by Developing Seeds in Planta
Two experiments were conducted to test the influence of light on seeds within siliques. In the first, B. napus L. cv Reston plants were grown outdoors in 25-cm pots. For measurement of oil synthesis in planta, siliques from four different plants were selected on a warm (+25°C), sunny day and at a developmental stage of 20 to 30 DAF. While still attached to the plant, either the base or the tip half of 13 siliques was covered with a 7-mm i.d. plastic tube wrapped with aluminum foil to shade half of the seeds. After 10 h, light- and dark-exposed seeds were harvested and fresh weight was determined. To assure similar developmental stage, seeds were selected for further analysis that had an average fresh weight per seed between 5 and 6 mg and that differed not more than 0.5 mg in fresh weight per seed between light and dark sections of the same silique.
FA and sugar content of the seeds was determined by extracting 10 to 15 seeds from each half-silique for 10 min with 2 mL of hot isopropanol (90°C). After cooling to room temperature, 4 mL hexane with 2 mg triheptadecanoine as internal standard were added and heated again for 10 min (90°C). After cooling, 0.5 volume of water was added. An aliquot of the upper hexane phase was analyzed by gas chromatography/flame ionization detector after direct transesterification of FAs to FA methyl esters (Browse et al., 1986). An aliquot of the water/isopropanol phase was used for enzymatic determination of Suc, Glc, and Fru (Stitt et al., 1989; Schwender and Ohlrogge, 2002).
In the second experiment, 3H2O was used as a radiolabeled precursor for FAS (Bao et al., 1998). In preliminary experiments, we determined that the water in seeds at different positions within the silique became similarly labeled with 3H2O after 20- to 24-h feeding. Eleven siliques at approximately 30 DAF were then removed from three greenhouse-grown plants, and the pedicle was immediately placed into a solution of 5 mCi/mL 3H2O, 50 mm Suc, and 3 mg mL−1 Murashige and Skoog salts. After 24 h in the light to allow movement of water into the silique (and its seeds) via transpiration, the base or tip half of each silique was covered with aluminum foil-covered tubes and the siliques were further incubated for 24 h with 600 to 800 μE light. During this 48 h, approximately 1 mL of solution was taken up by each silique. Five seeds from each light section and five seeds from each dark section were then removed, placed into individual screw-cap tubes, and heated with 0.1 mL isopropanol for 10 min at +85°C. After cooling, seeds were homogenized and 0.15 mL hexane were added and allowed to extract overnight at room temperature. After adding 0.8 mL of hexane and 0.2 mL of 0.1 m acetic acid, 0.5 mL of the upper-phase lipid extract were transferred to a scintillation vial, evaporated at 80° C for 15 min, and counted to determine 3H incorporation into FAs. Total 3H incorporation into each seed was also determined by counting an aliquot of the aqueous phase of the lipid extract. Results are expressed as 100 × (dpm in lipid extract/dpm in aqueous extract of seeds).
Acknowledgments
We acknowledge L. Tian, M. Magallanes-Lundback, and D. DellaPenna for assistance with pigment analysis and access to HPLC equipment, W. Hillier for help with transmittance measurements, as well as K. Fischer and C. Ohlrogge for excellent technical assistance with B. napus field study. We also thank Drs. B. Furbank and A. Weber for their comments on the manuscript.
This work was supported by the Department of Energy (grant no. DE–FG02–87ER13729) and the National Science Foundation (grant no. MCB 98–17882). Acknowledgment is also made to the Michigan Agricultural Experiment Station for its support of this research.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.047977.
References
- Aach H, Heise KP (1997) On the compartmentation of triacylglycerol synthesis in developing seeds of Brassica napus. Bot Acta 111: 123–129 [Google Scholar]
- Asokanthan PS, Johnson RW, Griffith M, Krol M (1997) The photosynthetic potential of canola embryos. Physiol Plant 101: 353–360 [Google Scholar]
- Bao XM, Pollard M, Ohlrogge J (1998) The biosynthesis of erucic acid in developing embryos of Brassica rapa. Plant Physiol 118: 183–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browse J, McCourt PJ, Somerville CR (1986) Fatty acid composition of leaf lipids determined after combined digestion and fatty acid methyl ester formation from fresh tissue. Anal Biochem 152: 141–145 [DOI] [PubMed] [Google Scholar]
- Browse J, Slack CR (1985) Fatty-acid synthesis in plastids from maturing safflower and linseed cotyledons. Planta 166: 74–80 [DOI] [PubMed] [Google Scholar]
- Collatz GJ (1978) The interaction between photosynthesis and ribulose-P2 concentration—effects of light, CO2, and O2. Carnegie Inst Wash Year Book 77: 248–251 [Google Scholar]
- Eastmond PJ, Kolacna L, Rawsthorne S (1996) Photosynthesis by developing embryos of oilseed rape (Brassica napus L.). J Exp Bot 47: 1763–1769 [Google Scholar]
- Eastmond PJ, Rawsthorne S (2000) Coordinate changes in carbon partitioning and plastidial metabolism during the development of oilseed rape embryo. Plant Physiol 122: 767–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JR (1999) Leaf anatomy enables more equal light and CO2 between chloroplasts. New Phytol 143: 93–104 [Google Scholar]
- Foyer C (1993) Interactions between electron transport and carbon assimilation in leaves: coordination of activities and control. In YP Abrol, P Mohanty, Govindjee, eds, Photosynthesis: Photoreactions to Plant Productivity. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 199–224
- Frank HA, Cogdell RJ (1996) Carotenoids in photosynthesis. Photochem Photobiol 63: 257–264 [DOI] [PubMed] [Google Scholar]
- Fuhrmann J, Johnen T, Heise KP (1994) Compartmentation of fatty acid metabolism in zygotic rape embryos. J Plant Physiol 143: 565–569 [Google Scholar]
- Geigenberger P, Merlo L, Reimholz R, Stitt M (1994) When growing potato-tubers are detached from their mother plant there is a rapid inhibition of starch synthesis, involving inhibition of ADP-Glucose pyrophosphorylase. Planta 193: 486–493 [Google Scholar]
- Goffman F, Ruckle M, Ohlrogge J, Shachar-Hill Y (2004) Carbon dioxide concentrations are very high in developing oilseeds. Plant Physiol Biochem (in press) [DOI] [PubMed]
- Heineke D, Riens B, Grosse H, Hoferichter P, Peter U, Flügge UI, Heldt HW (1991) Redox transfer across the inner chloroplast envelope membrane. Plant Physiol 95: 1131–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane HJ, Wilkin JM, Portis AJ, Andrews TJ (1998) Potent inhibition of ribulose-bisphosphate carboxylase by an oxidized impurity in ribulose-1,5-bisphosphate. Plant Physiol 117: 1059–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang F, Rawsthorne S (1994) Starch and fatty acid synthesis in plastids from developing embryos of oilseed rape (Brassica napus L.). Plant J 6: 795–805 [Google Scholar]
- King SP, Badger MR, Furbank RT (1998) CO2 refixation characteristics of developing canola seeds and silique wall. Aust J Plant Phys 25: 377–386 [Google Scholar]
- Lorimer GH, Badger MR, Andrews TJ (1976) The activation of ribulose-1,5-bisphosphate carboxylase by carbon dioxide and magnesium ions. Equilibria, kinetics, a suggested mechanism and physiological implications. Biochemistry 15: 529–536 [DOI] [PubMed] [Google Scholar]
- Mate CJ, Hudson GS, von Caemmerer S, Evans JR, Andrews TJ (1993) Reduction of ribulose bisphosphate carboxylase activase levels in tobacco (Nicotiana tabacum) by antisense RNA reduces ribulose bisphosphate carboxylase carbamylation and impairs photosynthesis. Plant Physiol 102: 1119–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möhlmann T, Scheibe R, Neuhaus HE (1994) Interaction between fatty-acid and starch synthesis in isolated amyloplasts from cauliflower floral buds. Planta 194: 492–497 [Google Scholar]
- Murphy DJ, Cummins I (1989) Biosynthesis of seed storage products during embryogenesis in rapeseed, Brassica napus. J Plant Physiol 135: 63–69 [Google Scholar]
- Norton G (1989) Nature and biosynthesis of storage proteins. In G Röbbelen, RK Downey, A Ashri, eds, Oil Crops of the World. McGraw-Hill, New York, pp 165–191
- Ögren E, Evans JR (1993) Photosynthetic light-response curves: I. The influence of CO2 partial pressure and leaf inversion. Planta 189: 182–190 [Google Scholar]
- Ohlrogge JB, Kuo TM (1984) Control of lipid synthesis during soybean seed development: enzymic and immunochemical assay of acyl carrier protein. Glycine max. Plant Physiol 74: 622–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul MJ, Knight JS, Habash D, Parry MAJ, Lawlor DW, Barnes SA, Loynes A, Gray JC (1995) Reduction in phosphoribulokinase activity by antisense RNA in transgenic tobacco: effect on CO2 assimilation and growth in low irradiance. Plant J 7: 535–542 [Google Scholar]
- Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta 975: 348–394 [Google Scholar]
- Porterfield DM, Kuang A, Smith PJS, Crispi ML, Musgrave ME (1999) Oxygen-depleted zones inside reproductive structures of Brassicaceae: implications for oxygen control of seed development. Can J Bot 77: 1439–1446 [PubMed] [Google Scholar]
- Ratcliffe RG (1995) Metabolic aspects of the anoxic response in plant tissue. In N Smirnoff, ed, Environmental Plant Metabolism. BIOS Scientific Publishers, Oxford, pp 111–127
- Rolletschek H, Borisjuk L, Koschorreck M, Wobus U, Weber H (2002) Legume embryos develop in a hypoxic environment. J Exp Bot 53: 1099–1107 [DOI] [PubMed] [Google Scholar]
- Roughan PG, Ohlrogge JB (1996) Evidence that isolated chloroplasts contain an integrated lipid-synthesizing assembly that channels acetate into long-chain fatty acids. Plant Physiol 110: 1239–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruuska SA, Badger MR, Andrews TJ, von Caemmerer S (2000) Photosynthetic electron sinks in transgenic tobacco with reduced amounts of Rubisco: little evidence for significant Mehler reaction. J Exp Bot 51: 357–368 [DOI] [PubMed] [Google Scholar]
- Ruuska SA, Girke T, Benning C, Ohlrogge JB (2002) Contrapuntal networks of gene expression during Arabidopsis seed filling. Plant Cell 14: 1191–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage RF, Sharkey TD, Seemann JR (1990) Regulation of ribulose-1,5-bisphosphate carboxylase activity in response to light intensity and CO2 in the C3 annuals Chenopodium album L. and Phaseolus vulgaris L. Plant Physiol 94: 1735–1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savchenko G, Wiese C, Neimanis S, Hedrich R, Heber U (2000) pH regulation in apoplastic and cytoplasmic cell compartments of leaves. Planta 211: 246–255 [DOI] [PubMed] [Google Scholar]
- Scheibe R (1987) NADP+ malate dehydrogenase in C3 plants: regulation and role of a light-activated enzyme. Physiol Plant 71: 393–400 [Google Scholar]
- Scheibe R, Anderson LE (1981) Dark modulation of NADP-dependent malate dehydrogenase and glucose 6 phosphate dehydrogenase. Biochim Biophys Acta 636: 58–64 [DOI] [PubMed] [Google Scholar]
- Scheibe R, Fickenscher K, Ashton A (1986) Studies on the mechanism of the reductive activation of NADP-malate dehydrogenase by thioredoxin m and low-molecular weight thiols. Biochim Biophys Acta 870: 191–197 [Google Scholar]
- Scheibe R, Jacquot JP (1983) NADP regulates the light activation of NADP-dependent malate dehydrogenase. Planta 157: 548–553 [DOI] [PubMed] [Google Scholar]
- Scheibe R, Stitt M (1988) Comparison of NADP-malate dehydrogenase activation, QA reduction and O2 reduction in spinach leaves. Plant Physiol Biochem 26: 473–481 [Google Scholar]
- Schwender J, Ohlrogge JB (2002) Probing in vivo metabolism by stable isotope labeling of storage lipids and proteins in developing Brassica napus embryos. Plant Physiol 130: 347–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwender J, Ohlrogge JB, Shachar-Hill Y (2003) A flux model of glycolysis and the oxidative pentose phosphate pathway in developing Brassica napus embryos. J Biol Chem 278: 29442–29453 [DOI] [PubMed] [Google Scholar]
- Siefermann-Harms D (1985) Carotenoids in photosynthesis. I. Location in photosynthetic membranes and light-harvesting function. Biochim Biophys Acta 811: 325–355 [Google Scholar]
- Singal HR, Talwar G, Dua A, Singh R (1995) Pod photosynthesis and seed dark CO2 fixation support oil synthesis in developing Brassica seeds. J Biosci 20: 49–58 [Google Scholar]
- Smith RG, Gauthier DA, Dennis DT, Turpin DT (1992) Malate- and pyruvate-dependent fatty acid synthesis in leucoplasts from developing castor bean endosperm. Plant Physiol 98: 1233–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M, Lilley RM, Gerhardt R, Heldt HW (1989) Metabolite levels in specific cells and subcellular compartments of plant leaves. Methods Enzymol 174: 518–552 [Google Scholar]
- Sun J, Nishio JN, Vogelmann TC (1998) Green light drives CO2 fixation deep within leaves. Plant Cell Physiol 39: 1020–1026 [Google Scholar]
- Thelen JJ, Mekhedov S, Ohlrogge JB (2001) Brassicaceae express multiple isoforms of biotin carboxyl carrier protein in a tissue-specific manner. Plant Physiol 125: 2016–2028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, DellaPenna D (2001) Characterization of a second carotenoid β-hydroxylase gene from Arabidopsis and its relationship to the LUT1 locus. Plant Mol Biol 47: 379–388 [DOI] [PubMed] [Google Scholar]
- Turnham E, Northcote DH (1983) Changes in the activity of acetyl-CoA carboxylase during rape-seed formation. Biochem J 212: 223–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigeolas H, van Dongen JT, Waldeck P, Hühn D, Geigenberger P (2003) Lipid storage metabolism is limited by the prevailing low oxygen concentrations within growing seeds of Brassica napus L. Plant Physiol 133: 2048–2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Caemmerer S, Edmondson DL (1986) Relationship between steady-state gas-exchange, in vivo ribulose bisphosphate carboxylase activity and some carbon-reduction cycle intermediates in Raphanus sativus. Aust J Plant Physiol 13: 669–688 [Google Scholar]
- von Caemmerer S, Evans JR, Hudson G, Andrews TJ (1994) The kinetics of ribulose 1,5 bisphosphate carboxylase/oxygenase in vivo inferred from measurements of photosynthesis in leaves of transgenic tobacco. Planta 195: 88–97 [Google Scholar]
- Wara-Aswapati O, Kemble RJ, Bradbeer JW (1980) Activation of glyceraldehyde-phosphate dehydrogenase (NADP) and phosphoribulokinase in Phaseolus vulgaris leaf extracts involves the dissociation of oligomers. Plant Physiol 66: 34–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JA, Todd J, Newman T, Focks N, Girke T, Martínez de Ilárduya O, Jaworski JG, Ohlrogge JB, Benning C (2000) A new set of Arabidopsis expressed sequence tags from developing seeds. The metabolic pathway from carbohydrates to seed oil. Plant Physiol 124: 1582–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willms JR, Salon C, Layzell DB (1999) Evidence for light-stimulated fatty acid synthesis in soybean fruit. Plant Physiol 120: 1117–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue LG, McCune LM, Kleppinger-Sparace KF, Brown MJ, Pomeroy MK, Sparace SA (1997) Characterization of the glycerolipid composition and biosynthetic capacity of pea root plastids. Plant Physiol 113: 549–557 [DOI] [PMC free article] [PubMed] [Google Scholar]