Abstract
The lsd1 mutant of Arabidopsis fails to limit the boundaries of hypersensitive cell death response during avirulent pathogen infection and initiates unchecked lesions in long day photoperiod giving rise to the runaway cell death (rcd) phenotype. We link here the initiation and propagation of rcd to the activity of photosystem II, stomatal conductance and ultimately to photorespiratory H2O2. A cross of lsd1 with the chlorophyll a/b binding harvesting-organelle specific (designated cao) mutant, which has a reduced photosystem II antenna, led to reduced lesion formation in the lsd1/cao double mutant. This lsd1 mutant also had reduced stomatal conductance and catalase activity in short-day permissive conditions and induced H2O2 accumulation followed by rcd when stomatal gas exchange was further impeded. All of these traits depended on the defense regulators EDS1 and PAD4. Furthermore, nonphotorespiratory conditions retarded propagation of lesions in lsd1. These data suggest that lsd1 failed to acclimate to light conditions that promote excess excitation energy (EEE) and that LSD1 function was required for optimal catalase activity. Through this regulation LSD1 can influence the effectiveness of photorespiration in dissipating EEE and consequently may be a key determinant of acclimatory processes. Salicylic acid, which induces stomatal closure, inhibits catalase activity and triggers the rcd phenotype in lsd1, also impaired acclimation of wild-type plants to conditions that promote EEE. We propose that the roles of LSD1 in light acclimation and in restricting pathogen-induced cell death are functionally linked.
The sessile nature of plants means that they must be able to adjust metabolic processes to a constantly fluctuating light environment. The amount of absorbed light energy in excess of that needed by plants for photosynthetic metabolism is termed excess excitation energy (EEE; Asada, 1999; Karpinski et al., 1999; Niyogi, 2000; Mullineaux and Karpinski, 2002; Fryer et al., 2003). Failure to dissipate EEE results in over-reduction of components of photosynthetic electron transport and increased production of reactive oxygen species (ROS) in the chloroplast. ROS include singlet oxygen, superoxide anion (O2−˙), hydrogen peroxide (H2O2), and the hydroxyl radical (*OH; Asada 1999). Their accumulation leads to photoinhibition, photooxidative damage and eventually death of the cell, manifested as bleaching or chlorosis of the photosynthetic tissues (Karpinski et al., 1999; Karpinska et al., 2000; Kasahara et al., 2002).
Light acclimation processes are aimed at dissipating EEE or minimizing its formation by diminishing the capacity of the leaf to capture light energy (Niyogi, 2000; Ort, 2001; Kasahara et al., 2002). Immediate and short-term responses to conditions that promote EEE include the rearrangement of light harvesting complexes, changing the stoichiometry between photosystem I (PSI) and photosystem II (PSII; Allen, 1995; Pfannschmidt et al., 1999; Wollman, 2001), and increases in thermal dissipation of excitation energy reflected by the changes in the nonphotochemical quenching parameter (NPQ; Niyogi, 2000; Muller et al., 2001; Ort, 2001).
There are many environmental conditions that promote EEE (Asada, 1999; Niyogi, 2000; Ort, 2001). Besides sudden increases in light intensity or a change in light quality, other environmental factors such as changes in temperature, CO2 availability, and water status can bring about an increase in EEE (van Rensen et al., 1999; Tsonev et al., 2003). In many of these situations, closure of stomata is a critical factor in the response of the leaf to stress (Fryer et al., 2003). Under such conditions, gas exchange with the external environment is curtailed, leading to a rapid fall in internal CO2 concentrations (Cornic and Fresneau, 2002; Noctor et al., 2002). This impairs the consumption of electrons by CO2 fixation creating the conditions for an increase in EEE and increased activity of the photorespiratory cycle (Ku and Edwards, 1978; Wingler et al., 2000; Noctor et al., 2002; Fryer et al., 2003).
Photorespiration results from the oxygenase reaction of the Rubisco. The oxygenase reaction is an important EEE dissipatory pathway (Kozaki and Takeba, 1996; Willekens et al., 1997; Wingler et al., 2000). However, during the necessary recycling of glycollate, the glycollate oxidase-catalyzed reaction in the peroxisomal part of the photorespiratory cycle generates considerable amounts of H2O2 that is scavenged principally by peroxisomal catalase (CAT; EC 1.11.1.6; Kozaki and Takeba, 1996; Willekens et al., 1997). In addition to CAT, other ROS-scavenging enzymes such as copper-zinc superoxide dismutase (CuZnSOD) may also be important in the peroxisome (del Rio et al., 2002). Any increase in photorespiration beyond the capacity of the prevailing ROS scavenging system limits the effectiveness of photorespiration as a means of dissipating EEE (Noctor et al., 2002).
The lesion simulating disease 1 (lsd1) mutant was first characterized for its O2−-dependent spreading chlorotic/necrotic phenotype that develops under long (>16 h) or continuous photoperiods or after infection with an avirulent pathogen (Dietrich et al., 1994; Jabs et al., 1996). The phenotype was indicative of a failure in processes that regulate cell death, not only to prevent its initiation but also to limit lesion spread (propagation), and was named runaway cell death (rcd). LSD1 has been suggested to be a negative regulator of cell death by acting as a ROS rheostat. Above a certain ROS threshold, the pro-death pathway would operate leading to cell death (Dietrich et al., 1997; Kliebenstein et al., 1999). Short day (SD) photoperiods (8 h) and low photosynthetically active photon flux density (PPFD; typically circa 100 μmol m−2 s−1) are permissive conditions for the growth of lsd1 (Dietrich et al., 1994; Jabs et al., 1996). These observations suggest a strong relationship between prevailing light conditions and the initiation of the lsd1 phenotype. Given this light-dependent phenotype for lsd1, we considered it possible that LSD1 may influence light acclimation processes in Arabidopsis. This paper reports that LSD1 is indeed required for acclimation to conditions that promote EEE.
RESULTS
LSD1 Is Required for Acclimation to Conditions Promoting EEE
Previous observations (Dietrich et al., 1994) showed that spreading lesions were initiated in lsd1 upon a shift from a short to a long photoperiod and this was confirmed in our growth conditions (data not shown). However, long day (LD) conditions and light intensities used in the present study were less than those described by Jabs et al. (1996; see “Materials and Methods”) and this affected the extent of lesion formation.
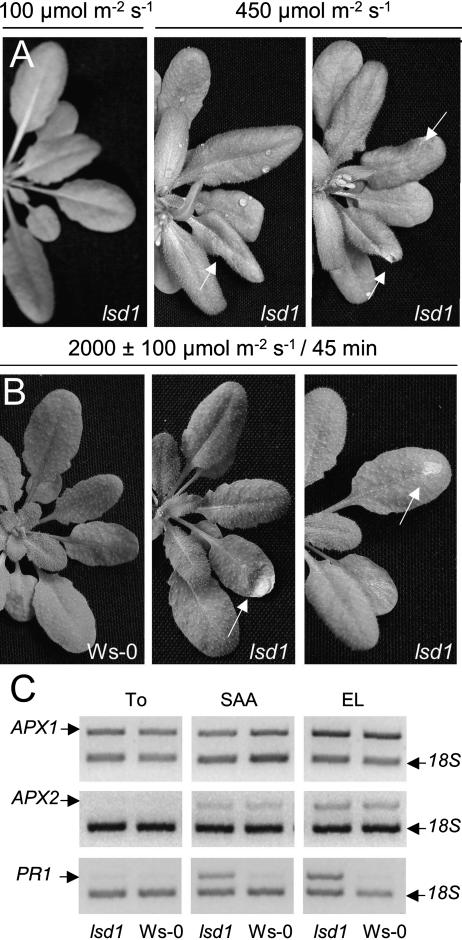
Arabidopsis ecotype Wassilewskija (Ws-0) and lsd1 plants grown in short days (8-h photoperiod) at a PPFD of 100 μmol m−2 s−1 (hereafter called permissive conditions) were exposed to higher light intensities (PPFD of 450 μmol m−2 s−1) under the same photoperiod. The increase in light intensity was sufficient to induce lesion formation in lsd1 after approximately 7 d (Fig. 1A). The response of lsd1 to more severe excess light (EL) treatments was also analyzed (Fig. 1B). Low light, SD-adapted Ws-0 and lsd1 rosettes were partially exposed to a 20-fold higher irradiance (2,000 μmol m−2 s−1) for 45 min. The substantial photoinhibition of PSII in such leaves was indicated by a sharp drop in the maximum and operating efficiencies of PSII (Fv/Fm and ΦPSII, respectively), photochemical quenching (qp), and further changes in thermal dissipation (NPQ) fluorescence parameters (Supplemental Fig. 1, available at www.plantphysiol.org). A moderate reduction of these chlorophyll fluorescence parameters was also detected in the low light-zone of partially exposed Ws-0 and lsd1 rosettes (Supplemental Fig. 1). No differences were observed between Ws-0 and lsd1 in these parameters in exposed leaves or in leaves undergoing systemic acquired acclimation (SAA, Supplemental Fig. 1; Karpinski et al., 1999). Messenger RNA levels of APX1 and APX2, encoding isoforms of cytosolic ascorbate peroxidase important in catalyzing the reduction of H2O2 arising directly from the chloroplast (Karpinski et al., 1997; Karpinska et al., 2000; Fryer et al., 2003), were similar in mutant and wild-type, both in SAA- and EL-leaves (Fig. 1C). Despite the apparent recovery of photosynthetic electron transport observed 2 and 24 h after the EL-induced photooxidative stress (Supplemental Fig. 2), lesions developed in exposed leaves (Fig. 1B). We concluded from the above set of experiments that it was the amount of light and not the photoperiod shift that triggers rcd in lsd1.
Figure 1.
EEE triggers the lsd1 runaway cell death phenotype. A, Three- to 4-week-old Ws-0 and lsd1 plants grown in standard conditions were exposed to HL (450 ± 20 μmol m−2 s−1). Lesion formation and runaway cell death started after approximately 1 week in those conditions (n = 15). B, Four- to 5-week-old rosettes of Ws-0 and lsd1 plants were partially exposed to EL (2,000 ± 100 μmol m−2 s−1) for 45 min (Karpinski et al., 1999) and then allowed to recover in SD until lesions were visible (2–4 d; n = 15). C, Relative quantification of the APX1, APX2, and PR1 transcripts in EL-exposed and in nonexposed leaves undergoing SAA (Karpinski et al., 1999). Gels are representative of three different replicates; see “Materials and Methods.”
PR1 (PATHOGENESIS RELATED 1) mRNA levels were strongly induced both in EL exposed and SAA-leaves of lsd1 (Fig. 1C). This was consistent with previous reports on induction of PR1 upon transfer of lsd1 plants from short to long photoperiods (Dietrich et al., 1994; Jabs et al., 1996). The data presented above show that lsd1 failed to acclimate to permanent or transitory changes in light intensity and induced defense gene expression as a consequence. We concluded that LSD1 is essential for acclimation of plants to EEE.
Excitation Energy from Photosystem II Affects the Light Sensitive Phenotype of lsd1
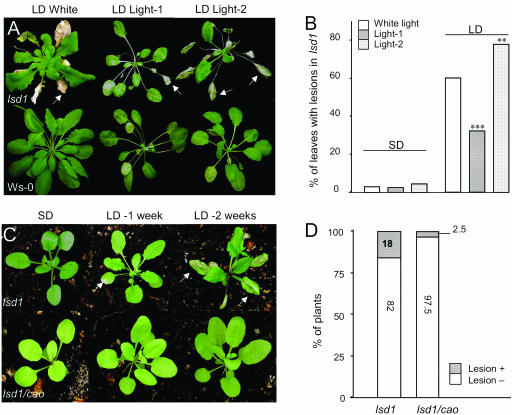
Wild-type and lsd1 plants were transferred to LD nonpermissive conditions enriched for light preferentially absorbed by PSI (700 nm wavelength, hereafter called light-1) or by PSII (680 nm wavelength, hereafter called light-2). The use of these light conditions has the advantage that the balance of light absorption between PSI and PSII, and the subsequent redox status of photosynthetic electron transport components can be manipulated in a noninvasive way (Pfannschmidt et al., 1999). No significant lesion formation was observed in lsd1 rosettes in either light condition in short days. However in long photoperiods, the severity of the lesion phenotype observed in light-2 and white light was significantly greater than in light-1 (Fig. 2, A and B). The differential effect of light-1 and light-2 on lesion formation suggested a specific role for increased excitation energy at PSII in the development of the mutant phenotype. Therefore, lsd1 was crossed with the chlorophyll a/b binding protein organelle specific (cao) knockout mutant (see “Materials and Methods”). CAO encodes the chloroplastic signal recognition particle protein, a chaperone necessary for the assembly of a large part of the PSII antenna encoded by light harvesting complex B (LHCB; Klimyuk et al., 1999). In cao, the size of the PSII antenna is much more reduced than that of PSI, leading to reduced absorption of light energy by PSII (Klimyuk et al., 1999). After a shift to long photoperiod, lsd1/cao double mutants showed a significant delay in the initiation as well as the extension of lesions compared to lsd1 plants (Fig. 2, C and D). Lower lesion formation in lsd1/cao was associated with a significantly higher thermal dissipation of EEE (NPQ) in those plants (Supplemental Fig. 3). We reasoned from these experiments that lesion formation in lsd1 is linked to the activity of PSII and/or PSII antennae organization.
Figure 2.
rcd triggered by nonpermissive light condition is reverted by reduction of PSII activity. Ws-0 and lsd1 plants acclimated to white-light, light-1, or light-2 for 2 weeks in SD (8 h) were shifted to nonpermissive LD (16 h) under the respective light quality. A, Pictures of representative rosettes 2 weeks after shift from SD to LD in given light conditions. B, Quantitative analysis of leaf damage in lsd1 after 2 weeks in LD in the stated light conditions. Bars represent the number of fully damaged leaves per rosette in each light quality (n = 50 rosettes from three independent experiments). Data were tested for significance by t test (P ≤ 0.01** and P ≤ 0.001***). C, Seedlings of lsd1 and lsd1/cao (see “Materials and Methods”), were grown in SD for 3 weeks and then shifted to LD. Picture of representative rosettes 1 and 2 weeks after shift from SD to LD are shown. D, Statistical reckoning of damaged rosettes after 10 d in LD. Data represent means of n = 200 rosettes from three independent experiments. White arrows indicate damaged leaves.
Role of Stomata and Photorespiration in Development of the lsd1 Phenotype
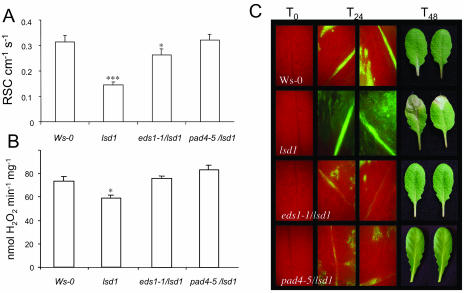
An examination of the chlorophyll a fluorescence parameters (Supplemental Fig. 1) did not reveal any effect of LSD1 on thermal dissipation or other nonphotochemical quenching processes. Therefore, attention was focused on EEE dissipatory processes that consume electrons, such as photorespiration. Closure of stomata in Arabidopsis leaves occurs rapidly in response to even small (as low as 3-fold) increases in PPFD and leads to increased photorespiration (Ku and Edwards, 1978; Wingler et al., 2000; Noctor et al., 2002; Fryer et al., 2003). We measured stomatal conductance in both Ws-0 and lsd1 grown under permissive conditions (Fig. 3A). Relative stomatal conductance values in lsd1 plants were 50% lower than in Ws-0 (0.146 ± 0.011 and 0.315 ± 0.025 cm s−1, respectively), indicating that stomata were more closed in the mutant than in wild-type plants. This lower stomata conductance was paralleled by a lower total catalase (CAT) activity (Fig. 3B). Mutations in PAD4 (PHYTOALEXIN DEFICIENT4) and EDS1 (ENHANCED DISEASE SUSCEPTIBILITY1) block lsd1-conditioned rcd, triggered by long photoperiods, pathogen inoculation, ROS provision, or supply of the phenolic signaling molecule, salicylic acid (SA; Rustérucci et al., 2001). The significantly lower stomatal conductance and CAT activity observed in lsd1 were not observed in the pad4-5/lsd1 or eds1-1/lsd1 double mutants (Fig. 3, A and B).
Figure 3.
Effects of lower stomata conductance and forced limitation of foliar gas exchange in lsd1 are reverted in pad4-5/lsd1 and eds1-1/lsd1. A, Relative stomatal conductance (RSC) and B, CAT activity in leaves of Ws-0, lsd1, pad4-5/lsd1, and eds1-1/lsd1 in SD permissive conditions. Note the approximately 50% significantly (P ≤ 0.001***) lower stomata conductance and lower CAT activity (P ≤ 0.05*) in lsd1 and the recovery of wild-type phenotype in the double mutants. Data represent means of n = 15 from three independent measurements. Vertical bars represent sd. C, Leaves of Ws-0 and lsd1 plants grown in SD were affixed a small amount of lanolin, and H2O2 monitored after 24 h (T24) treatment by DCF-2 yellow-green fluorescence (see “Materials and Methods”). Runaway cell death was observed in lsd1 but not in Ws-0 nor in pad4-5/lsd1 and eds1-1/lsd1 after 48 h (T48). Representative pictures of treated leaves are shown (n = 15 from three different experiments).
We reasoned that impairment in stomatal conductance and lower CAT activity could be an important contributory factor in the rcd phenotype in lsd1. If this were the case, artificial blocking of stomatal pores and gas exchange by smearing lanolin on the lower surface of lsd1 leaves would promote lesion formation under otherwise permissive light conditions. After 24 h, a readily detectable increase in foliar H2O2 was observed in lanolin treated leaves of lsd1 by dichlorofluorescein staining. This was followed by rcd after 48 h (Fig. 3C). The increase in foliar H2O2 and rcd as a consequence of limiting gas exchange was not observed in Ws-0, the pad4-5/lsd1 or eds1-1/lsd1 double mutants (Fig. 3C). While dichlorofluorescein staining is only a semiquantitative measure of H2O2 accumulation, the data suggest that localized increases in photorespiration caused by blocking gas exchange (during stress or artificially) can induce rcd in lsd1 and these observations link the phenotype to stomata conductance and production of photorespiratory H2O2. Attempts to quantify H2O2 in foliar extracts more accurately as described in Karpinski et al. (1997, 1999) were unsuccessful due to interference from the lanolin.
Attenuation of rcd in Nonphotorespiratory Conditions
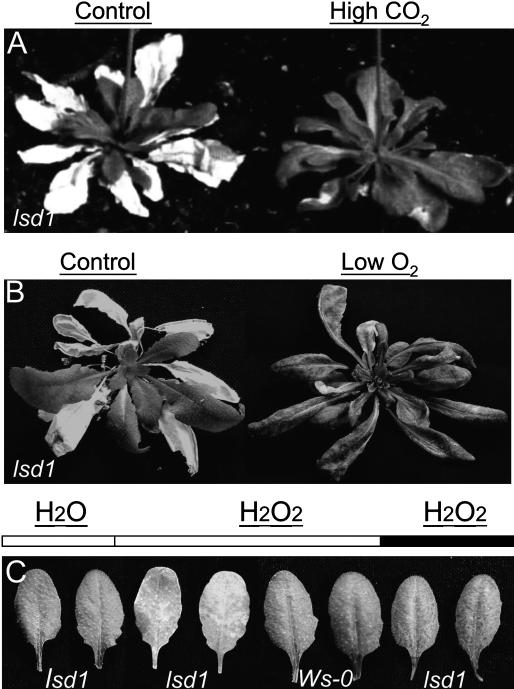
If photorespiration is a major source of H2O2 promoting rcd in lsd1, we reasoned that preventing the oxygenase reaction of Rubisco by incubating plants under high CO2 or low O2 tensions should mitigate the lesion phenotype in lsd1. Results in Figure 4, A and B, show that placing lsd1 plants either in an atmosphere of 0.12% (v/v) CO2 (3-fold above ambient concentration) or 2% (v/v) O2 (10% of ambient concentration) substantially attenuated the lesion phenotype under nonpermissive LD conditions.
Figure 4.
Reactive oxygen species (ROS) originating from photorespiration are critical for runaway cell death (rcd) in lsd1. A, Four-week-old lsd1 plants cultivated in LD and which had initiated lesion formation were fumigated either with a 0.12% CO2 and 21% O2 (high CO2) atmosphere LD (16 h) for a period of 2 weeks or B, with a 2% O2 and 0.04% CO2 (low O2) atmosphere in continuous light (CL) for a period of 1 week. Note the attenuation of lsd1 runaway cell death phenotype in high CO2 and low O2 atmospheres. The number of fully damaged leaves per rosette was counted and tested for significance by t test (P ≤ 0.01 for both experiments). C, Pictures of representative leaves of Ws-0 and lsd1 after 2 d incubation either in light or dark in a solution of 20 mm H2O2 (n = 40 from three independent experiments). Control leaves were incubated in water.
In higher plants acclimation to EEE is thought to be driven by significant increases in H2O2 levels (Karpinski et al., 1997, 1999; Karpinska et al., 2000; Fryer et al., 2003). Treatment of lsd1 leaves with 20 mm H2O2 under permissive light conditions also caused spreading lesions after 48 h (Fig. 4C). This was not observed on H2O2 treated Ws-0 leaves or in lsd1 leaves kept in the dark (Fig. 4C).
CAT1 Activity Is Reduced in lsd1
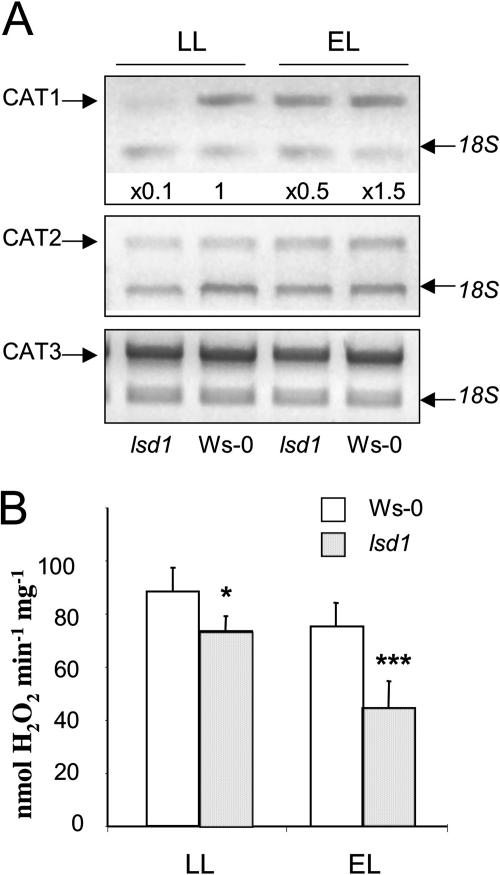
Increased stomatal closure, enhanced sensitivity to exogenously supplied H2O2 and partial reversion of the lsd1 phenotype in the lsd1/cao double mutant or by non-photorespiratory conditions, suggested that ROS scavenging may have been compromised in lsd1. Since APX transcripts were not affected in lsd1 (Fig. 1C) and peroxisomal catalase is primarily responsible for removal of photoproduced H2O2 (Kozaki and Takeba, 1996; Willekens et al., 1997; Asada, 1999), mRNA levels for the three genes encoding subunits of catalases in Arabidopsis (CAT1, CAT2, and CAT3; Frugoli et al., 1996) and total foliar catalase activity were assessed in lsd1 and Ws-0 control plants cultivated in permissive conditions (LL) and after EL treatment. CAT1 but not CAT2 and CAT3 transcript was strongly diminished in lsd1 compared to Ws-0 (Fig. 5A) in LL-acclimated plants. Reduced CAT1 mRNA in lsd1 was associated with significantly (P < 0.01) lower foliar CAT activity and higher H2O2 levels compared to wild type (Figs. 5B and 3C). Although, CAT1 gene expression was induced in lsd1 after 1 h exposure to EL (5-fold compared to LL) levels were nevertheless still lower than in wild type (Fig. 5A). Interestingly, the increased expression of CAT1 in EL-stressed lsd1 and wild-type leaves was associated with a further decrease in total foliar CAT activity, which was more pronounced in the mutant (P < 0.001; Fig. 5B). From these observations we concluded that LSD1 is important for regulation of the CAT1 expression and total foliar CAT activity.
Figure 5.
Reduced foliar catalase activity and inhibited expression of CAT1 gene in lsd1. A, Relative PCR quantification of the CAT1, CAT2, and CAT3 transcript levels in Ws-0 and lsd1 plants in SD LL and after EL treatment. Gels are representative of three different replicates. Fold induction is stated for CAT1. CAT2 and CAT3 expressions were similar to wild type. Observed differences were statistically significant (P ≤ 0.05*). B, Total foliar catalase activity in Ws-0 and lsd1 plants in SD permissive conditions and in EL-exposed plants. Catalase activity measurements are representative of seven different replicates from three independent experiments for each treatment (n = 21). Vertical bars represent sd (P ≤ 0.05* and P ≤ 0.001***).
Salicylic Acid, EEE, and Stomatal Conductance
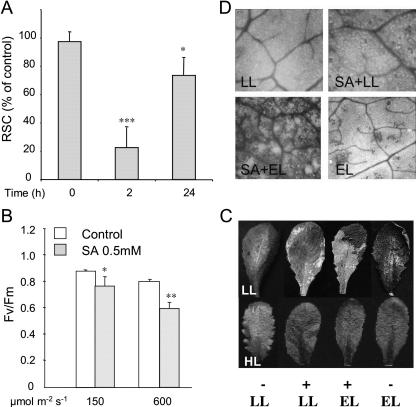
SA treatment induces rcd in lsd1 under otherwise permissive conditions (Jabs et al., 1996). Interestingly, rapid closure of stomata and an increase in foliar H2O2 have been shown to occur upon SA treatment of leaves (Manthe et al., 1992; Chen et al., 1993; Rao et al., 1997; Shirasu et al., 1997; Lee, 1998; Mori et al., 2001). This may be associated with SA-dependent inhibition of CAT activity (Sanchez-Casas and Klessig, 1994; Conrath et al., 1995; Durner and Klessig, 1996; Chen et al., 1997). If peroxisomal CAT levels prior to a change in the light environment are crucial to successful acclimation or recovery, we anticipated that wild-type leaves treated with SA would generally behave in a manner similar to lsd1. Treatment of wild-type Arabidopsis leaves with SA under SD conditions induced rapid stomatal closure within 2 h (Fig. 6A). Furthermore, such leaves showed photoinhibition of photosynthetic electron transport under low light conditions that was intensified in high light (HL; Fig. 6B). Photobleaching in wild type leaves exposed to EL was characterized by the appearance of a delimited area of cell death revealed by lactophenol-trypan blue staining (TB; Fig. 6D). These effects were strongly accelerated by a combined effect of SA-treatment and EL exposure (Fig. 6, C and D). It is important to note here that stomatal gas exchange in SA-treated wild-type leaves before EL exposure was reduced approximately by 4-fold (Fig. 6A), while in combined treatments (SA + EL) it was by 12-fold (0.027 ± 0.019 cm s−1). SA treatment of lsd1 leaves in low light caused a reduction of stomatal gas exchange by 14-fold (0.024 ± 0.016 cm s−1) in comparison to wild-type control values. Failure to tolerate EL treatment ensued. This effect of SA on acclimation was reinforced by the observation that HL-acclimated leaves, already dissipating or limiting EEE, were tolerant to such SA treatment (Fig. 6C).
Figure 6.
SA impairs acclimation to EEE in low light-acclimated plants. A, Relative stomata conductance (RSC) in wild-type leaves of rosette grown in SD treated with SA (0.4 mm) in comparison to control leaves treated with water. B, Photosynthetic parameter Fv/Fm in LL-acclimated leaves treated with 0.5 mm SA and then exposed to LL (100 ± 25 μmol m−2 s−1) or HL (450 ± 25 μmol m−2 s−1) for 2 h. C, LL- and HL-acclimated leaves treated with 0.4 mm SA (+) for several hours and exposed to EL (2,200 ± 200 μmol m−2 s−1, 90 min exposure). D, TB staining of representative leaves. Pictures were taken 2 h after treatments and represents of n = 15 from three independent experiments (P ≤ 0.05*, P ≤ 0.01**, and P ≤ 0.001***).
Light Dependence of rcd in lsd1 Leaves Inoculated with Avirulent Peronospora parasitica
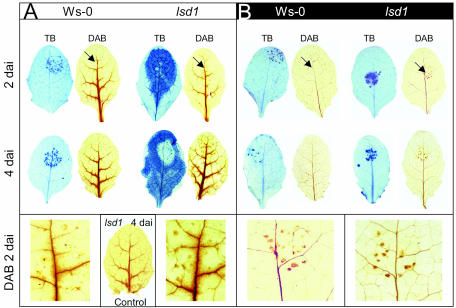
The above data, suggesting a positive role of LSD1 in acclimation to EEE, led us to consider whether this is linked to the established role of LSD1 as a negative regulator of cell death (Jabs et al., 1996; Dietrich et al., 1997; Aviv et al., 2002; Epple et al., 2003). Infection of avirulent pathogens onto lsd1 leaves under permissive light conditions triggers rcd (Dietrich et al., 1994; Jabs et al., 1996). We tested to what extent hypersensitive cell death response formation in wild type and lsd1 and lesion propagation in lsd1 are light-dependent processes. When challenged with avirulent Peronospora parasitica (Noco2) both lsd1 and Ws-0 plants initiated a hypersensitive cell death response 2 d after inoculation (dai) irrespective of light conditions (Fig. 7, 2-dai sections). Hypersensitive cell death response was characterized by the appearance of a delimited area of cell death revealed by TB staining and H2O2 formation at sites of infection detected by 3,3-diaminobenzidine (DAB) precipitation. In the light, infected leaves of both genotypes accumulated H2O2 around the veins to higher levels than control leaves, consistent with previous observations (Fig. 7, DAB staining; Fryer et al., 2003). While hypersensitive cell death response in Ws-0 was strictly restricted to the area around pathogen infection sites, lesions were larger in light-incubated leaves than those kept in the dark at all time points (Fig. 7). In the light lsd1 leaves developed spreading lesions phenotype (Fig. 7A, 2- and 4-dai, TB staining). In the dark, lsd1 exhibited strongly diminished rcd (Fig. 7B, TB staining). These data show that initiation of the hypersensitive cell death response and production of ROS in both genotypes is light independent. However, light causes an increase in the size of hypersensitive cell death response lesions in Ws-0 and promotes lesion propagation in lsd1.
Figure 7.
rcd but not initial hypersensitive cell death (HR) after P. parasitica (Pp) inoculation is light-dependent in lsd1 leaves. Leaves of 4-week-old Ws-0 and lsd1 were inoculated by placing a 10-μL droplet of Pp isolate Noco2 spores or H2O as control on the top one-half of each leaf and then incubated in either a SD light regime (A) or in the dark (B). Two and 4 dai leaves were analyzed for cell death and H2O2 accumulation by TB- and DAB-staining, respectively. Accumulation of H2O2 at interaction sites 2 dai in the light or in the dark is marked with black arrows and shown at 10×magnification (bottom). Note the accumulation of H2O2 around the leaf veins in both Ws-0 and lsd1 leaves incubated in the light, paralleled by rcd in lsd1. No H2O2 accumulation was observed in the dark-incubated leaves and no rcd was visible. Images are representative of four independent experiments using at least five leaves per genotype and experiment.
DISCUSSION
The increased sensitivity of lsd1 to changes in photoperiod and light intensities is manifested as induction of rcd. Our data suggest that lsd1 fails to dissipate EEE effectively and thus accumulates ROS. The above data also suggests that LSD1 controls PAD4-, EDS1-, and SA-dependent stomatal closure and subsequent photorespiratory production of ROS; thus, we concluded that LSD1 prevents photooxidative damage.
LSD1 and the Regulation of CAT Expression
The data presented in this paper also show that LSD1 can control the activity of CAT in Arabidopsis, presumably by controlling CAT1 transcription levels in LL conditions (Fig. 5, A and B). In Arabidopsis, catalase is encoded by a multi-gene family consisting of three genes (CAT1, CAT2, and CAT3). They encode individual subunits that associate to form at least six isozymes (Frugoli et al., 1996). The subunit composition of the isozymes is variable depending on the developmental and physiological stage of the plant (Robertson McClung, 1997). Both the steady state levels of catalase mRNA, protein synthesis and activity are tightly regulated in a number of plant species (Hertwig et al., 1992; Robertson McClung, 1997; Schmidt et al., 2002). Furthermore, peroxisomal catalase has been suggested to be photosensitive. Thus, under conditions of increased light intensity the enzyme required to maintain the functioning of the photorespiratory cycle may become progressively impaired unless replaced by active synthesis (Feierabend et al., 1992; Hertwig et al., 1992; Schmidt et al., 2002).
These observations were confirmed by reduction of foliar CAT activity in wild-type plants exposed to EL, although transcript levels for CAT were induced (Fig. 5). Significantly stronger reduction of CAT activity was observed in lsd1 plants exposed to the same EL blast, although transcript levels for CAT1 were also induced. Lower CAT1 transcripts levels in LL-acclimated and EL exposed lsd1 mutant than in wild-type plants suggests that CAT1 expression in LL-acclimated lsd1 could not be sufficiently induced upon exposure to EL.
LSD1 was also proposed to participate in an SA-dependent signaling pathway for the activation of a CuZnSOD that would allow a limited accumulation of superoxide during the hypersensitive cell death response (Kliebenstein et al., 1999). Those observations and data presented here point to a primary role of LSD1 as a regulator of ROS levels. Control of ROS metabolism at key points would have a decisive impact on the plant response to environmental cues. These include light acclimation mechanisms described here (Fig. 1), local and systemic signaling for the induction of defense gene expression during EEE (Prasad et al., 1994; Karpinski et al., 1997, 1999; Karpinska et al., 2000; Mullineaux et al., 2000; Desikan et al., 2001), and pathogen attack (Lamb and Dixon, 1997; Alvarez et al., 1998; Maleck et al., 2000; Beers and McDowell, 2001). Elevated levels of H2O2 confer disease resistance to virulent pathogens but can also elicit programmed cell death as part of the hypersensitive cell death response (Levine et al., 1994; Alvarez et al., 1998; Dat et al., 2003; Yoda et al., 2003). Finally, H2O2-mediated defense gene induction has been observed after applying H2O2 by various means, including infiltration of Arabidopsis cultured cells (Desikan et al., 1996), silencing of a tobacco (Nicotiana tabacum) catalase gene (Takahashi et al., 1997; Willekens et al., 1997), overexpression a gene encoding H2O2-generating oxalate oxidase (Hu et al., 2003), ectopically expressing a Glc oxidase in potato (Solanum tuberosum) plants (Wu et al., 1997), and through polyamine degradation in tobacco plants (Yoda et al., 2003).
The lsd1 Phenotype Resembles that of Catalase Deficient Plants
The increased sensitivity of lsd1 to changes in photoperiod and light intensities is manifested as induction of rcd. Our data suggest that lsd1 fails to dissipate EEE effectively and thus accumulates ROS. Chlorophyll a fluorescence parameters measured in lsd1 leaves and the induction of APX1 and APX2 expression revealed that low light-acclimated lsd1 plants (chloroplasts) were able to respond to transient EL exposure in a similar manner to the parental Ws-0 line (Fig. 1D; Supplemental Fig. 1). These data imply that long-term acclimatory responses rather than the immediate or short-term responses to EEE (photooxidative stress) are regulated by LSD1. This was confirmed when lsd1 plants with a reduced PSII activity, induced environmentally by changing light quality (Fig. 2, A and B) or genetically by using cao (Fig. 2, C and D), showed diminution of the spreading lesion phenotype under nonpermissive conditions. In the lsd1/cao double mutant, the attenuation of the rcd was accompanied by an increase in NPQ (Supplemental Fig. 3). In contrast to photorespiration NPQ is an immediate mechanism that dissipates EEE at the PSII antenna, thus preventing over-reduction of PSII. A higher NPQ thus conveys a lower EEE pressure on PSII and a decrease in operation of alternative electron sinks such as photorespiration. We conclude that in wild-type plants light energy absorbed through PSII substantially drives acclimation processes involving LSD1. The involvement of photorespiration in the light sensitive lsd1 rcd phenotype was further revealed by the attenuation of spreading lesion in conditions that inhibit photorespiration (high CO2 or low O2; Fig. 4).
In wild-type plants, failure to acclimate to EEE leads to necrotic cell death manifested at the whole leaf level as bleaching (Karpinski et al., 1999; Karpinska et al., 2000; Kasahara et al., 2002). The expression of PR1 in EL and SAA-leaves of lsd1 (Fig. 1C) is an indicator of failed acclimation to the challenging light environment and the switch into a cell death mode (Takahashi et al., 1997). Similar phenotypes have been observed in transgenic tobacco plants deficient in peroxisomal catalase. When such plants were exposed to increased light intensities they displayed hypersensitive cell death response-like lesions at least superficially resembling those of lsd1, increased sensitivity to H2O2 and induction of PR1 expression (Chamnongpol et al., 1996; Takahashi et al., 1997; Willekens et al., 1997; Mittler et al., 1999). Furthermore, both the catalase-deficient transgenic tobacco plants and lsd1 show hypersensitivity to avirulent pathogens (Jabs et al., 1996; Mittler et al., 1999) and enhanced resistance to virulent pathogens (Chamnongpol et al., 1998; Aviv et al., 2002). The above considerations lead us to conclude that lsd1 behaves similarly to a catalase-deficient plant.
Role of Stomata in Acclimation to a Changing Light Environment
The 50% reduction in stomatal conductance and lower catalase activity in lsd1 plants in permissive conditions provides an important clue to how the response to EEE may be linked to stomatal behavior, photoproduced H2O2, and the rcd observed in lsd1. Artificial limitation of gas exchange by sealing stomata with lanolin induced a very large increase in H2O2 levels in lsd1 under permissive light (Fig. 3C). It is noteworthy that both stomatal conductance and total CAT activity were restored to wild-type levels in the genetic revertants of the lsd1 phenotype (pad4-5/lsd1 and eds1-1/lsd1) and that this was mirrored by a relative decrease in H2O2 accumulation during forced limitation of gas exchange in these lines (Fig. 3C). These results point to a role of LSD1 in reducing cell ROS content by controlling PAD4- and EDS1-dependent stomatal closure and consequent foliar (peroxisomal) H2O2 production during EEE. A requirement for both EDS1 and PAD4 in driving stomatal closure and lanolin-induced H2O2 accumulation in lsd1 is consistent with their previously defined roles in processing or interpreting ROS-derived signals in a pro-death pathway (Rustérucci et al., 2001) and suggests functions of EDS1 and PAD4 beyond regulation of plant defense.
While ABA-dependent stomatal closure was proposed to be mediated in guard cells by H2O2 generated by a NADPH oxidase (Pei et al., 2000; Kwak et al., 2003), other sources of H2O2 in the leaf cannot be excluded. It is significant that stomatal closure was reduced by the NADPH oxidase inhibitor diphenyl iodonium (Pei et al., 2000; Zhang et al., 2001). Attenuation of rcd by diphenyl iodonium in LD-grown lsd1 plants (Jabs et al., 1996) may therefore be influenced by the effect of this inhibitor on stomatal guard cells. This notion is further supported by the inhibition of stomatal closure by exogenous catalase, indicating that external H2O2 can impact on guard cell function (Zhang et al., 2001). Moreover, xanthine, which has been used as part of the xanthine-xanthine oxidase system (X-XO) to trigger O2− dependent lesion formation in lsd1 (Jabs et al., 1996), itself induces stomata closure (Mori et al., 2001).
Application of SA to Ws-0 leaves rapidly induced stomatal closure and photoinhibition that ended up in photodamage (Fig. 6). SA has also been shown to inhibit CAT activity (Sanchez-Casas and Klessig, 1994; Conrath et al., 1995; Durner and Klessig, 1996; Chen et al., 1997). Whatever the mechanism involved, SA reduces the capability of photosynthetic tissue to acclimate to conditions that promote EEE. We suggest it is by this means that lesion formation is favoured in lsd1.
Role of Stomata and Photorespiration in the Hypersensitive Cell Death Response of Wild-Type Plants
Recent data suggest that hypersensitive cell death response needs functional chloroplasts although their precise role in programmed cell death has not been resolved (Genoud et al., 2002; Karpinski et al., 2003). Such a requirement is consistent with chloroplasts playing a pivotal role in photorespiration. However, the hypersensitive cell death response has also been observed in root tissue upon challenge with pathogens or elicitors (Hermanns et al., 2003). In such nonphotosynthesizing cells the major sources of ROS include membrane-bound NADPH oxidases, amine oxidases and cell wall peroxidases. Use of inhibitors and studies on mutants compromised in ROS production indicate that ROS play a major role in hypersensitive cell death response formation in leaves (Desikan et al., 1996; Blee et al., 2001; Bolwell et al., 2002; Torres et al., 2002; Epple et al., 2003). From our data, it is clear that both rcd in lsd1 and maximum development hypersensitive cell death response lesions in wild-type plants are light-dependent and associated with a large increase in foliar H2O2 levels (Fig. 7). However, it is important to note that lesion formation was initiated in the dark (Fig. 7B). DAB staining revealed that H2O2 accumulated at infection foci (Fig. 7B). Since this occurred in the dark, neither the initiated lesions nor the H2O2 accumulation could have been driven by light and EEE. We propose that initiation of cell death and the initial oxidative burst upon first contact with an avirulent pathogen is light- and EEE-independent. However, photoproduced ROS as a consequence of EEE strongly stimulates lesion propagation.
Closure of stomata during challenge with elicitors and an inverse relation between humidity and hypersensitive cell death response development has been described elsewhere (May et al., 1996; Lee et al., 1999; Jambunathan et al., 2001; Yoshioka et al., 2001) and has led to the suggestion that a “humidity sensing factor” is important in the generation of the hypersensitive cell death response (Yoshioka et al., 2001). The results of those studies are consistent with a role for stomata in promoting photorespiration. Failure to close stomata due to high humidity would slow down ROS accumulation as demonstrated in the Cf-9/Avr9 hypersensitive cell death response in tomato leaves (May et al., 1996). Based on the arguments presented here, we would interpret this as a diminished contribution of photorespiration to the total accumulation of ROS. We propose that a humidity sensing factor (Yoshioka et al., 2001) is a manifestation of stomatal guard cell function (Talbott et al., 2003).
MATERIALS AND METHODS
Plant Material and Growth Conditions
Arabidopsis ecotypes and mutants were cultivated under SD (8 h) or LD (16 h), with a mixture of lights (fluorescence tubes L30W/77-fluora and 30W41-827 lumilux; OSRAM, Berlin), light intensity of 100 ± 20 μmol m−2 s−1, day/night temperature of 22/18°C and a relative humidity of 50%. Conventional soil (Topstar-Economa Garden AB, Sweden) was complemented with a thin layer of autoclaved clay.
Light Stress Conditions
During EL experiments, 4- to 5-week-old Ws-0 and lsd1 plants grown in SD were partially or fully exposed to EL (2,000 ± 100 μmol m−2 s−1) with an extra light source (HMI 1200 W/GS photo optic lamp; OSRAM, Germany) for 45 min. This causes rapid inhibition of photosynthetic electron transport (Karpinski et al., 1997, 1999). After EL-treatment, plants were returned to SD growth conditions for recovery. For the HL experiment, 3- to 4-week-old Ws-0 and lsd1 plants grown in standard conditions were afterward exposed to HL (450 ± 20 μmol m−2 s−1) with a supplementary light source (OSRAM Powerstar HQI-E 250W; Germany) until lesions were visible. Temperature and humidity for excess and HL experiments were the same as in SD conditions.
Chlorophyll a fluorescence parameters were determined with a portable fluorescence monitoring system (FMS1, TECHTUM Lab AB) and the manufacturer's software (Hansatech, Kings Lynn, UK). Images of the chlorophyll a parameters for Figure 6 were generated as described by Barbagallo et al. (2003) using a FluorImager and its associated software (Technologica, Colchester, UK). Chlorophyll fluorescence terminology is explained in detail elsewhere (Maxwell and Johnson, 2000). Stomatal conductance was measured in growth room conditions with a portable AP4 porometer (Delta-T Devices, Cambridge, UK).
Catalase Activity and Statistical Treatment of the Data
The activity of CAT was measured spectrophotometrically according to Aebi (1984) by monitoring H2O2 disappearance at 240 nm in 50 mm phosphate buffer, pH 7.0, containing initially 13 mm H2O2. Catalase activity measurements are representative of 5 or 7 different replicates (Figs. 3 and 6, respectively) from three independent experiments (n = 15 or n = 21). Vertical bars represent sd.
Unless stated otherwise, data were statistically treated for significance by ANOVA.
Experiments with Light-1 and -2
Two-week-old seedlings grown under SD standard conditions were exposed to either light-1 or light-2 or kept in conventional light for 2 weeks. For these treatments, plant trays were covered with either a red filter of half-maximal transmission at 650 nm (light-1, medium red 027; LEE Filters, Andover, UK), or by an orange filter of half-maximal transmission at 560 nm (light-2, orange no. 405; Strand Lighting, Isleworth, UK). Light-1 was generated with white fluorescent tubes (30W41-827 lumilux; OSRAM, Berlin) while fluorescent tubes (L30W/77-fluora Sylvania, Germany) were used for light-2. The same lights as the standard growth conditions were used for the controls. Due to the filters' opacity, all three lights' irradiances were adjusted to 50 μmol m−2 s−1. After 2 weeks in SD conditions under the respective lights, plants were shifted to an LD regime, other parameters remaining unchanged. Pictures were taken and the number of fully damaged leaves per rosette computed 2 weeks after the shift from short to long photoperiods (n = 50 rosettes from three independent experiments).
Double Mutant lsd1/cao
The lsd1/cao double mutant was selected from a cross between lsd1 (Ws-0) and cao (ecotype Landsberg erecta; Klimyuk et al., 1999) parental lines. From the F2 pale green plants reflecting the cao mutation and dark green plants reflecting wild-type phenotype were selected. A DNA PCR screen for homozygosity of the lsd1 mutation was performed with the primer set 5′-GTGTGTGTTTGGATGAAAGTAGCAG-3′ and 5′-GCTAAATGACAACAGCTTAGACGC-3′ and both lsd1/cao and lsd1 lines identified. Selected single and double mutants were backcrossed 5 times to Ws-0 to create comparable genetic backgrounds for lsd1/cao and lsd1.
Four-week-old lsd1/cao and lsd1 plants grown in SD conditions were transferred to LD nonpermissive conditions. Chlorophyll fluorescence parameters were measured in leaves 5 d after the shift in photoperiods (n = 15 plants from two different experiments) and the number of lsd1 plants with lesions assessed after 10 d in these conditions (n = 200 rosettes from three independent experiments).
Gas Exchange Experiments
For the high CO2 and low O2 experiments, 4-week-old lsd1 plants cultivated in LD and having developed lesions were transferred either to a 0.12% (v/v) CO2 and 21% (v/v) O2 atmosphere (AGA gas AB, Sweden) in LD conditions for a period of 2 weeks, or to a 2% (v/v) O2 and 0.04% (v/v) CO2 atmosphere (AGA gas AB, Sweden) in continuous light for a period of 1 week. Control plants were kept in a conventional atmosphere either in LD or in continuous light, respectively (n = 15 plants). The treatment with H2O2 was performed according to Karpinski et al. (1999) and for a period of 2 d (n = 40 leaves from two different experiments). Artificial limitation of gas exchange was performed with Lanolin (ROTH, Karlsruhe, Germany) smeared on the adaxial side of lsd1 and Ws-0 leaves. Part of the treated leaves were excised after 24 h and H2O2 detected as yellow-green fluorescence with the 2′,7′-dichlorodihydrofluorescein (DCF-2) staining according to Cathcart et al. (1983) and monitored with a fluorescence microscope facility (Olympus DP50). Representative pictures of treated leaves are shown (Fig. 3B; n = 10 from two different experiments).
Relative Quantification of PR1, APX1, APX2, CAT1, CAT2, and CAT3 mRNA
Rosettes of Ws-0 and lsd1 control plants and partially exposed to EL (2,000 ± 100 μmol m−2 s−1) for 45 min were harvested. After the light challenge, local (EL), systemic (nonexposed) as well as control (T0) leaves were excised and frozen in liquid nitrogen. RNA extraction was performed using a Qiagen Rneasy plant mini kit (Qiagen GmbH, Hilden). First strand cDNA was synthesized with a RETROscript First Strand Synthesis kit (Ambion, Austin, TX). PCRs were performed using primers from Invitrogen using 18SRNA as a standard (QuantumRNA 18S internal standard; Ambion): APX1, 5′-CTCTGCTGGAACTTTCGATTG-3′ and 5′-TGTGGGCCTCAGCGTAATCAG-3′; APX2, 5′-AAGAAAGCTGTTCAGAGATGC-3′ and CGGTTGGTAGTTGAAGTC; PR1, 5′-ATTTTACTGGCTATTCTCGATTT-3′ and 5′-TTAGTATGGCTTCTCGTTACAT-3′; CAT1, 5′-CGGATCAAAATTGTCTTCAAGCATCATGG-3′ and 5′-GATAGCTTCCTCATCCGACAGGCAT-3′; CAT2, 5′-CCAGCTAGTTCTTACAACTCTCCCTTCTT-3′ and 5′-CCAACAAGAATTGCATCTTCTTCCAAAAGAGAC-3′; CAT3, 5′-AGCCTATTTGGGGGATCATCAACCTTCTA-3′ and 5′-CAACCTTGGCCTCTTCATCAGTCAGATTC-3′.
Histochemical Detection of H2O2 at Pathogen Interaction Sites and Pathogen Infections
Detection of H2O2 was by endogenous peroxidase-dependent in situ histochemical staining using 3,3-diaminobenzidine (DAB) in a protocol modified from Thordal-Christensen et al. (1997). Leaves of 4-week-old plants were inoculated with a 10-L droplet of Pp conidiospores placed on the leaf surface. Leaves were then excised and supplied through the cut petiole with a solution of 1 mg/mL DAB for 8 h in light (100–160 ± 20 μmol m−2 s−1) or in darkness under the same conditions. Subsequently, the DAB solution was replaced with water, and leaves were maintained under the same conditions as before. Material was mounted on a slide in 60% glycerol and examined using a light microscope (Axiophot; Zeiss, Jena, Germany). H2O2 was detectable as reddish brown coloration (n = 24 leaves from four independent experiments).
Lactophenol-Trypan Blue Staining
Plant cell necrosis induced by Peronospora parasitica (Noco 2) inoculation as well as the development of the hypersensitive cell death response and photodamage in leaf tissues were monitored by staining with lactophenol-trypan blue and destaining in saturated chloral hydrate as described (Koch and Slusarenko, 1990). Material was mounted on a slide in 60% glycerol and examined using a light microscope (Axiophot; n = 24 leaves from three independent experiments). For the development of the hypersensitive cell death response, excised leaves were manipulated in parallel with those used for detection of H2O2 and maintained under the same conditions (see below).
SA Treatment
Four- to 5-week-old wild-type plants grown in standard conditions or in HL were sprayed in the abaxial and adaxial sides of the leaves with a solution of 0.4 mm SA. Control leaves were sprayed with water. Data represent means ± sd (vertical bars) of n = 24 from three independent experiments. Plants were afterward left in the growth conditions or exposed to EL (Karpinski et al., 1997, 1999). The maximum efficiency of PSII electron transport (Fv/Fm) of leaves treated with 0.5 mm SA and then set at 100 ± 25 μmol m−2 s−1 or 450 ± 25 μmol m−2 s−1 for 2 h were generated using a FluorImager software (Technologica, Colchester, UK).
Supplementary Material
Acknowledgments
We wish to thank Professor Jaakko Kangasjärvi and Drs. Dietmar Funck and Mats Ellerstrom for the critical reading of the manuscript. Further acknowledgments are extended to Dr. E. Niewiadomska for assessment with CAT activity assays.
This work was supported by the Department of Botany at Stockholm University, by the Swedish Research Councils (VR and FORMAS), by Carl Tryggers Foundation and the Swedish Council for International Cooperation in Research and Higher Education (STINT), and by the Wallenberg Consortium North (to S.K. and B.K.). P.M.M. acknowledges the support of the UK Biotechnology and Biological Sciences Research Council. C.R. and J.E.P. are grateful to The European Commission for a Marie-Curie postdoctoral training fellowship and The Alexander von Humboldt Foundation for funding. Z.M. is grateful to The European Commission for funding from project (QLAM–2001–00424).
In memory of Dr. Anna Siedlecka and members of her family who tragically died on May 26, 2004.
The online version of this article contains Web-only data.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.043646.
References
- Aebi H (1984) Catalase in vitro. Methods Enzymol 105: 121–126 [DOI] [PubMed] [Google Scholar]
- Allen JF (1995) Thylakoid protein phosphorylation, state1-state 2 transition, and photosystems stoichiometry adjustment: redox control at multiple levels of gene expression. Physiol Plant 9: 196–205 [Google Scholar]
- Alvarez ME, Pennell RI, Meijer PJ, Ishikawa A, Dixon RA, Lamb C (1998) Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell 92: 773–784 [DOI] [PubMed] [Google Scholar]
- Asada K (1999) The water-water cycle in chloroplasts: scavenging of active oxygen species and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50: 601–639 [DOI] [PubMed] [Google Scholar]
- Aviv DH, Rustérucci C, Iii BF, Dietrich RA, Parker JE, Dangl JL (2002) Runaway cell death, but not basal disease resistance, in lsd1 is SA- and NIM1/NPR1-dependent. Plant J 29: 381–391 [DOI] [PubMed] [Google Scholar]
- Barbagallo RP, Oxborough K, Pallett KE, Baker NR (2003) Rapid non-invasive screening for perturbations of metabolism and plant growth using chlorophyll fluorescence imaging. Plant Physiol 132: 485–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beers EP, McDowell JM (2001) Regulation and execution of programmed cell death in response to pathogens, stress and developmental cues. Curr Opin Plant Biol 4: 561–567 [DOI] [PubMed] [Google Scholar]
- Blee KA, Jupe SC, Richard G, Zimmerlin A, Davies DR, Bolwell GP (2001) Molecular identification and expression of the peroxidase responsible for the oxidative burst in French bean (Phaseolus vulgaris L.) and related members of the gene family. Plant Mol Biol 47: 607–620 [DOI] [PubMed] [Google Scholar]
- Bolwell GP, Bindschedler LV, Blee KA, Butt VS, Davies DR, Gardner SL, Gerrish C, Minibayeva F (2002) The apoplastic oxidative burst in response to biotic stress in plants: a three-component system. J Exp Bot 53: 1367–1376 [PubMed] [Google Scholar]
- Cathcart R, Schwiers E, Ames BN (1983) Detection of picomole levels of hydroperoxides using a fluorescent dichlorofluorescein assay. Anal Biochem 134: 111–116 [DOI] [PubMed] [Google Scholar]
- Chamnongpol S, Willekens H, Langebartels C, Van Montagu M, Inzé D, Van Camp W (1996) Transgenic tobacco with a reduced catalase activity develops necrotic lesions and induces pathogenesis-related expression under high light. Plant J 10: 491–503 [Google Scholar]
- Chamnongpol S, Willekens H, Moeder W, Langebartels C, Sandermann H Jr, Van Montagu M, Inzé D, Van Camp W (1998) Defense activation and enhanced pathogen tolerance induced by H2O2 in transgenic tobacco. Proc Natl Acad Sci USA 12: 5818–5823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Iyer S, Caplan A, Klessig DF, Fan B (1997) Differential accumulation of salicylic acid and salicylic acid-sensitive catalase in different rice tissues. Plant Physiol 114: 193–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Silva S, Klessig DF (1993) Active oxygen species in the induction of plant systemic acquired-resistance by salicylic acid. Science 262: 1883–1886 [DOI] [PubMed] [Google Scholar]
- Conrath U, Chen Z, Ricigliano JR, Klessig DF (1995) Two inducers of plant defense responses, 2,6-dichloroisonicotinec acid and salicylic acid, inhibit catalase activity in tobacco. Proc Natl Acad Sci USA 92: 7143–7147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornic G, Fresneau C (2002) Photosynthetic carbon reduction and carbon oxidation cycles are the main electron sinks for photosystem II activity during a mild drought. Ann Bot (Lond) 89: 887–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dat JF, Pellinen R, Beeckman T, Van De Cotte B, Langebartels C, Kangasjärvi J, Inzé D, Van Breusegem F (2003) Changes in hydrogen peroxide homeostasis trigger an active cell death process in tobacco. Plant J 33: 621–632 [DOI] [PubMed] [Google Scholar]
- Del Rio LA, Corpas FJ, Sandalio LM, Palma JM, Gomez M, Barroso JB (2002) Reactive oxygen species, antioxidant systems and nitric oxide in peroxisomes. J Exp Bot 53: 1255–1272 [PubMed] [Google Scholar]
- Desikan R, Hancock JT, Coffey MJ, Neill SJ (1996) Generation of active oxygen in elicited cells of Arabidopsis thaliana is mediated by NADPH oxidase-like enzyme. FEBS Lett 382: 213–217 [DOI] [PubMed] [Google Scholar]
- Desikan R, Mackerness SA-H, Hancock JT, Neill SJ (2001) Regulation of the Arabidopsis transcriptome by oxidative stress. Plant Physiol 127: 159–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich RA, Delaney TP, Uknes SJ, Ward ER, Ryals JA, Dangl JL (1994) Arabidopsis mutants simulating disease resistance response. Cell 77: 565–577 [DOI] [PubMed] [Google Scholar]
- Dietrich RA, Richberg MH, Schmidt R, Dean C, Dangl JL (1997) A novel zinc finger protein is encoded by the Arabidopsis LSD1 gene and functions as a negative regulator of plant cell death. Cell 88: 685–694 [DOI] [PubMed] [Google Scholar]
- Durner J, Klessig DF (1996) Salicylic acid is a modulator of tobacco and mammalian catalases. J Biol Chem 271: 28492–28501 [DOI] [PubMed] [Google Scholar]
- Epple P, Mack AA, Morris VRF, Dangl JL (2003) Antagonistic control of oxidative stress-induced cell death in Arabidopsis by two related, plant-specific zinc finger proteins. Proc Natl Acad Sci USA 100: 6831–6836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feierabend J, Schaan C, Hertwig B (1992) Photoinactivation of catalase occurs under both high- and low-temperature stress conditions and accompanies photoinhibition of photosystem II. Plant Physiol 100: 1554–1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frugoli JA, Zhong HH, Nuccio ML, McCourt P, McPeek MA, Thomas TL, McClung CR (1996) Catalase is encoded by a multigene family in Arabidopsis thaliana (L.) Heynh. Plant Physiol 112: 327–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer MJ, Ball L, Oxborough K, Karpinski S, Mullineaux PM, Baker NR (2003) Control of ascorbate peroxidase 2 expression by hydrogen peroxide and leaf water status during excess light stress reveals a functional organisation of Arabidopsis leaves. Plant J 33: 691–705 [DOI] [PubMed] [Google Scholar]
- Genoud T, Buchala AJ, Chua N-H, Métraux J-P (2002) Phytochrome signalling modulates the SA-perceptive pathway in Arabidopsis. Plant J 31: 87–95 [DOI] [PubMed] [Google Scholar]
- Hermanns M, Slusarenko AJ, Schlaich NL (2003) Organ-specificity in a plant disease is determined independently of R gene signalling. Mol Plant Microbe Interact 16: 752–759 [DOI] [PubMed] [Google Scholar]
- Hertwig B, Streb P, Fieirabend J (1992) Light dependence of catalase synthesis and degradation in leaves and the influence of interfering stress conditions. Plant Physiol 100: 1547–1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Bidney DL, Yalpani N, Duvick JP, Crasta O, Folkerts O, Lu G (2003) Overexpression of a gene encoding hydrogen peroxide-generating oxalate oxidase evokes defense responses in sunflower. Plant Physiol 133: 170–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabs T, Dietrich RA, Dangl JL (1996) Initiation of runaway cell death in an Arabidopsis mutant by extracellular superoxide. Science 27: 1853–1856 [DOI] [PubMed] [Google Scholar]
- Jambunathan N, Siani JM, McNellis TW (2001) A humidity-sensitive Arabidopsis copine mutant exhibits precocious cell death and increased disease resistance. Plant Cell 13: 2225–2240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpinska B, Wingsle G, Karpinski S (2000) Antagonistic effects of hydrogene peroxide and glutathione on acclimation to excess excitation energy in Arabidopsis. IUBMB Life 50: 21–26 [DOI] [PubMed] [Google Scholar]
- Karpinski S, Escobar C, Karpinska B, Creissen G, Mullineaux PM (1997) Photosynthetic electron transport regulates the expression of cytosolic ascorbate peroxidase genes in Arabidopsis during excess light stress. Plant Cell 9: 627–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpinski S, Reynolds H, Karpinska B, Wingsle G, Creissen G, Mullineaux PM (1999) Systemic signaling and acclimation in response to excess excitation energy in Arabidopsis. Science 23: 654–657 [DOI] [PubMed] [Google Scholar]
- Karpinski S, Gabrys H, Mateo A, Karpinska B, Mullineaux PM (2003) Light perception in plant disease defence signaling. Curr Opin Plant Biol 6: 390–396 [DOI] [PubMed] [Google Scholar]
- Kasahara M, Kagawa T, Oikawa K, Suetsugu N, Miyao M, Wada M (2002) Chloroplast avoidance movement reduces photodamage in plants. Nature 420: 829–832 [DOI] [PubMed] [Google Scholar]
- Kliebenstein DJ, Dietrich RA, Martin AC, Last RL, Dangl JL (1999) LSD1 regulates salicylic acid induction of copper zinc superoxide dismutase in Arabidopsis thaliana. Mol Plant Microbe Interact 12: 1022–1026 [DOI] [PubMed] [Google Scholar]
- Klimyuk VI, Persello-Cartieaux F, Havaux M, Contard-David P, Schuenemann D, Meiherhoffd K, Gouete P, Jones JDG, Hoffman NE, Nussaume L (1999) A chromodomain protein encoded by the Arabidopsis CAO gene is a plant-specific component of the chloroplast signal recognition particle pathway that is involved in LHCP targeting. Plant Cell 11: 87–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch E, Slusarenko A (1990) Arabidopsis is susceptible to infection by a downy mildew fungus. Plant Cell 2: 437–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozaki A, Takeba G (1996) Photorespiration protects C3 plants from photooxidation. Nature 384: 557–560 [Google Scholar]
- Ku SB, Edwards GE (1978) Oxygen inhibition of photosynthesis. III. Temperature dependence of quantum yield and its relation to O2/CO2 solubility ratio. Planta 140: 1–6 [DOI] [PubMed] [Google Scholar]
- Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL, Bloom RE, Bodde S, Jones JD, Schroeder JI (2003) NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J 22: 2623–2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb C, Dixon RA (1997) The oxidative burst in plant disease resistance. Annu Rev Plant Physiol Plant Mol Biol 48: 251–275 [DOI] [PubMed] [Google Scholar]
- Lee JS (1998) The mechanism of stomatal closing by salicylic acid in Commelina communis L. J Plant Biol 41: 97–102 [Google Scholar]
- Lee S, Choi H, Suh S, Doo IS, Oh KY, Choi EJ, Taylor ATS, Low PS, Lee Y (1999) Oligogalacturonic acid and chitosan reduce stomatal aperture by inducing the evolution of reactive oxygen species from guard cells of tomato and Commelina communis L. Plant Physiol 121: 147–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A, Tenhaken R, Dixon R, Lamb CJ (1994) H2O2 from the oxidative burst orchestrate the plant hypersensitive disease resistance response. Cell 79: 583–593 [DOI] [PubMed] [Google Scholar]
- Maleck K, Levine A, Eulgem T, Morgan A, Schmidt J, Lawton KA, Dangl JL, Dietrich RA (2000) The transcriptome of Arabidopsis thaliana during systemic acquired resistance. Nat Genet 26: 403–409 [DOI] [PubMed] [Google Scholar]
- Manthe B, Schulz M, Schnable H (1992) Effects of salicylic acid on growth and stomatal movements on Vicia faba L: evidence for salicylic acid metabolism. J Chem Ecol 18: 1525–1539 [DOI] [PubMed] [Google Scholar]
- May MJ, Hammond-Kosack KE, Jones JDG (1996) Involvement of reactive oxygen species, glutathione metabolism, and lipid peroxidation in the Cf-gene-dependent defense response of tomato cotyledons induced by race-specific elicitors of Cladosporium fulvum. Plant Physiol 110: 1367–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell K, Johnson GN (2000) Chlorophyll fluorescencea practical guide. J Exp Bot 51: 659–668 [DOI] [PubMed] [Google Scholar]
- Mittler R, Herr EH, Orvar BL, van Camp W, Willekens H, Inzé D, Ellis BE (1999) Transgenic tobacco plants with reduced capability to detoxify reactive oxygen intermediates are hyperresponsive to pathogen infection. Proc Natl Acad Sci USA 96: 14165–14170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori IC, Pinontoan R, Kawano T, Muto S (2001) Involvement of superoxide generation in salicylic acid-induced stomatal closure in Vicia faba. Plant Cell Physiol 42: 1383–1388 [DOI] [PubMed] [Google Scholar]
- Muller P, Li XP, Niyogi KK (2001) Non-photochemical quenching. A response to excess light energy. Plant Physiol 125: 1558–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullineaux P, Ball L, Escobar C, Karpinska B, Creissen G, Karpinski S (2000) Are diverse signalling pathways integrated in the regulation of Arabidopsis antioxidant defence gene expression in response to excess excitation energy? Philos Trans R Soc Lond B Biol Sci 335: 1531–1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullineaux P, Karpinski S (2002) Signal transduction in response to excess light: getting out of the chloroplast. Curr Opin Plant Biol 5: 43–48 [DOI] [PubMed] [Google Scholar]
- Niyogi KK (2000) Safety valves for photosynthesis. Curr Opin Plant Biol 3: 455–460 [DOI] [PubMed] [Google Scholar]
- Noctor G, Veljovic-Jovanovic S, Driscoll S, Novitskaya L, Foyer Ch (2002) Drought and oxidative load in the leaves of C3 plants: a predominant role for photorespiration? Ann Bot (Lond) 89: 841–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ort DR (2001) When there is too much light? Plant Physiol 125: 29–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei ZM, Murata Y, Benning G, Thomine S, Klüsener B, Allen GJ, Grill E, Schroeder JI (2000) Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406: 731–734 [DOI] [PubMed] [Google Scholar]
- Pfannschmidt T, Nilsson A, Allen JF (1999) Photosynthetic control of chloroplast gene expression. Nature 397: 625–628 [Google Scholar]
- Prasad TK, Anderson MD, Martin BA, Stewart CR (1994) Evidence for chilling-induced oxidative stress in maize seedlings and regulatory role of hydrogen peroxide. Plant Cell 6: 65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MV, Paliyath G, Ormrod DP, Murr DP, Watkins CB (1997) Influence of salicylic acid on H2O2 production, oxidative stress, and H2O2-metabolizing enzymes. Salicylic acid-mediated oxidative damage requires H2O2. Plant Physiol 115: 137–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson McClung C (1997) Regulation of catalases in Arabidopsis. Free Radic Biol Med 23: 489–496 [DOI] [PubMed] [Google Scholar]
- Rustérucci C, Aviv DH, Holt BF, Dangl JL, Parker JE (2001) The disease resistance signaling components EDS1 and PAD4 are essential regulators of the cell death pathway controlled by LSD1 in Arabidopsis. Plant Cell 13: 2211–2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Casas P, Klessig DF (1994) A salicylic acid-binding activity and a salicylic acid-inhibitable catalase activity are present in a variety of plant species. Plant Physiol 106: 1675–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M, Dehne S, Feierabend J (2002) Post-transcriptional mechanisms control catalase synthesis during its light-induced turnover in rye leaves through the availability of the hemin cofactor and reversible changes of the translation efficiency of mRNA. Plant J 31: 601–613 [DOI] [PubMed] [Google Scholar]
- Shirasu K, Nakajima H, Rajasekhar VK, Dixon RA, Lamb C (1997) Salicylic acid potentiates an agonist-dependent gain control that amplifies pathogen signals in the activation of defense mechanisms. Plant Cell 9: 261–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbott LD, Rahveh E, Zeiger E (2003) Relative humidity is a key factor in the acclimation of the stomatal response to CO2. J Exp Bot 54: 2141–2147 [DOI] [PubMed] [Google Scholar]
- Takahashi H, Chen Z, Du H, Liu Y, Klessig DF (1997) Development of necrosis and activation of disease resistance in transgenic tobacco plants with severely reduced catalase levels. Plant J 11: 993–1005 [DOI] [PubMed] [Google Scholar]
- Thordal-Christensen H, Zhang Z, Yangdou W, Collinge DB (1997) Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J 11: 1187–1194 [Google Scholar]
- Torres MA, Dangl JL, Jones JD (2002) Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc Natl Acad Sci USA 99: 517–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsonev T, Velikova V, Georgieva K, Hyde PF, Jones HG (2003) Low temperature enhances photosynthetic down-regulation in French bean (Phaseolus vulgaris L.) plants. Ann Bot (Lond) 91: 343–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rensen JJS, Govindjee CX (1999) Role of bicarbonate in photosystem II, the water-plastoquinone oxido-reductase of plant photosynthesis. Physiol Plant 105: 585–593 [Google Scholar]
- Willekens H, Chamnongpol S, Davey M, Schraudner M, Langebartels C, Van Montagu M, Inzé D, Van Camp W (1997) Catalase is a sink for H2O2 and is indispensable for stress defence in C3 plants. EMBO J 16: 4806–4816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingler A, Lea PJ, Quick WP, Leegood RC (2000) Photorespiration: metabolic pathways and their role in stress protection. Philos Trans R Soc Lond B Biol Sci 355: 1517–1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollman FA (2001) State transitions reveal the dynamics and flexibility of the photosynthetic apparatus. EMBO J 20: 3623–3630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Shortt BJ, Lawrence EB, Leon J, Fitzsimmons KC, Levine EB, Raskin I, Shah DM (1997) Activation of host defense mechanisms by elevated production of h2o2 in transgenic plants. Plant Physiol 115: 427–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoda H, Yamaguchi Y, Sano H (2003) Induction of Hypersensitive Cell Death by Hydrogen Peroxide Produced through Polyamine Degradation in Tobacco Plants. Plant Physiol 132: 1973–1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka K, Kachroo P, Tsui F, Sharma SB, Shah J, Klessig DF (2001) Environmentally sensitive, SA-dependent defense responses in the cpr22 mutant of Arabidopsis. Plant J 26: 447–459 [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhang L, Dong F, Gao J, Galbraith DW, Song CP (2001) Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Plant Physiol 126: 1438–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.