Abstract
Aims
To assess the impact of a reduction in the nicotine content of cigarettes on estimated consumption of reduced nicotine cigarettes and usual brand cigarettes at a variety of hypothetical prices.
Design
Double blind study with participants randomly assigned to receive cigarettes for six weeks that were either usual brand or an investigational cigarette with one of five nicotine contents.
Setting
Ten sites across the United States
Participants
839 eligible adult smokers randomized from 2013 to 2014
Intervention and comparator
Participants received their usual brand or an investigational cigarette with one of five nicotine contents: 15.8 (primary control), 5.2, 2.4, 1.3, or 0.4 mg/g.
Measurements
The Cigarette Purchase Task completed at Baseline and at Week 6 post-randomization visit.
Findings
Compared with normal nicotine content controls, the lowest nicotine content (0.4 mg/g) reduced the number of study cigarettes participants estimated they would smoke at a range of prices (mean reduction relative to 15.8 mg/g at a price of $4.00/pack: 9.5, 95% CI: 5.61,12.19). The lowest nicotine content also reduced the maximum amount of money allocated to study cigarettes and the price at which participants reported they would stop buying study cigarettes [median reduction relative to 15.8 mg/g (95% CI): $8.21 (4.27,12.15) per day and, and $0.44 (0.17,0.71) per cigarette, respectively]. A reduction in nicotine content to the lowest level also reduced the maximum amount of money allocated to usual brand cigarettes (median reduction relative to 15.8 mg/g: $4.39 per day, 95% CI: 1.88,6.90).
Conclusions
In current smokers, a reduction in nicotine content may reduce cigarette consumption, reduce the reinforcement value of cigarettes, and increase cessation if reduced-nicotine content cigarettes were the only cigarette available for purchase.
Keywords: end game, nicotine, harm reduction, public policy, taxation
INTRODUCTION
In 2009, the Food and Drug Administration (FDA) was given the authority to regulate tobacco in the United States (US), including the content of nicotine within cigarettes, to any nonzero amount (1). Additionally, the WHO Framework Convention on Tobacco Control, which has been ratified by over 170 countries, includes an agreement to establish guidelines for the emissions of tobacco products (2). A recently completed clinical trial in which 839 smokers were randomly assigned to cigarettes with varying nicotine contents showed that after six weeks, smokers assigned to cigarettes with 2.4 mg nicotine/g tobacco or less smoked fewer cigarettes per day than individuals assigned to cigarettes with a normal nicotine content (15.8 mg/g nicotine) (3). This study suggests that reducing the nicotine content of cigarettes could be an effective regulatory approach to reducing cigarette use (4, 5).
All clinical trials investigating nicotine reduction to date have provided cigarettes free of charge, but the average cost of cigarettes in the US is $6.36/pack (6). Thus, the impact of a nicotine reduction policy within the context of normally priced cigarettes is unknown. Given that cigarette price affects smoking rates (7), understanding the impact of cost on the effectiveness of a nicotine reduction policy is important. In the most recent clinical trial (3), smokers in the lowest nicotine content groups (2.4 mg/g nicotine or less) smoked significantly fewer cigarettes per day than the control group at Week 6 post-randomization, but no groups showed decreases in the rate of smoking compared to their own rates at baseline. This is likely because all participants were provided with free cigarettes throughout the trial, which inflated the number of cigarettes smoked per day relative to baseline, when cigarettes were purchased by the participants. However, if a nicotine reduction policy were implemented within the context of normally priced cigarettes, there may be a significant reduction in smoking behavior as compared to current smoking, and regions with higher prices may see the largest reductions in smoking.
Behavioral economics is a hybrid field of economics and psychology that assesses changes in reinforcer consumption as a function of changes in reinforcer cost (8). One behavioral economics assessment that has been shown to be useful for assessing sensitivity to cost in smokers is the Cigarette Purchase Task (CPT) (9, 10). The CPT is a hypothetical task in which smokers estimate the number of cigarettes they would smoke under a range of cigarette costs. From these data, a demand curve can be produced for each participant that characterizes changes in cigarette consumption as a function of price (11-13). Demand indices of interest include: Intensity (Q0), or the number of cigarettes that smokers report they would smoke if cigarettes were provided for free, Omax, or the maximum expenditure on cigarettes across all prices, Pmax, or the price that produces Omax, Breakpoint, or the lowest price per cigarette which suppresses consumption to zero, and α, or sensitivity to cost. Omax, Pmax, Breakpoint, and α are thought to reflect reinforcement value (11, 14).
The present paper describes the impact of nicotine reduction on the CPT after six weeks of smoking reduced nicotine content cigarettes. The analyses assessed the impact of a reduction in the nicotine content of cigarettes on estimated cigarette consumption at a variety of hypothetical prices, on demand parameters for study cigarettes, and on demand parameters for usual brand cigarettes. Understanding how nicotine reduction impacts demand for usual brand cigarettes may be useful for estimating effects of a nicotine reduction regulatory policy on the reinforcement value of conventional cigarettes if normal nicotine content cigarettes were still available (e.g., through a black market or under regulatory conditions that allowed access to both normal and reduced nicotine products). The primary data from this clinical trial have been reported elsewhere (3). We hypothesized that those assigned to cigarettes with lower nicotine contents for 6 weeks would have lower cigarette demand indices for their assigned study cigarettes.
METHODS
Participants
Adult daily smokers were recruited through community advertisements to one of 10 sites (University of Pittsburgh, Brown University, Johns Hopkins University, University of Minnesota Twin Cities, University of Minnesota Duluth, Duke University, MD Anderson Cancer Center, University of California San Francisco, Moffitt Cancer Center, and University of Pennsylvania). Inclusion criteria included: at least 18 years old, smoking at least five cigarettes per day (CPD), expired carbon monoxide (CO) > 8 ppm or urine cotinine > 100 ng/ml. Exclusion criteria included: intention to quit smoking in the next 30 days, use of other tobacco products on more than 9 days per month, binge drinking more than 9 days per month, significant or unstable medical or psychiatric conditions as determine by a licensed medical professional, positive illicit drug screen for drugs other than cannabis, pregnant or breastfeeding, or exclusively smoking “roll your own” cigarettes. 839 participants were eligible and randomized following completion of phone-screening and in-person screening assessments.
Study Design
All participants purchased and smoked their usual brand of cigarettes during a two-week baseline period. Participants were then randomly assigned, in a double blind manner, to one of seven groups: they either received their usual brand cigarette or investigational cigarettes (Spectrum, produced for NIDA by 22nd Century Group, Inc.) with one of five nicotine contents: 15.8 mg/g, 5.2 mg/g, 2.4 mg/g, 1.3, or 0.4 mg/g. Two groups of participants received the 0.4 mg/g cigarette, and these individuals either received a cigarette with a tar yield similar to the other investigational cigarettes (8-10 mg ISO) or a high-tar cigarette (13 mg ISO). All cigarettes were available in both menthol and nonmenthol versions depending on smoker preference. Participants were asked not to use other cigarettes or tobacco products, but were encouraged to be honest about their use of other products. Participants reported the number of study and non-study cigarettes smoked separately during the trial using an interactive voice-response system (InterVision Media) which called participants daily. During the six-week experimental period, participants visited the lab each week to complete a battery of assessments and receive a 14-day supply of their assigned cigarette. At the end of the six-week experimental period, participants were compensated $80.00 to abstain from smoking for 24 hours, and those with biochemically-confirmed abstinence (CO < 6 ppm or < 50% of Week 6 visit) completed additional assessments. More detail on the study procedures, including the CONSORT flow chart, can be found in the primary manuscript for this trial (3).
Participants completed the CPT during a randomization visit, the Week 2 post-randomization visit (not reported here for brevity), the Week 6 post-randomization visit, and the abstinence visit. Participants remained blind to their assigned nicotine content for all of the outcomes reported in this paper. This task was adapted from Mackillop et al.,(15) and asked participants to estimate how many cigarettes they would smoke under a range of prices. At the baseline visit, participants completed a version of the task that asked about usual-brand cigarettes. At the Week 2 post-randomization visit, the Week 6 post-randomization visit, and the abstinence visit, participants completed two versions: one that asked about the assigned study cigarettes and another that asked about usual-brand cigarettes. Participants who were randomized to their usual brand completed only that version of the task throughout the study. Participants were told to imagine they had the same income/savings that they had right now, no access to any cigarettes or nicotine products other than those offered at these prices, they could smoke without any restrictions for the next 24 hours, and they would smoke the cigarettes they requested at this time and could not save or stockpile cigarettes for a later date. The prices per cigarette included $0.00, $0.02, $0.05, $0.10-$1.00 in $0.10 increments, and from $1.00-$5.00 in $1.00 increments. At each price, participants were also informed about the corresponding price per pack of cigarettes in addition to the price per cigarette. As an additional measure of how nicotine reduction would impact smoking behavior if participants were required to purchase cigarettes, at the Week 6 post-randomization visit participants were asked “Starting today, if the study cigarette was the only type of cigarette available for purchase, by a year from now I would (stop smoking/smoke less/smoke same/smoke more).”
STATISTICAL ANALYSES
We characterized the impact of nicotine reduction on smoking behavior at a range of prices. We used linear mixed model regression to test whether the groups differed on the number of study cigarettes that participants reported they would smoke at $4/pack, $10/pack, and $20/pack. For the study cigarette version of the CPT, participants sometimes reported that they would not smoke the study cigarette at all, or not at any price greater than $0.00. To assess whether nicotine content impacted this outcome, we used random effects logistic regression to examine whether the proportion of people who reported they would not smoke at any price ≥ $0.00. We also used logistic regression to examine whether there were differences between groups in the proportion of people who said they would stop smoking 1 year from now if the cigarette used during the study was the only cigarette type available for purchase.
We assessed the impact of nicotine reduction on demand parameters for both study cigarettes and usual brand cigarettes using mixed effects median regression (i.e., mixed effects quantile regression with τ = 0.5) because it is more robust against outliers relative to ordinary least squares regression. The outcomes of interest included Intensity (Q0), Omax, Pmax, Breakpoint, and α. All except for α could be empirically obtained from the data. To estimate α, a non-linear least square (NLS) regression was fit for each participant (12):
| (1) |
except when a participant's data did not follow the assumed exponential curve, specifically, when (1) the number of cigarettes smoked increased from one price to the next higher price by > 10 cigarettes and > 100%, (2) the number of reported cigarettes was the same across all prices, (3) R-square ≤ 0.20, or (4) participants reported they would smoke 0 cigarettes at all prices (including $0.00) or all prices > $0.00. Excluding participants in category (4) excludes those who were most impacted by a reduction in nicotine content. Thus, results focus on empirical parameters whenever possible. The parameter k was determined by subtracting the log10-transformed average consumption at the highest price ($100/pack) from that at the lowest price ($0) used in curve fitting. We report Spearman correlations for empirical and derived demand indices.
For all analyses, the 5.2, 2.4, 1.3, and 0.4 mg/g nicotine groups were compared to the 15.8 mg/g group (primary control), using a Bonferonni correction for the four comparisons (type I error rate=0.0125). The usual brand group was not included in analyses but is included in the figures for comparison purposes. Analyses comparing the 0.4 mg/g-High Tar (HT) group to the 15.8 mg/g group were defined a priori as exploratory and used the same type I error (3). We controlled for the baseline value of each outcome variable by including it in the regression analyses as a covariate, except when these values were invalid (described below). For each outcome, we tested heterogeneity across sites in a fixed effects model. There was evidence of heterogeneity associated with study site for the question about predicted smoking in one year and for many outcomes associated with the CPT (p-value of type III test for site < 0.05 in a fixed effect only model), so study site was included as a random effect in all regressions. A secondary analysis not reported here included age, gender, race, and log-transformed salivary nicotine metabolite ratio as covariates, and the pattern of results is similar.
In the primary paper for this trial, the impact of nicotine content on cigarettes smoked per day differed for nicotine contents at or above 5.2 mg/g and contents at or below 2.4 mg/g (3): participants assigned to nicotine contents 2.4, 1.3, and 0.4 mg/g smoked significantly fewer cigarettes than those in the 15. 8 mg/g group, and cigarette consumption in the 5.2 mg/g group was similar to consumption in the 15.8 mg/g group. Thus, we also report in text the results from a secondary analysis in which we test the hypothesis that the “below threshold groups” (2.4, 1.3, and 0.4 mg/g) are different from the “above threshold groups” (15.8 and 5.2 mg/g) after controlling for baseline as a covariate and study site as a random effect. These analyses use a type 1 error rate of 0.05.
To assess whether hypothetical cigarette consumption on the CPT is related to self-reported cigarette consumption, we examined Spearman correlations between the number of free study cigarettes smoked per day during Week 6 in the investigational cigarette groups and the empirical study Q0 (estimated study cigarette consumption if cigarettes were free) on the CPT.
Mixed-effects quantile regression which was performed using the R package lqmm (16), and remaining analyses were performed using SAS® (SAS Institute Inc., Cary, NC, USA).
RESULTS
Of the 839 randomized subjects, 773 (92%) completed the usual brand cigarette version of the CPT at Week 6 and 637 (76%) after 24 hours of abstinence; among the 721 subjects who were randomized to investigational cigarettes groups, 660 (92%) completed to the study cigarette version of the CPT at Week 6 and 540 (75%) after 24 hours of abstinence. At Week 6, 663 (92%) participants completed the question about predicted smoking behavior in one year if the study cigarette were the only cigarette available for purchase.
Impact of nicotine reduction on smoking behavior at a range of prices
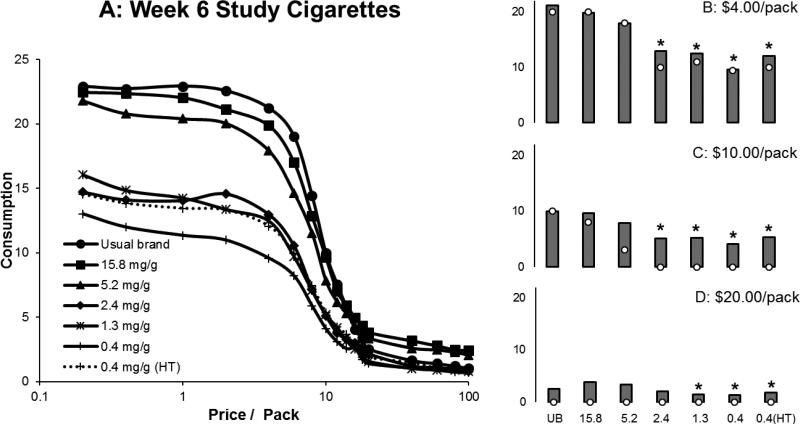
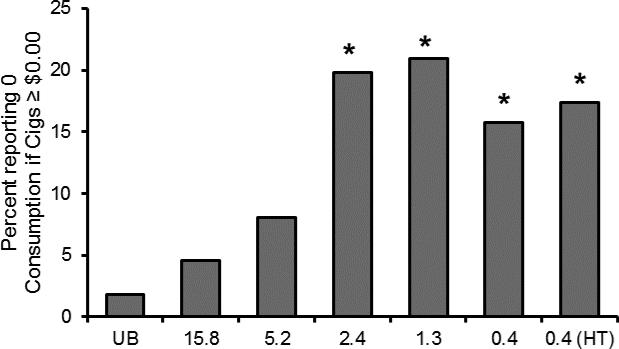
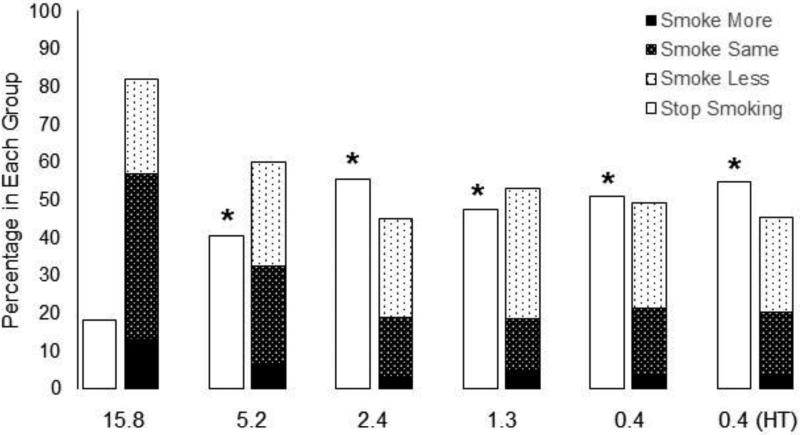
The estimated number of free study cigarettes participants reported they would smoke on the CPT was highly correlated with the actual number of free cigarettes smoked during Week 6 of the trial (Pearson correlation for investigational cigarette groups = 0.68, 95% CI: 0.64-0.72). Estimated study cigarette consumption decreased as hypothetical price increased, and was systematically decreased in response to decreases in the nicotine content of cigarettes (Figure 1, Table 1). Consumption at $4.00 per pack and $10.00 per pack was lower in the 2.4, 1.3, 0.4, and 0.4 (HT) mg/g nicotine content groups compared to the control group (15.8 mg/g nicotine). Consumption at $20.00 per pack was lower in the 1.3, 0.4, and 0.4 (HT) mg/g nicotine content groups compared to the control. Consumption was significantly different between below and above threshold groups for all three price points (Estimated difference in CPD (95% CI, p value): $4.00/pack: 6.26 (4.53-8.00, p<0.01), $10.00/pack: 4.00 (2.60-5.40, p<0.01), $20.00/pack: 1.72 (0.69-2.74, p<0.01)). The percentage of people who reported that they would smoke 0 cigarettes at any price ≥ $0.00 was also higher in the 2.4, 1.3, 0.4, and 0.4 (HT) mg/g nicotine content groups (Odds ratio relative to 15.8 mg/g groups (95% CI, p value): 5.14 (1.86-14.23, p <0.01), 5.50 (2.00-15.10, p <0.01), 3.89 (1.38-10.97, p = 0.01), and 4.38 (1.58-12.15, p < 0.01), respectively) (Figure 2). There was also a significant difference between below and above threshold groups (Odds ratio (95% CI, p value): 3.47 (1.88-6.40, p<0.01)) Significantly more participants in the 5.2 mg/g, 2.4 mg/g, 1.3 mg/g, 0.4 mg/g, and 0.4 (HT) mg/g groups compared to the control group said that, in a year, they would stop smoking if the study cigarette was the only cigarette available for purchase (Odds ratio relative to 15.8 mg/g group (95% CI, p value): 3.10 (1.67-5.78, p<0.01), 5.84 (3.13-10.91, p <0.01), 4.24 (2.28-7.88, p <0.01), 4.86 (2.61-9.05, p < 0.01), 5.84 (3.15-10.82, p <0.01)). There was a significant difference between below and above threshold groups (Odds ratio (95% CI, p value): 2.54 (1.77- 3.64, p <0.01) (Figure 3).
Figure 1.
Estimated study cigarette consumption across a range of prices (A) after six weeks. Figures B, C, and D plot estimated consumption at three prices: $4.00/pack, $10.00/pack, and $20.00/pack. The bars plot the mean and the open symbols plot the median. A significant reduction compared the control group (15.8 mg/g group) after adjusting for multiple comparisons is represented by *.
Table 1.
Regression analyses for effects of nicotine content on estimated cigarette consumption at $4.00, $10.00, and $20.00/pack.
| $4.00/pack | $10.00/pack | $20.00/pack | ||||
|---|---|---|---|---|---|---|
| Group | Estimated reduction from the 15.8 mg/g group (95% CI) | p | Estimated reduction from the 15.8 group (95% CI) | p | Estimated reduction from the 15.8 group (95% CI) | p |
| 5.2 | 2.61 (−0.06, 5.28) | 0.06 | 2.05 (−0.06, 4.15) | 0.06 | 0.88 (−0.62, 2.38) | 0.25 |
| 2.4 | 6.16 (3.45, 8.86) | <.01* | 4.52 (2.39, 6.66) | <.01* | 1.53 (0.01, 3.05) | 0.05 |
| 1.3 | 7.52 (4.85, 10.20) | <.01* | 5.02 (2.91, 7.14) | <.01* | 2.63 (1.12, 4.14) | <0.01* |
| 0.4 | 9.50 (6.81, 12.19) | <.01* | 5.55 (3.43, 7.67) | <.01* | 2.28 (0.77, 3.80) | <0.01* |
| 0.4 (HT) | 8.26 (5.61, 10.91) | <.01* | 4.73 (2.64, 6.82) | <.01* | 2.37 (0.88, 3.86) | <0.01* |
An asterisk indicates p<0.0125 for the comparison with the 15.8 mg/g group.
Figure 2.
The percentage of participants who reported they would not smoke any study cigarettes at prices greater than $0.00. A significant increase compared to the control group (15.8 mg/g group) after adjusting for multiple comparisons is indicated by *.
Figure 3.
The percentage of participants in each group who reported that if the study cigarette were the only cigarette available for purchase, one year from now they would smoke more, smoke the same, smoke less, or stop smoking. A significant difference from the control group (15.8 mg/g group) in the percentage of participants who reported that they would stop smoking in comparison to all other options, after adjusting for multiple comparisons, is indicated by *.
Impact of nicotine reduction on study cigarette demand parameters
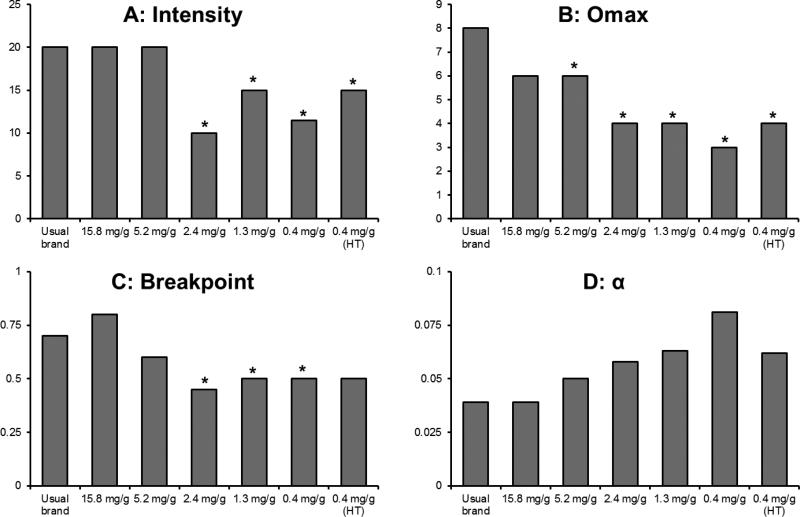
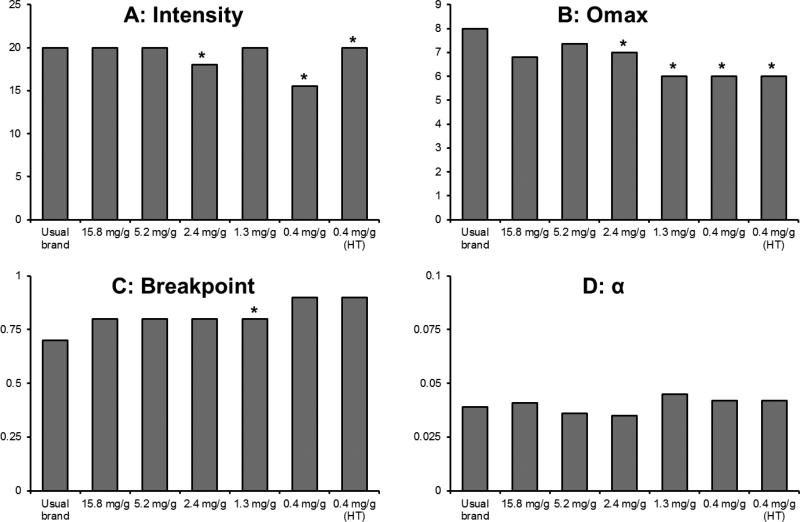
122 and 135 participants were excluded from the derived parameters due to criteria described in the Statistical Analyses section at Week 6 and after 24 hours of abstinence, respectively. For the remaining data, fits for derived parameters were satisfactory (Week 6: R2 mean=0.85, median =0.86; 24-hour abstinence: R2 mean=0.86, median =0.86). k was 1.12 and 1.17 for the Week 6 visit and after 24 hours of abstinence, respectively. Results from regression analyses are reported in Table 2. Effects of nicotine content on Intensity, Omax, Breakpoint, and α for study cigarettes at Week 6 are shown in Figure 4, and all medians are reported in Supplementary Table 1. Intensity (i.e., the number of cigarettes participants estimated they would smoke if cigarettes were free) was significantly lower at Week 6 and following 24 hours of abstinence for the 2.4, 1.3, 0.4, and 0.4 (HT) mg/g groups. There was a significant difference between below and above threshold groups at Week 6 and after 24 hours of abstinence (Estimated difference in CPD (CI, p value): Week 6 6.25 (4.79-7.72, p <0.01), 24 hour abstinence 7.20 (5.32-9.07, p <0.01)). Omax (amount of money participants would spend on cigarettes per day) was significantly lower than in the control group at Week 6 for the 5.2, 2.4, 1.3, 0.4, and 0.4 (HT) mg/g groups and after 24 hours of abstinence for the 2.4, 1.3, and 0.4 mg/g groups. There was a significant difference between above and below threshold groups (Estimated difference (CI, p value): Week 6 = $6.17 ($1.89-$10.45, p =0.01), 24 hour abstinence = $5.43 ($1.86-$8.99, p <0.01)). Pmax (price of cigarettes that corresponds to Omax) was reduced at Week 6 and after 24-hours of abstinence in the 1.3 mg/g group. There was a significant difference between below and above threshold groups at Week 6 (Estimated difference (CI, p value): Week 6 =0.10 ($0.06-$0.15, p <0.01)), but not after 24 hours of abstinence. Breakpoint (lowest price per cigarette which suppresses consumption to zero) was lower at Week 6 for the 2.4, 1.3, and 0.4 mg/g group, and after 24 hours of abstinence for the 5.2, 1.3 and 0.4 mg/g groups. There was a significant difference between below and above threshold groups at Week 6 and after 24 hours of abstinence (Estimated difference (CI, p value): Week 6 = $0.25 ($0.13-$0.37, p <0.01), 24 hour abstinence = $0.34 ($0.16-$0.52, p <0.01)). A reduction in nicotine content increased overall sensitivity to cost (α) after 24-hours of abstinence in the 1.3, 0.4, and 0.4 (HT) mg/g groups. There was a significant difference between below and above threshold groups at Week 6 and after 24 hours of abstinence (Estimated difference (CI, p value): Week 6 = 0.02 (0.01-0.03, p <0.01), 24 hours abstinence = 0.03 (0.01-0.04), p <0.01). Empirical demand parameters (Intensity, Omax, Pmax) were highly correlated with those derived from Equation 1. Spearman correlations between derived and empirical parameters were all >0.99 for Intensity, and ranged from 0.85-0.90 for Pmax, and 0.95-0.96 for Omax (all ps < 0.001).
Table 2.
Regression analyses for demand parameters for the study cigarette at Week 6 and after 24 hours of abstinence.
| Week 6 Study Cigarette | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intensity | Omax | Pmax | Breakpoint | α | ||||||
| Group | Estimated reduction from the 15.8 mg/g group (95% CI) | p | Estimated reduction from the 15.8 mg/g group (95% CI) | p | Estimated reduction from the 15.8 mg/g group (95% CI) | p | Estimated reduction from the 15.8 mg/g group (95% CI) | p | Estimated increase from the 15.8 mg/g group (95% CI) | p |
| 5.2 | 1.85 (−0.20, 3.90) | 0.08 | 6.78 (2.63, 10.94) | <.01* | 0.12 (−0.07, 0.32) | 0.21 | 0.23 (0.01, 0.44) | 0.04 | 0.00 (−0.02, 0.03) | 0.79 |
| 2.4 | 7.07 (4.96, 9.18) | <.01* | 7.32 (3.42, 11.22) | <.01* | 0.20 (0.01, 0.38) | 0.04 | 0.33 (0.10, 0.55) | 0.01* | 0.02 (−0.01, 0.04) | 0.25 |
| 1.3 | 6.60 (3.51, 9.69) | <.01* | 7.85 (4.00, 11.69) | <.01* | 0.27 (0.11, 0.42) | <.01* | 0.59 (0.30, 0.87) | <.01* | 0.02 (0.00, 0.04) | 0.09 |
| 0.4 | 8.44 (5.55, 11.32) | <.01* | 8.21 (4.27, 12.15) | <.01* | 0.23 (0.05, 0.41) | 0.01 | 0.44 (0.17, 0.71) | <.01* | 0.04 (−0.01, 0.08) | 0.14 |
| 0.4 (HT) | 8.13 (4.12, 12.15) | <.01* | 7.31 (2.96, 11.65) | <.01* | 0.20 (−0.01, 0.40) | 0.06 | 0.37 (−0.01, 0.75) | 0.06 | 0.02 (−0.01, 0.05) | 0.19 |
| 24 Hour Abstinence Study Cigarette | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intensity | Omax | Pmax | Breakpoint | α | ||||||
| Group | Estimated reduction from the 15.8 mg/g group (95% CI) | p | Estimated reduction from the 15.8 mg/g group (95% CI) | p | Estimated reduction from the 15.8 mg/g group (95% CI) | p | Estimated reduction from the 15.8 mg/g group (95% CI) | p | Estimated increase from the 15.8 mg/g group (95% CI) | p |
| 5.2 | 2.54 (0.20, 4.88) | 0.03 | 5.12 (1.74, 11.97) | 0.14 | 0.18 (−0.15, 0.52) | 0.28 | 0.43 (0.10, 0.76) | 0.01* | 0.02 (0.00, 0.03) | 0.04 |
| 2.4 | 8.79 (5.77, 11.81) | <.01* | 6.78 (2.08, 11.48) | <.01* | 0.22 (−0.10, 0.54) | 0.17 | 0.56 (0.10, 1.03) | 0.02 | 0.02 (0.00, 0.05) | 0.07 |
| 1.3 | 9.06 (6.24, 11.88) | <.01* | 7.54 (3.49, 11.58) | <.01* | 0.40 (0.12, 0.67) | <.01* | 0.73 (0.42, 1.04) | <.01* | 0.04 (0.02, 0.05) | <.01* |
| 0.4 | 7.86 (5.47, 10.25) | <.01* | 7.02 (2.94, 11.11) | <.01* | 0.28 (0.02, 0.54) | 0.04 | 0.60 (0.23, 0.98) | <.01* | 0.05 (0.03, 0.07) | <.01* |
| 0.4 (HT) | 9.01 (5.67, 12.36) | <.01* | 6.88 (1.10, 12.66) | 0.02 | 0.25 (−0.03, 0.53) | 0.08 | 0.52 (0.06, 0.97) | 0.03 | 0.03 (0.01, 0.05) | <.01* |
An asterisk indicates p<0.0125 for the comparison with the 15.8 mg/g group.
Figure 4.
Median demand parameters (Intensity, Omax, Breakpoint, α) at Week 6. A significant difference from the control group (15.8 mg/g group) after controlling for baseline, study site, and multiple comparisons is represented by *.
Impact of nicotine reduction on demand for Usual Brand Cigarettes
60 and 66 participants were excluded from the derived parameters for usual brand cigarette demand due to criteria described in the Statistical Analyses section at Week 6 and after 24 hours of abstinence, respectively. For the remaining data, fits for derived parameters were satisfactory (Week 6: R2 mean=0.86, median =0.87; 24-hr abstinence: R2 mean=0.85, median=0.86). k was 1.16 and 1.18 for the Week 6 visit and after 24 hours of abstinence, respectively. Results of regression analyses are reported in Table 3, Week 6 Intensity, Omax, Breakpoint, and α for usual-brand cigarettes are shown in Figure 5, and all medians are reported in Supplementary Table 2. Use of reduced-nicotine cigarettes during the study reduced the number of usual-brand cigarettes participants estimated that they would smoke if these cigarettes were free at Week 6 and after 24-hours of abstinence in the 2.4, 0.4, and 0.4 (HT) groups. There was a significant difference in Intensity between below and above threshold groups at Week 6 and after 24 hours of abstinence (Estimated difference in CPD (CI, p value): Week 6 = 2.88 (0.97-4.80, p <0.01), 24 hour abstinence = 3.16 (1.09-5.22, p <0.01)). Furthermore, use of reduced-nicotine cigarettes reduced the maximum amount people reported being willing to spend on usual-brand cigarettes at Week 6 in the 2.4, 1.3, 0.4, and 0.4 (HT) groups (Omax,), but not after 24 hours of abstinence. There was a significant difference between below and above threshold groups after 24 hours of abstinence but not at Week 6 (Estimated difference in CPD (CI, p value): 24 hour abstinence = 5.97 (1.94-10.00, p <0.01)). Use of reduced-nicotine content cigarettes did not reduce the price at which that maximum occurred (Pmax). There was no difference between above and below threshold groups. Relative to 15.8 mg/g, use of 1.3 mg/g cigarettes significantly decreased the maximum price at which people report they would continue to smoke (Breakpoint) usual brand cigarettes at Week 6 and after 24-hours of abstinence, but there was no significant difference in breakpoint when groups were divided by the hypothesized threshold. Nicotine reduction did not significantly affect sensitivity to cost (α) of usual brand cigarettes at Week 6 or after 24 hours of abstinence, and this remained true when groups were divided by the hypothesized threshold. As with study cigarettes, empirical demand parameters (Intensity, Omax, Pmax) for usual-brand cigarettes were highly correlated with those derived from Equation 1. Spearman correlations ranged from 0.98-0.99 for Intensity, 0.72-0.86 for Pmax, and 0.92-0.96 for Omax (all ps < 0.01).
Table 3.
Regression analyses for demand parameters for usual brand cigarette at Week 6 and after 24 hours of abstinence.
| Week 6 Usual Brand Cigarette | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intensity | Omax | Pmax | Breakpoint | α | ||||||
| Group | Estimated reduction from the 15.8 mg/g group (95% CI) | p | Estimated reduction from the 15.8 mg/g group (95% CI) | p | Estimated reduction from the 15.8 mg/g group (95% CI) | p | Estimated reduction from the 15.8 mg/g group (95% CI) | p | Estimated increase from the 15.8 mg/g group (95% CI) | p |
| 5.2 | 1.68 (−0.28, 3.64) | 0.09 | 3.59 (0.33, 6.85) | 0.03 | −0.06 (−0.22, 0.10) | 0.44 | 0.09 (−0.14, 0.32) | 0.44 | 0.00 (−0.01, 0.01) | 0.91 |
| 2.4 | 2.82 (0.68, 4.96) | 0.01* | 3.80 (1.48, 6.12) | <.01* | −0.05 (−0.20, 0.10) | 0.51 | 0.00 (−0.18, 0.18) | 1.00 | 0.00 (−0.01, 0.01) | 0.94 |
| 1.3 | 3.61 (0.76, 6.45) | 0.01 | 4.50 (2.04, 6.96) | <.01* | 0.02 (−0.11, 0.15) | 0.72 | 0.31 (0.11, 0.51) | <.01* | 0.01 (0.00, 0.01) | 0.07 |
| 0.4 | 5.06 (2.52, 7.60) | <.01* | 4.39 (1.88, 6.90) | <.01* | −0.02 (−0.16, 0.12) | 0.75 | 0.14 (−0.08, 0.36) | 0.22 | 0.00 (0.00, 0.01) | 0.31 |
| 0.4 (HT) | 3.81 (2.18, 5.44) | <.01* | 4.36 (2.33, 6.38) | <.01* | −0.02 (−0.13, 0.10) | 0.79 | 0.26 (0.04, 0.48) | 0.02 | 0.00 (0.00, 0.01) | 0.44 |
| 24 Hour Abstinence Usual Brand Cigarette | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intensity | Omax | Pmax | Breakpoint | α | ||||||
| Group | Estimated reduction from the 15.8 mg/g group (95% CI) | p | Estimated reduction from the 15.8 mg/g group (95% CI) | p | Estimated reduction from the 15.8 mg/g group (95% CI) | p | Estimated reduction from the 15.8 mg/g group (95% CI) | p | Estimated increase from the 15.8 mg/g group (95% CI) | p |
| 5.2 | 0.66 (−1.91, 3.22) | 0.61 | 4.26 (−2.26, 10.77) | 0.20 | 0.23 (−0.08, 0.54) | 0.15 | 0.12 (−0.21, 0.46) | 0.47 | 0.01 (−0.02, 0.04) | 0.47 |
| 2.4 | 3.55 (0.83, 6.27) | 0.01* | 4.76 (−0.72, 10.25) | 0.09 | 0.18 (−0.12, 0.47) | 0.24 | 0.09 (−0.18, 0.37) | 0.49 | 0.01 (−0.02, 0.03) | 0.60 |
| 1.3 | 3.54 (0.37, 6.72) | 0.03 | 5.86 (0.14, 11.58) | 0.04 | 0.27 (0.00, 0.55) | 0.05 | 0.42 (0.12, 0.73) | <.01* | 0.01 (−0.01, 0.04) | 0.38 |
| 0.4 | 3.68 (1.43, 5.93) | <.01* | 4.96 (−0.03, 9.96) | 0.05 | 0.21 (−0.06, 0.49) | 0.12 | 0.20 (−0.12, 0.52) | 0.22 | 0.01 (−0.01, 0.03) | 0.44 |
| 0.4 (HT) | 3.84 (1.54, 6.15) | <.01* | 5.48 (−2.11, 13.08) | 0.15 | 0.28 (−0.06, 0.62) | 0.10 | 0.23 (−0.14, 0.61) | 0.22 | 0.01 (−0.01, 0.03) | 0.32 |
An asterisk indicates p<0.0125 for the comparison with the 15.8 mg/g group.
Figure 5.
Median demand parameters (Intensity, Omax, Breakpoint, α) for usual-brand cigarettes at Week 6. A significant difference from the control group (15.8 mg/g group) after controlling for individual baseline, site, and multiple comparisons is represented by *. Note that significant differences between groups can result without differences between unadjusted medians (e.g., Intensity, 0.4 (HT) mg/g group, Breakpoint 1.3 mg/g group) as a result of adjusting for individual baseline demand parameters and study site.
DISCUSSION
The present paper investigated relationships between nicotine content of cigarettes and cigarette price on hypothetical cigarette purchase behavior using the CPT. The main findings are as follows: 1) Reducing the nicotine content of cigarettes reduced the number of cigarettes that people reported they would smoke across a range of prices. 2) Reducing the nicotine content of cigarettes increased the proportion of people who reported that they would quit smoking if the study cigarette were the only cigarette available for purchase, decreased the number of cigarettes people estimated they would smoke if cigarettes were free (Intensity), decreased the maximum dollar amount people reported being willing to spend on cigarettes (Omax), decreased the lowest price per cigarette which suppresses consumption to zero (Breakpoint), and increased sensitivity to cost (α) following 24-hr abstinence; 3) Using low nicotine cigarettes for six weeks also reduced the number of usual brand cigarettes participants estimate they would smoke if cigarettes were free, and reduced the maximum amount of money participants were willing to spend on usual brand cigarettes.
These data provide important information for regulatory bodies considering mandating a reduction in the nicotine content of cigarettes. In the clinical trial in which these data were collected (3), individuals assigned to cigarettes with 5.2 mg/g nicotine or more increased the number of cigarettes they smoked over a 6-week period when cigarettes were provided for free as part of the trial. Individuals assigned to cigarettes with ≤ 2.4 mg/g nicotine did not change the number of cigarettes they smoked compared to baseline, but smoked fewer cigarettes at Week 6 post randomization compared to the control group. Thus, it was unclear whether a reduction in nicotine content would result in reduced smoking behavior within the context of normally-priced cigarettes. The present data suggest that across a range of prices, a reduction in nicotine content to ≤ 2.4 mg/g nicotine will decrease the number of cigarettes smoked. We also found that increases in the cost of cigarettes resulted in even fewer estimated CPD. For example, at a price of $10/pack (near the minimum price of $11.02 in New York City, NY, USA) (17), smokers in the lowest nicotine content group reported that they would smoke 4 cigarettes per day, while smokers in the control group reported they would smoke 10 cigarettes per day. At very high prices (e.g., $20.00/pack) consumption was suppressed in all groups, but participants in the 0.4 mg/g nicotine group still reported they would smoke significantly fewer cigarettes than participants in the control group (1 vs 4 cigarettes). The data also suggest that if a nicotine reduction policy were enacted, there would be an increase in smoking cessation. Significantly more people in the lowest nicotine content group reported that they would not smoke the study cigarettes at any price ≥ than $0.00. When asked directly how their smoking behavior would change if the study cigarette were the only cigarette available for purchase, participants in all reduced nicotine groups were more likely than participants in the control group to report that they would stop smoking completely.
A reduction in nicotine content significantly altered the demand parameters for study cigarettes. A decrease in Intensity, or the number of cigarettes participants estimated they would smoke if they were free, is consistent with the primary outcome data from the clinical trial in which cigarettes were provided free of charge (3). Indeed, cigarette consumption during Week 6 of the trial and estimated study cigarette consumption on the CPT were highly correlated. Changes in the other parameters are thought to reflect changes in related but dissociable aspects of the reinforcement value of a product (11, 14), and these changes may have important behavioral implications. Previous research has shown that Intensity, Breakpoint, Omax Pmax, and α are significantly correlated with smoking behavior (18), severity of nicotine dependence (15, 19, 20), treatment motivation (21), and treatment success (22). Thus, the observed changes in this paper suggest that the reduction of nicotine in cigarettes decreases the reinforcement value of cigarettes and is likely to impact the intensity and persistence of smoking. Changes in sensitivity to cost (α) were not significantly different from the 15.8 mg/g group at Week 6 likely because we were only able to obtain α values for participants who reported they would smoke across at least two prices, and a substantial proportion of people in low nicotine groups reported that they would not smoke any study cigarettes at prices greater than $0.00. Thus, those participants who were most impacted by nicotine reduction were not included in the analysis of α, reducing our power to detect differences. Combining groups above and below a hypothesized threshold for maintaining behavior (between 5.2 and 2.4 mg/g) revealed significant differences in sensitivity to cost (α). Observed differences in breakpoint are also consistent with a change in cost sensitivity. Demand parameters from the abstinence visit were generally consistent with the demand parameters from the Week 6 visit, although there was a significant impact of nicotine reduction on α during the abstinence visit. These data suggest that the reduced reinforcement value associated with lower nicotine content cigarettes is maintained during brief abstinence.
Questions related to choice between usual brand and reduced nicotine products when they are concurrently available are best addressed in paradigms that assess cross-price elasticity. The present study demonstrated that nicotine reduction reduced some demand parameters related to usual brand cigarettes, although the change in demand was generally smaller than what was observed for study cigarettes (Tables 2 and 3). The change in Intensity for usual brand cigarettes is consistent with data reported in the primary paper for this trial showing that nicotine reduction decreased the number of usual brand cigarettes smoked per day following the end of the clinical trial. Importantly, the smaller impact of nicotine reduction on demand for usual brand relative to the change in demand for study cigarettes suggests that if reduced nicotine content cigarettes are made available in a marketplace that continues to offer normal-nicotine content cigarettes, smokers are likely to continue to use normal-nicotine content cigarettes, especially if the price of both products is the same (23). Thus, for nicotine-reduction to be maximally effective at reducing nicotine exposure and dependence, access to normal-nicotine content cigarettes would need to be limited by establishing a differential price structure (i.e., higher taxes for cigarettes with higher nicotine contents (24, 25)) or by setting product standards that require all cigarettes to have reduced nicotine. Relatedly, if a product standard requiring all cigarettes to have reduced nicotine is enacted, smokers may seek our alternative sources of nicotine, either by purchasing normal-nicotine content cigarettes through an illegal source (i.e., a black market) or by purchasing alternative tobacco products like e-cigarettes. The relatively persistent demand for usual brand cigarettes suggests that in order to reduce the influence of a black market, it will be important for less harmful sources of nicotine to remain widely available (26, 27).
Conclusions
A nicotine reduction policy has been suggested as a potential regulatory policy in the United States and other countries (28). However, because all clinical trials that have investigated the impact of nicotine reduction to date have provided cigarettes free of charge, it is unknown how nicotine reduction will affect smoking behavior when cigarettes must be purchased. Improvements in public health are likely to be largest if nicotine reduction leads to a decrease in the prevalence of smoking. The present paper shows that a reduction in nicotine content reduced the number of cigarettes people estimated they would smoke across a range of prices and increased the number of smokers who reported they would not smoke at any price. In fact, 51% of participants in the lowest nicotine content group reported that they would stop smoking within a year if the study cigarette was the only cigarette available for purchase. Nicotine reduction also decreased breakpoint (lowest price per cigarette which suppresses consumption to zero). These data suggest that a policy mandating a reduction in the nicotine content of cigarettes would likely decrease both the rate and prevalence of smoking. Furthermore, a reduction in nicotine content decreased the reinforcing value of cigarettes as measured by multiple demand parameters. This decrease in reinforcement value may lead to an increase in cessation attempts and an increase in the success of cessation attempts. Overall, these data provide support for nicotine reduction as a strategy for improving public health.
Supplementary Material
Acknowledgements
The authors would like to thank all of the individuals who were instrumental in conducting the clinical trial including Rachel L. Denlinger-Apte, Neal L. Benowitz, Ryan G. Vandrey, Mustafa al’ Absi, Steven G. Carmella, Paul M. Cinciripini, David J. Drobes, Steve Hecht, Joni Jensen, Joseph Koopmeiners, Tonya Lane, F. Joseph McClernon, Sharon Murphy, Jason D. Robinson, Maxine L. Stitzer, Andrew A. Strasser, Hilary Tindle, and all the students, fellows, and staff involved in the Center for the Evaluation of Nicotine in Cigarettes.
Funding: Research reported in this publication was supported by the National Institute on Drug Abuse and FDA Center for Tobacco Products (CTP) (U54 DA031659). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Food and Drug Administration. Tracy Smith was supported by the National Cancer Institute (T32 CA186783).
Footnotes
Portions of these data were presented as part of the Vermont Conference on Behavior and Health on October 1st, 2015.
Clinicaltrials.gov Registration: NCT01681875, Project 1, Study 1: Investigating the Impact of Nicotine Using Spectrum Cigarettes (P1S1)
Conflict of Interest Declaration: None
Contributor Information
Tracy T. Smith, University of Pittsburgh Cancer Institute, Pittsburgh, PA
Rachel N. Cassidy, Center for Alcohol and Addiction Studies, Brown University, Providence, RI, USA
Jennifer W. Tidey, Center for Alcohol and Addiction Studies, Brown University, Providence, RI, USA
Xianghua Luo, Division of Biostatistics, School of Public Health, University of Minnesota, Minneapolis, MN, USA.
Chap T. Le, Division of Biostatistics, School of Public Health, University of Minnesota, Minneapolis, MS, USA
Dorothy K. Hatsukami, Department of Psychiatry, University of Minnesota, Minneapolis, MS, USA
Eric C. Donny, Department of Psychology, University of Pittsburgh, Pittsburgh, PA, USA
References
- 1.Congress The Family Smoking Prevention and Tobacco Control Act. 2009 [Google Scholar]
- 2.WHO Framework Convention on Tobacco Control. Geneva: 2003. [Google Scholar]
- 3.Donny EC, Denlinger RL, Tidey JW, Koopmeiners JS, Benowitz NL, Vandrey RG, et al. Randomized Trial of Reduced-Nicotine Standards for Cigarettes. N Engl J Med. 2015;373(14):1340–9. doi: 10.1056/NEJMsa1502403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benowitz NL, Henningfield JE. Establishing a nicotine threshold for addiction. The implications for tobacco regulation. N Engl J Med. 1994;331(2):123–5. doi: 10.1056/NEJM199407143310212. [DOI] [PubMed] [Google Scholar]
- 5.Benowitz NL, Henningfield JE. Reducing the nicotine content to make cigarettes less addictive. Tob Control. 2013;22(Suppl 1):i14–7. doi: 10.1136/tobaccocontrol-2012-050860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eriksen M, Mackay J, Schluger N, Gomeshtapeh FI, Drope J. In: The Tobacco Atlas. Society AC; Foundation WL, editor. Atlanta, GA: 2015. [Google Scholar]
- 7.Chaloupka FJ, Straif K, Leon ME. Working Group IAfRoC. Effectiveness of tax and price policies in tobacco control. Tob Control. 2011;20(3):235–8. doi: 10.1136/tc.2010.039982. [DOI] [PubMed] [Google Scholar]
- 8.Hursh SR, Roma PG. Behavioral economics and empirical public policy. J Exp Anal Behav. 2013;99(1):98–124. doi: 10.1002/jeab.7. [DOI] [PubMed] [Google Scholar]
- 9.Murphy JG, MacKillop J. Relative reinforcing efficacy of alcohol among college student drinkers. Exp Clin Psychopharmacol. 2006;14(2):219–27. doi: 10.1037/1064-1297.14.2.219. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs EA, Bickel WK. Modeling drug consumption in the clinic using simulation procedures: demand for heroin and cigarettes in opioid-dependent outpatients. Exp Clin Psychopharmacol. 1999;7(4):412–26. doi: 10.1037//1064-1297.7.4.412. [DOI] [PubMed] [Google Scholar]
- 11.Hursh SR, Silberberg A. Economic demand and essential value. Psychol Rev. 2008;115(1):186–98. doi: 10.1037/0033-295X.115.1.186. [DOI] [PubMed] [Google Scholar]
- 12.Koffarnus MN, Franck CT, Stein JS, Bickel WK. A modified exponential behavioral economic demand model to better describe consumption data. Exp Clin Psychopharmacol. 2015;23(6):504–12. doi: 10.1037/pha0000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hursh SR, Raslear TG, Shurtleff D, Bauman R, Simmons L. A cost-benefit analysis of demand for food. J Exp Anal Behav. 1988;50(3):419–40. doi: 10.1901/jeab.1988.50-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bickel WK, Marsch LA, Carroll ME. Deconstructing relative reinforcing efficacy and situating the measures of pharmacological reinforcement with behavioral economics: a theoretical proposal. Psychopharmacology (Berl) 2000;153(1):44–56. doi: 10.1007/s002130000589. [DOI] [PubMed] [Google Scholar]
- 15.MacKillop J, Murphy JG, Ray LA, Eisenberg DT, Lisman SA, Lum JK, et al. Further validation of a cigarette purchase task for assessing the relative reinforcing efficacy of nicotine in college smokers. Exp Clin Psychopharmacol. 2008;16(1):57–65. doi: 10.1037/1064-1297.16.1.57. [DOI] [PubMed] [Google Scholar]
- 16.Geraci M. Linear Quantile Mixed Models: The 1qmm Package for Laplace Quantile Regression. Journal of Statistical Software. 2014;57(13):1–29. [Google Scholar]
- 17.Sensible Tobacco Enforcement Act. New York City Administrative Code; USA: 2013. [Google Scholar]
- 18.MacKillop J, Tidey JW. Cigarette demand and delayed reward discounting in nicotine- dependent individuals with schizophrenia and controls: an initial study. Psychopharmacology (Berl) 2011;216(1):91–9. doi: 10.1007/s00213-011-2185-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chase HW, Mackillop J, Hogarth L. Isolating behavioural economic indices of demand in relation to nicotine dependence. Psychopharmacology (Berl) 2013;226(2):371–80. doi: 10.1007/s00213-012-2911-x. [DOI] [PubMed] [Google Scholar]
- 20.O'Connor RJ, Heckman BW, Adkison SE, Rees VW, Hatsukami DK, Bickel WK, et al. Persistence and amplitude of cigarette demand in relation to quit intentions and attempts. Psychopharmacology. 2016;233(12):2365–71. doi: 10.1007/s00213-016-4286-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy JG, MacKillop J, Tidey JW, Brazil LA, Colby SM. Validity of a demand curve measure of nicotine reinforcement with adolescent smokers. Drug Alcohol Depend. 2011;113(2-3):207–14. doi: 10.1016/j.drugalcdep.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mackillop J, Murphy CM, Martin RA, Stojek M, Tidey JW, Colby SM, et al. Predictive Validity of a Cigarette Purchase Task in a Randomized Controlled Trial of Contingent Vouchers for Smoking in Individuals With Substance Use Disorders. Nicotine Tob Res. 2016;18(5):531–7. doi: 10.1093/ntr/ntv233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kozlowski LT. Let actual markets help assess the worth of optional very-low-nicotine cigarettes before deciding on mandatory regulations. Addiction. 2016 doi: 10.1111/add.13515. [DOI] [PubMed] [Google Scholar]
- 24.Laugesen M, Grace RC. Excise, electronic cigarettes and nicotine reduction to reduce smoking prevalence in New Zealand by 2025. N Z Med J. 2015;128(1420):72–4. [PubMed] [Google Scholar]
- 25.Laugesen M, Grace RC. Excise, electronic cigarettes and nicotine reduction to reduce smoking prevalence in New Zealand by 2025. N Z Med J. 2016;129(1430):94–5. [PubMed] [Google Scholar]
- 26.Donny EC, Walker N, Hatsukami D, Bullen C. Reducing the nicotine content of combusted tobacco products sold in new zealand. Tob Control. doi: 10.1136/tobaccocontrol-2016-053186. In Press. [DOI] [PubMed] [Google Scholar]
- 27.Benowitz NL, Donny EC, Hatsukami DK. Reduced nicotine content cigarettes, e- cigarettes and the cigarette end game. Addiction. 2016 doi: 10.1111/add.13534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reg) WSGoTPRT Advisory Note: Global Nicotine Reduction Strategy. 2015. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.