Abstract
Brucella abortus is the causative agent of brucellosis, which causes abortion in domestic animals and undulant fever in humans. This bacterium infects and proliferates mainly in macrophages and dendritic cells where is recognized by pattern recognition receptors (PRRs) including Nod-like receptors (NLRs). Our group recently demonstrated the role of AIM2 and NLRP3 in Brucella recognition. Here, we investigated the participation of NLRP12 in innate immune response to B. abortus. We found that NLRP12 inhibits the early production of IL-12 in bone marrow-derived macrophages upon B. abortus infection. We also observed that NLRP12 suppresses in vitro NF-κB and MAPK signaling in response to Brucella. Moreover, we showed that NLRP12 modulates caspase-1 activation and IL-1β secretion in B. abortus infected-macrophages. Furthermore, we observed that mice lacking NLRP12 were more resistant in the early stages of B. abortus infection. NLRP12−/− infected-mice presented reduced bacterial burdens in the spleens and increased production of IFN-γ and IL-1β compared to wild-type controls. In addition, NLRP12 deficiency leads to reduction in granuloma number and size in mouse livers. Altogether, our findings suggest that NLRP12 plays an important role in regulating negatively the early inflammatory responses against B. abortus.
Keywords: Innate immunity, Nod-like receptors, inflammasome, NLRP12, Brucella abortus
Introduction
Brucella abortus is a Gram-negative facultative intracellular bacterium that induces brucellosis, a major worldwide zoonotic disease. In cattle, B. abortus infection leads to infertility and abortion, resulting in considerable economic loss [1]. In humans, B. abortus causes undulant fever, endocarditis, arthritis, osteomyelitis and neurologic disorders [2]. Human brucellosis occurs through inhalation of aerosols containing the pathogen, contact with infected animals, or, more often, consumption of unpasteurized milk or dairy products [3].
In hosts, B. abortus survives and replicates predominantly in macrophages and dendritic cells, manipulating host cell vesicular-trafficking pathways and creating a Brucella-containing vacuole (BCV). To establish an intracellular replication niche, Brucella delivers effector proteins into the host cytosol through a type IV secretion system (T4SS) encoded by the virB operon [4]. Brucella is recognized by the host using germline-encoded pattern recognition receptors (PRRs) such as TLRs (Toll-like receptors) and NLRs (Nod-like receptors). These sensors trigger the production of pro-inflammatory cytokines leading to the development of a type 1 pattern of immune response that is critical for bacterial clearance and infection control [5]. TLRs are transmembrane receptors, that recognize and bind pathogen-associated molecular patterns (PAMPs), resulting in signal transduction and translocation of NF-κB transcription factor to the nucleus and phosphorylation of mitogen- activated protein (MAP) kinases p38, JNK and ERK [6]. Our laboratory and others have described the involvement of several TLRs and TLRs-associated pathways, such as TLR9 and MyD88, in the recognition of B. abortus [7–13].
NLRs are cytoplasmic receptors that sense different PAMPs and DAMPS (danger-associated molecular patterns), and serve as regulators of gene expression by modulating signaling pathways of MAPK and NF-κB as well as participate in the formation of inflammasomes. As cytosolic sensors, NLRs play a critical role in immune response to intracellular pathogens [14]. The NLRs NOD1 and NOD2 recognize bacterial peptidoglycan fragments and recruit the adaptor protein Rip2 to induce a proinflammatory response [15]. Our group demonstrated that bone-marrow-derived macrophages (BMDMs) from NOD1, NOD2, and Rip2 deficient mice possess reduced production of TNF-α compared to wild-type (WT) animals infected with B. abortus. However, these proteins had no role in resistance to Brucella infection in vivo [16]. Some NLRs such as NLRP1, NLRC4, NLRP3, NLRP6, NLRP12 and AIM2 assemble a multimeric complex with the adapter protein ASC and pro-caspase-1 called inflammasome [17, 18]. After being recruited for the inflammasome, caspase-1 is activated, and promotes processing of pro-IL-1β and pro-IL-18 to their mature forms as well as an inflammatory cell death known as pyroptosis [19, 20]. In particular, NLRP3 responds to a variety of pathogens and stimuli [21] and AIM2 recognizes cytoplasmic dsDNA [22, 23]. Our laboratory recently described that both NLRP3 and AIM2 receptors are involved in in vitro IL-1β secretion in response to B. abortus, and knockout (KO) mice of each receptor are more susceptible to murine brucellosis compared to WT animals [24]. However, it remains unclear if other NLRs are involved in the recognition of B. abortus.
NLRP12 (also known as Nalp12, Monarch-1 and Pypaf 7) is a NLR member expressed in immune cells, and its ligand is unknown [25]. NLRP12 was initially described as an activator of caspase-1 and NF-κB signaling in overexpression studies [18]. However, subsequent reports describe NLRP12 as a suppressor of pro-inflammatory signaling and suggest inflammasome-independent functions. NLRP12 has been implicated in autoinflammatory disorders, colon inflammation and tumorigenesis and host resistance to infectious diseases [25]. Nonetheless, the function of NLRP12 in immune responses against bacterial infections is still not fully addressed.
Herein, we investigate the role of NLRP12 in response to B. abortus infection. We observed that NLRP12 inhibits NF-κB and MAPK signaling and caspase-1 activation in BMDMs. Furthermore, in a model of murine brucellosis the absence of NLRP12 conferred host resistance to Brucella infection. Collectively, our results suggest an important role of NLRP12 in modulating the early inflammatory responses against B. abortus.
Results
NLRP12 negatively regulates IL-12 production in B. abortus-infected macrophages
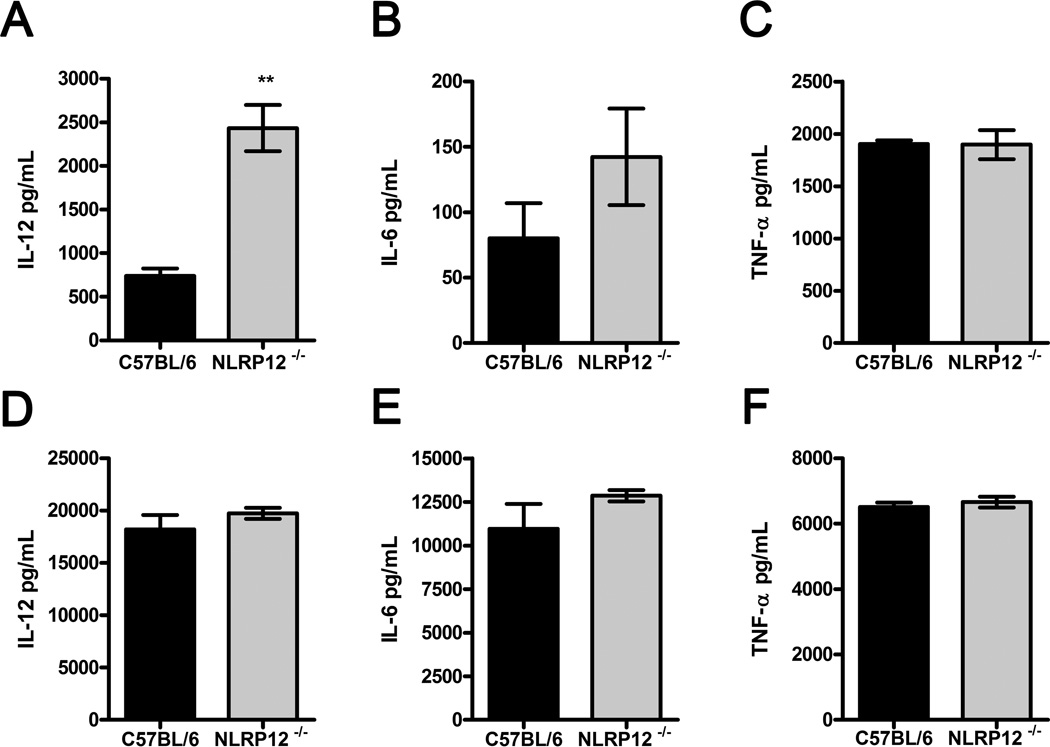
Upon recognition by innate immune receptors, B. abortus triggers the production of proinflammatory cytokines such as IL-12 and TNF-α [26]. We first investigated whether NLRP12 participates in the regulation of in vitro proinflammatory cytokine production in B. abortus-infected BMDMs. After 5 hours of infection, BMDMs from NLRP12−/− mice infected with B. abortus S2308 displayed an increased production of IL-12, compared with WT BMDMs (Figure 1A). At the same time, infected NLRP12−/− BMDMs presented no statistically difference in IL-6 and TNF-α production (Figure 1B, 1C), but a slightly elevated IL-6 level was observed relative to WT counterparts. After 24 hours of infection, no difference was observed in cytokine production between infected macrophages from NLRP12−/− and WT mice (Figure 1 D–F), suggesting that NLRP12 plays a role in the early innate immune response against B. abortus in vitro. Additionally, we measured the level of NLRP12 expression by qPCR in unmatured bone marrow cells and BMDM. As shown in Supplementary Figure 1, there is a small reduction in NLRP12 mRNA levels in BMDM compared to unmatured bone marrow cells; however, NLRP12 mRNA transcripts were also detected in 10 days matured bone marrow cells (BMDMs).
Figure 1.
NLRP12 dampens IL-12 production in B. abortus-infected macrophages. BMDMs from C57BL/6 and NLRP12−/− mice were infected with B. abortus S2308 at a MOI of 100:1. After five (A–C) and 24 hours (D–F) of infection culture supernatants were analyzed for IL-12, IL-6 and TNF-α production by ELISA. Data are expressed as mean ± SEM of three samples per group analyzed in triplicate from one experiment representative of four independent experiments. ** P ≤ 0.005 in comparison to C57BL/6 BMDMs using Student’s t-test.
NLRP12 antagonizes NF-κB and MAPK signaling in response to B. abortus
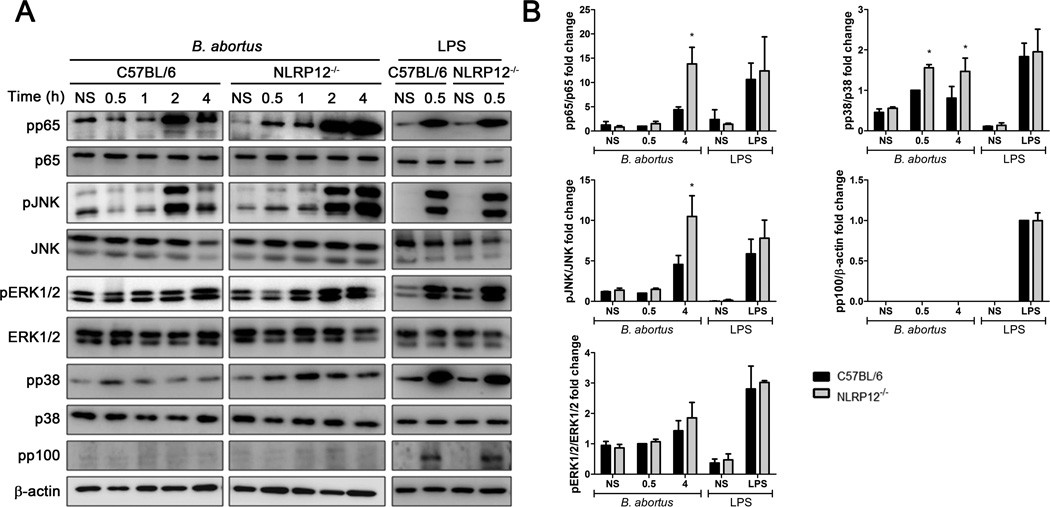
The recognition of B. abortus by innate immune receptors results in the activation of different signaling pathways, culminating in the expression of several proinflammatory genes [13, 27]. We next assessed the potential role of NLRP12 in regulating NF-κB and MAPK signaling pathways in BMDMs upon B. abortus infection. B. abortus induced phosphorylation of p65, JNK, ERK1/2 and p38 in both C57BL/6 and NLRP12−/− macrophages (Figure 2A). However, NLRP12 participates in the regulation of phosphorylation of p65, JNK and p38 in infected-BMDMs, since the lack of NLRP12 leads to increased phosphorylation of those kinases particularly at 4 hours postinfection (Figure 2B). We further investigated if non-canonical NF-κB signaling pathway was altered in NLRP12−/− cells, by examining phosphorylation of p100. We did not detect phosphorylation of this kinase in WT and NLRP12 KO macrophages in response to B. abortus. Also, we did not observe any modulation of p38 signaling by NLRP12 in response to LPS from Escherichia coli. These data are in agreement with the augmented IL-12 production observed in B. abortus NLRP12−/− BMDMs and suggest that NLRP12 dampens cytokine production by inhibiting NF-κB and MAPK phosphorylation.
Figure 2.
NLRP12 negatively regulates NF- κB and MAPK signaling in response to B. abortus. BMDMs from C57BL/6 and NLRP12−/− mice were non-stimulated (NS) or infected with B. abortus S2308 at a MOI of 1000:1 for the indicated times or stimulated with LPS (1µg/ml) for 30 min and lysed for western blot analysis. (A) Cell lysates were then separated by SDS-PAGE, blotted, and probed with the indicated antibodies. β-actin was used as loading control. (B) Bar graphs show densitometry analysis of pp65, pJNK, pERK1/2 and pp38 relative to their respective total proteins or pp100 relative to β-actin. Data shown in all panels are expressed as mean ± SEM of three independent experiments. * P ≤ 0.05 in comparison to C57BL/6 BMDMs using Two-way ANOVA.
NLRP12 modulates inflammasome activation in response to B. abortus
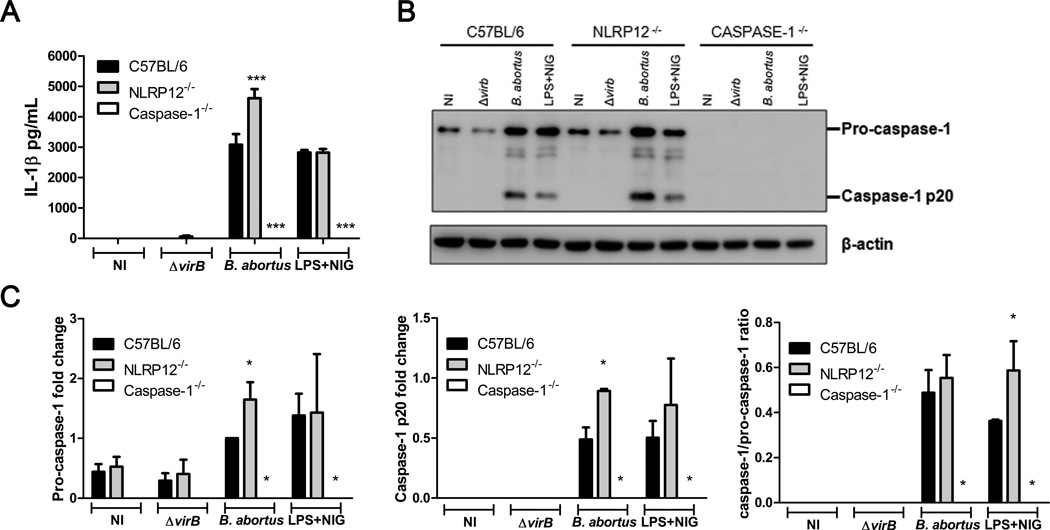
Our group recently showed that the adaptor protein ASC is essential for caspase-1 activation and IL-1β secretion during B. abortus infection. In addition, the B. abortus T4SS virB is necessary for inflammasome activation [24]. To determine whether NLRP12 has a role in inflammasome activation upon B. abortus infection, we infected C57BL/6, NLRP12−/− and caspase-1−/− BMDMs with B. abortus S2308 or virB mutant for 17 hours. IL-1β secretion induced by B. abortus S2308 was significantly increased in NLRP12−/− macrophages compared to WT cells (Figure 3A). Furthermore, we confirmed that Brucella T4SS is important for IL-1β release, as we observed a drastic reduction of IL-1β secretion in macrophages infected with virB mutant strain. Deficiency in NLRP12 did not affect the production of IL-1β triggered by nigericin, used as positive control. As expected, no IL-1β secretion was detected in caspase-1−/− BMDMs. We further investigated whether NLRP12 modulates inflammasome activation by the cleavage of caspase-1 as determined by immunoblotting of the supernatant from those macrophages. No cleaved caspase-1 was detected from virB-infected BMDMs. Also, NLRP12−/− macrophages infected with B. abortus S2308 presented more pro-caspase-1 and active caspase-1 compared to WT BMDMs (Figure 3B and 3C). Interestingly, we did not observe modulation of ASC and IL-18 expression by NLRP12 in B. abortus-infected BMDMs (Supplementary Figure 2). Taken together these results indicate that in response to B. abortus, NLRP12 modulates in vitro pro-caspase-1 expression leading to reduced caspase-1 level and IL-1β secretion.
Figure 3.
NLRP12 inhibits B. abortus-induced IL-1β secretion and caspase-1 activation. BMDMs from C57BL/6, NLRP12−/− and caspase-1−/− mice were non-infected (NI) or infected with B. abortus S2308 or ΔvirB at a MOI of 100:1 for 17 hours or stimulated with LPS and nigericin. (A) IL-1β levels in culture supernatants were quantified by ELISA. Data are shown as mean ± SEM of three samples per group analyzed in triplicate. *** P ≤ 0.001 between knockout BMDMs and C57BL/6 BMDMs, Two-way ANOVA. (B) Culture supernatants were separated by SDS-PAGE, blotted, and probed with anti-caspase-1. Detection of β-actin in cell lysates was used as loading control. (C) Bar graphs show densitometry analysis of pro-caspase-1, caspase-1 p20 and caspase-1/pro-caspase-1 ratio. Data shown in all panels are expressed as mean ± SEM of three independent experiments. * P ≤ 0.05 in comparison to C57BL/6 BMDMs using Two-way ANOVA.
NLRP12 deficiency protects mice from B. abortus infection
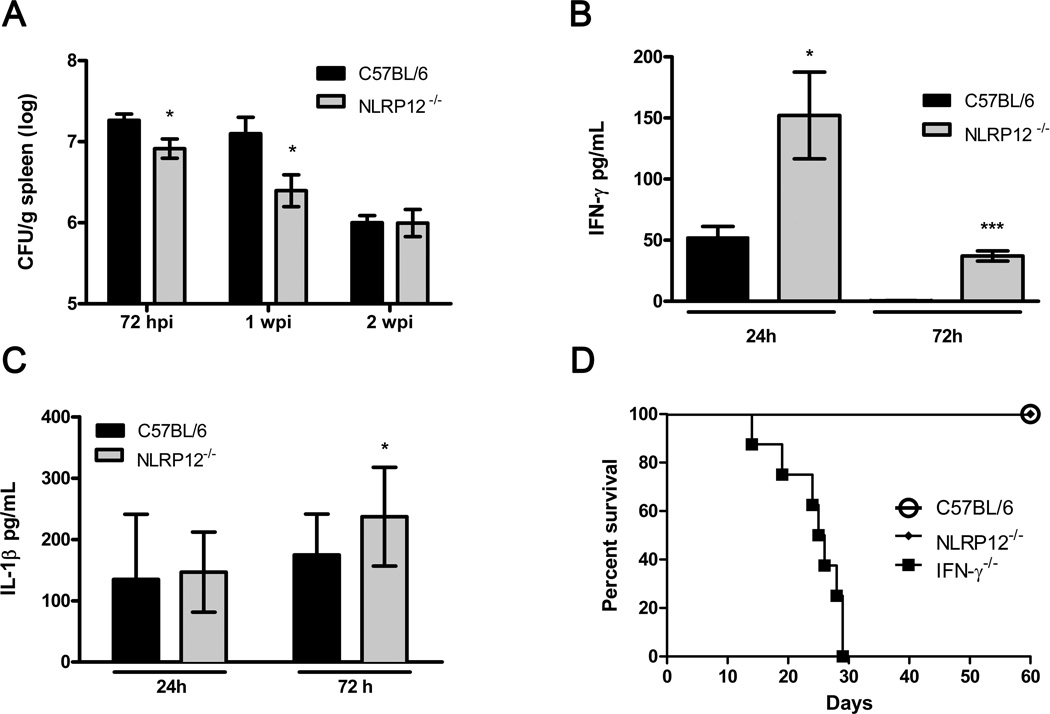
To determine the role of NLRP12 in vivo following B. abortus infection, C57BL/6 and NLRP12−/− mice were infected intraperitoneally with virulent B. abortus S2308. After 72 hours of infection, the bacterial burden from spleens was determined. NLRP12−/− mice were significantly more resistant to B. abortus infection and presented a reduced bacterial load compared to WT controls (Figure 4A). Lack of NLRP12 also enhanced host resistance to B. abortus at one week postinfection. Conversely, no differences were observed between both mouse groups after two weeks postinfection. Taken together, these results strongly suggest an important early role of NLRP12 in modulating host susceptibility to B. abortus in vivo.
Figure 4.
NLRP12 deficiency partially protects mice from B. abortus infection. C57BL/6 (n=5) and NLRP12−/− (n=5) mice were infected i.p. with (A) 1×106 units of B. abortus S2308, and at 72 hours (hpi), 1 or 2 weeks postinfection (wpi) bacterial loads in the spleens were determined and expressed as log colony forming units (CFU); or with (B,C) 1×109 B. abortus S2308 and sera were collected at indicated times to determine (B) IFN-γ and (C) IL-1β levels by ELISA. (D) C57BL/6 (n=8), NLRP12−/− (n=8) and IFN-γ−/− (n=8) mice were infected i.p with 1×106 B. abortus S2308. Survival of the mice was monitored daily. All data are the mean ± SEM. Data are representative of two independent experiments. * P≤0.05 and *** P ≤ 0.0001 compared to C57BL/6, Student’s t-test.
Previously, in vivo protection against B. abortus infection was shown to require the induction of a Th1-type immune response, where IFN-γ is a pivotal cytokine for host control of brucellosis [28, 29]. Thus, to investigate the role of NLRP12 in regulating in vivo Th1 response upon B. abortus infection, IFN-γ levels in sera of infected mice were evaluated. After 24 hours of infection, NLRP12−/− mice displayed increased systemic production of IFN-γ compared to WT controls (Figure 4B). After 72 hours of infection, augmented production of IFN-γ persisted in mice lacking NLRP12. At this same time interval (72 hrs), we also detected modest but elevated levels of IL-1β in NLRP12−/− mice sera compared to C57BL/6 (Figure 4C). To further investigate the contribution of NLRP12 in host susceptibility against B. abortus, we infected WT, NLRP12−/− and IFN-γ−/− mice and monitored survival. IFN-γ−/− mice were used as positive control, due to their enhanced susceptibility to brucellosis. Following infection, all IFN-γ-deficient mice succumbed within 29 days of infection, whereas no mortality was observed in C57BL/6 and NLRP12−/− mice (Figure 4D).
NLRP12 regulates liver granuloma during B. abortus infection
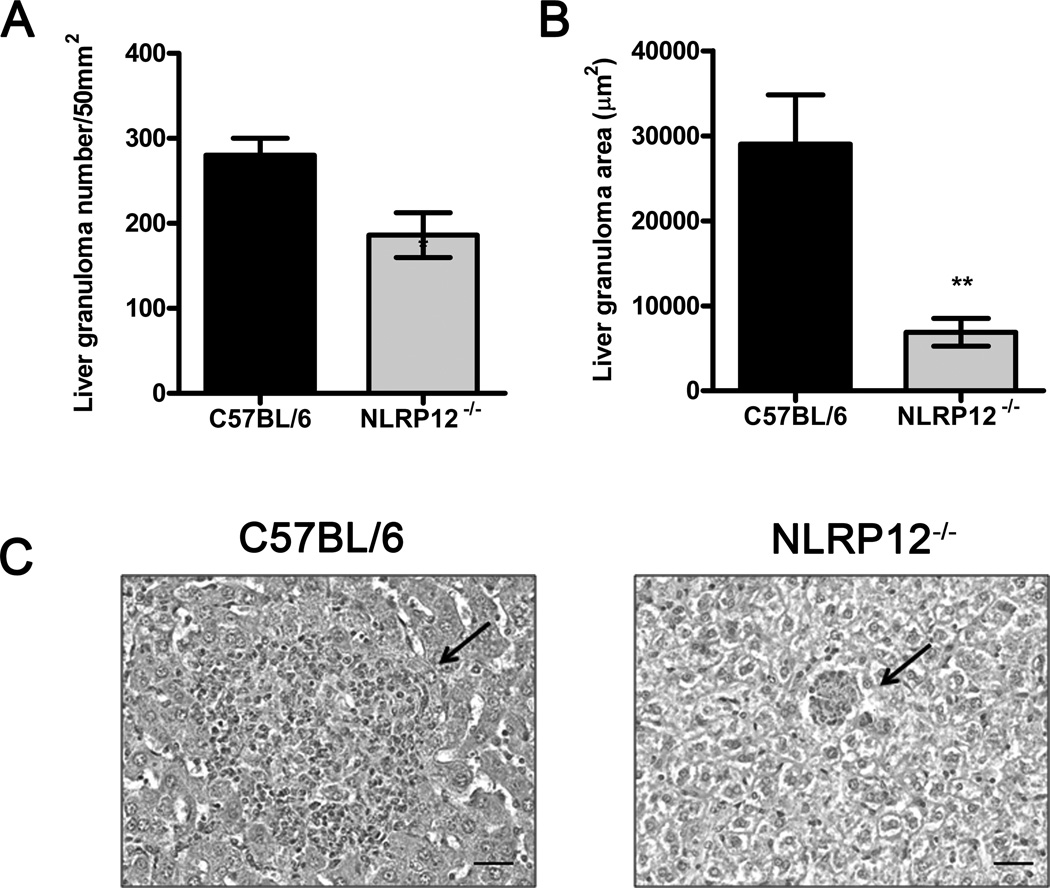
Infection with B. abortus results in the formation of liver and spleen granulomas, where inflammatory cells aggregate to restrain bacterial growth. After 1 week of infection, granulomas in the liver are conspicuous [30]. At this time, NLRP12−/− mice displayed a significant reduction in granuloma number and size when compared to WT counterparts (Figure 5 A–C). These data suggest that NLRP12 also modulates host liver pathology in the early stages of Brucella infection.
Figure 5.
NLRP12 modulates granuloma formation during early B. abortus infection. C57BL/6 (n=5) and NLRP12−/− (n=5) mice were infected i.p. with 1×106 B. abortus S2308 for 1 week. Livers were paraffin imbedded for histological analysis and (A) granuloma numbers (B) and surface area of granulomas were determined (µm2). Bars show mean ± SEM. Data are representative of two independent experiments. * P ≤ 0.05 and **, P ≤ 0.005 compared to C57BL/6, Student’s t-test. (C) Representative H&E staining of hepatic tissue of infected mice. Arrows indicate granulomas in the liver of C57BL/6 (left) and NLRP12−/− (right) mice. Digital images were captured using 20× magnification. Scale bars, 20 µm.
Discussion
The innate immune response against B. abortus begins with the recognition of bacterial components by PRRs such as TLRs and NLRs. Several studies have explored the involvement of TLRs and their signaling pathways in response to Brucella; however, the participation of NLRs in brucellosis is not fully understood [27]. Recently, our group demonstrated the importance of NLRP3 and AIM2 in host susceptibility against B. abortus [24]. In this study, we described an anti-inflammatory role of NLRP12 in response to B. abortus infection.
B. abortus triggers antigen-presenting cells to produce several proinflammatory cytokines such as TNF-α, IL-6, IL-12, IL-1β and type I IFNs. IL-12 is an important cytokine that drives Th0 cells to differentiate into Th1 effector cells that secrete IFN-γ, a cytokine essential to control Brucella infection [31]. In our in vitro experiments with BMDMs, we observed that NLRP12 is a negative regulator of IL-12 production upon B. abortus infection. Interestingly, we found that NLRP12 attenuates IL-12 secretion at 5 hours but not at 24 hours after infection. Also using BMDMs, Zaki and colleagues demonstrated an early negative regulation promoted by NLRP12 in proinflammatory cytokine production in Salmonella typhimurium infection [32]. Another report using NLRP12−/− BMDCs described the role of this receptor in attenuating early cytokine production in response to PAMPs associated with Escherichia coli, Klebsiella pneumoniae and Mycobacterium tuberculosis [33]. Altogether, these findings suggest that NLRP12 plays an important role as an anti-inflammatory mediator in the early innate immune response against different bacterial pathogens.
Multiple signaling pathways lead to proinflammatory cytokine production in response to B. abortus [13]. Here, we showed that NLRP12 modulates phosphorylation of NF-κB and MAPK components in BMDMs infected with B. abortus. We hypothesized that NLRP12 dampens IL-12 production by modulating these transduction signaling pathways (Figure 6). Potentially, the negative regulation in signaling mediated by NLRP12 also interferes with the expression of other essential inflammatory molecules. In our model of infection, we did not detect activation of the non-canonical (alternative) NF-κB pathway in response to B. abortus. However, Brucella may activate this alternative pathway in other cell types than macrophages and during longer infection kinetics. In S. typhimurium-infected BMDMs, NLRP12 regulates ERK phosphorylation and activation of canonical NF-κB signaling, with no role in the non-canonical pathway [32]. NLRP12 has been implicated to regulate both canonical and alternative NF-κB signaling. However, the regulatory role of NLRP12 in non-canonical NF-κB has been described mainly in biochemical assays, dendritic cells and colon cancer models [34, 35]. Due to the slow kinetics and dependence of de novo protein synthesis for the alternative NF-κB pathway [36], we speculate that canonical signaling plays a much important role in the early response to bacterial infections in murine macrophages.
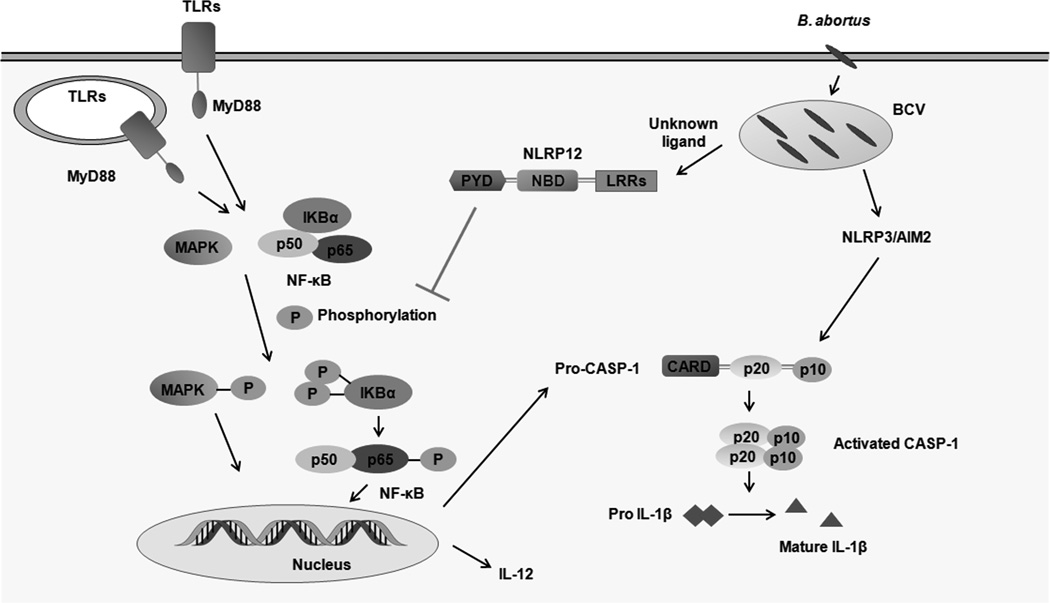
Figure 6.
Model of NLRP12-mediated modulation of proinflammatory response during B. abortus infection in murine macrophages. Upon recognition of B. abortus components, TLRs via MyD88 trigger activation of NF-κB and MAPK signaling. NLRP12 inhibits phosphorylation of MAPK and the p65 subunit of NF-κB and IL-12 secretion. NLRP12 also modulates pro-caspase-1 expression and mature IL-1β secretion. At present, the NLRP12 agonist is unknown, and it is unclear whether NLRP12 cooperates with other NLRs members to regulate innate immune response. TLRs, Toll-like receptors; MyD88, Myeloid differentiation primary response gene 88; LRRs, leucine-rich repeats; NBD, nucleotide-binding domain; CARD, caspase recruitment domain; PYD, pyrin domain; BCV, Brucella-containing vacuole; Casp-1, Caspase-1.
Inflammasomes play a central role in host defense against pathogens and endogenous danger signals. Inflammasome formation leads to caspase-1 activation and IL-1β maturation, contributing to a robust proinflammatory response. During B. abortus infection, ASC inflammasome is indispensable for inducing the activation of caspase-1 and secretion of IL-1β, whereas NLRP3 and AIM2 are partially required for IL-1β maturation [24]. In this study, we observed that mature IL-1β, pro-caspase-1 and active caspase-1 were markedly increased in B. abortus-infected NLRP12−/− BMDMs. Our data demonstrates that NLRP12 does not affect the expression of ASC and pro-IL-18 (Figure S2). Therefore, we hypothesize that NLRP12 acts interfering in pro-caspase-1 expression and caspase-1 cleavage in response to Brucella probably inhibiting signal 1 via NF-κB (Figure 6). Further experiments are required to elucidate the underlying mechanisms by which NLRP12 modulates expression of some inflammasome components and IL-1β secretion following B. abortus infection. In contrast to our findings, other studies reported that NLRP12 does not contribute to IL-1β maturation in response to derived-microbial PAMPs or S. typhimurium [32]. Another study determined that NLRP12 is an inflammasome component involved in the recognition of Yersinia pestis and positively regulates IL-1β and IL-18 production [37]. To the best of our knowledge, this is the first study to describe a negative regulation of caspase-1 activation mediated by NLRP12 in response to a bacterial infection. NLRP12-mediated suppression of proinflammatory signaling was also shown to play a central role in the attenuation of colon inflammation and tumorigenesis in mice [35, 38]. More recently, an unexpected role for NLRP12 was defined as intrinsic negative regulator of pathogenic T cell responses in autoinflammatory disease [39]. In this model, dysregulated production of IL-4 promoted atypical neuroinflammatory disease in NLRP12−/− mice. Overall, the function of NLRP12 appears to be dependent on the type of cells, pathogens, and stimuli analyzed.
The protective host response against B. abortus requires Th1-type cytokines such as IFN-γ that activates macrophage microbicidal mechanisms. In fact, IFN-γ−/− mice fail to control Brucella replication and succumb to infection [28]. In the present study, absence of NLRP12 leads to protection against B. abortus infection in vivo. Mice deficient for NLRP12 had reduced bacterial counts in the spleen and higher levels of serum IFN-γ at 72 hours after infection. Moreover, a greater reduction in granuloma number and size was detected in NLRP12−/− mice at 1 week of infection. The formation of granulomas is an important component of coordinated antibacterial defenses, in which lymphocytes cooperate with macrophages to restrain bacterial growth. Previous studies have described hepatic microgranulomas during systemic infections with pathogenic Brucella spp. in the mouse [40]. The granulomas induced by Brucella are mainly composed of CD11b+ F4/80+ MHC-II+ cells and a fraction of these cells also expressed CD11c marker and appeared similar to inflammatory DCs [41]. Since, NLRP12 is important in maintaining neuthophils and DCs in a migration-competent state [42] and this sensor also affected macrophages content in BALF from Klebsiella penumoniae infected mice [33], we hypothesize that lack of NLRP12 in Brucella infected animals might have influenced DCs and macrophages migration during granuloma formation. These data are consistent with our in vitro findings that demonstrate the negative regulation of immune response promoted by NLRP12. Conversely, mice lacking ASC, caspase-1, AIM2, and NLRP3 are more susceptible to B. abortus [24]. Because the present in vivo data indicate that NLRP12 has a role in attenuating inflammation at the early stages of Brucella infection, we hypothesize that NLRP12 likely acts upstream of these inflammasome components.
In summary, our findings demonstrated that NLRP12 is a negative regulator of proinflammatory response against B. abortus. NLRP12 inhibits in vitro production of IL-12, modulates expression of some inflammasome components and IL-1β secretion upon B. abortus infection. NLRP12 also plays a role in vivo, attenuating IFN-γ response and contributing to host susceptibility in the early immune response to murine brucellosis. Nevertheless, the B. abortus ligands required for NLRP12 recognition remain to be identified.
Material and methods
Mice
Wild-type C57BL/6 (WT) mice were purchased from the Federal University of Minas Gerais (UFMG), NLRP12−/−, caspase-1−/− and IFN-γ−/− were described previously [29, 43, 44]. Mice 6 to 8 week of age were used for in vivo experiments and/or to obtain macrophages from bone marrow cells. Food and water were provided ad libitum and all procedures performed in this study were approved by the local ethical committee (CETEA # 128/2014).
Bacteria
Bacteria used included B. abortus strain (S) 2308 obtained from our laboratory collection and the B. abortus virB operon mutant strain kindly provided by Dr. Renato de Lima Santos (UFMG). Bacteria were grown in BB liquid medium (Difco, Detroit, MI, USA) at 37°C under constant agitation for 72 hours. Bacterial cultures were pelleted and suspended in phosphate-buffered saline (PBS) containing 25% of glycerol, and then aliquoted and stored at −80 C until use. Aliquots were serially diluted and plated in BB medium containing 1.5 % bacteriological agar (BB agar). After incubation for 72 hours at 37°C, bacterial concentration was determined by counting CFUs.
Bacterial counting in B. abortus infected mice
Five mice from each group (C57BL/6 or NLRP12−/−) were infected intraperitoneally (i.p.) with 1×106 virulent B. abortus S2308 in 100µl of PBS. After 72h, 1 and 2 weeks postinfection, mice were sacrificed and spleens were used to determine the number of bacteria by CFU counting. Spleens harvested from each animal were weighed and macerated in 10mL of saline (NaCl 0.9%). To determine bacterial burden, spleens were serially diluted in saline and plated in duplicate on BB agar. Plates were incubated for 3 days at 37°C and CFU number was determined.
Survival analysis of B. abortus infected mice
Eight mice from each group (C57BL/6, NLRP12−/− and IFN-γ−/−) were infected i.p with 1×106 virulent B. abortus S2308 as described above and were monitored daily for 60 days.
Bone marrow-derived macrophages (BMDMs)
Bone marrow cells were flushed from femurs and tibias of C57BL/6, NLRP12−/− or caspase-1−/− mice and were differentiated into macrophages as previously described [24]. Briefly, cells were seeded in 24-well plates (5×105 cells/well) and cultured in DMEM (Gibco, Carlsbad, CA, USA) containing 10% L929 cell-conditioned medium (LCCM), 10% FBS (HyClone, Logan, UT, USA), 1% penicillin-streptomycin and 1% HEPES, at 37°C in an atmosphere of 5% CO2. At day 4 of differentiation, LCCM was added (100uL/well) and at day 7, culture medium was replaced with fresh medium containing 10% LCCM. At day 10, cells were completely differentiated into macrophages and culture medium was replaced with antibiotic-free DMEM plus 1% FBS. NLRP12 expression in unmatured bone marrow cells and macrophages was evaluated by Real-time PCR (Supplementary Figure 1).
Real-time PCR (RT-PCR)
BMDMs from C57BL/6 and NLRP12−/−mice were infected with B. abortus S2308 (multiplicity of infection [MOI] 100:1) for 24 hours and total RNA was extracted with TRIzol reagent (Invitrogen) according to the manufacturer's instructions. cDNA was synthesized by reverse transcription (RT) from 1µg of total RNA, and was used to perform RT-PCR in a final volume of 10µl containing SYBR green PCR Master Mix (Applied Biosystems, Carlsbad, CA, USA) and 20µM of primers. RT-PCR was performed in triplicate, on an ABI 7900 Real-time PCR system (Applied Biosystems). The primers used for the NLRP12 [32], ASC [45] and IL-18 [46] and β-actin [12] genes were as follows: NLRP12 forward, 5′-CCTCTTTGAGCCAGACGAAG-3′; NLRP12 reverse, 5’- GCCCAGTCCAACATCACTTT-3’; ASC forward, 5’- CAGAGTACAGCCAGAACAGGACAC-3’: ASC reverse, 5’-GTGGTCTCGCACGAACTGCCTG-3’: IL-18 forward, 5’-GCCTCAAACCTTCCAAATCA-3’: IL-18 reverse, 5’- TGGATCCATTTCCTCAAAGG-3’: β-actin forward, 5’-AGGTGTGCACCTTTTATTGGTCTCAA-3’; and β-actin reverse, 5’- TGTATGAAGGTTTGGTCTCCCT-3’. The levels of mRNAs are presented as relative expression units after normalization to the β-actin gene.
Cytokines measurement
To assay in vitro production of IL-12, IL-6 and TNF-α, BMDMs were infected with Brucella abortus S2308 (MOI 100:1) for 5 or 24 hours and supernatants were harvested. For in vivo determination of IFN-γ and IL-1β levels, C57BL/6 or NLRP12−/− mice were infected i.p. with 1×109 virulent B. abortus S2308 and sacrificed at the indicated times. Blood was collected and purified sera were used for cytokine analysis. All cytokines were measured using commercially available ELISA Duoset kits (R&D Systems, Minneapolis, MN, USA).
Western blotting for cell signaling events
To detect phosphorylation of MAPK and NF-κB, BMDMs were serum starved for 16 hours and infected with B. abortus S2308 (MOI 1000:1) or stimulated with E.coli LPS (1µg/ml) for 30 min. At the indicated times, cells were lysed with M-PER™ Mammalian Protein Extraction Reagent (Thermo Fisher Scientific) supplemented with protease and phosphatase inhibitors (Roche). Protein concentration was determined using Pierce™ BCA Protein Assay Kit (ThermoFisher Scientific). Equal amounts of proteins were separated on 15% SDS-PAGE gels and transferred to nitrocellulose membranes (Amersham Biosciences, Uppsala, Sweden) in transfer buffer (50mM Tris, 40mM glycine, 10% methanol). Membranes were blocked for 1 hour in TBS with 0.1% Tween-20 containing 5% nonfat dry milk and incubated overnight with primary antibodies (ERK1/2, p38, JNK, p65, phospho-ERK1/2, phospho-p38, phospho-JNK, phospho-p65, phospho-p100, and β-actin [Cell Signaling Technology, Danvers, MA, USA]) at 4°C. Membranes were incubated with horseradish peroxidase-conjugated secondary antibody and Luminol chemiluminescent HRP substrate (Millipore, Billerica, MA, USA) was used for antibody detection. Densitometry analysis was performed using ImageQuant TL Software (GE Healthcare, Buckinghamshire, United Kingdom), and band intensities were normalized to total proteins or β-actin. Data were obtained relative to the level of C57BL/6 BMDMs infected with B. abortus for 30 min assigned arbitrarily with the value of 1.0.
Detection of activated caspase-1 and secreted IL-1β
BMDMs were infected with Brucella abortus S2308 or virB mutant (MOI 100:1) for 17 hours. As a positive control, cells were primed with 1 µg/ml of E. coli LPS (Sigma-Aldrich, St. Louis, MO, USA) for 4h and stimulated with 20µM nigericin sodium salt (Sigma-Aldrich) for 30 minutes. Culture supernatants were collected and cells were lysed with M-PER™ Mammalian Protein Extraction Reagent (Thermo Fisher Scientific) for western blotting analysis as described above. Processed p20 subunit of caspase-1 (caspase-1 p20) and unprocessed caspase-1 (pro-caspase-1) were detected using primary antibody anti-caspase-1 (p20) (Adipogen, San Diego, CA, USA). Densitometry analysis was performed using ImageQuant TL Software (GE Healthcare). Band intensities were normalized to the level of pro-caspase-1 related to C57BL/6 BMDMs infected with B. abortus. IL-1β was measured from culture supernatants using IL-1β ELISA Duoset kit (R&D Systems) according to the manufacturer’s instructions.
Histopathology
Five mice (C57BL/6 or NLRP12−/−) from each group were infected i.p. as described above. Liver medial lobes from 1 week B. abortus-infected mice were fixed in 10% buffered formaldehyde solution and embedded in paraffin by standard techniques. Histological sections (5µM thick) were stained with hematoxylin and eosin (HE). Total number of granulomas was unbiasedly determined using an Olympus CX31 microscope with a 20× objective. Digital images of 15 granulomas/animal were acquired using an Olympus SC30 camera. The area of histological sections and the size of granulomas were calculated using the Image Tool 3.0 software, and total numbers of granulomas were normalized for a 50mm2 tissue area.
Statistical analysis
Statistical analyses were performed using GraphPad Prism software, version 5 (GraphPad Software, San Diego, CA, USA). Data were analyzed using Two-way ANOVA or Student’s t test to calculate the significance differences. Data are presented as mean ± SEM, and a value of P ≤ 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa do estado de Minas Gerais (FAPEMIG), CAPES/PVE, CAPES/PNPD, CNPq/CT-Biotec, CNPq/CBAB and National Institute of Health R01 AI116453.
Abbreviations
- BMDMs
bone-marrow-derived macrophages
- LCCM
L929 cell-conditioned medium
- NLRs
Nod-like receptors
- MOI
multiplicity of infection
- PAMPs
pathogen-associated molecular patterns
- PRRs
pattern recognition receptors
- T4SS
type IV secretion system
- TLRs
Toll-like receptors
- WT
wild-type
Footnotes
Conflict of interest disclosure
The authors have no financial or commercial conflicts of interest.
References
- 1.Pappas G. The changing Brucella ecology: novel reservoirs, new threats. Int J Antimicrob Agents. 2010;36(Suppl 1):S8–S11. doi: 10.1016/j.ijantimicag.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 2.Franco MP, Mulder M, Gilman RH, Smits HL. Human brucellosis. Lancet Infect Dis. 2007;7:775–786. doi: 10.1016/S1473-3099(07)70286-4. [DOI] [PubMed] [Google Scholar]
- 3.Godfroid J, Scholz HC, Barbier T, Nicolas C, Wattiau P, Fretin D, Whatmore AM, Cloeckaert A, Blasco JM, Moriyon I, Saegerman C, Muma JB, Al Dahouk S, Neubauer H, Letesson JJ. Brucellosis at the animal/ecosystem/human interface at the beginning of the 21st century. Prev Vet Med. 2011;102:118–131. doi: 10.1016/j.prevetmed.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 4.de Jong MF, Tsolis RM. Brucellosis and type IV secretion. Future Microbiol. 2012;7:47–58. doi: 10.2217/fmb.11.136. [DOI] [PubMed] [Google Scholar]
- 5.Oliveira SC, de Almeida LA, Carvalho NB, Oliveira FS, Lacerda TL. Update on the role of innate immune receptors during Brucella abortus infection. Vet Immunol Immunopathol. 2012;148:129–135. doi: 10.1016/j.vetimm.2011.05.036. [DOI] [PubMed] [Google Scholar]
- 6.Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- 7.Campos MA, Rosinha GM, Almeida IC, Salgueiro XS, Jarvis BW, Splitter GA, Qureshi N, Bruna-Romero O, Gazzinelli RT, Oliveira SC. Role of Toll-like receptor 4 in induction of cell-mediated immunity and resistance to Brucella abortus infection in mice. Infect Immun. 2004;72:176–186. doi: 10.1128/IAI.72.1.176-186.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giambartolomei GH, Zwerdling A, Cassataro J, Bruno L, Fossati CA, Philipp MT. Lipoproteins, not lipopolysaccharide, are the key mediators of the proinflammatory response elicited by heat-killed Brucella abortus. J Immunol. 2004;173:4635–4642. doi: 10.4049/jimmunol.173.7.4635. [DOI] [PubMed] [Google Scholar]
- 9.Huang LY, Ishii KJ, Akira S, Aliberti J, Golding B. Th1-like cytokine induction by heat-killed Brucella abortus is dependent on triggering of TLR9. J Immunol. 2005;175:3964–3970. doi: 10.4049/jimmunol.175.6.3964. [DOI] [PubMed] [Google Scholar]
- 10.Weiss DS, Takeda K, Akira S, Zychlinsky A, Moreno E. MyD88, but not toll-like receptors 4 and 2, is required for efficient clearance of Brucella abortus. Infect Immun. 2005;73:5137–5143. doi: 10.1128/IAI.73.8.5137-5143.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Macedo GC, Magnani DM, Carvalho NB, Bruna-Romero O, Gazzinelli RT, Oliveira SC. Central role of MyD88-dependent dendritic cell maturation and proinflammatory cytokine production to control Brucella abortus infection. J Immunol. 2008;180:1080–1087. doi: 10.4049/jimmunol.180.2.1080. [DOI] [PubMed] [Google Scholar]
- 12.de Almeida LA, Macedo GC, Marinho FA, Gomes MT, Corsetti PP, Silva AM, Cassataro J, Giambartolomei GH, Oliveira SC. Toll-like receptor 6 plays an important role in host innate resistance to Brucella abortus infection in mice. Infect Immun. 2013;81:1654–1662. doi: 10.1128/IAI.01356-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomes MT, Campos PC, Pereira GS, Bartholomeu DC, Splitter G, Oliveira SC. TLR9 is required for MAPK/NF-kappaB activation but does not cooperate with TLR2 or TLR6 to induce host resistance to Brucella abortus. J Leukoc Biol. 2015 doi: 10.1189/jlb.4A0815-346R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaw MH, Reimer T, Kim YG, Nunez G. NOD-like receptors (NLRs): bona fide intracellular microbial sensors. Curr Opin Immunol. 2008;20:377–382. doi: 10.1016/j.coi.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caruso R, Warner N, Inohara N, Nunez G. NOD1 and NOD2: signaling, host defense, and inflammatory disease. Immunity. 2014;41:898–908. doi: 10.1016/j.immuni.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oliveira FS, Carvalho NB, Zamboni DS, Oliveira SC. Nucleotide-binding oligomerization domain-1 and-2 play no role in controlling Brucella abortus infection in mice. Clin Dev Immunol. 2012;2012:861426. doi: 10.1155/2012/861426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sollberger G, Strittmatter GE, Garstkiewicz M, Sand J, Beer HD. Caspase-1: the inflammasome and beyond. Innate Immun. 2014;20:115–125. doi: 10.1177/1753425913484374. [DOI] [PubMed] [Google Scholar]
- 18.Wang L, Manji GA, Grenier JM, Al-Garawi A, Merriam S, Lora JM, Geddes BJ, Briskin M, DiStefano PS, Bertin J. PYPAF7, a novel PYRIN-containing Apaf1-like protein that regulates activation of NF-kappa B and caspase-1-dependent cytokine processing. J Biol Chem. 2002;277:29874–29880. doi: 10.1074/jbc.M203915200. [DOI] [PubMed] [Google Scholar]
- 19.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 20.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vanaja SK, Rathinam VA, Fitzgerald KA. Mechanisms of inflammasome activation: recent advances and novel insights. Trends Cell Biol. 2015;25:308–315. doi: 10.1016/j.tcb.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burckstummer T, Baumann C, Bluml S, Dixit E, Durnberger G, Jahn H, Planyavsky M, Bilban M, Colinge J, Bennett KL, Superti-Furga G. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol. 2009;10:266–272. doi: 10.1038/ni.1702. [DOI] [PubMed] [Google Scholar]
- 23.Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, Fitzgerald KA. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gomes MT, Campos PC, Oliveira FS, Corsetti PP, Bortoluci KR, Cunha LD, Zamboni DS, Oliveira SC. Critical role of ASC inflammasomes and bacterial type IV secretion system in caspase-1 activation and host innate resistance to Brucella abortus infection. J Immunol. 2013;190:3629–3638. doi: 10.4049/jimmunol.1202817. [DOI] [PubMed] [Google Scholar]
- 25.Tuncer S, Fiorillo MT, Sorrentino R. The multifaceted nature of NLRP12. J Leukoc Biol. 2014;96:991–1000. doi: 10.1189/jlb.3RU0514-265RR. [DOI] [PubMed] [Google Scholar]
- 26.Golding B, Scott DE, Scharf O, Huang LY, Zaitseva M, Lapham C, Eller N, Golding H. Immunity and protection against Brucella abortus. Microbes Infect. 2001;3:43–48. doi: 10.1016/s1286-4579(00)01350-2. [DOI] [PubMed] [Google Scholar]
- 27.Gomes MT, Campos PC, de Almeida LA, Oliveira FS, Costa MM, Marim FM, Pereira GS, Oliveira SC. The role of innate immune signals in immunity to Brucella abortus. Front Cell Infect Microbiol. 2012;2:130. doi: 10.3389/fcimb.2012.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy EA, Sathiyaseelan J, Parent MA, Zou B, Baldwin CL. Interferon-gamma is crucial for surviving a Brucella abortus infection in both resistant C57BL/6 and susceptible BALB/c mice. Immunology. 2001;103:511–518. doi: 10.1046/j.1365-2567.2001.01258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brandao AP, Oliveira FS, Carvalho NB, Vieira LQ, Azevedo V, Macedo GC, Oliveira SC. Host susceptibility to Brucella abortus infection is more pronounced in IFN-gamma knockout than IL-12/beta2-microglobulin double-deficient mice. Clin Dev Immunol. 2012;2012:589494. doi: 10.1155/2012/589494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grillo MJ, Blasco JM, Gorvel JP, Moriyon I, Moreno E. What have we learned from brucellosis in the mouse model? Vet Res. 2012;43:29. doi: 10.1186/1297-9716-43-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oliveira SC, Soeurt N, Splitter G. Molecular and cellular interactions between Brucella abortus antigens and host immune responses. Vet Microbiol. 2002;90:417–424. doi: 10.1016/s0378-1135(02)00225-0. [DOI] [PubMed] [Google Scholar]
- 32.Zaki MH, Man SM, Vogel P, Lamkanfi M, Kanneganti TD. Salmonella exploits NLRP12-dependent innate immune signaling to suppress host defenses during infection. Proc Natl Acad Sci U S A. 2014;111:385–390. doi: 10.1073/pnas.1317643111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allen IC, McElvania-TeKippe E, Wilson JE, Lich JD, Arthur JC, Sullivan JT, Braunstein M, Ting JP. Characterization of NLRP12 during the in vivo host immune response to Klebsiella pneumoniae and Mycobacterium tuberculosis. PLoS One. 2013;8:e60842. doi: 10.1371/journal.pone.0060842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lich JD, Williams KL, Moore CB, Arthur JC, Davis BK, Taxman DJ, Ting JP. Monarch-1 suppresses non-canonical NF-kappaB activation and p52-dependent chemokine expression in monocytes. J Immunol. 2007;178:1256–1260. doi: 10.4049/jimmunol.178.3.1256. [DOI] [PubMed] [Google Scholar]
- 35.Allen IC, Wilson JE, Schneider M, Lich JD, Roberts RA, Arthur JC, Woodford RM, Davis BK, Uronis JM, Herfarth HH, Jobin C, Rogers AB, Ting JP. NLRP12 suppresses colon inflammation and tumorigenesis through the negative regulation of noncanonical NF-kappaB signaling. Immunity. 2012;36:742–754. doi: 10.1016/j.immuni.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun SC. The noncanonical NF-kappaB pathway. Immunol Rev. 2012;246:125–140. doi: 10.1111/j.1600-065X.2011.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vladimer GI, Weng D, Paquette SW, Vanaja SK, Rathinam VA, Aune MH, Conlon JE, Burbage JJ, Proulx MK, Liu Q, Reed G, Mecsas JC, Iwakura Y, Bertin J, Goguen JD, Fitzgerald KA, Lien E. The NLRP12 inflammasome recognizes Yersinia pestis. Immunity. 2012;37:96–107. doi: 10.1016/j.immuni.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zaki MH, Vogel P, Malireddi RK, Body-Malapel M, Anand PK, Bertin J, Green DR, Lamkanfi M, Kanneganti TD. The NOD-like receptor NLRP12 attenuates colon inflammation and tumorigenesis. Cancer Cell. 2011;20:649–660. doi: 10.1016/j.ccr.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lukens JR, Gurung P, Shaw PJ, Barr MJ, Zaki MH, Brown SA, Vogel P, Chi H, Kanneganti TD. The NLRP12 Sensor Negatively Regulates Autoinflammatory Disease by Modulating Interleukin-4 Production in T Cells. Immunity. 2015;42:654–664. doi: 10.1016/j.immuni.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Corsetti PP, de Almeida LA, Carvalho NB, Azevedo V, Silva TM, Teixeira HC, Faria AC, Oliveira SC. Lack of endogenous IL-10 enhances production of proinflammatory cytokines and leads to Brucella abortus clearance in mice. PLoS One. 2013;8:e74729. doi: 10.1371/journal.pone.0074729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Copin R, Vitry MA, Hanot Mambres D, Machelart A, De Trez C, Vanderwinden JM, Magez S, Akira S, Ryffel B, Carlier Y, Letesson JJ, Muraille E. In situ microscopy analysis reveals local innate immune response developed around Brucella infected cells in resistant and susceptible mice. PLoS Pathog. 2012;8:e1002575. doi: 10.1371/journal.ppat.1002575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arthur JC, Lich JD, Ye Z, Allen IC, Gris D, Wilson JE, Schneider M, Roney KE, O'Connor BP, Moore CB, Morrison A, Sutterwala FS, Bertin J, Koller BH, Liu Z, Ting JP. Cutting edge: NLRP12 controls dendritic and myeloid cell migration to affect contact hypersensitivity. J Immunol. 2010;185:4515–4519. doi: 10.4049/jimmunol.1002227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ataide MA, Andrade WA, Zamboni DS, Wang D, Souza Mdo C, Franklin BS, Elian S, Martins FS, Pereira D, Reed G, Fitzgerald KA, Golenbock DT, Gazzinelli RT. Malaria-induced NLRP12/NLRP3-dependent caspase-1 activation mediates inflammation and hypersensitivity to bacterial superinfection. PLoS Pathog. 2014;10:e1003885. doi: 10.1371/journal.ppat.1003885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mayer-Barber KD, Barber DL, Shenderov K, White SD, Wilson MS, Cheever A, Kugler D, Hieny S, Caspar P, Nunez G, Schlueter D, Flavell RA, Sutterwala FS, Sher A. Caspase-1 independent IL-1beta production is critical for host resistance to mycobacterium tuberculosis and does not require TLR signaling in vivo. J Immunol. 2010;184:3326–3330. doi: 10.4049/jimmunol.0904189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yim HC, Wang D, Yu L, White CL, Faber PW, Williams BR, Sadler AJ. The kinase activity of PKR represses inflammasome activity. Cell Res. 2016;26:367–379. doi: 10.1038/cr.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lupfer CR, Anand PK, Liu Z, Stokes KL, Vogel P, Lamkanfi M, Kanneganti TD. Reactive oxygen species regulate caspase-11 expression and activation of the non-canonical NLRP3 inflammasome during enteric pathogen infection. PLoS Pathog. 2014;10:e1004410. doi: 10.1371/journal.ppat.1004410. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.