Abstract
Cannabinoid stability in oral fluid (OF) is important for assuring accurate results since OF has become a valid alternative matrix of choice for drug testing. We previously published OF cannabinoid stability studies using Quantisal™, Oral-Eze®, and StatSure™ devices stored at room temperature for 1 week, 4°C for up to 4 weeks and in −20°C up to 24 weeks. Extending refrigerated stability up to 3 months would be helpful for clinical and forensic testing, for reanalysis of OF samples and for batching research analyses. Individual authentic OF pools were prepared after controlled smoking of a 6.9% Δ9-tetrahydracannabinol cannabis cigarette; the Quantisal™ device was utilized for OF collection. Fifteen healthy volunteers participated in the Institutional Review Board approved study. Stability for THC, 11-nor-9-carboxy-THC (THCCOOH), Δ9-tetrahydrocannabivarin (THCV), cannabidiol (CBD) and cannabigerol (CBG) were determined after storage at 4°C for 1, 2 and 3 months. Results within ±20% of baseline concentrations were considered stable. All analytes were stable for up to 2 months at 4°C for all participants with positive baseline concentrations. Baseline concentrations were highly variable. In total, THC, THCCOOH, THCV, CBD and CBG were stable for 3 months at 4°C for pooled positive specimens from 14 of 15, 8 of 9, 7 of 8, 8 of 9 and 9 of 10 participants, respectively. In conclusion, Quantisal™ collected OF specimens should be stored at 4°C for no more than 2 months to assure accurate THC, THCCOOH, THCV, CBD and CBG quantitative results; only one participant's OF was unstable at 3 months.
Keywords: oral fluid, cannabinoid, stability, liquid chromatography-mass spectrometry
INTRODUCTION
Oral fluid (OF) is a common matrix for drug testing in clinical and forensic settings. The US Substance Abuse Mental Health Services Administration (SAMHSA) recently approved OF testing for federally mandated workplace drug testing that will likely lead to expansion of OF analysis1. Compared to urine and blood collection, OF is less invasive and easier to collect while adulteration is more difficult because OF collection is easily observed2. Infection risk is also reduced compared to blood and OF reflects recent drug exposure better than urine2.
Cannabinoids are the most commonly abused illicit drug globally2. Determination of cannabinoids in OF is important during drug treatment, pain management, workplace testing, driving under the influence of drugs (DUID) and in sports anti-doping testing programs2. Therefore, cannabinoid OF stability is important for assuring accurate and reliable results.
Lund et. al. found THC concentrations in Intercept-collected OF were within ±26% of baseline after 1 week at 4°C and within ±20% for up to 11 months at −20°C3. We previously published OF cannabinoid stability data for the Quantisal™, Oral-Eze®, and StatSure™ devices stored at room temperature for 1 week, 4°C for up to 4 weeks and at −20°C up to 24 weeks4, 5. Cannabinoid concentrations were stable for 4 weeks at 4°C for all devices and all participants. StatSure™ OF samples also were stable for 24 weeks at −20°C, while Quantisal™ and Oral-Eze® cannabinoid concentrations were unstable under these conditions, which presents problems when retesting is required for Quantisal™ and Oral-Eze® specimens stored for >4 weeks.
In this report, we examined stability up to 3 months at 4°C for Δ9-tetrahydrocannabinol (THC), 11-nor-9-carboxy-THC (THCCOOH), Δ9-tetrahydrocannabivarin (THCV), 11-hydroxy-THC (11-OH-THC), cannabidiol (CBD) and cannabigerol (CBG) in Quantisal™-collected OF to determine if cannabinoids were stable in OF beyond one month at 4°C. We also for the first time studied stability of two additional minor cannabinoids present in cannabis plants, THCV and CBG. Detection of multiple cannabinoids improves cannabinoid test interpretation since the presence of minor cannabinoids may help identify recent cannabis intake6-8. These data provide OF stability information for forensic and clinical toxicology laboratories to guide timelines for accurate initial analysis and assist interpretation when defense/court requested OF re-analysis is necessary.
MATERIAL AND METHODS
Participants
A National Institute on Drug Abuse (NIDA) Institutional Review Board approved study was conducted with healthy male and female volunteers between the ages of 18-50 years old. Seven frequent users with a smoking frequency of ≥5 times per week and 8 occasional users with a smoking frequency of ≤3 times per week but ≥2 times per month provided written informed consent. Participants resided on a closed research unit the night before drug administration. Participants smoked a cigarette containing 6.9% THC (54mg total), 0.08% Δ8-THC, 0.20% CBD and 0.44% CBN ad libitum over 10 min.
OF stability sample collection and analysis
All OF was collected with the Quantisal™ (Immunalysis, Pomona, CA, USA) device with a 1 mL OF collection volume indicator. The collection pad is placed immediately into the accompanying tube containing 3 mL elution and stabilization buffer, yielding a 4-fold dilution. Individual stability pools were created for 15 participants by combining portions of OF specimens collected at −1.5, 1.5, 3.5, 5, 8, 10, 12 and 14h. After vortexing, each pool was aliquoted into Nunc cryotubes. Duplicate aliquots were analyzed within 24h of collection and 2 aliquots for each time point were stored at 4 °C and analyzed after 1, 2 and 3 months. Stability pools were created for each of the 15 participants.
Six cannabinoids: THC, THCCOOH, THCV, 11-OH-THC, CBD and CBG were analyzed employing 1 mL of Quantisal™ OF-buffer mixture with a previously published LCMS/MS method6. Samples were extracted via solid phase extraction on Strata X-C cation exchange columns (3mL/30mg, Phenomenex Inc., Torrance, CA, USA). Liquid chromatography tandem mass spectrometry was used for quantification with atmospheric pressure chemical ionization (APCI) and multiple reaction monitoring on a SCIEX 6500 QTRAP and Shimadzu UFLCxr system. Limits of quantification (LOQ) were 15 ng/L for THCCOOH and 200 ng/L for all other analytes. Inter-assay accuracy and imprecision were 88.1-106% and 5.8-8.2% coefficient of variation, respectively (n=92)
Data analysis
OF stability pool samples analyzed within 24h of collection and pooling established baseline concentrations. Since, method inter-assay imprecision was <8.2% we chose to consider concentrations within 80-120% of baseline concentrations stable. If baseline concentrations were below LOQ, no stability data could be obtained.
RESULTS
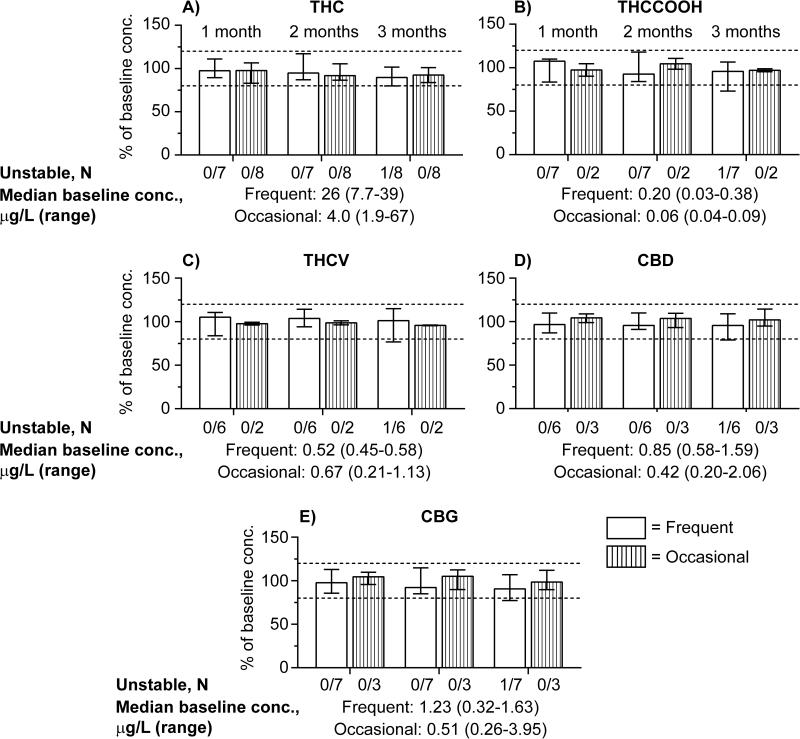
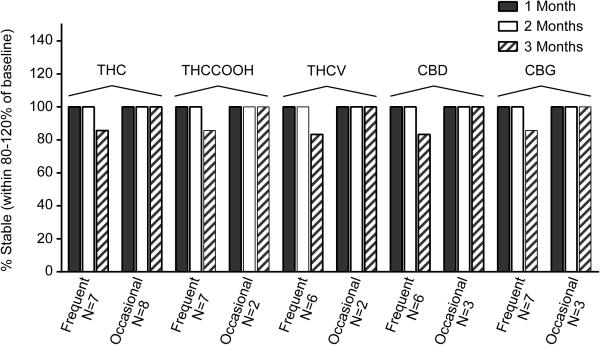
THC, THCCOOH, THCV, CBD and CBG concentrations were quantified in pooled Quantisal™ OF samples from 7 frequent and 8 occasional cannabis users (single pool for each participant). The OF samples were analyzed in duplicate within 24h or stored for 1, 2 or 3 months at 4°C; stability as %baseline concentrations (median and ranges) are shown in Figure 1. Percentages of stable samples for each cannabinoid after 1-3 months at 4°C are shown in Figure 2. 11-OH-THC was not detected in any sample above the LOQ; therefore, no stability data could be obtained. Baseline OF concentrations were <LOQ for THCCOOH in 6/8 occasional users, THCV in 1/7 frequent user and 6/8 occasional users, CBD in 1/7 frequent user and 5/8 occasional users, and CBG in 5/8 occasional users.
Figure 1.
Baseline cannabinoid concentrations and cannabinoids stability in Quantisal™ collected OF: (A) Δ9-tetrahydrocannabinol (THC), (B) 11-nor-9-carboxy-Δ9-tetrahydrocannabinol (THCCOOH), (C) Δ9-tetrahydrocannabivarin (THCV), (D) Cannabidiol (CBD) and (E) Cannabigerol (CBG). Median %baseline cannabinoid concentrations for occasional and frequent participants when stored 1, 2 and 3 months at 4°C; error bars depict ranges.
Figure 2.
Percentage of participant OF pools with concentrations remaining within ±20% of initial baseline concentrations after 1-3 months 4°C storage. Δ9-tetrahydrocannabinol (THC); 11-nor-9-carboxy-Δ9-tetrahydrocannabinol (THCCOOH); Δ9-tetrahydrocannabivarin (THCV); Cannabidiol (CBD) and Cannabigerol (CBG).
THC stability
THC concentrations were stable in OF collected with the Quantisal™ device in 7 frequent and 8 occasional users for 3 months at 4°C, except for samples from participant A, a frequent cannabis smoker, whose stability results were just below 80% (79.9%) at 3 months (Fig. 1A).
THCCOOH stability
In OF collected with the Quantisal™ device, THCCOOH concentrations were stable in all frequent users for 1 and 2 months and in 6/7 frequent users for 3 months at 4°C. For participant A, median %baseline concentration was 73% at 3 months (Fig. 1B) but 107% baseline concentration for both 1 and 2 months. THCCOOH OF concentrations were within 90.1-110.7% baseline for 1, 2 and 3 months in 2 occasional users with measureable baseline THCCOOH.
THCV stability
In OF collected with the Quantisal™ device, THCV concentrations were stable in all frequent users for 1 and 2 months and in 5/6 users for 3 months at 4°C. THCV OF was 76.8% baseline after 3 months at 4°C for frequent user A (Fig.1C). One frequent user's OF had baseline THCV concentration <LOQ. THCV was stable in the 2 occasional users’ OF with baseline concentrations >LOQ for 1, 2 and 3 months with %baseline concentrations of 96.1-99.3%, 96.3-101% and 95.3-96.1%, respectively.
CBD stability
CBD baseline concentrations were stable in 6 frequent users’ OF for 1 and 2 months and in 5/6 participants for 3 months at 4°C. CBD was 78.8% baseline after 3 months at 4°C for frequent user A (Fig.1D). CBD was stable in 3 occasional users whose baseline concentrations were >LOQ for 1, 2 and 3 months with %baseline concentrations of 98.8-109%, 93.1-110% and 94.9-114%, respectively.
CBG stability
CBG was stable in all frequent users’ OF for 1 and 2 months and in 6/7 participant pools for 3 months at 4°C. CBG was 77.2% baseline for participant A, after 3 months at 4°C (Fig.1E). Three occasional users had stable CBG OF concentrations for 1, 2 and 3 months with %baseline concentrations of 95.7-110%, 89.9-112% and 89.7-112%, respectively.
DISCUSSION
We present cannabinoid stability data in authentic OF samples stored at 4°C following controlled cannabis smoking. Individual pooled OF samples collected with the Quantisal™ device from 7 frequent and 8 occasional cannabis smokers permitted evaluation of inter-subject stability variability while stored at 4°C for 1, 2 and 3 months in OF. This study has a wide range of baseline OF cannabinoid concentrations. Baseline OF concentrations for THC were 7.7-39.2 μg/L and 1.9-67 μg/L for frequent and occasional users, respectively (Figure 1-A), while THCCOOH concentrations were 30-380 ng/L and 40-90 ng/L for frequent and occasional users, respectively (Figure 1-B). THC and THCCOOH baseline concentrations are similar to those observed during our previous cannabinoids stability studies4, 5.
Overall, all analytes were stable for up to 2 months at 4°C for all participants with positive baseline concentrations. Analytes were considered stable if the concentration change was less than ±20% of baseline to account for analytical imprecision9. We only observed instability issues for one frequent user after 3 months storage with decreases of −20.1, −27.0, −23.2, −21.2 and −22.8% baseline for THC, THCCOOH, THCV, CBD and CBG, respectively. Baseline concentrations for this participant fell within those of other participants. No instability (>±20%) was observed for any analyte in any other of the participants for up to 3 months at 4°C.
Blood is the most common matrix in driving under the influence of drugs (DUID) cases due to the good correlation between this matrix's drug concentrations and pharmacological effects10. OF correlates better to blood than urine and is a valid alternative matrix for DUID testing11. Langel et al suggested that instead of analyzing THC alone, multiple cannabinoids should be monitored to identify recent use10, as we previously suggested for blood and oral fluid cannabinoids6-8, 12. Measuring multiple cannabinoids and metabolites is important; THCCOOH presence unequivocally documents that drug exposure was not from environmental exposure, since THCCOOH is only formed in vivo and is not present in cannabis smoke13. Analysis of CBN and CBD in OF were suggested as differentiating between residual cannabinoid excretion and recent drug use14, 15, although, these plant constituents’ concentrations vary based upon cannabis plant cultivation16. We hypothesize that CBG may also provide value for identifying recent cannabis intake. THCV is a cannabis plant cannabinoid that was targeted for distinguishing illegal cannabis use from synthetic THC administration17.
Choice of OF collection device may greatly impact cannabinoid concentrations in oral fluid. The elution and stabilization buffer effects drug recovery from the collection pad5, 18, may prevent cannabinoid degradation by stabilizing OF pH or inhibiting enzymatic degradation19, and may retain analytes in solution by reducing adsorption to collection tube surfaces and/or precipitants5, 18. We previously observed differing stability results for various OF devices, with StatSure™ OF samples being stable for 24 weeks at −20°C, while Quantisal™ and Oral-Eze® cannabinoid concentrations were unstable under these conditions4, 5; therefore, extrapolating our current Quantisal™ stability observations to other OF collection devices is not suggested.
In general, frequent and occasional users’ OF cannabinoid concentrations collected with the Quantisal™ device were stable for 3 months at 4°C (Figure 2). In total, THC, THCCOOH, THCV, CBD and CBG were stable for up to 3 months for pooled positive specimens from 14/15, 8/9, 7/8, 8/9, 9/10 participants, respectively (Figure 1). There was no distinct difference in OF stability between frequent and occasional users for THC. However, OF baseline concentrations were generally lower for occasional users with only 2/8, 2/8, 3/8 and 3/8 pooled positive samples from participants containing baseline concentrations >LOQ for THCCOOH, THCV, CBD and CBG, respectively, making comparisons between frequent and occasional users less robust for these analytes. Participant A had greater than ±20% instability for all analytes beyond 2 months. Participant A's mean OF specimen concentrations decreased 20.1, 27.0, 23.2, 21.2 and 22.8% after 3 months for THC, THCCOOH, THCV, CBD and CBG, respectively. This inter-individual effect may depend on Participant A's OF pH or enzyme activity.
CONCLUSION
Quantisal™-collected OF specimens should be stored at 4°C for no more than 2 months to assure accurate quantitative results for THC, THCCOOH, THCV, CBD and CBG. However, if only THC is monitored, OF results were within 79% of baseline at 3 months for all participants. These data clarify OF cannabinoid stability and should be helpful for clinical and forensic drug testing laboratories in timing analyses and assisting interpretation of results for court/defense requested OF sample re-analyses after 1-3 months storage. Given the inconsistency of cannabinoids storage stability reported for other devices3-5, we caution extrapolating our findings for Quantisal™-collected OF to other collection devices.
ACKNOWLEDGEMENT
The research was supported by the Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health. A Material Transfer Agreement with Immunalysis Inc., provided the Quantisal™ collection devices for the study, but Immunalysis Inc. had no role in study design, data analysis or presentation of results.
REFERENCES
- 1.Substance Abuse and Mental Health Services Administration Mandatory Guidelines for Federal Workplace Testing Programs. Federal Register. 2015;80:28054–28101. [Google Scholar]
- 2.Lee D, Huestis MA. Current knowledge on cannabinoids in oral fluid. Drug Test Anal. 2014;6:88–111. doi: 10.1002/dta.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lund HM, Oiestad EL, Gjerde H, Christophersen AS. Drugs of abuse in oral fluid collected by two different sample kits--stability testing and validation using ultra performance tandem mass spectrometry analysis. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:3367–3377. doi: 10.1016/j.jchromb.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Lee D, Milman G, Schwope DM, Barnes AJ, Gorelick DA, Huestis MA. Cannabinoid stability in authentic oral fluid after controlled cannabis smoking. Clin Chem. 2012;58:1101–1109. doi: 10.1373/clinchem.2012.184929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anizan S, Bergamaschi MM, Barnes AJ, Milman G, Desrosiers N, Lee D, Gorelick DA, Huestis MA. Impact of oral fluid collection device on cannabinoid stability following smoked cannabis. Drug Test Anal. 2015;7:114–120. doi: 10.1002/dta.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desrosiers NA, Scheidweiler KB, Huestis MA. Quantification of six cannabinoids and metabolites in oral fluid by liquid chromatography-tandem mass spectrometry. Drug Test Anal. 2015;7:684–694. doi: 10.1002/dta.1753. [DOI] [PubMed] [Google Scholar]
- 7.Lee D, Vandrey R, Mendu DR, Murray JA, Barnes AJ, Huestis MA. Oral fluid cannabinoids in chronic frequent cannabis smokers during ad libitum cannabis smoking. Drug Test Anal. 2015;7:494–501. doi: 10.1002/dta.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newmeyer MN, Desrosiers NA, Lee D, Mendu DR, Barnes AJ, Gorelick DA, Huestis MA. Cannabinoid disposition in oral fluid after controlled cannabis smoking in frequent and occasional smokers. Drug Test Anal. 2014;6:1002–1010. doi: 10.1002/dta.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peters FT, Drummer OH, Musshoff F. Validation of new methods. Forensic Sci Int. 2007;165:216–224. doi: 10.1016/j.forsciint.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 10.Langel K, Gjerde H, Favretto D, Lillsunde P, Oiestad EL, Ferrara SD, Verstraete AG. Comparison of drug concentrations between whole blood and oral fluid. Drug Test Anal. 2014;6:461–471. doi: 10.1002/dta.1532. [DOI] [PubMed] [Google Scholar]
- 11.Musshoff F, Hokamp EG, Bott U, Madea B. Performance evaluation of on-site oral fluid drug screening devices in normal police procedure in Germany. Forensic Sci Int. 2014;238:120–124. doi: 10.1016/j.forsciint.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Schwope DM, Karschner EL, Gorelick DA, Huestis MA. Identification of recent cannabis use: whole-blood and plasma free and glucuronidated cannabinoid pharmacokinetics following controlled smoked cannabis administration. Clin Chem. 2011;57:1406–1414. doi: 10.1373/clinchem.2011.171777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moore C, Coulter C, Uges D, Tuyay J, van der Linde S, van Leeuwen A, Garnier M, Orbita J., Jr. Cannabinoids in oral fluid following passive exposure to marijuana smoke. Forensic Sci Int. 2011;212:227–230. doi: 10.1016/j.forsciint.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 14.Anizan S, Milman G, Desrosiers N, Barnes AJ, Gorelick DA, Huestis MA. Oral fluid cannabinoid concentrations following controlled smoked cannabis in chronic frequent and occasional smokers. Anal Bioanal Chem. 2013;405:8451–8461. doi: 10.1007/s00216-013-7291-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee D, Milman G, Barnes AJ, Goodwin RS, Hirvonen J, Huestis MA. Oral fluid cannabinoids in chronic, daily Cannabis smokers during sustained, monitored abstinence. Clin Chem. 2011;57:1127–1136. doi: 10.1373/clinchem.2011.164822. [DOI] [PubMed] [Google Scholar]
- 16.Wille SM, Baumgartner MR, Fazio VD, Samyn N, Kraemer T. Trends in drug testing in oral fluid and hair as alternative matrices. Bioanalysis. 2014;6:2193–2209. doi: 10.4155/bio.14.194. [DOI] [PubMed] [Google Scholar]
- 17.ElSohly MA, deWit H, Wachtel SR, Feng S, Murphy TP. Delta9-tetrahydrocannabivarin as a marker for the ingestion of marijuana versus Marinol: results of a clinical study. J Anal Toxicol. 2001;25:565–571. doi: 10.1093/jat/25.7.565. [DOI] [PubMed] [Google Scholar]
- 18.Crouch DJ. Oral fluid collection: the neglected variable in oral fluid testing. Forensic Sci Int. 2005;150:165–173. doi: 10.1016/j.forsciint.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 19.Samano KL, Anne L, Johnson T, Tang K, Sample RH. Recovery and Stability of Delta9-Tetrahydrocannabinol Using the Oral-Eze(R) Oral Fluid Collection System and Intercept(R) Oral Specimen Collection Device. J Anal Toxicol. 2015;39:648–654. doi: 10.1093/jat/bkv093. [DOI] [PubMed] [Google Scholar]