Abstract
Purpose
We describe 7 years of follow-up for the energy/vitality outcome in early-stage Hodgkin's disease patients treated on a randomized clinical trial that compared subtotal lymphoid irradiation (STLI) with combined modality treatment (CMT) (SWOG 9133). Survivorship research questions involved the extent to which symptoms/side effects endured over a follow-up period of 7 years for this early-stage patient group.
Methods
Two hundred thirty-nine patients participated in the quality of life (QOL) companion study (SWOG 9208) and completed the SF-36 vitality scale, SF-36 health perception item, Cancer Rehabilitation Evaluation System-Short Form (CARES-SF), and symptom distress scale. This paper reports vitality outcome results obtained from randomization, 6 months, and annually for 7 years. To assess changes in vitality over time, we used linear mixed models with patient as a random effect.
Results
Patients receiving CMT had lower observed vitality at 6 months than did the STLI patients (p < .0001). However, beginning at year 1, vitality results did not differ significantly by treatment over the 5-year (p = .13) and 7-year (p = .16) follow-up periods. Vitality only slightly improved over baseline in either group after treatment. The results were similar after accounting for patterns of recurrence and missing data.
Conclusions
This study demonstrated that patients with early-stage Hodgkin's disease experience a short-term (at 6 months) decrease in vitality with treatment, which is more severe with CMT, but that after the first year, vitality scores were similar between the two treatment groups. Enduring fatigue results for patients receiving these therapies were not observed.
Implications for cancer survivors
These data provide comprehensive 7-year follow-up vitality information, an important symptom for early-stage lymphoma survivors.
Keywords: Hodgkin's disease, Combined modality treatment, Quality of life, Symptoms, Vitality
Introduction
Fatigue has been identified as one of the adverse events of those individuals diagnosed with cancer [1, 2] as well as specific types of cancer such as Hodgkin's lymphoma (HL) [3, 4]. Cancer-related fatigue is “a distressing, persistent, subjective sense of physical, emotional, and/or cognitive tiredness or exhaustion related to cancer and/or cancer treatment that is not proportional to recent activity and interferes with usual functioning” (p. 1844) [2, 5]. This type of fatigue occurs in 60–96 % of treated cancer patients [6]. Moreover, fatigue has a greater impact on quality of life (QOL) than do other cancer-/treatment-related symptoms (such as nausea, pain, or depression) [3]. Aside from fatigue's physical impacts, it has important emotional, social, psychological, social, and financial effects for both patients and family members [3, 6]. Therefore, to understand the effect of treatment on QOL for patients with HL, a fatigue outcome is essential. The American Society of Clinical Oncology (ASCO) recently reported guidelines for monitoring cancer patient fatigue [2].
We previously reported SF-36 vitality scale results for the first 2 years of follow-up of patients registered to S9208 [7, 8], the QOL companion study to S9133 (Phase III Randomized Intergroup Trial of Subtotal Lymphoid irradiation Versus Doxorubicin, Vinblastine, and Subtotal Lymphoid Irradiation for Stage IA to IIA Hodgkin's Disease) [9]. We found that patients with early-stage disease had less vitality than the general population at baseline. Mean vitality scores were 5.8 points lower for males and 5.4 points lower for females than for similarly aged males and females from the general cancer population, but these differences were not statistically significantly different [7, 8, 10]. However, at 6 months after randomization, patients receiving the combined radiation/chemotherapy regimen reported statistically significantly less vitality and worse CARES-SF, symptom distress scale, and SF-36 health perception scores than did patients receiving radiation alone. STLI vitality levels were slightly lower than baseline levels at years 1 and 2 postrandomization; year 1 CMT vitality levels were similar to baseline levels but slightly higher at year 2 [8].
A literature review of HL survivors addressed associated factors, severity, and prevalence of prior to 2012 [11]; studies used a variety of fatigue measures including the SF-36 vitality scale. Comparisons with normative population data indicated a wide prevalence range for fatigue in patients with HL (11–76 %) versus the general population (10 %). The authors reported that higher levels of fatigue were reported by older patients in four of seven studies. Daniëls et al. [12] conducted a study of chronic fatigue in 180 HL survivors with a mean time of 4.6 years from diagnosis. These data indicated significant differences in fatigue level for HL survivors versus the normal population sample. In addition, the authors found an association between higher levels of fatigue and the presence of anxiety and depression. Heutte et al. reported results of two studies indicating the high prevalence of fatigue and its impact on activities of daily living; however, the authors also noted the more general effects of fatigue on QOL such as emotional and social functioning [13]. Although it is not clear what the cause of long-term fatigue might be, and what the underlying and contributing factors are, it is important to continue to monitor this important symptom with prospective, longitudinal studies [13, 14]. In this manuscript, we report fatigue results for 7 years of follow-up in a prospective randomized clinical trial comparing two different treatment strategies for early-stage HL. Data indicating long-term fatigue effects support the examination of the SWOG 5-year (primary longitudinal comparison) and 6/7-year (exploratory comparison) analyses provided in this manuscript.
Materials and methods
Study design
SWOG 9133 randomized patients with clinically staged stage IA, IEA, IIA, and IIEA HL. The trial excluded patients with unfavorable status (e.g., those with “B” symptoms). Patients with a SWOG/Zubrod performance status of 0 to 2 were eligible [9]. The two treatment arms were subtotal lymphoid irradiation (STLI) and combined-modality therapy (CMT) (chemotherapy plus radiation). Treatment regimens are described in more detail in Press et al. [9]. The companion QOL study, SWOG 9208, opened 19 months after the therapeutic trial was activated. Eligibility criteria for the QOL companion study in addition to criteria required for the therapeutic trial included that patients give informed consent, completed QOL forms in English, and provided the baseline QOL form at the time of trial registration. The human subjects' mechanism at each institution approved both SWOG 9133 and SWOG 9208. This report addresses vitality results from baseline to the last follow-up assessment at 7 years.
QOL assessment
Fatigue was assessed with the SF-36 vitality scale score [15–17], the dependent variable in this analysis; vitality was measured at baseline (prior to randomization), 6 months, and annually through 7 years. [7] The SF-36 vitality scale contains four items; each item has six response options (ranging from “all of the time” to “none of the time”). Results for all SF-36 scales are reported on a 0–100 scale, with higher scores reflecting better status (in this case, more vitality). Evaluable patients were required to be trial eligible and to have had a baseline vitality score.
Deng et al. [18] supported the unidimensionality of a vitality construct as measured by the SF-36 vitality scale. Bifactor [19] along with other model approaches were used to evaluate unidimensionality with an item bank composed of items from a number of vitality and fatigue measures. Of interest was the determination of whether a measure was essentially unidimensional defined when a general factor explained the majority of item variance; group factors were allowed [19]. Although Deng et al. [18] noted the need for additional research, they recommended that vitality (energy/fatigue) could be treated as unidimensional.
We included baseline levels of the following variables in the current longitudinal models: the physical score from the Cancer Rehabilitation Evaluation System-Short Form (CARES-SF) [20]; the symptom distress scale total score [21, 22]; the single-item health perception measure from the SF-36 [23]; and SWOG/Zubrod performance status [24].
Analytic plan
Submission rates were calculated based on the number of patients alive and on study long enough to reach each assessment time. T tests were used to identify potential differences in observed vitality scores at each assessment time by treatment group and sex.
Linear mixed models were used to analyze the effect of the treatment intervention on energy/vitality over time [25]. This approach accounts for correlation between observations with-in each patient. Each model included main effects for intervention assignment (CMT vs. STLI) and time. In addition, given that both the intercept and slope may be a function of the baseline vitality score, this score was included in the regression as a covariate. Including baseline vitality scores in the model is also supported by our prior observation that the baseline vitality scores for these patients were worse than levels reported by males and females of similar age from the general population [7, 8, 10]. This finding suggested an effect of disease on fatigue [7, 8]. The interaction between intervention and time was tested, as was a potential quadratic relationship of intervention over time. Interaction term and quadratic terms were assessed for model inclusion by comparing the log-likelihood differential between nested models, which is distributed chi-square with degrees of freedom equal to the difference in the number of variables between models. The clustering variable was patient, treated as a random effect.
Linear mixed models will give unbiased estimates if missing data are missing at random (MAR). Sensitivity to the MAR assumption was examined by using pattern mixture models as an alternative analytic approach. Pattern mixture models condition on the type of missingness pattern through covariate adjustment in the regression model and thereby allows modeling under not missing at random (NMAR) [26]. Fixed and random effects were included as noted above for the mixed model approach, along with a binary indicator variable as a covariate representing differential dropout patterns for patients with dropout at ≤3 years after registration vs. dropout >3 years after registration. The 3-year cut point was based on observed patterns. Both the main effect of the dropout pattern and its interaction with each of the fixed effects was included in the model.
Interpretation of model results
In the analysis of vitality, CMT treatment was coded as a 1, and STLI treatment was coded as a 0; time was coded as a continuous variable in years. The nature of the best fitting models is described as follows.
-
&
Linear model with no interaction: Where there is no interaction and a simple linear model represents the best model fit, the fitted models will be represented by parallel straight lines for the treatment arms, indicating a relationship between treatment and vitality that is constant over time. A positive coefficient would indicate that STLI treatment generated higher vitality scores than CMT did and a negative coefficient would indicate that CMT treatment generated higher vitality scores than STLI did. In addition, a positive coefficient for time would indicate increasing vitality scores for both arms as time increases, and a negative coefficient for time would indicate decreasing vitality scores as time increases.
-
&
Linear interaction model: Models with a statistically significant linear interaction term would indicate that the treatment effect for STLI vs. CMT was linear but varied over time, and the fitted regression lines would be nonparallel.
-
&
Quadratic model with no interaction: If the relationship between vitality scores and time follows a quadratic relationship, then the fitted model will be represented by curved lines. If there is no interaction of treatment and (quadratic) time, the curved lines will be parallel to each other.
-
&
Quadratic interaction model: If the relationship between vitality scores and time is quadratic and there is an interaction with treatment, then the curved fitted model regression lines representing the change in vitality scores over time would differ (i.e., be non-parallel) by STLI vs. CMT treatment.
Of note, in instances where there are significant interactions, the coefficients for treatment and time cannot be interpreted independently but must be considered in combination with the coefficients for the interaction terms. The prespecified primary assessment time point was at 5 years after randomization; however, models were also included using the entire 7 years of available follow-up data. Although the model-based analysis represents the definitive analysis, observed values are provided in the figures for illustration and comparison.
QOL follow-up assessment data were collected through May, 2010. All analyses were conducted in SAS®. The SAS® procedure “proc mixed” was used to conduct linear mixed models and pattern mixture models [27].
Results
Evaluable patients, response rates, and baseline characteristics
There were a total of N = 120 evaluable patients with QOL forms randomized to the CMT arm and N = 119 evaluable patients randomized to the STLI arm. Response rates for submission of the form containing the vitality scale items across both arms combined was 100 % at randomization (as required for study enrollment) and 82 % at 6 months; annual submission rates were 80 % (year 1), 69 % (year 2), 66 % (year 3), 61 % (year 4), 59 % (year 5), 51 % (year 6), and 46 % (year 7) (Online Resource 1). Sample sizes and response rates by arm are shown in Figure 1. Overall, the most common reasons for non-submission of forms at follow-up were patient refusal (45 %) and institution error (25 %) (Online Resource 1).
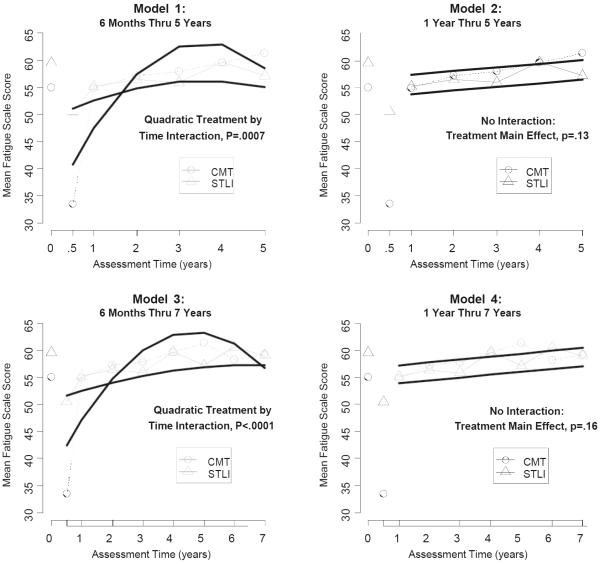
Fig. 1.
Fitted and observed mean energy/vitality scale scores by treatment over time. The upper left panel (Model 1) shows the results for vitality data from 6 months through 5 years, indicating a statistically significant quadratic treatment by time interaction (p = .0007). The upper right panel (Model 2) shows the results for vitality data from 1 year through 5 years. With the 6-month scores excluded, there was no evidence of a quadratic or linear treatment by time interaction, and there was no treatment effect over time (p = .13). The lower left panel (Model 3) shows the results for vitality data from 6 months through all 7 years of follow-up. Similar to the 6-month through 5-year data (Model 1), Model 3 showed a statistically significant quadratic treatment by time interaction (p < .0001). The lower right panel (Model 4) shows the results for vitality data from 1 year through 7 years. Similar to the 1-year through 5-year data (Model 2), with the 6-months scores excluded, there was no evidence of a quadratic or linear treatment by time interaction, and there was no treatment effect over time (p = .16)
Baseline characteristics are shown by intervention in Table 1. The proportion with performance status equal to one was higher on the CMT arm (9 vs. 4 %, p = .0001). There were no statistically significant differences by arm in the female proportion or in the mean baseline vitality, CARES-physical, or SDS scores. Other factors were well-balanced as shown in Table 1.
Table 1.
Baseline characteristics by treatment arm
| CMT (n = 120) | STLI (n = 119) | P value | |
|---|---|---|---|
| Sex | .94 | ||
| Female | 43 % | 42 % | |
| Male | 57 % | 58 % | |
| Mean age (range) | 35.1 (17–71) | 35.5 (18–85) | .78 |
| Performance status | .34 | ||
| 0 | 91 % | 94 % | |
| 1 | 9 % | 6 % | |
| 2 | 0 % | 0 % | |
| Involved lymph node sites | .85 | ||
| 0–1 | 33 % | 34 % | |
| ≥2 | 66 % | 66 % | |
| Histology | .97 | ||
| NS/LP | 81 % | 81 % | |
| Other | 19 % | 19 % | |
| High neck presentation | .62 | ||
| Yes | 77 % | 74 % | |
| No | 23 % | 26 % | |
| Type of disease | .62 | ||
| NED | 9 % | 8 % | |
| Bidimensionally measurable | 83 % | 82 % | |
| Assessable | 8 % | 11 % | |
| Lymphangiogram performed | .31 | ||
| Yes | 17 % | 22 % | |
| No | 83 % | 78 % | |
| Mean vitality total score | 55.0 | 59.5 | .13 |
| Mean CARES physical score | 0.54 | 0.52 | .74 |
| Mean SDS total score | 20.5 | 19.8 | .27 |
CMT combined-modality treatment, STLI subtotal lymphoid irradiation, NS nodular sclerosis, LP lymphocyte predominant, NED no evidence of disease, CARES Cancer Rehabilitation Evaluation System-Short Form, SDS symptom distress scale
Observed vitality scale scores
Table 2 shows observed vitality scale scores by intervention assignment and by sex over 7 years. By arm, observed mean vitality scale scores generally ranged between 50 and 60 across the entire range of follow-up with the exception of the 6-month assessment for CMT patients, where the mean vitality scale score was 33.4 and was much lower than the score for STLI patients (50.5; p < .0001).
Table 2.
Mean energy/vitality scale scores over time
| Mean energy/vitality scale score by intervention |
|||||||
|---|---|---|---|---|---|---|---|
| CMT (N = 120) |
STLI (N = 119) |
||||||
| Assessment time | N | Mean Observed (SD) | Fitteda | N | Mean Observed (SD) | Fitteda | p valueb |
| Baseline | 120 | 55.0 (22.5) | 119 | 59.5 (22.8) | .13 | ||
| 6 months | 97 | 33.4 (20.2) | 42.4 | 98 | 50.5 (22.3) | 51.6 | <.0001 |
| 1 year | 96 | 54.9 (21.2) | 47.2 | 95 | 55.2 (24.8) | 62.5 | .94 |
| 2 years | 80 | 57.2 (21.8) | 54.8 | 86 | 56.4 (23.3) | 54.0 | .82 |
| 3 years | 77 | 57.9 (23.8) | 60.0 | 80 | 55.9 (25.2) | 55.3 | .61 |
| 4 years | 67 | 59.6 (22.6) | 62.8 | 78 | 59.7 (23.5) | 56.2 | .99 |
| 5 years | 74 | 61.3 (21.3) | 63.2 | 67 | 57.2 (25.0) | 56.8 | .30 |
| 6 years | 59 | 58.2 (23.0) | 61.2 | 62 | 60.6 (20.1) | 57.2 | .55 |
| 7 years | 57 | 59.2 (20.3) | 56.8 | 52 | 59.0 (23.6) | 57.2 | .97 |
CMT combined modality treatment, STLI subtotal lymphoid irradiation
Fitted scores from model including a quadratic interaction term (Model 3) through year 7 are shown in columns 4 and 7
Reflects comparison of observed scores
Observed mean vitality scale scores by sex are also shown in Table 2 with results combined over treatment. Mean baseline vitality scale scores were somewhat higher for males and remained higher than scores for females through year 1. However, beyond year 1, no consistent pattern in vitality scores by sex was evident.
Linear mixed model results
Table 3 shows fitted model results according to four different models, distinguished by final assessment times (5 vs. 7 years) and the initial assessment time point included in the model (6 months vs. 1 year). The fitted results are plotted by treatment and time in Fig. 1 for models 1–4, along with the observed mean scale scores for vitality.
Table 3.
Model coefficients for longitudinal analysis
| 5 years of follow-up |
7 years of follow-up |
|||||||
|---|---|---|---|---|---|---|---|---|
| Model 1: 6 months thru 5 years |
Model 2: 1 year thru 5 years |
Model 3: 6 months thru 7 years |
Model 4: 1 year thru 7 years |
|||||
| Model term | Coefficient (95 % CI) | P value | Coefficient (95 % CI) | P value | Coefficient (95 % CI) | P value | Coefficient (95 % CI) | P value |
| Intercept | 52.59 (35.90 to 69.28) | <.0001 | 59.04 (41.42 to 76.65) | <.0001 | 55.61 (39.32 to 71.89) | <.0001 | 60.89 (43.70 to 78.08) | <.0001 |
| Treatment (main effect) | −16.58 (−23.69 to −9.47) | <.0001 | 3.59 (−1.05 to 8.24) | .13 | −13.51 (−19.65 to −7.37) | <.0001 | 3.28 (−1.24 to 7.81) | .16 |
| Time (main effect) | 3.60 (−0.23 to 7.94) | .07 | 0.66 (−0.12 to 1.44) | .10 | 2.00 (−0.43 to 4.43) | .11 | 0.53 (0.04 to 1.01) | .03 |
| Treatment by time (linear interaction) | 13.25 (7.43 to 19.06) | <.0001 | 9.22 (5.74 to 12.69) | <.0001 | ||||
| Time by Time (quadratic term) | −0.54 (−1.29 to 0.21) | .16 | −0.15 (−0.49 to 0.18) | .37 | ||||
| Treatment by time × time (quadratic interaction) | −1.84 (−2.91 to −0.78) | .0007 | −1.05 (−1.53 to −0.57) | <.0001 | ||||
| Baseline energy/vitality | 0.23 (0.10 to 0.35) | .0004 | 0.22 (0.09 to 0.36) | .002 | .22 (0.10 to 0.34) | .0006 | 0.21 (0.08 to 0.34) | .002 |
| Baseline PS | 1.07 (−7.83 to 9.97) | .81 | 0.58 (−9.19 to 10.36) | .91 | 1.55 (−7.25 to 10.34) | .73 | 0.92 (−8.67 to 10.51) | .85 |
| Baseline CARESPHYS | −4.13 (−10.48 to 2.22) | .20 | −5.32 (−12.43 to 1.80) | .14 | −3.73 (−9.97 to 2.52) | .24 | −4.73 (−11.65 to 2.20) | .18 |
| Baseline SDS | −0.70 (−1.29 to −0.12) | .02 | −0.79 (−1.42 to −0.15) | .02 | −0.77 (−1.35 to −0.20) | .009 | −0.84 (−1.47 to −0.22) | .009 |
PS performance status, CARESPHYS Cancer Rehabilitation Evaluation System-Short Form Physical score, SDS symptom distress scale
Model 1 includes follow-up from 6 months thru 5 years, emphasizing follow-up assessment times with response rates ranging from 100 % at baseline (both arms) to 62 % (CMT) and 56 % (STLI) at 5 years. Figure 1 shows how, at 6 months after registration, the observed mean scaled score for vitality on the CMT arm is much lower than that on the STLI arm. Model 1, in attempting to fit the 6-month assessment along with the other follow-up assessments, generates a statistically significant quadratic interaction term. The model fit suggests that estimates of vitality for CMT patients start out low but eventually plateau at a mean scaled vitality score of approximately 62, then drop off slightly by year 5. On the other hand, according to this model, estimates for vitality on the STLI arm start out higher than for patients on the CMT arm, increase only gradually over time, but by year 2 and beyond are somewhat lower than the modeled estimates on the CMT arm.
To assess the extent to which the inclusion of 6-month scores influenced the results, a secondary sensitivity analysis (Model 2) excluded the 6-month vitality scores and thus assessed the relative behavior of vitality scores over time beginning 1 year after study registration. The exclusion of the observed 6 month vitality scores resulted in a model fit with no statistically significant quadratic effects. Also, there was no evidence of a linear interaction of intervention and time. Therefore, the model fit is indicated by two parallel lines. As noted in Table 3, there was no evidence of a treatment effect (p = .13). Observed estimates of vitality scores for years 1 through 5 ranged from 55 to approximately 61.
Additionally, we analyzed data through the full 7 years of follow-up. Results are shown as models 3 and 4 in Table 3 and Fig. 1. Modeled patterns of vitality over time were generally similar: for the 6-month through 7-year data (Model 3), the strength of the quadratic interaction of treatment and time was similarly strong (p < .0001); for 1-year through 7-year data (Model 4), there was no evidence of a treatment by time interaction, and the strength of the treatment effect was very similar to data through 5 years (p = .16). The 7-year data also indicate a statistically significant increase in the vitality total score for both arms over time (p = .03).
Pattern mixture model results
We used cohort plots to assess whether patterns of vitality differed according to dropout time. Results indicated that patients with dropout at year 3 or earlier tended to report declining vitality scale scores prior to dropout, whereas patients with dropout at 4 years or later did not show this pattern prior to dropout. We constructed an indicator variable indicating time of dropout and included it as a covariate in the model. Results were consistent with results from the linear mixed models, with strong evidence of a treatment by quadratic time interaction for data from 6 months through 5 years (p < .0001). There was no evidence of an interaction or main effect of treatment for data from 1 year through 5 years (p = .18). There was repeated strong evidence of a treatment by quadratic time interaction for data from 6 months through 7 y ears (p < .0001), and there was no evidence of an interaction or main effect of treatment for data from 1 year through 7 years (p = .19) (Data not shown.)
Recurrence as time-dependent covariate
To each of the four linear mixed models, a time-dependent covariate representing recurrence was included. Recurrence was a highly significant predictor of vitality in each model and indicated a reduction in the vitality total score of ~8 points (Model 1, 8.7-point reduction, p = .006; Model 2, 8.4-point reduction, p = .01; Model 3, 7.5-point reduction, p = .006; Model 4, 7.5-point reduction, p = .009). However, the addition of recurrence did not substantially impact the results of the models with respect to treatment, time, or their interactions, perhaps due to its infrequent occurrence in this dataset (Data not shown). Indeed only 7 % of the observed vitality assessments across 7 years of follow-up occurred after a recurrence. Consistent with our main study results showing greater recurrence for STLI patients [9], more observed vitality assessments occurred after a recurrence on the STLI arm than on the CMT arm (10 vs. 4 %, p < .0001).
Discussion
S9208 study results suggest lower levels of vitality at baseline for both study arms compared to population normative data for the SF-36 vitality scale. At 6 months, we observed a substantial negative treatment impact on vitality for patients randomized to the chemotherapy plus radiation (CMT) arm; patients assigned to this treatment had a mean vitality score at 6 months that was 17 points lower than the mean score for patients receiving radiation alone. However, consistent with our data reported earlier [7, 8] for treatment arm comparisons at 1 and 2 years postrandomization, there was no treatment arm effect on vitality over 5 or 7 years of follow-up. Also, although recurrence was a statistically significant predictor of energy/vitality in both the 5- and 7-year models, adjustment for recurrence did not substantively change the results by treatment arm. Our longitudinal findings are strengthened by the similar results obtained with pattern mixture models; we added these analyses to examine the sensitivity of the results to missing data over this long follow-up period of 7 years.
A limitation of this report is the length of time from the start of the study in 1992 to the publication of these fatigue results. The treatment of early-stage HL has evolved considerably during this period [28–31], but there is still disagreement about the nature of these changes. For example, Radford et al. [31] favor a PET/CT-guided approach that eliminates radiotherapy from the regimen for the majority of patients with early-stage HL, whereas Raemaekers et al. [30] favor a combined modality approach including radiotherapy for this patient group. None of the four recent studies included QOL measures. Our data, given the 7 years of follow-up, are useful for gaining a better sense of the chronicity of disease- and treatment-related symptoms such as fatigue. As noted in the “Materials and methods” section, these data were not fully collected until 2010. This is the challenge of doing survivorship research, particularly when trying to provide patients with information about long-term effects of their disease and its various treatments.
Given the good performance status of the study population, we designed the study to evaluate the persistence of treatment effects on patients who were expected to become long-term survivors and therefore chose to follow patients for 7 years. Although baseline mean vitality scores for patients in both treatment arms were lower than those of the general population [], we found continued recovery of patient vitality across the 7 years of observation. Also, although there was a substantial effect of CMT on fatigue at 6 months, beginning at year 1, there was no evidence of a treatment effect on fatigue. Our 7-year data suggest that for good performance level, in patients treated with these two regimens, fatigue is not the long-term problem, and we speculated it might be after examining the first 2 years of follow-up in an earlier publication. [7, 8]
Conclusion
The important survivorship question in this study pertained to whether the short-term worsening of QOL associated with CMT would be offset by a lower recurrence rate and longer disease-free survival in that group, without sacrificing long-term functioning as represented here by vitality. The earlier published treatment outcomes from S9133 supported improved survival for the CMT arm, and the QOL companion study (S9208) results indicate that this occurred without sustained impairment in vitality over 7 years of observation. Fatigue is one of the most enduring and bothersome posttreatment sequelae in cancer survivors and was a major anticipated concern in Hodgkin's disease survivors [6, 13]. Based on the findings from this study, in early-stage, low-risk HL, patients who received CMT did not sacrifice QOL for improved survival outcomes. However, patients with recurrent disease (only 7 % of the vitality sample) were observed to have decrements in vitality most likely as a result of later treatment for recurrence; these decrements occurred significantly less frequently in the CMT treatment arm.
Supplementary Material
Acknowledgments
We thank the patients and research staff at SWOG and CALGB institutions for providing the QOL outcome data that allowed us to monitor for effects of treatment on vitality over a 7-year period.
Funding This investigation was supported by NIH/NCI NCTN grants CA180888, CA180819, CA180821, CA180801, CA180818, CA180834, CA180835, and CA180828; NIH/NCI NCORP grants CA189974, CA189954, CA189953, CA189952, CA189853, CA189854, CA189830, CA189872, CA189804, CA189957, and CA189808; and legacy grants NIH/NCI grants CA32102, CA38926, CA37429, CA46282, CA46368, CA22433, CA58415, CA13612, CA76447, CA46113, CA76132, CA58723, CA12644, CA35128, CA12213, CA52654, CA58658, CA35996, CA76429, CA16385, CA74647, CA76462, CA37981, CA58348, CA35119, and CA35262. Clinical trials registration identification number NCT00002563.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s11764-016-0559-y) contains supplementary material, which is available to authorized users.
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflicts of interest.
Ethical approval All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent Informed consent was obtained from all individual participants included in this study.
References
- 1.Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J Clin Oncol. 2000;18(4):743–53. doi: 10.1200/JCO.2000.18.4.743. [DOI] [PubMed] [Google Scholar]
- 2.Bower JE, Bak K, Berger A, Breitbart W, Escalante CP, Ganz PA, et al. Screening, assessment, and management of fatigue in adult survivors of cancer: an American Society of Clinical oncology clinical practice guideline adaptation. J Clin Oncol. 2014;32(17):1840–50. doi: 10.1200/JCO.2013.53.4495. doi:10.1200/JCO.2013.53.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roper K, McDermott K, Cooley ME, Daley K, Fawcett J. Health-related quality of life in adults with Hodgkin's disease: the state of the science. Cancer Nurs. 2009;32(6):E1–17. doi: 10.1097/NCC.0b013e3181aa4a33. doi:10.1097/NCC.0b013e3181aa4a33. [DOI] [PubMed] [Google Scholar]
- 4.Oerlemans S, Mols F, Nijziel MR, Lybeert M, van de Poll-Franse LV. The impact of treatment, socio-demographic and clinical characteristics on health-related quality of life among Hodgkin's and non-Hodgkin's lymphoma survivors: a systematic review. Ann Hematol. 2011;90(9):993–1004. doi: 10.1007/s00277-011-1274-4. doi:10.1007/s00277-011-1274-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Network NCC . NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Survivorship. NCCN; Fort Washington: [Accessed 14 March 2013]. 2013. http://www.nccn.org/about/news/newsinfo.aspx?NewsID=333. [Google Scholar]
- 6.Stasi R, Abriani L, Beccaglia P, Terzoli E, Amadori S. Cancer-related fatigue: evolving concepts in evaluation and treatment. Cancer. 2003;98(9):1786–801. doi: 10.1002/cncr.11742. doi:10.1002/cncr.11742. [DOI] [PubMed] [Google Scholar]
- 7.Ganz PA, Moinpour CM, Pauler DK, Kornblith AB, Gaynor ER, Balcerzak SP, et al. Health status and quality of life in patients with early-stage Hodgkin's disease treated on Southwest Oncology Group Study 9133. J Clin Oncol. 2003;21(18):3512–9. doi: 10.1200/JCO.2003.01.044. doi:10.1200/JCO.2003.01.044. [DOI] [PubMed] [Google Scholar]
- 8.Author Corrections. J Clin Oncol. 2013;31(17):2231–2. Authors. [Google Scholar]
- 9.Press OW, LeBlanc M, Lichter AS, Grogan TM, Unger JM, Wasserman TH, et al. Phase III randomized intergroup trial of subtotal lymphoid irradiation versus doxorubicin, vinblastine, and subtotal lymphoid irradiation for stage IA to IIA Hodgkin's disease. J Clin Oncol. 2001;19(22):4238–44. doi: 10.1200/JCO.2001.19.22.4238. [DOI] [PubMed] [Google Scholar]
- 10.Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 health survey: manual and interpretation guide. The Health Institute, New England Medical Center; Boston: 1993. New England Medical Center Hospital. Health Institute. [Google Scholar]
- 11.Daniëls LA, Oerlemans S, Krol AD, van de Poll-Franse LV, Creutzberg CL. Persisting fatigue in Hodgkin lymphoma survivors: a systematic review. Ann Hematol. 2013;92(8):1023–32. doi: 10.1007/s00277-013-1793-2. doi:10.1007/s00277-013-1793-2. [DOI] [PubMed] [Google Scholar]
- 12.Daniëls LA, Oerlemans S, Krol AD, Creutzberg CL, van de Poll-Franse LV. Chronic fatigue in Hodgkin lymphoma survivors and associations with anxiety, depression and comorbidity. Br J Cancer. 2014;110(4):868–74. doi: 10.1038/bjc.2013.779. doi:10.1038/bjc.2013.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heutte N, Flechtner HH, Mounier N, Mellink WA, Meerwaldt JH, Eghbali H, et al. Quality of life after successful treatment of early-stage Hodgkin's lymphoma: 10-year follow-up of the EORTC-GELA H8 randomised controlled trial. Lancet Oncol. 2009;10(12):1160–70. doi: 10.1016/S1470-2045(09)70258-X. doi:10.1016/S1470-2045(09)70258-X. [DOI] [PubMed] [Google Scholar]
- 14.Evens AM, Wagner LI. Curing Hodgkin's lymphoma: quantity and quality. Lancet Oncol. 2009;10(12):1134–5. doi: 10.1016/S1470-2045(09)70339-0. doi:10.1016/S1470-2045(09)70339-0. [DOI] [PubMed] [Google Scholar]
- 15.Ware JE., Jr The SCD, MOS. 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–83. [PubMed] [Google Scholar]
- 16.McHorney C, Ware JE., Jr The RA, MOS. 36-item short-form health survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31(3):247–63. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 17.McHorney CA, Ware JE, Jr, Lu JF. The SCD, MOS. 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;32(1):40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Deng N, Guyer R, Ware JE., Jr Energy, fatigue, or both? A bifactor modeling approach to the conceptualization and measurement of vitality. Qual Life Res. 2015;24(1):81–93. doi: 10.1007/s11136-014-0839-9. doi:10.1007/s11136-014-0839-9. [DOI] [PubMed] [Google Scholar]
- 19.Reise SP, Morizot J, Hays RD. The role of the bifactor model in resolving dimensionality issues in health outcomes measures. Qual Life Res. 2007;16(Supplement 1):19–31. doi: 10.1007/s11136-007-9183-7. doi:10.1007/s11136-007-9183-7. [DOI] [PubMed] [Google Scholar]
- 20.Schag CAC, Ganz PA, Heinrich RL. Cancer Rehabilitation Evaluation System—Short Form (CARES-SF): a cancer specific rehabilitation and quality of life instrument. Cancer. 1991;68:1406–13. doi: 10.1002/1097-0142(19910915)68:6<1406::aid-cncr2820680638>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 21.McCorkle R, Benoliel JQ. Symptom distress, current concerns, and mood disturbance after diagnosis of life threatening disease. Soc Sci Med. 1983;17:431–8. doi: 10.1016/0277-9536(83)90348-9. [DOI] [PubMed] [Google Scholar]
- 22.McCorkle R, Cooley ME, Shea JA. A user's manual for the symptom distress scale. University of Pennsylvania School of Nursing; Philadelphia: 1998. [Google Scholar]
- 23.Ware JE., Jr Monitoring and evaluating health services. Med Care. 1985;23(5):705–9. [PubMed] [Google Scholar]
- 24.Zubrod CG, Schneiderman M, Frei E, Brindley C, Gold GL, Shnider B, et al. Appraisal of methods for the study of chemotherapy in man: comparative therapeutic trial of nitrogen mustard and thiophosphoramide. J Chronic Diseases. 1960;11:7–33. [Google Scholar]
- 25.Breslow NE, Clayton DG. Approximate Inference in generalized linear mixed models. J Am Stat Assoc. 1993;88(421):9–25. doi:10.2307/2290687. [Google Scholar]
- 26.Little RJA, Wang YX. Pattern-mixture models for multivariate incomplete data with covariates. Biometrics. 1996;52(1):98–111. doi:10.2307/2533148. [PubMed] [Google Scholar]
- 27.SAS Institute Inc . Base SAS® 9.4 Procedures Guide: Statistical Procedures. 2nd edition NC2013; Cary: [Google Scholar]
- 28.Eich HT, Diehl V, Görgen H, Pabst T, Markova J, Debus J, et al. Intensified chemotherapy and dose-reduced involved-field radiotherapy in patients with early unfavorable Hodgkin's lymphoma: final analysis of the German Hodgkin Study Group HD11 trial. J Clin Oncol. 2010;28(27):4199–206. doi: 10.1200/JCO.2010.29.8018. doi:10.1200/JCO.201029.8018. [DOI] [PubMed] [Google Scholar]
- 29.Engert A, Plütschow A, Eich HT, Lohri A, Dörken B, Borchmann P, et al. Reduced treatment intensity in patients with early-stage Hodgkin's lymphoma. New Engl J Med. 2010;363(7):640–52. doi: 10.1056/NEJMoa1000067. [DOI] [PubMed] [Google Scholar]
- 30.Raemaekers JMM, André MPE, Federico M, Girinsky T, Oumedaly R, Brusamolino E, et al. Omitting radiotherapy in early positron emission tomography-negative stage I/II Hodgkin lymphoma is associated with an increased risk of early relapse: clinical results of the preplanned interim analysis of the randomized EORTC/LYSA?FIL H10 trial. J Clin Oncol. 2014;32(12):1188–94. doi: 10.1200/JCO.2013.51.9298. doi:10.1200/JCO.2013.51.9298. [DOI] [PubMed] [Google Scholar]
- 31.Radford J, Illidge T, Counsell N, Hancock B, Pettengell R, Johynson P, et al. Results of a trial of PET_directed therapy for early-stage Hodgkin's lymphoma. N Engl J Med. 2015;372(17):1598–607. doi: 10.1056/NEJMoa1408648. doi:10.1056/NEJMoa1408648. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.