Abstract
Background
Zika virus (ZIKV) was first detected in Brazil in May 2015 and the country experienced an explosive epidemic. However, recent studies indicate that the introduction of ZIKV occurred in late 2013. Cases of microcephaly and deaths associated with ZIKV infection were identified in Brazil in November, 2015.
Objectives
To determine the etiology of three fatal adult cases.
Study design
Here we report three fatal adult cases of ZIKV disease. ZIKV infection in these patients was confirmed by cells culture and/or real-time reverse transcriptase polymerase chain reaction (RT-qPCR) and by antigen detection using immunohistochemical assay. Samples of brain and other selected organs taken at autopsy from three patients were also analyzed by histopathological and immunohistological examination.
Results
The first patient, a 36-year-old man with lupus and receiving prednisone therapy, developed a fulminant ZIKV infection. At autopsy, RT-qPCR of blood and tissues was positive for ZIKV RNA, and the virus was cultured from an organ homogenate. The second patient, a previously healthy female, 16 years of age, presented classic symptoms of Zika fever, but later developed severe thrombocytopenia, anemia and hemorrhagic manifestations and died. A blood sample taken on the seventh day of her illness was positive RT-PCR for ZIKV RNA and research in the serum was positive for antinuclear factor fine speckled (1/640), suggesting Evans syndrome (hemolytic anemia an autoimmune disorder with immune thrombocytopenic purpura) secondary to ZIKV infection. The third patient was a 20-year-old woman hospitalized with fever, pneumonia and hemorrhages, who died on 13 days after admission. Histopathological changes were observed in all viscera examined. ZIKV antigens were detected by immunohistochemistry in viscera specimens of patients 1 and 3. These three cases demonstrate other potential complications of ZIKV infection, in addition to microcephaly and Guillain-Barre syndrome (GBS), and they suggest that individuals with immune suppression and/or autoimmune disorders may be at higher risk of developing severe disease, if infected with ZIKV.
Keywords: Zika virus, Erythematous lupus, Evans syndrome, Autoimmune disorders, Immunohistochemistry, Histopathology
1. Background
Zika virus (ZIKV) is an emerging mosquito-borne pathogen belonging to the family Flaviviridae, genus Flavivirus. ZIKV was first isolated in 1947 from the blood of a sentinel monkey during studies of sylvan yellow fever in Uganda [1]. During the next 60 years, sporadic isolations of ZIKV were made from mosquitoes and from humans in various countries in sub-Saharan Africa and Southeast Asia. The clinical manifestations of reported human cases of ZIKV infection during this period were relatively mild: a self-limited illness of 3–5 days duration, characterization by fever, myalgia, headache, conjunctivitis and rash. Given the clinical similarity of ZIKV infection to dengue fever and other human pathogenic flaviviruses endemic or epidemic in Africa and Southeast Asia and the extensive cross-reactivity of ZIKV antibodies with these other flaviviruses, it seems likely that many cases of ZIKV infection were unrecognized or misdiagnosed in the past. But in general, ZIKV has not been considered to be a dangerous pathogen or a serious public health threat.
This perception changed in 2007, when a ZIKV outbreak occurred on Yap island in Micronesia [2]. Subsequent outbreaks occurred on a number of other small island nations in the South Pacific, culminating in a large outbreak in French Polynesia in 2013–2014 [2,3]. An estimated 28,000 cases occurred during the French Polynesian outbreak (approximately 11% of the population); and for the first time, cases of Guillain-Barre syndrome (GBS), an autoimmune disease causing acute or subacute flaccid paralysis, was observed in patients following ZIKV infection [3]. In early 2015, ZIKV was detected in Northeast Brazil and the virus rapidly spread to other regions of the country. The epidemic has continued to spread and by May 2016, autochthonous transmission of ZIKV had been reported in 42 countries and territories in the Region of the Americas [4]. Associated with the ZIKV epidemic, there has been a dramatic increase in the incidence of microcephaly, as well as many cases of GBS. The purpose of this report is to describe three fatal cases of ZIKV infection, none of which had microcephaly or GBS, suggesting that additional severe clinical presentations and pathologies may be associated with ZIKV infection.
2. Study design
2.1. Case reports
2.1.1. Case 1
A 36-year-old butcher from São Luís, Maranhão State, northeast Brazil. His relevant medical history included past alcohol abuse, rheumatoid arthritis and erythematous lupus (EL). He had been taking prednisone, 50 mg/day, for two years for EL. His current illness began suddenly with fever, malaise, nausea, vomiting, diarrhea and dyspnea. He visited a local first aid unit, where was given acetaminophen, and sent home. Two days later, he returned to an emergency care unit (ECU) complaining of intense abdominal pain, difficulty walking and shortness of breath. The clinical impression at that time was that the patient had severe dengue, and he was admitted to the ECU. He died within a few hours of respiratory distress. An intra-cardiac blood sample as well as fragments of brain, spleen and liver and a pool of heart, lung and kidney were obtained at autopsy and frozen at −70 °C for virologic studies (virus isolation [5] and RT-qPCR [6,7]). Other tissue samples were formalin-fixed for histopathologic and immunohistochemical examination, using protocols published before [8–10].
2.1.2. Case 2
A previously healthy 16-year-old female from Benevides, Para State, northern region. She presented at a local ECU with a history of fever, rash, malaise, headache and generalized myalgia of 7 days duration. A blood sample was taken during her initial visit and was submitted for testing, and the patient was sent home. Her condition worsened and she returned several days later, because of hemorrhagic phenomena: epistaxis, bleeding gums, hematuria, vaginal bleeding and cutaneous ecchymoses. She was transferred to a tertiary hospital where her laboratory findings on admission were hemoglobin 7.9 g/dL, platelets 8000/mm3, white blood count 10,600/uL, bilirubin (total 2.3 mg/dL; indirect 1.6 mg/dL), and lactic acid dehydrogenase 1229 u/L. Physical examination on admission to the hospital revealed an afebrile, alert but lethargic, well oriented young woman with moderate jaundice and ecchymoses and petechiae on her trunk and extremities. Cardiopulmonary auscultation was normal except for tachycardia (150 bpm). Slight (level-1) hepatosplenomegaly was detected but no edema. Because of her hemorrhages, anemia and thrombocytopenia, the patient received 5 units of platelets and several units of whole blood during her hospitalization; but her condition deteriorated, and she died of hypovolemic shock 25 days after onset of her illness. No autopsy was done, but studies were performed on samples of blood and serum for arboviruses (DENV, CHIKV, YFV, ZIKV), Chagas disease, syphilis, HIV-1 and –2, HTLV I/II and viral hepatitis (A, B and C). Further, aspiration of bone marrow was performed for myelogram, and markers for antibody research of autoimmune disease (antinuclear factor, anti-RNP, anti-Sm, anti-Ro/SS-A, anti-La/SS-B and anti-DNA native).
2.1.3. Case 3
A 20-year-old woman from a rural area of the municipality of Serrinha, State of Rio Grande do Norte, northeast region, was admitted to a tertiary hospital with fever, dry cough, chest pain and dyspnea. She also presented with oral hemorrhages. Hemogram showed pancytopenia (500 neutrophils per u/L) and low platelets (100,000 per u/L). A chest X-ray revealed a diffuse bilateral pulmonary infiltrate. Thirteen days after admission, the patient died. During the autopsy, bilateral pulmonary abcesses were observed, and specimens of lung, kidney and liver were collected for histopathological exam.
3. Laboratory tests
3.1. Real time RT-PCR (RT-qPCR) and virus isolation
Virus isolation attempts were done in cultures of the C6/36 clone of Aedes albopictus cells [5]. Briefly, blood and tissue fragments were triturated in phosphate-buffered saline (PBS) containing 10% fetal bovine serum (FBS) and antibiotics. After 10 min of centrifugation at 2100 × g at 4 °C, 100uL of the supernatant was inoculated into a 25-cm2 tissue culture flask with a monolayer of the mosquito cells. After incubation for 2 h at 28 °C, medium was added and the cells were incubated at ambient temperature (~25 °C) for 10 days. The cells were initially examined by indirect immunofluorescent assay (IFA), using a flavivirus group hyperimmune polyclonal mouse antibody. Subsequently the IFA-positive cultures were tested by RT-qPCR, as described previously [6,7].
Supernatants of tissue homogenates and serum samples were used for RNA extraction using Trizol Plus RNA Extraction kit (Ambion) according to manufacturer’s instructions. For Real Time PCR (RT-qPCR) reactions were carried out in 7500 Real Time PCR System (Applied Biotechnologies) using Superscript III Platinum One-Step RT-qPCR kit (Invitrogen) and two different primer/probe sets targeting both NS5 [6] and E [7] regions of ZIKV genome.
Viral RNA of case 1 was extracted using the QI Amp Viral RNA Mini Kit (Qiagen) and amplified using a next generation platform (Ion Torrent), following the manufacturer’s instructions and recommendations of a previously published protocol [11].
3.2. Histopathology and immunohistochemistry
Tissue samples were processed for histopathology using paraffin block inclusion and the hematoxylin and eosin (H&E) method [8–10] and an adapted immunohistochemical Streptavidin Alkaline Phosphatase (SAAP) assay [12–14] and stained using an anti-ZIKV polyclonal antibody serum.
4. Results
4.1. Case 1
4.1.1. RT-qPCR and virus isolation
All samples (blood and tissues) were positive for Zika virus by RT-qPCR. Zika virus was also isolated from the sample of pooled viscera (heart, lung and kidney) but not from blood or fragments of brain, liver and spleen. Blood and tissue samples of this case were negative in molecular tests done for dengue, chikungunya, St. Louis encephalitis, West Nile and yellow fever viruses as well as rotavirus and viral hepatitis A, B and C. No serologic test was done due the absence of serum sample.
4.1.2. Histopathology
4.1.2.1. Brain
Neuronal necrosis of basal nuclei in the brain was observed together with intense edema in the white matter as well as focal gliosis, neuronophagia, and perivascular hemorrhages. In the cortex, an inflammatory infiltrate consisting predominantly of mononuclear cells was seen. The conclusion was encephalopathy with intense edema, characteristic of viral encephalitis (Fig. 1A–C).
Fig. 1.

Histopathological aspects of Case 1. Fig. 1A–C – Brain showing neuronal necrosis especially in basal nuclei, edema, gliosis, neuronophagia and perivascular hemorrhage. Fig. 1D and E – Liver with cellular edema, lytic necrosis (circle), macro and micro steatosis (rectangles) and acidophilic bodies (arrows) associated with mononuclear inflammatory response. Fig. 1F – Spleen with congestion and hemorrhage. Fig. 1G – Heart with interstitial mononuclear cell infiltration, edema and fibrosis of undetermined infarct. Fig. 1H – Lung shows edema, congestion and hemorrhage (HE, Magnification: ×40).
4.1.2.2. Liver
Necrotic lesions and acidophilic bodies (similar to Councilman bodies) were seen especially in the midzone (Zone 2 of Rappapart), generally accompanied by multifocal steatosis. The portal space exhibited inflammatory infiltrates and vascular congestion. The microscopic picture was suggestive of viral hepatitis (Fig. 1D and E).
4.1.2.3. Spleen
Multiple hemorrhagic foci, vascular congestion and arteriolar hyalinosis were seen (Fig. 1F).
4.1.2.4. Heart
Interstitial edema and fibrosis with rare focal hemorrhages and inflammatory cells and vascular congestion were observed (Fig. 1G).
4.1.2.5. Lung
Intense edema and focal hemorrhages with much vascular congestion were observed (Fig. 1H).
4.1.2.6. Kidney
The most important findings were acute membranous proliferative glomerulonephritis and acute tubular necrosis with focal hemorrhages (not shown).
4.1.3. Immunohistochemistry
Large amounts of ZIKV antigen were observed in the brain, and sporadic ZIKV antigens were found in liver, kidney, heart and lung (Fig. 2). Viral antigens were more frequently observed in apoptotic cells, but were also found in cells with apparent normal morphology. Immunohistochemical analysis for dengue and yellow fever viral antigens, using the respective monoclonal antibodies, was negative.
Fig. 2.

Immunohistochemistry of Case 1. Fig. 2A–C – Areas expressing ZIKV antigens in the brain, especially in the neuronal bodies with (arrows) and without (arrow heads) nucleus and proximal dendrites. Fig. 2D – ZIKV antigens observed in pulmonary alveoli (ellipses). Fig. 2E – ZIKV antigens observed in myocardial tissue (rectangles). Fig. 2F and G – ZIKV antigens observed in hepatocytes (rectangle). Fig. 2H and I – ZIKV antigens in epithelia of renal tubules (circles) (IHC SAAP, Magnification: ×40, except Fig C – IHC SAAP, Magnification: ×100).
4.2. Case 2
4.2.1. RT-qPCR, virus isolation, myelogram and autoimmune disease markers
The sample was positive for ZIKV RNA by RT-qPCR, but negative upon culture in C6/36 cells. No autopsy was done on this patient, when she died 25 days after onset of her febrile illness. Serologic tests (IgM-ELISA) done on a serum sample taken during the patient’s first clinic visit (day 7 of illness) and tested for zika, Dengue, yellow fever, Saint Louis encephalitis, West Nile and chikungunya viruses, were negative (absence of detecting of specific IgM), as well as to Chagas disease, syphilis, HIV-1 and –2, HTLV I/II, and viral hepatitis A, B and C.
The myelogram showed hypercellularity with preserved cell maturation without evidence of anomalous elements of tissue. Anti-nuclear factor was the only reagent marker for nuclear fine speckled pattern (1/640); all other markers investigated were negative (anti-RNP, anti-Sm, anti-Ro/SS-A, anti-La/SS-B and anti-DNA native).
4.3. Case 3
4.3.1. RT-qPCR and virus isolation
The tissue samples (liver, kidney and lung) taken at autopsy were all RT-qPCR-positive for ZIKV RNA. The samples were negative (RT-qPCR) to dengue, chikungunya and yellow fever viruses. ZIKV was not isolated from tissues cultured on C6/36 cells. No serologic test was done due the absence of serum sample.
4.3.2. Histopathology
4.3.2.1. Lung
The presence of fibrin exudates, areas with alveolar hemorrhage and others with hyalin membrane were also observed. Vascular congestion, characteristic of pneumonia, was seen in diffuse areas of pulmonary tissues (Fig. 3A).
Fig. 3.

Histopathological aspects of Case 3. Fig. 3A – Lobar pneumonia: hemorrhage, fibrin leukocyte exudates and hyaline membrane (HE, Magnification: ×10). Fig. 3B – Kidney: glomerulus presenting cell hyperplasia and tubules with cell edema (arrows) (HE, Magnification: ×40). Fig. 3C – Liver with areas of necrosis, apoptosis, Councilman bodies (large arrows) and steatosis (thin arrows) (HE, Magnification: ×40). Fig. 3D – Liver with large area of necrosis, confluent and forming bridges (arrows) between the lobes (HE, Magnification: ×10).
4.3.2.2. Kidney
Acute tubular necrosis and cell tumefaction were observed, as well as acute glomerulonephritis with glomerular cell hyperplasia (Fig. 3B).
4.3.2.3. Liver
Large areas of both necrosis and apoptosis in zones 2 and 3 were observed. These areas were confluent and formed bridges between hepatic lobules and showed several acidophilic corpuscles (Councilman bodies), characteristic of severe hepatic injury (Fig. 3C and D).
4.3.3. Immunohistochemistry
ZIKV antigens were found in liver, kidney and lung (Fig. 4) of case 3. Studies were conducted to test for bacteria in immunohistochemical specimens, using Gram staining of lung tissue fragments. The result was positive for Gram positive bacteria. Tests performed for dengue and yellow fever viruses by Immunohistochemical assay were negative.
Fig. 4.
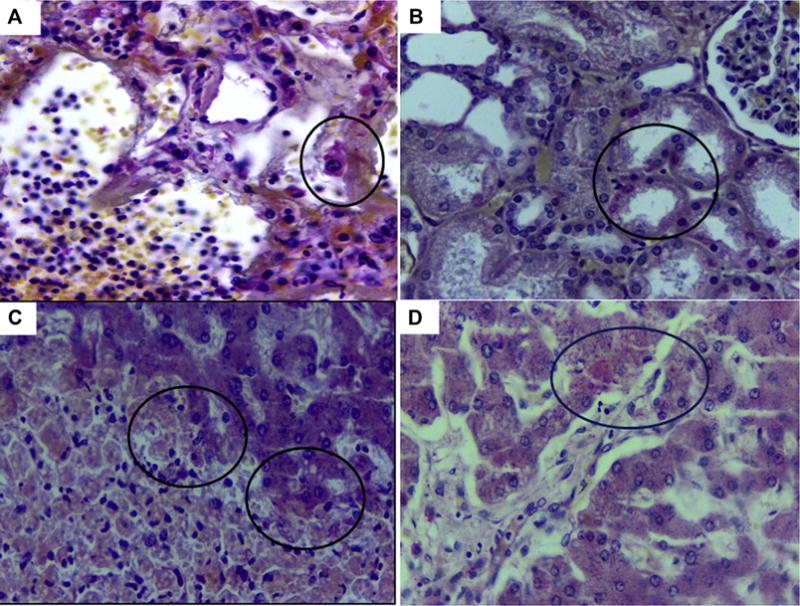
Immunohistochemistry of Case 3. Fig. 4A – Positive immunostaining for ZIKV antigens in alveolar lining cells (circle) (IHC SAAP, Magnification: ×40). Fig. 4B – Positive immunostaining for ZIKV antigens in renal tubule (circle) (IHC SAAP, Magnification: ×40). Fig. 4C – Positive immunostaining for ZIKV antigens in hepatocytes of necrotic margins and apopototic cells, Councilmann bodies (circles) (IHC SAAP, Magnification: ×40). Fig. 4D – Positive immunostaining for ZIKV antigens in hepatocytes (circle) characterized by acidophilic and granular appearance in the cytoplasm (IHC SAAP, Magnification: ×40).
5. Discussion
ZIKV was originally isolated in 1947 from the blood of a sentinel rhesus monkey in Uganda, during studies on the ecology of sylvan yellow fever [15]. Over the next 65 years, the virus was occasionally isolated from mosquitoes and febrile humans in various countries in sub-Saharan Africa and Southeast Asia [3,16,17]. Most (~80%) human infections during this period were probably asymptomatic. When symptomatic, the clinical disease was a generally self-limited dengue-like illness with fever, rash, headache, myalgia and conjunctivitis of 3–5 days duration [3,18]. It was not until the Zika fever outbreak in French Polynesia in 2013–2014, that it became apparent that ZIKV infection was not entirely benign or a self-limited illness [3,18]. During that outbreak, cases of GBS were first reported in persons with ZIKV infection [3]. When ZIKV cases appeared in Brazil in early 2015, there was also a dramatic increase in cases of microcephaly and GBS; many of these cases were associated with ZIKV infection [3,18]. The three cases reported in this communication add yet another dimension to ZIKV infection and its potential complications. In addition, all three of these cases were fatal.
Case 1 was a 47-year-old man receiving prednisone for EL, who died with a fulminant ZIKV infection. The pathological findings and extent of organ involvement and viral antigen in his tissues were similar to that seen in fatal cases of West Nile virus infection in immunosuppressed patients and animals [19–21]. A nearly complete ZIKV genome was obtained from the isolate from this patient. It was obtained using both Ion semi conduction and pyrosequencing methods [11,22] implemented in the Ion PGM (Life Technologies) and GS FLX 454 (Roche Life Sciences) devices. As shown in Fig. 5, the isolate (BeH 818305) falls within the ZIKV Asian genotype clade (bootstrap = 99%) and is closely related to other recently reported strains obtained from humans in Brazil, Surinam, French Polynesia and Haiti. These findings are in agreement with other recently published phylogenetic studies, namely that ZIKV strains currently circulating in Brazil and other regions of tropical America are all very similar and are within the Asian genotype [23,24].
Fig. 5.
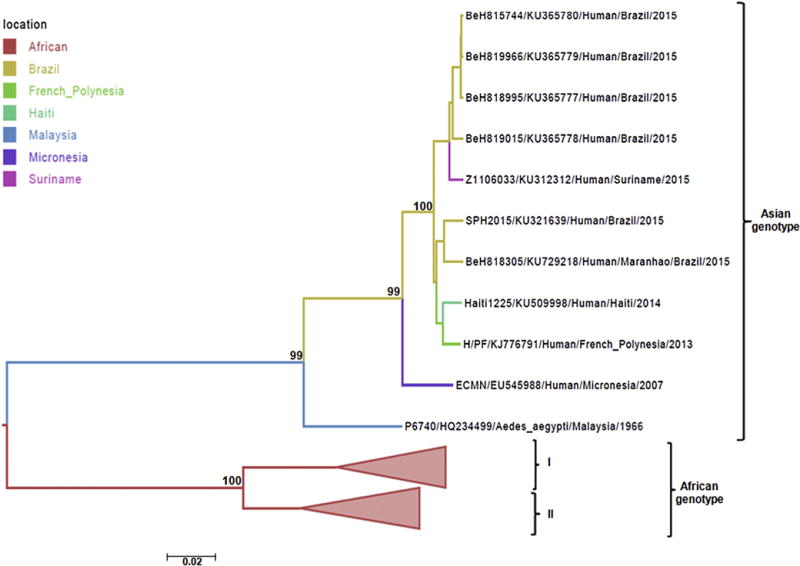
Phylogeny of the Env gene region ZIKV Case 1 (BeH818305, Accession Number: KU729218). ML bootstrap scores are shown next to nodes. The tree was estimated using PhyML. Sequence names include accession number, strain name, location of sampling and date of sampling. Isolates in this study: Strain BeH815744 – self-limited clinical infection in patient from João Pessoa, Paraíba State, Northeast Brazil; Strains BeH819966, BeH818995 BeH819015- self-limited clinical infection in patient from Belém, Pará State, North Brazil; Strain Z1106033 – self-limited clinical infection in patient from Paramaribo, Suriname; Strain SPH2015 (death) blood donor patient from Sumaré, São Paulo State, Southeast Brazil; Strain Haiti1225 – undifferentiated febrile illness in patient from Gressier/Leogane region of Haiti; Strain H/PF/KJ77679 case with fever, headache, myalgia, arthralgia, and rash in patient returning from French Polynesia; Strain ECMN/EU545988 – sample collected in April 2007 during an outbreak of rash, conjunctivitis, and arthralgia in state of Yap, Micronesia; P6740/HQ234499 – sample obtained from Aedes aegypti mosquitoes in Malaysia, 1966.
Case 2 was a previously healthy young woman who developed thrombocytopenia, anemia and hemorrhagic manifestations following ZIKV infection. Laboratory findings were consistent with hemolytic anemia and thrombocytopenia that were considered for the diagnosis of Evans syndrome post ZIKV infection. During her hospitalization, she received multiple units of platelets and whole blood; consequently, her hematologic profile was altered. Unfortunately no autopsy was done, so the exact cause of her death could not be determined. A similar case was recently reported of a woman infected in Suriname, who developed thrombocytopenia, anemia and subcutaneous bleeding following ZIKV infection; however she recovered [25]. Four other fatal Zika infections with similar clinical manifestations and laboratory findings were recently reported from Puerto Rico and Colombia [26,27]. A recent report from Colombia also described a young woman with sickle cell disease who also died following ZIKV infection [28].
Case 3 was a young woman who developed thrombocytopenia, hemorrhages and pneumonia. ZIKV antigens were detected in the patient’s tissues, but an immunohistochemical assay also demonstrated a positive reaction to gram-positive bacteria, suggesting that the patient’s death may have been due primarily to a bacterial infection, rather than to her ZIKV infection. However, she also had hemorrhagic manifestations with pancytopenia on admission to the hospital. Collectively, our three cases and the six other aforementioned cases suggested that immune suppressed individuals or persons with autoimmune disorders may be at higher risk of developing severe disease, if infected with ZIKV.
Until recently, ZIKV was considered to be an exotic agent and not a subject of much interest or study. However, the recent epidemics in French Polynesia and the Americas have dramatically changed the perception of its public health importance[29–32]. As more clinical, epidemiological and population-based studies are carried out, undoubtedly additional clinical manifestations and pathologies will be found to be associated with ZIKV infection.
Acknowledgments
We are grateful for people of Departments of Pathology and Arbovirology and Hemorrhagic Fevers for their technical support, as well as Public Health Laboratories of Maranhão and Rio Grande do Norte States.
Funding
This study was partially supported by the Ministry of Health through the Evandro Chagas Institute official budget and by CNPq (PFCV by grants 573739/2008-0, 301641/2010-2 and 457664/2013-4), CAPES Zika Fast-track, the CNPq Zika fund, and FINEP Zika and other aarboviruses fund; RBT is supported by the grant R24 AT 120992 from National Institute of Health.
Footnotes
Ethics statement
Biological samples of patients were obtained and processed in the context of the emergency definition by the Ministry of Health during surveillance activities of the ZIKV epidemic in Brazil and, accordingly, no need informed consent of patients.
Competing interests
Authors declare they have not conflict of interest.
References
- 1.Dick GW. Zika virus (II). Pathogenicity and physical properties. Trans R Soc Trop Med Hyg. 1952;46:521–534. doi: 10.1016/0035-9203(52)90043-6. http://dx.doi.org/10.1016/0035-9203(52)90043-6. [DOI] [PubMed] [Google Scholar]
- 2.Duffy MR, Chen TH, Hancock WT, Powers AM, Kool JL, Lanciotti RS, Pretrick M, Marfel M, Holzbauer S, Dubray C, Guillaumot L, Griggs A, Bel M, Lambert AJ, Laven J, Kosoy O, Panella A, Biggerstaff BJ, Fischer M, Hayes EB. Zika virus outbreak on yap island, federated states of Micronesia. N Engl J Med. 2009;360:2536–2543. doi: 10.1056/NEJMoa0805715. http://dx.doi.org/10.1056/NEJMoa0805715. [DOI] [PubMed] [Google Scholar]
- 3.Musso D, Gubler DJ. Zika virus. Clin Microbiol Rev. 2016;29:487–524. doi: 10.1128/CMR.00072-15. http://dx.doi.org/10.1128/CMR.00072-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.P.A.H.O. PAHO. Zika Epidemiological Update – 29 Jul 2016. 2016 http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=35555&lang=pt.
- 5.Tesh RB. A method for the isolation and identification of dengue viruses, using mosquito cell cultures. Am J Trop Med Hyg. 1979;28:1053–1059. doi: 10.4269/ajtmh.1979.28.1053. [DOI] [PubMed] [Google Scholar]
- 6.Faye O, Faye O, Dupressoir A, Weidmann M, Ndiaye M, Sall A Alpha. One-step RT-PCR for detection of Zika virus. J Clin Virol. 2008;43:96–101. doi: 10.1016/j.jcv.2008.05.005. http://dx.doi.org/10.1016/j.jcv.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ, Stanfield SM, Duffy MR. Genetic and serologic properties of Zika virus associated with an epidemic, yap state, Micronesia, 2007. Emerg Infect Dis. 2008;14:1232–1239. doi: 10.3201/eid1408.080287. http://dx.doi.org/10.3201/eid1408.080287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall WC, Crowell TP, Watts DM, Barros VLR, Kruger H, Pinheiro F, Peters CJ. Demonstration of yellow fever and dengue antigens in formalin-fixed paraffin-embedded human liver by immunohistochemical analysis. Am J Trop Med Hyg. 1991;45:408–417. doi: 10.4269/ajtmh.1991.45.408. [DOI] [PubMed] [Google Scholar]
- 9.Quaresma JAS, Barros VLRS, Fernandes ER, Pagliari C, Takakura C, da Costa Vasconcelos PF, de Andrade HF, Duarte MIS. Reconsideration of histopathology and ultrastructural aspects of the human liver in yellow fever. Acta Trop. 2005;94:116–127. doi: 10.1016/j.actatropica.2005.03.003. http://dx.doi.org/10.1016/j.actatropica.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Quaresma JAS, Barros VLRS, Pagliari C, Fernandes ER, Guedes F, Takakura CFH, Andrade HF, Vasconcelos PFC, Duarte MIS. Revisiting the liver in human yellow fever: virus-induced apoptosis in hepatocytes associated with TGF-β, TNF-α and NK cells activity. Virology. 2006;345:22–30. doi: 10.1016/j.virol.2005.09.058. http://dx.doi.org/10.1016/j.virol.2005.09.058. [DOI] [PubMed] [Google Scholar]
- 11.Rothberg JM, Hinz W, Rearick TM, Schultz J, Mileski W, Davey M, Leamon JH, Johnson K, Milgrew MJ, Edwards M, Hoon J, Simons JF, Marran D, Myers JW, Davidson JF, Branting A, Nobile JR, Puc BP, Light D, Clark Ta, Huber M, Branciforte JT, Stoner IB, Cawley SE, Lyons M, Fu Y, Homer N, Sedova M, Miao X, Reed B, Sabina J, Feierstein E, Schorn M, Alanjary M, Dimalanta E, Dressman D, Kasinskas R, Sokolsky T, Fidanza Ja, Namsaraev E, McKernan KJ, Williams A, Roth GT, Bustillo J. An integrated semiconductor device enabling non-optical genome sequencing. Nature. 2011;475:348–352. doi: 10.1038/nature10242. http://dx.doi.org/10.1038/nature10242. [DOI] [PubMed] [Google Scholar]
- 12.Hsu SM, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981;29:577–580. doi: 10.1177/29.4.6166661. http://dx.doi.org/10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- 13.Pagliari C, Quaresma JAS, Fernandes ER, Stegun FW, Brasil RA, de Andrade HF, Barros V, Vasconcelos PFC, Duarte MIS. Immunopathogenesis of dengue hemorrhagic fever: contribution to the study of human liver lesions. J Med Virol. 2014;86:1193–1197. doi: 10.1002/jmv.23758. [DOI] [PubMed] [Google Scholar]
- 14.Pagliari C, Simões Quaresma JA, Kanashiro-Galo L, de Carvalho LV, Vitoria WO, da Silva WLF, Penny R, Vasconcelos BCB, da Costa Vasconcelos PF, Duarte MIS. Human kidney damage in fatal dengue hemorrhagic fever results of glomeruli injury mainly induced by IL17. J Clin Virol. 2016;75:16–20. doi: 10.1016/j.jcv.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Dick GW, Kitchen S, Haddow A. Zika virus (I). isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46:509–520. doi: 10.1016/0035-9203(52)90042-4. http://dx.doi.org/10.1016/0035-9203(52)90042–4. [DOI] [PubMed] [Google Scholar]
- 16.Haddow AD, Schuh AJ, Yasuda CY, Kasper MR, Heang V, Huy R, Guzman H, Tesh RB, Weaver SC. Genetic characterization of zika virus strains: geographic expansion of the asian lineage. PLoS Negl Trop Dis. 2012;6:e1477. doi: 10.1371/journal.pntd.0001477. http://dx.doi.org/10.1371/journal.pntd.0001477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faye O, Freire CCM, Iamarino A, Faye O, de Oliveira JVC, Diallo M, Zanotto PMA, Sall AA. Molecular evolution of zika virus during its emergence in the 20th century. PLoS Negl Trop Dis. 2014;8:e2636. doi: 10.1371/journal.pntd.0002636. http://dx.doi.org/10.1371/journal.pntd.0002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lazear HM, Diamond MS. Zika virus: new clinical syndromes and its emergence in the western hemisphere. J Virol. 2016;90:4864–4875. doi: 10.1128/JVI.00252-16. http://dx.doi.org/10.1128/JVI.00252-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armah HB, Wang G, Omalu BI, Tesh RB, Gyure Ka, Chute DJ, Smith RD, Dulai P, Vinters HV, Kleinschmidt-DeMasters BK, Wiley Ca. Systemic distribution of West Nile virus infection: postmortem immunohistochemical study of six cases. Brain Pathol. 2007;17:354–362. doi: 10.1111/j.1750-3639.2007.00080.x. http://dx.doi.org/10.1111/j.1750-3639.2007.00080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kleinschmidt-DeMasters BK, Marder BA, Levi ME, Laird SP, McNutt JT, Escott EJ, Everson GT, Tyler KL. Naturally acquired West Nile virus encephalomyelitis in transplant recipients: clinical, laboratory, diagnostic, and neuropathological features. Arch Neurol. 2004;61:1210–1220. doi: 10.1001/archneur.61.8.1210. http://archneur.jamanetwork.com/article.aspx?articleid=786449. [DOI] [PubMed] [Google Scholar]
- 21.Mateo R, Xiao SY, Guzman H, Lei H, Travassos Da Rosa APA, Tesh RB. Effects of immunosuppression on West Nile virus infection in hamsters. Am J Trop Med Hyg. 2006;75:356–362. 75/2/356 [pii] [PubMed] [Google Scholar]
- 22.Margulies M, Egholm M, Altman WE, Attiya S, Bader JS, Bemben La, Berka J, Braverman MS, Chen Y-J, Chen Z, Dewell SB, Du L, Fierro JM, Gomes XV, Godwin BC, He W, Helgesen S, Ho CH, Irzyk GP, Jando SC, Alenquer MLI, Jarvie TP, Jirage KB, Kim J-B, Knight JR, Lanza JR, Leamon JH, Lefkowitz SM, Lei M, Li J, Lohman KL, Lu H, Makhijani VB, McDade KE, McKenna MP, Myers EW, Nickerson E, Nobile JR, Plant R, Puc BP, Ronan MT, Roth GT, Sarkis GJ, Simons JF, Simpson JW, Srinivasan M, Tartaro KR, Tomasz A, Vogt Ka, Volkmer Ga, Wang SH, Wang Y, Weiner MP, Yu P, Begley RF, Rothberg JM. Genome sequencing in microfabricated high-density picolitre reactors. Nature. 2005;437:376–380. doi: 10.1038/nature03959. http://dx.doi.org/10.1038/nature03959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faria NR, Azevedo RdSdS, Kraemer MUG, Souza R, Cunha MS, Hill SC, Theze J, Bonsall MB, Bowden TA, Rissanen I, Rocco IM, Nogueira JS, Maeda AY, Vasami FGSd, Macedo FLLd, Suzuki A, Rodrigues SG, Cruz ACR, Nunes BT, Medeiros DBAd, Rodrigues DSG, Nunes Queiroz AL, Silva EVPd, Henriques DF, Travassos da Rosa ES, de Oliveira CS, Martins LC, Vasconcelos HB, Casseb LMN, Simith DDB, Messina JP, Abade L, Lourenco J, Alcantara LCJ, Lima MMd, Giovanetti M, Hay SI, de Oliveira RS, Lemos PSd, De Oliveira LF, de Lima CPS, da Silva SP, Vasconcelos JMd, Franco L, Cardoso JF, Vianez JLdSG, Junior, Mir D, Bello G, Delatorre E, Khan K, Creatore M, Coelho GE, de Oliveira WK, Tesh R, Pybus OG, Nunes MRT, Vasconcelos PFC. Zika virus in the Americas: early epidemiological and genetic findings. Science. 2016;352(80):345–349. doi: 10.1126/science.aaf5036. http://dx.doi.org/10.1126/science.aaf5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lanciotti RS, Lambert AJ, Holodniy M, Saavedra S, del CC Signor L. Phylogeny of Zika virus in western hemisphere, 2015. Emerg Infect Dis. 2016;22:933–935. doi: 10.3201/eid2205.160065. http://dx.doi.org/10.3201/eid2205.160065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karimi O, Goorhuis A, Schinkel J, Codrington J, Vreden SGS, Vermaat JS, Stijnis C, Grobusch MP. Thrombocytopenia and subcutaneous bleedings in a patient with Zika virus infection. Lancet. 2016;387:939–940. doi: 10.1016/S0140-6736(16)00502-X. http://dx.doi.org/10.1016/S0140-6736(16)00502-X. [DOI] [PubMed] [Google Scholar]
- 26.Dirlikov E, Ryff KR, Torres-Aponte J, Thomas DL, Perez-Padilla J, Munoz-Jordan J, Caraballo EV, Garcia M, Segarra MO, Malave G, Simeone RM, Shapiro-Mendoza CK, Reyes LR, Alvarado-Ramy F, Harris AF, Rivera A, Major CG, Mayshack M, Alvarado LI, Lenhart A, Valencia-Prado M, Waterman S, Sharp TM, Rivera-Garcia B. Update: ongoing Zika virus transmission—Puerto Rico November 1 2015–April 14 2016. MMWR Morb Mortal Wkly Rep. 2016;65:451–455. doi: 10.15585/mmwr.mm6517e2. http://dx.doi.org/10.15585/mmwr.mm6517e2. [DOI] [PubMed] [Google Scholar]
- 27.Sarmiento-Ospina A, Vásquez-Serna H, Jimenez-Canizales CE, Villamil-Gómez WE, Rodriguez-Morales AJ. Zika virus associated deaths in Colombia. Lancet Infect Dis. 2016;16:523–524. doi: 10.1016/S1473-3099(16)30006-8. http://dx.doi.org/10.1016/S1473-3099(16)30006-8. [DOI] [PubMed] [Google Scholar]
- 28.Arzuza-Ortega L, Polo A, Pérez-Tatis G, López-García H, Parra E, Pardo-Herrera LC, Rico-Turca AM, Villamil-Gómez W, Rodríguez-Morales AJ. Fatal sickle cell disease and Zika virus infection in girl from Colombia. Emerg Infect Dis. 2016;22:925–927. doi: 10.3201/eid2205.151934. http://dx.doi.org/10.3201/eid2205.151934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heymann DL, Hodgson A, Sall AA, Freedman DO, Staples JE, Althabe F, Baruah K, Mahmud G, Kandun N, Vasconcelos PFC, Bino S, Menon KU. Zika virus and microcephaly: why is this situation a PHEIC? Lancet. 2016;387:719–721. doi: 10.1016/S0140-6736(16)00320-2. http://dx.doi.org/10.1016/S0140-6736(16)00320-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plourde AR, Bloch EM. A literature review of Zika virus. Emerg Infect Dis. 2016;22:1185–1192. doi: 10.3201/eid2207.151990. http://dx.doi.org/10.3201/eid2207.151990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vasconcelos PFDC. Doença pelo vírus Zika: um novo problema emergente nas Américas? Rev Pan-Amazônica Saúde. 2015;6:9–10. http://dx.doi.org/10.5123/S2176-62232015000200001. [Google Scholar]
- 32.Vasconcelos PFC, Calisher CH. Emergence of human arboviral diseases in the americas, 2000–2016. Vector-Borne Zoonotic Dis. 2016;16:295–301. doi: 10.1089/vbz.2016.1952. http://dx.doi.org/10.1089/vbz.2016.1952. [DOI] [PubMed] [Google Scholar]


