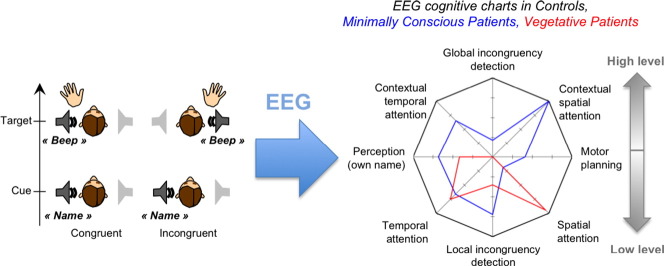
Abstract
The use of cognitive evoked potentials in EEG is now part of the routine evaluation of non-communicating patients with disorders of consciousness in several specialized medical centers around the world. They typically focus on one or two cognitive markers, such as the mismatch negativity or the P3 to global auditory regularity. However it has become clear that none of these markers in isolation is at the same time sufficiently specific and sufficiently sensitive to be taken as the unique gold standard for diagnosing consciousness. A good way forward would be to combine several cognitive markers within the same test to improve evaluation. Furthermore, given the diversity of lesions leading to disorders of consciousness, it is important not only to probe whether a patient is conscious or not, but also to establish a more general and nuanced profile of the residual cognitive capacities of each patient using a combination of markers.
In the present study we built a unique EEG protocol that probed 8 dimensions of cognitive processing in a single 1.5 h session. This protocol probed variants of classical markers together with new markers of spatial attention, which has not yet been studied in these patients. The eight dimensions were: (1) own name recognition, (2) temporal attention, (3) spatial attention, (4) detection of spatial incongruence (5) motor planning, and (6,7,8) modulations of these effects by the global context, reflecting higher-level functions. This protocol was tested in 15 healthy control subjects and in 17 patients with various etiologies, among which 13 could be included in the analysis. The results in the control group allowed a validation and a specific description of the cognitive levels probed by each marker. At the single-subject level, this combined protocol allowed assessing the presence of both classical and newly introduced markers for each patient and control, and revealed that the combination of several markers increased diagnostic sensitivity. The presence of a high-level effect in any of the three tested domains distinguished between minimally conscious and vegetative patients, while the presence of low-level effects was similar in both groups. In summary, this study constitutes a validated proof of concept in favor of probing multiple cognitive dimensions to improve the evaluation of non-communicating patients. At a more conceptual level, this EEG tool can help achieve a better understanding of disorders of consciousness by exploring consciousness in its multiple cognitive facets.
Abbreviations: ADAN, Anterior Directing Attention Negativity; CNV, contingent negative variation; DOC, disorders of consciousness; LRP, lateralized readiness potential; MCS, minimally conscious state; VS, vegetative state
Keywords: Disorders of consciousness, Non-communicating patients, EEG, Event related potentials, Attention, Cognition
Graphical abstract
Highlights
-
•
This new EEG protocol probes 8 cognitive functions within a single 1.5 h session.
-
•
It allows a complete neuropsychological evaluation only based on brain activity.
-
•
It increases sensitivity in detecting both low-level and high-level functions in patients.
-
•
Only the high-level functions distinguish minimally conscious from vegetative states.
-
•
Multidimensional EEG testing is feasible in patients and can improve evaluation.
1. Introduction
Patients who survive severe brain injury sometimes remain in states where they show either no signs of consciousness (vegetative state, VS) or fluctuating signs of consciousness (minimally conscious state, MCS (Giacino et al., 2002)). Careful clinical examination alone is often insufficient to detect signs of mental activity in these patients, because of cognitive or motor limitations due to brain lesions (Rohaut et al., 2013, Schnakers et al., 2008). An extreme example is the case of patients with locked-in syndrome, who are fully conscious of themselves and their environment but suffer from paralysis following lesions to the ventral pons, impeding behavioral responses (Patterson and Grabois, 1986, Plum and Posner, 1972). Therefore it is essential to supplement behavioral evaluation by neuroimaging methods such as functional magnetic resonance imagery (fMRI) or electroencephalographic recordings (EEG), with which we can infer the patients' preserved cognition directly from their brain activity. EEG recordings in particular are of prime importance because they can be recorded at bedside and offer an optimal time-resolution to capture the respective dynamics of unconscious and conscious cognitive processes.
Several EEG protocols are now routinely used in some clinical centers to inform the diagnosis of patients with disorders of consciousness (DOC). Each protocol typically targets one or two EEG markers. Some of these markers are very specific but not very sensitive, while others show the converse profile. One such marker is the mismatch negativity (or MMN), which signs the detection of a local deviance, e.g. one odd tone within a series of identical tones (AAAAB) (Naatanen, 1995). This marker is not specific to consciousness, since some comatose patients show this effect (Fischer et al., 1999), but it is useful in predicting good functional outcome (Luaute et al., 2005). In contrast, the detection of a global deviance, such as detecting an odd series within repetitions of identical series (for example a series of identical tones AAAAA within repeated series containing one odd tone AAAAB) is only observed when the subject is conscious and voluntarily attends to global regularities in the auditory environment, thus qualifying this global effect as specific of consciousness (Bekinschtein et al., 2009, Faugeras et al., 2012, Faugeras et al., 2011). However this marker is not very sensitive, since it is absent in conscious but inattentive healthy volunteers. Other important markers include response to own name (Perrin et al., 1999), or markers of motor preparation (Bekinschtein et al., 2008, Bekinschtein et al., 2011, Cruse et al., 2011, Cruse et al., 2012, Goldfine et al., 2011). Although each of these ERP markers shows some relevance in the evaluation of DOC patients, none of them stands out as the single “gold standard”. Also, none of them can summarize the others: they all test different, possibly complementary, dimensions.
While the prevalent approach so far has been to search for a hypothetical unique marker of consciousness, here we would like to argue that a decisive advance could come from actually combining several markers. The present study tries to establish a proof of concept: that it is possible in practice to build a single protocol that probes many cognitive functions within a single recording session to improve the evaluation of DOC patients.
We built a single experimental protocol from which we could derive most of the classical markers described above, as well as markers of spatial attention, which has not been tested in patients so far. We included spatial attention because decades of research on healthy volunteers suggest that conscious perception is tightly related to the allocation of attentional resources (Cohen et al., 2011, Mack and Rock, 1998, O'Regan et al., 1999, Rensink et al., 1997, Sergent et al., 2005, Sergent and Dehaene, 2004). Recent studies even suggest that a key event that makes a stimulus become conscious is the reactivation of sensory representations by the attentional system (Sergent et al., 2013, Thibault et al., 2016). While many current ERP markers call upon general attention one way or another (Bekinschtein et al., 2009, Chennu et al., 2013), to our knowledge no previous study has probed such “outward oriented” attention in these patients. It could be particularly revealing of whether a patient can become conscious of their environment.
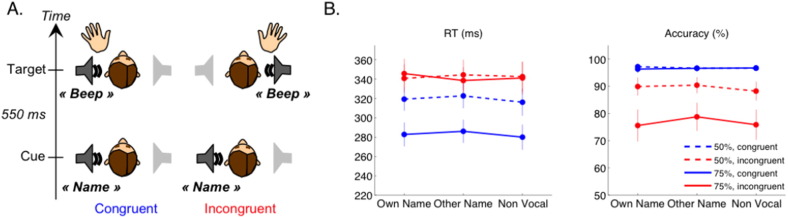
Our protocol was an auditory adaptation of the standard Posner cueing protocol (Posner et al., 1980, Schroger and Eimer, 1996). Healthy controls and patients were instructed to move the hand, or imagine moving the hand, on the side of presentation of a target tone (motor planning). Just before each target tone, a cue was played either on the same or on the opposite side (temporal attention and spatial attention), and this cue could be the participant's own name, another name, or a non-verbal control (own name recognition) (Fig. 1A). In different sessions the cue either predicted the future target's side with 75% validity, or was not predictive of the target's side (global versus local detection of incongruence). So in total, this single protocol probed 8 different cognitive dimensions: (1) own name recognition, (2) temporal attention (3) spatial attention, (4) detection of spatial incongruence between the cue and the target, (5) motor planning in response to the target and (6,7,8) modulation of these effects by the global regularities (predictive value of the cue).
Fig. 1.
Experimental design and behavioral results in controls.
(A) Graphical summary of the experimental design: an auditory cue (either the subject's own name, another name or a non vocal control) was played on the left or on the right, followed by a target «beep» on the same side (congruent) or on the other side (incongruent). The control participants were asked to press a button with the hand corresponding with the target side, the patients were instructed to imaging squeezing their hand on the target's side. (B) Behavioral results for the control group: the left panel shows average reaction times to the target across participants for correct responses, in the non-predictive context (50% of congruent trials, dotted lines) and in the predictive context (75% of congruent trials, plain lines) as a function of the cue type (x axis) and spatial congruence between the cue and the target (blue for congruent, red for incongruent). Error bars represent ± the standard error of the mean (SEM). The right panel shows the results on accuracy, with the same conventions.
With this multidimensional test, we tackled two important issues. First, a better marker of consciousness might arise from multidimensional probing. Secondly, while most research so far has focused on detecting consciousness, in practice clinicians are in need for tools that not only test whether a patient is conscious or not, but also establish a more general and nuanced profile of the residual cognitive capacities of this patient. This would be a precious tool for orienting care and rehabilitation strategies, beyond the question of consciousness per se.
2. Materials and methods
2.1. Control participants
Fifteen healthy individuals participated in the control study (6 women, 9 men, aged 23 ± 3, 4 left handed). All participants gave written informed consent before taking part in the experiment and received monetary compensation for their participation. The protocol conformed to French regulation and the Declaration of Helsinki and was approved by the ethics committee CPP Ile de France 1 (Paris, France). All control participants were included in the analysis.
2.2. Patients
Patients were included following written informed consent from their legal representative. The experiment was conducted under the approval of the ethics committee CPP Ile de France 1 (Paris, France). The patients were recorded without sedation for at least 24 h in order to maximize their arousal and their level of cognitive performance during the auditory task. All the EEG markers described here were derived from the same multidimensional protocol, acquired within a single EEG session lasting 1.5 h. Behavioral evaluation of each patient with the CRS-R (details below) was done just before this EEG session. Patients also underwent the original version of the “Local-Global” EEG test, either the day before or on the same day (Bekinschtein et al., 2009, Faugeras et al., 2012, Faugeras et al., 2011). The markers of local or global incongruence detection described here do not come from the routine “Local/Global” EEG test but from the new protocol.
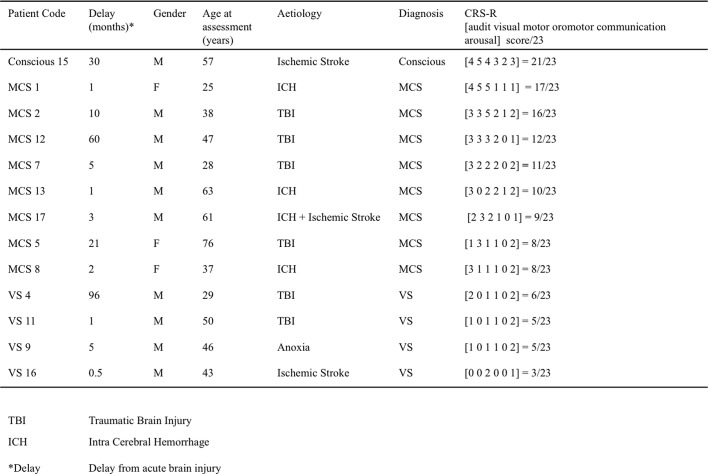
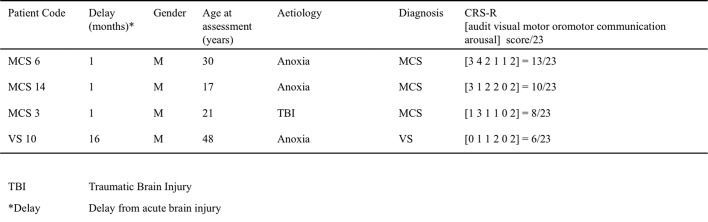
Among the 17 patients who underwent this protocol, 4 were discarded from the analysis because of insufficient quality of the EEG recording (patients details in Supplementary Table 1, Supplementary Table 2, EEG exclusion criteria below). Thirteen patients were retained in the present analysis (SI Table S1): 3 women and 10 men, with ages ranging from 25 to 63 years at the time of recording (mean = 46.1 years, sd = 14.6). The delay since injury varied from 15 days to 8 years (mean delay = 19.6 months, sd = 29.7). The etiologies were traumatic brain injuries (TBI) for six patients, stroke (ischemic and/or hemorrhagic) for six patients, and anoxia for one patient.
Supplementary Table 1.
Details of the patients included in the analysis.
Supplementary Table 2.
Details of the patients discarded from the analysis.
Clinical evaluation of consciousness was based on the French version of the CRS-R scale (Schnakers et al., 2008), after careful neurological examination by trained neurologists (FF, BR, LN). This scale consists of 23 items divided in six subscales addressing auditory, visual, motor, oromotor, communication and arousal functions. CRS-R subscales are comprised of hierarchically arranged items. The lowest item on each subscale represents reflexive activity while the highest items represent cognitively mediated behaviors. This scoring enables a distinction to be drawn between conscious (or exit MCS), minimally conscious (MCS) and vegetative states (VS) (Schnakers et al., 2009) also called “unresponsive wakefulness” (Laureys et al., 2010). One patient was conscious (patient number 15), 8 patients were MCS and 4 were VS at the time of recording.
2.3. Stimuli and experimental procedure
All the EEG markers described here were derived from the same multidimensional protocol, acquired within a single EEG session lasting 1.5 h. Auditory stimuli were presented via headphones. Each trial consisted of a cue followed by a target with a fixed stimulus onset asynchrony (SOA) of 550 ms (Fig. 1A). The target was a brief tone composed of three superimposed sinusoidal tones (500, 1000 and 2000 Hz), lasting 50 ms with a 7 ms rise and a 7 ms fall time. There were three randomly interleaved cue types: the participant or patient's own first name, another first name, and a non-vocal control matched to the participant's own name. The participants' own names and the other names were all digitally recorded with the same female voice in the same “calling” intonation, using the software Audacity. For each participant, the “other name” was matched in gender, number of syllables (1 2 or 3) and duration to the participant's own name, but with no similar syllables. Names from close relatives or friends were excluded. Each non-vocal control was created in Matlab by modulating a characteristic frequency of the corresponding own name (mean frequency of the spectrum) and two of its harmonics with the amplitude envelope of the own name stimulus. In this way, the non-vocal stimulus preserved some physical characteristics of the own name but did not sound like a voice (Holeckova et al., 2006). For each participant, the three cues were matched in peak amplitude and duration, and lasted between 400 ms and 500 ms across participants.
Each participant performed 3 blocks with a predictive context (75% of congruent trials) and 3 blocks with non-predictive context (50% of congruent trials). The two block types were alternated, and the starting condition was randomized across participants. Each block consisted of 192 trials, 64 for each cue type (own name, other name or non-vocal control), among which 32 were congruent in the 50% blocks, and 48 in the 75% blocks. This resulted in a total of 96 trials for each cue type and congruence condition in the 50% context, and 144 congruent versus 48 incongruent trials for each cue type in the 75% context.
2.4. EEG Acquisition and Preprocessing
We recorded high-density EEG sampled at 250 Hz and referenced to the vertex using a 257-channel system (Electrical Geodesics Inc., Oregon, USA). Preprocessing was performed using the Fieldtrip software (Oostenveld et al., 2011) and custom scripts in Matlab. Continuous data were band-pass filtered between 0.1 and 20 Hz for control subjects, and between 0.5 and 20 Hz for patients, because patients showed important low-frequency noise. Data were then segmented from 750 ms before to 1000 ms after each target “beep”. In a first pass of semi-automatic artifact rejection, trials and channels whose variance across a session exceeded a certain threshold were rejected, using the Fieldtrip artifact rejection tool (3000 μV2 for control subjects, 5000 μV2 for patients, corresponding to standard deviations of 55 μV and 71 μV respectively). Rejected electrodes (7% of the 257 electrodes on average) were reconstructed by interpolating their neighbors in Fieldtrip. Data were then visually inspected for remaining artifacts. In patients with excessive eye-movements or blinks, these artifacts were removed from the signal using a PCA procedure prior to segmentation: an ERP of the artifact was derived by averaging the EEG signal around the time of the blinks or the saccades detected in EOG. The first principal component of this ERP, selective of the artifact, was removed from the continuous EEG in components space and the remaining components were reconverted in the native space. The preprocessed epochs were averaged in synchrony with the target onset, transformed to an average reference, and corrected for baseline signal during a 200 ms period before the onset of the cue. Four of the 17 patients had to be discarded from further analysis because there had > 30% of bad trials.
2.5. Statistics
2.5.1. Individual statistics of the GFP
For each experimental contrast, the presence of an effect was tested on the Global Field Power (GFP) which is the root summed square of the voltage of all electrodes at each time point (Lehmann and Skrandies, 1980). The GFP of the contrast between two conditions provides a compact summary of the time course of an effect. GFPs were corrected for a baseline period of 200 ms before cue onset.
To test for the significance of an effect in GFP, we performed two-steps statistics: at the level of individual samples, and then at the level of temporal clusters, based on the non-parametric cluster-level statistics developed in the fieldtrip software (Maris and Oostenveld, 2007) adapted to temporal clusters (Chennu et al., 2013). At the first level we estimated the p-value of the effect at each time point using Monte Carlo permutations (1000 randomizations). Only the time points with a significant effect at p < 0.05 were retained. Significant time points were then clustered based on temporal contiguity, and only the clusters that were within the time window of interest were retained (see below). Finally the cluster-level significance (pclust, one-sided) was estimated based on the sum of the GFPs within each cluster, using Monte Carlo permutations (1000 randomizations). These cluster-level statistics were intrinsically corrected for multiple comparison (Maris and Oostenveld, 2007). The analysis on control participants revealed that a classical threshold at pclust < 0.05 was too conservative and produced false negatives (SI Fig. 1). To date there is no consensus on the best criteria for individual statistics in ERPs, as demonstrated in a recent paper by Gabriel and collaborators (Gabriel et al., 2016). Here we decided to use the analysis on controls to set a pragmatic cutoff at pclust < 0.075, which was the best compromise to prevent false negatives while keeping a low level of false positives. So, to summarize, an effect was deemed present if and only if there was at least one cluster of significant effects (p < 0.05 at each sample) within the time window of interest, with pclust < 0.075.
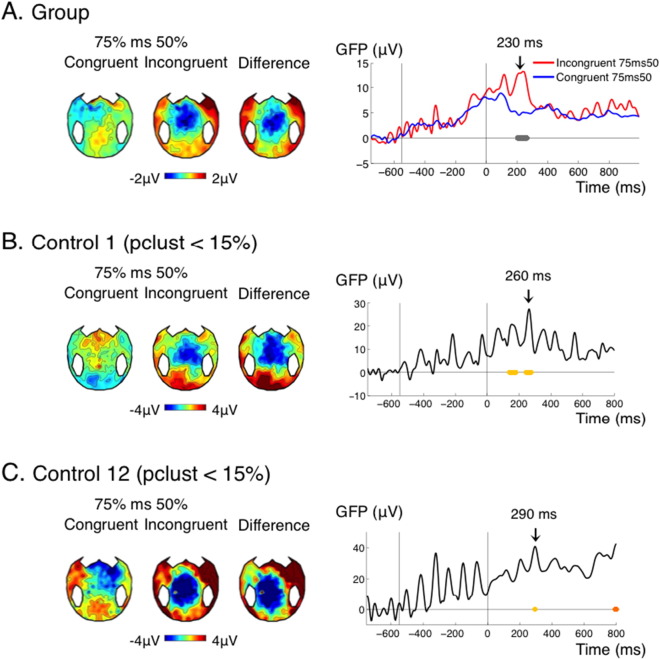
Supplementary Fig. 1.
False negatives in controls argue for a pragmatic threshold of pclust < 0.075 in cluster-level statistics.
(A) effect of global incongruence detection for the control group. (B) and (C) effect of global incongruence detection in two controls who show the effect in their temporal profile and in their topographies, but for whom pclust, the pvalue at the cluster level, does not satisfy the classical threshold at 0.05. To prevent such false negatives, based on the analysis of the controls, we set a pragmatic threshold at pclust < 0.075. With this slightly higher threshold, Control 1 and 12 are correctly detected as having the effect.
The time window of interest varied with the different contrasts, based on minimal a priori assumptions over the time period where the effect should occur. For own name versus other name it was the interval between cue and target i.e. − 550 to 0 ms; for 75% context versus 50% context it was − 500 ms to 500 ms; for spatial congruence effect and its strategic modulation it was 100 ms to 800 ms; for the simple “target Right versus Left” contrast it was 100 ms to 800 ms; for the effect of cue side, revealing spatial attention and motor anticipation before actual response to the target, the time interval was − 400 to + 200 ms (any effect beyond 200 ms could reflect actual motor response to the target); the same time window was used for the strategic modulation of this effect by context.
2.5.2. Group-level GFP statistics in control subjects
The GFP statistics for the control group followed the same logic as the individual statistics, using surrogate distributions through randomization of the condition labels across subjects. In contrast with the individual statistics, the first level threshold for the group GFP statistics was calculated at each time and not across all time points, so that the first-level threshold varied with time. Indeed, at the group level, randomizations still retained general trends with time such as Contingent Negative Variations.
2.5.3. Analysis of the contingent negative variation (temporal attention)
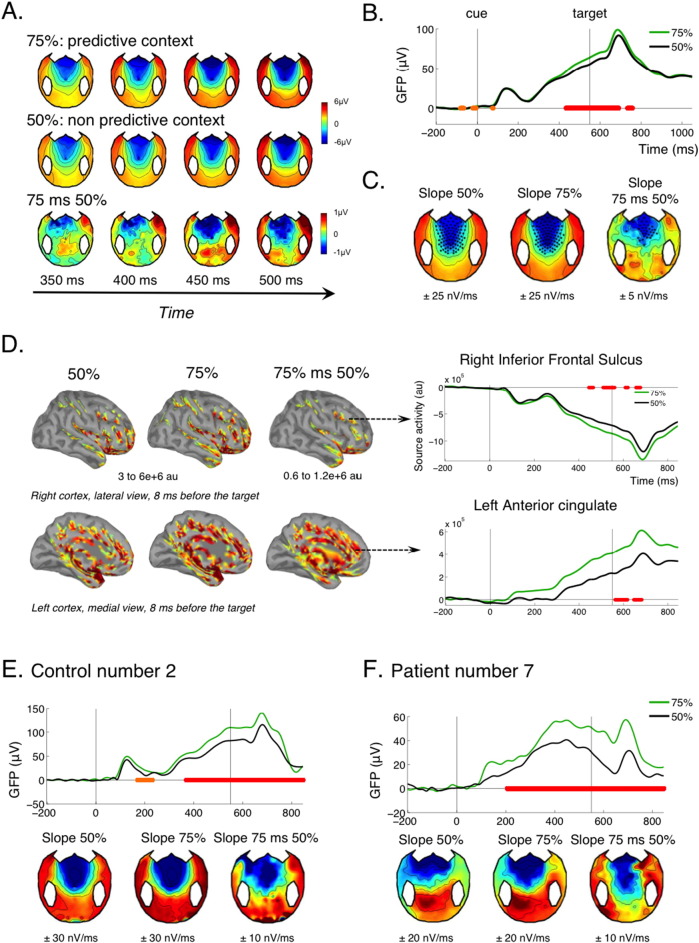
The Contingent Negative Variation (CNV) is a classical waveform that is observed when a first event makes the subject anticipate a second event (Walter et al., 1964). It is characterized by a fronto-central negativity whose intensity gradually builds up as the anticipated time of the second event approaches. In the present design, the onset of the target is entirely predictable following the cue, since the SOA is fixed at 550 ms. A strong CNV is thus expected in all experimental conditions. Furthermore, the CNV is modulated by factors that increase the predictable character of the second target. In the present protocol, we have such a manipulation: in separate session, the side of the probe either predicts the side of the target (75% sessions) or not (50% sessions). If subjects realize this spatial regularity, we should observe an additional strategic modulation of the CNV, with a steeper slope in predictive than in non-predictive sessions.
The topography of the CNV for the control group was used as a mask for probing the presence of a CNV and modulation of CNV for each individual. In the control group analysis, we first estimated for each control subject the slope of the evoked potential across all trials between − 300 ms and − 100 ms before target at each electrode site, using a linear regression of voltage over time. Electrodes showing a significant negative slope at the group level (t-test of the slopes against zero, p < 0.05, Bonferroni corrected) were considered as the CNV specific electrodes (SI Fig. 2, top of the left column) and used as electrodes of interest for individual patients and controls.
Supplementary Fig. 2.
Individual topographies of the CNV and modulation of CNV.
For each patient and each control, we estimated the slope between − 300 ms and − 100 ms for each electrode of interest in each trial, using a linear regression of voltage against time. An individual was considered having a CNV effect if > 10 of the electrodes of interest showed a significant negative slope across all experimental trials (t-test of the slopes against zero, p < 0.05). Furthermore, an individual was considered having a strategic modulation of the CNV if > 10 of the electrodes of interest showed a more negative slope in predictive (75%) than in non-predictive (50%) sessions (two-sample t-test of the slopes in both conditions, p < 0.05). A “significant” CNV modulation was not considered if the primary CNV effect was absent.
2.5.4. Group comparisons statistics
We tested whether the outcome of our cognitive assessments varied according to the clinical categories by computing the Bayes factor of the corresponding contingency tables (Gunel and Dickey, 1974) using the JASP software (JASP, 2016). We report the Bayes factors using the Raftery terminology (Raftery, 1995).
2.5.5. Source reconstruction
The cortical sources of the grand average effects observed in the control group were reconstructed using the Brainstorm software (Tadel et al., 2011). The reconstruction was based on the MNI anatomical template “Colin 27”. The overlapping spheres method was used for computing the forward model. The dSPM method was used for source reconstruction (Dale et al., 2000). For cortical sources visualization in the figures, source activity cutoff was set at half of the maximum on the scale, with a minimum size of 10 vertices.
3. Results and discussion
3.1. Behavioral results in the control group
In the control study, participants had to press a button as fast as possible on the side of the target, allowing a behavioral analysis. For each participant and each of our 12 experimental conditions we excluded reaction times (RTs) below 90 ms and above 1000 ms and extracted the median reaction time across the remaining trials (a similar pattern of results was obtained when using the mean instead of the median). Fig. 1B shows the group average RT and accuracy. Statistical significance of the behavioral effects were assessed through repeated measures ANOVAs on RTs and accuracy, with factors of Context (50% or 75%), Cue Type (Own name, Other name, Non vocal), and Spatial Congruence (Incongruent or Congruent), and separate repeated measures ANOVAs within each Context.
In the 50% context, although the cue was not predictive of the target side, participants were faster at reporting the target's side when it was preceded by a spatially congruent cue, irrespective of the cue type (ANOVA restricted to 50%: F(1.00,14.00) = 16.8, p = 0.001). They were also more accurate for congruent than incongruent cues (ANOVA restricted to 50%: F(1.00,14.00) = 7.9, p = 0.014). So both RT and accuracy indicated that the irrelevant cue automatically attracted spatial attention, speeding and improving the response to the target when it was on the same side. In sessions where the cue side predicted the target side with 75% probability, reaction times for congruent cues further improved by approximately 40 ms, while staying at a similar level for incongruent cues. This yielded an even stronger difference in RTs between spatially congruent and incongruent trials in the 75% context, irrespective of cue type (ANOVA restricted to 75%, main effect of congruence: F(1.00,14.00) = 37.5, p < 0.001; general ANOVA, context x congruence interaction: F(1.00,14.00) = 27.3, p < 0.001). This massive improvement in RT for congruent cues in the 75% context was mirrored by an important drop in accuracy for incongruent trials, yielding an even greater difference in accuracy between congruent and incongruent cues in the predictive than in the non-predictive context (ANOVA restricted to 75%, main effect of congruence: F(1.00,14.00) = 17.9, p = 0.001; general ANOVA context x congruence interaction: F(1.00,14.00) = 19.2, p = 0.001).
In contrast, there was no indication of an effect of the cue type, neither in RTs nor in accuracy.
In conclusion, this pattern of results suggests that control subjects showed automatic orienting of attention in the 50% context. In the 75% context they strategically used the predictability of the cue to prepare their response to the target, thus yielding faster responses in congruent trials, at a cost of a higher error rate in the 25% of trials where the target was not on the same side as the cue.
3.2. Own name recognition
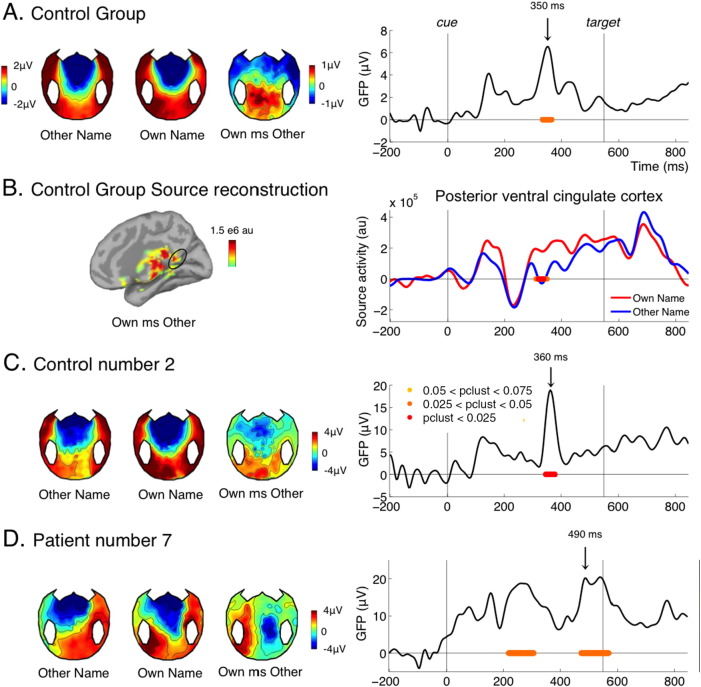
Contrasting the processing of the participant's own name versus the other name in the control group revealed the classical P300 to own name: a positive waveform over centro-parietal and occipital electrodes occurring around 300 ms after stimulus presentation (Berlad and Pratt, 1995, Folmer and Yingling, 1997) (Fig. 2A). The separate topographies for own name and other name were dominated by the CNV (fronto-central negativity, detailed in next section) so that the P300 to own names was only visible in the subtraction. According to source reconstruction (Fig. 2B.), an important source of the effect was located in midline structures, particularly in the posterior ventral cingulate cortex, as identified in Destrieux parcellation (Destrieux et al., 2010). This matches the literature on self-referential processes, which identifies the posterior cingulate cortex as a key component (Buckner and Carroll, 2007). One study directly testing the PET activity correlating with the P300 to own name also pointed to two midline structures: the precuneus (slightly above the posterior cingulate cortex identified here) and the medial prefrontal cortex (Perrin et al., 2005).
Fig. 2.
Own name perception.
(A) ERP contrast between trials where the cue was the participant's own name versus the other name for the control group. The left panel shows the topographies evoked by the other name, the own name, and the difference at the time of peak of the group effect (350 ms). The right panel shows the time course of the GFP of the own name versus other name difference for the control group. (B) source reconstruction of the effect for the control group at the time of the peak (350 ms), and time course of reconstructed activity in the posterior ventral cingulate cortex (Destrieux parcellation). (C and D) Topographies and GFP time course of the effect for one individual control (C) and for one individual patient (D). For all GFP time courses, periods where the cluster-level p-value was below 0.075 are indicated under the curve by red to yellow lines with the following convention: red for pclust < 0.025, orange for 0.025 < pclust < 0.05, yellow for 0.05 < pclust < 0.075 (as indicated in C, right panel). For the source activity (B, right panel) the same convention applies but for uncorrected p values (standard sample by sample t-test with no correction).
The P300 response to own name has been shown to occur even in the absence of consciousness, notably during sleep stage II, although with a delayed latency, around 600 ms (Perrin et al., 1999). Previous studies on patients have shown that a P300 to the patient's own name can be elicited in most MCS patients and even in some VS patients, although again the latency was around 600 ms (Perrin et al., 2006). In the present study we observed a significant P300 effect in most but not all control subjects (9/15, Table 1 and Fig. 2C for one sample control subject). The effect was present in 4/8 MCS patients, and 1/4 VS patients (Table 1). It was absent in the conscious patient. As expected from the literature, for most patients showing the effect, the latency of the P300 to own name was shifted in time. It was the case for our sample patient, MCS7 (Fig. 2D). For this patient, the P300 also showed an atypical left lateralization, which might be due to the patient's right craniotomy and diffuse lesions over the right hemisphere.
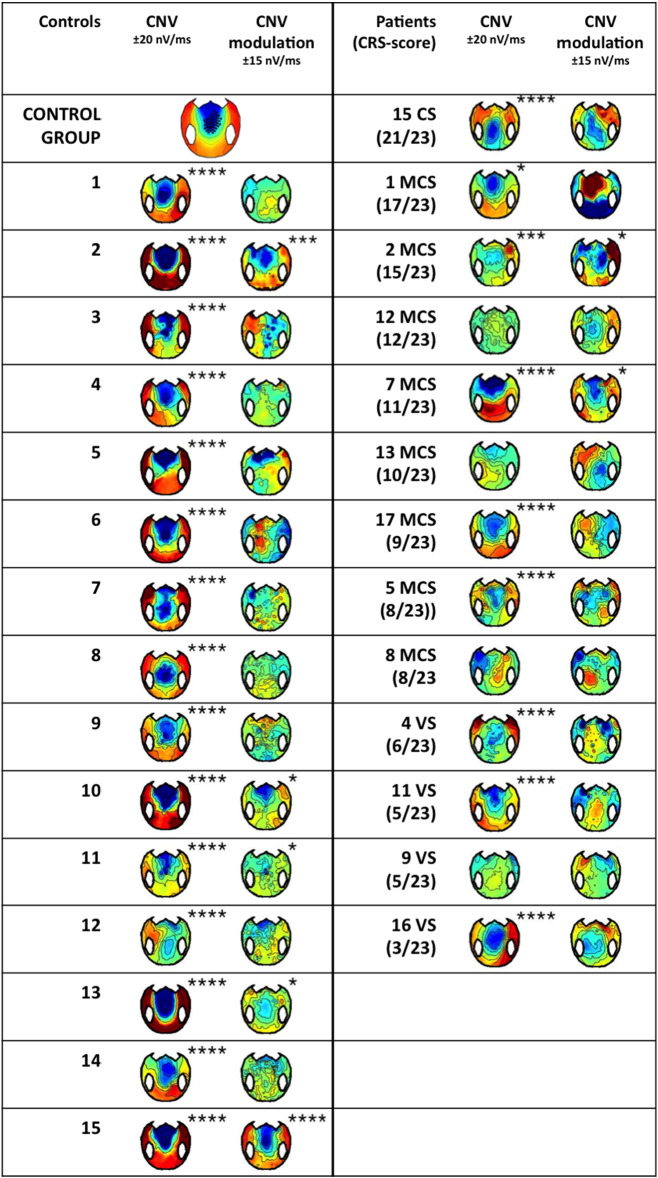
Table 1.
Individual EEG results.
An effect was considered as present in one individual (“Yes”) if and only if there was at least one cluster of significant effects (p < 0.05 at each sample) within the time window of interest, with a cluster level p-value (pclust) lower than 0.075 (see Materials and methods). For the effects indicated as “present” with no further precision pclust < 0.05.
3.3. Temporal attention (CNV) and contextual modulation of temporal attention
Since there was a fixed temporal interval of 550 ms between the cue and the target, the cue predicted the moment of appearance of the target. This gave rise to a typical signature of temporal attention or “anticipation”: the Contingent Negative Variation or CNV (Walter et al., 1964). Because of the relatively short temporal interval between cue and target, the present paradigm only allowed to observe the early part of the CNV (Rohrbaugh et al., 1976), whose amplitude is thought to relate to physiological noradrenergic arousal (Brunia et al., 2012). In the control group, a typical CNV developed between the cue and the target across all conditions, in the form of a fronto-central negativity whose intensity increased gradually towards the expected time of the target (Fig. 3A–C.). This effect was already observable in the 50% sessions, where the cue only predicted the time of the target but not its side. It was further modulated by the context: in the blocks where the cue also predicted the side of the target at 75%, the slope of the CNV was steeper than in non-predictive blocks (50%) (Fig. 3A–C). This modulation was significant at the group level. Importantly, it was present before the target, during a time period where the stimulation was the same in the 75% or 50% context, and thus necessarily reflected knowledge of the general context. Contextual modulations of anticipation have been described in healthy subjects in many previous studies (Faugeras and Naccache, 2016, Scheibe et al., 2009, Scheibe et al., 2010). The contextual modulation observed here might be directly linked to the strategic switch observed in behavior: when the cue was predictive, control subjects prepared in advance their response to the target based on the cue side.
Fig. 3.
Temporal attention (CNV) and contextual modulation.
(A–D) CNV effect in the control group. (A) shows the evolution of the topography evoked by the cue, irrespective of its type, in the 200 ms preceding the target's expected time (at 550 ms). The frontal negativity characteristic of the CNV increases with time both in the unpredictive and predictive context, but with a steeper slope in the predictive context, as can be seen on the topographies of the difference. (B) Accordingly, the GFP gradually increased between the cue and the target, with a steeper slope in the predictive context (75% of congruent trials). Corrected p-value of the contextual effect (GFP of the difference between 75 and 50% context) are indicated on this graph, with the same convention as in Fig. 2. (C) Group averaged topographies of the slope (nV/ms) estimated using a linear regression of the voltage of each electrode between 300 ms and 100 ms before target for the 75% context, the 50% context and for the difference 75 minus 50%. Electrodes showing a significant negative slope are highlighted (p < 0.05 uncorrected). (D) Reconstructed sources are shown for the 75% context, the 50% context and the difference at a time point where CNV culminates, just before target onset (− 8 ms). The graphs on the right show the activity of two selected sources in the right inferior frontal sulcus and in the left anterior cingulate (Destrieux parcellation). (E–F) GFP time courses and slope topographies for an individual control (E) and an individual patient (F). Conventions for statistical significance as in Fig. 2.
Previous MEG studies have identified the anterior cingulate cortex as a major cortical source of the CNV (Ioannides et al., 1994, Liu et al., 1996). This was confirmed with joint fMRI and EEG recording: fMRI activity in the anterior cingulate cortex correlates with trial-by-trial variations in the amplitude of the CNV (Nagai et al., 2004). Accordingly, source reconstruction revealed strong activation of the anterior cingulate as well as dorsolateral prefrontal cortex just before target onset, with a temporal profile that mirrored the CNV (Fig. 3D).
One previous study in our group pioneered the use of the CNV for probing temporal attention in non-communicating patients (Faugeras et al., 2012). This study used the local-global protocol (Bekinschtein et al., 2009): each trial consisted of a series of 5 regularly spaced sounds played over a period of 650 ms. In control participants, these series triggered a CNV that started with the first sound and resolved shortly after the last sound. A CNV was observed for some MCS as well as some VS patients, suggesting that this “first level” CNV reflected an “automatic” anticipation effect. However, its presence was associated with a higher chance of also showing a “global effect”, a specific marker of consciousness. So, although not specific of conscious processing, the presence of a CNV might sign the preservation of important precursors of consciousness. Indeed, it reflects the preservation of at least some functions of the anterior cingulate and prefrontal cortex, two regions that are often considered as essential nodes in conscious processing (Dehaene et al., 2006, Dehaene et al., 2003, Rees et al., 2002).
In the present study, a significant CNV was observed for each and every control subject, for the conscious patient, for 5/8 MCS patients and 3/4 VS patients (Table 1 and SI Fig. 2) confirming that a significant “first level” CNV can be observed in some VS patients.
The present protocol further probed whether the CNV was modulated by the strategic context. Although the strategic modulation of the CNV was significant at the group level for control subjects, only 5 out of 15 control subjects showed a significant modulation at the individual level (Table 1, Fig. 3E, SI Fig. 2). Two MCS patients showed a significant modulation (Fig. 3F), the conscious patient did not, and none of the VS patient did (Table 1, Fig. 3F, SI Fig. 2). As noticed before, this modulation denoted an understanding of the context over a large time scale. It could come both from the instructions at the beginning of each session, and from the integration of the contingencies over several trials, in both cases reflecting the preservation of high-level cognitive abilities (Bekinschtein et al., 2009, Faugeras et al., 2012, Faugeras et al., 2011). Interestingly, patient MCS 7, who showed a particularly neat profile of CNV modulation (Fig. 3F), also showed the best outcome from our pool of patients, with a GOS-E of 5 at 12 months after the accident, meaning that he was able to look after himself at home (Table 1). This might hint towards a potential prognostic value of this new marker, which will be further investigated in future studies.
3.4. Lateralized Potential to the cue: spatial attention and motor anticipation
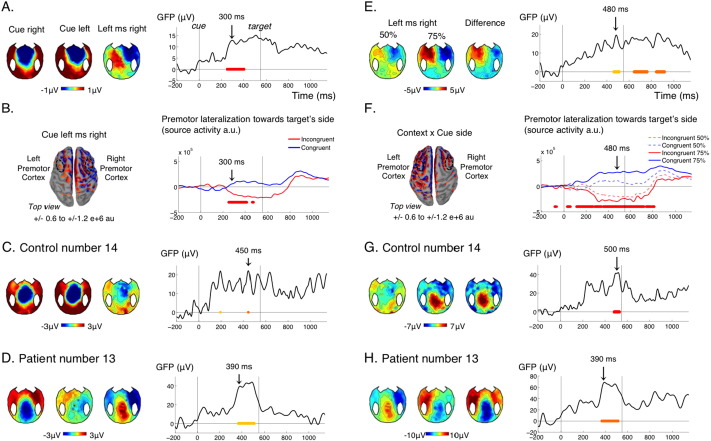
Another major novelty of the present protocol is the probing of spatial attention. In the 50% context, there was no strategic advantage in orienting attention to the cue or prepare a response to it, because the cue side was unrelated to the target side. Still, the reaction times to the target were influenced by whether the preceding cue had been presented on the same or the other side (Fig. 1B.), denoting an automatic orienting of spatial attention by the cue. In the control group, the cues elicited a negative bias over the controlateral fronto-central electrodes around 300 ms after the cue, such that the “left minus right cues difference” showed a bipolar topography over fronto-central electrodes (Fig. 4A). This effect cannot be attributed to the slightly asymmetrical processing of lateralized sounds by the contralateral auditory cortex: if it had been the case, it would have been observed at the time of the auditory evoked N1 (100 ms after the cue) with a less anterior focus. Instead, the latency and topography of this effect were typical of an Anterior Directing Attention Negativity (ADAN), which reflects a shift of attention induced by spatial cueing (Eimer and Driver, 2001, Eimer and Van Velzen, 2002, Seiss et al., 2007). Source reconstruction indicated lateralized activations in the frontal eye field and premotor cortices (Fig. 4B.). These sources were in accordance with the generators of the ADAN (Praamstra et al., 2005). Furthermore, since participants were asked to produce a lateralized response on the same side as the lateralized stimulation, the present ERPs probably showed a mixture of ADAN and cue-related lateralized readiness potential (LRP), a motor preparation component (Deecke et al., 1976, Kutas and Donchin, 1980). We derived an index of lateralization of activity in the premotor cortex in favor of the correct response to the target by performing the following subtraction: Right Premotor (Target Right - Left) – Left Premotor (Target Right - Left). As expected, this index showed a positive bump after the target and just before the actual button press (Fig. 4B right panel). What happened to this index before the target depended on the spatial congruence of the cue: in congruent trials (blue), the cue biased in advance this index towards the future side of the target, whereas in incongruent trials (red), the cue biases this index in the opposite direction. We can see that this incorrect attentional and premotor bias introduced a slight delay in the correct motor preparation in response to the target (around 200 ms post-target), which probably explains the delay in reaction times (Fig. 1B).
Fig. 4.
Spatial attention (ADAN) and contextual modulation.
(A–D) Effect of cue side in the non-predictive context (50%). (A) GFP time course of the effect of cue side (right panel) and corresponding topographies at 300 ms (left panel) for the control group. (B) Source reconstruction for the control group at 300 ms (left panel) and lateralization of the reconstructed activity in premotor cortices towards the future target's side (premotor activity ipsilateral minus contralateral to the future target's side) when the cue is on the same side as the future target (congruent, blue) or on the other side (incongruent, red). The premotor cortices correspond to the left and right middle frontal gyri of Destrieux parcellation. (C–D) Topographies and time course of the effect of cue side in an individual control (C) and an individual patient (D). Time of the topographies is indicated with an arrow on the corresponding GFP time course. (E–H) Modulation of the effect of cue side by the predictive context in the control group (E–F) and in the two individuals (G–H). Conventions for statistical significance as in Fig. 2.
This attention shift and action anticipation to the cue side in the 50% context was observed in only 8 out of 15 control subjects (Fig. 4C, Table 1). It was not present in the conscious patient. It was the only marker that was present in a greater proportion of VS patients (2/4) than MCS patients (1/8) (Fig. 4D, Table 1). It is noteworthy that, in the present protocol, lateralized solicitations were very frequent, which could induce habituation in the attentional orienting system. This marker might paradoxically detect patients with a deficient habituation mechanism, a hypothesis that remains to be formally tested.
3.5. Contextual modulation of lateralized potential to the cue
The lateralized potential to the cue (ADAN) was further modulated by the strategic context. In the 75% context, in contrast with the 50% context, there was a strong strategic advantage in shifting attention to the cue side and planning a movement based on it, since the prepared response could readily be applied to the target on 75% of the trials. So, if participants understood the task, the attention shift and action anticipation triggered by the cue should be much stronger in the 75% context. This contextual modulation was indeed observed in the control group: the lateralized ERP triggered by the cue was similar in topography but significantly stronger in the 75% than in the 50% context (Fig. 4E). In sources reconstruction, activity in premotor cortices before the target was even more biased by the cue side in the 75% sessions, resulting in a significant interaction of cue side with context on the lateralization index in premotor cortex (Fig. 4F).
Although this strategic effect was significant at the group level for controls, in individual tests it was found significant in only 3 out of 15 control subjects, (Fig. 4G, Table 1). This suggests that our correction for multiple comparisons at the individual level might be too drastic. Significant contextual modulation was observed in the conscious patient, in one MCS patient but no VS patient (Fig. 4H, Table 1).
3.6. Local incongruence detection
When the target appeared it could be on the same side as the preceding cue (congruent) or on the other side (incongruent). For spatially incongruent targets, in contrast with congruent ones, we expected activations linked to the reorientation of attention as well as the monitoring of response conflicts (Petersen and Posner, 2012, Van Veen and Carter, 2002). Indeed, in incongruent trials, the cue attracted attention to one side of space, and the target triggered a reorientation to the other side; furthermore, the cue primed a motor response that was opposite to the response to the target, a conflict that needed to be resolved in order to produce the correct response. Both aspects reflect some incongruence detection.
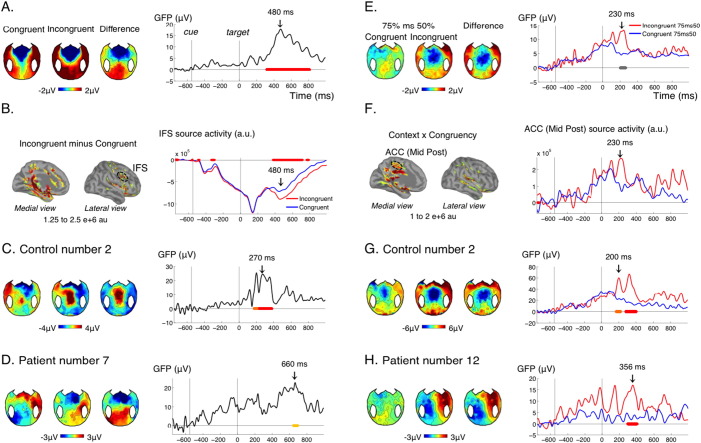
We first examined the spatial congruence effect in the 50% sessions, where it denoted “automatic” orienting to the cue side despite its irrelevance to the task. In the control group, this contrast resulted in a strong and sustained effect between 300 and 800 ms after the target (Fig. 5A). The topography of this effect showed a strong negative focus over frontocentral electrodes, and a strong positive focus over centro-occipital electrodes, which is reminiscent of a P300. This result is compatible with previous findings on spatial auditory attention in control subjects (Schroger and Eimer, 1996). In reconstruction, we found important sources of this effect in the right inferior frontal sulcus, a key area of the network involved in reorienting attention (Corbetta et al., 2002, Corbetta and Shulman, 2002, Petersen and Posner, 2012) (Fig. 5B). We also found activation in the anterior cingulate as expected from previous studies on conflict monitoring (Van Veen and Carter, 2002). This incongruence detection effect (reorienting and conflict monitoring) was present in 11 of our 15 control subjects (Table 1, Fig. 5C). It was present in the conscious patient, in 4 of the 8 MCS patients, and 1 of the 4 VS patients (Table 1, Fig. 5D).
Fig. 5.
Local and global incongruence detection.
(A–D) Effect of cue-target incongruence in the non-predictive context (50%), reflecting local incongruence detection (A) GFP time course of the effect of cue-target congruence (right panel) and corresponding topographies at 480 ms (left panel) for the control group. (B) Source reconstruction of the effect for the control group at 480 ms (left panel) and activity in the Inferior Frontal Sulcus (IFS, Destrieux) in the congruent and incongruent conditions. (C–D) Topographies and time course of the effect in an individual control (C) and an individual patient (D). (E–H) Interaction between context and cue-target congruence. (E) In the control group, incongruent trials were processed differently in the predictive versus non-predictive context, while congruent trials were processes similarly in the two contexts, reflecting global incongruence detection. (F) In source reconstruction this effect could be seen for example in the middle posterior part of the anterior cingulated cortex (ACC). (G–H) Topographies and time course of the effect in an individual control (G) and an individual patient (H). None of the patients showed both the local and global incongruence effect, hence different individual patients were chosen to illustrate these two effects. Conventions for statistical significance as in Fig. 2. In (E) right panel, the cluster shown in grey had a corrected p value of 0.25.
3.7. Global incongruence detection
Again, our protocol allowed investigating how the strategic context interacted with spatial incongruence detection. In the control group, this analysis revealed a short period of interaction (~ 70 ms) around 230 ms after the target (Fig. 5E). Although this effect was significant for uncorrected p-values, after correction for multiple comparisons with our procedure (see Materials and methods) its cluster p value was 0.12. This could be explained by the relatively short-lived nature of this effect, such that variations in latency across participants could have prevented observing a strong effect at the group level. Breaking down this interaction into its different subparts revealed that incongruent targets received a different treatment in the 75% context compared to the 50% context: although stimulation was physically the same in both contexts, incongruent targets in the 75% context induced a stronger transient central negativity around 230 ms, just before the response (Fig. 5A). This central negativity could reflect the detection of a “global deviant”: incongruent targets were rare events in the 75% context, but not in the 50% context. In line with this interpretation, congruent targets, which were frequent in both contexts, did not evoke such differential activity. Although the topography was reminiscent of the MMN, it is important to note that the detection of deviance across 75% and 50% context necessarily implied integrating probabilities over several trials, i.e. several tens of seconds, a time scale for which the classical “automatic” MMN disappears. In studies using a “local-global” paradigm, the processing of global deviants starts with a central negativity around 200 ms, and is followed by a strong and sustained positivity (Bekinschtein et al., 2009, Faugeras et al., 2012, Faugeras et al., 2011). It seems that in the present protocol, only the early part of this global deviance detection effect was observed. Source reconstruction suggested the involvement of the caudal part of the anterior cingulate cortex (Fig. 5F). This effect was found significant in 8/15 controls (Fig. 5G, Table 1), 1 MCS patient and 0/5 VS patients (Fig. 5H, Table 1).
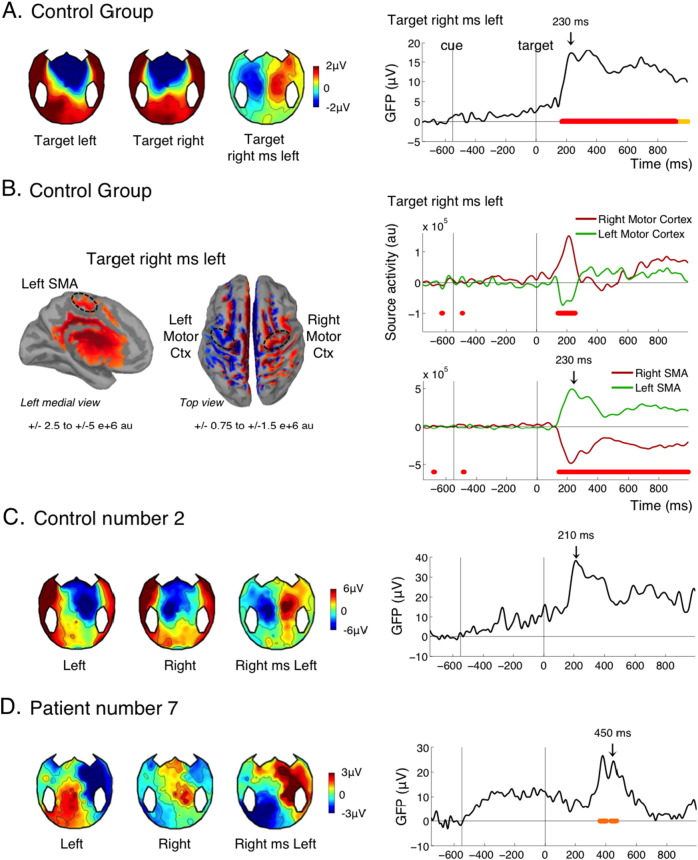
3.8. Lateralized readiness potential to the target: motor planning
As mentioned before, the present experiment was not passive listening: all healthy volunteers were required to indicate the side of the target by pressing a button with their right or left hand. Similarly, patients were instructed verbally, and reminded at the beginning of each block, to squeeze or imagine squeezing their right of left hand in accordance to the side of the target. In control subjects we observed, as expected, a typical lateralized readiness potential, or LRP (Deecke et al., 1976, Kutas and Donchin, 1980): around 200 ms after the target, just before the actual button press, the potential was more negative over the motor cortex controlateral to the response, so that the contrast “right minus left target” yielded a typical lateralized topography (Fig. 6A). Accordingly, source reconstruction revealed lateralized activations notably in the motor cortex and supplementary motor area (SMA; Fig. 6B). A significant LRP was found in 8 out of 15 controls (Table 1), suggesting that our threshold for individual statistics was too strict in this instance, since all control participants did perform the motor task behaviorally. As an example, control number 2 clearly showed a LRP with a standard time course and topography, however this effect was not found significant after our correction for multiple comparisons (Fig. 6C). A significant LRP was found in 2/8 MCS patients, but none of the VS patients. Importantly, this means that these two MCS patients were trying to perform the instructed task, and were imagining squeezing their hand on the side of the target. One of these two MCS patients was the patient with the best outcome (Fig. 6D). The slightly atypical topography can be attributed to the right injury and craniotomy of this patient.
Fig. 6.
Motor planning (LRP).
(A) Effect of target side in the control group, mostly reflecting a lateralized readiness potential (LRP). Topographies of the LRP at 230 ms are shown on the left panel. The time course of the GFP is shown on the right. (B) Source reconstruction of the target left minus right difference in the control group at 230 ms (left panel) and time course of the activity in the left and right motor cortex and SMA. Left and Right motor cortex were selected using the Destrieux parcellation (left and right precentral gyri). For selecting the supplementary motor areas (SMAs), we used the left and right paracentral regions from the Desikan-Killiany parcellation, the Destrieux parcellation being less precise in this region. (C-D) Topographies and time course of the effect in an individual control (C) and an individual patient (D). Conventions for statistical significance as in Fig. 2.
Note that we failed to observe evidence of command following in the 4 VS patients we tested here using the LRP marker. Obviously our effective is too small to draw any conclusion, but this observation may be discussed in the light of previous diverging reports about the ability to detect motor preparation in VS patients using EEG spectral power modulations (Cruse et al., 2011, Cruse et al., 2012, Goldfine et al., 2013, Goldfine et al., 2011). Other studies, using electromyography or fMRI, hinted towards the possibility to detect motor planning in some presumably misdiagnosed VS patients (Bekinschtein et al., 2008, Bekinschtein et al., 2011), but this issue is still under debate.
3.9. Synthesizing the multi-dimensional effects
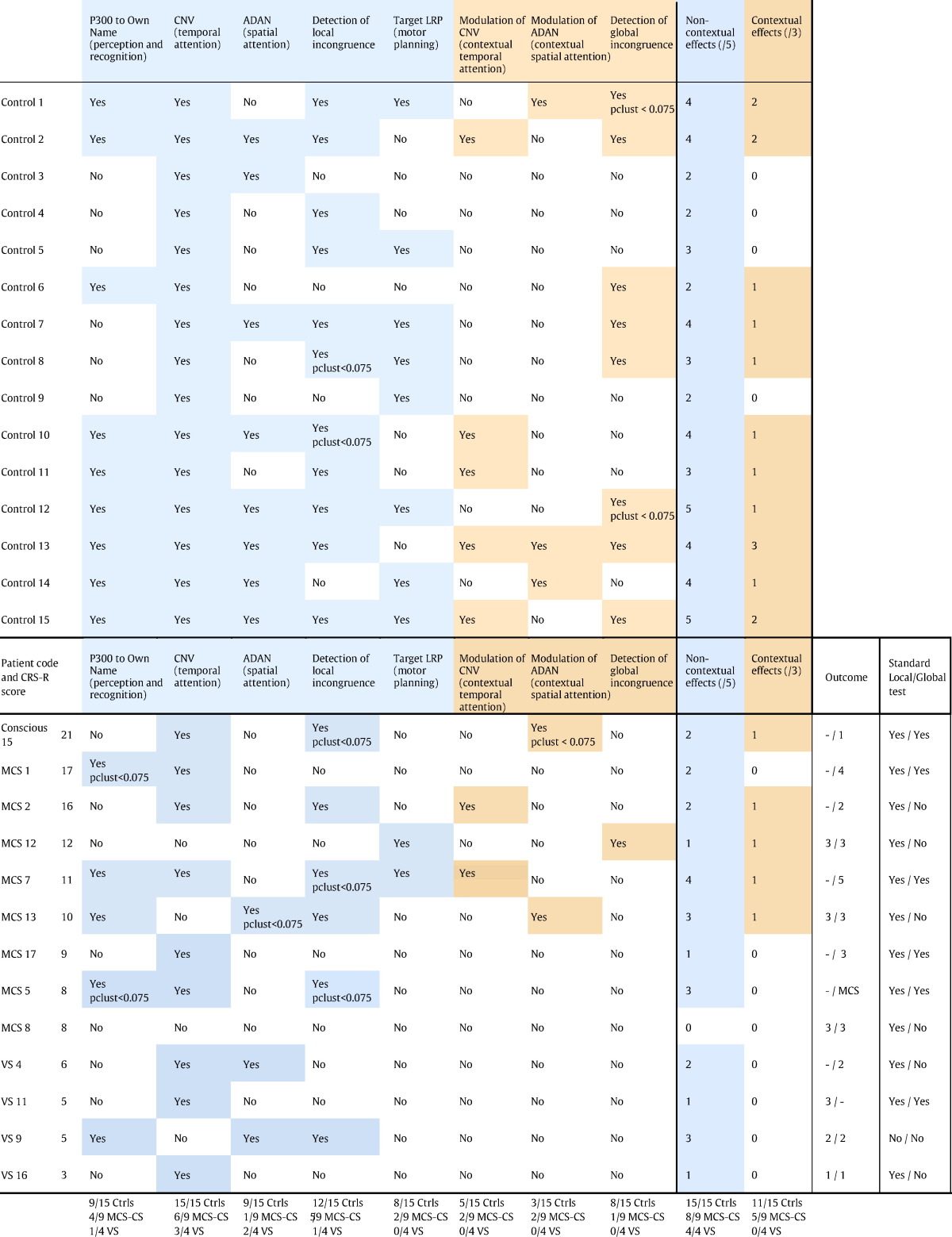
In summary, we could derive, from a single protocol, 8 different ERP indexes probing multiple cognitive domains with different levels of complexity (Table 1, Fig. 7). We had 5 low-level effects and 3 high-level effects, which were modulations of a low-level effect by the general context (75% or 50%). The different dimensions were:
-
1.
Perception (Own name recognition)
-
2.
Temporal attention (CNV)
-
3.
Spatial attention and anticipation of future action (ADAN to the cue)
-
4.
Local incongruence detection (effect of spatial incongruence in the 50% context)
-
5.
Motor planning (LRP to the target)
-
6.
Contextual modulation of temporal attention (modulation of the CNV)
-
7.
Contextual modulation of spatial attention (modulation of ADAN to the cue)
-
8.
Global incongruence detection (modulation of spatial incongruence detection by the global context)
Fig. 7.
EEG cognitive charts.
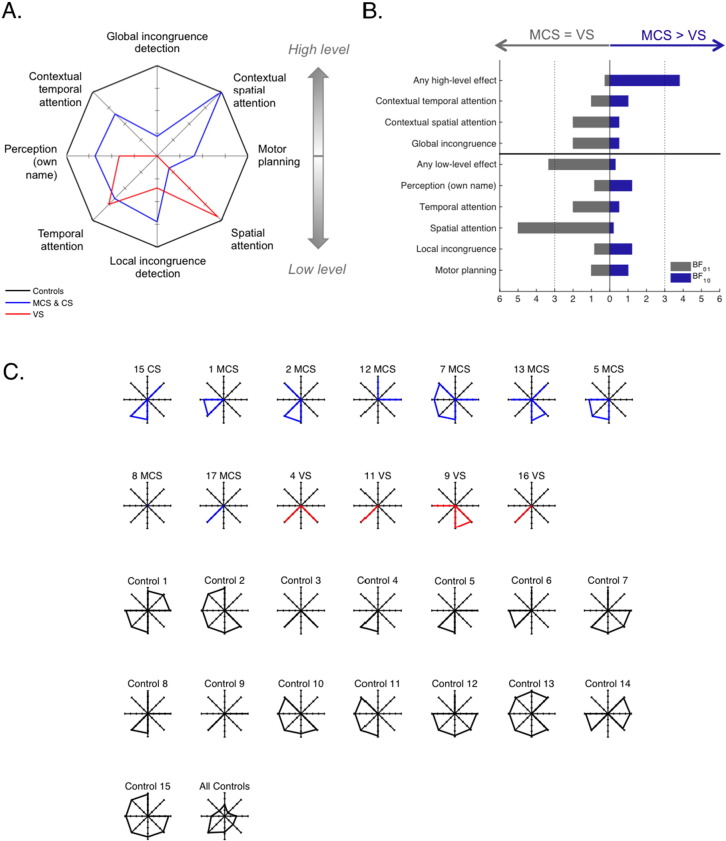
(A) Average statistical scores for the MCS + Conscious group and the VS group over the 8 cognitive dimensions tested represented on a radar/spider plot. On this graph, the average scores in the control group were set to 100%, and the scores in the other groups were normalized according to this reference. (B) Bayes Factors in favor of hypothesis 1 (MCS > VS) over hypothesis 0 (MCS = VS) and conversely (BF10 and BF01, with BF10 = 1/BF01) on the different cognitive dimensions. The dotted lines denote the value beyond which there is positive evidence in favor of one of the hypothesis (BF > 3) according to Raftery's terminology (Raftery, 1995) (C) Cognitive chart of each individual, without normalization.
Following the same logic as clinical evaluation using a CRS-R scale, the present protocol aimed at establishing for each patient a chart of their residual cognitive capacities using ERPs. We represented these ERP cognitive charts by plotting the statistical level of each ERP effect (1-pclust in %) on a 8 axes spider plot, one for each ERP index (Fig. 7) with the first level effects at the bottom, and the high-level effects at the top. Fig. 7A shows the averaged statistical scores for the VS group and the MCS group (including the conscious patient), normalized to the control. The high level effects, on top of the chart, were only present in the MCS group, not in the VS group, while there was no obvious difference between groups for the low-level effects. This was confirmed by Bayesian statistics. Fig. 7.B. shows the Bayes factors for the distinction between the MCS and VS patients along the different cognitive dimensions. When taken individually, none of the cognitive dimensions was conclusively more represented in MCS than in VS patients (all BF10 < 3, (Raftery, 1995)), and there was positive evidence that low-level spatial attention was similar in the two groups (BF01 = 5). However, when combining dimensions, there was positive evidence that MCS patients were more susceptible that VS patients to show at least one high-level effect, any of them (BF10 = 3.8). Conversely there was positive evidence that the two groups were similar when considering the presence of at least one low-level effect, any of them (BF01 = 3.3). This illustrates that combining several cognitive dimensions allowed improvement in the patients' assessment.
The ERP cognitive charts for each individual are represented in Fig. 7C (see also Table 1). The absence of any effect in patient 8 (MCS) is suggestive of a recording problem that was not detected during preprocessing. The importance of combining several indexes, as revealed by the Bayesian statistics, is clearly understandable when looking at individual results. None of the MCS or conscious patients showed more than one strategic effect, but the type of index that revealed strategic processing varied across patients. If we had only used one high-level index, we would have detected at most 2 patients with indications of high-level processing, instead of the 5 patients detected here (Table 1).
Interestingly, the patient who showed the best outcome, and regained functional communication at 12 months (MCS7 in Table 1) not only showed a strategic effect at the time of the test, 12 month before, he also was the patient with the greatest number of low-level effects, including motor planning. This underlines another reason why considering multiple indices might be important: although strategic effects are probably good signs that central executive processors are partially working, preserved lower level functions such as own name recognition or local incongruence detection might be essential for constantly stimulating remaining central processes. It seems reasonable to assume that the potential for recovery relies on the conjunction of multiple preserved functions, in which case a multidimensional assessment of cognition might be interesting also for prognosis.
It is noteworthy that, even in control subjects, the detection of the different cognitive indexes was not perfect. This problem is not specific to the present protocol or methods: individual statistics in ERPs are notoriously difficult, and, to date, there is no consensus on the best criteria that should be used to detect an effect (Gabriel et al., 2016). Here, combining the different dimensions drastically improved detection rate, even in controls. So multidimensional testing might also be useful for compensating this methodological problem.
4. Conclusion
Both pragmatic and theoretical reasons now point to multidimensional testing as an obvious way forward for improving the evaluation of DOC patients, and advancing our understanding of these deficits (Bayne et al., 2016). The challenge was to build a practical test that efficiently probed multiple cognitive dimensions within a single protocol and a reasonable time slot, and to construct the corresponding analysis pipeline. In particular, we had to define technical landmarks to transform complex spatio-temporal EEG signals into simple neuro-cognitive scores. The results of this initial study suggest that the challenge has been met: for each of 15 individual controls and 13 individual patients, we could derive from a relatively complex experimental design, an interpretable chart of their cognitive abilities along 8 dimensions (Fig. 7). These charts come as direct echo of the theoretical proposition recently made by Bayne and collaborators (Bayne et al., 2016).
Although some improvements can still be made to the protocol and analysis in terms of statistical power, the advantages of multidimensional testing were already potent in this initial proof of concept. Probing three high-level dimensions instead of one boosted the sensitivity in detecting patients with some preserved central executive functions (5 patients instead of 2). Motor planning in response to instructions was only found in MCS, not VS patients, in accordance with the clinical evaluation. When tested on larger populations, this dimension offers the potential of detecting misdiagnosed VS patients who can covertly follow instructions (Bekinschtein et al., 2011, Cruse et al., 2011). Finally, there is some interesting indication that the potential for recovery might rely on the combination of some preserved central function (at least one high-level effect) with a large panel of preserved low-level functions.
The present multidimensional approach is compatible with the global workspace model of consciousness (Dehaene and Changeux, 2011, Dehaene et al., 2006): in this model, conscious access to a representation relies on the establishment of a stable phase of communication and reinforcement between the areas primarily holding the representation (e.g. sensory areas), and more central areas that are able to maintain and use this information in a purposeful, non-stereotyped manner (executive fronto-parietal areas). Therefore, although this model postulates the existence of a single core mechanism for consciousness, namely global sharing, this mechanism typically manifests itself in multiple cognitive dimensions. In this model, the prerequisites for conscious access to emerge would be some preserved attentional and executive functions, some preserved lower-level functions and preserved connectivity among these nodes. The protocol presented here probes several executive and lower-level functions, but it does not focus on connectivity per se. Our protocol can easily be complemented with tools evaluating various aspects of spontaneous dynamics of EEG complexity and functional connectivity (King et al., 2013, Sitt et al., 2014), which can be acquired “for free” during the resting phases between test sessions.
In conclusion, although it stands to reason that a better evaluation of patients should emerge from testing multiple cognitive dimensions in EEG, in practice there has been no attempt so far in this direction, presumably because of practical obstacles. Here we propose a compact protocol that probes 8 cognitive dimensions in one go, and demonstrate that combining these dimensions does improve the patients' evaluation compared to isolated dimensions. We hope that it can constitute a basis for improving the evaluation of DOC patients in the future.
The following are the supplementary data related to this article.
Acknowledgments
We thank Dominique Morlet and Sebastien Marti for methodological support and advice. We thank Viola Störmer, Paolo Bartolomeo, Sebastien Marti, Athena Demertzi and two anonymous reviewers for useful comments. This study was supported by a Marie Curie Reintegration Grant to Claire Sergent (FP7-PEOPLE-ERG-2008), the ANR grant “CogniComa” (ANR-14-CE-0013-03), the Fondation pour la Recherche Médicale (‘Equipe FRM 2015’ grant), the program ‘Investissements d'avenir’ ANR-10-IAIHU-06, the ANR 14-CE15-0013-04, and the ‘Recovery of consciousness after severe brain injury Phase II’ grant of the James S. McDonnell Foundation.
References
- Bayne T., Hohwy J., Owen A.M. Are there levels of consciousness? Trends Cogn. Sci. 2016 doi: 10.1016/j.tics.2016.03.009. [DOI] [PubMed] [Google Scholar]
- Bekinschtein T.A., Coleman M.R., Niklison J., 3rd, Pickard J.D., Manes F.F. Can electromyography objectively detect voluntary movement in disorders of consciousness? J. Neurol. Neurosurg. Psychiatry. 2008;79(7):826–828. doi: 10.1136/jnnp.2007.132738. [DOI] [PubMed] [Google Scholar]
- Bekinschtein T.A., Dehaene S., Rohaut B., Tadel F., Cohen L., Naccache L. Neural signature of the conscious processing of auditory regularities. Proc. Natl. Acad. Sci. U. S. A. 2009;106(5):1672–1677. doi: 10.1073/pnas.0809667106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekinschtein T.A., Manes F.F., Villarreal M., Owen A.M., Della-Maggiore V. Functional imaging reveals movement preparatory activity in the vegetative state. Front. Hum. Neurosci. 2011;5:5. doi: 10.3389/fnhum.2011.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlad I., Pratt H. P300 in response to the subject's own name. Electroencephalogr. Clin. Neurophysiol. 1995;96(5):472–474. doi: 10.1016/0168-5597(95)00116-a. [DOI] [PubMed] [Google Scholar]
- Brunia C.H., Van Boxtel G.M., Böcker K.B., Luck S., Kappenman E. The Oxford Handbook of Event-related Potential Components. 2012. Negative slow waves as indices of anticipation: the Bereitschaftspotential, the contingent negative variation, and the stimulus-preceding negativity; pp. 189–207. [Google Scholar]
- Buckner R.L., Carroll D.C. Self-projection and the brain. Trends Cogn. Sci. 2007;11(2):49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Chennu S., Finoia P., Kamau E., Monti M.M., Allanson J., Pickard J.D.…Bekinschtein T.A. Dissociable endogenous and exogenous attention in disorders of consciousness. Neuroimage Clin. 2013;3:450–461. doi: 10.1016/j.nicl.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M.A., Alvarez G.A., Nakayama K. Natural-scene perception requires attention. Psychol. Sci. 2011;22(9):1165–1172. doi: 10.1177/0956797611419168. [DOI] [PubMed] [Google Scholar]
- Corbetta M., Kincade J.M., Shulman G.L. Neural systems for visual orienting and their relationships to spatial working memory. J. Cogn. Neurosci. 2002;14(3):508–523. doi: 10.1162/089892902317362029. [DOI] [PubMed] [Google Scholar]
- Corbetta M., Shulman G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Cruse D., Chennu S., Chatelle C., Bekinschtein T.A., Fernandez-Espejo D., Pickard J.D.…Owen A.M. Bedside detection of awareness in the vegetative state: a cohort study. Lancet. 2011;378(9809):2088–2094. doi: 10.1016/S0140-6736(11)61224-5. [DOI] [PubMed] [Google Scholar]
- Cruse D., Chennu S., Fernandez-Espejo D., Payne W.L., Young G.B., Owen A.M. Detecting awareness in the vegetative state: electroencephalographic evidence for attempted movements to command. PLoS ONE. 2012;7(11) doi: 10.1371/journal.pone.0049933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale A.M., Liu A.K., Fischl B.R., Buckner R.L., Belliveau J.W., Lewine J.D., Halgren E. Dynamic statistical parametric mapping: combining fMRI and MEG for high-resolution imaging of cortical activity. Neuron. 2000;26(1):55–67. doi: 10.1016/s0896-6273(00)81138-1. [DOI] [PubMed] [Google Scholar]
- Deecke L., Grozinger B., Kornhuber H.H. Voluntary finger movement in man: cerebral potentials and theory. Biol. Cybern. 1976;23(2):99–119. doi: 10.1007/BF00336013. [DOI] [PubMed] [Google Scholar]
- Dehaene S., Changeux J.P. Experimental and theoretical approaches to conscious processing. Neuron. 2011;70(2):200–227. doi: 10.1016/j.neuron.2011.03.018. [DOI] [PubMed] [Google Scholar]
- Dehaene S., Changeux J.P., Naccache L., Sackur J., Sergent C. Conscious, preconscious, and subliminal processing: a testable taxonomy. Trends Cogn. Sci. 2006;10(5):204–211. doi: 10.1016/j.tics.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Dehaene S., Sergent C., Changeux J.P. A neuronal network model linking subjective reports and objective physiological data during conscious perception. Proc. Natl. Acad. Sci. U. S. A. 2003;100:8520–8525. doi: 10.1073/pnas.1332574100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destrieux C., Fischl B., Dale A., Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. NeuroImage. 2010;53(1):1–15. doi: 10.1016/j.neuroimage.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eimer M., Driver J. Crossmodal links in endogenous and exogenous spatial attention: evidence from event-related brain potential studies. Neurosci. Biobehav. Rev. 2001;25(6):497–511. doi: 10.1016/s0149-7634(01)00029-x. [DOI] [PubMed] [Google Scholar]
- Eimer M., Van Velzen J. Crossmodal links in spatial attention are mediated by supramodal control processes: evidence from event-related potentials. Psychophysiology. 2002;39(4):437–449. doi: 10.1017.S0048577201393162. (10.1017.S0048577201393162) [DOI] [PubMed] [Google Scholar]
- Faugeras F., Naccache L. Dissociating temporal attention from spatial attention and motor response preparation: a high-density EEG study. NeuroImage. 2016;124(Pt A):947–957. doi: 10.1016/j.neuroimage.2015.09.051. [DOI] [PubMed] [Google Scholar]
- Faugeras F., Rohaut B., Weiss N., Bekinschtein T., Galanaud D., Puybasset L.…Naccache L. Event related potentials elicited by violations of auditory regularities in patients with impaired consciousness. Neuropsychologia. 2012;50(3):403–418. doi: 10.1016/j.neuropsychologia.2011.12.015. [DOI] [PubMed] [Google Scholar]
- Faugeras F., Rohaut B., Weiss N., Bekinschtein T.A., Galanaud D., Puybasset L.…Naccache L. Probing consciousness with event-related potentials in the vegetative state. Neurology. 2011;77(3):264–268. doi: 10.1212/WNL.0b013e3182217ee8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer C., Morlet D., Bouchet P., Luaute J., Jourdan C., Salord F. Mismatch negativity and late auditory evoked potentials in comatose patients. Clin. Neurophysiol. 1999;110(9):1601–1610. doi: 10.1016/s1388-2457(99)00131-5. [DOI] [PubMed] [Google Scholar]
- Folmer R.L., Yingling C.D. Auditory P3 responses to name stimuli. Brain Lang. 1997;56(2):306–311. doi: 10.1006/brln.1997.1828. [DOI] [PubMed] [Google Scholar]
- Gabriel D., Muzard E., Henriques J., Mignot C., Pazart L., Andre-Obadia N.…Moulin T. Replicability and impact of statistics in the detection of neural responses of consciousness. Brain. 2016;139(Pt 6) doi: 10.1093/brain/aww065. [DOI] [PubMed] [Google Scholar]
- Giacino J.T., Ashwal S., Childs N., Cranford R., Jennett B., Katz D.I.…Zasler N.D. The minimally conscious state: definition and diagnostic criteria. Neurology. 2002;58(3):349–353. doi: 10.1212/wnl.58.3.349. [DOI] [PubMed] [Google Scholar]
- Goldfine A.M., Bardin J.C., Noirhomme Q., Fins J.J., Schiff N.D., Victor J.D. Reanalysis of “bedside detection of awareness in the vegetative state: a cohort study”. Lancet. 2013;381(9863):289–291. doi: 10.1016/S0140-6736(13)60125-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfine A.M., Victor J.D., Conte M.M., Bardin J.C., Schiff N.D. Determination of awareness in patients with severe brain injury using EEG power spectral analysis. Clin. Neurophysiol. 2011;122(11):2157–2168. doi: 10.1016/j.clinph.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunel E., Dickey J. Bayes factors for independence in contingency-tables. Biometrika. 1974;61(3):545–557. [Google Scholar]
- Holeckova I., Fischer C., Giard M.H., Delpuech C., Morlet D. Brain responses to a subject's own name uttered by a familiar voice. Brain Res. 2006;1082(1):142–152. doi: 10.1016/j.brainres.2006.01.089. [DOI] [PubMed] [Google Scholar]
- Ioannides A.A., Fenwick P.B., Lumsden J., Liu M.J., Bamidis P.D., Squires K.C.…Fenton G.W. Activation sequence of discrete brain areas during cognitive processes: results from magnetic field tomography. Electroencephalogr. Clin. Neurophysiol. 1994;91(5):399–402. doi: 10.1016/0013-4694(94)90125-2. [DOI] [PubMed] [Google Scholar]
- JASP . 2016. JASP (Version 0.8.0.0) [Google Scholar]
- King J.R., Sitt J.D., Faugeras F., Rohaut B., El Karoui I., Cohen L.…Dehaene S. Information sharing in the brain indexes consciousness in noncommunicative patients. Curr. Biol. 2013;23(19):1914–1919. doi: 10.1016/j.cub.2013.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutas M., Donchin E. Preparation to respond as manifested by movement-related brain potentials. Brain Res. 1980;202(1):95–115. [PubMed] [Google Scholar]
- Laureys S., Celesia G.G., Cohadon F., Lavrijsen J., Leon-Carrion J., Sannita W.G.…European Task Force on Disorders of, C Unresponsive wakefulness syndrome: a new name for the vegetative state or apallic syndrome. BMC Med. 2010;8:68. doi: 10.1186/1741-7015-8-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann D., Skrandies W. Reference-free identification of components of checkerboard-evoked multichannel potential fields. Electroencephalogr. Clin. Neurophysiol. 1980;48(6):609–621. doi: 10.1016/0013-4694(80)90419-8. [DOI] [PubMed] [Google Scholar]
- Liu M.J., Fenwick P.B., Lumsden J., Lever C., Stephan K.M., Ioannides A.A. Averaged and single-trial analysis of cortical activation sequences in movement preparation, initiation, and inhibition. Hum. Brain Mapp. 1996;4(4):254–264. doi: 10.1002/(SICI)1097-0193(1996)4:4<254::AID-HBM3>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Luaute J., Fischer C., Adeleine P., Morlet D., Tell L., Boisson D. Late auditory and event-related potentials can be useful to predict good functional outcome after coma. Arch. Phys. Med. Rehabil. 2005;86(5):917–923. doi: 10.1016/j.apmr.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Mack A., Rock I. MIT Press; Cambridge, Mass.: 1998. Inattentional Blindness. [Google Scholar]
- Maris E., Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods. 2007;164(1):177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Naatanen R. The mismatch negativity: a powerful tool for cognitive neuroscience. Ear Hear. 1995;16(1):6–18. [PubMed] [Google Scholar]
- Nagai Y., Critchley H.D., Featherstone E., Fenwick P.B., Trimble M.R., Dolan R.J. Brain activity relating to the contingent negative variation: an fMRI investigation. NeuroImage. 2004;21(4):1232–1241. doi: 10.1016/j.neuroimage.2003.10.036. [DOI] [PubMed] [Google Scholar]
- O'Regan J.K., Rensink R.A., Clark J.J. Change-blindness as a result of ‘mudsplashes’. Nature. 1999;398(6722):34. doi: 10.1038/17953. [DOI] [PubMed] [Google Scholar]
- Oostenveld R., Fries P., Maris E., Schoffelen J.M. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput. Intell. Neurosci. 2011;2011:156869. doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson J.R., Grabois M. Locked-in syndrome: a review of 139 cases. Stroke. 1986;17(4):758–764. doi: 10.1161/01.str.17.4.758. [DOI] [PubMed] [Google Scholar]
- Perrin F., Garcia-Larrea L., Mauguiere F., Bastuji H. A differential brain response to the subject's own name persists during sleep. Clin. Neurophysiol. 1999;110(12):2153–2164. doi: 10.1016/s1388-2457(99)00177-7. [DOI] [PubMed] [Google Scholar]
- Perrin F., Maquet P., Peigneux P., Ruby P., Degueldre C., Balteau E.…Laureys S. Neural mechanisms involved in the detection of our first name: a combined ERPs and PET study. Neuropsychologia. 2005;43(1):12–19. doi: 10.1016/j.neuropsychologia.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Perrin F., Schnakers C., Schabus M., Degueldre C., Goldman S., Bredart S.…Laureys S. Brain response to one's own name in vegetative state, minimally conscious state, and locked-in syndrome. Arch. Neurol. 2006;63(4):562–569. doi: 10.1001/archneur.63.4.562. [DOI] [PubMed] [Google Scholar]
- Petersen S.E., Posner M.I. The attention system of the human brain: 20 years after. Annu. Rev. Neurosci. 2012;35:73–89. doi: 10.1146/annurev-neuro-062111-150525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plum F., Posner J.B. The diagnosis of stupor and coma. Contemp. Neurol. Ser. 1972;10:1–286. [PubMed] [Google Scholar]
- Posner M.I., Snyder C.R., Davidson B.J. Attention and the detection of signals. J. Exp. Psychol. 1980;109(2):160–174. [PubMed] [Google Scholar]
- Praamstra P., Boutsen L., Humphreys G.W. Frontoparietal control of spatial attention and motor intention in human EEG. J. Neurophysiol. 2005;94(1):764–774. doi: 10.1152/jn.01052.2004. [DOI] [PubMed] [Google Scholar]
- Raftery A.E. Bayesian model selection in social research. Sociol. Methodol. 1995;25(25):111–163. [Google Scholar]
- Rees G., Kreiman G., Koch C. Neural correlates of consciousness in humans. Nat. Rev. Neurosci. 2002;3(4):261–270. doi: 10.1038/nrn783. [DOI] [PubMed] [Google Scholar]
- Rensink R.A., O'Regan J.K., Clark J.J. To see or not to see: the need for attention to perceive changes in scenes. Psychol. Sci. 1997;8(5):368–373. [Google Scholar]
- Rohaut B., Faugeras F., Naccache L. Brain Disorders in Critical Illness: Mechanisms, Diagnosis, and Treatment. Vol. 59. 2013. Neurology of consciousness impairments. [Google Scholar]
- Rohrbaugh J.W., Syndulko K., Lindsley D.B. Brain wave components of the contingent negative variation in humans. Science. 1976;191(4231):1055–1057. doi: 10.1126/science.1251217. [DOI] [PubMed] [Google Scholar]
- Scheibe C., Schubert R., Sommer W., Heekeren H.R. Electrophysiological evidence for the effect of prior probability on response preparation. Psychophysiology. 2009;46(4):758–770. doi: 10.1111/j.1469-8986.2009.00825.x. [DOI] [PubMed] [Google Scholar]
- Scheibe C., Ullsperger M., Sommer W., Heekeren H.R. Effects of parametrical and trial-to-trial variation in prior probability processing revealed by simultaneous electroencephalogram/functional magnetic resonance imaging. J. Neurosci. 2010;30(49):16709–16717. doi: 10.1523/JNEUROSCI.3949-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnakers C., Majerus S., Giacino J., Vanhaudenhuyse A., Bruno M.A., Boly M.…Laureys S. A French validation study of the coma recovery scale-revised (CRS-R) Brain Inj. 2008;22(10):786–792. doi: 10.1080/02699050802403557. [DOI] [PubMed] [Google Scholar]
- Schnakers C., Vanhaudenhuyse A., Giacino J., Ventura M., Boly M., Majerus S.…Laureys S. Diagnostic accuracy of the vegetative and minimally conscious state: clinical consensus versus standardized neurobehavioral assessment. BMC Neurol. 2009;9:35. doi: 10.1186/1471-2377-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroger E., Eimer M. Effects of lateralized cues on the processing of lateralized auditory stimuli. Biol. Psychol. 1996;43(3):203–226. doi: 10.1016/0301-0511(96)05192-7. doi:0301051196051927 [pii] [DOI] [PubMed] [Google Scholar]
- Seiss E., Gherri E., Eardley A.F., Eimer M. Do ERP components triggered during attentional orienting represent supramodal attentional control? Psychophysiology. 2007;44(6):987–990. doi: 10.1111/j.1469-8986.2007.00591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergent C., Baillet S., Dehaene S. Timing of the brain events underlying access to consciousness during the attentional blink. Nat. Neurosci. 2005;8(10):1391–1400. doi: 10.1038/nn1549. [DOI] [PubMed] [Google Scholar]
- Sergent C., Dehaene S. Is consciousness a gradual phenomenon? Evidence for an all-or-none bifurcation during the attentional blink. Psychol. Sci. 2004;15(11):720–728. doi: 10.1111/j.0956-7976.2004.00748.x. [DOI] [PubMed] [Google Scholar]
- Sergent C., Wyart V., Babo-Rebelo M., Cohen L., Naccache L., Tallon-Baudry C. Cueing attention after the stimulus is gone can retrospectively trigger conscious perception. Curr. Biol. 2013;23(2):150–155. doi: 10.1016/j.cub.2012.11.047. [DOI] [PubMed] [Google Scholar]
- Sitt J.D., King J.R., El Karoui I., Rohaut B., Faugeras F., Gramfort A.…Naccache L. Large scale screening of neural signatures of consciousness in patients in a vegetative or minimally conscious state. Brain. 2014;137(Pt 8):2258–2270. doi: 10.1093/brain/awu141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadel F., Baillet S., Mosher J.C., Pantazis D., Leahy R.M. Brainstorm: a user-friendly application for MEG/EEG analysis. Comput Intell Neurosci. 2011;2011:879716. doi: 10.1155/2011/879716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault L., van den Berg R., Cavanagh P., Sergent C. Retrospective attention gates discrete conscious access to past sensory stimuli. PLoS ONE. 2016;11(2) doi: 10.1371/journal.pone.0148504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Veen V., Carter C.S. The timing of action-monitoring processes in the anterior cingulate cortex. J. Cogn. Neurosci. 2002;14(4):593–602. doi: 10.1162/08989290260045837. [DOI] [PubMed] [Google Scholar]
- Walter W.G., Cooper R., Aldridge V.J., McCallum W.C., Winter A.L. Contingent negative variation: an electric sign of sensorimotor association and expectancy in the human brain. Nature. 1964;203:380–384. doi: 10.1038/203380a0. [DOI] [PubMed] [Google Scholar]