Abstract
Total 25(OH)D levels were determined to assess bone health in elderly populations; however, the bioavailability of 25(OH)D is regulated by the albumin and vitamin D binding protein (DBP) levels and DBP variations. Whether bioavailable 25(OH)D level is a superior biomarker for vitamin D than total 25(OH)D level regarding the BMD and the bone metabolism were not yet fully understood. With a community based cross-sectional study of 967 postmenopausal women, we found that the variant rs7041, but not rs4588, of DBP was significantly associated with the blood DBP level, which was positively correlated with the total 25(OH)D level but negatively associated with bioavailable 25(OH)D levels. Both total and bioavailable 25(OH)D levels were significantly correlated with the BMD value in postmenopausal women; however, only the bioavailable 25(OH)D level was an independent determinant of the BMD values when adjusted for age, body mass index and bone turnover biomarkers (OST and β-CTX). The bioavailable and total 25(OH)D were negatively correlated with bone formation biomarkers (OST, PINP and ALP) and PTH levels, while they were positively correlated with osteoprotegerin (OPG) level; however, the bone resorption biomarker (β-CTX) was not correlated with the 25(OH)D levels. An increment of PTH level, along with reduced bioavailable 25(OH)D levels, was evident when the bioavailable 25(OH)D level was < 5 ng/mL, which may be the optimal cutpoint for sufficient vitamin D in Chinese elderly women. The blood calcium, magnesium, ALP, TSH, FGF23, and phosphorus levels were not correlated with the total or the bioavailable 25(OH)D levels. These results suggested that high bioavailable 25(OH)D levels were correlated with reduced bone turnover processes and were a biomarker superior to total 25(OH)D for vitamin D in assessing the risks of bone-related diseases. The results indicate that the bioavailable 25(OH)D level should be determined in assessing the bone health.
Keywords: Total 25(OH)D, Bioavailable 25(OH)D, Bone mineral density, Bone turnover biomarkers, Postmenopausal women
Highlights
-
•
DBP levels and variants on DBP were associated with the total and bioavailable 25(OH)D levels in the elderly populations.
-
•
Both the total and bioavailable 25(OH)D levels were correlated with the BMD in postmenopausal women.
-
•
Multivariate analyses suggested that the bioavailable but not total 25(OH)D was an independent determinant for the BMD.
-
•
Higher bioavailable 25(OH)D levels were correlated with reduced bone turnover and lower PTH in postmenopausal women.
With a cross-sectional community study, we found that the variant rs7041, but not rs4588, of DBP was significantly associated with the blood DBP level, which was positively correlated with the total 25(OH)D levels but negatively associated with the bioavailable 25(OH)D levels. The bioavailable 25(OH)D level was an independent determinant for BMD but not total 25(OH)D. Higher vitamin D levels were correlated with the reduced bone turnover process and lower PTH levels, which might lead to the higher BMD value in postmenopausal women. These results suggested that bioavailable 25(OH)D was a superior biomarker than total 25(OH)D regarding the bone metabolism, and that vitamin D intervention may improve the bone health in elderly populations.
1. Introduction
Over 50% of elderly people aged 50 years or older suffer from osteoporosis, which is a common and complex heath problem manifested by a decreased BMD and collateral damage to the bone microarchitecture (Wu and Du, 2016). The BMD value is measured in diagnosing of osteopenia and osteoporosis, and populations with a lower BMD may exhibit impaired skeletal strength and increased risk of fragility fractures. Epidemiological studies have identified age, sex, body weight, body mass index (BMI), living habits, dietary factors (including calcium, vitamin D, caffeine and alcohol etc.), physical activity levels, and genetic variations might influence the individual BMD status (Kumar et al., 2010, Waugh et al., 2009, Karasik et al., 2016, Nguyen et al., 2000). Intervention strategies that have been developed based on these findings may increase the BMD and subsequently reduce osteoporosis or risks of fragility fractures have been widely recommended by clinical physicians.
Vitamin D, an essential nutrient for humans, is obtained from the ingestion of vitamin D-containing foods or supplements or is synthesized from 7-dehydrocholesterol by skin cells following exposure to sunlight (Li et al., 2014). After absorption or synthesis, vitamin D can be converted into 25(OH)D by the 25-hydroxylase (CYP2R1) in the liver, and further converted into its biologically active form, 1α,25(OH)2D by the renal 25-hydroxyvitamin D-1α-hydroxylase (CYP27B1). 1α,25(OH)2D regulates about 3–5% of human genes through binding to its widely expressed nuclear receptor, VDR, which belongs to the steroid-thyroid-retinoid receptor superfamily of ligand-activated transcription factors (Feldman et al., 2014). In osteoblasts, 1α,25(OH)2D stimulates osteoblast differentiation and accelerates mineralization through both autocrine and paracrine pathways (Woeckel et al., 2010). Vitamin D promotes the gut absorption of calcium and phosphate, which stimulate bone mineralization and increase the BMD (Holick, 2007). In addition, vitamin D also decreases the production of parathyroid hormone (PTH), a hormone that enhances bone turnover and bone loss (Frost et al., 2010). As the major storage form of vitamin D, the total serum or plasma 25(OH)D concentration has usually been recognized as a biomarker of the vitamin D status in the clinical setting (Holick, 2007, Hossein-nezhad and Holick, 2013). It is widely accepted that vitamin D deficiency [total blood 25(OH)D < 30 ng/mL] is associated with the classical skeletal disorders, including rickets, osteopenia, osteoporosis and the risk of fractures (Holick, 2007, Theodoratou et al., 2014). Epidemiological studies have evaluated the correlation between the circulating 25(OH)D level and the BMD value, but the results were controversial. Some studies have reported a positive association between the serum 25(OH)D level and the BMD at the hip and spine (Saquib et al., 2006, von Muhlen et al., 2005), while others found no such association (Videhult et al., 2016, Sayed-Hassan et al., 2016, Garnero et al., 2007). The associations between the total 25(OH)D level and the biomarkers of bone turnover were also suggested to be affected by the sex, age, disease status and medication use of the study participants (Garnero et al., 2007, Lu et al., 2012, Hernandez et al., 2014).
In the human body, about 85–90% of the total circulating 25(OH)D is bound to its carrier protein, the vitamin D binding protein (DBP, encoded by the GC gene), which transports it between tissues. The DBP-bound 25(OH)D is biologically inactive, not acting on target cells (Safadi et al., 2012). Another 10–15% is bound to albumin, and < 1% is present as free-circulating 25(OH)D (Bikle et al., 1986), which is recognized as bioavailable 25(OH)D. Genetic variants of DBP, including rs7041 and rs4588, affect the circulating DBP level and the affinity of DBP for 25(OH)D (Powe et al., 2013). Thus, the level of bioavailable 25(OH)D depends not only on the total 25(OH)D, but also on the levels of DBP and albumin and the genetic background of the individual. However, the effects of distinct types of circulating 25(OH)D on BMD values and bone metabolism have not been systematically evaluated. Herein, we determined the correlations between the bioavailable 25(OH)D level, the BMD values, and the biomarkers of bone turnover in postmenopausal women, who are prone to suffer from osteoporosis and fractures. The present findings provide insights into the biological properties of vitamin D and its activities in bone metabolism and health.
2. Participants and Methods
2.1. Recruitment of the Participants
A cross-sectional study was performed in an elderly Chinese population (defined as those aged 50 and older). From September to December of 2015, female residues living in three communities of the Xuihui distinct of Shanghai, China were invited to receive a physical examination in order to determine their risks of bone fractures. All postmenopausal women were asked to complete a pre-validated questionnaire including their basic characteristics and a history of diagnosed diseases. We excluded those participants with a history of cancer, neoplasia, stoke, autoimmune disorders, or AIDS or had taken vitamin D supplements or vitamin D analogs (including calciferol, cholecalciferol, and alfacalcidol) in the past three months. None of the participants had received other medical treatments related to bone diseases. A total of 967 eligible participants fully meeting the inclusion criteria were recruited, with a response rate of 84.4%, and all provided written consent for their participation. The study was approved by the Ethics Committee of Longhua Hospital Affiliated with Shanghai University of Traditional Chinese Medicine (No. 2014LCSY12).
2.2. Data Collection
A face-to-face interview was performed by the physicians from the Longhua Hospital to collect the basic characteristics and the disease history of the participants. Height was determined without shoes using a portable stadiometer, and the weight was measured with the subject wearing indoor clothing without shoes. The participants reported their detailed previous medical history, including previous fractures, diagnosed diabetes, chronic kidney diseases, thyroid diseases and their hepatitis status. For each participant, we determined the lumbar spine BMD using dual-energy X-ray absorptiometry with a mobile research vehicle.
2.3. Blood Sample Collection and Processing
Each participant provided a total of 7 mL of venous blood after an overnight fast. The blood samples were collected in both EDTA anticoagulation and non-EDTA tubes to collect the plasma and serum samples, respectively. The serum samples were collected within 2 h after the blood collection at room temperature. After being collected in EDTA-containing tubes, the blood was gently mixed and centrifuged at 3000 rpm for 15 min at room temperature to separate the plasma. The plasma and serum were stored in aliquots at − 80 °C until use. The genomic DNA was extracted from the lymphoid cells using the standard phenol-chloroform method. The purified DNA was stored at − 20 °C until use.
2.4. Laboratory Analysis
The levels of total 25-hydroxyvitamin D (D2 and D3) were measured with a Shimadzu series HPLC (Shimadzu Corporation, Japan) instrument connected to an API 5500 LC-MS/MS system (Applied Biosystems Inc., USA). The plasma DBP concentrations were measured with human DBP Quantikine Enzyme-Linked Immuno Sorbent Assay (ELISA) kits (R&D Systems, Minneapolis, Minnesota, USA) according to the manufacturer's instructions. The serum human fibroblast growth factor-23 (FGF23) and osteoprotegerin (OPG) levels were determined with ELISA kits provided by CUSABIO (Wuhan, China). The thyroid-stimulating hormone (TSH), N-terminal propeptide of type I procollagen (PINP), β-CrossLaps of type I collagen containing cross-linked C-telopeptide (β-CTX), osteocalcin (OST) and parathyroid hormone (PTH) levels were determined with the Roche electrochemiluminescence system (Roche Diagnosis Elecsys, Roche Diagnostics). The serum calcium and magnesium levels were determined with the o-cresolphthalein complexone (oCPC) method. The albumin concentration was tested using a bromocresol green (BCG) dye binding method on a modular P-800 autoanalyzer (Roche Diagnostics). The serum phosphorus level was analyzed with the ammonium phosphomolybdate volumetric method. Plasma creatinine was analyzed with the enzymatic Roche Creatinine Plus assay. The plasma alkaline phosphatase (ALP) level was analyzed using enzymatic colorimetric assays based on a Roche modular P-800 autoanalyzer. All the biochemical analyses were performed in batches with all samples from individual runs included in one assay. The intraassay coefficient of variation (CV) was < 5.0% and the interassay CV was < 12.0%.
2.5. DNA Genotyping Methods
The single nucleotide polymorphisms rs4588 and rs7041 of the vitamin D-binding protein gene were genotyped with the Taqman SNP genotyping assays (Thermo Fisher Scientific, USA) following the manufacturer's guidelines. These two variants were chosen because that they have a relative high prevalence in the general Chinese population, and they were previously reported to be associated with the circulating DBP level and the affinity of DBP for 25(OH)D (Powe et al., 2013).
2.6. Calculation of the Bioavailable 25-Hydroxyvitamin D Level
Bioavailable 25-hydroxyvitamin D was recognized as that not bound to the DBP, including the free circulating 25(OH)D and the 25(OH)D that was bound to albumin in blood. The concentration of bioavailable 25(OH)D was dependent on the total 25(OH)D, DBP, the albumin levels and the amino acid variants of DBP. We calculated the bioavailable 25(OH)D level according to the methods reported by Powe et al. (2013). The affinity constant between vitamin D and albumin was 6 × 105 M− 1. The affinity constant was 1.12 × 109 M− 1 for the subjects homozygous for the Gc1F variant (TT for rs7041 and GG for rs4588) of DBP, 0.60 × 109 M− 1 for the homozygous Gc1S variant (GG for rs7041 and GG for rs4588), 0.36 × 109 M− 1 for the homozygous Gc2 variant (TT for rs7041 and TT rs4588) and 0.70 × 109 M− 1 for any other genotype.
2.7. Statistical Analysis
The characteristics of the study participants were presented as the medians and the interquartile range (IQR) or the number of participants and the corresponding proportion. A comparison of the distributions of the biochemical parameters between the groups was performed using an ANOVA (analysis of variance), and post-hoc testing for the differences between pairs of genotype groups was performed with Tukey's method. For the genotyping results, we used the χ2 test [with one degree of freedom] to test whether the genotype distribution was consistent with the Hardy-Weinberg equilibrium (HWE). The correlation between the variants was determined with Pearson's coefficient of correlation. The relationships between the total 25(OH)D or bioavailable 25(OH)D levels and DBP level or BMD values of the participants were examined using univariate linear models. The locally weighted regression smoothing (LOESS) regression and LOESS smoothing scatterplots were obtained to investigate the relationship between the bioavailable and total 25(OH)D levels and PTH levels. To examine the extent to which the total or bioavailable 25(OH)D, bone metabolism factors and bone turnover biomarkers influence the BMD value, univariate and multivariate linear regression analyses were performed. All parameters were normality scaled with a mean of 0 and standard deviation (SD) of 1, and the linear regression coefficients indicate the expected change in the response variable BMD in SD units per one SD increment of the prediction variants, holding the other predictor variables constant. For the multivariate analyses, the best fit of the final model was selected by the backward step-down method with the Akaike information criterion (AIC) setting the BMD as the dependent variable and the patient age, body mass index (BMI), ALP, P1NP, β-CTX, OST, total and bioavailable 25(OH)D levels as the independent variables. The variation inflation factor (VIF) was used to determine the multicollinearity problems for the predictor variables. Two-tailed P-values < 0.05 were recognized as statistically significant. All of the analyses were performed with the R software (version 3.3.1) and related packages (www.r-project.org).
3. Results
3.1. Basic Characteristics of the Participants
A total of 967 postmenopausal women fully met the recruitment criteria for inclusion in the community cross-sectional population study. The age of the participants ranged from 50.00 to 82.00 years old with a median value of 63.00 (Table 1). The BMI of the participants ranged from 16.65 to 42.42 kg/m2 with a median value of 23.51 kg/m2. Of the participants, 433 were overweight or obese (44.8%). 102 (10.5%) participants reported a history of diabetes. A relatively large proportion (166 cases, 17.2%) of women had been diagnosed with a thyroid disease. Including (hypothyreosis, hyperthyroid, and thyroid cyst). A few of the subjects were positive for chronic hepatitis and a history of bone fractures (Table 1). For the genotyping results, neither of the two variants (rs4588 and rs7041) was departed from the HWE test (P > 0.05, respectively).
Table 1.
The baseline characteristics of the postmenopausal women (N = 967).
| Characteristics | Postmenopausal women (N = 967) |
|---|---|
| Age, median (IQR, years) | 63 (59–68) |
| BMI, median (IQR, kg/m2) | 23.51 (21.64–25.68) |
| Under weight (≤ 18.4 kg/m2) | 14 (1.4%) |
| Normal (18.5–23.9 kg/m2) | 520 (53.8%) |
| Overweight (24.0–26.9 kg/m2) | 288 (29.8%) |
| Obese (≥ 27.0 kg/m2) | 145 (15.0%) |
| History of bone fracture | |
| Positive (%) | 65 (6.7%) |
| Negative (%) | 902 (93.3%) |
| Diagnosis of diabetes | |
| Positive (%) | 102 (10.5%) |
| Negative (%) | 865 (89.5%) |
| History of thyroid diseases | |
| Positive (%) | 166 (17.2%) |
| Negative (%) | 801 (82.8%) |
| History of chronic kidney disease | |
| Positive (%) | 24 (2.5%) |
| Negative (%) | 943 (97.5%) |
| Chronic hepatitis status | |
| Positive (%) | 16 (1.7%) |
| Negative (%) | 951 (98.3%) |
| rs7041⁎ | |
| GG (%) | 72 (7.4%) |
| TG (%) | 370 (38.3%) |
| TT (%) | 525 (54.3%) |
| rs4588⁎ | |
| TT (%) | 114 (11.8%) |
| GT (%) | 422 (43.6%) |
| GG (%) | 431 (44.6%) |
Abbreviations: BMI, body mass index; IQR, interquartile range.
Hardy-Weinberg equilibrium test: P = 0.125 for rs7041 and P = 0.827 for rs4588.
3.2. Biochemical Analysis of the Total 25(OH)D and Bioavailable 25(OH)D Levels and the Genotypes of the Participants
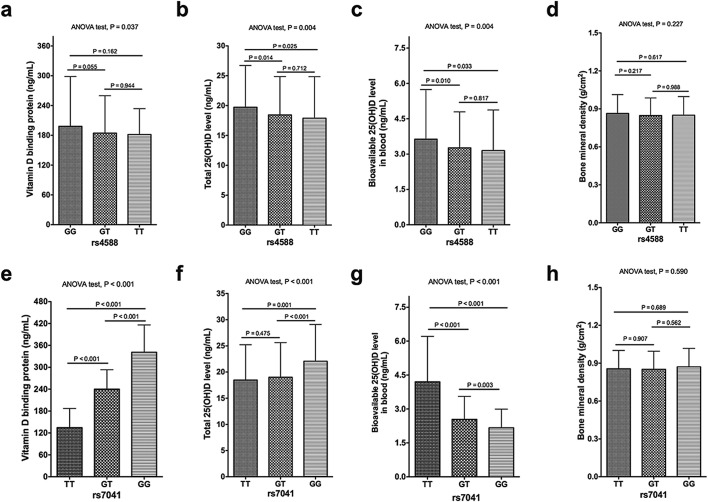
The BMD ranged from 0.43 to 1.68 g/cm2 with a median value of 0.85 g/cm2 (Table 2). The median of the total 25(OH)D level was 17.90 ng/mL, with 591 (61.1%) patients having severe vitamin D deficiency (< 20 ng/mL), 302 (31.2%) having a vitamin D deficiency (20 to 30 ng/mL) and 74 (7.7%) having sufficient vitamin D (> 30 ng/mL) levels. The median concentration of bioavailable 25(OH)D was 2.91 ng/mL (ranged from 0.71–11.45 ng/mL; Table 2), and this was about 5.9% to 47.9% of the total 25(OH)D level. For rs4588, we found no significant differences in the DBP levels of the participants carrying the TT, GT or GG genotypes (P > 0.05 for Tukey's multiple comparisons, Fig. 1a). But compared to wide type GG carriers, the TT or GT carriers had lower total 25(OH)D levels and bioavailable 25(OH)D levels (Fig. 1b and c). Carrying the GG or GT genotypes for rs7041 was correlated with a higher DBP level compared to TT carriers (P < 0.001, Fig. 1e). For rs7041, GG carriers had a higher total 25(OH)D level compared to the GT or TT carriers (P < 0.001; Fig. 1f); however, the GT and GG carriers had a significantly lower bioavailable 25(OH)D level compared to the TT participants (P < 0.025; Fig. 1g). No significant difference was noted for the BMD for between distinct types of the two variants, rs4588 and rs7041 (P > 0.05; Fig. 1d and h).
Table 2.
The results of the biochemical analyses for postmenopausal women (N = 967).
| Parameters | Total participants (N = 967)a | Minimum | Maximum |
|---|---|---|---|
| BMD (g/cm2) | 0.85 (0.76–0.95) | 0.43 | 1.68 |
| FGF23 (pg/mL)b | 1.48 (1.03–2.50) | 0.04 | 73.37 |
| OPG (ng/mL) | 0.12 (0.08–0.22) | 0.03 | 51.55 |
| β-CTX (ng/mL) | 0.39 (0.29–0.50) | 0.04 | 1.58 |
| TSH (IU/mL) | 2.12 (1.50–3.08) | 0.01 | 88.94 |
| OST (ng/mL) | 17.22 (13.98–21.29) | 3.79 | 83.51 |
| PTH (pmol/L) | 3.88 (3.18–4.92) | 1.11 | 24.67 |
| ALP (U/L) | 76 (65–89) | 23 | 163 |
| Phosphorous (mmol/L) | 1.15 (1.07–1.23) | 0.65 | 1.77 |
| Serum magnesium (mmol/L) | 0.89 (0.86–0.93) | 0.69 | 1.20 |
| Serum calcium (mmol/L) | 2.31 (2.25–2.38) | 1.69 | 2.85 |
| P1NP (ng/mL) | 43.85 (35.11–56.26) | 9.39 | 115.40 |
| Albumin (mg/mL) | 46.9 (45.3–48.4) | 38.3 | 62.7 |
| DBP (μg/mL) | 177.0 (121.0–250.5) | 37.9 | 564.0 |
| Total 25(OH)D (ng/mL) | 17.9 (13.9–23.1) | 4.5 | 40.8 |
| Bioavailable 25(OH)D (ng/mL) | 2.91 (2.11–4.17) | 0.71 | 11.45 |
Abbreviations: ALP, alkaline phosphatase; DBP, vitamin D binding protein; FGF23, fibroblast growth factor-23 (FGF23); OPG, osteoprotegerin; TSH, thyroid-stimulating hormone; PINP, N-terminal propeptide of type I procollagen; β-CTX, β-CrossLaps of type I collagen containing crosslinked C-telopeptide; OST, osteocalcin; PTH, parathyroid hormone.
Data are shown as the medians (interquartile range).
84 participants lacked information about the FGF23 level.
Fig. 1.
The levels of DBP, total 25(OH)D, bioavailable 25(OH)D and BMD of the participants with different genetic types for rs4588 and rs7041. The DBP levels (a,e); total 25(OH)D level (b,f); the bioavailable 25(OH)D level (c,g); and the BMD values of the participants (d,h).
3.3. Correlations between the Biomarkers of the Vitamin D Status and the Lumbar Spine BMD
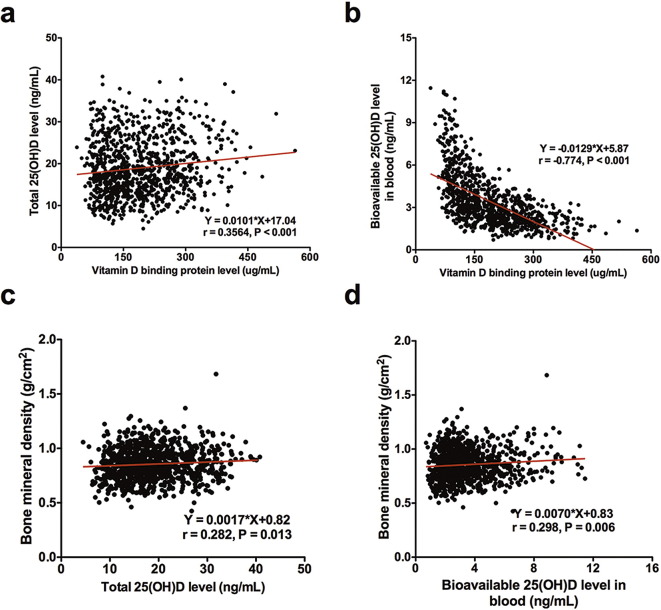
A positive correlation was noted for the total 25(OH)D level and the DBP level (r = 0.356, P < 0.001; Fig. 2a), while the DBP level was negatively correlated with the bioavailable 25(OH)D level (r = − 0.774, P < 0.001; Fig. 2b). However, we noticed that the BMD increased with an increasing total 25(OH)D level (r = 0.282, P = 0.013; Fig. 2c), and the correlation was more prominent for the bioavailable 25(OH)D level (r = 0.298, P = 0.006; Fig. 2d), but the correlation between the total or bioavailable 25(OH)D levels were not statistically significant (P > 0.05).
Fig. 2.
The correlation between DBP concentrations and the total 25(OH)D (a) or the bioavailable 25(OH)D levels (b). The correlations between the total 25(OH)D level (c) and the bioavailable 25(OH)D level (d) and the BMD are presented.
3.4. Correlations between Biomarkers for the Vitamin D Status and the Bone Metabolism Biomarkers
The total 25(OH)D level was negatively correlated with the bone formation biomarkers ALP, OST and PINP levels (Fig. 3 and Supplementary Table 1), whereas the bioavailable 25(OH)D level was significantly correlated with OST and PINP but not ALP (Fig. 3 and Supplementary Table 1). Interestingly, the bone resorption biomarker β-CTX was positively correlated with the bone formation biomarkers, including ALP, OST and PINP (Fig. 3; Supplementary Table 1), but it was not correlated with the total or bioavailable 25(OH)D levels. No significant correlation was noted for the serum magnesium, calcium and phosphorus levels with the biomarkers of the vitamin D status (Fig. 3 and Supplementary Table 1). Interestingly, we found that OPG and FGF23 were positively correlated with each other (P < 0.001) but neither was correlated with the BMD values of the participants (Fig. 3 and Supplementary Table 1). The bioavailable 25(OH)D and total 25(OH)D levels were positively correlated with the OPG levels but not correlated with FGF23 levels (Fig. 3 and Supplementary Table 1)
Fig. 3.
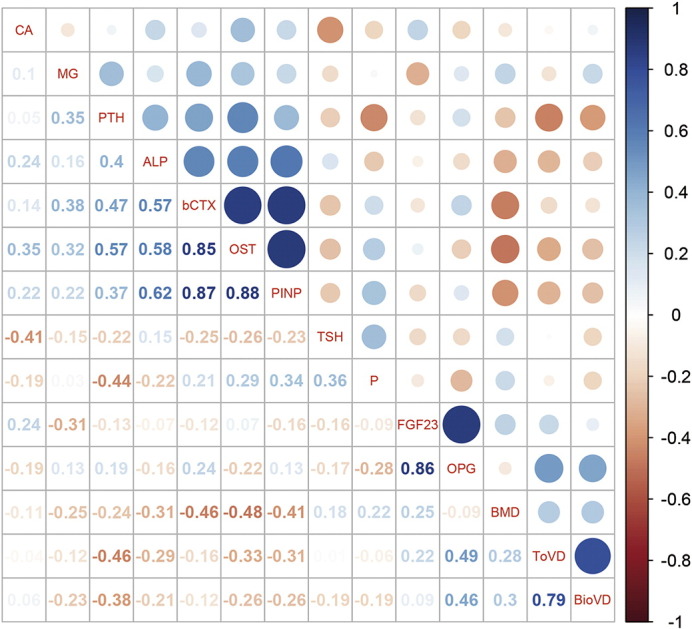
The correlation matrix for the total 25(OH)D, bioavailable 25(OH)D, bone turnover biomarkers, BMD, and other bone metabolism biomarkers in the postmenopausal women. The numbers in the squares and colored circles represent the Spearman's r values between the variants. The scale bar on the left indicated the Spearman's r values for the colored circles. ALP, alkaline phosphatase; b-CTX, β-CrossLaps of type I collagen containing crosslinked C-telopeptide; BioVD, bioavailable 25(OH)D; CA, calcium; MG, magnesium; DBP, vitamin D binding protein; FGF23, fibroblast growth factor-23 (FGF23); OPG, osteoprotegerin; OST, osteocalcin; P, phosphorous; PINP, N-terminal propeptide of type I procollagen; PTH, parathyroid hormone; TSH, thyroid stimulating hormone; ToVD, total 25(OH)D.
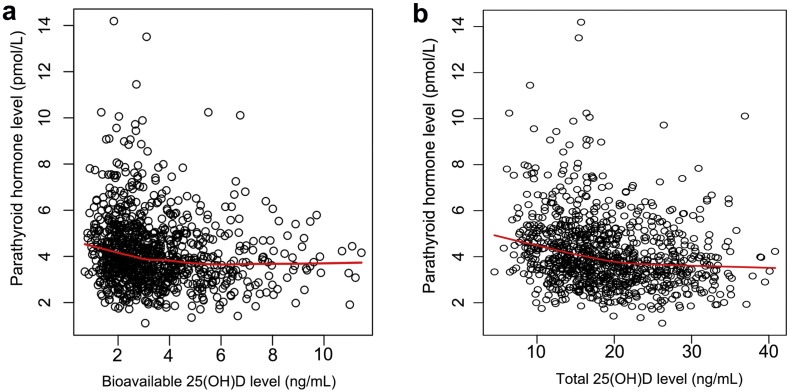
Both total and bioavailable 25(OH)D levels were negatively correlated with PTH levels in the postmenopausal women (Fig. 3 and Supplementary Table 1), and the correlation between total 25(OH)D (Spearman's r = − 0.46) and PTH was slightly stronger than bioavailable 25(OH)D (Spearman's r = − 0.38). The LOESS plots for the relationships for bioavailable and total 25(OH)D levels and PTH levels were presented as Fig. 4. The LOESS plot exhibited a relatively steep decrease of PTH in women with bioavailable 25(OH)D < 5.0 ng/mL and a relatively stationary phase in those with > 5.0 ng/mL (Fig. 4a). According to this standard, 165 of the 967 (17.1%) participants were recognized with sufficient vitamin D levels. The LOESS plot suggested that PTH level decreased along with the increment of total 25(OH)D level for women with total 25(OH)D level < 22.0 ng/mL and a slower decrease of PTH was noticed in women with total 25(OH)D > 22 ng/mL (Fig. 4b). On the basis of the current guidelines, as the vitamin D-sufficient threshold was 25(OH)D > 30 ng/mL, only 74 of the 967 (7.7%) participants would be classified as vitamin D-sufficient. The mean PTH level was 3.78 ± 1.39 pmol/L for participants with bioavailable 25(OH)D-sufficient group, which was similar to participants with total 25(OH)D sufficient group with the mean PTH was 3.87 ± 1.45 pmol/L (P = 0.655) (Table 2).
Fig. 4.
Relationship between bioavailable 25(OH)D level (a) or total 25(OH)D level (b) and the PTH levels in the participants. The red line was the LOESS regression plots show the trends of PTH levels changing along with bioavailable or total 25(OH)D levels.
3.5. The Impacts of the Blood Biomarkers for Bone Metabolisms on the BMD in Postmenopausal Women
To determine the extent to which the blood biomarker levels and basic characteristics of the subjects are responsible for the variations in the BMD values, standardized linearity regression analyses were conducted using univariate and multivariate models. The univariate analyses showed that the patient age (standardized β = − 0.196, P < 0.001), BMI (standardized β = 0.250, P < 0.001), ALP (standardized β = − 0.098, P = 0.002), P1NP (standardized β = − 0.168, P < 0.001), OST (standardized β = − 0.231, P < 0.001), β-CTX (standardized β = − 0.213, P < 0.001), total 25(OH)D (standardized β = 0.080, P = 0.013) and bioavailable 25(OH)D (standardized β = 0.089, P = 0.006) might be determinants of the BMD in postmenopausal women (Table 3).
Table 3.
The results of the univariate and multivariate linear regression analyses of the total and bioavailable 25(OH)D levels, bone metabolism biomarkers, and BMD for the participants (N = 967).
| Variables | Standardized β-coefficients (se)a | P-valuea | Standardize β-coefficients (se)b | P-valueb | Standardized β-coefficients (se)c | P-valuec |
|---|---|---|---|---|---|---|
| Age (years) | − 0.196 (0.032) | < 0.001 | − 0.228 (0.030) | < 0.001 | − 0.231 (0.030) | < 0.001 |
| BMI (kg/m2) | 0.250 (0.031) | < 0.001 | 0.251 (0.030) | < 0.001 | 0.247 (0.030) | < 0.001 |
| FGF23 (pg/mL) | 0.065 (0.034) | 0.056 | ||||
| OPG (ng/mL) | − 0.008 (0.032) | 0.796 | ||||
| DBP (μg/mL) | − 0.039 (0.032) | 0.233 | ||||
| TSH (IU/mL) | 0.034 (0.032) | 0.288 | ||||
| Albumin (mg/mL) | 0.004 (0.032) | 0.905 | ||||
| PTH (pmol/L) | − 0.059 (0.032) | 0.066 | ||||
| Phosphorous (mmol/L) | 0.047 (0.032) | 0.141 | ||||
| Magnesium (mmol/L) | − 0.061 (0.032) | 0.056 | ||||
| Calcium (mmol/L) | − 0.011 (0.032) | 0.725 | ||||
| ALP (U/L) | − 0.098 (0.032) | 0.002 | ||||
| P1NP (ng/mL) | − 0.168 (0.032) | < 0.001 | ||||
| OST (ng/mL) | − 0.231 (0.031) | < 0.001 | − 0.117 (0.044) | 0.008 | − 0.120 (0.043) | 0.005 |
| β-CTX (ng/mL) | − 0.213 (0.031) | < 0.001 | − 0.130 (0.043) | 0.003 | − 0.128 (0.043) | 0.003 |
| Total 25(OH)D (ng/mL) | 0.080 (0.032) | 0.013 | 0.054 (0.030) | 0.070 | ||
| Bioavailable 25(OH)D (ng/mL) | 0.089 (0.032) | 0.006 | 0.065 (0.030) | 0.029 |
Abbreviations: ALP, alkaline phosphatase; BMI, body mass index; DBP, vitamin D binding protein; FGF23, fibroblast growth factor-23 (FGF23); OPG, osteoprotegerin; TSH, thyroid stimulating hormone; P1NP, N-terminal propeptide of type I procollagen; β-CTX, β-CrossLaps of type I collagen containing cross linked C-telopeptide; OST, osteocalcin; PTH, parathyroid hormone.
Univariate linear regression model without adjustment.
Stepwise-selected multivariate regression model 1 with adjustment for age, BMI, β-CTX, OST and the total 25(OH)D level as independent variables.
Stepwise-selected multivariate regression model 2 with adjustment for age, BMI, β-CTX, OST and the bioavailable 25(OH)D level as independent variables.
Multivariate stepwise regression analyses suggested that the bioavailable 25(OH)D level (standardized β = 0.065, P = 0.029), but not the total 25(OH)D level (standardized β = 0.054, P = 0.070), was an independent determinant of the BMD value in postmenopausal women after adjustment for the patient age, BMI, and levels of OST and β-CTX (Table 3). In the multivariate models, the VIF was < 2.1 for each variable, suggesting that there was no multicollinearity effect.
4. Discussion
In the current cross-sectional study, we found that both the bioavailable 25(OH)D and total 25(OH)D levels were positively correlated with the BMD values for postmenopausal women and that they were correlated with the levels of bone formation biomarkers (OST, P1NP and ALP) and factors related bone metabolism including PTH and OPG. For postmenopausal women, the bioavailable 25(OH)D level, but not the total 25(OH)D level, was an independent determinant of the BMD after adjustments for age, BMI, and levels of bone turnover biomarkers (OST and β-CTX). These results suggest that, in the elderly population, vitamin D increases the BMD through inhibiting bone turnover, maintaining calcium and phosphorus homeostasis, and reducing osteoclastogenesis. In addition, the variant rs7041, but not rs4588, was significantly associated with the circulating DBP levels. The circulating DBP level was positively correlated with total 25(OH)D level but negatively correlated with the bioavailable 25(OH)D level. These results suggest that not only the total 25(OH)D level, but also the DBP level and its variants, should be considered when assessing the vitamin D status of patients, and that the bioavailable 25(OH)D level may be a more reliable biomarker in bone health assessment.
At present, the total blood 25(OH)D level is widely used as a biomarker for the vitamin D status in various populations, and the threshold for 25(OH)D deficiency (< 30 ng/mL) was defined by significantly increased PTH level or decreased calcium absorption (Sai et al., 2011, Rosen et al., 2012). However, because the results from experimental and epidemiological studies were inconclusive, the optimal cut-off point for the vitamin D level has not been clearly defined (Zhang et al., 2016). Here, we found that both the total 25(OH)D and the bioavailable 25(OH)D levels were negatively correlated with PTH concentrations, and there was a slightly stronger correlation between the PTH concentrations and total 25(OH)D than for the bioavailable 25(OH)D levels. The LOESS plot suggested that the optimal cutpoint for sufficient bioavailable 25(OH)D level was 5.0 ng/mL, and 17.1% of the participants were recognized with sufficient vitamin D. However, only 7.7% of the participants were defined with sufficient vitamin D according to the current guidelines for total 25(OH)D level of > 30 ng/mL. Between the two groups, there was no significant difference for the blood PTH levels and the BMD values (data not shown). Powe et al. reported that, although the total 25(OH)D level was higher in whites than in black Americans, the bioavailable 25(OH)D level was similar between the two groups in each quintile separated based on the PTH level (Powe et al., 2013). Here, we also found that the PTH level was negatively correlated with the BMD value, which may be due to the fact that PTH stimulates bone turnover in elderly populations. The results were consistent in that PTH levels were positively correlated with bone turnover biomarkers, including β-CTX, OST, ALP, and PINP. These results suggest that the bioavailable 25(OH)D but not the total 25(OH)D tightly regulates the change of PTH levels and that PTH influences the BMD values through regulating the bone turnover in postmenopausal women. Nevertheless, more studies are warranted to verify these findings.
The total 25(OH)D level was influenced by genetic factors. In the current study, we found that rs7041, but not rs4588, influences the DBP level; however, both variants were significantly associated with the total and bioavailable 25(OH)D levels. A positive correlation was found between the circulating DBP level and the total 25(OH)D level, which is consistent with previous reports demonstrating that circulating DBP prolongs the half-life of 25(OH)D by serving as a reservoir and aiding in the reabsorption of filtered vitamin D in the kidneys (Safadi et al., 2012, Nykjaer et al., 1999). The 25(OH)D bound to DBP has lower biological activity, and there is a negative correlation between the DBP level and bioavailable 25(OH)D level (Safadi et al., 2012). The DBP level and the presence of variants rs4588 and rs7041 were not associated with the BMD for the participants, which may be due to the fact that the variants account for only a small proportion of the bioavailable 25(OH)D variation or to the relatively smaller sample size in the current study, which made it difficult to detect the weak correlation between the DBP concentration and the BMD values in the postmenopausal women.
Several epidemiological studies have determined the correlation between levels of different forms of 25(OH)D and BMD in healthy populations, but inconclusive results were noticed. A study performed by Johnsen et al. found that, for 168 postmenopausal women not taking the vitamin D or calcium supplements, the bioavailable or free 25(OH)D was stronger correlated with BMD than total 25(OH)D; however, the correlation between different forms of 25(OH)D and the bone metabolism biomarkers and whether bioavailable 25(OH)D level was an independent determinant of PTH for BMD were not determined (Johnsen et al., 2014). Another study, with 304 adults aged between 21 and 81 years, found no significant association between any form of 25(OH)D and BMD, which might due to the relatively small sample size, the larger range of the age for the participants, or the mixture of the participants races (Jemielita et al., 2016). In the current study, we found that levels of the total and bioavailable 25(OH)D were significantly correlated with the bone metabolism biomarkers in addition to the BMD values. With a relatively larger sample size, we found that the bioavailable 25(OH)D and total 25(OH)D levels were positively correlated with the bone metabolism biomarker, OPG, a soluble decoy for the Receptor Activator For Nuclear Factor kB Ligand (RANKL). RANKL induces the differentiation and activation of osteoclasts, prolongs their life, and strengthens their adhesion to the bone surface through binding to its receptor, RANK (Receptor Activator for Nuclear Factor kB), which is expressed on osteoclasts (Hofbauer et al., 2004). Many studies have confirmed the important roles of the OPG/RANK/RANKL axis in various bone diseases and treatments (LaCroix et al., 2013, Tat et al., 2008, Stuss et al., 2013). The current study also found negative correlations between OST, PINP and the total or bioavailable 25(OH)D levels. These two bone formation biomarkers were highly correlated with each other, and both of them were negatively correlated with the BMD. Another bone formation biomarker, ALP, was also negatively correlated with the BMD, as well as with the total 25(OH)D level. No significant correlation was noted between ALP and the bioavailable 25(OH)D level, suggesting that ALP was not only regulated by vitamin D but also other factors such as PTH in the elderly populations. Postmenopausal women were characterized with increased bone turnover process. Both the bone formation and bone resorption levels might be enhanced in the elderly postmenopausal women in our study, as suggested by the fact that the bone resorption biomarker, β-CTX, was positively correlated with the ALP, OST and P1NP levels in the current study. It has been widely accepted that the net bone gain or loss of the skeleton is determined by the balance of the formation and resorption of the bone (Shieh et al., 2016, Nakatoh, 2016). However, the biomarkers of resorption and formation alone have poor predictive ability for the BMD, since both the bone resorption and formation were increased in subjects with increased bone turnover, regardless of whether there is a net gain or loss of bone. Here, we found that the bone formation biomarker, β-CTX, was negatively correlated with the BMD in the postmenopausal women, which was consistent with previous studies (Allali et al., 2009, He et al., 2014). These results suggested that the bone resorption is higher than the bone formation, which leads to a decrease in the BMD in postmenopausal women. In the current study, we found that a higher circulating 25(OH)D level was correlated with decreased levels of bone formation biomarkers, which suggested that vitamin D may reduce the bone turnover process in postmenopausal women and thus reduce the loss of the BMD.
The multivariate stepwise regression analyses suggested that, for postmenopausal women, bioavailable 25(OH)D, age, BMI, bone formation biomarker OST, and bone resorption biomarker β-CTX were independent determinants of the BMD values. The coefficients for age, OST, and β-CTX were negative, suggesting that increased age and bone turnover levels led to a decrease of BMD. After adjustment of covariates, a positive coefficient was noted for levels of the bioavailable 25(OH)D but not for the total 25(OH)D, suggesting that only bioavailable 25(OH)D was an independent determinant for BMD. A significantly positive correlation between the BMI and BMD values was noticed (data not shown), and the coefficient for the multivariate regression model was positive, suggesting that women with higher BMI have higher BMD values. This is consistent with a recent study reporting that bone turnover was lower and the BMD was higher in obese subjects than in normal people (Walsh et al., 2016). Although the results were sometimes controversial, many epidemiological studies have reported that postmenopausal women with higher BMI had a reduced risk for hip or pelvis fracture (DiPietro et al., 1993, Gnudi et al., 2009, Prieto-Alhambra et al., 2012, Sogaard et al., 2015). However, it was estimated that, for obese populations, there is a U-shape relationship between BMI and fracture risk and that the protective effects of weight on bone are reduced along with the increment of BMI (Palermo et al., 2016). The PTH was elevated in the obese women, which might damage the bone architecture and reduce the BMD values (Goldner et al., 2008, Bolland et al., 2006). The inflammation factors and adipokines secreted from the white adipose tissue could stimulate bone resorption, and abdominal/visceral obesity is usually associated with lower BMD values (Palermo et al., 2016). Moreover, a higher BMI is associated with the reduced bioavailability of 25(OH)D (Wortsman et al., 2000, Vimaleswaran et al., 2013). However, most of the studies that determined relationships between the BMI and BMD values in human subjects were observational. The role of BMI in the determination of BMD values needs further evaluation.
There were several advantages for the current study. First, all the participants were postmenopausal women not taking vitamin D or calcium supplements, which reduced influences of the menopausal status and vitamin supplements on the outcomes of the study. Second, we determined the associations between the total and bioavailable 25(OH)D levels and bone turnover biomarkers in addition to the BMD value, which could lead to elucidation of the underlying mechanisms for the effects of bioavailable 25(OH)D on bone health. Third, the LOESS plot was applied to define the optimal cutpoint for the sufficient bioavailable 25(OH)D level, which could provide guidelines for future clinical use.
Nevertheless, several limitations associated with the current study should be acknowledged. First, the postmenopausal women were more likely to be suffering from bone loss due to the fact that, in these individuals, the rate of bone resorption is faster than the rate of bone formation. Whether the results found here are similar for pre- or peri-menopausal women or men should be addressed in future studies. Second, we calculated the bioavailable 25(OH)D level based on the total 25(OH)D level, DBP level, albumin level, and the genetic background of the participants according to methods reported in previous studies (Powe et al., 2013). Thus, the extent to which the calculated 25(OH)D actually reflects the bioavailable 25(OH)D level in the circulation needs careful interpretation. Further, the methods used in determination of DBP might influence the outcomes of the bioavailable 25(OH)D levels. A recent study compared four DBP assays, including the polyclonal radial immunodiffusion (pRID) assay, two polyclonal assays (Genway Biotech, San Diego, CA and Immunodiagnostik AG, Bensheim, Germany) and one monoclonal ELISA (mELISA, R&D Systems, Minneapolis, MN) (Nielson et al., 2016). They reported that the DBP levels determined with these assays were dependent on the population race, but the underlying mechanisms are unknown. The deviation for free 25(OH)D from that measured by mELISA was larger than for pRID or polyclonal assays in comparing the results of proteomics in the African populations, but the results between the assays were similar in the US non-Hispanic whites or UK whites. The mean DBP concentrations between the assays were similar for all subjects, but the possibility that there was assay-specific bias in determination of the DBP concentrations could not be excluded (Nielson et al., 2016). The influence of the assays on DBP determinations in the Chinese population is unknown and needs to be evaluated in future studies. Third, since the current study was based on a cross-sectional examination, we could not determine the effects of the bioavailable 25(OH)D level or DBP level on the bone fracture risk for the postmenopausal women. Prospective cohort studies are warranted to determine whether the bioavailable 25(OH)D level has higher predictive ability than the total 25(OH)D level for the bone fracture risk or BMD loss in elderly women. Finally, we only determined the BMD of the lumbar spine, and the correlations between the biomarkers of vitamin D with the BMD values at other sites, including the femoral neck, were not determined.
In conclusion, we found that the bioavailable 25(OH)D level, but not the total 25(OH)D level, was an independent determinant for BMD in postmenopausal women. In elderly women, bioavailable vitamin D enhances the BMD value through modulating the bone turnover process, inhibiting PTH excretion, and stimulating OPG production. Factors that influence the bioavailable 25(OH)D levels should be taken into consideration when assessing the relationship between the vitamin D and bone health; however, these results should be validated in intervention studies with larger sample sizes.
The following are the supplementary data related to this article.
The P-values of the correlations between different forms of 25(OH)D, bone metabolism related biomarkers and bone mineral density (BMD) in the postmenopausal women (N = 967).
Conflicts of Interest
None.
Author Contributions
CL, PC, XD and JW contributed equally to this work. CL, PC, YW and HW conceived and designed the study. CL, JW, BS and YW recruited the participants and provided the blood samples. PC, XD, XL, QB and JL performed the biochemical analysis and data acquisition. CL, PC and XD performed the statistical analyses. YW and HW supervised and guided the study. CL, PC, XD and JW wrote the manuscript; all authors approved the final manuscript.
Acknowledgment
This study was supported by grants from the Ministry of Science and Technology of China (2014AA020524), the National Natural Science Foundation of China (81630086, 91529305, 81302507, 81328022, 81574001 and 81472280), the Strategic Priority Research Program (XDA12020319) of the Chinese Academy of Sciences, and the Science and Technology Commission of Shanghai Municipality (16411966800, 16391903700, 14391901800, 14140902100, 13XD1400200 and 13140902902). The study also supported by Innovation Team Project in Priority Areas of MOST (2015RA4002), the Major Diseases of Shanghai's Joint Research Project (2013ZYJB0701), Key Laboratory of theory and therapy of muscles and bones, Ministry of Education (Shanghai University of Traditional Chinese Medicine), major international cooperation project of national nature science foundation of China (81220108027), the Three Year Plan of Shanghai TCM (ZY3-CCCX-2-1002) and the National Clinical Research Base of TCM Project (JDZX2015075). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Yongjun Wang, Email: yjwang8888@126.com.
Hui Wang, Email: huiwang@sibs.ac.cn.
References
- Allali F., El Aichaoui S., Khazani H., Benyahia B., Saoud B., El Kabbaj S., Bahiri R., Abouqal R., Hajjaj-Hassouni N. High prevalence of hypovitaminosis D in Morocco: relationship to lifestyle, physical performance, bone markers, and bone mineral density. Semin. Arthritis Rheum. 2009;38:444–451. doi: 10.1016/j.semarthrit.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Bikle D.D., Gee E., Halloran B., Kowalski M.A., Ryzen E., Haddad J.G. Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. J. Clin. Endocrinol. Metab. 1986;63:954–959. doi: 10.1210/jcem-63-4-954. [DOI] [PubMed] [Google Scholar]
- Bolland M.J., Grey A.B., Ames R.W., Horne A.M., Gamble G.D., Reid I.R. Fat mass is an important predictor of parathyroid hormone levels in postmenopausal women. Bone. 2006;38:317–321. doi: 10.1016/j.bone.2005.08.018. [DOI] [PubMed] [Google Scholar]
- DiPietro L., Welch G.A., Davis D.R., Drane J.W., Macera C.A. Body mass and risk of hip fracture among a national cohort of postmenopausal white women: a reanalysis. Obes. Res. 1993;1:357–363. doi: 10.1002/j.1550-8528.1993.tb00013.x. [DOI] [PubMed] [Google Scholar]
- Feldman D., Krishnan A.V., Swami S., Giovannucci E., Feldman B.J. The role of vitamin D in reducing cancer risk and progression. Nat. Rev. Cancer. 2014;14:342–357. doi: 10.1038/nrc3691. [DOI] [PubMed] [Google Scholar]
- Frost M., Abrahamsen B., Nielsen T.L., Hagen C., Andersen M., Brixen K. Vitamin D status and PTH in young men: a cross-sectional study on associations with bone mineral density, body composition and glucose metabolism. Clin. Endocrinol. 2010;73:573–580. doi: 10.1111/j.1365-2265.2010.03847.x. [DOI] [PubMed] [Google Scholar]
- Garnero P., Munoz F., Sornay-Rendu E., Delmas P.D. Associations of vitamin D status with bone mineral density, bone turnover, bone loss and fracture risk in healthy postmenopausal women. The OFELY study. Bone. 2007;40:716–722. doi: 10.1016/j.bone.2006.09.026. [DOI] [PubMed] [Google Scholar]
- Gnudi S., Sitta E., Lisi L. Relationship of body mass index with main limb fragility fractures in postmenopausal women. J. Bone Miner. Metab. 2009;27:479–484. doi: 10.1007/s00774-009-0056-8. [DOI] [PubMed] [Google Scholar]
- Goldner W.S., Stoner J.A., Thompson J., Taylor K., Larson L., Erickson J., McBride C. Prevalence of vitamin D insufficiency and deficiency in morbidly obese patients: a comparison with non-obese controls. Obes. Surg. 2008;18:145–150. doi: 10.1007/s11695-007-9315-8. [DOI] [PubMed] [Google Scholar]
- He J., Zhang H., Wang C., Zhang Z., Yue H., Hu W., Gu J., Fu W., Hu Y., Li M., Liu Y., Zheng H., Zhang Z. Associations of serum sclerostin and polymorphisms in the SOST gene with bone mineral density and markers of bone metabolism in postmenopausal Chinese women. J. Clin. Endocrinol. Metab. 2014;99:E665–E673. doi: 10.1210/jc.2013-2086. [DOI] [PubMed] [Google Scholar]
- Hernandez J.L., Olmos J.M., Romana G., Llorca J., Martinez J., Castillo J., de Juan J., Perez-Pajares I., Ruiz S., Gonzalez-Macias J. Influence of vitamin D status on the effect of statins on bone mineral density and bone turnover markers in postmenopausal women. J. Clin. Endocrinol. Metab. 2014;99:3304–3309. doi: 10.1210/jc.2014-1102. [DOI] [PubMed] [Google Scholar]
- Hofbauer L.C., Kuhne C.A., Viereck V. The OPG/RANKL/RANK system in metabolic bone diseases. J. Muscoskelet. Neuronal Interact. 2004;4:268–275. [PubMed] [Google Scholar]
- Holick M.F. Vitamin D deficiency. N. Engl. J. Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- Hossein-nezhad A., Holick M.F. Vitamin D for health: a global perspective. Mayo Clin. Proc. 2013;88:720–755. doi: 10.1016/j.mayocp.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemielita T.O., Leonard M.B., Baker J., Sayed S., Zemel B.S., Shults J., Herskovitz R., Denburg M.R. Association of 25-hydroxyvitamin D with areal and volumetric measures of bone mineral density and parathyroid hormone: impact of vitamin D-binding protein and its assays. Osteoporos. Int. 2016;27:617–626. doi: 10.1007/s00198-015-3296-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsen M.S., Grimnes G., Figenschau Y., Torjesen P.A., Almas B., Jorde R. Serum free and bio-available 25-hydroxyvitamin D correlate better with bone density than serum total 25-hydroxyvitamin D. Scand. J. Clin. Lab. Invest. 2014;74:177–183. doi: 10.3109/00365513.2013.869701. [DOI] [PubMed] [Google Scholar]
- Karasik D., Rivadeneira F., Johnson M.L. The genetics of bone mass and susceptibility to bone diseases. Nat. Rev. Rheumatol. 2016;12:323–334. doi: 10.1038/nrrheum.2016.48. [DOI] [PubMed] [Google Scholar]
- Kumar A., Mittal S., Orito S., Ishitani K., Ohta H. Impact of dietary intake, education, and physical activity on bone mineral density among North Indian women. J. Bone Miner. Metab. 2010;28:192–201. doi: 10.1007/s00774-009-0118-y. [DOI] [PubMed] [Google Scholar]
- LaCroix A.Z., Jackson R.D., Aragaki A., Kooperberg C., Cauley J.A., Chen Z., Leboff M.S., Duggan D., Wactawski-Wende J. OPG and sRANKL serum levels and incident hip fracture in postmenopausal Caucasian women in the Women's Health Initiative Observational Study. Bone. 2013;56:474–481. doi: 10.1016/j.bone.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Chen P., Li J., Chu R., Xie D., Wang H. Review: the impacts of circulating 25-hydroxyvitamin D levels on cancer patient outcomes: a systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2014;99:2327–2336. doi: 10.1210/jc.2013-4320. [DOI] [PubMed] [Google Scholar]
- Lu H.K., Zhang Z., Ke Y.H., He J.W., Fu W.Z., Zhang C.Q., Zhang Z.L. High prevalence of vitamin D insufficiency in China: relationship with the levels of parathyroid hormone and markers of bone turnover. PLoS One. 2012;7:e47264. doi: 10.1371/journal.pone.0047264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatoh S. The importance of assessing the rate of bone turnover and the balance between bone formation and bone resorption during daily teriparatide administration for osteoporosis: a pilot study. J. Bone Miner. Metab. 2016;34:216–224. doi: 10.1007/s00774-015-0665-3. [DOI] [PubMed] [Google Scholar]
- Nguyen T.V., Center J.R., Eisman J.A. Osteoporosis in elderly men and women: effects of dietary calcium, physical activity, and body mass index. J. Bone Miner. Res. 2000;15:322–331. doi: 10.1359/jbmr.2000.15.2.322. [DOI] [PubMed] [Google Scholar]
- Nielson C.M., Jones K.S., Chun R.F., Jacobs J.M., Wang Y., Hewison M., Adams J.S., Swanson C.M., Lee C.G., Vanderschueren D., Pauwels S., Prentice A., Smith R.D., Shi T., Gao Y., Schepmoes A.A., Zmuda J.M., Lapidus J., Cauley J.A., Bouillon R., Schoenmakers I., Orwoll E.S., Osteoporotic Fractures in Men Research G Free 25-Hydroxyvitamin D: impact of vitamin D binding protein assays on racial-genotypic associations. J. Clin. Endocrinol. Metab. 2016;101:2226–2234. doi: 10.1210/jc.2016-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nykjaer A., Dragun D., Walther D., Vorum H., Jacobsen C., Herz J., Melsen F., Christensen E.I., Willnow T.E. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell. 1999;96:507–515. doi: 10.1016/s0092-8674(00)80655-8. [DOI] [PubMed] [Google Scholar]
- Palermo A., Tuccinardi D., Defeudis G., Watanabe M., D'Onofrio L., Lauria Pantano A., Napoli N., Pozzilli P., Manfrini S. BMI and BMD: the potential interplay between obesity and bone fragility. Int. J. Environ. Res. Public Health. 2016;13 doi: 10.3390/ijerph13060544. (pii: E544) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powe C.E., Evans M.K., Wenger J., Zonderman A.B., Berg A.H., Nalls M., Tamez H., Zhang D., Bhan I., Karumanchi S.A., Powe N.R., Thadhani R. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N. Engl. J. Med. 2013;369:1991–2000. doi: 10.1056/NEJMoa1306357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto-Alhambra D., Premaor M.O., Fina Aviles F., Hermosilla E., Martinez-Laguna D., Carbonell-Abella C., Nogues X., Compston J.E., Diez-Perez A. The association between fracture and obesity is site-dependent: a population-based study in postmenopausal women. J. Bone Miner. Res. 2012;27:294–300. doi: 10.1002/jbmr.1466. [DOI] [PubMed] [Google Scholar]
- Rosen C.J., Abrams S.A., Aloia J.F., Brannon P.M., Clinton S.K., Durazo-Arvizu R.A., Gallagher J.C., Gallo R.L., Jones G., Kovacs C.S., Manson J.E., Mayne S.T., Ross A.C., Shapses S.A., Taylor C.L. IOM committee members respond to Endocrine Society vitamin D guideline. J. Clin. Endocrinol. Metab. 2012;97:1146–1152. doi: 10.1210/jc.2011-2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safadi F.F., Thornton P., Magiera H., Hollis B.W., Gentile M., Haddad J.G., Liebhaber S.A., Cooke N.E. Osteopathy and resistance to vitamin D toxicity in mice null for vitamin D binding protein. J. Clin. Invest. 2012;103:239–251. doi: 10.1172/JCI5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sai A.J., Walters R.W., Fang X., Gallagher J.C. Relationship between vitamin D, parathyroid hormone, and bone health. J. Clin. Endocrinol. Metab. 2011;96:E436–E446. doi: 10.1210/jc.2010-1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saquib N., von Muhlen D., Garland C.F., Barrett-Connor E. Serum 25-hydroxyvitamin D, parathyroid hormone, and bone mineral density in men: the Rancho Bernardo study. Osteoporos. Int. 2006;17:1734–1741. doi: 10.1007/s00198-006-0191-1. [DOI] [PubMed] [Google Scholar]
- Sayed-Hassan R., Abazid N., Koudsi A., Alourfi Z. Vitamin D status and parathyroid hormone levels in relation to bone mineral density in apparently healthy Syrian adults. Arch. Osteoporos. 2016;11:18. doi: 10.1007/s11657-015-0245-0. [DOI] [PubMed] [Google Scholar]
- Shieh A., Han W., Ishii S., Greendale G.A., Crandall C.J., Karlamangla A.S. Quantifying the balance between total bone formation and total bone resorption: an index of net bone formation. J. Clin. Endocrinol. Metab. 2016;101:2802–2809. doi: 10.1210/jc.2015-4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogaard A.J., Holvik K., Omsland T.K., Tell G.S., Dahl C., Schei B., Falch J.A., Eisman J.A., Meyer H.E. Abdominal obesity increases the risk of hip fracture. A population-based study of 43,000 women and men aged 60–79 years followed for 8 years. Cohort of Norway. J. Intern. Med. 2015;277:306–317. doi: 10.1111/joim.12230. [DOI] [PubMed] [Google Scholar]
- Stuss M., Rieske P., Ceglowska A., Stepien-Klos W., Liberski P.P., Brzezianska E., Sewerynek E. Assessment of OPG/RANK/RANKL gene expression levels in peripheral blood mononuclear cells (PBMC) after treatment with strontium ranelate and ibandronate in patients with postmenopausal osteoporosis. J. Clin. Endocrinol. Metab. 2013;98:E1007–E1011. doi: 10.1210/jc.2012-3885. [DOI] [PubMed] [Google Scholar]
- Tat S.K., Pelletier J.P., Lajeunesse D., Fahmi H., Duval N., Martel-Pelletier J. Differential modulation of RANKL isoforms by human osteoarthritic subchondral bone osteoblasts: influence of osteotropic factors. Bone. 2008;43:284–291. doi: 10.1016/j.bone.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodoratou E., Tzoulaki I., Zgaga L., Ioannidis J.P.A. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ. 2014;348:g2035. doi: 10.1136/bmj.g2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videhult F.K., Ohlund I., Hernell O., West C.E. Body mass but not vitamin D status is associated with bone mineral content and density in young school children in northern Sweden. Food Nutr. Res. 2016;60:30045. doi: 10.3402/fnr.v60.30045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vimaleswaran K.S., Berry D.J., Lu C., Tikkanen E., Pilz S., Hiraki L.T., Cooper J.D., Dastani Z., Li R., Houston D.K., Wood A.R., Michaelsson K., Vandenput L., Zgaga L., Yerges-Armstrong L.M., McCarthy M.I., Dupuis J., Kaakinen M., Kleber M.E., Jameson K., Arden N., Raitakari O., Viikari J., Lohman K.K., Ferrucci L., Melhus H., Ingelsson E., Byberg L., Lind L., Lorentzon M., Salomaa V., Campbell H., Dunlop M., Mitchell B.D., Herzig K.H., Pouta A., Hartikainen A.L., Genetic Investigation of Anthropometric Traits G.C, Streeten E.A., Theodoratou E., Jula A., Wareham N.J., Ohlsson C., Frayling T.M., Kritchevsky S.B., Spector T.D., Richards J.B., Lehtimaki T., Ouwehand W.H., Kraft P., Cooper C., Marz W., Power C., Loos R.J., Wang T.J., Jarvelin M.R., Whittaker J.C., Hingorani A.D., Hypponen E. Causal relationship between obesity and vitamin D status: bi-directional Mendelian randomization analysis of multiple cohorts. PLoS Med. 2013;10:e1001383. doi: 10.1371/journal.pmed.1001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Muhlen D.G., Greendale G.A., Garland C.F., Wan L., Barrett-Connor E. Vitamin D, parathyroid hormone levels and bone mineral density in community-dwelling older women: the Rancho Bernardo Study. Osteoporos. Int. 2005;16:1721–1726. doi: 10.1007/s00198-005-1910-8. [DOI] [PubMed] [Google Scholar]
- Walsh J.S., Evans A.L., Bowles S., Naylor K.E., Jones K.S., Schoenmakers I., Jacques R.M., Eastell R. Free 25-hydroxyvitamin D is low in obesity, but there are no adverse associations with bone health. Am. J. Clin. Nutr. 2016;103:1465–1471. doi: 10.3945/ajcn.115.120139. [DOI] [PubMed] [Google Scholar]
- Waugh E.J., Lam M.A., Hawker G.A., McGowan J., Papaioannou A., Cheung A.M., Hodsman A.B., Leslie W.D., Siminoski K., Jamal S.A., Perimenopause, B. M. D. G. S. o. O. C Risk factors for low bone mass in healthy 40–60 year old women: a systematic review of the literature. Osteoporos. Int. 2009;20:1–21. doi: 10.1007/s00198-008-0643-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woeckel V.J., Alves R.D., Swagemakers S.M., Eijken M., Chiba H., van der Eerden B.C., van Leeuwen J.P. 1Alpha,25-(OH)2D3 acts in the early phase of osteoblast differentiation to enhance mineralization via accelerated production of mature matrix vesicles. J. Cell. Physiol. 2010;225:593–600. doi: 10.1002/jcp.22244. [DOI] [PubMed] [Google Scholar]
- Wortsman J., Matsuoka L.Y., Chen T.C., Lu Z., Holick M.F. Decreased bioavailability of vitamin D in obesity. Am. J. Clin. Nutr. 2000;72:690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- Wu S.F., Du X.J. Body mass index may positively correlate with bone mineral density of lumbar vertebra and femoral neck in postmenopausal females. Med. Sci. Monit. 2016;22:145–151. doi: 10.12659/MSM.895512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Shi L., Peng N., Xu S., Zhang M., Zhang S., Li H., Zhuang H., Gong M., Wu D., Wang R. Serum concentrations of 25-hydroxyvitamin D and its association with bone mineral density and serum parathyroid hormone levels during winter in urban males from Guiyang, Southwest China. Br. J. Nutr. 2016;115:960–966. doi: 10.1017/S0007114515005383. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The P-values of the correlations between different forms of 25(OH)D, bone metabolism related biomarkers and bone mineral density (BMD) in the postmenopausal women (N = 967).