Abstract
The origin of the carbon atoms in the CO2 respired by French bean (Phaseolus vulgaris) leaves in the dark has been studied using 13C/12C isotopes as tracers. The stable isotope labeling was achieved through a technical device that uses an open gas-exchange system coupled online to an elemental analyzer and linked to an isotope ratio mass spectrometer. The isotopic analysis of the CO2 respired in the dark after a light period revealed that the CO2 was labeled, but the labeling level decreased progressively as the dark period increased. The pattern of disappearance depended on the amount of carbon fixed during the labeling and indicated that there were several pools of respiratory metabolites with distinct turnover rates. We demonstrate that the carbon recently assimilated during photosynthesis accounts for less than 50% of the carbon in the CO2 lost by dark respiration and that the proportion is not influenced by leaf starvation in darkness before the labeling. Therefore, most of the carbon released by dark respiration after illumination does not come from new photosynthates.
Photosynthesis provides the carbohydrate substrate upon which plants depend. By contrast, glycolysis and respiration are responsible for the release of energy stored in carbohydrates. However, the amount of carbon assimilated during photosynthesis and immediately respired in leaves is unknown. In general, the respiratory carbon metabolism and its relation to recent photosynthates in leaves are poorly understood.
For many years, studies on carbon metabolism after a period of light used carbon isotopes as tracers. 14C labeling in the light was first applied in pioneering studies by Calvin and others to identify the fate of the carbon atoms fixed by photosynthesis (for review, see Rabinowitch, 1956). Subsequent studies using pulse-chase techniques with 14C were mainly used to examine allocation, that is, the partitioning of assimilates between distinct parts of the plants (Pearen and Hume, 1981; Fondy and Geiger, 1982; Soja et al., 1989; Kuzyakov et al., 2001) or the distribution of the 14C in different metabolites of the same organ (Sharkey et al., 1985; Li et al., 1992). Refixation of CO2 produced in the light by photorespiration is probably not a problem in the above studies, although it can obscure the results when studying fluxes in a given pathway, i.e. the photorespiratory pathway, where knowledge of the specific radioactivity of the carbon feeding the pathway must be accurately known (Biehler and Fock, 1996).
In darkened leaves, the measurements of 14C labeling in respired CO2 are scarce: Birecka et al. (1969) found that the radioactivity of the respired CO2 from the main shoot increased in wheat plants deprived of the ear, indicating that carbon losses in the last period of wheat development are due to more intensive respiration; Bort et al. (1996), feeding ears and flag leaves of durum wheat (Triticum durum) with 14C-labeled Suc and trapping the 14CO2 released by respiration, concluded that the apparent refixation of respiratory CO2 in the ears was double that measured in the flag leaves; Goren et al. (2000) showed that the radioactivity in the respired CO2 was lower than 2% in citrus juice cells fed with [14C]Suc and [14C]Fru; and Pärnik et al. (2002) showed that the total rate of respiration (as the sum of decarboxylation of stores and primary photosynthates) was not affected by light in cereals.
Labeling techniques using 13CO2 have been used to study plant accumulation of assimilates and its relationship with seed storage mobilization (Cliquet et al., 1990a, 1990b; Maillard et al., 1994) or to examine the refixation of respiratory CO2 in wheat ear (Gebbing and Schnyder, 2001, although CO2 respired was not measured directly). At the whole-plant scale, Avice et al. (1996) used the 13C labeling in alfalfa (Medicago sativa) to show that the main 13CO2 loss involved root respiration.
Respiratory metabolism has also been studied in heterotrophic cells using 13C-enriched Glc labeling coupled to NMR (Bligny et al., 1989; Aubert et al., 1996; Dieuaide-Noubhani et al., 1997). This technique was used to show that, in root tips of maize (Zea mays), exogenous Glc enters the Suc synthesis/degradation futile cycle, starch synthesis, the pentose phosphate cycle, and the Krebs cycle but, again, the 13C enrichment in the respired CO2 was not measured (Dieuaide-Noubhani et al., 1997). In detached leaves under CO2-free air conditions, 13C-enriched Glc labeled day-respired CO2 and leaf-emitted isoprene, indicating that the labeled Glc was incorporated into leaf metabolites and used as a respiratory substrate (Affek and Yakir, 2003). Nevertheless, data on the respiration of intact leaves after illumination are scarce.
In general, the CO2 used for steady-state labeling is artificially enriched with either 14CO2 or 13CO2, and labeling is performed in closed systems (Kouchi and Yoneyama, 1984; Geiger and Shieh, 1988). Long-term labeling studies are difficult to conduct in closed systems and require large amounts of heavy carbon isotopes (Schnyder, 1992). An alternative method, based on the difference in 13CO2 abundance between atmospheric CO2 and commercially available compressed, 12C-enriched CO2, was proposed by Deléens et al. (1983). This method allows the exposure of plants or parts of plants to CO2 with a constant carbon isotope composition (δ13C) in an open system. Using this method in a long-term experiment, Schnyder et al. (2003) have shown in whole plants that at least two pools were involved in feeding dark respiration.
The aim of this study was to determine the origin of the carbon atoms in the CO2 respired by French bean (Phaseolus vulgaris) leaves in the dark immediately after the photoperiod using 13C/12C isotopes as tracers, with an online open system that consists of a LI-6400 open gas-exchange system directly coupled to an elemental analyzer (EA) and to an isotope ratio mass spectrometer (IRMS). This system takes advantage of the difference in δ13C between atmospheric CO2 (−9.5‰) and commercially available (12C-enriched) CO2 (−51.2‰), and consequently the 13C abundance in the CO2 used for the labeling is in the same order of magnitude as that found in nature. This allows picking up the contribution of already stored carbon having only a slight 13C content, which would not have been possible if heavily labeled carbon (several percent as usual) was used. We demonstrate that the carbon recently assimilated during photosynthesis accounts for less than 50% of the carbon in the CO2 lost by dark respiration and that the proportion is not influenced by leaf starvation in darkness before the labeling. Therefore, most of the carbon released by dark respiration after illumination does not come from new photosynthates.
RESULTS
Plants were removed from the greenhouse, and the δ13C of the dark-respired CO2 (unlabeled) of the intact leaf was immediately measured in the closed online system. Then the leaf was placed in the open online system for isotopic labeling (at a δ13C of −51.2‰). After labeling, the leaflet was placed back to the closed online system to measure the δ13C of the dark-respired CO2 (labeled).
δ13C Evolution of Respired CO2 after Labeling
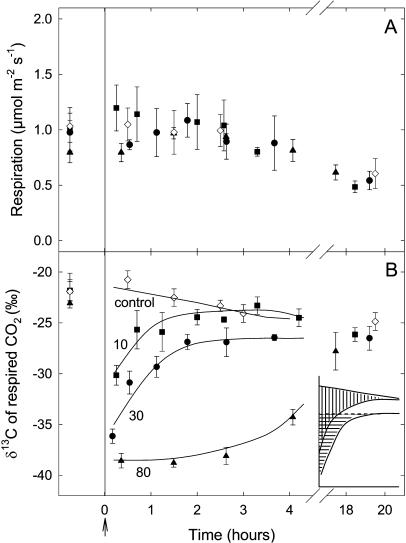
Leaf respiration did not change during the first 4 h of darkness after labeling but decreased slightly after 20 h (Fig. 1A). The δ13C value of the dark-respired CO2 was about −22‰ before labeling (Fig. 1B), and decreased to −30.1, −36.1, and −38.6‰ after labeling with CO2 depleted in 13C (−51.2‰) for 20 min (adding 10 mmol C m−2), 75 min (adding 30 mmol C m−2), and 180 min (adding 80 mmol C m−2), respectively (Fig. 1B, squares, circles, and triangles, respectively).
Figure 1.
Changes in the respiration rate (μmol m−2 s−1; A) and δ13C (‰; B) of CO2 respired in the dark after isotopic labeling in French bean leaves. Leaves were labeled with commercial CO2 with δ13C of −51.2‰ for 20 min (10 mmol C fixed per m2; squares), 75 min (30 mmol C fixed per m2; circles), and 180 min (80 mmol C fixed per m2; triangles), respectively. The arrow indicates the beginning of the dark period. The δ13C value of the dark-respired CO2 for a 20-h dark period for unlabeled leaves is also shown (diamonds). Data are the means of three replicates (se values are shown when larger than the symbols). At the beginning of the dark measurements, the percent of new carbon in respired CO2 was 18%, 29%, and 34% for the 10, 30, and 80 mmol C m−2 labeling curves, respectively. Inset, Schematic drawing of the surfaces delimited by (1) the control δ13C and the δ13C of the 20-min labeling (10 mmol C fixed per m2) curves; and (2) the δ13C of the 75-min labeling (30 mmol C fixed per m2) curve and the steady-rate line (δ13C = −27‰).
After 20 min of labeling (10 mmol C m−2), the δ13C value of the dark-respired CO2 rapidly increased during the dark period (reaching about −24‰ after 3 h). After 20 h, the δ13C of the dark-respired CO2 of these leaves reached a value similar to unlabeled leaves (−27‰; Fig. 1B, squares). After 75 min of labeling (30 mmol C m−2), the δ13C increased rapidly and reached about −27‰ in 2 h and then remained constant (Fig. 1B, circles). Interestingly, after 180 min of labeling (80 mmol C m−2), the δ13C value remained at about −38‰ during the first 3 h of darkness and slowly recovered to the expected value after 20 h (i.e. −27‰; Fig. 1B, triangles).
The surface delimited by the control δ13C curve and the δ13C curve after 20 min of labeling (i.e. 10 mmol C fixed per m2), on one hand (Fig. 1B, inset; redrawn with vertical bars), and the surface delimited by the δ13C curve after 75 min of labeling (i.e. 30 mmol C fixed per m2) and the steady-rate line (δ13C = −27‰), on the other hand (Fig. 1B, inset, horizontal bars), are similar. These surfaces represent the emptying of a respiratory pool during the 150 min of darkness immediately after the photosynthetic assimilation period.
Respiratory Metabolites after Labeling
Immediately after labeling, Suc and starch were labeled (e.g. δ13C of Suc and starch were −40.7‰ and −31.4‰, respectively, after 80 mmol C m−2 labeling; Table I). As the photosynthetic isotope discrimination of these leaves was online carbon isotopic discrimination (ΔO) = 20‰, the δ13C of the soluble sugars and starch should have approached −71‰ [=(−51 − ΔO)/(1 + ΔO) ∼ −71] if the metabolites had been fully labeled (with a CO2 of δ13C of −51‰), but this was not the case (Table I). At the end of the dark period (20 h), the labeling decreased in both metabolites.
Table I.
δ13C (‰) of Suc and starch immediately after labeling or 20-h dark period in French bean leaves
| After Labeling
|
After 20 h in Darkness
|
|||||
|---|---|---|---|---|---|---|
| Amount of C Fixed | δ13C of Suc | δ13C of Starch | RQ | δ13C of Suc | δ13C of Starch | RQ |
| mmol C m−2 | ||||||
| 10 | −30.4 ± 0.8 | −28.7 ± 0.8 | 1.0 ± 0.1 | −28.4 ± 1.3 | −27.8 ± 0.5 | 0.8 ± 0.1 |
| 30 | −35.2 ± 2.0 | −29.2 ± 0.8 | 1.1 ± 0.1 | −27.9 ± 0.8 | −27.4 ± 0.7 | 0.8 ± 0.2 |
| 80 | −40.7 ± 0.2 | −31.4 ± 1.3 | 1.0 ± 0.1 | −29.8 ± 0.8 | −28.1 ± 0.8 | 0.8 ± 0.1 |
RQ is given. Data are the means of three replicates ± se. δ13C (‰) of Suc and starch were −27.2 ± 0.5 and −28.4‰ ± 0.2‰, respectively, before the labeling.
The nature of the respiratory substrate can be determined by the respiratory quotient (RQ), which is the ratio of CO2 production to oxygen consumption. This quotient is close to 1 when carbohydrates are consumed and less than 1 when less-oxygenated substrates are used for respiration (e.g. 0.6 for fatty acids). Immediately after labeling, RQ was 1 and decreased to 0.8 at the end of the dark period (Table I), indicating that compounds other than carbohydrates are oxidized during respiration.
Effect of the Amount of Carbon Fixed by Photosynthesis on δ13C of Dark-Respired CO2
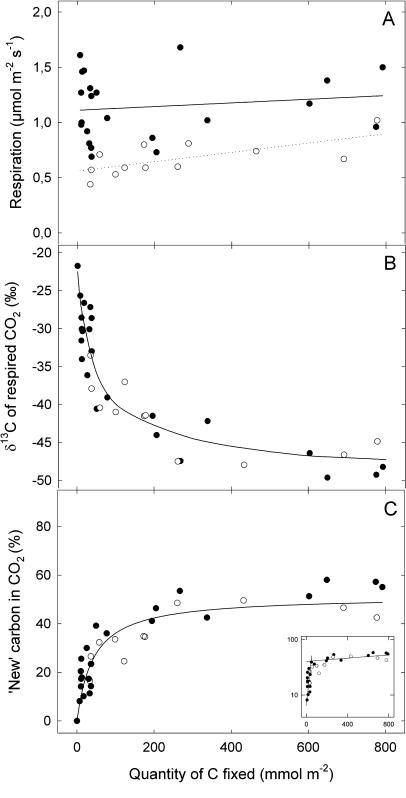
As expected, the δ13C of the dark-respired CO2 decreased with the increasing amount of labeled carbon assimilated by leaves, reflecting the global increase in the amount of labeled carbon in the leaf (Fig. 2). However, the δ13C began to saturate at about 80 mmol C m−2 and eventually reached a plateau, with values close to −48‰ (Fig. 2B, black symbols). Interestingly, the response of the δ13C of CO2 to the amount of carbon was the same for nonstarved leaves and leaves of plants previously starved in darkness for 3 d before the labeling experiments (Fig. 2B, white symbols).
Figure 2.
Changes of the respiration rate (μmol m−2 s−1; A), the δ13C value of dark-respired CO2 (‰; B), and the proportion (%; C) of new carbon in the dark-respired CO2 as a function of the amount of labeled (−51.2‰) carbon that is fixed by French bean leaves. The different plots correspond to different labeling times from 20 min to 24 h. Plants were taken directly from the greenhouse (nonstarved plants, black circles) or kept in the dark for 3 d (starved plants, white circles) before the labeling. Each point represents data from a separate leaf. Inset, Semilogarithmic plot of the data in Figure 2C.
The δ13C of the dark-respired CO2 was used to calculate the proportion of new carbon (i.e. recently fixed during the labeling) in the CO2 respired (see “Materials and Methods” for calculation details) and was plotted as a function of the amount of carbon fixed by leaves (Fig. 2C). The proportion of new carbon rose with increasing amounts of carbon assimilated by the leaves until about 80 mmol C m−2, for both nonstarved and starved leaves. This proportion then saturated to about 50% at 800 mmol m−2 of fixed C (i.e. around 24 h of labeling). Only about 50% of the carbon of the CO2 respired was labeled in nonstarved and starved plants, respectively (Fig. 2C). Figure 2C, inset, represents the semilogarithmic plot of the data in this figure, showing that at least two pools of substrates are feeding respiration.
Effect of the Amount of Carbon Fixed on Leaf Metabolites
The total amount of carbon and the calculated amount of new carbon coming from recent photosynthetic activity in Suc and starch (the two main photosynthetic products) after different amounts of carbon fixed during the labeling experiments in starved and nonstarved leaves are shown in Table II. The calculated values are obtained using the δ13C (see Fig. 3) and the total amount of each carbohydrate (see “Materials and Methods” for calculation details). The percentage of the assimilated carbon used to synthesize Suc and starch in the leaf is shown in brackets.
Table II.
Total amount of carbon and the calculated amount of new carbon in Suc and starch after different amounts of carbon were fixed during the labeling experiments in starved and nonstarved French bean leaves
| Total Amount
|
Calculated Amount
|
|||
|---|---|---|---|---|
| Amount of C fixed | Suc | Starch | Suc | Starch |
| mmol C m−2 | mmol C m−2 | mmol C m−2 | ||
| Nonstarved | ||||
| 30 | 11.7 ± 0.1 | 27.4 ± 6.9 | 3.9 (13.0) | 3.0 (10.1) |
| 250 | 10.7 ± 1.2 | 49.5 ± 9.3 | 3.4 (1.4) | 15.5 (6.2) |
| 600 | 11.1 ± 1.5 | 74.9 ± 5.0 | 5.5 (0.9) | 24.7 (4.1) |
| Starved | ||||
| 30 | 0.3 ± 0.2 | 6.7 ± 3.3 | 0.1 (0.3) | 1.5 (4.9) |
| 250 | 30.9 ± 1.3 | 45.4 ± 9.5 | 20.4 (8.2) | 22.4 (8.9) |
| 600 | 23.4 ± 2.5 | 32.4 ± 9.0 | 15.6 (2.6) | 16.5 (2.7) |
The percent of the carbon fixed during photosynthesis used to synthesize Suc and starch is shown in brackets. The calculated values are obtained using the δ13C (Fig. 3) and the total amount of each carbohydrate (see “Materials and Methods” for calculation details).
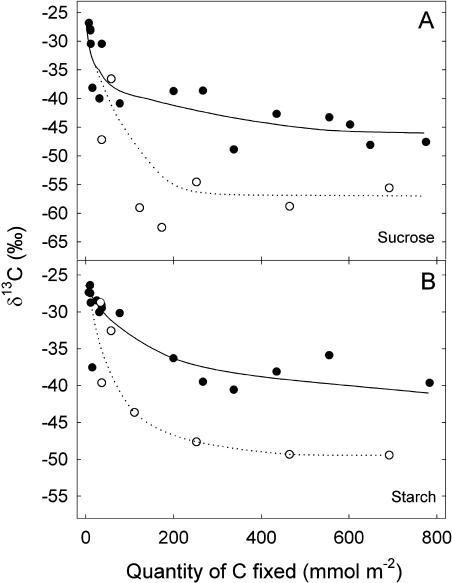
Figure 3.
Changes in the δ13C (‰) of Suc (A) and starch (B) as a function of the amount of carbon that is fixed by French bean leaves. Plants were taken directly from the greenhouse (nonstarved plants, black symbols) or kept in the dark for 3 d (starved plants, white symbols) before the labeling. Each point represents data from a separate leaf.
Suc content remained quite stable in nonstarved leaves while it peaked after 250 mmol C assimilated in starved leaves. Starch content increased in the first case and reached a maximum after 250 mmol C assimilated in starved plants. Variations in the amounts of new carbon in starch and Suc were similar to those of the total amount during the different labeling periods in both starved and nonstarved plants.
The δ13C of Suc and starch decreased when the amount of carbon assimilated increased, and the δ13C of these two compounds was lower in starved (about −55‰ and −50‰, respectively) than in nonstarved leaves (about −45‰ and −40‰, respectively; Fig. 3). Therefore, the labeling level in Suc and starch reached about 60% and 50% in starved plants and 40% and 30% in nonstarved plants, respectively.
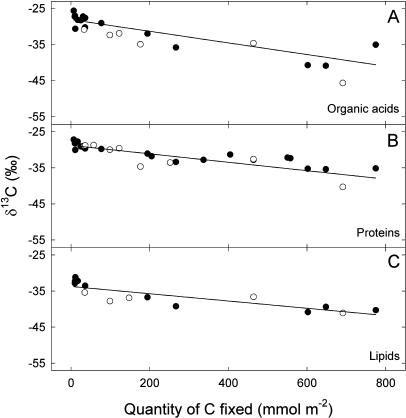
Organic acids, heat-precipitated proteins, and lipids, which have a slow turnover, were less labeled: Their isotope composition decreased very slowly when the amount of carbon increased, reaching values of around −40‰ only with a carbon quantity as high as up to 800 mmol C m−2 (Fig. 4).
Figure 4.
Changes in the δ13C (‰) of the organic acids (A), heat-precipitated proteins (B), and lipids (C) as a function of the quantity of carbon fixed by French bean leaves. Plants were taken directly from the greenhouse (nonstarved plants, black symbols) or kept in the dark for 3 d (starved plants, white symbols) before the labeling. Each point represents data from a separate leaf.
DISCUSSION
The CO2 respired in the dark by French bean leaves immediately after a light period originates from the oxidation of carbohydrates and has a δ13C of typically about −22‰ (Duranceau et al., 1999; Ghashghaie et al., 2003; Tcherkez et al., 2003). However, the relative contribution of the newly fixed carbon by photosynthetic activity versus that from stored carbohydrates to night respiration is unknown. We addressed this question by using stable carbon isotopes at a natural abundance in order to label the photosynthates and by manipulating the amount of carbohydrates stored in the leaves by starving the leaves in the dark.
Several Metabolic Pools Feed Dark Respiration after Illumination
After 20 and 75 min of labeling, the δ13C of the dark-respired CO2 rapidly increased and reached a steady-state value after about 150 min in the dark. But the δ13C of the CO2 produced was higher in the former labeling treatment (−24‰ versus −27‰; Fig. 1B). In both cases, a pool of respiratory carbon was rapidly filled by photosynthesis and rapidly emptied when the leaf was returned to darkness. As the surface delimited by the control δ13C and the δ13C of the 20-min labeling (10 mmol C fixed per m2) curves was similar to that of the surface delimited by the 75-min labeling (30 mmol C fixed per m2) curve and the steady-rate line (δ13C = −27‰; Fig. 1B, inset), we conclude that the kinetics of labeling disappearance in respired CO2 was the same in both cases and that the respiratory pool was already filled by a 20-min photosynthetic period during which 10 mmol C m−2 were fixed. Consequently, the size of this first respiratory pool was 10 mmol C m−2 or less. In both cases, the loss of labeled carbon from this compartment fits a first-order reaction with the same t1/2 of around 45 min (data not shown). The mean rate of dark respiration during the first 150 min after the light period was 1.1 μmol m−2 s−1 (Fig. 1A). Thus, the amount of carbon lost by respiration during this period was around 10 mmol C m−2, which is the (maximal) size of the first respiratory pool, indicating that all the carbon lost by respiration passes through this compartment first.
After 75 min of photosynthesis, a significant amount of low-turnover carbon compounds was synthesized and used by respiration, since, as mentioned above, the δ13C value of the evolved CO2 (i.e. −27‰) remained lower than that of the unlabeled (control) leaves (Fig. 1B). These compounds may be carbohydrates since the RQ value was still 1 after 4 h of darkness. In beet (Beta vulgaris), starch is mobilized after 75 min in darkness after a light period (Fondy and Geiger, 1982). Remobilization of the Suc stored in the vacuole could also contribute to respiration feeding (Dubinina et al., 2001). After 180 min of photosynthesis, this response was amplified, thereby explaining why the δ13C of the respiratory CO2 remained low (i.e. −38‰) 3 h in the dark following a light period and then increased slowly to reach the control value in about 18 h (Fig. 1B).
After 20 h in the dark, the respired CO2 was not labeled: It reached a δ13C value of about −27‰, similar to the unlabeled (control) leaves. The RQ value was then 0.8 (Table I), showing sugar starvation and use of low-oxygenated substrates (e.g. proteins or lipids) by respiration (Devaux et al., 1984; Aubert et al., 1996; Tcherkez et al., 2003). This response may be paralleled by a cytoplasmic regression that induces a progressive decrease in respiratory function, as observed in starved cultured cells in vitro (Aubert et al., 1996).
Dark Respiration after Illumination Is Fed Only Partly by Current Photosynthates
Interestingly, with plants taken from the greenhouse after 10 h of light, the δ13C value of the CO2 respired immediately after the labeling decreased progressively when the amount of carbon fixed increased and reached a steady value of −48‰ (Fig. 2B, black symbol). It was not possible to label all the respiratory substrates since, in this case, the δ13C of the CO2 produced should have approached the theoretical value of −71‰; that is, the δ13C of the CO2 in the air (−51‰) used for the labeling minus the photosynthetic isotope discrimination of the leaves (ΔO = 20‰). This implies that nonlabeled low-turnover compounds were still feeding dark respiration after 24 h of labeling in the light, during which 800 mmol of C were fixed per m2 of leaves. At this time, there was only about 50% of new carbon (given by recent photosynthetic activity) in respired CO2 (Fig. 2C). Thus, immediately after illumination, and also for approximately 2 h (see Fig. 1B), respiration in French bean leaves was fed by a mixture of recent photosynthates and old organic molecules, mainly carbohydrates (since RQ = 1).
Surprisingly, the relationship between the δ13C in the CO2 produced and the amount of carbon fixed by photosynthesis was the same in leaves of nonstarved and starved plants (Fig. 2B). Thus, if we assume that the low-turnover respiratory substrates have a similar δ13C value in both starved and nonstarved plants before labeling, we conclude that the dilution of low-turnover respiratory substrates by recent photosynthetic compounds was the same in both experimental groups.
Suc and Starch Synthesis Are Also Fed Only Partly by Current Photosynthates
The δ13C value of Suc in nonstarved leaves (−45‰) was very close to that of the CO2 respired for high amounts of fixed carbon (−48‰), indicating that the isotope composition of the CO2 reflects that of the respiratory metabolites. In this case, the results in Figure 3A indicate that the carbon also came from a nonlabeled source to feed the Suc pool (otherwise the δ13C of Suc would have also approached the theoretical value of −71‰).
By contrast, in starved plants, Suc was more labeled than the respiratory CO2 and reached a stable δ13C value between −55 and −60‰ when 200 mmol C m−2 was fixed. It is very unlikely that the difference of δ13C value in Suc and CO2 in starved plants is related to any carbon isotope fractionation during dark respiration because this process, which is expected to be about −6‰ (negative sign indicates 13C enrichment in respired CO2 compared to the substrate pool) at most, was not observed in the nonstarved leaves. Given the strong relationship between respiratory metabolism, shown by the RQ, and fractionation during dark respiration (Tcherkez et al., 2003), we conclude that there was no difference in respiratory fractionation between starved and nonstarved plants.
Thus, the steady δ13C value of Suc in starved plants indicates that some carbon skeletons were provided from pools of nonlabeled substrates to feed Suc synthesis also in this condition. The lower δ13C steady value of Suc in starved compared to nonstarved plants is presumably due to a lower dilution of carbon from current photosynthates by preexisting carbon because of the shortage of stored carbon after 3 d in the dark. However, previous experiments showed that the δ13C in Suc, expected from online measurements of ΔO, satisfactorily equaled that effectively measured after leaf extraction (Brugnoli et al., 1988). Note that normal air was used as a CO2 source in these experiments; consequently, the effect of the slow-turnover carbon reserves was not detected in the final isotope composition value.
As noted above, the δ13C of the CO2 evolved in both starved and nonstarved leaves is the same, while that of Suc is very different: The low respiration rates in starved leaves presumably induced a low in-draft flux of carbon from recent photosynthesis into the respiratory metabolic pool, with more recent photosynthates directed to feed other processes. As a result, the dilution of the respiratory carbon by recent photosynthesis in starved plants was the same as that in nonstarved plants, while the labeling in Suc was larger. Other metabolic pathways, such as lipolysis and gluconeogenesis (Kim and Smith, 1994; Chen et al., 2000; Rylott et al., 2003), may feed respiration. Nevertheless, it is very unlikely as the RQ value after illumination was 1.
Comparison of results shown in Table II also highlights the contribution of low-turnover carbon pools in feeding respiration. In nonstarved leaves, the increase in total amount of starch as a function of the amount of new carbon assimilated was about twice the increase in starch coming directly from photosynthetic activity. A similar ratio between the increase in the total amount and the amount newly derived from recently assimilated carbon of both starch and Suc was also observed in starved leaves after 250 mmol C m−2 had been assimilated. Interestingly, both total amount of starch and Suc decreased when 600 mmol C m−2 had been assimilated in starved leaves. New carbon coming from photosynthetic activity in these two compounds also decreased, although this decrease was about one-half that of the total amount. Taken together, these data suggest a tight link during the light period between the direct (from photosynthesis) and indirect (from low-turnover compounds) way of increasing starch and Suc pools in the leaves.
In nonstarved leaves, the total amount of Suc is larger than the amount of Suc newly synthesized from photosynthetic activity. Moreover, it does not change or changes only slightly with the amount of carbon fixed during photosynthesis, showing that it is equally synthesized (from photosynthesis) and used (i.e. for synthesis, for exportation, and by respiration). It is not possible in this condition to see an indirect way of Suc synthesis.
Of course, only a small fraction of newly assimilated carbon is used for synthesis of carbohydrates. For example, in nonstarved leaves, after the assimilation of 30 mmol C m−2, only 13% and 10% of the carbon fixed is used to synthesize Suc and starch, respectively, while in starved leaves, much less is used (see Table II, numbers in brackets). This fraction is even smaller in other metabolites that we have analyzed (i.e. lipids and heat-precipitated proteins; data not shown). The total fraction of the new carbon used for synthesis of major metabolites and for respiration does not exceed 30% in both types of plants (data not shown), indicating that a large fraction of new carbon is exported to the other organs.
Carbon Allocation and Respiratory Substrate Pools
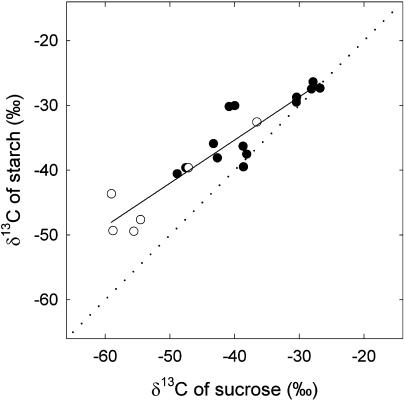
When the amount of carbon assimilated by the leaves was higher than about 400 mmol m−2, the labeling in starch also reached a stable value (Fig. 3), which in both cases was lower than that measured in Suc, indicating that new carbon preferentially fed Suc synthesis. The δ13C relationship in Suc and in starch deviated substantially from 1:1 and was the same in starved and nonstarved leaves (Fig. 5). The depletion of carbohydrate reserves did not change this relationship (in other words, there is no or nondetectable effect of the dilution of new carbon by preexisting Suc and starch pools). Thus, the data of Figure 5 suggest that the partitioning of new carbon between Suc and starch synthesis is, in both cases, about 40% to starch synthesis, as indicated by the slope of the relationship (about 0.6; Fig. 5). This estimation is consistent with that of Sharkey et al. (1985) and Fondy and Geiger (1982) on bean and sugar beet, respectively.
Figure 5.
δ13C of starch and Suc after labeling of French bean leaves. Plants were taken directly from the greenhouse (nonstarved plants, black symbols) or kept in the dark for 3 d (starved plants, white symbols) before the labeling. Each point represents data from a separate leaf. The regression line is obtained with all data in the plot. The regression is y = 0.66x − 8.69, r2 = 0.85. The regression is significant (F = 105.2; P < 0.0001).
The evolution of CO2 isotope composition and the proportion of new carbon in CO2 as a function of the amount of carbon obtained in this study indicate that respiratory carbon in the leaf has (at least) two sources with distinct turnover rates (Fig. 2). This can be easily seen with a semilogarithmic plot of the data (Fig. 2C, inset) in which two linear relations appear, with time constants (turnover delay) around 6 h (that is, 170 mmol C m−2 with net photosynthesis at 7.5 μmol m−2 s−1) and more than 10 d. Recently, at the mesocosm level, Schnyder et al. (2003) also proposed that two distinct substrate pools are used during dark respiration: a fast pool that is exchanged within hours and a slow pool that accounts for about 60% of total respiration and has a mean residence time of approximately 3.6 d. More data are now needed to further compare those two pools in both studies.
The occurrence of two kinetically distinct pools of Suc has already been reported in parenchyma cells of Vicia faba (Fisher and Outlaw, 1979). Nevertheless, the nature of the compartmentation of carbohydrate (Suc) pools in the leaf remains unclear. One can assume the involvement of the vacuole as a Suc pool with a slow turnover. For example, in suspended cultured cells using the 14C isotope, the vacuole sequestrates Suc (Gerhardt et al., 1987; Dubinina et al., 2001). Presumably, the slow turnover of Suc in the vacuole coupled to efficient export to phloem of current Suc in the light may explain the saturation δ13C value in nonstarved plants. In starved plants, this effect may be exaggerated by the fact that (1) the vacuole contains some metabolites from the autophagic processes subsequent to starvation (Bligny et al., 1989); (2) the renewal of the respiratory metabolic pool is modulated by respiration rate; and (3) the soluble sugars are composed of a mixture of old gluconeogenetic and new carbon.
The δ13C value of the CO2 respired in the dark is the result of a composite structure of the carbohydrate (Suc) pool in addition to a possible metabolic compartmentation effect that prevents current photosynthates from entering the glycolysis pathway. As an example, experimental data have shown that the level of the allosteric effector Fru-2,6-P2 decreases when the photoassimilate load (triose phosphate level) is high, relieving the inhibition of the cytosolic Fru-1,6-P2 phosphatase (Stitt, 1990).
In conclusion, the isotope-labeling technique with 13C abundance close to the natural one allowed us to study the origin of the carbon atoms in the CO2 respired in darkness by intact leaves. Although respiration after a light period globally oxidizes carbohydrates, our results show that this respiratory substrate is a mixture in which the current photosynthetic product is not the main component in physiological conditions. Similarly, Suc and starch synthesis are fed both by recent photosynthates and low-turnover carbon compounds. Further experiments are now needed to determine the basis of the metabolic compartmentation responsible for this effect.
MATERIALS AND METHODS
Plant Material
French bean (Phaseolus vulgaris L. cv Contender) plants were grown from seeds in 1-L pots of potting mix in a greenhouse, as described by Tcherkez et al. (2003). Minimum photosynthetic photon flux density (PPFD) during a 16-h photoperiod was maintained at approximately 500 μmol m−2 s−1 by supplementary lighting. Temperature and vapor pressure deficit were maintained at approximately 25.5/18.5°C and 1.4/1.2 kPa day/night, respectively. δ13C of CO2 in the greenhouse air was −9.5‰ ± 0.3‰. The first trifoliar fully expanded leaves were used for all measurements.
Gas Exchange and 13C/12C-Labeling Procedures
Plants were removed from the greenhouse after a light period of about 10 h and the attached leaflet was placed for 45 min in a respiration chamber for online measurements of dark-respired CO2 (unlabeled). After the initial measurement of dark-respired CO2, the attached leaflet was removed from the respiration chamber of the closed system and placed in a specially designed gas-exchange labeling chamber built in the laboratory for online isotope labeling in an open system. After labeling, the attached leaflet was removed from the labeling chamber of the open system, and half of it (still attached) was returned to the respiration chamber of the closed system for online measurements of dark-respired CO2 (labeled). The other half (and other unlabeled leaflets) was immediately frozen in liquid nitrogen, lyophilized, and powdered for metabolite analysis.
Closed Online System for Dark Respiration
The respiration chamber was placed in a closed system, which was directly coupled to an EA NA-1500 (Carlo-Erba, Milan) through a 15-mL loop, as described by Tcherkez et al. (2003). Briefly, molar fractions of respiratory CO2 were measured with an infrared gas analyzer (IRGA; Finor, Maihak, Germany) placed in the closed system that was first flushed with CO2-free air. The loop was shunted when CO2 reached around 300 μL L−1 and the gas inside the loop was introduced into the EA with helium for gas chromatography. The connection valve between the EA and the IRMS (VG Optima; Micromass, Villeurbanne, France) was opened when the CO2 peak emerged from the EA.
δ13Cwas calculated as the deviation of the carbon-to-isotope ratio (13C/12C, called R) from international standard (Pee Dee Belemnite):
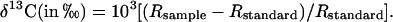 |
Open Online System for Isotopic Labeling
The assimilation chamber was connected in parallel to the sample air hose of the LI-6400 (LI-COR, Lincoln, NE). This aluminum chamber ([20 × 12 × 6] 10−6 m3) has a clear plastic lid that allows us to accommodate the middle leaflet of attached leaves (typical leaf surface about 0.01 m2). Two fans were placed in the chamber and gave a boundary layer conductance to water of about 6.7 mol m−2 s−1. Leaf temperature was controlled at 20°C with circulating water from a cooling water bath to the jacket of the leaf chamber and measured with a copper-constantan thermocouple plugged into the thermocouple sensor connector of the LI-6400 chamber/IRGA. Ingoing air was dried (at about 1 mmol water mol−1) and passed through the chamber at a rate of 1 L min−1, monitored by the LI-6400. Molar fractions of CO2 were measured with the IRGA of the LI-6400. This open online system is similar to the one described by Gillon and Yakir (2000).
Light was supplied by a 500-W halogen lamp (Massive N.V., Kontich, Belgium). The lamp was placed about 30 cm above the chamber and 5 cm of deionized water and 1 cm of glass in the container filtered the radiation. The PPFD at the leaf level inside the chamber was maintained at 500 μmol m−2 s−1 during the labeling period. The rest of the plant received a radiation of about 300 μmol m−2 s−1. For the labeling, CO2 was obtained from a bottle (Air Liquide, Grigny, France) with a δ13C of −51.2‰ ± 0.1‰.
After the photosynthetic measurements, the outgoing air of the chamber was shunted and the air with a CO2 content of about 300 μL L−1 was sent to the loop to measure ΔO. The gas inside the loop was introduced into the EA for gas chromatography, as described above. ΔO during photosynthesis was measured following the method described by Evans et al. (1986):
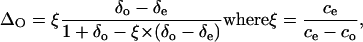 |
and δe and δo are the isotope compositions of CO2 in air entering and leaving the chamber, respectively, and ce and co are the CO2 concentrations (i.e. molar fractions, μL L−1) at a standard humidity of air entering and leaving the chamber, respectively. The measured photosynthetic discrimination was 20‰ ± 1.5‰.
The RQ was calculated from the ratio of carbon production [ν(CO2)] to oxygen consumption [ν(O2)]: RQ = ν(CO2)/ν(O2). CO2 production in darkness was measured using the IRGA of the closed system, as described above. Oxygen consumption of leaf discs (from the half-leaflet used for the metabolic analysis) was measured with an oxygen electrode (Hansatech, Norfolk, England).
Metabolite Extraction and Quantification
The extraction procedures for starch, Suc, Glc, and Fru were similar to those described by Tcherkez et al. (2003). Leaf powder was suspended with 1 mL of distilled water in an Eppendorf tube (Eppendorf Scientific, Hamburg, Germany). After centrifugation, starch was extracted from the pellet by HCl solubilization. Soluble proteins of the supernatant were heat denatured and precipitated, and soluble sugars and organic acids of the proteinless extract were separated by HPLC. After lyophilization, purified metabolites were suspended in distilled water, transferred to tin capsules (Courtage Analyze Service, Mont Saint-Aignan, France), and dried for isotope analysis.
Lipids were extracted as previously described (Deléens et al., 1984; Tcherkez et al., 2003). In brief, fatty acids were methyl esterified with 2 mL of methanol-BF3; 0.5 mL of water and 3 mL of pentane were then added for chlorophyll/lipid separation. The upper phase was transferred into another glass tube, and pentane was evaporated at 50°C with a nitrogen stream. Methyl esters were dissolved in 1.5 mL of methanolic sodium hydroxide. After 1 h at 40°C, pH was neutralized with 0.2 mL of HCl, and free fatty acids were separated with 2 mL of pentane. After pentane evaporation, fatty acids were dissolved in 70 μL of pentane and transferred to tin capsules for isotope analysis. Isotope analysis of metabolites was conducted using the same EA and IRMS as described above.
Calculation of the Percent of New Carbon in Respired CO2 and Metabolites
The CO2 respired in the dark after the labeling comes from old carbon that was already in the leaf before the labeling and new carbon that was recently fixed during the labeling. The proportion of new carbon in dark-respired CO2, denoted as p, was calculated using δ13C. The δ13C value resulting from respiration of old carbon is assumed to equal that observed before the labeling treatment (δb = −22.2‰). The δ13C value of CO2 from respiratory oxidation of new carbon takes into account two successive discriminations: the photosynthetic discrimination ΔO (=20‰) that applies to the δ13C of labeling CO2 (δL = −51.2‰), and the dark respiratory discrimination edark. The δ13C of dark-respired CO2 taken as a whole is then the combination of the two sources (old and new carbon); that is:
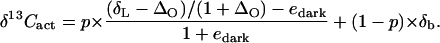 |
A rearrangement gives:
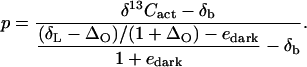 |
The edark value is quite variable (Ghashghaie et al., 2003) and could be at most −6‰ when calculated with Suc as a reference material. In this study, a comparison between Figures 2 and 3 (data obtained in nonstarved leaves) shows that it is close to zero. Moreover, the p values obtained assuming edark = −6‰ would only differ by about 3% from that obtained with edark = 0‰. Thus, next we will assume edark = 0‰.
Although the expression above was used in the calculations (see “Results”), it is worth noting that the term 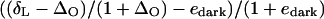 can be approximated by δL − ΔO − edark because ΔO and edark are small compared to 1. This approximation does not generally exceed 1‰ and introduces a proxy in the value of p of about 1% only.
can be approximated by δL − ΔO − edark because ΔO and edark are small compared to 1. This approximation does not generally exceed 1‰ and introduces a proxy in the value of p of about 1% only.
A similar relationship was used to calculate the proportion of new carbon in metabolites. The proportion of labeled carbon contained by a given metabolite pool, denoted as ρ, was then calculated by multiplying p by the amount of metabolite (denoted as c, in g m−2) and the proportion of carbon in it (denoted as x, in g g−1), and dividing by the amount of carbon fixed during the labeling (denoted as q, in mmol m−2), multiplied by 10−3 of the molar mass of carbon (M = 12 g mol−1):
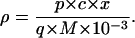 |
Acknowledgments
We thank Richard Bligny, Franz Badeck, Jean Vidal, and Joaquin Azcon-Bieto for critical reading of the manuscript, and Marc Berry for setting up the gas exchange-IRMS coupling. The authors also thank Max Hill for technical assistance on the HPLC procedure.
This work was supported by the European Community's Human Potential Program (contract no. HPRN–CT–1999–00059, NETCARB, to S.N.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.048470.
References
- Affek HP, Yakir D (2003) Natural abundance carbon isotope composition of isoprene reflects incomplete coupling between isoprene synthesis and photosynthetic carbon flow. Plant Physiol 131: 1727–1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert S, Gout E, Bligny R, Marty-Mazars D, Barrieu F, Alabouvette J, Marty F, Douce R (1996) Ultrastructural and biochemical characterization of autophagy in higher plant cells subjected to carbon deprivation: control by the supply of mitochondria with respiratory substrate. J Cell Biol 133: 1251–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avice JC, Ourry A, Lemaire G, Boucaud J (1996) Nitrogen and carbon flows estimated by 15N and 13C pulse-chase labelling during regrowth of alfalfa. Plant Physiol 112: 281–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biehler K, Fock H (1996) Evidence for the contribution of the Mehler-peroxidase reaction in dissipating excess electrons in drought-stressed wheat. Plant Physiol 112: 265–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birecka H, Skiba T, Kozlowska Z (1969) Translocation and redistribution of 14C-assimilates in cereal plants deprived of the ear. III. Assimilate distribution in root and respiration of Culm in wheat plants. Bull Acad Polon Sci Sér Sci Biol 17: 121–127 [PubMed] [Google Scholar]
- Bligny R, Foray M-F, Roby C, Douce R (1989) Transport and phosphorylation of choline in higher plant cells. J Biol Chem 264: 4888–4895 [PubMed] [Google Scholar]
- Bort J, Brown RH, Araus JL (1996) Refixation of respired CO2 in the ears of C3 cereals. J Exp Bot 47: 1567–1575 [Google Scholar]
- Brugnoli E, Hubick KT, von Caemmerer S, Wong SC, Farquhar GD (1988) Correlation between the carbon isotope discrimination in leaf starch and sugars of C3 plants and the ratio of intercellular and atmospheric partial pressures of carbon dioxide. Plant Physiol 88: 1418–1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HJ, Hou WC, Jane WN, Lin YH (2000) Isolation and characterisation of an isocitrate lyase from senescent leaves of sweet potato (Ipomoea batatas cv. Tainong 57). J Plant Physiol 157: 669–676 [Google Scholar]
- Cliquet JB, Deléens E, Bousser A, Martin M, Lescure JC, Prioul JL, Mariotti A, Morot-Gaudry JF (1990. a) Estimation of carbon and nitrogen allocation during stalk elongation by 13C and 15N tracing in Zea mays L. Plant Physiol 92: 79–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliquet JB, Deléens E, Mariotti A (1990. b) C and N mobilization from stalk and leaves during kernel filling by 13C and 15N tracing in Zea mays L. Plant Physiol 94: 1547–1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deléens E, Pavlidès D, Queiroz O (1983) Application du marquage isotopique naturel par le 13C à la mesure du renouvellement de la matière foliaire chez les plantes en C3. Physiol Veg 21: 723–729 [Google Scholar]
- Deléens E, Schwebel-Dugué N, Trémolières A (1984) Carbon isotope composition of lipidic classes isolated from tobacco leaves. FEBS Lett 178: 55–58 [Google Scholar]
- Devaux C, Baldet P, Joubes J, Dieuaide-Noubhani M, Just D, Chevalier C, Raymond P (1984) Physiological, biochemical and molecular analysis of sugar starvation responses in tomato roots. J Exp Bot 54: 1143–1151 [DOI] [PubMed] [Google Scholar]
- Dieuaide-Noubhani M, Canioni P, Raymond Ph (1997) Sugar-starvation-induced changes of carbon metabolism in excised maize root tips. Plant Physiol 115: 1505–1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubinina IM, Burakhanova EA, Kudryavtseva LF (2001) Vacuoles of mesophyll cells as a transient reservoir for assimilates. Russ J Plant Physiol 48: 32–37 [Google Scholar]
- Duranceau M, Ghashghaie J, Badeck F, Deléens E, Cornic G (1999)δ13C of CO2 respired in the dark in relation to δ13C of leaf carbohydrates in Phaseolus vulgaris L. under progressive drought. Plant Cell Environ 22: 515–523 [Google Scholar]
- Evans JR, Sharkey TD, Berry JA, Farquhar GD (1986) Carbon isotope discrimination measured concurrently with gas exchange to investigate CO2 diffusion in leaves of higher plants. Aust J Plant Physiol 13: 281–292 [Google Scholar]
- Fisher DB, Outlaw WH (1979) Sucrose compartmentation in the palisade parenchyma of Vicia faba L. Plant Physiol 64: 481–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fondy BR, Geiger DR (1982) Diurnal pattern of translocation and carbohydrate metabolism in source leaves of Beta vulgaris L. Plant Physiol 70: 671–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebbing T, Schnyder H (2001) 13C labelling kinetics of sucrose in glumes indicates significant refixation of respiratory CO2 in the wheat ear. Aust J Plant Physiol 28: 1047–1053 [Google Scholar]
- Geiger DR, Shieh W-J (1988) Analysis partitioning of recently fixed and of reserve carbon in reproductive Phaseolus vulgaris L. plants. Plant Cell Environ 11: 777–783 [Google Scholar]
- Gerhardt R, Stitt M, Heldt HW (1987) Subcellular metabolite in spinach leaves. Plant Physiol 83: 399–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghashghaie J, Badeck FW, Lanigan G, Nogués S, Tcherkez G, Deléens E, Cornic G, Griffiths H (2003) Carbon isotope fractionation during dark respiration and photorespiration in C3 plants. Phytochemistry Rev 2: 145–161 [Google Scholar]
- Gillon JS, Yakir D (2000) Internal conductance to CO2 diffusion and C18OO discrimination in C3 leaves. Plant Physiol 123: 201–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goren R, Hubermann M, Zehavi U, Chen-Zion M, Echevarria E (2000) Sugar utilization by citrus juice cells as determined by [14C]-sucrose and [14C]-fructose feeding analyses. Plant Physiol Biochem 38: 507–515 [Google Scholar]
- Kim DJ, Smith SM (1994) Molecular cloning of cucumber phosphoenolpyruvate carboxykinase and development regulation of gene expression. Plant Mol Biol 26: 423–434 [DOI] [PubMed] [Google Scholar]
- Kouchi H, Yoneyama T (1984) Dynamics of carbon photosynthetically assimilated in nodulated soya bean plants under steady-state conditions. 1. Development and application of 13CO2 assimilation system at a constant 13C abundance. Ann Bot (Lond) 53: 875–882 [Google Scholar]
- Kuzyakov Y, Ehrensberger H, Stahr K (2001) Carbon partitioning and below-ground translocation by Lolium perenne. Soil Biol Biochem 33: 61–74 [Google Scholar]
- Li B, Geiger DR, Shieh WJ (1992) Evidence for circadian regulation of starch and sucrose synthesis in sugar beet leaves. Plant Physiol 99: 1393–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillard P, Deléens E, Daudet FA, Lacointe A, Frossard JS (1994) Carbon and nitrogen partitioning in walnut seedlings during the acquisition of autotrophy through simultaneous 13CO2 and 15NO3 long-term labelling. J Exp Bot 45: 203–210 [Google Scholar]
- Pärnik TR, Voronin PY, Ivanova HN, Keerberg OF (2002) Respiratory CO2 fluxes in photosynthesising leaves of C3 species varying in rates of starch synthesis. Russ J Plant Physiol 49: 729–735 [Google Scholar]
- Pearen JR, Hume DJ (1981) 14C-labelled assimilate utilisation by soybeans grown with three nitrogen sources. Crop Sci 21: 938–942 [Google Scholar]
- Rabinowitch EI (1956) Photosynthesis, Vol 2. Interscience Publishers, New York
- Rylott EL, Gilday AD, Graham IA (2003) The gluconeogenic enzyme phosphoenolpyruvate carboxykinase in Arabidopsis is essential for seedling establishment. Plant Physiol 131: 1834–1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnyder H (1992) Long-term steady-state labelling of wheat plants by use of natural 13CO2/12CO2 mixtures in an open, rapidly turned-over system. Planta 187: 128–135 [DOI] [PubMed] [Google Scholar]
- Schnyder H, Schäufele R, Lötscher M, Gebbing T (2003) Disentangling CO2 fluxes: direct measurements of mesocosm-scale natural abundance 13CO2/12CO2 gas exchange, 13C discrimination, and labelling of CO2 exchange flux components in controlled environments. Plant Cell Environ 26: 1863–1874 [Google Scholar]
- Sharkey TD, Berry JA, Rascke K (1985) Starch and sucrose synthesis in Phaseolus vulgaris as affected by light, CO2, and abscisic acid. Plant Physiol 77: 617–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soja G, Haunold E, Praznik W (1989) Translocation of 14C-assimilates in Jerusalem artichoke (Helianthus tuberosus L.). J Plant Physiol 134: 218–223 [Google Scholar]
- Stitt M (1990) Fructose-2,6-bisphosphate as a regulatory molecule in plants. Annu Rev Plant Physiol Plant Mol Biol 41: 153–185 [Google Scholar]
- Tcherkez G, Nogués S, Bleton J, Cornic G, Badeck F, Ghashghaie J (2003) Metabolic origin of carbon isotope composition of leaf dark-respired CO2 in French bean. Plant Physiol 131: 237–244 [DOI] [PMC free article] [PubMed] [Google Scholar]