Abstract
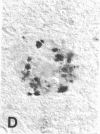
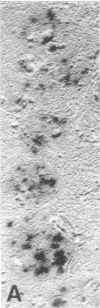
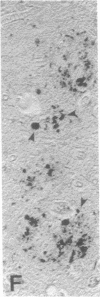
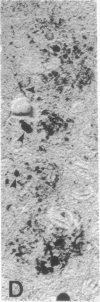
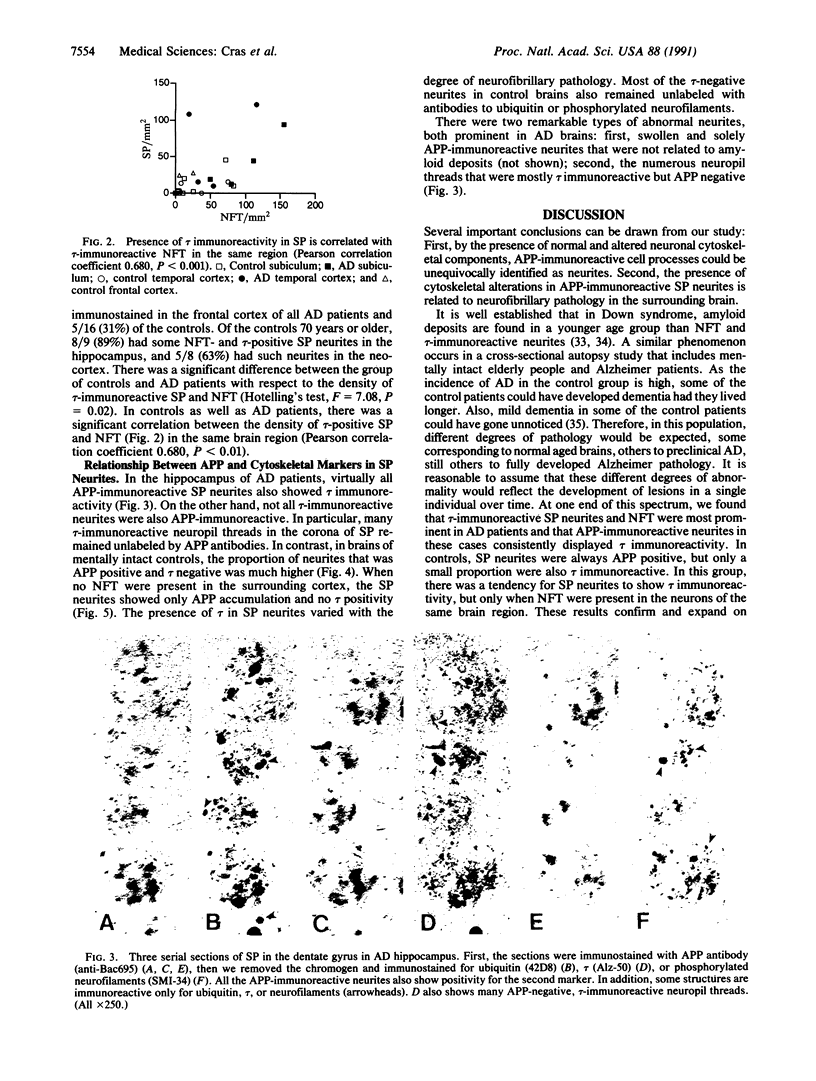
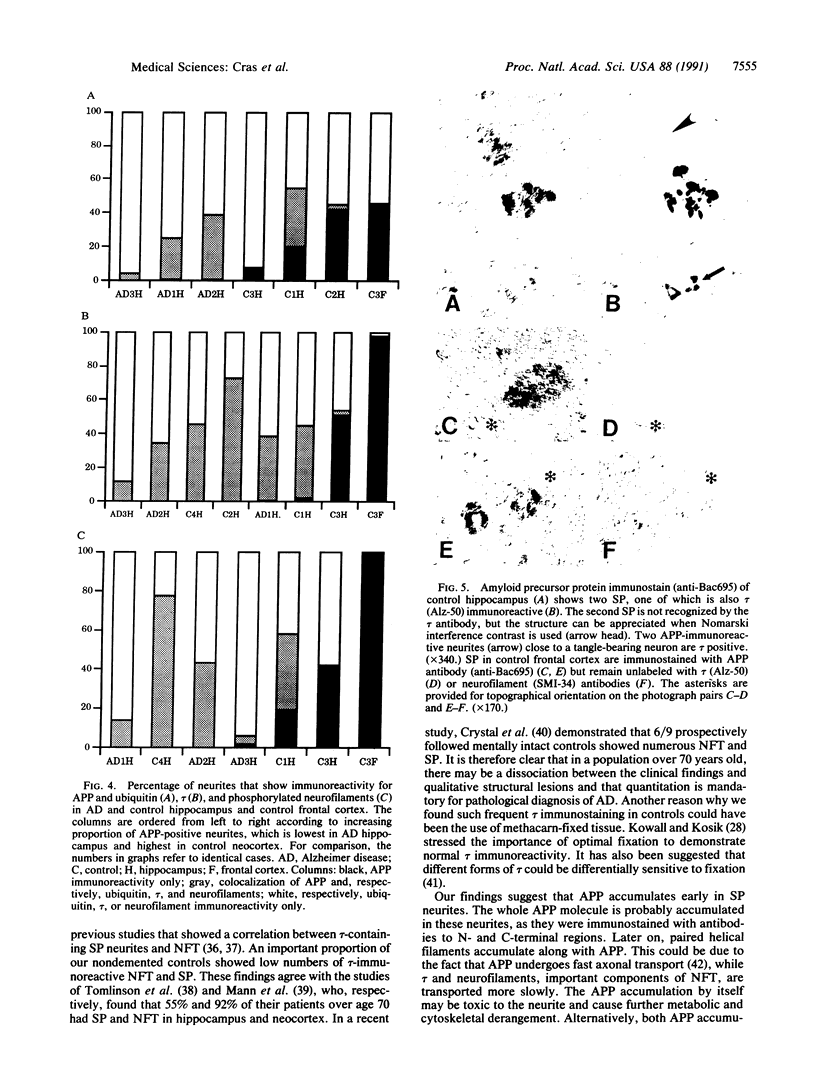
Senile plaques are polymorphous beta-amyloid protein deposits found in the brain in Alzheimer disease and normal aging. This beta-amyloid protein is derived from a larger precursor molecule of which neurons are the principal producers in brain. We found that amyloid precursor protein (APP)-immunoreactive neurites were involved in senile plaques and that only a subset of these neurites showed markers for the abnormal filaments characteristic of neurofibrillary pathology. In the neocortex of nondemented individuals with senile plaques but spared of neurofibrillary pathology, dystrophic neurites in senile plaques showed only APP accumulation. In contrast, in the brains of Alzheimer patients, virtually all APP-immunoreactive neurites also showed immunoreactivity with ubiquitin, tau, and phosphorylated neurofilaments. The presence of tau and neurofilament epitopes in dystrophic neurites in senile plaques was correlated with the extent of neurofibrillary pathology in the surrounding brain tissue. Accumulation of APP and the formation of neurofibrillary pathology in senile plaque neurites are therefore distinct phenomena. Our findings suggest that APP accumulation in senile plaque neurites occurs prior to tau accumulation and is therefore more closely related to appearance of neuritic dystrophy.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allsop D., Haga S. I., Haga C., Ikeda S. I., Mann D. M., Ishii T. Early senile plaques in Down's syndrome brains show a close relationship with cell bodies of neurons. Neuropathol Appl Neurobiol. 1989 Nov-Dec;15(6):531–542. doi: 10.1111/j.1365-2990.1989.tb01252.x. [DOI] [PubMed] [Google Scholar]
- Autilio-Gambetti L., Crane R., Gambetti P. Binding of Bodian's silver and monoclonal antibodies to defined regions of human neurofilament subunits: Bodian's silver reacts with a highly charged unique domain of neurofilaments. J Neurochem. 1986 Feb;46(2):366–370. doi: 10.1111/j.1471-4159.1986.tb12977.x. [DOI] [PubMed] [Google Scholar]
- Barcikowska M., Wisniewski H. M., Bancher C., Grundke-Iqbal I. About the presence of paired helical filaments in dystrophic neurites participating in the plaque formation. Acta Neuropathol. 1989;78(3):225–231. doi: 10.1007/BF00687751. [DOI] [PubMed] [Google Scholar]
- Braak H., Braak E., Ohm T., Bohl J. Alzheimer's disease: mismatch between amyloid plaques and neuritic plaques. Neurosci Lett. 1989 Aug 14;103(1):24–28. doi: 10.1016/0304-3940(89)90479-5. [DOI] [PubMed] [Google Scholar]
- Bugiani O., Giaccone G., Verga L., Pollo B., Ghetti B., Frangione B., Tagliavini F. Alzheimer patients and Down patients: abnormal presynaptic terminals are related to cerebral preamyloid deposits. Neurosci Lett. 1990 Oct 30;119(1):56–59. doi: 10.1016/0304-3940(90)90754-w. [DOI] [PubMed] [Google Scholar]
- Cataldo A. M., Nixon R. A. Enzymatically active lysosomal proteases are associated with amyloid deposits in Alzheimer brain. Proc Natl Acad Sci U S A. 1990 May;87(10):3861–3865. doi: 10.1073/pnas.87.10.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataldo A. M., Thayer C. Y., Bird E. D., Wheelock T. R., Nixon R. A. Lysosomal proteinase antigens are prominently localized within senile plaques of Alzheimer's disease: evidence for a neuronal origin. Brain Res. 1990 Apr 16;513(2):181–192. doi: 10.1016/0006-8993(90)90456-l. [DOI] [PubMed] [Google Scholar]
- Cras P., Kawai M., Siedlak S., Mulvihill P., Gambetti P., Lowery D., Gonzalez-DeWhitt P., Greenberg B., Perry G. Neuronal and microglial involvement in beta-amyloid protein deposition in Alzheimer's disease. Am J Pathol. 1990 Aug;137(2):241–246. [PMC free article] [PubMed] [Google Scholar]
- Crystal H., Dickson D., Fuld P., Masur D., Scott R., Mehler M., Masdeu J., Kawas C., Aronson M., Wolfson L. Clinico-pathologic studies in dementia: nondemented subjects with pathologically confirmed Alzheimer's disease. Neurology. 1988 Nov;38(11):1682–1687. doi: 10.1212/wnl.38.11.1682. [DOI] [PubMed] [Google Scholar]
- Dickson D. W., Wertkin A., Mattiace L. A., Fier E., Kress Y., Davies P., Yen S. H. Ubiquitin immunoelectron microscopy of dystrophic neurites in cerebellar senile plaques of Alzheimer's disease. Acta Neuropathol. 1990;79(5):486–493. doi: 10.1007/BF00296107. [DOI] [PubMed] [Google Scholar]
- Giaccone G., Tagliavini F., Linoli G., Bouras C., Frigerio L., Frangione B., Bugiani O. Down patients: extracellular preamyloid deposits precede neuritic degeneration and senile plaques. Neurosci Lett. 1989 Feb 13;97(1-2):232–238. doi: 10.1016/0304-3940(89)90169-9. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Wong C. W. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984 May 16;120(3):885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- Ishii T., Kametani F., Haga S., Satoh M. Subsequences of amyloid precursor protein in Alzheimer brain. Prog Clin Biol Res. 1989;317:965–970. [PubMed] [Google Scholar]
- Itagaki S., McGeer P. L., Akiyama H., Zhu S., Selkoe D. Relationship of microglia and astrocytes to amyloid deposits of Alzheimer disease. J Neuroimmunol. 1989 Oct;24(3):173–182. doi: 10.1016/0165-5728(89)90115-x. [DOI] [PubMed] [Google Scholar]
- Joachim C., Games D., Morris J., Ward P., Frenkel D., Selkoe D. Antibodies to non-beta regions of the beta-amyloid precursor protein detect a subset of senile plaques. Am J Pathol. 1991 Feb;138(2):373–384. [PMC free article] [PubMed] [Google Scholar]
- Kang J., Lemaire H. G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K. H., Multhaup G., Beyreuther K., Müller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987 Feb 19;325(6106):733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Kawai M., Kalaria R. N., Harik S. I., Perry G. The relationship of amyloid plaques to cerebral capillaries in Alzheimer's disease. Am J Pathol. 1990 Dec;137(6):1435–1446. [PMC free article] [PubMed] [Google Scholar]
- Khachaturian Z. S. Diagnosis of Alzheimer's disease. Arch Neurol. 1985 Nov;42(11):1097–1105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- Koo E. H., Sisodia S. S., Archer D. R., Martin L. J., Weidemann A., Beyreuther K., Fischer P., Masters C. L., Price D. L. Precursor of amyloid protein in Alzheimer disease undergoes fast anterograde axonal transport. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1561–1565. doi: 10.1073/pnas.87.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowall N. W., Kosik K. S. Axonal disruption and aberrant localization of tau protein characterize the neuropil pathology of Alzheimer's disease. Ann Neurol. 1987 Nov;22(5):639–643. doi: 10.1002/ana.410220514. [DOI] [PubMed] [Google Scholar]
- Lampert P. W. A comparative electron microscopic study of reactive, degenerating, regenerating, and dystrophic axons. J Neuropathol Exp Neurol. 1967 Jul;26(3):345–368. doi: 10.1097/00005072-196707000-00001. [DOI] [PubMed] [Google Scholar]
- Mann D. M., Brown A. M., Prinja D., Jones D., Davies C. A. A morphological analysis of senile plaques in the brains of non-demented persons of different ages using silver, immunocytochemical and lectin histochemical staining techniques. Neuropathol Appl Neurobiol. 1990 Feb;16(1):17–25. doi: 10.1111/j.1365-2990.1990.tb00928.x. [DOI] [PubMed] [Google Scholar]
- Mann D. M., Yates P. O., Marcyniuk B., Ravindra C. R. The topography of plaques and tangles in Down's syndrome patients of different ages. Neuropathol Appl Neurobiol. 1986 Sep-Oct;12(5):447–457. doi: 10.1111/j.1365-2990.1986.tb00053.x. [DOI] [PubMed] [Google Scholar]
- Miyakawa T., Shimoji A., Kuramoto R., Higuchi Y. The relationship between senile plaques and cerebral blood vessels in Alzheimer's disease and senile dementia. Morphological mechanism of senile plaque production. Virchows Arch B Cell Pathol Incl Mol Pathol. 1982 Aug;40(2):121–129. doi: 10.1007/BF02932857. [DOI] [PubMed] [Google Scholar]
- Morris J. C., McKeel D. W., Jr, Storandt M., Rubin E. H., Price J. L., Grant E. A., Ball M. J., Berg L. Very mild Alzheimer's disease: informant-based clinical, psychometric, and pathologic distinction from normal aging. Neurology. 1991 Apr;41(4):469–478. doi: 10.1212/wnl.41.4.469. [DOI] [PubMed] [Google Scholar]
- Motte J., Williams R. S. Age-related changes in the density and morphology of plaques and neurofibrillary tangles in Down syndrome brain. Acta Neuropathol. 1989;77(5):535–546. doi: 10.1007/BF00687256. [DOI] [PubMed] [Google Scholar]
- Mulvihill P., Perry G. Immunoaffinity demonstration that paired helical filaments of Alzheimer disease share epitopes with neurofilaments, MAP2 and tau. Brain Res. 1989 Apr 10;484(1-2):150–156. doi: 10.1016/0006-8993(89)90357-0. [DOI] [PubMed] [Google Scholar]
- Perry G., Friedman R., Shaw G., Chau V. Ubiquitin is detected in neurofibrillary tangles and senile plaque neurites of Alzheimer disease brains. Proc Natl Acad Sci U S A. 1987 May;84(9):3033–3036. doi: 10.1073/pnas.84.9.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry G., Lipphardt S., Mulvihill P., Kancherla M., Mijares M., Gambetti P., Sharma S., Maggiora L., Cornette J., Lobl T. Amyloid precursor protein in senile plaques of Alzheimer disease. Lancet. 1988 Sep 24;2(8613):746–746. doi: 10.1016/s0140-6736(88)90219-x. [DOI] [PubMed] [Google Scholar]
- Pollock N. J., Wood J. G. Differential sensitivity of the microtubule-associated protein, tau, in Alzheimer's disease tissue to formalin fixation. J Histochem Cytochem. 1988 Sep;36(9):1117–1121. doi: 10.1177/36.9.2841371. [DOI] [PubMed] [Google Scholar]
- Ponte P., Gonzalez-DeWhitt P., Schilling J., Miller J., Hsu D., Greenberg B., Davis K., Wallace W., Lieberburg I., Fuller F. A new A4 amyloid mRNA contains a domain homologous to serine proteinase inhibitors. Nature. 1988 Feb 11;331(6156):525–527. doi: 10.1038/331525a0. [DOI] [PubMed] [Google Scholar]
- Probst A., Anderton B. H., Brion J. P., Ulrich J. Senile plaque neurites fail to demonstrate anti-paired helical filament and anti-microtubule-associated protein-tau immunoreactive proteins in the absence of neurofibrillary tangles in the neocortex. Acta Neuropathol. 1989;77(4):430–436. doi: 10.1007/BF00687379. [DOI] [PubMed] [Google Scholar]
- Probst A., Langui D., Lautenschlager C., Ulrich J., Brion J. P., Anderton B. H. Progressive supranuclear palsy: extensive neuropil threads in addition to neurofibrillary tangles. Very similar antigenicity of subcortical neuronal pathology in progressive supranuclear palsy and Alzheimer's disease. Acta Neuropathol. 1988;77(1):61–68. doi: 10.1007/BF00688244. [DOI] [PubMed] [Google Scholar]
- Shelton E. R., Cohn R., Fish L., Obernolte R., Tahilramani R., Nestor J. J., Chan H. W. Characterization of beta-amyloid precursor proteins with or without the protease-inhibitor domain using anti-peptide antibodies. J Neurochem. 1990 Jul;55(1):60–69. doi: 10.1111/j.1471-4159.1990.tb08821.x. [DOI] [PubMed] [Google Scholar]
- Shin R. W., Ogomori K., Kitamoto T., Tateishi J. Increased tau accumulation in senile plaques as a hallmark in Alzheimer's disease. Am J Pathol. 1989 Jun;134(6):1365–1371. [PMC free article] [PubMed] [Google Scholar]
- Shoji M., Hirai S., Yamaguchi H., Harigaya Y., Kawarabayashi T. Amyloid beta-protein precursor accumulates in dystrophic neurites of senile plaques in Alzheimer-type dementia. Brain Res. 1990 Mar 26;512(1):164–168. doi: 10.1016/0006-8993(90)91187-l. [DOI] [PubMed] [Google Scholar]
- Sisodia S. S., Koo E. H., Beyreuther K., Unterbeck A., Price D. L. Evidence that beta-amyloid protein in Alzheimer's disease is not derived by normal processing. Science. 1990 Apr 27;248(4954):492–495. doi: 10.1126/science.1691865. [DOI] [PubMed] [Google Scholar]
- Suenaga T., Hirano A., Llena J. F., Ksiezak-Reding H., Yen S. H., Dickson D. W. Modified Bielschowsky and immunocytochemical studies on cerebellar plaques in Alzheimer's disease. J Neuropathol Exp Neurol. 1990 Jan;49(1):31–40. doi: 10.1097/00005072-199001000-00004. [DOI] [PubMed] [Google Scholar]
- Tabaton M., Mandybur T. I., Perry G., Onorato M., Autilio-Gambetti L., Gambetti P. The widespread alteration of neurites in Alzheimer's disease may be unrelated to amyloid deposition. Ann Neurol. 1989 Dec;26(6):771–778. doi: 10.1002/ana.410260614. [DOI] [PubMed] [Google Scholar]
- Tanzi R. E., Gusella J. F., Watkins P. C., Bruns G. A., St George-Hyslop P., Van Keuren M. L., Patterson D., Pagan S., Kurnit D. M., Neve R. L. Amyloid beta protein gene: cDNA, mRNA distribution, and genetic linkage near the Alzheimer locus. Science. 1987 Feb 20;235(4791):880–884. doi: 10.1126/science.2949367. [DOI] [PubMed] [Google Scholar]
- Tomlinson B. E., Blessed G., Roth M. Observations on the brains of non-demented old people. J Neurol Sci. 1968 Sep-Oct;7(2):331–356. doi: 10.1016/0022-510x(68)90154-8. [DOI] [PubMed] [Google Scholar]
- Whitson J. S., Selkoe D. J., Cotman C. W. Amyloid beta protein enhances the survival of hippocampal neurons in vitro. Science. 1989 Mar 17;243(4897):1488–1490. doi: 10.1126/science.2928783. [DOI] [PubMed] [Google Scholar]
- Wisniewski H. M., Bancher C., Barcikowska M., Wen G. Y., Currie J. Spectrum of morphological appearance of amyloid deposits in Alzheimer's disease. Acta Neuropathol. 1989;78(4):337–347. doi: 10.1007/BF00688170. [DOI] [PubMed] [Google Scholar]
- Wisniewski H. M., Wegiel J., Wang K. C., Kujawa M., Lach B. Ultrastructural studies of the cells forming amyloid fibers in classical plaques. Can J Neurol Sci. 1989 Nov;16(4 Suppl):535–542. doi: 10.1017/s0317167100029887. [DOI] [PubMed] [Google Scholar]
- Wolozin B. L., Pruchnicki A., Dickson D. W., Davies P. A neuronal antigen in the brains of Alzheimer patients. Science. 1986 May 2;232(4750):648–650. doi: 10.1126/science.3083509. [DOI] [PubMed] [Google Scholar]
- Yankner B. A., Dawes L. R., Fisher S., Villa-Komaroff L., Oster-Granite M. L., Neve R. L. Neurotoxicity of a fragment of the amyloid precursor associated with Alzheimer's disease. Science. 1989 Jul 28;245(4916):417–420. doi: 10.1126/science.2474201. [DOI] [PubMed] [Google Scholar]