Abstract
Background:
Data on the longitudinal association of walking pace with the risk of cognitive decline and dementia are inconsistent and inconclusive. Therefore, researchers conducted a meta-analysis of prospective cohort studies to quantitatively assess the association of walking pace with the risk of cognitive decline and dementia among elderly populations.
Methods:
Eligible studies were searched in PubMed and EMBASE through April 22, 2016. Additional information was retrieved through Google Scholar or hand review of the reference lists from the relevant studies. Prospective cohort studies were included if they reported relative risk (RR) and the corresponding 95% confidence interval (CI) of cognitive decline or dementia in relation to walking pace.
Results:
Seventeen studies were identified, including 10 studies reporting the RR of cognitive decline (9,949 participants and 2,547 events) and 10 presenting the RR of dementia (14,140 participants and 1,903 events). Comparing the lowest to the highest category of walking pace, the pooled RR was 1.89 (95% CI = 1.54–2.31) for cognitive decline and 1.66 (95% CI = 1.43–1.92) for dementia. With every 1 dm/s (360 m/h) decrement in walking pace, the risk of dementia was increased by 13% (RR = 1.13; 95% CI = 1.08–1.18).
Conclusions:
This meta-analysis provides accumulated evidence supporting that slow or decreased walking pace is significantly associated with elevated risk of cognitive decline and dementia in elderly populations.
Keywords: Cognitive decline, Dementia, “Meta-analysis”, Walking pace
Cognitive decline and dementia are common outcomes of aging that considerably affect the quality of life for the elderly people. In addition to increasing the cost of health care, cognitive decline and dementia may generate enormous physical and emotional pressure for the patient’s family. Because there are currently no cures for these health disorders, identifying risk factors and/or early indicators will be of substantial benefit to the patient, family, and society.
Gait variables have been considered important factors related to mental health among elderly people. In the past decade, numerous studies aimed to assess the associations between gait variables and cognitive decline or dementia; some (1–4), but not all, studies (5–8) reported significant associations. Walking pace, as a measure that has easier application and interpretation than other gait parameters (eg, stride length variability and stride time variability), has drawn researchers’ and public health professionals’ attention. Although previous qualitative systematic review suggested an inverse association between walking pace and cognitive dysfunction (9–11), the magnitude of the association and the dose–response relationship have not been systematically investigated and remain unclear. Therefore, we aimed to quantitatively summarize the up-to-date literature examining the overall association and the dose–response relationship between walking pace and the risk of cognitive decline and dementia in elderly populations.
Materials and Methods
Search Strategy
This study attempted to follow the criteria of “Meta-analyses of Observational Studies in Epidemiology guidelines” (12). The relevant prospective cohort studies published in the English language and reporting the associations between walking pace and the risk of cognitive decline or dementia were identified by searching the PubMed and EMBASE through April 22, 2016, using the terms “walking speed,” “gait speed,” “walking,” “gait” cross-referenced to “cognition disorders,” “cognitive decline,” “mild cognitive impairment,” “dementia,” “Alzheimer’s disease,” “Lewy bodies,” or “Frontal lobe”. In addition, Google Scholar using the same key words and the reference lists of identified studies were searched for additional citations.
Study Selection
Two reviewers (M.Q. and P.X.) independently reviewed the literature and identified all relevant studies. Disagreements were resolved by group discussion with a third reviewer (K.H.). Original studies were included if they met all the following criteria:
(1) Prospective cohort study design; (2) relative risk (RR) estimates of cognitive decline (with a specific definition for cognitive decline) or dementia in relation to walking pace and the corresponding 95% confidence interval (CI) or p value were reported, or such information could be derived from the published results; and (3) published in the English language.
We retrieved 403 studies from PubMed and EMBASE, and 391 of them were excluded due to one of the following reasons (Supplementary Figure 1): (1) not published in English; (2) not original studies; (3) did not include walking pace as exposure or cognitive decline or dementia as the outcome of interest; (4) did not report the association of interest; and (5) not the most recent publications. Five additional studies were identified through Google Scholar or the reference lists of the relevant studies. In sum, a total of 17 studies were identified.
Assessment of Study Quality
The quality of included studies was assessed based on the guidelines developed by the U.S. Preventive Task Force as well as the modified checklist used in the previous studies (13,14). Specifically, the following criteria were used to evaluate the quality of the study: (1) adjustment for potential confounders (ie, at least considered four factors: age, sex, education, and baseline cognitive function test scores); (2) information on loss of follow-up rate; (3) outcome ascertainment blind to exposure status; (4) clear and proper definition of exposure and outcome of interest; (5) temporality (ie, walking pace prior to outcome ascertainment); and (6) at least 5-year follow-up. Studies were graded as good quality if they met 5–6 criteria; fair if met 2–4; and poor if met <2 criteria. According to the criteria, 13 and 4 studies were considered good and fair quality, respectively.
Data Extraction
Information was extracted independently by two investigators (M.Q. and P.X.). Discrepancies were resolved by discussion with the third investigator (K.H.). We extracted the following information from each included study: the first author’s last name, publication year, study name (if available), country where the study was conducted, number of events/participants, age at baseline, proportion of male participants, length of follow-up, outcome ascertainment method, exposure measurement method, categories of walking pace (if available), and covariates in the final model.
Statistical Analysis
The RR and corresponding 95% CI extracted from each individual study were transformed to natural logarithms (ln) for stabilizing the variances, normalizing their distribution, and calculating its standard error. Hazard ratio and odds ratio were considered to be equivalent to RR as general measures of risk in this meta-analysis. The overall associations between walking pace and the risk of cognitive decline and dementia were expressed as weighted RRs using a random effects model, comparing the lowest with the highest category of walking pace. For the dose–response relationship estimate, all units from available studies were standardized into decimeter per second (dm/s). Subgroup analyses were conducted by geographic region, follow-up duration, study sample sizes, outcome assessment or definition, study quality, and dementia subtype to examine the effect modification.
Statistical heterogeneity was examined using the I 2 statistic along with Cochran’s Q test. None, low, moderate, and high degrees of heterogeneity were defined as <25%, 25%–<50%, 50%–<75%, and ≥75%, respectively. Publication bias was assessed using Egger’s regression asymmetry test (if the number of studies ≥3) or Begg’s adjusted rank correlation test (when the number of studies <3). Two sensitivity analyses were performed to test the robustness of the results: (1) replacing a random effects model with a fixed effects model and (2) removing one primary study from the pooled analysis each time.
All analyses were conducted using STATA statistical software (version 13.1; STATA Corporation LP, College Station, TX). A two-sided p value ≤0.05 was considered statistically significant.
Results
Study Characteristics
A total of 17 prospective cohort studies (1–8,15–23) were included. Of them, 12 studies were conducted in the United States (2–8,15,16,18,22,23), and the rest of the studies were conducted in Italy (17), Sweden (19), China (20), Japan (21), and Israel (1). The sample size ranged from 108 (2) to 2,776 (22) and the follow-up period ranged from 2 (1) to 11 (23) years. All studies except two (5,8) included both genders. The quality assessment indicated that the included studies generally had good or fair quality.
Seven studies reported results on cognitive decline, seven on dementia, and other three studies reported results on both. Of the 10 studies with data on cognitive decline, including 9,949 participants and 2,547 events (Supplementary Table 1), 7 presented categorical association and 3 reported linear relationship. Similarly, 10 studies with data on dementia were comprised of 14,140 participants and 1,903 events (Supplementary Table 2). Of them, seven examined categorical association and three assessed linear relationship.
Walking Pace and the Risk of Cognitive Decline
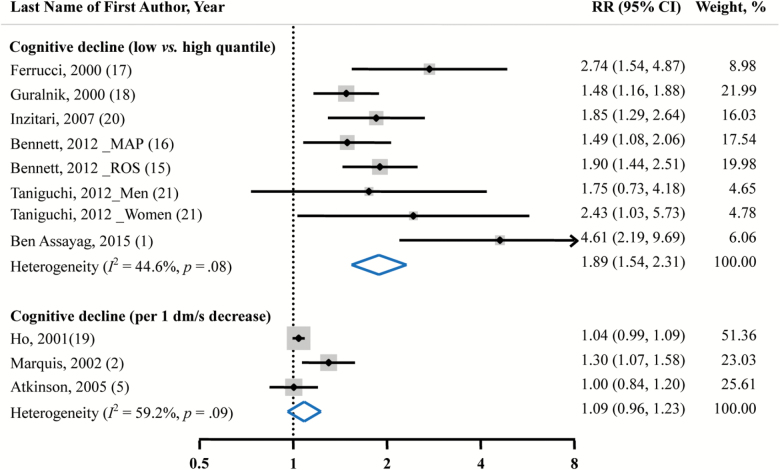
Walking pace was inversely associated with the risk of cognitive decline. Comparing the lowest with the highest level of walking pace, the risk of cognitive decline was increased by 89% (pooled RR = 1.89; 95% CI = 1.54–2.31; Figure 1). The heterogeneity was marginally significant among primary studies (I 2 = 44.6%, p = 0.08). Also, marginally significant publication bias was observed (Egger’s test, p = .06). No significant linear relationship between walking pace and the risk of cognitive decline was observed (RR = 1.09; 95% CI = 0.96–1.23).
Figure 1.
Pooled RRs and 95% CIs of cognitive decline in relation to walking pace. We obtained pooled estimates by using a random-effects model. Dots indicate the adjusted RRs by comparing the lowest with the highest category or every 1 dm/s (360 m/h) decrease in walking pace. The size of the shaded square is proportional to the percentage weight of each study. The horizontal lines represent 95% CIs. The diamond data markers indicate the summary RRs with the 95% CIs. CI = confidence interval; MAP = Memory and Aging Project; ROS = Religious Orders Study; RR = relative risk.
Walking Pace and the Risk of Dementia
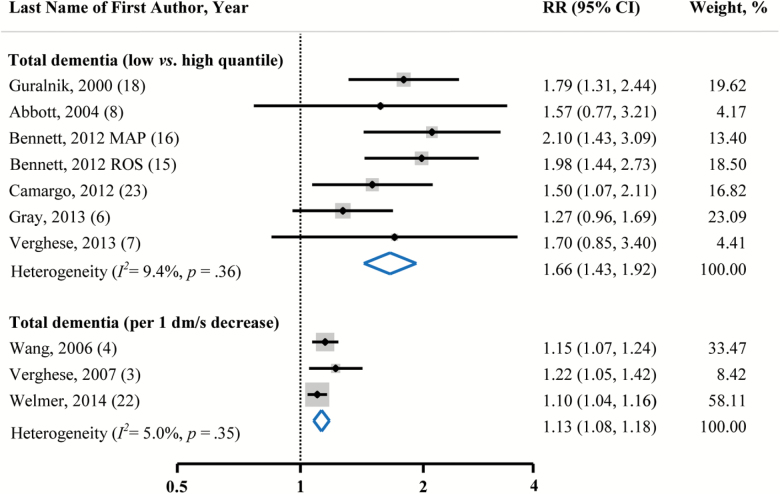
The pooled RR indicated that individuals with slow walking pace had a significantly higher risk of dementia as compared with those who had relatively fast walking pace (RR = 1.66; 95% CI = 1.43–1.92; Figure 2). No substantial heterogeneity (I 2 = 9.4%, p = .36) and publication bias (Egger’s test, p = .69) were observed.
Figure 2:
Pooled RRs and 95% CIs of total dementia in relation to walking pace. We obtained pooled estimates using a random-effects model. Dots indicate the adjusted RRs by comparing the lowest with the highest category or every 1 dm/s (360 m/h) decrease in walking pace. The size of the shaded square is proportional to the percentage weight of each study. The horizontal lines represent 95% CIs. The diamond data markers indicate the summary RRs with the 95% CIs. CI = confidence interval; MAP = Memory and Aging Project; ROS = Religious Orders Study; RR = relative risk.
According to available data from three studies, a significant dose–response relationship was found (RR = 1.13; 95% CI = 1.08–1.18, with every 1 dm/s decrement in walking pace). Heterogeneity (I 2 = 5.0%, p = .35) and publication bias (Egger’s test, p = .23) were not evident.
Subgroup and Sensitivity Analyses
Comparing the lowest with the highest category of walking pace, the inverse associations between walking pace and risk of cognitive decline and dementia were not materially modified by geographic region (United States, Europe, or Asia), follow-up period (above or below the average [5.8 years for cognitive decline and 6.9 years for dementia]), study sample size (above or below the average [1,185 for cognitive decline and 1,701 for dementia]), cognitive function assessment method (Mini-Mental State Examination or others), definition of cognitive decline (Mini-Mental State Examination decrease ≥4 or others), study quality assessment (good or fair), and dementia subtype (Alzheimer’s disease, vascular dementia or non-Alzheimer’s disease dementia; Supplementary Table 3).
Similarly, the inverse linear association between walking pace and the risk of dementia was not appreciably modified by geographic region, study sample size, and dementia subtype (Supplementary Table 3).
To test the robustness of our results, we replaced a random effects model with a fixed effects model. Although most of the results remained, the pooled linear association between walking pace and cognitive decline changed from statistically nonsignificant (RR = 1.09, 95% CI = 0.96–1.23) to significant (RR = 1.05, 95% CI = 1.01–1.10). Further analyses examining the influence of single study on the pooled associations were performed by removing one primary study from the meta-analysis each time. No single study substantially affected the overall estimates.
Discussion
This meta-analysis quantitatively accumulated evidence from longitudinal studies suggesting that walking pace was inversely associated with the risk of cognitive decline and dementia in elderly populations. These inverse associations seemingly persisted across different geographic areas, follow-up periods, sample size, cognitive function assessment methods, definition of cognitive decline, primary study quality, and subtypes of dementia.
Findings from this meta-analysis are supported by three previous systematic reviews. One review (10) focusing on the associations between objective measures of physical capability and health end points concluded that people with slow walking pace had a higher risk of cognitive dysfunction based only on two prospective cohort studies. Similarly, another review suggested that walking pace might be able to predict the occurrence of dementia among elderly population using data from seven studies (9). This rationale has been further supported by a recent review (11). Of note, all previous reviews are narrative reviews. The present meta-analysis provides additional information to the literature by quantitatively estimating both categorical association and dose–response relationship between walking pace and cognitive decline and/or dementia with data from 17 prospective cohort studies.
Several strengths of this study deserve to be highlighted. First, to the best of our knowledge, this is the first study to quantitatively assess the overall association between walking pace and the risk of cognitive decline and dementia. Second, all the included studies are prospective cohort studies that eliminate some potential biases such as recall bias. Third, our conclusions are strengthened by the consistent findings from both categorical and linear analyses.
Some limitations also need to be considered. First, the definition and assessment of cognitive decline, as well as the categorization of walking pace vary across studies, may result in misclassification bias. Nevertheless, our stratified analysis does not find material modification effects by the assessment method of cognitive function. Also, sensitivity analysis does not suggest any single study appreciably affects the combined associations of interest. Second, a potential publication bias caused by excluding studies published in other languages cannot be completely ruled out, though statistical tests do not suggest publication bias in the present meta-analysis. Third, there are not enough studies that enable us to examine the potential modification effects of sex and race on the associations of interest. However, this meta-analysis has combined the findings from the most comprehensive and up-to-date literature.
Potential Mechanisms
A body of evidence suggests that walking pace is significantly associated with muscle strength (24–26), and muscle loss is highly correlated with inflammation, oxidative stress, and sex corticosteroid levels (27,28), which have been linked to cognitive dysfunction (29,30). Also, observational studies (31,32) and randomized controlled trials (33) indicate that gait is considered not only as automatic activity but also as the activity that requires a seamless coordination of several neurologic systems including motor, sensory, and cerebellar activities. Consequently, walking pace can be an indicator of current cognitive function that is associated with future cognitive function.
Because of the prospective cohort study design, the participants at baseline had no diagnosed dementia or cognitive decline. However, those who had slow walking pace might have had some preclinical conditions that may put them at high risk of cognitive dysfunction and lead to dementia or cognitive decline later in life. In addition, slow walking pace as a component of poor physical performance may contribute to physical inactivity and, consequently, increase the risk of cognitive decline or dementia. For example, studies suggest that physical inactivity is independently positively associated with cognitive decline (34). Conversely, physical activity can increase the levels of brain-derived neurotrophic factor and insulin like growth factor 1, which may lead to enhance or maintain cognition through the life span (35).
Conclusions
In conclusion, this meta-analysis of longitudinal studies accumulates evidence supporting that slow or decreased walking pace is significantly associated with the risks of cognitive decline and dementia among elderly populations. In light of its characteristics of safety, cost-effectiveness, and ease to test and interpret, walking pace may be an effective indicator of the development of cognitive decline and dementia in elderly people. Since a randomized clinical trial on walking pace and cognitive function may not be feasible due to practical considerations, future well-designed, large-scale, prospective cohort studies are needed to determine the age-, sex-, and population-specified cutoff values for walking pace, in order to enhance the effectiveness and efficiency of this early indicator of cognitive decline and dementia.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
This work was supported by grants from the Shanghai Science and Technology Committee, China (12XD1404500 to P.C.) and National Institute of Health, USA (R01ES021735 to K.H.). M.Q. was supported by the Graduate Abroad Visiting Program from Shanghai University of Sport, China (STFX20150101), and the Humanity and Social Science Youth foundation of Ministry of Education of China (15YJC890029).
Conflict of Interest
None of the authors stated a conflict of interest.
Supplementary Material
Acknowledgments
M.Q., P.X., P.C., and K.H. were responsible for conception and design of the study. M.Q., P.X., and K.H. were responsible for literature reviewing. C.C., P.X., M.Q., K. H. were responsible for analysis and interpretation of the data. M.Q., P.X., C.C., and K.H. were responsible for drafting the manuscript. M.Q., P. X., C.C., J.W., Y.W., R.W., P.C., and K.H. were responsible for critical revision of the article for important intellectual content and final approval of the manuscript.
References
- 1. Ben Assayag E, Shenhar-Tsarfaty S, Korczyn AD, et al. Gait measures as predictors of poststroke cognitive function: Evidence from the TABASCO study. Stroke. 2015;46:1077–1083. doi:10.1161/STROKEAHA.114.007346 [DOI] [PubMed] [Google Scholar]
- 2. Marquis S, Moore MM, Howieson DB, et al. Independent predictors of cognitive decline in healthy elderly persons. Arch Neurol. 2002;59:601–606. doi:10.1001/archneur.59.4.601 [DOI] [PubMed] [Google Scholar]
- 3. Verghese J, Wang C, Lipton RB, Holtzer R, Xue X. Quantitative gait dysfunction and risk of cognitive decline and dementia. J Neurol Neurosurg Psychiatry. 2007;78:929–935. doi:10.1136/jnnp.2006.106914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang L, Larson EB, Bowen JD, van Belle G. Performance-based physical function and future dementia in older people. Arch Intern Med. 2006;166:1115–1120. doi:10.1001/archinte.166.10.1115 [DOI] [PubMed] [Google Scholar]
- 5. Atkinson HH, Cesari M, Kritchevsky SB, et al. Predictors of combined cognitive and physical decline. J Am Geriatr Soc. 2005;53:1197–1202. doi:10.1111/j.1532-5415.2005.53362.x [DOI] [PubMed] [Google Scholar]
- 6. Gray SL, Anderson ML, Hubbard RA, et al. Frailty and incident dementia. J Gerontol A Biol Sci Med Sci. 2013;68:1083–1090. doi:10.1093/gerona/glt013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Verghese J, Wang C, Lipton RB, Holtzer R. Motoric cognitive risk syndrome and the risk of dementia. J Gerontol A Biol Sci Med Sci. 2013;68:412–418. doi:10.1093/gerona/gls191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abbott RD, White LR, Ross GW, Masaki KH, Curb JD, Petrovitch H. Walking and dementia in physically capable elderly men. JAMA. 2004;292:1447–1453. doi:10.1001/jama.292.12.1447 [DOI] [PubMed] [Google Scholar]
- 9. Cooper R, Kuh D, Cooper C, et al. Objective measures of physical capability and subsequent health: a systematic review. Age Ageing. 2011;40:14–23. doi:10.1093/ageing/afq117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13:881–889. [DOI] [PubMed] [Google Scholar]
- 11. Kikkert LH, Vuillerme N, van Campen JP, Hortobagyi T, Lamoth CJ. Walking ability to predict future cognitive decline in old adults: A scoping review. Ageing Res Rev. 2016;27:1–14. doi:10.1016/j.arr.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 12. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi:10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 13. Lee M, Saver JL, Chang KH, Liao HW, Chang SC, Ovbiagele B. Impact of microalbuminuria on incident stroke: A meta-analysis. Stroke. 2010;41:2625–2631. doi:10.1161/STROKEAHA.110.581215 [DOI] [PubMed] [Google Scholar]
- 14. Lee M, Saver JL, Chang KH, Liao HW, Chang SC, Ovbiagele B. Low glomerular filtration rate and risk of stroke: meta-analysis. BMJ. 2010;341:c4249. doi:10.1136/bmj.c4249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bennett DA, Schneider JA, Arvanitakis Z, Wilson RS. Overview and findings from the religious orders study. Curr Alzheimer Res. 2012;9:628–645. doi:10.2174/156720512801322573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview and findings from the rush Memory and Aging Project. Curr Alzheimer Res. 2012;9:646–663. doi:10.2174/156720512801322663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: Bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625. doi:10.1111/j.1532-5415.2000.tb03873.x [DOI] [PubMed] [Google Scholar]
- 18. Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: Consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–M231. doi:10.1093/gerona/55.4.M221 [DOI] [PubMed] [Google Scholar]
- 19. Ho SC, Woo J, Sham A, Chan SG, Yu AL. A 3-year follow-up study of social, lifestyle and health predictors of cognitive impairment in a Chinese older cohort. Int J Epidemiol. 2001;30:1389–1396. doi:10.1093/ije/30.6.1389 [DOI] [PubMed] [Google Scholar]
- 20. Inzitari M, Newman AB, Yaffe K, et al. Gait speed predicts decline in attention and psychomotor speed in older adults: the health aging and body composition study. Neuroepidemiology. 2007;29:156–162. doi:10.1159/000111577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Taniguchi Y, Yoshida H, Fujiwara Y, Motohashi Y, Shinkai S. A prospective study of gait performance and subsequent cognitive decline in a general population of older Japanese. J Gerontol A Biol Sci Med Sci. 2012;67:796–803. doi:10.1093/gerona/glr243 [DOI] [PubMed] [Google Scholar]
- 22. Welmer AK, Rizzuto D, Qiu C, Caracciolo B, Laukka EJ. Walking speed, processing speed, and dementia: a population-based longitudinal study. J Gerontol A Biol Sci Med Sci. 2014;69:1503–1510. doi:10.1093/gerona/glu047 [DOI] [PubMed] [Google Scholar]
- 23. Camargo E, Beiser A, Tan Z, et al. Walking speed, handgrip strength and risk of dementia and stroke: The Framingham Offspring Study (S24. 003). Neurology. 2012;78:S24. 003. doi:10.1212/WNL.78.1_MeetingAbstracts.S24.003 [Google Scholar]
- 24. Marsh AP, Miller ME, Saikin AM, et al. Lower extremity strength and power are associated with 400-meter walk time in older adults: The InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2006;61:1186–1193. doi:10.1093/gerona/61.11.1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rantanen T, Guralnik JM, Izmirlian G, et al. Association of muscle strength with maximum walking speed in disabled older women. Am J Phys Med Rehabil. 1998;77:299–305. doi:10.1097/00002060-199807000-00008 [DOI] [PubMed] [Google Scholar]
- 26. Tiedemann A, Sherrington C, Lord SR. Physiological and psychological predictors of walking speed in older community-dwelling people. Gerontology. 2005;51:390–395. doi:10.1159/000088703 [DOI] [PubMed] [Google Scholar]
- 27. Cesari M, Penninx BW, Pahor M, et al. Inflammatory markers and physical performance in older persons: The InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2004;59:242–248. doi:10.1093/gerona/59.3.M242 [DOI] [PubMed] [Google Scholar]
- 28. Hansen RD. Hormonal and lifestyle determinants of appendicular skeletal muscle mass in men: The MINOS Study. Am J Clin Nutr. 2005;81:1180–1181. [DOI] [PubMed] [Google Scholar]
- 29. Hogervorst E, Bandelow S, Combrinck M, Smith AD. Low free testosterone is an independent risk factor for Alzheimer’s disease. Exp Gerontol. 2004;39:1633–1639. doi:10.1016/j.exger.2004.06.019 [DOI] [PubMed] [Google Scholar]
- 30. Weaver JD, Huang MH, Albert M, Harris T, Rowe JW, Seeman TE. Interleukin-6 and risk of cognitive decline: MacArthur studies of successful aging. Neurology. 2002;59:371–378. doi:10.1212/WNL.59.3.371 [DOI] [PubMed] [Google Scholar]
- 31. Callisaya ML, Blizzard CL, Wood AG, Thrift AG, Wardill T, Srikanth VK. Longitudinal relationships between cognitive decline and gait slowing: The Tasmanian Study of Cognition and Gait. J Gerontol a-Biol. 2015;70:1226–1232. doi:10.1093/gerona/glv066 [DOI] [PubMed] [Google Scholar]
- 32. Thorvaldsson V, MacDonald SWS, Fratiglioni L, et al. Onset and rate of cognitive change before dementia diagnosis: Findings from two Swedish population-based longitudinal studies. J Int Neuropsych Soc. 2011;17:154–162. doi:10.1017/S1355617710001372 [DOI] [PubMed] [Google Scholar]
- 33. Liu-Ambrose T, Davis JC, Nagamatsu LS, Hsu CL, Katarynych LA, Khan KM. Changes in executive functions and self-efficacy are independently associated with improved usual gait speed in older women. Bmc Geriatr. 2010;10:25. doi:10.1186/1471-2318-10-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Willey JZ, Gardener H, Caunca MR, et al. Leisure-time physical activity associates with cognitive decline: The Northern Manhattan Study. Neurology. 2016. Advance online access. doi:10.1212/WNL.0000000000002582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Prakash RS, Voss MW, Erickson KI, Kramer AF. Physical activity and cognitive vitality. Annu Rev Psychol. 2015;66:769–797. doi:10.1146/annurev-psych-010814-015249 [DOI] [PubMed] [Google Scholar]
- 36. Verghese J, Annweiler C, Ayers E, et al. Motoric cognitive risk syndrome: multicountry prevalence and dementia risk. Neurology. 2014;83:718–726. doi:10.1212/WNL.0000000000000717 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.