Abstract
When spores of the anaerobic bacterium Clostridium novyi-NT are systemically injected into animals, they germinate exclusively within the hypoxic regions of cancers. The germinated bacteria destroy adjacent tumor cells but spare a rim of well oxygenated tumor cells that subsequently expand. Surprisingly, we found that ≈30% of mice treated with such spores were cured of their cancers despite the viable tumor rim initially remaining after spore germination. The mechanism underlying this effect was shown to be immune-mediated, because cured animals rejected a subsequent challenge of the same tumor. Similar effects were observed in rabbits with intrahepatic tumors. It was particularly notable that the induced immune response, when combined with the bacteriolytic effects of C. novyi-NT, could eradicate large established tumors.
In the 1890s, William B. Coley (1) observed that a fraction of cancer patients who developed postoperative bacterial infections went into remission and were cured of their tumors. Although the mechanisms underlying Coley's observations were unclear, it was known even then that bacteria are immunostimulatory. Coley's work was thereby a major stimulus to the burgeoning field of tumor immunology. Coley's toxin and bacillus Calmette-Guérin are two offshoots of this stimulus; bacillus Calmette-Guérin is still used today for the treatment of human bladder cancers (2).
The studies described herein are based on serendipitous observations in cancer-bearing animals that are reminiscent of Coley's observations in patients. For the past few years, our group has been attempting to treat experimental tumors in mice with spores of a bacterial strain called Clostridium novyi-NT (3-5). The rationale for this treatment is that strict anaerobes can proliferate only in severely hypoxic tissues of live mammals; the only regions in healthy nontraumatized animals that are sufficiently hypoxic to allow anaerobic bacterial growth and accessible to the bacteria are those within tumors (3). Although tumors are angiogenic (6, 7), blood vessel growth does not keep pace with tumor cell growth in the majority of human cancers, leading to pronounced hypoxia often accompanied by necrosis (8). The capacity of anaerobic bacteria to target tumors was recognized >50 years ago and led to early clinical trials with one anaerobic strain (Clostridium sporogenes) (9). Interest in microbe-based approaches to cancer therapy has recently re-emerged with the development of methods to genetically engineer bacteria, reducing their toxicity and arming them with genes encoding prodrug-metabolizing enzymes (10-13).
C. novyi was chosen for research in our laboratory on the basis of an extensive screen of anaerobic bacterial species for those with optimal properties (3). C. novyi proved to be particularly oxygen-sensitive as well as highly mobile within tumors due, in part, to their numerous peritrichous flagellae (4). This mobility endowed them with the capacity to rapidly spread to hypoxic areas within tumors once they had germinated within a small necrotic focus. By removing the major systemic toxin (α-toxin) gene from C. novyi, an attenuated derivative named C. novyi-NT was created that could be more safely administered to mice (3).
When C. novyi-NT spores are injected intravenously into immunodeficient mice bearing human xenografts, the spores quickly germinate within necrotic regions of the tumors. Hypoxic and necrotic regions are generally localized within the central parts of tumors, with well perfused tumor cells occupying the rim. Because of the exquisite sensitivity of C. novyi-NT to oxygen, bacterial germination and spread halt when the bacteria reach the well oxygenated rim. It was shown that conventional chemotherapy and radiation therapy could be used to destroy the well oxygenated cells in this rim, and that the combination of C. novyi-NT with these therapies [combination bacteriolytic therapy (COBALT)] provided substantial antitumor activity in several xenograft models (3-5).
As described below, we have now attempted to more carefully assess the effects of C. novyi-NT in immunocompetent mice, using syngeneic murine tumors rather than human xenografts. We were particularly interested in whether an immune response to the bacteria might diminish the effects of combination bacteriolytic therapy (COBALT). Surprisingly, we observed that a significant fraction of immunocompetent animals treated with C. novyi-NT spores exhibited complete tumor regression in the absence of any additional chemotherapy or radiation, resulting in long-term cures. Because such cures had been observed only rarely in nude mice treated with C. novyi-NT spores alone, we sought to investigate the basis and generality of this phenomenon.
Methods
Animals. All animal experiments were overseen and approved by the Animal Welfare Committee of Johns Hopkins University and were in compliance with university standards. Six- to 8-wk-old BALB/c mice, purchased from Harlan Breeders (Indianapolis), were used for tumor implantation of CT26 and RENCA. Eight- to nine-pound New Zealand White rabbits, purchased from Myrtle's Rabbitry (Thompson Station, TN), were used for VX2 tumor implantation.
Cell Lines. CT26 and RENCA were purchased from American Type Culture Collection. Both cell lines were grown in McCoy's 5A medium (Invitrogen) supplemented with 5% FCS at 37°C in the presence of 5% carbon dioxide. VX2 was provided by J. A. Hilton at the Johns Hopkins Medical Institutions and passaged intramuscularly in the hind leg of donor rabbits.
Tumor Inoculation. Five hundred thousand to 5 million cells of CT26 or RENCA were injected subcutaneously into the flank of each mouse. Tumor volume was calculated as length × width2 × 0.5. Tumors were allowed to grow for ≈14 days, at which time the animals were treated either surgically or with C. novyi-NT spores. The average size of tumors when treatment was initiated was ≈350 mm3. VX2 tumors were implanted into New Zealand White rabbits after a midline subxiphoid incision. Minced tumor was injected into the left lobe of the liver by using a 16-gauge angiocatheter. The abdomen was closed in two layers by using vicryl. The tumors were allowed to grow for 14-22 days, at which time computed tomography (CT) imaging was performed and treatment was initiated. The average diameter of tumors when treatment was initiated was ≈2 cm. Tumor sections were processed through routine histopathology or stained with 2% Pico Green (a specific stain for DNA; Stratagene) in 10 mM Tris·HCl/1 mM EDTA, pH 8.0.
Treatment. C. novyi-NT spores were prepared as described (3). Mice were injected with 300 million spores suspended in 200 μl of PBS (Invitrogen) via the tail vein. Rabbits received 2 billion spores of C. novyi-NT suspended in 3 ml of PBS via the ear vein.
Cytokine Assays. The RayBio Mouse Cytokine Array II was purchased from RayBiotech (Norcross, GA) and used according to the manufacturer's instructions. Briefly, after blocking, membranes were incubated for 2 h with 10-fold diluted sera. The membranes were washed and then incubated with biotin-conjugated antibodies for 1 h. The membranes were washed again and incubated with horseradish peroxidase-conjugated streptavidin for 30 min, washed, and then developed.
Adoptive Transfer. Splenocytes and lymphocytes were isolated from naïve or cured mice that had rejected a subsequent tumor challenge. CD4+ and CD8+ cells were purified by magnetic separation (Miltenyi Biotec, Auburn, CA). The purity of cells ranged from 82% to 96%. Twenty million total cells, purified CD4+, or purified CD8+ cells were adoptively transferred into BALB/c mice. Two days later, the mice were challenged with 5 × 105 tumor cells. The χ2 test was used to compare the responses to tumor challenge among the various groups.
Imaging. CT and positron emission tomography (PET/CT) (14) images were obtained with a clinical scanner [Siemens Volume Zoom (Siemens Medical, Malvern, PA) and Discovery LS (GE Healthcare, Waukesha, WI), respectively]. Three-millimeter CT slices were obtained in the following sequence: noncontrast, arterial dominant contrast phase with 10 ml of Omnipaque administered at a rate of 1 ml/sec, and portal venous delayed phase. PET images were obtained 1 h after administration of ≈37 MBq (1 mCi) of [18F]-fluorodeoxyglucose (FDG). A whole-body emission PET scan was performed in the two-dimensional mode with 10 min of acquisition.
Results
C. novyi-NT Treatment of CT26 Tumors. The first model tested involved subcutaneous tumors of CT26 colorectal cancer cells in BALB/c mice. This model is representative of minimally to moderately immunogenic tumors in that tumors are formed when relatively small numbers of cells are injected into syngeneic mice but do not form in allogeneic mice even when large numbers of cells are injected (15). When C. novyi-NT spores were intravenously injected into mice with CT26 tumors, bacteria germinated exclusively within the tumors. By 24 h after treatment, hemorrhagic necrosis could be observed at the centers of most of the tumors. Three to four weeks later, the tumors had regrown from the small nonnecrotic region at the periphery in 66% (31 of 47) of the animals. In the other 34% of the mice, the tumors had completely regressed, and the mice remained tumor free until the end of the experiment (>60 days).
Morphological analysis of the tumors showed a striking accumulation of inflammatory cells at the periphery, already present at 24 h after spore injection (Fig. 1). The inflammatory cells appeared to be restraining the bacterial infection, because a ring of bacteria was surrounded by a dense concentric ring of inflammation (Fig. 1). Standard histopathological analysis (Fig. 1) demonstrated that the infiltrate was largely composed of neutrophils. Measurements of serum cytokines showed the presence of polypeptides associated with neutrophil chemotaxis and activation, such as IL-6, MIP-2, G-CSF, TIMP-1, and KC (Fig. 2). Seventy-two hours after spore injection, the cellular characteristics of the inflammatory ring had changed to include more monocytes and lymphocytes (data not shown).
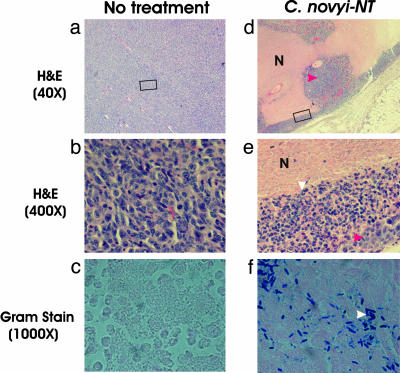
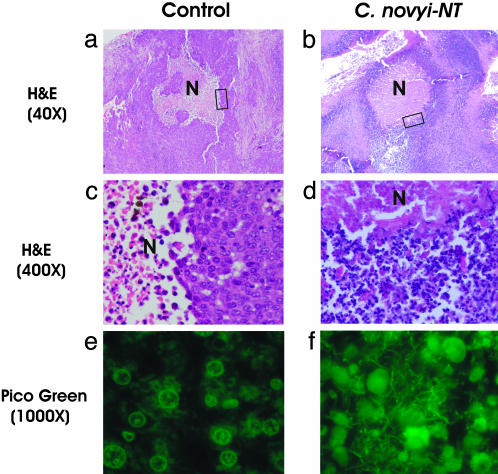
Fig. 1.
Tumor inflammation after i.v. injection with C. novyi-NT spores. Untreated tumors (a-c) and tumors treated with C. novyi-NT spores (d-f) were examined histopathologically after hematoxylin/eosin staining. Extensive areas of necrosis (N) were present within CT26 tumors 24 h after systemic injection of spores, and a ring of inflammation surrounded the tumors (e). The boxed areas in a and d are magnified in b and e, respectively, showing neutrophilic infiltrate in the treated lesions (e) but only tumor cells in the untreated control (b). The great majority of the cells in d and e were inflammatory cells (white arrow), with only a few nests of tumor cells remaining (red arrows). Gram stains (c and f) showed that the necrotic regions of only the treated lesions were filled with vegetative C. novyi-NT bacteria (white arrow).
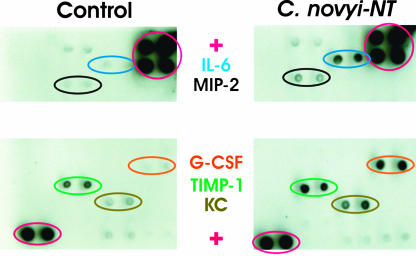
Fig. 2.
Serum cytokines 24 h after treatment with C. novyi-NT. Il-6, MIP-2, G-CSF, TIMP-1, and KC were up-regulated. Antibodies to each cytokine were spotted twice on each array. Levels of tumor necrosis factor α and 26 other cytokines did not change significantly. Positive (+) controls are circled in red. Very similar patterns to those observed (Right and Left) were depicted in this figure in the sera of two treated mice and two control mice, respectively.
To determine whether this inflammatory response to C. novyi-NT germination had stimulated an immune response against the tumors, mice that had been cured of their tumors were challenged with 5 × 106 CT26 cells in the opposite flank. Eight of ten such mice were resistant to this relatively large challenge (Figs. 3a and 4). As expected, tumors formed in all 10 naive mice injected with 5 × 106 cells (Fig. 3a), as well as in all naive mice injected with 10-fold fewer cells (data not shown).
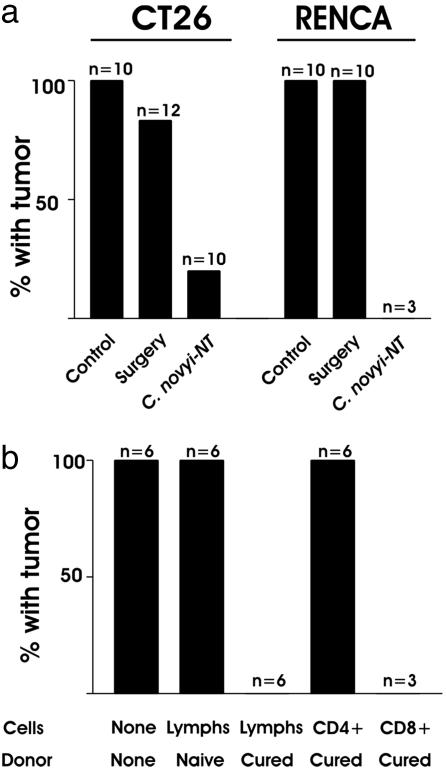
Fig. 3.
Rechallenge experiments. (a) Mice with the indicated tumors cured by treatment with C. novyi-NT or by surgical excision were challenged with a s.c. injection of 5 × 106 tumor cells. The responses were compared to those in mice that had not previously been injected with tumor cells (“Control”). (b) Lymphocytes were purified from naive mice or from mice that were cured of their CT26 tumors by C. novyi-NT treatment. Forty-eight hours after i.v. injection of the lymphocytes, the mice were challenged with a s.c. injection of 5 × 105 tumor cells. The y axes in both a and b represent the fraction of animals that formed tumors after tumor challenge. The number (n) of mice used in each group is indicated.
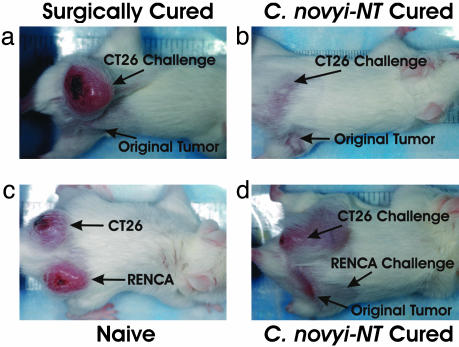
Fig. 4.
Photographs of mice used for challenge experiments. A mouse surgically cured of a CT26 tumor was not resistant to a challenge of CT26 cells in the opposite flank (a), whereas a mouse cured with C. novyi-NT was resistant (b). (c) CT26 tumors and RENCA tumors formed in naive mice when the corresponding cells were injected into one flank. (d) When mice cured of their RENCA tumors with C. novyi-NT were similarly injected, only CT26 tumors formed.
The immune responses that developed in these mice could theoretically be explained in two ways. First, it was possible that CT26 cells naturally elicit an immune response during tumori-genesis that was not strong enough to eradicate all tumor cells. The bacteriolytic effects of C. novyi-NT could simply have reduced the tumor load, thereby permitting the normal immune response to deal with the few remaining tumor cells and curing the animals (the permissive model). Second, it was possible that CT26 tumor cells could not initiate a robust immune response on their own, and that the infection with C. novyi-NT acted as an adjuvant to stimulate the response (the stimulant model). To distinguish between these two models, tumor-bearing mice were treated either with C. novyi-NT or with surgical excision. One to three months later, when it was clear that the animals were cured, they were rechallenged with 5 × 106 CT26 cells. In these experiments, 83% of the surgically cured mice but only 20% of the C. novyi-NT-treated mice developed tumors, supporting the stimulant model (P < 0.01, Fig. 3a).
Treatment of RENCA Tumors in Mice. To determine whether these results could be reproduced in another murine system, we repeated the experiment using RENCA cells, derived from a murine renal cell carcinoma that is also minimally to moderately immunogenic (16). Results obtained with this model were very similar to those observed with CT26. C. novyi-NT treatment of RENCA tumors routinely resulted in large regions of central necrosis, generally leaving a small viable rim. Of 15 treated mice, five (33%) were cured. Cured mice were completely resistant to challenge with 5 × 106 RENCA cells (Fig. 3a). The stimulant vs. permissive model for immunity was tested by comparing these results with surgically cured mice: none of 10 surgically cured mice were resistant to challenge with 5 × 106 RENCA tumor cells.
To demonstrate the specificity of the immune response, mice that were cured of RENCA tumors by C. novyi-NT treatment were rechallenged with both RENCA cells (on the right flank) and CT26 cells (on the left flank). These mice developed CT26 tumors at the same rate and of the same size as naive mice but did not develop RENCA tumors (Fig. 4).
Adoptive Transfer. We next sought to determine what cells were responsible for the immune response in C. novyi-NT-treated mice. Lymphocytes were isolated from the lymph nodes and spleens of mice cured with C. novyi-NT and were used to purify CD4+ or CD8+ cells, which were then injected into naive BALB/c mice. Two days later, all of the mice were inoculated s.c. with 5 × 105 live CT26 tumor cells. Tumors grew in all mice that had not been preinjected with lymphocytes (Fig. 3b). Similarly, tumors grew in all mice that had been injected with lymphocytes from naive control mice. In contrast, lymphocytes from mice cured with C. novyi-NT prevented tumor growth in each of six mice tested. The cells responsible were CD8+, because adoptive transfer of these cells, but not CD4+ cells, completely prevented tumor growth (Fig. 3b).
Treatment of Tumors in Rabbits. To determine whether these results were species-specific, as well as to assess the capacity of C. novyi-NT to germinate within internal tumors, we used the VX2 tumor model in rabbits. These tumors are weakly (if at all) immunogenic, as documented by their ability to cause tumors in outbred strains of rabbits. When inoculated intrahepatically, VX2 tumors are highly aggressive, spreading rapidly throughout the liver, abdominal cavity, and pleural space, killing the animals within 6-8 weeks. Fig. 9, which is published as supporting information on the PNAS web site, illustrates the typical appearance of the abdomen at necropsy, showing widespread invasion and metastases in the liver and adjacent anatomical structures.
To determine whether the inflammatory responses observed in s.c. tumors in mice were also induced in liver tumors in rabbits, animals were euthanized 4 days after administering C. novyi-NT. Fig. 5 shows a section of part of a representative tumor in a control rabbit, with large numbers of viable tumor cells surrounding central regions of necrosis. Four days after treatment with C. novyi-NT, the necrotic areas expanded, and the tumor cells were largely replaced with inflammatory infiltrates. Pico green staining showed that the necrotic regions were filled with germinated bacteria (Fig. 5).
Fig. 5.
C. novyi-NT germinates within VX2 tumors. (a) Small regions of necrosis (N) were found with the VX2 tumors of a control rabbit. (b) In rabbits treated with C. novyi-NT for 4 days, the necrotic regions enlarged, and the tumor was largely replaced with inflammatory cells, as indicated in the high-power view (d) of the boxed region in b. (f) Pico Green staining showed that the necrotic regions of the treated lesions were filled with vegetative C. novyi-NT.
A total of 23 rabbits were treated with a single i.v. injection of C. novyi-NT spores after their liver tumors became visible in pretreatment imaging studies. Tumor growth was assessed through serial CT with arterial-dominant phase-contrast agent enhancement. As shown in Fig. 6, untreated tumor nodules had hyperattenuated peripheries reflecting the high blood flow to actively proliferating tumor cells. If no treatment was administered, the tumors progressively grew, replacing much of the liver within 5-6 weeks. In contrast, the appearance of tumors decidedly changed 12 days after i.v. injection of C. novyi-NT spores (Fig. 6, day 34). Although the tumors had swelled, there was little or no peripheral hyperattenuation. Moreover, a gas pocket could sometimes be observed in the central necrotic region of the tumors, a classic feature of Clostridial infections. The gas deposits gradually disappeared, and in some cases no tumor could subsequently be detected radiologically (Fig. 6, day 77).
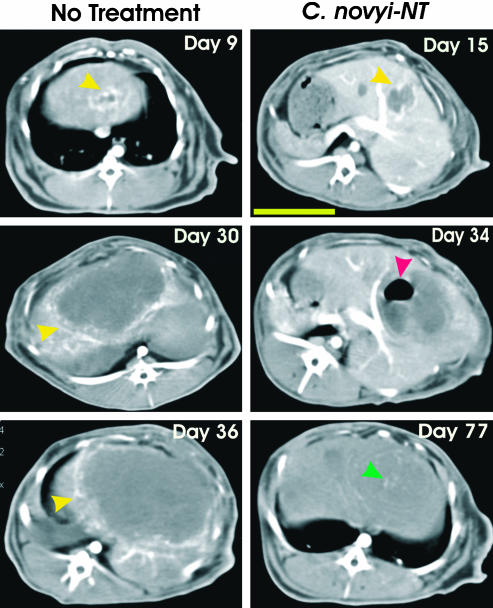
Fig. 6.
Serial CT scans of VX2 tumors. Arterial-dominant axial high-resolution CT images at the level of the primary tumor are shown in representative cases at the indicated days. Nine days after tumor implantation, there was peripheral enhancement of the tumor in an untreated rabbit (Left, yellow arrows). Twenty-one days later (day 30), the tumor had grown considerably in size, and prominent peripheral enhancement could still be observed. Six days later (day 36), the tumor had replaced most of the normal liver in this untreated rabbit. By 15 days after tumor implantation in another rabbit (Right), the tumor had established and exhibited peripheral enhancement (yellow arrow). This animal was treated on day 22, and 12 days later (day 34), there was diminished peripheral enhancement and gas appeared within the tumor (red arrow). By day 77 (55 days after treatment), there was no peripheral enhancement, and the gas had completely resolved. The small hyperintense foci (green arrow) on day 77 represent regions of calcification. This rabbit remains alive and healthy at 650 days after tumor inoculation. The yellow bar in the day 15 image (Right) represents 5 cm.
Necropsies revealed C. novyi-NT germination and extensive necrosis of tumors in every rabbit examined, leading to substantial increases in survival (P < 0.005; Fig. 10, which is published as supporting information on the PNAS web site). No germinated bacteria or necrosis was evident in any of the normal tissues of necropsied rabbits.
Of 23 rabbits treated with C. novyi-NT, 7 were apparently cured, because they exhibited no clinical or radiologic evidence of tumors at the end of the experiment (Fig. 10). Two observations suggested that these cures were in part due to an induced immune response. First, one rabbit that underwent PET in addition to CT scanning was particularly informative. There was a single liver tumor present on day 14, 1 day before treatment with C. novyi-NT (Fig. 7, day 14). Treatment with C. novyi-NT destroyed this lesion, as is evident by the lack of FDG uptake 1 week later (Fig. 7, day 22). But by that time, a second tumor not visible on day 14 had become apparent as a result of local extension or metastasis. This second lesion presumably arose after the initial germination event and was not effectively colonized by the bacteria, as is evident by its robust PET signal (Fig. 7, day 22). A third lesion, an abdominal wall metastasis that also contained viable tumor cells evident on PET, was observed several weeks later (Fig. 7, day 51). Yet all lesions eventually disappeared in the absence of any further treatment with C. novyi-NT (Fig. 7, day 455), suggesting (but not proving) that they might have been eradicated by an immune response that occurred after the initial germination.
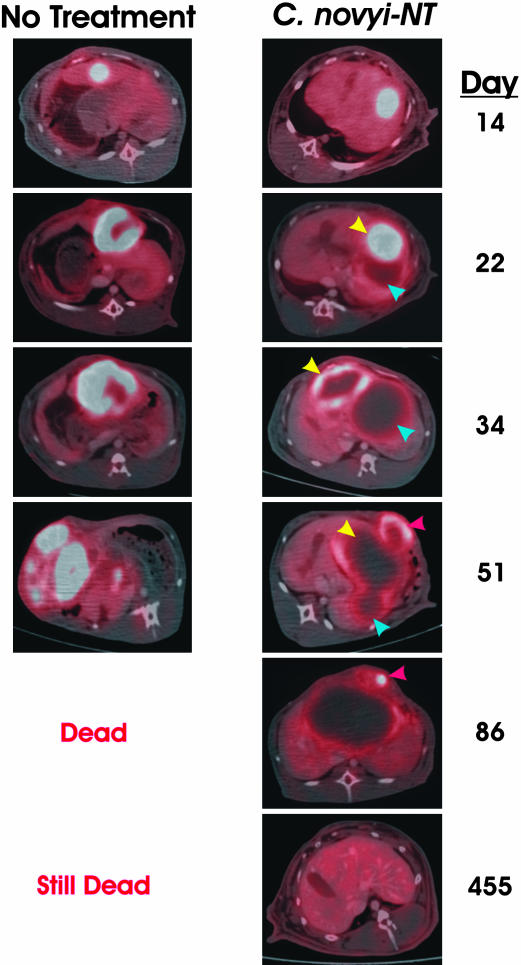
Fig. 7.
PET/CT images of lesions after C. novyi-NT therapy. (Left) Fused PET/CT images of an untreated rabbit. Fourteen days after implantation of VX2 cells, there was intense uptake of FDG in the tumor. Twenty-two days after implantation, the region incorporating FDG was much larger and contained a central area of decreased uptake, suggesting a necrotic focus. Continued tumor growth and progressive FDG uptake were evident on the images at days 34 and 51, and the animal died on day 52 with widespread FDG-positive metastatic disease in the mediastinum. (Right) Analogous images of a rabbit treated with C. novyi-NT. There was intense uptake of FDG 14 days after tumor implantation, similar to that in the untreated rabbit. An i.v. injection of C. novyi-NT spores was administered on day 15. Seven days later (day 22), two lesions were visible. One had considerably decreased uptake of FDG, suggesting C. novyi-NT germination (blue arrow), and the other, which appeared to be budding from the first, exhibited intense FDG uptake (yellow arrow). Twelve days later (day 34), both lesions had less FDG uptake (yellow and blue arrows). Seventeen days later (day 51), the first two lesions were resolving (yellow and blue arrows), but a new FDG-incorporating lesion was evident in the abdominal wall (red arrow). The FDG uptake in the abdominal wall lesion was substantially decreased by day 86 (red arrow). On day 455, the animal was alive and healthy, and there was no abnormal FDG uptake in the liver or elsewhere.
To obtain more direct evidence of the development of tumor immunity in these rabbits, we selected three that had been cured of their hepatic tumors by C. novyi-NT and challenged them with an intramuscular (quadriceps) inoculation of 1 × 106 tumor cells. In 12 naive animals, such inoculations always led to rapid tumor growth that destroyed the muscle and surrounding cutaneous tissues within a few weeks. However, no tumor growth occurred in C. novyi-NT-cured rabbits after identical intramuscular injections, even though the injection sites were far from the site of the original hepatic tumors (data not shown). This systemic immunity was apparently long-lasting, because the three rabbits were challenged 3, 5, or 11 months, respectively, after their initial treatment with C. novyi-NT.
Discussion
The results described above are notable for at least three reasons. They demonstrate that a purely bacteriolytic approach, in the absence of any additional forms of therapy, can cure experimental tumors. Second, they demonstrate that even large tumors are susceptible. Immunotherapeutic approaches have generally been found to be effective only against relatively small tumors, both experimentally and in patients (17-20). And third, the data show that both internal (hepatic) and external (s.c.) tumors in different species are responsive.
Our working hypothesis to explain these results is depicted in Fig. 8. Systemically administered C. novyi-NT spores are distributed throughout the body, but due to their strict anaerobic growth requirements, germinate only within anoxic or markedly hypoxic regions of tumors. Once germinated, the bacteria destroy adjacent cancer cells through the secretion of lipases, proteases, and other degradative enzymes. At the same time, the host reacts to this localized infection, producing cytokines such as IL-6, MIP-2, G-CSF, TIMP-1, and KC (Fig. 2) that attract a massive influx of inflammatory cells, initiated largely by neutrophils and followed within a few days by monocyte and lymphocyte infiltration. The inflammatory reaction restrains the spread of the bacterial infection, providing a second layer of control in addition to that provided by the requisite anaerobic environment. The inflammation may also directly contribute to the destruction of tumor cells through the production of reactive oxygen species, proteases, and other degradative enzymes. Moreover, it stimulates a potent cellular immune response that can subsequently destroy residual tumor cells not lysed by the bacteria. The cure rate is determined by the balance between bacteriolysis, angiogenesis, regrowth of residual tumor cells, and the rate of development of the immune response. In the experiments reported here, the host “wins” in ≈30% of cases, in either mice or rabbits, and the tumor is victorious in the remainder.
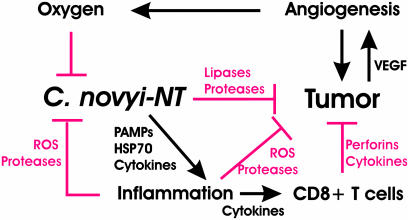
Fig. 8.
Working model. C. novyi-NT spores localize in hypoxic/anoxic areas of tumors, where they germinate and cause lysis of tumor cells. The resulting inflammation, as well as the oxygen present in adjacent areas, inhibits the proliferation and spread of C. novyi-NT. However, the inflammation may also directly contribute to tumor destruction through the release of reactive oxygen species, proteases, pore-forming agents, and tumoricidal cytokines. In addition, the inflammatory response stimulates a specific cellular antitumor immune response that constrains subsequent tumor growth.
Several new and previously recorded observations support this model. C. novyi is well known for its capacity to induce massive leukocytosis and inflammation, whereas many other species of Clostridia do not induce this level of response (21). The inflammatory reaction is classic in many ways, including the observed increase in neutrophil-directed cytokines in serum and the cellular nature and time course of the infiltrate (22). The antitumor effects of inflammation are well documented (23). That such a striking level of inflammation is associated with the subsequent development of a potent immune response is not surprising. In fact, the adaptive immune system evolved specifically to follow such inflammation, the first stages of which represent the innate immune system's response to bacterial infections. It is relevant that the active ingredient of the standard adjuvant (Freund's) used to induce experimental immunity is composed of inactivated bacteria (24). After administration of Freund's adjuvant, an immune response is directed to the bacteria not only within the adjuvant but also to other components within the mixture. This experimental finding is consistent with clinical observations, suggesting that infections are often associated with the development of autoimmunity (25).
At the molecular level, bacterial infections like those of C. novyi are associated with the release of pathogen-associated molecular patterns (PAMPs) from bacteria and Hsp70 from necrotic cells (26). Hsp70 induces maturation of dendritic cells, professional antigen-presenting cells that are essential for the production of potent immune responses. PAMPs interact with Toll-like receptors, leading to up-regulation of costimulatory molecules such as CD40 and proinflammatory cytokines such as IL-12. These in turn induce the production of IFN-γ and initiate a Th1-dependent cell-mediated response, primarily effected by CD8+ cytolytic T cells (27). The demonstration that CD8+ T cells from C. novyi-NT-cured mice can confer adoptive immunity in a tumor-specific fashion (Fig. 3b) is consistent with this scenario.
Could this therapeutic approach be successful in humans? The effects we observed in animals are contingent on both bacteriolysis and immunity. There are three reasons to believe that systemic injection of C. novyi-NT into humans would lead to bacteriolysis of tumors. First, C. novyi-NT germinates within the tumors of all three species tested (rabbits, rats, and mice), whether the tumors are s.c., intramuscular, or intrahepatic (Figs. 1 and 5 and unpublished data). Second, C. novyi-NT can germinate within human tumor xenografts in the nude mouse host (although complete regressions and cures are not generally observed as there is minimal T cell-mediated immunity). And third, there are many case reports of C. novyi germination and gangrene developing in penetrating wounds or after illicit drug injection (28). These reports demonstrate that the parental strain of C. novyi, differing from C. novyi-NT only in that the latter is devoid of the lethal α-toxin gene, can proliferate within hypoxic regions in humans.
Whether C. novyi-NT infection of cancers in humans will induce tumor immunity is more difficult to predict. There are many studies indicating that human tumors are immunogenic, as assessed by the presence of specific antibodies or reactive T cells in untreated patients (29, 30). Furthermore, it has been shown that stronger immune responses can be elicited through the administration of various vaccines in several clinical trials (31). But there are also many studies indicating that human tumor cells can protect themselves against potential immune responses through a variety of direct and indirect mechanisms (20, 32). As similar observations, both with respect to the potential of tumors to elicit an immune response and their ability to evade such responses, have been recorded in animals, there is reason to hope that the immune therapeutic effects stimulated by C. novyi-NT germination might be obtainable in carefully selected patients.
Supplementary Material
Acknowledgments
We thank L. Mezler and C. Lengauer for advice and assistance with photographic imaging and Carolyn McGee, Lauri Pipitone, Paul Eyabi, and E. Lattice Watson for assistance with the experiments in animals. This work was supported by the Miracle Foundation, the Clayton Fund, and National Institutes of Health Grants CA 062924, CA 43460, RR 00171, T32 DC 00027, and CA 92871.
Abbreviations: CT, computed tomography; PET, positron emission tomography; FDG, [18F]fluorodeoxyglucose.
References
- 1.Wiemann, B. & Starnes, C. O. (1994) Pharmacol. Ther. 64, 529-564. [DOI] [PubMed] [Google Scholar]
- 2.Peyromaure, M. & Zerbib, M. (2004) BJU Int. 93, 60-63. [DOI] [PubMed] [Google Scholar]
- 3.Dang, L. H., Bettegowda, C., Huso, D. L., Kinzler, K. W. & Vogelstein, B. (2001) Proc. Natl. Acad. Sci. USA 98, 15155-15160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bettegowda, C., Dang, L. H., Abrams, R., Huso, D. L., Dillehay, L., Cheong, I., Agrawal, N., Borzillary, S., McCaffery, J. M., Watson, E. L., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 15083-15088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dang, L. H., Bettegowda, C., Agrawal, N., Cheong, I., Huso, D., Frost, P., Loganzo, F., Greenberger, L., Barkoczy, J., Pettit, G. R., et al. (2004) Cancer Biol. Ther. 3, 326-337. [DOI] [PubMed] [Google Scholar]
- 6.Folkman, J. & Kalluri, R. (2000) in Cancer Medicine, eds. Holland, J. F. & Frei, E. (Decker, Hamilton, ON, Canada), pp. 161-194.
- 7.Kerbel, R. S. (2000) Carcinogenesis 21, 505-515. [DOI] [PubMed] [Google Scholar]
- 8.Jain, R. K. & Forbes, N. S. (2001) Proc. Natl. Acad. Sci. USA 98, 14748-14750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heppner, F. & Mose, J. R. (1978) Acta Neurochir. 42, 123-125. [DOI] [PubMed] [Google Scholar]
- 10.Matsumura, H., Takeuchi, A. & Kano, Y. (1997) Biosci. Biotechnol. Biochem. 61, 1211-1212. [DOI] [PubMed] [Google Scholar]
- 11.Low, K. B., Ittensohn, M., Le, T., Platt, J., Sodi, S., Amoss, M., Ash, O., Carmichael, E., Chakraborty, A., Fischer, J., et al. (1999) Nat. Biotechnol. 17, 37-41. [DOI] [PubMed] [Google Scholar]
- 12.Liu, S. C., Minton, N. P., Giaccia, A. J. & Brown, J. M. (2002) Gene Ther. 9, 291-296. [DOI] [PubMed] [Google Scholar]
- 13.Theys, J., Barbe, S., Landuyt, W., Nuyts, S., Van Mellaert, L., Wouters, B., Anne, J. & Lambin, P. (2003) Curr. Gene Ther. 3, 207-221. [DOI] [PubMed] [Google Scholar]
- 14.Tatsumi, M., Nakamoto, Y., Traughber, B., Marshall, L. T., Geschwind, J. F. & Wahl, R. L. (2003) Cancer Res. 63, 6252-6257. [PubMed] [Google Scholar]
- 15.Fearon, E. R., Pardoll, D. M., Itaya, T., Golumbek, P., Levitsky, H. I., Simons, J. W., Karasuyama, H., Vogelstein, B. & Frost, P. (1990) Cell 60, 397-403. [DOI] [PubMed] [Google Scholar]
- 16.Weiss, J. M., Shivakumar, R., Feller, S., Li, L. H., Hanson, A., Fogler, W. E., Fratantoni, J. C. & Liu, L. N. (2004) Cancer Gene Ther. 11, 346-353. [DOI] [PubMed] [Google Scholar]
- 17.Verra, N., Jansen, R., Groenewegen, G., Mallo, H., Kersten, M. J., Bex, A., Vyth-Dreese, F. A., Sein, J., van de Kasteele, W., Nooijen, W. J., et al. (2003) Br. J. Cancer 88, 1346-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun, X., Vale, M., Leung, E., Kanwar, J. R., Gupta, R. & Krissansen, G. W. (2003) Gene Ther. 10, 1728-1734. [DOI] [PubMed] [Google Scholar]
- 19.Rosenberg, S. A. (2004) N. Engl. J. Med. 350, 1461-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drake, C. G. & Pardoll, D. M. (2002) Semin. Cancer Biol. 12, 73-80. [DOI] [PubMed] [Google Scholar]
- 21.McGuigan, C. C., Penrice, G. M., Gruer, L., Ahmed, S., Goldberg, D., Black, M., Salmon, J. E. & Hood, J. (2002) J. Med. Microbiol. 51, 971-977. [DOI] [PubMed] [Google Scholar]
- 22.Nathan, C. (2002) Nature 420, 846-852. [DOI] [PubMed] [Google Scholar]
- 23.Hernandez-Ilizaliturri, F. J., Jupudy, V., Ostberg, J., Oflazoglu, E., Huberman, A., Repasky, E. & Czuczman, M. S. (2003) Clin. Cancer Res. 9, 5866-5873. [PubMed] [Google Scholar]
- 24.Means, T. K., Wang, S., Lien, E., Yoshimura, A., Golenbock, D. T. & Fenton, M. J. (1999) J. Immunol. 163, 3920-3927. [PubMed] [Google Scholar]
- 25.Eriksson, U., Ricci, R., Hunziker, L., Kurrer, M. O., Oudit, G. Y., Watts, T. H., Sonderegger, I., Bachmaier, K., Kopf, M. & Penninger, J. M. (2003) Nat. Med. 9, 1484-1490. [DOI] [PubMed] [Google Scholar]
- 26.Gelman, A. E. & Turka, L. A. (2003) Nat. Med. 9, 1465-1466. [DOI] [PubMed] [Google Scholar]
- 27.Kay, A. B. (2001) N. Engl. J. Med. 344, 30-37. [DOI] [PubMed] [Google Scholar]
- 28.Finn, S. P., Leen, E., English, L. & O'Briain, D. S. (2003) Arch. Pathol. Lab. Med. 127, 1465-1470. [DOI] [PubMed] [Google Scholar]
- 29.Old, L. J. (2003) Cancer Immun. 3, Suppl. 1, 1-8. [Google Scholar]
- 30.Van Der Bruggen, P., Zhang, Y., Chaux, P., Stroobant, V., Panichelli, C., Schultz, E. S., Chapiro, J., Van Den Eynde, B. J., Brasseur, F. & Boon, T. (2002) Immunol. Rev. 188, 51-64. [DOI] [PubMed] [Google Scholar]
- 31.Emens, L. A. & Jaffee, E. M. (2003) Cancer Biol. Ther. 2, S161-168. [PubMed] [Google Scholar]
- 32.Mapara, M. Y. & Sykes, M. (2004) J. Clin. Oncol. 22, 1136-1151. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.