Abstract
We measured the oxygen isotope composition (δ18O) of CO2 respired by Ricinus communis leaves in the dark. Experiments were conducted at low CO2 partial pressure and at normal atmospheric CO2 partial pressure. Across both experiments, the δ18O of dark-respired CO2 (δR) ranged from 44‰ to 324‰ (Vienna Standard Mean Ocean Water scale). This seemingly implausible range of values reflects the large flux of CO2 that diffuses into leaves, equilibrates with leaf water via the catalytic activity of carbonic anhydrase, then diffuses out of the leaf, leaving the net CO2 efflux rate unaltered. The impact of this process on δR is modulated by the δ18O difference between CO2 inside the leaf and in the air, and by variation in the CO2 partial pressure inside the leaf relative to that in the air. We developed theoretical equations to calculate δ18O of CO2 in leaf chloroplasts (δc), the assumed location of carbonic anhydrase activity, during dark respiration. Their application led to sensible estimates of δc, suggesting that the theory adequately accounted for the labeling of CO2 by leaf water in excess of that expected from the net CO2 efflux. The δc values were strongly correlated with δ18O of water at the evaporative sites within leaves. We estimated that approximately 80% of CO2 in chloroplasts had completely exchanged oxygen atoms with chloroplast water during dark respiration, whereas approximately 100% had exchanged during photosynthesis. Incorporation of the δ18O of leaf dark respiration into ecosystem and global scale models of C18OO dynamics could affect model outputs and their interpretation.
Variations in the oxygen isotope composition (δ18O) of CO2 in the atmosphere have the potential to reveal vital information about the global carbon cycle (Francey and Tans, 1987; Farquhar et al., 1993; Ciais et al., 1997). Furthermore, measurements of oxygen isotope composition of CO2 in canopy air may allow differentiation of CO2 fluxes into photosynthetic and respiratory components (Yakir and Wang, 1996). It was also recently suggested that nighttime measurements of δ18O in canopy air could be used to partition nocturnal ecosystem respiration between leaves and soil (Bowling et al., 2003a, 2003b). Leaf dark respiration is an important component of carbon cycling between vegetation and the atmosphere. An understanding of the factors controlling the δ18O of CO2 respired by leaves in the dark could therefore be important for interpreting the δ18O of atmospheric CO2 at local, regional, and global scales.
The net rate of CO2 efflux from a leaf in the dark can be thought of as the difference between two one-way diffusional fluxes, one from the atmosphere to the leaf and the other from the leaf to the atmosphere. For example, if the net respiratory CO2 efflux (ℜn) is defined as ℜn = gc(ci − ca), where gc is the leaf conductance to CO2, and ci and ca are CO2 mole fractions in the intercellular air spaces and atmosphere, respectively, the one-way flux from leaf to atmosphere becomes gcci and that from atmosphere to leaf becomes gcca. The difference between ℜn and gcci will depend on the magnitude of the CO2 concentration difference between ci and ca; this difference will in turn depend on the leaf conductance to CO2 and the CO2 production rate inside the leaf. If the CO2 concentration difference between ci and ca is very large, then the magnitude of the net CO2 efflux will approach that of the one-way CO2 efflux from leaf to atmosphere. However, if the CO2 concentration inside the leaf is only a little larger than that in the atmosphere, the net CO2 efflux from the leaf will be much smaller than the one-way CO2 efflux from the leaf.
It has previously been recognized that one of the primary controls over the δ18O of CO2 diffusing out of leaves in the dark should be the δ18O of leaf water (Flanagan et al., 1997, 1999). This is because gaseous CO2 exchanges oxygen atoms with water during interconversion between CO2 and bicarbonate. In plant tissues, this interconversion is catalyzed by the enzyme carbonic anhydrase. The rate constant for carbonic anhydrase is very fast, such that CO2 diffusing out of leaves is expected to reflect nearly complete oxygen isotope exchange with leaf water. There is an equilibrium fractionation that takes place during the exchange reaction, such that at 25°C, the δ18O of CO2 will be enriched by approximately 41‰ compared to the δ18O of water with which it has equilibrated.
In this article, we present measurements of the δ18O of CO2 respired by Ricinus communis leaves in the dark. We theorized that it should be the one-way flux of CO2 out of a respiring leaf that is labeled with the leaf water δ18O signal, rather than the net CO2 efflux. This led us to hypothesize that the effect of a respiring leaf on the δ18O of CO2 in air passing over the leaf could be much greater than predicted by considering the net CO2 efflux alone.
THEORY
Interpretation of the Oxygen Isotope Composition of Dark-Respired CO2
Natural abundance oxygen isotope ratios are commonly expressed relative to the value of a standard:
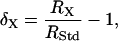 |
(1) |
where δx represents the proportional deviation of RX, the 18O/16O of material X, from RStd, the 18O/16O of a standard. Using δ notation, we present the following equation for the δ18O of CO2 respired by leaves in the dark (δR):
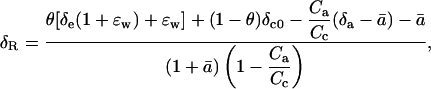 |
(2) |
where θ is the proportion of CO2 in the chloroplast that has completely exchanged oxygen atoms with chloroplast water, δe is the oxygen isotope composition of water at the evaporating sites within the leaf, ɛw is the equilibrium fractionation between water and CO2, δc0 is the oxygen isotope composition of CO2 in the chloroplast that has not exchanged oxygen atoms with chloroplast water, Ca is the ambient carbon dioxide partial pressure, Cc is the chloroplastic CO2 partial pressure, δa is the oxygen isotope composition of ambient CO2, and 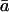 is the weighted mean isotopic discrimination against C18OO during diffusion from the chloroplast to the atmosphere. A summary of all symbols used in the text is given in Table I. A derivation of Equation 2 is presented (see “Derivation 1” in text). As described for photosynthesizing leaves by Gillon and Yakir (2000b), we make the assumption that CO2 inside the leaf comprises a mixture of CO2 completely equilibrated with leaf water (of proportion θ) and CO2 that has undergone no equilibration with leaf water (of proportion 1 − θ). We further assume that chloroplasts are appressed against intercellular air spaces in the mesophyll cells (Evans and von Caemmerer, 1996), such that CO2 evolved from mitochondria interacts with chloroplasts during diffusion out of the cells. Because carbonic anhydrase resides primarily in chloroplasts in C3 leaves (Everson, 1970; Jacobson et al., 1975; Tsuzuki et al., 1985), the chloroplastic CO2 concentration becomes the relevant parameter for modeling δR.
is the weighted mean isotopic discrimination against C18OO during diffusion from the chloroplast to the atmosphere. A summary of all symbols used in the text is given in Table I. A derivation of Equation 2 is presented (see “Derivation 1” in text). As described for photosynthesizing leaves by Gillon and Yakir (2000b), we make the assumption that CO2 inside the leaf comprises a mixture of CO2 completely equilibrated with leaf water (of proportion θ) and CO2 that has undergone no equilibration with leaf water (of proportion 1 − θ). We further assume that chloroplasts are appressed against intercellular air spaces in the mesophyll cells (Evans and von Caemmerer, 1996), such that CO2 evolved from mitochondria interacts with chloroplasts during diffusion out of the cells. Because carbonic anhydrase resides primarily in chloroplasts in C3 leaves (Everson, 1970; Jacobson et al., 1975; Tsuzuki et al., 1985), the chloroplastic CO2 concentration becomes the relevant parameter for modeling δR.
Table I.
Symbols used in text
| A | Net photosynthesis rate |
| ā | Weighted mean discrimination against C18OO for diffusion from chloroplast to atmosphere |
| a | Discrimination against C18OO during diffusion through stomata |
| ab | Discrimination against C18OO during diffusion through leaf boundary layer |
| aw | Summed discriminations against C18OO during liquid phase diffusion and dissolution |
| a13 | Discrimination against 13CO2 during diffusion through stomata |
| ab13 | Discrimination against 13CO2 during diffusion through leaf boundary layer |
| aw13 | Summed discrimination against 13CO2 during dissolution and liquid phase diffusion |
| αw | Equilibrium oxygen isotope effect between CO2 and water |
| b | Discrimination against 13CO2 by carboxylating enzymes |
| b18 | Discrimination against C18OO by Rubisco |
| C | Molar concentration of water |
| Ca | Partial pressure of CO2 in atmosphere |
| Cc | Partial pressure of CO2 in chloroplast |
| Ccs | Partial pressure of CO2 at the chloroplast surface |
| Ci | Partial pressure of CO2 in leaf intercellular air spaces |
| Cin | Partial pressure of CO2 in dry air entering leaf chamber |
| Cs | Partial pressure of CO2 at the leaf surface |
| ci | Mole fraction of CO2 in intercellular air spaces |
| ca | Mole fraction of CO2 in atmosphere |
| D | Diffusivity of H218O in water |
| ΔA | Discrimination against 13C or 18O during net CO2 uptake by photosynthesis |
| Δca | 18O enrichment of CO2 in chloroplast compared to atmosphere |
| Δe | 18O enrichment at evaporative sites in leaves compared to source water |
| Δea | 18O enrichment of CO2 in chloroplast compared to atmosphere when chloroplast CO2 is in full equilibrium with chloroplast water |
| Δi | Discrimination against 13CO2 that would occur if gi were infinite and photorespiration and day respiration did not discriminate |
| ΔL | 18O enrichment of average lamina leaf water compared to source water |
| Δobs | Observed discrimination against 13CO2 during photosynthesis |
| Δv | 18O enrichment of vapor in atmosphere compared to source water |
| δA | δ18O of CO2 taken up by net photosynthesis (VSMOW scale) |
| δa | δ18O of CO2 in atmosphere (VSMOW scale) |
| δc | δ18O of CO2 in chloroplast (VSMOW scale) |
| δc0 | δ18O of CO2 in chloroplast that has not equilibrated with chloroplast water (VSMOW scale) |
| δe | δ18O of water at evaporative sites in leaves (VSMOW scale) |
| δin | δ18O of CO2 in air entering leaf chamber (VSMOW scale) |
| δL | δ18O of average lamina leaf water (VSMOW scale) |
| δR | δ18O of CO2 efflux during dark respiration (VSMOW scale) |
| δs | δ18O of source water (VSMOW scale) |
| E | Transpiration rate |
| e | Discrimination against 13C during day respiration |
| ea | Vapor pressure in atmosphere |
| ei | Vapor pressure in leaf intercellular air spaces |
| ɛ+ | Equilibrium 18O fractionation between liquid water and vapor |
| ɛk | Kinetic fractionation during diffusion of H218O from leaf intercellular air spaces to atmosphere |
| ɛw | Equilibrium 18O fractionation between CO2 and water |
| f | Discrimination against 13C during photorespiration |
| gt | Conductance to H2O from leaf intercellular air space to atmosphere |
| gi | Conductance to CO2 from leaf intercellular air spaces to sites of carboxylation |
| gtc | Conductance to CO2 from chloroplast to atmosphere |
| Γ* | CO2 compensation point in absence of day respiration |
| k | Carboxylation efficiency |
| L | Scaled effective path length for calculation of ℘ |
| Λ | Area of leaf in leaf chamber |
| m13 | Slope of the relationship between Δi − Δobs and A/Ca |
| m | Slope of the relationship between δc and δe |
| n | Number of measurements in each experiment |
| ℘ | Péclet number |
| θ | Propotion of chloroplast CO2 isotopically equilibrated with chloroplast water |
| P | Atmospheric pressure |
| RA | 13C/12C or 18O/16O of net CO2 uptake by photosynthesis |
| Ra | 13C/12C or 18O/16O of CO2 in atmosphere |
| Rce | 18O/16O of CO2 in equilibrium with chloroplast water |
| Rc0 | 18O/16O of chloroplast CO2 that has not equilibrated with chloroplast water |
| Re | 18O/16O of water at evaporative sites in leaves |
| RR | 18O/16O of net CO2 efflux during dark respiration |
| RStd | 18O/16O of VSMOW standard |
| rs | Stomatal resistance to water vapor diffusion |
| rb | Leaf boundary layer resistance to water vapor diffusion |
| ℜd | Day respiration rate |
| ℜn | Net CO2 efflux rate during dark respiration |
| ρ | Ratio of rates of carboxylation and CO2 hydration in chloroplast |
| Tleaf | Leaf temperature |
| u | Flow rate of air through leaf chamber |
| wi | Mole fraction of water vapor in the leaf intercellular air spaces |
| W | Leaf lamina water concentration |
The diffusional discrimination, 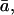 can be calculated as (Farquhar and Lloyd, 1993)
can be calculated as (Farquhar and Lloyd, 1993)
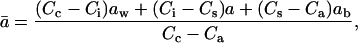 |
(3) |
where Ci is the CO2 partial pressure in the intercellular air spaces, and Cs is that at the leaf surface. The term aw describes the summed discrimination against C18OO during liquid-phase diffusion and dissolution (0.8‰); a is the discrimination during diffusion through the stomata (8.8‰); and ab is the discrimination during diffusion through the leaf boundary layer (5.8‰). We note that Equation 3 is precisely the same as the equation given for 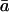 by Farquhar and Lloyd (1993); we have simply multiplied both their numerator and denominator by −1. The equilibrium fractionation between water and CO2 can be calculated as (Brenninkmeijer et al., 1983)
by Farquhar and Lloyd (1993); we have simply multiplied both their numerator and denominator by −1. The equilibrium fractionation between water and CO2 can be calculated as (Brenninkmeijer et al., 1983)
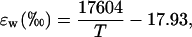 |
(4) |
where T is leaf temperature in K.
The oxygen isotope composition of CO2 in the chloroplast of a respiring leaf (δc) can be calculated from the following equation:
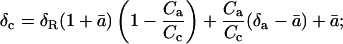 |
(5) |
a derivation of equation 5 is presented (see “Derivation 1” in text). Equations 23 and 24 can be combined, and, after dividing through by RStd, give
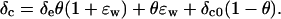 |
(6) |
For a series of measurements made at different values of δe, δc can be calculated from Equation 5 and plotted against δe. According to Equation 6, the slope of the relationship between δc and δe (m) is then equal to θ(1 + ɛw), such that θ can be calculated as θ = m/(1 + ɛw). The intercept of the relationship, I, is equal to θɛw + δc0(1 − θ), such that δc0 can be calculated as δc0 = (I − θɛw)/(1 − θ). We note that such an analysis assumes that only δe varies across the series of measurements; thus, θ, ɛw, and δc0 are assumed invariant.
The oxygen isotope enrichment at the evaporative sites in leaves (Δe) can be calculated as (Craig and Gordon, 1965; Dongmann et al., 1974; Farquhar and Lloyd, 1993)
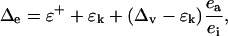 |
(7) |
where ɛ+ is the equilibrium fractionation that occurs during the phase change from liquid to vapor, ɛk is the kinetic fractionation that occurs during diffusion of vapor from the leaf intercellular air space to the atmosphere, Δv is the isotopic enrichment of vapor in the atmosphere, and ea/ei is the ratio of ambient to intercellular vapor pressures. The Δe and Δv are defined with respect to source water, such that Δe = Re/Rs − 1 and Δv = Rv/Rs − 1, where Re is 18O/16O of water at the evaporating sites, Rs is 18O/16O of source water, and Rv is 18O/16O of vapor in the atmosphere. The term δe can be calculated from Δe as
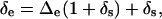 |
(8) |
where δs is the oxygen isotope composition of source water relative to a standard. The parameter Δv in Equation 6 can be calculated from measurements of the oxygen isotope composition of vapor in the atmosphere (δv) and source water as Δv = (δv − δs)/(1 + δs). The equilibrium fractionation between liquid and vapor, ɛ+, can be calculated as (Bottinga and Craig, 1969)
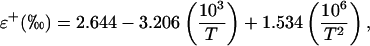 |
(9) |
where T is leaf temperature in K. The kinetic fractionation, ɛk, can be calculated as (Farquhar et al., 1989)
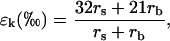 |
(10) |
where rs and rb are the stomatal and boundary layer resistances to water vapor diffusion (m2 s mol−1), and 32 and 21 are associated fractionation factors scaled to per mil. These fractionation factors have been revised up from values of 28 and 19, respectively, based on recent measurements showing the isotope effect for diffusion of H218O in air to be 1.032 (Cappa et al., 2003), rather than 1.028 (Merlivat, 1978).
Measurement of the Oxygen Isotope Composition of Dark-Respired CO2
For our first dark respiration experiment, in which air entering the leaf chamber was free of CO2, we calculated the oxygen isotope composition of respired CO2, δR, simply as the oxygen isotope composition of CO2 exiting the chamber, δa. In our second dark respiration experiment, where air entering the leaf chamber had a CO2 concentration sufficient to bring that inside the chamber close to that normally found in the atmosphere, we calculated δR with a modified form of the equation presented previously by Evans et al. (1986):
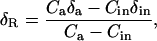 |
(11) |
where Ca is the CO2 partial pressure (μbar) of air within the chamber when dried, δa is δ18O of CO2 within the chamber, Cin is the CO2 partial pressure (μbar) of dry air entering the chamber, and δin is the δ18O of CO2 entering the chamber. A derivation of Equation 11 is provided (see “Derivation 2” in text). The terms Ca and δa are measured in gas exiting the leaf chamber, due to effective stirring of air within the chamber.
Calculation of Photosynthetic Discrimination against 13C and 18O
For measurements in the light, we calculated carbon and oxygen isotope discrimination during photosynthesis as described by Evans et al. (1986):
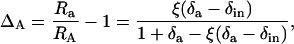 |
(12) |
where Ra is 13C/12C or 18O/16O of CO2 within the leaf chamber, RA is 13C/12C or 18O/16O of CO2 removed from the chamber by photosynthesis, δa is δ13C or δ18O of CO2 within the leaf chamber, δin is δ13C or δ18O of CO2 entering the chamber, and ξ is defined as Cin/(Cin − Ca), where Cin and Ca refer to CO2 partial pressures in dry air. We calculated the oxygen isotope composition of chloroplast CO2 during photosynthesis by rearranging the C18OO discrimination equation presented by Farquhar and Lloyd (1993):
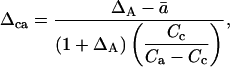 |
(13) |
where ΔA is discrimination against C18OO during photosynthesis, as defined above, and Δca is defined as (Rc/Ra) − 1, where Rc is 18O/16O of chloroplast CO2. We then calculated δc as δc = Δca(1 + δa) + δa.
For the photosynthesis measurements that comprised our third experiment, we compared the regression approach to calculating θ, as described above in the theory relating to dark respiration, to the method suggested by Gillon and Yakir (2000b), whereby θ can be calculated separately for each individual photosynthesis measurement:
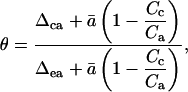 |
(14) |
where Δea is the value of Δca expected if chloroplastic CO2 were in full oxygen isotope equilibrium with δe. The Δea was calculated as
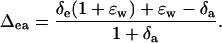 |
(15) |
Equation 14 incorporates an assumption that is not applied in the regression approach to calculating θ that we described above for dark respiration. The assumption is that the δ18O of CO2 in the chloroplast that has not equilibrated with leaf water can be calculated from the equation Rc0 = Ra[1 − ā(1 − Cc/Ca)] (Gillon and Yakir, 2000b), which can be replaced, to a close approximation, by 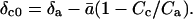 Defining δc0 in this way assumes no discrimination against C18OO by Rubisco; it also ignores any possible effect of photorespiration or day respiration on δc0.
Defining δc0 in this way assumes no discrimination against C18OO by Rubisco; it also ignores any possible effect of photorespiration or day respiration on δc0.
Calculation of the Conductance from Ci to Cc
The CO2 conductance from leaf intercellular air spaces to the sites of carboxylation in chloroplasts (gi) was calculated from 13C discrimination measurements during photosynthesis using the method of Evans et al. (1986):
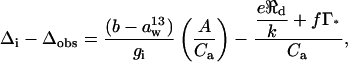 |
(16) |
where Δobs is the observed 13C discrimination, b is the discrimination against 13CO2 during carboxylation (taken as 29‰), 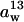 is the sum of discriminations against 13CO2 during dissolution of CO2 and liquid phase diffusion (1.8‰), A is the net photosynthetic rate (μmol CO2 m−2 s−1), Ca is the ambient CO2 partial pressure (μbar), ℜd is day respiration (μmol CO2 m−2 s−1), e is the associated discrimination against 13CO2, k is the carboxylation efficiency (mol m−2 s−1 bar−1), Γ* is the CO2 compensation point in the absence of ℜd (μbar), and f is the discrimination against 13CO2 associated with photorespiration. The term Δi represents the discrimination that would occur if gi were infinite, and if photorespiration and day respiration did not discriminate (Farquhar et al., 1982):
is the sum of discriminations against 13CO2 during dissolution of CO2 and liquid phase diffusion (1.8‰), A is the net photosynthetic rate (μmol CO2 m−2 s−1), Ca is the ambient CO2 partial pressure (μbar), ℜd is day respiration (μmol CO2 m−2 s−1), e is the associated discrimination against 13CO2, k is the carboxylation efficiency (mol m−2 s−1 bar−1), Γ* is the CO2 compensation point in the absence of ℜd (μbar), and f is the discrimination against 13CO2 associated with photorespiration. The term Δi represents the discrimination that would occur if gi were infinite, and if photorespiration and day respiration did not discriminate (Farquhar et al., 1982):
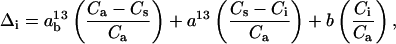 |
(17) |
where 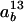 is the discrimination against 13CO2 during diffusion through the boundary layer (2.8‰), Cs is the CO2 partial pressure at the leaf surface, and a13 is the discrimination against 13CO2 during diffusion through the stomata (4.4‰). The term (
is the discrimination against 13CO2 during diffusion through the boundary layer (2.8‰), Cs is the CO2 partial pressure at the leaf surface, and a13 is the discrimination against 13CO2 during diffusion through the stomata (4.4‰). The term (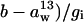 was calculated from the slope, m13, of a plot of Δi − Δobs against A/Ca. The term gi was then calculated as (
was calculated from the slope, m13, of a plot of Δi − Δobs against A/Ca. The term gi was then calculated as (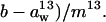 The value of m13 is independent of values assigned to f and e in Equation 16 because varying these parameters affects the intercept of the regression rather than the slope. Therefore, there is no need to assign values to f and e for calculation of gi.
The value of m13 is independent of values assigned to f and e in Equation 16 because varying these parameters affects the intercept of the regression rather than the slope. Therefore, there is no need to assign values to f and e for calculation of gi.
Calculation of the Oxygen Isotope Composition of Average Lamina Leaf Water
We estimated the average lamina leaf water 18O enrichment (ΔL) of leaves during CO2 collections from a model relating ΔL to Δe (Farquhar and Lloyd, 1993):
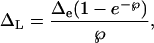 |
(18) |
where Δe is as calculated in Equation 7, and ℘ is a lamina radial Péclet number (Farquhar and Gan, 2003). The term ℘ is defined as EL/(CD), where E is transpiration rate (mol m−2 s−1), L is a scaled effective path length (m), C is the molar concentration of water (5.55 × 104 mol m−3), and D is the diffusivity of H218O in water (2.66 × 10−9 m−2 s−1). In a previous experiment, we found that the scaled effective path length for R. communis, grown and measured under the same conditions as in the present experiment, was 15.0 ± 3.5 mm (mean ± 1 sd; n = 5; Cernusak et al., 2003). This mean value was used to calculate ΔL. The term δL was calculated as δL = ΔL(1 + δs) + δs. Cernusak et al. (2003) also found that the ethanol-dry ice traps on the bypass drying loop of the gas exchange system were not quite efficient enough to remove all of the water vapor from the air cycling back to the chamber. Due to fractionation during condensation of the vapor in the traps, vapor in the air returning to the chamber was slightly enriched compared to that retained in the traps. As a result, Δv for the air exiting the chamber was found to be 1.2 ± 0.5‰ (mean ± 1 se; n = 5). This mean value was used in calculations of Δe.
Derivation 1: Equation for Predicting the δ18O of Dark-Respired CO2
We begin by writing an equation for the total CO2 flux from the leaf interior to the atmosphere in the dark in the steady state:
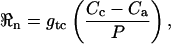 |
(19) |
where ℜn is the net CO2 efflux (μmol m−2 s−1); gtc is the total conductance to CO2 from chloroplast to atmosphere (mol m−2 s−1); Cc and Ca are the CO2 partial pressures in the chloroplast and atmosphere, respectively (μbar); and P is atmospheric pressure (bar). We make the assumption that, in C3 plants, carbonic anhydrase resides primarily in the chloroplast (Everson, 1970; Jacobson et al., 1975; Tsuzuki et al., 1985) and that it is therefore the chloroplastic CO2 concentration that should be considered when calculating the C18OO efflux from the leaf. We further assume that the chloroplasts in C3 plants are appressed against the intercellular air spaces in the leaf and that CO2 evolved in mitochondria interacts with chloroplasts during diffusion out of the leaf. These assumptions may need to be reassessed for application of the model to C4 plants. Equation 19 can be written for C18OO as
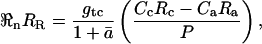 |
(20) |
where RR is the 18O/16O of dark-respired CO2, ā is the weighted mean diffusional fractionation from chloroplast to atmosphere (calculated as described in Equation 3 above), Rc is 18O/16O of chloroplastic CO2, and Ra is 18O/16O of ambient CO2. Equations 19 and 20 can be combined to give
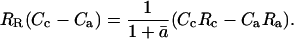 |
(21) |
Dividing Equation 21 by the 18O/16O of a standard, RStd, and applying the relationship RX/RStd = δX + 1 leads to
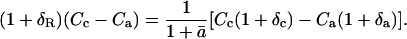 |
(22) |
Solving Equation 22 for δc leads to Equation 5 above:
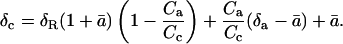 |
To write an expression for predicting δR, we apply an assumption proposed by Gillon and Yakir (2000b), under which the CO2 within the chloroplast can be divided into two pools: one pool, of proportion θ, has completely exchanged oxygen atoms with chloroplast water and therefore has an 18O/16O composition of Rce; the other pool, of proportion 1 − θ, has not exchanged oxygen atoms with chloroplast water and retains its initial 18O/16O composition of Rc0. We note that the term Rc0 could describe a mixture of mitochondrial CO2 and CO2 that has diffused into the leaf from the ambient air. Therefore, we do not define Rc0 solely as a function of CO2 diffusing into the leaf from the atmosphere, as was done previously for photosynthesis (Gillon and Yakir, 2000b). The term Rc is then written as
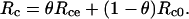 |
(23) |
The term Rce can be calculated from the equilibrium fractionation between CO2 and water:
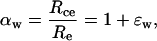 |
(24) |
where Re is 18O/16O of chloroplast water, which we assume to be equal to 18O/16O of water at the evaporative sites. Combining Equations 21, 23, and 24 leads to
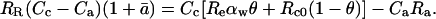 |
(25) |
Dividing through by RStd, and substituting 1 + ɛw for αw, gives
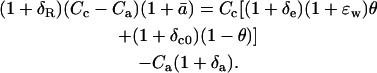 |
(26) |
Solving Equation 26 for δR leads to Equation 2 above, which is
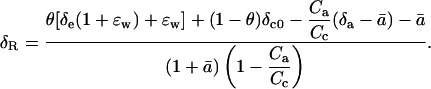 |
Derivation 2: Calculating δR From Online Gas-Exchange Measurements
Under steady-state conditions, the increase in CO2 concentration in air flowing through a gas-exchange cuvette containing a respiring leaf can be described as
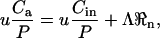 |
(27) |
where u is the flow rate through the cuvette (mol s−1), Λ is the area of the leaf in the cuvette (m2), Ca and Cin are CO2 partial pressures of dry air exiting and entering the cuvette (μbar), P is atmospheric pressure (bar), and ℜn is the respiration rate of the leaf (μmol CO2 m−2 s−1). The corresponding mass balance for C18OO can be written as
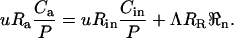 |
(28) |
Combining Equations 27 and 28 gives
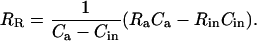 |
(29) |
Dividing through by the isotope ratio of a standard, RStd, and substituting from the relationship RX/RStd = δX + 1 gives
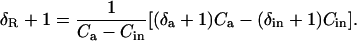 |
(30) |
Canceling common terms leads to Equation 11 above, which is
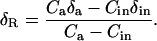 |
We note that the equations derived in this and the previous section can also be applied in the light. Thus, for photosynthesis, the term δR in Equations 2, 5, and 11 above can simply be replaced with the term δA. The term δA relates to ΔA by the relationship ΔA = (δa − δA)/(1 + δA).
RESULTS
Dark Respiration with CO2 Free Air Entering the Leaf Chamber
In the first dark respiration experiment, air entering the leaf chamber was free of CO2, and air exiting the leaf chamber had a mean CO2 partial pressure of 47 μbar. The CO2 exiting the leaf chamber was collected and analyzed for its isotopic composition. A summary of gas exchange parameters measured just prior to each CO2 collection is presented in Table II. The dark respiration rates of the leaves ranged from 0.8 to 2.0 μmol CO2 m−2 s−1 on a projected leaf area basis, with a mean value of 1.5. The Ca/Ci values ranged from 0.46 to 0.93, with a mean value of 0.81.
Table II.
Gas exchange and isotopic characteristics for R. communis leaves
| Parameter | Experiment 1: Dark Respiration at Low CO2 Concentration | Experiment 2: Dark Respiration at Atmospheric CO2 Concentration | Experiment 3: Photosynthesis at Atmospheric CO2 Concentration |
|---|---|---|---|
| n | 11 | 10 | 8 |
| gs (mol m−2 s−1) | 0.28 (0.04 to 0.55) | 0.13 (0.03 to 0.28) | 0.50 (0.18 to 0.77) |
| E (mmol m−2 s−1) | 4.4 (1.2 to 7.8) | 2.7 (1.3 to 4.0) | 8.8 (5.7 to 15.1) |
| Tleaf (°C) | 29.3 (27.3 to 31.2) | 30.2 (29.3 to 30.8) | 27.2 (24.0 to 30.1) |
| Ca (μbar) | 47 (24 to 66) | 347 (324 to 365) | 363 (328 to 395) |
| Ci (μbar) | 63 (27 to 125) | 357 (328 to 401) | 285 (250 to 317) |
| Cc (μbar) | 66 (28 to 128) | 360 (330 to 403) | 249 (207 to 287) |
| ea/ei | 0.53 (0.13 to 0.92) | 0.41 (0.11 to 0.73) | 0.51 (0.30 to 0.78) |
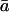 (‰) (‰) |
6.6 (5.4 to 8.4) | 5.9 (3.7 to 8.3) | 6.0 (5.0 to 7.5) |
| δa (‰ versus VSMOW) | 51.6 (43.8 to 59.0) | 43.5 (37.7 to 51.2) | 42.3 (40.1 to 45.4) |
| δe (‰ versus VSMOW) | 16.9 (5.0 to 29.2) | 20.5 (10.8 to 29.8) | 17.5 (9.4 to 23.9) |
| δL (‰ versus VSMOW) | 12.4 (4.3 to 26.5) | 17.2 (8.4 to 27.4) | 9.5 (4.1 to 16.4) |
| δc (‰ versus VSMOW) | 53.3 (44.8 to 63.2) | 51.4 (44.6 to 60.7) | 59.6 (50.4 to 70.7) |
| δR (‰ versus VSMOW) | 51.6 (43.8 to 59.0) | 277 (233 to 324) | |
| Modeled δR | 51.9 (34.0 to 64.4) | 291 (149 to 476) | |
| ΔA (‰ versus VSMOW) | 44.6 (32.4 to 78.5) | ||
| Modeled ΔA | 43.4 (30.6 to 62.9) |
Values are given as the mean, with the total range in parentheses, for the three experiments conducted. Symbols are as defined in Table I. Modeled δR values were calculated using Equation 2 and the empirically determined coefficients for θ and δc0 for experiments 1 and 2. Modeled ΔA values were calculated using Equation 13 and assuming Δca = Δea; the term Δea was calculated as in Equation 15.
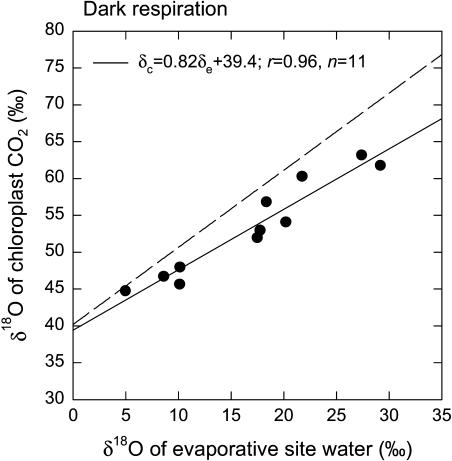
Isotopic parameters derived by combining the results of the gas exchange measurements with results of analyses of the isotopic composition of CO2 exiting the leaf chamber, and of irrigation water fed to the plants, are given in Table II; these parameters are δe, δL, and δc. Results for δa, the δ18O of CO2 exiting the leaf chamber, are also given in Table II. The observed δR values, which are equal to δa in the first experiment, ranged from 43.8‰ to 59.0‰, with a mean value of 51.6‰. All δ18O values in this paper are reported relative to Vienna Standard Mean Ocean Water (VSMOW). The δc values were significantly, positively correlated with corresponding values of δe (Fig. 1); the Pearson correlation coefficient (r) between the two was 0.96 (P < 0.0001, n = 11). The δc values were also significantly correlated with values of δL (r = 0.90, P = 0.0001, n = 11), but the relationship was not as strong as that between δc and δe. The slope of the regression relating δc to δe was 0.82, yielding an estimate for θ of 0.79. Thus, we estimated, by applying Equation 6, that 79% of the CO2 in the chloroplasts had equilibrated with chloroplast water during dark respiration in the first experiment. The intercept of the regression relating δc to δe was 39.4‰; this intercept yields an estimate for δc0 of 36.2‰. This is the mean δ18O estimated for CO2 not equilibrated with chloroplast water.
Figure 1.
The δ18O of chloroplast CO2 plotted against the δ18O of water at evaporative sites in R. communis leaves during dark respiration. In this experiment, air entering the leaf chamber was free of CO2, and the CO2 partial pressure of air exiting the chamber averaged 47 μbar. The δ18O of chloroplast CO2 was calculated from measurements of the δ18O of CO2 exiting the chamber and gas exchange parameters, as described in the theory section of the main text. The broken line on the graph represents the relationship expected if chloroplast CO2 were in full oxygen isotope equilibrium with water at evaporative sites. The δ18O values are presented relative to VSMOW. The δ18O of irrigation water fed to the plants was −7.2‰.
By applying the mean value of gi derived from carbon isotope discrimination measurements during photosynthesis (see results below), we generated estimates of Cc and 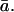 These values are detailed in Table II. Estimates of Ca/Cc ranged from 0.45 to 0.88, with a mean value 0.78. When these values for Ca/Cc and
These values are detailed in Table II. Estimates of Ca/Cc ranged from 0.45 to 0.88, with a mean value 0.78. When these values for Ca/Cc and 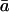 were inserted into Equation 2, along with the values of θ and δc0 described above, a mean modeled δR of 51.9‰ was predicted, in good agreement with the mean observed δR of 51.6‰. The range of modeled δR can be compared with the range of observed δR in Table II.
were inserted into Equation 2, along with the values of θ and δc0 described above, a mean modeled δR of 51.9‰ was predicted, in good agreement with the mean observed δR of 51.6‰. The range of modeled δR can be compared with the range of observed δR in Table II.
Dark Respiration at Atmospheric CO2 Concentration
In the second dark respiration experiment, the partial pressure of CO2 in the air entering the leaf chamber was adjusted such that the air exiting the chamber had a partial pressure of approximately 350 μbar. Under these conditions, leaf dark respiration rates were similar to those observed in the first experiment, ranging from 1.0 to 2.0 μmol CO2 m−2 s−1, with a mean value of 1.4. Stomatal conductance was lower than in the first experiment, having a mean value less than half that observed in the first experiment (Table II). This presumably reflects a response to the increased CO2 partial pressure within the leaf chamber. Although stomatal conductance was lower, Ca/Ci values were higher than in the first experiment due to the increase in Ca; values ranged from 0.91 to 0.99, with a mean of 0.97. The δ18O of CO2 in air entering the leaf chamber was 19.1 ± 0.1‰ (mean ± 1 se; n = 5). The mean δ18O of CO2 exiting the chamber was 43.5‰.
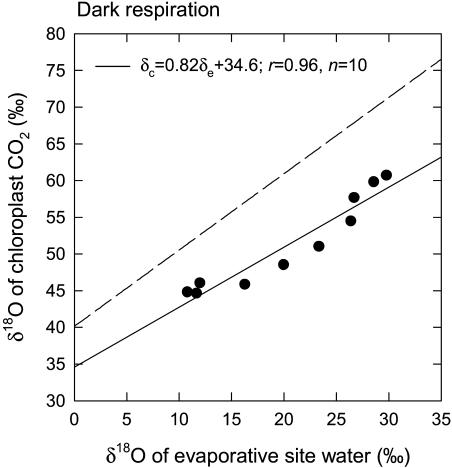
The most striking difference between the first and second dark respiration experiments was the difference in observed δR. The mean observed δR in the second experiment was 277‰, which can be compared with 52‰ for the first experiment (Table II). Mean values for δe, δL, and δc were similar between the two experiments (Table II). Differences between δe and δL in the second experiment were slightly less than in the first experiment, reflecting the lower transpiration rates (Table II). As in the first experiment, variation in δc was significantly correlated with variation in δe (Fig. 2), showing an r value of 0.95 (P < 0.0001, n = 10). It was also correlated with δL, with a slightly lower correlation coefficient (r = 0.94, P < 0.0001, n = 10). The regression slope of the relationship between δc and δe was 0.82, resulting in an estimate for θ of 0.79, suggesting that 79% of the CO2 in chloroplasts had equilibrated with chloroplast water during dark respiration in the second experiment. This θ value is the same as the value of 0.79 estimated in the first experiment. The value of the intercept of the regression of δc on δe was 34.6‰, yielding an estimate for δc0 of 14.3‰; this value is lower than the δc0 of 36.2‰ estimated in the first experiment.
Figure 2.
The δ18O of chloroplast CO2 plotted against the δ18O of water at evaporative sites in R. communis leaves during dark respiration. In this experiment, air entering the leaf chamber had an average CO2 partial pressure of 314 μbar, and air exiting the chamber had an average CO2 partial pressure of 347 μbar. The broken line on the graph represents the relationship expected if chloroplast CO2 were in full oxygen isotope equilibrium with water at the evaporative sites. The δ18O values are presented relative to VSMOW. The δ18O of irrigation water fed to the plants was −7.2‰.
Values of Ca/Cc in the second experiment did not differ from values for Ca/Ci when calculated to two decimal places; the range was from 0.91 to 0.99, with a mean of 0.97. This mean of 0.97 is considerably higher than the mean Ca/Cc of 0.78 observed in the first experiment. Mean estimates for 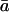 were similar between the two experiments (Table II). When the empirically determined coefficients for θ and δc0 for the second experiment were inserted into Equation 2, along with the other relevant parameters, the mean value of modeled δR was 291‰, which compares reasonably well with the mean observed δR of 277‰. The relatively small difference between the two presumably reflects variation around the regression line in Figure 2, which was used to estimate θ and δc0.
were similar between the two experiments (Table II). When the empirically determined coefficients for θ and δc0 for the second experiment were inserted into Equation 2, along with the other relevant parameters, the mean value of modeled δR was 291‰, which compares reasonably well with the mean observed δR of 277‰. The relatively small difference between the two presumably reflects variation around the regression line in Figure 2, which was used to estimate θ and δc0.
A comparison of modeled δR values across both experiments with observed δR showed that modeled δR accounted for 80% of variation in observed δR. The regression line relating the two was δR(observed) = 0.72δR(modeled) + 39.5 (R2 = 0.80, P < 0.0001, n = 21).
Carbon and Oxygen Isotope Discrimination during Photosynthesis
In the third experiment, R. communis leaves were placed in the leaf chamber in the light, and gas exchange and isotopic analyses were conducted. Photosynthesis rates ranged from 8.5 to 30.9 μmol CO2 m−2 s−1, with a mean value of 20.4. The CO2 partial pressure of air exiting the chamber ranged from 328 to 395 μbar, whereas the CO2 partial pressure of incoming air ranged from 533 to 967 μbar; this gave rise to ξ values ranging from 1.5 to 3.0. Stomatal conductance was approximately 4-fold larger in the light than in the dark at similar CO2 partial pressure (Table II). The Ci/Ca ranged from 0.66 to 0.90, with a mean of 0.79. The δ18O of CO2 entering the leaf chamber was 19.1 ± 0.1‰ (mean ± 1 se; n = 5); the δ13C of CO2 entering the leaf chamber was −33.1 ± 0.2‰ (mean ± 1 se; n = 5). The δ18O of CO2 exiting the leaf chamber ranged from 40.1‰ to 45.4‰; the δ13C of CO2 exiting the chamber ranged from −25.3‰ to −19.3‰.
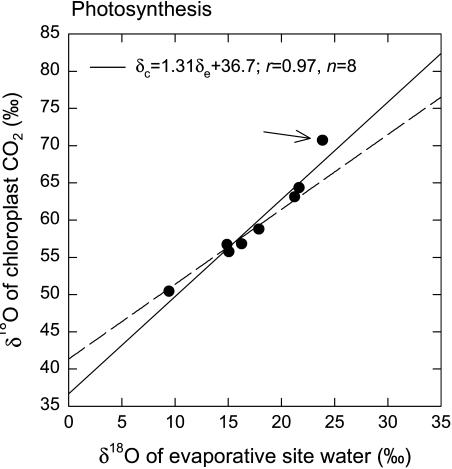
The mean observed oxygen isotope discrimination during photosynthesis (ΔA) was 44.6‰; the range is given in Table II. The δc values for the photosynthesis experiment were somewhat higher than for the dark respiration experiment at similar CO2 concentration, presumably reflecting a higher proportion of chloroplast CO2 equilibrated with chloroplast water (i.e. higher θ). Differences between δe and δL were larger in the photosynthesis experiment than in the dark respiration experiments, reflecting the higher transpiration rates (Table II). Variation in δc was significantly correlated with variation in δe (r = 0.97, P < 0.0001, n = 8), as shown in Figure 3. The δc was also correlated with δL (r = 0.91, P = 0.001, n = 8), but the correlation was not as strong as with δe. The slope of the relationship between δc and δe was 1.31; using Equation 6, this indicates a value for θ of 1.25. However, this slope estimate was strongly influenced by one outlying data point; this datum is identified by an arrow in Figure 3. If this outlying datum is excluded from the analysis, the slope of the relationship between δc and δe becomes 1.11, yielding an estimate for θ of 1.06. The individual θ values calculated according to the method of Gillon and Yakir (2000b) ranged from 0.93 to 1.24, with a mean value of 1.02. If the outlying data point identified with the arrow in Figure 3 is excluded, these individual θ estimates ranged from 0.93 to 1.06, with a mean of 0.99. Because the θ values were very close to 1.0, we did not estimate a δc0 value for the photosynthesis experiment.
Figure 3.
The δ18O of chloroplast CO2 plotted against the δ18O of water at evaporative sites in R. communis leaves during photosynthesis. In this experiment, air entering the leaf chamber had an average CO2 partial pressure of 833 μbar, and air exiting the chamber had an average CO2 partial pressure of 363 μbar. Irradiance varied from 300 to 800 μmol PAR m−2 s−1, and chamber air temperature varied between 25 and 30°C. The δ18O of chloroplast CO2 was calculated as described in the theory section of the main text. The broken line on the graph represents the relationship expected if chloroplast CO2 were in full oxygen isotope equilibrium with water at the evaporative sites. The δ18O values are presented relative to VSMOW. The δ18O of irrigation water fed to the plants was −7.2‰. The arrow on the graph indicates an outlying datum that strongly influenced the slope of the regression between δc and δe. Excluding this datum resulted in a slope between δc and δe of 1.10.
Observed carbon isotope discrimination values, Δobs, ranged from 19.4‰ to 25.2‰, whereas values predicted for infinite gi and no discrimination by photorespiration or day respiration, Δi, ranged from 20.6‰ to 26.5‰. The slope of the relationship between Δi − Δobs and A/Ca was 47.8 ± 14.6 (slope ± 1 se), yielding a mean gi estimate of 0.57 mol m−2 s−1 bar−1.
DISCUSSION
The most important result of this study is that we have shown that it is the one-way CO2 efflux from a respiring leaf that is labeled with the leaf water δ18O signal, rather than the net CO2 efflux. The one-way efflux can be calculated as gtcCc/P, where gtc is the total conductance to CO2 from chloroplast to atmosphere (mol m−2 s−1), and P is atmospheric pressure (bar). In our second dark respiration experiment, where Ca averaged 347 μbar, values for gtcCc/P ranged from 7.4 to 50.1 μmol CO2 m−2 s−1, whereas the net respiratory efflux, ℜn, ranged from 1.0 to 2.0 μmol CO2 m−2 s−1; the ratio of gtcCc/P to ℜn averaged 16.9. Thus, in cases where the CO2 diffusing out of a respiring leaf has a δ18O different from CO2 in canopy air, the effect of δR on δa could be significantly underestimated if one assumes that only the net CO2 efflux is influenced by the isotopic composition of leaf water. The analogous requirement for considering one-way CO2 fluxes when calculating the effect of photosynthesizing leaves on δ18O of atmospheric CO2 was discerned by Farquhar et al. (1993).
Previous attempts to model the effect of leaf dark respiration on the δ18O of CO2 in canopy air have considered only the net respiratory CO2 efflux. We will refer to this method as the net flux model. In the net flux model, δR is calculated as δR = δe + ɛw − a, where a is usually taken as 8.8‰. The C18OO isoflux is then calculated as the product of ℜn and δR. For the purposes of this discussion, we define an isoflux as the product of a net CO2 flux and its δ18O. The net flux model has been used to interpret nighttime measurements of δ18O in canopy CO2 (Flanagan et al., 1997, 1999; Mortazavi and Chanton, 2002; Bowling et al., 2003a, 2003b) and in global simulations of δ18O dynamics in atmospheric CO2 (Cuntz et al., 2003a, 2003b). Earlier global studies did not differentiate leaf respiration from soil respiration, and thus did not define δR for leaves (Farquhar et al., 1993; Ciais et al., 1997). A slightly different version of the net flux model, with a modified term for diffusional fractionation, has also been applied at the leaf level (Yakir et al., 1994; Yakir, 1998). If we apply the net flux model to data from our second experiment, where Ca was near that found in the atmosphere, predicted values for δR range from 42‰ to 61‰. These values can be compared to observed δR values ranging from 233‰ to 324‰. Thus, in the second dark respiration experiment, the net flux model underestimated the observed δR by 180‰ to 266‰. Note that these observed δR values are effective values that result when one treats the modification of δ18O of CO2 in air passing over the leaf as if it resulted from the net CO2 efflux alone. Thus, using these observed δR values, the C18OO isoflux is still calculated as ℜnδR, and the large difference between ℜn and gtcCc/P becomes manifested in the δR term.
If we apply the net flux model to our first experiment, where air entering the leaf chamber was free of CO2, it predicts δR values ranging from 36‰ to 60‰. Observed δR values in this experiment ranged from 44‰ to 59‰, in good agreement with predictions from the net flux model. The difference in the performance of the net flux model between the first and second dark respiration experiments can be effectively understood by examining an alternative formulation of Equation 2. If the definition of δc from Equation 6 is substituted into Equation 2, and the term (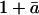 ) in the denominator of Equation 2 is assumed equal to unity, Equation 2 can be rewritten as
) in the denominator of Equation 2 is assumed equal to unity, Equation 2 can be rewritten as
 |
Equation 31 is informative in that terms I and II on the right side are analogous to the net flux model; the difference is that in Equation 31 δc is defined as in Equation 6, whereas in the net flux model δc is defined as δe + ɛw. Term III on the right side of Equation 31 reflects the proportion of CO2 that diffuses into the leaf and equilibrates with leaf water, then diffuses out of the leaf, thereby altering the isotopic composition of CO2 in the leaf chamber while leaving the net CO2 efflux rate unaltered. This process is analogous to the invasion effect that has been described for soil respiration (Tans, 1998; Miller et al., 1999; Stern et al., 2001). In the first dark respiration experiment, where air entering the leaf chamber was free of CO2, this process was also occurring, but had a much smaller impact on δR than in the second experiment. This is because (δc − δa) was small in the first experiment, having a mean value of 1.8‰; in contrast, (δc − δa) in the second experiment had a mean value of 7.9‰. Additionally, [Ca/(Cc − Ca)] was much smaller in the first experiment than in the second, having a mean value of 4.8 in the former versus 47.7 in the latter. As a result, the mean value for term III in Equation 31, which can be thought of as the invasion term, was 5.2‰ for the first dark respiration experiment, and 234‰ for the second dark respiration experiment.
Equation 31 can be used to highlight the conditions under which large departures in δR from values predicted by the net flux model can be expected at the ecosystem level under natural conditions. For example, if δc is very similar to δa, term III will be small. Additionally, if stomata are tightly closed, [Ca/(Cc − Ca)] will be small, and term III will also be small. Thus, the largest departures in δR from the predictions of the net flux model should occur when there is a relatively large difference between δc and δa, and when stomata are relatively open, such that [Ca/(Cc − Ca)] is large. The approximation in Equation 31 that (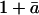 ) equals unity introduces a very small bias into calculations with this equation; however, this bias is less than 1% and is therefore negligible. Thus, Equation 31, in combination with Equation 6, can be used in place of Equation 2, if so desired.
) equals unity introduces a very small bias into calculations with this equation; however, this bias is less than 1% and is therefore negligible. Thus, Equation 31, in combination with Equation 6, can be used in place of Equation 2, if so desired.
Photosynthesis enriches the atmosphere in C18OO due to exchange of CO2 with evaporatively enriched leaf water in the chloroplast, whereas soil respiration is generally thought of as depleting the atmosphere in C18OO, because soil CO2 exchanges with water in soil that has generally not been enriched by evaporation (Flanagan and Ehleringer, 1998). In this study, we have observed that leaf dark respiration is capable of enriching air passing over a leaf in C18OO to as great an extent as photosynthesis. The mean δ18O value of CO2 exiting the leaf chamber in the respiration measurements at atmospheric CO2 partial pressure was 43.5‰; the mean value for photosynthesis measurements at similar Ca was 42.3‰. The δ18O of incoming CO2 in both experiments was 19.1‰, and flow rates through the chamber were similar between the two experiments. Thus, dark respiration had as marked an effect as photosynthesis on the δ18O of CO2 passing over the leaves, even though the net exchange of CO2 between the leaf and ambient air is roughly an order of magnitude less, and in the opposite direction, during dark respiration.
The effect of both photosynthesis and respiration on δ18O of CO2 in canopy air is partly controlled by the isotopic composition of leaf water. In natural systems, nighttime leaf water δ18O is typically intermediate between daytime leaf water δ18O and the δ18O of source water (Dongmann et al., 1974; Förstel, 1978; Zundel et al., 1978; Förstel and Hützen, 1983; Flanagan and Ehleringer, 1991; Flanagan et al., 1993, 1999; Cernusak et al., 2002; Mortazavi and Chanton, 2002). We therefore expect nighttime leaf respiration to impart a C18OO signal on the atmosphere that is intermediate between the soil respiration signal and the photosynthesis signal.
Accurate prediction of the oxygen isotope composition of leaf water is important for interpreting vegetation effects on δ18O of atmospheric CO2. Equation 7 can be used to calculate δe under steady state conditions. However, leaf water δ18O is unlikely to be at steady state at night (Flanagan and Ehleringer, 1991; Harwood et al., 1998; Cernusak et al., 2002). Cernusak et al. (2002) applied a non-steady state equation for δ18O in leaf water, derived by G.D. Farquhar and L.A. Cernusak (unpublished theory), and found good agreement between predicted and observed nighttime values. The combination of the non-steady state leaf water equation and the model that we have provided here for δR should allow reasonable predictions to be made of the impact of leaf dark respiration on δ18O of atmospheric CO2.
Stomatal conductance will be an important parameter in the prediction of both δe and δR during the night. However, little attention has been paid historically to nighttime stomatal conductance. Snyder et al. (2003) recently observed nighttime stomatal conductance to water vapor ranging from 10 to 150 mmol m−2 s−1 for 17 plant species in the western United States. However, a mechanistic framework for interpreting such variation does not currently exist. Further investigation into the patterns and processes controlling nighttime stomatal conductance will lead to more accurate prediction of nighttime δe and δR. We note that the mean stomatal conductance that we observed in the dark for R. communis at normal atmospheric CO2 concentration was 130 mmol m−2 s−1 (Table II), near the high end of values observed by Snyder et al. (2003) at night in the field. Our measurements were made during the day, and it is likely that stomatal conductance was influenced by circadian rhythms, causing it to be higher than it would be in the dark at night.
The mean value of θ for the photosynthesis experiment calculated by the method described by Gillon and Yakir (2000b) was very close to 1.0. If the outlying data point, indicated by an arrow in Figure 3, was excluded from the analysis, the regression method resulted in a similar estimate of 1.06. Thus, both calculations suggested θ values close to unity for photosynthesizing R. communis leaves. A quick examination of Figure 3 shows that observed δc estimates lie very close to those expected for full equilibrium, with the exception of the one outlier, which is several per mil above the value expected for full equilibrium. We are unable to find a satisfactory explanation for why this particular datum should differ so markedly from the others. Results have been reported for a number of other C3 species in which the CO2 diffusing out of photosynthesizing leaves appeared to be very close to full equilibrium with δe (Farquhar et al., 1993; Gillon and Yakir, 2001). Interestingly, the θ values that we observed during dark respiration in R. communis were lower than those observed during photosynthesis, having values close to 0.80. Further research is necessary to determine the cause of this apparent discrepancy between θ in the light and in the dark.
Gillon and Yakir (2000b) suggested that during photosynthesis δc0, the δ18O of CO2 in the chloroplast not equilibrated with chloroplast water, can be calculated, to a close approximation, as δc0 = δa − ā(1 − Cc/Ca). This definition assumes no discrimination against C18OO by Rubisco during photosynthesis, and neglects any influence of photorespiration or day respiration on δc0. The latter statement is tantamount to saying that CO2 evolved from the mitochondria in the light has the same oxygen isotope composition as CO2 in the chloroplast. In that case, any addition of mitochondrial CO2 will have no impact upon the δ18O of chloroplast CO2. The photosynthesis data set that we collected for R. communis did not allow us to test these assumptions because θ was very close to 1.0; thus, the δc0 signal was completely washed out by the activity of carbonic anhydrase.
However, this was not the case for dark respiration, during which θ was approximately 0.80. The method of Gillon and Yakir (2000b) leads to mean δc0 values for the first and second dark respiration experiments of 55.2 and 43.7‰, respectively. These values can be compared to the mean δc0 values generated by the regression method of 30.8 and 14.3‰, respectively. Although the regression method makes no a priori assumptions about the controls on δc0, we caution against over-interpretation of these latter values for the following reason: the regression analysis, as summarized in Equation 6, assumes no variation in θ and δc0 among individual measurements in each experiment. The δa values varied among measurements according to how the leaf was modifying the δ18O of CO2 in the leaf chamber. Therefore, to the extent that δc0 is controlled by δa, δc0 could also have varied among individual measurements.
Nonetheless, the large variation between δc0 calculated as suggested by Gillon and Yakir (2000b) and the apparent δc0 values observed in the dark respiration experiments warrants some discussion. There are three possible sources for the oxygen in CO2 evolved in mitochondria during either dark respiration or photosynthesis: atmospheric O2, organic oxygen from respiratory substrates, and oxygen from leaf water. Atmospheric O2 has a δ18O near 23.5‰ (VSMOW scale), and discrimination against 18OO during respiration in plant tissues ranges from about 17‰ to 26‰ (Guy et al., 1992). We would therefore expect the δ18O of respiratory CO2 deriving its oxygen atoms from O2 to be in the range of 0‰ to 5‰. Assuming the O2 tank used in our experiments had a δ18O similar to atmospheric O2, this range of values would apply. Organic oxygen in phloem sap sugars of the R. communis plants that we studied had a mean δ18O of 27.5 ± 0.6‰ (mean ± 1 sd; n = 10). Generally, this oxygen pool is expected to have a δ18O enriched by 27‰ compared to δL at the time of photosynthesis (Cernusak et al., 2003). Oxygen atoms derived from water during respiratory reactions would also be expected to be enriched by 27‰ compared to the δ18O of the water source. The difference between the δ18O of CO2 derived from any of these three sources and that of CO2 diffusing into the leaf from the atmosphere, prior to equilibration with leaf water, would depend on δa and, in the case of organic oxygen and oxygen from water, δL. However, it seems likely that under most circumstances the effect of incomplete equilibration between CO2 evolved from mitochondria and leaf water would be to decrease δc0 below the value predicted by the formulation given by Gillon and Yakir (2000b). More experiments like those conducted by Yakir et al. (1994) would be helpful for resolving this issue.
Farquhar and Lloyd (1993) discussed the departure of δc from that predicted for equilibrium with δe during photosynthesis in terms of the ratio of the rate of carboxylation by Rubisco to the rate of CO2 hydration by carbonic anhydrase. This ratio was termed ρ. A simplified non-equilibrium equation for discrimination against C18OO during photosynthesis, neglecting the possible effects of photorespiration and day respiration, was presented as (Farquhar and Lloyd, 1993)
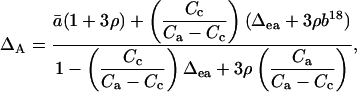 |
(32) |
where b18 is discrimination against C18OO by Rubisco. Using this equation, and assuming b18 = 0, we calculated a mean ρ value for our photosynthesis measurements of −0.002 ± 0.009 (mean ± 1 sd; n = 8); if the outlier in Figure 3 is excluded, the mean ρ value becomes 0.001 ± 0.006 (mean ± 1 sd; n = 7). These values can be compared to a mean ρ value calculated for Phaseolus vulgaris of 0.025 (Flanagan et al., 1994). Thus, the ρ values that we observed for R. communis were somewhat smaller than those observed previously for P. vulgaris. These values can be compared to a theoretical prediction for ρ of approximately 0.05 (Cowan, 1986).
In our calculations we have assumed that the δ18O of chloroplast water is equivalent to δe. One might expect chloroplast water to be slightly less enriched than δe due to the Péclet effect (Farquhar and Lloyd, 1993), which describes the interplay between advection of water toward the evaporative sites and diffusion of heavy isotopes away from the evaporative sites. We found that correlations between δc and δe were generally stronger than between δc and δL. This agrees with previous results (Flanagan et al., 1994), and suggests that δe is a more relevant parameter for predicting δ18O of CO2 diffusing out of leaves than δL.
Gillon and Yakir (2000a) suggested that the CO2 partial pressure at the chloroplast surface (Ccs) is a more appropriate parameter for predicting discrimination against C18OO during photosynthesis than that at the sites of carboxylation by Rubisco (Cc). They reconstructed Ccs by combining measurements of C18OO discrimination and carbonic anhydrase activity. We did not measure carbonic anhydrase activity directly, and so could not modify our calculations to take into account Ccs. In cases where the total resistance from the chloroplast to the atmosphere in the dark is dominated by the stomatal resistance, use of Ccs in place of Cc will likely not alter predictions of δR to a very large extent. However, if stomata are relatively open and (δc − δa) is large, such that the invasion term in Equation 31 is large, a variation between Cc and Ccs of as little as 2 μbar could have a significant effect on predicted δR. In such cases it may prove helpful to use Ccs in place of Cc, if possible.
Farquhar et al. (1993) found that a globally averaged leaf water δ18O of 4.4‰ satisfactorily balanced the global budget for δ18O of atmospheric CO2. In the most recent study of the global budget for δ18O of atmospheric O2, a globally averaged leaf water δ18O of between 6.1 and 6.8‰ was estimated (Hoffmann et al., 2004). Gillon and Yakir (2001) suggested that the globally averaged leaf water δ18O could be as much as 3‰ more than the estimate of Farquhar et al. (1993), in agreement with the requirement for balancing the Dole effect (global 18OO budget); the global C18OO budget could then be maintained by incomplete equilibration of chloroplast CO2 with chloroplast water (i.e. θ < 1). They estimated a globally averaged θ of 0.80. The results presented in this study provide an additional reason that the apparent leaf water signals required to balance the global C18OO and 18OO budgets should not be expected to resolve into a single value. The apparent leaf water signal relevant to the global δ18O budget for O2 is the average daytime leaf water δ18O, weighted by diurnal (daytime) variation in photosynthetic oxygen evolution rates. In contrast, the apparent leaf water signal relevant to the global δ18O budget for CO2 is the 24-h average leaf water δ18O, weighted by diel (day and night) variation in gtcCc/P. Thus, the apparent leaf water δ18O signals relevant to the global C18OO and 18OO budgets are fundamentally different.
CONCLUSION
We observed a very large variation in the δ18O of CO2 respired by leaves in the dark, with observed values ranging from 44‰ to as high as 324‰. We have shown that this large range of δR values can be satisfactorily explained by taking into account the flux of CO2 that enters the leaf, equilibrates with leaf water, and diffuses out of the leaf without affecting the net CO2 efflux. Incorporation of the correct expression for δ18O of leaf dark respiration into ecosystem and global scales models of C18OO dynamics could affect model outputs and their interpretation.
MATERIALS AND METHODS
Plant Material and Gas Exchange Measurements
Ricinus communis plants were grown from seeds in 10-L pots for 8 to 12 weeks in a temperature and humidity controlled glasshouse. Growth conditions were essentially the same as those described by Cernusak et al. (2003). Daytime temperature and humidity were 27°C ± 2°C and 40% ± 10%, respectively. Nighttime temperature was 20°C, with the same humidity as during the day. Measurements were made on fully expanded leaves of plants that were approximately 1 m tall. Projected areas of measured leaves ranged from approximately 400 to 800 cm2. The configuration of the gas exchange system was recently described (Cernusak et al., 2003). The through-flow rate of air in the leaf chamber was approximately 3 L min−1. Chamber air cycled continuously through a bypass drying loop to remove water vapor. The flow rate through the bypass drying loop was varied between 5 and 45 L min−1 to achieve different vapor pressures within the chamber, and therefore different values of ea/ei, and consequently of δe. Air entering the leaf chamber was generated by mixing 79% dry nitrogen with 21% dry oxygen using two mass flow controllers. Carbon dioxide was added to this air stream from a cylinder of 10% CO2 in air. Leaf temperature was measured with eight thermocouples arrayed across the underside of the leaf, and the average of these measurements used in gas-exchange and isotopic calculations. Gas-exchange calculations were performed according to the equations of Caemmerer and Farquhar (1981).
After gas exchange conditions in the leaf chamber stabilized for a time period judged long enough for leaf water to reach isotopic steady state, CO2 was cryogenically trapped from air exiting the chamber, as described previously (Evans et al., 1986; Caemmerer and Evans, 1991). Trapping continued until approximately 50 μmol of CO2 was obtained. The time period sufficient for leaf water to reach isotopic steady state was assumed to be three times the residence time of lamina leaf water (Förstel, 1978). The residence time of lamina leaf water was calculated as W/gtwi, where W is the lamina water concentration (mol m−2), gt is the total conductance of boundary layer plus stomata to water vapor (mol m−2 s−1), and wi is the mole fraction of water vapor in the leaf intercellular air spaces (mol mol−1). The term W was determined to be 6.3 ± 0.4 mol m−2 (mean ± 1 sd) from measurements of the difference between fresh weight and dry weight for one leaf from each of five plants. This mean value of W was assumed for all leaves in the experiment; gt and wi were calculated continuously for each leaf being measured. Time periods calculated in this way for leaf water to reach isotopic steady state after a step change in humidity ranged from approximately 0.5 to 3.5 h.
Three experiments were conducted, two in the dark and one in the light. In the first dark experiment, air entering the leaf chamber was free of CO2. All CO2 in the air exiting the chamber was therefore derived from the leaf. Measurements were conducted on one leaf from each of five plants. Each leaf was subject to two or three different chamber vapor pressures, and CO2 collected after gas exchange had stabilized for the requisite amount of time at each vapor pressure. Chamber air temperature was maintained at approximately 30°C. The second dark experiment was similar to the first, but differed in that CO2 was added to the air entering the chamber, such that the partial pressure within the chamber was approximately 350 μbar. The third experiment was in the light. Irradiance varied between 300 and 800 μmol PAR m−2 s−1, and chamber air temperature varied between 25°C and 30°C. The CO2 partial pressure within the chamber was approximately 350 μbar.
Isotope Measurements
The carbon and oxygen isotope composition of CO2 exiting the leaf chamber was determined on an Isoprime mass spectrometer (Micromass, Manchester, UK) operating in dual inlet mode. Repeated analyses of the same gas sample generally showed a precision of better than 0.1‰ (1 sd, n = 10) for δ13C and δ18O. The carbon and oxygen isotopic composition of the gas used as a reference for the dual inlet measurements was calibrated against standard gases supplied by the International Atomic Energy Agency (Vienna). Oxygen isotope ratios in this paper are presented relative to VSMOW; carbon isotope ratios are presented relative to the Vienna Pee Dee Belemnite standard (VPDB). The oxygen isotope composition of irrigation water fed to the plants was determined with an Isochrom mass spectrometer (Micromass) operating in continuous flow mode (Farquhar et al., 1997). The water samples were pyrolyzed in a custom-built furnace at 1,300°C prior to entering the mass spectrometer. Precision of analyses, based on repeated measurements of a laboratory standard water sample, was 0.3‰ (1 sd, n = 10). The δ18O of the irrigation water was found to be −7.2 ± 0.2‰ (mean ± 1 se; n = 6).
We assumed that the only source of N2O in the leaf chamber was the compressed air that the CO2 was mixed into, and that the concentration of N2O in this air was 300 nmol mol−1. The CO2 concentration was 10%, giving a ratio of N2O to CO2 of 3 × 10−6. This ratio could have been doubled during photosynthesis measurements, when the CO2 concentration exiting the chamber was as little as one-half that entering it, giving a ratio of 6 × 10−6. Using the empirical equations of Mook and van der Hoek (1983), this ratio of N2O to CO2 would result in measurement biases of 0.002‰ for both δ13C and δ18O. This bias was considered negligible, and no attempt was made to account for contamination of CO2 samples by N2O.
Acknowledgments
We thank Matthias Cuntz, Roger Gifford, Ricardo Marenco, and Francesco Ripullone for helpful comments on earlier versions of the manuscript.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.040758.
References
- Bottinga Y, Craig H (1969) Oxygen isotope fractionation between CO2 and water, and the isotopic composition of marine atmospheric CO2. Earth Planet Sci Lett 5: 285–295 [Google Scholar]
- Bowling DR, McDowell NG, Welker JM, Bond BJ, Law BE, Ehleringer JR (2003. a) Oxygen isotope content of CO2 in nocturnal ecosystem respiration. 1. Observations in forests along a precipitation transect in Oregon, USA. Global Biogeochem Cycles 17: 1120 [Google Scholar]
- Bowling DR, McDowell NG, Welker JM, Bond BJ, Law BE, Ehleringer JR (2003. b) Oxygen isotope content of CO2 in nocturnal ecosystem respiration. 2. Short-term dynamics of foliar and soil component fluxes in an old-growth ponderosa pine forest. Global Biogeochem Cycles 17: 1124 [Google Scholar]
- Brenninkmeijer C, Kraft P, Mook W (1983) Oxygen isotope fractionation between CO2 and H2O. Isot Geosci 1: 181–190 [Google Scholar]
- Caemmerer S, Evans JR (1991) Determination of the average partial pressure of CO2 in chloroplasts from leaves of several C3 plants. Aust J Plant Physiol 18: 287–305 [Google Scholar]
- Caemmerer S, Farquhar GD (1981) Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153: 376–387 [DOI] [PubMed] [Google Scholar]
- Cappa CD, Hendricks MB, DePaulo DJ, Cohen RC (2003) Isotopic fractionation of water during evaporation. J Geophys Res 108: 4525 [Google Scholar]
- Cernusak LA, Pate JS, Farquhar GD (2002) Diurnal variation in the stable isotope composition of water and dry matter in fruiting Lupinus angustifolius under field conditions. Plant Cell Environ 25: 893–907 [Google Scholar]
- Cernusak LA, Wong S-C, Farquhar GD (2003) Oxygen isotope composition of phloem sap in relation to leaf water in Ricinus communis. Funct Plant Biol 30: 1059–1070 [DOI] [PubMed] [Google Scholar]
- Ciais P, Denning AS, Tans PP, Berry JA, Randall DA, Collatz GJ, Sellers PJ, White JWC, Trolier M, Meijer HAJ, et al (1997) A three dimensional synthesis study of δ18O in atmospheric CO2. 1. Surface fluxes. J Geophys Res 102: 5857–5872 [Google Scholar]
- Cowan IR (1986) Economics of carbon fixation in higher plants. In TJ Givnish, ed, On the Economy of Plant Form and Function. Cambridge University Press, Cambridge, UK, pp 133–170
- Craig H, Gordon LI (1965) Deuterium and oxygen-18 variations in the ocean and the marine atmosphere. In E Tongiorgi, ed, Proceedings of a Conference on Stable Isotopes in Oceanographic Studies and Palaeotemperatures. Lischi and Figli, Pisa, Italy, pp 9–130
- Cuntz M, Ciais P, Hoffmann G, Allison CE, Francey R, Knorr W, Tans P, White JWC, Levin I (2003. a) A comprehensive global three-dimensional model of δ18O in atmospheric CO2: 2. Mapping the atmospheric signal. J Geophys Res 108: 4528 [Google Scholar]
- Cuntz M, Ciais P, Hoffmann G, Knorr W (2003. b) A comprehensive global three-dimensional model of δ18O in atmospheric CO2: 1. Validation of surface processes. J Geophys Res 108: 4527 [Google Scholar]
- Dongmann G, Nurnberg HW, Förstel H, Wagener K (1974) On the enrichment of H218O in the leaves of transpiring plants. Radiat Environ Biophys 11: 41–52 [DOI] [PubMed] [Google Scholar]
- Evans JR, Sharkey TD, Berry JA, Farquhar GD (1986) Carbon isotope discrimination measured concurrently with gas exchange to investigate CO2 diffusion in leaves of higher plants. Aust J Plant Physiol 13: 281–292 [Google Scholar]
- Evans JR, von Caemmerer S (1996) Carbon dioxide diffusion inside leaves. Plant Physiol 110: 339–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everson RG (1970) Carbonic anhydrase and CO2 fixation in isolated chloroplasts. Phytochemistry 9: 25–32 [Google Scholar]
- Farquhar GD, Gan KS (2003) On the progressive enrichment of the oxygen isotopic composition of water along leaves. Plant Cell Environ 26: 1579–1597 [PubMed] [Google Scholar]
- Farquhar GD, Henry BK, Styles JM (1997) A rapid on-line technique for determination of oxygen isotope composition of nitrogen-containing organic matter and water. Rapid Commun Mass Spectrom 11: 1554–1560 [Google Scholar]
- Farquhar GD, Hubick KT, Condon AG, Richards RA (1989) Carbon isotope discrimination and water-use efficiency. In PW Rundel, JR Ehleringer, KA Nagy, eds, Stable Isotopes in Ecological Research. Springer-Verlag, New York, pp 21–46
- Farquhar GD, Lloyd J (1993) Carbon and oxygen isotope effects in the exchange of carbon dioxide between terrestrial plants and the atmosphere. In JR Ehleringer, AE Hall, GD Farquhar, eds, Stable Isotopes and Plant Carbon-Water Relations. Academic Press, San Diego, pp 47–70
- Farquhar GD, Lloyd J, Taylor JA, Flanagan LB, Syvertsen JP, Hubick KT, Wong SC, Ehleringer JR (1993) Vegetation effects on the isotope composition of oxygen in atmospheric CO2. Nature 363: 439–443 [Google Scholar]
- Farquhar GD, O'Leary MH, Berry JA (1982) On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves. Aust J Plant Physiol 9: 121–137 [Google Scholar]
- Flanagan LB, Brooks JR, Varney GT, Ehleringer JR (1997) Discrimination against C18O16O during photosynthesis and the oxygen isotope ratio of respired CO2 in boreal forest ecosystems. Global Biogeochem Cycles 11: 83–98 [Google Scholar]
- Flanagan LB, Ehleringer JR (1991) Effects of mild water stress and diurnal changes in temperature and humidity on the stable oxygen and hydrogen isotopic composition of leaf water in Cornus stolonifera L. Plant Physiol 97: 298–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan LB, Ehleringer JR (1998) Ecosystem-atmosphere CO2 exchange: interpreting signals of change using stable isotope ratios. Trends Ecol Evol 13: 10–14 [DOI] [PubMed] [Google Scholar]
- Flanagan LB, Kubien DS, Ehleringer JR (1999) Spatial and temporal variation in the carbon and oxygen stable isotope ratio of respired CO2 in a boreal forest ecosystem. Tellus 51B: 367–384 [Google Scholar]
- Flanagan LB, Marshall JD, Ehleringer JR (1993) Photosynthetic gas exchange and the stable isotope composition of leaf water—comparison of a xylem-tapping mistletoe and its host. Plant Cell Environ 16: 623–631 [Google Scholar]
- Flanagan LB, Phillips SL, Ehleringer JR, Lloyd J, Farquhar GD (1994) Effect of changes in leaf water oxygen isotopic composition on discrimination against C18O16O during photosynthetic gas exchange. Aust J Plant Physiol 21: 221–234 [Google Scholar]
- Förstel H (1978) Contribution of oxygen isotope fractionation during the transpiration of plant leaves to the biogeochemical oxygen cycle. In WE Knumbein, ed, Proceedings of the 3rd International Symposium on Environmental Biogeochemistry and Geomicrobiology. Ann Arbor Press, Ann Arbor, MI, pp 811–824
- Förstel H, Hützen H (1983) 18O/16O ratio of water in a local ecosystem as a basis of climate record. In Palaeoclimates and Palaeowaters: A Collection of Environmental Isotope Studies. IAEA, Vienna, pp 67–81
- Francey RJ, Tans PP (1987) Latitudinal variation in oxygen-18 of atmospheric CO2. Nature 327: 495–497 [Google Scholar]
- Gillon JS, Yakir D (2000. a) Internal conductance to CO2 diffusion and C18OO discrimination in C3 leaves. Plant Physiol 123: 201–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillon JS, Yakir D (2000. b) Naturally low carbonic anhydrase activity in C4 and C3 plants limits discrimination against C18OO during photosynthesis. Plant Cell Environ 23: 903–915 [Google Scholar]
- Gillon JS, Yakir D (2001) Influence of carbonic anhydrase activity in terrestrial vegetation on the 18O content of atmospheric CO2. Science 291: 2584–2587 [DOI] [PubMed] [Google Scholar]
- Guy RD, Berry J, Fogel ML, Turpin DH, Weger HG (1992) Fractionation of the stable isotopes of oxygen during respiration by plants: the basis of a new technique to estimate partitioning to the alternative path. In H Lambers, LHW van der Plas, eds, Molecular, Biochemical, and Physiological Aspects of Plant Respiration. Academic Publishing, The Hague, The Netherlands, pp 443–453
- Harwood KG, Gillon JS, Griffiths H, Broadmeadow MSJ (1998) Diurnal variation of Δ13CO2, ΔC18O16O, and evaporative site enrichment of δH218O in Piper aduncum under field conditions in Trinidad. Plant Cell Environ 21: 269–283 [Google Scholar]
- Hoffmann G, Cuntz M, Weber C, Ciais P, Friedlingstein P, Heimann M, Jouzel J, Kaduk J, Maier-Reimer E, Seibt U, et al (2004) A model of the Earth's Dole effect. Global Biogeochem Cycles 18: 1008 [Google Scholar]
- Jacobson BS, Fong F, Heath RL (1975) Carbonic anhydrase of spinach. Plant Physiol 55: 468–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlivat L (1978) Molecular diffusivities of H216O, HD16O, and H218O in gases. J Chem Phys 69: 2864–2871 [Google Scholar]
- Miller JB, Yakir D, White JWC, Tans PP (1999) Measurement of 18O/16O in the soil-atmosphere CO2 flux. Global Biogeochem Cycles 13: 761–774 [Google Scholar]
- Mook W, Van der Hoek S (1983) The N2O correction in the carbon and oxygen isotopic analysis of atmospheric CO2. Isot Geosci 1: 237–242 [Google Scholar]
- Mortazavi B, Chanton JP (2002) Carbon isotopic discrimination and control of nighttime canopy δ18O-CO2 in a pine forest in the southeastern United States. Global Biogeochem Cycles 16: 1008 [Google Scholar]
- Snyder KA, Richards JH, Donovan LA (2003) Night-time conductance in C3 and C4 species: do plants lose water at night? J Exp Bot 54: 861–865 [DOI] [PubMed] [Google Scholar]
- Stern LA, Amundson R, Baisden WT (2001) Influence of soils on oxygen isotope ratio of atmospheric CO2. Global Biogeochem Cycles 15: 753–759 [Google Scholar]
- Tans PP (1998) Oxygen isotopic equilibrium between carbon dioxide and water in soils. Tellus 50B: 163–178 [Google Scholar]
- Tsuzuki M, Miyachi S, Edwards GE (1985) Localization of carbonic anhydrase in mesophyll cells of terrestrial C3 plants in relation to CO2 assimilation. Plant Cell Physiol 26: 881–891 [Google Scholar]
- Yakir D (1998) Oxygen-18 of leaf water: a crossroad for plant associated isotopic signals. In H Griffiths, ed, Stable Isotopes: Integration of Biological, Ecological, and Geochemical Processes. BIOS Scientific Publishers, Oxford, pp 147–188
- Yakir D, Berry JA, Giles L, Osmond CB (1994) Isotopic heterogeneity of water in transpiring leaves: identification of the component that controls the δ18O of atmospheric O2 and CO2. Plant Cell Environ 17: 73–80 [Google Scholar]
- Yakir D, Wang X-F (1996) Fluxes of CO2 and water between terrestrial vegetation and the atmosphere estimated from isotope measurements. Nature 380: 515–517 [Google Scholar]
- Zundel G, Miekeley W, Grisi BM, Förstel H (1978) The H218O enrichment in the leaf water of tropic trees: Comparison of species from the tropical rain forest and the semi-arid region in Brazil. Radiat Environ Biophys 15: 203–212 [DOI] [PubMed] [Google Scholar]