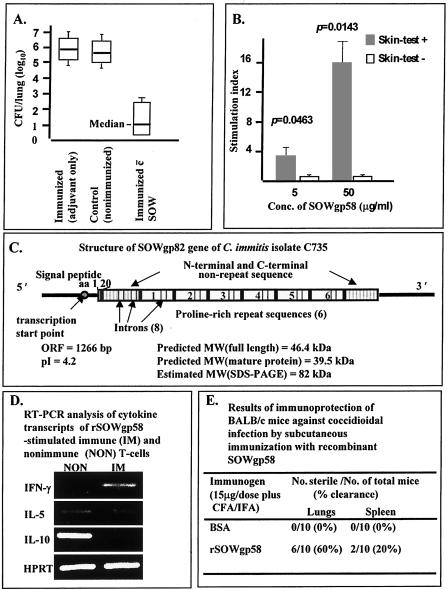
Abstract
Coccidioidomycosis is caused by the dimorphic fungi in the genus Coccidioides. These fungi live as mycelia in the soil of desert areas of the American Southwest, and when the infectious spores, the arthroconidia, are inhaled, they convert into the parasitic spherule/endospore phase. Most infections are mild, but these organisms are frank pathogens and can cause severe lethal disease in fully immunocompetent individuals. While there is increased risk of disseminated disease in certain racial groups and immunocompromised persons, the fact that there are hosts who contain the initial infection and exhibit long-term immunity to reinfection supports the hypothesis that a vaccine against these pathogens is feasible. Multiple studies have shown that protective immunity against primary disease is associated with T-helper 1 (Th-1)-associated immune responses. The single best vaccine in animal models, formalin-killed spherules (FKS), was tested in a human trial but was not found to be significantly protective. This result has prompted studies to better define immunodominant Coccidioides antigen with the thought that a subunit vaccine would be protective. These efforts have defined multiple candidates, but the single best individual immunogen is the protein termed antigen 2/proline-rich antigen (Ag2/PRA). Studies in multiple laboratories have shown that Ag2/PRA as both protein and genetic vaccines provides significant protection against mice challenged systemically with Coccidioides. Unfortunately, compared to the FKS vaccine, it is significantly less protective as measured by both assays of reduction in fungal CFU and assays of survival. The capacity of Ag2/PRA to induce only partial protection was emphasized when animals were challenged intranasally. Thus, there is a need to define new candidates to create a multivalent vaccine to increase the effectiveness of Ag2/PRA. Efforts of genomic screening using expression library immunization or bioinformatic approaches to identify new candidates have revealed at least two new protective proteins, expression library immunization antigen 1 (ELI-Ag1) and a β-1,3-glucanosyltransferase (GEL-1). In addition, previously discovered antigens such as Coccidioides-specific antigen (CSA) should be evaluated in assays of protection. While studies have yet to be completed with combinations of the current candidates, the hypothesis is that with increased numbers of candidates in a multivalent vaccine, there will be increased protection. As the genome sequences of the two Coccidioides strains which are under way are completed and annotated, the effort to find new candidates can increase to provide a complete genomic scan for immunodominant proteins. Thus, much progress has been made in the discovery of subunit vaccine candidates against Coccidioides and there are several candidates showing modest levels of protection, but for complete protection against pulmonary challenge we need to continue the search for additional candidates.
INTRODUCTION
Coccidioidomycosis (San Joaquin Valley fever) is a mycotic disease caused by Coccidioides immitis (68, 98, 125, 214) and the newly proposed phylogenetic species C. posadasii (116). The fungus propagates in soil in the semiarid regions of the southwestern United States, Mexico, and Central and South America, in a region corresponding to the Lower Sonoran Life Zone. The saprobic phase is characterized by mycelia that give rise to infectious arthroconidia, which become aerosolized when the soil is disturbed. Humans acquire the infection by inhalation of the arthroconidia, which differentiate into large, endosporulating spherules once they are in the host.
Coccidioides is a formidable pathogen, capable of causing progressive pulmonary and/or disseminated disease in previously healthy individuals. The disease presents a diverse clinical spectrum that includes inapparent infection, primary respiratory disease (usually with uncomplicated resolution), stabilized or progressive chronic pulmonary disease, and extrapulmonary dissemination which can be acute, chronic, or progressive. The degree of severity varies considerably within each category and depends, in part, on the dose of inhaled arthroconidia, the genetic predisposition of the host, and their immunologic status.
Between 25,000 and 100,000 new cases occur each year in the areas of endemic infection in the United States, but marked increases have occurred during sporadic epidemics (125, 248, 268). Among those who acquire primary infection, persons of African, Asian, and, to a lesser extent, Hispanic descent are more likely to develop disseminated disease than are Caucasians. This genetic predisposition, the geographically localized areas of endemicity, and the resistance of persons who experienced a benign, self-limiting primary infection document the feasibility of developing a vaccine against Coccidioides (16).
Coccidioidomycosis is considered to be a reemerging disease owing to the dramatic increase in the number of cases during the past decade. Major outbreaks occurred in southern California in 1977 and late 1991 through 1994 (105, 221). A new resurgence is indicated by the increase in coccidioidomycosis cases during the past year in Arizona (50). These outbreaks may be linked to climatic conditions, and the 1994 cases occurred after heavy rains, when the fungus propagated in the soil, followed by hot, dry, and windy periods that resulted in the aerosolization of mycelium-derived arthroconidia. The resulting high morbidity and mortality associated with these outbreaks prompted community and health-related organizations to seek funding for intensifying efforts to develop a vaccine for coccidioidomycosis. Financial support from the California HealthCare Foundation, the State of California Department of Health Services, and the Valley Fever Research Foundation led to a coordinated research program involving investigators located in California (Demosthenes Pappagianis and Theo Kirkland), Arizona (John Galgiani), Texas (Rebecca Cox), and Ohio (Garry Cole). New and fundamentally important discoveries have emerged from these research studies, and it is reasonable to predict that a vaccine, composed of multiple immunogens, will be entered into phase I and II clinical trials within the near future.
It is the intent of this review to focus on progress in vaccine development and host responses that are crucial for protective immunity.
HISTORICAL PERSPECTIVES
Coccidioidomycosis has been a recognized infection since 1892 (235, 303). The first reported case was in an Argentinian soldier who had exhibited skin lesions for 4 years; the causative organism was thought to be a protozoan. The first two cases in the United States heralded the protean manifestations of this infection, with marked differences in clinical presentation between the two patients (245). The first patient presented with a slowly progressing disease, which lead to his death approximately 9 years after the first appearance of symptoms. In contrast, the second patient presented with a rapidly progressing disease leading to death within approximately 4 months of the onset of symptoms. The species name of Coccidioides was proposed for the infectious agent, which was identified as the same protozoan described by Posadas and Wernicke. Because of the differences in clinical presentation and lesion development, it was proposed that each of these initial U.S. cases was initiated by different species, and it was proposed that the species name of immitis (meaning “not mild”) be used for the causative organism of the first case and pyogenes be used for the causative organism for the second case. The fungal nature of the organism was delineated four years later by Ophuls and Moffitt (209), who discovered that cultures of tissues always yielded a mold and that when the mold was injected into the ear vein of a rabbit, the rabbit developed “typical tubercle-like” nodules in the lungs, spleen, and kidneys. Microscopic examination of the nodules revealed protozoan bodies and no mycelium. They further showed the organism was dimorphic, undergoing a change from a mold to the “protozoan” phase in tissue and, conversely, sprouting hyphae when the tissue was examined in a coverslip preparation.
Until 1929, disseminated severe coccidioidomycosis, termed coccidioidal granuloma, was the only recognized form of the disease. However, a laboratory accident provided the first insight into milder forms of infection. Harold Chope, a 26-year-old Stanford University medical student who was doing research on Coccidioides in the laboratory of Ernest Dickson, accidentally opened a petri dish containing the mold form of Coccidioides. Chope became ill within 9 days of the incident, with an acute pneumonia, with pleuritic pains, fever, cough, hemoptysis, and a 15-lb weight loss over an 8-day period (114). Four weeks later, erythematous nodules erupted on his shins and endosporulating spherules were observed in the sputum. A diagnosis of coccidioidal granuloma was made, and the prognosis was considered to be grave, but Chope soon recovered completely. In 1937, Dickson presented Chope's case and four additional cases of this newly recognized benign form of this disease to the annual meeting of the California Medical Association. All five cases were characterized by acute pulmonary symptoms and, with one exception, erythema nodosum. Dickson's paper, published in California and Western Medicine (93), was entitled “Valley Fever” of the San Joaquin Valley and fungus Coccidioides, and in it he stated that the cases ‘prove conclusively that fungus Coccidioides is sometimes the cause of a symptom complex of acute illness identical with what has been known locally in the San Joaquin Valley as “Valley Fever.” ' Dickson proposed the term “coccidioidomycosis” to include all forms of infection by Coccidioides.
BIOLOGY OF COCCIDIOIDES
Ecology
Coccidioides is a haploid ascomycete classified in the family Onygenaceae (order Onygenales), along with the human respiratory pathogens Histoplasma. Blastomyces, and Paracoccidioides (84, 211, 269). The fungus is dimorphic, having a saprobic phase characterized by mycelia that produce enterothallic arthroconidia and a parasitic phase characterized by endosporulating spherules. The cytologic and ultrastructural details of the morphogenetic conversion have been described by several investigators (58,148, 163, 286). The arthroconidia, each with at least two nuclei, are derived by disarticulation of the septate hyphae. This process involves sequential and coordinated events: arrest of apical growth, progressive septation of the hyphae, condensation of the cytoplasm in certain hyphal compartments, autolysis of adjacent cells, and synthesis of new inner wall layer. Depending on the strain, arthroconidia are typically barrel-shaped, measuring 2.5 to 4 μm in width and 3 to 6 μm in length, and thus are small enough to reach the alveoli of the lungs when inhaled.
Early conversion of the arthroconidia into spherule-phase cells begins with isotropic growth characterized by a rounding up and swelling of the cells followed by synchronous nuclear divisions and segmentation, which is initiated by synchronized, centripetal growth of the spherule wall at multiple points (163). The central portion of the young spherule is occupied by a vacuole. Progressive compartmentalization of the cytoplasm that surrounds the vacuole gives rise to uninucleate compartments which round up and differentiate into endospores. The mature spherule measures 30 to 60 μm and can contain 200 to 300 endospores. At maturity, the spherule ruptures, releasing the endospores, which typically measure 2 to 4 μm in diameter. Each of these first-generation spherules is capable of developing into a mature, endosporulating spherule, thereby repeating the parasitic phase cycle. Thus, at any given point in time, the infected host is exposed to immature, mature, and rupturing spherules and newly released endospores, which differ quantitatively, if not qualitatively, in their cell wall and cytoplasmic composition.
An understanding of the regulatory events that underlie the induction of morphogenetic conversion in Coccidioides is rudimentary at best. The development of a defined liquid medium by Converse (59-61) enabled studies to delineate the absolute requirements for spherule induction and maintenance in vitro (29, 59, 60, 63). Increased temperature (between 34 and 41°C) and CO2 concentration (10 to 20%) induce spherulation. The addition of a surface-active agent such as Tamol N also stimulates spherulation. At 41°C, 100% of arthroconidia convert into spherules, whereas at lower temperatures under the same conditions, the arthroconidia give rise to hyphae. The differentiation of arthroconidia into endosporulating spherules in vitro appears to be identical to that observed in vivo (100) and, in both environments, typically takes between 72 and 96 h.
Phylogenetic Species
Until recently, C. immitis was the sole etiologic agent of coccidioidomycosis. Phylogenetic analyses using single-nucleotide polymorphisms, genes, and microsatellites have showed the existence of two genetically different C. immitis clades, California and non-California (116). The cumulative results have led to the proposal by Fisher et al. for a new species designation of C. immitis for the California clade and C. posadasii, in honor of Alejandro Posadas, who described the first case of coccidioidomycosis, for the non-California clade. Recognition of two rather than a single species of Coccidioides could have an impact on future studies regarding vaccine preparation and testing, as well as clinical, epidemiologic, and genetic studies of coccidioidomycosis. For this reason, this section will present details of the studies that led to the proposed species.
Molecular-genetic analyses of C. immitis strains began with the use of restriction fragment length polymorphism to analyze and compare the genomes of 14 isolates from California and 1 isolate from Venezuela. In this study, Zimmermann et al. (326) demonstrated that C. immitis contains at least two subgroups. Of the 14 isolates, 2 isolates, including 1 of the standard laboratory strains, Silveira, were placed in group I and the rest were placed in group II. There were no discernible differences in the group I and II isolates in terms of the geographic region where they were isolated or whether they were isolated from a patient with pulmonary or disseminated disease. The investigators noted, however, that group II isolates could be subdivided into additional subgroups. Subsequently, Burt et al. (32-34) identified polymorphic loci and examined 12 of these polymorphic loci in clinical isolates collected from 25 patients in Bakersfield, Calif., 25 patients in Tucson, Ariz., and 20 patients in San Antonio, Tex. Substantial genetic differentiation was observed between the isolates from California and those from Arizona or Texas, with little to no gene flow. There was also a significantly reduced gene flow between isolates from Arizona and Texas, but not as much as was observed between the isolates from these two states and those from California. Koufopanou et al. (176, 177), in a comparison of gene genealogies from 350- to 650-bp fragments of five nuclear genes, separated 17 isolates of Coccidioides that had been collected from California, Texas, Arizona, Mexico, and Argentina into two strains: one from California and the other from all other geographic locations. Strain Silveira was a notable exception in that it did not share polymorphisms with the California species, which corroborated the findings of Zimmermann et al. (326). Exceptions were also observed, since isolates from three patients from California showed the non- California genotype.
Fisher et al. (117, 119) identified seven microsatellite-containing loci for C. immitis, four of which were originally isolated from the California strains and three of which were isolated from the non-California strains. Analyses of 20 clinical isolates from the southwestern United States revealed that six of the seven microsatellites showed nonoverlapping allele distributions between the California and non-California strains. In a subsequent study, these investigators (118) examined 161 isolates from the areas of endemic infection in California, Arizona, Texas, Mexico, and South America. Using nine microsatellite-containing loci, the investigators confirmed that the isolates comprised two primary subgroups, i.e., the California and non-California phylogenetic strains. Subclades were observed with divergence between isolates from Central Valley, Calif., and those from the rest of southern California, which is delineated by the Tehachapi mountain range. Likewise, there was phylogeographic divergence within the non-California clade, with the Texas and South American isolates grouping into a subclade.
Perhaps the strongest argument for the two species is the lack of evidence of genetic exchange between C. immitis and C. posadasii. It appears, however, that the vast majority of studies performed to date have been done with the same isolates of C. immitis, and at the time of this writing, only 167 strains have been examined. There appears to be considerable overlap in the number of non-California stains (C. posadasii) in the California group (C. immitis) and, conversely, the number of California strains (C. immitis) in the non-California group (C. posadasii) (116). These collective results prompt the question whether posadasii should be used to designate a variety of C. immitis as opposed to a new species of Coccidioides.
As an argument against separation of the species, Pappagianis (213, 220) has emphasized that immunization with one C. immitis strain provides protection against respiratory challenge with a phenotypically or genotypically different C. immitis strain. Although differences in the virulence of C. immitis strains have been documented, those differences do not correlate with differences in their immunogenicity. For example, immunization of mice with viable arthroconidia of strain 46 protected them from intranasal (i.n.) challenge with strain Silveira. Strains 46 and Silveira differ when analyzed by restriction fragment length polymorphism (326) and by single-strain conformational polymorphism analysis. Strain 46 is classified as a California strain and strain Silveira is classified as a non-California strain by genotypic markers (116). Huppert et al. (146) showed that vaccination of mice with killed spherules of strain Silveira protected against i.n. challenge with strain 46, strain Woodville, and five other strains of C. immitis that were considered to be phenotypically atypical. Further support that immunizing strains of C. immitis protect against challenge with other strains is evinced by the solid immunity of persons who recovered from a primary benign coccidioidal pneumonia to exogenous reinfection. That is, such persons are likely to have been exposed to other strains of C. immitis as residents in regions of endemic infection.
While the differences in genotype between C. immitis and C. posadasii are strong, differences in phenotype are not remarkable and also argue against separation of the species. Although C. posadasii was reported to grow more slowly on yeast extract-glucose agar medium with high salt concentration, there was considerable overlap between the C. posadasii and C. immitis isolates, and hence the phenotype could not be used to distinguish the two (116). Minor differences in amino acid sequence of proteins of C. posadasii and C. immitis were reported by Koufopanou et al. (177) and Peng et al. (229), but as yet no differences have been detected in antigenicity, virulence, or morphology of the two groups. To begin to answer this latter question, we collected 12 isolates of Coccidioides (7 C. posadasii strains and 5 C. immitis strains) to compare them in an animal model of virulence (D. M. Magee and R. A. Cox, unpublished data). Since these isolates had been collected from various investigators over time, they were passed through mice before use in the present experiment. Groups of 10 BALB/c mice were infected intranasally with each strain and then monitored for survival over 45 days (Table 1). The analysis of this initial experiment shows that there are three patterns of survival after challenge, showing high, intermediate, and low virulence. Strain Silveira, our standard laboratory isolate, which is included with the proposed C. posadasii designation, exhibited intermediate virulence, and mice challenged with this isolate began to succumb on day 13, with a median 50% survival on day 14. In contrast, strain RMSCC 1040 (from the Roche Molecular Services Culture Collection [RMSCC]), also in the C. posadasii designation, exhibited increased virulence, and mice challenged with this strain began to succumb on day 9, with a median 50% survival on day 10.5. Comparison of the survival curves revealed that survival was significantly decreased compared to that of mice infected with strain Silveira (P < 0.002, Mantel-Haenszel log-rank survival analysis). On the other extreme, strain RMSCC 1038, also in the C. posadasii designation, exhibited low virulence, with 100% survival for over 45 days after challenge. The animals were sacrificed at that time, and all animals contained C. immitis in their lungs; therefore, the increased survival is not due to failure of infection. Thus, while we have Coccidioides strains with different levels of virulence in mice, variation in virulence between the proposed species designations was not demonstrable.
TABLE 1.
Virulence comparison of various Coccidioides strains
| Strain | Proposed species | Challenge dose (no. of arthroconidia) | Median time to death of 50% of mice (days) |
|---|---|---|---|
| Silveiraa | C. posadasii | 27 | 14 |
| 634b | C. posadasii | 29 | 14 |
| 735b | C. posadasii | 25 | 15 |
| RMSCC 1037c | C. posadasii | 34 | 10.5 |
| RMSCC 1038c | C. posadasii | 35 | >45 |
| RMSCC 1040c | C. posadasii | 29 | 11.5 |
| RMSCC 3700c | C. posadasii | 35 | >45 |
| RSd | C. immitis | 30 | 15 |
| RMSCC 2008c | C. immitis | 24 | 14 |
| RMSCC 2010c | C. immitis | 31 | 14.5 |
| RMSCC 2012c | C. immitis | 26 | 15.5 |
| RMSCC 2013c | C. immitis | 19 | 16 |
Current laboratory strain.
Kindly provided by Garry Cole.
Kindly provided by John Taylor through Gina Koenig and the RMSCC.
Kindly provided by Theo Kirkland.
Classification of Coccidioides as a Select Agent
Coccidioides was classified as a select agent of bioterrorism in the Final Rule on Additional Requirements for Facilities Transferring or Receiving Select Agents in response to the U.S. Antiterrorism and Effective Death Penalty Act of 1996 (94). This has been further refined; in the Federal Register on 23 August 2002, the Department of Human and Health Services requested comments regarding whether changes should be made to the list of select agents. A recently issued Interim Final Rule, effective as of 7 February 2003, includes C. immitis and C. posadasii as select agents (51, 90). The Select Agent Rule was enacted to “establish a system of safeguards to be followed when specific agents are transported; collect and provide information concerning the location where certain potentially hazardous agents are transferred; track the acquisition and transfer of these specific agents; and establish a process for alerting appropriate authorities if an unauthorized attempt is made to acquire these agents.”
Fierer and Kirkland have questioned the rationale and justification for classifying the fungus as a select agent (110). These investigators argue that (i) it would be relatively easy to isolate Coccidioides from the soil (as would also be the case for Bacillus anthracis); (ii) most primary infections are benign; (iii) the incubation period (usually 10 to 16 days) is too long to disable a military unit; (iv) no vaccine is available to protect those who are using Coccidioides as a biothreat agent; and (v) Coccidioides is not contagious. Although it is difficult to perceive Coccidioides itself as a biothreat, unless a very high inoculum of the fungus could be delivered, one could visualize transfected Coccidioides arthroconidia as vectors for delivering biothreat agent toxins, in which case the availability of a vaccine becomes essential.
Critical Comments
The separation of the dimorphic Coccidioides genera into multiple species is somewhat controversial, and to date, there has not been any debate in the literature. While the genetic evidence is compelling, the biological relevance of the genetic variability is not convincing. Clearly, much work needs to be done to determine if there are differences in antigenic variation and virulence between C. immitis and the proposed C. posadasii species. Until the biological evidence is firmly established, we propose that a variety designation, be used with C. immitis var. immitis and C. immitis var. posadasii. For the rest of this review, we limit the nomenclature of this organism to the genus level.
The inclusion of Coccidioides as a potential bioweapon, combined with the failure of the National Institutes of Health to include it on the Category A/B/C list for prioritization, may impede the research on this organism. The result is that this organism falls under the strict federal regulations but does not warrant targeted research funding. The cumulative effect could be an overall reduction in the effort to understand the biology and pathogenesis of Coccidioides.
CLINICAL MANIFESTATIONS
Epidemiology
The distribution of Coccidioides in nature has been established on the basis of skin testing with coccidioidin or spherulin, recognition of clinical cases, and ecologic investigations during epidemics. These results reveal that coccidioidomycosis is endemic in the Western Hemisphere, with the areas of highest endemicity being in southwestern United States and the bordering regions of northern Mexico and with regions of lower endemicity in Central and South America (216, 217). More recently, areas of endemicity have been documented in the Brazilian states of Piauí, Bahia, Ceará, and Maranhão (297). In the United States, areas of endemicity include the south central portion of Arizona, particularly Tucson and Phoenix, the southern one-third of California, notably the San Joaquin Valley, southwestern Texas, and New Mexico. The fungus is also found in scattered foci in southern Nevada and Utah (179). The regions of endemicity for coccidioidomycosis correspond to the Lower Sonoran Life Zone, and the distribution of the fungus in the soil is notoriously spotty (194). This zone is characterized by plants such as the creosote bush, mesquites, palo verde, and yuccas; a semiarid climate characterized by hot summers and few winter freezes; and a soil that is highly alkaline. Cumulative evidence has documented an association between climatic conditions and outbreaks of coccidioidomycosis (170, 171, 192, 216, 217, 278). The fungus propagates as mycelia in moist soils, and when the soil dries, the arthroconidia form and become airborne as a result of the action of wind or some other disturbance of the soil. The highest incidence of the disease occurs in late summer and early fall, when the soil is the driest.
Coccidioidomycosis is considered to be a reemerging disease because of the dramatic increase in the number of cases during the early part of the past decade (47, 52, 105, 157, 207, 219, 260, 282). Between 1991 and 1994, there was a notable increase of new cases in California, in particular in Kern and Tulare counties in the southern part of the San Joaquin Valley (219). During that time, there were 8,435 reported cases in Kern County, representing a sevenfold increase. This epidemic was attributable to an unusually rainy spring in March 1991 and February-March 1992 following a 5-year drought, the migration of persons previously unexposed to Coccidioides into areas of endemicity in southern California, and new construction projects. In a review of medical records in Kern County by the Centers for Disease Control as Prevention, medical bills totaled $45 million for hospitalization and outpatient care (283). A similar increase in coccidioidomycosis incidence occurred in Arizona between 1990 and 1995, from 7.0 per 100,000 population in 1990 to 14.9 per 100,000 in 1995 (10). The increase was thought to be attributable to the influx of older persons (65 years or older), who were nonimmune upon arrival. There was no apparent correlation between the increased number of cases and climatic conditions. After a period of quiescence, the number of cases is increasing in Arizona, which may herald the onset of a new epidemic (50).
More frequent travel, both domestic and international, to areas of endemicity has contributed significantly to this increase (37, 46, 48, 49, 53, 92, 212, 300). In 2000, nearly 28 million travelers visited Arizona, and of these, nearly 1 million were from abroad. Even greater numbers of travelers visit California and other areas of endemicity. In 2001, more than 300 persons from 30 countries participated in the World Championship of Model Airplane Flying in Lost Hills, Calif., an area where the fungus is highly endemic (46). Cases of coccidioidomycosis in participants who returned to Australia, Finland, New Zealand, and the United Kingdom were reported. Between 1992 and 1997, New York State had a total of 161 cases of coccidioidomycosis, all of which occurred in patients who had traveled to a region of endemicity, mostly in the southwestern United States (53). The Cleveland Clinic had 23 cases between 1980 and 1998 among patients who had traveled to an area where C. immitis was endemic (92). Travelers from the United States to areas of endemicity in Central and South America have also acquired coccidioidal infection. In 1996, members of a church congregation from Washington State traveled to Tecate, Mexico, to assist with construction projects (37); 21 (17%) of the members were diagnosed with coccidioidomycosis following their return. A similar incident occurred with church members from Pennsylvania, who acquired primary infection after traveling to Hermosillo, Mexico, to assist in a church construction project (48).
Categories of Disease
Primary pulmonary infection.
Since the early epidemiological studies by Smith et al. (274, 277, 279), it has become almost axiomatic that fully 60% of primary infections with Coccidioides are asymptomatic, being evident only by conversion of skin test reactivity. Notable exceptions have occurred during outbreaks during archeological excavations, construction projects, and military exercises (83, 180, 260, 282, 302, 307), where symptomatic infections have been documented in 90% or more of persons. This increased incidence of symptomatic infection following primary exposure to arthroconidia is probably attributable to exposure to an unusually high dose of arthroconidia (37). Of the remaining 40% of cases, the majority of patients exhibit only mild flu-like symptoms with an incubation period of 10 to 16 days (which can range from 7 to 20 days). The most common symptoms include cough, fever, chest pain, headache, fatigue, chills, malaise, and anorexia. Chest radiographs typically show pulmonary infiltrates, which may be single or multiple and are most often hilar or basal in location. Hilar adenopathy and pleural effusion may also be present. A diffuse erythematous rash termed toxic erythema occurs in 10 to 30% of individuals, usually within the first few days of clinical illness, and usually disappears shortly thereafter. This rash appears to be nonspecific, being thought to be associated with an acute febrile illness, and it usually covers the trunk and extremities.
Cutaneous infection.
Coccidioidomycosis can be acquired via a percutaneous route. Most of these occur in laboratory workers as a result of a hypodermic injection of Coccidioides (99, 310, 312). Primary cutaneous coccidioidomycosis is characterized by a painful suppurative lesion at the site of inoculation, often with regional lymphadenopathy. Of the 18 cases reported as of 1977, all but 2 have remained localized (41).
Valley fever.
Approximately 5% of all primary infections develop what is termed as the Valley fever complex, usually coincident with the development of delayed-type hypersensitivity reactions (99,114, 275). Erythema nodosum and erythema multiforme comprise the principal manifestations of the valley fever complex and may be accompanied by arthralgias (desert rheumatism) and a mild conjunctivitis. These are considered to be specific cutaneous lesions of acute coccidioidomycosis, in contrast to the nonspecific toxic erythema noted above, and are temporally associated with the acquisition of delayed-type hypersensitivity to Coccioides. Of the two syndromes, erythema nodosum is the more extensively studied. Initially, the lesions appear as bright reddish nodules, but within days they become livid red or purplish, and on healing they appear as bruises. They are commonly limited to the lower extremities. Erythema nodosum occurs most often in Caucasian females and has long been regarded to denote a good prognosis, although exceptions have been documented (99, 122, 157). Curiously, the predisposition of females to developing erythema nodosum is not manifest before puberty. Arthritis involving the joints, most commonly the ankle and knee, develops in approximately one-third of persons with erythema nodosum and/or erythema multiforme. These clinical manifestations of primary coccidioidomycosis are thought to be attributable to a hypersensitivity to the fungus, a concept that is supported by the hyperreactivity of the patient to skin testing with coccidioidin. Fungal cells are not present in the lesions of erythema nodosum or multiforme, nor have they been demonstrated in coccidioidal arthritis or conjunctivitis; as yet, the underlying basis of the valley fever complex has not been determined. Erythema nodosum in other diseases, notably tuberculosis, leprosy, sarcoidosis, and autoimmune disorders, is considered to be a hypersensitivity response, and in many cases, circulating immune complexes, C3, and immunoglobulin G (IgG) are demonstrable in the walls of venules (242). Although circulating immune complexes, composed of anti-Coccidioides IgG and coccidioidal antigen(s), have been demonstrable in the sera of patients with coccidioidomycosis (81, 317), studies have not been done to assess immune complexes in persons with valley fever complex.
Pulmonary coccidioidomycosis.
Approximately 5% of persons with primary coccidioidomycosis develop persistent pulmonary coccidioidomycosis, manifested by chronic progressive pneumonia, miliary disease, pulmonary nodules, or pulmonary cavitation (99, 311, 313, 314). Pulmonary nodules are usually benign but can become cavitary. A classic radiologic finding is the presence of a “thin-walled cavity,” which typically fails to show a surrounding tissue reaction. Although the latter is not pathognomonic, it is strongly suggestive of coccidioidomycosis (114). Most patients have only a single cavity, whereas in others the cavities are multiple or multilocular (276). In a study of 211 cases, Hyde (149) reported that half of cavities eventually close spontaneously, requiring neither surgery nor chemotherapy. Possible complications of cavitation include hemorrhage, secondary infection, progressive increase in size, and, if located peripherally, bronchopleural fistulae. A few patients develop chronic progressive pulmonary involvement, with symptoms of cough, weight loss, fever, hemoptysis, dyspnea, and chest pain that may persist for years. Radiographic results include inflammatory infiltrates, biapical fibronodular lesions, and multiple cavities.
Peripheral blood eosinophilia has been found in many patients with primary coccidioidomycosis and patients with disseminated disease (106, 114, 135, 259, 306). In one study, 66 of 75 patients with acute symptomatic pulmonary coccidioidomycosis showed eosinophil counts ranging from 3 to 26% of the total peripheral blood count (135). However, one patient was observed to have a peripheral blood eosinophilia as high as 89% (306). Peak eosinophilia generally occurs between the second and third weeks of clinical illness. Schermoly and Hinthorn (259) reported a patient with both 48% eosinophilia in his peripheral blood and 91% eosinophilia in his cerebrospinal fluid. The investigators further noted that a bone marrow biopsy specimen showed a marked proliferation of eosinophils. The patient was treated with amphotericin B, and after 2 weeks of therapy her eosinophil counts were normal. The basis for the increased eosinophilia is not known, but it does not appear to be associated with erythema multiforme or erythema nodosum.
Disseminated coccidioidomycosis.
The early epidemiological studies by Smith et al. (277) established that approximately 1% of patients with primary coccidioidomycosis developed disseminated disease. This incidence has been higher in more recent studies. In an epidemiologic investigation of the outbreak that occurred in Ventura County following the earthquake in Northridge, Calif., in 1994, Schneider et al. (260) reported that 3.7% of patients developed disseminated disease. Pappagianis and Einstein (221) reported a 4.2% incidence of dissemination as a result of the 1977 dust storm in California, and Pappagianis reported a 5.7% rate in military personnel and their families at Lemore U.S. Naval Air Station between 1961 and 1977 (216). Similar findings were observed in the epidemic of coccidioidomycosis in the San Joaquin Valley between September 1991 and January 1994 (157).
Dissemination, when it occurs, is usually an early event and may occur in the absence of any clinical or radiographic evidence of previous pulmonary infection (99, 114, 122), although exceptions have been noted (252). The process may be acute, subacute, or chronic. Extrapulmonary spread may consist of a single lesion in the skin and subcutaneous tissues, bone, meninges, lymph nodes, spleen, liver, kidneys, pleura, or virtually any part of the body, with the general exception of the gastrointestinal tract (122). If only a single lesion develops, unless it is in the meninges, prognosis is generally favorable. If dissemination is multifocal, the overall mortality rate is greater than 50%.
Lesions in the skin and subcutaneous tissues occur in more than 65% of cases of disseminated disease and may present as small papular nodules, ulcerated nodules, or verrucous granuloma. When bone is involved, complicated sinuses may form communications between the bone and the adjacent soft tissue, leading to a draining sinus through the skin. Sinus tracts also originate in subcutaneous tissues and the viscera. Meningitis occurs in 30 to 50% of cases of disseminated disease, and in some patients this is the only site of extrapulmonary disease. In the absence of treatment, the disease is invariably fatal, with death usually occurring within 2 years of primary infection. Acute miliary dissemination, in which seeding of the fungus is thought to occur early after primary infection, is also almost invariably fatal, with death occurring within 3 to 4 months.
Risk Factors for Severe, Disseminated Coccidioidomycosis
Genetically determined susceptibility.
In no other mycosis is the racial predisposition toward developing severe, disseminated disease more conclusive. Gifford et al. (134) were among the first to document the increased susceptibility of Filipinos, African Americans, and Mexican Americans to developing disseminated coccidioidomycosis. On the basis of the number of cases occurring in Kern County, Calif., between 1901 and 1936, Filipinos were 176 times more likely to develop disseminated disease than were Caucasians; African Americans and Mexican Americans were, respectively, 14 and 3 times more likely to develop dissemination than were Caucasians. Sievers (267) evaluated risk factors in the American Indian population residing in the Phoenix Area of the Indian Health Service. During a 16-year investigation period from 1959 through 1974, both American Indians and Mexicans were three times more likely to develop disseminated coccidioidomycosis than were Caucasians. The mortality rate was also increased, by fivefold, in American Indians and Mexicans compared to Caucasians. Some investigators raised the argument that these differences could be ascribed to occupational exposure or socioeconomic conditions (144, 265). The results of more recent outbreaks, however, wherein exposure was less likely to be biased by occupation, have borne out a racial predisposition to dissemination (108, 217, 225). In analyses of cases occurring after the California dust storm in 1977, Pappagianis and Einstein (221) reported that 54% of African American patients and 38% of Asians had disseminated disease, compared to only 11.2% of whites. A similar increase in incidence in African American and Asians was noted in studies of patients treated at the Naval Hospital in Lemoore, Calif., following the same dust storm (308). Rosenstein et al. (248) conducted a population-based study for coccidioidomycosis in Kern County, Calif., for the period from January 1995 through December 1996. The patient population consisted of 380 subjects, divided into 270 persons with mild primary coccidioidomycosis (designated the case control group), 77 patients with severe pulmonary coccidioidomycosis as judged by radiologic findings and hospitalization, and 38 patients with disseminated coccidioidomycosis. The percentage of patients with disseminated disease was increased in African Americans but not Asian or Hispanics. No association was detected between severe pulmonary or disseminated disease and occupation or outdoor activities.
Although the genetic basis for this increased susceptibility remains elusive, investigators have conducted studies to assess genetic polymorphisms that may control or be associated with the predisposition of certain ethnic populations to Coccidoides. Human leukocyte antigen (HLA) molecules are highly polymorphic and present antigenic peptides to α/β T lymphocytes. Scheer et al. reported an increased phenotype frequency of the HLA-A9 and -B9 antigens in patients with disseminated coccidioidomycosis (M. Scheer, G. Opelz, P. Tarasaki, and W. Hewitt; Program Abstr. 13th Intersci Conf. Antimicrob. Agents Chemother., 1973). Persons with the ABO blood group B phenotype have also been reported to be at risk for developing disseminated disease (91). These findings are consistent with, and may merely reflect, the increased phenotype frequencies of the HLA-A9 and blood group B in persons of Filipino and Black descent (2, 204, 228).
In a case-control study of persons from Kern County, Louie et al. (191) compared HLA class II alleles and haplotypes and ABO phenotypes in mild versus severe (disseminated) coccidioidomycosis. No differences were observed in the ABO blood types for the Caucasians or African Americans with regard to whether their disease was mild. Among Hispanics, A and B phenotypes were significantly more frequent in patients with disseminated disease than those with mild, uncomplicated pulmonary disease. The investigators reported that the HLA class II DRB1*1301 allele marks a predisposition to severe disseminated disease in all patients, regardless of their ethnic or racial background. It bears comment that the incidence this allele was increased in Caucasian, Hispanic, and African American patients compared with the ethnicity-matched controls. There was not, however, an increase in the incidence of this allele in patients with disseminated disease versus those with mild pulmonary disease within any of the ethnic groups, as would be expected if the DRB1*1301 allele were truly associated with an increased risk for dissemination. Identification of host genetic variants that predispose persons to severe disseminated coccidioidomycosis would be of great value in vaccine development and should be pursued, particularly in light of the growing availability of intragenic single-nucleotide polymorphisms for mapping human genome sequence variations (250).
While it is assumed that the increased susceptibility of African Americans, Asians, and Hispanics has an immunologenetic basis, studies have not yet documented that supposition. If an immunologic basis exists, it does not appear to reside in an inherent inability of these persons to mount a cellular immune response to Coccidioides. Gifford and Catanzaro (133), in a comparison of Coccidioides antigen skin testing in patients with active coccidioidomycosis, noted that although African Americans were more likely to have disseminated disease than were Caucasians, their skin test reactivity was comparable to those among other ethnic groups. Along this same line, Williams et al. (309) reported that African Americans and Filipinos acquired T-cell reactivity in response to vaccination with the formalin-killed spherule (FKS) vaccine at levels comparable to that observed in Caucasians.
Gender.
Males are reported to have a 3.5- to 5-fold-increased occurrence of disseminated coccidioidomycosis compared to females (99, 217). Following the major 4-year epidemic in San Joaquin Valley, Johnson et al. (157) studied 535 patients, 25 of whom had disseminated disease, and found that male gender was a risk factor for dissemination when the data were analyzed by univariate (P < 0.05) but not multivariate analysis. However, Arsura et al. (13), in analyses of 536 cases in Kern County between September and December 1991, found that 76% of the 25 patients who had disseminated disease were male, compared to 52% of males in the total population; this was significant by chi-square analysis (P < 0.02; odds ratio, 2.9). It appears that males are more susceptible than females, but the differences may not be as high as noted in earlier studies.
Age.
Sievers (266), in his study of disseminated coccidioidomycosis in the American Indian population, found that children younger than 5 years and adults older than 50 years were significantly more susceptible than persons aged 6 through 49 years. An increased susceptibility of older persons was observed in the outbreak in Ventura County following the Northridge earthquake (260), in the 3-year coccidioidomycosis epidemic that started in San Joaquin Valley in 1991 (12, 157) as well as a subsequent follow-up covering the period from January 1995 through December 1996 (248), and in analyses of cases of coccidioidomycosis in Arizona (182). In the last study, elderly persons who had recently relocated to Arizona appeared to be at the highest risk.
Pregnancy.
Until recently, the increased susceptibility associated with pregnancy was considered to be unequivocal (99, 136, 137, 238, 274). In areas of high endemicity, approximately 0.1% of pregnancies have been complicated by coccidioidomycosis, with a resulting mortality rate of 88% (291). In a review of 65 women who had coccidioidomycosis during pregnancy, VanBergen et al. (291) reported that 37 (57%) developed disseminated disease and, of these, 29 (78%) died. The later the gestation stage, the more likely it is that dissemination will occur (217). These studies have prompted some to consider abortion or early delivery in gravid females with coccidioidomycosis, in particular those in the third trimester of pregnancy. More recent studies suggest that the risk of dissemination and death in pregnancy have been overstated (15, 38, 293). Wack et al. (293) examined 47,120 pregnancies among three health care centers in Tucson, Ariz., during a 6-year period and reported that only 10 were complicated by coccidioidomycosis. None of these were fatal. The two women who developed fulminant, disseminated coccidioidomycosis were thought to have acquired their coccidioidal infection during the third trimester. In a retrospective analysis of coccidioidomycosis in pregnant women during the California 1991 to 1994 outbreak, Caldwell et al. (38) reported that disseminated disease occurred in 3 (9%) of 32 patients. None of the patients died. The investigators noted that although the 9% incidence of disseminated coccidioidomycosis in pregnant women was lower than that reported in earlier studies, it is higher than that observed in the general population and in females of reproductive age. One consistent finding is that the increased risk of developing disseminated coccidioidomycosis occurs in women who acquired their primary infection during pregnancy and not in those who had a previous coccidioidal infection (99).
It has been theorized that the high risk of severe coccidioidomycosis in pregnant females is attributed to the immunosuppressive state that accompanies gestation. Another mechanism was offered by Drutz et al. (101) and Powell et al. (236, 237), who reported that progesterone and 17 β-estradiol, at levels comparable to those in the sera of pregnant females, stimulate the growth of the spherule/endospore phase in vitro. While this offers an attractive and plausible explanation to account for the unique susceptibility of gravid females to coccidioidomycosis, there have been no reports of whether these hormones have a stimulatory effect on fungal growth in vivo.
Immunocompromising diseases or conditions.
Coccidioidomycosis is a frequent complication for persons who are immunologically compromised by human immunodeficiency virus (HIV) infection (3, 6, 115, 197, 315). A prospective study conducted during a 4-year period in Phoenix and Tucson indicated that the risk of active coccidioidomycosis in HIV-infected persons ranged from 8% to 41% (6). It is not known what percentage of HIV-infected persons with coccidioidomycosis have a primary infection with Coccidioides or reactivation of a previously quiescent infection as a consequence of their pronounced immunodeficiency. In support of the former, Ampel (3) did not observe any association between the length of time the HIV-infected person resided in a coccidioidomycosis-endemic area and a history of a previous infection with the fungus. The course of coccidioidomycosis in HIV-infected persons can vary widely, ranging from a median survival of only 1 month in patients with a diffuse bilateral reticulonodular infiltrates to several months or longer in persons who have unilateral focal pulmonary infiltrates (125).
Persons with other immunosuppressive diseases or conditions, such as Hodgkin's disease, malignant neoplasms, and collagen vascular disease, recipients of immunosuppressive drug therapy, and organ transplant recipients also have a high risk of dissemination of disease and death (30, 95, 292). There have been several reports of reactivation of previous coccidioidal infection in organ transplant recipients (30, 95, 292).
It is also possible that an acute bacterial or viral infection can reactivate a quiescent coccidioidal infection. To cite one example, coccidioidomycosis was diagnosed in a 48-year-old Caucasian man who reactivated a prior benign coccidioidal infection while in England after developing a group A, beta-hemolytic Streptococcus infection (307).
Critical Comments
Coccidioides is a true primary human pathogen, causing significant morbidity and mortality for those living in or visiting the areas of endemicity. There are clear genetic influences that affect the development of severe disseminated disease, but the mechanisms of the genetic control of dissemination have not been characterized. A population-based study is needed to determine genetic polymorphisms in patients with infections of different severities to delineate the gene(s) involved in genetically determined resistance to coccidioidomycosis.
HOST DEFENSES IN HUMANS
Innate Immunity
Polymorphonucleur leukocytes.
Polymorphonucleur leukocytes (PMNL) comprise the earliest cellular influx to arthroconidia (122, 256). This response may be attributable to chemotactic components released by the fungus, as suggested by Forbus and Bestebreurtje (122) in histologic studies of tissues from coccidioidomycosis patients and subsequently confirmed by Galgiani et al. (129) in chemotactic assays using human PMNL stimulated with coccidioidin or spherulin. The interaction of Coccidioides with PMNL has been examined using PMNL from humans (100, 123, 126-130), rhesus macaques (23), dogs (301), and mice (31). Phagocytosis of arthroconidia is enhanced in the presence of immune serum (100, 123, 301). Ingestion of the arthroconidia is followed by a respiratory burst (31, 123), and, although the fungal cells are sensitive to the products of the respiratory burst (24, 127) and to cationic peptides (defensins) (263), fewer than 20% of the arthroconidia are killed by the encounter (23, 31, 100, 123). Indeed, some studies suggest that PMNL may promote the maturation of arthroconidia into endosporulating spherules (14, 128).
Transformation of arthroconidia into spherules renders the latter impervious to phagocytosis and killing by PMNL (123, 126), owing in part to the increased size of the spherules (60 to 80 μm) relative to PMNL (12 μm). Although Galgiani (126) reported that PMNL appear to directly adhere to spherules in vitro, Frey and Drutz (123) reported that spherules possess an extracellular fibrillar matrix that impedes their physical contact with PMNL. Rupture of the spherules and release of the endospores triggers an influx of PMNL (122, 123). The newly released endospores are encased in a fibrillar matrix, but over time they become single cells that are readily phagocytized by PMNL. Ingestion of the endospores triggers an oxidative burst, albeit to a lesser extent than that induced by arthroconidia (23, 123), and the level of intracellular killing is no more impressive than that observed in the interaction of PMNL and arthroconidia (23, 31, 123).
Monocytes/macrophages.
Kashkin et al. (158) reported that peritoneal macrophages from nonimmune guinea pigs phagocytized but did not kill arthroconidia. The relative inefficacy of nonimmune macrophages in killing Coccidioides was confirmed by Beaman et al. (20-24). These investigators examined the in vitro interaction between arthroconidia and endospores with alveolar macrophages from nonimmune rhesus macaques, resident peritoneal macrophages from DBA/2 mice, and peripheral blood monocytes from healthy, skin test-negative persons. Both arthroconidia and endospores are phagocytized by monocytes/macrophages, but fewer than 1% of the phagocytized cells were killed. In contrast, Ampel and Galgiani (7) reported that peripheral blood monocytes from healthy, skin test-positive or -negative persons inhibited or killed arthroconidia. The differences in the results of these studies could be attributable to differences in the methods used to quantify fungal viability. Beaman et al. (22-24) used conventional assays for determining fungal CFU, whereas Ampel and Galgiani (7) used a newly developed microtiter system for determining fungal CFU (102) and a radiolabeled N-acetylglucosamine (precursor to chitin) uptake experiment to assess the inhibition of fungal viability.
One mechanism that Coccidioides might use to survive intracellularly is the inhibition of phagosome-lysosome fusion (21, 24), a strategy used by many intracellular pathogens to evade the antimicrobial effects of phagocytes (11, 206, 232, 249, 284, 305). Coincubation of monocytes/macrophages with immune T lymphocytes or gamma interferon (IFN-γ) significantly enhanced their anticoccidioidal activity (18, 19) (see below).
Natural killer cells.
Natural killer (NK) cells are a major component of innate immunity. Under normal conditions, NK cells are confined primarily to the peripheral blood, spleen, and bone marrow, but they can migrate to sites of inflammation in response to chemokines. On activation, NK cells secrete cytokines, notably IFN-γ, and chemokines that induce inflammatory responses and control the growth of monocytes and granulocytes (203). Before adaptive immunity has fully developed, NK cells are thought to the main source of IFN-γ, in response to macrophage-derived interleukin-12 (IL-12) and IL-18.
Petkus and Baum (231) reported that incubation of spherule/endospore-phase cells for 4 h with human peripheral blood lymphocytes, depleted of monocytes/macrophages, significantly reduced the viability of the fungal cells. Incubation of the lymphocytes with Leu-11 (CD56) antibody, which binds the Fc receptor of NK cells, and complement reduced the anticoccidioidal effect of the lymphocytes by 80%. Supernatants from peripheral blood lymphocytes coincubated with K562 cells or Coccidioides were cytotoxic for Coccidioides, as judged by an inhibition of fungal CFU. These results suggest a direct cytotoxicity of NK cells and NK-cell factor. However, because Leu-11 is not specific to NK cells, additional studies are needed to confirm that NK cells are directly toxic to Coccidioides. One approach might be to utilize the NK-92 cell line (CDRL-2407; American Type Culture Collection), which is a human NK-cell line that is highly toxic to a broad range of NK-cell targets (193, 289).
Dendritic cells.
Dendritic cells (DCs) are potent antigen-presenting cells (APCs) and play a pivotal role in innate immunity and adaptive immunity (190). On initial infection, precursor DCs are recruited from the blood to inflammatory sites, where they transform to immature DCs. In the initial interaction, the pathogen binds to pattern recognition receptors, notably Toll-like receptors (TLR), which recognize structurally conserved pathogen-associated microbial products. This initial recognition and binding leads to the induction of proinflammatory cytokines, which include tumor necrosis factor alpha (TNF-α), IL-1, IL-6, and IL-8.
Recent studies by Richards et al. (243) showed that a spherule-phase antigen, toluene spherule lysate (TSL), induced maturation of peripheral blood-derived DCs from healthy, nonimmune subjects. DC maturation was demonstrated by increased cell surface expression of HLA-DR, CD40, CD54, CD80, CD83, and CD86. The TSL-pulsed DCs also stimulated proliferation in allogeneic lymphocytes, to a greater level than did nonpulsed (immature) DCs, and they stimulated autologous nonadherent peripheral blood mononuclear cells to produce IFN-γ. The potential immunotherapeutic use of DCs was established by Richards et al. (244), who showed that the anergy demonstrated by peripheral blood lymphocytes from patients with disseminated coccidioidomycosis could be reversed by the addition of DCs pulsed with coccidioidal antigen. Although the latter studies were conducted in vitro, additional studies of the restoration of immunity by DC immunotherapy in animal models could reveal a new avenue for adjunctive therapy in severe coccidioidomycosis.
Adaptive Immunity
Activation of immature DCs leads to their secretion of chemokines such as CCL3 and CXCL8 and maturation of the DCs into highly efficient APCs, which function to regulate T- and B-cell responses, a role in the immune response that distinguishes DCs from other APCs such as macrophages and B cells (241). The antigen-bearing DCs travel from peripheral tissue via afferent lymphatic channels to secondary lymphoid organs, such as the spleen and lymph nodes, and complete their maturation at these sites. The mature DCs lose their endocytic activity by downregulating receptors that interact with antigen, and they upregulate major histocompatibility complex molecules; CD83; the costimulatory molecules such as CD40, CD58, CD80, and CD86; and the chemokine receptors CCR7 and CXCR5 (45, 107, 253). The upregulation of the chemokine receptors CCR7 and CXCR5 is strategic in that it effects the localization of DCs to appropriate sites within the lymph nodes and secondary lymphoid organs, where they interact with T cells and B cells (251, 253). By producing cytokines that polarize the Th response, the mature DCs effectively induce and orchestrate the adaptive immune response (107).
Beginning with the early studies by Smith et al. (276, 279-281), a profile of adaptive immune responses in persons with different entities of coccidioidomycosis emerged. Persons with primary, asymptomatic, or benign disease characteristically have strong skin test reactivity to coccidioidin and low or nondemonstrable levels of anti-Coccidioides complement fixation (CF) antibody. The converse pattern develops in patients who develop severe, chronic, or progressive pulmonary or disseminated disease. Typically, these persons, in particular those with disease involving two or more organ systems (lungs, central nervous system, bones and/or joints), are hyporesponsive or anergic to coccidioidal skin testing but have high levels of anti-Coccidioides IgG antibody to the CF antigen. Recovery from active disease, either spontaneous or in response to antifungal therapy, is in many patients associated with a reacquisition of T-cell reactivity to Coccidioides antigens and decreased CF antibody titers. However, the responses of patients with inactive disease do not coincide with those of persons who were able to overcome their primary infection without consequence; instead, they tend to be intermediate between those of the latter patients and patients with active disseminated disease.
Cellular immunity. (i) Cutaneous delayed-type hypersensitivity.
The classical antigen preparation that was used in the early skin test and serologic studies was coccidioidin. This antigen was prepared by Smith (276) as a soluble broth culture filtrate of mycelial cells grown for 2 months in a synthetic asparagine-glycerol-salts medium. With the development of a medium for the cultivation of the spherule-endospore phase in vitro (61), Levine et al. (184) produced an aqueous lysate of spherules of strain Silveira that had been grown in Converse medium and then incubated in distilled water for up to 40 days at 34°C. The soluble aqueous lysate was designated spherulin (SPH) and used as a skin test antigen at a dose of 2.8 μg (Usual Test Strength), which corresponded to coccidioidin 1:100. Skin test reactivity persists in most persons who recover from primary infection, and such persons are endowed with immunity to exogenous reinfection (274, 276, 279). The persistence of coccidioidin reactivity in persons who have recovered from their primary infection may be attributable to reexposure to the fungus, as would probably occur in persons who reside in areas of endemicity, or to the presence of viable Coccidioides cells in calcified lesions (36). Although skin test reactivity persists in most persons, some gradually lose their coccidioidin sensitivity (215, 266). Whether this is accompanied by a loss of resistance to Coccidioides is not known but is a question of immense importance since it may relate to long-term protection in response to vaccination.
As many as 80% of persons who develop solitary pulmonary lesions manifest skin test reactivity to coccidioidin, whereas less than one-third of those who develop progressive or chronic pulmonary disease manifest reactivity (44, 82, 254, 274, 276, 304). Skin test reactivity in persons with extrapulmonary disease varies, depending on the extent of disease involvement. Approximately 70% of those who have a single extrapulmonary site of involvement manifest reactivity to coccidioidin 1:100, whereas fewer than 30% of patients with multifocal disease are reactive to coccidioidin or SPH. Low or nondemonstrable skin test reactivity denotes a poor prognosis for recovery. Smith (274, 276) reported that 75% of patients who were skin test reactive to coccidioidin recovered from their disease whereas only 17% of patients who were skin test negative recovered.
The specificity of the cutaneous anergy has been examined in a number of studies (44, 82, 133, 274). In most patients, the anergy is specific to Coccidioides, as evidenced by skin test reactivity to a panel of recall antigens such as Candidin, mumps antigen, trichophyton, and streptodornase-streptokinase. The exceptions occur in patients who have severe disseminated disease involving multiple foci of infection. Approximately half of these patients fail to respond to recall antigens. In addition, Rea et al. (239, 240) reported that some patients fail to respond to contact sensitization with dinitrochlorobenzene, a result that documents a pronounced suppression of cell-mediated immune responsiveness.
(ii) Cytokine production.
TNF-α is a cytokine produced by a large variety of cells, including macrophages, DCs, CD4+ and CD8+ T cells, and B cells (27, 109, 285, 299, 320). Cumulative evidence has established that TNF-α is responsible for many of the biological and physiological consequences of acute infection, immunological reactions, and tissue injury (181). In addition to its oncolytic activity and ability to induce cachexia, TNF-α can activate neutrophils, enhance the cytolytic activity of macrophages, augment NK-cell activity, promote T- and B-cell proliferation, and modulate endothelial cell surface antigens. TNF-α plays multiple roles in immune and pathologic responses in tuberculosis (120, 132, 141, 162, 202, 246, 295). On one hand, TNF-α is required for the control of acute infection and the formation and maintenance of granulomas, but on the other hand, it has been implicated as a major component in host-mediated destruction of lung tissue.
Ampel (4) reported that autoclaved spherules and arthroconidia of Coccidioides induced the production of TNF-α by adherent mononuclear cells from healthy human donors. TNF-α production was increased in cells from healthy, skin test-positive persons when the supernatants were assayed for cytotoxicity against the TNF-α-susceptible L929 cell line. No differences were evident, however, when the supernatants were assayed by an enzyme-linked immunosorbent assay (ELISA), which, unlike the L929 assay, is specific for TNF-α (198). Dooley et al. (97) reported that both FKS and live spherules induced TNF-α, IL-1β, and IL-6 production by peripheral blood mononuclear cells and plastic-adherent monocytes/macrophages of healthy persons and coccidioidomycosis patients. The production of the proinflammatory cytokines was comparable in 15 healthy SPH skin test-positive subjects, 13 healthy skin-test negative persons, and 16 patients with active coccidioidomycosis.
Studies have also been done to assess the production of the Th1-asociated cytokines IL-2 and IFN-γ. In a study of 20 healthy subjects who were skin test positive to SPH and 15 healthy, skin-test negative persons, Ampel et al. (5) showed that peripheral blood mononuclear cells from skin test-positive but not skin test-negative donors secreted both IL-2 and IFN-γ in response to in vitro stimulation with a toluene-induced lysate of spherules, designated TSL (Table 2). Comparisons of cytokine production, lymphocyte proliferation, and skin test reactivity revealed that there was a tendency toward a direct correlation, but the heterogeneity of responses precluded a significant correlation. Of note, lymphocytes from five subjects who were skin test positive to SPH were essentially nonresponsive to TSL in vitro. The basis for this unexpected deviation between in vivo and in vitro T-cell responses is not known and, as noted by the investigators, is unlikely to be attributable to differences in the antigenic composition of SPH and TSL, given that the other 15 skin test-positive subjects were reactive to both preparations. An ensuing study by Corry et al. (66) compared the production of the Th1 cytokines IFN-γ and IL-12 and the Th2 cytokines IL-4 and IL-10 by lymphocytes from healthy, SPH skin test-positive and -negative subjects and patients with active pulmonary or disseminated coccidioidomycosis. The results established that IFN-γ production was significantly lower in cells from the patients with disseminated disease than in those from healthy, skin test-positive persons. By contrast, lymphocytes from patients with pulmonary disease secreted levels that were comparable to those of healthy, SPH-reactive donors. The study groups did not differ in their production of the Th1 cytokine IL-12 or the Th2 cytokines IL-4 or IL-10. Additional studies are clearly needed to further examine the production of Th1 and Th2 cytokines in coccidioidomycosis, with emphasis on relating cytokine responses to clinical progression or regression. It would be at least as important to address the question whether genetically determined susceptibility is associated with, and perhaps attributable to, an inability to maintain Th1 responses, as has been shown in the murine model (discussed below).
TABLE 2.
Percentage of CD3+ lymphocytes producing intracellular IFN-γ after incubation with tissue culture medium alone, IL-12, the Coccidioides antigen TSL, or TSL plus IL-12a
| Subject (n) | % of lymphocytes producing IFN-γ after incubation with:
|
|||
|---|---|---|---|---|
| Controlb | IL-12 | TSL | TSL + IL-12 | |
| Nonimmune (5) | 0.05 ± 0.02 | 0.27 ± 0.08 | 0.11 ± 0.04 | 0.46 ± 0.25 |
| Immune (7) | 0.09 ± 0.03 | 2.08 ± 1.01 | 1.31 ± 0.42 | 5.72 ± 1.38 |
| P value | 0.38 | 0.71 | 0.04 | 0.01 |
Reprinted from reference 5 with permission of the publisher.
Incubated with tissue culture medium alone.
Ampel et al. (5, 8, 9) recently analyzed cytokine responses of peripheral blood from healthy immune and nonimmune persons and patients with active coccidioidomycosis by using flow cytometry. Incubation of the peripheral blood specimens with the Coccidioides antigen T27K induced CD3+ T cells to produce IFN-γ. Of the CD3+ T cells from immune donors, 0.43% were positive for intracellular IFN-γ, compared to 0.01% of the CD3+ T cells from nonimmune donors, 0.11% of the CD3+ T cells from patients with pulmonary disease, and 0.09% of the CD3+ T cells from patients with disseminated disease. Ampel et al. (9) subsequently quantified the expression of CD69, a glycoprotein that is expressed by activated T cells and NK cells (270). Using flow cytometry, the percentage of CD3 blood lymphocytes expressing CD69 in blood specimens incubated with and without T27K was determined. After subtracting the background level of CD69 expression, i.e., on nonstimulated blood lymphocytes, the mean fluorescence intensities of CD69 expression on CD3 lymphocytes from healthy immune subjects and patients with active disease were significantly increased compared to those on cells from healthy, nonimmune persons. There was no difference in the mean fluorescence intensity of CD69 expression on CD3 lymphocytes from the 20 healthy immune donors and 70 patients with active disease. However, within the patient group, those with pulmonary disease showed a significantly higher mean fluorescence intensity of CD69 expression in response to T27K than did those with disseminated disease. The investigators observed a significant direct association between the mean fluorescence intensity of CD69 on CD3 lymphocytes and the production of the cytokines IFN-γ, IL-2, and TNF-α.
(iii) Cytokine activation of monocytes.
Beaman and Pappagianis (24) reported that human peripheral blood monocytes phagocytized but did not kill Coccidioides arthroconidia or endospores and that the lack of killing was associated with an inhibition of phagosome-lysosome fusion by the fungal cells. When monocytes were incubated in the presence of lymphocytes from immune persons, there was a significant increase in phagosome-lysosome fusion and killing of the fungal cells. Incubation of the monocytes with recombinant human IFN-γ or recombinant TNF-α augmented the fungicidal capabilities of the monocytes (18). The mechanism by which IFN-γ or TNF-α activate human monocytes to an anti-Coccidioides level is not known, but in studies of human alveolar macrophages from tuberculosis patients, IFN-γ and TNF-α activate the macrophages to generate nitric oxide and related reactive nitrogen intermediates via nitric oxide synthase, using l-arginine as the substrate (296).
Humoral immunity. (i) Antibodies.
Chronic or progressive coccidioidomycosis is associated with a polyclonal B-lymphocyte activation, as evidenced by elevated levels of IgG, IgA, and IgE in serum (39, 69, 70, 226). Antibodies reactive with coccidioidal antigens have been demonstrable within each of these Ig classes. Serum IgG levels directly correlate with disease involvement, being highest in patients with multifocal involvement. The serum IgA level is elevated in approximately 20% of patients, being manifested most often in patients with chronic pulmonary disease (69). To our knowledge, secretory IgA levels have not been reported. Hyperproduction of IgE would be consistent with a Th2 response and has been demonstrated in approximately 23% of patients with active disease, with the highest incidence occurring in patients with disseminated disease and, within this group, in patients who have disease involving two or more organ systems (for example, lungs, bones and/or joints, skin, and central nervous system) (69). Anti-Coccidioides IgE was demonstrable in most patients with elevated IgE levels; however, IgE reactivity was also demonstrable against common allergens, such as bermuda grass and ragweed. Longitudinal studies of coccidioidomycosis patients with excessive IgE levels revealed that, in most patients, IgE production diminished to normal or near normal levels after clinical remission, suggesting that IgE hyperproduction is a consequence of the disease. This interpretation is countered, however, by the report that atopic persons are at greater risk of developing symptomatic coccidioidomycosis than are persons who are nonatopic (96).
(ii) Immune complexes.
Circulating C1q-binding immune complexes have been detected in sera from coccidioidomycosis patients and shown to correlate with disease severity (81, 317). Whereas 33% of sera from patients with disease involving a single organ system had elevated immune complex levels, 67% of sera from patients with disseminated multifocal disease showed circulating immune complexes. Analyses of immune complexes in serum from a patient with severe disseminated disease revealed Coccidioides antigen, C1q and anti-Coccidioides IgG antibody.
The role, if any, of immune complexes in the immunopathogenesis of coccidioidomycosis is not known. Investigators reported suppression of lymphocyte proliferation responses when lymphocytes from healthy coccidioidin skin test-positive persons were assayed in the presence of patient sera and, conversely, augmentation of the responses of patient lymphocytes when assayed in sera from healthy subjects versus autologous serum (80, 208). Immunoaffinity chromatography of patient sera with Staphylococcus protein A ablated the suppressive effect of the sera, a result that would be consistent with suppression by antibody, alone or complexed with antigen. However, addition of immune complexes formed in vitro (by the addition of coccidioidin to a serum sample with high levels of anti-Coccidioides IgG) to cultured mononuclear cells from healthy, coccidioidin skin test-positive persons did not suppress their proliferation response to coccidioidin (80). These results, taken together, argue against suppression by immune complexes and raise the question whether the suppression observed with patient sera was merely attributable to the neutralization of coccidioidin in such a manner that it was not available to stimulate lymphocytes.
Critical Comments
Protection against human coccidioidomycosis has been convincingly related to induction of Th1-associated immune responses. The cumulative response includes processing and presentation of critical antigens by macrophages and/or DCs, leading to the induction of T cells to produce IFN-γ and other Th1-associated cytokines. These cytokines, in turn, provide the signals for recruiting and activating immune effector cells. The work by Richards et al. provides the best hope for developing an immunotherapy for patients with progressive disseminated disease. If the reversal of T-cell anergy, as shown is in vitro assays utilizing DCs pulsed with Coccidioides antigens, can be extrapolated and expanded, there will be a significant increase in the therapeutic armamentarium.
MURINE MODEL OF COCCIDIOIDOMYCOSIS
The vast majority of studies of Coccidioides vaccines have been conducted using the murine model. The focus on this model has been based largely on the relatively low expense of using mice compared with that of using larger animal models and on the availability of immunologic and molecular reagents to delineate the host response to the fungus. Also, the availability of inbred mouse strains, which differ in their susceptibility to Coccidioides, is immensely important in evaluating the protective capacity of Coccidioides vaccines.
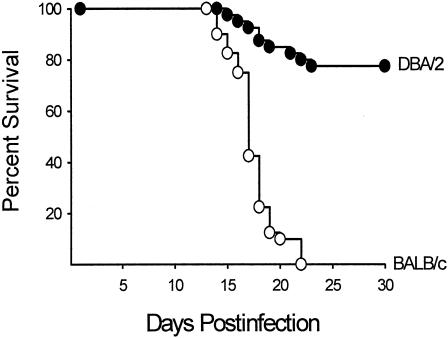
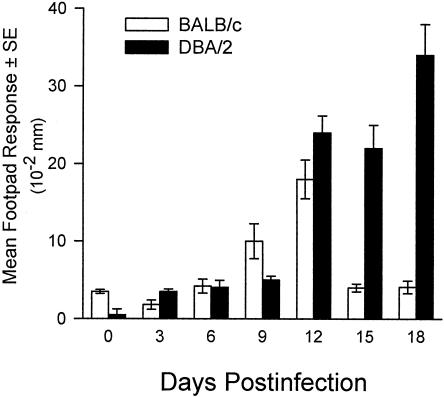
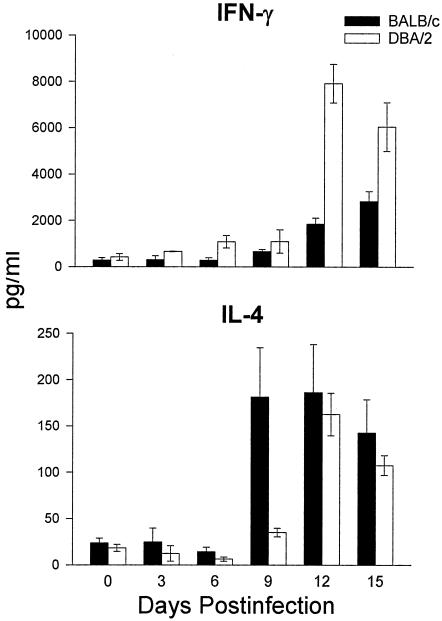
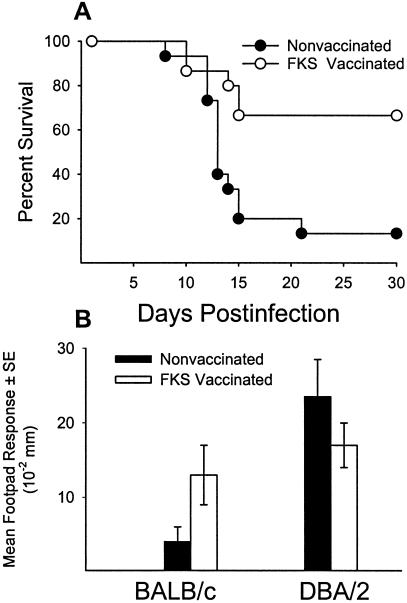
Despite the utility of the mouse model, there are some conspicuous differences in the course of the disease in mice and humans. First, the disease disseminates to the spleen and liver in both the relatively resistant DBA/2 mouse strain and the highly susceptible BALB/c mouse strain after pulmonary challenge with only 10 arthroconidia (77). This is in contrast to low incidence of dissemination in humans, except in persons exposed to a high infectious dose. Second, in contrast to the direct correlation between CF antibody and disease severity in human coccidioidomycosis, infected (nonimmunized) mice do not have detectable CF antibody levels (26, 175). On the other hand, similarities have been demonstrated in the correlation between resistance and the production of Th1 cytokines in the murine and human models and, conversely, between susceptibility and the production of Th2 cytokines. The extent to which the murine model can be used to validate the potential of vaccine candidates for human disease simply is not known at this time. Inbred mouse strains also provide a model for studying genetically determined susceptibility to Coccidioides. Studies by Kirkland and Fierer (165, 166) compared the susceptibility of inbred mouse strains to intraperitoneal (i.p.) infection with gradient doses of Coccidioides arthroconidia and established that BALB/cAnN mice were the most susceptible whereas DBA/2N mice were the most resistant (Table 3). C3H/HeN mice were of intermediate susceptibility. Differences in the susceptibility of BALB/c and DBA/2 mice were also demonstrable when the challenge was performed via the i.n. route (77) (Fig. 1). On the basis that both the susceptible BALB/c and resistant DBA/2 mouse strains are of the H-2d haplotype, susceptibility is not controlled primarily by the H-2 locus. A cross between BALB/c and DBA/2 mice yielded an F1 progeny that was resistant to challenge, whereas the progenies of matings between susceptible strains were susceptible. Hence, resistance is a dominant phenotype.
TABLE 3.
Lethality of Coccidioides for inbred mouse strains challenged by an i.p. routea
| Mouse strain | Log10 LD50 (SE)b |
|---|---|
| BALB/cAnN | 1.67 (0.60) |
| C57BL/10N | 2.77 (0.33) |
| C57BL/6N | 2.83 (0.23) |
| C57L/J | 1.65 (0.56) |
| (BALB/cAnN × C57BL/6)F1 | 1.82 (0.45) |
| (C57BL/6 × DBA/2N)F1 | 4.20 (0.19)c |
| (BALB/cAnN × DBA/2N)P1 | 4.95 (0.18)c |
| DBA/2N | 5.25 (0.36)c |
Reprinted from reference 166 with permission.
LD50, 50% lethal dose; SE, standard error.
Significantly different from all other strains (P < 0.01) but not significantly different from each other.
FIG. 1.
Percent survival of BALB/c and DBA/2 mice on days 1 through 30 after i.n. infection with 10 arthroconidia. Each group consisted of ≥22 mice. Reprinted from reference 77 with permission.
Analysis of a backcross between (BALB/c × DBA/2)F1 × BALB/c yielded a 1:1 distribution of susceptible and resistant offspring, suggesting that a single gene accounts for the difference in resistance (164). This gene was given the designation CmS and, on the basis of the relative susceptibilities of the various inbred mouse strains, differs from the Bcg-Ity-Lsh gene, designated NRAMP (natural resistance- associated macrophage protein), that controls natural resistance to Salmonella enterica serovar Typhimurium, Leishmania donovani. Mycobacterium species, and other intracellular pathogens (35). The CmS gene is not phenotypically expressed until 10 to 12 days after i.p. infection, as evidenced by the presence of comparable numbers of fungal CFU in BALB/c and DBA/c mice 10 days after i.p. (164) or i.n. (77) challenge. Thereafter, the fungal CFU in the susceptible BALB/c mice are significantly increased compared to the resistant DBA/2 strain.
Fierer et al. (113) used a set of 26 recombinant inbred (RI) mouse lines derived from a cross between susceptible B6 mice and resistant DBA/2 mice to map the genes responsible for resistance to this fungus. Each of the 26 RI lines has either the B6 or the DBA/2 allele at every genetic locus; hence, analysis of resistance provides a means of searching for linkage. Comparisons of the fungal load in the lungs of the recombinant inbred mice after i.p. infection showed that 4 of the 26 RI lines had a fungal load that was greater than 10 times the load found in the susceptible B6 parent; 5 had less than 10% of the fungal load found in the resistant DBA/2 strain. Similar results were obtained in comparisons of fungal load in the spleens. These data indicate that resistance is a polygenic trait and may be linked to two unlinked loci, as opposed to their earlier report that a single gene, CmS, determines resistance (164). One of the resistance loci is on chromosome 4 near the Lv gene (aminolevulinate dehydratase); the other locus is on chromosome 6 near Tnfr1 (tumor necrosis factor receptor 1). A backcross between (B6 × DBA/2)F1 × B6 mice showed that 87% of the progeny that were heterozygous for both loci were resistant whereas only 25% of the progeny that were resistant were homozygous at both loci. Mice that were heterozygous at only one of the two loci showed intermediate resistance, which would be consistent with the additive effect of the two loci. The investigators also found a direct correlation between expression of IL-10 mRNA and increased fungal load in the 26 RI lines, suggesting that the gene that influences the IL-10 response may be linked near Lv and Tnfr1 on chromosomes 4 and 6, respectively. In an ensuing study, Fierer et al. (112) compared the susceptibility of C57BL/6 and C57BL/10 mouse strains, which are nearly congenic for the Lv locus. At 14 days after i.p. challenge with 500 arthroconidia, 79% of the B6 mice were dead, compared to only 8% of the B10 mice. However, there was no difference in the survival rate of B6 and B10 mice by day 28. The investigators concluded from these results that a gene near the Lv locus is involved in the early, innate response to Coccidioides infection. The Lv locus is not the only locus implicated in the resistance to Coccidioides, however, since BXD 29, which is one of the RI lines derived from a cross between B6 and DBA/2 mice, is resistant to Coccidioides even though this line is B6 at Lv. B6 mice were also found to produce a 100-fold-lower level of IL-10 than did B10 mice, which argues against the earlier supposition that a locus near Lv was responsible for modulating IL-10 production. That is, if IL-10 production were modulated by a locus near Lv, then B6 mice would have produced increased levels of IL-10 compared with B10 mice.
Host Defenses in Experimentally Infected Mice
Role of T lymphocytes in protective immunity.
Unequivocal evidence from studies of murine models has established that cellular immunity is crucial to host defense against Coccidioides. A series of early investigations established that neonatal or adult thymectomized mice and congenitally athymic BALB/c mice are highly susceptible to challenge with Coccidioides compared with their T-cell-sufficient counterparts (25, 54, 140). Immunization of DBA/2 mice with the FKS vaccine induced protection against challenge, which could be adoptively transferred via splenic T cells but not via B cells or serum from immunized mice (25, 26). Depletion of T cells from the immune spleen cells ablated protective transfer (25). More recently, adoptive transfer of the protective immunity by spleen cells from FKS-immunized DBA/2 mice was shown to be dependent primarily on CD4+ T cells, but optimal transfer was achieved with both CD4+ and CD8+ T cells (79).
One mechanism by which immune T cells function as effectors of protective immunity is by activating macrophages to inhibit the growth of arthroconidia and endospores (20, 21). Murine alveolar or peritoneal macrophages effectively phagocytized arthroconidia and endospores in vitro, but the cells were not killed. Rather, within 24 to 30 h, the phagocytized endospores began to develop into spherules and the arthroconidia germinated and formed hyphae. When the macrophages were coincubated with immune lymphocytes, 37% of the phagocytized arthroconidia and 25% of phagocytized endospores were killed (21). The immune lymphocytes alone did not kill arthroconidia or endospores. Rather, the effect of immune lymphocytes was to activate the antifungal activity of the macrophages by increasing phagosome-lysosome fusion. Whereas phagosome-lysosome fusion occurred with only 13% of phagocytized spores when macrophages were cultured alone or in the presence of nonimmune lymphocytes, it increased to 61% when the macrophages were cocultured with immune lymphocytes. Increased phagosome-lysosome fusion was directly correlated with antifungal activity. Depletion of T cells in the immune lymphocyte preparation decreased phagosome-lysosome fusion to 26%. It was found that activation of macrophages occurred via a soluble factor produced by immune T cells. This soluble factor was most probably IFN-γ, since antibody to murine IFN-γ reduced the activation of macrophages by immune lymphocytes and since macrophage activation was effected by the addition of IFN-γ.
The differences in the hereditary patterns of disease severity in human and murine coccidioidomycosis, coupled with the protective role of cellular immunity in host defense against Coccidioides, suggests an immunologic basis for genetically determined susceptibility to this fungus. To address this possibility, Cox et al. (77) infected BALB/c and DBA/2 mice with 10 arthroconidia of Coccidioides strain Silveira via an i.n. instillation and, at 3 day intervals, footpad tested separate groups of mice from each strain. Both mouse strains mounted a significant footpad hypersensitivity to coccidioidin by day 9 postinfection, and this hypersensitivity increased in magnitude by day 12 (Fig. 2). Thereafter, the susceptible BALB/c mice developed anergy whereas delayed-type hypersensitivity persisted in the resistant DBA/2 mice. Footpad responses were not measured beyond day 18 because of the excessive mortality in the BALB/c mouse group.
FIG. 2.
Footpad hypersensitivity responses in BALB/c and DBA/2 mice at 3-day intervals after i.n. infection with 10 arthroconidia. Bars depict means and standard errors (SE) obtained with groups of nine or more mice. Reprinted from reference 77 with permission.
Role of cytokines in host defense.
Since cytokines are critical for the induction and expression of protective immunity, Cox and Magee (78) examined the in vivo production of the proinflammatory cytokines TNF-α, IL-1α, and IL-6 in BALB/c and DBA/2 mice at various times after pulmonary challenge. The levels of these cytokines in lung homogenates of the two mouse strains were essentially comparable in the two mouse strains when measured by ELISAs and PCR for cytokine mRNA. An ensuing study was conducted to examine the induction and expression of IFN-γ and IL-4 during active coccidioidomycosis in BALB/c and DBA/2 mice (196). Quantitation of cytokines in lung homogenates obtained from groups of mice at 3-day intervals after pulmonary challenge revealed that the resistant DBA/2 mice produced increased levels of the Th1-associated cytokine IFN-γ as early as day 6 postinfection whereas IFN-γ was not detected in the lungs of BALB/c mice until day 9 postinfection (Fig. 3A). Although the levels of IFN-γ increased in both strains thereafter, the magnitude of the response was significantly greater in the resistant mouse strain on days 12 and 15 postinfection. A reciprocal pattern was observed in the kinetics of the Th2-associated IL-4 cytokine production (Fig. 3B). IL-4 levels were significantly elevated in lung homogenates from BALB/c mice 9 days after pulmonary challenge, at which time DBA/2 mice showed only a modestly elevated IL-4 level. Thereafter, on days 12 and 15, both strains showed increased and comparable levels of IL-4 in lung tissues.
FIG. 3.
Levels of IFN-γ (A) and IL-4 (B) in homogenates of lung tissues obtained before and at various times after i.n. challenge. The bars depict means and standard errors obtained with groups of seven or more mice. Reprinted from reference 196 with permission.
The role of IFN-γ in host resistance to Coccidioides was determined by pretreating the susceptible BALB/c mice with murine recombinant IFN-γ (rIFN-γ) beginning on the day before challenge, again on the day of challenge, and then at daily intervals for 12 days. As shown in Fig. 4, rIFN-γ-treated BALB/c mice were protected against i.p. challenge. Conversely, neutralization of endogenous IFN-γ in the resistant DBA/2 mouse strain by administering anti-IFN-γ effected a significant decrease in their ability to control the fungus after i.n. or i.p. challenge. These data establish that IFN-γ plays a pivotal role in resistance to Coccidioides whereas IL-4 downregulates protective immunity against this fungus. Since IL-12 is one cytokine that can act early during host defenses to promote the differentiation of cytokine production toward IFN-γ, Magee and Cox (195) examined the effect of administering rIL-12 to BALB/c mice. Treatment of mice with rIL-12 beginning on the day before challenge and at daily intervals for 11 days after i.p. challenge significantly protected the treated mice compared with the ontreated mice (Fig. 5). The protective efficacy of rIL-12 was associated with a shift in the expression of Th1- and Th2-associated cytokines, as evidenced by the finding that IFN-γ expression predominated in the lungs of IL-12-treated infected BALB/c mice whereas IL-4 predominated in lungs of nontreated infected mice. As a complementary approach to assessing the immunoprotective role of IL-12, endogenous IL-12 in the resistant DBA/2 mice was neutralized by treatment with rat anti-mouse IL-12 monoclonal antibody beginning on the day before and continuing on days 3 and 6 after i.p. challenge with arthroconidia. Rat IgG was used as a control. Treatment of the resistant DBA/c mice with rat anti-IL-12 treated rendered them significantly more susceptible to the disease. Two further comments are relevant to these studies. First, rIFN-γ and rIL-12 protected BALB/c mice against i.p. challenge but had no effect when the mice were challenged via the i.n. route. Second, rIFN-γ and rIL-12 immunotherapy was effective in ameliorating the course of disease in BALB/c mice if the cytokines were administered starting 1 day prior to infection and continuing daily for the first 11 days after challenge, but there was no increase in resistance if immunotherapy was initiated after day 5 post-challenge (R. A. Cox and D. M. Magee, unpublished data).
FIG. 4.
Therapeutic effects of rIFN-γ treatment of BALB/c mice. The bars depict means and standard errors of log10 CFU per gram of lungs, livers, and spleens from groups of 13 mice treated with 105 U of rIFN- γ or buffer alone at daily intervals beginning on the day before infection and continuing through 12 days postinfection. The mice were sacrificed 13 days after challenge. Reprinted from reference 196 with permission.
FIG. 5.
Protective effect of rIL-12 against i.p. challenge in BALB/c mice. The bars depict the means and standard errors of log10 CFU per gram of lungs, livers, and spleens obtained from groups of 9 or 10 BALB/c mice treated with 0.1 μg of rIL-12 or buffer alone beginning on the day before and continuing for 11 days after challenge. The mice were sacrificed 12 days after i.p. challenge. Reprinted from reference 195 with permission.
To determine if altering the method of delivery of IL-12 might protect against pulmonary challenge, Jiang et al. (152) constructed a single-chain IL-12 retroviral construct and expressed the construct in the BALB/c-derived J774 cell line. Treatment of BALB/c mice with the IL-12 cDNA-transduced J774 cells inhibited Coccidioides growth in tissues from mice challenged by the i.n. route, as evidenced by significant reductions in the fungal load in the lungs, livers, and spleens at day 12 postinfection compared to the loads in recipients of nontransduced J774 cells. Whereas the recipients of nontransduced J774 cells contained 200 pg of IFN-γ per 100 mg of lung tissue, recipients of IL-12-transduced J774 cells contained 1,300 pg of IFN-γ per 100 mg.
Other studies have corroborated the increased production of Th2 cytokines in susceptible inbred mouse strains. Fierer et al. (111) reported that BALB/c, C57BL/6, and CAST/Ei strains infected with Coccidioides express more IL-10 and IL-4 mRNA than do resistant strains. Of interest, IL-10 knockout mice on a C57BL/6 background were as resistant to Coccidioides as were DBA/2 mice, which is consistent with a role of IL-10 in suppression of cellular immune responses.
Role of antibody in host defense.
Little attention has been given to the role of antibody in the protection of Coccidioides since Kong et al. (175) reported that passive transfer of serum from mice vaccinated with FKS did not protect recipients. To the contrary, they suggested that the serum may have promoted the disease. Beaman et al. (25, 26) extended that finding to show that neither serum nor B cells from immune mice transferred protection against challenge in mice. Beaman et al. (26) also showed that preincubation of arthroconidia with serum from immune mice failed to neutralize the infectivity of the arthrospores. Additional data that argue against a protective role of antibody were obtained in a study by Segal and Catanzaro (262), who showed that the susceptible C57BL/6 mouse produced high titers of antibody against coccidioidin after intramuscular (i.m.) injection of Coccidioides arthroconidia whereas the resistant A/J strain did not. While the evidence for antibody-mediated protection in coccidioidomycosis is not strong, it is possible that there are protective antibodies that could be identified by using monoclonal antibody technology, as has been described for Cryptococcus neoformans (42, 205, 233). Additional studies need to be performed to identify potential protective antibodies in coccidioidomycosis.
Critical Comments
The murine model is the best-studied animal model of coccidioidomycosis and has provided corroboration of the basic protective immune mechanisms with those seen in humans. The advantage of inbred mouse strains is that they provide models for assessing genetic susceptibility against progressive disease. However, this model fails to faithfully reflect human disease in that all mouse strains, regardless of the level of genetic susceptibility, experience disseminated disease early after pulmonary challenge. While the advantages of the mouse model include a wealth of immunological reagents and the availability of mice with selective gene deletions, addition of animal models that more accurately reflect human disease would be of benefit.
VACCINE CANDIDATES
A large body of evidence documents the feasibility of developing a vaccine for coccidioidomycosis. First, persons who recover from a benign or asymptomatic infection with Coccidioides are resistant to exogenous reinfection (114, 213, 220, 277, 279); hence, the fungus has immunizing capacity. Second, the fungus is geographically restricted, thereby delineating the areas of potential infection. Third, the target population is well defined and includes persons who are genetically predisposed to developing disseminated disease and persons who have a high probability of exposure by virtue of their occupation.
Viable Cells
Rixford and Gilchrist (245), in 1896, reported that experimental cutaneous infection of a dog with the exudate of lesions from a coccidioidomycosis patient induced immunity to cutaneous challenge. The immunizing capacity of a sublethal infection was subsequently confirmed by several groups of investigators (62, 65, 224, 227). Converse and Besemer (62) reported that vaccinating cynomolgous monkeys by subcutaneous (s.c.) injection of 101 to 108 viable arthroconidia protected the monkeys against respiratory challenge with approximately 7,000 viable arthroconidia, as judged by their healthy appearance throughout a 4-month observation period, normal chest X rays, only a minor histopathologic changes at autopsy, and negative lung cultures in 80% of the animals.
The use of viable cells posed a major limitation, however, in that organisms persisted at the site of vaccination and not infrequently were demonstrable at distal sites, even when the vaccine was administered by the s.c. route (65, 173). In an effort to mitigate the risk of disease from vaccination with live Coccidioides cells, Pappagianis et al. (224) vaccinated mice by using a riboflavin-requiring auxotrophic mutant created by Foley et al. (121) by X irradiation of Coccidioides strain Silveira. The mutant strain engendered immunity to challenge but reverted in vivo to a prototrophic state that was accompanied by a reacquisition of virulence. Similar findings were obtained with a cobalt-irradiated mutant of Coccidioides and with spherules that had been attenuated by in vitro passage (174). Efforts to reduce the risks imposed by viable attenuated vaccines included prevaccination with killed organisms (62) and treatment of vaccinated mice with amphotericin B (43). Both approaches ameliorated the disease but were not applicable in humans because of the potential adverse consequences.
Nonviable Cells
Owing to the lack of a suitable medium for culturing spherules, most of the early work with nonviable vaccines was conducted with mycelium-phase cells (64, 124, 173, 185, 186). Following the development of a medium for culturing spherules (61), Levine and coworkers (173, 185) compared the vaccine capacity of the parasitic and saprobic forms of Coccidioides. Mice vaccinated i.m. with 1.5 mg of FKS survived an i.n. challenge dose of 104 arthroconidia, whereas only 50% of those vaccinated with killed mycelia survived challenge with Coccidioides. Immunogenicity of the parasite-phase cells increased with maturation, with killed spherules being more protective than endospores. Whether these differences are attributed to qualitative or quantitative differences in immunogens that comprise the morphogenetic phases of Coccidioides is not known.
The effect of the dose, schedule, and route of immunization with killed spherules was delineated in studies by Levine et al. (187) using the murine model. Mice (NAMRU strain) immunized with 2 to 2.7 mg of FKS were more resistant to i.n. challenge than were mice given lower doses. Administration of the vaccine in three or more injections over a period of 7 days engendered a higher level of protection than did a single injection, and protection was enhanced when the vaccine was given at multiple sites (187). Although demonstrable levels of immunity were established as early as 7 days after the last vaccination, protection was more pronounced at 21 to 30 days. The route of vaccine delivery proved to be a major determinant of protective efficacy. Both the i.m. and s.c. routes were effective in eliciting protection against i.n. challenge, whereas immunization via an i.n. route was less protective and intravenous (i.v.) immunization offered the least protection. Indeed, i.v. injection of 120 μg of killed spherules prior to, concomitant with, or within 35 days of i.m. vaccination with killed spherules diminished the protective efficacy of the latter. On the basis of the foregoing studies, studies of the murine model have used 2.1 mg of the FKS vaccine, administered in three i.m. injections of 0.7 mg at weekly intervals. Using this protocol, we examined the efficacy of the FKS vaccine in the genetically susceptible BALB/c mouse strain (77). As shown in Fig. 6A, BALB/c mice were protected against pulmonary challenge with 30 arthroconidia of Coccidioides strain Silveira. Also, in contrast to the anergy that develops in nonimmunized BALB/c mice (Fig. 2), FKS-vaccinated BALB/c mice were able to maintain their footpad hypersensitivity response to coccidioidin at a level comparable to that is vaccinated or nonvaccinated DBA/2 mice (Fig. 6B).
FIG. 6.
(A) Survival of FKS-vaccinated and nonvaccinated BALB/c mice on days 1 through 30 after i.n. infection with 100 arthroconidia. Each group consisted of 15 mice. (B) Footpad hypersensitivity in FKS-vaccinated and nonvaccinated BALB/c and DBA/2 mice 15 days after i.n. infection with 10 arthroconidia. The bars depict means and standard errors (SE) obtained with groups of 15 mice. Reprinted from reference 77 with permission.
Levine et al. (188) evaluated the FKS vaccine in cynomolgous monkeys. Nine female monkeys, with an average weight of 1.8 kg, were given 1.5 mg of FKS s.c. on day 1, 1.5 mg i.m. on day 11, 3 mg s.c. on day 28, and 3 mg i.m. on day 54. Nine control monkeys were treated with corresponding volumes of saline. The monkeys were challenged on day 107 (53 days after the completion of the vaccination or placebo immunization) with arthroconidia of strain Silveira. The arthroconidia were suspended in physiologic saline at a concentration of 5 × 103 viable particles per ml, and a cloud was generated from the suspension by using an atomizer in a modified Henderson apparatus. The calculated dose was 200 arthroconidia per animal, except for one of the vaccinated monkeys who was thought to have been exposed to 400 arthroconidia. During the 265-day observation period, five of the control monkeys died (days 39, 44, 63, 116, and 244) whereas only one of the vaccinated monkeys died of the disease. The latter monkey was the one that had inadvertently received 400 spores. One other vaccinated monkey was killed accidentally during cardiac puncture on day 63 postinfection. Survivors on day 262 after challenge were skin tested with coccidioidin. Two of seven vaccinated monkeys and all four of the controls were skin test negative. It was noted that the two vaccinated monkeys had shown sensitivity after vaccination and before challenge; hence, the subsequent negative skin test reactivity represented an acquired anergy. CF antibody titers were increased in both vaccinated and control monkeys, and the median titers were similar in the two groups. At necropsy, both vaccinated and control monkeys showed pulmonary and disseminated involvement, but the disease was judged to be less severe in the vaccinated monkeys than in the control monkeys.
The efficacy of the FKS vaccine in the murine and monkey models, coupled with the severity of coccidioidomycosis in patients with disseminated disease, prompted clinical trials to assess its toxicity and potential use in humans. Recipients of a total of 2.7 mg of killed spherules, given i.m. in one to three injections, showed localized tenderness or induration at the site(s) of injection but otherwise tolerated the vaccine well. Doses greater than 10 mg produced marked and unacceptable levels of toxicity, an effect that could be attributable to the induction of proinflammatory cytokines by the FKS (4, 273). Based on these and other trials (309), a regimen of three doses of 1.75 mg each was chosen for use. Approximately 7% of recipients showed adverse reactions, ranging from local inflammation to systemic toxicity. Skin test conversions to coccidioidin and/or spherulin occurred in approximately half of vaccine recipients and generally persisted for longer than 6 months; 16% showed seroconversion with reactivity to CF, tube precipitin (TP), or both (309). It is noteworthy that no differences were observed in the immunologic responses of Caucasians, African Americans, or Filipinos.
The protective efficacy of the spherule vaccine was evaluated in a double-blind multicenter study between 1980 and 1985 (218, 223). A total of 2,867 healthy persons who were skin test negative to both coccidioidin and spherulin were randomized into placebo and vaccine groups. The vaccine recipients were given three injections of 1.75 mg of the FKS; the placebo group was given saline injections. After a 5-year follow-up period, no significant difference was observed in the incidence or severity of coccidioidal cases that developed in the two groups of subjects (218). The incidence of coccidioidomycosis was low while this study was being conducted, with only 21 cases occurring in the two study groups; this low incidence probably contributed to the lack of detectable vaccine-induced protection. Given the marked increase in infections that occurred during 1991 and into 1994, it would be of great interest to retrospectively determine if subjects enrolled in the FKS vaccine study might have acquired coccidioidomycosis during the epidemic and, if so, whether differences exist in the incidence of the disease in those who received the vaccine and those who received the placebo vaccine.
It has been suggested that the ineffectiveness of the FKS vaccine in the clinical trials may be attributable to the low dose of the vaccine used, owing to its toxicity (218, 220). On a body weight basis, the quantity of the FKS vaccine that was used in the human trials was less than 0.1% of the dose needed to immunize mice. These discouraging results obtained with the FKS vaccine in humans prompted investigators to seek subcellular vaccines. On the basis that Kong et al. (172) reported that the protective capacity of killed spherules resided primarily in their cell walls, most investigators have focused on the identification and isolation of immunoprotective cell wall components.
Cell-Derived Antigens
Antigens, either native extracts, recombinant proteins, or genes, have been reported to induce protection and could be candidates for a univalent or, most probably, multivalent vaccine. A summary of the major results obtained with leading vaccine candidates is presented in Table 4, which focuses on the strain of mice, with BALB/c being the most genetically susceptible; the route of challenge, with pulmonary challenge being the most relevant and rigorous; the number of arthroconidia; and whether protection was measured as a reduction in CFU or increased survival or both.
TABLE 4.
Experiments evaluating vaccine candidatesa
| Immunogen | Immunization | Adjuvant or vector | Challenge
|
CFU in lungs | CFU in spleen | Survival | Reference | ||
|---|---|---|---|---|---|---|---|---|---|
| Mouse strain | Route | Dose (no. of arthroconidia) | |||||||
| FKS | Whole killed cells | None | NAMRU | i.n. | 49 | ND | ND | ↑ | 186 |
| FKS | Whole killed cells | None | BALB/c | i.n. | 30 | ND | ND | ↑ | 77 |
| FKS | Whole killed cells | None | BALB/c | i.n. | ↓ | ↓ | 154 | ||
| 27K | Cell extract | Alum | Swiss Webster | i.n. | 500 | ND | ND | NS | 325 |
| 27K | Cell extract | Alum | Swiss Webster | i.n. | 5,000 | ND | ND | ↑ | 325 |
| 27K | Cell extract | Alum | Swiss Webster | i.n. | 15,000 | ND | ND | ↑ | 325 |
| 27K | Cell extract | Alum | Swiss Webster | i.v. | 500 | ND | ND | ↑ | 325 |
| 27K | Cell extract | Alum | Swiss Webster | i.v. | 5,000 | ND | ND | ↑ | 325 |
| 27K | Cell extract | CFA | BALB/c | i.n. | 30 | ↓ | ↓ | ND | This review |
| Ag2/PRA | rProtein | IFA/CFA | BALB/c | i.p. | 50 | ↓ | ↓ | ND | 167 |
| Ag2/PRA | rProtein | RIBI (730/700) | BALB/c | i.p. | 250 | NS | ↓ | NS | 154 |
| Ag2/PRA | rProtein | RIBI MPL | BALB/c | i.p. | 50 | ↓ | ↓ | ND | 1 |
| Ag2/PRA | DNA | pVR1012 | BALB/c | i.p. | 2,500 | ↓ | ↓ | ↑ | 1 |
| Ag2/PRA | DNA | pVR1020 | BALB/c | i.p. | 50 | NS | NS | ND | 1 |
| Ag2/PRA | rProtein | RIBI MPL-SE | BALB/c | i.n. | 7 | ND | ND | ↑ | 264 |
| Ag2/PRA | rProtein | RIBI MPL-SE | BALB/c | i.n. | ≥10 | ND | ND | NS | 264 |
| Ag2/PRA | rProtein | RIBI MPL-AF | BALB/c | i.n. | ≤97 | ND | ND | ↑ | 264 |
| Ag2/PRA | rProtein | RIBI MPL-AF | BALB/c | i.n. | >97 | ND | ND | NS | 264 |
| Ag2/PRA | rProtein | RIBI MPL | C57BL/6 | i.p. | 500 | ↓ | ↓ | ND | 1 |
| Ag2/PRA | rProtein | RIBI MPL-SE | C57BL/6 | i.n. | ≤145 | ND | ND | ↑ | 264 |
| Ag2/PRA | DNA | pVR1020 | C57BL/6 | i.p. | 500 | ↓ | NS | ND | 264 |
| Urease | rProtein | CpG-ODN/IFA | BALB/c | i.p. | 100 | ↓ | ↓ | ↑ | 189 |
| Urease | DNA | pSecTagA2 | BALB/c | i.p. | 100 | ND | ND | ↑ | 189 |
| SOWgp | rProtein | IFA/CFA | BALB/c | i.p. | 50 | ↓ | ↓ | ND | 163 |
| CSA | rProtein | CpG/MPL | C57BL/6 | i.p. | 49 | ↓ | ↓ | ↑ | 318 |
| GEL-1 | rProtein | IFA/CpG-ODN | BALB/c | i.p. | 100 | ↓ | ↓ | ND | 89 |
| GEL-1 | rProtein | IFA/CpG-ODN | C57BL/6 | i.n. | 80 | ND | ND | ↑ | 89 |
| ELI-Ag1 | DNA | pBK-CMV | BALB/c | i.p. | 2,500 | ND | ND | ↑ | 150 |
r, recombinant; MPL-SE, monophosphoryl lipid—stable emulsion; MPL-AF, monophosphoryl lipid—aqueous formulation; ND, not done; NS, not significant; ↓, decreased; ↑ increased.
PBS extract of spherule cell walls.
Extraction of an immunoprotective component(s) from cell walls of spherules was first reported by Pappagianis et al. (222). These investigators isolated the cell walls by mechanical disruption of spherules of strain Silveira. Incubation of the cell walls for 5 days with constant agitation in phosphate-buffered saline (PBS) containing 2% chloroform as a preservative released a wall fraction which, when admixed with complete Freund's adjuvant (CFA) or alum, protected NAMRU mice against pulmonary challenge with 103 arthroconidia. The immunoprotective cell wall fraction contained some fragments, and, when the latter were removed by centrifugation, the opalescent supernatant was protective. It was not determined if the immunizing component(s) was soluble or present as a colloid; as of this writing, there have been no further reports of the spherule wall-derived PBS extract.
27K.
On the basis that FKS protects experimental-animal models against pulmonary challenge with Coccidioides, Zimmermann et al. (325) sought to isolate the protective immunogen(s) directly from FKS. The whole killed spherules of strain Silveira were mechanically disrupted, and the supernatant obtained after centrifugation of the lysate at 27,000 × g was designated 27K. When suspended in saline to a concentration of 3.5 mg/ml, 27K was slightly opalescent, which is reminiscent of the PBS wall fraction discussed above. Three weekly immunizations of outbred Swiss-Webster mice with 1 mg of the 27K vaccine with alum induced strong protective immunity against i.n. challenge with 5,000 arthroconidia (P = 0.003) and 15,000 arthroconidia (P = 0.04), as judged by survival over a 13-week period after challenge (Fig. 7). Of particular importance, the immunity induced by the 27K vaccine was equal to that induced by the FKS vaccine. In a one study, we have assessed the protective capacity of the 27K vaccine in inbred mice (Magee and Cox, unpublished). Groups of susceptible BALB/c mice were immunized three times at weekly intervals with 250 μg of the 27K vaccine in Freund's adjuvant. CFA was used for the first immunization, and incomplete Freund's adjuvant (IFA) was used for the last two injections. The animals were challenged i.n. with 30 arthroconidia 2 weeks after the last immunization and sacrificed 12 days after challenge for determination of total fungal CFU in the lungs and spleens. The results show that there was greater than a 2 log and 3 log reduction in fungal CFU in the lungs and spleens, respectively, in 27K-immunized mice compared to adjuvant-immunized mice (P < 0.001) (Fig. 8). The cumulative results of all the reports to date show that the 27K vaccine provides the best protection by a subcellular fraction of Coccidioides.
FIG. 7.
Vaccine efficacy of 27K against i.n. challenge. Swiss Webster mice were immunized with 27K in alum, 27K alone, or alum alone and challenged with 5,000 arthroconidia via an i.n. route. Survival was monitored daily for 75 days post challenge. Reprinted from reference 325 with permission.
FIG. 8.
Vaccine efficacy of 27K against i.n. challenge in susceptible mice. BALB/c mice were immunized with 27K in CFA/IFA or alum CFA/IFA alone and challenged with 30 arthroconidia via an i.n. route. Survival was monitored daily for 45 days after the challenge.
It is curious that the high level of protection engendered by the 27K vaccine against pulmonary challenge was achieved using alum, since this adjuvant enhances antibody but not Th-cell responses. It is even more curious that the vaccine alone, without alum adjuvant, induced protection, albeit at a lower level than that provided by 27K in alum. Perhaps there is a particulate component(s) in the 27K vaccine which serves as an antigen depot for presentation and processing by APCs.
Ag2/PRA.
In 1975, Ward et al. (298) isolated an alkali-soluble, water-soluble fraction from trypsin-treated cell walls of Coccidioides mycelia and showed that the cell wall extract elicited delayed-type footpad hypersensitivity response in mice immunized with Coccidioides. Lecara et al. (183) showed that the C-ASWS extract from mycelium-phase cells induced protective immunity in mice. Immunization of DBA/2 mice with a total of 0.5 mg of the mycelium-derived C-ASWS in CFA afforded a significant degree of protection against i.p. challenge with 1,500 arthroconidia of strain Silveira. Mice challenged with 50 arthroconidia by an i.n. route were also protected. Of 24 DBA/2 mice vaccinated with a total of 1 mg of C-ASWS in CFA, 20 (83%) were significantly protected, compared to the survival of 14 (56%) of 25 mice given CFA alone (P < 0.05). The C-ASWS extract also protected DBA/2 mice against i.n. challenge with 500 arthroconidia, with a 53% survival on day 30 postinfection versus 30% in control mice (P < 0.01). Protection was not obtained against challenge with 1,500 arthroconidia. Subsequent analyses showed that the spherule-derived C-ASWS was more protective than the mycelium-derived extract and that while the mycelium- and spherule-derived C-ASWS extracts protected DBA/2 mice against i.n. challenge, neither induced protective immunity against i.n. challenge in the highly susceptible BALB/c mice (Cox and Magee, unpublished). In addition to its protective capacity, C-ASWS was shown to be reactive in eliciting in vitro lymphocyte proliferation responses in healthy, coccidioidin skin test-positive persons and patients with various stages of coccidioidomycosis (75, 82). C-ASWS was also shown to react with TP antibody (74) and anti-Coccidioides IgG and IgE antibodies (69).
Antigenic analyses of C-ASWS by two-dimensional immunoelectrophoresis (2D-IEP) revealed that it is a high-molecular-weight polysaccharide-protein complex containing a polymeric antigen which has antigenic identity to the coccidioidin precipitinogen that Huppert et al. (147) designated as antigen 2 (Ag2) (71). The extract also contained a second component that showed an incomplete precipitin antigen in 2D-IEP. The latter was subsequently identified as the TP antigen (71). Solid- phase immunoadsorption of coccidioidin with goat antiserum against C-ASWS affected the adsorption of Ag2 and trace amounts of the TP antigen. The desorbed; Ag2-enriched fraction elicited significant footpad hypersensitivity responses in Coccidioides-infected mice, to a level comparable to the responses elicited by coccidioidin (67). Attempts to purify Ag2 from the polysaccharide-enriched TP antigen were unsuccessful. Rather, both antigens coeluted on lectin affinity, molecular-sieve, and ion-exchange columns, suggesting that the two antigens are physically or chemically complexed (67, 71, 74). Studies were undertaken, therefore, to clone the gene that encodes Ag2. Using a polyclonal goat antiserum prepared against the Ag2-enriched solid-phase immunoadsorption fraction and a monoclonal antibody directed against Ag2 (76) to identify Ag2-positive clones, Zhu et al. (324; Y. Zhu, C. Yang, D. M. Magae, and R. A. Cox, Abstr. Third Natl. Inst. Allergy Infect. Dis. Workshop Med. Mycol., 1993.) cloned a 1,255-bp gene encoding Ag2 (Fig. 9). The cloned Ag2 cDNA encodes a 194-amino-acid protein having an 18-amino-acid N-terminal signal sequence and a C-terminal glycosylphosphatidylinositol (GPI) anchor. The cloned Ag2 gene encodes a product that contains a region with 10 tetrapeptide repeats TXX′T, where X is alanine, histidine, or glutamic acid and X′ is alanine, valine, or glutamic acid. Whereas no N-glycosylation sites were predicted, several O-glycosylation sites were predicted within a Thr-rich region (Thr97-Thr165). In an ensuing study, Zhu et al. (323) cloned the Ag2 gene from genomic DNA and showed that it contains two introns, 78 and 101 bp long.
FIG. 9.
Nucleotide sequence and deduced amino acid sequence of cloned Ag2 cDNA. DNA base numbers are on the left, and amino acid numbers are on the right. The underlined amino acid region at the beginning of the N terminus of the Ag2 protein is the signal sequence. The doubly underlined amino acid region contains the tetrapeptide repeats. The underlined amino acid region at the C terminus is the GPI anchor. Reprinted from reference 324 with permission.
The cloned Ag2 cDNA was expressed in Escherichia coli as a glutathione S-transferase (GST) fusion peptide and shown to elicit footpad hypersensitivity tests of FKS-immunized mice at a level comparable to that of C-ASWS (324). For determinations of vaccine efficacy, Jiang et al. (154) immunized BALB/c mice with three weekly injections of 100 μg of recombinant Ag2 (rAg2) fusion protein. The first immunization was given in RIBI 730 adjuvant via an i.m. route; the two subsequent immunizations were given in RIBI 700 adjuvant via an s.c. route, and the mice were challenged i.p. with 250 arthroconidia of strain Silveira 1 month after the last immunization. The rAg2 fusion protein induced a significant reduction in the fungal load in the livers and spleens by day 12 postinfection (Fig. 10A) but did not increase survival compared to that control of mice immunized with the GST peptide alone (Fig. 10B). The low protective efficacy of the rAg2 peptide, expressed and isolated from E. coli, was thought to be attributable to a lack of posttranslational modification(s) required for optimal presentation and/or processing to APCs. Since genetic immunization provides a means of producing native antigen in vivo and has been documented to be highly effective in inducing protective cellular and humoral immune responses (261), studies were done to evaluate genetic immunization with Ag2 cDNA ligated to the plasmid pVR1012 (154). The pVR1012 DNA plasmid vector (Vical, Inc.) contains a kanamycin resistance gene and the human cytomegalovirus promoter and lacks a signal (leader) sequence. BALB/c mice were immunized by three weekly i.m. immunizations each with 50 μg of Ag2 cDNA-pVR1012 and challenged i.p. with Coccioides. As shown in Fig. 11A, vaccinated BALB/mice showed a 2-log or greater reduction in fungal load on day 12 after infection with 2,500 arthroconidia, a dose 10-fold greater than that used in protection studies of rAg2. The decreased fungal load in the lungs, livers, and spleens was accompanied by increased survival, with 20 of 20 mice vaccinated with Ag2 cDNA-pVR1012 surviving a for 40-day period postinfection, compared to the survival of only 1 (9%) of 20 mice vaccinated with plasmid pVR1012 alone (Fig. 11B). Protection was associated with the induction of footpad hypersensitivity and the production of IFN-γ. Genetic immunization with Ag2 cDNA-pVR1012 reduced the fungal load in the livers and spleens of BALB/c mice challenged with 50 arthroconidia via an i.n. instillation but did not reduce the fungal load in the lungs (Fig. 12) or decrease mortality. In our experience, failure to protect at the lung level predicts failure to prolong survival in vaccinated mice (Magee and Cox, unpublished). In contrast to the results obtained with Ag2 cDNA, mice vaccinated with the FKS vaccine were strongly protected against i.n. challenge. The latter is an important finding, since it establishes that BALB/c mice can be protected against i.n. challenge.
FIG. 10.
Vaccine efficacy of rAg2, expressed as a GST fusion peptide, in BALB/c mice challenged by the i.p. route with 250 arthroconidia. Results are expressed as the numbers of CFU (means and standard errors) in the lungs, livers, and spleens on day 12 postinfection (A) and the percent survival in mice on days 1 through 30 postinfection (B). Reprinted from reference 154 with permission.
FIG. 11.
Vaccine efficacy of pVR1012-Ag2 cDNA against i.p. challenge. BALB/c mice were immunized with pVR1012-Ag2 cDNA or pVR1012 alone and then challenged with 2,500 arthroconidia. (A) Fungal load, expressed as log10 CFU/g of tissue, was determined 12 days after challenge. Bars depict means and standard errors obtained with groups of 20 mice. (B) Percent survival was determined on days 1 through 40 for groups of 11 mice. Reprinted from reference 154 with permission.
FIG. 12.
Vaccine efficacy of pVR1012-Ag2 cDNA and FKS against i.n. challenge. BALB/c mice were immunized with pVR1012-Ag2 cDNA, pVR1012 alone, or FKS and then challenged with 50 arthroconidia via the i.n. route. Fungal load, expressed as log10 CFU per gram of tissue, was determined 12 days after challenge. Reprinted from reference 154 with permission.
Jiang et al. (151) reported that administration of Ag2 cDNA-pVR1012 with single-chain IL-12 cDNA (p40-L-p35), each subcloned into the pVR1012 plasmid, significantly enhanced protection against i.p. challenge with 2,500 arthroconidia over that conferred by Ag2 cDNA alone. The enhanced protection was associated with increased IFN-γ production, at levels higher than those induced in mice vaccinated with Ag2-pVR1012 or IL-12-pVR1012 alone. Analyses of the anti-Coccidioides IgG isotype response revealed a predominant IgG1 response, which is associated with a Th2 response, and a low IgG2a response. While it is recognized that antibody isotype responses may vary during the course of immunization and infection, owing to the changing dynamics of Th1 and Th2 responses, the predominance of IgG1 both before and after challenge is paradoxical, particularly in the absence of detectable IL-4, and merits further study. When mice immunized with Ag2 pVR1012 and IL-12 cDNA were challenged with 50 arthroconidia via the i.n. route and evaluated for fungal load 12 days later, there was a significant decrease in the fungal load in the spleens and livers but not in the lungs.
Recently, Jiang et al. (153) reported that the signal sequence encoded in the first 18 amino acids of the translated Ag2 cDNA protected mice against i.p. challenge with 2,500 arthroconidia, as measured by fungal load and survival. The level of protection induced by the signal sequence was lower than that induced by the full-length Ag2(1-194) cDNA, but the difference was not statistically significant. A synthetic peptide corresponding to the Ag2 cDNA signal sequence also induced protection, albeit at a lower level. The protection induced by the signal sequence cDNA was not attributable to a nonspecific immunopotentiation form nucleotide sequences, as establishing by the finding that immunization of BALB/c mice with a frameshift mutation of the Ag2(1-18) cDNA, created with PCR by deleting the first nucleotide in the second codon, did not induce protection. The protection induced by the signal sequence vaccine correlated with an IFN-γ response and was not associated with the production of anti-Coccidioides antibody. The lack of an antibody response to Ag2(1-18) cDNA is consistent with an earlier report showing that a synthetic Ag2(1-18) peptide was not detected by hyperimmune goat anticoccidioidin or anti-Ag2 antisera or sera from patients with active coccidioidomycosis (321). The Ag2(19-194) cDNA construct, which is devoid of the signal sequence, induced protection, but to a significantly lower level than the signal sequence or the full-length Ag2(1-194) cDNA.
In addition to its T-cell reactivity and vaccine capacity, the rAg2 fusion peptide showed reactivity with sera of patients with active coccidioidomycosis. Using PCR-generated Ag2 truncations to identify the B-cell reactive epitopes, Zhu et al. (321) established that rAg2 peptide expressed both linear and conformational B-cell-reactive epitopes localized to a domain composed of amino acids 19 to 96 [Ag2(19-96)]. Truncations designed to identify epitopes within the Ag2(19-96) domain yielded fragments that were either nonreactive [Ag2(62-194), Ag2(19-61), and Ag2(49-79)] or showed reduced reactivity [Ag2(19-70)] when assayed by ELISA and immunoblotting. When assayed against sera from 28 patients with active coccidioidomycosis, 79% of the sera showed reactivity to the rAg2(19-96) fusion peptide. No reactivity was demonstrable with sera from histoplasmosis or blastomycosis patients.
In 1991, Dugger et al. (103) deglycosylated TSL by using anhydrous fluoride and then isolated a 33-kDa peptide from the deglycosylated antigen by molecular sieve chromatography. The 33-kDa peptide showed antigenic identity to the anodal precipitin leg of Ag2 when assayed by 2D-IEP in tandem with coccidioidin and was reactive with an IgG2a monoclonal antibody specific to Ag2 (76). The purified 33-kDa antigen elicited proliferation in peripheral blood mononuclear cells of healthy, skin test-positive persons and reacted with sera from coccidioidomycosis patients (131). Amino acid analysis of the 33-kDa peptide revealed an unusually high proline (17%) and threonine (15%) content. Immunoelectron microscopy using affinity-purified human antibodies revealed that the antigen was localized to the inner cell wall and cleavage planes of developing spherules. In endospore preparations, the 33-kDa antigen appeared to be exposed on the wall surface and the interconnecting glycocalyx (131).
Almost contemporaneously with the cloning of Ag2 by Zhu et al. (323, 324; Zhu et al., Abstr. Third NIAID Workshop, 1995), Dugger et al. (104) cloned the gene that encodes the 33-kDa peptide by screening a spherule/endospore phase lambda ZAP cDNA expression library with goat anti-33-kDa antiserum. Nucleic acid sequence analyses revealed a 582-bp open reading frame (ORF) encoding a 194-amino-acid protein with a predicted molecular mass of 19.5 kDa. The deduced amino acid composition showed a high proline and threonine content (12.9 and 11.3 mol%, respectively), and, on that basis, the 33-kDa peptide was given the designation “proline-rich antigen” (PRA).
Kirkland et al. (167) ligated the gene encoding PRA into pET32a and expressed the construct in E. coli as a histidine-tagged recombinant protein. After removal of the histidine tag by thrombin cleavage, the rPRA elicited a T-cell proliferation in lymph node T cells from mice immunized with recombinant PRA (rPRA) and a weak but statistically significant response in mice immunized with FKS or live attenuated Coccidioides strain 95-291 (294). Immunization of BALB/c mice with 5 μg of rPRA in IFA, followed by CFA, protected the mice against i.p. challenge with 50 arthroconidia of Coccidioides (R.S. strain), as judged by a reduced fungal load in the lungs and spleens compared to the fungal load in mice immunized with CFA alone. Abuodeh et al. (1) extended these studies to a comparison of the vaccine efficacy of rPRA and PRA cDNA. BALB/c and C57BL/6 mice, which are less susceptible to Coccidioides than are BALB/c mice (164, 166), were immunized with 5 μg of rPRA emulsified in MPL adjuvant (see “Adjuvants” below) and then given a booster injection 4 weeks later. The mice were challenge i.p. using 50 arthroconidia for BALB/c mice and 500 arthroconidia for C57BL/6 mice. The rPRA in MPL adjuvant induced protection against i.p. challenge in both BALB/c and C57BL/6 mice, as judged by a reduced fungal load compared to that in mice given saline alone. It is noted that the appropriate control group for this experiment would have been mice given the MPL adjuvant alone, as opposed to saline, since adjuvant alone can increase the host response.
For genetic vaccination, BALB/c and C57BL/6 mice were immunized i.m. with 100 μg of PRA cDNA ligated to pVR1020. This DNA vector is similar to pVR1012, except that it contains the tissue plasminogen activator signal peptide upstream of the BamHI cloning site. At 4 weeks after the first immunization, the BALB/c and C57BL/6 mice were boosted; they were challenged 4 weeks later with 100 and 500 arthroconidia, respectively, via an i.p. route (1). When the DNA vaccine was used, there was a reduction in fungal CFU in mice vaccinated with the PRA cDNA-pVR1020 construct compared with that in mice vaccinated with saline alone. However, and most importantly, there was not a significant difference in the fungal load in the lungs of BALB/c mice vaccinated with PRA cDNA-pVR1020 and those vaccinated with the pVR1020 vector alone, the latter being a more appropriate control than saline. In contrast to the results obtained with the highly susceptible BALB/c mice, immunization of C57BL/6 mice with the PRA cDNA-pVR1020 construct significantly reduced the fungal load in the lungs compared to that in mice given the vector alone (P = 0.017). Splenocytes from both BALB/c and C57BL/6 mice immunized with the recombinant or PRA gene vaccine showed a proliferative response to rPRA and secreted IFN-γ in vitro in response to rPRA. IL-4 production was not observed. The predominant IgG isotype response to PRA was IgG1, which is consistent with the findings by Jiang et al. (151) and raises the question why the antibody response is a Th2 rather than a Th1 response.
Whereas the preceding studies evaluated the protective capacity of rPRA and the PRA gene vaccine against i.p. challenge, Shubitz et al. (264) examined the protective capacity of rPRA in BALB/c and C57BL/6 mice challenged by i.n. infection. BALB/c and C57BL/6 mice, vaccinated s.c. with 1 or 5 μg of rPRA in monophosphoryl lipid A (MPL-AF [see “Adjuvants” below]) and boosted 4 weeks later, were protected against i.n. challenge with 7 arthroconidia but not 10 or more arthroconidia. Whereas 20 (59%) of 34 BALB/c mice immunized with rPRA in MPL-AF survived infection, only 4 (17%) of 24 mice immunized with the MPL-AF adjuvant alone survived (P < 0.005). The investigators noted, however, that none of the survivors in either the PRA-vaccinated or adjuvant control group showed evidence of infection at necropsy, which raises the question whether they had been infected. This is an important point, since even the FKS vaccine does not induce sterilizing immunity against pulmonary challenge with Coccidioides arthroconidia (325; Cox and Magee, unpublished). Studies with the more resistant C57BL/6 strain showed that rPRA-vaccinated mice were resistant to i.n. challenge with as many as 145 arthroconidia, which is a 21-fold-increased dose compared to that given the BALB/c mice. Of particular interest, the investigators reported that i.n. immunization was much more protective than s.c. immunization. This is an important finding, with implications for vaccine trials in humans.
Peng et al. (230) generated overlapping subunits of PRA to determine which region might express the protective epitope(s). Comparison of plasmids encoding PRA(1-106), PRA(27-106), PRA(90-151), or PRA(90-194) showed that mice vaccinated with PRA(1-106) or PRA(27-106) had a significantly reduced-fungal load (P < 0.05) after i.p. challenge with 50 arthroconidia compared to mice immunized with vector alone. Identical results were obtained when mice were immunized with recombinant proteins encoding the same PRA subunits. Neither a plasmid nor a recombinant product encoding the signal sequence alone, which was shown by Jiang et al. (153) to be protective, was evaluated.
The genes encoding Ag2 and PRA have identical nucleic acid sequences and, for that reason, are now collectively designated Ag2/PRA (153, 264). The combined studies discussed above establish that rAg2/PRA will protect BALB/c mice against an i.p. challenge and a low dose given by the i.n. route when the rAg2/PRA is used with the MPL adjuvant. The more resistant C57BL/6 strain can be protected against both routes of challenge. While the ability to protect C57BL/6 against pulmonary challenge is an important and valuable finding, the low level of protection in the genetically susceptible BALB/c mice leads to the question whether the Ag2/PRA vaccine will be able to protect persons who are known to have a genetic predisposition to severe, disseminated coccidioidomycosis.
T-cell-reactive protein.
Cole et al. (56) isolated a water-soluble fraction from arthroconidia which had been stripped of their hydrophobic outer wall layer and showed that the soluble conidial wall fraction elicited lymphocyte proliferation in lymph node cells from (BALB/c × DBA/2)F1 (CD2F1) mice that had been immunized with live spherules of the attenuated strain 95-271 and then boosted with FKS. When analyzed by advancing-line immunoelectrophoresis with a reference coccidioidin/burro anti-coccidioidin system that had been developed by Huppert and et al. (147), at least 10 antigens were identified, notably Ag2 and an antigen designated AgCS. When analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), at least 14 bands were detected. The soluble conidial wall fraction showed reactivity in immunodiffusion assays for antibodies against the CF and TP antigens.
Kirkland et al. (169) subjected the soluble conidial wall fraction to SDS-PAGE and electrotransferred the bands to nitrocellulose membranes. The antigen-bearing nitrocellulose membranes were cut into strips and tested for their reactivity in a T-cell proliferation assay using a soluble conidial wall fraction-specific murine T-cell line derived from lymph node cells of immunized (BALB/c × DBA/2)F1 mice. A 43- to 66-kDa region was found to be the most stimulatory. In concomitant experiments, the investigators developed a lambda gt11 mycelium-phase cDNA library and, using antiserum to the soluble, conidial wall fraction, cloned a 200-bp cDNA insert that encoded a protein which was shown to induce proliferation in the soluble conidial wall fraction-specific T-cell line. This T-cell-reactive protein (TCRP) was cloned by Wyckoff et al. (316). The tcrP gene contains a 1,197-bp ORF that encodes a hydrophobic 45.2-kDa protein with 50% identity and 70% homology to a mammalian cytoplasmic enzyme, 4-hydroxyphenylpyruvate dioxygenase, which is involved in the degradation of phenylalanine to tyrosine, and a similar identity and homology to mammalian F antigen, which is a major epitope of the mammalian liver. The gene does not contain a signal sequence or GPI anchor site.
Kirkland et al. (168) ligated the tcrP gene with the pET28b plasmid and expressed the construct in E. coli. The 48-kDa recombinant fusion protein was used to induce anti-TCRP serum in rabbits, and experiments using the antiserum showed that the antigen was expressed in the cytosolic fraction of parasite-phase cells. The rTCRP stimulated proliferation and production of IFN-γ in lymph node T cells from FKS-immunized BALB/c mice. However, the rTCRP was only weakly protective when evaluated for protective efficacy in rTCRP-immunized BALB/c mice that had been challenged by the i.p. route.
The relatively low protective capacity of rTCRP and its homology to mammalian proteins argue against its use as a component of a vaccine against Coccidioides.
Spherule outer wall.
When spherules are grown in liquid Converse medium, they shed a lipid-rich, membranous outer wall material which may be similar to the extracellular matrix described by Frey and Drutz (123). Cole et al. (57) extracted the spherule outer wall (SOW) fraction of Coccidioides strains C634 and C735 by detergent extraction with the nonionic detergent N-octyl-d-glucopyranoside (OG). Tandem 2D-IEP of the OG-soluble fraction revealed the presence of Ag2, AgCS, and at least two other unidentified components. When assayed by SDS-PAGE, three Coomassie blue-stained polypeptide bands of 66, 58, and 14 kDa were detected. A fourth band, determined to have a size of 19 kDa, was not well stained. In immunoblot assays, the OG-soluble fraction showed two bands that were reactive with sera from coccidioidomycosis patients. These bands corresponded to the 66- and 19-kDa components observed in SDS-PAGE. The isolated SOW and OG-soluble fraction elicited proliferation in lymphocytes from immunized (BALB/c × DBA/2)F1 mice, and in a separate study, SOW was found to protect BALB/c mice and C3H mice against i.p. challenge with 50 arthroconidia (168). The extract was also reactive with sera from coccidioidomycosis patients in an ELISA and showed reactivity with the TP antigen in the IDTP assay (57).
Subsequently, Hung et al. (142) extracted the SOW material of Coccidioides strain C735 with Triton X-114 and subjected the aqueous phase to cold-acetone precipitation. Neither Ag2 nor AgCS was detected in the aqueous extract of the Triton X-114 fraction of SOW; instead, two major glycoproteins, with approximate molecular masses of 58 and 82 kDa in SDS-PAGE, were detected. When the SOW preparation from Coccidioides strains C634 and Silveira were extracted with Triton X-114, the aqueous phase of the Triton X-114 extracts showed bands of 66 and 58 kDa, respectively. N-terminal amino acid sequencing showed that the bands all had identical sequences, and periodic acid-Schiff staining showed that all were glycopeptides. Recombinant SOW glycoprotein (SOWgp) protected BALB/c mice against i.p. infection, stimulated the proliferation of human peripheral mononuclear cells from healthy skin test-positive persons, and induced IFN-γ production (Fig. 13). Immunofluorescence using antibody prepared against the 58- kDa SOWgp of strain Silveira reacted with SOWgp of other Coccidioides strains and, when used in immunofluorescence microscopy, detected SOWgp58 in spherules but not mycelium phase cells. The latter results indicate that the SOWgp is parasite-phase specific. The SOWgp detected antibody in the sera of coccidioidomycosis patients and were also reactive with sera from patients with histoplasmosis or blastomycosis, although the heterologous reactions were weaker than those obtained with sera from coccidioidomycosis patients.
FIG. 13.
Evaluation of SOW and SOWgp as candidate vaccine antigens. (A) Fungal load, expressed as log10 CFU, in lung tissue of SOW- immunized and nonimmunized BALB/c mice. (B) Proliferation response of peripheral blood mononuclear cells from healthy skin test-positive or negative persons after in vitro stimulation with SOWgp58. (C) Structure of the SOWgp gene isolated from strain C735. (D) Expression of IFN-γ mRNA but not IL-5 or IL-10 mRNAs in spleen cells from SOWgp-immunized versus nonimmunized mice. (E) Ability of rSOWgp58 to induce sterilizing immunity in BALB/c mice. The mice were immunized with rSOWgp58 in IFA and then CFA and challenged with 50 arthroconidia via the i.p. route 42 days after the last immunization. Reprinted from reference 163 with permission of the publisher.
The gene that encodes SOWgp from strain C735 was cloned from a Coccidioides genomic library by using a PCR-generated probe (142, 163). The SOWgp gene contains an ORF of 1,266 bp, which translates into a 422-amino-acid protein with a 20 amino-acid-signal sequence on the N terminus and a GPI anchor signal consensus sequence. The predicted molecular size for the translated mature protein is 39.5 kDa, which is much smaller than the 82 kDa for the native glycoprotein, a difference thought to be attributable to the high proline content. When the cDNA sequences of the SOWgp genes from three Coccidioides isolates were compared, differences were noted in the number of proline-rich tandem repeats, which ranged from four to six. The SOWgp gene was expressed in E. coli, and antiserum to the rSOWgp was used in immunoblot analyses to examine the expression of SOWgp during the growth of Coccidioides in vitro. The highest level of expression was observed in presegmented spherules, with lower expression observed during spherule maturation and none detected in mycelium-phase cells. Northern hybridization confirmed that the SOWgp gene is parasite-phase specific (163).
Recombinant SOWgp was used to immunize BALB/c mice. Subcutaneous immunization with 15 μg of rSOWgp in IFA was followed by two booster immunizations, given 2 and 4 weeks later in CFA. When challenged with 50 arthroconidia via the i.p. route and sacrificed 2 weeks later, 6 of the 10 mice vaccinated with rSOWgp58 were reported to have cleared Coccidioides from the lungs, 2 of 10 mice had cleared the fungus from the spleen, whereas none of the control mice immunized with bovine serum albumin in IFA/CFA had cleared their infection (Fig. 13). The rSOWgp-vaccinated BALB/c mice expressed IFN-γ but not IL-5 or IL-10 mRNA in their spleen cells. Hung et al. (143) recently reported on the role of SOWgp as an adhesin to host extracellular matrix proteins (laminin > fibronectin > collagen) and a possible virulence factor in Coccidioides.
Although rSOW appeared to have potential as an immunoprotective antigen, no further studies of its vaccine capacity have been reported. Rather, Cole (55) recently reported that SOWgp probably will not play a useful role in vaccine development because it stimulates a biased Th2 pathway of immune response, as indicated by cytokine analyses. This new information is difficult to interpret in light of the reported Th1 cytokine responses and protection induced in SOWgp-vaccinated BALB/c mice (163).
Urease.
In vitro growth of mycelium- and spherule-phase cells of Coccidioides results in the release ammonia and a concomitant increase in the pH of the culture medium (163), which may result from urea hydrolysis. It has been speculated that the enhanced production of ammonia by endospores in an acidic environment may be related to their ability to survive within the phagolysosome. Another hypothesis implicates urease in the pathogenicity of Coccidioides on the basis that colonization of host tissue by the fungus results in the formation of abscesses with an alkaline pH, which could compromise host defenses (163, 200).
Yu et al. (319) cloned the gene that encodes urease by using degenerate sense and antisense oligonucleotide primers designed on the basis of consensus sequences for URE genes from other organisms. The cloned Coccidioides URE gene is composed of a 2,517-bp ORF that encodes an 839-amino-acid protein with a predicted molecular size of 91.5 kDa. The translated URE protein showed a high sequence similarity to reported URE proteins of Cryptococcus neoformans. Schizosaccharomyces pombe. Canavalia ensiformis (jack bean), Helicobactor pylori. Klebsiella aerogenes. Bacillus pasteurii, and Proteus mirabilis. Expression of the URE gene is highest during the endosporulation phase of the parasitic cycle (163).
BALB/c mice were immunized with rURE, together with synthesized unmethylated CpG-containing oligonucleotides (CpG-ODN), which functions as a potent adjuvant in the induction of Th1 responses (discussed below), and IFA. Splenic and lymph node T cells from rURE-immunized, noninfected mice proliferated in response to in vitro stimulation with rURE (189). Control mice, immunized with bovine serum albumin plus CpG-ODN and IFA, did not show a proliferative response. In vitro-stimulated T cells from mice immunized with rURE and CpG-ODN plus IFA-immunized mice also expressed IFN-γ and IL-2 mRNAs, but not the Th2-associated cytokines IL-10, IL-5, and IL-4. Production of the Th1-associated cytokines IFN-γ and IL-2 was also demonstrable in lungs of immunized infected mice. BALB/c mice immunized with rURE plus CpG-ODN in IFA and then challenged by the i.p. route with 100 arthroconidia of strain C735 were significantly protected, with a 44% survival, in contrast to mice that were immunized with bovine serum albumin plus CpG-ODN in IFA, which all died. Protection was dose dependent, with survival on day 40 post-challenge of only 25% of mice immunized with 5 μg of rURE, in contrast to 44% of mice immunized with 30 μg (189). It is particular noteworthy that sera from mice immunized with rURE plus CpG-ODN in IFA showed a predominant Th1-associated IgG2a antibody response when assayed 12 days after i.p. challenge. This is in contrast to the predominant Th2-associated IgG1 response to Ag2/PRA, FKS, and SOWgp.
Immunization of BALB/c mice with the URE gene, expressed by the pSecTag2A.URE plasmid construct, induced an even higher level of protection, with survival of 10 (83%) of 12 mice on day 40 after i.p. challenge with 100 arthroconidia, compared to survival of 2 (17%) of the 12 mice immunized with the vector alone (Fig. 14). Moreover, 8 of the 10 survivors immunized with the URE cDNA showed sterile lungs and spleens whereas both of the control mice that survived the challenge were culture positive for Coccidioides. As with the rURE, mice immunized with the URE gene showed strong T-cell proliferation in vitro, in response to rURE, and produced IFN-γ and IL-2.
FIG. 14.
Vaccine efficacy of rURE against i.p. challenge. BALB/c mice were immunized with rURE plus CpG-ODN and IFA or, for a control, with bovine serum albumin (BSA) plus CpG-ODN and IFA. The mice were challenged with 100 arthroconidia via the i.p. route. Survival was determined on days 1 through 40 postinfection for groups of 12 mice. Reprinted from reference 189 with permission.
Native urease is a cytosolic enzyme (200) but is detected in broth culture filtrates of the saprobic and parasitic phases after 5 to 6 days of growth. The URE gene does not contain a signal sequence; hence, urease release most probably occurs when the fungal cells undergo autolysis. Mirbod (200) examined the effect of the urease inhibitors, which included acetohydroxamic acid, boric acid, and thiourea, on the urease activity of the purified enzyme. All of these inhibited urease activity. However, when acetohydroxamic acid was added directly to fresh media inoculated with mycelial mat obtained from 4-day-old cultures in glucose-yeast extract, urease activity was decreased but ammonium production was only slightly decreased. Hence, urease activity does not account for all of the ammonia produced by the fungus. Importantly, urease inhibitors did not alter the growth of the fungus. These results, coupled with the high sequence homology between Coccidioides urease and ureases from other sources, including eukaryotic ureases, generate skepticism that rURE or the URE DNA will be a safe and effective vaccine.
HSP60.
Thomas et al. (290) cloned and expressed the gene that encodes Coccidioides heat shock protein 60 (HSP60) by using degenerate sense and antisense oligonucleotide primers designed on the basis of consensus sequences from HSPs from Histoplasma capsulatum and Saccharomyces cerevisiae. The cloned hsp60 gene has a 1,782-bp ORF which encodes a 594-amino-acid protein of 62.4 kDa. The latter has 78 to 83% sequence homology to HSP60 proteins from other fungi. By using antiserum raised against the rHSP60 as a probe in immunoblots and immunofluorescence microscopy, HSP60 was detected in the cell wall and cytosolic fractions of parasitic cells. The translated protein showed a 72% identity to the HSP60 proteins of H. capsulatum. Saccharomyces cerevisiae. Schizosaccharomyces pombe, and Homo sapiens.
In vitro T-cell proliferation assays of rHSP60-immunized BALB/c mice revealed that the rHSP60 was a potent T-cell stimulant (290). Li et al. (189) examined the protective capacity of the rHSP60 in comparison with rURE, both administered with CpG-ODN and IFA. rHSP60 was much less effective as a protective antigen, with only a 16% survival after i.p. challenge. Moreover, the rHSP60 fusion protein elicited a predominant Th2 response in immunized infected BALB/c mice, as judged by expression of IL-4, IL-5, and IL-10 mRNAs in lung homogenates.
Coccidioides-specific antigen.
Early studies by Kaufman and Standard (159) established that Coccidioides produces a heat-stable exoantigen that is specific for this fungus. The exoantigen was demonstrable in culture filtrates of all Coccidioides strains examined and was not detected in extracts derived from heterologous fungi, including such pathogens as Histoplasma capsulatum and Blastomyces dermatitidis. Cox and Britt (73) purified the exoantigen and produced monospecific antiserum. When examined by line immunoelectrophoresis, the exoantigen was identified as antigen 11 in the CDN/burro anti-CDN reference system described by Huppert et al. (145) and was present in culture filtrates of both mycelia and spherules (72). In 1995, Pan and Cole (210) reported cloning the gene that encodes this Coccidioides-specific antigen (CSA) and established that the antigen was a 19-kDa serine proteinase.
Recently, Yu et al. (J. Yu, L. F. Shubitz, T. Peng, K. Osborn, T. N. Kirkland, G. T. Cole, and J. N. Galgiani, Abstr. 103rd Gen. Meet. Am. Soc. Microbiol. 2003, poster F-111, 2003) expressed the CSA gene in E. coli and reported that the recombinant antigen administered with CpG in monophosphoryl lipid-oil (MPL) protected C57BL/6 mice against i.p. challenge. More importantly, 1 μg of rCSA given with CpG/MPL also protected C57BL/6 mice against i.n. challenge, with a 30% survival at 60 days postinfection. Coimmunization of the mice with 1 μg of rCSA and rAg2/PRA(1-106) increased survival to 90%. Although this is a preliminary report, it provides evidence to support the contention that a multivalent recombinant vaccine would be more effective than a univalent vaccine.
GEL-1.
With the advent of the genetic sequence of one Coccidioides strain (C. posadasii, strain 735) being determined at The Institute for Genomic Research (TIGR), it is possible to utilize the predicted proteins to evaluate motifs that might be indicative of potential vaccine candidates. Delgado et al. (89) have recently reported the discovery of a new vaccine candidate by using a strategy to identify proteins with GPI anchors to characterize potential cell wall-associated proteins. This line of experiments follows results by Kong et al. indicating that the most protective subfraction of FKS was associated with the cell walls as opposed to the cytosol fraction (172). With this method, a β-1,3 glucanosyltransferase, termed GEL-1, was identified and cloned and tested for immunogenic capacity. The predicted structure consisted of a 447-amino-acid protein with an 18-amino-acid signal peptide and a 34- amino-acid GPI anchor with two N-glycosylation sites. An E. coli-expressed recombinant protein was produced and used to immunize BALB/c and C57Bl/6 mice with CpG oligonucleotides in IFA. Immunization with as little as 1 μg of rGel1 protein protected BALB/c mice against i.p. challenge with 100 arthroconidia. The protective capacity of rGel1 in BALB/c mice challenged with higher doses of arthroconidia was not tested, nor did the investigators determine whether rGel1 protected BALB/c mice against the more rigorous pulmonary route of challenge. The latter test was conducted with the more resistant C57BL/6 strain, however. Immunization of C57Bl/6 with 1 μg of rGel-1 with CpG-ODN in IFA led to increased survival after pulmonary challenge with 80 arthroconidia, in contrast to control mice immunized with PBS plus CpG in IFA. The investigators reported that rGel1 elicited IFN-γ and a predominant IgG2a antibody response, consistent with the induction of a protective Th1 pathway of immune response.
Coccidioides Gel1 shows a 90% similarity to the translated sequence of Aspergillus fumigatus Gel1. Similarities were also observed with glucanosyltransferase sequences of other fungi, included Candida albicans and C. glabrata. The Coccidioides Gel1 mRNA was expressed at the highest level during the endosporulation stage of the parasitic phase; by immunostaining, the protein was localized to the endospore cell surface, consistent with its presence in the cell wall.
ELI-Ag1.
Our efforts at new vaccine candidate discovery have utilized expression library immunization (ELI) (17). This is a method for identifying genes within the genome of a pathogen that are capable of inducing protective immunity in the host. The procedure capitalizes on the facts that all the protein antigens of a pathogen are encoded by the genome and, second, that genetic immunization is a highly efficacious method for inducing immune responses. The overall strategy is to divide the genome into sibling sublibraries, test each sublibrary for its ability to induce immunity, and then divide protective sublibraries and repeat the process until the gene or genes are identified. We modified the original process describe by Barry et al. and utilized RNA from Coccidioides spherules cultured in RPMI 1640 tissue culture medium to create a cDNA library. To ensure that the results were not attributed to Ag2/PRA, the initial unamplified library was plated at approximately 100 plaques per 150-mm plate and plaque lifts were screened for the presence of Ag2/PRA-positive clones. From the initial 15 100-plaque libraries, 3 contained Ag2/PRA and were not included in further testing. The plaques from the remaining plates were amplified, excised into the pBKCMV plasmid vector, and stored for analysis. An initial series of experiments screened 10 of the initial pools, and 2 of these pools provided significant protection against i.p. challenge. The pool providing the highest level of protection (59% survival over a 35-day survival curve; P < 0.001) was subsequently subdivided and used to immunize mice with sequentially smaller pools of genes, leading to the discovery of a single gene, termed ELI antigen 1 (ELI-Ag1) (150) (Fig. 15).
FIG. 15.
Identification of ELI-Ag1 as a vaccine candidate. BALB/c mice were immunized with individual clones from a pool of genes shown to protect mice from a systemic challenge of 2,500 arthroconidia. The screen started with a pool containing 100 genes from a cDNA library derived from endosporulating spherules of Coccidioides. The most protective clone, designated ELI-Ag1 (▴), is shown in comparison. Pool 7-3-5 (▪), from whence ELI Ag1 was derived, and the vector control (•) are depicted by black lines. The remaining nonprotective clones are depicted by gray circles. Reprinted from reference 150 with permission of the publisher.
The deduced amino acid sequence of the ORF of the cDNA showed that the protein, of unknown function or homology to other known proteins in GenBank, is a 224-amino-acid protein with an 18-amino-acid signal peptide and a 15-amino-acid GPI anchor. There are four potential O-glycosylation sites, four N- glycosylation sites, and a chitin binding domain between amino acids 141 and 155. The cumulative data support the notion that this protein would be a cell wall-associated protein. The results in Fig. 15 show the survival curve of the fourth-level screen, representing mice immunized with the individual genes from the last protective pool of 10 genes. The highest level of protection, equivalent to the complete pool of 10 genes, was provided by the single clone encoding ELI-Ag1. To our knowledge, this was the first report of ELI leading to the identification of a single protective gene for vaccination against a fungal disease.
Studies are under way to express ELI-Ag1 in a eucaryotic expression system and to test the recombinant protein for its vaccine capacity, alone and in combination with other vaccine candidates, in BALB/c mice challenged by a pulmonary route. Studies are also needed to assess the host response to ELI-Ag1.
Critical Comments
Of the vaccine candidates tested in the murine model, none surpass the FKS vaccine and only the 27K cellular extract approaches the high level of protection of FKS in the most susceptible BALB/c mouse strain. We think that a vaccine candidate should be taken to human clinical trials only after it is shown to protect the most susceptible mouse strain against pulmonary challenge. Of the single-antigen candidates, Ag2/PRA is the most extensively studied and its protective capacity has been reproduced in multiple laboratories. However, as a recombinant protein vaccine, Ag2/PRA-induced protection has been shown convincingly only against pulmonary challenge in intermediately susceptible C57BL/6 mice. Other antigens are in the early phases of discovery and testing, and it is hoped that new candidates will soon be proven in multiple laboratories to the level of that seen for Ag2/PRA. The current thought is that the next human coccidioidomycosis vaccine will be a multivalent composite of several immunodominant proteins. Therefore, new candidates need to be discovered. While DNA-based immunization is an efficient tool for vaccine discovery and rapid testing, it is doubtful that a DNA-based vaccine will be used against coccidioidomycosis. Thus, the eventual vaccine product will require the production of a recombinant protein(s). In addition to the vaccine candidate(s) itself, the adjuvant system used for immunization has a profound impact on the protection observed (see below). It would also be of great benefit to observe a protective response in an animal model other than the mouse. The cumulative results are that significant progress has been made for vaccine discovery against Coccidioides but that much work is left before we can reliably and reproducibly produce protection in the most susceptible animals.
SPECIAL CONSIDERATIONS
Adjuvants
Emphasis on vaccine development has shifted in recent years from the use of live attenuated organisms and purified subcellular components to the use of recombinant protein subunits, synthetic peptides, protein polysaccharide conjugates, and plasmid DNA. These new-generation vaccines are likely to be less reactogenic than traditional vaccines but are also less immunogenic. Given that a soluble protein (or peptide) or recombinant peptide vaccine will not by itself induce cellular immune responses, it is imperative that particular attention be paid to selecting the optimal adjuvant for delivering the peptide to APCs. Consideration must be given to selecting an adjuvant that is approved, or under consideration for approval, for use in humans. Equally important, the vaccine must be able to induce immunity against pulmonary challenge. The vast majority of studies conducted with coccidioidal antigens or gene vaccines have been performed using the i.p. route. While these experiments provide valuable information in regard to selecting potential vaccine candidates, the rigorous test will be to establish that protection is engendered against pulmonary challenge.
Currently, the only adjuvants licensed for human use by the U.S. Food and Drug Administration are aluminum-based mineral salts (generically called alum), namely, aluminum hydroxide and aluminum phosphate. Aluminum compounds preferentially skew the immune response toward a Th2 response characterized by the secretion of IL-4 and IL-5 and the generation of IgG1 and IgE. Th1 responses are required, however, for host defense against diseases that are dependent on cell-mediated immunity for protection, for example, coccidioidomycosis, tuberculosis, malaria, HIV infection, leishmaniasis, and histoplasmosis. Although CFA has been a standard and effective adjuvant for driving Th1 responses in experimental-animal models, it is reactogenic and thus is unacceptable for use in humans. More recently developed Th1-type adjuvants, notably MPL, CpG-ODN, and QS-21, have not been licensed for use in humans but have been evaluated in preclinical trials with humans; in experimental-animal models, these adjuvants have proved to be superior to alum and superior or comparable to CFA in the induction of humoral and cell-mediated immune responses (28, 40, 85, 156, 160, 201).
The identification of TLRs and their importance in innate and adaptive immunity have provided new insights into adjuvant design. Within minutes of recognizing pathogen-associated molecular patterns, an array of cell types initiate defense mechanisms, including the production of reactive oxygen intermediates and secretion of inflammatory cytokines, including IL-1, TNF-α, IL-12, and IFN-γ. These cytokines activate NK cells and also initiate a cascade of signals to cells of the adaptive immune response. Pharmacological manipulation of innate immune responses might lead to more effective vaccines, as well as novel therapeutic strategies. While it is not within the scope of this review to present an in-depth discussion of adjuvants, a brief overview is provided of adjuvants that have efficacy in potentiating cellular immune responses and are currently in, or close to being in, clinical trials with humans.
MPL adjuvant.
The first microbial product discovered to bind TLRs was lipopolysaccharide (LPS) (234). Although LPS is a potent adjuvant for protein and carbohydrate antigens and induces both humoral and cellular immunity, its toxicity precludes its use in human vaccines (155). Investigators chemically modified lipid A by uncoupling its toxic effects while retaining its immunostimulatory effects (156). The resulting product, monophosphoryl lipid A (MLA), was further modified by hydrolysis of the β-hydroxymyristoyl residue attached to the 3-position of the reducing sugar, and the product was designated MPL. This adjuvant has been administered to over 12,000 persons and has resulted in minimal side effects. Evidence suggests that LPS, lipid A, MLA, and MPL bind TLR4 (156, 234). Two formulations of MPL that have been used are MPL-SE, which is MPL, a naturally derived disaccharide adjuvant of Salmonella enterica serovar Minnesota plus squalene, and MPL-AF, which is an aqueous micellar suspension of MPL dispersed in dipalmitoyl phosphatidylcholine. MPL-AF augments humoral and cellular immune responses to vaccines administered by the i.n. route. In preclinical trials with over 10,000 healthy persons, MPL was shown to have an acceptable tolerability and was effective in inducing IL-2, IFN-γ, and cytotoxic T cells (272). In a direct comparison of the vaccine efficacy of rAg2/PRA in MPL-SE versus rAg2/PRA in CFA, mice immunized with the former were significantly more protected than mice immunized with the latter after i.n. challenge with 30 arthroconidia (unpublished data). Moreover, administration of rAg2/PRA in MPL-AF adjuvant significantly protected BALB/c and C57BL/6 mice against pulmonary challenge with Coccidioides (discussed above).
CpG-OGN.
Microbial but not vertebrate DNA contains unmethylated cytosine-phosphate-guanine (CpG) dinucleotides with specific flanking regions that are recognized by cells of the innate immune system to allow the discrimination of pathogen-derived DNA from self DNA. In mice, the optimal stimulatory CpG motif consists of two 5′ purines and two 3′ pyrimidines, with the most potent being 5′-GACGTT-3′ (178). These motifs rapidly stimulate murine macrophages, DCs, and B cells to secrete proinflammatory cytokines such as IL-1, TNF-α, IL-6, IL-12, and IL-18 and stimulate the IL-12 secondarily activated murine NK cells to secrete IFN-γ and to have increased cytotoxic activity. Recent studies have shown that unmethylated CpG triggers innate immunity through TLR9 (139). Thus, one mechanism by which CpGs trigger Th1 responses is by bringing about the maturation of immature DCs into mature APCs.
Although CpGs have been evaluated mainly in rodents, recent investigations by Hartmann et al. (138) showed that a CpG phosphorothioate ODN with a TpC dinucleotide at the 5′ end followed by three 6-mer CpG motifs (5′-GTCGTT-3′) separated by TpT dinucleotides was most effective in stimulating primate immune cells. Preclinical trials with humans have shown impressive immunomodulatory effects and limited toxicity with CpG-OGN-formulated cancer vaccines (40). Another notable feature of CpG-OGN adjuvants is that they are useful for delivering both recombinant (or native) antigens and gene vaccines. This provides a means of directly comparing the level of protection achieved by a given peptide vaccine versus its DNA. Thus far, the only published study using CpG-OGNs in studies of Coccidioides vaccine candidates was by Li et al. (189). As discussed above, both rURE and the URE gene vaccine induced protective immunity when administered with CpG-OGN, but the level of immunity was significantly higher with the gene vaccine. Unfortunately, the protective effect of the vaccine in CpG-OGN was not assessed by using a pulmonary challenge.
QS-21.
QS-21 is a natural saponin adjuvant from the bark of the Chilean tree Quillaja saponaria. It is a water-soluble triterpene glycoside with amphiphilic character and can be mixed with a soluble antigen, resulting in a fully soluble vaccine, or it can be combined with emulsion or mineral salts adjuvants. The adjuvant is highly effective in enhancing both Th1 cytokines (IL-2 and IFN-γ), antibodies of the IgG2a isotype, and cytotoxic T-lymphocyte (CTL) activity (161). Saponins intercalate into cell membranes, which is thought to allow antigens to gain access to the cytoplasm for the induction of CTL. The capacity of QS-21 to induce CTL activity makes it an optimal candidate for vaccines against pathogens that require a potent CTL response. Although CTL activity has not been demonstrated in coccidioidomycosis, the intracellular parasitism of macrophages by Coccidioides endospores warrants studies to explore this response.
QS-21 has been tested in more than 3,000 patients in 60 clinical trials, with transient severe pain being noted in four trials where 200 μg of adjuvant was used but fewer side effects at lower doses (272). Studies are under way to reduce the side effects by chemical alterations or reformulation. QS-7 is one such reformulation and has been shown to be active in stimulating IgG2a and CTL responses. ASO2 is a proprietary emulsion containing MPL and QS-21; while it has been highly effective in clinical trials, it has several side effects, including headaches, myalgia, local pain, and systemic chills (160). As yet, neither QS-21 nor ASO2 has been used in studies of Coccidioides vaccines.
Microparticles.
Microparticles composed of polylactide-coglycolides (PLGs) are biodegradable and biocompatible polyesters that have been used in humans as suture material and for controlled release of drugs (272). PLG microparticles appear to mediate their immunopotentiating effect as a consequence of their uptake by DCs and macrophages, and they can be used with recombinant peptide or DNA vaccines. Anionic microparticles are used for the delivery of adsorbed proteins, whereas cationic microparticles are used for the delivery of DNA vaccines alone or with CpG-OGN (271). These protein- or DNA-adsorbed microparticles have proven effective in inducting Th1 and CTL responses in mice, guinea pigs, and nonhuman primates. The biodegradation rate of PLG microspheres is a major advantage since it allows a single-dose immunization (247).
Route of Immunization
Although most vaccines have been administered by an i.m. or s.c. route, administration by the mucosal route has a number of important advantages. Immunization by the i.n. route is still in exploratory stages but offers the advantage of targeted delivery of the vaccine directly to lung cells, where the specialized bronchus-associated lymphoid tissue, the nasal-associated lymphoid tissue, and the intraepithelial DCs within the lower airways would be directly exposed to the vaccine. Another important advantage of mucosal delivery is that it avoids the use of needles, which is highly desirable for human vaccines.
While vaccines delivered by an i.n. route are effective in inducing antibody, oral or i.n. vaccines rarely stimulate effective cellular immune responses. Since most adjuvants have an unacceptable level of toxicity for i.n. immunization, alternative delivery systems are needed. One strategy that has promise is delivery of the protein or DNA vaccine to the lung via i.n. instillation of the vaccine coupled to PLG microparticles. Another strategy to optimize the stimulation of immunity at the lung level, as well as systemic immunity, has been to vaccinate via an i.m. route and then use the i.n. route for boosting.
CURRENT AND FUTURE DIRECTIONS
Of all the fungal diseases, coccidioidomycosis is the best suited for the development and application of a vaccine. This is based on the geographically defined regions of endemicity, the well-defined target populations, and the immunizing capacity of the fungus. The advances in technologies and application of genomics, proteomics, and bioinformatics, together with rapid throughput of identifying immunoreactive epitopes, will greatly accelerate the achievement of this goal. While a number of vaccine candidates have been identified and cloned, only the 27K preparation induces protection against pulmonary challenge to the level achieved with the FKS vaccine. The 27K vaccine is, however, antigenically heterogeneous, and the protective component has yet to be identified. Therefore, there continues to be a need to identify other vaccine candidates for use in a monovalent vaccine or, more probably, a multivalent component vaccine.
The genome of C. posadasii is currently being sequenced at TIGR and, at the time of this writing, has about an 8× coverage (i.e., the sequence of the genome was covered to a theoretical eight times) of the estimated 29-Mb of chromosomal DNA. There are approximately 6,000 predicted coding regions within the genome, and approximately 75% of the genes have a significant match in the GenBank nonredundant protein database (T. Kirkland, personal communication). Bioinformatics tools will accelerate the progress from genome sequencing to vaccine design (86-88, 199, 257, 261). EpiMer and EpiMatrix are computer-driven pattern-matching algorithms that serve to identify T-cell-reactive epitopes. Conservatrix and BlastiMer permit the identification of protein sequences for highly conserved regions and homology to other known proteins (257). Although homology-based genomic analysis can yield misleading results in the assignment of gene function (255), it can narrow the list of putative epitopes to be tested. By using this “epitope-driven vaccine design” (86), the epitopes can be screened directly for reactivity with immune T cells and T-cell subsets to identify those to be further evaluated in animal models.
Another approach to identifying potential vaccine candidates in the Coccidioides genome would be to clone the ORFs and evaluate their protective capacity. One way to avoid this colossal task would be to construct linear expression elements (287), which consist of PCR-amplified ORFs linked to a eucaryotic promoter and terminator, and then evaluate the protective efficacy of the constructs by using ELI (17, 288). Another method would be to utilize a bioinformatic strategy to search for proteins with known immunogenic motifs or GPI anchors that would indicate that they were cell wall associated. Once antigens have been identified by this strategy, studies can be undertaken to evaluate their immunoreactivity and protective capacity when administered to experimental-animal models.
One potential problem that may impede vaccine development in coccidioidomycosis is the possible requirement for posttranslational modification for optimal presentation and processing. This may be circumvented by expressing the genes in a yeast system. However, in the case of Ag2/PRA which contains 3-O-methylmannose, the machinery for producing this glycosylated peptide, in a manner corresponding to that of the native antigen, may be lacking. An attractive alternative strategy would be to overexpress the gene in Coccidioides (55), using a tagged gene construct which will enable the translated peptide to be purified from other coccidioidal components.
REFERENCES
- 1.Abuodeh, R. O., L. F. Shubitz, E. Siegel, S. Snyder, T. Peng, K. I. Orsborn, E. Brummer, D. A. Stevens, and J. N. Galgiani. 1999. Resistance to Coccidioides immitis in mice after immunization with recombinant protein or a DNA vaccine of a proline-rich antigen. Infect. Immun. 67:2935-2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albert, E. D., M. R. Mickey, and P. I. Terasaki. 1973. Genetics of the HLA system in four populations: American-Caucasians, Japanese-Americans, American Negroes, and Mexican Americans, p. 233-240. In J. Dausett and J. Colombani (ed.), Histocompatibility testing. The Williams & Wilkins Co., Baltimore, Md.
- 3.Ampel, N. M. 1996. Emerging disease issues and fungal pathogens associated with HIV infection. Emerg. Infect. Dis. 2:109-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ampel, N. M. 1994. In vitro production of tumor necrosis factor-alpha by adherent human peripheral blood mononuclear cells incubated with killed coccidioidal arthroconidia and spherules. Cell. Immunol. 153:248-255. [DOI] [PubMed] [Google Scholar]
- 5.Ampel, N. M., and L. Christian. 2000. Flow cytometric assessment of human peripheral blood mononuclear cells in response to a coccidioidal antigen. Med. Mycol. 38:127-132. [DOI] [PubMed] [Google Scholar]
- 6.Ampel, N. M., C. L. Dols, and J. N. Galgiani. 1993. Coccidioidomycosis during human immunodeficiency virus infection: results of a prospective study in a coccidioidal endemic area. Am. J. Med. 94:235-240. [DOI] [PubMed] [Google Scholar]
- 7.Ampel, N. M., and J. N. Galgiani. 1991. Interaction of human peripheral blood mononuclear cells with Coccidioides immitis arthroconidia. Cell Immunol. 133:253-262. [DOI] [PubMed] [Google Scholar]
- 8.Ampel, N. M., L. A. Kramer, K. M. Kerekes, S. M. Johnson, and D. Pappagianis. 2001. Assessment of the human cellular immune response to T27K, a coccidioidal antigen preparation, by flow cytometry of whole blood. Med. Mycol. 39:315-320. [DOI] [PubMed] [Google Scholar]
- 9.Ampel, N. M., L. A. Kramer, L. Li, D. S. Carroll, K. M. Kerekes, S. M. Johnson, and D. Pappagianis. 2002. In vitro whole-blood analysis of cellular immunity in patients with active coccidioidomycosis by using the antigen preparation T27K. Clin. Diagn. Lab. Immunol. 9:1039-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ampel, N. M., D. G. Mosley, B. England, P. D. Vertz, K. Komatsu, and R. A. Hajjeh. 1998. Coccidioidomycosis in Arizona: increase in incidence from 1990 to 1995. Clin. Infect. Dis. 27:1528-1530. [DOI] [PubMed] [Google Scholar]
- 11.Armstrong, J. A., and P. D'Arcy Hart. 1971. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes and phagosomes. J. Exp. Med. 134:713-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arsura, E. L. 1997. The association of age and mortality in coccidioidomycosis. J. Am. Geriatr. Soc. 45:532-533. [DOI] [PubMed] [Google Scholar]
- 13.Arsura, E. L., W. B. Kilgore, J. W. Caldwell, J. C. Freeman, H. E. Einstein, and R. H. Johnson. 1998. Association between facial cutaneous coccidioidomycosis and meningitis. West. J. Med. 169:13-16. [PMC free article] [PubMed] [Google Scholar]
- 14.Baker, D., and A. I. Braude. 1956. A study of stimuli leading to the production of spherules in coccidioidomycosis. J. Lab. Clin. Med. 47:169-181. [PubMed] [Google Scholar]
- 15.Barbee, R. A., M. J. Hicks, D. Grosso, and C. Sandel. 1991. The maternal immune response in coccidioidomycosis. Is pregnancy a risk factor for serious infection? Chest 100:709-715. [DOI] [PubMed] [Google Scholar]
- 16.Barnato, A. E., G. D. Sanders, and D. K. Owens. 2001. Cost-effectiveness of a potential vaccine for Coccidioides immitis. Emerg. Infect. Dis. 7:797-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barry, M. A., W. C. Lai, and S. A. Johnston. 1995. Protection against mycoplasma infection using expression-library immunization. Nature 377:632-635. [DOI] [PubMed] [Google Scholar]
- 18.Beaman, L. 1991. Effects of recombinant gamma interferon and tumor necrosis factor on in vitro interactions of human mononuclear phagocytes with Coccidioides immitis. Infect. Immun. 59:4227-4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beaman, L. 1987. Fungicidal activation of murine macrophages by recombinant gamma interferon. Infect. Immun. 55:2951-2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beaman, L., E. Benjamini, and D. Pappagianis. 1983. Activation of macrophages by lymphokines: enhancement of phagosome-lysosome fusion and killing of Coccidioides immitis. Infect. Immun. 39:1201-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beaman, L., E. Benjamini, and D. Pappagianis. 1981. Role of lymphocytes in macrophage-induced killing of Coccidioides immitis. in vitro. Infect. Immun. 34:347-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beaman, L., and C. A. Holmberg. 1980. In vitro response of alveolar macrophages to infection with Coccidioides immitis. Infect. Immun. 28:594-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beaman, L., and C. A. Holmberg. 1980. Interaction of nonhuman primate peripheral blood leukocytes and Coccidioides immitis in vitro. Infect. Immun. 29:1200-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beaman, L., and D. Pappagianis. 1985. Fate of Coccidioides immitis arthroconidia in human peripheral blood monocyte cultures in vitro, p. 170-180. In H. Einstein and A. Catanzaro (ed.), Coccidioidomycosis. Proceedings of the 4th International Conference. National Foundation for Infectious Disease, Washington, D.C.
- 25.Beaman, L., D. Pappagianis, and E. Benjamini. 1977. Significance of T cells in resistance to experimental murine coccidioidomycosis. Infect. Immun. 17:580-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beaman, L. V., D. Pappagianis, and E. Benjamini. 1979. Mechanisms of resistance to infection with Coccidioides immitis in mice. Infect. Immun. 23:681-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Becker, S., R. B. Devlin, and J. S. Haskill. 1989. Differential production of tumor necrosis factor, macrophage colony stimulating factor, and interleukin 1 by human alveolar macrophages. J. Leukoc. Biol. 45:353-361. [PubMed] [Google Scholar]
- 28.Brazolot Millan, C. L., R. Weeratna, A. M. Krieg, C. A. Siegrist, and H. L. Davis. 1998. CpG DNA can induce strong Th1 humoral and cell-mediated immune responses against hepatitis B surface antigen in young mice. Proc. Natl. Acad. Sci. USA 95:15553-15558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Breslau, A. M., and M. Y. Kubota. 1964. Continuous in vitro cultivation of spherules of Coccidioides immitis. J. Bacteriol. 87:468-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Britt, R. H., D. R. Enzmann, and J. S. Remington. 1981. Intracranial infection in cardiac transplant recipients. Ann. Neurol. 9:107-119. [DOI] [PubMed] [Google Scholar]
- 31.Brummer, E., L. Beaman, and D. A. Stevens. 1985. Killing of endospores, but not arthroconididia of Coccidioides immitis by immunologically activated polymorphoneclear neutrophils, p. 201-213. In H. Einstein and A. Catanzaro (ed.), Coccidioidomycosis. Proceedings of the 4th International Conference. National Foundation for Infectious Disease, Washington, D.C.
- 32.Burt, A., D. A. Carter, G. L. Koenig, T. J. White, and J. W. Taylor. 1996. Molecular markers reveal cryptic sex in the human pathogen Coccidioides immitis. Proc. Natl. Acad. Sci. USA 93:770-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burt, A., D. A. Carter, T. J. White, and J. W. Taylor. 1994. DNA sequencing with arbitrary primer pairs. Mol. Ecol. 3:523-525. [DOI] [PubMed] [Google Scholar]
- 34.Burt, A., B. M. Dechairo, G. L. Koenig, D. A. Carter, T. J. White, and J. W. Taylor. 1997. Molecular markers reveal differentiation among isolates of Coccidioides immitis from California, Arizona and Texas. Mol. Ecol. 6:781-786. [DOI] [PubMed] [Google Scholar]
- 35.Buschman, E., and E. Skamene. 2001. From Bcg/Lsh/Ity to Nramp1: three decades of search and research. Drug. Metab. Dispos. 29:471-473. [PubMed] [Google Scholar]
- 36.Butt, E. M., and A. M. Hoffman. 1945. Healed or arrested pulmonary coccidioidomycosis; correlation of coccidioidin skin tests with autopsy findings. Am. J. Pathol. 21:485-505. [PMC free article] [PubMed] [Google Scholar]
- 37.Cairns, L., D. Blythe, A. Kao, D. Pappagianis, L. Kaufman, J. Kobayashi, and R. Hajjeh. 2000. Outbreak of coccidioidomycosis in Washington state residents returning from Mexico. Clin. Infect. Dis. 30:61-64. [DOI] [PubMed] [Google Scholar]
- 38.Caldwell, J. W., E. L. Arsura, W. B. Kilgore, A. L. Garcia, V. Reddy, and R. H. Johnson. 2000. Coccidioidomycosis in pregnancy during an epidemic in California. Obstet. Gynecol. 95:236-239. [DOI] [PubMed] [Google Scholar]
- 39.Calhoun, D. L., E. O. Osir, K. O. Dugger, J. N. Galgiani, and J. H. Law. 1986. Humoral antibody responses to specific antigens of Coccidioides immitis. J. Infect. Dis. 154:265-272. [DOI] [PubMed] [Google Scholar]
- 40.Carpentier, A. F., G. Auf, and J. Y. Delattre. 2003. CpG-oligonucleotides for cancer immunotherapy: review of the literature and potential applications in malignant glioma. Front. Biosci. 8:e115-e127. [DOI] [PubMed] [Google Scholar]
- 41.Carroll, G. F., L. D. Haley, and J. M. Brown. 1977. Primary cutaneous coccidioidomycosis: a review of the literature and a report of a new case. Arch. Dermatol. 113:933-936. [DOI] [PubMed] [Google Scholar]
- 42.Casadevall, A., M. Feldmesser, and L. A. Pirofski. 2002. Induced humoral immunity and vaccination against major human fungal pathogens. Curr. Opin. Microbiol. 5:386-391. [DOI] [PubMed] [Google Scholar]
- 43.Castleberry, M. W., J. L. Converse, and P. J. Soto, Jr. 1964. Antibiotic control of tissue reactions in dogs vaccinated with viable cells of Coccidioides immitis. J. Bacteriol. 87:1216-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Catanzaro, A., L. E. Spitler, and K. M. Moser. 1975. Cellular immune response in coccidioidomycosis. Cell. Immunol. 15:360-371. [DOI] [PubMed] [Google Scholar]
- 45.Cella, M., A. Engering, V. Pinet, J. Pieters, and A. Lanzavecchia. 1997. Inflammatory stimuli induce accumulation of MHC class II complexes on dendritic cells. Nature 388:782-787. [DOI] [PubMed] [Google Scholar]
- 46.Centers for Disease Control and Prevention. 2001. Coccidioidomycosis among persons attending the world championship of model airplane flying—Kern County, California, October 2001. Morb. Mortal. Wkly. Rep. 50:1106-1107. [PubMed] [Google Scholar]
- 47.Centers for Disease Control and Prevention. 1994. Coccidioidomycosis following the Northridge earthquake—California, 1994. Morb. Mortal. Wkly. Rep. 43:194-195. [PubMed] [Google Scholar]
- 48.Centers for Disease Control and Prevention. 2000. Coccidioidomycosis in travelers returning from Mexico—Pennsylvania, 2000. Morb. Mortal. Wkly. Rep. 49:1004-1006. [PubMed] [Google Scholar]
- 49.Centers for Disease Control and Prevention. 2001. Coccidioidomycosis in workers at an archeologic site—Dinosaur National Monument, Utah, June-July 2001. Morb. Mortal. Wkly. Rep. 50:1005-1008. [PubMed] [Google Scholar]
- 50.Centers for Disease Control and Prevention. 2003. Increase in coccidioidomycosis—Arizona, 1998-2001. Morb. Mortal. Wkly. Rep. 52:109-112. [PubMed] [Google Scholar]
- 51.Centers for Disease Control and Prevention. 2002. Laboratory security and emergency response guide for laboratories working with select agents. Morb. Mortal. Wkly. Rep. 51:1-6. [PubMed] [Google Scholar]
- 52.Centers for Disease Control and Prevention. 1994. Update: coccidioidomycosis—California, 1991-1993. Morb. Mortal. Wkly. Rep. 43:421-423. [PubMed] [Google Scholar]
- 53.Chaturvedi, V., R. Ramani, S. Gromadzki, B. Rodeghier, H. G. Chang, and D. L. Morse. 2000. Coccidioidomycosis in New York State. Emerg. Infect. Dis. 6:25-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clemons, K. V., C. R. Leathers, and K. W. Lee. 1985. Systemic Coccidioides immitis infection in nude and beige mice. Infect. Immun. 47:814-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cole, G. T., C.-Y. Hung, and N. Delgado. 2002. Parasite phase-specific gene expression in Coccidioides. ASM News 68:603-610. [Google Scholar]
- 56.Cole, G. T., T. N. Kirkland, and S. H. Sun. 1987. An immunoreactive, water-soluble conidial wall fraction of Coccidioides immitis. Infect. Immun. 55:657-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cole, G. T., K. R. Seshan, M. Franco, E. Bukownik, S. H. Sun, and V. M. Hearn. 1988. Isolation and morphology of an immunoreactive outer wall fraction produced by spherules of Coccidioides immitis. Infect. Immun. 56:2686-2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cole, G. T., and S. H. Sun. 1985. Arthroconidium-spherule-endospore transformation in Coccidioides immitis, p. 281-333. In P. J. Szaniszlo (ed.), Fungal dimorphism: with emphasis on fungi pathogenic for humans. Plenum Publishing Corp., New York, N.Y.
- 59.Converse, J. L. 1956. Effect of physio-chemical environment of spherulation of Coccidioides immitis in a chemically defined medium. J. Bacteriol. 72:784-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Converse, J. L. 1957. Effect of surface active agents on endosporulation of Coccidioides immitis in a chemically defined medium. J. Bacteriol. 74:106-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Converse, J. L. 1955. Growth of spherules of Coccidioides immitis in a chemically defined liquid medium. Proc. Soc. Exp. Biol. Med. 90:709-711. [DOI] [PubMed] [Google Scholar]
- 62.Converse, J. L., and A. R. Besemer. 1959. Experimental primary cutaneous coccidioidomycosis in the monkey. J. Bacteriol. 87:81-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Converse, J. L., and A. R. Besemer. 1959. Nutrition of the parasitic phase of Coccidioides immitis in a chemically defined medium. J. Bacteriol. 78:231-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Converse, J. L., M. W. Castleberry, A. R. Besemer, and E. M. Snyder. 1962. Immunization of mice against coccidioidomycosis. J. Bacteriol. 84:46-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Converse, J. L., M. W. Castleberry, and E. M. Snyder. 1963. Experimental viable vaccine against pulmonary coccidioidomycosis in monkeys. J. Bacteriol. 86:1041-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Corry, D. B., N. M. Ampel, L. Christian, R. M. Locksley, and J. N. Galgiani. 1996. Cytokine production by peripheral blood mononuclear cells in human coccidioidomycosis. J. Infect. Dis. 174:440-443. [DOI] [PubMed] [Google Scholar]
- 67.Cox, R. A. 1989. Antigenic structure of Coccidioides immitis. Immunol. Ser. 47:133-170. [PubMed] [Google Scholar]
- 68.Cox, R. A. 1989. Coccidioidomycosis, p. 165-197. In R. A. Cox (ed.), Immunology of fungal diseases. CRC Press, Inc., Boca Raton, Fla.
- 69.Cox, R. A., and D. R. Arnold. 1979. Immunoglobulin E in coccidioidomycosis. J. Immunol. 123:194-200. [PubMed] [Google Scholar]
- 70.Cox, R. A., B. S. Baker, and D. A. Stevens. 1982. Specificity of immunoglobulin E in coccidioidomycosis and correlation with disease involvement. Infect. Immun. 37:609-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cox, R. A., and L. A. Britt. 1985. Antigenic heterogeneity of an alkali-soluble, water-soluble cell wall extract of Coccidioides immitis. Infect. Immun. 50:365-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cox, R. A., and L. A. Britt. 1987. Antigenic identity of biologically active antigens in coccidioidin and spherulin. Infect. Immun. 55:2590-2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cox, R. A., and L. A. Britt. 1986. Isolation and identification of an exoantigen specific for Coccidioides immitis. Infect. Immun. 52:138-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cox, R. A., and L. A. Britt. 1986. Isolation of a coccidioidin component that reacts with immunoglobulin M precipitin antibody. Infect. Immun. 53:449-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cox, R. A., E. Brummer, and G. Lecara. 1977. In vitro lymphocyte responses of coccidioidin skin test- positive and -negative persons to coccidioidin, spherulin, and a coccidioides cell wall antigen. Infect. Immun. 15:751-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cox, R. A., M. J. Dolan, D. M. Magee, and J. N. Galgiani. 1993. Production of a murine monoclonal antibody that recognizes an epitope specific to Coccidioides immitis antigen 2. Infect. Immun. 61:1895-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cox, R. A., W. Kennell, L. Boncyk, and J. W. Murphy. 1988. Induction and expression of cell-mediated immune responses in inbred mice infected with Coccidioides immitis. Infect. Immun. 56:13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cox, R. A., and D. M. Magee. 1995. Production of tumor necrosis factor alpha, interleukin-1 alpha, and interleukin-6 during murine coccidioidomycosis. Infect. Immun. 63:4178-4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cox, R. A., and D. M. Magee. 1998. Protective immunity in coccidioidomycosis. Res. Immunol. 149:417-428, 506-507. [DOI] [PubMed] [Google Scholar]
- 80.Cox, R. A., and R. M. Pope. 1987. Serum-mediated suppression of lymphocyte transformation responses in coccidioidomycosis. Infect. Immun. 55:1058-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cox, R. A., R. M. Pope, and D. A. Stevens. 1982. Immune complexes in coccidioidomycosis. Correlation with disease involvement. Am. Rev. Respir. Dis. 126:439-443. [DOI] [PubMed] [Google Scholar]
- 82.Cox, R. A., and J. R. Vivas. 1977. Spectrum of in vivo and in vitro cell-mediated immune responses in coccidioidomycosis. Cell. Immunol. 31:130-141. [DOI] [PubMed] [Google Scholar]
- 83.Crum, N., C. Lamb, G. Utz, D. Amundson, and M. Wallace. 2002. Coccidioidomycosis outbreak among United States Navy SEALs training in a Coccidioides immitis-endemic area—Coalinga, California. J. Infect. Dis. 186:865-868. [DOI] [PubMed] [Google Scholar]
- 84.Currah, R. S. 1985. Taxonomy of Onygenales: Arthrodermataceae. Gymnoascacease. Myxotrichaceae, and Onygenaceae. Mycotaxon 24:1-216. [Google Scholar]
- 85.Davis, H. L., R. Weeratna, T. J. Waldschmidt, L. Tygrett, J. Schorr, A. M. Krieg, and R. Weeranta. 1998. CpG DNA is a potent enhancer of specific immunity in mice immunized with recombinant hepatitis B surface antigen. J. Immunol. 160:870-876. [PubMed] [Google Scholar]
- 86.De Groot, A. S., A. Bosma, N. Chinai, J. Frost, B. M. Jesdale, M. A. Gonzalez, W. Martin, and C. Saint-Aubin. 2001. From genome to vaccine: in silico predictions, ex vivo verification. Vaccine 19:4385-4395. [DOI] [PubMed] [Google Scholar]
- 87.De Groot, A. S., B. M. Jesdale, E. Szu, J. R. Schafer, R. M. Chicz, and G. Deocampo. 1997. An interactive Web site providing major histocompatibility ligand predictions: application to HIV research. AIDS Res. Hum. Retroviruses 13:529-531. [DOI] [PubMed] [Google Scholar]
- 88.De Groot, A. S., H. Sbai, C. S. Aubin, J. McMurry, and W. Martin. 2002. Immuno-informatics: mining genomes for vaccine components. Immunol. Cell Biol. 80:255-269. [DOI] [PubMed] [Google Scholar]
- 89.Delgado, N., J. Xue, J. J. Yu, C. Y. Hung, and G. T. Cole. 2003. A recombinant beta-1,3- glucanosyltransferase homolog of Coccidioides posadasii protects mice against coccidioidomycosis. Infect. Immun. 71:3010-3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Department of Health and Human Services. 2002. Possession, use, and transfer of select agents and toxins. 42 CFR, Part 73. Fed. Regist. 240:76886-76905. [Google Scholar]
- 91.Deresinski, S. C., D. Pappagianis, and D. A. Stevens. 1979. Association of ABO blood group and outcome of coccidioidal infection. Sabouraudia 17:261-264. [DOI] [PubMed] [Google Scholar]
- 92.Desai, S. A., O. A. Minai, S. M. Gordon, B. O'Neil, H. P. Wiedemann, and A. C. Arroliga. 2001. Coccidioidomycosis in non-endemic areas: a case series. Respir. Med. 95:305-309. [DOI] [PubMed] [Google Scholar]
- 93.Dickson, E. C. 1937. “Valley Fever” of the San Joaquin Valley and fungus Coccidioides. Calif. West. Med. 47:151-155. [PMC free article] [PubMed] [Google Scholar]
- 94.Dixon, D. M. 2001. Coccidioides immitis as a select agent of bioterrorism. J. Appl. Microbiol. 91:602-605. [DOI] [PubMed] [Google Scholar]
- 95.Dodd, L. G., and S. D. Nelson. 1990. Disseminated coccidioidomycosis detected by percutaneous liver biopsy in a liver transplant recipient. Am. J. Clin. Pathol. 93:141-144. [DOI] [PubMed] [Google Scholar]
- 96.Dodge, R. R., M. D. Lebowitz, R. A. Barbee, and B. Burrows. 1985. Immediate reactions to coccidioidin and spherulin: the relation of atopy to coccidioidomycosis, p. 214-223. In H. Einstein and A. Catanzaro (ed.), Coccidioidomycosis. Proceedings of the 4th International Conference. National Foundation for Infectious Disease, Washington, D.C.
- 97.Dooley, D. P., R. A. Cox, K. L. Hestilow, M. J. Dolan, and D. M. Magee. 1994. Cytokine induction in human coccidioidomycosis. Infect. Immun. 62:3980-3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Drutz, D. J., and A. Catanzaro. 1978. Coccidioidomycosis. Part I. State of the art. Am. Rev. Respir. Dis. 117:559-585. [DOI] [PubMed] [Google Scholar]
- 99.Drutz, D. J., and A. Catanzaro. 1978. Coccidioidomycosis. Part II. State of the art. Am. Rev. Respir. Dis. 117:727-770. [DOI] [PubMed] [Google Scholar]
- 100.Drutz, D. J., and M. Huppert. 1983. Coccidioidomycosis: factors affecting the host-parasite interaction. J. Infect. Dis. 147:372-390. [DOI] [PubMed] [Google Scholar]
- 101.Drutz, D. J., M. Huppert, S. H. Sun, and W. L. McGuire. 1981. Human sex hormones stimulate the growth and maturation of Coccidioides immitis. Infect. Immun. 32:897-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dugger, K. O., and J. N. Galgiani. 1989. Neutrophil killing of single microorganisms as measured by a new method. Diagn. Microbiol. Infect. Dis. 3:199-203. [DOI] [PubMed] [Google Scholar]
- 103.Dugger, K. O., J. N. Galgiani, N. M. Ampel, S. H. Sun, D. M. Magee, J. Harrison, and J. H. Law. 1991. An immunoreactive apoglycoprotein purified from Coccidioides immitis. Infect. Immun. 59:2245-2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dugger, K. O., K. M. Villareal, A. Ngyuen, C. R. Zimmermann, J. H. Law, and J. N. Galgiani. 1996. Cloning and sequence analysis of the cDNA for a protein from Coccidioides immitis with immunogenic potential. Biochem. Biophys. Res. Commun. 218:485-489. [DOI] [PubMed] [Google Scholar]
- 105.Durry, E., D. Pappagianis, S. B. Werner, L. Hutwagner, R. K. Sun, M. Maurer, M. M. McNeil, and R. W. Pinner. 1997. Coccidioidomycosis in Tulare County, California, 1991: reemergence of an endemic disease. J. Med. Vet. Mycol. 35:321-326. [DOI] [PubMed] [Google Scholar]
- 106.Echols, R. M., D. L. Palmer, and G. W. Long. 1982. Tissue eosinophilia in human coccidioidomycosis. Rev. Infect. Dis. 4:656-664. [DOI] [PubMed] [Google Scholar]
- 107.Edwards, A. D., S. P. Manickasingham, R. Sporri, S. S. Diebold, O. Schulz, A. Sher, T. Kaisho, S. Akira, and C. Reis e Sousa. 2002. Microbial recognition via Toll-like receptor-dependent and -independent pathways determines the cytokine response of murine dendritic cell subsets to CD40 triggering. J. Immunol. 169:3652-3660. [DOI] [PubMed] [Google Scholar]
- 108.Einstein, H. E., and R. H. Johnson. 1993. Coccidioidomycosis: new aspects of epidemiology and therapy. Clin. Infect. Dis. 16:349-354. [DOI] [PubMed] [Google Scholar]
- 109.Endres, R., M. B. Alimzhanov, T. Plitz, A. Futterer, M. H. Kosco-Vilbois, S. A. Nedospasov, K. Rajewsky, and K. Pfeffer. 1999. Mature follicular dendritic cell networks depend on expression of lymphotoxin beta receptor by radioresistant stromal cells and of lymphotoxin beta and tumor necrosis factor by B cells. J. Exp. Med. 189:159-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fierer, J., and T. Kirkland. 2002. Questioning CDC's “Select Agent” criteria. Science 295:43. [DOI] [PubMed] [Google Scholar]
- 111.Fierer, J., L. Walls, L. Eckmann, T. Yamamoto, and T. N. Kirkland. 1998. Importance of interleukin- 10 in genetic susceptibility of mice to Coccidioides immitis. Infect. Immun. 66:4397-4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fierer, J., L. Walls, and T. N. Kirkland. 2000. Genetic evidence for the role of the Lv locus in early susceptibility but not IL-10 synthesis in experimental coccidioidomycosis in C57BL mice. J. Infect. Dis. 181:681-685. [DOI] [PubMed] [Google Scholar]
- 113.Fierer, J., L. Walls, F. Wright, and T. N. Kirkland. 1999. Genes influencing resistance to Coccidioides immitis and the interleukin-10 response map to chromosomes 4 and 6 in mice. Infect. Immun. 67:2916-2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fiese, M. J. 1958. Coccidioidomycosis. Charles C Thomas, Springfield, Il.
- 115.Fish, D. G., N. M. Ampel, J. N. Galgiani, C. L. Dols, P. C. Kelly, C. H. Johnson, D. Pappagianis, J. E. Edwards, R. B. Wasserman, R. J. Clark, and et al. 1990. Coccidioidomycosis during human immunodeficiency virus infection. A review of 77 patients. Medicine (Baltimore) 69:384-391. [DOI] [PubMed] [Google Scholar]
- 116.Fisher, M. C., G. Koenig, T. J. White, and J. W. Taylor. 2002. Molecular and phenotypic description of Coccidioides posadasii sp.nov., previously recognized as the non-California population of Coccidoides immitis. Mycologia 94:73-84. [PubMed] [Google Scholar]
- 117.Fisher, M. C., G. Koenig, T. J. White, and J. W. Taylor. 2000. A test for concordance between the multilocus genealogies of genes and microsatellites in the pathogenic fungus Coccidioides immitis. Mol. Biol. Evol. 17:1164-1174. [DOI] [PubMed] [Google Scholar]
- 118.Fisher, M. C., G. L. Koenig, T. J. White, G. San-Blas, R. Negroni, I. G. Alvarez, B. Wanke, and J. W. Taylor. 2001. Biogeographic range expansion into South America by Coccidioides immitis mirrors New World patterns of human migration. Proc. Natl. Acad. Sci. USA 98:4558-4562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fisher, M. C., T. J. White, and J. W. Taylor. 1999. Primers for genotyping single nucleotide polymorphisms and microsatellites in the pathogenic fungus Coccidioides immitis. Mol. Ecol. 8:1082-1084. [DOI] [PubMed] [Google Scholar]
- 120.Flynn, J. L., M. M. Goldstein, J. Chan, K. J. Triebold, K. Pfeffer, C. J. Lowenstein, R. Schreiber, T. W. Mak, and B. R. Bloom. 1995. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 2:561-572. [DOI] [PubMed] [Google Scholar]
- 121.Foley, J. M., R. J. Berman, and C. E. Smith. 1960. X-ray irradiation of Coccidioides immitis arthrospores: survival curves and avirulent mutants isolated. J. Bacteriol. 79:480-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Forbus, W., and A. M. Bestebreurtje. 1946. Coccidioidomycosis: a study of 95 cases of the disseminated type with special reference to the pathogenesis of the disease. Mil. Surg. 5:653-719. [PubMed] [Google Scholar]
- 123.Frey, C. L., and D. J. Drutz. 1986. Influence of fungal surface components on the interaction of Coccidioides immitis with polymorphonuclear neutrophils. J. Infect. Dis. 153:933-943. [DOI] [PubMed] [Google Scholar]
- 124.Friedman, L., and C. E. Smith. 1956. Vaccination of mice against Coccidioides immitis. Am. Rev. Tuber. Pulm. Dis. 74:245-248. [DOI] [PubMed] [Google Scholar]
- 125.Galgiani, J. N. 1993. Coccidioidomycosis. West. J. Med. 159:153-171. [PMC free article] [PubMed] [Google Scholar]
- 126.Galgiani, J. N. 1986. Inhibition of different phases of Coccidioides immitis by human neutrophils or hydrogen peroxide. J. Infect. Dis. 153:217-222. [DOI] [PubMed] [Google Scholar]
- 127.Galgiani, J. N. 1985. Potential role of human polymorphonuclear leukocytes in the early host response to Coccidioides immitis, p. 181-190. In H. Einstein and A. Catanzaro (ed.), Coccidioidomycosis. Proceedings of the 4th International Conference. National Foundation for Infectious Diseases, Washington, D.C.
- 128.Galgiani, J. N., R. Hayden, and C. M. Payne. 1982. Leukocyte effects on the dimorphism of Coccidioides immitis. J. Infect. Dis. 146:56-63. [DOI] [PubMed] [Google Scholar]
- 129.Galgiani, J. N., R. A. Isenberg, and D. A. Stevens. 1978. Chemotaxigenic activity of extracts from the mycelial and spherule phases of Coccidioides immitis for human polymorphonuclear leukocytes. Infect. Immun. 21:862-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Galgiani, J. N., C. M. Payne, and J. F. Jones. 1984. Human polymorphonuclear-leukocyte inhibition of incorporation of chitin precursors into mycelia of Coccidioides immitis. J. Infect. Dis. 149:404-412. [DOI] [PubMed] [Google Scholar]
- 131.Galgiani, J. N., S. H. Sun, K. O. Dugger, N. M. Ampel, G. G. Grace, J. Harrison, and M. A. Wieden. 1992. An arthroconidial-spherule antigen of Coccidioides immitis: differential expression during in vitro fungal development and evidence for humoral response in humans after infection or vaccination. Infect. Immun. 60:2627-2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Garcia, I., Y. Miyazaki, G. Marchal, W. Lesslauer, and P. Vassalli. 1997. High sensitivity of transgenic mice expressing soluble TNFR1 fusion protein to mycobacterial infections: synergistic action of TNF and IFN-gamma in the differentiation of protective granulomas. Eur. J. Immunol. 27:3182-3190. [DOI] [PubMed] [Google Scholar]
- 133.Gifford, J., and A. Catanzaro. 1981. A comparison of coccidioidin and spherulin skin testing in the diagnosis of coccidioidomycosis. Am. Rev. Respir. Dis. 124:440-444. [DOI] [PubMed] [Google Scholar]
- 134.Gifford, M. A., W. C. Buss, and R. J. Douds. 1937. Data on Coccidioides fungus infection, Kern County, 1900-1936. Kern County Health Department Annual Report, p. 39-54. Kern County Health Department, Bakersfield, Calif.
- 135.Goldstein, D. M., and S. Louie. 1943. Primary pulmonary coccidioidomycosis. Report of an epidemic of seventy five cases. War Med. 4:299-317. [Google Scholar]
- 136.Harris, R. E. 1966. Coccidioidomycosis complicating pregnancy. Report of three cases and review of the literature. Obstet. Gynecol. 28:401-405. [PubMed] [Google Scholar]
- 137.Harrison, H. N. 1958. Fatal maternal coccidioidomycosis: a case report and review of sixteen cases from the literature. Am. J. Obstet. Gynecol. 75:813-820. [PubMed] [Google Scholar]
- 138.Hartmann, G., R. D. Weeratna, Z. K. Ballas, P. Payette, S. Blackwell, I. Suparto, W. L. Rasmussen, M. Waldschmidt, D. Sajuthi, R. H. Purcell, H. L. Davis, and A. M. Krieg. 2000. Delineation of a CpG phosphorothioate oligodeoxynucleotide for activating primate immune responses in vitro and in vivo. J. Immunol. 164:1617-1624. [DOI] [PubMed] [Google Scholar]
- 139.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408:740-745. [DOI] [PubMed] [Google Scholar]
- 140.Hicks, H. R., and W. T. Northey. 1967. Studies on the response of thymectomized mice to infection with Coccidioides immitis, p. 183-187. In L. Ajello (ed.), Proceedings of Second Coccidioidomycosis Symposium. University of Arizona Press, Tucson.
- 141.Hodge-Dufour, J., M. W. Marino, M. R. Horton, A. Jungbluth, M. D. Burdick, R. M. Strieter, P. W. Noble, C. A. Hunter, and E. Pure. 1998. Inhibition of interferon gamma induced interleukin 12 production: a potential mechanism for the anti-inflammatory activities of tumor necrosis factor. Proc. Natl. Acad. Sci. USA 95:13806-13811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hung, C. Y., N. M. Ampel, L. Christian, K. R. Seshan, and G. T. Cole. 2000. A major cell surface antigen of Coccidioides immitis which elicits both humoral and cellular immune responses. Infect. Immun. 68:584-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hung, C. Y., J. J. Yu, K. R. Seshan, U. Reichard, and G. T. Cole. 2002. A parasitic phase-specific adhesin of Coccidioides immitis contributes to the virulence of this respiratory fungal pathogen. Infect. Immun. 70:3443-3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Huppert, M. 1978. Racism in coccidioidomycosis? Am. Rev. Respir. Dis. 118:797-798. [DOI] [PubMed] [Google Scholar]
- 145.Huppert, M., J. P. Adler, E. H. Rice, and S. H. Sun. 1979. Common antigens among systemic disease fungi analyzed by two-dimensional immunoelectrophoresis. Infect. Immun. 23:479-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Huppert, M., H. B. Levine, S. H. Sun, and E. T. Peterson. 1967. Resistance of vaccinated mice to typical and atypical strains of Coccidioides immitis. J. Bacteriol. 94:924-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Huppert, M., N. S. Spratt, K. R. Vukovich, S. H. Sun, and E. H. Rice. 1978. Antigenic analysis of coccidioidin and spherulin determined by two- dimensional immunoelectrophoresis. Infect. Immun. 20:541-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Huppert, M., S. H. Sun, and J. L. Harrison. 1982. Morphogenesis throughout saprobic and parasitic cycles of Coccidioides immitis. Mycopathologia 78:107-122. [DOI] [PubMed] [Google Scholar]
- 149.Hyde, L. 1968. Coccidioidal pulmonary cavitation. Dis. Chest 54(Suppl. 1):273-277. [DOI] [PubMed] [Google Scholar]
- 150.Ivey, F. D., D. M. Magee, M. D. Woitaske, S. A. Johnston, and R. A. Cox. 2003. Identification of a protective antigen of Coccidioides immitis by expression library immunization. Vaccine 21:4359. [DOI] [PubMed] [Google Scholar]
- 151.Jiang, C., D. M. Magee, and R. A. Cox. 1999. Coadministration of interleukin 12 expression vector with antigen 2 cDNA enhances induction of protective immunity against Coccidioides immitis. Infect. Immun. 67:5848-5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jiang, C., D. M. Magee, and R. A. Cox. 1999. Construction of a single-chain interleukin-12-expressing retroviral vector and its application in cytokine gene therapy against experimental coccidioidomycosis. Infect. Immun. 67:2996-3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jiang, C., D. M. Magee, F. D. Ivey, and R. A. Cox. 2002. Role of signal sequence in vaccine-induced protection against experimental coccidioidomycosis. Infect. Immun. 70:3539-3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Jiang, C., D. M. Magee, T. N. Quitugua, and R. A. Cox. 1999. Genetic vaccination against Coccidioides immitis: comparison of vaccine efficacy of recombinant antigen 2 and antigen 2 cDNA. Infect. Immun. 67:630-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Johnson, A. G. 1994. Molecular adjuvants and immunomodulators: new approaches to immunization. Clin. Microbiol. Rev. 7:277-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Johnson, D. A., D. S. Keegan, C. G. Sowell, M. T. Livesay, C. L. Johnson, L. M. Taubner, A. Harris, K. R. Myers, J. D. Thompson, G. L. Gustafson, M. J. Rhodes, J. T. Ulrich, J. R. Ward, Y. M. Yorgensen, J. L. Cantrell, and V. G. Brookshire. 1999. 3-O-Desacyl monophosphoryl lipid A derivatives: synthesis and immunostimulant activities. J. Med. Chem. 42:4640-4649. [DOI] [PubMed] [Google Scholar]
- 157.Johnson, R. H., J. W. Caldwell, G. Welch, and H. E. Einstein. 1996. The great coccidioidomycosis epidemic: clinical features, p. 77-87. In H. Einstein and A. Catanzaro (ed.), Coccidioidomycosis. Proceedings of the 5th International Conference. National Foundation for Infectious Diseases, Washington, D.C.
- 158.Kashkin, K. P., S. M. Likholetov, and A. V. Lipnitsky. 1977. Studies on mediators of cellular immunity in experimental coccidioidomycosis. Sabouraudia 15:59-68. [DOI] [PubMed] [Google Scholar]
- 159.Kaufman, L., and P. Standard. 1978. Immuno-identification of cultures of fungi pathogenic to man. J. Clin. Microbiol. 1:135-140. [DOI] [PubMed] [Google Scholar]
- 160.Kenney, R. T., N. Regina Rabinovich, S. Pichyangkul, V. L. Price, and H. D. Engers. 2002. 2nd meeting on novel adjuvants currently in/close to human clinical testing. World Health Organization—Organization Mondiale de la Sante Fondation Merieux, Annecy, France, 5-7 June 2000. Vaccine 20:2155-2163. [DOI] [PubMed] [Google Scholar]
- 161.Kensil, C. R. 1996. Saponins as vaccine adjuvants. Crit. Rev. Ther. Drug Carrier Syst. 13:1-55. [PubMed] [Google Scholar]
- 162.Kindler, V., A. P. Sappino, G. E. Grau, P. F. Piguet, and P. Vassalli. 1989. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell 56:731-740. [DOI] [PubMed] [Google Scholar]
- 163.Kirkland, T., and G. T. Cole. 2002. Coccidioidomycosis: pathogenesis, immune response, and vaccine development, p. 365-400. In R. A. Calderone and R. L. Cihlar (ed.), Fungal pathogenesis: principles and clinical applications. Marcel Dekker, Inc., New York, N.Y.
- 164.Kirkland, T. N., and J. Fierer. 1985. Genetic control of resistance to Coccidioides immitis: a single gene that is expressed in spleen cells determines resistance. J. Immunol. 135:548-552. [PubMed] [Google Scholar]
- 165.Kirkland, T. N., and J. Fierer. 1986. Genetics of resistance to coccidioidomycosis, p. 169-171. In L. Leive (ed.), Microbiology—1986. American Society for Microbiology, Washington, D.C.
- 166.Kirkland, T. N., and J. Fierer. 1983. Inbred mouse strains differ in resistance to lethal Coccidioides immitis infection. Infect. Immun. 40:912-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kirkland, T. N., F. Finley, K. I. Orsborn, and J. N. Galgiani. 1998. Evaluation of the proline-rich antigen of Coccidioides immitis as a vaccine candidate in mice. Infect. Immun. 66:3519-3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kirkland, T. N., P. W. Thomas, F. Finley, and G. T. Cole. 1998. Immunogenicity of a 48-kilodalton recombinant T-cell-reactive protein of Coccidioides immitis. Infect. Immun. 66:424-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kirkland, T. N., S. W. Zhu, D. Kruse, L. L. Hsu, K. R. Seshan, and G. T. Cole. 1991. Coccidioides immitis fractions which are antigenic for immune T lymphocytes. Infect. Immun. 59:3952-3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kolivras, K. N., and A. C. Comrie. 2003. Modeling valley fever (coccidioidomycosis) incidence on the basis of climate conditions. Int. J. Biometeorol. 47:87-101. [DOI] [PubMed] [Google Scholar]
- 171.Kolivras, K. N., P. Johnson, A. C. Comrie, and S. R. Yool. 2001. Environmental variability and coccidioidomycosis (valley fever). Aerobiologia 17:31-42. [Google Scholar]
- 172.Kong, Y. M., H. B. Levine, and C. E. Smith. 1963. Immunogenic properties of nondisrupted and disrupted spherules of Coccidioides immitis in mice. Sabouraudia 2:131-142. [PubMed] [Google Scholar]
- 173.Kong, Y. M., and H. B. Levine. 1967. Experimentally induced immunity in the mycoses. Bacteriol. Rev. 31:35-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kong, Y. M., and H. B. Levine. 1967. Loss and recovery of virulence of arthrospores and spherule- endospores of Coccidioides immitis in live vaccines, p. 189-195. In L. Ajello (ed.), Proceedings of the Second Coccidioidomycosis Symposium. University of Arizona Press, Tucson.
- 175.Kong, Y. M., D. C. Savage, and H. B. Levine. 1965. Enhancement of immune responses in mice by a booster injection of Coccidioides spherules. J. Immunol. 95:1048-1056. [PubMed] [Google Scholar]
- 176.Koufopanou, V., A. Burt, T. Szaro, and J. W. Taylor. 2001. Gene genealogies, cryptic species, and molecular evolution in the human pathogen Coccidioides immitis and relatives (Ascomycota, Onygenales). Mol. Biol. Evol. 18:1246-1258. [DOI] [PubMed] [Google Scholar]
- 177.Koufopanou, V., A. Burt, and J. W. Taylor. 1997. Concordance of gene genealogies reveals reproductive isolation in the pathogenic fungus Coccidioides immitis. Proc. Natl. Acad. Sci. USA 94:5478-5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Krieg, A. M., A. K. Yi, J. Schorr, and H. L. Davis. 1998. The role of CpG dinucleotides in DNA vaccines. Trends Microbiol. 6:23-27. [DOI] [PubMed] [Google Scholar]
- 179.Lacy, G. H., and F. E. Swatek. 1977. Coccidioides in California, p. 107-114. In L. Ajello (ed.), Coccidioidomycosis: current clinical and diagnostic status. Symposia Specialists, Miami, Fla.
- 180.Lacy, G. H., and F. E. Swatek. 1974. Soil ecology of Coccidioides immitis at Amerindian middens in California. Appl. Microbiol. 27:379-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Le, J., and J. Vilcek. 1987. Tumor necrosis factor and interleukin 1: cytokines with multiple overlapping biological activities. Lab. Investig. 56:234-248. [PubMed] [Google Scholar]
- 182.Leake, J. A., D. G. Mosley, B. England, J. V. Graham, B. D. Plikaytis, N. M. Ampel, B. A. Perkins, and R. A. Hajjeh. 2000. Risk factors for acute symptomatic coccidioidomycosis among elderly persons in Arizona, 1996-1997. J. Infect. Dis. 181:1435-1440. [DOI] [PubMed] [Google Scholar]
- 183.Lecara, G., R. A. Cox, and R. B. Simpson. 1983. Coccidioides immitis vaccine: potential of an alkali-soluble, water-soluble cell wall antigen. Infect. Immun. 39:473-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Levine, H. B., J. M. Cobb, and G. M. Scalarone. 1969. Spherule coccidioidin in delayed dermal sensitivity reactions of experimental animals. Sabouraudia 7:20-32. [DOI] [PubMed] [Google Scholar]
- 185.Levine, H. B., J. M. Cobb, and C. E. Smith. 1960. Immunity to coccidioidomycosis induced in mice by purified spherule, arthrospore, and mycelial vaccines. Trans. N. Y. Acad. Sci. 22:436-449. [DOI] [PubMed] [Google Scholar]
- 186.Levine, H. B., J. M. Cobb, and C. E. Smith. 1961. Immunogenicity of spherule-endospore vaccines of Coccidioides immitis for mice. J. Immunol. 87:218-227. [PubMed] [Google Scholar]
- 187.Levine, H. B., Y. C. Kong, and C. Smith. 1965. Immunization of mice to Coccidioides immitis: dose, regimen and spherulation stage of killed spherule vaccines. J. Immunol. 94:132-142. [PubMed] [Google Scholar]
- 188.Levine, H. B., R. L. Miller, and C. E. Smith. 1962. Influence of vaccination on respiratory coccidioidal disease in cynomologous monkeys. J. Immunol. 89:242-251. [PubMed] [Google Scholar]
- 189.Li, K., J. J. Yu, C. Y. Hung, P. F. Lehmann, and G. T. Cole. 2001. Recombinant urease and urease DNA of Coccidioides immitis elicit an immunoprotective response against coccidioidomycosis in mice. Infect. Immun. 69:2878-2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Liu, Y. J. 2001. Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell 106:259-262. [DOI] [PubMed] [Google Scholar]
- 191.Louie, L., S. Ng, R. Hajjeh, R. Johnson, D. Vugia, S. B. Werner, R. Talbot, and W. Klitz. 1999. Influence of host genetics on the severity of coccidioidomycosis. Emerg. Infect. Dis. 5:672-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lubarsky, R., and O. A. Plunkett. 1955. Some ecologic studies of Coccidioides immitis in soil, p. 308-310. In T. H. Sternberg and V. D. Newcomer (ed.), Therapy of fungus diseases. An international symposium. Little, Brown, & Co., Boston, Mass.
- 193.MacLeod, R., S. Nagel, M. Kaufmann, K. Greulich-Bode, and H. Drexler. 2002. Multicolor-FISH analysis of a natural killer cell line (NK-92). Leukoc. Res. 26:1027-1033. [DOI] [PubMed] [Google Scholar]
- 194.Maddy, K. 1957. Ecological factors possibly relating to the geographic distribution of coccidioidomycosis. U.S. Public Health Service Publication 575. U.S. Public Health Service, Washington, D.C.
- 195.Magee, D. M., and R. A. Cox. 1996. Interleukin-12 regulation of host defenses against Coccidioides immitis. Infect. Immun. 64:3609-3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Magee, D. M., and R. A. Cox. 1995. Roles of gamma interferon and interleukin-4 in genetically determined resistance to Coccidioides immitis. Infect. Immun. 63:3514-3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.McNeil, M. M., and N. M. Ampel. 1995. Opportunistic coccidioidomycosis in patients infected with human immunodeficiency virus: prevention issues and priorities. Clin. Infect. Dis. 21(Suppl.1):S111-S113. [DOI] [PubMed] [Google Scholar]
- 198.Meager, A., H. Leung, and J. Woolley. 1989. Assays for tumour necrosis factor and related cytokines. J. Immunol. Methods 116:1-17. [DOI] [PubMed] [Google Scholar]
- 199.Meister, G. E., C. G. Roberts, J. A. Berzofsky, and A. S. De Groot. 1995. Two novel T cell epitope prediction algorithms based on MHC-binding motifs; comparison of predicted and published epitopes from Mycobacterium tuberculosis and HIV protein sequences. Vaccine 13:581-591. [DOI] [PubMed] [Google Scholar]
- 200.Mirbod, F., R. A. Schaller, and G. T. Cole. 2002. Purification and characterization of urease isolated from the pathogenic fungus Coccidioides immitis. Med. Mycol. 40:35-44. [DOI] [PubMed] [Google Scholar]
- 201.Moldoveanu, Z., L. Love-Homan, W. Q. Huang, and A. M. Krieg. 1998. CpG DNA, a novel immune enhancer for systemic and mucosal immunization with influenza virus. Vaccine 16:1216-1224. [DOI] [PubMed] [Google Scholar]
- 202.Moreira, A. L., L. Tsenova-Berkova, J. Wang, P. Laochumroonvorapong, S. Freeman, V. H. Freedman, and G. Kaplan. 1997. Effect of cytokine modulation by thalidomide on the granulomatous response in murine tuberculosis. Tubercle Lung. Dis. 78:47-55. [DOI] [PubMed] [Google Scholar]
- 203.Moretta, A., C. Bottino, M. C. Mingari, R. Biassoni, and L. Moretta. 2002. What is a natural killer cell? Nat. Immunol. 3:6-8. [DOI] [PubMed] [Google Scholar]
- 204.Mourant, A. E., A. C. Kopec, and D. S. Kazmiera. 1959. The ABO blood groups. Blackwell Scientific Publishing, Oxford, United Kingdom.
- 205.Mukherjee, J., M. D. Scharff, and A. Casadevall. 1992. Protective murine monoclonal antibodies to Cryptococcus neoformans. Infect. Immun. 60:4534-4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Naroeni, A., N. Jouy, S. Ouahrani-Bettache, J. P. Liautard, and F. Porte. 2001. Brucella suis-impaired specific recognition of phagosomes by lysosomes due to phagosomal membrane modifications. Infect. Immun. 69:486-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Olivere, J. W., P. A. Meier, S. L. Fraser, W. B. Morrison, T. W. Parsons, and D. M. Drehner. 1999. Coccidioidomycosis—the airborne assault continues: an unusual presentation with a review of the history, epidemiology, and military relevance. Aviat. Space. Environ. Med. 70:790-796. [PubMed] [Google Scholar]
- 208.Opelz, G., and M. I. Scheer. 1975. Cutaneous sensitivity and in vitro responsiveness of lymphocytes in patients with disseminated coccidioidomycosis. J. Infect. Dis. 132:250-255. [DOI] [PubMed] [Google Scholar]
- 209.Ophuls, W., and H. C. Moffitt. 1900. A new pathogenic mould (formerly described as a protozoon: Coccidioides immitis pyogenes) preliminary report. Philadelphia Med. J. 5:1471-1472. [Google Scholar]
- 210.Pan, S., and G. T. Cole. 1995. Molecular and biochemical characterization of a Coccidioides immitis-specific antigen. Infect. Immun. 63:3994-4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Pan, S., L. Sigler, and G. T. Cole. 1994. Evidence for a phylogenetic connection between Coccidioides immitis and Uncinocarpus reesii (Onygenaceae). Microbiology 140:1481-1494. [DOI] [PubMed] [Google Scholar]
- 212.Panackal, A. A., R. A. Hajjeh, M. S. Cetron, and D. W. Warnock. 2002. Fungal infections among returning travelers. Clin. Infect. Dis. 35:1088-1095. [DOI] [PubMed] [Google Scholar]
- 213.Pappagianis, D. 1999. Coccidioides immitis antigen. J. Infect. Dis. 180:243-244. [DOI] [PubMed] [Google Scholar]
- 214.Pappagianis, D. 1993. Coccidioidomycosis. Semin. Dermatol. 12:301-309. [PubMed] [Google Scholar]
- 215.Pappagianis, D. 1967. Epidemiological aspects of respiratory mycotic infections. Bacteriol. Rev. 31:25-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Pappagianis, D. 1988. Epidemiology of coccidioidomycosis. Curr. Top. Med. Mycol. 2:199-238. [DOI] [PubMed] [Google Scholar]
- 217.Pappagianis, D. 1980. Epidemiology of coccidioidomycosis, p. 63-85. In D. A. Stevens (ed.), Coccidioidomycosis: a Text. Plenum Publishing Corp., New York, N.Y.
- 218.Pappagianis, D, and The Valley Fever Vaccine Study Group. 1993. Evaluation of the protective efficacy of the killed Coccidioides immitis spherule vaccine in humans. Am. Rev. Respir. Dis. 148:656-660. [DOI] [PubMed] [Google Scholar]
- 219.Pappagianis, D. 1994. Marked increase in cases of coccidioidomycosis in California: 1991, 1992, and 1993. Clin. Infect. Dis. 19(Suppl. 1):S14-S18. [DOI] [PubMed] [Google Scholar]
- 220.Pappagianis, D. 2001. Seeking a vaccine against Coccidioides immitis and serologic studies: expectations and realities. Fungal Genet. Biol. 32:1-9. [DOI] [PubMed] [Google Scholar]
- 221.Pappagianis, D., and H. Einstein. 1978. Tempest from Tehachapi takes toll or Coccidioides conveyed aloft and afar. West. J. Med. 129:527-530. [PMC free article] [PubMed] [Google Scholar]
- 222.Pappagianis, D., R. Hector, H. B. Levine, and M. S. Collins. 1979. Immunization of mice against coccidioidomycosis with a subcellular vaccine. Infect. Immun. 25:440-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Pappagianis, D., and H. B. Levine. 1975. The present status of vaccination against coccidioidomycosis in man. Am. J. Epidemiol. 102:30-41. [DOI] [PubMed] [Google Scholar]
- 224.Pappagianis, D., H. B. Levine, C. E. Smith, R. J. Berman, and G. K. Kobayashi. 1961. Immunization of mice with viable Coccidioides immitis. J. Immunol. 86:28-34. [PubMed] [Google Scholar]
- 225.Pappagianis, D., S. Lindsay, S. Beall, and P. Williams. 1979. Ethnic background and the clinical course of coccidioidomycosis. Am. Rev. Respir. Dis. 120:959-961. [DOI] [PubMed] [Google Scholar]
- 226.Pappagianis, D., N. J. Lindsey, C. E. Smith, and M. T. Saito. 1965. Antibodies in human coccidioidomycosis: immunoelectrophoretic properties. Proc. Soc. Exp. Biol. Med. 118:118-122. [DOI] [PubMed] [Google Scholar]
- 227.Pappagianis, D., R. L. Miller, C. E. Smith, and G. S. Kobayashi. 1960. Response of monkeys to respiratory challenge following subcutaneous inoculation with Coccidioides immitis. Am. Rev. Respir. Dis. 82:244-250. [DOI] [PubMed] [Google Scholar]
- 228.Payne, R., K. K. Kidd, P. Rodvany, and H. H. Perkins. 1973. HL-A and other polymorphisms of the Filipino people, p. 203-208. In J. Dausett and J. Colombani (ed.), Histocompatibility testing. The Williams & Wilkins Co., Baltimore, Md.
- 229.Peng, T., K. I. Orsborn, M. J. Orbach, and J. N. Galgiani. 1999. Proline-rich vaccine candidate antigen of Coccidioides immitis: conservation among isolates and differential expression with spherule maturation. J. Infect. Dis. 179:518-521. [DOI] [PubMed] [Google Scholar]
- 230.Peng, T., L. Shubitz, J. Simons, R. Perrill, K. I. Orsborn, and J. N. Galgiani. 2002. Localization within a proline-rich antigen (Ag2/PRA) of protective antigenicity against Infection with Coccidioides immitis in mice. Infect. Immun. 70:3330-3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Petkus, A. F., and L. L. Baum. 1987. Natural killer cell inhibition of young spherules and endospores of Coccidioides immitis. J. Immunol. 139:3107-3111. [PubMed] [Google Scholar]
- 232.Pieters, J., and J. Gatfield. 2002. Hijacking the host: survival of pathogenic mycobacteria inside macrophages. Trends Microbiol. 10:142-146. [DOI] [PubMed] [Google Scholar]
- 233.Pirofski, L. A., and A. Casadevall. 1996. Cryptococcus neoformans: paradigm for the role of antibody immunity against fungi? Zentbl. Bakteriol. 284:475-495. [DOI] [PubMed] [Google Scholar]
- 234.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. Van Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 235.Posadas, A. 1892. Un nuevo caso de micosis fungoidea con psorospermias. An. Circ. Med. Argent. 15:585-597. [Google Scholar]
- 236.Powell, B. L., and D. J. Drutz. 1984. Identification of a high-affinity binder for estradiol and a low- affinity binder for testosterone in Coccidioides immitis. Infect. Immun. 45:784-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Powell, B. L., D. J. Drutz, M. Huppert, and S. H. Sun. 1983. Relationship of progesterone- and estradiol-binding proteins in Coccidioides immitis to coccidioidal dissemination in pregnancy. Infect. Immun. 40:478-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Purtilo, D. T. 1975. Opportunistic mycotic infections in pregnant women. Am. J. Obstet. Gynecol. 122:607-610. [DOI] [PubMed] [Google Scholar]
- 239.Rea, T. H., H. Einstein, R. Johnson, and N. E. Levan. 1977. Further study of dinitrochlorobenzene responsivity in disseminated coccidioidomycosis, p. 365-370. In L. Ajello (ed.), Coccidioidomycosis: current clinical and diagnostic status. Symposia Specialists, Miami, Fla.
- 240.Rea, T. H., H. Einstein, and N. E. Levan. 1976. Dinitrochlorobenzene responsivity in disseminated coccidioidomycosis: an inverse correlation with complement-fixing antibody titers. J. Investig. Dermatol. 66:34-37. [DOI] [PubMed] [Google Scholar]
- 241.Reis e Sousa, C. 2001. Dendritic cells as sensors of infection. Immunity 14:495-498. [DOI] [PubMed] [Google Scholar]
- 242.Requena, L., and C. Requena. 2002. Erythema nodosum. Dermatol. Online J. 8:1-32. [PubMed] [Google Scholar]
- 243.Richards, J. O., N. M. Ampel, J. N. Galgiani, and D. F. Lake. 2001. Dendritic cells pulsed with Coccidioides immitis lysate induce antigen-specific naive T cell activation. J. Infect. Dis. 184:1220-1224. [DOI] [PubMed] [Google Scholar]
- 244.Richards, J. O., N. M. Ampel, and D. F. Lake. 2002. Reversal of coccidioidal anergy in vitro by dendritic cells from patients with disseminated coccidioidomycosis. J. Immunol. 169:2020-2025. [DOI] [PubMed] [Google Scholar]
- 245.Rixford, E., and T. C. Gilchrist. 1896. Two cases of protozoan (coccidioidal) infection of the skin and other organs. Johns Hopkins Hosp. Rev. 1:209-268. [Google Scholar]
- 246.Rook, G. A., J. Taverne, C. Leveton, and J. Steele. 1987. The role of gamma-interferon, vitamin D3 metabolites and tumour necrosis factor in the pathogenesis of tuberculosis. Immunology 62:229-234. [PMC free article] [PubMed] [Google Scholar]
- 247.Rosas, J. E., J. L. Pedraz, R. M. Hernandez, A. R. Gascon, M. Igartua, F. Guzman, R. Rodriguez, J. Cortes, and M. E. Patarroyo. 2002. Remarkably high antibody levels and protection against P. falciparum malaria in Aotus monkeys after a single immunisation of SPf66 encapsulated in PLGA microspheres. Vaccine 20:1707-1710. [DOI] [PubMed] [Google Scholar]
- 248.Rosenstein, N. E., K. W. Emery, S. B. Werner, A. Kao, R. Johnson, D. Rogers, D. Vugia, A. Reingold, R. Talbot, B. D. Plikaytis, B. A. Perkins, and R. A. Hajjeh. 2001. Risk factors for severe pulmonary and disseminated coccidioidomycosis: Kern County, California, 1995-1996. Clin. Infect. Dis. 32:708-715. [DOI] [PubMed] [Google Scholar]
- 249.Russell, D. 1995. Mycobacterium and Leishmania: stowaways in the endosomal network. Trends Cell Biol. 5:125-128. [DOI] [PubMed] [Google Scholar]
- 250.Sachidanandam, R., D. Weissman, S. C. Schmidt, J. M. Kakol, L. D. Stein, G. Marth, S. Sherry, J. C. Mullikin, B. J. Mortimore, D. L. Willey, S. E. Hunt, C. G. Cole, P. C. Coggill, C. M. Rice, Z. Ning, J. Rogers, D. R. Bentley, P. Y. Kwok, E. R. Mardis, R. T. Yeh, B. Schultz, L. Cook, R. Davenport, M. Dante, L. Fulton, L. Hillier, R. H. Waterston, J. D. McPherson, B. Gilman, S. Schaffner, W. J. Van Etten, D. Reich, J. Higgins, M. J. Daly, B. Blumenstiel, J. Baldwin, N. Stange-Thomann, M. C. Zody, L. Linton, E. S. Lander, and D. Altshuler. 2001. A map of human genome sequence variation containing 1.42 million single nucleotide polymorphisms. Nature 409:928-933. [DOI] [PubMed] [Google Scholar]
- 251.Saeki, H., M. T. Wu, E. Olasz, and S. T. Hwang. 2000. A migratory population of skin-derived dendritic cells expresses CXCR5, responds to B lymphocyte chemoattractant in vitro, and co-localizes to B cell zones in lymph nodes in vivo. Eur. J. Immunol. 30:2808-2814. [DOI] [PubMed] [Google Scholar]
- 252.Salkin, D. 1967. Clinical examples of reinfection in coccidioidomycosis, p. 11-18. In L. Ajello (ed.), Proceedings of the Second Coccidioidomycosis Symposium. University of Arizona Press, Tucson.
- 253.Sallusto, F., C. R. Mackay, and A. Lanzavecchia. 2000. The role of chemokine receptors in primary, effector, and memory immune responses. Annu. Rev. Immunol. 18:593-620. [DOI] [PubMed] [Google Scholar]
- 254.Sarosi, G. A., J. D. Parker, I. L. Doto, and F. E. Tosh. 1970. Chronic pulmonary coccidioidomycosis. N. Engl. J. Med. 283:325-329. [DOI] [PubMed] [Google Scholar]
- 255.Saunders, N. J., and E. R. Moxon. 1998. Implications of sequencing bacterial genomes for pathogenesis and vaccine development. Curr. Opin. Biotechnol. 9:618-623. [DOI] [PubMed] [Google Scholar]
- 256.Savage, D. C., and S. H. Madin. 1968. Cellular responses in lungs of immunized mice to intranasal infection with Coccidioides immitis. Sabouraudia 6:94-102. [DOI] [PubMed] [Google Scholar]
- 257.Schafer, J. R., B. M. Jesdale, J. A. George, N. M. Kouttab, and A. S. De Groot. 1998. Prediction of well-conserved HIV-1 ligands using a matrix-based algorithm, EpiMatrix. Vaccine 16:1880-1884. [DOI] [PubMed] [Google Scholar]
- 258.Reference deleted.
- 259.Schermoly, M. J., and D. R. Hinthorn. 1988. Eosinophilia in coccidioidomycosis. Arch. Intern. Med. 148:895-896. [PubMed] [Google Scholar]
- 260.Schneider, E., R. A. Hajjeh, R. A. Spiegel, R. W. Jibson, E. L. Harp, G. A. Marshall, R. A. Gunn, M. M. McNeil, R. W. Pinner, R. C. Baron, R. C. Burger, L. C. Hutwagner, C. Crump, L. Kaufman, S. E. Reef, G. M. Feldman, D. Pappagianis, and S. B. Werner. 1997. A coccidioidomycosis outbreak following the Northridge, Calif, earthquake. JAMA 277:904-908. [PubMed] [Google Scholar]
- 261.Seder, R. A., and S. Gurunathan. 1999. DNA vaccines—designer vaccines for the 21st century. N. Engl. J. Med. 341:277-278. [DOI] [PubMed] [Google Scholar]
- 262.Segal, G. P., and A. Catanzaro. 1985. Genetic regulation of antibody responses to Coccidioides immitis: the role of antibody in mediated immunity, p. 191-200. In H. Einstein and A. Catanzaro (ed.), Coccidioidomycosis. Proceedings of the 4th International Conference. National Foundation for Infectious Disease, Washington, D.C.
- 263.Segal, G. P., R. I. Lehrer, and M. E. Selsted. 1985. In vitro effect of phagocyte cationic peptides on Coccidioides immitis. J. Infect. Dis. 151:890-894. [DOI] [PubMed] [Google Scholar]
- 264.Shubitz, L., T. Peng, R. Perrill, J. Simons, K. Orsborn, and J. N. Galgiani. 2002. Protection of mice against Coccidioides immitis intranasal infection by vaccination with recombinant antigen 2/PRA. Infect. Immun. 70:3287-3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Sievers, M. L. 1979. Coccidioidomycosis and race. Am. Rev. Respir. Dis. 119:839. [DOI] [PubMed] [Google Scholar]
- 266.Sievers, M. L. 1974. Disseminated coccidioidomycosis among southwestern American Indians. Am. Rev. Respir. Dis. 109:602-612. [DOI] [PubMed] [Google Scholar]
- 267.Sievers, M. L. 1977. Prognostic factors in disseminated coccidioidomycosis among southwestern American Indians, p. 63-78. In L. Ajello (ed.), Coccidioidomycosis: current clinical and diagnostic status. Symposia Specialists, Miami, Fla.
- 268.Sievers, M. L. 1980. Racial susceptibility to coccidioidomycosis. N. Engl. J. Med. 302:58-59. [DOI] [PubMed] [Google Scholar]
- 269.Sigler, L., and J. W. Carmichael. 1976. Taxonomy of Malbranchea and some other hyphomycetes with arthroconidia. Mycotaxon 4:349-488. [Google Scholar]
- 270.Simms, P. E., and T. M. Ellis. 1996. Utility of flow cytometric detection of CD69 expression as a rapid method for determining poly- and oligoclonal lymphocyte activation. Clin. Diagn. Lab. Immunol. 3:301-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Singh, M., M. Briones, G. Ott, and D. O'Hagan. 2000. Cationic microparticles: a potent delivery system for DNA vaccines. Proc. Natl. Acad. Sci. USA 97:811-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Singh, M., and D. T. O'Hagan. 2002. Recent advances in vaccine adjuvants. Pharm. Res. 19:715-728. [DOI] [PubMed] [Google Scholar]
- 273.Slagle, D. C., R. A. Cox, and U. Kuruganti. 1989. Induction of tumor necrosis factor alpha by spherules of Coccidioides immitis. Infect. Immun. 57:1916-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Smith, C. E. 1951. Annual progress report. Commission on Acute Respiratory Diseases. Armed Forces Epidemiological Board, Washington, D.C.
- 275.Smith, C. E. 1940. Epidemiology of acute coccidioidomycosis with erythema nodosum (“San Joaquin” or “Valley Fever”). Am. J. Public Health 30:600-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Smith, C. E. 1948. The use of coccidioidin. Am. Rev. Tuberc. 57:330-360. [DOI] [PubMed] [Google Scholar]
- 277.Smith, C. E., R. R. Bard, E. G. Whiting, and H. G. Rosenberger. 1946. Varieties of coccidioidal infection in relation to the epidemiology and control of the diseases. Am. J. Public Health 36:1394-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Smith, C. E., R. R. Bard, H. G. Rosenberger, and E. G. Whiting. 1946. Effect of season and dust control on coccidioidomycosis. JAMA 132:833-838. [DOI] [PubMed] [Google Scholar]
- 279.Smith, C. E., D. Pappagianis, H. B. Levine, and M. I. Saito. 1961. Human coccidioidomycosis. Bacteriol. Rev. 25:310-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Smith, C. E., M. T. Saito, R. R. Beard, R. M. Kepp, R. W. Clark, and B. U. Eddie. 1950. Serological tests in the diagnosis and prognosis of coccidioidomycosis. Am. J. Hyg. 52:1-20. [DOI] [PubMed] [Google Scholar]
- 281.Smith, C. E., M. T. Saito, and S. A. Simons. 1956. Pattern of 39,500 serologic tests in coccidioidomycosis. JAMA 160:546-552. [DOI] [PubMed] [Google Scholar]
- 282.Standaert, S. M., W. Schaffner, J. N. Galgiani, R. W. Pinner, L. Kaufman, E. Durry, and R. H. Hutcheson. 1995. Coccidioidomycosis among visitors to a Coccidioides immitis-endemic area: an outbreak in a military reserve unit. J. Infect. Dis. 171:1672-1675. [DOI] [PubMed] [Google Scholar]
- 283.Sternberg, S. 1994. The emerging fungal threat. Science 266:1632-1634. [DOI] [PubMed] [Google Scholar]
- 284.Strasser, J. E., S. L. Newman, G. M. Ciraolo, R. E. Morris, M. L. Howell, and G. E. Dean. 1999. Regulation of the macrophage vacuolar ATPase and phagosome-lysosome fusion by Histoplasma capsulatum. J. Immunol. 162:6148-6154. [PubMed] [Google Scholar]
- 285.Strieter, R. M., D. G. Remick, J. P. Lynch, 3rd, M. Genord, C. Raiford, R. Spengler, and S. L. Kunkel. 1989. Differential regulation of tumor necrosis factor-alpha in human alveolar macrophages and peripheral blood monocytes: a cellular and molecular analysis. Am. J. Respir. Cell Mol. Biol. 1:57-63. [DOI] [PubMed] [Google Scholar]
- 286.Sun, S. H., and M. Huppert. 1976. A cytological study of morphogenesis in Coccidioides immitis. Sabouraudia 14:185-198. [PubMed] [Google Scholar]
- 287.Sykes, K. F., and S. A. Johnston. 1999. Linear expression elements: a rapid, in vivo, method to screen for gene functions. Nat. Biotechnol. 17:355-359. [DOI] [PubMed] [Google Scholar]
- 288.Sykes, K. F., M. G. Lewis, B. Squires, and S. A. Johnston. 2002. Evaluation of SIV library vaccines with genetic cytokines in a macaque challenge. Vaccine 20:2382-2395. [DOI] [PubMed] [Google Scholar]
- 289.Tam, Y. K., B. Miyagawa, V. C. Ho, and H. G. Klingemann. 1999. Immunotherapy of malignant melanoma in a SCID mouse model using the highly cytotoxic natural killer cell line NK-92. J. Hematother. 8:281-290. [DOI] [PubMed] [Google Scholar]
- 290.Thomas, P. W., E. E. Wyckoff, E. J. Pishko, J. J. Yu, T. N. Kirkland, and G. T. Cole. 1997. The hsp60 gene of the human pathogenic fungus Coccidioides immitis encodes a T-cell reactive protein. Gene 199:83-91. [DOI] [PubMed] [Google Scholar]
- 291.VanBergen, W. S., F. J. Fleury, and E. L. Cheatle. 1976. Fatal maternal disseminated coccidioidomycosis in a nonendemic area. Am. J. Obstet. Gynecol. 124:661-663. [DOI] [PubMed] [Google Scholar]
- 292.Vartivarian, S. E., P. E. Coudron, and S. M. Markowitz. 1987. Disseminated coccidioidomycosis. Unusual manifestations in a cardiac transplantation patient. Am. J. Med. 83:949-952. [DOI] [PubMed] [Google Scholar]
- 293.Wack, E. E., N. M. Ampel, J. N. Galgiani, and D. A. Bronnimann. 1988. Coccidioidomycosis during pregnancy. An analysis of ten cases among 47,120 pregnancies. Chest 94:376-379. [DOI] [PubMed] [Google Scholar]
- 294.Walch, H. A., and A. Kalvoda. 1971. Immunization of mice with induced mutants of Coccidioides immitis. I. Characterization of mutants and preliminary studies of their use as viable vaccines. Sabouraudia 9:173-184. [PubMed] [Google Scholar]
- 295.Wang, C. H., H. C. Lin, C. Y. Liu, K. H. Huang, T. T. Huang, C. T. Yu, and H. P. Kuo. 2001. Upregulation of inducible nitric oxide synthase and cytokine secretion in peripheral blood monocytes from pulmonary tuberculosis patients. Int. J. Tuberc. Lung. Dis. 5:283-291. [PubMed] [Google Scholar]
- 296.Wang, C. H., C. Y. Liu, H. C. Lin, C. T. Yu, K. F. Chung, and H. P. Kuo. 1998. Increased exhaled nitric oxide in active pulmonary tuberculosis due to inducible NO synthase upregulation in alveolar macrophages. Eur. Respir. J. 11:809-815. [DOI] [PubMed] [Google Scholar]
- 297.Wanke, B., M. Lazera, P. C. Monteiro, F. C. Lima, M. J. Leal, P. L. Ferreira Filho, L. Kaufman, R. W. Pinner, and L. Ajello. 1999. Investigation of an outbreak of endemic coccidioidomycosis in Brazil's northeastern state of Piaui with a review of the occurrence and distribution of Coccidioides immitis in three other Brazilian states. Mycopathologia 148:57-67. [DOI] [PubMed] [Google Scholar]
- 298.Ward, E. R., Jr., R. A. Cox, J. A. Schmitt, Jr., M. Huppert, and S. H. Sun. 1975. Delayed-type hypersensitivity responses to a cell wall fraction of the mycelial phase of Coccidioides immitis. Infect. Immun. 12:1093-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Ware, C. F., P. D. Crowe, M. H. Grayson, M. J. Androlewicz, and J. L. Browning. 1992. Expression of surface lymphotoxin and tumor necrosis factor on activated T, B, and natural killer cells. J. Immunol. 149:3881-3888. [PubMed] [Google Scholar]
- 300.Warnock, D. W., B. Dupont, C. A. Kauffman, and T. Sirisanthana. 1998. Imported mycoses in Europe. Med. Mycol. 36(Suppl. 1):87-94. [PubMed] [Google Scholar]
- 301.Wegner, T. N., R. E. Reed, R. J. Trautman, and C. D. Beavers. 1972. Some evidence for the development of a phagocytic response by polymorphonuclear leukocytes recovered from the venous blood of dogs inoculated with Coccidioides immitis or vaccinated with an irradiated spherule vaccine. Am. Rev. Respir. Dis. 105:845-849. [DOI] [PubMed] [Google Scholar]
- 302.Werner, S. B., and D. Pappagianis. 1973. Coccidioidomycosis in Northern California. An outbreak among archeology students near Red Bluff. Calif. Med. 119:16-20. [PMC free article] [PubMed] [Google Scholar]
- 303.Wernicke, R. 1892. Ueber einen Protozoenbefund bei Mycosis fungoides. Zentbl. Bakteriol. 12:859-861. [Google Scholar]
- 304.Wiant, J. R., and J. W. Smith. 1973. Coccidioidin skin reactivity in pulmonary coccidioidomycosis. Chest 63:100-102. [DOI] [PubMed] [Google Scholar]
- 305.Wiater, L. A., K. Dunn, F. R. Maxfield, and H. A. Shuman. 1998. Early events in phagosome establishment are required for intracellular survival of Legionella pneumophila. Infect. Immun. 66:4450-4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Willett, F. M., and A. Weiss. 1945. Coccidioidomycosis in southern California: report of a new endemic area with a review of 100 cases. Ann. Intern. Med. 23:349-375. [Google Scholar]
- 307.Williams, F. M., V. Markides, J. Edgeworth, and A. J. Williams. 1998. Reactivation of coccidioidomycosis in a fit American visitor. Thorax 53:811-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Williams, P. L., D. L. Sable, P. Mendez, and L. T. Smyth. 1979. Symptomatic coccidioidomycosis following a severe natural dust storm. An outbreak at the Naval Air Station, Lemoore, Calif. Chest 76:566-670. [DOI] [PubMed] [Google Scholar]
- 309.Williams, P. L., D. L. Sable, S. P. Sorgen, D. Pappagianis, H. B. Levine, S. K. Brodine, B. W. Brown, F. C. Grumet, and D. A. Stevens. 1984. Immunologic responsiveness and safety associated with the Coccidioides immitis spherule vaccine in volunteers of white, black, and Filipino ancestry. Am. J. Epidemiol. 119:591-602. [DOI] [PubMed] [Google Scholar]
- 310.Wilson, J. W., C. E. Smith, and O. A. Plunkett. 1953. Primary cutaneous coccidioidomycosis: criteria for diagnosis and report of a case. Calif. Med. 79:233-239. [PMC free article] [PubMed] [Google Scholar]
- 311.Winn, W. A. 1968. A long term study of 300 patients with cavitary-abscess lesions of the lung of coccidioidal origin. An analytical study with special reference to treatment. Dis. Chest. 54(Suppl. 1):268. [DOI] [PubMed] [Google Scholar]
- 312.Winn, W. A. 1965. Primary cutaneous coccidioidomycosis- reevaluation of its potentiality based on study of three new cases. Arch. Dermatol. 92:215-220. [DOI] [PubMed] [Google Scholar]
- 313.Winn, W. A. 1967. A working classification of coccidioidomycosis and its application to therapy, p. 3-9. In L. Ajello (ed.), Proceedings of the Second Coccidioidomycosis Symposium. University of Arizona Press, Tucson.
- 314.Winn, W. A., S. M. Finegold, and R. W. Huntington. 1967. Coccidioidomycosis with fungemia, p. 93-109. In L. Ajello (ed.), Proceedings of the Second Coccidioidomycosis Symposium. University of Arizona Press, Tucson.
- 315.Woods, C. W., C. McRill, B. D. Plikaytis, N. E. Rosenstein, D. Mosley, D. Boyd, B. England, B. A. Perkins, N. M. Ampel, and R. A. Hajjeh. 2000. Coccidioidomycosis in human immunodeficiency virus-infected persons in Arizona, 1994-1997: incidence, risk factors, and prevention. J. Infect. Dis. 181:1428-1434. [DOI] [PubMed] [Google Scholar]
- 316.Wyckoff, E. E., E. J. Pishko, T. N. Kirkland, and G. T. Cole. 1995. Cloning and expression of a gene encoding a T-cell reactive protein from Coccidioides immitis: homology to 4-hydroxyphenylpyruvate dioxygenase and the mammalian F antigen. Gene 161:107-111. [DOI] [PubMed] [Google Scholar]
- 317.Yoshinoya, S., R. A. Cox, and R. M. Pope. 1980. Circulating immune complexes in coccidioidomycosis. Detection and characterization. J. Clin. Investig. 66:655-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Reference deleted.
- 319.Yu, J. J., S. L. Smithson, P. W. Thomas, T. N. Kirkland, and G. T. Cole. 1997. Isolation and characterization of the urease gene (URE) from the pathogenic fungus Coccidioides immitis. Gene 198:387-391. [DOI] [PubMed] [Google Scholar]
- 320.Zhou, L. J., and T. F. Tedder. 1995. A distinct pattern of cytokine gene expression by human CD83+ blood dendritic cells. Blood 86:3295-3301. [PubMed] [Google Scholar]
- 321.Zhu, Y., V. Tryon, D. M. Magee, and R. A. Cox. 1997. Identification of a Coccidioides immitis antigen 2 domain that expresses B-cell-reactive epitopes. Infect. Immun. 65:3376-3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Reference deleted.
- 323.Zhu, Y., C. Yang, D. M. Magee, and R. A. Cox. 1996. Coccidioides immitis antigen 2: analysis of gene and protein. Gene 181:121-125. [DOI] [PubMed] [Google Scholar]
- 324.Zhu, Y., C. Yang, D. M. Magee, and R. A. Cox. 1996. Molecular cloning and characterization of Coccidioides immitis antigen 2 cDNA. Infect. Immun. 64:2695-2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Zimmermann, C. R., S. M. Johnson, G. W. Martens, A. G. White, B. L. Zimmer, and D. Pappagianis. 1998. Protection against lethal murine coccidioidomycosis by a soluble vaccine from spherules. Infect. Immun. 66:2342-2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Zimmermann, C. R., C. J. Snedker, and D. Pappagianis. 1994. Characterization of Coccidioides immitis isolates by restriction fragment length polymorphisms. J. Clin. Microbiol. 32:3040-3042. [DOI] [PMC free article] [PubMed] [Google Scholar]